- 1The Skaraborg Institute for Research and Development, Skövde, Sweden
- 2Department of Epidemiology and Global Health, Umeå University, Umeå, Sweden
- 3Department of Clinical Laboratory, Kilimanjaro Christian Medical Centre, Moshi, Tanzania
- 4Department of Radiology, Kilimanjaro Christian Medical Centre, Moshi, Tanzania
- 5Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden
- 6Institute of Microbiology, Infectious Diseases and Immunology, Charité University Medicine, Berlin, Germany
Objectives: The presence of schistosomal eggs in the urine is a sufficient but not necessary condition for an individual to be diagnosed with urogenital schistosomiasis. The absence of eggs does not prove that a person is disease-free. Thus, when examining populations using egg occurrence, there is a real risk of underestimating the prevalence. The aim is to develop an easy to use model for improved prevalence estimates of urogenital schistosomiasis.
Design and methods: Urine samples were taken from 161 schoolchildren and 124 adults on three different days for each individual. The probands were recruited from two areas in northern Tanzania with varying prevalence of urogenital schistosomiasis. The presence of eggs by microscopy and haematuria by dipstick were recorded for each sample and the measurements combined using the discordance of the outcomes.
Result: As a consequence of applying the developed model, a substantial increase in the prevalence estimate was noted for groups displaying a low egg occurrence.
Conclusion: By using the biological relationship that exists between the presence of eggs and blood in urine of an infected individual, we provide a way of adjusting the prevalence estimates of urogenital schistosomiasis, using the observed prevalence of haematuria, in the absence of competing causes.
Introduction
Schistosomiasis is classified as a neglected tropical disease (NTD) and it afflicts some of the world’s poorest people (1). The World Health Organization (WHO) has endorsed a strategy for the prevention and control of the morbidity of schistosomiasis (2). The causal parasite – a dioecious trematode – can infest an individual in contact with contaminated freshwater, where snails act as intermediate hosts. In its second larval stage, the trematode penetrates intact skin and enters the human body. The larvae migrate through several layers of tissue and undergo stages of development. As adults, the worms form couples which begin to produce eggs while residing in the veins at various locations in the abdominal cavity. These locations seem to be specific for each schistosome species. The three main schistosoma species infecting humans are Schistosoma haematobium, S. japonicum, and S. Mansoni (3).
In the case of S. haematobium, the eggs penetrate the mucosal layers of the urogenital tract to end up in the urine with potential to contaminate water sources and continue the parasite’s lifecycle (4). Eggs are central in the pathogenesis and diagnosis. If left untreated, a worm-pair is said to persist at an average of two to five years, but can probably live longer (5). Parasites do not multiply in one and the same host. Hence the infection status will be the effect of an accumulation of consecutive infestations. According to estimates, urogenital schistosomiasis (UGS) affects more than 100 million people in sub-Saharan Africa (6).
In 1986 the US researchers Smith & Christie made a simplified classification of schistosomiasis haematobium in an active and an inactive phase on its pathobiology. The active phase is characterized by living adult worm pairs, oviposition and a granulomatous host response (4). The active phase is epidemiologically important because of its role in transmission of the parasite. Inactive schistosomiasis occurs after the adult worms have died and is characterised by absence of viable eggs in the tissues and urine of the host. Calcified eggs, however, remain in the tissues and will constitute possible niduses for the development of sequelae. In an endemic context, these phases will overlap and are dependent on e.g. frequency of exposure, host immunology and treatment response (7). Schistosomiasis should be considered a chronic inflammatory disease in endemic settings (8).
Haematuria is a sign of a mucosal impact brought on by the egg-shedding process (4, 9). This is an inefficient process, however, since very many eggs produced never leave the human body, but remain trapped in host tissues with a potential for causing serious sequelae in the urogenital system (8, 10, 11).
Urogenital schistosomiasis is commonly diagnosed by the detection of eggs in a light microscope after filtration of urine. Specific antigens detected in blood or urine samples are also indications of infection, as well as directly visible blood in the urine or blood detectable with reagent strips (12, 13). Macroscopic haematuria is considered a good indicator of UGS in areas with highly snail-infested freshwaters (14). Haematuria detected by urine dipsticks has been shown to be very common together with UGS, but with varying levels of sensitivity and specificity when the gold standard is based on egg detection in urine (15–17).
To be diagnosed with UGS, the occurrence of eggs in the urine is a sufficient but not necessary condition. The absence of eggs does not prove that a person is disease-free. Oviposition does not occur until six to eight weeks after cercariae penetrate the skin, after which egg production varies in intensity depending on, among other things, host factors and number of worm-pairs (18). Consequently, a one-to-one relationship does not exist between the presence of eggs in the urine and the diagnosis of UGS. Tests for antigens reflecting the presence of worm-pairs in the human body are available. However, no commercially low-cost equipment or procedure enabling a reliable diagnosis of the disease status under field conditions is at hand (13, 16).
Praziquantel is the standard drug for treating UGS and has the strongest evidence base so far (19). This is also the standard treatment recommended by WHO for mass treatment campaigns and thus the main and so far the most effective treatment for the control and elimination of schistosomiasis (20). Mass treatment with praziquantel consists of a single oral dose aiming at 40 mg/kg. It is in fact mostly decided according to body length (21, 22).
