- 1Department of Clinical Microbiology, Harvard Medical School, Boston, MA, United States
- 2Faculty of Pharmacy, Mansoura University, Mansoura, Egypt
- 3Institute of Global Health, Heidelberg University, Heidelberg, Germany
- 4Department of Biology, University of Salahaddin-Erbil, Erbil, Iraq
- 5Department of Microbiology, All India Institute of Medical Sciences, Gorakhpur, India
- 6Department of Community Medicine and School of Public Health, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- 7Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Edfina, El-Beheira, Egypt
- 8Department of Veterinary Sciences and Animal Husbandry, Amrita School of Agricultural Sciences, Amrita Vishwa Vidyapeetham University, Coimbatore, Tamil Nadu, India
- 9Department of Pharmacy, BGC Trust University Bangladesh, Chittagong, Bangladesh
- 10Department of Pharmacy, Faculty of Allied Health Sciences, Daffodil International University, Dhaka, Bangladesh
- 11Department of Biomedical Sciences, College of Medicine, University of Zakho, Kurdistan Region, Iraq
- 12Division of Pathology, Indian Council of Agricultural Research (ICAR)-Indian Veterinary Research Institute, Bareilly, Izatnagar, Uttar Pradesh, India
- 13Department of Virology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Introduction
Eastern equine encephalitis (EEE), caused by the mosquito-borne Eastern equine encephalitis virus (EEEV), is an important, high-mortality disease affecting equines, humans, and other vertebrate hosts (1, 2). EEE is one of the most severe forms of arboviral encephalitis in the USA, with a mortality rate of 30%–40%, and neurological sequelae are observed in 50% of survivors (3). The enzootic cycle between Culiseta melanura (Coquillett) and passerine birds is crucial to the maintenance of EEEV. EEEV causes intermittent outbreaks in the east and midwest of the USA, and has the highest recorded case fatality rate (CFR) among arboviruses in the Americas (4). It is an uncommon vector-borne disease, and approximately 6–8 cases, on average, are reported annually in the USA. There has been a rise in virus activity over the past decade, with major outbreaks in both human and equine populations. It is anticipated that the range of mosquitoes in the Americas, especially vectors of EEEV, may be impacted by predicted climate change, which may modify disease risk and constitute a public health problem (5). The consistent rise in incidence, seen across a wider region and population, demonstrates that EEE is an emerging disease. Notably, EEEV is also considered a potential bioterrorism weapon owing to its airborne transmissibility. This article presents an overview of EEEV and EEE, the current emerging scenario of increasing incidence, and salient prevention and control measures.
Virology
Eastern equine encephalitis virus (EEEV) is a single-stranded positive-sense RNA virus that belongs to the Alphavirus genus of the Togaviridae family. Its genetic structure has two main parts, responsible for the structural and non-structural proteins, respectively: the 5′ end is responsible for four non-structural proteins (i.e., nsP1, nsP2, nsP3, and nsP4) and the 3′ tail for three structural proteins, comprising the capsid and E1 and E2 glycoproteins (6). EEEV is considered the most pathogenic among viruses in the same genus, which was formerly named the South American EEE (Madariaga) virus and was changed by the new classification of the virus. The CFR of EEE ranges from 30% to 70% in humans and from 75% to 90% in equines (7–9). EEEV has been divided into two types, North American and South American, based on its antigenic properties, and, with the new classification in 2010, it was divided into four lineages. Lineage 1 is mainly present in North America and the Caribbean, and lineages 2–4 are present in Central and South America (9).
Routes of viral transmission
The transmission cycle of EEEV is similar to that of other arboviruses. It depends on the presence of enzootic vectors (C. melanura) that feed on the amplifying hosts, commonly bird species, and then with the aid of bridge vectors, such as Aedes albopictus, Ochlerotatus japonicus japonicus, Coquillettidia perturbans, and Culex erraticus, transmit the disease to the end hosts of humans and horses, as shown in Figure 1. Humans and horses are considered dead-end hosts, as they do not form a high viral load, which in turn facilitates disease transmission to other hosts; birds, in contrast, do form a high viral load (4, 10). The North American type of the disease is associated with the typical cycle of mosquitoes and birds, leading to sporadic cases in humans, equines, and other animals (9).
