
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 09 January 2023
Sec. Neglected Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.1016176
This article is part of the Research Topic Diagnostics for Neglected Tropical Diseases View all 5 articles
 Glory Ngongeh Amambo1,2
Glory Ngongeh Amambo1,2 Ngong Innocentia1,2
Ngong Innocentia1,2 Raphael Awah Abong1,2
Raphael Awah Abong1,2 Fanny Fri Fombad1,2
Fanny Fri Fombad1,2 Abdel Jelil Njouendou1,3
Abdel Jelil Njouendou1,3 Franck Nietcho1
Franck Nietcho1 Relindis Ekanya1,2
Relindis Ekanya1,2 Chi Anizette Kien1,2
Chi Anizette Kien1,2 Rene Ebai1,2
Rene Ebai1,2 Benjamin Lenz4
Benjamin Lenz4 Manuel Ritter4
Manuel Ritter4 Mathias Eyong Esum1,2
Mathias Eyong Esum1,2 Kebede Deribe5,6
Kebede Deribe5,6 Jerome Fru Cho1,2
Jerome Fru Cho1,2 Amuam Andrew Beng1,2
Amuam Andrew Beng1,2 Peter Ivo Enyong1,2
Peter Ivo Enyong1,2 Zhiru Li7
Zhiru Li7 Marc P. Hübner4,8
Marc P. Hübner4,8 Kenneth Pfarr4,8*
Kenneth Pfarr4,8* Achim Hoerauf4,8,9
Achim Hoerauf4,8,9 Clotilde Carlow7
Clotilde Carlow7 Samuel Wanji1,2*
Samuel Wanji1,2*Conventional diagnosis of filarial infections is based on morphological identification of microfilariae using light microscopy and requires considerable expertise, is time-consuming, and can be subjective. Loop-mediated isothermal amplification (LAMP) has advantages over microscopy or PCR because of its operational simplicity, rapidity and versatility of readout options. LAMP assays represent a major step forward in improved filarial diagnostic tools suitable for low resource settings and field applicability. The study goal was to retrospectively evaluate the performance and suitability of the O-150, RF4, and Mp419 LAMP assays for diagnosing Onchocerca volvulus, Loa loa and Mansonella perstans infections, respectively, in humans and vectors under experimental and natural field conditions. Surveys were conducted in four health districts of Cameroon using skin snip and thick blood film methods to detect skin (O. volvulus) and blood (L. loa and M. perstans) dwelling microfilaria in humans. Engorged vectors (Simulium spp., Chrysops spp., and Culicoides spp.) were evaluated by LAMP. Dissected, wild-caught vectors were also analyzed. LAMP showed a prevalence of 40.4% (O. volvulus), 17.8% (L. loa) and 36.6% (M. perstans) versus 20.6% (O. volvulus), 17.4% (L. loa) and 33.8% (M. perstans) with microscopy. Simulium spp. were dissected for microscopy and pooled for LAMP. The O-150 LAMP assay infection rate was 4.3% versus 4.1% by microscopy. Chrysops spp. were dissected and analyzed individually in the LAMP assay. The RF4 LAMP assay infection rate was 23.5% versus 3.3% with microscopy. The RF4 LAMP assay also detected parasites in Chrysops spp. fed on low microfilaremic volunteers. The Mp419 LAMP assay infection rate was 0.2% for C. milnei and 0.04% for C. grahamii, while three other species were LAMP-negative. The sensitivity, species specificity, rapidity and ease of its use of these filarial LAMP assays, and validation of their performance in the field support use as alternatives to microscopy as diagnostic and surveillance tools in global health programs aimed to eliminate onchocerciasis.
Onchocerciasis, a.k.a. river blindness, is a debilitating vector-borne (Simulium spp.) disease caused by Onchocerca. volvulus (1, 2). The disease can present as dermatitis, skin nodules and ocular lesions, resulting from the migration of the first stage larvae (microfilariae, mf) through the skin and eyes, leading to an intense, papular dermatitis associated, itch and/or even severe ocular damage that can result in blindness (2). The disease is endemic in Africa, Yemen and Latin America; affecting over 37 million people of which 99% live in Africa (1). The mass drug administration (MDA) programs to control onchocerciasis have implemented community-wide treatment of the endemic populations with ivermectin (CDTI). However, despite more than 20 years of CDTI, onchocerciasis is still a major public health and socioeconomic problem for several endemic countries, including Cameroon (1).
In many areas, onchocerciasis MDA programs have run for several years and are entering a period where important decisions as to their continuation need to be made. In order to evaluate their success, certify elimination and guide the decision to stop MDA, careful monitoring of infection in human hosts and vectors with sensitive and specific diagnostic tools is required. Conventionally, diagnosis is based on identification of microfilariae (mf, first stage larvae) in skin biopsies (onchocerciasis) or blood (for the related co-endemic infections mansonelliosis and loiasis) as well as insect vectors using light microscopy (1). To achieve the World Health Organization goal of interrupting transmission of onchocerciasis in support of the UN Sustainable Development Goals (1), MDA should be extended to all endemic regions, including those that have been exempted due to co-endemicity with loiasis. The latter requires new diagnostics that can rapidly identify and differentiate co-endemic filarial nematode infections, e.g., Loa loa or Mansonella perstans.