As of 2022 WHO has adopted a comprehensive guideline with the overall aim “to provide evidence-based recommendations to countries in their efforts to accomplish schistosomiasis morbidity control and elimination as a public health problem, and to move towards interruption of transmission.” In this guideline the prevalence threshold of schistosomiasis infection for annual preventive chemotherapy is set at 10 percent (12). These recommendations comprise “all age groups from 2 years old, including adults, pregnant women after the first trimester and lactating women.”
The WHO also calls for the development of diagnostic tests, including standardized point-of-care diagnostic, and develop new interventions, including alternatives to praziquantel and methods of snail control (1).
Irrespective of purpose - control or elimination - the methods for estimating the prevalence of the disease must not underestimate the prevailing prevalence. The risk of underestimation is real when population groups are examined, since currently available field methods certainly do not have 100 percent sensitivity.
Aim
Our aim is to explore a way of obtaining improved estimates of the prevalence of urogenital schistosomiasis to facilitate decisions regarding if, how and when mass treatment is optimal for its control and elimination.
The specific objective is to compare the occurrence of haematuria and S. haematobium eggs in urine of schoolchildren and adults from two field surveys and two areas with varying prevalence in northern Tanzania.
Subjects and methods
Study site
Kileo and Kivulini are villages in the Kilimanjaro region, Tanzania with populations of 5 300 and 2 500, respectively (23). S. haematobium is prevalent in both villages, where the main cash crop is rice farmed in a traditional irrigation system. Around half of the households own plots of land in the irrigated fields. People from Kileo tend to hire seasonal workers to cultivate their fields, while people from Kivulini work in rice fields both as owners and hired hands (24) (own observations 2008-2016). The irrigation canals are used for washing and swimming. Most households have a temporary or permanent pit latrine. A governmental dispensary is situated in Kileo and there are two primary schools in each village with 1 015 children altogether (local school registry, 2013).
The SPES project
The project Sustainable Prevention of Endemic Schistosomiasis (SPES) has been active with surveys and prevention of schistosomiasis in schoolchildren in the above mentioned villages Kileo and Kivulini since 1996 (24–26). In the 2013 survey a scheme was introduced, where a midday urine sample was taken on three consecutive days from each participant. The goal was to improve the accuracy of the diagnosis and to reduce the suspected underestimation of UGS prevalence (27). All schoolchildren had been exposed to praziquantel via a mass treatment campaign in 2008 and treatments via the SPES survey activities. The same applies to the majority of the participating post-schooling individuals, in the following referred to as adults.
Study design
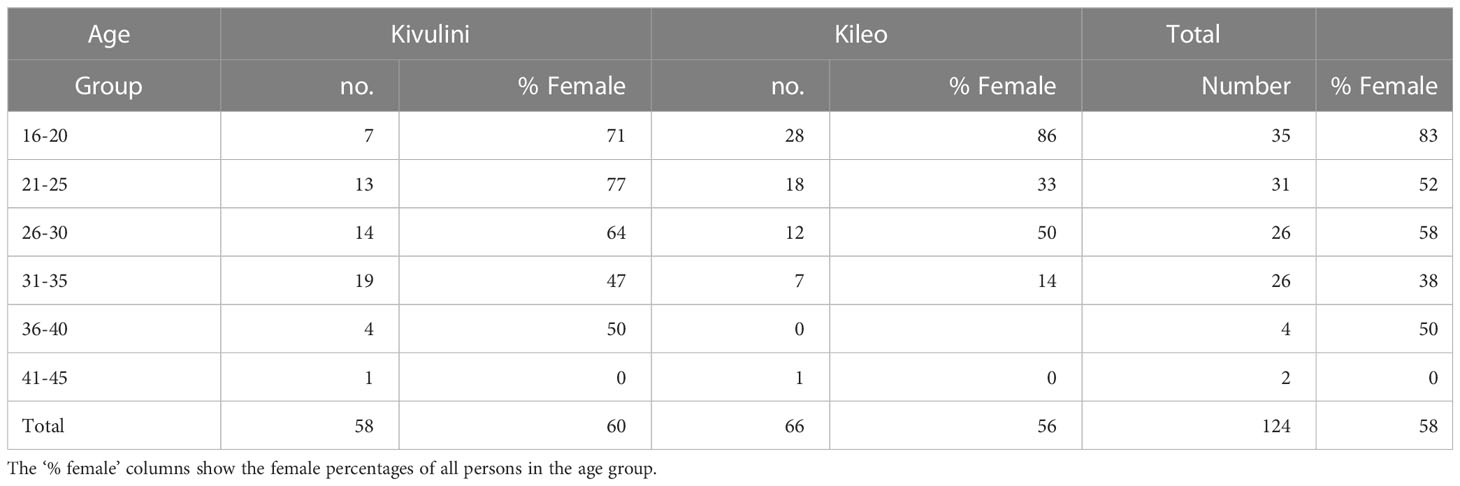
This is a comparative study based on two cross-sectional data-sets. The first dataset is from the 2013 triple urine examination of schoolchildren for S. haematobium eggs and haematuria of the four schools in Kileo and Kivulini. The children were selected from classes four and seven with stated ages 9-15 years. The second dataset consists of former schoolchildren, who now are adults and who had participated in some of the earlier SPES school surveys prior to 2013. In 2015, they were invited to be examined with the same procedure as the children, with three consecutive urine samples. The numbers and demographic characteristics of the study population are given in Table 1.

