
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 17 October 2022
Sec. Major Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.957166
Background: Anemia is a major public health problem, affecting nearly one-quarter of the world’s population. It is defined as a reduction in the hemoglobin level in the peripheral blood to below the normal threshold set for a particular population. Very often in the subtropics, helminths or malaria co-infect an individual, causing morbidities that vary by age and region. This study aims to characterize the type of anemia observed in children under 7 years of age infected with malaria in the western region of Côte d’Ivoire, to recommend a better strategy of care.
Methods: The study was carried out from March 2020 to May 2021 in 22 villages in Man, Tonkpi Region, with a cohort of 451 children, both male and female, aged from 3 months to 6 years. The children provided venous blood samples for the diagnosis and characterization of anemia (full blood count), and Giemsa staining (GS) (thick and thin smears) and rapid diagnostic tests (RDTs) were used for the diagnosis of malaria. Risk factors and morbidity profiles were assessed using a questionnaire. Logistic regressions models were employed to identify independent risk factors and morbidity patterns associated with Plasmodium falciparum mono-infection and co-infections.
Results: Of the 451 children who completed the study, 221 (49.0%) were female and 230 (51.0%) were male. The prevalence of anemia was 55.0%, distributed as 30.7% mild, 66.1% moderate, and 3.2% severe anemia. The characterization of anemia revealed that hypochromic microcytic anemia (HMA) was the predominant type, being found in 195 (78.63%) children. It was followed by normochromic microcytic anemia (29 children, 11.69%), normochromic normocytic anemia (14 children, 5.65%), and, finally, hypochromic normocytic anemia (10 children, 4.03%). The prevalence of malaria was 66.7% and 78.3% based on GS and RDTs, respectively. The closed association between malaria (Plasmodium) and anemia led to P. falciparum alone causing 56.7% of mild, 51.3% of moderate, and 37.5% of severe anemia in children.
Conclusion: Malaria infection was highly prevalent among children aged ≤ 7 years in both sex and in different age groups, although the number of Plasmodium parasites present during infections was greatest in younger children. Similarly, the prevalence of anemia was high, with moderate anemia and HMA being more prevalent in children ≤ 7 years of age in the western region of Côte d’Ivoire.
Parasitic infections such as malaria are common in the tropical and subtropical regions. They lead to high rates of morbidity and mortality, especially in children (1). In 2020, approximately 241 million people worldwide were infected with malaria, and an estimated 627,000 of these individuals died (2). Furthermore, the World Health Organization (WHO) African Region has been the most affected by malaria, accounting for 95% of malaria cases and 96% of malaria deaths worldwide. Children under 5 years of age in the same region accounted for approximately 80% of all deaths from malaria (3). Malaria is responsible for acute or chronic anemia in populations in the tropics (4). A 2022 WHO report on anemia estimated that the global prevalence of anemia was 39.8% in children aged 6–59 months and 29.9% in women of childbearing age (defined as women aged 15–49 years) (4). This equates to 269 million anemic children and over half a billion anemic women aged 15–49 years (4).
In Côte d’Ivoire, the prevalence of malaria in 2017 was estimated at 49% and 52% based on Giemsa staining (GS) of a thick blood smear and rapid diagnostic testing (RDT), respectively (5). The prevalence of anemia in 2019 among children aged 6–59 months was 72.2%, and among women of childbearing age it was 51% (6). In most cases, polyparasitism is the rule, and these conditions affect the same people, causing morbidities that vary by age and region (7, 8). Hence, it seems appropriate, even essential, to conduct research to treat these morbidities, which represent public health problems. This community-based research study aims to characterize the type of anemia observed in children under 7 years of age infected with malaria in Man, Tonkpi Region, in the western region of Côte d’Ivoire, to recommend a better strategy of care.
This observational clinical research study was carried out from March 2020 to May 2021 in 22 villages in Man, Tonkpi Region. The target sample size of 451 was calculated using Schwartz’s formula:
where p is the expected prevalence of anemia in the target population, estimated at 73.4% (9), e an accuracy of 5%, c a corrective coefficient for the cluster effect of 1.5, and z a confidence level of 95% (z = 1.96).
Children aged 3 months to< 7 years were enrolled and provided blood samples for the diagnosis of anemia and malaria. Study participants belonged to different ethnic groups (Akan, Gour, Mandé, Krou, and others from neighboring countries such as Burkina Faso, Mali, and Guinea), resided in a rural area, had similar lifestyle patterns, and had parents who earned their living mostly as farmers or commercial traders.
Sociodemographic data, including age, sex, height (to the nearest cm), weight (to the nearest 0.5 kg), and parents’ profession, were also collected using a questionnaire administered to each enrolled participant who provided a signed written informed consent or gave a fingerprint (if illiterate).
A total of 2 ml of venous blood was drawn in the morning into ethylenediaminetetraacetic acid (EDTA)-treated evacuated tubes from each of the 451 consented study participants. Blood samples were kept on ice until they were transported to the central laboratory at the centre hospitalier régional (CHR), Man, for the hematology testing [i.e., full blood count (FBC)].
In addition, approximately 10–20 µl of blood was collected from the children’s pricked fingers for use in malaria RDTs.
Approximately 10 µl of the 2 ml of venous blood that was collected in the EDTA-treated tube was used to prepare GS thick and thin blood smear on a single slide for the count of malaria parasitemia (10, 11). The slides were analyzed and quality controlled by well-trained and experienced laboratory technicians. Slides were considered positive when asexual forms and/or gametocytes of any Plasmodium species were observed on the blood film. Parasite density per microliter of blood was determined by the number of malarial parasites per 200 leukocytes on a thick blood film, assuming a white blood cell (WBC) count of 8000 leucocytes/µl of blood and then transformed into malaria parasite density by multiplying by a factor of 40. Malaria parasite density was classified as low (< 5,000 parasites/µl of blood) or high (≥ 5,000 parasites/µl of blood) (10, 11).
The GS (thick and thin blood smear) slides were scored as negative or positive. Positive scores were further classified as Plasmodium falciparum, P. malariae, P. vivax, or P. ovale, or as undetermined if the technician experienced difficulty clearly identifying the species.