The filarial worm L. Loa (African eye worm) causes loiasis and can be co-endemic with onchocerciasis in Cameroon, Central African Republic, Democratic Republic of Congo, Gabon, Nigeria, and the Republic of Congo, (3). The parasite is transmitted to humans by the tabanid flies Chrysops silacea and C. dimidiate (4, 5) in rainforest and savannah areas of Central and Western Africa (3, 6); where circa 3-13 million people are affected (7). Despite transient, localized angioedema and sub-conjunctival migration of the adult worm (8), L. loa infection has been considered a benign condition. However, ivermectin treatment of persons infected/co-infected with L. loa, especially those with high microfilarial loads (>30,000 mf per milliliter, mf/mL), may experience severe adverse events (encephalopathy) (9–11). Hence, MDA cannot be safely carried out in these areas of co-endemicity (12–14).
Mansonellosis is a filarial infection caused by M. perstans, Mansonella. sp. DEUX, M. ozzardi, M. streptocerca and M. rodhaini. M. perstans has the widest geographic distribution; 33 countries in sub-Saharan Africa and in South America (15–17). Mansonellosis is probably the most prevalent of all the human filarial infections with prevalence rates of 3–96% (15, 17, 18), nevertheless, there are no MDA programs for this infection. An estimated 114 million people in Africa are infected with M. perstans and 580 million are at risk. Infected individuals may suffer non-specific symptoms of infection: chronic pruritis, fever, headache, joint pain, lymphadenopathy, and rashes (15), while some may also experience ocular lesions due to adult M. perstans in the eyes (19). Mansonellosis, particularly due to M. perstans, may down regulate immune responses (20), enhancing susceptibility to other infections such as tuberculosis and malaria (21), as well as negatively affecting the efficacy of vaccines (17).
Mansonella spp. infection lack specific immunodiagnostic tests (17), but there are several immunological methods for detection of either antibody or antigen for L. loa (22) and O. volvulus (2). However, low levels of sensitivity (23) and cross-reactivity (24–26) have been reported. Monitoring infections in the vectors is essential for assessing transmission and recrudescence (27). Vector transmission assessments requires microscopic identification of parasites in captured vectors; requiring expertise to morphologically identify the parasites and is a slow process that is not practical for large-scale surveys. Nucleic acid-based assays provide alternative assays with higher sensitivity than parasitological or immunological methods that can be used on samples from humans and vectors.
Polymerase chain reaction (PCR)-based methods have been developed to detect Mansonella spp. (28–30), but do not distinguish M. perstans from M. ozzardi without an additional step of sequencing the amplicon (29), L. loa (29, 31), and O. volvulus (2, 32, 33). Generally, PCR assays are more sensitive than the conventional parasitological technique, yet they require trained personnel and relatively expensive equipment; which can limit their widespread use (34). A simpler method that circumvents some of the current limitations to specifically diagnose filarial infections in humans and vectors would overcome the technical barriers of in field or low-resource settings,
Loop-mediated isothermal amplification (LAMP) is a simple to perform alternative to PCR that does not require expensive equipment and offers a colorimetric readout particularly suitable for low resource settings (35–37). In addition, LAMP is not as sensitive to inhibitors commonly found in clinical specimens and insects that inhibit the Taq polymerases used in PCR (38). Our consortium has developed highly specific and sensitive LAMP assays for L. loa (36), O. volvulus (39), and M. perstans (40). The present study evaluated the performance and suitability of the O. volvulus O-150, L. loa RF4 and M. perstans Mp419 LAMP assays for the detection of these filarial infections in humans and different vector species under experimental and field conditions.
For O. volvulus infections, a total of 670 skin snips samples were collected from four health districts (HD) in the Littoral region of Cameroon that were subjected to microscopy and the O-150 LAMP (Table 1). Overall, 138 samples (20.6%) were positive by microscopy, while 271 samples (40.4%) were positive by LAMP. The proportion of microfilaridermia positivity detected by the LAMP assay was significantly higher than by microscopy in all HDs.
