- 1Department of Internal Medicine, University of Health and Allied Sciences, Ho, Ghana
- 2Department of Microbiology, University of Health and Allied Sciences, Ho, Ghana
Onchocerciasis affects predominantly rural communities in Africa, and with small foci in South America and the Yemen. The disease is a major cause of blindness and other significant morbidity and mortality. Control programs have achieved a major impact on the incidence and prevalence of onchocerciasis by interrupting transmission with vector control programs, and treatment with mass drug administration using the microfilaricide ivermectin. Over the last few decades, several microfilaricides have been developed. This initially included diethylcarbamazine, which had significant side effects and is no longer used as such. Ivermectin which is a safe and highly effective microfilaricide and moxidectin which is a longer acting microfilaricide are presently recognized therapies. Suramin was the first effective macrofilaricide but was prohibitively toxic. Certain antibiotics including doxycycline can help eliminate adult worms by targeting its endosymbiont bacteria, Wolbachia pipientis. However, the dosing regimens may make this difficult to use as part of a mass disease control program in endemic areas. It is now widely recognized that treatments that are able to kill or permanently sterilize adult filarial worms should help achieve the elimination of this disease. We summarize in detail the historic drug development in onchocerciasis, including prospective future candidate drugs.
Introduction
Onchocerciasis was first described in 1875, about a century and a half ago, by John O’Neill, a clinician, who worked with the British Navy, and who isolated the microfilariae (mf) of Onchocerca volvulus from the skin of people living West African suffering from an intensely itchy skin disorder (1). The adult worms were first described in subcutaneous nodules by Leuckart in 1893 (2). These represent the repository of adult worms which produce the mf responsible for many of the severe clinical manifestations. Rodolfo Robles, a Guatemalan physician, first linked the parasite to visual impairment in 1917 (3). In 1926, D. B. Blacklock, subsequently demonstrated that it was transmitted by insects, belonging to the genus Simulium (4). These insects breed near fast-flowing streams and rivers and infective larvae are inoculated during the bite of the infected female blackfly vectors (5). Nigeria is responsible for 25% of the global prevalence of Onchocerciasis which is also found in 31 other African countries. It has additionally been found in Guatemala, Brazil, Colombia, Yemen, Ecuador, Venezuela and Mexico, probably due to the accidental importation of the nematode (6). While humans are the known host of O. volvulus, experimental infection of chimpanzees with third stage larvae result in their development into adult worms which produce mf that remain detectable for 6.5-9 years (7). It is not clear if zoonotic infections occur naturally, as this may make the complete eradication of the disease challenging (6).
There are a number of strains of this parasite which have differing degrees of infectivity to the various blackflies species and differences in their clinical features (8–11). Although natural infections only occur in the tropics, specifically in Africa, South America, and the Yemen (12), the disease is occasionally also seen in immigrants and travellers from endemic regions who migrate to temperate regions including Europe and the North America, often with a link to war and or poverty (13).
In 1995, Onchocerciasis was estimated to affect about 17.7million people worldwide with two hundred and seventy thousand reported blind and about half a million with severe visual impairment (14). It affects predominantly rural communities, and promotes poverty and considerable socioeconomic consequences. It is ranked second as a cause of avoidable blindness (hence its synonym river blindness), and may reduce the life span of sufferers by up to 15 years (15). It causes other ocular manifestations including punctate or sclerosing keratitis, uveitis, chorioretinitis, and optic atrophy. The prevalence of blindness has decreased substantially following the success of control programmes (16). However, approximately 200 million people reside in areas at risk of transmission and are as such at potential risk of infection (17). Further manifestations of onchocerciasis include skin disease comprising severe itching, papular onchodermatitis, lichenified onchodermatitis, skin depigmentation, skin atrophy and subcutaneous nodules. It is associated with a hanging groin, inguinal and femoral hernias (18), and has been associated with epilepsy (19).
Onchocerca parasites have a long and complicated life cycle. During a blood meal, infected Simulium blackflies introduce third-stage larvae onto the skin of the human host which enter the bite wound, and develop into adult filarial worms. Adult worms typically reside in nodules in subcutaneous or deep tissue locations, and can live for up to a decade and a half and produce mf for many years. The mf are found predominantly in the skin where they live for 1-2 years. An uninfected blackfly ingests these during a blood meal, and the mf then migrate from the midgut of the blackfly, through the hemocoel to reach the thoracic muscles where they develop initially into first-stage larvae and subsequently into infective third-stage larvae. The third-stage infective larvae reach the proboscis of the blackfly and are then able to infect another person (11).
To date, it has not been possible to maintain the O. volvulus parasite cyclically through their complete developmental stages in convenient laboratory hosts or by in vitro cultivation. As such research on drug development has required investigators to depend on material collected from natural infections in the field and implanting or injecting these parasites stages into suitable laboratory hosts or by using in vitro systems of parasite cultivation (11, 20). As such, surrogate models are often used for drug screening. These include infection of BALB/c mice with infective Litomosoides sigmodontis third-stage larvae, resulting in patent infections with circulating mf. Alternative surrogate models include Acanthochieloma viteae infections of jirds and Brugia pahangi infections in a rodent host. Additionally, a number of cattle parasites including Onchocerca gutturosa, Onchocerca linealis and Onchocerca Ochengi have been used as surrogates for drug screening (21).
The blackfly vector of O. volvulus includes several species of Simulium which lay their eggs in fast flowing water. In Africa, the parasite is transmitted predominantly by the S. damnosum complex and other species such as S. sirbanum (22). Some anthropophilic blackfly vectors in South America which transmit the filarial nematode include S. ochraceum, S. incrustatum, S. exiguum, S. guianense, S. metallicum, S. oyapockense, and S. minusculum (23). Blackfly eggs deposited in fast flowing water hatch and release larvae which develop into pupae. The eggs, larvae and pupae are aquatic stages which attach to rocks and plants in fast flowing rivers and streams, and filter out suspended particles. The aquatic stages have been found aquatic vegetation including Cynodon dactylon, Kanahia laniflora and also Digitaria ciliaris. Additionally, manmade constructions such as concrete-lined stream channels and dams provide good locations for the development of blackfly larvae and pupae (24).
Onchocerciasis control began with vector control in West Africa in 1975. This program successfully interrupted the transmission of the parasite in many areas (25), and was then followed by The African Program for Onchocerciasis Control, which used ivermectin mass distribution annually in sub-Saharan Africa, as a means ending onchocerciasis as a disease of public health importance. This program now aims to eliminate transmission where feasible, and has achieved a significant reduction in prevalence, and has now incorporated a target of eliminating onchocerciasis, by 2025 in the majority (80%) of African countries (26). The Onchocerciasis Elimination Program for the Americas, was launched in 1993, as an international partnership and regional initiative based on mass ivermectin administration twice or four times each year, aiming to cover at least 85% of eligible populations. Between 1989 and 2016, at least 11.5 million ivermectin doses have been given in South America and has eliminated transmission in 11 out of 13 endemic foci (27).
Although ivermectin continues to be highly effective, there have been a few reports of sub-optimal responses raising concerns of possible future drug resistance (25, 28), as such it remains essential to develop a safe alternate therapy including an effective macrofilaricidal agent. The new WHO roadmap acknowledges that new medicines or treatment regimens that are able permanently sterilize or kill onchocerca macrofilariae would shorten elimination timelines considerably and hasten the realization of the ultimate goal of disease elimination (29).
Drugs evaluated for anti onchocercal activity
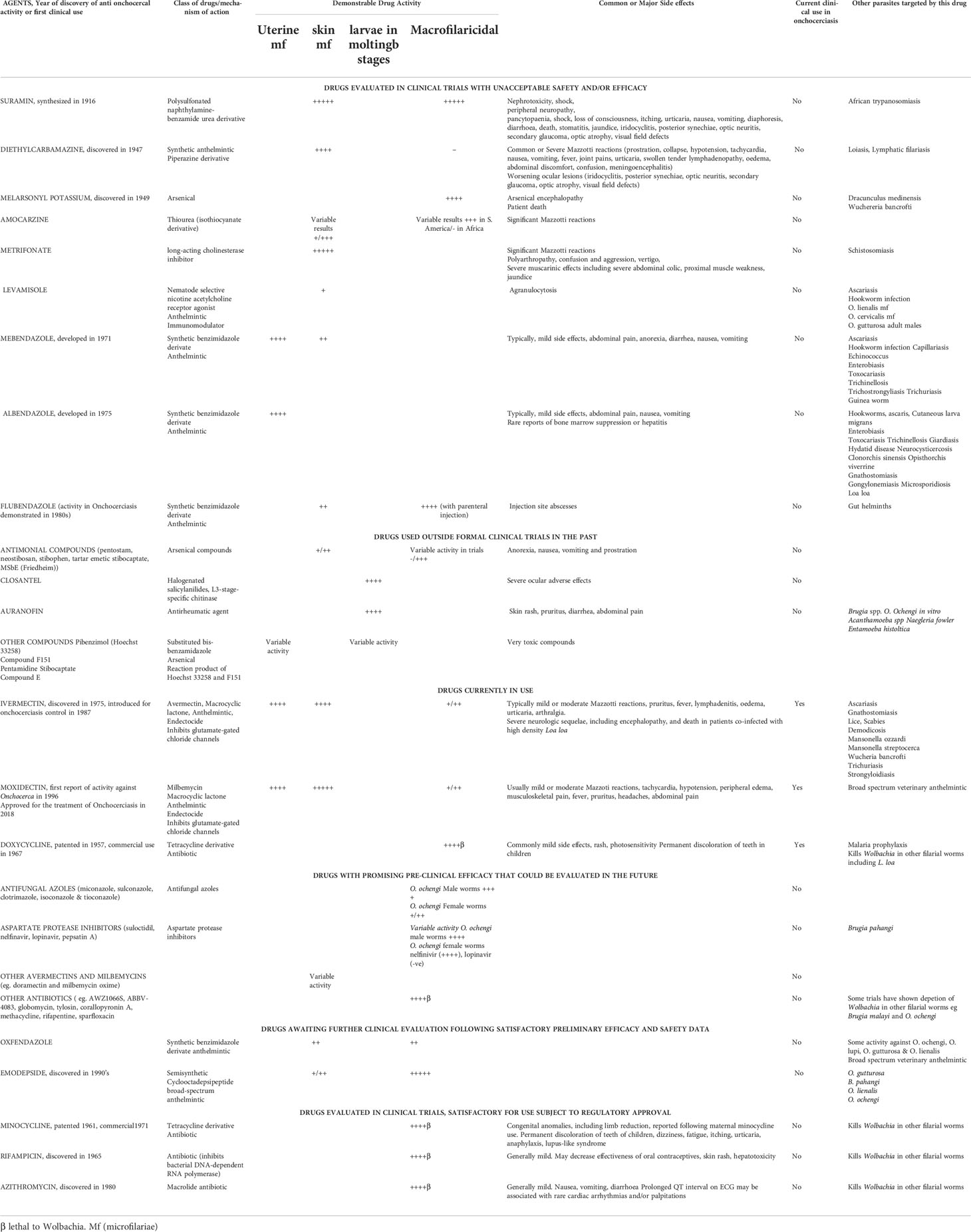
Over the last century, several prospective drugs have been evaluated for anti-Onchocera activity. These drugs are of varying efficacy, and some have been associated with unpleasant or severe side effects including patient death. The drugs that have been evaluated for or used to treat onchocerciasis are described below, including those not in use (Table 1).
Drugs evaluated in clinical trials with unacceptable safety and/or efficacy
Suramin
Paul Ehrlich initially synthesized the dye trypan blue in 1904. It is a polysulfonated naphthylamine which was subsequently found to have antitrypanosomal activity. The pharmaceutical firm Bayer then made a series of derivatives of this dye which included the drug suramin. This was synthesized in 1916 by a team comprising Richard Kothe, Oskar Dressel, and Bernhard Heymann. Suramin was the first drug used to treat onchocerciasis and was found to have both microfilaricidal and macrofilaricidal activity against Onchocerca volvulus (30, 31). The drug was unfortunately prohibitively toxic and requires hospitalization. As such it is no longer in routine use. Side effects of suramin include peripheral neuropathy, renal and bone marrow toxicity, hypersensitivity, dermatitis, anemia, and deteriorating ocular lesions (31, 32).
