- 1Department of Veterinary Public Health, College of Veterinary Science, Assam Agricultural University, Guwahati, India
- 2Department of Veterinary Microbiology, College of Veterinary Science, Assam Agricultural University, Guwahati, India
- 3Animal Health Division, National Research Centre on Pig, Guwahati, India
- 4Department of Clinical Sciences, Division of Reproduction, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 5Department of Biosciences, International Livestock Research Institute, Nairobi, Kenya
- 6Department of Medical Biochemistry and Microbiology, Uppsala University, Uppsala, Sweden
West Nile virus (WNV) is a zoonotic, emerging mosquito-borne virus which can cause severe disease in the form of encephalitis and acute flaccid paralysis in humans. In Assam, northeast India, arboviruses seem to be re-emerging, however, WNV has been little studied. The present investigation was carried out from April, 2018 to March, 2019 to study sero-positivity of WNV in chicken in urban and peri-urban areas of Guwahati, the capital city of Assam. Four urban and four peri-urban areas of Guwahati were selected. A total of 864 chicken serum samples (72 samples per month) were screened by ELISA and further confirmed by haemagglutination inhibition (HI), which revealed that 3.13% of the chickens had been exposed to WNV, with 0.69% sero-positivity in urban areas compared to 5.56% in peri-urban. Peak sero-prevalence of WNV were reported during the month of July and August with 8.33% each with lowest sero-prevalence being recorded in November (1.39%) and no sero-positive birds from December to April. These results indicate that WNV is one of the actively circulating flaviviruses in Assam, and human febrile and encephalitic cases should be screened for the disease.
Introduction
West Nile virus (WNV) is an arthropod-borne virus of the genus Flavivirus and belongs to the Japanese encephalitis virus (JEV) antigenic complex under family Flaviviridae (1). Since the first isolation of WNV in 1937 in the West Nile district of Uganda from a febrile woman (2), the virus has been spreading and is considered an emerging zoonosis (3). WNV can cause encephalitis in severe cases and a few patients develop acute flaccid paralysis (4, 5). Although WNV is transmitted in a cycle involving different birds and mosquitoes (6), domestic birds are unlikely to play a major role in the virus transmission as they usually do not develop sufficient viraemia (7, 8). However, seroconversion in poultry in any geographical region indicates the existence of the virus and also the consequent threat to human population, and therefore poultry can be used as sentinel for disease surveillance (9).
Assam is a state in North-eastern India, situated south of the eastern Himalayas along the Brahmaputra and Barak River valleys, covering an area of 78,438 km2. To its north lies Bhutan and Arunachal Pradesh with Nagaland and Manipur to the East; Meghalaya, Tripura, Mizoram and Bangladesh to the South and West Bengal to the West. The state extends from 89°42′E to 96°E longitude and 24°8′ N to 28°2′ N latitude. It is the most populated state with about 50% of the 38.8 million inhabitants of Northeast India (10). Recognition of WNV as the causative agent of acute encephalitis syndrome in Assam was first confirmed in 2006, and the study by Khan et al. (10) was the first report of this flavivirus from eastern parts of India. Although JEV has been reported to cause epidemics in Assam since 1976, studies have shown that only 53.7% of persons with acute encephalitis syndrome (AES) were infected with this virus and most cases remain undiagnosed (11). Since many patients with symptoms of neurotropic viral infection are JEV negative, there is likely circulation of some other virus, which might be WNV, as signs and symptoms produced by JEV and WNV are similar, but little is known about WNV circulation in the state, although the virus has been confirmed in some cases of AES (12).
In Assam, a total of 52 mosquito species under 11 genera have been detected, the most predominant being Anopheles followed by Culex, Aedes, Mansonia, Armigeres, Mimomyia, Ochlerostatus, Malaya, Toxorhynchites, Ficalbia and Aedeomyia (13). Mosquitoes belonging to Ma. uniformis and Cx. vishnui group comprising of Cx. tritaeniorhynchus, Cx. vishnui and Cx. pseudovishnui were reported to be highly abundant, breeding predominantly in paddy fields (14).
Assam is a state with many national reserves and wetlands, and hence a diverse avifauna (15), but in addition to all wild birds, poultry is also very common, and there has been substantial development of poultry farming in India (16). Agriculture and allied activities play a major role in the economy and the farmers are considered to be the backbone of agriculture in Assam. Poultry has become a vital component of the farm economy as it generates additional income and employment in the rural area (17). Poultry rearing is traditionally popular in this state, especially in rural and tribal areas for meat and egg production and poultry is the primary source of animal protein and supplementary income for the rural people.
In Assam, WNV has been detected by reverse transcriptase-PCR in Cx. vishnui, Cx. whitmorei, Cx. tritaeniorhynchus, Cx. quinquefasciatus, Cx. pseudovishnui and Mansonia uniformis, and the viruses detected were found to be WNV lineage 5, and 99-100% similar to a WNV strain earlier found in a human sample in Assam (18).WNV has been identified from various regions of Assam to which JEV is endemic indicating that WNV might be a substantial contributor to the acute encephalitis syndrome (AES) cases in this region.
Rice is the main crop of Assam and occupies about two-thirds of the total cultivated area of the state. However, abundance of rainfall, rice fields, animal reservoirs and diverse mosquito species makes Assam the most vulnerable state for spread of vector-borne diseases. Circulation of highly pathogenic neuro-invasive WNV has also been reported from this part of the country (12), and it is therefore urgent to identify ways to protect the Assamese population, using a one-health approach (19). Considering the above facts, the present study was conducted to better understand WNV epidemiology and to map serological evidence of WNV infection in poultry in urban and peri-urban areas of Guwahati.
Methods
This repeated cross-sectional study was conducted from April, 2018 to March, 2019.
Selection of Study Area and Location of Poultry Farms
Based on a pilot survey and retrospective data on the availability of poultry in urban and peri-urban farms of Guwahati, the area of study was identified. Urban farms were defined as within the official city boundaries and peri-urban as within 10 km of the official city boundaries of Guwahati, the capital city of Assam (20). All villages in that circle were mapped and poultry farms identified. Thereafter, four locations each from urban (Chandmari, Hatigaon, Noonmati and Khanapara) and peri-urban (Jugukuchi, Kamalajari, Garal and Gadebari) areas of the city were purposely selected as representing different parts of the city. From each study locations, three farms were selected randomly encompassing in total 24 farms. Coordinates of farms were recorded using GPS tools (Figure 1). A suitable questionnaire was prepared for collecting baseline information from urban and peri-urban poultry farmers of Guwahati which was validated for its legitimacy and consistency. Consent was obtained from the farmers before collection of information.