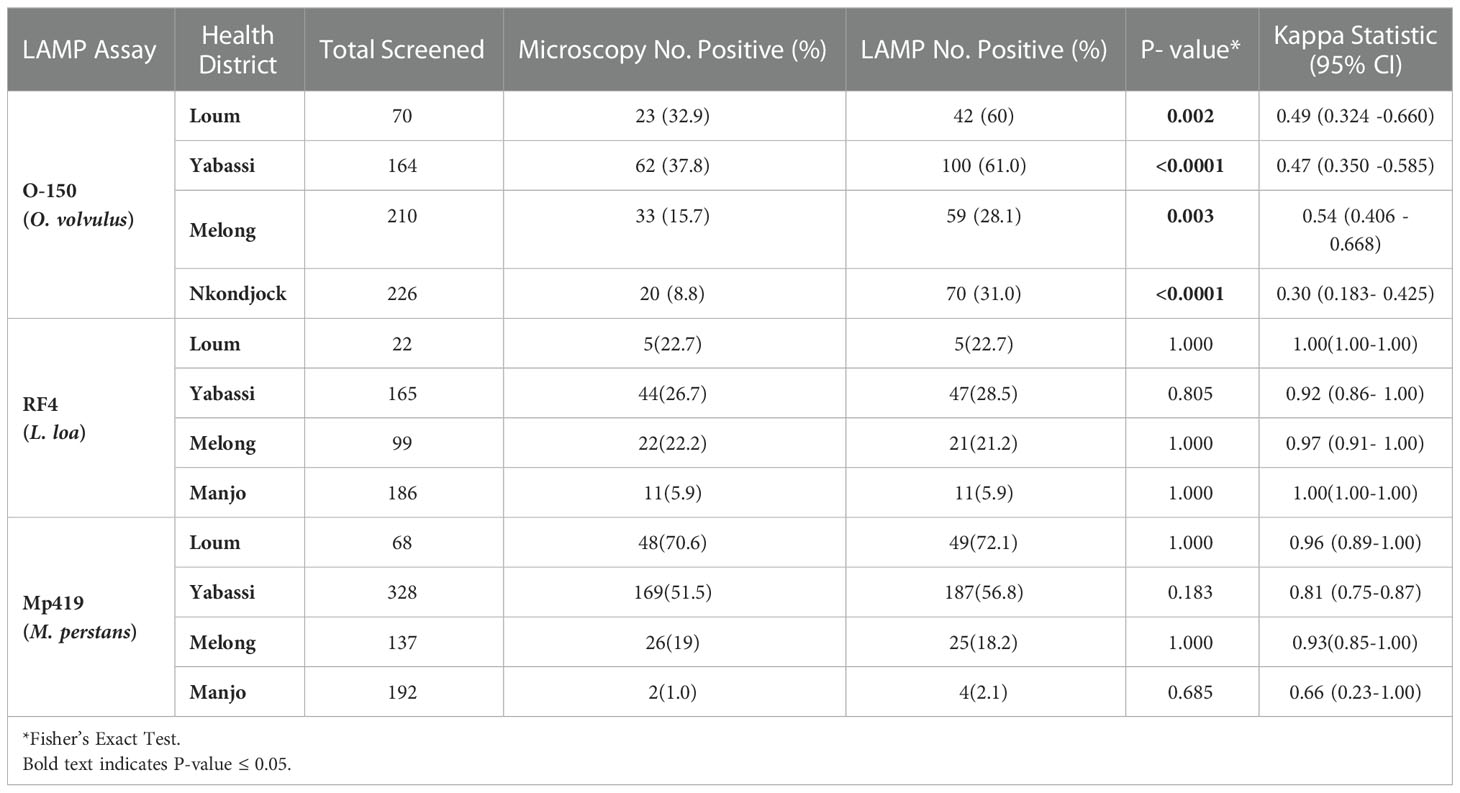
Table 1 Prevalence of O. volvulus, L. loa, and M. perstans infections in the health districts as determined by microscopy and LAMP assays.
For L. loa infections, 472 blood samples were collected from 4 HDs of the Littoral region of Cameroon and analyzed by the RF4 LAMP assay (Table 1). Overall, 82 samples (17.4%) were positive by microscopy, while 84 samples (17.8%) were positive by LAMP. The number of samples scored positive by LAMP assay were similar to those scored by microscopy.
For M. perstans infections, 725 human blood samples from participants in 4 HDs of the Littoral regions of Cameroon were examined for M. perstans infection using microscopy and the Mp419 LAMP assay (Table 1). Overall, 245 samples (33.8%) were positive by microscopy, while 265 samples (36.5%) were positive by LAMP. The number of samples scored positive by LAMP assay were similar to those scored by microscopy.
The accuracy (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and the Kappa index) of all three LAMP assays using microscopy as reference diagnostic method were calculated (Table 2). The O-150 LAMP assay detected 133 more O. volvulus positive skin snips than microscopy. The RF4 LAMP detected 80/82 microscopy positive samples; 4 microscopy negative samples were RF4 LAMP-positive and 2 microscopy positive samples were negative. The Mp419 LAMP assay detected 28 more positive samples than microscopy; 7 microscopy-positive samples were negative by the LAMP assay.
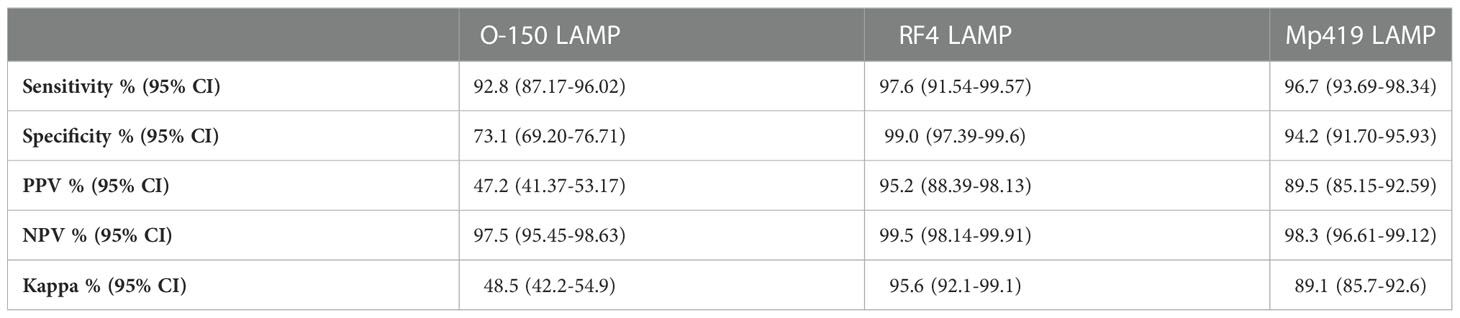
Table 2 Performance characteristics of the three LAMP methods evaluated using microscopy as reference method.
