
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Trop. Dis. , 08 September 2022
Sec. Vaccines for Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.988665
This article is part of the Research Topic Rising Stars in Vaccines for Tropical Diseases Research View all 4 articles
Africa bears the greatest burden of malaria with more than 200 million clinical cases and more than 600,000 deaths in 2020 alone. While malaria-associated deaths dropped steadily until 2015, the decline started to falter after 2016, highlighting the need for novel potent tools in the fight against malaria. Currently available tools, such as antimalarial drugs and insecticides are threatened by development of resistance by the parasite and the mosquito. The WHO has recently approved RTS,S as the first malaria vaccine for public health use. However, because the RTS,S vaccine has an efficacy of only 36% in young children, there is need for more efficacious vaccines. Indeed, based on the global goal of licensing a malaria vaccine with at least 75% efficacy by 2030, RTS,S is unlikely to be sufficient alone. However, recent years have seen tremendous progress in vaccine development. Although the COVID-19 pandemic impacted malaria control, the rapid progress in research towards the development of COVID-19 vaccines indicate that harnessing funds and technological advances can remarkably expedite vaccine development. In this review, we highlight and discuss current and prospective trends in global efforts to discover and develop malaria vaccines through leveraging mRNA vaccine platforms and other systems optimized during COVID-19 vaccine studies.
In 2020, over 200 million clinical cases of malaria and more than 600,000 malaria deaths were reported, with the vast majority occurring in sub-Saharan Africa (1). Importantly, malaria, which is the leading cause of death in young children, also poses great danger to pregnant mothers (2). The emergence of the Coronavirus disease 2019 (COVID-19) pandemic and related transmission control measures, such as social distancing and travel restrictions, disrupted and complicated the implementation of previously ongoing malaria control and intervention strategies (3). Importantly, malariology experts have highlighted the need to develop more tools and strategies towards achieving malaria elimination and eventual eradication (4). It is anticipated that a highly effective malaria vaccine might further reduce the global burden of malaria. Although RTS,S, the recently approved circumsporozoite protein (CSP)-based malaria vaccine has low efficacy, it has renewed hope for the development and deployment of effective malaria vaccines (5).
Although the number of COVID-19 cases in Africa has been lower than predicted, its effect on other tropical diseases such as malaria, which pose significant burdens to a significant portion of African population cannot be underestimated (1, 3). Nevertheless, the fast-tracked vaccine discovery efforts against severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) highlighted new opportunities for malaria vaccine development and deployment in malaria holoendemic regions of Africa.
Currently, several strategies are being used to develop vaccines against various parasite developmental stages. Indeed, in the last 10 years, there has been an exponential growth in the number of studies aiming to develop such vaccines [as reviewed (6)]but these have been hampered by several challenges, some of which can be overcome using strategies that have been used to rapidly develop vaccines against COVID-19 (7). We believe that such a vaccine will alleviate the current malaria related problems in Africa that are now compounded by the emergence of the SARS-CoV-2 virus.
Plasmodium falciparum, the main causative agent of malaria, has a complex life cycle alternating between human and anopheles mosquitoes. Current malaria vaccine candidates generally target pre-erythrocytic, erythrocytic and sexual stage parasites. Some of the antigens under consideration are shown in Figure 1 (6). Vaccines targeting the pre-erythrocytic stage prevent infection progression to symptomatic malaria (6). Phase III vaccine trials of the CSP-based RTS,S showed that it modestly reduced clinical malaria episodes (by approximately 36%) in young African children and by about 26% among infants who received four vaccine doses with statistically significant efficacy against severe malaria in young children (8). Pilot rollout of the RTS,S vaccine is now underway in Malawi, Ghana, and Kenya to assess protective benefits and safety during routine use in real-life settings (9). The WHO has recently approved the widespread deployment of the RTS,S vaccine (5). In addition, a phase 2b trial involving CSP-based R21 in Matrix-M adjuvant was observed to be safe and very immunogenic with a protective efficacy of more than 74% in children aged 5–17 months drawn from a highly seasonal malaria transmission setting of Burkina Faso (10). Phase 3 trials in different malaria transmission settings are now required. PfCelTOS, a micronemal secreted-protein, is the only non-CSP based recombinant protein be evaluated in a clinical trial (Clinicaltrials.gov NCT02174978).