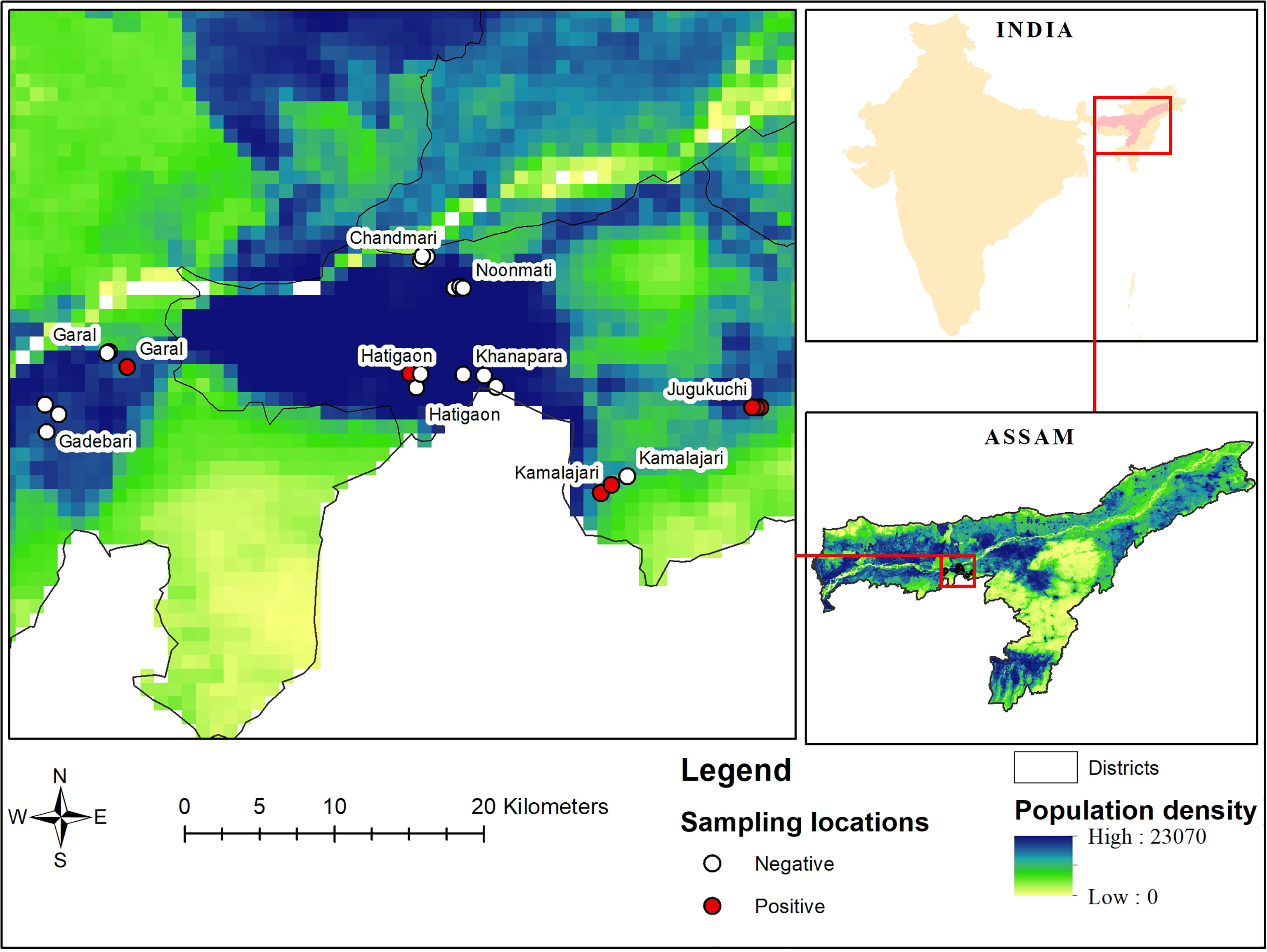
Figure 1 Locations of sampled urban and peri-urban poultry farms of Guwahati, together with population density (people/square km) and serological status of the farms (positive or negative).
Serum Sample Collection
The farms were visited every month for a period of 1 year (April, 2018, to March, 2019), and at each visit, three birds were randomly selected from each of the 24 farms, comprising of 72 serum samples per month. Sample size was decided after budget considerations and discussions with farmers on how many birds they would allow to have sampled. The farmers consented to the blood collection procedure of the chickens and from each bird, 2 ml of whole blood was collected using sterilized disposable syringes. The blood samples were transferred to a vacutainer and left to clot at room temperature. The samples collected were then brought to laboratory in sample carrier box using ice packs. The serum samples separated from the clot were then stored at -20°C until analysis.
Serological Analyses
Serum samples were screened for WNV antibodies using a commercially available competitive enzyme-linked immunosorbent assay (ELISA) kit (7) (ID Screen® West Nile Competition, IDVet, Grabels, France) following the manufacturer’s instructions, which allows species-independent recognition of WNV antibodies against the Pr-E envelope protein. However, flavivirus ELISA are prone to cross-reaction with other flaviviruses (21–24), and the results of the ELISA can thus only be interpreted as having antibodies against a flavivirus. The most specific test to identify WNV antibodies is through virus neutralization tests (VNT) (21, 25), but this method is less sensitive and requires high biosafety level of laboratories which is why ELISA is more commonly used (26).
Since VNT was not possible in the lab, the ELISA positive samples in this study were confirmed for WNV specific antibodies using haemagglutination inhibition (HI) test as recommended by OIE (27) and as per the method described previously (28, 29).
Acetone extraction of the serum was carried out to remove non-specific HA inhibitors. Cold acetone (2.5 ml) was added to serum (50 µl) samples in test tubes which were then capped with rubber stoppers. The solution was mixed well and extracted for 5 min in an ice-bath. Centrifugation was carried out at 1500×g for 5 min at 4°C and thereafter, the supernatant was decanted. The sediments thus obtained were spread inside tubes and vacuum dried at room temperature for 1 hour. Borate saline (pH 9.0; 500 µl) was added to each tube, capped with a rubber stopper, and sediments were allowed to dissolve overnight at 4°C to make 1/10 dilution of the sera.
To each of the acetone extracted sera, 1/50 volume of packed goose red blood cells (RBC) were added and adsorbed for 20 minutes in an ice bath. Then centrifugation was done at 800×g for 10 min. The supernatant obtained was thus made ready to carry out HI test (1/10 dilution).
