- 1Department of Pharmacy, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates
- 2Division of Internal Medicine, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates
- 3Division of Tropical and Infectious Diseases, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates
- 4Division of General Paediatrics, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates
- 5Department of Molecular Biology and Genetics, Khalifa University, Abu Dhabi, United Arab Emirates
- 6Department of Pathology and Infectious Diseases, College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates
- 7Biotechnology Center, Khalifa University, Abu Dhabi, United Arab Emirates
Background: Antimicrobial stewardship (AMS) is a crucial tool for rationalizing the use of antimicrobial agents and reducing the burden of antimicrobial resistance. We aimed to assess the impact of AMS interventions on antimicrobial utilization and adherence to antimicrobial guidelines.
Methods: We conducted a prospective quasi-experimental study at a major tertiary hospital in the United Arab Emirates. Using standardized World Health Organization’s methodology, point-prevalence surveys (PPS) were performed in November 2019 and January 2022. Core AMS interventions consisted of proactive bloodstream infection service, proactive and reactive infectious diseases consult service, prospective audit and feedback by clinical pharmacists, development of antimicrobial guidelines based on cumulative antibiograms, and implementation of induction programs for new clinical staff. Days of therapy (DOT) per 1000 patient days present and rate of compliance with antimicrobial guidelines were compared before and after the core interventions. Multiple logistic regression analysis was carried out to adjust for the potential confounding effects of age, gender, hospitalization within 90 days, central or peripheral line insertion, urinary catheterization, and mechanical ventilation. P-value<0.05 was considered statistically significant.
Results: Pre- and post-intervention PPSs included 292 and 370 patients, respectively. Both had similar age and gender distribution. Patients receiving antimicrobials were 51% (149/292) in 2019 and 45% (166/370) in 2022 (p 0.12). Univariate analysis showed a reduced post-intervention DOT per 1000 patients present (6.1 +/- 16.2 vs 2.4 +/-5.1, p<0.01) and an improved post-intervention guideline compliance (59% vs 67%, p 0.23). Following multiple logistic regression, the reduction in post-intervention DOT remained statistically significant (co-efficient -0.17 (95% CI -8.58 to -1.94, p<0.01), and the improvement in guideline adherence became statistically significant (adjusted odds ratio 1.91 (95% CI 1.05 to 3.45, p 0.03).
Conclusion: Coordinated and sustained AMS interventions have a significant impact on improving antimicrobial utilisation and adherence to guidelines.
1. Introduction
The advent of antimicrobial agents has drastically altered daily medical practice. Antimicrobials are advanced medical treatments; therefore, once fatal infections are now treatable and even preventable (1).
The antimicrobial stewardship program (ASP) consists of well-studied measurements and interventions to facilitate the optimal use of antimicrobial agents, aiming to help clinicians improve clinical outcomes and optimize the appropriate use of antimicrobials while minimizing the harm caused by unnecessary or suboptimal use of antimicrobial therapy. Consequently, reducing microbial resistance and decreasing the spread of infections caused by multidrug-resistant organisms (2).
Multiple interventions are being carried out worldwide to improve antimicrobial stewardship, increase compliance and adherence to antibiotics usage, and improve clinical/patient outcomes. Implementing frequent audits and feedback to the prescriber (either by the clinical pharmacist or by infectious disease specialist) has proven to reduce the usage and duration of antibiotics (3). In addition, adopting preauthorization systems for dispensing restricted broad-spectrum antibiotics may help reduce the burden of antimicrobial resistance (4). Furthermore, clinical education is considered a cornerstone for any successful antimicrobial stewardship, and it was found that it can lower the annual antimicrobial prescriptions rates through training sessions or telephone consultation (5, 6). The ASP implements institution-specific guidelines for common infectious diseases and effectively facilitates proper antimicrobial prescription. They can aid in significantly increasing the use of appropriate initial antimicrobial agents, de-escalation of treatment, and shorter duration of antimicrobial therapy (2).