Diethylcarbamazine
Diethylcarbamazine was discovered in 1947, and was the first active solely microfilaricidal agent. The drug is a piperazine derivative and has the chemical formula of N,N-diethyl-4-methylpiperazine-1-carboxamide. Unfortunately, it does not kill adult worms and as such was not useful in curing the disease. Furthermore, reactions due to the hosts immunological response to the dead and dying mf were rather common and often severe. These reactions were first described by the Mexican parasitologist Luigi Mazzotti in 1948 (33), and include tachycardia, low blood pressure, fever, joint pains, urticaria, swollen tender lymphadenopathy, oedema, and abdominal discomfort. When used to treat heavily infected individuals these reactions could be severe and life-threatening. Furthermore, ocular lesions were often aggravated (34). Deaths were reported in 7 out of 327 patients treated with diethylcarbamazine (35). As such this product has become redundant in the treatment of onchocerciasis.
Melarsonyl potassium
The arsenical drug melarsonyl potassium was discovered in 1949 and used in the treatment of African trypanosomiasis. This drug showed initial promise in the treatment of onchocerciasis. A clinical trial in Cameroon demonstrated that an intramuscular injection of this drug, comprising 4 consecutive daily doses of 200 mg, with the same 4 daily doses repeated after a 10–14-day interval, appeared to kill or sterilize the female adult worms in the treated patients (36).
Following the death of the adult worms, the remaining population of mf gradually falls, over the ensuing 2 years from natural death. Typically, the drug had only little or no immediate activity on mf (37). Despite its efficacy, this drug unfortunately commonly caused arsenical encephalopathy, resulting in the death of some patients. A single trial utilizing a dose of 7.5mg/kg in patients with Onchocerciasis resulted in 4 deaths out of 4287 treated individuals despite the availability of antidote dimercaprol (36). These side effects preclude the use of melarsonyl potassium in the treatment of this filarial disease (38).
Amocarzine (CGP 6140)
Initial Studies in South America suggested that amocarzine (4-methyl-N-[4-(4-nitroanilino) phenyl] piperazine-1-carbothioamide) might be useful in the treatment of onchocerciasis (39).
Following the treatment of 272 Guatemalan patients with amocarzine, the examination of nodules excised four months after treatment showed that more than half (57%) of the female parasites were dead, with nearly a quarter (24%) necrobiotic. Nineteen percent of the female parasites were still alive. Male nematodes were significantly more necrobiotic than females. Following treatment, cutaneous mf decreased rapidly to about 10% of the initial level within one week, and around 20% of pre-treatment levels after one year. In this study, a low dose drug regimen showed activity against adult worms and mf with good drug tolerability in patients with moderate to heavy onchocerciasis. The dose used in this trial was 3 mg/kg body weight, twice daily after food for three days. The drug was well absorbed with predictable plasma levels (40). A similar study carried out in Ecuador and Guatemala treated 312 patients with a dose of 3 mg/kg twice daily for 3 days. The regimen killed 73% of the 1477 female O. volvulus evaluated following the excision of nodules four months after treatment. The average mf skin count was greatly reduced within a week and remained low for at least 6 months. The mf counts were 6-11% of the day 0 value when assessed on day 8 and 14-18% when assessed on day 180. In this study, mf clearance was poor in patients who took the drug while fasting (41).
A study of amorcazine in Mali demonstrated a significant reduction in plasma concentrations and urinary recovery of the drug and of the N-oxide metabolite in fasted patients compared to patients who have been fed beforehand. They authors concluded that the higher solubility or amocarzine at acidic pH compared to poor solubility at neutral pH might explain the increase in bioavailability after meals (42).
A clinical trial in Ghana compared a single dose of 150 µg/kg body weight of ivermectin pre-treatment on day 1 followed by amocarzine (3 mg/kg body weight) taken after meals on days 8, 9 and 10. Thirty-four patients received this and were compared with 33 patients who received ivermectin alone or 33 patients who received amocarzine alone. All palpable nodules were excised on day 120 and were then fixed, sectioned, stained, and examined by two blind researchers. Mazzotti-type reactions, including pruritus, a cutaneous rash, peripheral sensory neuropathy and swellings, were more frequent and more severe with amocarzine than with ivermectin. Pre-treatment with ivermectin suppressed some reactions to amocarzine significantly, but did not affect other symptoms such as dizziness or gaze-evoked nystagmus (43). In this trial, amocarzine did not affect male parasites or the intra-uterine stages, and was a less potent microfilaricide. Ivermectin with amocarzine showed similar efficacy to ivermectin alone. The results were different from the findings from the South America and it was concluded that amocarzine was unlikely to be useful in treating onchocerciasis in Africa (43).
Additional studies in Ghana could only demonstrate a significant microfilaricidal effect at doses greater than 12 mg/kg with the maximum tolerated dose being 20mg/kg and no macrofilaricidal activity at any dose (44). Overall, Mazzotti-type reactions, were noticeably more severe and frequent following treatment with amocarzine.
Metrifonate
Metrifonate demonstrated activity against Onchocerca lienalis mf inoculated into CBA/Ca mice when this model was used as a drug screen for onchocerciasis (45). Additionally, metrifonate had some activity against adult male Onchocerca gutturosa worms in vitro (46).
Metrifonate has also been used in a clinical study for the treatment of infections with O. volvulus infections in persons in West Africa. Using doses of 10 mg/kg daily for a total of six days, metrifonate was effective against the mf, but produced unpleasant reactions due mf death, and the muscarinic effects of this drug (47).
Metrifonate has no action on the adult worms (48). This drug is not used for treating Onchocerciasis due to its adverse side effect profile and lack of macrofilaricidal activity (49).
Levamisole
Preliminary studies in a mouse model suggested that levamisole had microfilaricidal activity against O. lienalis mf (45) and O. gutturosa adult males (46). Furthermore, levamisole has previously been used to treat Onchocerca cervicalis dermatitis in horses (50).
Subsequent human clinical trials in Mexico and in Ghana showed that levamisole did not offer promise in treating human onchocerciasis (51, 52). When given alone or given with albendazole, levamisole had little activity against O. volvulus. Additionally, levamisole combined with ivermectin was not macrofilaricidal and was not more effective against the mf or the adult parasites than ivermectin alone.
Benzimidazoles (mebendazole, albendazole and flubendazole)
Benzimidazole anthelmintics are in routine use for the control of soil-transmitted helminth infections. Clinical studies at the Onchocerciasis Chemotherapy Research Centre in Tamale and subsequently in Hohoe, Ghana showed that the benzimidazole carbamates, mebendazole and albendazole, had different effects on O. volvulus. Mebendazole was microfilaricidal and is also toxic to developing embryos still within an egg shell but not the stretched mf. Albendazole has no activity against mf but was toxic to all uterine stages. The reasons for these differences remain uncertain. In this Ghanaian study, none of the drugs tested had effects against macrofilariae (44).
Flubendazole was approved for the treatment of gastrointestinal nematode infections in 1980 and has been used in by veterinarians and physicians. Unfortunately, this drug has very low oral bioavailability, as such intramuscular injections were employed as the route of delivery in early research (53). The parenteral administration of flubendazole can lead to high macrofilaricidal efficacy. A clinical trial in Latin America in the using weekly intramuscular doses of 750mg of flubendazole in the 1980s demonstrated complete macrofilaricidal efficacy against O. volvulus but unfortunately caused injection site abscess (54). In order to stimulate further research into the discovery and development of new macrofilaricides, for onchocerciasis, recent studies have focused on the development of new formulations with higher oral bioavailability, potentially permitting its usefulness in the treatment of onchocerciasis. When compared with diethylcarbamazine, skin mf levels in the flubendazole group fell slowly, and at 6 and 12 months were significantly lower than in the diethylcarbamazine treated group (0·2 vs 7·3 mf/mg at 12 months). By 6 months none of the flubendazole treated subjects had mf in the cornea, and only one individual had mf in the anterior chamber of the eye, while the numbers of intraocular mf in the diethylcarbamazine group had returned to pre-treatment levels (55). Ivermectin showed consistently superior activity against mf than flubendazole (54).
Drugs used outside formal clinical trials in the past
Antimonial drugs
Several antimonial preparations have been evaluated for the treatment of Onchocerciasis, including pentostam, neostibosan, stibophen, and tartar emetic with little or no usefulness in treating onchocerciasis. Two antimonial preparations of alleged reduced toxicity, TWSb (stibocaptate) and MSbE (Friedheim) were also tested against O. volvulus. These drugs demonstrated inconsistent results, including the complete elimination of all parasites in a few patients on one hand, and no detectable action in other subjects.
Microfilaricidal activity was demonstrable in many persons, especially after treatment with TWSb, which was used at higher doses than MSbE. There was inconsistent killing or sterilizing of adult female worms observed in some patients. Unfortunately, these drugs were rather toxic, and often this made it necessary to stop treatment. Interest in using these antimonial drugs for treating onchocerciasis decreased because of common severe side effects including anorexia, nausea, vomiting and prostration (56).
Closantel
The development of O. volvulus parasites requires a L3-stage-specific chitinase called OvCHT1. Closantel is a halogenated salicylanilides and is used as a veterinary anthelmintic drug that targets this chitinase.
Closantel is a potent and specific proton ionophore and a OvCHT1 inhibitor (57, 58). It inhibits filarial chitinases and inhibits the molting of L3 larvae (59). Closantel, however did not have appreciable macrofilaricidal activity and unfortunately caused severe adverse ocular side effects (60), which have limited its usefulness.
Auranofin
Auranofin, is an orally administered, gold-containing compound. This drug has been used for the treatment of rheumatoid arthritis since at least 1985 when it was approved by the United States Food and Drug Administration (FDA) for treating this condition. This drug has been shown to have antiparasitic properties (61).
It has been shown to kill both Brugia spp. and Onchocerca. ochengi adult parasites in vitro and has been demonstrated to inhibit the molting of third-stage O. volvulus larvae. Half-maximal inhibitory concentrations are low and in the micromolar to nanomolar range (62). There are no clinic trials in patients with Onchocerciasis to date.
Other compounds
Shortly after the discovery of Suramin, Brian Duke demonstrated that pibenzimol (Hoechst 33258; Molecular formula: C25H24N6O), a substituted bis-benzamidazole, showed activity against mf and adult O. volvulus worms in experimentally infected chimpanzees. Additionally, an arsenical compound F151 (Molecular formula: C19H29AsN8O4S2) showed a singular macrofilaricidal action. Furthermore, Compound E (a reaction product of Hoechst 33258 and F151) demonstrated both micro- and macrofilaricidal activity. However, the overall toxicity of these compounds was high, precluding their clinical utility, and as such, further trials were not made. They also tested pentamidine and stibocaptate, but these drugs showed no antifilarial activity (63).
Drugs currently in use
Ivermectin
Ivermectin is a widely used broad-spectrum antiparasitic drug that interferes with glutamate-gated chloride channels found in invertebrates, but not vertebrates. A Japanese biochemist, Satoshi Omura, discovered ivermectin from Streptomyces avermitilis, a soil actinomycete (64). Together with William Campbell, an Irish parasitologist, they were jointly awarded the Nobel Prize (in Physiology or Medicine) for their role in the discovery of avermectins and ivermectin, an acknowledgement of the enormous impact of this discovery in the control of this disease.
Ivermectin is the world’s first endectocide and a safe and highly effective microfilaricide for onchocerciasis (65). Ivermectin was demonstrated to be an effective microfilaricide in the early 1980’s and introduced for onchocerciasis control in 1987 (53). It is a semisynthetic macrocyclic lactone derivative. It is generally better tolerated and safer than diethylcarbamazine in treating Onchocerciasis. Several large-scale programs, especially in endemic areas in Africa employ ivermectin treatment (150 micrograms/kg) once a year for disease control and treatment. This dose paralyses the mf, which are then carried away via lymphatics and destroyed in lymph nodes. It reduces the load of mf by 90% and suppresses the production of mf for about two or three years. Frequent doses of ivermectin can also prevent the development of embryo parasites in female worms, and as such reduces the number of adults with time by attrition. Ivermectin substantially reduces of cures lesions in the anterior segment of the eye. Furthermore, regular treatment prevents severe lesions in the posterior segment (66). There are advocates for repeating treatment every three to six months in symptomatic patients (67), and thereafter, repeating treatment based on a recurrence of symptoms such as pruritus, the presence of the typical rash, or eosinophilia.