West Nile virus reference strain WN_0304 (Israel 2000) was used as antigen and prepared by diluting virus culture stored on Whatman® FTA® Classic cards with a borate saline solution with 0.4% bovine albumin (BABS). The antigen was tested for its haemagglutinating activity before performing the HI test. The highest dilution of the virus causing haemagglutination was taken as 1 HA unit. Four times more concentrated 1 HA unit virus was considered as 4 HA unit.
Serial two-fold dilution of the test serum samples was made with BABS in the microtitre plate. An equal volume of known viral antigen using 4 HA unit was added to each of the serum containing wells. The plates were shaken gently and kept at room temperature for 1 h. To all the wells double the volume of the washed RBC solution was added. The plates were then shaken gently and incubated at 37°C for 1 h. In each of the test, serum control containing BABS and diluted antigen; and RBC control containing BABS and RBC were kept.
Data Analyses
Data were entered into MS Excel and statistical comparisons conducted by STATA 14.2 (STATAcorp Ltd, College Station, Texas) using Exact Fisher’s test. Other studies have also found higher sensitivity of ELISA (97.9%) compared to HI (87.5%), while the specificity of ELISA and HI have been reported as 95.7% and 100%, respectively (30). Due to the higher specificity, HI results are used for the descriptive statistics. Coordinates were entered into Google Earth Pro to understand how densely populated the area was where chickens were found positive. Four climatological seasons (pre-monsoon, monsoon, post-monsoon and winter) were defined as by India Meteorological Department, Ministry of Earth Sciences, Government of India.
Results
Based on the questionnaire data, it was found that the purpose of rearing chicken in the present study was mainly for earning livelihood by selling the birds, while only a small number of farmers reared the birds for home consumption and egg production.
In the present study, 25% of the total farms were under integrated production system and all of these belonged to peri-urban areas. In integrated systems the farmers only invest by providing the sheds, equipment and the existing land and the integrator provides all other requisites including day-old chick, feed, litter (bedding), medications, vaccines, technical support etc. for raising the birds. No farm had an abattoir and the farmers sold the birds directly in the market, or the birds were collected by the company in case of integrated farms. The farmers and their families were all living in the same compound at walking distance from the poultry house.
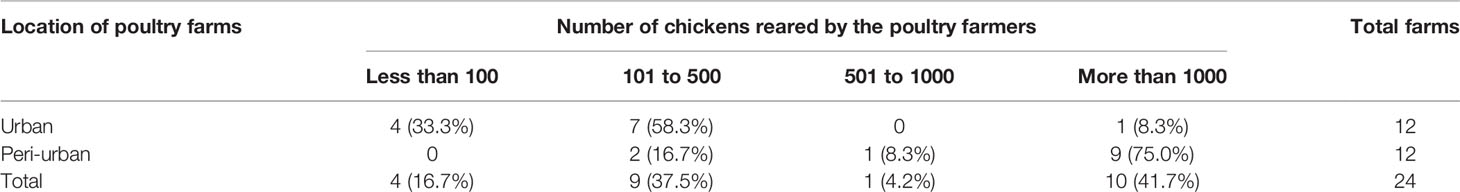
Out of the 24 visited farms, four had indigenous, which means local breeds or non-descript birds, with between 5 and 11 birds per farm. The other 20 farms kept broilers, and reported keeping between 200 and 100,000 birds (Table 1). Farmers in the peri-urban areas of Guwahati reared comparatively more birds than the urban farmers. In the present study, 75.0% of peri-urban farms had more than 1000 birds followed by 16.7% farms with 101-500 birds. In contrast to peri-urban farms, 58.3% of urban farms had 101-500 birds followed by 33.3% farms with less than 100 birds. Most farms were deemed to have moderately clean shed (15/24 farms, defined as: Bedding dry or little bit damp. Feeders and water sources may have some contamination), and similarly most farms had moderately clean birds (16/24 farms, defined as: Majority of birds having plumage color more towards brownish or dusty).
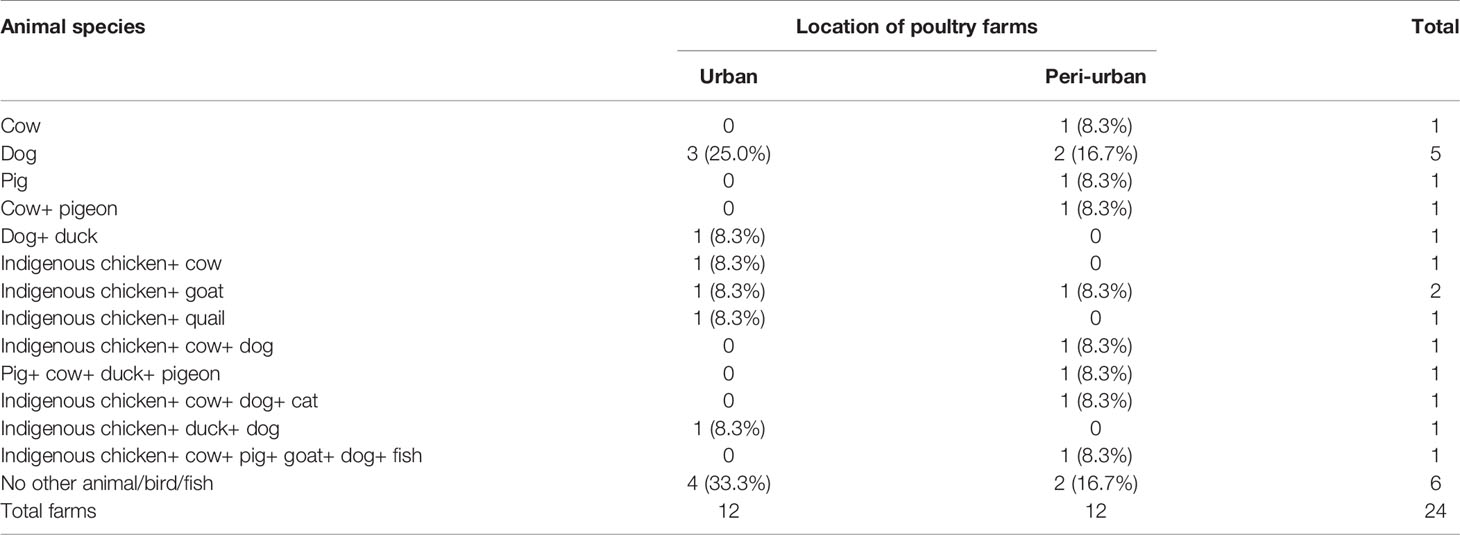
The poultry farmers of urban and peri-urban Guwahati also reared cow, pig, goat, duck, quail, pigeon, and fishes (Table 2). Out of the 24 farms, 66.7% farms were found to have at least one other animal species. Household of eight farms, five in urban and three in peri-urban areas, were only kept poultry. Other animals in contact with chickens were recorded to be comparatively higher in peri-urban (55.6%) than urban (44.4%) poultry farms of Guwahati. Presences of pigs were recorded only in peri-urban poultry farms and multiple in-contact of other animals with chickens were recorded from both urban and peri-urban farms.