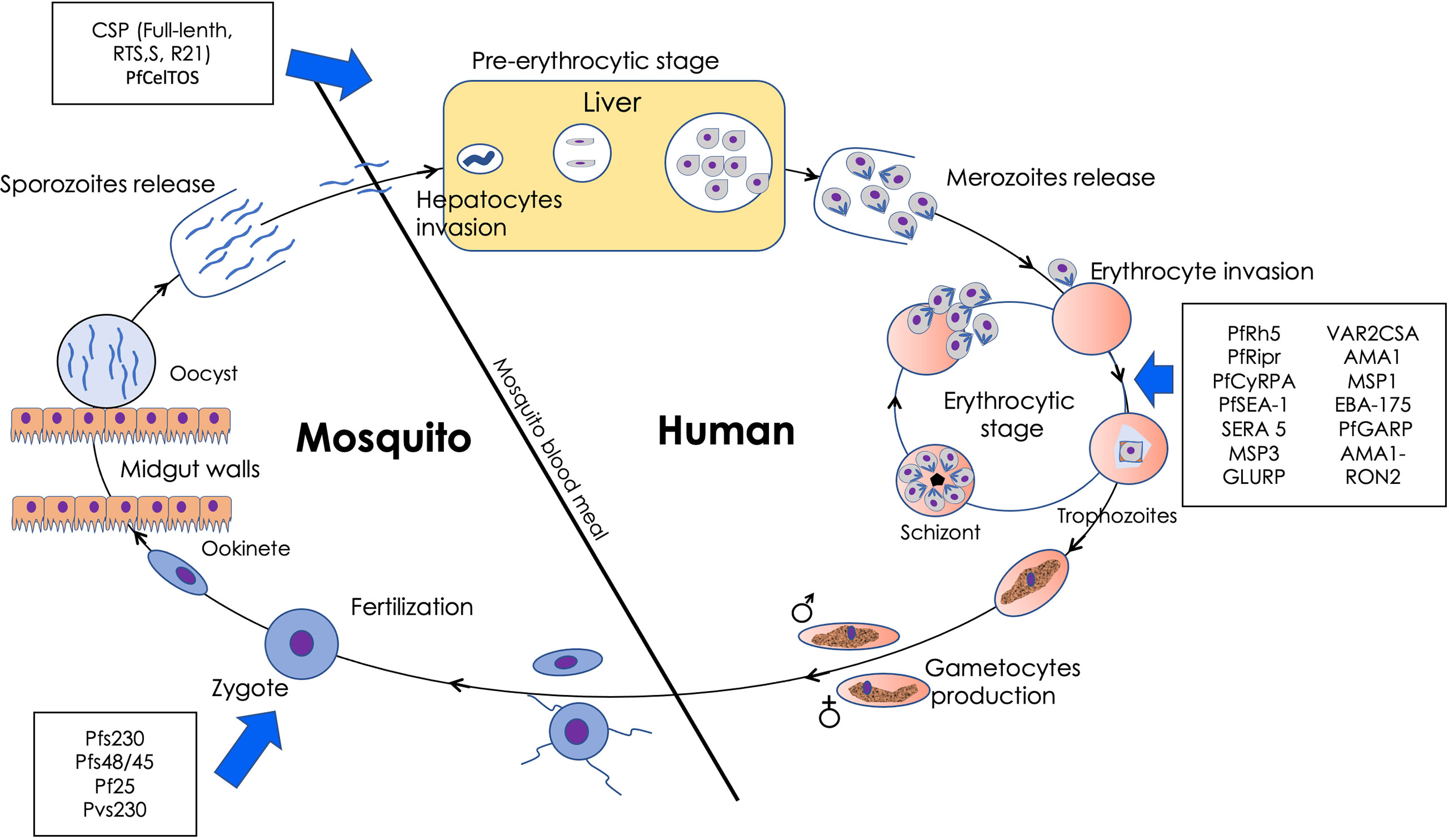
Figure 1 Plasmodium falciparum lifecycle in human and anopheles mosquitoes. In brief, infective P. falciparum sporozoites develop in liver cells and are subsequently released as merozoites into the blood circulation. Merozoites, the asexual erythrocytic stage of the parasite, infect erythrocytes to start the cyclic erythrocytic stage. Some of the merozoites differentiate into gametocytes in the bone marrow. Upon re-entry into the circulation, they may be picked by feeding mosquitoes. The gametocytes undergo fertilization in mosquitoes’ mid-gut after mature male and female gametes egresses from the erythrocytes in response to the change in temperature and environment to form zygotes. Zygotes transform into infective ookinetes, which traverse the mosquito mid-gut epithelium before developing into oocysts. Sporozoites which emerge from the oocysts, migrate to reach the mosquito’s salivary glands for transmission to the next human host. The steps targeted by the leading vaccine candidates are as indicated with blue allows.
Erythrocytic or blood-stage vaccines are aimed at inducing high antibody levels targeting specific parasite antigens in order to inhibit erythrocyte invasion by merozoites, thereby controlling parasite replication in the host. Vaccine antigens like Reticulocyte-binding Protein Homolog 5 (PfRh5) and PfRh5-interacting protein (PfRipr) are very promising, with clear functional activity (Table 1) (12, 21). PfRh5 binds to basigin and forms an essential stable merozoite invasion complex with PfRipr and Cysteine-Rich Protective Antigen (PfCyRPA) (12). The three proteins elicit invasion-inhibitory antibodies in experimental animals and naturally acquired antibodies in humans correlate with protection from clinical malaria (12, 21, 22). Other blood-stage antigens in the clinical development phase include MSP3, Glutamic-acid-rich protein (PfGARP) and Schizont Egress Antigen 1 (PfSEA-1). A randomized phase 2 study involving children in Burkina Faso, Gabon, Ghana, and Uganda found that although the GMZ vaccine, a fusion of MSP3 and GLURP, exhibited good tolerability, immunogenicity, and some protection from malaria infection, its efficacy was low (23). However, because other studies have shown that MSP3 is effective, improved formulation or the use of alternative adjuvants that may enhance the GMZ vaccine’s immunogenicity and the duration of protection should be explored (24, 25). PfGARP is expressed on the exofacial surface of erythrocytes infected in the early–late trophozoite stage. Anti-PfGARP activates programmed cell death of parasites via caspase-like proteases and fragmentation of parasite DNA (13). PfSEA-1 is expressed in schizont-infected erythrocytes and anti-PfSEA-1 antibodies block parasite egress from RBCs (14). Other promising vaccine antigens include Serine rich antigen 5 (SERA 5) (18) and Plasmodium-encoded Macrophage Inhibitory Factor (PMIF) (19).
On the other hand, transmission blocking vaccines (TBVs) present an excellent path to stopping population transmission although with no direct benefits to the vaccinee (26–28). TBVs are based on the principle that when antibodies against a specific antigen expressed in the sexual stages of the malaria parasite (gametocyte, gamete, zygote and ookinete) are taken up along with gametocytes during a blood meal, they can reduce the number of oocysts in mosquito vectors (26). Potential TBV target antigens include Pfs230, Pfs48/45, Pf25, and Pfs28, which are expressed on the surface of gametocytes, gametes, zygotes, and ookinete (20, 28, 29). The advantages of TBVs are summarized elsewhere and their advancement has progressed to clinical trials (26–28, 30). While these efforts have mainly focused on antigens like Pfs25, Pfs230, and Pfs48/45, which were identified in the pre-genome era, new antigen discovery approaches promise to change this (28).