One of the established methodologies within antimicrobial stewardship programs to evaluate antimicrobial use at an institutional level is point prevalence surveys. Point Prevalence survey is a practical, standardized tool to measure hospital antimicrobial prescribing. It collects antimicrobial prescription data, reflects on the population receiving the antimicrobials, and defines the most common infections while conducting the survey (7, 8). Therefore, the barriers to enhancing the appropriate use of antimicrobials and decreasing microbial resistance can be identified through PPS to optimize the clinical outcomes (7–9).
A point prevalence survey was conducted before and after implementing ASP interventions to compare the study outcomes. The goals are to assess the impact of ASP team interventions on antimicrobial days of therapy and compliance with local antimicrobial guidelines at the hospital.
2. Methods
2.1. Setting
This study was conducted at Sheikh Shakhbout Medical City (SSMC), a 750-bed governmental tertiary hospital providing medical, surgical and ICU services.
2.2. Study design
We conducted a prospective non-randomised quasi-experimental study assessing antimicrobial stewardship program (ASP) interventions and their impact on key performance indicators (e.g., days of therapy and compliance with hospital antimicrobial guidelines) through point-prevalence surveys.
WHO provides a standardised methodology for point prevalence surveys to support hospitals worldwide in collecting antimicrobial use data to evaluate the impact of the local antimicrobial stewardship programmes (ASP) and facilitates comparisons of antibiotic use over time and between hospitals (10).
The same methodology was adopted at SSMC, with a baseline survey conducted in September 2019 and a follow-up survey completed in February 2022. The survey was conducted for three weeks for both periods, considering a set of variables defined in WHO PPS guidelines.
2.3. Inclusion and exclusion criteria
Patients who were eligible for the PPS include adult and paediatric patients hospitalised on the day of the survey whether they are receiving antibiotics or not, patients admitted to the ward before or at 8 a.m., patients who are on antimicrobials at 8 a.m. on the day of the survey, and patients who have been prescribed surgical antimicrobials prophylaxis before 8 a.m. on the day of the study. In addition, the survey covered all antimicrobials administered through oral, parenteral, rectal or through inhalation.
On the other hand, patients attending outpatient clinics, renal dialysis units, day-surgery wards, and the Emergency Department are excluded. Furthermore, antimicrobial orders initiated after 8 a.m. on the day of the survey, antimicrobial orders stopped before 8 a.m. on the survey day, and dosage forms including topical antibiotics (i.e. ear drops, eye drops, or vaginal suppositories) were precluded from the analysis.
2.4. Interventions
After the PPS survey was conducted in 2019, the ASP team performed multiple interventions to improve clinical outcomes. The ASP team consisted of infectious disease (ID) physicians, clinical pharmacists, Infection prevention and control (IPC) nurses, and physicians from different specialities. ASP interventions included:
2.4.1. Bacteraemia services
ID physicians proactively reviewed cases identified with positive cultures collected from sterile samples (blood) and consequently defined a treatment plan.
2.4.2. ASP stewardship queries
ID team initiated ID consult service where active on-call ID physicians received consultation over the phone for queries related to antimicrobials preauthorisation and consultation for complex cases.
2.4.3. Prospective audit and feedback by clinical pharmacists
Clinical pharmacists’ teams perform prospective audits for antimicrobials prescribed for patients in the assigned wards, they review cases for antimicrobials appropriateness and intervene with the primary team physicians, and the feedback is delivered directly to them.
2.4.4. Guidelines development and implementation
Local antimicrobial guidelines were developed and shared with the hospital staff. Common indications for antibiotic use have been included, e.g. community-acquired pneumonia, urinary tract infection, intra-abdominal infection, skin and soft tissue infection and surgical prophylaxis.
The recommendations reflected in the local guidelines were based on hospital treatment preferences, susceptibilities, formulary options, and patient mix.
2.4.5. ASP induction program for new joiners (physicians, pharmacists, nurses)
Education is a crucial component of comprehensive efforts to improve hospital antibiotic use. The induction module is an effective tool to introduce and reinforce antimicrobial stewardship program objectives, highlight the key performance indicators, and emphasise the roles of clinicians, nurses, and pharmacists.