Ivermectin may also have a mild effect on adult worms, but is incapable of eradicating infection (68). Multiple doses of ivermectin demonstrate an eventual macrofilaricidal effect and a moderate sterilizing effect after multiple treatments, and a halving of the life expectancy of adult worm after three years of annual ivermectin treatments (69). Treatment with ivermectin may be necessary for 10 years or more until attrition of pre-treatment adult worms which are known to otherwise live for an average of 15 years (70). However, any new infections may make eradication difficult to achieve within this time frame using a yearly ivermectin mass drug administration strategy. Presently, mass drug administration programs consist of ivermectin administration at 6 to 12 monthly intervals for 10 to 16 years (71).
In the setting of dual infection with Loa loa, the treatment of onchocerciasis with ivermectin can facilitate entry of L. loa mf into the central nervous system, leading to severe neurologic sequelae, including encephalopathy, and death. This occurs typically in persons with very high-density L. loa microfilaremia (≥ 30,000 mf/mL blood) (72). As such, in areas where both conditions are endemic, blood should be obtained to test for the presence of L. loa mf prior to ivermectin administration in patients with Onchocerciasis (73, 74). The LoaScope is helpful for quickly determining mf density of L. loa infections in the field (72). This is a novel cell phone–based microscope reader that gives L. loa mf density readings within 3 minutes of placing uncoagulated blood (obtained by finger prick) in a special cubical capillary tube under the lens. The LoaScope permits rapid, point-of-care identification of patients at risk for severe adverse events following therapy. This strategy allows the identification and treatment of patients in L. loa endemic areas who have onchocerciasis but do not have high L. loa microfilarial loads (75).
Adverse effects following ivermectin administration are usually mild and may in part be related to the host immune response to released Wolbachia antigens (76) or the filarial parasite itself (77). Symptoms usually develop within three days of treatment and include a rash, dizziness, pruritus, myalgia, arthralgia, and tender lymphadenopathy. A rise in pulse rate and respiratory rate, and an increase in body temperature or hypotension tends to accompany more severe reactions. These are typically mild or moderate Mazotti reactions. However, severe systemic reactions including postural hypotension may also occur. The incidence of these symptoms correlates with burden of infection prior to treatment. Mazzoti reactions have been graded according to severity (grades 0-4), with grade 0 having no symptoms and grade 4 having the most severe symptoms. With regards to postural hypotension, decreases in mean arterial pressure of >20mmHg, >25mmHg, >30mmHg, or either >35mmHg or the inability to stand long enough for blood pressure to be taken on standing due to dizziness are considered grade 1, 2, 3 and 4 reactions respectively (78). These symptoms can usually be managed with rest, analgesics and antihistamines.
The development of ivermectin resistance has been suggested in Ghana (79). In some communities despite continued excellent microfilaricidal activity, the suppressive effects of ivermectin on the reproductive capacity of adult female O. volvulus worms waned in following multiple drug treatments (80) with these worms becoming unresponsive or increasingly resistant to the anti-fecundity effects of multiple treatments (81). It has been suggested that suboptimal responses may be attributable to host factors, including polymorphisms in host multidrug resistance genes (82). Macrocyclic lactone drug resistance in Dirofilariae immitis is well recognized and making it plausible that drug resistance may develop against other helminths (83). Some authorities consider that anthelmintic resistance in general is inevitable (84).
The ideal approach to the treatment of coinfection with onchocerciasis and loiasis remains uncertain; however, the efficacy of doxycycline and albendazole in eliminating L. loa infections (73, 85) may make these drugs useful precursors to ivermectin treatment in areas with coinfection.
One potential additional benefit of ivermectin mass drug administration is that this drug is an endectocide. Mosquitoes exposed to the drug during feeding on the blood of treated individuals may subsequently die, and has been demonstrated in randomized trials. This effect has bred interest in using ivermectin as a means of malaria control although it is not yet clear if this will result in a reduction in malaria transmission (86, 87).
Ivermectin has also been used for the treatment of scabies, head lice, cutaneous larva migrans and gnathostomiasis. However, there is some concern about the safety of the drug and its use in children under 5 years or in children weighing less than 15 kg. Admittedly, ivermectin has been occasionally used in children younger than 5 years. Nevertheless, the safety of ivermectin in young children and pregnant women remains uncertain (88). A systematic review and meta-analysis of the safety of oral ivermectin during pregnancy has concluded that so far, no study has reported major side effects such as neonatal deaths, preterm births, low birthweight or maternal morbidity. However, it is still unclear if ivermectin increases the risk of congenital abnormalities, spontaneous abortions or stillbirths (89).
Ivermectin combination therapy
The addition of albendazole to ivermectin does not appear to confer treatment benefit. In a randomized trial including more than 270 patients with onchocerciasis in Ghana, combination therapy with ivermectin and albendazole did not improve sterilizing female worms, killing adult parasites, or achieving sustained mf clearance compared with ivermectin alone (90).
Moxidectin
Moxidectin is a semisynthetic macrocyclic lactone and a member of the milbemycin family. It is derived from nemadectin isolated from the bacterium Streptomyces cyanogriseus subsp. Noncyanogenus. This class of drugs are chemically related to the avermectins.
In response to early reports of potential Onchocerca resistance to ivermectin, Tagboto & Townson (91, 92) carried out in vivo studies which demonstrated that moxidectin was more active than ivermectin in eliminating O. volvulus and O. lienalis mf from experimentally infected mice in 1996. They also demonstrated that moxidectin activity against Onchocerca mf in mice persisted for long periods and interrupted laboratory infection of mice several weeks after a single dose of this drug (93). This effect is potentially because this drug is highly lipophilic (94).
Moxidectin has a longer half-life than ivermectin (20-43 days versus approximately 1 day), and is more efficacious than ivermectin in reducing skin microfilarial loads. It may be capable of interrupting the transmission cycle within six rounds of annual treatment. Similar to the prior laboratory-based studies, moxidectin appeared to exhibit greater and more persistent microfilaricidal activity against Onchocerca than ivermectin with a similar side effect profile (95, 96). The adverse events reported following treatment with moxidectin and ivermectin are mostly related to the inflammatory reactions to the dead and dying mf, including pruritus, rashes, tender lymphadenopathy, muscle discomfort, fever, low blood pressure, tachycardia, and eosinophilia. These Mazzotti reactions were more common following treatment with moxidectin, and likely due to the greater efficacy of this drug causing more pronounced killing of mf. Adverse events were self-limiting and mild or moderate, and did not necessitate medical intervention (96).
Moxidectin is now recognized as a reputable veterinary drug for the treatment of a range of endo and ectoparasites and has been proposed as an appropriate treatment for human scabies (97, 98).
It has been suggested that annual moxidectin treatments as an onchocerciasis control strategy would enable a similar reduction in programme duration as biannual ivermectin treatment, with a likely improvement in cost effectiveness. This may have significant connotations where resource limitations are a major issue. Additionally, the need to time treatment to peak transmission periods is not as important for control programmes using moxidectin and should simplify distribution programmes (99).
Moxidectin, has been approved in 2018 by the United States FDA for treatment of onchocerciasis in person who are at least 12 years old (https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210867lbl.pdf).
Doxycycline
O. volvulus parasites carry endosymbiotic bacteria (Wolbachia sp.) required for embryogenesis and survival (100, 101). A number of in vivo and in vitro studies utilizing potential candidate antibiotics were carried out to compare the effect of killing Wolbachia on the subsequent viability of Onchocerca (102–104) and other filarial parasites (103) with doxycycline showing particular promise.
Clinical studies have demonstrated that Doxycycline has excellent activity against Wolbachia and induces sterility in female parasites with a macrofilaricidal effect that is dose dependent (105, 106). For rapid relief of symptoms caused by the mf stage, it may not be unreasonable to treat with ivermectin a week or so prior to starting doxycycline. The safety of simultaneous treatment is unknown. Tetracycline antibiotics including doxycycline may unfortunately cause permanent tooth discoloration in children younger than 8 years and for this reason, this drug is generally avoided in pregnant women and children under 9 years.
Doxycycline may be effective for treatment in communities where onchocerciasis and loiasis are co-endemic. In a study in Cameroon assessing the effectiveness of community-directed treatment with a 6-week course of doxycycline among 104 persons in communities with both onchocerciasis and loiasis, treatment solely with doxycycline was effective in reducing microfilaremia and adult worm viability (107).
A small clinical study in Ghana compared oral doxycycline 200mg once daily for 4 weeks (N = 33), and a shortened duration of treatment with oral minocycline at 200mg once daily for 3 weeks (N=30). On completion of the treatment regimes, histological analysis of the adult parasites in excised nodules revealed the absence of Wolbachia in 98.8% of the doxycycline treated and 72.7% of the minocycline treated female nematodes (108).
Drugs with promising pre-clinical efficacy that could be evaluated in the future
Antifungal azoles and aspartate protease inhibitors
Recent in vitro screening against adult O. ochengi male and female worms obtained from naturally infected cattle, and juvenile O. volvulus (maintained in vitro) confirmed the activity of 13 compounds including candidate drugs that are antifungal azoles or aspartic protease inhibitors. The azole drugs miconazole, sulconazole, clotrimazole, isoconazole and tioconazole were very effective (causing more than 99% motility inhibition) against male O. ochengi (utilizing a 5-day in vitro assay), but not highly active against the viability of female worms. Additional compounds that showed great activity against male O. ochengi worms (with more than 96% motility inhibition) included suloctidil, pimozide and primaquine. IC50 values for suloctidil and primaquine were 5.5 µM and 0.4 µM respectively. Suloctidil and primaquine showed significant efficacy against female O. ochengi (>87% viability inhibition) (109).
Suloctidil is an aspartate protease inhibitor that showed consistently high potency across worm genders. Two aspartate inhibitors, nelfinavir and lopinavir (30 µM), inhibited the motility of female B. pahangi by more than 98%, male O. ochengi by more than 88%, and O. volvulus L4 by more than 56%. In Additional, nelfinavir was demonstrated to be highly effective against the viability of female O. ochengi (100%), while lopinavir was not active against O. ochengi female parasites. Pepstatin A is a different aspartate protease inhibitor that has demonstrable action against O. ochengi, with preferential activity against male worms (100% motility inhibition) compared to female (50% viability inhibition) parasites (109).
Other avermectins and milbemycins
Townson, McCall & Tagboto (1998) tested a range of avermectins and milbemycins including doramectin and milbemycin oxime, and demonstrated variable, but good microfilaricidal activity in all member of this group of drugs, but with moxidectin being superior to other drugs in this group (110).
Eventually subsequent clinical studies have led to the approval of moxidectin as an effective and safe microfilaricide in the treatment of Onchocerciasis (99). Further clinical trials of the other avermectins and milbemycins have not been carried out.
Other antibiotics
The anti-Wolbachia Consortium have been active in identifying novel anti-Wolbachia drugs. A cell-based assay for detecting activity against Wolbachia has permitted approximately 2600 drugs to be screened, that have already approved for human use, with the view to repurposing them for use in treating onchocerciasis. Several drugs have been thus identified as being equal to or more efficacious than doxycycline, with 69 of these drugs being available for oral administration. Following subsequent screening in a filarial model involving Litomosoides sigmodontis infection in mice, four of these were deemed active. These antibiotics were predominantly from the fluoroquinolone, tetracycline and rifamycin drug classes (111).
Following several years of screening potential antibiotics by the anti-Wolbachia consortium, new antibiotics including AWZ1066S and ABBV-4083 are presently going through clinical trials with the aim of delivering a safe and effective macrofilaricide which may support the goal of the elimination of onchocerciasis and lymphatic filariasis (112). AWZ1066S in particular shows superior efficacy to current anti-Wolbachia therapies in validated preclinical models of infection and has properties that are felt to be compatible with a short treatment duration of 7 days or less (113).
Although not yet tested against Onchocerca, globomycin, a compound that is a signal peptidase II inhibitor, has demonstrated action against Wolbachia. It has been shown to reduce the motility and the viability of adult Brugia malayi worms in vitro (114). This drug is worth testing against onchocerciasis. Additional drugs with anti-Wolbachia effects that have not been tested in onchocerciasis include tylosin, an antibiotic used in veterinary practice but not approved for human use, and the drugs corallopyronin A, methacycline, rifapentine and sparfloxacin all of which have depleted Wolbachia in animal filarial models (115).
