
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Trop. Dis., 05 January 2023
Sec. Neglected Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.1068364
This article is part of the Research TopicRe-emergence of neglected tropical diseases amid the COVID-19 pandemic: Epidemiology, transmission, mitigation strategies, and recent advances in chemotherapy and vaccinesView all 13 articles
 Md. Aminul Islam1,2*†
Md. Aminul Islam1,2*† Shuaibu Suleiman Adeiza3†
Shuaibu Suleiman Adeiza3† Mohammad Ruhul Amin1
Mohammad Ruhul Amin1 Fatema Hasan Kaifa1
Fatema Hasan Kaifa1 Jose M. Lorenzo4,5
Jose M. Lorenzo4,5 Prosun Bhattacharya6*
Prosun Bhattacharya6* Kuldeep Dhama7
Kuldeep Dhama7Marburg virus (MARV) is a pathogenic zoonotic RNA virus etiologic for Marburg virus disease (MVD), a severe hemorrhagic fever. This is a rare disease, with a high fatality rate, that spreads via infected blood or body fluids or indirectly via fomites (contaminated objects and substances such as clothed, beds, personal protective equipment, or medical equipments). A few vaccines to protect against MARV are undergoing clinical trials, but there is not yet an approved vaccine against this disease. Eventually, prevention and control guidelines should be adhered to rigorously to alleviate this infection. This bibliometric analysis aimed to harness narrative evaluation, emphasizing the significance of quantitative approaches and delineating the most thought-provoking concerns for researchers using VOSviewer software (Centre for Science and Technology Studies, Leiden University, the Netherlands). “Marburg Virus” OR “MARV” AND “Diseases” search criteria were used for the analysis of articles published between 1962 and 2022. Co-occurrence analysis was carried out, which characterized different thematic clusters. From this analysis, we found that 1688 published articles, and the number of publications increased across that period annually, with a growth rate of 8.78%. It is also conspicuous that the number of publications in the United States reached its acme during this period (i.e., 714 publications, accounting for 42.29% of the total), and the United States Army Medical Research Institute of Infectious Diseases published the most literature (i.e., 146 papers). Our study found that the three pre-eminent authors of Marburg virus papers were “FELDMANN, HEINZ“ of the National Institute of Allergy and Infectious Diseases, United States, “BECKER, STEPHAN” of the Philipps University of Marburg, Germany, and “GEISBERT, THOMAS W” of the University of Texas Medical Branch, United States. In this study we found that “JOURNAL OF VIROLOGY” has published the most pertinent literature, totaling 88 articles, followed by “The journal of Infectious Diseases”, which published 76 relevant papers, and “VIRUSES”, which published 52 corresponding papers. The most cited paper on the Marburg virus was published in Nature Medicine, with 522 total citations and 29 citations/year. Studies of the changing epidemiology and evolving nature of the virus and its ecological niche are required; breakthrough and implementation of the efficacious vaccine candidate(s), prophylaxis and therapeutic alternatives and supervision strategies, unveiling awareness-raising programs, and developing apposite and timely preparedness, prevention, and proactive control strategies are of utmost importance.
Marburg virus (MARV) is a deadly zoonotic virus and the WHO identified it as a risk group 4 pathogen (WHO 2022) (https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease). It is a leading member of the family that includes Ebolavirus, i.e., Filoviridae, it belongs to the genus Marburgvirus and species Marburg arburgvirus, and it causes diseases in both humans and non-human primates (1). Other viruses of the same family as Marburgvirus—Filoviridae—are Ebolavirus, Cuevavirus, Striavirus, and Thamnovirus (2). MARV is enveloped and pleomorphic, consisting of filamentous, non-segmented, rod-shaped, cobra-like, circular/ring-like, and branch-shaped particles of uniform diameter but variable length (≈ 19.1 kb). The viral genome, negative-sense single-stranded RNA(–ssRNA), contains seven Open Reading Fragments (ORFs): (1) nucleoprotein (NP); (2) virion protein 35 (VP35); (3) VP40; (4) VP40; (5) VP24; (6) glycoprotein (GP); and (7) large-viral polymerase (L) (3, 4). In 1967, in Germany (Marburg and Frankfurt) and Serbia (Belgrade), MARV was first detected in laboratories. Recently, two MARV-confirmed deaths were reported from the southern Ashanti region of Ghana (1). Since the first detection of Marburg virus disease (MVD), outbreaks have occurred around the world (5). In 2008, cases were recorded in the USA and the Netherlands; the Democratic Republic of Congo (DRC), Uganda, Kenya, and South Africa also confirmed MARV cases, and the largest outbreak was in Angola in 2005 [374 positive tests and 329 deaths, with an 88% care fatality rate (CFR)] (Figure 1 and Supplementary Table 1) (6, 7). According to previous research, the incubation time of MARV is 3–21 days (typically 5–10 days) from exposure (8, 9). The range was reported as 3–13 days for filoviruses (Zaire ebolavirus and Marburg virus) in one study (10) and 2–26 days in another (11). After virus entry to the host cell through damaged skin and mucosal surfaces, it infects the immune cells (e.g., monocytes, macrophages, and dendritic cells). Viral entry into the host body includes three distinct steps—attachment, macropinocytosis, and fusion—and early replication disseminates to the hepatocytes, endothelial cells, fibroblasts, and epithelial cells, targeting the spleen, liver, and secondary lymphoid organs, with inhibition of type-I interferon (IFN-1) synthesis (12). Among a few entry mechanisms, with subsequent MRV through cellular attachment or fusion, endocytosis (clathrin mediated) and macropinocytosis are notably reported in previous studies, involving GP, tyrosine kinase, and C-like lectins in receptor binding sites (12). Sometimes the severity of MARV is higher than Ebola virus (EBOV), while the most modifications are affiliated with immune cells and gene expression against the viral cell. Phagocytes, monocytes, macrophages, Kupffer cells, dendritic cells, and endothelial cells are decimated by this virus (13). Various organs, such as the liver, kidney, stomach, heart, brain, spleen, and lymph nodes, are conspicuous in swelling during infection, with hemorrhage and necrosis. Predominantly, hepatic, lymphatic, and liver tissue, testes, and ovaries are affected more severely, with a paucity of lymphocytes (2).
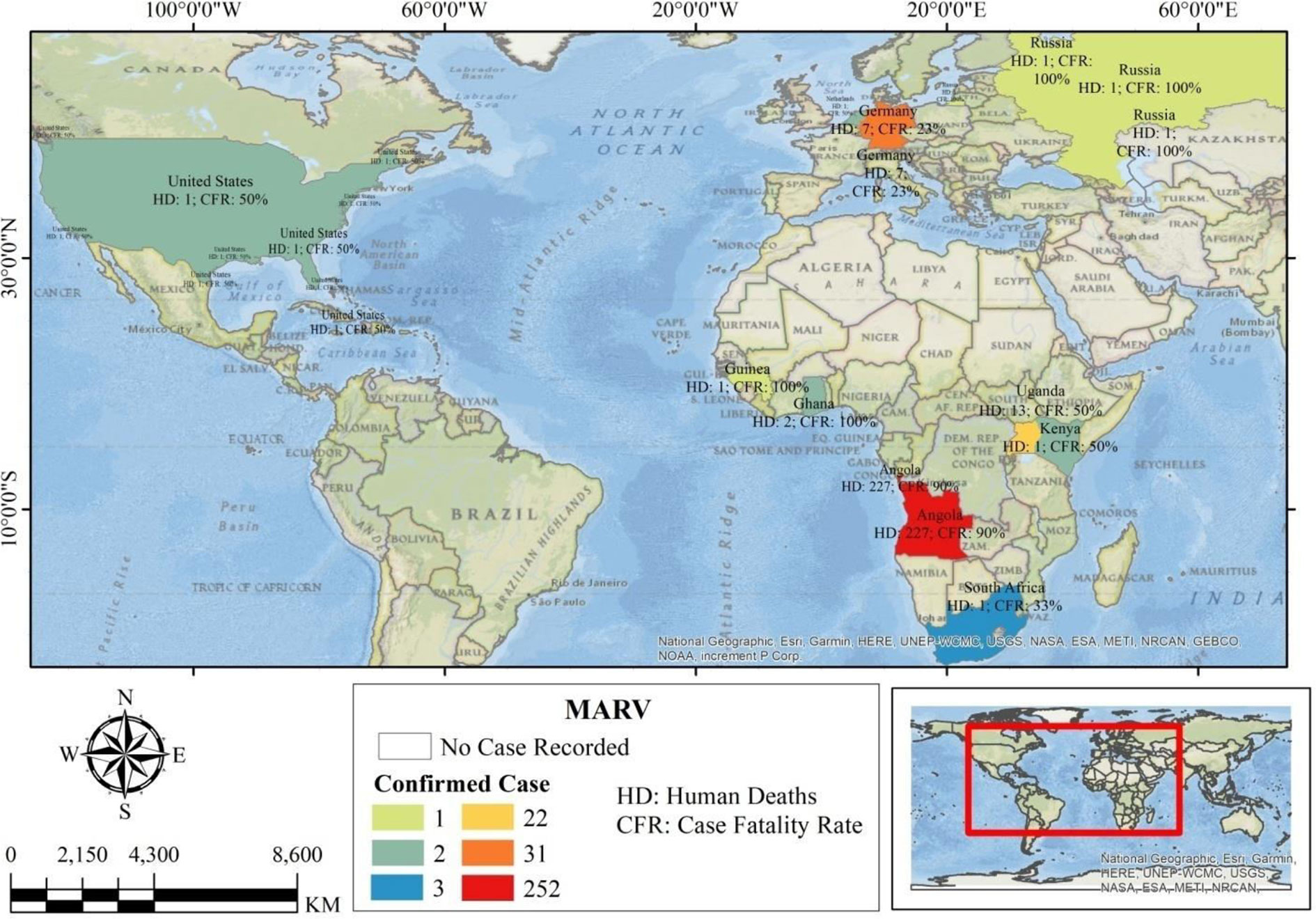
Figure 1 The global map indicating the epidemiology of Marburg virus, showing Angola as a highly affected area.