2.4.6. ID rounds in critical care units
ID physicians conduct regular reviews of antimicrobial therapy in critical care areas and provide patient-specific recommendations to optimise antimicrobial therapy during face-to-face meetings with physicians and pharmacists based in intensive care units (ICU).
2.5. Ethical statement
The study was approved by the antimicrobial stewardship subcommittee and was registered as a quality improvement project with the quality department at SSMC. (Registration no. SSMC/CA/2022/002). Data were anonymised and de-identified to preserve patient confidentiality.
2.6. Data collection
The KoBo toolbox® was used to design a password-protected and standardised data collection tool to gather de-identified and anonymised demographic, clinical and antimicrobial information. The database included demographic data (e.g. age, gender), clinical data (e.g. recent hospital admission in 90 days, length of hospitalisation, surgical intervention, and presence of vascular, urinary or tracheal lines) and antimicrobial data (e.g. receipt of antimicrobial agent, indication, empirical/directed, dose, route of administration, duration, antimicrobial-related interventions, agent review status within 48-72 hours of administration, and local guideline compliance). In addition, detailed microbiological information (e.g. culture collection, type of specimen, pathogen, and antimicrobial susceptibility) was also collected.
2.7. Study outcomes
The study outcomes comprise days of antimicrobial therapy (DOT) and local antimicrobial guidelines compliance rate. Since it is a point prevalence survey, we couldn’t calculate the full antibiotic treatment days, however, DOT was calculated by counting the number of the days the patient had been on antimicrobial (s) until the date of the survey. If the patient was on more than one antimicrobial, the DOT for each antimicrobial will be summed to give one DOT product for all the antimicrobials that the patient had been on.:
The local antimicrobial guidelines compliance rate was calculated by dividing the number of patients whose antimicrobial therapy complied with hospital guidelines by the total number of patients on antibiotic therapy. The product was multiplied by 100 as per the following equation:
2.8. Statistical analysis
SPSS® version 26 has been used for data analysis. Patients’ baseline characteristics and clinical outcomes were summarised by means and standard deviations for continuous data, while frequencies and percentages summarised categorical variables. Chi-squared (χ2) test was used for comparing categorical variables and independent Sample t-test for comparing continuous variables. A p-value of< 0.05 was considered statistically significant. Outcomes with a p-value<0.25 were chosen for a backward multiple regression analysis adjusted for multiple infection risk factors to remove the effect of the possible confounders on the study outcomes. The square roots of continuous variables that were not normally distributed were used to meet the independent t-test and regression analysis assumptions of normality for the continuous variables.
3. Results
Patient’s baseline characteristics from pre- and post-intervention groups are shown in Table 1. The pre-intervention group (first group) had 292 patients, whereas the post-intervention group included 370 patients who were questioned following the intervention (second group). Both groups had a similar average age of 30.3 and male gender predominated.

Table 1 Patients’ characteristics, risk factors for infection and antimicrobial stewardship outcomes at baseline in 2019 and post-antimicrobial stewardship interventions in 2022.
During the pre-intervention stage of the research, the adult medical ward contributed the most (31%) patients to the study. While the adult surgical ward made the most contribution (35%) of patients to the research’s post-intervention phase.
Antimicrobials were given to 149 patients in the first group, with 219 antimicrobials, and 166 patients in the second group, with 215 antimicrobials. Central lines, peripheral lines, hospitalisation within 90 days, urine catheters, and mechanical ventilation were all risk factors for infection in both groups of patients (11–14) (Table 1).
As indicated in Table 1, the proportion of patients who were on antibiotics was 51% (149/292) for the pre-intervention group and 45% (166/370) for the post-intervention group. The mean DOT measured in the pre-intervention group was 6.1 days ( ± SD 16.2) while 2.4 days in the post-intervention group ( ± SD 5.1), and the difference was statistically significant (p< 0.01). Compliance with the hospital’s local antimicrobial guidelines was found to be 59% in the pre-intervention group compared to 67% in the post-intervention group, which is considered statistically insignificant (P= 0.230) (Table 2).