Out of 864 samples, 36 were positive for antibodies against WNV by ELISA (4.2%, 95% confidence interval (CI) 2.9-5.7%). Further confirmation of these 36 ELISA positive samples by HI revealed 27 to be positive for WNV with an overall apparent prevalence of 3.1% (95% CI 2.07-4.5%) (Table 3). Location-wise sero-prevalence of WNV in chicken was recorded to be 0.9% and 0.7% in urban areas and 7.4% and 5.6% in peri-urban areas, as evidenced by ELISA and HI, respectively, which was comparatively higher in peri-urban than urban areas of Guwahati. Half of the peri-urban farms (50%) had at least one positive bird, compared to one (8.3%) of the urban farms (p=0.07). The sero-prevalence was recorded from Hatigaon in urban Guwahati and three peri-urban locations namely, Jugukuchi, Kamalajari and Garal. Location-wise, highest WNV sero-prevalence was recorded in Jugukuchi (11.1%), followed by Kamalajari (8.3%) with lowest in Hatigaon and Garal, showing 2.8% sero-positivity each. No WNV sero-positivity in chicken was found in the other sites (Figures 1 and 2).

Table 3 Sero-prevalence of West Nile virus in urban and peri-urban poultry farms by enzyme-linked immunosorbent assay (ELISA) and haemagglutination inhibition (HI).
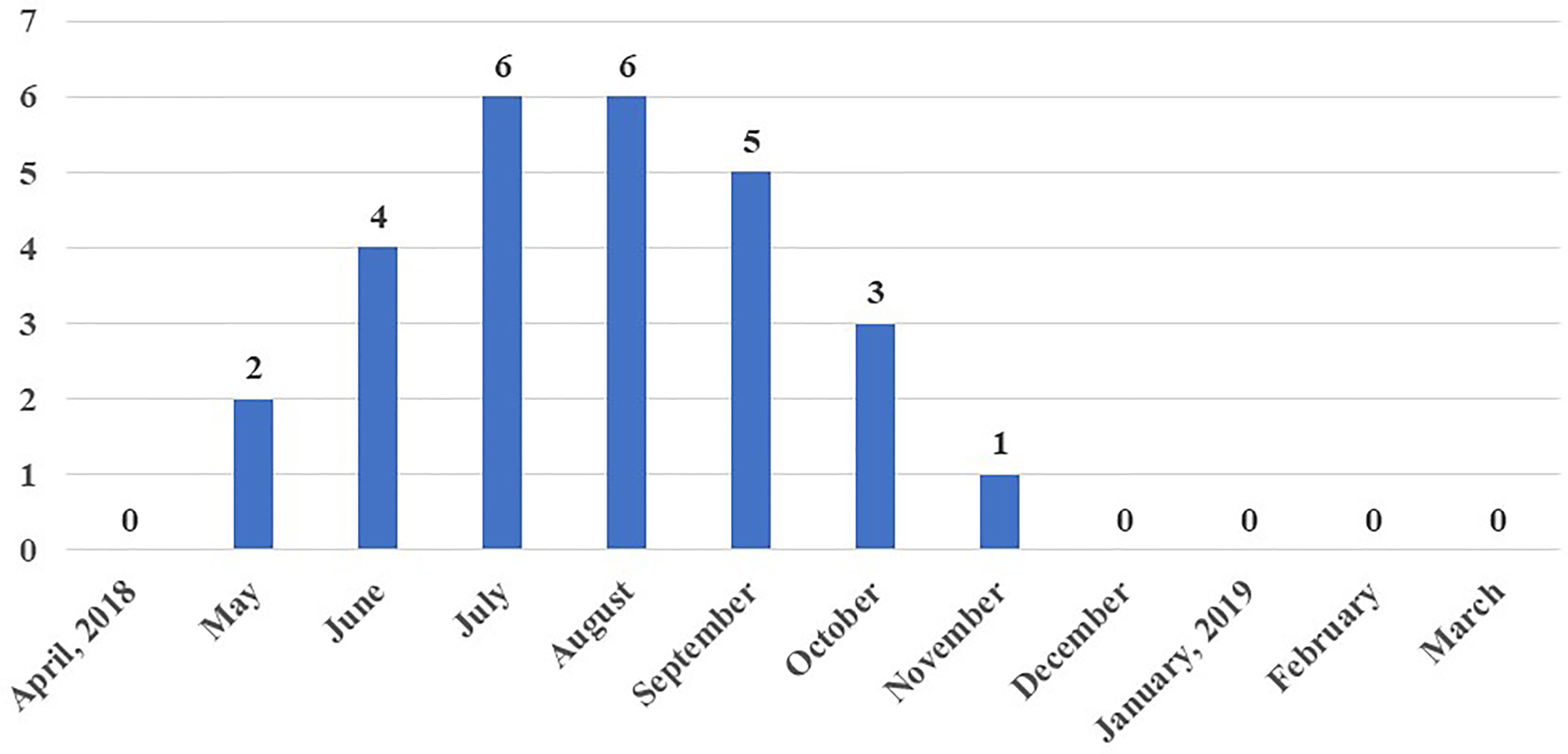
Figure 2 Accumulated sero-prevalence of West Nile virus in chicken samples over one year in different parts of Guwahati, Assam, India.
WNV antibodies were detected in one poultry farm of urban Guwahati compared to six sero-positive farms belonging to peri-urban areas (Figure 1). Through mapping of the farm coordinates, it was possible to see that positive farms were both in densely populated as well as in more peri-urban locations. More farms with seropositive birds were observed among those that kept pigs (2/3, 66.7% (the negative farm had only one pig)) compared to farms not keeping pigs (5/21, 23.8%), and the same was observed for farms keeping any kind of livestock apart from poultry (4/8, 50%) compared to farms with only poultry (3/16, 18.8%), but the difference was not significant (p>0.1). None of the small farms with indigenous poultry had any seropositive birds.
Maximum sero-positivity of WNV was reported in July and August, 2018 with 8.3% prevalence each. Lowest sero-positivity was recorded in November (1.4%) and no positive birds were recorded from December to April (Figure 3). Out of a total of 27 WNV sero-positive samples, three were from urban farms, one each in the months of July, August and September 2018. The remaining 24 sero-positive samples from May to November 2018 were from peri-urban farms.