Clinical symptom onset is sudden, with high fever, fatigue, chills, headache, diarrhea, and myalgia. Moreover, a maculopapular rash is noticeable after 5–6 days and severe hemorrhagic symptoms within 7 days (14). The disease is categorized into three phases: an initial phase, with flu-like symptoms, persisting for 0–4 days; the organ phase, lasting 5–13 days, with neurological symptoms; and the convalescence phase, from day 13 onward (1). Multiorgan failure, pharyngitis, nausea, vomiting, chest pain, a sore throat, and abdominal pain may appear, and sometimes jaundice, inflammation of the pancreas, and severe weight loss are also conspicuous. In fatal cases death occurs after the onset of the clinical symptoms (15). Contact with positive patients’ saliva, sweat, stool, urine, tears, or breast milk can result in direct human-to-human transmission, and contaminated objects and substances can result in indirect transmission.
MARV can be introduced to human populations through inhalation of the contaminated excreta from bats, with occasional secondary human-to-human transmission (16). It can also persist in immune-privileged sites in individuals who have recovered from the disease. Mines or caves were the main sources of spreading this disease initially, usually where Rousettus bats were found. Direct human-to-human transmission is also possible through damaged/broken skin, secretions, body fluids (e.g., vaginal secretions, amniotic fluid, semen, and vomit), blood, and contaminated objects and substances such as beds, clothes, needles, and medical equipment (2). However, as Marburgvirus and Ebolavirus belong to the same family, it can be predicted that MARV persists in a patient’s body fluids. One study reported the highest level of MARV shedding in oral secretions from a bat (13). Interestingly, (17) detected MARV RNA in the urine of infected Egyptian rousette bats (Rousettus aegyptiacus), which indicated that infectious urine may be another route of transmission. Public health workers are a major risk group for MVD because of direct contact with patients. Moreover, people who are involved with burial ceremonies need to follow precautions to avoid direct contact with the body (18).
To fulfill sensitivity and quantification of viral titer, qRT-PCR using nucleocapsid protein can be used to detect MARV, whereas blood serum, plasma, or whole blood can be used to detect viral antibodies or antigens using the ELISA approach (19). In addition, patients’ tissues, serum, and plasma are preferable for the conventional polymerase chain reaction (PCR) method where RT-PCR is not available. It is recommended to collect blood samples without anticoagulant agents such as heparin or EDTA (20). Microscopy can predict viral morphology from tissue specimens that have been previously fixed in formalin.
To date, there is no approved treatment or vaccine available against MARV. As larger outbreaks are rare, available clinical data are often insufficient for finding out appropriate treatment regimens. Therefore, the treatment is solely based on supportive care, which needs to be given without delay. This encompasses rehydration with oral or intravenous fluids, regulating oxygen status and blood pressure, reinstating lost blood, rendering treatment as per the patient’s symptoms, and scrutinizing complicating infections, which could help in improving the survival of MVD patients. To delineate current knowledge, knowledge gaps, and hidden research trends, a bibliometric analysis is necessary. The central targets of the current research are to identify the outbreak effects of MVD and focus on the essential lessons that can be learned about this deadly disease. This study is designed to identify research trends, the number of published articles, highly cited articles, and the pre-eminent countries, institutions, and authors of Marburg virus papers.
The first stage primarily focuses on identifying and categorizing the current literature. The overall objective involved five sub-categories aiming to:
● clarify the attributes and interrelatedness of appropriate investigations
● constitute a well-structured organization of related studies concepts and findings
● validate the restrictions and gaps in current information, thus establishing the scope of potential areas of the study
● develop a conceptual mindset on the environmental issues along with the effect of the Marburg virus situation
● pursue the pathway of hot themes and potential future study trends.
The bibliographic data gathered from published research articles was parsed using bibliometric analysis in order to investigate patterns and trends in MARV literature and identify the core scientific networks (21). Previous research has employed bibliometric approaches to investigate the mutuality, subject classification, and future research directions of scientific disciplines (22).
The trend, which is the critical informative statistics of the appropriate form of knowledge, its effects, and the evolutionary process of the topic’s high recurrence rate, with a discernible analysis of co-occurrence, could be illustrated by bibliometrics and literature systematization. The bibliometric analysis makes some facilities for the researcher, such as obtaining true data from several scientific publications and the suppliers of this information and the publications.
Two researchers (AI and SSA) reviewed all titles and abstracts to choose publications that met the inclusion criteria (Figure 2). Before reaching a consensus, no concerns were discussed. Once the following criteria were met, data were retrieved from the literature: (a) the primary topic had to be linked to the study; (b) the literature could take any form (such as an article, review, editorial piece, or meeting abstract); and (c) the literature had to cover other topics that were also connected to the study. Duplicate entries, titles, abstracts of papers unrelated to the topic, and inaccessible abstracts were the exclusion criteria.
The Dimensions database is ranked among the top repositories for bibliometric and patentometric analysis because of its thorough coverage of millions of research works, grants, clinical trials, data sets, policy documents, and patents (23, 24). A title and abstract search for the terms “Marburg Virus” OR “MARV” AND “Diseases” was used to search for articles published in between 1962 and 2022. To account for changes in publication and citation count, all metadata were gathered and evaluated by two researchers in one sitting. Bibliometrix and ggplot2 R packages (25) were used to examine, analyze, and visualize data gathered from the Dimension database for bibliometric pointers. Bibliometric indicators included authors, affiliated countries and institutions, keywords, and citation counts. Bradford’s law of publication scattering was used to evaluate the distribution of literature in preferred journals.
VOSviewer software (Centre for Science and Technology Studies, Leiden University, the Netherlands) was used to create networks and visualize links of metadata (VOS viewer). The size of the circle reflects the degree of connection between documents. Coupling analysis was applied to view the country and institutional co-operation. Co-occurrence clustering was used to visualize research themes and identify research trends and current hot spots (26).
Significant challenges in the purification process were the ambiguity in papers’ titles, keywords, abstracts, and their topic incorporation. However, some addressed the Marburg virus and included “MARV” or its synonyms. The situation was assigned to a temporal relationship with the MARV crisis rather than evaluating its environmental impacts. During data cleanup, some generic terms, such as human, humans, article, and study, were removed.