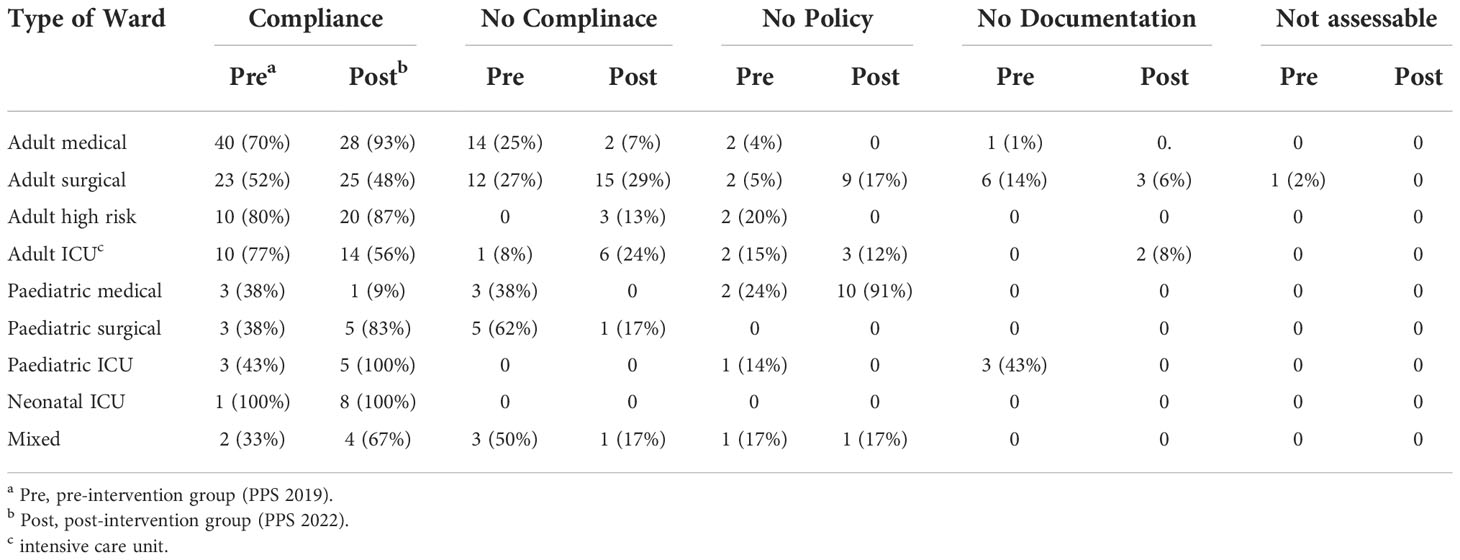
Table 2 Antimicrobial guide compliance rate comparison between pre and post-intervention groups distributed as per ward type.
Table 2 illustrates the compliance status to local hospital antimicrobial guidelines in each hospital unit. For adults, medical wards had the highest compliance rate compared to other wards. For paediatrics, there is a high percentages of ‘‘no policy’’ represented by 91% in the paediatric wards, Table 3.