Table 1 Absolute numbers of schoolchildren, adults and percentages of females, by school and living location.
In the adult group, there was a difference in the age distribution. Participants from Kileo had a mean age of 23 years, and from Kivulini 28.5 years. Adult female participants were more common than males, especially in the youngest age group (Table 2).
Initially, 476 individuals were asked to participate. Twenty-nine schoolchildren and 51 adults, in total 80 individuals, never appeared and were therefore dropped. The number of eligible subjects was 396 and remained the same for each of the three examinations. Some individuals failed to appear on all test occasions. Figure 1 shows how many individuals that appeared for each round. In this study we included those 285 individuals, 161 shoolchildren and 124 adults, with three completed test rounds.

Figure 1 Flow-chart of the number of participating individuals and their participation in the urine examinations.
Three of the 15 women who were menstruating at the time of the investigation, likewise did not have any detectable blood or eggs. Of the remaining 12 women, the urine test of one exhibited eggs. For testing purposes, all the urinary blood testing values of these 12 women were set as negative.
Urine tests
Urine was collected around noon, in 0.3 litre cups to minimize the circadian variation of egg excretion in a field laboratory specially set up for the occasion and powered by a portable generator (28). Around thirty minutes before micturition, 300 ml of beverage was given. The urine samples were thoroughly mixed, tested with a dipstick (Multistix 5 50/FP, Siemens, Germany) and analyzed visually. A dipstick result was considered positive for haematuria when the number of erythrocytes was 25 or more per µL. Fifty ml of urine was filtrated through a polycarbonate membrane of 12 μm pore size (Millipore, Merck, Germany). The membranes were then examined in a microscope for S. haematobium eggs using the trypan-blue filtration technique (29, 30). Intensity of the infection is expressed as number of eggs/10 ml urine.
Rationale and calculation of adjusted prevalence
With three samples available, it is possible to calculate a prevalence using either the first sample, the two first samples or all three samples. The combined results from three consecutive urine filtrations performed on three separate urine samples can be used to define a criterion for the presence of schistosomiasis in an individual; a result is considered positive when at least one of the three consecutive tests contains at least one egg. Thus the prevalence will increase with each added, independent urine sample (15).
The prerequisite for usefulness of the dipstick test for haematuria is that the individuals in the examined group meet the above criterion of eggs in the urine. In order to examine the effects of different definitions of what constitutes a positive haematuria test, we calculated haematuria prevalence based on different requirements: with only one urine sample available, the outcome is either positive or negative; for two samples, a positive haematuria test means that either one of them or both has to be positive; for three separate samples, an overall positive haematuria test means that either one of three, two of three or all three samples show haematuria. In this way, we can control the sensitivity of the haematuria results to a certain degree.
Finally, we combined these results into an overall assessment of schistosomiasis prevalence in the examined group using the observed discordance between the egg and the haematuria occurrences. The rationale for this is based on two facts. Firstly, in the case when a person is infected with S. haematobium, eggs in the urine are a sufficient but not necessary prerequisite for determining whether infection exists. Secondly, haematuria is often present when a person is infected with S. haematobium and as such could serve as an indicator of infection (16).
Statistical methods
The statistical methods comprised descriptive statistics: means, proportions and counts. Ninety-five percent confidence intervals (CIs) are given.
Ethical considerations
The surveys were approved by the Kilimanjaro Christian Medical Centre/Kilimanjaro Christian Medical College Ethics Committee, Moshi, Tanzania (KCMC/C: 71, 1999-09-23; 019, 2007-09-24) and the Regional Ethical Review Board, Göteborg, Sweden (382-08).The local school authorities and all adult participants gave their consent after having been informed of the purpose and the procedures of the study. All schoolchildren were invited to participate after informed consent from their parents or guardians had been obtained. All persons with schistosome eggs in the urine were treated free of charge with praziquantel (single oral dose of 40 mg/kg). Persons with other suspected pathological findings were referred to local health authorities.
Results
The results consist of two parts, (I) a development of a new model to improve the UGS prevalence and (II) the ensuing prevalence estimates based on empirical data:
A model for how to determine UGS prevalence
The relationship between schistosomiasis infected individuals, haematuria positive ones and those with eggs is depicted in Figure 2. All urine positive individuals (U+) will by definition be a proper subset of all schistosomiasis positive individuals (S+). The haematuria positive individuals (H+), on the other hand, are neither necessarily contained within the U+ group, nor in the S+ one. This means that there is a potential for improving the schistosomiasis prevalence estimation on a group level, i.e. how many of the haematuria positive individuals which have no detected eggs in the urine but are in fact schistosomiasis infected.