In addition, whole organism vaccines, such as the radiation-attenuated P. falciparum sporozoite (PfSPZ) vaccine have also been pursued. For instance, the vaccine was confirmed to be safe, well tolerated, and showed significant protection against P. falciparum infection in Malian adults in a randomized, double-blind phase 1 trial (31). The PfSPZ vaccine could also induce a strain-transcending protection against homologous and heterologous parasites in American adult (35–45 years) volunteers enrolled in a controlled human malaria infection (32). Similar observations were reported in a multi-arm, double-blind, randomized, placebo-controlled trial involving 336 Kenyan infants (5–12 months) albeit with no significant protective efficacy (33). Nonetheless, the sourcing of sporozoites that require mosquito colonies, and the need for aseptic cryopreserved delivery of sporozoites remains a major problem (34). On the other hand, because the in vitro culturing of the blood stage parasites is well-established and would allow easy vaccine manufacturing, efforts are currently ongoing to develop and evaluate genetically or chemically attenuated whole-parasite blood-stage vaccines (35, 36).
The COVID-19 vaccine discovery and development landscape developed at an unprecedented scale. The relatively short duration (10 months) between publication of the first SARS-CoV-2 sequences to phase 3 trials was remarkable when compared with the typical vaccine development timeline of 3–9 years (37, 38). The COVID-19 vaccine pipeline is made of a broad range of technology platforms, including traditional and novel approaches (39). Although most of these technologies, including a mRNA and nanotechnologies, had been developed over the previous decade, the development of COVID-19 vaccines indicate that leveraging such approaches along with other genomic era technologies to identify effective vaccine candidate molecules can accelerate the development of vaccines against other tropical diseases, including malaria (40). The mRNA-based platforms are highly flexible and quick, while retaining the capacity to induce robust immune responses against specific targets. This has renewed the hope of quickly developing effective vaccines against malaria, a topic we discuss below. Leveraging the strategies and gains made during COVID-19 vaccine development will significantly accelerate the development of such promising mRNA malaria vaccines.
Early clinical trials showed that two doses of BNT162b2 (BioNTech/Pfizer) elicited high SARS-CoV-2 neutralizing antibody titers and robust antigen-specific CD8+ and Th1-type CD4+ T-cell responses (41). BNT162b2 is a lipid nanoparticle-formulated, nucleoside-modified RNA encoding the SARS-CoV-2 full-length spike protein modified by two proline mutations (41). On December 11, 2020, the U.S. Food and Drug Administration issued the first emergency use authorization for a vaccine for the prevention of COVID-19 in healthy individuals (7). The use of mRNA vaccine platform is currently being tested for several strains of SARS-CoV-2 as well as in other diseases including HIV (42).
Presently, there are two types of mRNA vaccines: conventional mRNA vaccines and self-amplifying mRNA vaccines (43). Both are derived from positive strand RNA viruses. Vaccine synthesis involves the production of mRNA, through a cell-free in-vitro enzymatic transcription reaction that consists of a linearized plasmid DNA template encoding the mRNA vaccine, a recombinant RNA polymerase, and nucleoside triphosphates, which provide essential components (43). A cap structure is enzymatically added to the transcriptional product at the end of the reaction or as a synthetic cap analog in a single step procedure. Finally, a poly(A) tail forms a mature mRNA sequence.
The vaccines induce a local inflammation which induces an infiltration of immune cells, such as neutrophils, monocytes, myeloid and plasmacytoid dendritic cells. This is followed by a quick uptake of the mRNA by these cells. Intracellularly, there are two types of RNA sensors – endosomal toll-like receptors (TLRs) and the retinoic acid-inducible gene (RIG)-I-like receptor family (44, 45). The former set is divided into TLR-3, TLR7, TLR8, and TLR9, which are localized in the endosomal compartment of professional immune surveillance cells, such as dendritic cells (DCs), macrophages and monocytes (44, 45). TLR3 recognizes dsRNA longer than 45 base pairs, as well as dsRNA resulting from single strand RNA (ssRNA) forming secondary structures or derived from viral replication intermediates (46). TLR7 and TLR8 are activated by RNAs rich in polyuridines, guanosines and/or uridines. TLR7 can bind both dsRNA and ssRNA, whereas TLR8 recognizes ssRNA only. TLR7 activation can increase antigen presentation, promote cytokine secretion and stimulate B-cell responses (45). RIG-I preferentially recognizes ssRNA and dsRNA bearing a 5′triphosphate, and stimulate IFN production. Subsequently, the immune cells migrate to the lymph nodes for clearance. It is at this point that antigen presentation to T cells and interactions of antigen and B cells takes place. The outcome is the formation of germinal centers, which results in the development of memory B cells and antibody-producing plasma cells in the bone marrow (45).
The mRNA revolution will make it possible to assess multiple vaccine candidates that target proteins involved in the pre-erythrocytic stage such as CSP, those essential in the erythrocytic stage such as Rh5, or those involved in the parasites sexual stage (summarized in Table 1). Indeed, the use of RNA vaccines against malaria had been explored even prior to the COVID-19 outbreak. A report proposing a malaria RNA replicon-based vaccine was published in 2018 (19) and a patent based on this technology (termed self-amplifying RNA, saRNA), originally requested in 2014.