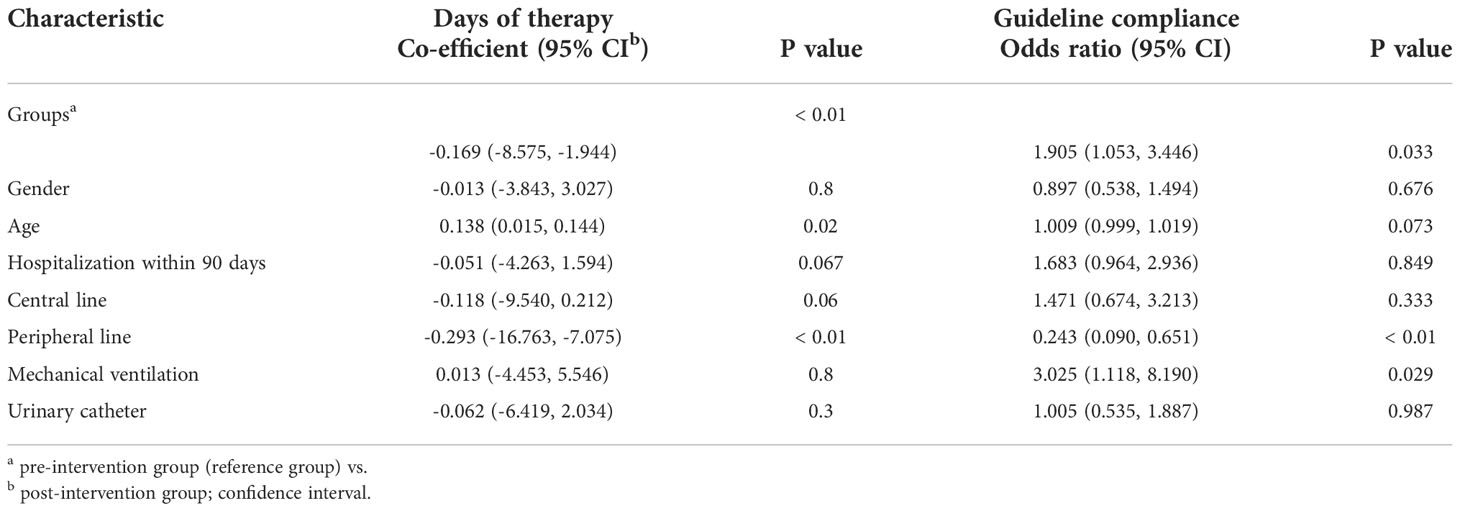
Table 3 Multiple regression analysis comparing Days of therapy and compliance with antimicrobial guidelines between the pre-intervention (PPS 2019) and post-intervention (PPS 2022) groups.
The results of the multiple regression analysis (adjusted for age; gender; the presence of central line, peripheral line and urinary catheter; mechanical ventilation; and 90-day hospitalisation) are illustrated in Table 3. The difference in the DOT between the first and second groups remained statistically significant (p< 0.01) with a negative co-efficient value (-0.169), indicating less DOT in the post-intervention group. On the other hand, there is a statistically significant improvement in compliance with the guidelines found in the post-intervention group (p= 0.033).
4. Discussion
Our findings show that adherence to SSMC Clinical Guidelines was improved in the post-intervention group, along with many factors that contributed to such improvement. The presence of an effective hospital antimicrobial stewardship program could be attributed to the successful implementation of guidelines and antibiotics policies, in addition to the quality improvement initiative projects conducted by ASP core members. This is in line with multiple recent studies (15–19).
The implementation of a wide range of ASP interventions between the two surveys resulted in a statistically significant decrease in the number of days of therapy in the post-intervention group. The rapid expansion of ID and clinical pharmacy services played a pivotal role in driving the successful implementation of ASP interventions. Of importance, the role of the clinical pharmacist intervention is always highlighted in improving compliance with hospital guidelines, improving antimicrobial stewardship outcomes, and reduction of days of therapy (20–22).
The observed prevalence of antibiotic use in the pre and post-intervention groups were higher than the findings of global PPS studies in countries like Northern Ireland (23), were the prevalence was 45%.Compared with low and middle income countries, including Brazil, Ghana, Uganda, Zambia, and Tanzania, overall prevalence was found to be approximately 50% (24, 25).
Numerous metrics are used to track antimicrobial use and ASP efficacy, but there is no consensus on which metric is preferred. DOT/1000 patient day metric was chosen for this study because, compared to other metrics, it offers the best balance of feasibility and applicability. Furthermore, adding patient days to the denominator helps to compare the impact of the interventions within the same facility over time (26, 27).
The results of our study are aligned with other antimicrobial stewardship programmes that evaluated the impact of ASP and showed that the interventions were associated with a shorter duration of antibiotic therapy and less inappropriate antimicrobial use (19, 28).
The lack of guidance on using antibiotics in paediatric services needs to be addressed. Our results identified areas of potential improvement for appropriate prescribing of antibiotics for paediatric patients (Table 2), highlighting the need to implement more guidelines and policies in the paediatric wards. Repeated PPS needs to be part of the paediatric antibiotic stewardship strategy to identify prescribing trends over time and evaluate the efficacy of ASP initiatives in the paediatric department.
