- 1Department of Tuberculosis, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China
- 2National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, Shenzhen, China
Central nervous system tuberculosis (CNSTB) is the most fatal type of tuberculosis (TB). Early administration of glucocorticoids can improve the prognosis of CNSTB patients and reduce mortality; however, some CNSTB patients do not respond well to anti-tuberculosis drugs and glucocorticoids. As an immunomodulatory drug, Thalidomide has been used under such circumstances. We retrospectively reviewed the drug to describe its clinical characteristics, efficacy, and safety in the treatment of four complicated CNSTB patients who responded well to thalidomide. Thalidomide may be an effective and well-tolerated drug for the treatment of CNSTB, and therefore requires further study.
Introduction
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (MTB) and remains a major cause of ill-health and mortality. Around 9.9 million people fell ill with TB in 2020 (1). Central nervous system tuberculosis (CNSTB) is the most fatal type of TB, which include tuberculous meningitis(TBM), tuberculoma without meningitis and spinal tuberculosis. It accounts for approximately 5–10% of all extrapulmonary TB cases and 1% of all TB cases (2). Although CNSTB is a crippling disease with a high rate of morbidity and mortality, early application of glucocorticoids can improve patient prognosis and reduce mortality (3, 4). However, some patients do not respond well to anti-tuberculosis drugs (ATD)(include isoniazid, rifampicin,ethionamide and pyrazinamide) and glucocorticoids (5). Studies have shown that the immunomodulatory drug thalidomide can help alleviate these problems, especially when glucocorticoids are ineffective (6, 7).
In the present study, we describe a case series of four patients with complicated CNSTB who were treated with adjunctive thalidomide between 2017 and 2020. We aim to report our experience of using thalidomide to treat a range of complicated CNSTB cases (Table 1).
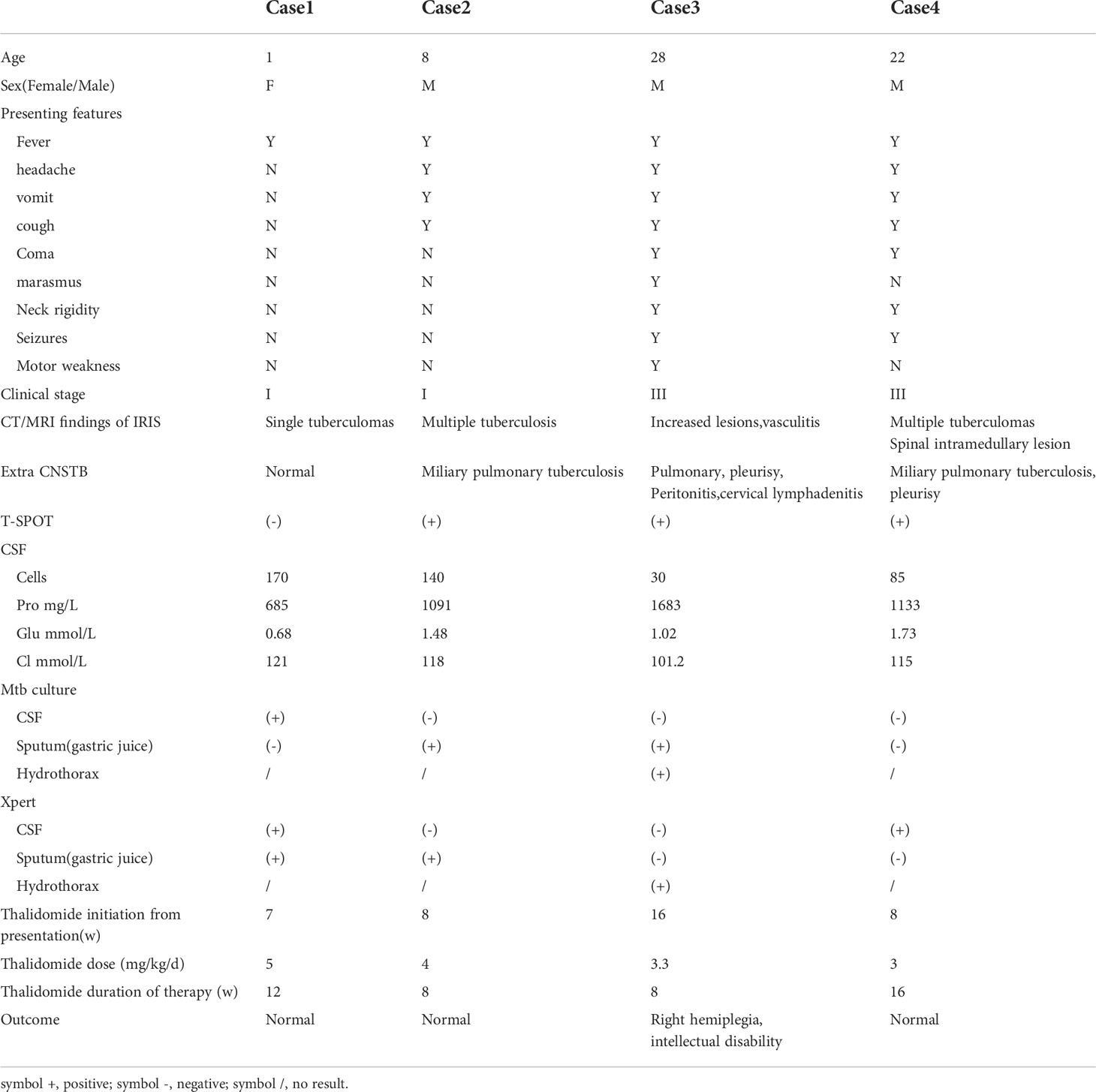