About 10–20 µl of blood collected from the child’s pricked finger was tested for malaria using an RDT, using the plastic capillary tube provided in the RDT kit, as per manufacturer instructions (Humasis Malaria Pf/Pan Antigen Test; Humasis Co. Ltd, Humasis, South Korea). The result was scored as negative (i.e., no P. falciparum detected) or mixed (i.e., P. falciparum and other species of Plasmodium detected).
The FBC was measured using the URIT 3000 PLUS analyzer (URIT Medical Electronic Co. Ltd, Guilin, China). The results were printed and analyzed further, and characterized on the basis of the mean corpuscular hemoglobin (MCH) (normal value 27–31 pg) and the mean corpuscular hemoglobin concentration (MCHC) (normal value 33–36 g/dl) as either normochromic or hypochromic, and on the basis of the mean cell volume (MCV) (normal value 80–100 fl) as normocytic, microcytic, or macrocytic.
Anemia was classified, depending on age and sex, according to hemoglobin (Hb) concentration, as per WHO guidelines (www.who.int/vmnis/indicators/haemoglobin.pdf) (12). Thus, children aged from 3 months to 6 years with a Hb level of< 11 g/dl were considered anemic, and those with a Hb level of ≥ 11 g/dl were considered normal (non-anemic). Anemia was also categorized as mild if the Hb level was< 10 g/dl, as moderate if Hb level was between 7 g/dl and 10 g/dl, and as severe if the Hb level was< 7 g/dl. The samples were further characterized based on the MCH and MCHC as either normochromic or hypochromic, and based on MCV as being normocytic, microcytic, or macrocytic.
Data were entered into a database using the double-entry system in EpiData version 3.1 (EpiData, Odense, Denmark). Inconsistencies were cleaned and, after validation, the data were exported for the analysis to SPSS version 20 [IBM Corp (13)] and Stata®, version 16 software (Stata Corp LP, College Station, TX, USA). Univariate analysis (chi-squared test), Wilcoxon rank-sum tests, and Kruskal–Wallis tests were used for comparison between groups. Logistic regression estimates were employed to identify independent risk factors and morbidity patterns associated with P. falciparum mono-infections and co-infections.
This study was conducted after obtaining ethics clearance from the Comité National d’Éthique des Sciences de la Vie et de la Santé (CNESVS) de la Côte d’Ivoire (N/Ref 024-21/MSHP/CNESVS-km). Further permission to conduct the study was obtained from the chief of each village visited. In essence, the full study details (i.e., the aims, procedures, and potential risks and benefits) were explained to the physicians, nurses, and assistant nurses of each involved health center and the villagers before the start of the study.
Given that all the participants were minors, consent from one parent was sought and obtained before the study procedure was completed. Only voluntarily consented participants were included in the study. Treatment was made available free of charge to all sick participants. Those who were diagnosed with mild or moderate anemia, with or without malarial infection, received an ambulatory treatment of antimalarial or antihelminthic and/or anti-anemic treatment based on their Hb level (with treatment via ferric hydroxide polymaltose complex syrups), and their RDT and GS slide (thick and thin blood smear) results (with treatment via artemether/lumefantrine 20/120 or 40/240 tablets). Those who required further assistance were referred to the local health center.
Participants were informed that their information would be anonymized (confidential) by using a coding system instead of their names.
A total of 451 children from 22 villages were enrolled in the study, of whom 230 (51.0%) were male and 221 (49.0%) were female (Supplemental Table 1).
Of the 451 children enrolled in the study, 248 (55.0%) were anemic and 203 (45.0%) were not anemic (Table 1). Further characterization of the observed anemia and its severity showed that 76 (30.7%) children had mild anemia, 164 (66.1%) had moderate anemia, and 8 (3.2%) had severe anemia. Female (56.1%) and male (53.8%) participants were equally affected by anemia. It was noted that anemia varied by age group, with infants aged 3 months to 2 years (66.0%) being at a higher risk of anemia than older children (3 to 6 years; 28.1%). In addition, the majority of observed cases of anemia were of moderate severity (66.1%), with most of the remainder being classified as mild (30.7%) and severe disease being relatively uncommon (3.2%). Details are available in Table 1.
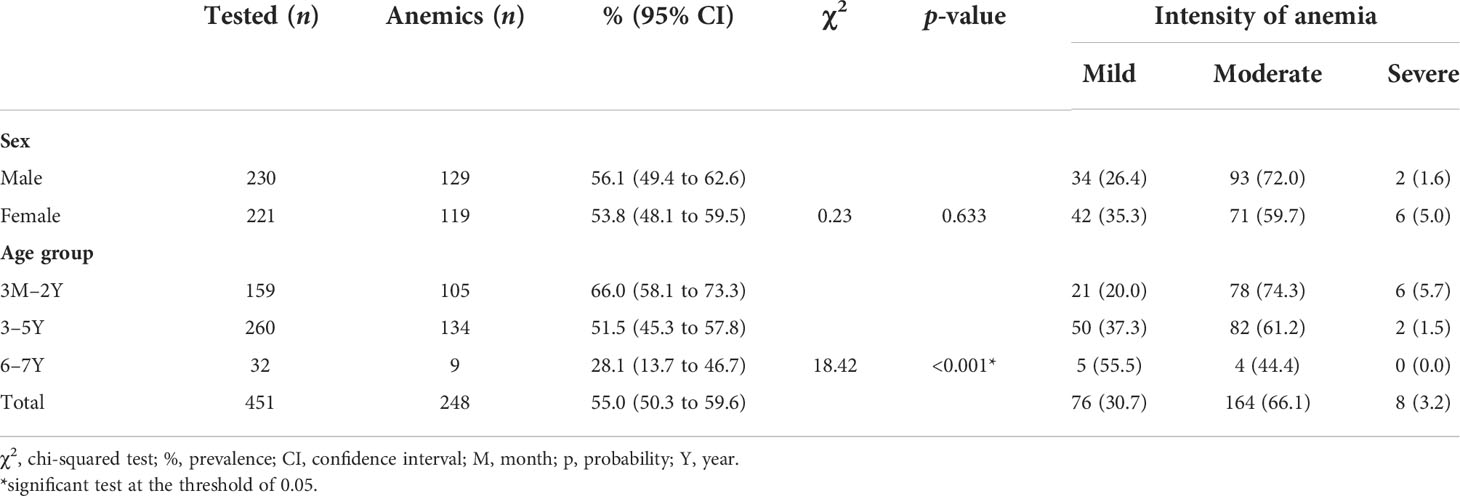
Table 1 Prevalence and intensity of anemia by sex and age, in the western region of Côte d’Ivoire, from March 2020 to May 2021.