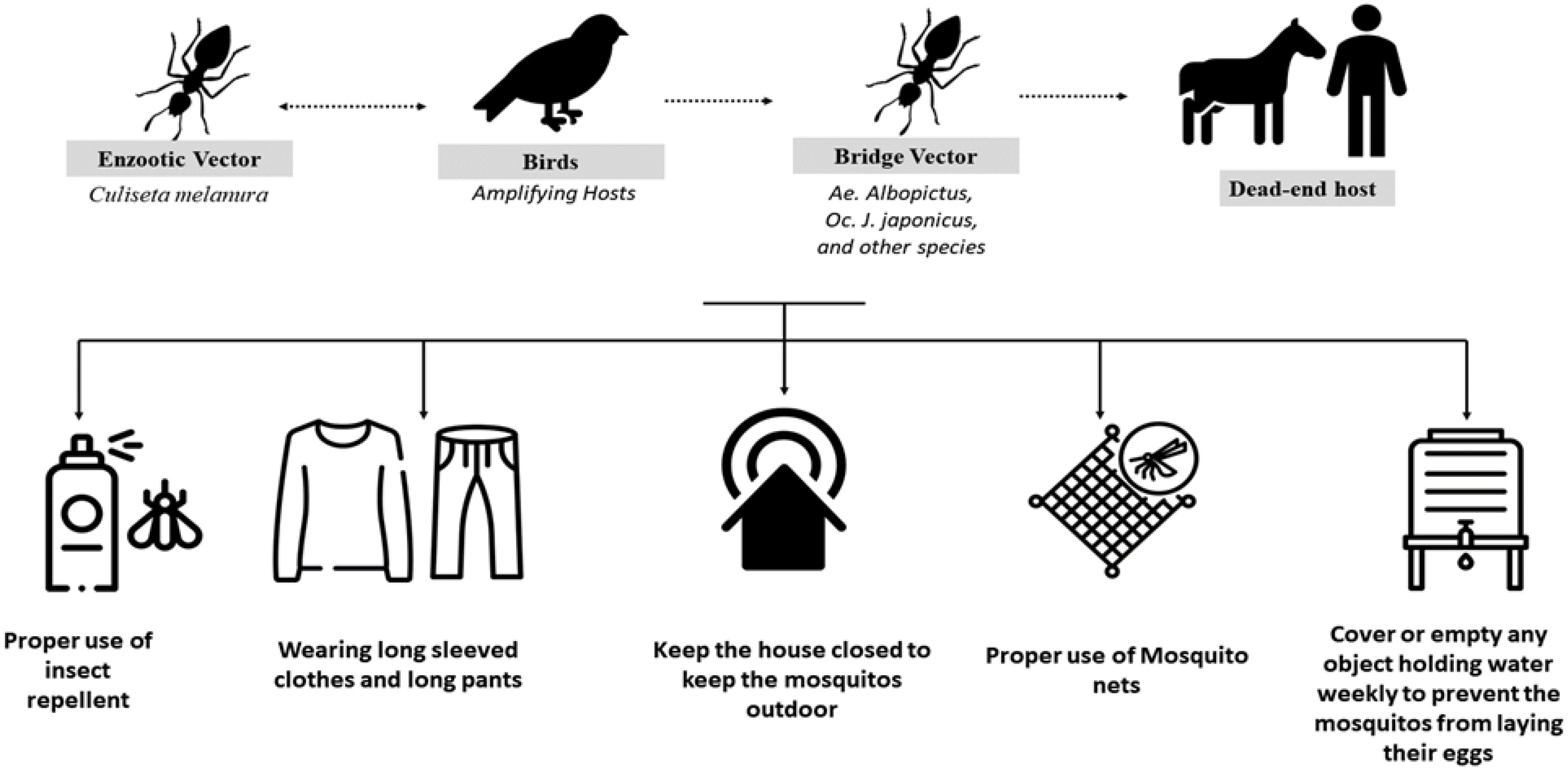
Figure 1 Transmission cycle of the Eastern equine encephalitis virus (EEEV) and the measures recommended by the Centers for Disease Control and Prevention (CDC) to disrupt this cycle and prevent infection.
The South American variant of EEEV, Madariaga virus (MADV), differs from the North American type in that it is found almost exclusively in animals. However, in an outbreak in Panama in 2010, a dozen cases of encephalitis in humans were recorded. And, in another serological study, in Panama and the Peruvian Amazon, it was found that 2%–5% of the general population had detectable serological evidence of the virus, which means that they had been exposed to an asymptomatic or mild infection in the past. Eight cases in children were reported in the period between 2015 and 2016, which shows the difference in the nature of infection between the two types of the virus (11).
The transmission cycle of the disease is considered the basis for the difference in the number of reported cases and in disease severity and mortality, as different enzootic vectors are associated with different numbers of dead-end host cases. Differences in bridge vectors are also linked to different feeding patterns in birds and correlated with the number of cases of infection in dead-end hosts (4).