Table 1 Demographic, clinical, laboratory and imaging findings of 4 patients of CNSTB treated with thalidomide.
Case 1
A one-year-old girl presented with fever and vomiting. No focal neurology was detected on examination. Magnetic resonance imaging (MRI) showed abnormally enhanced nodular lesions near the right cerebral horn. Cerebrospinal fluid (CSF) and gastric juice (GJ) were positive for X-pert MTB/RIF assay with no rifampicin resistance (RR). CSF culture was positive for MTB. The patient was treated with antitubercular therapy (ATT) and dexamethasone. Fever and vomiting improved after 20 days in the hospital, but symptoms repeated on the 50th day. With her guardian’s consent, thalidomide was initiated at a dose of 5 mg/kg/d. The patient’s temperature returned to normal after five days of thalidomide administration and continued for three months without side effects. ATT was discontinued after one year. The girl responded well to this treatment and had no central nervous system sequelae.
Case 2
A nine-year-old boy presented with fever, headache, vomiting, and cough. No focal neurology was identified. An MRI (Figures 1A–C) revealed multiple rounded lesions in the left occipital lobe with peripheral enhancement. The proximal end of the right middle cerebral artery was thin, and tuberculous vasculitis was considered. GJ was positive for X-pert MTB/RIF assay and MTB culture with no drug resistance. The patient was started on ATT and dexamethasone for four weeks. Although gradual improvement was observed initially, the patient complained of headache, vomiting, and fever following the reduction of glucocorticoids. Repeat MRI (Figures 1D–F) showed that the left occipital lobe lesion was significantly larger than the anterior lesion. As GJ MTB culture with drug susceptibility testing had been obtained, the possibility of drug-resistant TB was not considered, which made the diagnosis of paradoxical reactions more likely. Therefore, the glucocorticoid dose was maintained and thalidomide was initiated at a dose of 4 mg/kg/d for two months,Thalidomide treatment improved the intracranial lesions (Figures 1G–I). ATT was maintained for one year. The patient has since recovered.