The prevalence of each type of anemia did not vary by sex or age. Both sexes and the different age groups were almost equally affected. The only noted variation was the high prevalence of hypochromic microcytic anemia (78.63%), relative to normochromic microcytic anemia (11.69%) and normochromic normocytic anemia (5.65%). No case of macrocytic anemia was detected in the study (Table 2).
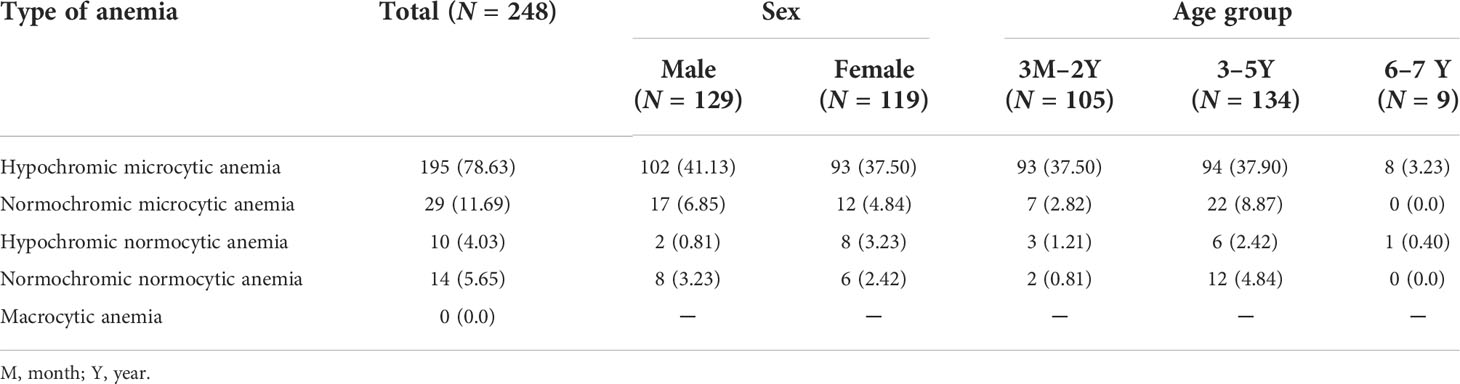
Table 2 Distribution of the type of anemia according to sex and age in the western region of Côte d’Ivoire, from March 2020 to May 2021.
Of the 451 children enrolled in the study, 233 (51.7%) were infected by P. falciparum and 120 (26.6%) by mixed species; 98 (21.7%) tested negative throughout using RDTs. Both sexes were almost equally infected (female 76.9% vs. male 79.6%), with younger children (66.7%) being infected less often than older children (84.6%).
Mono-infection with P. falciparum (51.7%) predominated, followed by co-infection with P. malariae (26.6%), and affected fewer males than females (ratio 1.6 vs. 2.4 respectively) (Table 3).
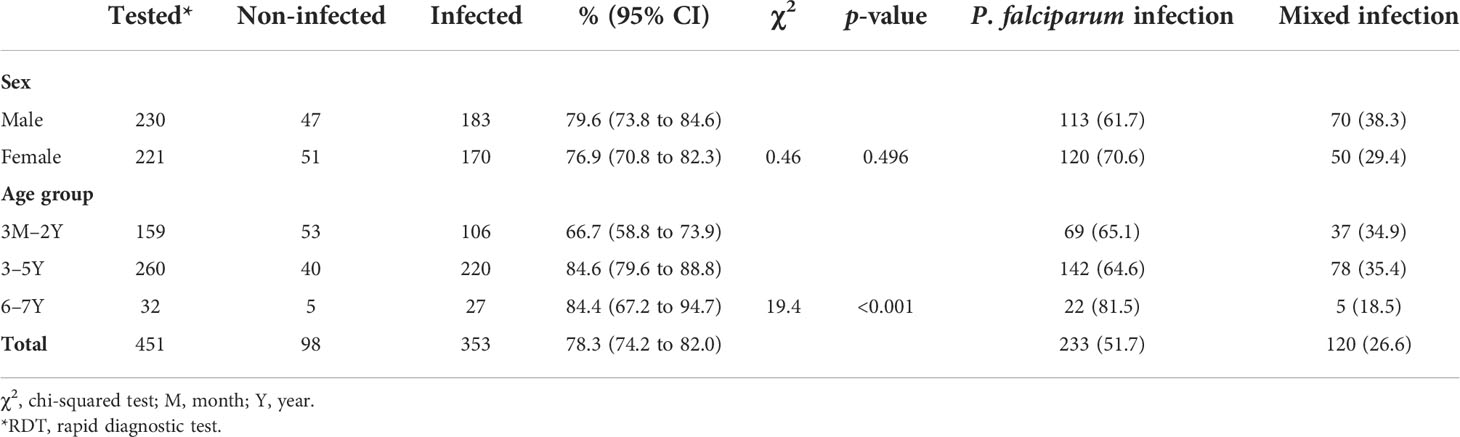
Table 3 Prevalence of malaria (RDT) by sex and age in the Western region of Côte d’Ivoire, from March 2020 to May 2021.
The GS thick and thin blood smear on one slide could be carried out for only 288 (63.9%) participants. Of the 288 slides examined, 192 (66.7%) were positive and 96 (33.3%) were negative (Table 4).