“Our second point prevalence survey was conducted during the coronavirus disease 2019 (COVID-19) pandemic, a time of profound hardship and stress on the healthcare system, promoting emergency measures such as early hospital discharge and community quarantine. Therefore, it is plausible that such interventions might have influenced the DOT estimate of the second survey. However, the formula we used to compute the DOT in this study utilized the number of days spent receiving antibiotics until the survey date rather than the discharge date. Therefore, we are confident that COVID-19 discharge interventions did not confound our DOT estimate.”
This study has some limitations. First, the PPS study design is restricted to assessing only inpatient antibiotic use, though it is our targeted setting in this study; consequently, the antibiotics used in outpatient clinics were not reviewed. However, we plan to expand the audit to cover the outpatient setting using an appropriate auditing tool. Second, the point-in-time nature of the PPS design further limits insight into seasonal patterns in antibiotic use, but it still can give a clue about the practices and habits. Third, the two surveys occurred in two different seasons, which likely has the potential to skew antimicrobial use results. The survey conducted during the Post-intervention period included winter months when antimicrobial use would be expected to be higher than in the summer. Last, our study followed a non-randomised design and, as per WHO PPS methodology, all admitted patients should be surveyed.
In conclusion. Several areas of practice deserve specific attention to optimize the prudent use of antimicrobial in the hospital. Future Antimicrobial stewardship initiatives should be directed towards updating paediatric infectious disease guidelines and to follow any deviation of therapy particularly in the use of broad-spectrum, non–oral antimicrobials, and surgical prophylaxis practices. There is an opportunity to enhance quality in documenting indications and reporting a stop/review date.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Study group members
Marleine Pierre Bejjani Moukarze, Department of Pharmacy, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates; Rizwan Ali, Department of Pharmacy, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates; Prameela Maniamma, Infection Prevention and Control, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates; Fouzia Jabeen, Microbiology department, Union 71 laboratory, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates; Ramesh Ganeshan, Division of Tropical and Infectious Diseases, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates; Ahmed Al Messabi, Division of Tropical and Infectious Diseases, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates; Robert Serafino Wani, Division of Tropical and Infectious Diseases, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates; Emmanuel Nsutebu, Division of Tropical and Infectious Diseases, Sheikh Shakhbout Medical City in partnership with Mayo Clinic, Abu Dhabi, United Arab Emirates.
Author contributions
JS, ZB contributed to the conception and design of the study. JS, AS, ME, KY, RAK, LY, ME, RE, RT, IE, AE, AA, NA, MA, RA, AA, NA, AA contributed to the data collection. AS, JS, ZB, ME performed data analysis JS, AS, ZB, SO’S, DE reviewed the drafts. MM, RA, PM, FJ, RG, AM, RW, EN Part of antimicrobial stewardship study group. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hwang S, Kwon KT. Core elements for successful implementation of antimicrobial stewardship programs. Infection & chemotherapy. (2021) 53(3):421.
