- 1Laboratory of Biotechnology, Environment and Health, University of Blida 1, Blida, Algeria
- 2Institute of Veterinary Sciences, University of Blida 1, Blida, Algeria
- 3Livestock Management, Production and Animal Health Division, Research Centre for Agropastoralism, Djelfa, Algeria
- 4Laboratory of Parasitic Eco-Epidemiology and Population Genetics, Pasteur Institute of Algeria, Algiers, Algeria
The present study aimed to determine the phenology and predilection sites of ticks infesting cattle in the western region of Djurdjura (North Algeria) from November 2018 and October 2020. Nineteen cattle farms located in thirteen localities within four provinces were visited monthly for two years. Among the 289 examined cattle, 64.36 ± 2.81% (n=189) were infested by ticks. Of the 10,243 collected ticks, the most abundant tick species was Rhipicephalus bursa (31.57 ± 0.46%), followed by R. annulatus (31.26 ± 0.45%), Hyalomma marginatum (10.67 ± 0.30%), H. lusitanicum (7.02 ± 0.25%), H. excavatum (5.52±%0.22), H. scupense (4.27 ± 0.19%) and H. impeltatum (3.32 ± 0.17%). Ticks of the Hyalomma species were present throughout the year but in a limited number during the winter. H. scupense and H. impletatum showed similar activity from March to October and peaked in April and July, respectively (P<0.001). H. lusitanicum and H. excavatum were active from April to November and both peaked in September and October respectively. Rhipicephalus bursa was collected from April to August and R. annulatus from July to October. R. turanicus was active between April and June. Hyalomma genus had an affinity of attachment for the inguinal region (P<0.001). All parasitic stages of R. annulatus had an affinity for the necks (P<0.001). Nymphs of R. bursa prefer the neck (P<0.001), the adults attach to the perennial and inguinal regions (P<0.001). R. turanicus attach to the ears (P<0.001). As far as we know, this is the first study on the phenology and predilection sites of cattle ticks in the west region of Djurdjura in Algeria.
Introduction
Ticks are the most important hematophagous ectoparasites of livestock. They attach to their hosts at preferred sites before taking their blood meals. Several factors influence this attachment, including coat colour, skin thickness, hair length, host odour, environmental factors related to seasonal changes, and the length of the tick’s mouthparts (1, 2). Rhipicephalus turanicus attaches to the head, immature stages of R. bursa attach to the thighs and tail, and adults prefer the udder quarters and the perineum. Hyalomma marginatum, H. excavatum, H. lusitanicum and H. scupense attach preferentially to the posterior udder quarters and perineum (3, 4). In tropical areas, R. sulcatus and R. senegalensis have a tropism for the auricular conch, while H. truncatum and H. rufipes prefer the ano-genital region (5).
The attachment of ticks to the animal’s body causes serious losses to the cattle industry (6). The direct effects of blood-sucking lead to reduced weight gain and anaemia, while the implantation of the rostrum also reduces hides quality. Indirect effects of ticks are the injection of salivary toxins, which cause toxicosis and transmit several pathogens (7).
Eight hundred and ninety-six species of ticks have been described worldwide; they are divided into three families. The Nuttalliellidae are monotropic, with only one species Nuttalliella namaqua, the Argasidae totalling 193 species and Ixodidae that consists of 702 species (8, 9). So far, 26 Ixodid species were reported to be present in Algeria (3, 10–14). Among these species, some transmit to cattle several pathogens belonging to Anaplasma, Babesia and Theileria genera (15–17). It is well established that several tick species transmit the Crimean-Congo haemorrhagic fever virus, which was reported in Algeria (18).
The humid Mediterranean climate of the Djurdjura region has four distinct seasons, which favours the development of a perennial flora. More than 42% of the total area of the region consists of forests of Atlas cedar, holm oak, cork oak, holly, mountain maple, Montpellier maple, field maple, Prunus avium, Zea oak, black pine, Aleppo pine, yew, and wild and common olive (19). This region is a habitat of a varied fauna of birds, rodents, lagomorphs, carnivores and other mammals (20). Senevet and Rossi (21) listed eight species of ticks that parasitize livestock. Sergent et al. (22) reported the seasonality of these species. These ticks are obligate parasites of animals with a complex developmental cycle, which requires the availability of several hosts (23). Djurdjura region is an agricultural area; livestock farming is the main activity of the population; the semi-intensive cattle system allows for meat and milk production. Natural grasslands and concentrates constitute the bulk of the diet of cattle. Ticks and tick-borne diseases, especially piroplasmosis, are a particular concern for farmers and veterinarians (19, 24). Effective tick control programmes require the determination of the phenology and predilection sites of ticks to use appropriate acaricides (7). Implementation of tick control programmes requires a good knowledge of the ecology of ticks and the epidemiology of tick-associated infections. However, to the best of our knowledge, no comprehensive survey of ticks infesting cattle has been conducted in the western part of the Djurdjura region of northern Algeria. Therefore, we aimed in the present study to determine the identity, seasonal activity, and predilection sites of ticks infesting cattle in the western part of Djurdjura, Algeria.
Material and Methods
Study Area
The study was conducted in the western part of the Djurdjura, North central Algeria. It is a large area (8,905 km2) with a landscape interspersed with valleys and mountains. Thirteen localities within four provinces were included in the present study, namely Blida, Boumerdes, Bouira and Tizi Ouzou (Figure 1).
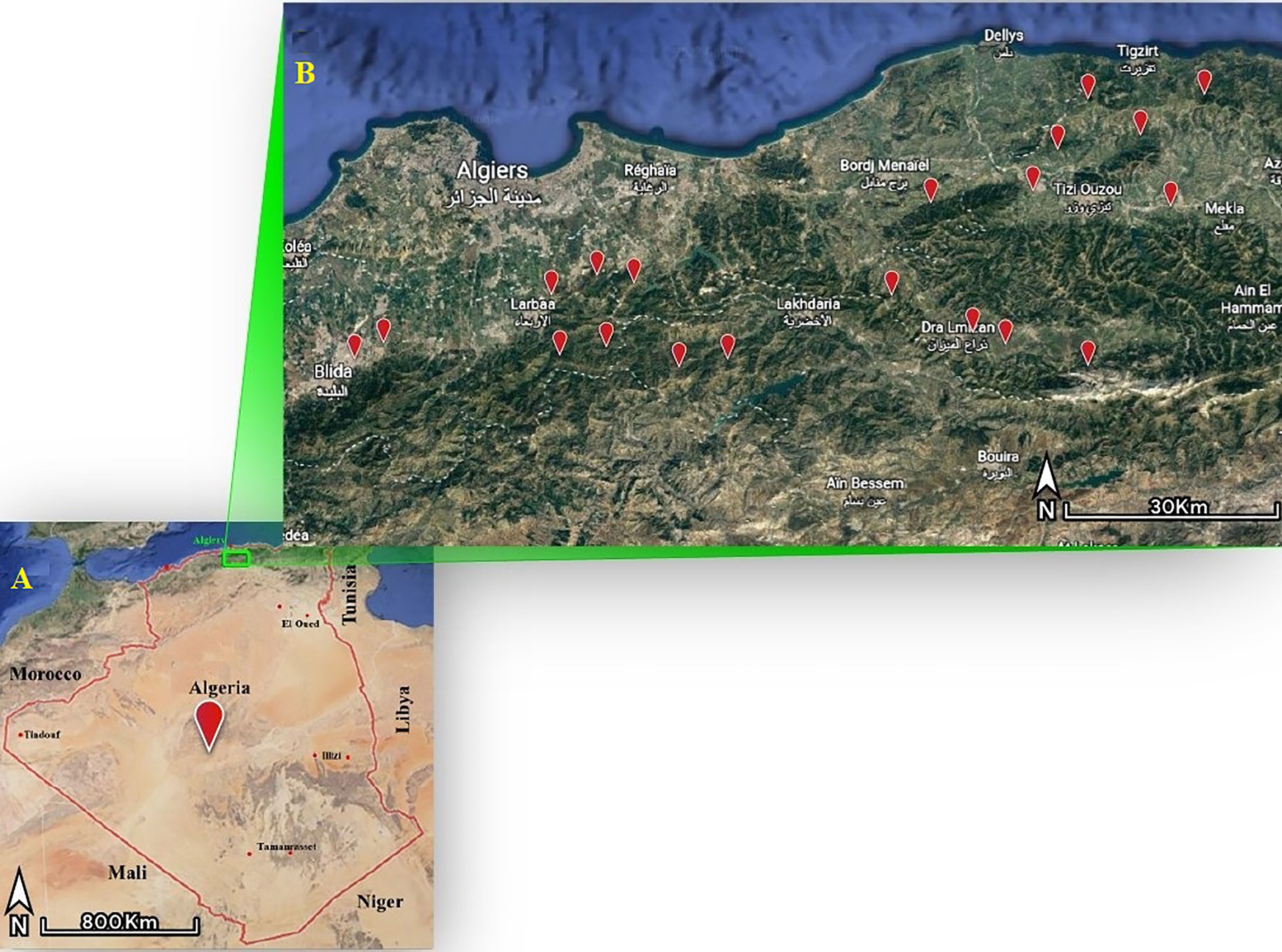
Figure 1 Geographic presentation of the study area. Map (A) show the location of the western parts of the Djurdjura. Map (B) shows the study area and the red points highlight the localities where ticks were collected.