The selected review papers were read in detail to delineate the essential data for the evaluations, especially the critical lessons presented in the following section. The papers were divided into several clusters based on commonalities. Top-cited papers were selected after examining the papers’ titles and abstracts and assigned to various clusters dependent on their thematic aims. This stage ended by refining the categorization by combining analogous clusters.
A total of 1688 documents were included, and the global volume of publications peaked at an annual growth rate of 8.78%. The years 2015 (112 documents) and 2020 (108 documents) marked the peaks of global publications, which accounted for 6.635% and 6.398% of the total number of publications, respectively. (Figure 3A). The highest number of average citations per year (6.5) occurred in 2011 (Figure 3B). The included document types were article (n=1,463, 86.61%), chapter (n=176, 10.43%), edited book (n=4, 0.25%), monograph (n=1, 0.06%), preprint (n=40, 2.4%), and conference proceeding (n=4, 0.25%).
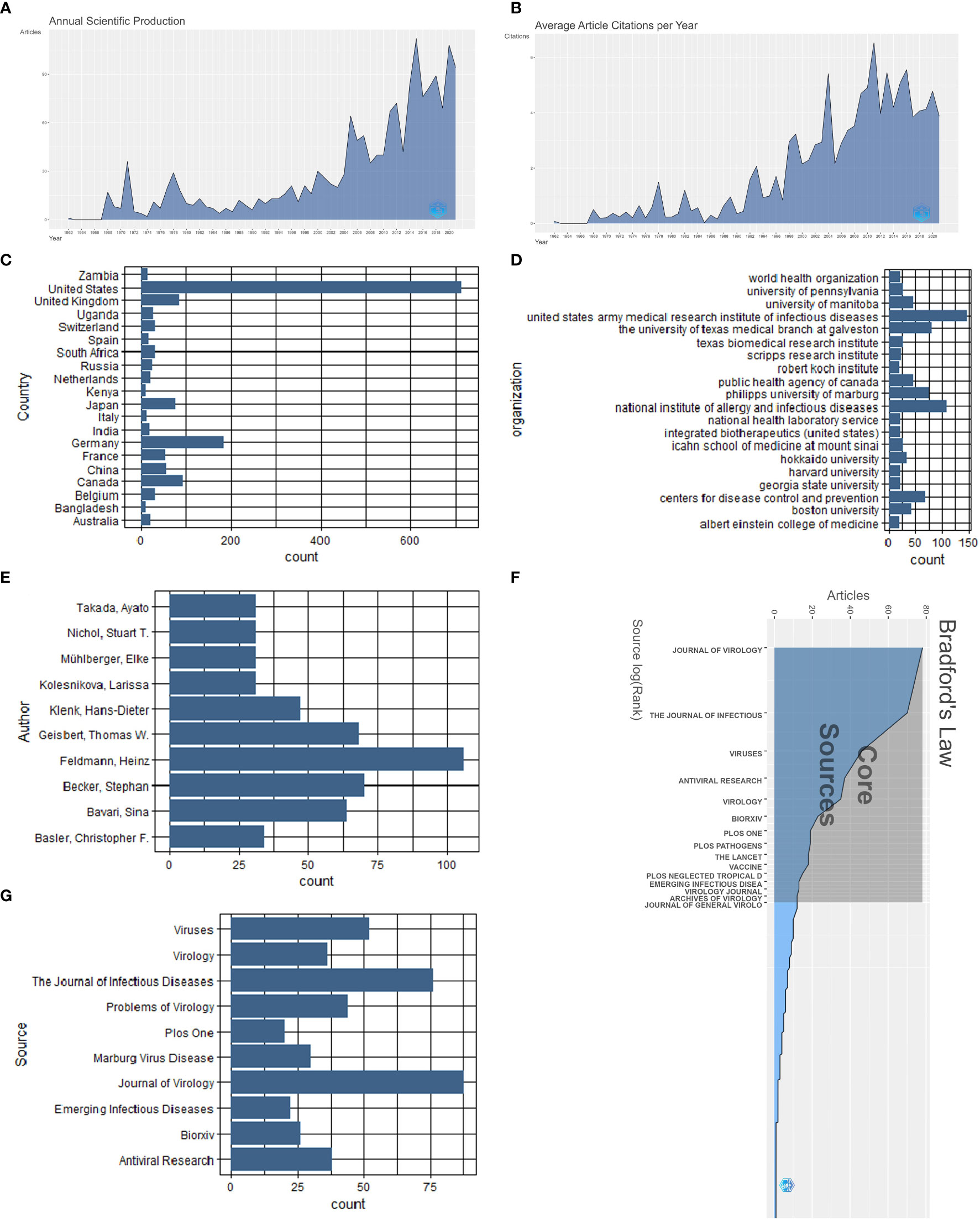
Figure 3 Marburg virus-related publications: (A) Annual scientific production; (B) Average citation per year; (C) Top 20 countries by publication volume; (D) Top 20 institutions by publication volume; (E) Top 10 authors by publication volume; (F) Bradford’s law of publication; (G) Top 10 journals by publication volume.
Figure 3C shows that, out of the 1688 documents, the United States published the most documents (714, accounting for 42.299%), followed by Germany (183, accounting for 10.841%) and the United Kingdom (85, accounting for 5.036%).
Figure 3D shows the top 20 institutions engaging in Marburg virus studies in terms of the volume of publications worldwide. Among them, the United States Army Medical Research Institute of Infectious Diseases has published the most literature (146 papers), the National Institute of Allergy and Infectious Diseases ranked second (108 papers), and the University of Texas Medical Branch at Galveston ranked third (80 papers).
Figure 3E shows the top 10 authors in this field. The top three authors of the papers were “FELDMANN, HEINZ” of the National Institute of Allergy and Infectious Diseases, United States, “BECKER, STEPHAN” of the Philipps University of Marburg, Germany, and “GEISBERT, THOMAS W” of the University of Texas Medical Branch at Galveston, the United States, publishing 106, 70, and 68 articles, respectively. The majority of Marburg virus-related publications were published in 15 core sources in accordance with Bradford’s law (Figure 3F).
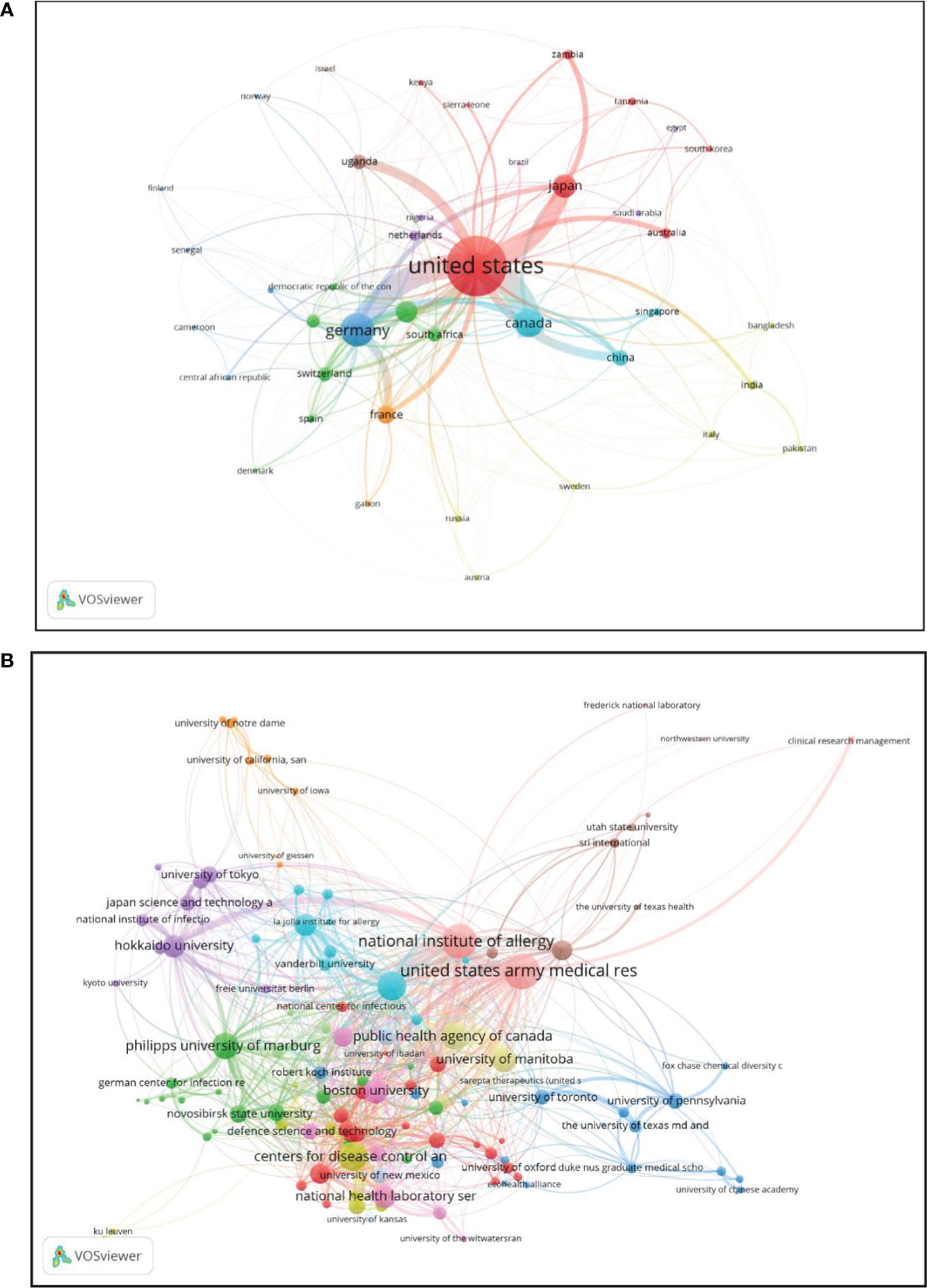
Figure 4 Coupling analysis: The size of the bubbles reflects the weight of the literature, and the color signifies clustering. The broadness of the lines is consistent with the intensity connection. (A) Country coupling analysis; (B) Institutional coupling analysis.