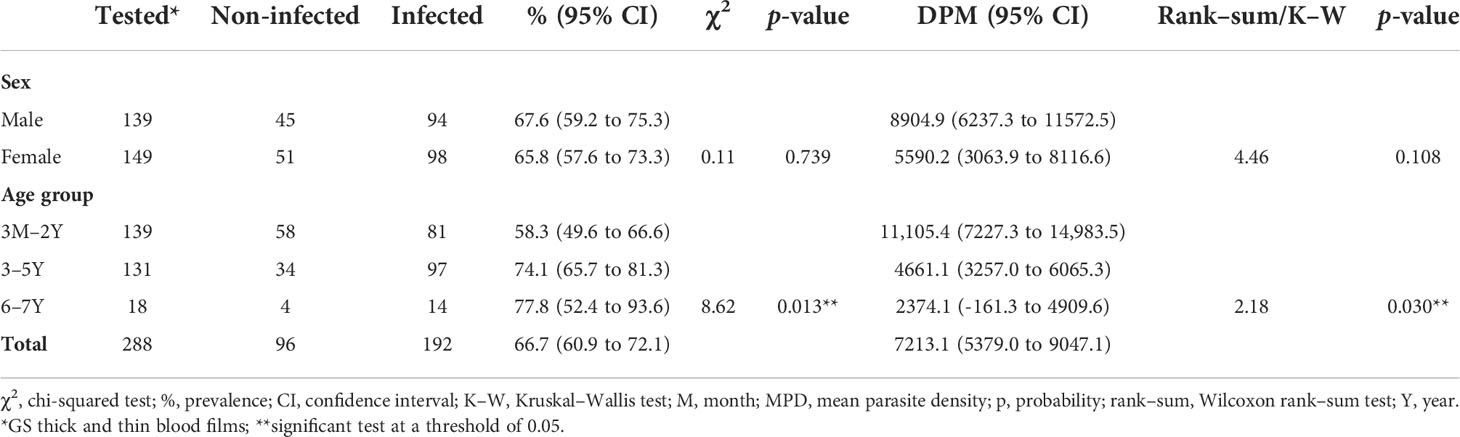
Table 4 Prevalence of malaria (Giemsa stained - thick and thin blood smear test) by sex and age in the Western region of Côte d’Ivoire, from March 2020 to May 2021.
There was no sex variation given that male participants (67.6%) were affected to almost the same extent as female participants (65.8%). Once again, the prevalence was higher among older children (aged 3–6 years) (77.8%) than among the youngest children (aged 3 months to 2 years) (58.3%), as shown in Table 4.
In terms of the mean parasite density in contrario, the study revealed that the youngest children (i.e., those aged 3 months to 2 years) had a higher level of parasitemia (11,105.4/µl of blood) than older children (aged 3-6 years) (2374.1/µl of blood) (Table 4).
Microscopic analysis indicated the presence of two Plasmodium species, which were identified as P. falciparum and P. malariae. Overall, 153 children were infected by P. falciparum (153/288, 53.1%), 13 by P. malariae (13/288, 4.5%), and 26 by a mixture (i.e., co-infection) of P. falciparum and P. malariae (26/288, 9.0%) among the examined slides of the infected persons (data not shown). It is important to emphasize that an RDT was carried out for each of the 451 participants, whereas the GS thick and thin blood smear was carried out for only 288 participants.
With regard to the severity of the anemia due to P. falciparum and P. malariae infections among the 248 anemic children (164 mild, 76 moderate, and 8 severe), the RDT results showed that 56.7% (93/164) of the children with mild anemia were infected with P. falciparum and 20.7% (34/164) co-infected with P. falciparum and P. malariae, and 22.6% (37/164) tested negative. Similarly, 51.3% (39/76) of the children with moderate anemia were infected with P. falciparum and 32.9% (25/76) co-infected with P. falciparum and P. malariae, and 15.8% (12/76) tested negative. Finally, 37.5% (3/8) of the children with severe anemia were infected with P. falciparum, 50.0% (4/8) co-infected with P. falciparum and P. malariae, and 12.5% (1/8) tested negative among the severe cases (data not shown).
In terms of the association between the Plasmodium species and other variables, such as type of anemia, sex, age, Hb levels, and severity of the anemia, the univariate and multivariate logistic regression results showed that there was no significant variation between malaria infection and sex, and that normochromic microcytic anemia was the only statistically significant type of anemia and was more prevalent irrespective of the Plasmodium species in children aged 3–5 years or older (ORa 2.75, 95% CI 1.72 to 4.41; p< 0.001). Furthermore, females are more protected than males against mixed Plasmodium species infections (ORb 0.52, 95% CI 0.29 to 0.94; p = 0.030) (Table 5).
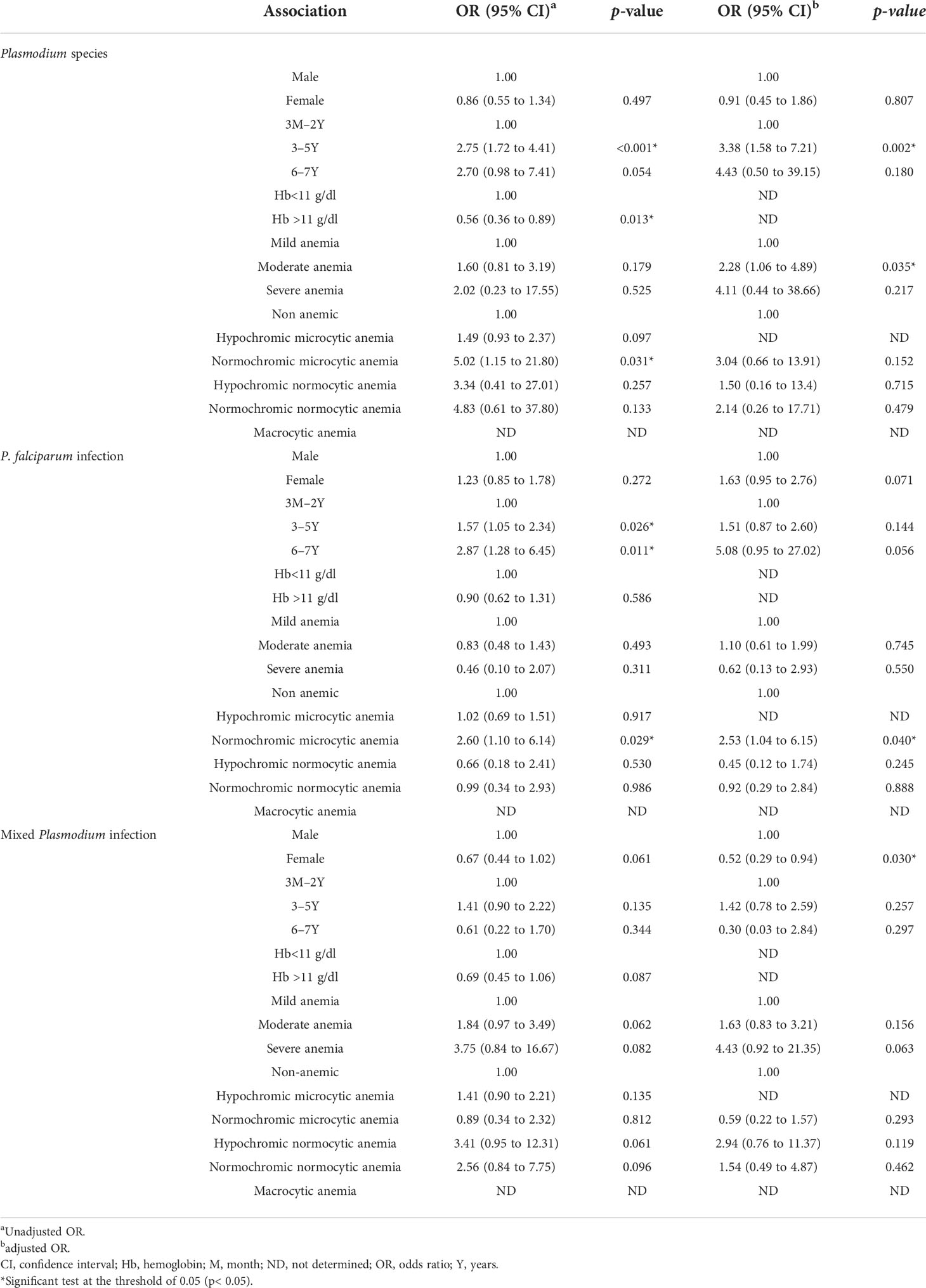
Table 5 Association between plasmodial infections, demographic factors, and anemia in the Western region of Côte d’Ivoire.
This research article reports the findings of an observational community-based research study conducted in children between 3 months and 6 years of age in 22 villages in Man, Tonpki Region, Côte d’Ivoire. The overall prevalence of anemia shows that more than half of the children observed were anemic. Both sexes (males and females) were equally affected, although children aged 3 months to 2 years were affected slightly often more than older children (i.e., aged 3– 6 years). In addition, mild and moderate anemia is more prevalent among the same age groups. These results are consistent with those reported in many low- to middle-income countries (LMICs), including Côte d’Ivoire (14, 15), and are far from the WHO’s target goal for 2025 (16). In fact, our results are in line with what is reported by WHO in West Africa, which is that 51% of males and 49% of females of preschool age children are anemic (17). In terms of severity, although the trends from mild to severe are also shared, the prevalence in our study is higher for mild and moderate anemias than the WHO-reported prevalence in West Africa: males — 8% vs. 1.6% severe anemia, 50% vs. 72% moderate anemia, and 21% vs. 26.4% mild anemia; females — 5% vs. 7% severe, 59.7% vs. 48% moderate, and 35.3% vs. 22% mild, respectively (17).