Synthetic analogues of the macrolide veterinary antibiotic, tylosin A with improved bioavailability when administered orally (A-1535469 and A-1574083) were compared to doxycycline and minocycline, in the treatment of O. ochengi infections. A one- or two-week course of A-1535469 or A-1574083 provided >90% Wolbachia depletion from nematodes in infected animals, causing an interruption of embryogenesis and the depletion of mf. The two analogues delivered equal or better efficacy compared to a three-to-four-week course of doxycycline or minocycline. A-1574083 (also called ABBV-4083) shows promise as an anti-Wolbachia macrolide with an efficacy, pharmacology, and safety profile that is compatible with a short-term oral drug use for onchocerciasis (116).
Drugs awaiting further clinical evaluation following satisfactory preliminary efficacy and safety data
Oxfendazole
Following the demonstration that the parenteral administration of flubendazole showed promise as a macrofilaricide in a variety of models and in humans. Further research has been conducted into benzimidazoles with improved oral bioavailability and a better side effect profile.
Oxfendazole is a benzimidazole anthelmintic with good oral bioavailability. It is metabolized to the less active metabolites, fenbendazole and fenbendazole sulfone. In a study in mice, serum levels were higher with oral than with subcutaneous administration. Unlike flubendazole which is poorly absorbed after oral dosing in humans, bioavailability after oral dosing in humans should exceed 50%. Oxfendazole has been demonstrated to exhibit modest to marginal motility inhibition of the adult filarial nematodes of O. gutturosa, pre-adult (5th stage larva) O. volvulus worms and O. lienalis mf in vitro and was comparable to the activity of flubendazole and albendazole, but less than the activity of melarsomin dihydrochloride (used as a positive control in this study). Clinical phase 1 results suggest that oxfendazole is likely to be nontoxic at doses thought to be effective in treating onchocerciasis (117).
A preliminary trial of oral oxfendazole in treating naturally acquired Onchocerca lupi infections in privately owned domestic dogs in Portugal was recently published. O. lupi infections have been found in dogs and cats in several European nations and in North America, causing mainly ocular lesions. The disease is increasingly being recognized as a zoonosis. In humans, O. lupi displays marked neurotropism and these nematodes are typically embedded in nodules localized in the cervical spine of infants, children and adults with severe consequences. This trial demonstrated a mean percentage of reduction of mf of 78% (n=4) and 12.5% (n=3) respectively 180 days following treatment with 50mg/kg of oxfendazole for 5 or 10 days compared with control animals (n=4). The reduction in skin microfilaria counts was statistically lower in the dogs treated for 5 days but not in the dogs treated for 10 days. By 6 months follow up, skin microfilaria counts were not significantly different from control animals. Additionally, there was a reduction in ocular lesions of treated dogs by ultrasound examination by 50% and 47.5% in the 2 treated groups respectively, but these differences did not reach statistical significance. One treated dog completely cleared its ocular lesions. Further study using a larger sample size will be invaluable (118).
Twenty O. ochengi male worms were implanted into the peritoneum of Mongolian gerbils (Meriones unguiculatus). These gerbils were then treated with subcutaneous oxfendazole (12 mg/kg, twice daily, for 5 days starting on the third day after implantation) and the worms recovered 35 days post implantation. Oxfenbendazole significantly affected worm motility in this study/However, the effects of flubendazole (used as a positive control) on worm burden and viability were more pronounced than that of oxfenbendazole (119). Oxfendazole has not yet been evaluated in a clinical trial in human onchocerciasis.
Drugs undergoing first clinical trials
Emodepside
Despite recent advances in treatment options for Onchocerciasis, important concerns remain. The cornerstone of disease control is based on mass ivermectin distribution programmes, with the drug donated by the pharmaceutical company Merck & Co. Inc. Since this drug is predominantly microfilaricidal, it needs to be given for extended periods to cover the reproductive lifespan of the adult worms and requires substantial population coverage to significantly disrupt transmission. Major limitations of mass drug distribution are the absence of a drug with macrofilaricidal action or permanent sterilizing activity, and concerns over the prospect of ivermectin drug resistance. As such, there remains an urgent need for a new macrofilaricidal drug, that is safe and active orally.
IN 1992, researchers from Meiji Seika Kaisha Ltd. discovered a novel family of N-methylated cyclooctadepsipeptides from the fungus imperfectus Mycelia sterilia (Rosellinia sp.). This fungus is part of the microflora of the leaves of Camellia japonica (120–122). Eight different cyclooctadepsipeptides, designated PF1022A, B, C, D, E, F, G and H were isolated with PF1022A being the most plentiful and the most potent anthelmintic among the members of this group (120, 123). Emodepside (bismorpholino-cyclooctadepsipeptide), a semisynthetic Cyclooctadepsipeptides (124) with broad-spectrum anthelmintic activity was subsequently developed. It is licensed for the treatment of nematodes and cestodes infections in cats and dogs. Emodepside has two morpholine rings in para-position of each of the two (R)-phenyllactic acids, which increase the solubility and improve the bioavailability of this drug, compared to its natural precursor PF1022A (125).
Emodepside is active against extraintestinal nematode stages such as migrating larvae or macrofilariae. The drug binds to the latrophilin-like receptor (LAT-1) of Haemonchus contortus (126). Despite this, Caenorhabditis elegans worms that have a double deletion of lat-1 and lat-2 still die when exposed to emodepside (127), suggesting that whilst latrophilin is one target of emodepside, other targets are also likely to be involved in its anthelmintic action. Emodepside has been shown to bind to ionotropic GABAA receptors from Ascaris suum muscle cells (128) and voltage-gated Ca2+-activated potassium channel SLO-1 (slowpoke big K+ conductance channel) (129, 130).
The fact that emodepside targets filariae life cycle stages including adult worms may be a possible advantage over ivermectin (129). As such, emodepside is thought to be one of the most promising candidates for evaluation as an adulticide for onchocerciasis. Accordingly, in 2014, Bayer AG and the Drugs for Neglected Diseases initiative started to collaborate to develop emodepside for treating persons with the disease.
Emodepside has been screened in vitro and in vivo against a variety of filarial worms including O. gutturosa (131). A direct microfilaricidal was shown in mice infected with mf of O. lienalis at doses of 5 × 1.56 mg/kg or higher (132). Adult O. gutturosa male parasites obtained from the nuchal ligament connective tissues of naturally infected cattle and were tested in a 5-day in vitro assay (concentration range 2.95 × 10-12 M to 1.25 × 10-5 M). Emodepside immobilized the worms at a concentration of more than 4.8 × 10-8 M, producing an EC50 for motility of 9 × 10-10 M (133). However, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)/formazan colorimetry results demonstrated that low emodepside concentrations (≤1.25 × 10-5 M) did not significantly affect macrofilarial viability, indicating that although the worms were paralyzed, they were not dead, after 5 days exposure to drug. However, a long-term assay in vitro confirmed that after a 5-day exposure to 1.25 × 10-5M, emodepside eventually killed O. gutturosa adult males after a total of 40 days of culture (measured both by motility and MTT colorimetry), indicating that worms did not recover following the initial 5-day exposure to drug (132). Emodepside has been shown to have a greater macrofilaricidal activity against O. gutturosa than Brugia pahangi (132). This study also evaluated the efficacy of this drug against O. lienalis mf in vitro and in mice (132). The in vitro motility of Onchocerca macrofilariae was affected at lower concentrations than that of the mf (129). We have previously demonstrated drug activity against male O. volvulus worms in vitro in a small trial (Tagboto & Townson, unpublished).
Targeting macrofilariae in this fashion is projected to result in a reduction in the number of treatment cycles required to break transmission and to cure patients. It is also hoped that emodepside treatment will be efficacious and safe in regions where L. loa co-infection is present, since treatment with ivermectin may be contraindicated in patients co-infected with L. loa. If this is proven, it would greatly enhance the goal of eliminating onchocerciasis in areas where O. volvulus and L. loa are co-endemic (134–137).
Emodepside has been evaluated against O. ochengi in cattle. This is the parasite most closely related to O. volvulus. Following the administration of a single dose of 0.15 mg/kg of emodepside, the density of mf in the skin of the cattle did not change. A single dose of 0.75 mg/kg and 0.15 mg/kg given daily for 7 days transiently reduced skin microfilarial density (138). In addition, 0.75 mg/kg emodepside given daily for one week resulted in an initial reduction, followed by a transient rise and then complete clearance, of mf in 4 out of 7 animals within 18 months of treatment. The motility of adult macrofilariae retrieved from excised nodules was significantly affected over time at all emodepside doses for females, but only at the highest dose for males (129). In 5 out of 7 cattle, including those with no mf, the 7 × 0.75 mg/kg treatment scheme caused death or sterility of female worms, showing slow-acting macrofilaricidal and sterilizing activity (138).
Emodepside is currently being evaluated for the treatment of human onchocerciasis within the scope of a drug development partnership between the Drugs for Neglected Diseases initiative (DNDi) and Bayer AG (136). Phase I clinical trials have been completed with acceptable safety and tolerability profiles, and with no major safety concerns after a single oral dose of 20 mg as liquid service formulations and after multiple oral doses over 10 days at 5 and 10 mg daily and at 10 mg twice daily in healthy volunteers (139).
A Phase II trial is presently ongoing in Hohoe, Ghana and started in 2021 (139). It is a randomized, double-blind, parallel group trial to investigate emodepside in subjects with O. volvulus infection, comprising two parts. In part 1, the safety, tolerability, pharmacodynamics, pharmacokinetics, and dose–response relationship for activity was investigated. In part 2, the efficacy of selected drug doses, and safety, tolerability, and pharmacokinetics will be studied.
Drugs evaluated in clinical trials, satisfactory for use subject to regulatory approval
Minocycline, rifamipcin, azithromycin
O. volvulus parasites carry endosymbiotic bacteria (Wolbachia sp.) required for embryogenesis and survival (100, 101). A number of in vivo and in vitro studies utilizing potential candidate antibiotics were carried out to compare the effect of killing Wolbachia on the subsequent viability of Onchocerca (102–104) and other filarial parasites (103).
Rifampin and azithromycin also have good in vitro activity against Wolbachia (103). Short five-day courses do not appear to be effective for the clinical management of onchocerciasis (140). Rifampicin is thought to be a potential drug for use in children who cannot be treated with doxycycline (141). However, further study to determine the optimal dose and duration of treatment is needed (142).
A small clinical study in Ghana compared oral doxycycline 200mg once daily for 4 weeks (N = 33), and a shortened duration of treatment with oral minocycline at 200mg once daily for 3 weeks (N=30). On the completion of treatment, histological analysis of the adult worms removed from the excised nodules revealed the absence of Wolbachia in 98.8% of the doxycycline treated and 72.7% of the minocycline treated female worms. This trial concluded that minocycline was also a useful antibiotic in treating onchocerciasis (108).
Medicinal plants with reported anti-onchocercal activity
A number of medicinal plant products from Cameroon have been claimed to have anti-Onchocercal activity. These plant products include Pachleasma tersmanii, Raphia faninifera, Capra procera, Erythrophleum ivorense & Hilleria latifolia, but good studies on efficacy are lacking (143). Recently, Attah et al., reported microfilaricidal effects of Euphorbia hirta & Rauwolfia vomitoria extracts (144). Adevuole et al. (145), recently reported similar effects from Morinda lucida extracts. None of these studies included clinical or toxicology studies and as such the value of these findings is uncertain. Lantana camara and Tamarindus indica are local Cameroonian plants apparently used to treat onchocerciasis locally. Twelve extracts were tested in vitro on mf, adult male and adult female O. ochengi parasites. All the extracts showed complete action against O. ochengi adult parasites and mf at a high dose of 500 μg/mL. Furthermore, a methylene chloride extract lantadene A from the leaves of L. camara, had an IC50s of 7.85 μg/mL for adult male worms, 10.38 μg/mL for adult female worms, and 10.84 μg/mL for O. ochengi mf (146). Microfilaricidal activity was demonstrated at fairly high concentrations compared to ivermectin and moxidectin and several of the extracts tested showed some cytotoxicity against LLCMK2 monkey kidney cells at concentrations that were required to kill the worms. Similarly, some activity against O. ochengi has been demonstrated from extracts of Craterispermum laurinum and Morinda lucida (147), Cyperus articulates (148), Margaritaria discoidea and Homalium africanum (149) from Cameroon.