Outbreak history
The first detection of EEEV was reported in 1831; the virus was isolated from horses in Massachusetts, USA, where 75 horses died with neurological disease sequelae. Thereafter the virus was isolated and identified as the cause of encephalitis during an outbreak in Delaware, Maryland, New Jersey, and Virginia in 1933 (12, 13). The disease was first detected in humans in 1938, in Massachusetts, USA, when 25 out of 38 infected individuals died (14). The major outbreak of the disease in a human was recorded in 1959, in which 32 encephalitis cases were detected in New Jersey, USA (3). The incidence of EEEV infection in the period from 1964 to 2002 is summarized in Figure 2. The numbers of confirmed or suspected cases of EEE are summarized in Table 1, which is adapted from the Centers for Disease Control and Prevention (CDC)’s resources for disease surveillance between 2003 and 2022 (15).
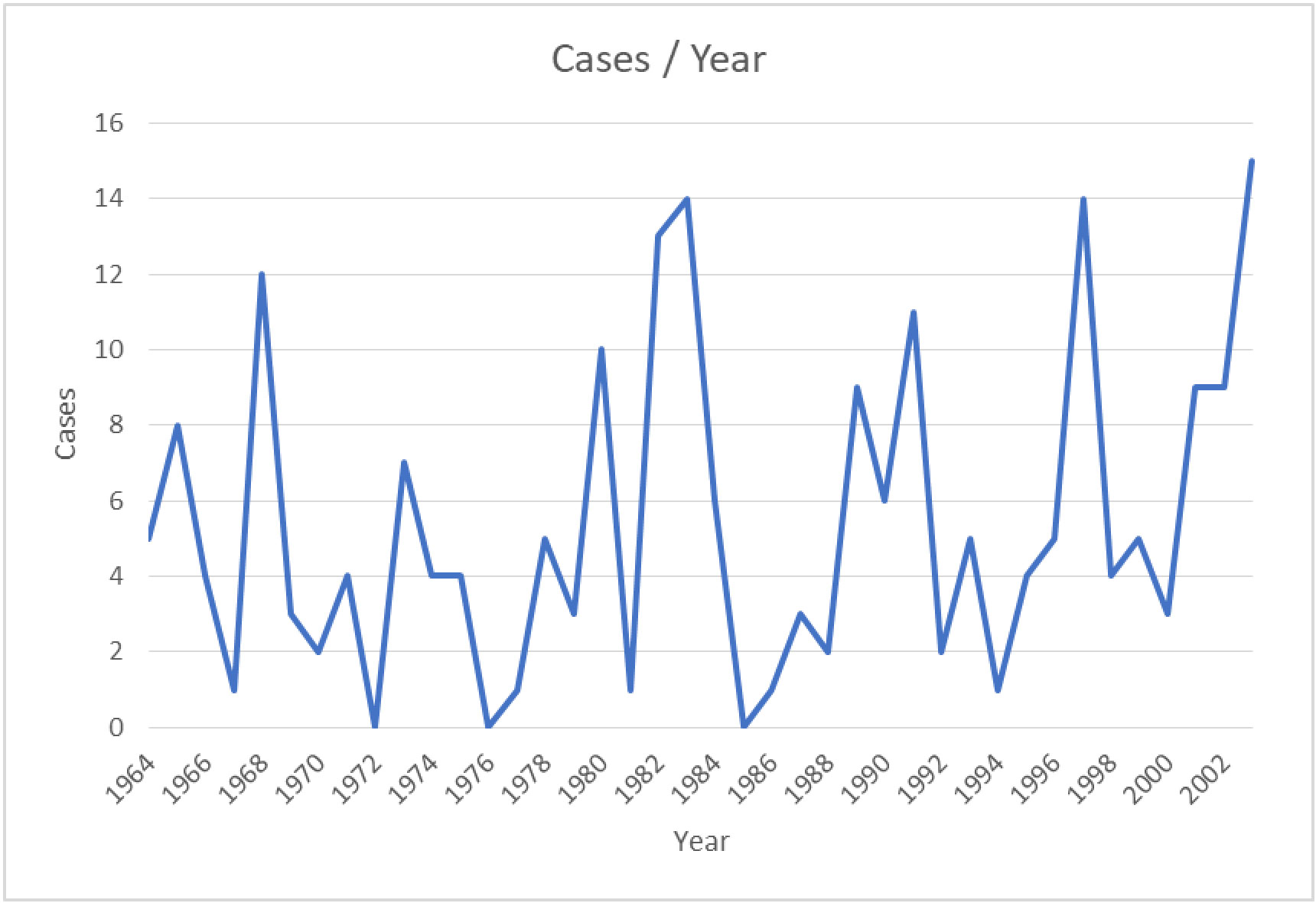
Figure 2 Confirmed and suspected cases of EEEV infection between 1964 and 2002 (based on data obtained from the CDC) (15).
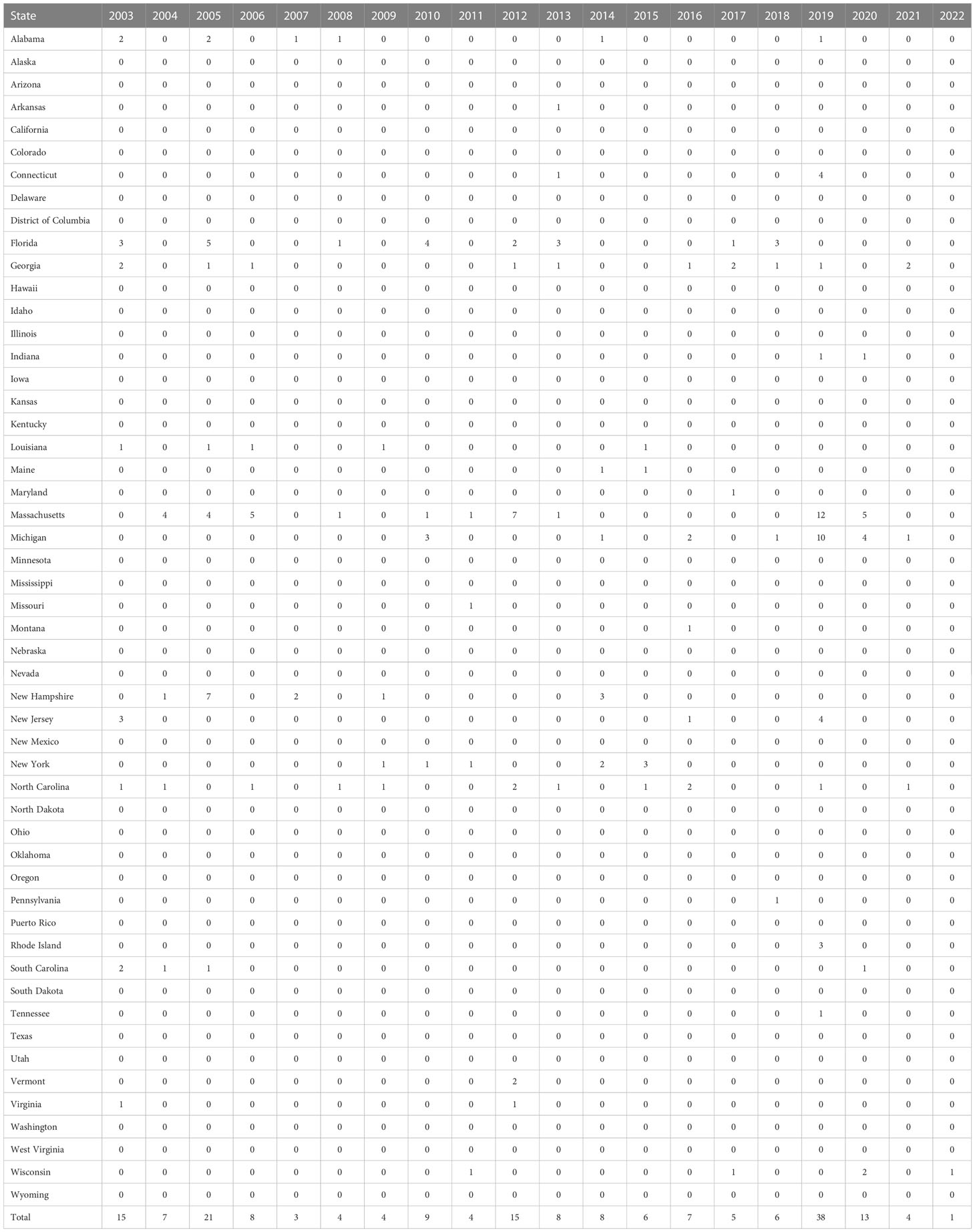
Table 1 Confirmed and suspected cases of Eastern equine encephalitis virus (EEV) infection between 2003 and 2022 (adapted from the ArboNET surveillance system of the CDC) (15).