Figure 2 The overlaps of schistosomiasis infected individuals (S+), dipstick haematuria positive individuals (H+) and urine filtration-diagnosed schistosomiasis infected individuals (U+). The yellow area represents the proportion added to get the adjusted prevalence. The grey area represents the non-pathological haematuria-proportion.
Valid global information of the prevalence of microscopic haematuria without a known pathological background is not easily obtained. Various sources have shown estimates in the range from around 2 to 20 percent in various non-African populations of all ages (31, 32). What could be a reasonable proportion in the investigated groups? Our study groups consist of schoolchildren and younger adults from a schistosomiasis-endemic area, which makes an underlying serious disorder other than UGS rather unlikely. There are of course other causes for microscopic haematuria than UGS, but our aim here is to achieve a reasonable adjustment without risk of serious under-estimation of UGS. Therefore, to avoid overestimation of the prevalence, we let this proportion assume an approximate middle value of 10 percent. Attributing all microscopic haematuria cases as cases of UGS would speak against reason concerning alternative causes.
We can now improve our prevalence estimate, i.e. reducing an unwanted underestimation of the schistosomiasis prevalence, by combining the verified egg-prevalence and the occurrence of haematuria to make an adjusted estimate of the unknown true proportion of schistosomiasis-infected individuals through a simple formula given in Box 1.
Box 1. Adjusted prevalence for schistosomiasis haematobium
Where
= number of schistosomiasis infested individuals determined by the occurrence of eggs in the urine
= proportion of non-pathological haematuria-positive individuals
= number of haematuria positive cases where no eggs in the urine were detected
= number of individuals in the examined population group
Application of the adjustment model on empirical data
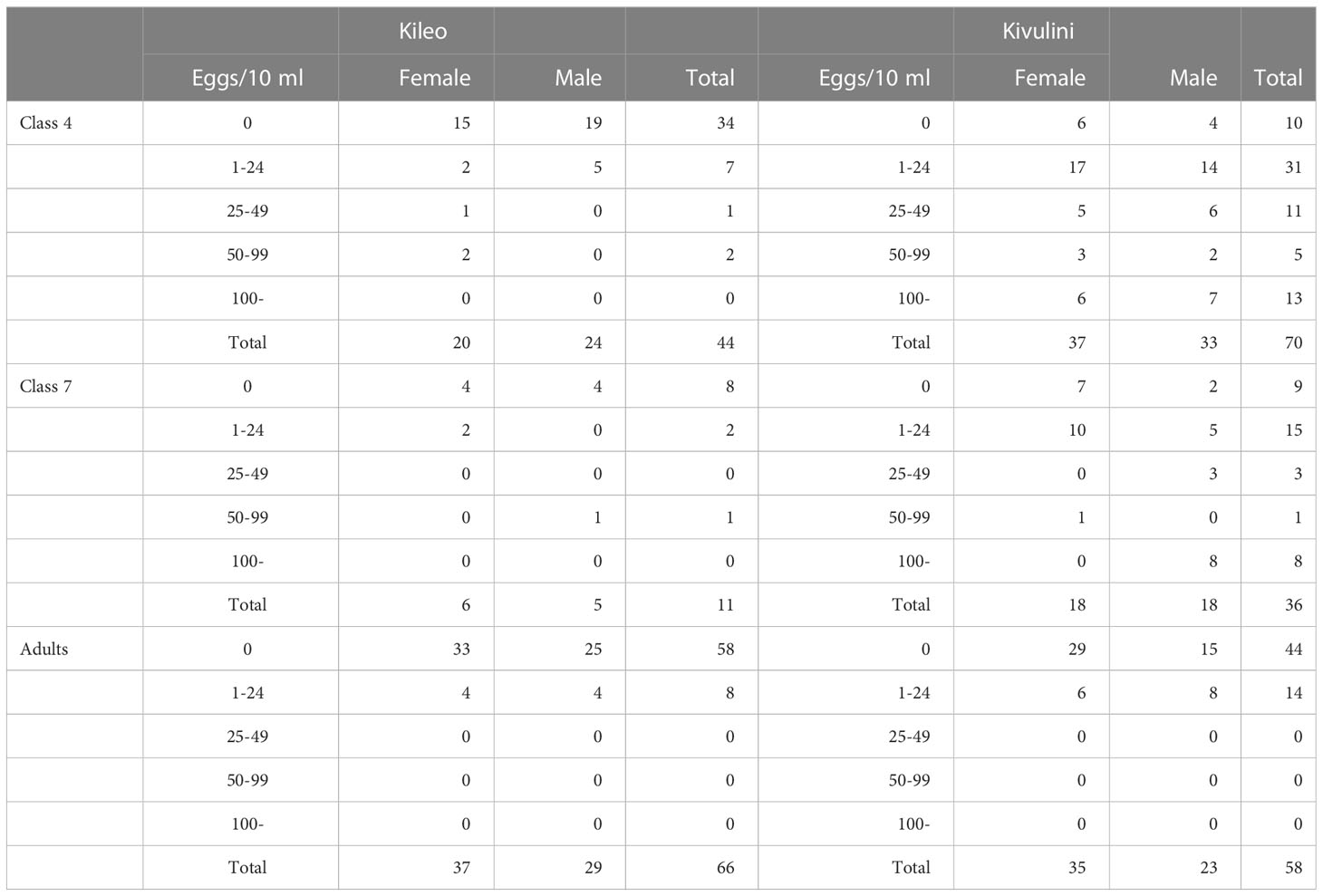
Egg count
The cumulative occurrence of eggs increased for each round of the three urine filtrations (Table 3). It is unclear how many samples that are needed to determine the true prevalence. Therefore the prevalence based on three samples can be considered an observed minimum prevalence. According to the table, there are some differences in occurrence with respect to sex. Compared to the differences found between the two villages, the overall sex differences are small. What is quite clear, however, is that for many groups, the number of positive cases increases with the number of filtrations performed. The groups not showing such an increase are in general rather small and therefore their cumulative results will be more vulnerable to random variations.