Twumasi et al. (150), tested a number of traditional herbal preparations from Ghana used locally for treating schistosomiasis and demonstrated activity against O. ochengi mf, adult male and female worms. An aqueous extract of Syzygium aromaticum, Xylopia aethiopica, Tapinanthus bangwensis and Phyllanthus niruri called NTD-B2-DCM, was the most active against female and male adult O. ochengi worms (IC50 = 76.2 μg/mL and 76.7 μg/mL, respectively). They also demonstrated significant killing of female worms by an extract used locally for treating onchocerciasis called NTD-01-DCM (an extract of Mangifera indica, Momordica charantia, Zingiber officinale, Xylopia aethiopica) and NTD-B6-DCM used locally for treating schistosomiasis (an extract of Alstonia boonei, Anthocleista nobilis, Ageratum conyzoides, Nauclea latifolia, and Rauwolfia vomitoria).
Ndjonka et al., carried out a review on medicinal plants and natural products with activity against onchocerca in 2018. They reported 13 plant families with anti-onchocercal compounds with Anacardium occidentale, Euphorbia hirta and Acacia nilotica showing particular promise. Isolated compounds with activity included polycarpol, voacamine, voacangine, ellagic acid, gallic acid, gentisic acid, 3-O-acetyl aleuritolic acid and (–)-epigallocatechin 3-O-gallate (151).
Drug resistance in onchocerciasis
Macrocyclic lactone resistance is now fairly common throughout in nematode parasites of small ruminants and has become of some concern in cattle, horses and dogs (152).
The responses of 268 individuals with Onchocerciasis and with persistent skin mf after 10-19 prior annual doses of ivermectin were studied. Skin snips were taken from these persons 364 days after ivermectin exposure to determine the mf recovery rate. Three categories of response were determined, namely good, intermediate or poor. Nodules were also excised and skin snips taken 90 days following a second study with ivermectin treatment. It was determined that approximately 25% of subjects carried adult female worms that responded suboptimally to ivermectin. Stratification of the female worms by morphological age and mf content showed that nearly 90% of the worms were older or middle aged and that most mf were produced by these previously exposed worms with little contribution from young worms derived from ongoing transmission. The results confirm that in some adult female worms were non-responsive or resistant to the anti-fecundity effects of multiple treatments with ivermectin (81).
Adult female O. volvulus worms were collected from the same individuals, before ivermectin exposure and after regular exposure. Following 13 three-monthly treatments, female parasites homozygotes with the less common genotype of the β-tubulin gene, appeared with increased frequency, providing some evidence for genetic selection. This gene has previously been linked to ivermectin selection and resistance in parasitic nematodes These worms appeared to be less fertile and had very few embryonic stages in their uteri than wild-type homozygotes, and raised concerns that ivermectin resistance may be occurring in some parasite populations (153).
Adult female worms obtained from nodules removed from individuals infected with O. volvulus before and 80 days after treatment with ivermectin were examined for their reproductive capacities. Worms from two groups of individuals were compared. Namely individuals who had been treated with repeated doses of ivermectin over 13 years and a control group with no repeated ivermectin exposure. Eighty days following treatment, the number of degenerating mf was significantly lower in the worms obtained from the frequently treated individuals. The authors conclude that the embryostatic effect of ivermectin was reduced in the frequently treated group, resulting in the reduced sequestration of mf in the adult female works from that group compared with the control population (154).
O. volvulus adult worms were obtained from 73 patients before and after treatment with ivermectin 3-monthly for 3 years. It was observed that the frequency of the common “aa” β-tubulin homozygote genotype reduced from 68.6% to 25.6%, while the “ab” heterozygotes increased from 20.9% to 69.2%. In this study, genotype frequencies also were determined to two other genes that are known to be not linked to drug resistance, namely, heat shock protein 60 and acidic ribosomal protein genes There was no significant selection for these genes. This β-tubulin genetic selection is associated with lower reproductive rates in the female parasites. This study suggests that a population of O. volvulus, which is more tolerant to ivermectin, is being selected and may have implications for the development of ivermectin resistance and for onchocerciasis control programmes (155).
Twenty-one individuals who had received at least 9 treatments with ivermectin and observed to be sub-optimal responders were matched, by age, weight, number of treatments, locality and skin mf counts, with 7 excellent responders with no detectable mf, and 14 subjects with no prior ivermectin exposure. Each subject was treated with ivermectin and plasma concentrations of the drug were then monitored for 72 h and found to be similar. The mf and adult-worm responses to treatment were assessed from skin mf counts before treatment and on days 8, 90 and 365 post-treatments. Embryograms on day-90, and the results of fly-feeding experiments were also compared. The results show that the persistence of mf despite multiple treatments with ivermectin is mainly due to the non-response of the adult female worms and not to inadequate drug exposure (79).
It has been observed that when parasite populations become resistant to macrocyclic lactones, moxidectin remains is more effective than the avermectins. This may well have implications for onchocerciasis control programmes if similarly observed in O. volvulus (156)
Conclusion
Annual ivermectin treatment has substantially impacted the prevalence and public health importance of onchocerciasis in sub-Saharan Africa. This has presently enabled the elimination of this tropical disease as an important public health problem. However, hypo-endemic areas remain a source of ongoing infection and may contribute to disease resurgence if annual ivermectin drug treatments are discontinued or interrupted in areas that may be hard to treat consequent to ongoing conflict, with financial challenges or areas with lethargic political interest. As such, other treatment options, especially safe and efficacious macrofilaricides that could help improve the management of onchocerciasis in the long term by helping to permanently interrupt parasite transmission altogether, improve elimination timelines and achieve the goal of disease eradication. There are several promising candidate drugs that require further evaluation to determine if they are able to fill this therapeutic need.
Author contributions
ST conceptualized the work. Both authors equally contributed to the design and writing of the article. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wish to thank Dr. Simon Townson for his invaluable suggestions and help in writing up this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. O’Neill J. On the presence of a filaria in "craw-craw". Lancet (1875) 105(2686):265–6. doi: 10.1016/S0140-6736(02)30941-3
2. Manson P. Filaria volvuloxus. In: Davidson AH, editor. Hygiene and diseases of warm climates. London: Y.J. Pentland (1893). p. 1016.
3. Marroquin HF. The discovery of Robles disease. Trop Med Health (2015) 43(Suppl):7–14. doi: 10.2149/tmh.2015-s04
4. Blacklock DB. The development of onchocerca volvulus in simulium damnosum. Ann Trop Med Parasitol (1926) 11:1–48. doi: 10.1080/00034983.1926.11684476
5. Townson S, Tagboto S. Development of techniques for measuring the viability of onchocerca lienalis in vitro and on their subsequent development in the blackfly vector simulium ornatum. Trans R Soc Trop Med Hyg (1991) 85:318.
6. Crump A, Morel CM, Omura S. The onchocerciasis chronicle: from the beginning to the end? Trends Parasitol (2012) 1:280–8. doi: 10.1016/j.pt.2012.04.005
7. Duke BO. Observations on onchocerca volvulus in experimentally infected chimpanzees. Tropenmedizin Und Parasitol (1980) 31(1):41–54.
8. Townson S, Tagboto SK, Castro J, Lujan A, Awadzi K, Titanji VP. Comparison of the sensitivity of different geographical races of onchocerca volvulus microfilariae to ivermectin: studies in vitro. Trans R Soc Trop Med Hygiene (1994) 88(1):101–6. doi: 10.1016/0035-9203(94)90521-5
9. Adukpo RO. Determination of onchocerca volvulus strains prevalent in the nkwanta north district of Ghana 2019.(Doctoral dissertation, university of Ghana) . Available at: http://ugspace.ug.edu.gh/bitstream/handle/123456789/28100/Determination%20of%20Onchocerca%20Volvulus%20Strains%20Prevalent%20in%20the%20Nkwanta%20North%20District%20of%20Ghana.pdf?sequence=1&isAllowed=y.
10. Kim YE, Remme JH, Steinmann P, Stolk WA, Roungou JB, Tediosi F. Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl Trop Dis (2015) 9(4):e0003664. doi: 10.1371/journal.pntd.0003664
11. Tagboto SK, Townson S, Titanji VP, Awadzi K, Castro J, Zea-Flores G. Comparison of the sensitivity of different geographical isolates of onchocerca volvulus microfilariae to ivermectin: effects of exposure to drug on development in the blackfly simulium ornatum. Trans R Soc Trop Med Hygiene (1994) 88(2):237–41. doi: 10.1016/0035-9203(94)90313-1
12. Guerrant RL, Walker DH, Weller PF. Tropical infectious diseases: Principles, pathogens and practice e-book. Elsevier Health Sci (2011) 741–9. doi: 10.1345/aph.1Q302
13. Schwartz RA, Al-Qubati Y, Zieleniewski Ł, Shah R, Kapila R. Onchocerciasis (river blindness): larva-induced eczema (onchodermatitis) from an important oculocutaneous tropical disease spilling over into north America and Europe. Int J Dermatol (2020) 59(9):1065–70. doi: 10.1111/ijd.14614
14. Boatin BA, Richards FO Jr. Control of onchocerciasis. Adv Parasitol (2006) 61:349–94. doi: 10.1016/S0065-308X(05)61009-3
15. Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Oral drug therapy for multiple neglected tropical diseases: a systematic review. JAMA (2007) 298(16):1911–24. doi: 10.1001/jama.298.16.1911
16. Coffeng LE, Stolk WA, Zouré HG, Veerman JL, Agblewonu KB, Murdoch ME, et al. African Programme for onchocerciasis control 1995-2015: model-estimated health impact and cost. PLoS Negl Trop Dis (2013) 7(1):e2032. doi: 10.1371/journal.pntd.0002032
17. Hill E, Hall J, Letourneau ID, Donkers K, Shirude S, Pigott DM, et al. A database of geopositioned onchocerciasis prevalence data. Sci Data (2019) 6(1):67. doi: 10.1038/s41597-019-0079-5
18. Nelson GS. “Hanging groin” and hernia, complications of onchocerciasis. Trans R Soc Trop Med Hygiene (1958) 52(3):272–5. doi: 10.1016/0035-9203(58)90087-7
19. Colebunders R, Siewe FN, Hotterbeekx A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol (2018) 34(3):208–16. doi: 10.1016/j.pt.2017.11.009
20. Patton JB, Bennuru S, Eberhard ML, Hess JA, Torigian A, Lustigman S, et al. Development of onchocerca volvulus in humanized NSG mice and detection of parasite biomarkers in urine and serum. PLoS Negl Trop Dis (2018) 12(12):e0006977. doi: 10.1371/journal.pntd.0006977
21. Ngwewondo A, Scandale I, Specht S. Onchocerciasis drug development: from preclinical models to humans. Parasitol Res (2021) 120(12):3939–64. doi: 10.1007/s00436-021-07307-4
22. Koala L, Nikièma AS, Paré AB, Drabo F, Toé LD, Belem AMG, et al. Entomological assessment of the transmission following recrudescence of onchocerciasis in the comoé valley, Burkina Faso. Parasites Vectors (2019) 12(1):1–9. doi: 10.1186/s13071-019-3290-5
23. Nascimento-Carvalho ÉS, Cesário RD, do Vale VF, Aranda AT, Valente AC, Maia-Herzog M. A new methodology for sampling blackflies for the entomological surveillance of onchocerciasis in Brazil. PLoS One (2017) 12(7):e0179754. doi: 10.1371/journal.pone.0179754
24. Zarroug IM, Elaagip A, Gumaa SG, Ali AK, Ahmed A, Siam HA, et al. Notes on distribution of simulium damnosum sl along atbara river in galabat sub-focus, eastern Sudan. BMC Infect Dis (2019) 19(1):1–5. doi: 10.1186/s12879-019-4113-1
25. Boatin B. The onchocerciasis control programme in West Africa (OCP). Ann Trop Med Parasitol (2008) 102(sup1):13–7. doi: 10.1179/136485908X337427
26. Kuesel AC. Research for new drugs for elimination of onchocerciasis in Africa. Int J Parasitol: Drugs Drug Resistance (2016) 6(3):272–86. doi: 10.1016/j.ijpddr.2016.04.002
27. Sauerbrey M, Rakers LJ, Richards FO. Progress toward elimination of onchocerciasis in the americas. Int Health (2018) 10(suppl_1):i71–8. doi: 10.1093/inthealth/ihx039
28. Winnen M, Plaisier AP, Alley ES, Nagelkerke NJ, van Oortmarssen G, Boatin BA, et al. Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull World Health Organ (2002) 80:384–91.