Climate and Vegetation
The western part of Djurdjura has a Mediterranean climate. Its annual rainfall varies between 600 and 1.200 mm, with a relative humidity of 50%–75% during the summer season. The region is sub-humid to humid with hot and dry summers and cool and rainy winters. The temperature reaches 35°C in summer and drops to 5°C in winter. The region has four seasons, winter (December to February), spring (March to May), summer (June to August) and autumn (September to November). The northwest winds generate heavy rainfall accompanied by cold waves. It is one of the most wooded regions in Algeria and is the shelter of varied flora and fauna (19, 20).
Farms and Animals
Nineteen semi-intensive farms with a herd size of more than 15 animals were randomly selected for tick’s collection. A total number of 289 cows; were monthly examined for the ticks collection between November 2018 and October 2020. The animals graze during the day, and in the evening, they return to the farm. The grazing season extends from February to November. The animals examined were Holstein (n=45, 15.6 ± 2.13%), Montbeliard (n=65, 22.5 ± 2.45%) and crossbred (n=179, 61.9 ± 2.85%). Among the 289 examined cows aged between 1 and 14 years, 109 (37.72 ± 2.85%) were less than 18 months old, and 180 (62.28 ± 2.85%) were over 18 months old. Only animals not treated with acaricides, and present at all visits were included in the study.
Tick Collection
The cattle body was divided into seven anatomical areas (Figure 2); the head (head and ears), the neck (neck, dewlap and brisket), the back (withers, back and rump), the thorax (thorax and flank), the inguinal region (belly and inguinal region), the perennial region (perineum, anus and tail) and the legs (four legs and two thighs). All ticks visible on each anatomical region were collected manually, and then stored in labelled tubes containing 70% ethanol. Ticks were transported to the Regional Veterinary Laboratory of Tizi Ouzou. They were identified to species level, developmental stages and sex, and counted accordingly to the key of Estrada Pena et al. (23, 25). All ticks with damaged mouthparts and morphological anomalies were discarded.
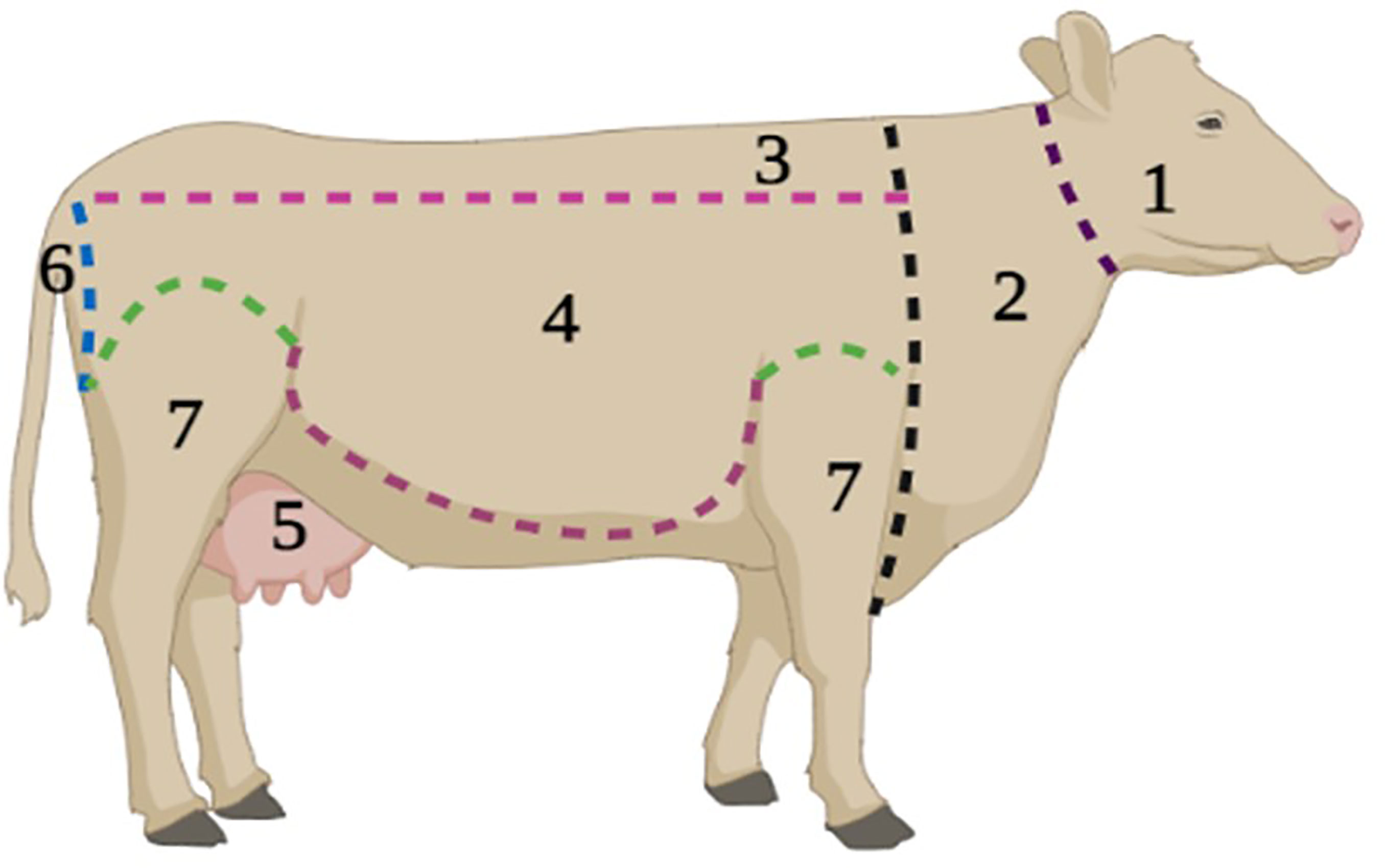
Figure 2 Anatomical subdivision of the cow body for tick’s collection in the western part of the Djurdjura, North Central Algeria, between November 2018 and October 2020. 1. Head, 2. Neck, 3. Back, 4. Thorax, 5. Inguinal, 6. Perennial region, 7. Legs.
Statistical Analysis
The statistical analyses were carried out in two steps using R software version 4.0.4 (The R Foundation, 1020 Vienna, Austria). The results obtained in this survey were estimated and compared using Bayesian approaches. The comparison of the tick’s infestation according to the seasons, months and predilections sites was performed by Chi-square and Fisher’s exact tests (the difference was considered significant at 5% threshold). The affinity groups of tick species according to the parasitic stage, sex, season and predilection sites was done by the multiple correspondence analysis (MCA). The MCA graphically represents the relationship between several categorical datasets.
Results
Of the 289 examined cattle, 186 animals were infested by ticks, corresponding to an overall infestation rate of 64.36 ± 2.81%. The rate of infestation is significantly higher (P<0.001) in cattle aged >18 months old (n= 5734, 55.98 ± 0.49) compared to those aged <18 months (n= 4509, 44.02 ± 0.49%). There was no significant difference in infestation rate between exotic (Montbeliard and Holstein) (n=83, 44.62 ± 3.64%) and crossbred (n=103, 55.38 ± 3.64%).