All three LAMP assays had higher sensitivity and specificity than microscopy. The RF4 and Mp419 LAMP assays had high PPV, while O-150 was intermediate; however, these findings need to be discussed against the fact that mf microscopy in onchocerciasis has a low sensitivity, in particular in hypoendemic settings (see discussion), and a latent class analysis would be helpful to determine the “true” (=higher) prevalence values (41). The NPV for all three LAMP assays was high. The Kappa indices of the RF4 and Mp419 LAMP assays indicated that the LAMP assays are equivalent to microscopy in these endemic settings. As the O-150 LAMP assay was more sensitive than microscopy, the Kappa index was, as expected, lower.
A total of 16 pools (2 flies/pool) of flies that had fed on a volunteer (mf load 50 mf/mL) were analyzed using the colorimetric O-150 LAMP assay. Positive (O. volvulus DNA) and negative (or molecular biology grade H2O) control samples were included. Of the 16 pools, which consisted of 32 engorged flies, all 16 (100%) were positive by the LAMP assay.
Equal numbers (18) of Chrysops flies that had fed on volunteers with low (<10 mf/mL) or high (>30,000 mf/mL) mf load were evaluated with the colorimetric RF4 LAMP assay. Positive (L. loa DNA) and negative (molecular biology grade H2O) control samples were included. Of the 18 flies that had fed on the volunteer with low parasitemia, 88.9% were positive, while 94.4% of the 18 flies that had fed on the volunteer with high parasitemia were positive (Table 3). Detection of infection in flies that fed on the volunteer with low parasitemia was limited to the abdomen up to 7 days post infection, while from day 10 post infection, parasites were also detected in the head and thorax. However, in flies fed on a volunteer with high parasitemia, the LAMP assay detected infection at all-time points in all parts of the flies.
Culicoides spp. which were fed on two volunteers with microfilarial loads of 80,000 mf/mL and 3,000 mf/mL, respectively, were evaluated using the colorimetric Mp419 LAMP assay. Of the 10 pools comprised of flies which had fed on a volunteer with 3,000 mf/mL, 4 were positive (40%), while 11 of 12 pools (91.7%) scored positive after feeding on a volunteer with 80,000 mf/mL.
Of the 22,274 black flies (S. damnosum) collected, 9,134 were dissected to compute the entomological indices and the remaining flies were stored in 80% ethanol (Supplementary Table S1). Of the flies stored in ethanol, 12,900 were divided into 129 pools for DNA extraction and LAMP assay. By microscopy, a parity of 24.7% (2,257/9,134), infection rate of 41.2/1,000 parous flies and an infective rate of 11.0/1,000 parous flies were observed (Table 4).
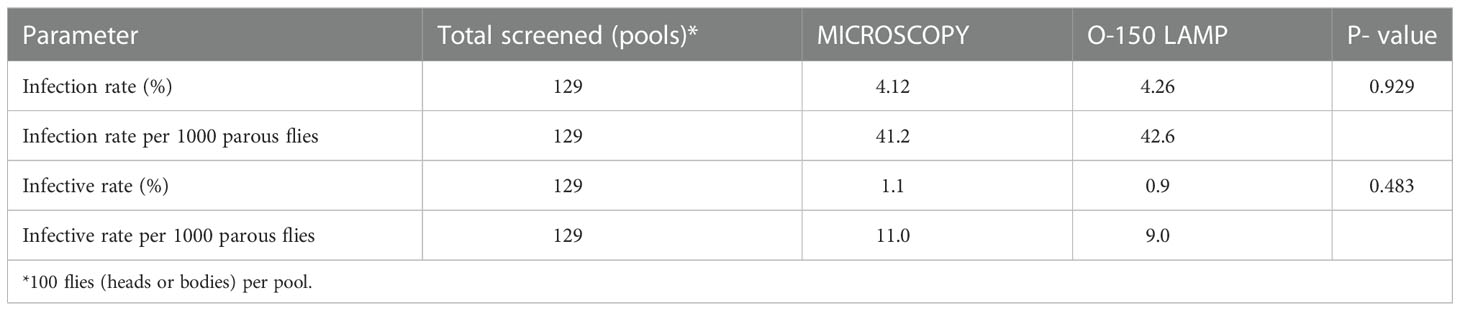
Table 4 O. volvulus infection rates in wild-caught Simulium flies as determined by microscopy and O-150 LAMP.
A total of 258 sub pools (129 head pools and 129 body pools) were analyzed with the LAMP assay. As per the World Health Organization (42), we assumed a parity of 50% in the fly pools to calculate infection/infective rates per 1,000 parous flies. Out of 129 head pools, 47 (36.4%) were positive, while 114 (88.4%) were positive for either body or head infection or both. The calculated infection and infective rates for the O-150 LAMP were 42.6 (n = 129 pools of 100 flies with 15 negative pools) and 9.0 (n = 129 pools of 100 flies with 82 negative pools) per 1,000 parous flies, respectively (Table 4).
A total of 7,841 wild-caught Chrysops dissected and examined by microscopy (Supplementary Table S2), 257 (3.3%) were infected with L. loa (Table 5). The non-MDA site Batouri HD had the highest infection rate of 4.4% (103/2365). Among the HDs receiving MDA, the infection rate ranged from 10/812 (1.2%) in South West 2 to 3.5% (17/485) in North West.