A study by Lindsey and colleagues revealed that over 14 years, from 2003 to 2016, 121 human cases of EEE were reported from 74 counties in 20 states of the USA. The majority of patients (119) had neuroinvasive disease, with only two having non-neuroinvasive disease, and almost all patients with neuroinvasive disease (110 out of 119) had encephalitis or meningoencephalitis. In total, 118 patients were hospitalized and there were 50 fatalities. The CFR was 75% in patients aged over 70 years and 31% in patients aged less than 70 years. Those aged less than 5 years or over 60 years were more likely to develop neuroinvasive disease (16).
According to the CDC, the total number of reported cases of EEE from different states in the USA in the period 2011–2020 was 110: 26 cases were reported from Massachusetts (owing to the presence of the Hockomock Swamp, which is a natural habitat for the birds and the mosquitoes, which in turn increases the likelihood of disease transmission), 18 from Michigan, nine from Florida, seven from North Carolina, seven from Georgia, six New York, five from New Jersey, and five from Connecticut; the remainder were sporadic cases from different states. This demonstrates that the disease has spread across different states, as shown in Figure 3 (18). The overall numbers of reported cases between 2011 and 2020 were as follows: four cases in 2011, 15 cases in 2012, eight cases in 2013, eight cases in 2014, six cases in 2015, seven cases in 2016, five cases in 2017, six cases in 2018, 38 cases in 2019, and 13 cases in 2020 (19). According to the ArboNET, the total number of cases of EEE in the USA in 2021 was four in three states: Michigan, North Carolina, and Georgia (Liberty and Camden counties) (17).
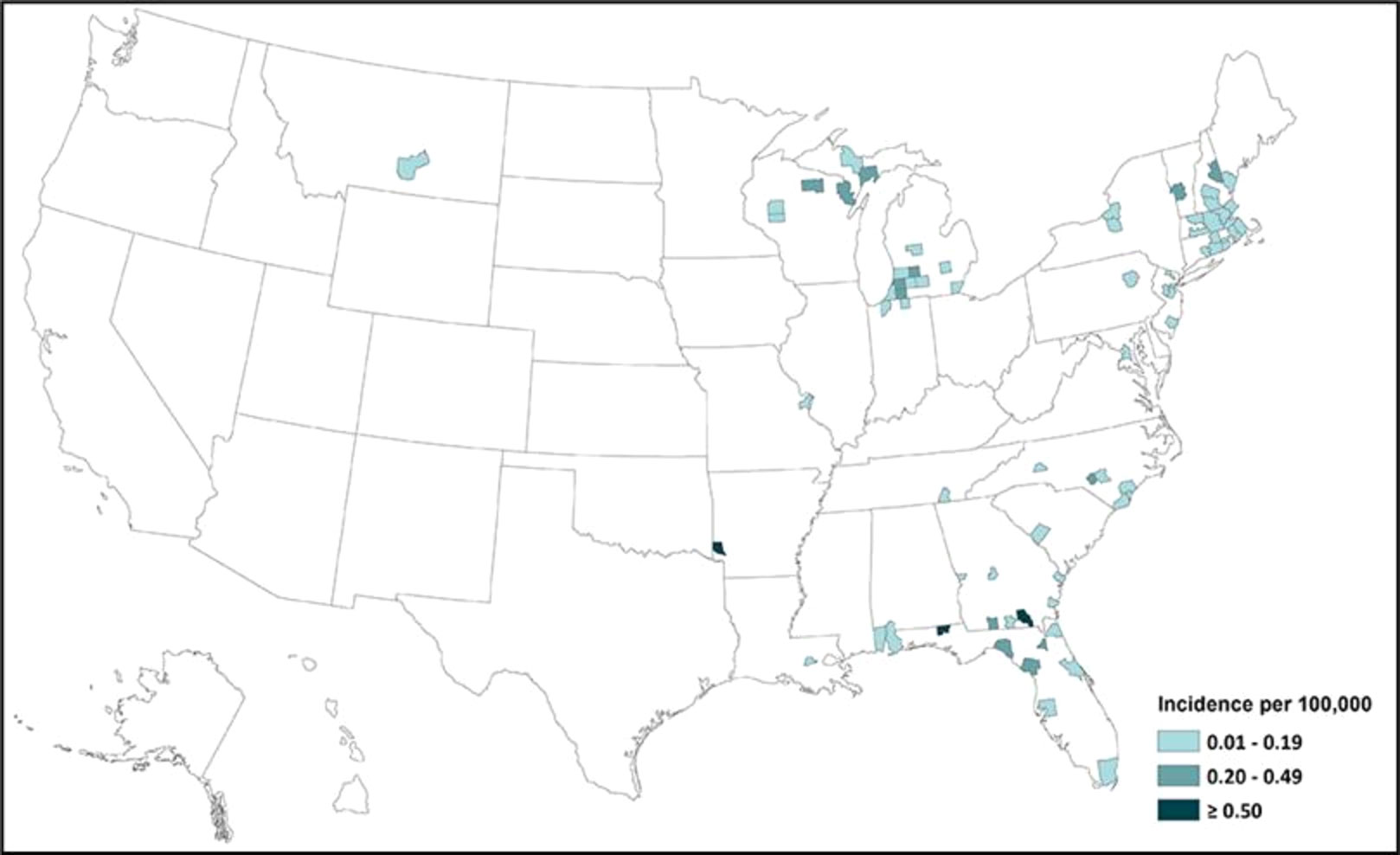
Figure 3 Incidence of EEEV infection in different states of the USA from 2011 to 2020 (adapted from the CDC) (17).
According to the CDC, the total number of fatal cases of EEE in the period between 2011 and 2020 was 47, giving a mortality rate of 43% (19).
In 2022, cases reported in animals included as a dead horse in Antwerp in New York, a dead dog in Albion (as reported by Oswego County Health Department), and a dead horse in Mexico; the EEE virus was also detected in sentinel chickens. Although there were no reported human cases, cases in animals present a risk for virus transmission to horses and humans (20–23).