Table 3 Percentages of individuals, children and adults, with eggs in the urine after observing one, two and three urine samples respectively, by school, village and sex.
In an attempt to show the underestimation due to chance in determining the prevalence, one-sided CIs were calculated based on the cumulative occurrence using three samples (Table 3).
Most of the infected individuals had 25 eggs/10 ml or less, while the younger male children from Kivulini tended to have higher counts, which means that the intensity of infection can be regarded as rather low (Table 4).
Haematuria
The proportion of individuals with haematuria-positive tests did show a high degree of variability. They ranged from 41 to 78 percent for both sexes after three tests (Table 5). Clearly, these values represent a significant proportion of all investigated subjects. Haematuria is evidently highly prevalent, with an average of 58 percent for all groups.

Table 5 The cumulative development of haematuria in percent after one, two and three urine samples per schoolchild and adult, as well as per school, village and sex.
The outcome of the adjustment model
By adopting the above assumptions and applying the proposed formula (Box 1) to our data, the groups containing the highest proportion haematuria-positives and egg-negatives exhibit the largest increase in adjusted prevalence (Table 6). This naturally coincides with the groups exhibiting low egg-verified prevalence.

Table 6 Estimated schistosomiasis prevalences, as verified (ver) using egg-counts (A) and prevalences adjusted (adj) using estimates of haematuria prevalence (B) by schoolchildren, adults and village.
Table 6 shows thirteen different scenarios with up to three urine filtrations, up to three dipstick tests and three different requirements for a positive haematuria finding. Each case is defined by the first three columns in the table. The diagnosis of haematuria depends on how many positive samples are required according to the chosen definition. We can therefore say that Table 6 shows the consequences on the schistosomiasis haematobium prevalence according to three criteria of sensitivity; number of urine filtrations 1, 2 or 3, number of haematuria tests performed 1, 2 or 3 and additionally the effect of requiring 1, 2 or 3 haematuria tests to be positive for a haematuria case to be counted as a suspect schistosomiasis haematobium case. It is worth noting that it is much easier to use a dipstick under field conditions than performing a urine filtration and counting eggs. The table therefore contains examples where more haematuria tests are performed than urine filtrations, but never the opposite.
An important message from Table 6 is that by adjusting the prevalence based on egg detection in a single urine sample by incorporating the result from just one haematuria test on that same sample will substantially increase the schistosomiasis prevalence estimate for those population groups displaying a low egg occurrence (blue bar). This seems to be a more parsimonious way of estimating the prevalence than repeating the egg counting procedure to obtain an improved minimum egg-verified prevalence of several independent samples.
It is also worthwhile noting the similarities between adjusted prevalences based on egg counts from three samples compared to estimates based on one egg count only, where both these estimates are obtained from adding information from three haematuria tests while only requiring one haematuria test to be positive (red bars).
Discussion
Annual mass chemotherapy of school-children with praziquantel, conceived during the 1980s, was meant to reduce the occurrence of severe schistosomiasis-associated morbidity later in life (33–35). Mass treatment in that vein was never intended to eliminate schistosomiasis. Even when UGS morbidity control still has to be prioritized in many areas, we have to consider and prepare valid and affordable tools and methods suitable for monitoring elimination efforts (36).
Praziquantel is only effective against adult worms and does not prevent re-infection (Doenhoff et al., 2009). Cure rates of up to 90 percent with single dose treatments of population groups have been recorded, but complete cures have seldom, if ever, been recorded in endemic areas (37). In each schistosomiasis-infected population, there is a wide range of individuals with different worm burdens, thus varying in their egg excretion patterns. As a consequence, after a local mass treatment with a single dose of praziquantel - even if the outcome is a reduced overall egg-prevalence - an unknown number of individuals will still continue to excrete eggs (or will excrete eggs released from worms that have matured in the meantime) and, hence, keeps transmission going. This means that the disease might be locally controlled, but the goal to eliminate UGS as a public health problem will be harder to accomplish (38). Our group-based prevalence adjustment procedure might be one way of keeping the ambition for transmission control alive when decisions are made whether and how to conduct mass treatment campaigns based on the WHO recommendations.