In Marburg virus research, “JOURNAL OF VIROLOGY” has published the most pertinent literature, totaling 88 articles, followed by “THE JOURNAL OF INFECTIOUS DISEASES”, which published 76 relevant papers, and “VIRUSES” published 52 related papers. Figure 3G shows the top 10 journals in terms of the number of publications on Marburg virus research worldwide.
A total of 81 countries were included by the criterion of at least five study records (Figure 4A). The top three countries in terms of the coupling strength of the literature in this field were the United States, whose total link strength (TLS) was 224, Germany (92), and Japan (52).
An analysis of all the materials included covered a total of 875 institutions, and 127 institutions according to the criterion of being mentioned at least five times in the literature (Figure 4B). The top three institutions in terms of coupling strength in the literature in this field were the United States Army Medical Research Institute of Infectious Diseases (TLS 216), National Institute of Allergy and Infectious Diseases (TLS 201), and University of Texas Medical Branch (TLS 144).
Research works relevant to the Marburg virus can be divided into three categories based on the results of keyword clustering: epidemiology and public health, pathogenicity and vaccine development, and immunology and molecular biology (Figures 5A, B). In epidemiology and public health (red circle), frequently used keywords were “Marburg”, “Virus”, “Ebola Disease”, “Outbreak”, “Case”, “Patient”, “Transmission”, “Pathogen”, “Viral hemorrhagic fever”, and “Bat”. Pathogenicity and vaccine development (blue circle) used “Infection”, “Treatment”, “Vaccine”, “Non human primate”, “Therapeutic”, “Response”, “Antibody”, “Protection”, and “Human” keywords very often. The frequent keywords applied by immunology and molecular biology (green circles) include “Marburg virus”, “EBV”, “Proteins”, “VP24”, “VP40”, “Expression”, “Mechanism”, “Genome”, “Domain”, “Inhibitor”, and “Cell”.
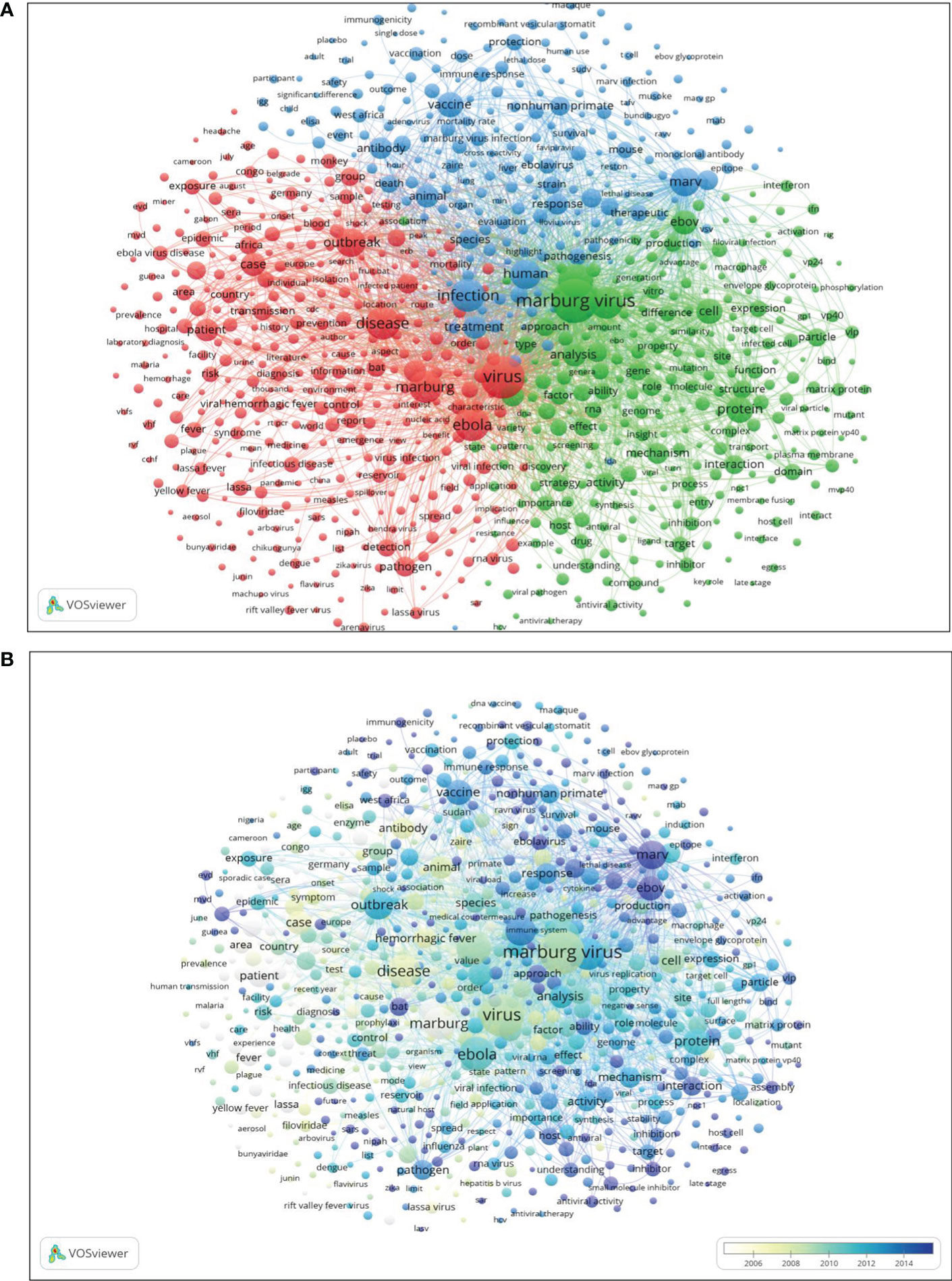
Figure 5 Text mining: (A) Research theme distribution map; (B) Breakdown of research hot spot trends. Temporal change in research hot spot is shown by the color gradient of white (early trends) to dark purple (future trends).
By dividing different keywords in terms of time, a distribution map of research priorities in different time periods was generated. Figure 5B shows that “Small molecule inhibitor”, “Protein analysis”, and “Immunogenicity” are future hot spots in the Marburg virus research field.