In a recent study, data confirmed that mice immunized with Plasmodium-encoded Macrophage Inhibitory Factor (PMIF) had intact splenic germinal centers (GC) architecture and showed B cell zonal expansion, an increase in the number of CD4 T follicular helper cells and GC B cells, and a higher anti-Plasmodium antibody titer (19, 47) (Table 1). Adoptive transfer of the CD4 T cells that develop in PMIF- immunized mice during Plasmodium blood-stage infection also conferred full protection to blood-stage infection in naive hosts (19). Upon transfer, these CD4 T cells showed enhanced Plasmodium-specific proliferation and IFN-γ production, and reduced exhaustion (19, 47). These findings demonstrated protection in murine model of malaria initiated by sporozoite or blood-stage infection thereby confirming the role of the Plasmodium-encoded factor PMIF in actively interfering with the adaptive immune response by a pro-inflammatory mechanism involving engagement of the host MIF receptor to suppress the differentiation of memory CD4 and CD8 T cell subsets (19).
Studies have revealed additional antigens that can be used as models of malaria mRNA vaccine research. P. falciparum glutamic-acid-rich protein (PfGARP) was recently identified as a potential mRNA erythrocytic stage vaccine target (13). PfGARP is expressed on the surface of infected erythrocytes where it naturally stimulates production of antibodies capable of inducing programmed cell death of the trophozoite-infected erythrocytes and attenuates severe malaria (18). Other erythrocytic stage proteins such as PfRh5, PfRipr and PfCyRPA, that form a stable merozoite invasion complex (12), should also be considered for mRNA vaccines. In similar vein, VAR2CSA, the major protein involved in placental sequestration of infected erythrocytes by binding to Chondroitin Sulfate A in the intervillous spaces of the placenta can be targeted (15–17). Recently, immunization with immunodominant PfCSP mRNA-LNP was observed to achieve sterile protection against infection with two P. berghei PfCSP transgenic parasite strains (11). Put together, protection observed by PMIF, PfGARP and PfCSP immunization support the usefulness of these and similar antigens as mRNA vaccine candidates, either as standalone immunogens or in combination for adequate robust protective responses.
Nevertheless, the choice of antigens to be developed into vaccines remains to be a major challenge due to the complexity of the malaria parasite, lack of clear correlates of protection both for humoral and cell-mediated immunity, difficulties in the identification of key antigen targets of protective immunity, polymorphisms of the known antigens and lack of robust adjuvants (48, 49). However, recent development of more robust antigen discovery platform such us the use of the wheat germ cell-free system has opened a new perspective in this direction that may help overcome these challenges (50).
One advantage of the mRNA is the ability to quickly change the vaccines to address new pathogen variants when compared with conventional vaccine platforms. Indeed, numerous SARS-CoV-2 variants have been reported globally with reduced efficacy of the existing vaccines (51, 52). These include the Alpha (B.1.1.7 and Q lineages), Beta (B.1.351 and descendent lineages), Gamma (P.1 and descendent lineages), Epsilon (B.1.427 and B.1.429), Eta (B.1.525), Iota (B.1.526), Omicron (B.1.1.529), among others variants (52). Pharmaceutical companies have quickly developed different types of vaccines that have been approved for use by regulatory authorities. Phase III clinical trials of the mRNA-based vaccine, BNT162b2 (Pfizer and BioNTech) showed 95% efficacy 28 days after the first dose (53), its effectiveness against the B.1.1.7 was affected by the emergence of the E484K substitution (53). Efficacy trials revealed that the Oxford/AstraZeneca vaccine based on a viral vector, reached 82.4% after a second dose in individuals who received the dose after a period of 12 weeks. Vaccine efficacy against symptomatic PCR positive patients was similar for B.1.1.7 and non-B.1.1.7 lineages (74.6% and 84% respectively (54). The UK government recently approved the use of Moderna Spikevax bivalent Original/Omicron vaccine that targets both the original version of SARS-CoV-2 and the omicron BA.1 variant (55), a demonstration of how multivalent may work.
Similarly, the field efficacies of leading malaria vaccine have been significantly hampered by genetic polymorphisms in target antigens. For instance, RTS,S the leading vaccine, has various polymorphisms in the pfcsp gene among global P. falciparum population which affect the effectiveness of the vaccines (56). Genetic polymorphism has also been reported on pfrh5 gene, with some peptides in PfRH5 identified as having high basigin affinities than others (57). These polymorphisms may alter the protein tertiary structures thus leading to loss of vaccine efficacy (58). The quick re-configuration of the COVID-19 vaccine development platform to handle emerging mutants has demonstrated that it may be possible to quickly configure second-generation malaria vaccines to accommodate new or existing parasite mutants or even combine mutants in multi-valent vaccines. In addition, as in the case of COVID-19, use of multivalent plasmodial immunogens containing sequences derived from different strains may confer potent and broader protective efficacy against circulating strains (58).
mRNA vaccines are noninfectious and non-integrating with all the advantages associated with nucleic acid vaccines [as reviewed (39)]. This includes a) ease of design, relying on understanding the genetic sequences of target genes, b) safety since mRNA vaccines have no potential risk of infection or insertional mutagenesis posing little risk of causing infection while minimizing the handling of pathogens, and c) low cost-effective production (59, 60). However, they also present many unknowns and challenges, including the lack of data on the long-term safety and their instability, which may lead to low immunogenicity and the need for repeat vaccination (59, 60). Although the rapid decline in antibody response that is seen after mRNA vaccine administration may potentially be mitigated using self-amplifying mRNA vaccines, there is concern that such vaccines may induce excessive inflammation via enhanced type I IFN activation (61). Nevertheless, the inherent immunogenicity can be down-modulated (62).