Clinical manifestations
The incubation period of EEEV ranges between 4 and 10 days; the carrier may be asymptomatic, febrile, or have neurological manifestation. The febrile period is associated with chills, aches, and joint pain, which can last from 1 to 2 weeks, with fewer than 5% of cases developing meningitis and encephalitis (19, 20). The neurological manifestations include encephalitis and meningitis along with other symptoms such as fever, vomiting, headache, diarrhea, seizure, behavioral change, drowsiness, and coma. One-third of encephalitis patients die, and those who survive have impairments that can be mild or severe, including seizures, paralysis, and coma (24, 25).
In 2019, four cases of the EEEV infection were detected in Connecticut, USA. All four patients experienced severe and progressive disease despite empiric treatment, and certain manifestations, such as fever, coma, weakness, confusion, and seizures, were common to all patients. Examination revealed pleocytosis in the cerebrospinal fluid (CSF), but initial immunoglobin M (IgM) testing was negative, so the patients were referred to the CDC, where EEE was diagnosed. This unexpected outbreak of EEE in this state shows the importance of public health departments being connected to the CDC and having the ability to administer diagnostic tests that will enable the detection of EEE in an evidence-based manner (26).
According to the CDC, the number of confirmed cases of EEE in 2019 was 38. Four patients (three men aged between 50 and 60 years and one girl aged 6 years) had neuroinvasive manifestations and presented to a hospital in New England. Some symptoms, such as fever, ataxia, dizziness, seizures, and mental changes, were common to all patients. Two patients diagnosed as having a severe form of EEE required ventilation. Two of the four patients died, and the other two experienced a full recovery. This shows the importance of early diagnosis of this disease, which should be suspected if there is pleocytosis in the CSF and hyperintensity of gray matter is detected on an MRI scan (27).
In a study that examined the risk factors associated with EEE, it was found that, in 15 cases of EEE in children occurring between 1970 and 2010, certain manifestations, such as fever, headache, seizure, leukocytosis, and pleocytosis in the CSF, were common to all. In terms of outcome, five patients experienced severe neurological slippage, two experienced mild neurological slippage, four patients died, and four made a full recovery. It was observed that a long prodromal period of the disease is associated with less severe outcomes and that, in children, EEE is associated with a characteristic pattern of multifocal lesions that are correlated with the high incidence of complex partial lesions (28).
Another reported case of EEE occurred in 42-year-old man who was working in a wooded area, which is considered a risk factor for EEEV, and was admitted to a New Jersey hospital. It was reported in this case that the patient had received multiple mosquito bites in the week before admission. The patient was admitted with intractable headache and facial paresthesia, and required to be ventilated. After 9 days the patient started to improve, which shows the importance of taking a history from patients and the importance of administering symptomatic treatment (29).
Diagnosis
EEE should be suspected in any patient presenting with a febrile disease or neurological disease in geographical regions where EEE is prevalent and who has a history of mosquito bites, blood transfusion, and organ transplantation. However, the disease also needs to be confirmed, as other viral infections have similar manifestations. Clinical evaluation, in the form of neuroimaging and scanning, can reveal encephalitis in the form of brain lesions, destruction of neurons, and vasculitis in different brain regions, such as the cortex, brain stem, and midbrain (30). The first diagnostic method to be used should be serological testing, which includes the detection of EEEV-specific IgM. Infection can then be confirmed by the detection of neutralizing antibodies at the CDC or another official health facility, as virus isolation from clinical samples is challenging. The diagnosis of EEE is therefore based on clinical manifestations and laboratory detection using a molecular technique, such as polymerase chain reaction (PCR) analysis, which is considered more accurate and sensitive (31, 32).
In a study of all cases of EEE occurring in the period from 1988 to 1994, 36 cases were identified and the neurological manifestations associated with the disease were reported. Confusion, somnolence, focal weakness, seizures, and meningeal signs were common, and in most cases were are followed by deterioration and coma. The period between the appearance of the symptoms and neuroimaging findings of lesions in different parts of the brain, such as basal ganglia, thalami, and cerebral cortex, ranged from 1 to 14 days (33).
The diagnosis of the disease includes the serological identification of the IgM antibodies, nucleic acid identification using reverse transcription-PCR (RT-PCR) analysis of a sample of blood and CSF, and neuroimaging using MRI and CT scanning. However, in the initial stage of the disease, serology may be negative, so tests must be repeated multiple times, supported, if still negative, byPCR; therefore, clinicians should test patients early with all available techniques, as this can be helpful in case identification with the help from the local branch of the CDC (3, 31).
Treatment and prevention
Disease management is mainly supportive, including the use of antipyretics for fever, pain relievers for headache, antiemetics and fluid replacements for nausea and vomiting, and close monitoring for encephalitis as a result of the increased risk of raised intracranial pressure (25). Multiple EEEV vaccines are now in research and development, but the infrequent, localized, and widely dispersed nature of outbreaks means that there may not be significant incentives to move through with development and licensing. Although a vaccine against EEEV is available for horses, there is as yet no human equivalent (2). However, an early-generation investigational EEEV vaccine is currently available through the US Army Investigational New Drug program, which may be useful for people who are at high occupational risk (such as laboratory workers). Vaccines made from mosquito saliva that would be effective against a wide variety of mosquito-borne diseases are still in the research and development phase. An expected benefit of these vaccinations is the incorporation of salivary proteins from mosquitoes chosen for their ability to transmit numerous human arboviruses (7, 34). Prevention measures mainly involve protection from mosquito bites, such as covering the skin and the clothes with an insect repellent, such as picaridin (with adherence to the product instructions on safe use); wearing long-sleeved tops, pants, and hats, paying particular attention to areas of the body most likely to experience mosquito bites; clearing standing water that is considered a source for mosquitoes gathering; and closing all the openings and holes in the home that might enable mosquitoes to enter (35).