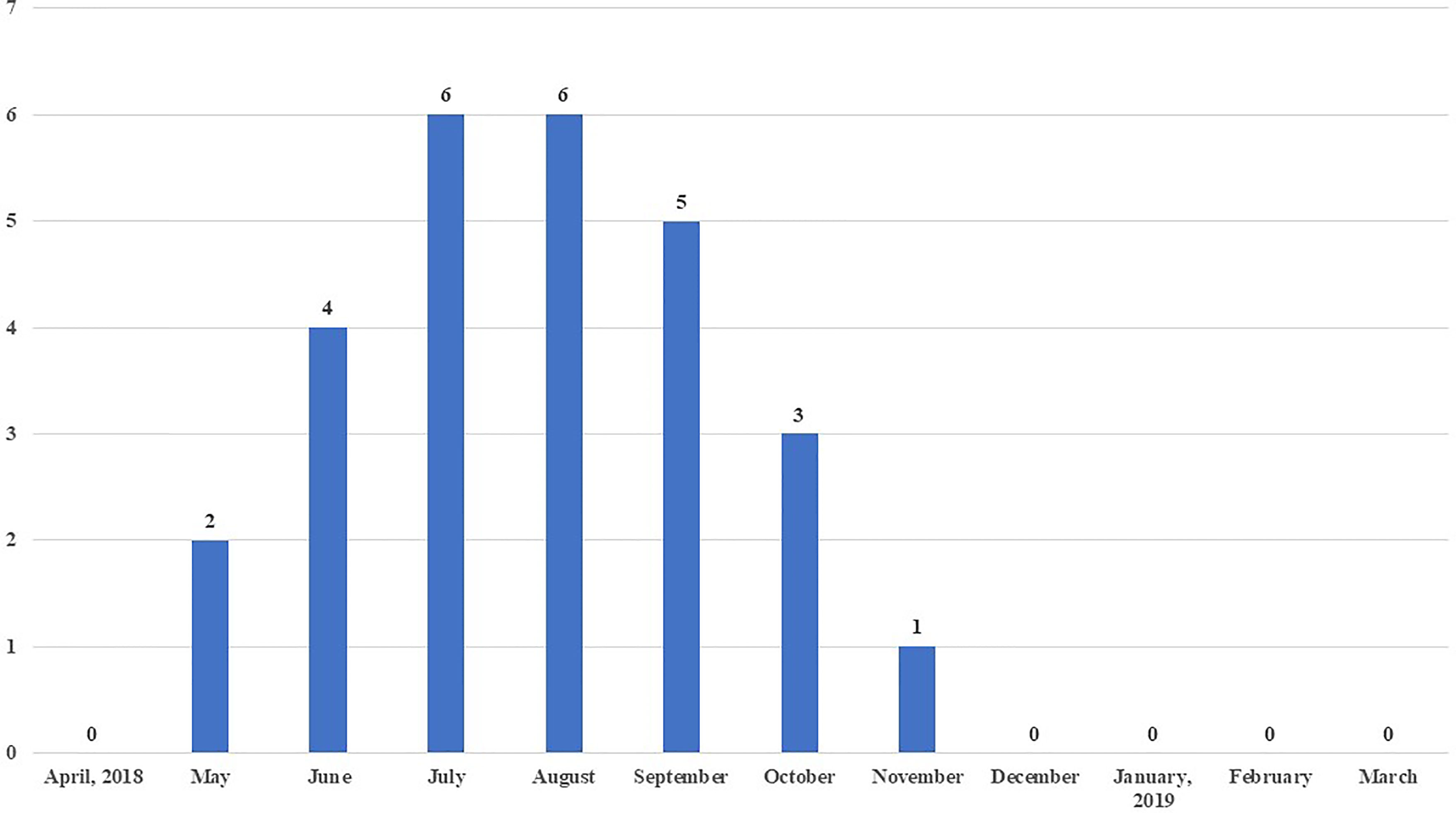
Figure 3 Month-wise number of chickens sero-positive to West Nile virus (WNV) out of 72 chicken sampled each month.
Most seroconversions were observed in monsoon (7.3%), followed by post-monsoon (2.8%) and pre-monsoon (0.9%) with no sero-positive birds detected in winter season (Table 4). All the sero-positive samples in urban areas of Guwahati were recorded during monsoon season.
In the present study, the most common disease symptoms in the poultry farmers or their house-hold members were reported to be fever (75.0%) followed by flu-like symptoms (70.8%), headache (66.7%), fatigue (50.0%), off-fed (29.2%), myalgia (25.0%), arthralgia (25.0%), nausea (16.7%) and vomiting (12.5%). No farmers reported drowsiness, neurological symptoms or motor dysfunction. Peri-urban farmers (41.7%) suffered more than urban farmers (8.3%) from myalgia (p=0.06). The only symptom with an association with serological status of the farm was myalgia (p=0.02), where 4/7 (7.1%) seropositive farms reported myalgia, while this was only reported by 2/17 (11.8%) seronegative farms.
Discussion
This study demonstrates that poultry in Assam, India, are exposed to WNV and that exposure is more common in peri-urban areas, and around the monsoon season. The higher positivity (4.17%) found in our study by ELISA compared to HI (3.13%) is likely due mainly to higher sensitivity, but could also be indicative of lower specificity, and more cross-reactions, maybe with JEV. Flaviviruses are prone to serological cross-reactions (31), and it may be difficult to conclude which virus an animal has been exposed to by only using one serological method, particularly in an area with more than one flavivirus circulating, like Assam.
Higher (29.40%) as well as lower (0.60-1.70%) WNV sero-positivity in domestic birds has been reported in other countries (7, 32). Domestic chickens do not usually show clinical signs of WNV, and therefore they are the most commonly used avian model for the study of immune responses to WNV infection. However, abnormal death and paralysis of domestic chickens has been found associated with sero-positivity against WNV (32).
There is expected variation in WNV sero-prevalence over the year, as the WNV epidemiology is determined by both abiotic and biotic factors, including climate (33–35). In this study, the sero-prevalence were higher during the months with high average monthly temperature and precipitation. Higher environmental temperature increases the ability of Culex mosquitoes to transmit WNV (36, 37), and in addition, higher temperatures facilities faster larval development and this was also shown in an earlier study in Assam, which found a peak of the relative mosquito abundance during monsoon and least mosquitoes during winter (38).