2. Abbo LM, MacDougall C, Schuetz AN, Septimus EJ. Implementing an antibiotic stewardship program: Guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis (2016) 62:e51. doi: 10.1093/cid/ciw118
3. Broom J, Broom A, Plage S, Adams S, Adams K, Post JJ, et al. Antimicrobial stewardship interventions: a practical guide. World Health Organization (2021) V96(4):266–80. doi: 10.2471/BLT.17.20348
4. Schuts E, Boyd A, Muller A, Mouton J, Prins J. The effect of antibiotic restriction programs on prevalence of antimicrobial resistance: A systematic review and meta-analysis. Open Forum Infect Dis. (2021) V8(4). doi: 10.1093/ofid/ofab070
5. Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clinic Proc (2011) 86(11):1113– 23. doi: 10.4065/mcp.2011.0358
6. World Health Organization, Regional Office for Europe.Antimicrobial stewardship interventions: a practical guide. World Health Organization. Regional Office for Europe (2021). https://apps.who.int/iris/handle/10665/340709
7. Vandal E, Latour K, Goossens H, Magerman K, Drapier N, Catry B, et al. Point prevalence survey of antimicrobial use and healthcare-associated infections in Belgian acute care hospitals: results of the global-PPS and ECDC-PPS 2017. Antimicrob Resist Infect Control (2020) 9:13. doi: 10.1186/s13756-019-0663-7
8. Guterres H, Nelwan E, Chen LK, Nugroho P. Point prevalence survey of antibiotics use among inpatient in national referral hospital in Indonesia. Int J Infect Diseases. (2020) 101:93. doi: 10.1016/j.ijid.2020.09.267
9. Pauwels I, Versporten A, Vermeulen H, Vlieghe E, Goossens H. Assessing the impact of the global point prevalence survey of antimicrobial consumption and resistance (Global-PPS) on hospital antimicrobial stewardship programmes: results of a worldwide survey. Antimicrob Resist Infect Control (2021) 10:138. doi: 10.1186/s13756-021-01010-w
10. World Health Organization. WHO methodology for point prevalence survey on antibiotic use in hospitals. World Health Organization (2018).
11. Miller A, Vujcich E, Brown J. Effect of central line duration and other risk factors on central line-associated bloodstream infection in severe adult burns patients at a Large tertiary referral burns centre: A 5-year retrospective study. Eur Burn J (2022) 3(1):18–26. doi: 10.3390/ebj3010003
12. Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect diseases. (2018) 66(7):1004–12. doi: 10.1093/cid/cix947
13. Letica-Kriegel AS, Salmasian H, Vawdrey DK, Youngerman BE, Green RA, Furuya EY, et al. Identifying the risk factors for catheter-associated urinary tract infections: a large cross-sectional study of six hospitals. BMJ Open (2019) 9(2):e022137. doi: 10.1136/bmjopen-2018-022137
14. Wu D, Wu C, Zhang S, Zhong Y. Risk factors of ventilator-associated pneumonia in critically III patients. Front Pharmacol (2019) 10:482. doi: 10.3389/fphar.2019.00482
15. Stenehjem E, Hersh AL, Buckel WR, Jones P, Sheng X, Evans RS, et al. Impact of implementing antibiotic stewardship programs in 15 small hospitals: a cluster-randomized intervention. Clin Infect Diseases. (2018) 67(4):525–32. doi: 10.1093/cid/ciy155
16. Shirazi OU, Ab Rahman NS, Zin CS. An overview of the hospitals’ antimicrobial stewardship programs implemented to improve antibiotics’ utilization, cost and resistance patterns. J Pharmacy. (2022) 2(1):16–30. doi: 10.31436/jop.v2i1.76
17. May L, Nguyen MH, Trajano R, Tancredi D, Aliyev ER, Mooso B, et al. A multifaceted intervention improves antibiotic stewardship for skin and soft tissues infections. Am J Emergency Med (2021) 46:374–81. doi: 10.1016/j.ajem.2020.10.017
18. Ahmed NJ, Almalki ZS, Alfaifi AA, Alshehri AM, Alahmari AK, Elazab E, et al. Implementing an antimicrobial stewardship programme to improve adherence to a perioperative prophylaxis guideline. Healthcare (2022) 10(3):464. doi: 10.3390/healthcare10030464