Table 1 lists 10 highly cited papers on the Marburg virus. The first paper was published in “NATURE MEDICINE” in 2005, and was titled “LIVE ATTENUATED RECOMBINANT VACCINE PROTECTS NONHUMAN PRIMATES AGAINST EBOLA AND MARBURG VIRUSES” (27). The article assessed the efficacy of replication-competent vaccines against EBOV and MARV based on attenuated recombinant vesicular stomatitis virus vectors expressing either the EBOV glycoprotein or MARV glycoprotein in nonhuman primates. “The study data suggested that the vaccine candidates were safe and highly efficacious in a relevant animal model because the EBOV vaccine induced humoral and apparent cellular immune responses in all vaccinated monkeys, but the MARV vaccine induced a stronger humoral than cellular immune response.” Published in the “JOURNAL OF CLINICAL MICROBIOLOGY” in 2002, the second study looked at “RAPID DETECTION AND QUANTIFICATION OF RNA OF EBOLA AND MARBURG VIRUSES, LASSA VIRUS, CRIMEAN-CONGO HEMORRHAGIC FEVER VIRUS, RIFT VALLEY FEVER VIRUS, DENGUE VIRUS, AND YELLOW FEVER VIRUS BY REAL-TIME REVERSE TRANSCRIPTION-PCR”. The study showed “six one-step, real-time reverse transcription-PCR assays for viral pathogen detection based on the SuperScript™ reverse transcriptase-platinum Taq polymerase enzyme mixture”. “The suitability of the assays was demonstrated by detection and of viral RNA in serum samples of VHF patients by the ≥95% detection limits observed” (28). The authors of the third quantification study published in “PLOS PATHOGENS” in 2011 focused on “DISTINCT PATTERNS OF IFITM-MEDIATED RESTRICTION OF FILOVIRUSES, SARS CORONAVIRUS, AND INFLUENZA A VIRUS”. The study showed that “IFITM proteins restricted infection mediated by the entry of glycoproteins (GP (1, 2) of Marburg and Ebola Filoviruses (MARV, EBOV)”. “The data indicated that IFITM-mediated restriction is localized to a late stage in the endocytic pathway.” “The results also showed, IFITM proteins differentially restrict the entry of a broad range of enveloped viruses, and modulate cellular tropism independently of viral receptor expression” (29).
A quick understanding of each area’s front lines is crucial, and the papers from pioneer authors and/or groups were tracked. To find out the themes of interest in paper data based on the Marburg virus (MARV) and Marburg virus disease (MVD), two approaches were employed to reach more reliable outcomes: title, keyword, and abstract based (30). From the start of the study to its end in 2021, 1688 articles were published in various scientific journals.
The MARV was largely ignored for many years, and a reasonable proportion of scientific papers have been published in the decades since. Controlling MARV rapidly and effectively appears to be a challenge. This is owing in part to an incomplete knowledge map of MARV research and a lack of understanding of current research status, hot spots, and development trends. This can lead to a considerable amount of repetitive, unimportant, or inefficient research, which can impede proper virus research and further hinder the development of targeted prevention and surveillance methods.
In consideration of transmission scale, MARV is less contagious than COVID-19, owing to the pathogen’s limited infectivity and protracted asymptomatic incubation (31). Hence, a mapping of MARV research was created through bibliometric and visualization techniques by selecting and evaluating over 1688 MARV-related scientific articles. The annual number of papers and cumulative citations of publications on MARV demonstrate the areas of interest and development over time. Between 1962 and 2022, the number of publications was modest and constant. Surprisingly, dramatic growth peaks occurred separately in 2015 and 2021, coinciding with the breakouts of MARV. This rapid increase demonstrates the substantial impact of growing MVD on public health. The number of publications is related to the size and spread of the infectious disease outbreak (32).
The United States, the United Kingdom, Canada, Germany, and Japan were the most active countries at the forefront of MARV research. Excluding Japan, our findings are consistent with other findings that the United States, the United Kingdom, Germany, and other European countries are typically the most active in scientific research (33).
The prominent contributors’ different backgrounds (scholars, clinicians, research experts, etc.) represent the interdisciplinary nature of MARV research. A unified approach to responding to and controlling new viral disease outbreaks is required; therefore, scientists, clinicians, and CDC experts must communicate and exchange information even though it is critical (34).
In this study, only a few journals accounted for the majority of MARV research publications. This is consistent with Bradford’s law. This reflects the authority of these journals, as well as their high level of interest in MARV research. Popular journals and their research trends in a specific topic provide dependable references for researchers. Furthermore, key journals facilitate faster search routes for scholars and can act as an important publication guide (33).
Keyword co-occurrence analysis can yield a wealth of useful information, allowing the identification of hot spots and trends and directing researchers to similar topics in their field (35). Three distinct MARV research topics can be identified, which mostly involve issues of epidemiology and public health, pathogenicity and vaccine development, and immunology and molecular biology, and commensurate effort by research scientists is compulsory for effective MVD prevention and control.
The highest number of confirmed cases of MARV was observed between 2004 and 2005, when the fatality rate was 90% (confirmed cases 252, deaths 227) in Angola. After that, various outbreaks were reported in Uganda from 2007 to 2017; the highest number of deaths recorded was 18 in 2012 and 100% CFR was documented in 2014 (Supplementary Table 1). The software used to evaluate the co-occurrence, bibliographic coupling, and clusters was VOSviewer version 1.6.18 (36). Standard weight features employed in this study are described as “Links attribute” and “Total link strength attribute” (37). With the increase of significance, the number of neighboring elements and the distance between these elements and the point of interest became smaller, and the elements’ density was higher. Furthermore, as the more significant the neighboring elements’ weight, the higher was the element density (36).
(28), established a new diagnosis protocol for detecting hemorrhagic fevers (VHFs) related to MARV with acute infections. (29), published an article in “PLOS PATHOGENS” reporting interferon-inducible transmembrane proteins (IFITM) originated from the MARV. (38), isolated Marburg viruses from Egyptian fruit bats (31/611, 5.1%), suggesting that they are a natural reservoir and source of the Marburg virus. One published article first reported that BCX4430 fights against MVD, acting on the RNA polymerase of the virus (39). From the country-based coupling analysis of MARV it can be concluded that the top 10 highest articles were published in the United States, Germany, Canada, Japan, France, South Africa, China, Switzerland, Uganda, and Spain. It also observed that among the top 10 highest institutes were the National Institute of Allergy, United States Army Medical Center for Disease Control, Philips University, National Health Laboratory, Boston University, Hokkaido University, University of New Mexico, University of Toronto, University of Pennsylvania. The availability of Marburg virus samples, case numbers, or research interest might be reasons behind this scenario. From research theme distribution map analysis, we detected several clusters where the Marburg virus, Ebola, Marburg, infection, human, outbreak, disease, MARV, and vaccine are notably observed. The first paper of MARV was published in 1962 and the top-cited article was accepted by “NATURE MEDICINE” in 2005, which discussed vaccination against two deadly viruses, Ebola and Marburg. Vaccine candidates formulated with either the EBOV or MARV glycoproteins expressed in attenuated recombinant virus vectors were safe and highly effective when tested in non-human primates. Although the database search was thorough, it is conceivable that some items were missed.
As an infectious disease, MVD with high CFR is able to create devastating outbreaks around the world at any time (40–45). It is high time for conducting research studies based on previous outbreaks and data regarding the virus (19). According to previous studies, it can be found that the clinical investigations of this disease are not inadequate (5, 46). In addition to that, there is no detailed knowledge about host and virus interaction for designing vaccines or therapeutic drugs to prevent and control MVD (47).
It is important to enhance awareness programs about MVD, educating people at greater risk, including healthcare workers. Proactive planning and highly collaborative efforts involving researchers, experts in public health, policymakers, and biologists are necessary to design suitable strategies to counteract MVD (46). Surveillance and monitoring need to be upgraded, along with the strengthening of rapid and confirmatory diagnosis of MVD cases and contact tracing and tracking in affected regions employing wastewater-based surveillance, with serological and molecular epidemiological investigations (48–50). Numerous publications show that circumspection, wastewater monitoring, and prognosis of the outbreak are important as well for this type of virus (51–53).
Indisputably, further analyses are necessary to inquire about the relationship between MARV and the environment (54–56). The public health sector should act on this knowledge gap to empower the community, supplying educational materials for epidemic preparedness in the future, using communication channels proposed by the communities. One Health rules implementation, the upgrade of Biosafety Level 4 Laboratories (BSL-4s), and conducting multidisciplinary research is necessary. The development of effective vaccines, antivirals, and other therapies, and adopting apposite mitigation strategies, are the current priorities in combating MARV as it poses a drastic global health concern and could cause a deadly pandemic due to its intense lethality (5, 50, 57).