Sero-prevalence may also depend on managemental and husbandry practices as well as genetic variation in disease resistance (39). Under poor housing and sanitary conditions, vectors proliferate in dirty water, filthy environment, thereby, having their highest impact on the underprivileged section of the society (40), making them especially vulnerable. Vector densities have been reported to be higher in rural areas compared to urban localities due to more favorable habitats for some mosquito species (41), which is similar to our finding of more common seroconversions in peri-urban compared to urban areas. Similarly, presence of potential factors facilitating vector-borne and zoonotic diseases such as the way of living (42), socio-economic status of the people (43), livestock dependence (44), cultural aspects of community (45), drainage system (46), may also contribute to higher sero-prevalence of WNV in peri-urban areas.
In disease epidemiology, GIS and Global Positioning System (GPS) helps in envisioning topographical distribution of disease, with respect to time and space, which is difficult to perform in any other system (47). Through mapping of the coordinates of the positive farms in this study into online tools such as Google Maps, or Google Earth, it was possible to show that seroconversions in birds occurred in densely populated as well as peri-urban areas.
WNV infections are asymptomatic in 80% of infected humans (48), while most symptomatic persons develop fever, headache, muscle pain, joint pain, rash, or gastrointestinal symptoms and very rarely (less than 1%) infected persons show neurological disorders, including encephalitis, meningitis, or acute flaccid paralysis (49). However, humans are dead-end host as there is no danger of WNV transmission from infected humans because of insufficient viremia to permit virus transmission through mosquitoes (50). None of the poultry farmers interviewed in this study reported neurological symptoms, but fever and headache were commonly reported.
Agriculture along with livestock farming was adopted as a source of livelihood by majority of the peri-urban farmers in contrary to the absence of agriculture in urban areas which might be due to lesser land holding by farmers in urban areas. Due to presence of numerous job avenues offered by different companies in urban areas, combining farming with private job was also another option for urban farmers which was non-existent in peri-urban areas of Guwahati. Agriculture plays an important role in the Indian economy and 70% of its rural households still depend primarily on agriculture for their livelihood, comprising of 82% of small and marginal farmers (51). All the poultry farmers during the present study were found to be either small or marginal and most of them were engaged in agriculture and allied sectors corroborating the above statement.
The noticeable difference in the number of birds reared in urban and peri-urban farms might be due to more conveniences of farming in peri-urban areas because of larger land holding by farmers, joint families extending help in farming, involvement of more household members in farm activities, availability of agricultural produce which can be used as feed stuffs, proximity to wholesale markets, less availability of other sources of livelihood as well as pro-activeness of different companies expanding integrated system of broiler production by involving the peri-urban farmers which was evident from the present study. Mixed farming is a commonly adopted farming practice in India involving combination of crop production with one or more type of animal species like rearing of cattle, sheep, goat, pigs and poultry as well as fishery, beekeeping, sericulture, etc. (52).
The limitations of this study include the lack of confirmation with VNT, as well as the relatively low number of birds sampled each month per farm. Given the known cross-reactions between different flaviviruses, particularly JEV and WNV, and the fact that the ELISA kit used cannot differentiate these viruses (23, 53), it is possible that some of these sero-conversions could be against JEV instead of WNV. The best method for distinguishing the causative virus would be VNT, but this would require a biosafety level 3 laboratory, which was not available. However, the study shows the use of serological testing of birds to understand the pattern of flavivirus infections in the city area, thereby indicating risks for humans.
After completion of the present study, all the farmers under study were included in an awareness program about mosquito-borne diseases and which precautions that can be done, including to remove the possible mosquito breeding sites like stagnant-standing water, empty containers, broken flower pots, tip over buckets, tyres, etc. The farmers of the sero-positive farms were informed about the presence of West Nile virus in their respective areas. The farmers were also advised to seek medical help if they developed fever, headache, muscle pain, joint pain, neurological disorder or any other symptoms common to WNV infection.
In conclusion, this study found circulation of WNV in urban and peri-urban Guwahati, indicating that this virus may be an important contributor to the undiagnosed cases of acute encephalitis that occurs every year. Understanding the distribution of WNV sero-prevalence in chicken can support future strategic planning of vector-borne disease control approaches by identifying the underlying environmental factors as well as demography of urban and peri-urban farmers in a city such as Guwahati. For improved understanding of the risks for WNV as well as other mosquito-borne flaviviruses, it would be recommended to set up surveillance programmes with a One Health approach, where sampling in animals can advise preventive measures in humans.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Ethics Committee, Assam Agricultural University, Khanapara (Approval Registration No. 770/ac/CPCSEA/FVSc/AAU/IAEC/17-18/584 dated 09.08.2017).
Author Contributions
Conceptualization, RH and JL. Methodology, JL, RH, and AT. Formal analysis, JL and AT. Investigation, AT, RH, JL, ND, NK, PT, and SM. Resources, JL. Writing—original draft preparation, AT, JL, and RH. Writing—review and editing, all authors. Supervision, JL, RH, SP, and DB. Project administration, RH and JL. Funding acquisition, RH and JL. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful to Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas, grant number 2016-00364) for funding the research. The authors are also grateful to Assam Agricultural University, Khanapara, Guwahati, NRC on Pig, Rani, Guwahati, Assam, the CGIAR system and Uppsala University, Sweden for the supports provided during the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge all participating poultry farmers and all students for their help in the survey, as well as Stephen Oloo for his help with generating the map.
References
1. Mackenzie JS, Barrett ADT, Deubel V. The Japanese Encephalitis Serological Group of Flaviviruses: A Brief Introduction to the Group. In: Mackenzie JS, Barrett ADT, Deubel V, editors. Japanese Encephalitis and West Nile Viruses. Berlin, Germany: Springer International Publishing (2002). p. 1–10.
2. Smithburn KC, Hughes TP, Burke AW, Paul JH, African A. A Neurotropic Virus Isolated From the Blood of a Native of Uganda. Am J Trop Med Hyg (1940) s1-20(4):471–92. doi: 10.4269/ajtmh.1940.s1-20.471
3. Hubálek Z, Halouzka J, Juřicová Z, Šikutová S, Rudolf I, Honza M, et al. Serologic Survey of Birds for West Nile Flavivirus in Southern Moravia (Czech Republic)(2008) (Accessed 2019 Dec 25).
4. George S, Gourie-Devi M, Rao JA, Prasad SR, Pavri KM. Isolation of West Nile Virus From the Brains of Children Who had Died of Encephalitis. Bull World Health Organ (1984) 62(6):879–82.
5. Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile Virus Infection: A New Acute Paralytic Illness. Neurology (2003) 61(1):55–9. doi: 10.1212/01.WNL.0000073617.08185.0A
6. Hayes C, Monath TP. West Nile Fever. In: The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: Taylor & Francis (1989). Available at: https://www.google.com/books?hl=sv&lr=&id=6kDCDwAAQBAJ&oi=fnd&pg=PT75&dq=West+Nile+virus+Hayes&ots=qssYh32ncM&sig=pQ-Dp7e9X5EMIPi0hvUZLtUb4RA.