We also noted in this study that the prevalence of anemia among children aged< 5 years was higher (45.3%) than the global prevalence (42.0%) (18). Furthermore, 66.0% of the younger children aged 3 months to 2 years in our study were anemic, and exhibited the highest prevalence in terms of intensity (i.e., 5.7% severe and 74.3% moderate) among the stratified age groups. This alarming status can be explained by the fact that iron is a major factor in anemia in the first 46 months; the interval from conception of the fetus to 2 years of life is a critical window when nutritional needs must be met. If these needs are not met, children may experience morbidity or mortality (19). We noted that, among the stratified age groups, children in the age group ≥6 to < 7 years exhibited the highest prevalence of mild anemia (55.5%) and the lowest prevalence of moderate (44.4%) and severe anemia (0.0%). This may be explained by the fact that children of this age are likely to be spending most of their time running errands or playing outside in various environmental settings in which there are additional foods, such as legumes, nuts and seeds, and fruits and vegetables, to be consumed in addition to their normal shared family food. These extra micronutrients may provide them with a healthy iron balance, reducing their absolute deficiency in iron. In fact, according to Gupta et al. in 2016, and the Physicians Committee for Responsible Medicine (PCRM)’s Guideline in 2022, iron deficiency anemia is usually preventable and highly treatable with the consumption of a fortified diet of whole grains, legumes, nuts and seeds, and fruits and vegetables (20, 21). Similar results were reported by Righetti et al., in 2012 in Côte d`Ivoire, where 78.1% of infants were anemic (61% mild, 32% moderate, and 7% severe), compared with 46.8% of children (94.6% mild, 5.4% moderate, and 0% severe) (22).
The consequences of anemia are well established, and it has been reported several times that iron deficiency anemia affects not only the intelligence of infants and children irreversibly, but also the rapid brain development, physical growth, and early learning capacity, with lifelong consequences on cognition that translate to the equivalent of a 5- to 10-point deficit in intelligence quotient (19, 23–28).
When the observed anemia was further characterized on the basis the MCH, MCHC, and MCV, the highest prevalence was te hypochromic microcytic anemia (78.6%) followed by normochromic microcytic anemia (11.69%). Indeed, iron deficiency is the most common cause of microcytic and hypochromic anemia (26). Iron deficiency anemia is classically described as a microcytic anemia and appears when the body’s iron demand is not met by iron absorption from the diet. In most cases, microcytosis is the result of impaired Hb synthesis. Disorders of iron metabolism and protoporphyrin and heme synthesis, as well as impaired globin synthesis, lead to defective Hb production and to the generation of microcytosis and microcytic anemia (23, 29). Iron deficiency anemia has previously been reported in Côte d’Ivoire by Franziska et al., in 2001, with an estimated 50% prevalence (30), as well as elsewhere in other studies (14, 31–34).