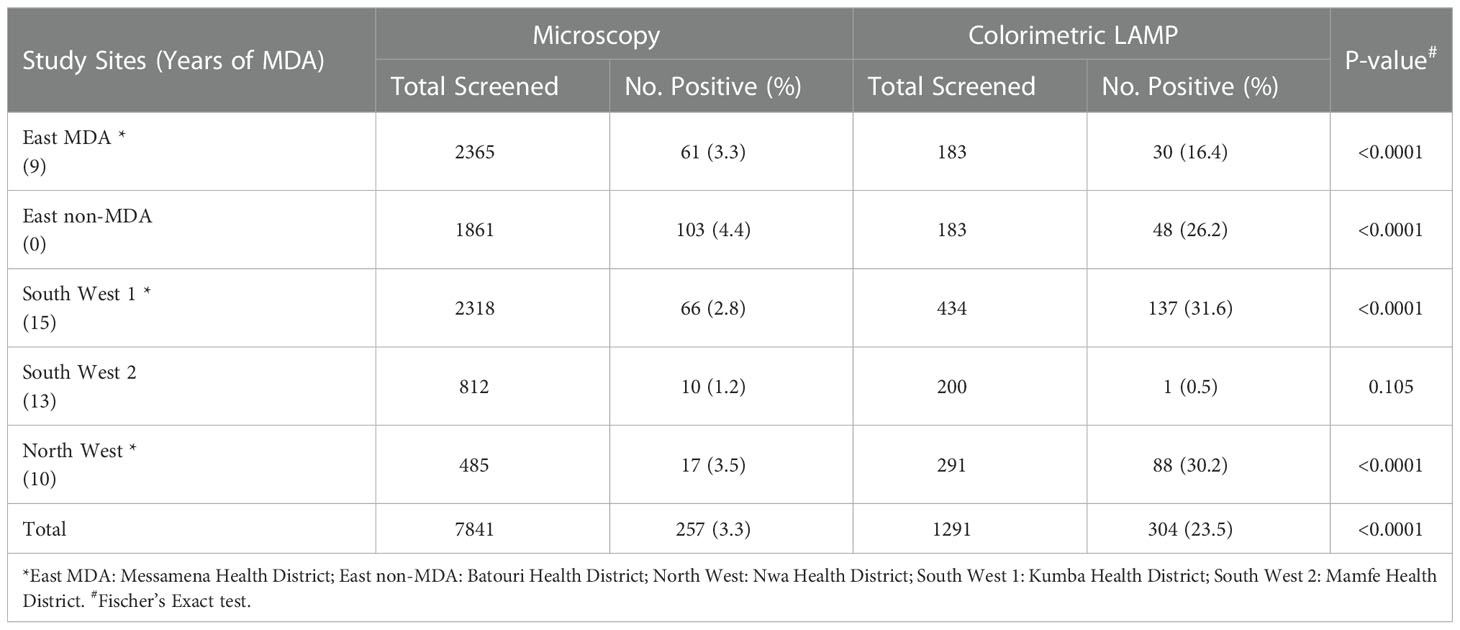
Table 5 Natural infection rates of wild-caught Chrysops in various study sites determined by microscopy and LAMP methods.
The RF4 LAMP assay using DNA extracted from 1291 wild-caught Chrysops flies identified 304 positive flies and an overall infection of 23.5% (Table 5). In the non-MDA site, the infection rate was 26.2% (48/183). Similar levels of infection of 30.2% (88/129) and 31.6% (138/434) were observed in the North West and SW 1 sites. In the Eastern MDA site (Messamena HD) and South West 2 CDTI site, the infection rates were lower; 16.5% (30/183) and 0.5% (1/200), respectively. The RF4 colorimetric LAMP assay was significantly more sensitive than microscopy in detecting L. loa infection in wild-caught Chrysops (P<0.001) (Table 5, with the exception of the South West 2 CDTI project site where the infection rate detected by the two methods was low (P = 0.105).
Wild-caught Culicoides spp. were identified morphologically and then divided into pools for DNA extraction and pool screening with the Mp419 LAMP assay: C. milnei, 181 pools of 10 flies/pool; C. grahamii, 225 pools of 50 flies/pool; C. fulvithorax, 51 pools of 50 flies/pool; C. innornatipennis, 2 pools of 50 flies/pool; and C. neavei, 1 pool of 50 flies. Infection was found only in C. milnei (0.2%) and C. grahamii (0.04%) flies (Table 6).