7. Maquart M, Boyer S, Rakotoharinome VM, Ravaomanana J, Tantely ML, Heraud J-M, et al. High Prevalence of West Nile Virus in Domestic Birds and Detection in 2 New Mosquito Species in Madagascar. PloS One (2016) 11(1):e0147589. doi: 10.1371/journal.pone.0147589. Coffey LL.
8. Langevin SA, Bunning M, Davis B, Komar N. Experimental Infection of Chickens as Candidate Sentinels for West Nile Virus. Emerg Infect Dis (2001) 7(4):726–9. doi: 10.3201/eid0704.017422
9. Komar N. West Nile Virus Surveillance Using Sentinel Birds. Ann N Y Acad Sci (2001) 951:58–73. doi: 10.1111/j.1749-6632.2001.tb02685.x
10. Khan SA, Dutta P, Khan AM, Chowdhury P, Borah J, Doloi P, et al. West Nile Virus Infection, Assam, India. Emerg Infect Dis (2011) 17(5):947. doi: 10.3201/eid1705.100479
11. Phukan A, Baruah PK, Mahanta J. Japanese Encephalitis in Assam, North East India. Southeast Asian J Trop Med Public Health (2004) 35(3):618–22.
12. Chowdhury P, Khan SA, Dutta P, Topno R, Mahanta J. Characterization of West Nile Virus (WNV) Isolates From Assam, India: Insights Into the Circulating WNV in Northeastern India. Comp Immunol Microbiol Infect Dis (2014) 37(1):39–47. doi: 10.1016/j.cimid.2013.10.006
13. Dutta K, Rangarajan PN, Vrati S, Basu A. Japanese Encephalitis: Pathogenesis, Prophylactics and Therapeutics. Curr Sci (2010) 98:326–34.
14. Dev V, Sharma V, Barman K. Mosquito-Borne Diseases in Assam, North-East India: Current Status and Key Challenges. WHO South-East Asia J Public Heal (2015) 4(1):20. doi: 10.4103/2224-3151.206616
16. Vetrivel SC, Chandrakumarmangalam S. The Role of Poultry Industry in Indian Economy. Rev Bras Cienc Solo (2013) 15:287–93.
17. Pandey RR, Bhardwaj SS, Mahajan VVK, World KN-P of the 20 th, 1996 U, Nirman KPS. Economic Study of Poultry Production in India. In: Proceedings of the 20th World Poultry Congress on Economic Study of Poultry Production in India. New Delhi, India: World’s Poultry Science Association (1996) p. 527–34.
18. Khan SA, Chowdhury P, Choudhury P, Dutta P. Detection of West Nile Virus in Six Mosquito Species in Synchrony With Seroconversion Among Sentinel Chickens in India. Parasites Vectors (2017) 10(1):13. doi: 10.1186/s13071-016-1948-9
19. Dhanze H, Bhilegaonkar KN, Rawat S, Chethan HB, Kerketta P, Dudhe N, et al. Seasonal Sero-Prevalence of Japanese Encephalitis in Swine Using Indirect IgG ELISA. J Vet Public Heal (2014) 12(2):103–5.
20. Mohakud SS, Hazarika RA, Sonowal S, Bora DP, Talukdar A, Tamuly S, et al. The Extent and Structure of Pig Rearing System in Urban and Peri-Urban Areas of Guwahati. Infect Ecol Epidemiology (2020) 10(1):12. doi: 10.1080/20008686.2020.1711576
21. Lustig Y, Sofer D, Bucris ED, Mendelson E. Surveillance and Diagnosis of West Nile Virus in the Face of Flavivirus Cross-Reactivity. Front Microbiol (2018) 9:1–10. doi: 10.3389/fmicb.2018.02421
22. Mansfield KL, Horton DL, Johnson N, Li L, Barrett ADT, Smith DJ, et al. Flavivirus-Induced Antibody Cross-Reactivity. J Gen Virol (2011) 92(12):2821–9. doi: 10.1099/vir.0.031641-0
23. Ruget A-S, Beck C, Gabassi A, Trevennec K, Lecollinet S, Chevalier V, et al. Japanese Encephalitis Circulation Pattern in Swine of Northern Vietnam and Consequences for Swine’s Vaccination Recommendations. Transbound Emerg Dis (2018) 65(6):1485–92. doi: 10.1111/tbed.12885
24. Rönnberg B, Gustafsson Å, Vapalahti O, Emmerich P, Lundkvist Å, Schmidt-Chanasit J, et al. Compensating for Cross-Reactions Using Avidity and Computation in a Suspension Multiplex Immunoassay for Serotyping of Zika Versus Other Flavivirus Infections. Med Microbiol Immunol (2017) 206(5):383–401. doi: 10.1007/s00430-017-0517-y
25. Weingartl HM, Drebot MA, Hubálek Z, Halouzka J, Andonova M, Dibernardo A, et al. Comparison of Assays for the Detection of West Nile Virus Antibodies in Chicken Serum. Can J Vet Res (2003) 67:128–32. Canadian Veterinary Medical Association.
26. Joó K, Bakonyi T, Szenci O, Sárdi S, Ferenczi E, Barna M, et al. Comparison of Assays for the Detection of West Nile Virus Antibodies in Equine Serum After Natural Infection or Vaccination. Vet Immunol Immunopathol (2017) 183:1–6. Elsevier B.V. doi: 10.1016/j.vetimm.2016.10.015
27. OIE. Japanese Encephalitis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Part Ii. Paris, France: Office International Des Epizooties (2010). p. 1–11.
28. Clarke DH, Casals J. Techniques for Hemagglutination and Hemagglutination-Inhibition With Arthropod-Borne Viruses. Am J Trop Med Hyg (1958) 7(5):561–73. doi: 10.4269/ajtmh.1958.7.561
29. Gulati BR, Singha H, Singh BK, Virmani N, Khurana SK, Singh RK. Serosurveillance for Japanese Encephalitis Virus Infection Among Equines in India. J Vet Sci (2011) 12(4):341–5. doi: 10.4142/jvs.2011.12.4.341
30. Williams R, Schoeman M, Vanwyk A, Roos K, Josemans EJ. Comparison of ELISA and HI for Detection of Antibodies Against Wesselsbron Disease Virus. Onderstepoort J Vet Res (1997) 64:245–50.