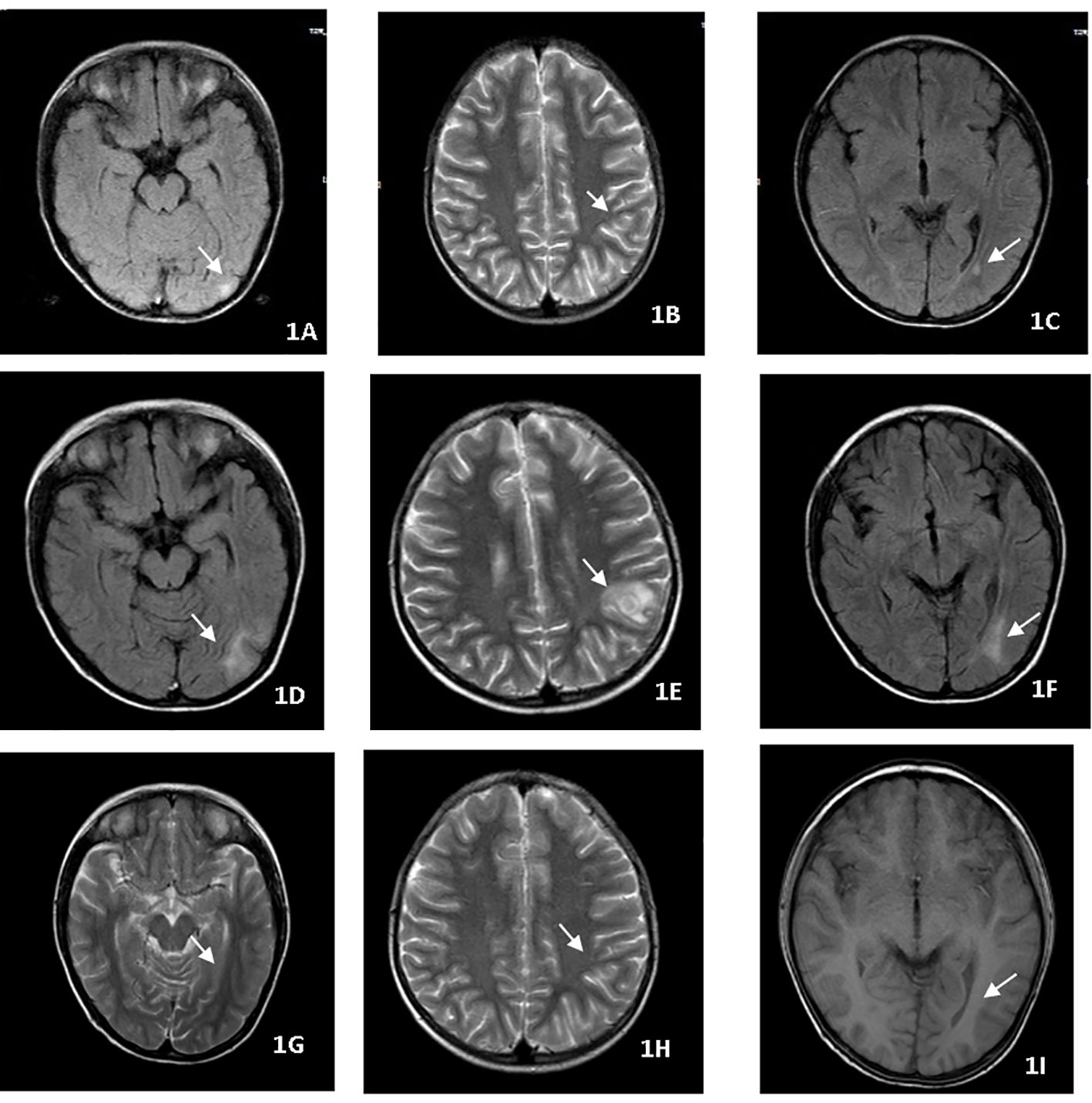
Figure 1 (A–C) MRI revealed multiple rounded lesions in the left occipital lobe with peripheral enhancement. (D–F) MRI showed that the left occipital lobe lesion was significantly larger than the anterior lesion. (G–I) Thalidomide treatment improved the intracranial lesions after 2 months.
Case 3
A 28-year-old male on treatment for pulmonary TB, pleurisy, and peritonitis for two weeks presented with fever, headache, vomiting, coma, and cough. Neurological examination revealed a dense right-sided hemiparesis in isolation and neck rigidity (Glasgow Coma Scale GCS 8/15). Brain CT imaging (Figures 2A–D) confirmed an abnormal signal in the right temporal lobe with associated meningeal thickening and enhancement, and multiple linear and nodular enhancement was observed in the area of the right middle cerebral artery. Hydrothorax was positive for X-pert MTB/RIF assay with no RR. The culture of sputum and hydrothorax was positive for MTB after three weeks. The patient commenced ATT and dexamethasone. After 16 weeks of therapy, the patient remained with right-sided hemiplegia evident on exercise and intellectual disability. The patient’s meningitis was complicated with vasculitis. So thalidomide was started at a dose of 3.3 mg/kg/d for two months to maximally inhibit inflammation. In the days following the initiation of thalidomide, the patient became more responsive and was able to follow simple one-stage commands and move his right limbs,and MRI showed obvious improvement of intracranial lesions (Figures 2E, F). After 18 months of ATT, the patient improved and was able to walk by himself with a mild limp. Computational power, cognitive ability and linguistic ability improved but did not reach the level of his peers.