Various methods have been used to produce EEEV vaccines; for example, the replicon particle-based vaccine removes the genes responsible for the structural proteins. These vaccines were observed to be effective as individual and trivalent based (36). Another type is the viral vector-based vaccine, in which multiple viruses are used as a vector for the transmission of the genetic material that helps the body generate an immunity against the virus. Examples of such vaccines include the EILV/EEEV vaccine, which uses the C-E3-E2-6K-E1 gene in an animal model (CD-1 mice); the EILV/EEEV vaccine, which uses the C-E3-E2-6K-E1 and C-E2-E1 genes in an animal model (CD-1 mice using the Eilat virus as a viral vector); the rISFV-EEEV vaccine, which uses the E3-E2-6K-E1 gene in an animal model (CD-1 mice, using the Isafahan virus as a viral vector); the SIN/NAEEEV vaccine, which uses the C-E3-E2-6K-E1 gene in an animal model (either NIH Swiss mice or Cynomolgus macaque, using the Sindbis virus as a viral vector); the MVA-BN-E vaccine, which uses the E3-E2-6K-E1 gene in an animal model (BALB/c mice); and the MVA-BN-W +E+V vaccine, which uses the E3-E2-6K-E1 gene in an animal model (BALB/c mice, using vaccinia virus as a viral vector) (37–41).
Another vaccine strategy is the plasmid DNA vaccine, which, among other advantages, is low cost, has high stability, can be manufactured on a large scale, and involves no live parts. Among the disadvantages include the possibility that the vaccine will induce autoimmunity and the fact that large amounts and multiple doses of the are needed to provide adequate protection. Examples include the pcDNA™3.1(+)-C-E vaccine, which uses the C-E3-E2-6K-E1 gene in a BALB/c mice animal model (42).
Another form of vaccine uses the inactivated form of the PE-6 strain of EEEV and the FY 06-31 protocol. The vaccine is administered on day 0, day 28, and month 6; patients with an inadequate immune response, prior EEEV vaccination, and other eligible conditions also receive booster doses. It was reported that the vaccine elicited a high immune response in the primary series and when administered on an annual basis to laboratory personnel at risk, which demonstrates that the vaccine is safe and immunogenic (43).
The safety and tolerability of a trivalent vaccine have been investigated in a phase 1, randomized, open-label clinical trial. Healthy volunteers aged 18 to 50 years were given the Venezuelan equine encephalitis (WEVEE) virus like particle (VLP) vaccine at doses 6, 30, and 60 μg at day 0 and week 8. It was reported that the vaccine was safe and well tolerated, with only a few reported side effects, such as injection site pain and tenderness (44).
Conclusion and future prospects
As most reported human cases of EEE are caused by the North American type of EEEV, we should focus our research on the prevention of the prevalence of this type of disease.
EEE is a rare but deadly disease, but it is anticipated that the risk of EEEV infection may vary as a result of the projected influence of climate change on mosquito populations, leading to a greater disease burden or the spread of the illness into previously unaffected geographical areas. Hence, there is a need to increase awareness of this disease and more research should be undertaken to bridge the knowledge gap and prevent this illness.
Local health departments in every endemic country can monitor equids, birds, and mosquitoes for signs of human illness in the absence of vaccinations or specialized treatments; nevertheless, underfunding of public health activities is a constant danger to even these crude prevention methods. Several American public health specialists have recently advocated for a national defense strategy against arboviruses and other vector-borne diseases, a concept that has been endorsed by specialists from other countries. Piecemeal efforts to combat arboviruses are unlikely to be successful. Throughout the USA and the rest of the world, multiple potentially lethal viruses are always present in virologically occult enzootic foci. An additional cause for concern is the potential for climatic and weather-related factors, such as variations in temperature and precipitation, to influence the life cycles and geographic distribution of arthropod vectors and viral transmission patterns. Such vectors pose a genuine and immediate threat, and there is high probability that further arbovirus emergencies will occur. Although EEE is not yet a global health emergency, the recent uptick in cases has highlighted our lack of preparedness for unexpected infectious disease outbreaks. It would be wise to follow proactive active control measures and increase vigilance in the face of these threats. There is also a need for enhanced awareness among public health and medical personnel concerning the increase in EEE cases, and the significance of adopting appropriate prevention and control measures, particularly in regions with high prevalence. Early diagnosis of the disease, followed by timely treatment and management of EEEV-infected patients, is essential.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berlin D, Gilani AI, Grewal AK, Fowkes M. “Eastern equine encephalitis,”. Pract Neurol (2017) 17(5):387–91. doi: 10.1136/PRACTNEUROL-2017-001659
2. Morens DM, Folkers GK, Fauci AS. Eastern Equine encephalitis virus - another emergent arbovirus in the united states. N Engl J Med (2019) 381(21):1989–92. doi: 10.1056/NEJMP1914328
3. Banda C, Samanta D. “Eastern equine encephalitis,” (2022). StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK557692/ (Accessed 19, 2022).
4. Petrucciani A, Yu G, Ventresca M. Multi-season transmission model of Eastern equine encephalitis. PloS One (2022) 17(8):e0272130. doi: 10.1371/JOURNAL.PONE.0272130
5. Corrin T, Ackford R, Mascarenhas M, Greig J, Waddell LA. Eastern Equine encephalitis virus: A scoping review of the global evidence. Vector Borne Zoonotic Dis (2021) 21(5):305. doi: 10.1089/VBZ.2020.2671
6. Strauss EG, Strauss JH. Structure and replication of the alphavirus genome. Togaviridae Flaviviridae (1986), 35–90. doi: 10.1007/978-1-4757-0785-4_3
7. Ciota AT. Eastern Equine encephalitis virus taxonomy, genomics, and evolution. J Med Entomol (2022) 59(1):14–9. doi: 10.1093/JME/TJAB079
8. Smith DR, Schmaljohn CS, Badger C, Ostrowski K, Zeng X, Grimes SD, et al. Comparative pathology study of Venezuelan, eastern, and western equine encephalitis viruses in non-human primates. Antiviral Res (2020) 182. doi: 10.1016/J.ANTIVIRAL.2020.104875
9. Arrigo NC, Adams AP, Weaver SC. Evolutionary patterns of eastern equine encephalitis virus in north versus south America suggest ecological differences and taxonomic revision. J Virol (2010) 84(2):1014–25. doi: 10.1128/JVI.01586-09
10. “Transmission | Eastern equine encephalitis. CDC. Available at: https://www.cdc.gov/easternequineencephalitis/transmission/index.html (Accessed 11, 2022).