31. Makino Y, Tadano M, Saito M, Maneekarn N, Sittisombut N, Sirisanthana V, et al. Studies on Serological Cross-Reaction in Sequential Flavivirus Infections. Microbiol Immunol (1994) 38:951–5. doi: 10.1111/j.1348-0421.1994.tb02152.x
32. Lefrançois T, Blitvich BJ, Pradel J, Molia S, Vachiéry N, Pallavicini G, et al. West Nile Virus Surveillance, Guadeloupe, 2003-2004. Emerg Infect Dis (2005) 11(7):1100–3. doi: 10.3201/eid1107.050105
33. Randolph SE, Rogers DJ. The Arrival, Establishment and Spread of Exotic Diseases: Patterns and Predictions. Nat Rev Microbiol (2010) 8:361–71. doi: 10.1038/nrmicro2336
34. Cornel AJ, Jupp PG, Blackburn NK. Environmental Temperature on the Vector Competence of Culex Univittatus (Diptera: Culicidae) for West Nile Virus. J Med Entomol (1993) 30(2):449–56. doi: 10.1093/jmedent/30.2.449
35. Paz S, Malkinson D, Green MS, Tsioni G, Papa A, Danis K, et al. Permissive Summer Temperatures of the 2010 European West Nile Fever Upsurge. PloS One (2013) 8(2):e56398. https://dx.plos.org/10.1371/journal.pone.0056398. Mores CN. doi: 10.1371/journal.pone.0056398
36. Paz S, Semenza JC. Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia–a Review. Int J Environ Res Public Health (2013) 10(8):3543–62. doi: 10.3390/ijerph10083543
37. Danforth ME, Reisen WK, Barker CM. The Impact of Cycling Temperature on the Transmission of West Nile Virus. J Med Entomol (2016) 53(3):681–6. doi: 10.1093/jme/tjw013
38. Baruah A, Hazarika RA, Barman NN, Islam S, Gulati BR. Mosquito Abundance and Pig Seropositivity as a Correlate of Japanese Encephalitis in Human Population in Assam, India. J Vector Borne Dis (2018) 55(4):291. doi: 10.4103/0972-9062.256564
39. Yogeshpriya S, Saravanan M, Ranjithkumar M, Pillai UN. Occurrence of Leptospirosis in an Organized Buffalo Farm. Int J Livest Res (2017) 7(8):117–21. doi: 10.5455/ijlr.20170527072615
40. WHO. Global Brief on Vector-Borne Diseases. Geneva, Switzerland: World Health Organization (2014).
42. Albert DP, Gesler W, Levergood B. Spatial Analysis, GIS and Remote Sensing: Applications in the Health Sciences. Chelsea; Michigan: Ann Arbor Press. Available at: https://books.google.se/books?hl=sv&lr=&id=Abj0LW4BlU0C&oi=fnd&pg=PP1&dq=Spatial+analysis,+GIS+and+remote+sensing+application+in+the+health&ots=VjIUEQn2IL&sig=HxVczDr2S31oCHzSOK6Q88Hk030&redir_esc=y#v=onepage&q=Spatial analysis%2CGISandremotesensinga.
43. Narain K, Rajguru SK, Mahanta J. Prevalence of Trichuris Trichuris in Relation to Socio-Economic and Behavioural Determinants of Exposure to Infection in Rural Assam. Indian J Med Res (2000) 112:140–6.
44. Mbogo CNM, Kabiru EW, Glass GE, Forster D, Snow RW, Khamala CPM, et al. Vector-Related Case-Control Study of Severe Malaria in Kilifi District, Kenya. Am J Trop Med Hyg (1999) 60(5):781–5. doi: 10.4269/ajtmh.1999.60.781
45. Roper MH, Carrion Torres RS, Cava Goicochea CG, Andersen EM, Aramburú Guarda JS, Calampa C, et al. The Epidemiology of Malaria in an Epidemic Area of the Peruvian Amazon. Am J Trop Med Hyg (2000) 62(2):247–56. doi: 10.4269/ajtmh.2000.62.247
46. Eisele TP, Keating J, Swalm C, Mbogo CM, Githeko AK, Regens JL, et al. Linking Field-Based Ecological Data With Remotely Sensed Data Using a Geographic Information System in Two Malaria Endemic Urban Areas of Kenya. Malar J (2003) 2(1):1–17. doi: 10.1186/1475-2875-2-44
47. Gandhi G, Chapla J, Gujju Gandhi C, Reddya Naik B. Data Mapping of Vector Borne Disease With Geographical Information System & Global Position System Technology: In Tribal Areas Khammam District, Telangana State. ~ 39 ~ Int J Mosq Res (2017) 4(2):39–43.
48. Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, et al. Epidemic West Nile Encephalitis, New York, 1999: Results of a Household-Based Seroepidemiological Survey. Lancet (2001) 358(9278):261–4. doi: 10.1016/S0140-6736(01)05480-0
49. Gray TJ, Webb CE. A Review of the Epidemiological and Clinical Aspects of West Nile Virus. Int J Gen Med (2014) 7:193. doi: 10.2147/IJGM.S59902
50. Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and Transmission Dynamics of West Nile Virus Disease. Emerg Infect Dis (2005) 11(8):1167. doi: 10.3201/eid1108.050289a
51. FAO. India at a Glance (2019). Available at: http://www.fao.org/india/fao-in-india/india-at-a-glance/en/.
52. Chitra B, Ramanna K. Knowledge Level of Farmers on Improved Practices in Selected Farming Systems of Mandya District in Karnataka. J Ext Educ (2012) 24(4):4917–22.
Keywords: vector-borne disease (VBD), zoonoses, emerging infectious diseases, Flavivirus, arboviruses, South Asia
Citation: Talukdar A, Hazarika RA, Bora DP, Pegu SR, Talukdar P, Kader NA, Mohakud SS, Deka NJ and Lindahl JF (2022) Sero-Prevalence of West Nile Virus in Urban and Peri-Urban Poultry Farms of Guwahati, India. Front. Trop. Dis 3:792857. doi: 10.3389/fitd.2022.792857
Received: 11 October 2021; Accepted: 20 January 2022;
Published: 28 February 2022.
Edited by:
Wilmer Villamil Gómez, University of Sucre, ColombiaReviewed by:
Shih Keng Loong, University of Malaya, MalaysiaJody Hobson-Peters, The University of Queensland, Australia
Copyright © 2022 Talukdar, Hazarika, Bora, Pegu, Talukdar, Kader, Mohakud, Deka and Lindahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johanna F. Lindahl, ai5saW5kYWhsQGNnaWFyLm9yZw==
 Archana Talukdar1
Archana Talukdar1 Razibuddin A. Hazarika
Razibuddin A. Hazarika Durlav P. Bora
Durlav P. Bora Johanna F. Lindahl
Johanna F. Lindahl