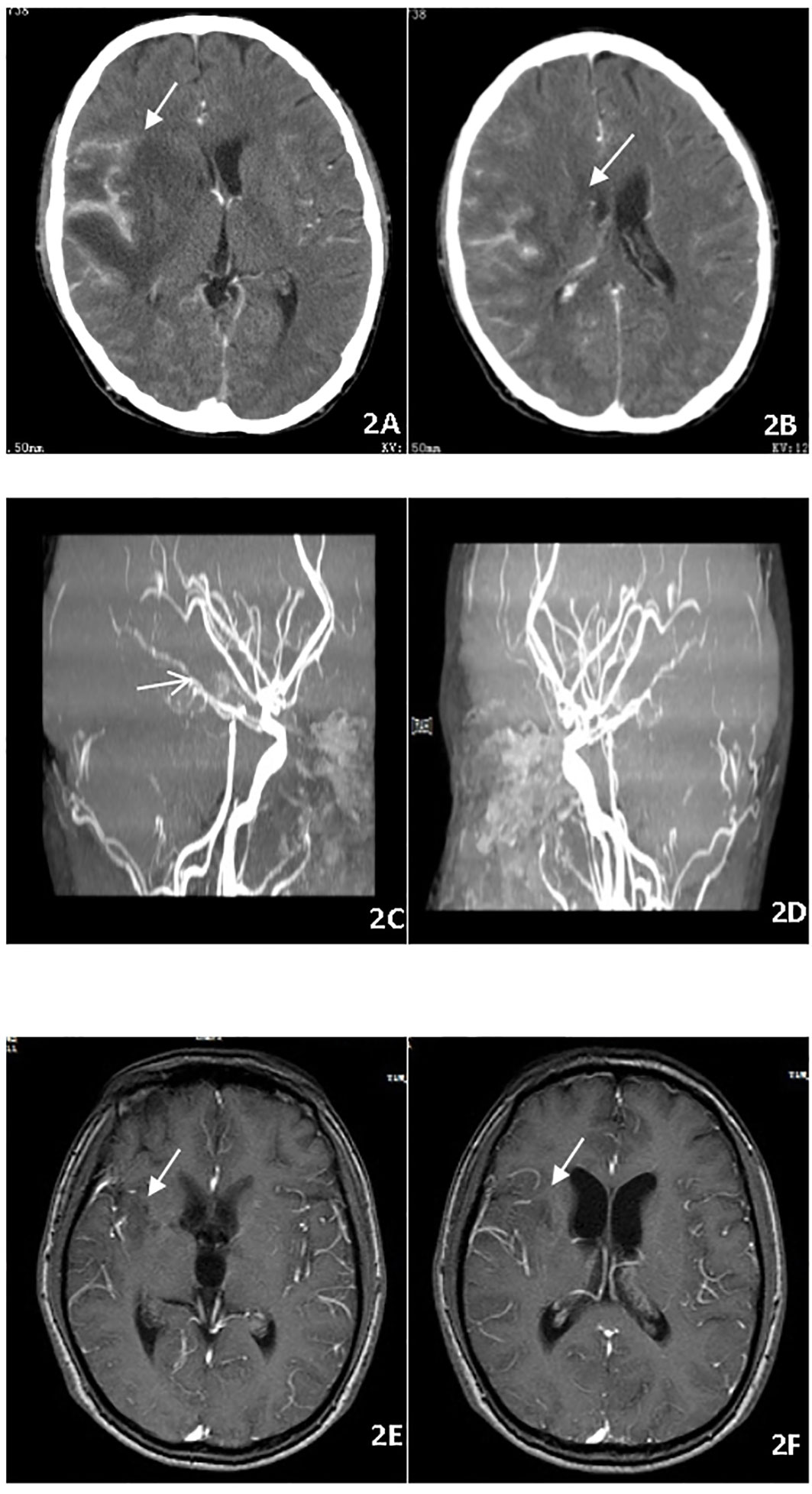
Figure 2 (A–D) Brain CT imaging before treatment, abnormal signal in the right temporal lobe with meningeal thickening and enhancement and multiple linear and nodular enhancement in the right middle cerebral artery. (E, F) Brain MRI showed thalidomide treatment improved the intracranial lesions after 2 months.