11. Lednicky JA, White SK, Mavian CN, Badry El MA, Telisma T, Salemi M, et al. Emergence of madariaga virus as a cause of acute febrile illness in children, Haiti, 2015-2016. PloS Negl Trop Dis (2019) 13(1). doi: 10.1371/JOURNAL.PNTD.0006972
12. HANSON RP. An epizootic of equine encephalomyelitis that occurred in Massachusetts in 1831. Am J Trop Med Hyg (1957) 6(5):858–62. doi: 10.4269/AJTMH.1957.6.858
13. Giltner: The 1933 outbreak of infectious equine encephalo. . . - Google scholar. Available at: https://scholar.google.com/scholar_lookup?title=The1933outbreakofinfectiousequineencephalomyelitisintheeasternstates&author=L.T.Giltner&author=M.S.Shahan&publication_year=1933&journal=NorthAmer.Vet&volume=14&pages=25-27 (Accessed 19, 2022).
14. Feemster RF, Director FAPHA. Outbreak of encephalitis in man due to the Eastern virus of equine encephalomyelitis. Am J Public Health Nations Health (1938) 28 doi: 10.2105/AJPH.28.12.1403
15. Confirmed and probable Eastern equine encephalitis cases, human, united states, 1964-2003, by state (1964). Available at: https://www.cdc.gov/easternequineencephalitis/resources/eee_humancases.pdf (Accessed 19, 2022).
16. Lindsey NP, Staples JE, Fischer M. “Eastern equine encephalitis virus in the united states, 2003–2016,”. Am J Trop Med Hyg (2018) 98(5):1472–7. doi: 10.4269/AJTMH.17-0927
17. ArboNET disease maps. Available at: https://wwwn.cdc.gov/arbonet/maps/ADB_Diseases_Map/index.html (Accessed 19, 2022).
18. Armstrong PM, Andreadis TG. Ecology and epidemiology of Eastern equine encephalitis virus in the northeastern united states: An historical perspective. J Med Entomol (2022) 59(1):1–13. doi: 10.1093/JME/TJAB077
19. Statistics & maps | Eastern equine encephalitis | CDC. Available at: https://www.cdc.gov/easternequineencephalitis/statistics-maps/index.html#casesbyincidence (Accessed 19, 2022).
20. First 2022 Delaware evidence of Eastern equine encephalitis detected in DNREC’s sentinel chickens - state of Delaware news. Available at: https://news.delaware.gov/2022/08/12/first-2022-delaware-evidence-of-eastern-equine-encephalitis-detected-in-dnrecs-sentinel-chickens/ (Accessed 19, 2022).
21. Eastern Equine encephalitis reported in Antwerp, NY horse - outbreak news today. Available at: http://outbreaknewstoday.com/eastern-equine-encephalitis-reported-in-antwerp-ny-horse-97417/ (Accessed 19, 2022).
22. Donkey in the town of Albion died from EEEV. Available at: https://www.oswegocounty.com/news_detail_T17_R1946.php (Accessed 19, 2022).
23. Horse dies of EEE virus in oswego county, officials plan aerial spraying - syracuse.com. Available at: https://www.syracuse.com/news/2022/08/horse-dies-of-eee-virus-in-oswego-county-officials-plan-aerial-spraying.html (Accessed 19, 2022).
24. Symptoms, diagnosis, & treatment | Eastern equine encephalitis | CDC. Available at: https://www.cdc.gov/easternequineencephalitis/symptoms-diagnosis-treatment/index.html (Accessed 19, 2022).
25. Eastern Equine encephalitis (EEE): Symptoms, treatment & prevention. Available at: https://my.clevelandclinic.org/health/diseases/21187-eastern-equine-encephalitis-eee#symptoms-and-causes (Accessed 19, 2022).
26. Brown SC, Cormier J, Tuan J, Lier AJ, McGuone D, Armstrong PM, et al. “Four human cases of Eastern equine encephalitis in Connecticut, USA, during a larger regional outbreak, 2019,”. Emerg Infect Dis (2021) 27,8:2042. doi: 10.3201/EID2708.203730
27. Montalvo M, Ayoub D, McGary M, Byrd K, Mahmoud L, Mermel L, et al. “Eastern equine encephalitis: Case series in southern new England and review of the literature,”. Neurol Clin Pract (2021) 11(5):e714. doi: 10.1212/CPJ.0000000000001079
28. Silverman MA, Misasi J, Smole S, Feldman HA, Cohen AB, Santagata S, et al. “Eastern equine encephalitis in children, Massachusetts and new Hampshire,USA, 1970–2010,”. Emerg Infect Dis (2013) 19(2):194. doi: 10.3201/EID1902.120039
29. Millet N, Faiek S, Gurrieri D, Kals K, Adams W, Hamaty E, et al. “Deadly neuroinvasive mosquito-borne virus: A case of Eastern equine encephalitis,”. Perm. J (2021) 25:20–288. doi: 10.7812/TPP/20.288
30. Clinical evaluation & disease | Eastern equine encephalitis | CDC. Available at: https://www.cdc.gov/easternequineencephalitis/healthcare-providers/clinical-evaluation-disease.html (Accessed 19, 2022).
31. Diagnostic testing | Eastern equine encephalitis. CDC. Available at: https://www.cdc.gov/easternequineencephalitis/healthcare-providers/diagnostic-testing.html (Accessed 19, 2022).