Collected Ticks
A total number of 10,243 ticks were collected during the 24 visits, consisting of 77 (0.75 ± 0.08%) larvae, 2,450 (23.92 ± 0.41%) nymphs and 7,716 (75.33 ± 0.42%) adults. The monthly infestation rate was high, ranging from 2.85% in January to 13.34% in June. The sex ratio of adult ticks (M: F) was 1.66. The number of ticks per animal ranged from zero to 61 and 95 in May and July, respectively. Five tick genera were collected; Rhipicephalus (66.74 ± 0.46%) and Hyalomma (30.83 ± 0.45%) were the most abundant ticks compared to Ixodes (1.64 ± 0.002%), Dermacentor (0.70 ± 0.001%) and Haemphysalis (0.09 ± 5.80%). Among the listed genera, twelve species were identified and showed a variable infestation rate according to the seasons, of which seven species are perennial and five seasonal (Tables 1, 2).
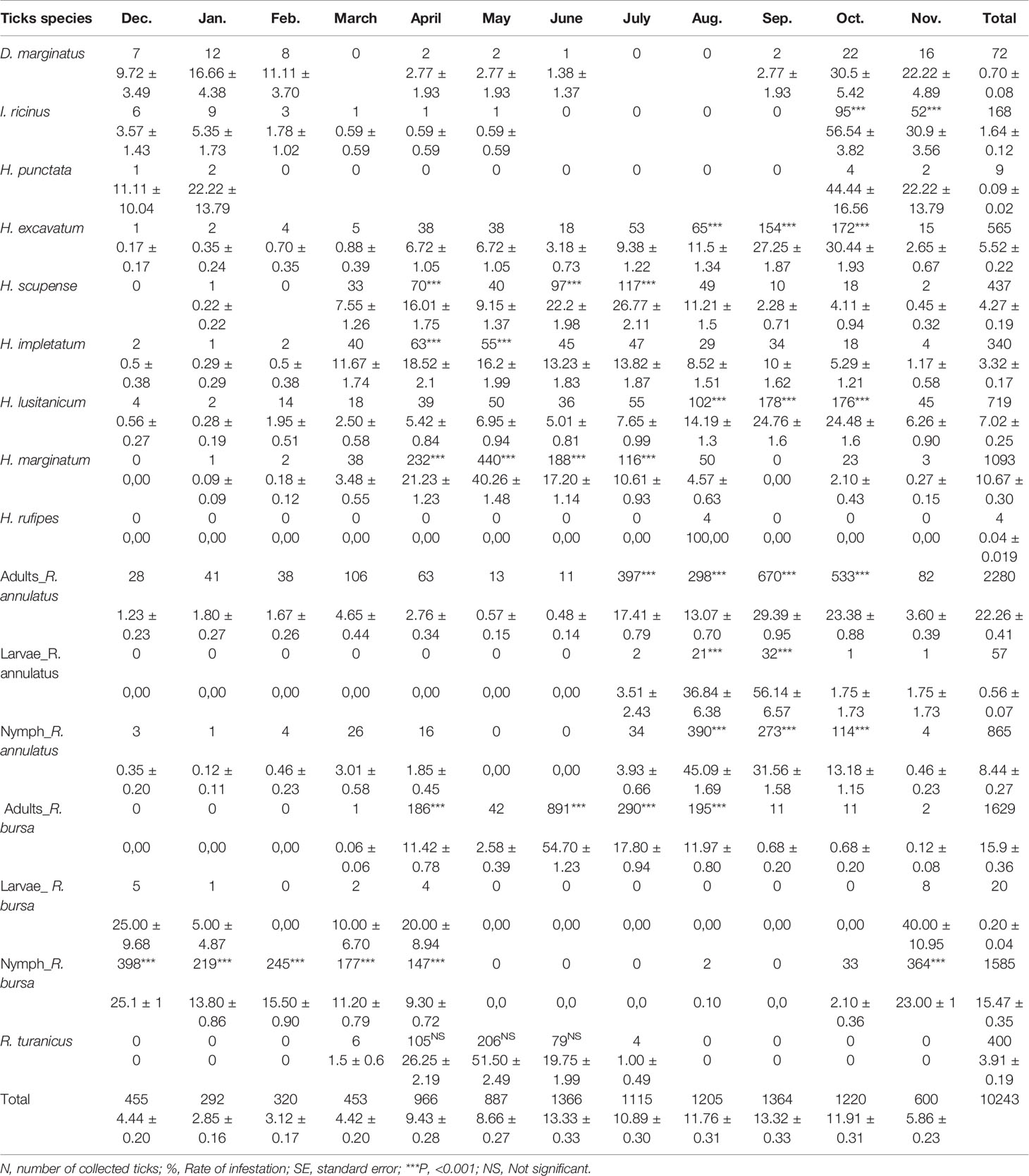
Table 1 Number and monthly distribution of tick species (number. % ± SE) identified in the western part of the Djurdjura, North Central Algeria, between November 2018 and October 2020.
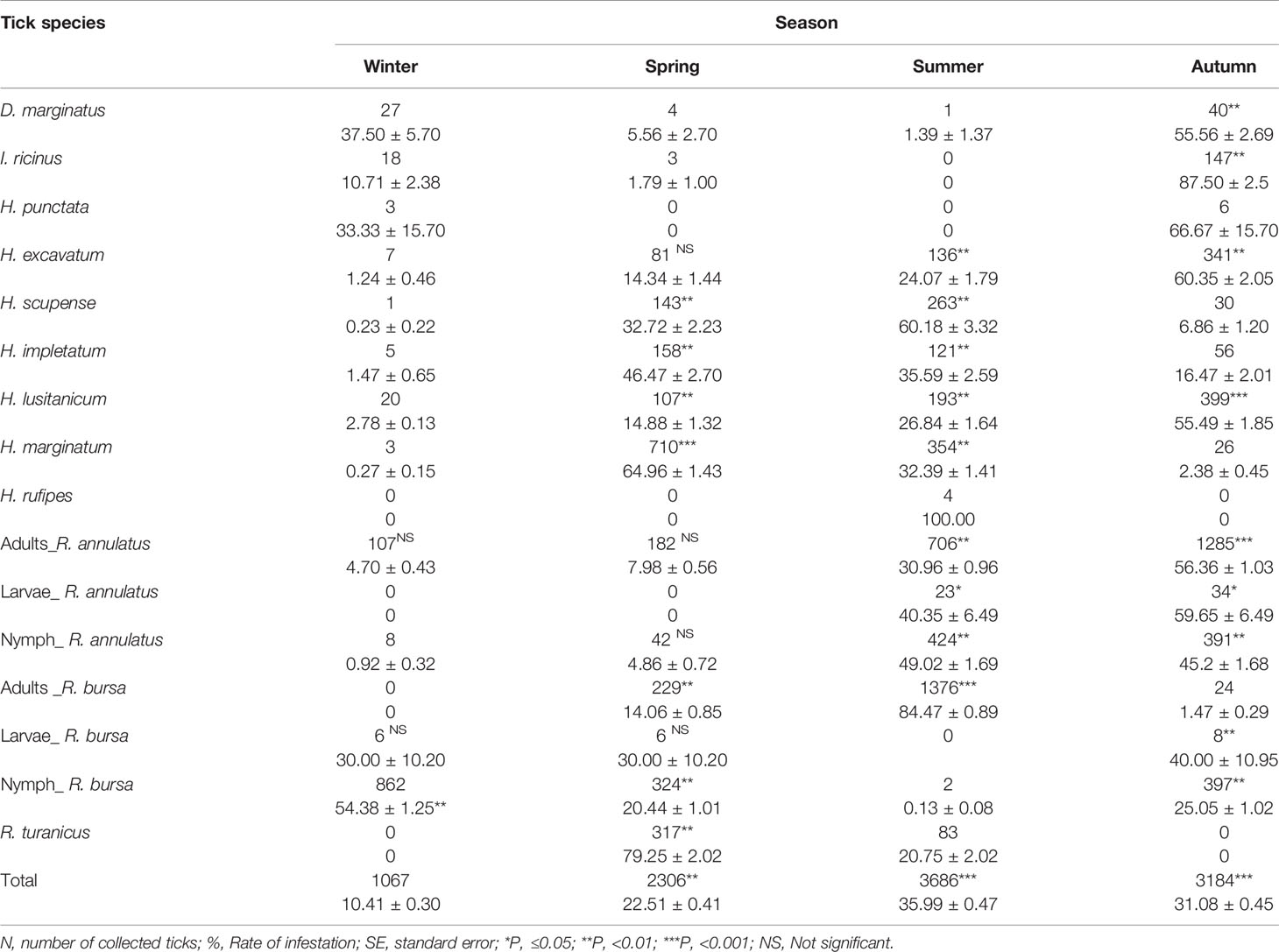
Table 2 Seasonal distribution of tick’s species (N, % ± SE) identified in the western part of the Djurdjura, North Central Algeria, between November 2018 and October 2020.