Case 4
A 22-year-old male presented with fever, headache, vomiting, and coma. Clinical examination revealed meningitis and lethargy (GCS 12/15). MRI (Figure 3A) showed multiple enhancing nodular lesions from the left cerebellar hemisphere and the right occipital lobe to the right frontal lobe. CSF was positive for X-pert MTB/RIF assay with no RR. The patient was started on standard ATT and dexamethasone. The patient improved quickly and returned to normal consciousness a week later. The dexamethasone dosage was reduced gradually. Two months later, the patient complained of backache. A repeat MRI (Figure 3B) showed cervical and thoracic spinal membrane thickening and enhancement with multiple rounded lesions. Oral glucocorticoids (prednisone) were administered at a dosage 10 mg/d. The extent of spinal cord swelling and risk of cord compression was felt to justify the simultaneous initiation of thalidomide in an attempt to maximally inhibit inflammation. Therefore, thalidomide (3 mg/kg/day) was administered for 4 months, while the TB regime was continued for an additional 12 months. The patient remains clinically well (Figure 3C).
Figure 3 (A) Brain MRI showed multiple enhancing nodular lesions in the left cerebellar hemisphere. (B) Spin MRI showed cervical and thoracic spinal membrane thickening and enhancement with multiple rounded lesions. (C) Spin MRI showed the thickening and enhancement of cervical and thoracic spinal membranes were significantly improved.
Discussion
Inflammation in the brain has always been regarded as an important determinant of the prognosis of CNSTB. It has been proven that the levels of TNF-α, IL-2, IL-6, and IFN-γ in the CSF play an important role in the pathogenesis and severity of tuberculous meningitis (TBM) (8). As early as 1950, it was found that glucocorticoids alleviated the meningitis reaction (9). However, in the treatment of CNSTB, an immune reconstitution inflammation syndrome (IRIS) often occurs after the glucocorticoid dosage is reduced. It showed that the initial treatment was effective with the clinical symptoms and imaging manifestations deteriorating after 2 weeks to 3 months of full treatment (10, 11). In our study (Table 1), IRIS occurred after 2 weeks to 4 months of full treatment. Three cases showed deterioration of clinical and neuroimaging manifestations, and one case only showed deterioration of clinical manifestations. Therefore, it is necessary to explore additional adjunctive anti-inflammatory drugs, which can better dampen excessive inflammation in CNSTB. Thalidomide is one of these drugs, especially when traditional high-dose corticosteroid therapy fails to control chronic inflammation and other neurological complications associated with TBM (12, 13).
As a potent TNF-α inhibitor, the clinical application of thalidomide in immune, neoplastic, and infectious diseases has expanded considerably in recent years. Using a rabbit model of TBM, Tsenova et al. (14) demonstrated that thalidomide treatment combined with antibiotics markedly reduced TNF-α levels, leukocytosis, and brain pathology, resulting in 100% survival of the infected rabbits. Studies suggest that thalidomide has a beneficial effect on TBM during antibiotic treatment in children (15). In our study, in Table 1, two children and two adults without HIV treated with adjunctive thalidomide for CNSTB-related complications (IRIS) had good prognoses. The IRIS in cases two, three, and four occurred during corticosteroid reduction, then thalidomide was initiated. Therefore, thalidomide may have had a role in allowing the safe reduction of steroids. Patients with high risk factors such as optochiasmatic arachnoiditis, tuberculomas (cases one and two), vasculitis leading to cerebral infarcts (cases two and three), and spinal arachnoiditis (case four) may need to use thalidomide earlier.
Van et al. (15) showed that thalidomide at a lower dosage (<5 mg/kg/d) may be efficacious with a favorable safety profile. However, a dose of 12 to 24 mg/kg/d can be used based on the patient’s condition,The course of the treatment can be extended to 8 months based on the duration of the disease (7). The thalidomide dosage used in our cases was 3–5 mg/kg/day, with a treatment duration of 2–4 months. The patients tolerated thalidomide well.Nevertheless, there are several side effects of thalidomide including teratogenicity,gastrointestinal reactions, hematological toxicity, cardiovascular toxicity, neurotoxicity, skin damage, pulmonary embolism, and others. Therefore, attention should be paid to the above side effects during the use of thalidomide.