With regard to the development of mRNA vaccines for malaria, several challenges still remain. These include the lack of robust criteria for the selection of the best antigen or antigen cocktails, potential modification (such as glycosylation) of some parasite proteins expressed in mammalian/human cells, which may reduce their immunogenicity (63, 64). The identifying and development of strategies for generating long-lasting malaria mRNA vaccine-mediated protective immunity is also an important challenge, and criteria for selecting the best malaria mRNA adjuvant is lacking (65). Additionally, polymorphisms in the antigens used for vaccines production may lead to immune escape, a key problem in various candidate vaccines, including RTS,S. Importantly, because malaria is a burden in low–middle income countries where most people earn less than US$1 a day, coupled with the realization of the need for local vaccine production by many African countries, innovative approaches are needed to improve the stability of the inherently unstable mRNA requiring ultralow temperature freezers storage. Thus development of a thermostable formulations such as SARS-CoV-2 ARCoV mRNA vaccine, that require no freezer storage will be key for mass vaccination campaigns in remote areas (66). In summary, mRNA vaccines have the potential for rapid, inexpensive and scalable production, primarily due to high yields in in vitro transcription reactions.
Strategies like the use of viral vectors, nanotechnology-based vaccines, and machine learning designed vaccines may offer effective alternatives or supplement mRNA vaccines. Viral vectors are modified, harmless viruses capable of infecting human cells and expressing an antigen of interest (67), triggering robust immune responses (68). Their advantages over conventional modes of vaccine delivery include; a) capacity for large inserts, which allows delivery of any antigen of choice, b) ability to deliver antigens with high fidelity as they are delivered as genetic instructions, and c) generation of potent immune reactions without need for adjuvants by mimicking normal infection (68, 69). Despite concerns about their recombination risk and unknown virulence (68, 69), viral vector vaccines have been developed for infections like Ebola (70) and COVID-19 (71). Although animal models and human trials (6, 72) indicate that viral vector vaccines may be efficacious against malaria, none are in clinical use. However, given that R21, a virus-like particle malaria vaccine that meets the ≥75% efficacy threshold recommended by the WHO has concluded phase 2b trial, viral vector vaccines may develop into effective alternatives to mRNA vaccines (10). In addition, during the COVID-19 pandemic, machine learning (ML) and artificial intelligence (AI) have been widely used (73), including in guiding the development of universal vaccines (74), designing multi-epitope vaccines (75), and predicting the best sites for trials and those likely to benefit from vaccines (76, 77). These applications highlight the potential value of ML and AI during the development and deployment of malaria mRNA vaccines (78, 79). Similarly, nanotechnology facilitates targeted vaccine delivery, stability, slow release, and immunogenicity (78, 79). The high efficacy of the COVID-19 mRNA vaccines is attributable to the lipid nanoparticles (LNPs) used to shield the mRNA from degradation by ribonucleases (80). This technology has the potential to significantly benefit the development of mRNA/nanotechnology-based malaria vaccines. Indeed, using spontaneous nanoliposome antigen particularization (SNAP), conjugated malaria antigens have been stably presented in uniformly-oriented display without covalent modification or disruption of antigen conformations (81). Immunizing mice and rabbits with SNAP carrying Pfs25 (a malaria transmission-blocking vaccine candidate) was well tolerated and triggered a robust antibody response with minimal local reactogenicity (81). Importantly, multiplexing up to four antigens triggered strong and balanced antibody production (81), highlighting the potential of nanotechnology in the development of multi-antigen/multi-stage particulate vaccines.
Current data suggest that developing of a malaria vaccine leveraging COVID-19 pipelines is a rational approach for strengthening global health. The preclinical trials of the pre-erythrocytic PfCSP and PMIF mRNA vaccine candidates have showed that it may be possible to achieve malaria protection, making this approach worth pursuing. Expanding these platforms to other vaccine targets deserve further investigation.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
BNK is an EDCTP Fellow under EDCTP2 programme supported by the European Union grant number TMA2020CDF-3203-EndPAMAL. FK is also an EDCTP Fellow supported by grant number TMA2019CDF-2736-IRMiP. JG is supported by the African Academy of Sciences and the Royal Society FLAIR grant number FLR\R1\201314. The funding sources had no role in study design, collection, analysis, interpretation of data, and publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Cutts JC, Agius PA, Zaw L, Powell R, Moore K, Draper B, et al. Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: A systematic review. BMC Med (2020) 18:14. doi: 10.1186/s12916-019-1467-6