Seasonal Ticks
The five seasonal ticks showed varying levels of infestation rate by month and season (Tables 1, 2). R. turanicus was most active between March and July (Table 1), with its peak occurring in May (P<0.001, Table 2). Four H. rufipes were collected during summer (Tables 1, 2). Infestation with I. ricinus peaked in October, following a period of significant activity between October and January (P<0.001 Tables 1, 2). Dermacentor marginatus was found on cattle between September and February and peaked in October (P<0.001, Table 1, 2). Only nine specimens of Hae. punctata were collected from October to January and peaked in October (Tables 1, 2).
Perennial Ticks
Monthly and seasonal infestation rates of the perennial ticks were presented in Tables 1 and 2. Rhipicephalus bursa (31.57 ± 0.46%) and R. annulatus (31.26 ± 0.45%) have the highest infestation rate (Table 1). The adult activity of R. bursa is from March to November, with a peak in June. The abundance of this stage is significantly high in April, June, July and August (P<0.001, Tables 1 and 2). The lower larval activity occurred between November to January and from March to April. However, the nymph activity was observed from October to April and peaked in December (P<0.001, Tables 1, 2). Adults R. annulatus occurs throughout the year with significant activity between July and October (P<0.001, Tables 1, 2); this stage peaked in July and September. Larvae were active from July to November and peaked in September. Nymphs peaked in August with high activity from July to October (P<0.001, Tables 1, 2).
Hyalomma species occurs all the year; each species has a monthly and seasonal infestation rate. They were active in spring, summer and autumn compared to the winter (P ≤ 0.05, Table 2). H. marginatum was the most collected Hyalomma species with significant activity from March to August and reached a peak in May (P<0.001, Tables 1, 2). H. lusitanicum and H. excavatum show a similar pattern of activity from February to October (P<0.001) and peaked respectively in September and October (Tables 1, 2). H. scupense and H. impletatum showed the same pattern of activity from March to October; both ticks peaked respectively in July and April (P<0.001, Tables 1, 2).
Distribution of Ticks on the Cattle Body
The 12 tick species collected share the same predilection sites with mixed infestations ranging from five to eleven tick species on the same anatomical site. According to the MCA, the tick population is homogeneous, and their spatial distribution on the cattle body depends on the season, the species and the parasitic stages (Figure 3).
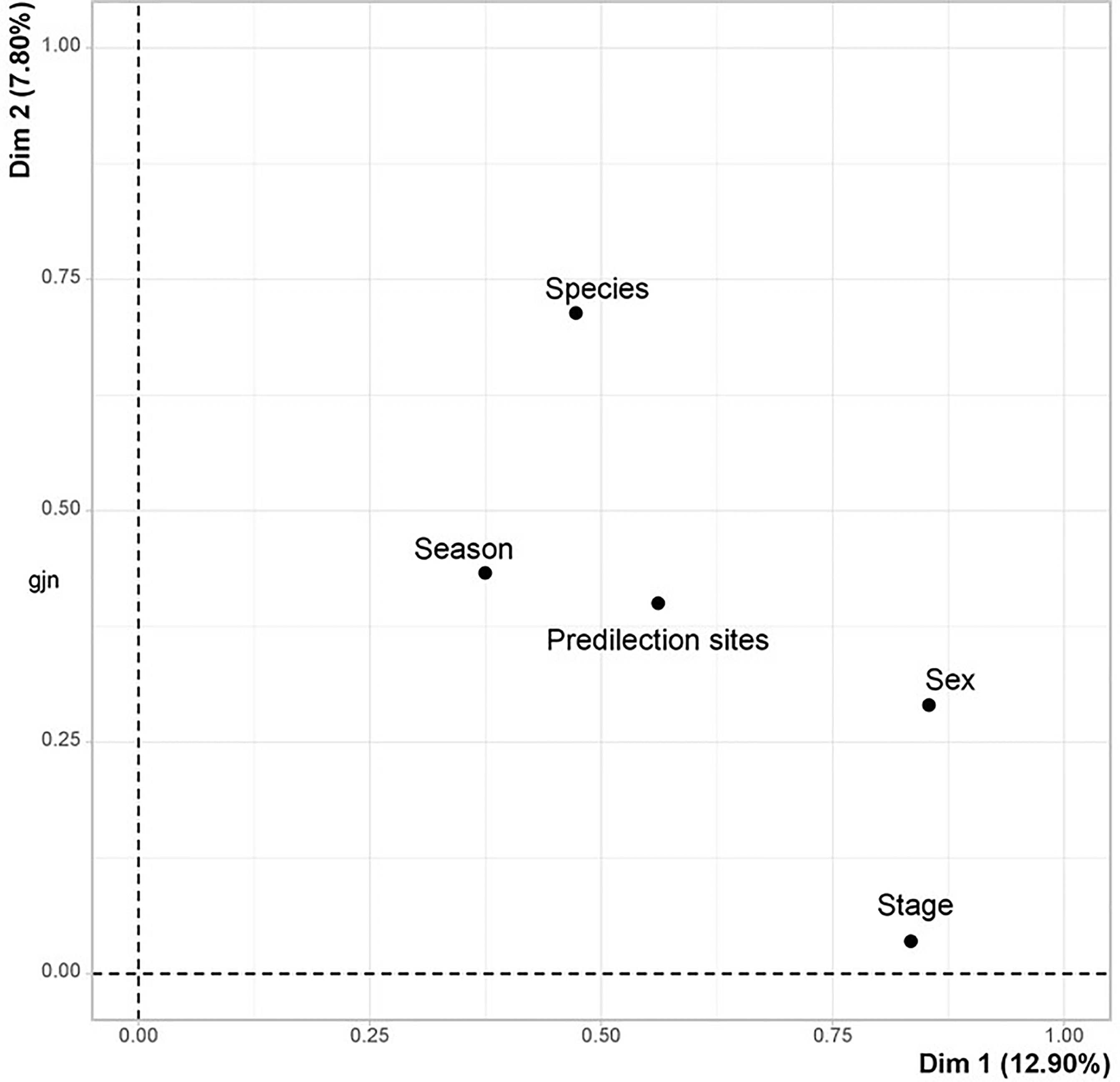
Figure 3 Factorial design of the qualitative variables studied in the western part of the Djurdjura, North Central Algeria, between November 2018 and October 2020.
The MCA identified three groups of ticks according to the qualitative variables studied (Figure 4). The larval and nymphal stages of R. annulatus attach to the neck in autumn (Table 3 and Figure 4). In contrast, adults of this species have a high frequency of attachment to the neck in autumn and the inguinal region in summer (Table 3).