29. World Health Organisation. Ending the neglect to attain the sustainable development. a road map for neglected tropical diseases 2021–2030. In: BYNC-SA 3.0 IGO. World Health Organization (2020).
30. Burch TA, Ashburn LL. Experimental therapy of onchocerciasis with suramin and hetrazan; results of a three-year study. Am J Trop Med (1951) 31(5):617–23. doi: 10.4269/ajtmh.1951.s1-31.617
31. Duke BO. The effects of drugs on onchocerca volvulus: 3. trials of suramin using different dosages and a comparison of the brands antrypol, moranyl and naganol. Bull World Health Org (1968) 39(2):157.
32. Thylefors B, Rolland A. The risk of optic atrophy following suramin treatment of ocular onchocerciasis. Bull World Health Org (1979) 57(3):479.
34. Awadzi K, Gilles HM. Diethylcarbamazine in the treatment of patients with onchocerciasis. Br J Clin Pharmacol (1992) 34(4):281. doi: 10.1111/j.1365-2125.1992.tb05632.x
35. Oomen AP. Fatalities after treatment of onchocerciasis with diethylcarbamazine. Trans R Soc Trop Med Hygiene (1969) 63(4):548. doi: 10.1016/0035-9203(69)90055-8
36. Duke BO. The effects of drugs on onchocerca volvulus: 4. trials of melarsonyl potassium. Bull World Health Org (1970) 42(1):115.
37. Duke BO. The intake and transmissibility of onchocerca volvulus microfilariae by similium damnosum fed on patients treated with diethylcarbamazine, suramin or Mel W. Bull World Health Org (1968) 39(2):169.
38. Awadzi K. Clinical picture and outcome of serious adverse events in the treatment of onchocerciasis. Filaria J (2003) 2(1):1–9. doi: 10.1186/1475-2883-2-S1-S6
39. Zak F, Guderian R, Zea-Flores G, Guevara A, Moran M, Poltera AA. Microfilaricidal effect of amocarzine in skin punch biopsies of patients with onchocerciasis from Latin America. Trop Med Parasitol: Off Organ Deutsche Tropenmedizinische Gesellschaft Deutsche Gesellschaft Fur Technische Zusammenarbeit (GTZ) (1991) 42(3):294–302.
40. Zea-Flores G, Beltranena F, Poltera AA, Lopez M, Moran M, Zea-Flores CE, et al. Amocarzine investigated as oral onchocercacidal drug in 272 adult male patients from guatemala. results from three dose regimens spread over three days. Trop Med Parasitol: Off Organ Deutsche Tropenmedizinische Gesellschaft Deutsche Gesellschaft Fur Technische Zusammenarbeit (GTZ) (1991) 1:240–62.
41. Poltera AA, Moran M, Zak F, Striebel HP, Zea-Flores G, Guderian R, et al. Onchocercacidal effects of amocarzine (CGP 6140) in Latin America. Lancet (1991) 9:583–4. doi: 10.1016/0140-6736(91)91642-8
42. Lecaillon JB, Dubois JP, Soula G, Pichard E, Poltera AA, Ginger CD. The influence of food on the pharmacokinetics of CGP 6140 (amocarzine) after oral administration of a 1200 mg single dose to patients with onchocerciasis. Br J Clin Pharmacol (1990) 30(4):629–33. doi: 10.1111/j.1365-2125.1990.tb03825.x
43. Awadzi K, Opoku NO, Attah SK, Addy ET, Duke BO, Nyame PK, et al. The safety and efficacy of amocarzine in African onchocerciasis and the influence of ivermectin on the clinical and parasitological response to treatment. Ann Trop Med Parasitol (1997) 91:281–96. doi: 10.1080/00034983.1997.11813141
44. Awadzi K. Research notes from the onchocerciasis chemotherapy research centre, Ghana. Ann Trop Med Parasitol (1997) 91:703–11. doi: 10.1080/00034983.1997.11813193
45. Townson S, Dobinson A, Connelly C, Muller R. Chemotherapy of onchocerca lienalis microfilariae in mice: a model for the evaluation of novel compounds for the treatment of onchocerciasis. J Helminthol (1988) 62(3):181–94. doi: 10.1017/S0022149X00011494
46. Townson S, Connelly C, Dobinson A, Muller R. Drug activity against onchocerca gutturosa males in vitro: a model for chemotherapeutic research on onchocerciasis. J Helminthol (1987) 61(4):271–81. doi: 10.1017/S0022149X00010178
47. Orme M, Awadzi K, Edwards G, Breckenridge AM. Drug treatment of onchocerciasis. QJM: Int J Med (1988) 66(3):195–201.
48. Büttner DW, Albiez EJ, Von Essen J, Erichsen J. Histological examination of adult onchocerca volvulus and comparison with the collagenase technique. Trop Med Parasitol: Off Organ Deutsche Tropenmedizinische Gesellschaft Deutsche Gesellschaft Fur Technische Zusammenarbeit (GTZ) (1988) 39:390–417.
49. Awadzi K, Orme ML, Breckenridge AM, Haddock D, Gilles HM. Studies of metrifonate in onchocerciasis. Acta Pharmacol Toxicol (1981) 49:131–6. doi: 10.1111/j.1600-0773.1981.tb03264.x
50. Lees MJ, Kleider N, Tuddenham T. Cutaneous onchocerciasis in the horse: Five cases in southwestern British Columbia. Can Veterinary J (1983) 24(1):3.
51. Rivas-Alcalá AR, Taylor H, Ruvalcaba-Macias AM, Mackenzie C, Greene B, Domiguez-Vazquez A, et al. Chemotherapy of onchocerciasis: a controlled comparison of mebendazole, levamisole, and diethylcarbamazine. Lancet (1981) 318(8245):485–90. doi: 10.1016/S0140-6736(81)90880-1
52. Awadzi K, Edwards G, Opoku NO, Ardrey AE, Favager S, Addy ET, et al. The safety, tolerability and pharmacokinetics of levamisole alone, levamisole plus ivermectin, and levamisole plus albendazole, and their efficacy against onchocerca volvulus. Ann Trop Med Parasitol (2004) 98(6):595–614. doi: 10.1179/000349804225021370
53. Higazi TB, Geary TG, Mackenzie CD. Chemotherapy in the treatment, control, and elimination of human onchocerciasis. Res Rep Trop Med (2014) 5:77. doi: 10.2147/RRTM.S36642
54. Geary TG, Mackenzie CD, Silber SA. Flubendazole as a macrofilaricide: History and background. PLoS Negl Trop Dis (2019) 13(1):e0006436. doi: 10.1371/journal.pntd.0006436
55. Dominguez-Vazquez A, Taylor HR, Greene BM, Ruvalcaba-Macias AM, Rivas-Alcala AR, Murphy RP, et al. Comparison of flubendazole and diethylcarbamazine in treatment of onchocerciasis. Lancet (1983) 1(8317):139–43. doi: 10.1016/S0140-6736(83)92753-8
56. Duke BO. The effects of drugs on onchocerca volvulus: 2. the antimonial preparations TWSb and MSbE. Bull World Health Org (1968) 39(2):147.
57. Garner AL, Gloeckner C, Tricoche N, Zakhari JS, Samje M, Cho-Ngwa F, et al. Design, synthesis, and biological activities of closantel analogues: Structural promiscuity and its impact on onchocerca volvulus. J Med Chem (2011) 54(11):3963–72. doi: 10.1021/jm200364n
58. Gloeckner C, Garner AL, Mersha F, Oksov Y, Tricoche N, Eubanks LM, et al. Repositioning of an existing drug for the neglected tropical disease onchocerciasis. Proc Natl Acad Sci (2010) 107(8):3424–9. doi: 10.1073/pnas.0915125107
59. Gooyit M, Tricoche N, Lustigman S, Janda KD. Dual protonophore–chitinase inhibitors dramatically affect o. volvulus molting. J Med Chem (2014) 57(13):5792–9. doi: 10.1021/jm5006435
60. Tabatabaei SA, Soleimani M, Mansouri MR, Mirshahi A, Inanlou B, Abrishami M, et al. Closantel; a veterinary drug with potential severe morbidity in humans. BMC Ophthalmol (2016) 16(1):1–5. doi: 10.1186/s12886-016-0387-x
61. Capparelli EV, Bricker-Ford R, Rogers MJ, McKerrow JH, Reed SL. Phase I clinical trial results of auranofin, a novel antiparasitic agent. Antimicrobial Agents Chemother (2017) 61(1):e01947–16. doi: 10.1128/AAC.01947-16
62. Bulman CA, Bidlow CM, Lustigman S, Cho-Ngwa F, Williams D, Rascón C.OMMAJr AA, et al. Repurposing auranofin as a lead candidate for treatment of lymphatic filariasis and onchocerciasis. PLoS Negl Trop Dis (2015) 9(2):e0003534. doi: 10.1371/journal.pntd.0003534
63. Duke BO. The effects of some drugs–pentamidine, stibocaptate, hoechst 33258, f 151, compound'E'and nifurtimox–on onchocerca volvulus in chimpanzees. Tropenmedizin Und Parasitol (1977) 28(4):447–55. doi: 10.1371/journal.pntd.0003534
64. Omura S, Iwai Y, Hirano A, Nakagawa A, Awaya J, Tsuchiya H, et al. A new alkaloid AM-2282 of streptomyces origin taxonomy, fermentation, isolation and preliminary characterization. J Antibiotics (1977) 30(4):275–82. doi: 10.7164/antibiotics.30.275
65. Campbell WC. Ivermectin: a reflection on simplicity (Nobel lecture). Angewandte Chem Int Ed (2016) 55(35):10184–9. doi: 10.1002/anie.201601492
66. Chippaux JP, Boussinesq M, Prod’hon J. Apport de l’ivermectine dans le contrôle de l’onchocercose. The Use Ivermectin Control Onchocerciasis]Cahiers D'études Recherches Francophones/Santé (1995) 5(3):149–58.
67. Gardon J, Boussinesq M, Kamgno J, Gardon-Wendel N, Duke BO. Effects of standard and high doses of ivermectin on adult worms of onchocerca volvulus: a randomised controlled trial. Lancet (2002) 360(9328):203–10. doi: 10.1016/S0140-6736(02)09456-4
68. Duke BO. Evidence for macrofilaricidal activity of ivermectin against female onchocerca volvulus: further analysis of a clinical trial in the republic of Cameroon indicating two distinct killing mechanisms. Parasitology (2005) 130(4):447–53. doi: 10.1017/S0031182004006766
69. Walker M, Pion SD, Fang H, Gardon J, Kamgno J, Basáñez MG, et al. Macrofilaricidal efficacy of repeated doses of ivermectin for the treatment of river blindness. Clin Infect Dis (2017) 65(12):2026–34. doi: 10.1093/cid/cix616
70. Hoerauf AM. Onchocerciasis, Tropical Infectious Diseases: Principles, Pathogens and Practice E-Book. Guerrant R, Walker DH, Weller PF (Eds), Elsevier Health Sciences (2011). p. 741.
71. Frempong KK, Walker M, Cheke RA, Tetevi EJ, Gyan ET, Owusu EO, et al. Does increasing treatment frequency address suboptimal responses to ivermectin for the control and elimination of river blindness? Clin Infect Dis (2016) 62(11):1338–47. doi: 10.1093/cid/ciw144
72. Emukah E, Rakers LJ, Kahansim B, Miri ES, Nwoke BEB, Griswold E, et al. In southern Nigeria loa loa blood microfilaria density is very low even in areas with high prevalence of loiasis: Results of a survey using the new LoaScope technology. Am J Trop Med Hyg (2018) 99(1):116–23. doi: 10.4269/ajtmh.18-0163
73. Twum-Danso NA, Meredith SE. Variation in incidence of serious adverse events after onchocerciasis treatment with ivermectin in areas of Cameroon co-endemic for loiasis. Trop Med Int Health (2003) 8(9):820–31. doi: 10.1046/j.1365-3156.2003.01091.x
74. Makenga Bof JC, Maketa V, Bakajika DK, Ntumba F, Mpunga D, Murdoch ME, et al. Onchocerciasis control in the democratic republic of Congo (DRC): challenges in a post-war environment. Trop Med Int Health (2015) 20(1):48–62.