3. Sherrard-Smith E, Hogan AB, Hamlet A, Watson OJ, Whittaker C, Winskill P, et al. The potential public health consequences of COVID-19 on malaria in africa. Nat Med (2020) 26:1411–6. doi: 10.1038/s41591-020-1025-y
4. Chen I, Cooney R, Feachem RGA, Lal A, Mpanju-Shumbusho W. The lancet commission on malaria eradication. Lancet (2018) 391(10130):1556–8. doi: 10.1016/S0140-6736(18)30911-5
5. The L. Malaria vaccine approval: A step change for global health. Lancet (2021) 398(10309):1381. doi: 10.1016/S0140-6736(21)02235-2
6. Duffy PE, Patrick Gorres J. Malaria vaccines since 2000: Progress, priorities, products. NPJ Vaccines (2020) 5:48. doi: 10.1038/s41541-020-0196-3
7. Kyriakidis NC, Lopez-Cortes A, Gonzalez EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines (2021) 6:28. doi: 10.1038/s41541-021-00292-w
8. White MT, Bejon P, Olotu A, Griffin JT, Bojang K, Lusingu J, et al. A combined analysis of immunogenicity, antibody kinetics and vaccine efficacy from phase 2 trials of the RTS,S malaria vaccine. BMC Med (2014) 12:117. doi: 10.1186/s12916-014-0117-2
9. Laurens MB. RTS,S/AS01 vaccine (Mosquirix): An overview. Hum Vaccin Immunother (2020) 16:480–9. doi: 10.1080/21645515.2019.1669415
10. Datoo MS, Natama MH, Some A, Traore O, Rouamba T, Bellamy D, et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant matrix-m, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet (2021) 397:1809–18. doi: 10.1016/S0140-6736(21)00943-0
11. Mallory KL, Taylor JA, Zou X, Waghela IN, Schneider CG, Sibilo MQ, et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines (2021) 6:84. doi: 10.1038/s41541-021-00345-0
12. Volz JC, Yap A, Sisquella X, Thompson JK, Lim NT, Whitehead LW, et al. Essential role of the PfRh5/PfRipr/CyRPA complex during Plasmodium falciparum invasion of erythrocytes. Cell Host Microbe (2016) 20:60–71. doi: 10.1016/j.chom.2016.06.004
13. Raj DK, Das Mohapatra A, Jnawali A, Zuromski J, Jha A, Cham-Kpu G, et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature (2020) 582:104–8. doi: 10.1038/s41586-020-2220-1
14. Raj DK, Nixon CP, Nixon CE, Dvorin JD, DiPetrillo CG, Pond-Tor S, et al. Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science (2014) 344:871–7. doi: 10.1126/science.1254417
15. Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate a in the human placenta. Science (1996) 272:1502–4. doi: 10.1126/science.272.5267.1502
16. Doritchamou JYA, Morrison R, Renn JP, Ribeiro J, Duan J, Fried M, et al. Placental malaria vaccine candidate antigen VAR2CSA displays atypical domain architecture in some Plasmodium falciparum strains. Commun Biol (2019) 2:457. doi: 10.1038/s42003-019-0704-z
17. Chene A, Gangnard S, Dechavanne C, Dechavanne S, Srivastava A, Tetard M, et al. Down-selection of the VAR2CSA DBL1-2 expressed in E. coli as a lead antigen for placental malaria vaccine development. NPJ Vaccines (2018) 3:28. doi: 10.1038/s41541-018-0064-6
18. Palacpac NM, Ntege E, Yeka A, Balikagala B, Suzuki N, Shirai H, et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36,” (in eng). PLoS One (2013) 8:e64073. doi: 10.1371/journal.pone.0064073
19. Baeza Garcia A, Siu E, Sun T, Exler V, Brito L, Hekele A, et al. Neutralization of the plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat Commun (2018) 9:2714. doi: 10.1038/s41467-018-05041-7
20. Singh K, Burkhardt M, Nakuchima S, Herrera R, Muratova O, Gittis AG, et al. Structure and function of a malaria transmission blocking vaccine targeting Pfs230 and Pfs230-Pfs48/45 proteins. Commun Biol (2020) 3:395. doi: 10.1038/s42003-020-01123-9
21. Nagaoka H, Kanoi BN, Ntege EH, Aoki M, Fukushima A, Tsuboi T, et al. Antibodies against a short region of PfRipr inhibit Plasmodium falciparum merozoite invasion and PfRipr interaction with Rh5 and SEMA7A. Sci Rep (2020) 10:6573. doi: 10.1038/s41598-020-63611-6
22. Payne RO, Silk SE, Elias SC, Miura K, Diouf A, Galaway F, et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight (2017) 2(21):e96381. doi: 10.1172/jci.insight.96381
23. Sirima SB, Mordmüller B, Milligan P, Ngoa UA, Kironde F, Atuguba F, et al. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children,” (in eng). Vaccine (2016) 34:4536–42. doi: 10.1016/j.vaccine.2016.07.041
24. Dassah S, Adu B, Tiendrebeogo RW, Singh SK, Arthur FKN, Sirima SB, et al. GMZ2 vaccine-induced antibody responses, naturally acquired immunity and the incidence of malaria in burkinabe children. Front Immunol (2022) 13:899223:899223. doi: 10.3389/fimmu.2022.899223
25. Alves KCS, Guimaraes JM, Almeida MEM, Mariuba LAM. Plasmodium falciparum merozoite surface protein 3 as a vaccine candidate: A brief review. Rev Inst Med Trop Sao Paulo (2022) 64:e23. doi: 10.1590/S1678-9946202264023
26. Yu S, Wang J, Luo X, Zheng H, Wang L, Yang X, et al. Transmission-blocking strategies against malaria parasites during their mosquito stages. Front Cell Infect Microbiol (2022) 12:820650:820650. doi: 10.3389/fcimb.2022.820650