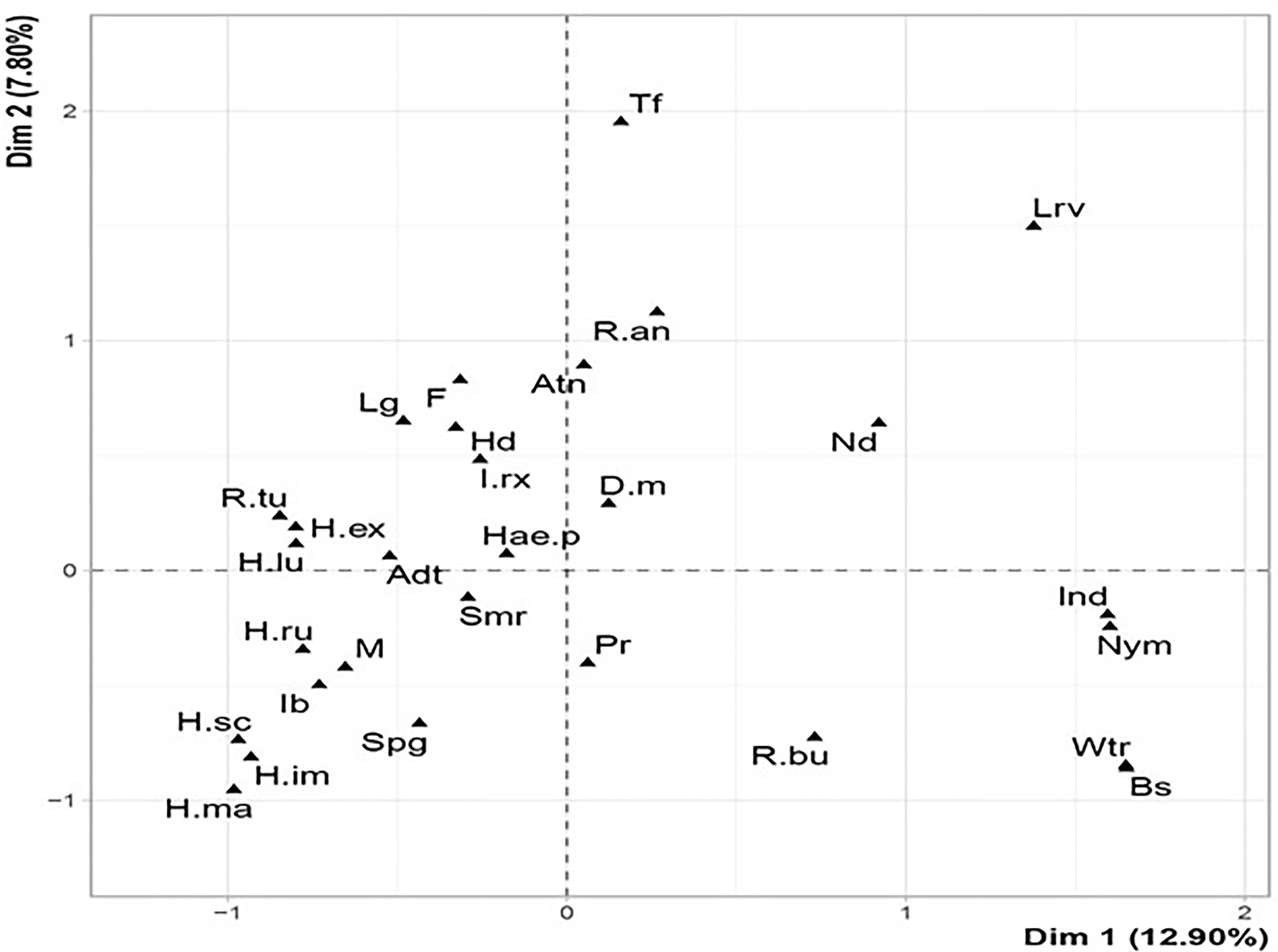
Figure 4 Factorial design of body distribution, seasonality, parasitic stage and tick species in the western part of the Djurdjura, North Central Algeria, between November 2018 and October 2020. Hd, Head; Pr, Perennial region; D.m., Dermacentor marginatus; H.ex., Hyalomma excavatum; H.ma., Hyalomma marginatum; R.an., Rhipicephalus annulatus; Adt, Adulte; Bs, Back; Atn, Autumn; Ib, Inguinal; Wtr, Winter; I.rx., Ixodes ricinus; H.sc, Hyalomma scupense; H.ru., Hyalomma rufipes; R.tu., Rhipicephalus turanicus; F, Female; Nd, Neck; Spg, Spring; Ind, Unidentified.
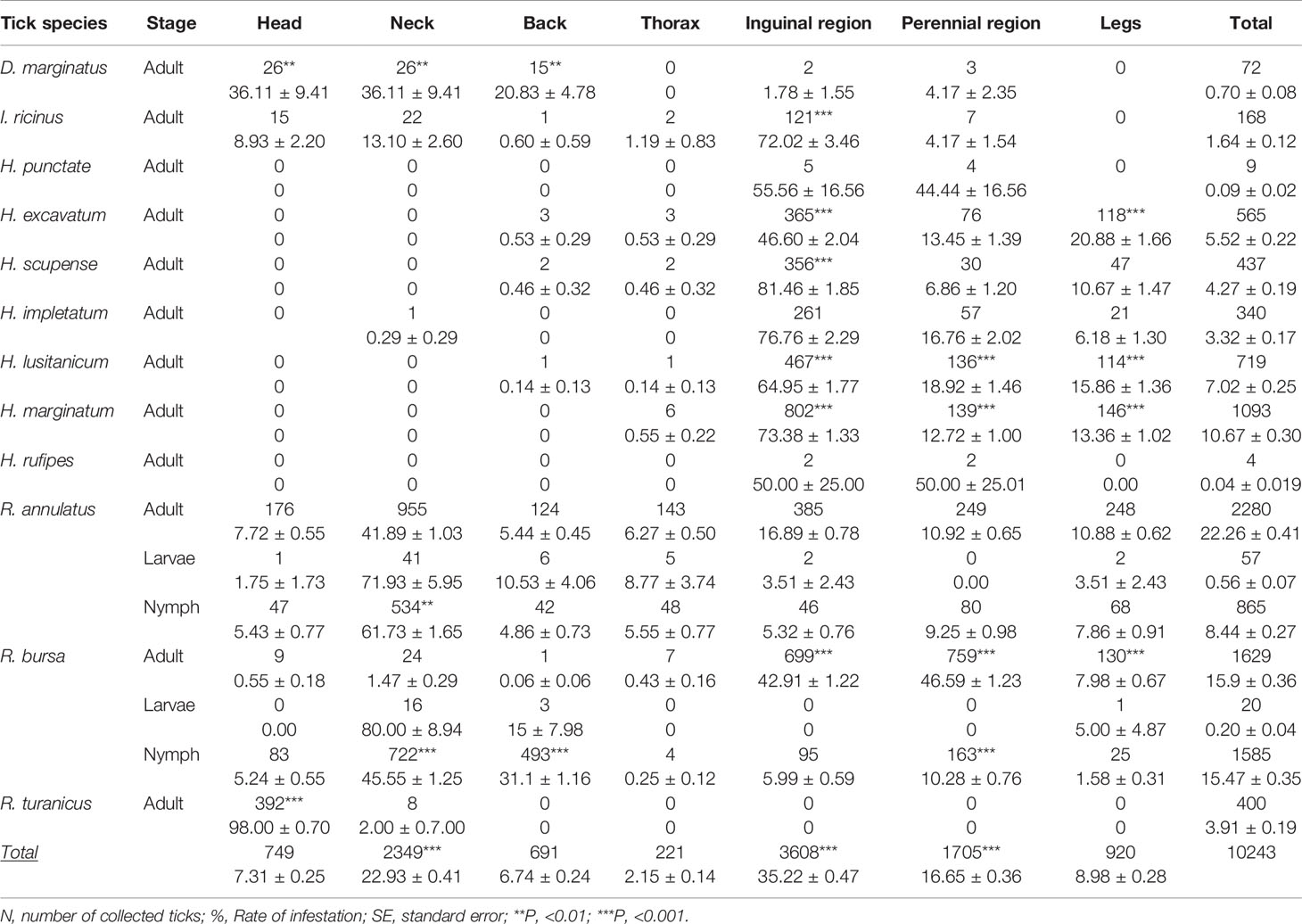
Table 3 Body distribution of tick species (number, % ± SE) identified in the western part of the Djurdjura, North Central Algeria, between November 2018 and October 2020.
The nymph of R. bursa has an affinity for attachment to the neck and back in winter (Table 3 and Figure 4). The adult attaches to the perineum in summer and the inguinal in spring (Table 3). All R. turanicus ticks were collected from the head region, especially the ears in spring (Table 3 and Figure 4).
Hyalomma species had a similar spatial distribution; they have a strong affinity for the inguinal region and a lesser degree for the perineum and legs. According to MCA, H. impeltatum and H. marginatum are spring ticks with the inguinal region as their predilection site (Figure 4 and Table 3). H. excavatum and H. lusitanicum are autumnal ticks with an affinity of attachment for legs (Table 3 and Figure 4). The adults of I. ricinus prefer the inguinal region, and D. marginatus prefer to attach to the head, neck and back (Table 3).
Discussion
Effective control measures against ticks and tick-borne diseases; requires a detailed account of the distribution, seasonal dynamics, and predilection sites of ticks on livestock. The latter is crucial because ticks choose areas that are non-accessible to grooming and licking, rich in blood vessels, dark and hidden to avoid predators and deceive the vigilance of owners (4, 7). The nature of the host coat can influence the attachment site preference of different tick species, as can the seasons (26); knowing the attachment site of ticks allows us to recommend the appropriate acaricide and application method with its optimal concentration (4, 27).