75. Kamgno J, Pion SD, Chesnais CB, Bakalar MH, D'Ambrosio MV, Mackenzie CD, et al. A test-and-Not-Treat strategy for onchocerciasis in loa loa-endemic areas. N Engl J Med (2017) 377(21):2044–52. doi: 10.1056/NEJMoa1705026
76. Keiser PB, Reynolds SM, Awadzi K, Ottesen EA, Taylor MJ, Nutman TB. Bacterial endosymbionts of onchocerca volvulus in the pathogenesis of posttreatment reactions. J Infect Dis (2002) 185(6):805–11. doi: 10.1086/339344
77. Wildenburg G, Korten S, Mainuka P, Büttner DW. Ivermectin influence on the mast cell activity in nodules of onchocerciasis patients. Trop Med Int Health: TM IH (1998) 3(11):918–25. doi: 10.1046/j.1365-3156.1998.00326.x
78. Opoku NO, Bakajika DK, Kanza EM, Howard H, Mambandu GL, Nyathirombo A, et al. Single dose moxidectin versus ivermectin for onchocerca volvulus infection in Ghana, Liberia, and the democratic republic of the Congo: a randomised, controlled, double-blind phase 3 trial. Lancet (2018) 392(10154):1207–16. doi: 10.1016/S0140-6736(17)32844-1
79. Awadzi K, Boakye DA, Edwards G, Opoku NO, Attah SK, Osei-Atweneboana MY, et al. An investigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol (2004) 98(3):231–49. doi: 10.1179/000349804225003253
80. Doyle SR, Bourguinat C, Nana-Djeunga HC, Kengne-Ouafo JA, Pion SDS, Bopda J, et al. Genome-wide analysis of ivermectin response by onchocerca volvulus reveals that genetic drift and soft selective sweeps contribute to loss of drug sensitivity. PLoS Negl Trop Dis (2017) 11(7):e0005816. doi: 10.1371/journal.pntd.0005816
81. Osei-Atweneboana MY, Awadzi K, Attah SK, Boakye DA, Gyapong JO, Prichard RK. Phenotypic evidence of emerging ivermectin resistance in onchocerca volvulus. PLoS Negl Trop Dis (2011) 5(3):e998. doi: 10.1371/journal.pntd.0000998
82. Kudzi W, Dodoo AN, Mills JJ. Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans? BMC med. Genet (2010) 11:111. doi: 10.1186/1471-2350-11-111
83. Martin RJ, Wolstenholme AJ, Caffrey CR. Anthelmintics: From discovery to resistance II (San Diego, 2016). Int J Parasitol Drugs Drug Resist (2016) 6(3):297–8. doi: 10.1016/j.ijpddr.2016.09.002
84. Wolstenholme AJ, Martin RJ. Anthelmintics - from discovery to resistance. Int J Parasitol Drugs Drug Resist (2014) 4(3):218–9. doi: 10.1016/j.ijpddr.2014.10.001
85. Klion AD, Horton J, Nutman TB. Albendazole therapy for loiasis refractory to diethylcarbamazine treatment. Clin Infect Dis (1999) 29:680. doi: 10.1086/598654
86. de Souza DK, Thomas R, Bradley J, Leyrat C, Boakye DA, Okebe J. Ivermectin treatment in humans for reducing malaria transmission. Cochrane Database Syst Rev (2021) 6(6):CD013117. doi: 10.1002/14651858.CD013117.pub2
87. Foy BD, Alout H, Seaman JA, Rao S, Magalhaes T, Wade M, et al. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): a cluster-randomised trial. Lancet (2019) 393(10180):1517–26. doi: 10.1016/S0140-6736(18)32321-3
88. del Mar Sáez-De-Ocariz M, McKinster CD, Orozco-Covarrubias L, Tamayo-Sánchez L, Ruiz-Maldonado R. Treatment of 18 children with scabies or cutaneous larva migrans using ivermectin. Clin Exp Dermatol (2002) 27(4):264–7. doi: 10.1046/j.1365-2230.2002.01050.x
89. Nicolas P, Maia MF, Bassat Q, Kobylinski KC, Monteiro W, Rabinovich NR, et al. Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis. Lancet Glob Health (2020) 8(1):e92–e100. doi: 10.1016/S2214-109X(19)30453-X
90. Batsa Debrah L, Klarmann-Schulz U, Osei-Mensah J, Dubben B, Fischer K, Mubarik Y, et al. Comparison of repeated doses of ivermectin versus ivermectin plus albendazole for the treatment of onchocerciasis: A randomized, open-label, clinical trial. Clin Infect Dis (2020) 71(4):933–43. doi: 10.1093/cid/ciz889
91. Tagboto SK, Townson S. Onchocerca volvulus and o. lienalis: the microfilaricidal activity of moxidectin compared with that of ivermectin in vitro and in vivo. Ann Trop Med Parasitol (1996) 90(5):497–505.
92. Townson S, Tagboto SK. Moxidectin is more active than ivermectin in eliminating onchocerca volvulus and O. lienalis microfilariae from experimentally infected mice. Trans R Soc Trop Med Hygiene (1996) 20:216.
93. Townson S, Tagboto S. Persistence of moxidectin activity against onchocerca microfilariae in mice. Eur Multicolloquium Parasitol (EMOP V11) Parma Italy Parassitol (1996) 38:227.
94. Lanusse CE, Imperiale FA, Lifschitz AL. Macrocyclic lactones: endectocide compounds. Wiley Blackwell Publishing, Inc:Hoboken, NJ, USA (2018) p. 1102–27.
95. Cotreau MM, Warren S, Ryan JL, Fleckenstein L, Vanapalli SR, Brown KR, et al. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol (2003) 43(10):1108–15. doi: 10.1177/0091270003257456
96. Awadzi K, Opoku NO, Attah SK, Lazdins-Helds J, Kuesel AC. A randomized, single-ascending-dose, ivermectin-controlled, double-blind study of moxidectin in onchocerca volvulus infection. PLoS Negl Trop Dis (2014) 8(6):e2953. doi: 10.1371/journal.pntd.0002953
97. Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl Trop Dis (2016) 10(3):e0004389. doi: 10.1371/journal.pntd.0004389
98. Bernigaud C, Fang F, Fischer K, Lespine A, Aho LS, Dreau D, et al. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: Efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl Trop Dis (2016) 10(10):e0005030. doi: 10.1371/journal.pntd.0005030
99. Turner HC, Walker M, Attah SK, Opoku NO, Awadzi K, Kuesel AC, et al. The potential impact of moxidectin on onchocerciasis elimination in Africa: an economic evaluation based on the phase II clinical trial data. Parasites Vectors (2015) 8(1):1–2. doi: 10.1186/s13071-015-0779-4
100. Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Autenrieth IB, Fleischer B, et al. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet (2000) 355(9211):1242–3. doi: 10.1016/S0140-6736(00)02095-X
101. Johnston KL, Taylor MJ. Wolbachia in filarial parasites: targets for filarial infection and disease control. Curr Infect Dis Rep (2007) 9(1):55–9. doi: 10.1007/s11908-007-0023-2
102. Townson S, Tagboto S. Onchocerca gutturosa: sensitivity to a range of antibiotic and antiparasitic drugs. In: The 18th international conference of the world association for the advancement of veterinary parasitology. Stresa, Italy: WAAVP (2001). p. 158
103. Townson S, Hutton D, Siemienska J, Hollick L, Scanlon T, Tagboto SK, et al. Antibiotics and wolbachia in filarial nematodes: antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against onchocerca gutturosa, onchocerca lienalis and brugia pahangi. Ann Trop Med Parasitol (2000) 8):801–16. doi: 10.1080/00034983.2000.11813605
104. Townson S, Tagboto S, McGarry HF, Egerton GL, Taylor MJ. Onchocerca parasites and wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against onchocerca gutturosa in vitro. Filaria J (2006) 5(1):1–9. doi: 10.1186/1475-2883-5-4
105. Walker M, Specht S, Churcher TS, Hoerauf A, Taylor MJ, Basáñez MG. Therapeutic efficacy and macrofilaricidal activity of doxycycline for the treatment of river blindness. Clin Infect Dis (2015) 60(8):1199–207. doi: 10.1093/cid/ciu1152
106. Debrah AY, Specht S, Klarmann-Schulz U, Batsa L, Mand S, Marfo-Debrekyei Y, et al. Doxycycline leads to sterility and enhanced killing of female onchocerca volvulus worms in an area with persistent microfilaridermia after repeated ivermectin treatment: a randomized, placebo-controlled, double-blind trial. Clin Infect Dis (2015) 61(4):517–26. doi: 10.1093/cid/civ363
107. Turner JD, Tendongfor N, Esum M, Johnston KL, Langley RS, Ford L, et al. Macrofilaricidal activity after doxycycline only treatment of onchocerca volvulus in an area of loa loa co-endemicity: a randomized controlled trial. PLoS Negl Trop Dis (2010) 4(4):e660. doi: 10.1371/journal.pntd.0000660
108. Klarmann-Schulz U, Specht S, Debrah AY, Batsa L, Ayisi-Boateng NK, Osei-Mensah J, et al. Comparison of doxycycline, minocycline, doxycycline plus albendazole and albendazole alone in their efficacy against onchocerciasis in a randomized, open-label, pilot trial. PLoS Negl Trop Dis (2017) 11(1):e0005156. doi: 10.1371/journal.pntd.0005156
109. Tyagi R, Bulman CA, Cho-Ngwa F, Fischer C, Marcellino C, Arkin MR, et al. An integrated approach to identify new anti-filarial leads to treat river blindness, a neglected tropical disease. Pathogens (2021) 10(1):71. doi: 10.3390/pathogens10010071
110. Townson S, McCall PJ, Tagboto S. The anti-onchocercal activity of a range of avermectins and milbemycins. In: Second European congress on tropical medicine, Liverpool, U.K.: Wiley Blackwell (1998). p. 153–4.