Overall, the prevalence of malaria did not differ significantly between sex in our study (male 79.6% vs. female 76.9%) and the younger children were infected less than the older children (3 months to 2 years: 66.7%; 3–5 years: 84.6%; and ≥ 6 years: 84.4%). We also observed a high prevalence of anemia amongst the same sex (male 56.1% vs. female 53.8%) and in almost the same age groups (3 months to 2 years: 66.0%; 3–5 years: 51.5%; ≥ 6 years: 28.1%) and that was significantly associated with a high levels of P. falciparum infection, as previously reported (35, 36). In fact, the RDT results revealed 51.7% of P. falciparum infections, 26.6% of mixed infections and 21.7% negatives in both sex while the malaria Giemsa stained -thick and thin blood smear slides showed 53.1% P. falciparum, 4.5% P. malariae, 9.0% mixed infections made of P. falciparum and P. malariae and 33.3% of negatives). Our findings highlight the relationship between malaria and anemia, that is the malaria-associated decrease in Hb concentrations in infected individuals, which are in line with previously reported studies in Côte d’Ivoire (14, 32, 37), in other West African countries (28, 38), and globally (19, 36, 39–41).
The analysis of the prevalence of malaria either from the GS thin and thick blood smear tests or from the RDTs by sex indicates that there is no statistical significance on the sex basis (χ2 = 0.11, p = 0.739, and χ2 = 0.46, p = 0.496, respectively). This may be explained by the use of insecticide-treated nets (ITNs), long-lasting insecticidal nets (LLINs), and indoor residual spraying (IRS) by parents following the yearly country-wide National Malaria LLINs distribution programyear, and also reported elsewhere by other authors (42–45).
However, when we consider the prevalence of malaria by age, there is a statistical significance (χ2 = 8.62, p< 0.013, and χ2 = 19.4, p< 0.001, respectively) indicating that the malaria parasites infect younger children (3 months to 2 years) much less than older children (3–5 years and 6 to< 7 years) (58.3% vs. 77.8%). Inversely, there is a low parasitemia in children aged 3 to 6 years compared to the children aged 3 months to 2 years (2374.1 vs. 11,105.4, respectively). This may be because of the maturity of the immune system of the children aged over 3 to 6 years compared with the youngest children (3 months to 2 years), with an immature immune system relying mainly on the mother`s transmitted immune protection (maternal antibodies) or the presence of fetal Hb (HbF), which is protective and causes poor parasite growth, as previously reported (46–50). According to the study by Marete et al., these high variations in the parasitemia may be attributed to various factors, such as geographic locations, sociodemographics, genetic variations among these populations, utilization of malaria preventive measures, or the nutritional and immunity status among the infants (51).
Finally, the multivariate logistic regression analysis showed that female children are more protected than male children against the mixed Plasmodium sp. infection. We could not find any previously reported scientific evidence with regard to the age of our study population. Nevertheless, this protector effect may be because female children sleep in most cases under the impregnated mosquito nets (LLINs) with their mother, whereas male children tend to avoid the nets, mimicking their father.
Furthermore, it has also been reported that, given equal exposure, adult men and women are equally vulnerable to malaria infection, except for pregnant women who are at greater risk of severe malaria in most endemic areas (52).
Our study has some limitations. First, we did not measure serum ferritin levels to assess iron status or reticulocyte counts for confirming if the anemia is regenerative or not. Second, the small sample size led to some undetermined statistical analysis results. Finally, we did not assess the presence of other parasites that may have co-infected the same participant and caused the observed anemia.
Based on our results, the prevalence of anemia among children aged< 7 years is still high, with the moderate hypochromic microcytic anemia being the only statistically significant type of anemia and more prevalent (78.63%) irrespective of the Plasmodium species in children aged 3–6 years in the western region of Côte d’Ivoire. Furthermore, malaria infection was highly prevalent in both sex (66.7% by GS vs. 78.3% by RDT), with the Plasmodium parasites infecting the younger (aged 3 months to 2 years) children much less than the older children (aged 3–6 years). P. falciparum infection was more prominent in older children, who had the lowest infestation rate (parasitemia), than in the younger children, who had the heaviest infestation rate. Therefore, continued and effective efforts are needed to improve the general health of the high-risk population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comité National d’Éthique des Sciences de la Vie et de la Santé (CNESVS) de la Côte d’Ivoire. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
ME and KN contributed to the conception of the study, data analysis and interpretation, drafting of the manuscript, and agreed to be accountable for all aspects of the work. GC was involved in the data analysis of the manuscript. All authors contributed to the article and approved the submitted version.
We are grateful to Dr Mamadou Ouattara, Dr. Diakité Nana Rose-N`Goran and Dr. Fidele Bassa for helpful assistance in the collection of some data. We thank the Bantegouin, Biakale, Blapleu, Bogouiné 1, Botongouiné, Bloleu, Douele-Dimba, Gbangbe Zélé, Gouimpleu, Guezon, Guianle, Guiapleu, Kabacouma, Kiélé, Kpanzaopleu, Kpata, Koutongouiné 2, Lamapleu, Plouba, Tiakeupleu, Tiapleu, and Zoba communities for their willing participation in field study and for all the study participants for their commitment and willingness to collaborate. We are grateful to Dr. Tra Bi Joel, Head of Laboratory at CHR of Man, and the laboratory technician for the processing of the clinical samples. Many thanks go to Dr. Bah Die Anicette, Dr. Aka Assande Ronald and Dr. Mougoh Ella for helpful clinical assistance in the management of the transferred children at CHR of Man.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.957166/full#supplementary-material
1. Snow RW. Global malaria eradication and the importance of plasmodium falciparum epidemiology in Africa. BMC Med (2015) 13:23. doi: 10.1186/s12916-014-0254-7
2. WHO (2021). Rapport 2021 sur le paludisme dans le monde. Available at: https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/world-malaria-report-2021-global-briefing-kit-fre.pdf?sfvrsn=8e5e915_23&download=true (Accessed 15 August 2022).
3. WHO. Malaria. Fact sheets. (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/malaria (Accessed 15 August 2022).
4. WHO. Prevalence of anaemia in children under 5 years (%) (2020). Geneva: World Health Organization. Available at: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (Accessed 10 January 2022).
5. WHO. World malaria report 2018 (2018). Available at: https://www.who.int/malaria/publications/country-profiles/profile_civ_en.pdf (Accessed 13 January 2022).