In circumstances where the prevalence of S. haematobium eggs was established as low (<20%) based on one standard urine filtration (10 ml), the result of a reagent strip for haematuria is said to constitute an “unstable proxy for UGS” if used alone and not in conjunction with urine filtration (39). In our proposed model, we require at least one filtration, not only to satisfy the adjustment model, but first and foremost, to insure that we are dealing with schistosomiasis.
Researchers Krauth et al. make three important observations from their very comprehensive material of over 1000 outcomes, which underpin our suggested adjustment model (39). 1) When the egg-prevalence of S. haematobium was lower than 21 percent, regardless of setting, 15-20 percent of the subjects had haematuria. 2) The percentage of ‘haematuria seemingly unrelated to S. haematobium’ was stable over different prevalence ranges, given that this class of haematuria becomes more invisible the higher the prevalence. 3) ‘Seemingly unrelated haematuria’ is not linked to sex to any significant degree.
Their first statement informs of a reasonably stable presence of background haematuria, just as used in our adjustment model. It is not clear, however, how large this proportion is. The interval 15 to 20 percent must also contain an entity of undetected disease, where the microscopic screening does not detect eggs, even if the urine sample belongs to an infected person. According to examples in the Krauth et al. article, this entity can vary to some extent, but cannot be dismissed altogether (39). This supports our assumption that the size of the parameter (i.e. proportion of non-pathological haematuria-positive individuals) should be somewhere in the range from 2 to 20 percent. Our estimated proportion of 10 percent therefore seems reasonable. The second point brings forward the fact that the ‘seemingly unrelated haematuria’, or background haematuria, becomes hidden when existing in parallel with high levels of egg excretion. Our adjustment model indirectly accounts for this feature. When the number of individuals that excrete eggs increases, the number of haematuria positive individuals without eggs will decrease as a consequence. The third point presented further supports the idea that there is no need to have separate models for males and females.
A next step with our prevalence adjustment model is to determine the inherent uncertainty of this rather complex statistical measure, which will allow for interval estimation and thereby clarifying the prevalence description. We therefore propose the following approximate confidence interval, given in Box 2, of the adjusted prevalence for schistosomiasis haematobium.
Box 2. A simplified confidence interval of the adjusted prevalence for schistosomiasis haematobium
Where
= number of schistosomiasis infested individuals determined by the occurrence of eggs in the urine
= proportion of non-pathological haematuria-positive individuals
= number of haematuria positive cases where no eggs in the urine were detected
= number of individuals in the examined population group
= is the 100 x (1-confidence level)/2 critical value for the standard normal distribution
The subjects in our study represent a typical target population for possible mass-treatment. The proportion of detected infected adults in both Kileo and Kivulini is generally lower than among the schoolchildren. Adults exhibit lower rates of egg excretion and will therefore, to an even higher degree than for children, run the risk of presenting an underestimation of the true prevalence of schistosomiasis (8, 14).
Diagnosing schistosomiasis haematobium is a difficult undertaking with any technique, since what we essentially are trying to detect is a viable worm-couple inside a human body and our methods all rely on indirect measurements of different kinds of markers (40). It is of course important to be aware of potential competing causes for haematuria, including e.g. urogenital infections and malignancy. Nevertheless, if several haematuria tests are performed, we would not need to demand that all of them must be positive. One positive should be sufficient, which gives a higher test sensitivity. Since we are dealing with young populations, competing causes for large numbers of haematuria positive cases should be rare. In practice we believe that our approach will be applied on just one urine sample; it might be considered impractical to ask for more than one urine sample. Another reason is that when our technique is used to adjust historical prevalence data, where both results from haematuria tests and egg counts are available, one urine sample will be the most common case. Future comparisons with the results from modern diagnostic techniques will provide guidance on the performance of this technique, for different number of samples used in the adjustment procedure (41).
If the goals and targets of the WHO mass treatment campaigns are to be reached, it is important to provide prevalence estimates which do not underestimate the true prevalence to any greater degree particularly after several rounds of mass chemotherapy. If an underestimate is used, the consequence could well be that too many infected individuals will go unnoticed, which will impede the stated goals of the mass treatment strategy and thwart a well-meant public health intervention.
Conclusions
By using the biologic affinity that exists between presence of eggs and blood in the urine of an individual infected by S. haematobium, we have devised a way of adjusting the prevalence estimates of urogenital schistosomiasis - in the absence of competing causes - using the observed prevalence of haematuria. The adjusted prevalence estimate is especially consequential for populations with a seemingly low level of prevalence, based on egg counts alone. The adjusted prevalence provides a feasible and easy-to-handle way to plan and determine how mass treatment can be launched when aiming at an interruption of the transmission cycle and ultimately elimination of urogenital schistosomiasis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Kilimanjaro Christian Medical Centre/Kilimanjaro Christian Medical College Ethics Committee, Moshi, Tanzania Regional Ethical Review Board, Göteborg, Sweden. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
PN and IK conceived the study. PN designed the study. PN, BA and IK performed the literature search. PN, EN, CK, BA and IK performed the field work and the data collection. PN analyzed and interpreted the data. PN and IK wrote the first version of the paper. PN, EN, CK, BA, HF and IK contributed to the critical revisions of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
The Swedish International Development Cooperation Agency/Department for Research Cooperation with Developing Countries (Sida/SAREC) has been the main sponsor of the SPES project (SWE 2002-234; SWE 2007-328). The Skaraborg Institute for Research and Development supported the Swedish members of the SPES team.