111. Johnston KL, Ford L, Umareddy I, Townson S, Specht S, Pfarr K, et al. Repurposing of approved drugs from the human pharmacopoeia to target wolbachia endosymbionts of onchocerciasis and lymphatic filariasis. Int J Parasitol: Drugs Drug Resist (2014) 4(3):278–86. doi: 10.1016/j.ijpddr.2014.09.001
112. Johnston KL, Hong WD, Turner JD, O’Neill PM, Ward SA, Taylor MJ. Anti-wolbachia drugs for filariasis. Trends Parasitol (2021) 37(12):1068–81. doi: 10.1016/j.pt.2021.06.004
113. Hong WD, Benayoud F, Nixon GL, Ford L, Johnston KL, Clare RH, et al. AWZ1066S, a highly specific anti-wolbachia drug candidate for a short-course treatment of filariasis. Proc Natl Acad Sci (2019) 116(4):1414–9. doi: 10.1073/pnas.1816585116
114. Rana AK, Misra-Bhattacharya S. Current drug targets for helminthic diseases. Parasitol Res (2013) 112(5):1819–31. doi: 10.1007/s00436-013-3383-6
115. Boussinesq M, Fobi G, Kuesel AC. Alternative treatment strategies to accelerate the elimination of onchocerciasis. Int Health (2018) 10(suppl_1):i40–8. doi: 10.1093/inthealth/ihx054
116. Taylor MJ, von Geldern TW, Ford L, Hübner MP, Marsh K, Johnston K, et al. Preclinical development of an oral anti-wolbachia macrolide drug for the treatment of lymphatic filariasis and onchocerciasis. Sci Trans Med (2019) 11(483):eaau2086. doi: 10.1126/scitranslmed.aau2086
117. Hübner MP, Martin C, Specht S, Koschel M, Dubben B, Frohberger SJ, et al. Oxfendazole mediates macrofilaricidal efficacy against the filarial nematode litomosoides sigmodontis in vivo and inhibits onchocerca spec. motility in vitro. PLoS Negl Trop Dis (2020) 14(7):e0008427. doi: 10.1371/journal.pntd.0008427.131
118. Colella V, Maia C, Pereira A, Gonçalves N, Caruso M, Martin C, et al. Evaluation of oxfendazole in the treatment of zoonotic onchocerca lupi infection in dogs. PLoS Negl Trop Dis (2018) 12(1):e0006218. doi: 10.1371/journal.pntd.0006218
119. Cho-Ngwa F, Mbah GE, Ayiseh RB, Ndi EM, Monya E, Tumanjong IM, et al. Development and validation of an onchocerca ochengi adult male worm gerbil model for macrofilaricidal drug screening. PLoS Negl Trop Dis (2019) 13(7). doi: 10.1371/journal.pntd.0007556
120. Krücken J, Harder A, Jeschke P, Holden-Dye L, O’Connor V, Welz C, et al. Anthelmintic cyclooctadepsipeptides: complex in structure and mode of action. Trends Parasitol (2012) 28(9):385–94. doi: 10.1016/j.pt.2012.06.005
121. Epe C, Kaminsky R. New advancement in anthelmintic drugs in veterinary medicine. Trends Parasitol (2013) 29(3):129–34. doi: 10.1016/j.pt.2013.01.001
122. Sasaki T, Takagi M, Yaguchi T, Miyadoh S, Okada T, Koyama M. A new anthelmintic cyclodepsipeptide, PF1022A. J Antibiot (Tokyo) (1992) 45(5):692–7. doi: 10.7164/antibiotics.45.692
123. Ohyama M, Iinuma K, Isogai A, Suzuki A. Total synthesis of the anthelmintic cyclodepsipeptide, PF1022A. Biosci Biotechnol Biochem (1994) 58(6):1193–4. doi: 10.1271/bbb.58.1193
124. Harder A, Schmitt-Wrede HP, Krücken J, Marinovski P, Wunderlich F, Willson J, et al. Cyclooctadepsipeptides–an anthelmintically active class of compounds exhibiting a novel mode of action. Int J Antimicrobial Agents (2003) 22(3):318–31. doi: 10.1016/S0924-8579(03)00219-X
125. Harder A, Holden–Dye L, Walker R, Wunderlich F. Mechanisms of action of emodepside. Parasitol Res (2005) 97(1):S1–0. doi: 10.1007/s00436-005-1438-z
126. Saeger B, Schmitt-Wrede HP, Dehnhardt M, Benten WP, Krücken J, Harder A, et al. Latrophilin-like receptor from the parasitic nematode haemonchus contortus as target for the anthelmintic depsipeptide PF1022A. FASEB J (2001) 15(7):1332–4. doi: 10.1096/fj.00-0664fje
127. Holden-Dye L, Crisford A, Welz C, von Samson-Himmelstjerna G, Walker RJ, O’Connor V. Worms take to the slo lane: a perspective on the mode of action of emodepside. Invertebrate Neurosci (2012) 12(1):29–36. doi: 10.1007/s10158-012-0133-x
128. Chen W, Terada M, Cheng JT. Characterization of subtypes of gamma-aminobutyric acid receptors in an ascaris muscle preparation by binding assay and binding of PF1022A, a new anthelmintic, on the receptors. Parasitol Res (1996) 82(2):97–101. doi: 10.1007/s004360050077
129. Kulke D, von Samson-Himmelstjerna G, Miltsch SM, Wolstenholme AJ, Jex AR, Gasser RB, et al. Characterization of the Ca2+-gated and voltage-dependent k+-channel slo-1 of nematodes and its interaction with emodepside. PLoS Negl Trop Dis (2014) 8(12):e3401. doi: 10.1371/journal.pntd.0003401
130. Crisford A, Ebbinghaus-Kintscher U, Schoenhense E, Harder A, Raming K, O'Kelly I, et al. The cyclooctadepsipeptide anthelmintic emodepside differentially modulates nematode, insect and human calcium-activated potassium (SLO) channel alpha subunits. PLoS Negl Trop Dis (2015) 9(10):e0004062. doi: 10.1371/journal.pntd.0004062
131. Hübner MP, Townson S, Gokool S, Tagboto S, Maclean MJ, Verocai GG, et al. Evaluation of the in vitro susceptibility of various filarial nematodes to emodepside. Int J Parasitol: Drugs Drug Resist (2021) 17:27–35.
132. Krücken J, Holden-Dye L, Keiser J, Prichard RK, Townson S, Makepeace BL, et al. Development of emodepside as a possible adulticidal treatment for human onchocerciasis–the fruit of a successful industrial–academic collaboration. PLoS Pathogens (2021) 17(7):e1009682. doi: 10.1371/journal.ppat.1009682
133. Nicolay F, Harder A, von Samson-Himmelstjerna G, Mehlhorn H. Synergistic action of a cyclic depsipeptide and piperazine on nematodes. Parasitol Res (2000) 86(12):982–92. doi: 10.1007/PL00008530
134. Pion SD, Clarke P, Filipe JA, Kamgno J, Gardon J, Basáñez MG, et al. Co-Infection with onchocerca volvulus and loa loa microfilariae in central Cameroon: are these two species interacting? Parasitology (2006) 132(Pt 6):843–54. doi: 10.1017/S003118200600984X
135. Lustigman S, Makepeace BL, Klei TR, Babayan SA, Hotez P, Abraham D, et al. Onchocerca volvulus: the road from basic biology to a vaccine. Trends Parasitol (2018) 34(1):64–79. doi: 10.1016/j.pt.2017.08.011
136. Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno J, Ngoumou P, et al. Three probable cases of loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hygiene (1998) 58(4):461–9. doi: 10.4269/ajtmh.1998.58.461
137. Chippaux JP, Boussinesq M, Gardon J, Gardon-Wendel N, Ernould JC. Severe adverse reaction risks during mass treatment with ivermectin in loiasis-endemic areas. Parasitol Today (1996) 12(11):448–50. doi: 10.1016/0169-4758(96)40006-0
138. Bah GS, Schneckener S, Hahnel SR, Bayang NH, Fieseler H, Schmuck GM, et al. Emodepside targets SLO-1 channels of onchocerca ochengi and induces broad anthelmintic effects in a bovine model of onchocerciasis. PLoS Pathogens (2021) 17(6):e1009601. doi: 10.1371/journal.ppat.1009601
139. Gillon JY, Dennison J, van den Berg F, Delhomme S, Dequatre Cheeseman K, Peña Rossi C, et al. Safety, tolerability and pharmacokinetics of emodepside, a potential novel treatment for onchocerciasis (river blindness), in healthy male subjects. Br J Clin Pharmacol (2021) 87(10):3949–60. doi: 10.1111/bcp.14816
140. Richards FO Jr, Amann J, Arana B, Punkosdy G, Klein R, Blanco C, et al. No depletion of wolbachia from onchocerca volvulus after a short course of rifampin and/or azithromycin. Am J Trop Med Hygiene (2007) 77(5):878–82. doi: 10.4269/ajtmh.2007.77.878
141. Specht S, Mand S, Marfo-Debrekyei Y, Debrah AY, Konadu P, Adjei O, et al. Efficacy of 2- and 4-week rifampicin treatment on the wolbachia of onchocerca volvulus. Parasitol Res (2008) 103(6):1303–9. doi: 10.1007/s00436-008-1133-y
142. Aljayyoussi G, Tyrer HE, Ford L, Sjoberg H, Pionnier N, Waterhouse D, et al. Short-course, high-dose rifampicin achieves wolbachia depletion predictive of curative outcomes in preclinical models of lymphatic filariasis and onchocerciasis. Sci Rep (2017) 7(1):1–1. doi: 10.1038/s41598-017-00322-5
143. Tagboto S, Townson S. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasitol (2001) 50:199–295. doi: 10.1016/s0065-308x(01)50032-9
144. Attah SK, Ayeh-Kumi PF, Sittie AA, Oppong IV, Nyarko AK. Extracts of euphorbia hirta Linn.(Euphorbiaceae) and rauvolfia vomitoria afzel (Apocynaceae) demonstrate activities against onchocerca volvulus microfilariae in vitro. BMC Compl Altern Med (2013) 13(1):1–0. doi: 10.1186/1472-6882-13-66
145. Adewole KE, Attah AF, Adebayo JO. Morinda lucida benth (Rubiaceae): A review of its ethnomedicine, phytochemistry and pharmacology. J Ethnopharmacol (2021) 276:114055. doi: 10.1016/j.jep.2021.114055
146. Ngwewondo A, Wang M, Manfo FPT, Samje M, Ganin's JN, Ndi E, et al. Filaricidal properties of lantana camara and tamarindus indica extracts, and lantadene a from l. camara against onchocerca ochengi and loa loa. PLoS Negl Trop Dis (2018) 12(6):e0006565. doi: 10.1371/journal.pntd.0006565
147. Samje M, Metuge J, Mbah J, Nguesson B, Cho-Ngwa F. In vitro anti-onchocerca ochengi activities of extracts and chromatographic fractions of craterispermum laurinum and morinda lucida. BMC Complement Altern Med (2014) 14:325. doi: 10.1186/1472-6882-14-325
148. Metuge JA, Nyongbela KD, Mbah JA, Samje M, Fotso G, Babiaka SB, et al. Anti-onchocerca activity and phytochemical analysis of an essential oil from cyperus articulatus l. BMC Complement Altern Med (2014) 14:223. doi: 10.1186/1472-6882-14-223
149. Cho-Ngwa F, Abongwa M, Ngemenya MN, Nyongbela KD. Selective activity of extracts of margaritaria discoidea and homalium africanum on onchocerca ochengi. BMC Complement Altern Med (2010) 10:62. doi: 10.1186/1472-6882-10-62
150. Twumasi EB, Akazue PI, Kyeremeh K, Gwira TM, Keiser J, Cho-Ngwa F, et al. Antischistosomal, antionchocercal and antitrypanosomal potentials of some ghanaian traditional medicines and their constituents. PLoS Negl Trop Dis (2020) 14(12):e0008919. doi: 10.1371/journal.pntd.0008919
151. Ndjonka D, Djafsia B, Liebau E. Review on medicinal plants and natural compounds as anti-onchocerca agents. Parasitol Res (2018) 117(9):2697–713. doi: 10.1007/s00436-018-6003-7
152. Prichard RK. Is anthelmintic resistance a concern for heartworm control? what can we learn from the human filariasis control programs? Vet Parasitol (2005) 133(2-3):243–53. doi: 10.1016/j.vetpar.2005.04.008
153. Nana-Djeunga H, Bourguinat C, Pion SD, Kamgno J, Gardon J, Njiokou F, et al. Single nucleotide polymorphisms in β-tubulin selected in onchocerca volvulus following repeated ivermectin treatment: possible indication of resistance selection. Mol Biochem Parasitol (2012) 185(1):10–8. doi: 10.1016/j.molbiopara.2012.05.005
154. Nana-Djeunga HC, Bourguinat C, Pion SD, Bopda J, Kengne-Ouafo JA, Njiokou F, et al. Reproductive status of onchocerca volvulus after ivermectin treatment in an ivermectin-naïve and a frequently treated population from Cameroon. PLoS Negl Trop Dis (2014) 8(4):e2824. doi: 10.1371/journal.pntd.0002824
155. Bourguinat C, Pion SD, Kamgno J, Gardon J, Duke BO, Boussinesq M, et al. Genetic selection of low fertile onchocerca volvulus by ivermectin treatment. PLoS Negl Trop Dis (2007) 1(1):e72. doi: 10.1371/journal.pntd.0000072
Keywords: onchocerciasis, macrofilaricide, microfilaricide, river blindness, drug developement
Citation: Tagboto S and Orish V (2022) Drug development for onchocerciasis-the past, the present and the future. Front. Trop. Dis 3:953061. doi: 10.3389/fitd.2022.953061
Received: 25 May 2022; Accepted: 27 June 2022;
Published: 25 July 2022.
Edited by:
Somia Iqtadar, King Edward Medical University, PakistanReviewed by:
Amit Sinha, New England Biolabs, United StatesHimashree Bhattacharyya, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, India
Copyright © 2022 Tagboto and Orish. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Senyo Tagboto, c2VueW8yQGhvdG1haWwuY29t
 Senyo Tagboto
Senyo Tagboto Verner Orish
Verner Orish