32. Kang X, Li Y, Liu H, Lin F, Cai X, Sun T, et al. “A duplex real-time reverse transcriptase polymerase chain reaction assay for detecting western equine and eastern equine encephalitis viruses,”. Virol J (2010) 7(1):1–5. doi: 10.1186/1743-422X-7-284/TABLES/2
33. Obert R, et al. Clinical and neuroradiographic manifestations of Eastern equine encephalitis (1997). doi:10.1056/NEJM199706263362604.
34. Powers AM. “Resurgence of interest in Eastern equine encephalitis virus vaccine development,”. J Med Entomol (2022) 59(1):20–6. doi: 10.1093/JME/TJAB135
35. Prevention | st. Louis encephalitis. CDC. Available at: https://www.cdc.gov/sle/prevention/index.html (Accessed 19, 2022).
36. Reed DS, Glass PJ, Bakken RR, Barth JF, Lind CM, da Silva L, et al. “Combined alphavirus replicon particle vaccine induces durable and cross-protective immune responses against equine encephalitis viruses,”. J Virol (2014) 88(20):12077. doi: 10.1128/JVI.01406-14
37. Erasmus JH, Seymour RL, Kaelber JT, Kim DY, Leal G, Sherman MB, et al. “Novel insect-specific eilat virus-based chimeric vaccine candidates provide durable, mono- and multivalent, single-dose protection against lethal alphavirus challenge,”. J Virol (2018) 92(4):1274–91. doi: 10.1128/JVI.01274-17
38. Nasar F, Matassov D, Seymour RL, Latham T, Gorchakov RV, Nowak RM, et al. “Recombinant isfahan virus and vesicular stomatitis virus vaccine vectors provide durable, multivalent, single-dose protection against lethal alphavirus challenge,”. J Virol (2017) 91(8):1729–45. doi: 10.1128/JVI.01729-16
39. Roy CJ, Adams AP, Wang E, Leal G, Seymour RL, Sivasubramani SK, et al. “A chimeric sindbis-based vaccine protects cynomolgus macaques against a lethal aerosol challenge of eastern equine encephalitis virus,”. Vaccine (2013) 31(11):1464. doi: 10.1016/J.VACCINE.2013.01.014
40. Wang E, Petrakova O, Adams AP, Aguilar PV, Kang W, Paessler S, et al. CHIMERIC SINDBIS/EASTERN EQUINE ENCEPHALITIS VACCINE CANDIDATES ARE HIGHLY ATTENUATED AND IMMUNOGENIC IN MICE. Vaccine (2007) 25(43):7573. doi: 10.1016/J.VACCINE.2007.07.061
41. Hu WG, Steigerwald R, Kalla M, Volkmann A, Noll D, Nagata LP. “Protective efficacy of monovalent and trivalent recombinant MVA-based vaccines against three encephalitic alphaviruses,”. Vaccine (2018) 36(34):5194–203. doi: 10.1016/J.VACCINE.2018.06.064
42. Boley PA, Alhamo MA, Lossie G, Yadav KK, Vasquez-Lee M, Saif LJ, et al. “Porcine deltacoronavirus infection and transmission in poultry, united states,”. Emerg Infect Dis (2020) 26(2):255. doi: 10.3201/EID2602.190346
43. Pierson BC, Cardile AP, Okwesili AC, Downs IL, Reisler RB, Boudreau EF, et al. “Safety and immunogenicity of an inactivated eastern equine encephalitis virus vaccine,”. Vaccine (2021) 39(20):2780–90. doi: 10.1016/J.VACCINE.2021.03.030
44. Coates EE, Edupuganti S, Chen GL, Happe M, Strom L, Widge A, et al. “Safety and immunogenicity of a trivalent virus-like particle vaccine against western, eastern, and Venezuelan equine encephalitis viruses: a phase 1, open-label, dose-escalation, randomised clinical trial,”. Lancet Infect Dis (2022) 22(8):1210–20. doi: 10.1016/S1473-3099(22)00052-4
Keywords: Eastern equine encephalitis, EEE, diagnosis, treatment, vaccine
Citation: Sah R, Siddiq A, Al-Ahdal T, Maulud SQ, Mohanty A, Padhi BK, El-Shall NA, Chandran D, Emran TB, Hussein NR, Dhama K and Satapathy P (2023) The emerging scenario for the Eastern equine encephalitis virus and mitigation strategies to counteract this deadly mosquito-borne zoonotic virus, the cause of the most severe arboviral encephalitis in humans—an update. Front. Trop. Dis 3:1077962. doi: 10.3389/fitd.2022.1077962
Received: 24 October 2022; Accepted: 30 December 2022;
Published: 30 January 2023.
Edited by:
Venkataramana Kandi, Kaloji Narayana Rao University of Health Sciences, IndiaReviewed by:
Yamilka Diaz, Gorgas Memorial Institute of Health Studies, PanamaCopyright © 2023 Sah, Siddiq, Al-Ahdal, Maulud, Mohanty, Padhi, El-Shall, Chandran, Emran, Hussein, Dhama and Satapathy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranjit Sah, cmFuaml0c2FoNTdAZ21haWwuY29t; Tareq Al-Ahdal, dGFyZXEuYWwtYWhkYWxAdW5pLWhlaWRlbGJlcmcuZGU=; Bijaya Kumar Padhi, YmtwYWRoaUBnbWFpbC5jb20=
†Present address: Ranjit Sah, Tribhuvan University Teaching Hospital, Institute of Medicine, Kathmandu, Nepal
 Ranjit Sah
Ranjit Sah Abdelmonem Siddiq
Abdelmonem Siddiq Tareq Al-Ahdal
Tareq Al-Ahdal Sazan Qadir Maulud
Sazan Qadir Maulud Aroop Mohanty
Aroop Mohanty Bijaya Kumar Padhi
Bijaya Kumar Padhi Nahed A. El-Shall
Nahed A. El-Shall Deepak Chandran
Deepak Chandran Talha Bin Emran
Talha Bin Emran Nawfal R. Hussein11
Nawfal R. Hussein11 Kuldeep Dhama
Kuldeep Dhama Prakasini Satapathy
Prakasini Satapathy