Rhipicepahlus turanicus was collected from the head with a relative abundance of 79.25 ± 2.02% of specimens in-ears in spring and 20.75 ± 2.02% in early summer (P<0.001; Table 1). Similar results were reported in western (3, 10) and eastern Algeria (12), Morocco (28), Tunisia (29), Egypt (30), Turkey (31), Republic of Guinea (32), Zimbabwe (33) and southern Europe (23). R. turanicus was reported with a relative abundance of 82.30%, and they likely prefer to feed on wild boar (34). This species is not known as the main vector of any pathogens for cattle (23) but transmits Hepatozoon canis (35). It causes a warm and painful oedematous reaction of the auricular concha in infested sheep (our unpublished data).
The activity of R. bursa in the western part of the Djurdjura is similar to that reported in Mila and El Tarf (12), north-central of Algeria (24) and Tiaret (10). However, these results are different from those reported in the western part of the country (3) and Morocco (28). The predilection site of R. bursa on cattle varies according to the season and the parasitic stage. Immatures are sensitive to desiccation, their activity starts from late autumn to beginning spring (Table 2), as was reported by Boulkaboul (10), they settle on sun-exposed areas such as the neck and back, as it seems that the low winter temperatures are unfavorable to their engorging. The adults are active from late spring to late summer and settle on the perineum and inguinal area, which corresponds to observations reported in the western part of the country (3).
The cattle tick R. annulatus is active all the year, shared between the different parasitic stages. This seasonal activity is favoured by the humid climate of the study area. Our results are similar to those reported in the eastern part of Algeria (12), Tunisia (36), Libya (37) and Southern Europe (23). However, the R. annulatus activity in the Djurdjura region is different from that reported in the western part of the country (3) and Morocco (28).
The number of larvae of R. annulatus and R. bursa collected from cattle is low because these parasitic stages prefer to feed on small mammals, which are numerous in the Djurdjura region (20), and their small size deceives our vigilance. The larvae and nymph of R. annulatus feed on the neck of cattle of the Djurdjura. Whereas they attached on the perineal region in the cattle of the western Algeria (3). This difference in predilection site is probably due to the hot-humid climate in the west and the cold-humid climate in the study area. In contrast, R. bursa immatures stages feed on the neck and back, similar to the results reported by Yousfi Monod et al. (3).
Gueye et al. (5) reported that the parasitic stages of R. decoloratus attach to the dewlap, while those of B. microplus prefer the neck of tropical cattle (7). Our results confirm the above findings and highlight that R. annulatus have an affinity for the neck and dewlap in the Mediterranean region (Table 3). The low temperatures slow down metamorphosis and development and increase the mortality of ticks, especially immature stages (38). Below a certain temperature threshold, these processes do not take place, and the ticks are in a lethargic state or die (38). The parasitic stages of R. annulatus have an affinity for the neck and dewlap because these anatomical areas are exposed to the sun. We have collected R. annulatus from the thighs of cattle as reported in the western part of the country (3).
Our results demonstrated that species belonging to the genus Hyalomma represent the most abundant ticks on cattle in the Djurdjura region. They were observed throughout the year and are abundant in the summertime than in winter; similar to reported results in the Maghreb (3, 4, 10, 12, 28, 39). Because of the climatic difference between regions in Algeria, H. marginatum reached its peak in May in the Djurdjura region in February in the west (10) and in April in the eastern part of Algeria (12). This species is the vector of the virus causing Crimean-Congo haemorrhagic fever in humans (23). H. marginatum is closely associated with wild animals, while larvae and nymphs feed on a wide range of ground-feeding birds and medium-sized mammals, and adults prefer large animals (40).
Hyalomma scupense appear on cattle in late spring with a peak in July and disappear by the end of August in Algeria (3, 10, 12, 24). We have observed synchronisation between the H. scupense activity and clinical cases of tropical theileriosis (15, 24, 41). A few adult males of H. scupense were collected from September to early November as reported in Turkey (31). The larvae and nymphs feed on cattle, in autumn, from September to November. This tick species is often associated with livestock barns, stables, sheds and pens (4). We could not find larvae and nymphs of H. scupense. Probably, these parasitic stages are engorged and detached for winter diapause. In addition, the farmers clean their stables twice a day and at the same time, they bring out the Immatures in the dung.
H. lusitanicum is significantly more abundant than H. excavatum (P<0.001). Both species have been reported in the Maghreb (3, 10, 12, 28). There is a period from the beginning of October to mid-November, during which we have confirmed many clinical cases of tropical theileriosis in Algeria (our unpublished data). This period coincides with the activity of both species (Tables 1 and 2). These two tick species are likely natural vectors of Theileria annulata, especially as the adults of H. scupense are not active during this period. H. lusitanicum was suspected to be a vector of T. annulata in Algeria (12) and Spain (42).
Hyalomma rufipes was reported for the first time in the study area. The immature feed on rodents, birds and lagomorphs, while the adults feed on herbivores (43). Therefore, it seems that the probability of detecting this species on domestic ruminants is very low for this broad host spectrum by taking one or two samples per month. It could also explain, at least in part, the relatively low number of H. rufipes reported in the present study, which is similar to results reported in the western part of Algeria (10) and Turkey (31).
H. impeltatum was reported in North Africa, the Arabian Peninsula, and the Middle East as far as Pakistan. It is also found in sub-Saharan Africa from Senegal and Mauritania in the west to Eritrea, Djibouti and Somalia in the east. It extends to Cameroon in central Africa and Tanzania in the east (44). We collected this species from March to October, and it peaked between April and May. In the western part of the country, H. impeltatum peaked in June (3). On the other hand, H. impeltatum was collected in dromedary with a relative abundance of 19.04% (11).
Dermacentor marginatus is found in the cold and humid parts of the Mediterranean region associated with the Atlas Mountains (45). We collected D. marginatus from September to February with a peak in January similar to results reported from Tlemcen (3) and Morocco (28). D. marginatus was collected in wild boars from Mostaganem to Tlemcen. This tick ecologically prefers areas with dense bushes and oak and pine trees (3). Ixodes ricinus appears on cattle for a short period in autumn and winter, with a peak in October similar to the results reported in Tlemcen (3) and in El Tarf (12).
Yousfi-Monod et al. (3) state that adult Hyalomma and Ixodes attach in the udder quarters of cows (4). The predilection sites of the six adults Hyalomma identified in this work is similar for all the species (Table 2). They have a marked preference for the inguinal region. In heavy infestations, H. marginatum, H. lusitanicum and H. impletatum prefer the perianal area. In contrast, H. excavatum and H. scupense prefer the legs. Our results corroborate the finding reported in the western part of Algeria (3). However, during heavy infestations of the inguinal region by adults of I. ricinus, the remaining individuals prefer to attach themselves to the head and neck (Table 2).
Some of the identified tick species in the western part of the Djurdjura have already been associated with the transmission of pathogens, such as Theileria annulata, T. buffeli, Babesia bovis, B. bigemina, Anaplasma marginale (46, 47) as well as in other regions of the country (12, 15, 17, 48) and North Africa in general (36, 49, 50).
Conclusion
Since the work conducted in the Pasteur Institute of Algeria during the last century (21, 22, 51), this is the first report describing the intensity, predilection sites, monthly and seasonal activity of twelve species of cattle ticks in the western part of the Djurdjura in Algeria.
Among the identified ticks, eight species are particularly frequent and widespread throughout the study area; H. marginatum, H. lusitanicum, H. excavatum, H. impeltatum are annual with medium infestations. However, R. bursa and R. annulatus achieve massive infestations.
The larvae and nymph of R. bursa parasitize cattle in autumn and winter, and those of R. annulatus are active in summer and autumn respectively. R. annulatus adults ticks are autumnal, and R. turanicus are spring ticks. In contrast, adults of H. scupense and R. bursa are summer ticks.
Three tick species, D. marginatus, I. ricinus and Hae. punctate are parasites of cattle in winter. These cold-climate ticks are adapted to the warm Mediterranean climate of North Africa and have reversed their seasonal activity pattern compared to Europe and have localized to the humid regions of northern Algeria (23).
All parasitic stages of R. annulatus had an affinity for the necks. Nymphs of R. bursa prefer the neck the adults attach to the perineum and inguinal region. R. turanicus attach to the ears. The Hyalomma species had an affinity for the inguinal and perennial region.
Despite the Covid 19 pandemic, we reported a high diversity of cattle ticks in the western parts of Djurdjura, and the molecular identification will support this work. Due to the bioclimatic gradient of Algeria, the study needs to be replicated in other biotopes of the country.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The Ethics Committee of the Directorate of Veterinary Services. Written informed consent for participation was not obtained from the owners because Veterinary clients made their animals available to us for tick collection. No handling was done.