It is pertinent to mention that robust scientific evidence for the use of any of above options is lacking; thus, it is essential to conduct large multicenter trials to provide guidelines for better management of patients with CNSTB. Few clinical studies of thalidomide in the treatment CNSTB currently exist, and it is hoped that these cases can provide clinicians with diagnosis and treatment ideas for the treatment of CNSTB.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PL and SL coordinated all work and did most of the writing. TL was responsible for evaluation of the medical records and imaging data. NP and LX, XX were responsible for the pathological data. WH and XL, YY were responsible for the biochemical and microbiological data. All authors reviewed and commented on the drafts and approved the final manuscript and the decision to submit for publication.
Funding
Construction of key clinical specialties in Shanghai (shslczdzk03002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Global tuberculosis report 2021. Geneva: WHO (2021). Available at: http://www.who.int/mediacentre/news/releases/2021/political-commitment-tb/en.
2. Katrak SM. Central nervous system tuberculosis. J Neurol Sci (2021) 421:117278. doi: 10.1016/j.jns.2020.117278
3. Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect Dis (2014) 14(10):947–57. doi: 10.1016/S1473-3099(14)70852-7
4. Critchley JA, Young F, Orton L, Gamer P. Corticosteroids for prevention of mortality in people with tuberculosis: Systemic review and meta-analysis. Lancet (2013) 13:223–37. doi: 10.1016/S1473-3099(12)70321-3
5. Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics (1997) 99:226–31. doi: 10.1542/peds.99.2.226
6. Lagier JC, Raoult D. Immune reconstitution inflammatory syndrome associated with bacterial infections. Expert Opin Drug Saf (2014) 13(3):341–50. doi: 10.1517/14740338.2014.887677
7. Liu P, Pei N, Liu X, Huang W, Lu S. Thalidomide in the treatment of human immunodeficiency virus-negative tuberculous meningitis: A case report. Med (Baltimore). (2020) 99(40):e22639. doi: 10.1097/MD.0000000000022639
8. Sharma S, Goyal MK, Sharma K, et al. Cytokines do play a role in pathogenesis of tuberculous meningitis: A prospective study from a tertiary care center in India. J Neurol Sci (2017) 379:131–6. doi: 10.1016/j.jns.2017.06.001
9. Shane SJ, Clowater RA, Riley C. The treatment of tuberculous meningitis with cortisone and streptomycin. Can Med Assoc J (1952) 67(1):13–5.
10. Thampi N, Stephens D, Rea E, et al. Unexplained deterioration during antituberculous therapy in children and adolescents: clinical presentation and risk factors. Pediatr Infect Dis J (2012) 31(2):129–33. doi: 10.1097/INF.0b013e318239134c
11. Van Rie A, Sawry S, Link-Gelles R, et al. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome in children. Pediatr Pulmonol (2016) 51(2):157–64. doi: 10.1002/ppul.23221
12. Kumar R, Kolloli A, Singh P, Vinnard C, Kaplan G, Subbian S. Thalidomide and phosphodiesterase 4 inhibitors as host directed therapeutics for tuberculous meningitis: Insights from the rabbit model. Front Cell Infect Microbiol (2020) 9:450. doi: 10.3389/fcimb.2019.00450
13. Thwaites G, Fisher M, Hemingway C, et al. British Infection society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect (2009) 59(3):167–87. doi: 10.1016/j.jinf.2009.06.011
14. Tsenova L, Sokol K, Freedman VH, Kaplan G. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis (1998) 177(6):1563–72. doi: 10.1086/515327
Keywords: thalidomide, central nervous system tuberculosis (CNSTB), tuberculous meningitis (TBM), immune reconstitution inflammation syndrome (IRIS), treatment
Citation: Liu P, Li T, Xi X, Pei N, Huang W, Liu X, Xia L, Yang Y and Lu S (2022) Case Report: Thalidomide in the treatment of complicated central nervous system tuberculosis. Front. Trop. Dis 3:983635. doi: 10.3389/fitd.2022.983635
Received: 01 July 2022; Accepted: 20 September 2022;
Published: 07 October 2022.
Edited by:
Theolis Barbosa, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Decheng Wang, China Three Gorges University, ChinaDiego Luis Costa, Department of Biochemistry and Immunology, University of São Paulo, Brazil
Amina A. Kamar, American University of Beirut, Lebanon
Copyright © 2022 Liu, Li, Xi, Pei, Huang, Liu, Xia, Yang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuihua Lu, bHVzaHVpaHVhNjZAMTI2LmNvbQ==
 Ping Liu
Ping Liu Tao Li1
Tao Li1 Xuhui Liu
Xuhui Liu Shuihua Lu
Shuihua Lu