Acknowledgments
The authors wish to thank all the team members from the Kilimanjaro Christian Medical Centre (KCMC) and the Kileo Dispensary for their dedication and participation in the SPES project. We are also indebted to the teachers, schoolchildren, former schoolchildren and village authorities of Kileo and Kivulini for their willingness to participate.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author BA declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization (2020).
2. WHO. Report of the World Health Assembly. Resolution N. 54.19 (2001). Geneva. Available at: https://apps.who.int/gb/archive/pdffiles/WHA54/ea54r19.pdf (Accessed 2023-07-06).
3. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet (2014) 383:2253–64. doi: 10.1016/S0140-6736(13)61949-2
4. Smith JH, Christie JD. The pathobiology of Schistosoma haematobium infection in humans. Hum Pathol (1986) 17(4):333–45. doi: 10.1016/S0046-8177(86)80456-7
5. Wilkins HA, Goll PH, Marshall TF, Moore PJ. Dynamics of Schistosoma haematobium infection in a Gambian community. III. Acquisition and loss of infection. Trans R Soc Trop Med Hyg (1984) 78(2):227–32. doi: 10.1016/0035-9203(84)90283-9
6. van der Werf MJ, de Vlas S, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop (2003) 86:125–39. doi: 10.1016/S0001-706X(03)00029-9
7. Mutapi F, Maizels R, Fenwick F, Woolhouse M. Human schistosomiasis in the post mass drug administration era. Lancet Infect Dis (2017) 17:e42–48. doi: 10.1016/S1473-3099(16)30475-3
8. King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn (2008) 4:65–79. doi: 10.1177/1742395307084407
9. Abdel-Salam E, Ehsan A. Cystoscopic picture of Schistosoma haematobium in Egyptian children correlated to intensity of infection and morbidity. Am J Trop Med Hyg (1978) 22(4):774–8. doi: 10.4269/ajtmh.1978.27.774
10. Warren KS. Schistosomiasis: a multiplicity of immunopathology. J Investig Dermatol (1976) 67:464–9. doi: 10.1111/1523-1747.ep12514738
11. Cheever AW, Kamel IA, Elwi AM, Mosimann JE, Danner R. Schistosoma mansoni and S. haematobium infections in Egypt. II. Quantitative parasitological findings at necropsy. Am J Trop Med Hyg (1977) 26(4):702–16. doi: 10.4269/ajtmh.1977.26.702
12. WHO. WHO guideline on control and elimination of human schistosomiasis. Geneva: World Health Organization (2022).
13. Hinz R, Schwarz NG, Hahn A, Frickmann H. Serological approaches for the diagnosis of schistosomiasis - A review. Mol Cell Probes (2017) 31:2–21. doi: 10.1016/j.mcp.2016.12.003
14. Savioli L, Hatz C, Dixon D, Kisumku UM, Mott KE. Control of morbidity due to Schistosoma haematobium on Pemba Island: Egg excretion and hematuria as indicators of infection. Am J Trop Med Hyg (1990) 43:289–95. doi: 10.4269/ajtmh.1990.43.289
15. Kosinski KC, Bosompem KM, Stadecker MJ, Wagner AD, Plummer J, Durant JL, et al. Diagnostic accuracy of urine filtration and dipstick tests for Schistosoma haematobium infection in a lightly infected population of Ghanaian schoolchildren. Acta Trop (2011) 118(2):123–7. doi: 10.1016/j.actatropica.2011.02.006
16. Ochodo EA, Gopalakrishna G, Spek B, Reitsma JB, van Lieshout L, Polman K, et al. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database Syst Rev (2015) 3:1–292. doi: 10.1002/14651858.CD009579.pub2
17. Mohammed H, Landeryou T, Chernet M, Liyew EF, Wulataw Y, Getachew B, et al. Comparing the accuracy of two diagnostic methods for detection of light Schistosoma haematobium infection in an elimination setting in Wolaita Zone, South Western Ethiopia. PloS One (2022) 17(4):e0267378. doi: 10.1371/journal.pone.0267378
19. Kramer CV, Zhang F, Sinclair D, Olliaro PL. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev (2014) 8). doi: 10.1002/14651858.CD000053.pub3
20. WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Geneva: World Health Organization (2020). Licence: CC BY-NC-SA 3.0 IGO.
21. Nordin P, Poggensee G, Mtweve S, Krantz I. From a weighing scale to a pole: a comparison of two different dosage strategies in mass treatment of Schistosomiasis haematobium. Glob Health Action (2014) 7(1):25351. doi: 10.3402/gha.v7.25351
22. Gazzinelli-Guimaraes PH, Dhanani N, King CH, Campbell CH, Aurelio HO, Ferro J, et al. Accuracy of the WHO praziquantel dose pole for large-scale community treatment of urogenital schistosomiasis in northern Mozambique: Is it time for an update? PloS Negl Trop Dis (2018) 12(11):e0006957. doi: 10.1371/journal.pntd.0006957
23. Tanzania Population and Housing Census 2002 . National Bureau of Statistics. Available at: http://ghdx.healthdata.org/record/tanzania-population-and-housing-census-2002 (Accessed 2023-07-06).