6. The World Bank. Prevalence of anemia among children (% of children ages 6-59 months) - cote d'Ivoire (2020). Available at: https://data.worldbank.org/indicator/SH.ANM.CHLD.ZS?locations=CI&most_recent_value_desc=true (Accessed 13 January 2022).
7. Darlan DM, Ananda FR, Sari MI, Arrasyid NK, Sari DI. Correlation between iron deficiency anemia and intestinal parasitic infection in school-age children in medan. Earth Environ Sci (2018) 125:1–6. doi: 10.1088/1755-1315/125/1/012059
8. Dioufa S, Folquetb M, Mbofungc K, Ndiayed O, Broue K, Dupontf C, et al. Prévalence et déterminants de l’anémie chez le jeune enfant en afrique francophone – implication de la carence en fer. Arch Pediatrie (2012) 22:1188–97. doi: 10.1016/j.arcped.2015.08.015
9. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in hemoglobin concentration and prevalence of total and severe anemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Global Health (2013) 1(1):16–21. doi: 10.1016/S2214-109X(13)70001-9
10. Trape JF. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans R Soc Trop Med Hyg (1985) 79:181–4. doi: 10.1016/0035-9203(85)90329-3
11. WHO. Diagnosis of malaria (1990). Geneva: PAHO Scientific Publication (Accessed 06 January 2022).
12. NHLBI and NIH (2022). What is anemia? . Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/anemia (Accessed 15 Mai 2020).
13. Available at: (http://www.stata.com/support/faqs/statistics/adjusted-means-after-anova).
14. Knoblauch AM, Winkler MS, Archer C, Divall MJ, Owuor M, Yapo RM, et al. The epidemiology of malaria and anaemia in the bonikro mining area, central côte d’Ivoire. Malaria J (2014) 13:194. doi: 10.1186/1475-2875-13-194
15. Prieto-Patron A, Hutton ZV, Fattore G, Sabatier M, Detzel P. Reducing the burden of iron deficiency anemia in cote D’Ivoire through fortification. J Health Population Nutr (2020) 39:1. doi: 10.1186/s41043-020-0209-x
16. WHO. Discussion paper the extension of the 2025 maternal , infant and young child nutrition targets to 2030 (2017). Available at: https://www.who.int/nutrition/global-target2025/discussion-paper-extension-targets-2030.pdf (Accessed 06 January 2022).
17. Bull World Health Organ. Spatial heterogeneity of haemoglobin concentration in preschool-age children in sub-Saharan Africa. Bull WHO (2011) 89:459–68. doi: 10.2471/BLT.10.083568
18. WHO. Health topic, anemia (2016). Geneva: World Health Organization. Available at: https://www.who.int/health-topics/anaemia#tab=-tab_1 (Accessed 06 January 2022).
19. Jiahong S, Wu H, Zhaob M, Magnussen CG, Xi Bo. Prevalence and changes of anemia among young children and women in 47 low- and middle-income countries 2000–2018. EClinicalMedicine (2021) 41:101136. doi: 10.1016/j.eclinm.2021.101136
20. Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the united states. Nutrients (2016) 8:330–6. doi: 10.3390/nu8060330
21. Physicians Committee for Responsible Medicine. Nutrition_Guide_for_Clinicians (2022). Available at: https://nutritionguide.pcrm.org/nutritionguide/view/Nutrition_Guide_for_Clinicians/1342090/all/Iron_Deficiency_Anemia#:~:text=Iron%20deficiency%20anemia%20is%20usually,iron%20supplementation%20may%20be%20needed (Accessed 15 July 2022).
22. Righetti AurélieA, Koua A-YG, Adiossan LG, Glinz D, Hurrell RF, N'Goran EliézerK, et al. Etiology of anemia among infants, school-aged children, and young non-pregnant women in different settings of south-central côte d'Ivoire. Am J Trop Med Hyg (2012) 87(3):425–34. doi: 10.4269/ajtmh.2012.11-0788
23. Grantham-McGregor S, Ani CA. Review of studies on the effect of iron deficiency on cognitive development in children. J Nutr (2001) 131:649S–66S. doi: 10.1093/jn/131.2.649S
24. Osorio MM, Lira PI, Batista-Filho M, Ashworth A. Prevalence of anemia in children 6-59 months old in the state of pernambuco, Brazil. Rev Panam Salud Pública (2001) 10:101–7. doi: 10.1590/S1020-49892001000800005
25. Pasricha S-R, Drakesmith H, Black J, Hipgrave D, Biggs B-A. Control of iron deficiency anemia in low-and middle-income countries. Blood (2013) 121:2607–17. doi: 10.1182/blood-2012-09-453522
26. Parul C, Mullany LC, Hurley KM, Katz J, Black RE. Nutrition and maternal, neonatal, and child health. Seminars in Perinatology (2015) 39(5):361–72. doi: 10.1053/j.semperi.2015.06.009
27. Sripriya S, Rabe H. Prevention of iron deficiency anemia in infants and toddlers. Pediatr Res (2021) 89:63–73. doi: 10.1038/s41390-020-0907-5
28. Starck T, Bulstra CA, Tinto H, Rouamba T, Sie A, Jaenisch T, et al. The effect of malaria on haemoglobin concentrations: a nationally representative household fixed−effects study of 17,599 children under 5 years of age in Burkina Faso. Malar J (2021) 20:416. doi: 10.1186/s12936-021-03948-z
29. Chang KH, Stevenson MM. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int J Parasitol (2004) 34:1501–16. doi: 10.1016/j.ijpara.2004.10.008
30. Franziska SA, Adou P, Davidsson L, Cook JD, Hurrell RF. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in côte d’Ivoire. Am J Clin Nutr (2001) 74:776–82. doi: 10.1093/ajcn/74.6.776
31. Rohner F, Zimmermann MB, Amon RJ, Vounatsou P, Tschannen AB, N'Goran EK, et al. In a randomized controlled trial of iron fortification, anthelmintic treatment, and intermittent preventive treatment of malaria for anemia control in ivorian children, only anthelmintic treatment shows modest benefit. J Nutr (2010) 140:635–41. doi: 10.3945/jn.109.114256