Given the recently reported MVD cases in Ghana, along with last year’s report in Guinea and outbreaks in preceding years, and feasible subsequent threats, there is an utmost need for discovering an effective vaccine and therapies for this devastating disease (58). As MARV can only be handled in BSL-4s, very few laboratories have the capability for basic and applied research for developing prophylactics and therapeutics against this lethal virus. Hence, strengthening research facilities with maximum containment laboratories is critical for handling MARV. From the SARS-CoV-2 pandemic situation, everyone should understand the lesson of One Health, which is that three major things (environment, animals, and humans) are involved in the prevention and inspection of zoonotic diseases such as MVD (59–62). Swift, multidisciplinary action is required to incorporate and inspect such incidences of MVD before an unwanted quick spread of MARV occurs in other regions and countries amid the ongoing COVID-19 pandemic and current global health concern that is the burgeoning cases of monkeypox (63, 64).
Although, there is no specific treatment (i.e., vaccines/antiviral drugs) for this disease, cardiac glycosides, antipyretics, and steroids have previously been prescribed (65). Currently, various drugs are being investigated; a recent study suggested that remdesivir may be effective against the Marburg virus in cynomolgus macaque models (66), and another research study identified that cholesterol-conjugated fusion inhibitors are efficacious against this virus (67). 4-(aminomethyl)benzamide, BCX4430, favipiravir, aloperine small molecules, monoclonal antibodies, and cytokines are feasible against MARV infection (15, 68). Several attempts have been made to develop a vaccine and appropriate treatment regimen to counteract MVD (69). The GP and VP40 matrix proteins have been identified as the most antigenic viral proteins to develop a new chimeric subunit vaccine (70). An inhibitor compound, FC-10696, has recently been discovered to suppress the egress of MARV (71). Also, AVI-7288 has been indicated to exhibit potential as post-exposure prophylaxis against MARV (72). Numerous experiments were methodized on rodent and Non Human Primates (NHP) models for testing vaccine efficacies against MARV. To date, some vaccines have been trialed for human use. Among them, the cAd3 vaccine, also known as chimpanzee adenovirus serotype 3 vectors, encoded with wild-type GP from MARV, is the subject of a Phase 1 clinical trial for human use (73). To maintain the patient’s electrolytes, fluids, oxygen status, and blood pressure, as well as to replace lost blood and clotting factors, and treatment for any complicating infections, supportive hospital therapy should be utilized (63, 64, 74).
In the present bibliometric study, we attempted to analyze studies published on MARV to identify the most cited published papers, delineate some topics for researchers for further research and identify knowledge gaps. To understand more about MARV and control outbreaks, analysis of published research articles plays a significant role in revealing the epidemiology, genomics, and signs and symptoms; this bibliometric study provides wider knowledge about the previously published article areas, authors, citations, and institutions related to this research. Among the countries that are potential clusters based on the review, the USA is the most prevalent, followed by Germany, Canada, and Japan. However, there is no balanced reporting of these clusters. This is likely to be due to data set availability for MARV research. Actions taken almost immediately are vital to avoid outbreaks of MARV in the future and determine whether or not the post-outbreak situation will be managed sustainably. By tackling this challenge, cities will be able to foster cycling culture and shift short-term cyclists into long-term ones.
MI: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Roles/Writing - original draft, Writing - review & editing. SA: Data curation, Formal analysis, Investigation, Methodology, Roles/Writing. MA: Data curation, Investigation, Visualization, Writing - review & editing, Funding. FK: review & editing. JL: Review & editing, Investigation, Visualization. PB: Conceptualization, Funding, Roles/Writing - original draft, Writing - review & editing, Supervision, Funding. KD: Review & editing, Investigation, Visualization, Supervision. All authors critically scrutinisedand approved the final version of the manuscript. The corresponding authors are responsible for confirming that the descriptions are accurate and agreed by all authors.
This study was supported by President Abdul Hamid Medical College, Noakhali Science and Technology University, Bangladesh and KTH Royal Institute of Technology, Sweden.
We acknowledge all the authors institutions. Authors of this manuscript also acknowledge handling editors and reviewer of this manuscript. We also thankful to Tahmid Anam from SUST for supporting map in this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.1068364/full#supplementary-material
1. Hussain Z. Ghana Declares its first outbreak of marburg virus disease after two deaths. BMJ (2022) o1797. doi: 10.1136/bmj.o1797
2. Fujita-Fujiharu Y, Sugita Y, Takamatsu Y, Houri K, Igarashi M, Muramoto Y, et al. Structural insight into marburg virus nucleoprotein–RNA complex formation. Nat Commun (2022) 13(1):1191. doi: 10.1038/s41467-022-28802-x
3. Shi M, Lin X-D, Chen X, Tian J-H, Chen L-J, Li K, et al. The evolutionary history of vertebrate RNA viruses. Nature (2018) 556(7700):197–202. doi: 10.1038/s41586-018-0012-7
4. Zhao H, Shi L, Li Z, Kong R, Ren X, Ma R, et al. The Yun/Prohibitin complex regulates adult drosophila intestinal stem cell proliferation through the transcription factor E2F1. Proc Natl Acad Sci (2022) 119(6). doi: 10.1073/pnas.2111711119
5. Chakraborty C, Bhattacharya M, Sharma AR, Roy SS, Islam MA, Chakraborty S, et al. Deep learning research should be encouraged for diagnosis and treatment of antibiotic resistance of microbial infections in treatment associated emergencies in hospitals. Int J Surg (2022) 105:106857. doi: 10.1016/j.ijsu.2022.106857
6. Koundouno FR, Kafetzopoulou LE, Faye M, Renevey A, Soropogui B, Ifono K, et al. Detection of marburg virus disease in Guinea. New Engl J Med (2022) 386(26):2528–30. doi: 10.1056/NEJMc2120183
7. Timen A. Response to imported case of marburg hemorrhagic fever, the Netherlands. Emerging Infect Dis (2009) 15(8):1171–5. doi: 10.3201/eid1508.090051
8. Brauburger K, Hume AJ, Mühlberger E, Olejnik J. Forty-five years of marburg virus research. Viruses (2012) 4(10):1878–927. doi: 10.3390/v4101878
9. Pavlin BI. Calculation of incubation period and serial interval from multiple outbreaks of marburg virus disease. BMC Res Notes (2014) 7(1):906. doi: 10.1186/1756-0500-7-906
10. Kortepeter MG, Dierberg K, Shenoy ES, Cieslak TJ. Marburg virus disease: A summary for clinicians. Int J Infect Dis (2020) 99:233–42. doi: 10.1016/j.ijid.2020.07.042
11. Ellis DS, Stamford S, Lloyd G, Bowen ETW, Platt GS, Way H, et al. Ebola And marburg viruses: I. some ultrastructural differences between strains when grown in vero cells. J Med Virol (1979) 4(3):201–11. doi: 10.1002/jmv.1890040306
12. Yu Z, Wu H, Huang Q, Zhong Z. Simultaneous detection of marburg virus and Ebola virus with TaqMan-based multiplex real-time PCR method. J Clin Lab Anal (2021) 35(6). doi: 10.1002/jcla.23786
13. Schuh AJ, Amman BR, Towner JS. Filoviruses and bats. Microbiol Aust (2017) 38(1):12. doi: 10.1071/MA17005
14. Janik E, Ceremuga M, Niemcewicz M, Bijak M. Dangerous pathogens as a potential problem for public health. Medicina (2020) 56(11):591. doi: 10.3390/medicina56110591
15. Hickman MR, Saunders DL, Bigger CA, Kane CD, Iversen PL. The development of broad-spectrum antiviral medical countermeasures to treat viral hemorrhagic fevers caused by natural or weaponized virus infections. PLos Negl Trop Dis (2022) 16(3):e0010220. doi: 10.1371/journal.pntd.0010220
16. Pigott DM, Golding N, Mylne A, Huang Z, Weiss DJ, Brady OJ, et al. Mapping the zoonotic niche of marburg virus disease in Africa. Trans R Soc Trop Med Hyg. (2015) 109(6):366–78. doi: 10.1093/trstmh/trv024
17. Paweska JT, Jansen van Vuren P, Masumu J, Leman PA, Grobbelaar AA, Birkhead M, et al. Virological and serological findings in rousettus aegyptiacus experimentally inoculated with vero cells-adapted hogan strain of marburg virus. PLos One (2012) 7(9):e45479. doi: 10.1371/journal.pone.0045479
18. Schmidt K, Mühlberger E. Marburg virus reverse genetics systems. Viruses (2016) 8(6):178. doi: 10.3390/v8060178