19. Mandelli G, Dore F, Langer M, Garbero E, Alagna L, Bianchin A, et al. Effectiveness of a multifaced antibiotic stewardship program: A pre-post study in seven Italian ICUs. J Clin Med (2022) 11(15):4409. doi: 10.3390/jcm11154409
20. Mas-Morey P, Valle M. A systematic review of inpatient antimicrobial stewardship programmes involving clinical pharmacists in small-to-medium-sized hospitals. Eur J Hosp. Pharm (2018) 25(e1):e69–73. doi: 10.1136/ejhpharm-2017-001381
21. Fukuda T, Tanuma K, Jio S, Saito J, Komura M, Yamatani A, et al. Impact of a pharmacist-led antimicrobial stewardship program on the number of days of antimicrobial therapy for uncomplicated gram-negative bacteraemia in a community hospital. Cureus (2021) 13(4):e14635. doi: 10.7759/cureus.14635
22. Kim YC, Kim EJ, Heo JY, Choi YH, Ahn JY, Jeong SJ, et al. Impact of an infectious disease specialist on an antimicrobial stewardship program at a resource-limited, non-academic community hospital in Korea. J Clin Med (2019) 8(9):1293. doi: 10.3390/jcm8091293
23. Elhajji FD, Al-Taani GM, Anani L, Al-Masri S, Abdalaziz H, Qabba’H SH, et al. Comparative point prevalence survey of antimicrobial consumption between a hospital in northern Ireland and a hospital in Jordan. BMC Health Serv. Res (2018) 18:849. doi: 10.1186/s12913-018-3656-y
24. Porto A, Goossens H, Versporten A, Costa S, Brazilian Global-PPS Working Group. Global point prevalence survey of antimicrobial consumption in Brazilian hospitals. J Hosp. Infect (2020) 104:165–71. doi: 10.1016/j.jhin.2019.10.016
25. D’Arcy N, Ashiru-Oredope D, Olaoye O, Afriyie D, Akello Z, Ankrah D, et al. Antibiotic prescribing patterns in Ghana, Uganda, Zambia and Tanzania hospitals: Results from the global point prevalence. survey (G-PPS) on antimicrobial use and stewardship interventions implemented. Antibiotics (2021) 10:1122. doi: 10.3390/antibiotics10091122
26. Momattin H, Al-Ali AY, Mohammed K, Al-Tawfiq JA. Benchmarking of antibiotic usage: an adjustment to reflect antibiotic stewardship program outcome in a hospital in Saudi Arabia. J Infection Public Health (2018) 11(3):310–3. doi: 10.1016/j.jiph.2017.08.008
27. Moehring RW, Anderson DJ, Cochran RL, Hicks LA, Srinivasan A, Dodds Ashley ES. Structured taskforce of experts working at reliable standards for stewardship (STEWARDS) panel. expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Diseases. (2017) 64(3):377–83. doi: 10.1093/cid/ciw787
Keywords: antimicrobial stewardship, antimicrobial utilization, antimicrobial guidelines, days of therapy, antimicrobial stewardship (AMS)
Citation: Shamseddine J, Sadeq A, Yousuf K, Abukhater R, Yahya LO, Espil MA, Hassan ME, Fadl RE, Ahmed RTE, Elkonaissi I, Abdelsalam AE, Naqbi AA, Nuaimi NA, Hosani MA, Marri RA, Abdouli AA, Alakhras AM, Masri NIA, O’Sullivan S, Everett D and Babiker ZOE (2023) Impact of antimicrobial stewardship interventions on days of therapy and guideline adherence: A comparative point-prevalence survey assessment. Front. Trop. Dis 3:1050344. doi: 10.3389/fitd.2022.1050344
Received: 21 September 2022; Accepted: 28 November 2022;
Published: 23 January 2023.
Edited by:
Gavin Barlow, Hull York Medical School, United KingdomReviewed by:
Veranja Chathurani Liyanapathirana, University of Peradeniya, Sri LankaLinus Olson, Karolinska Institutet (KI), Sweden
Copyright © 2023 Shamseddine, Sadeq, Yousuf, Abukhater, Yahya, Espil, Hassan, Fadl, Ahmed, Elkonaissi, Abdelsalam, Naqbi, Nuaimi, Hosani, Marri, Abdouli, Alakhras, Masri, O’Sullivan, Everett and Babiker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinan Shamseddine, jshamseddine@ssmc.ae
†These authors share first authorship
‡These authors share last authorship
 Jinan Shamseddine
Jinan Shamseddine Ahmed Sadeq1†
Ahmed Sadeq1† Siobhan O’Sullivan
Siobhan O’Sullivan Dean Everett
Dean Everett Zahir Osman Eltahir Babiker
Zahir Osman Eltahir Babiker