Author Contributions
AB: conceptualization, data curation, methodology, and writing-original draft. RK: conceptualization and investigation. BM: software, methodology, and visualization. TK: conceptualization, investigation, methodology, and visualization. FS: validation and visualization. GT: validation and visualization. HZ: conceptualization, data curation, formal analysis, methodology, funding acquisition, project administration, software, supervision, and writing-review & editing. All authors analysed, reviewed, and edited the manuscript’s final version and approved it for publication.
Funding
This study was financed by the FNRSDT/DGRSDT of Algeria and the LEAP-AGRI European Community within the framework of the H2O2O ERA.Net program, project N° 727715 (MeTVAC-LEAP-AGRI_220).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Kechih Saliha and Dr. Djerbal Mouloud from the Regional Veterinary Laboratory of Draa Ben Kheda, Tizi Ouzou, Algeria. We are grateful to Miss Messaoudi Zahra and Nabi Ranyia for scientific help. We are grateful to the Veterinary Department of the Ministry of Agriculture as well as those of Blida, Bouira, Boumerdes and Tizi Ouzou for their support of this work. We thank the farmers for making their animals available to us for tick collection. We kindly thank Gharbi Mohamed (Professor at the National Veterinary School, Tunis, Tunisia) for valuable comments and the revising of the manuscript.
References
1. Opara MN, Ezeh NO. Ixodid Ticks of Cattle in Borno and Yobe States of Northeastern Nigeria: Breed and Coat Colour Preference. Anim Res Int (2011) 88:1359–65.
2. Kaur D, Jaiswal K, Mishra S. Epidemiological Study of Ixodid Ticks Infesting Cattle Reared by Small Holder Farmers. J Entomol Zool Stud (2017) 4:284–91. doi: 10.22271/j.ento
3. Yousfi-Monod A, Aeschlimann R. Recherche Sur Les Tiques (Acarina, Ixodidae), Parasites Des Bovidés Dans L’ouest Algérien. Ann Parasitol Hum Comparée (1986) 61:341–58. doi: 10.1051/parasite/1986613341
4. Gharbi M, Darghouth MA. A Review of Hyalomma Scupense (Acari, Ixodidae) in the Maghreb Region: From Biology to Control. Parasite (2014) 21:1–12. doi: 10.1051/parasite/2014002
5. Gueye A, Mbengue M, Diouf A, Seye M. Tiques Et Hémoparasitoses Du Bétail Au Sénégal. I La région Des Niayes Rev d"elevage Med Vet Des pays Trop (1986) 39:381–93. doi: 10.19182/remvt.8568
6. Jongejan F, Uilenberg G. Ticks and Control Methods. Rev Sci Tech (1994) 13:1201–26. doi: 10.20506/rst.13.4.818
7. Barré N, Uilenberg G. Pathogenicity and Control of Ticks. In: Chermette R, Uilenberg G, Lefèvre PC, Blancou J, editors. Infection and Parasitic Diseases of Livestock. Bacterial Disease Fungal Disease Parasitic Disease. Paris (2010). p. 125–36.
8. Camicas JL, Hervy JP, Adam F, Morel PC. The Ticks of the World (Acarida, Ixodida): Nomenclature, Described Stages, Hosts, Distribution. Ticks World (Acarida Ixodida) Nomencl Descr Stages Hosts Distrib (1998) 233.
9. Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, et al. Article The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the World: A List of Valid Species Names. Zootaxa (2010) 2528:1–28. doi: 10.11646/zootaxa.2528.1.1
10. Boulkaboul A. Parasitisme Des Tiques (Ixodidae) Des Bovins a Tiaret, Algérie. Rev D’élevage Médecine Vétérinaire Des pays Trop (2003) 56:157. doi: 10.19182/remvt.9858
11. Bouhous A, Aissi M, Harhoura KH. Etude Des Ixodidae Chez Le Dromadaire Dans Le Sud Algérien, Région D’adrar. Ann Med Vet (2008) 152:52–8.
12. Benchikh Elfegoun MC, Gharbi M, Djebir S, Kohil K. Dynamique D’activité Saisonnière Des Tiques Ixodidés Parasites Des Bovins Dans Deux Etages Bioclimatiques Du Nord-Est Algérien. Rev D’élevage Médecine Vétérinaire Des pays Trop (2013) 66:117. doi: 10.19182/remvt.10150
13. Boucheikhchoukh M, Laroche M, Aouadi A, Dib L, Benakhla A, Raoult D, et al. MALDI-TOF MS Identification of Ticks of Domestic and Wild Animals in Algeria and Molecular Detection of Associated Microorganisms. Comp Immunol Microbiol Infect Dis (2018) 57:39–49. doi: 10.1016/j.cimid.2018.05.002
14. Lafri I, Benredjem W, Neffah-Baaziz F, Lalout R, Abdelouahed K, Gassen B, et al. Inventory and Update on Argasid Ticks and Associated Pathogens in Algeria. New Microbes New Infect (2018) 23:110–4. doi: 10.1016/j.nmni.2018.02.009
15. Rouina AD. Clinical Study of Bovine Theileriosis Based on 327 Cases in Algeria (North-West Region, Mascara). Maghreb Vétérinaire (1984) 1:23–7.
16. Ziam H, Benaouf H. Prevalence of Blood Parasites in Cattles From Wilayates of Annaba and El Tarf East Algeria. Arch Inst Pasteur Tunis (2004) 81:1–4.
17. Foughali AA, Ziam H, Aiza A, Boulkrout H, Berber A, Bitam I, et al. Cross-Sectional Survey of Cattle Haemopathogens in Constantine, Northeast Algeria. Vet Med Sci (2021) 7(4):1237–44. doi: 10.1002/vms3.459
18. Kautman M, Tiar G, Papa A, Široký P. Ap92-Like Crimean-Congo Hemorrhagic Fever Virus in Hyalomma Aegyptium Ticks, Algeria. Emerg Infect Dis (2016) 22:354–6. doi: 10.3201/eid2202.151528
19. Meddour R, Meddour-Sahar O, Derridj A, Gehu JM. Synopsis Commenté Des Groupements Végétaux Forestiers Et Préforestiers De La Kabylie Djurdjuréenne (Algérie). Rev For (2010) 62:295–308. doi: 10.4267/2042/38944
20. Amrouche-Larabi L, Denys C, Boukhemza M, Bensidhoum M, Hamani A, Nicolas V, et al. Inventaire Des Petits Vertébrés Terrestres De Quelques Localités Du Nord Algérien. Trav l’Institut Sci (2015) 8:85–95.
21. Senevet G, Rossi P. Contribution a L’étude Des Ixodidés (XIIe Note) Etudes Saisonnière Des Ixodidés De La Région De Bouira (Algérie). Arch Inst Pasteur d’Algérie (1924) 2:223–32.
22. Sergent E, Poncet A. Tableau De La Répartition Saisonnière Des Tiques Les Plus Répandues En Algérie. Arch Inst Pasteur d’Algérie (1937) 15:220–4.
23. Estrada-Peña A, Bouattour A, Camicas JL, Walker AR. Ticks of Domestic Animals in the Mediterranean Region. Alger: University of Zaragoza (2004). Available at: https://www.academia.edu/download/45161360/Ticks_of_Domestic_Animals_in_the_Mediter20160428-17980-mg36yy.pdf.
24. Ziam H, Saidani K, Aissi M. Prevalence of Bovine Piroplasmosis and Anaplasmosis in North- Central Algeria. Sci Parasitol (2017) 18:7–15.
25. Estrada-Peña A, Andrei DM, Petney TN. Ticks of Europe and North Africa A Guide to Species Identification. Cham: Switzerland (2017). doi: 10.1007/978-3-319-63760-0.