19. Nyakarahuka L, Skjerve E, Nabadda D, Sitali DC, Mumba C, Mwiine FN, et al. Knowledge and attitude towards Ebola and marburg virus diseases in Uganda using quantitative and participatory epidemiology techniques. PLos Negl Trop Dis (2017) 11(9):e0005907. doi: 10.1371/journal.pntd.0005907
20. Geisbert TW, Jaax NK. Marburg hemorrhagic fever: Report of a case studied by immunohistochemistry and electron microscopy. Ultrastructural Pathol (1998) 22(1):3–17. doi: 10.3109/01913129809032253
21. Yin X, Wang H, Wang W, Zhu K. Task recommendation in crowdsourcing systems: A bibliometric analysis. Technol Soc (2020) 63:101337. doi: 10.1016/j.techsoc.2020.101337
22. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update, (2000 – 2014). Expert Opin Biol Ther (2014) 14(9):1295–317. doi: 10.1517/14712598.2014.920813
24. Hook DW, Porter SJ, Herzog C. Dimensions: Building context for search and evaluation. Front Res Metrics Analytics (2018) 3:23. doi: 10.3389/frma.2018.00023
25. Aria M, Cuccurullo C. bibliometrix : An r-tool for comprehensive science mapping analysis. J Informetrics (2017) 11(4):959–75. doi: 10.1016/j.joi.2017.08.007
26. Huang T, Wu H, Yang S, Su B, Tang K, Quan Z, et al. Global trends of researches on sacral fracture surgery. Spine (2020) 45(12):E721–8. doi: 10.1097/BRS.0000000000003381
27. Jones SM, Feldmann H, Ströher U, Geisbert JB, Fernando L, Grolla A, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and marburg viruses. Nat Med (2005) 11(7):786–90. doi: 10.1038/nm1258
28. Drosten C, Goöttig S, Schilling S, Asper M, Panning M, Schmitz H, et al. Rapid detection and quantification of RNA of Ebola and marburg viruses, lassa virus, Crimean-Congo hemorrhagic fever virus, rift valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol (2002) 40(7):2323–30. doi: 10.1128/JCM.40.7.2323-2330.2002
29. Huang I-C, Bailey CC, Weyer JL, Radoshitzky SR, Becker MM, Chiang JJ, et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza a virus. PLos Pathog (2011) 7(1):e1001258. doi: 10.1371/journal.ppat.1001258
30. Kaur P, Dhir A, Tandon A, Alzeiby EA, Abohassan AA. A systematic literature review on cyberstalking. an analysis of past achievements and future promises. Technological Forecasting Soc Change (2021) 163:120426. doi: 10.1016/j.techfore.2020.120426
31. Okonji OC, Okonji EF, Mohanan P, Babar MS, Saleem A, Khawaja UA, et al. Marburg virus disease outbreak amidst COVID-19 in the republic of Guinea: A point of contention for the fragile health system? Clin Epidemiol Global Health (2022) 13:100920. doi: 10.3201/eid2306.170047
32. Qi S, Hua F, Xu S, Zhou Z, Liu F. Trends of global health literacy research, (1995–2020): Analysis of mapping knowledge domains based on citation data mining. PLos One (2021) 16(8):e0254988. doi: 10.1371/journal.pone.0254988
33. Sweileh WM, Moh’d Mansour A. Bibliometric analysis of global research output on antimicrobial resistance in the environment, (2000–2019). Global Health Res Policy (2020) 5(1):37. doi: 10.1186/s41256-020-00165-0
34. Xiao P, Wu D, Wang J. Bibliometric analysis of global research on white rot fungi biotechnology for environmental application. Environ Sci pollut Res (2022) 29(1):1491–507. doi: 10.1007/s11356-021-15787-1
35. Adeiza S, Shuaibu A. Trends in monkeypox research: A sixty year bibliometric analysis. Microbes Infect Dis (2022) 3(3):500–13. doi: 10.21608/mid.2022.147680.1334
36. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
37. Stephan P, Veugelers R, Wang J. Reviewers are blinkered by bibliometrics. Nature (2017) 544(7651):411–2. doi: 10.1038/544411a
38. Towner JS, Amman BR, Sealy TK, Carroll SAR, Comer JA, Kemp A, et al. Isolation of Genetically Diverse Marburg Viruses from Egyptian Fruit Bats. PLoS Pathogens (2009) 5(7):e1000536. doi: 10.1371/journal.ppat.1000536
39. Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature (2014) 508(7496):402–405. doi: 10.1038/nature13027
40. Haque MA, Wang F, Chen Y, Hossen F, Islam MA, Hossain MA, et al. Bacillus spp. contamination: A novel risk originated from animal feed to human food chains in south-Eastern Bangladesh. Front Microbiol (2022) 12:78310. doi: 10.3389/fmicb.2021.78310
41. Hossain FE, Islam S, Islam MA, Islam S, Ahmed F. Detection of virulence genes of APEC (avian pathogenic escherichia coli) isolated from poultry in noakhali, Bangladesh. Biores Commun (2021) 7(1):967–72. doi: 10.3329/brc.v7i1.54253
42. Rakib SH, Masum SM, Farhana A, Islam MA, Islam MF, Reza MT, et al. Design of a cost-effective ultraviolet disinfection unit to minimize the cross-contamination of COVID-19 in transport. 2022 International Conference on Advancement in Electrical and Electronic Engineering (ICAEEE) (2022), 1–6. doi: 10.1109/ICAEEE54957.2022.9836348
43. Rakib SH, Masum S, Patwari MRI, Fahima RA, Farhana A, Islam MA. esign and Development of a low cost Ultraviolet Disinfection system to reduce the cross infection of SARS-CoV-2 in ambulances, in: 2021 International Conference on Electronics, Communications and Information Technology (ICECIT), (2021), 1–4. doi: 10.1109/ICECIT54077.2021.9641131
44. Roy S, Bhowmik DR, Begum R, Amin MT, Islam MA, Ahmed F, et al. Aspirin attenuates the expression of adhesion molecules, risk of obesity, and adipose tissue inflammation in high-fat diet-induced obese mice. Prostaglandins Other Lipid Mediators (2022) 162:106664. doi: 10.1016/j.prostaglandins.2022.106664
45. Roy S, Ripon MAR, Begum R, Bhowmik DR, Amin MT, Islam MA, et al. Arachidonic acid supplementation attenuates adipocyte inflammation but not adiposity in high fat diet induced obese mice. Biochem Biophys Res Commun (2022) 608:90–5. doi: 10.1016/j.bbrc.2022.03.089
46. Dhama K, Chandran D, Chakraborty S, Yatoo MI, Islam MA, Bhattacharya M, et al. Zoonotic concerns of marburg virus: Current knowledge and counteracting strategies including one health approach to limit animal-human interface: An update. Int J Surg (2022) 106:106941. doi: 10.1016/j.ijsu.2022.106941
47. Mohapatra RK, Sarangi AK, Kandi V, Chakraborty S, Chandran D, Alagawany M, et al. Recent re-emergence of marburg virus disease in an African country Ghana after Guinea amid the ongoing COVID-19 pandemic: Another global threat? current knowledge and strategies to tackle this highly deadly disease having feasible pandemic potential. Int J Surg (2022) 106:106863. doi: 10.1016/j.ijsu.2022.106863
48. Chakraborty S, Chandran D, Mohapatra RK, Yatoo MI, Islam A, Sharma AK, et al. Marburg virus disease – a mini-review. J Exp Biol Agric Sci (2022a) 10(2320):689–96. doi: 10.18006
49. Chakraborty S, Chandran D, Mohapatra RK, Islam MA, Alagawany M, Bhattacharya M, et al. Langya virus, a newly identified henipavirus in China - zoonotic pathogen causing febrile illness in humans, and its health concerns: Current knowledge and counteracting strategies – correspondence. Int J Surg (2022b) 105:106882. doi: 10.1016/j.ijsu.2022.106882
50. Chakraborty S, Mohapatra RK, Chandran D, Alagawany M, Sv P, Islam MA, et al. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. an update – correspondence. Int J Surg (2022c) 105:106869. doi: 10.1016/j.ijsu.2022.106869
51. Islam A, Hossen F, Rahman A, Sultana KF, Hasan MN, Haque A, et al. An opinion on wastewater-based epidemiological monitoring (WBEM) with clinical diagnostic test (CDT) for detecting high-prevalence areas of community COVID-19 infections. Curr Opin Environ Sci Health (2022b), 100396. doi: 10.1016/j.coesh.2022.100396
52. Islam A, Rahman A, Jakariya, Bahadur NM, Hossen F, Mukharjee SK, et al. A 30-day follow-up study on the prevalence of SARS-COV-2 genetic markers in wastewater from the residence of COVID-19 patient and comparison with clinical positivity. Sci Total Environ (2022c), 159350. doi: 10.1016/j.scitotenv.2022.159350
53. Tiwari A, Adhikari S, Kaya D, Islam MA, Malla B, Sherchan SP, et al. Monkeypox outbreak: Wastewater and environmental surveillance perspective. Sci Total Environ (2023) 856:159166. doi: 10.1016/j.scitotenv.2022.159166
54. Islam MA, Haque MA, Rahman MA, Hossen F, Reza M, Barua A, et al. A review on measures to rejuvenate immune system: Natural mode of protection against coronavirus infection. Front Immunol (2022) 13:837290. doi: 10.3389/fimmu.2022.837290
55. Ahmed F, Islam MA, Kumar M, Hossain M, Bhattacharya P, Islam MT, et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation centre in Bangladesh: Variation along the sewer network. Science of the total environment. 776 (2021) 145724. doi: 10.1016/j.scitotenv.2021.145724
56. Jakariya M, Ahmed F, Islam MA, Ahmed T, Marzan A, Hossain M, et al. Wastewater based surveillance system to detect SARS-CoV-2 genetic material for countries with on-site sanitation facilities: an experience from Bangladesh. MedRxiv (2021) 8852000:2021.07.30.21261347.