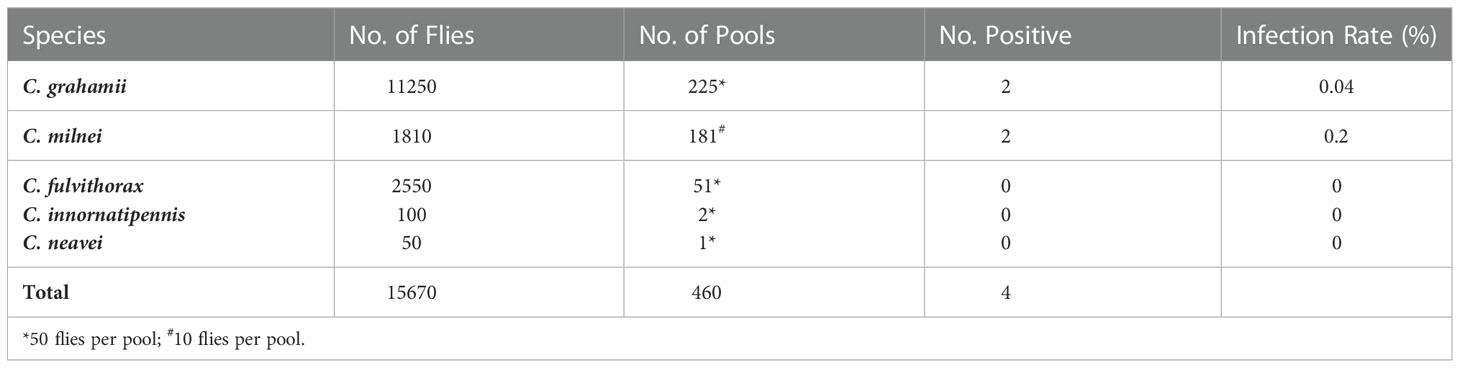
Table 6 Natural infection rates in different species of wild-caught Culicoides spp. determined by Mp419 LAMP.
Sensitive and specific diagnostics are needed for assessing pathogen population changes in both human and vector hosts so that MDA interventions can be certified for achieving elimination goals. However, when prevalence is reduced due to successful control measures, e.g., MDA, monitoring of parasites in human or vector samples by conventional techniques, i.e., microscopy, can be challenging. Recently, a panel of new highly sensitive and rapid LAMP tests with a simple color readout have become available for the specific detection of O. volvulus (39), L. loa (43) and M. perstans (40). The goal of this study was to perform a comprehensive evaluation of the performance of these promising methods by testing samples collected from humans and the filarial nematode vectors.
The O-150 colorimetric LAMP assay was used in comparison to microscopy to identify O. volvulus infected individuals from different study sites. In each geographic location, significantly higher prevalences were detected using O-150 LAMP. Yabassi, Loum, and Nkondjock HDs recorded the highest prevalence with LAMP (60.4%, 60.0%, and 31.0%, respectively) while the Melong HD recorded the lowest prevalence of 28.1%. The greater number of positives using LAMP is consistent with the greater sensitivity of LAMP previously reported in populations that have received MDA in which the low sensitivity of microscopy was exacerbated (39, 44). This is also consistent with our observation of far greater number of LAMP positives in the Nkondjock HD, an area of low prevalence resulting from MDA. A study to model the sensitivity of the skin snip method showed that the degree of aggregation of mf in skin increases as worm burden declines (45). As such, it is expected that in settings of hypoendemicity, mf are more aggregated and skin snips becomes less sensitive (46, 47). This explains why skin snipping may fail to diagnose light infections as the chance that a snip contains few mf is increased. Interestingly, we also observed a few samples (10) that were positive for microscopy but negative for LAMP. It is possible that these mf are a different filarial species (e.g., M. streptocerca) as demonstrated in other studies (17), and highlights the importance of having a highly specific molecular test for skin snip analyses. The “true” prevalence might be better defined by latent class analysis (41), as it may be closer to the LAMP results; this is being done currently.
To further evaluate the suitability of the LAMP assay to monitor O. volvulus infection in vectors, we assayed DNA isolated from experimentally infected Simulium black flies. All 16 (100%) pools from the 32 flies engorged on the volunteer were positive by the LAMP. When the assay was further used to analyze pools of wild-caught black flies LAMP detected a similar number of positives compared with dissection and microscopy. It is important to note that the infection and infective rates from pool screening were calculated with an assumed parity of 50% as recommended (42). However, for the >9000 flies dissected we observed just 24.7% parous flies. If we were to use our observed parity to compute the pool screening infection and infective rates, the rates per 1000 parous flies for the LAMP would have generated twice as many positives, and thus LAMP would be more sensitive than microscopy. A higher level of sensitivity of LAMP was also previously observed in studies using pools containing 200 black flies spiked with 0.1 ng O. volvulus DNA (48).
The L. loa RF4 LAMP assay was evaluated on blood samples collected from 4 different health districts: Yabassi, Loum, Melong and Manjo. Interestingly, both LAMP and microscopy showed a good concordance and a kappa index of 95.6% in all regions. In Yabassi, Loum, and Melong, the prevalence of infection was similar (21.2 – 28.5%), while substantially lower in Manjo (5.9%).
The suitability and sensitivity of LAMP to detect infection in infected Chrysops was clearly demonstrated using experimentally infected Chrysops which had fed on individuals with either high (>30,000 mf/ml, 94.4% sensitivity) or low parasitemia (<10 mf/ml, 88.9% sensitivity). After ingestion by the vector, L. loa microfilariae require 7-14 days to develop into infective stage larvae (49, 50). In experimentally infected Chrysops that had fed on a volunteer with a low level of infection, parasites were detected solely in the abdomen as early as day 1 and up to day 7 post infection with the LAMP assay. Parasites were found throughout the flies from day 10 onwards. In contrast, infection was detected in the head, thorax and abdomen on days 1-14 post infection in flies that had fed on an individual with a high parasitemia. The ability of the RF4 LAMP assay to detect all developmental stages of the parasite in flies that had fed on a volunteer with low (10 mf/ml) parasitaemia suggests the suitability of this method for identification of Chrysops with extremely low levels of infection that may be missed using microscopy. Significantly higher rates of infection were detected using LAMP, with the East MDA, East non-MDA, South West 1, and North West sites having the highest infection rates with LAMP (16.4%-31.6%) while the South West 2 CDTI project site recorded the lowest infection rate of 0.5%.