26. MacLeod J, Mwanaumo B. Ecological Studies of Ixodid Ticks (Acari: Ixodidae) in Zambia. IV. Some Anomalous Infestation Patterns in the Northern and Eastern Regions. Bull Entomol Res (1978) 68:409–29. doi: 10.1017/S0007485300009391
27. Barré N, Camus E, Borel G, Aprelon R. Sites of Fixation of Amblyomma Variegatum Ticks on Their Hosts in Guadeloupe (French West Indies). Rev Elev Med Vet Pays Trop (1991) 44:453–8. doi: 10.19182/remvt.9152
28. Laamari A, Kharrim KE, Mrifag R, Boukbal M, Belghyti D. Dynamique Des Populations De Tiques Parasites Des Bovins De La Région Du Gharb Au Maroc; Population Dynamics of Cattle Ticks in Gharb Region in Morocco. Rev D’élevage Médecine Vétérinaire Des pays Trop (2012) 65(3-4):57–62. doi: 10.19182/remvt.10123
29. Bouattour A. Dichotomous Identification Keys of Ticks (Acari: Ixodidae), Livestock Parasites in North Africa. Arch Inst Pasteur Tunis (2002) 79:43–50.
30. Okely M, Anan R, Gad-Allah S, Samy AM. Hard Ticks (Acari: Ixodidae) Infesting Domestic Animals in Egypt: Diagnostic Characters and a Taxonomic Key to the Collected Species. Med Vet Entomol (2021) 35(3):333–51. doi: 10.1111/mve.12502
31. Bakirci S, Sarali H, Aydin L, Eren H, Karagenc T. Distribution and Seasonal Activity of Tick Species on Cattle in the West Aegean Region of Turkey. Exp Appl Acarol (2012) 56:165–78. doi: 10.1007/S10493-011-9502-0
32. Tomassone L, Camicas JL, Pagani P, Diallo OT, Mannelli A, De Meneghi D. Monthly Dynamics of Ticks (Acari: Ixodida) Infesting N’Dama Cattle in the Republic of Guinea. Exp Appl Acarol (2004) 32:209–18. doi: 10.1023/B:APPA.0000021771.34520.ab
33. Sungirai M, Abatih EN, Moyo DZ, De Clercq P, Madder M. Shifts in the Distribution of Ixodid Ticks Parasitizing Cattle in Zimbabwe. Med Vet Entomol (2017) 31:78–87. doi: 10.1111/mve.12215
34. Zeroual F, Bitam I, Ouchene N, Leulmi H, Aouadi A, Benakhla A. Identification and Seasonal Dynamics of Ticks on Wild Boar (Sus Scrofa) in the Extreme North-East of Algeria. Bull la Soc Zool Fr (2014) 139:247–55.
35. Giannelli A, Lia RP, Annoscia G, Buonavoglia C, Lorusso E, Dantas-Torres F, et al. Rhipicephalus Turanicus, a New Vector of Hepatozoon Canis. Parasitology (2017) 144:730–7. doi: 10.1017/S003118201600250X
36. Bouattour A, Darghouth MA, Ben Miled L. Cattle Infestation by Hyalomma Ticks and Prevalence of Theileria in Hyalomma Detritum Species in Tunisia. Vet Parasitol (1996) 65:233–45. doi: 10.1016/S0304-4017(96)00951-X
37. Beesley WN, Gabay MM. New Records for Rhipicephalus Bursa, Boophilus Microplus, B.decoloratus and Hypoderma Lineatum From Libya. Med Vet Entomol (1991) 5:259–60. doi: 10.1111/j.1365-2915.1991.tb00549.x
38. Zamora EJ, Leal B, Thomas DB, Dearth RK. Survival of Off-Host Unfed Rhipicephalus (Boophilus) Annulatus (Acari: Ixodidae) Larvae in Study Arenas in Relation to Climatic Factors and Habitats in South Texas, USA. Ticks Tick Borne Dis (2020) 11:101317. doi: 10.1016/j.ttbdis.2019.101317
39. Bouattour A, Darghouth MA, Daoud A. Distribution and Ecology of Ticks (Acari: Ixodidae) Infesting Livestock in Tunisia: An Overview of Eigth Years Field Collections. Parassitologia (1999) 41:5–10.
40. Ruiz-Fons F, Fernández-de-Mera IG, Acevedo P, Höfle U, Vicente J, de la Fuente J, et al. Ixodid Ticks Parasitizing Iberian Red Deer (Cervus Elaphus Hispanicus) and European Wild Boar (Sus Scrofa) From Spain: Geographical and Temporal Distribution. Vet Parasitol (2006) 140:133–42. doi: 10.1016/j.vetpar.2006.03.033
41. Sergent E, Donatien A, Parrot L, Lestoquard F. Etudes Sur Les Piroplasmoses Bovines. Arch l’Institut Pasteur Tunis Inst Pasteur Algérie (1945) 1:1–816.
42. Viseras J, Hueli LE, Adroher FJ, García-Fernández P. Studies on the Transmission of Theileria Annulata to Cattle by the Tick Hyalomma Lusitanicum. Zentralbl Veterinarmed B (1999) 46:505–9. doi: 10.1111/J.1439-0450.1999.TB01242.X
43. Vatansaver Z. Hyalomma Rufipes. In: Petney T, Estrada-Pena A, Mihalca AD, editors. Ticks of Europe and North Africa. Cham, Switzerland (2017). p. 355–9.
44. Estrada-Peña A, Mihalca AD, Petney TN. Genus Hyalomma Koch, 1844. In: Estrada-Peña A, Mihalca AD, Petney TN, editors. Tick of Europe and North Africa. A Guide to Species Identifcation. Cham: Springer (2017). p. 343–8. doi: 10.1007/978-3-319-63760-0_65
45. Walker A, Bouattour A, Camicas J, Estrada-peña A, Horak I, Latif A, et al. Ticks of Domestic Animals in Africa: A Guide to Identification of Species (2003). Available at: http://www.researchgate.net/publication/259641898_Ticks_of_domestic_animals_in_Africa_a_guide_to_identification_of_species/file/5046352d0429878d7f.pdf.
46. Ziam H, Kelanamer R, Aissi M, Ababou A, Berkvens D, Geysen D. Prevalence of Bovine Theileriosis in North Central Region of Algeria by Real-Time Polymerase Chain Reaction With a Note on Its Distribution. Trop Anim Health Prod (2015) 47:787–96. doi: 10.1007/s11250-015-0772-0
47. Ziam H, Tahar K, Khelaf S, Rabah K, Zoheir H, Dirk G. Bovine Piroplasmosis-Anaplasmosis and Clinical Signs of Tropical Theileriosis in the Plains of Djurdjura (North Algeria). Vet Med Sci (2020) 00:1–10. doi: 10.1002/vms3.305
48. Ziam H, Ababou A, Kazadi JM, Harhoura KH, Aissi M, Geysen D, et al. Prévalences Et Signes Cliniques Associés Des Piroplasmoses Bovines Dans Les Wilayates D’Annaba Et El Tarf, Algérie. Rev Med Vet (Toulouse) (2016) 167:241–9.
49. Al-Hosary A, Rǎileanu C, Tauchmann O, Fischer S, Nijhof AM, Silaghi C. Epidemiology and Genotyping of Anaplasma Marginale and Co-Infection With Piroplasms and Other Anaplasmataceae in Cattle and Buffaloes From Egypt. Parasites Vectors (2020) 13:1–11. doi: 10.1186/s13071-020-04372-z
50. Mossaad E, Gaithuma A, Mohamed YO, Suganuma K, Umemiya-shirafuji R, Ohari Y, et al. Molecular Characterization of Ticks and Tick-Borne Pathogens in Cattle From Khartoum State and East Darfur State, Sudan. Pathogens (2021) 10:1–16. doi: 10.3390/pathogens10050580
Keywords: predilection sites, cattle, Djurdjura, phenology, tick, Algeria
Citation: Bedouhene A, Kelanemer R, Medrouh B, Kernif T, Saidi F, Tail G and Ziam H (2022) Seasonal Dynamics and Predilection Sites of Ticks (Acari: Ixodidae) Feeding on Cows in the Western Parts of the Djurdjura, Algeria. Front. Trop. Dis 3:856179. doi: 10.3389/fitd.2022.856179
Received: 16 January 2022; Accepted: 21 March 2022;
Published: 20 April 2022.
Edited by:
Felix Nchu, Cape Peninsula University of Technology, South AfricaReviewed by:
Munyaradzi Marufu, University of Pretoria, South AfricaShawgi Hassan, University of Khartoum, Sudan
Copyright © 2022 Bedouhene, Kelanemer, Medrouh, Kernif, Saidi, Tail and Ziam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hocine Ziam, emlhbWhvY2luZTE5NjdAZ21haWwuY29t
 Amina Bedouhene1
Amina Bedouhene1 Bachir Medrouh
Bachir Medrouh Tahar Kernif
Tahar Kernif Hocine Ziam
Hocine Ziam