57. Chandran D, Dhama K, M K MA, Chakraborty S, Mohapatra RK, Yatoo MI, et al. Monkeypox : An update on current knowledge and research advances. J Exp Biol Agric Sci (2022) 10(2320):679–88. doi: 10.18006/2022.10(4).679.688
58. Dulin N, Spanier A, Merino K, Hutter JN, Waterman PE, Lee C, et al. Systematic review of marburg virus vaccine nonhuman primate studies and human clinical trials. Vaccine (2021) 39(2):202–8. doi: 10.1016/j.vaccine.2020.11.042
59. Ahmed F, Aminul Islam M, Kumar M, Hossain M, Bhattacharya P, Tahmidul Islam M, et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation centre through wastewater surveillance in Bangladesh. MedRxiv (2020). doi: 10.1101/2020.09.14.20194696
60. Islam MA, Marzan AA, Islam MS, Sultana S, Parvej MI, Hossain MS, et al. Sex-specific epidemiological and clinical characteristics of COVID-19 patients in the southeast region of Bangladesh. medRxiv (2021). doi: 10.1101/2021.07.05.21259933
61. Jakariya M, Ahmed F, Islam MA, Al Marzan A, Hasan MN, Hossain M, et al. Wastewater-based epidemiological surveillance to monitor the prevalence of SARS-CoV-2 in developing countries with onsite sanitation facilities. Environ pollut (2022), 119679. doi: 10.1016/j.envpol.2022.119679
62. Sakib MMH, Nishat AA, Islam MT, Raihan Uddin MA, Iqbal MS, Bin Hossen FF, et al. Computational screening of 645 antiviral peptides against the receptor-binding domain of the spike protein in SARS-CoV-2. Comput Biol Med (2021) 136:104759. doi: 10.1016/j.compbiomed.2021.104759
63. Islam MA, Hasan MN, Ahammed T, Anjum A, Majumder A, Siddiqui MNEA, et al. Association of household fuel with acute respiratory infection (ARI) under-five years children in Bangladesh. Frontiers in Public Health, 10. doi: 10.3389/fpubh.2022.985445.
64. Islam MA, Sangkham S, Tiwari A, Vadiati M, Hasan MN, Noor STA, et al. Association between global monkeypox cases and meteorological factors. Int J Environ Res Public Health (2022) 19(23):15638. doi: 10.3390/ijerph192315638
65. Zhao F, He Y, Lu H. Marburg virus disease: A deadly rare virus is coming. BioSci. Trends (2022), 01333. doi: 10.5582/bst.2022.01333
66. Porter DP, Weidner JM, Gomba L, Bannister R, Blair C, Jordan R, et al. Remdesivir (GS-5734) is efficacious in cynomolgus macaques infected with marburg virus. J Infect Dis (2020) 222(11):1894–901. doi: 10.1093/infdis/jiaa290
67. Cross RW, Bornholdt ZA, Prasad AN, Borisevich V, Agans KN, Deer DJ, et al. Combination therapy protects macaques against advanced marburg virus disease. Nat Commun (2021) 12(1):1891. doi: 10.1038/s41467-021-22132-0
68. Saeed U. Contemplating potential chemical therapeutic options to combat marburg virus. Am J Biomed Sci Res (2021) 13(5):561–70. doi: 10.34297/AJBSR.2021.13.001915
69. Sami SA, Marma KKS, Mahmud S, Khan MAN, Albogami S, El-Shehawi AM, et al. Designing of a multi-epitope vaccine against the structural proteins of marburg virus exploiting the immunoinformatics approach. ACS Omega (2021) 6(47):32043–71. doi: 10.1021/acsomega.1c04817
70. Hasan M, Azim KF, Begum A, Khan NA, Shammi TS, Imran AS, et al. Vaccinomics strategy for developing a unique multi-epitope monovalent vaccine against marburg marburgvirus. Infection Genet Evol (2019) 70:140–57. doi: 10.1016/j.meegid.2019.03.003
71. Thi EP, Mire CE, Ursic-Bedoya R, Geisbert JB, H. Lee AC, Agans KN, et al. Marburg virus infection in nonhuman primates: Therapeutic treatment by lipid-encapsulated siRNA. Sci Trans Med (2014) 6(250). doi: 10.1126/scitranslmed.3009706
72. Warren TK, Whitehouse CA, Wells J, Welch L, Charleston JS, Heald A, et al. Delayed time-to-Treatment of an antisense morpholino oligomer is effective against lethal marburg virus infection in cynomolgus macaques. PLos Negl Trop Dis (2016) 10(2):e0004456. doi: 10.1371/journal.pntd.0004456
73. Iversen P, Warren T, Wells J, Garza N, Mourich D, Welch L, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and marburg virus infections. Viruses (2012) 4(11):2806–30. doi: 10.3390/v4112806
Keywords: marburg virus (MARV), marburg virus disease (MVD), hemorrhagic fever, bibliometric analysis, prevention and control, re-emerging zoonotic disease, vaccine, prevention and treatment
Citation: Islam MA, Adeiza SS, Amin MR, Kaifa FH, Lorenzo JM, Bhattacharya P and Dhama K (2023) A bibliometric study on Marburg virus research with prevention and control strategies. Front. Trop. Dis 3:1068364. doi: 10.3389/fitd.2022.1068364
Received: 12 October 2022; Accepted: 25 November 2022;
Published: 05 January 2023.
Edited by:
Somia Iqtadar, King Edward Medical University, PakistanReviewed by:
Pranab Kishor Mohapatra, C. V. Raman Global University, IndiaCopyright © 2023 Islam, Adeiza, Amin, Kaifa, Lorenzo, Bhattacharya and Dhama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Aminul Islam, YW1pbnVsbWJnQGdtYWlsLmNvbQ==; YW1pbnVsQHBhaG1jLmVkdS5jb20=; Prosun Bhattacharya, cHJvc3VuQGt0aC5zZQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.