Despite more than a decade of onchocerciasis CDTI in the South West 1 CDTI project, infection rates have remained high. This may be due to the persistence of a permanent parasite reservoir. Alternatively, a study by Wanji et al. has identified increased apathy towards ivermectin intake in the study area (51). Low adherence in meso- and hyper endemic areas may exacerbate infection transmission and maybe the result of the fear of side effects to ivermectin (52, 53).
The Mp419 LAMP had excellent sensitivity (96.7%), specificity (94.2%) and Kappa index (89%), when using blood samples, indicating that the LAMP assay is equivalent to microscopy. The utility of the Mp419 LAMP assay was also demonstrated in the Culicoides vectors, providing a new tool for entomological screening for M. perstans.
In summary, we demonstrate the suitability and usefulness of the panel of simple LAMP tests to detect O. volvulus, L. loa and M. perstans in humans and different vector species under experimental and field conditions. These new tools should facilitate surveillance and control efforts for important filarial diseases.
The parasitological survey of the study was conducted from the month of May 2020 to January 2021 in the Littoral region of Cameroon, in the Loum (4°42ˊ58˝N, 9°44´47˝E), Melong (5°7′16″N, 9°57′10″E), Yabassi (4°27′16″N, 9°57′56″E), Nkondjock (4°10′06″N, 10°04′51″E) and Manjo health districts (HDs), belonging to the Littoral 2 Community-Directed Treatment with Ivermectin (CDTI) project (Figure 1). For the skin snip samples, individuals were recruited from; Loum, Melong, Yabassi and Nkondjock HDs. While for blood samples, individuals were recruited from Loum, Melong, Yabassi and Manjo HDs. These HDs have different levels of filarial endemicity and had been under CDTI for over 16 years prior to the study.
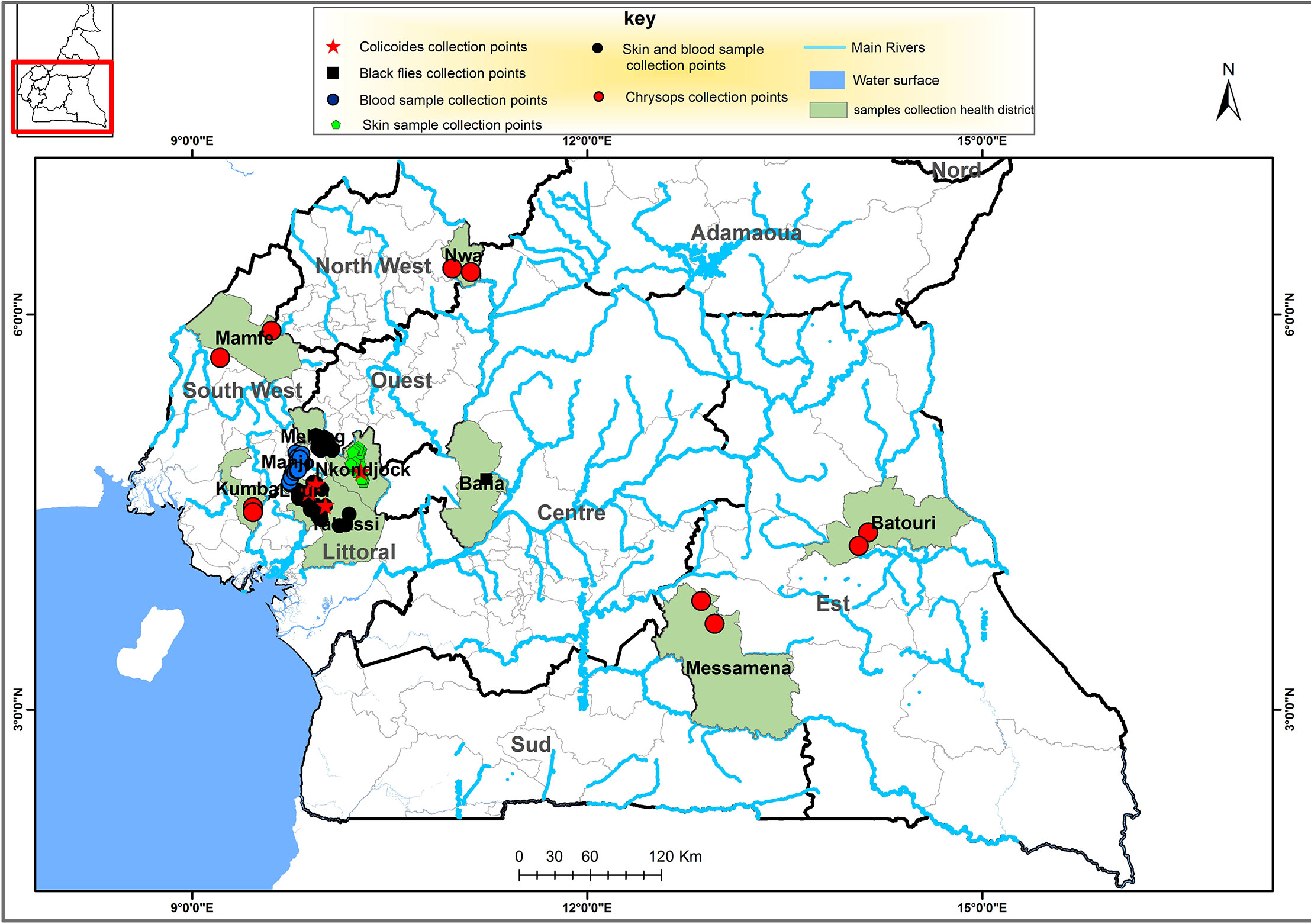
Figure 1 Map of the southern portion Cameroon depicting the regions in which the human and vector samples were collected, as indicated in the key. This map was created using ArcGIS (ArcMap v10.5.1) software by Esri.