27. Miura K, Tachibana M, Takashima E, Morita M, Kanoi BN, Nagaoka H, et al. Malaria transmission-blocking vaccines: Wheat germ cell-free technology can accelerate vaccine development. Expert Rev Vaccines (2019) 18:1017–27. doi: 10.1080/14760584.2019.1674145
28. Takashima E, Tachibana M, Morita M, Nagaoka H, Kanoi BN, Tsuboi T. Identification of novel malaria transmission-blocking vaccine candidates. Front Cell Infect Microbiol (2021) 11:805482:805482. doi: 10.3389/fcimb.2021.805482
29. Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One (2008) 3:e2636. doi: 10.1371/journal.pone.0002636
30. Ishino T, Tsuboi T. Progress toward a transmission-blocking vaccine against malaria. Lancet Infect Dis (2018) 18:927–8. doi: 10.1016/S1473-3099(18)30358-X
31. Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: A randomised, double-blind phase 1 trial. Lancet Infect Dis (2017) 17:498–509. doi: 10.1016/S1473-3099(17)30104-4
32. Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, DeZure A, Enama ME, et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci USA (2017) 114:2711–6. doi: 10.1073/pnas.1615324114
33. Oneko M, Steinhardt LC, Yego R, Wiegand RE, Swanson PA, Kc N, et al. Safety, immunogenicity and efficacy of PfSPZ vaccine against malaria in infants in western Kenya: A double-blind, randomized, placebo-controlled phase 2 trial. Nat Med (2021) 27:1636–45. doi: 10.1038/s41591-021-01470-y
34. Prinz H, Sattler JM, Roth A, Ripp J, Adams JH, Frischknecht F. Immunization efficacy of cryopreserved genetically attenuated plasmodium berghei sporozoites. Parasitol Res (2018) 117:2487–97. doi: 10.1007/s00436-018-5937-0
35. Good MF, Stanisic DI. Whole parasite vaccines for the asexual blood stages of plasmodium. Immunol Rev (2020) 293:270–82. doi: 10.1111/imr.12819
36. Cai J, Chen S, Zhu F, Lu X, Liu T, Xu W. Whole-killed blood-stage vaccine: Is it worthwhile to further develop it to control malaria?. Front Microbiol (2021) 12:670775:670775. doi: 10.3389/fmicb.2021.670775
37. Le TT, Cramer JP, Chen R, Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov (2020) 19:667–8. doi: 10.1038/d41573-020-00151-8
38. Heaton PM. The covid-19 vaccine-development multiverse. N Engl J Med (2020) 383:1986–8. doi: 10.1056/NEJMe2025111
39. Verdecia M, Kokai-Kun JF, Kibbey M, Acharya S, Venema J, Atouf F. COVID-19 vaccine platforms: Delivering on a promise?. Hum Vaccin Immunother (2021) 17:2873–93. doi: 10.1080/21645515.2021.1911204
40. Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat Rev Drug Discov (2021) 20:817–38. doi: 10.1038/s41573-021-00283-5
41. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
42. Valentin A, Bergamaschi C, Rosati M, Angel M, Burns R, Agarwal M, et al. Comparative immunogenicity of an mRNA/LNP and a DNA vaccine targeting HIV gag conserved elements in macaques. Front Immunol (2022) 13:945706:945706. doi: 10.3389/fimmu.2022.945706
43. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov (2018) 17:261–79. doi: 10.1038/nrd.2017.243
44. Rehwinkel J, Gack MU. RIG-i-like receptors: Their regulation and roles in RNA sensing. Nat Rev Immunol (2020) 20:537–51. doi: 10.1038/s41577-020-0288-3
45. De Beuckelaer A, Grooten J, De Koker S. Type I interferons modulate CD8(+) T cell immunity to mRNA vaccines. Trends Mol Med (2017) 23:216–26. doi: 10.1016/j.molmed.2017.01.006
46. Botos I, Liu L, Wang Y, Segal DM, Davies DR. The toll-like receptor 3:dsRNA signaling complex. Biochim Biophys Acta (2009) 1789:667–74. doi: 10.1016/j.bbagrm.2009.06.005
47. Sun T, Holowka T, Song Y, Zierow S, Leng L, Chen Y, et al. A plasmodium-encoded cytokine suppresses T-cell immunity during malaria. Proc Natl Acad Sci USA (2012) 109:E2117–26. doi: 10.1073/pnas.1206573109
48. Beeson JG, Kurtovic L, Dobano C, Opi DH, Chan JA, Feng G, et al. Challenges and strategies for developing efficacious and long-lasting malaria vaccines. Sci Transl Med (2019) 11(474). doi: 10.1126/scitranslmed.aau1458
49. Bonam SR, Renia L, Tadepalli G, Bayry J, Kumar HMS. Plasmodium falciparum malaria vaccines and vaccine adjuvants. Vaccines (Basel) (2021) 9(10):1072. doi: 10.3390/vaccines9101072
50. Kanoi BN, Nagaoka H, Morita M, Tsuboi T, Takashima E. Leveraging the wheat germ cell-free protein synthesis system to accelerate malaria vaccine development. Parasitol Int (2021) 80:102224. doi: 10.1016/j.parint.2020.102224
51. Malik JA, Ahmed S, Mir A, Shinde M, Bender O, Alshammari F, et al. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J Infect Public Health (2022) 15:228–40. doi: 10.1016/j.jiph.2021.12.014
52. Krause PR, Fleming TR, Longini IM, Peto R, Briand S, Heymann DL, et al. SARS-CoV-2 variants and vaccines. N Engl J Med (2021) 385:179–86. doi: 10.1056/NEJMsr2105280
53. Collier DA, De Marco A, Ferreira I, Meng B, Datir RP, Walls AC, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature (2021) 593:136–41. doi: 10.1038/s41586-021-03412-7
54. Muik A, Wallisch AK, Sanger B, Swanson KA, Muhl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science (2021) 371:1152–3. doi: 10.1126/science.abg6105
55. Mahase E. Covid-19: UK will roll out moderna’s omicron BA.1 vaccine as part of autumn booster programme. BMJ (2022) 378:o2038. doi: 10.1136/bmj.o2038