24. Ahlberg BM, Mwangi R, Poggensee G, Feldmeier H, Krantz I. “Better infection than hunger” A sociological study of illness perceptions with special focus on urinary schistosomiasis in Northern Tanzania. Afr Soc Rev (2003) 7(1):18–34.
25. Poggensee G, Krantz I, Nordin P, Mtweve S, Ahlberg B, Mosha G, et al. A six-year follow-up of schoolchildren for urinary and intestinal schistosomiasis and soil-transmitted helminthiasis in Northern Tanzania. Acta Trop (2005) 93:131–40. doi: 10.1016/j.actatropica.2004.10.003
26. Freudenthal S, Ahlberg BM, Mtweve S, Nyindo P, Poggensee G, Krantz I. School-based prevention of schistosomiasis: initiating a participatory action research project in northern Tanzania. Acta Trop (2006) 100(1-2):79–87. doi: 10.1016/j.actatropica.2006.09.013
27. King CH, Galvani AP. Underestimation of the global burden of schistosomiasis. Lancet (2017) 391:307–8. doi: 10.1016/S0140-6736(18)30098-9
28. Doehring E, Feldmeier H, Daffalla AA. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann Trop Med Parasitol (1983) 77(6):587–94. doi: 10.1080/00034983.1983.11811757
29. Feldmeier H, Bienzle U, Dietrich M. Combination of viability test and quantification method for Schistosoma haematobium eggs. Tropenmedizin und Parasitol (1979) 30:417–22.
30. Feldmeier H, Doehring E, Daffalla AA. Simultaneous use of a sensitive filtration technique and reagent strips in urinary schistosomiasis. Trans R Soc Trop Med Hyg (1982) 76(3):416–21. doi: 10.1016/0035-9203(82)90204-8
31. Kelly JD, Fawcett DP, Goldberg LC. Assessment and management of non-visible haematuria in primary care. BMJ (2009) 338:227–32. doi: 10.1136/bmj.a3021
32. Jimbo M. Evaluation and management of haematuria. Prim Care (2010) 37(3):461–72. doi: 10.1016/j.pop.2010.04.006
33. Mott KE, Dixon H, Osei-Tutu E, England EC. Relation between intensity of Schistosoma haematobium infection and clinical haematuria and proteinuria. Lancet (1983) 321(8332):1005–8. doi: 10.1016/S0140-6736(83)92641-7
34. WHO. The control of schistosomiasis: report of a WHO Expert Committee. WHO Technical Report Series 728 (1985). Geneva: WHO. Available at: https://apps.who.int/iris/handle/10665/39529 (Accessed 2023-07-06).
35. Feldmeier H, Poggensee G. Diagnostic techniques in schistosomiasis control. A review. Acta Trop (1993) 52(4):205–20. doi: 10.1016/0001-706X(93)90009-Z
36. Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop (2013) 128:423–40. doi: 10.1016/j.actatropica.2012.04.013
37. Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, Botros S, et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology (2009) 136:1825–35. doi: 10.1017/S0031182009000493
38. Molyneux DH, Hopkins DR, Zagaria N. Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol (2004) 20(8):347–51. doi: 10.1016/j.pt.2004.06.004
39. Krauth S, Greter H, Stete K, Coulibaly JT, Traoré SI, Ngandolo BN, et al. All that is blood is not schistosomiasis: experiences with reagent strip testing for urogenital schistosomiasis with special consideration to very-low prevalence settings. Parasites Vectors (2015) 8:584–94. doi: 10.1186/s13071-015-1165-y
40. Barbour AD. Schistosomiasis. In: Anderson R, editor. Population dynamics of infectious diseases, theory and applications. London: Chapman and Hall (1982).
Keywords: Schistosomiasis haematobium, haematuria, control, elimination, prevalence
Citation: Nordin P, Nyale E, Kalambo C, Ahlberg BM, Feldmeier H and Krantz I (2023) Determining the prevalence of urogenital schistosomiasis based on the discordance between egg counts and haematuria in populations from northern Tanzania. Front. Trop. Dis 4:1100139. doi: 10.3389/fitd.2023.1100139
Received: 16 November 2022; Accepted: 10 July 2023;
Published: 02 August 2023.
Edited by:
Patrick Lammie, Task Force for Global Health, United StatesReviewed by:
William Evan Secor, Centers for Disease Control and Prevention (CDC), United StatesMoses Adriko, MoH, Uganda
Copyright © 2023 Nordin, Nyale, Kalambo, Ahlberg, Feldmeier and Krantz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Per Nordin, cGVyLm5vcmRpbkBza2FyYWJvcmctaW5zdGl0dXRlLnNl
 Per Nordin
Per Nordin Edwin Nyale3
Edwin Nyale3 Beth Maina Ahlberg
Beth Maina Ahlberg