32. Righetti AA, Adiossan LG, Ouattara M, Glinz D, Hurrell RF, N'Goran EK, et al. Dynamics of anemia in relation to parasitic infections, micronutrient status, and increasing age in south-central côte d'Ivoire. J Infect Dis (2013) 207:1604–15. doi: 10.1093/infdis/jit066
33. Muriuki JM, Mentzer AJ, Mitchell R. Malaria is a cause of iron deficiency in African children. Nat Med (2021) 27:653–8. doi: 10.1038/s41591-021-01238-4
34. Prieto-Patron A, Hutton ZV, Fattore G, Sabatier M, Detze P. Reducing the burden of iron deficiency anemia in Cote D’Ivoire through fortification. J Health Popul Nutr (2020) 39:1. doi: 10.1186/s41043-020-0209-x
35. Calis CJ, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anemia in Malawian children. N Engl J Med (2008) 358:888–99. doi: 10.1056/NEJMoa072727
36. Safari MKinung’hi, Mazigo HD, Dunne DW, Kepha S, Kaatano G, Kishamawe C, et al. Coinfection of intestinal schistosomiasis and malaria and association with haemoglobin levels and nutritional status in school children in Mara region, northwestern Tanzania: a cross-sectional exploratory study. BMC Res Notes (2017) 10:583. doi: 10.1186/s13104-017-2904-2
37. Koudou BG, Tano Y, Keiser J, Vounatsou P, Girardin O, Klero K, et al. Effect of agricultural activities on prevalence rates, and clinical and presumptive malaria episodes in central côte d'Ivoire. Acta Trop (2009) 111:268–74. doi: 10.1016/j.actatropica.2009.05.006
38. Magalhäes SRJ, Clements ACA. Mapping the risk of anaemia in preschool-age children: the contribution of malnutrition, malaria, and helminth infections in West Africa. PloS Med (2011) 8:e1000438. doi: 10.1371/journal.pmed.1000438
39. Kotepui M, Phunphuech B, Phiwklam N, Chupeerach C, Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malar J (2014) 13:218. doi: 10.1186/1475-2875-13-218
40. Fanello C, Onyamboko M, Lee SJ, Woodrow C, Setaphan S, Chotivanich K, et al. Post-treatment haemolysis in African children with hyperparasitaemic falciparum malaria; a randomized comparison of artesunate and quinine. BMC Infect Dis (2017) 17:575. doi: 10.1186/s12879-017-2678-0
41. Nobelle S, Bigoga J, Ngondi J, Njeambosay B, Esemu L, Kouambeng l, et al. Relationship between malaria, anaemia, nutritional and socio-economic status amongst under-ten children, in the north region of Cameroon: A cross-sectional assessment. PloS One (2019) 14(6):e0218442. doi: 10.1371/journal.pone.0218442.
42. Hetzel MW, Morris H, Tarongka N, Barnadas C, Pulford J, Makita L, et al. Prevalence of malaria across Papua new Guinea after initial roll-out of insecticide-treated mosquito nets. Trop Med Int Health (2015) 20:1745–55. doi: 10.1111/tmi.12616
43. Heggenhougen KH, Hackethal V, Vivek P, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). The behavioural and social aspects of malaria and its control: An introduction and annotated bibliography Vol. 118. . Geneva (2003).
44. Molyneux CS, Murira G, Masha J, Snow RW. Intra-household relations and treatment decision-making for childhood illness: a Kenyan case study. J Biosocial Sci (2002) 34(1):109–31. doi: 10.1017/S0021932002001098
45. Sorge F, Imbert P, Laurent C, Banerjee A, F Khelfaoui F, Guérin N, et al. Protection antivectorielle de l’enfant: insecticides et insectifuges. Arch Pediatr (2007) 14:1442–50. doi: 10.1016/j.arcped.2007.08.022
46. Perlmann P, Troye-Blomberg M. Malaria and the immune system in humans. Chem Immunol (2002) 80:229–42. doi: 10.1159/000058846
47. Yeka A, Nankabirwa J, Mpimbaza A, Kigozi R, Arinaitwe E, Drakeley C, et al. Factors associated with malaria parasitemia, anemia and serological responses in a spectrum of epidemiological settings in Uganda. PloS One (2015), 10. doi: 10.1371/journal.pone.0118901
48. Bostrom S, Giusti P, Arama C, Persson JO, Dara V, Traore B, et al. Changes in the levels of cytokines, chemokines and malaria-specific antibodies in response to plasmodium falciparum infection in children living in sympatry in Mali. Malar J (2012) 11(1):109. doi: 10.1186/1475-2875-11-109
49. Wanzira H, Katamba H, Okullo AE, Agaba B, Kasule M, Rubahika D. Factors associated with malaria parasitaemia among children under 5 years in Uganda: a secondary data analysis of the 2014 malaria indicator survey dataset. Malar J (2017) 16:191. doi: 10.1186/s12936-017-1847-3
50. Gemperli A, Vounatsou P, Kleinschmidt I, Bagayoko M, Lengeler C, Smith T. Spatial patterns of infant mortality in Mali: the effect of malaria endemicity. Am J Epidemiol (2004) 159:64–72. doi: 10.1093/aje/kwh001
51. Marete I, Koskei PK, Koskei A, Nyandiko W. Malaria parasitemia among asymptomatic infants seen in a malaria endemic region of western Kenya. East African Medical Journal (2016) 93:198–204.
Keywords: anemia, prevalence, children under 7 years, malaria, Côte d’Ivoire
Citation: Ehouman MA, N’Goran KE and Coulibaly G (2022) Malaria and anemia in children under 7 years of age in the western region of Côte d’Ivoire. Front. Trop. Dis 3:957166. doi: 10.3389/fitd.2022.957166
Received: 30 May 2022; Accepted: 12 September 2022;
Published: 17 October 2022.
Edited by:
Aparup Das, ICMR-National Institute of Research in Tribal Health, IndiaReviewed by:
Olusola Ojurongbe, Ladoke Akintola University of Technology, NigeriaCopyright © 2022 Ehouman, N’Goran and Coulibaly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mocket Adolphe Ehouman, b2xvcGFtMkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.