56. Pringle JC, Carpi G, Almagro-Garcia J, Zhu SJ, Kobayashi T, Mulenga M, et al. RTS,S/AS01 malaria vaccine mismatch observed among Plasmodium falciparum isolates from southern and central Africa and globally. Sci Rep (2018) 8:6622. doi: 10.1038/s41598-018-24585-8
57. Arevalo-Pinzon G, Curtidor H, Munoz M, Patarroyo MA, Bermudez A, Patarroyo ME. A single amino acid change in the Plasmodium falciparum RH5 (PfRH5) human RBC binding sequence modifies its structure and determines species-specific binding activity. Vaccine (2012) 30:637–46. doi: 10.1016/j.vaccine.2011.11.012
58. DeGrace MM, Ghedin E, Frieman MB, Krammer F, Grifoni A, Alisoltani A, et al. Defining the risk of SARS-CoV-2 variants on immune protection. Nature (2022) 605:640–52. doi: 10.1038/s41586-022-04690-5
59. Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther (2008) 16:1833–40. doi: 10.1038/mt.2008.200
60. Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release (2016) 240:227–34:pp. doi: 10.1016/j.jconrel.2015.12.032
61. Minnaert AK, Vanluchene H, Verbeke R, Lentacker I, De Smedt SC, Raemdonck K, et al. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across. Adv Drug Deliv Rev (2021) 176:113900. doi: 10.1016/j.addr.2021.113900
62. Kariko K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res (2011) 39:e142. doi: 10.1093/nar/gkr695
63. Goddard-Borger ED, Boddey JA. Implications of plasmodium glycosylation on vaccine efficacy and design. Future Microbiol (2018) 13:609–12. doi: 10.2217/fmb-2017-0284
64. Favuzza P, Blaser S, Dreyer AM, Riccio G, Tamborrini M, Thoma R, et al. Generation of Plasmodium falciparum parasite-inhibitory antibodies by immunization with recombinantly-expressed CyRPA. Malaria J (2016) 15:161. doi: 10.1186/s12936-016-1213-x
65. Pulendran B, Arunachalam PS, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov (2021) 20:454–75. doi: 10.1038/s41573-021-00163-y
66. Zeng G. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine. Lancet Microbe (2022) 3:e561. doi: 10.1016/S2666-5247(22)00150-1
67. Bouard D, Alazard-Dany D, Cosset FL. Viral vectors: From virology to transgene expression. Br J Pharmacol (2009) 157:153–65. doi: 10.1038/bjp.2008.349
68. Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol (2018) 9:1963:1963. doi: 10.3389/fimmu.2018.01963
69. Choi Y, Chang J. Viral vectors for vaccine applications. Clin Exp Vaccine Res (2013) 2:97–105. doi: 10.7774/cevr.2013.2.2.97
70. Dolzhikova IV, Tokarskaya EA, Dzharullaeva AS, Tukhvatulin AI, Shcheblyakov DV, Voronina OL, et al. Virus-vectored Ebola vaccines. Acta Naturae (2017) 9:4–11.
71. Lundstrom K. Viral vectors for COVID-19 vaccine development. Viruses (2021) 13. doi: 10.3390/v13020317
72. Yusuf Y, Yoshii T, Iyori M, Mizukami H, Fukumoto S, Yamamoto DS, et al. A viral-vectored multi-stage malaria vaccine regimen with protective and transmission-blocking efficacies. Front Immunol (2019) 10:2412:2412. doi: 10.3389/fimmu.2019.02412
73. Keshavarzi Arshadi A, Webb J, Salem M, Cruz E, Calad-Thomson S, Ghadirian N, et al. Artificial intelligence for COVID-19 drug discovery and vaccine development. Front Artif Intell (2020) 3:65. doi: 10.3389/frai.2020.00065
74. Malone B, Simovski B, Moline C, Cheng J, Gheorghe M, Fontenelle H, et al. Artificial intelligence predicts the immunogenic landscape of SARS-CoV-2 leading to universal blueprints for vaccine designs. Sci Rep (2020) 10:22375. doi: 10.1038/s41598-020-78758-5
75. Yang Z, Bogdan P, Nazarian S. An in silico deep learning approach to multi-epitope vaccine design: A SARS-CoV-2 case study. Sci Rep (2021) 11:3238. doi: 10.1038/s41598-021-81749-9
76. Wedlund L, Kvedar J. New machine learning model predicts who may benefit most from COVID-19 vaccination. NPJ Digit Med (2021) 4:59. doi: 10.1038/s41746-021-00425-4
77. Tomic A, Tomic I, Rosenberg-Hasson Y, Dekker CL, Maecker HT, Davis MM. SIMON, an automated machine learning system, reveals immune signatures of influenza vaccine responses. J Immunol (2019) 203:749–59. doi: 10.4049/jimmunol.1900033
78. Kheirollahpour M, Mehrabi M, Dounighi NM, Mohammadi M, Masoudi A. Nanoparticles and vaccine development. Pharm Nanotechnol (2020) 8:6–21. doi: 10.2174/2211738507666191024162042
79. Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol (2013) 3:13. doi: 10.3389/fcimb.2013.00013
80. Khurana A, Allawadhi P, Khurana I, Allwadhi S, Weiskirchen R, Banothu AK, et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today (2021) 38:101142. doi: 10.1016/j.nantod.2021.101142
Keywords: malaria, P. falciparum, mRNA vaccines, Africa, COVID-19, reverse vaccinology, immunoinformatics
Citation: Kanoi BN, Maina M, Likhovole C, Kobia FM and Gitaka J (2022) Malaria vaccine approaches leveraging technologies optimized in the COVID-19 era. Front. Trop. Dis 3:988665. doi: 10.3389/fitd.2022.988665
Received: 07 July 2022; Accepted: 19 August 2022;
Published: 08 September 2022.
Edited by:
Kwadwo Asamoah Kusi, University of Ghana, GhanaReviewed by:
Irfan Zaidi, National Institutes of Health (NIH), United StatesCopyright © 2022 Kanoi, Maina, Likhovole, Kobia and Gitaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernard N. Kanoi, Ymthbm9pQG1rdS5hYy5rZQ==; Jesse Gitaka, amdpdGFrYUBta3UuYWMua2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.