The cross-sectional entomological survey was conducted in the Centre region (Simulium), South West, North West and East region (Chrysops) and the Littoral region (Culicoides) in Cameroon. Blackflies were collected in the village of Biatsotsa located on the River Mbam in the Bafia HD, part of the Mbam drainage basin. The Bafia HD belongs to the Centre 1 CDTI project area that, despite over 20 rounds of annual CDTI, is still meso-endemic for onchocerciasis (54).
The South West 1 (kumba HD) and South West 2 (Mamfe HD) are situated in areas of mild L. loa endemicity that had received CDTI for more 12-14 years by the time of the study (55). The Eastern and North West project sites are situated in high L. loa endemicity areas that had received CDTI for 10 and 9 years, respectively, prior to the study (55, 56).
The study was comprised of two main parts: parasitological survey and an entomological survey. The parasitological survey involved the use of skin/blood samples to detect skin-dwelling and blood-dwelling mf infection and the entomological survey involved the use of experimentally-infected flies to determine sensitivity and a field phase using wild-caught insects.
The examination for the presence or absence of nodules was done following the WHO standard protocol as previously conducted (51). Consenting participants were examined in a well-illuminated and enclosed room to maintain participant privacy (3); with emphasis being laid on the bony prominences. The number of nodules was recorded and their location marked on anatomical diagrams in the participant recruitment forms.
Skin snipping was done as previously described (51). Prior to skin snipping, the iliac crest of each participant was cleaned with a 70% alcohol pad and allowed to air dry. Two bloodless skin biopsies from the posterior left and right iliac crest of each participant were taken using a sterile 2 mm corneo-scleral punch (CT 016 Everhards 2218-15 C, Meckenheim, Germany). Baneocin antibiotic powder was applied to the snipped areas to prevent infection. The two skin samples from each participant were placed in two separate wells of a 96 well microtiter plate containing 100 uL of saline and incubated at room temperature for a period of 24 hours to allow maximum emergence of mf from the skin. The plates were sealed with parafilm to prevent any spill-over or evaporation of the saline (51). Emerged mf were counted using a light microscope at 10x magnification and expressed per skin snip (51). For each individual, the skin biopsies were placed in the same 1.5 mL Eppendorf tube (Eppendorf AG, Hamburg, Germany) containing 80% ethanol (GAPUMA UK Limited) and stored at -20°C for subsequent DNA extraction and LAMP analysis.
DNA from skin biopsies was extracted using the QIAGEN DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s instruction. Using sterilized forceps, skin biopsies were placed into 2 mL Eppendorf tubes (Eppendorf AG) containing 160 μL of 1X Phosphate Buffered Saline (Sigma-Aldrich, USA) and 18–20 (1.0–1.3 mm) glass beads (VWR International, Darmstadt, Germany). The skin snips were homogenized at 7000 rpm for 180s using a MagNA Lyser Instrument (Roche Diagnostics GmbH, Mannheim, Germany). After homogenization, the Qiagen protocol was followed and DNA was eluted in 200 μL of elution buffer and stored at -20°C until use for LAMP assays.
Diurnal blood collections were performed between 8am-4pm. The thick blood film (TBF) was prepared by spreading 50 μL non-heparinized finger-prick blood on clean, labeled and dry slides covering an area of 1.5×2.5 cm. The smears were allowed to air-dry and then packaged for transport back to the base for staining with 10% Giemsa using standard procedures (25).
The stained smears were read at a 10× magnification using a light microscope by trained technicians. Parasites (L. loa and M. perstans) were identified using microfilarial identification keys (57). Counts were quantified and expressed as microfilariae per milliliter (mf/mL) of blood.
A prominent vein was located and disinfected using a cotton swab soaked with 70% alcohol. A sterile 5 mL syringe was used to collect 2 mL of venous blood. The blood was transferred to labelled sterile EDTA tubes. Blood samples were placed in a cooled box containing ice packs and transported to the laboratory for DNA extraction and LAMP assay.
Blood samples were allowed to thaw at room temperature prior to analyses. DNA from blood pellets was extracted using QIAGEN QIAmp DNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturers’ instructions. Blood samples were homogenized with the use of a pipette prior to dispensing 200 μL of the blood samples into the respective labelled 1.5 mL tubes containing 20 μL of Protease. To these tubes, 200 µL of Buffer AL was added and thoroughly homogenized by pulse vortexing for 15 seconds and incubated at 56°C for 10min. Following incubation, 200 μL of 96% ethanol was added and vortexed thoroughly before pipetting into a QIAmp DNA Mini spin column which was placed in a 2 mL collection tube. The remaining steps were performed as described above for skin snip DNA extraction.
Female blood-seeking S. damnosum flies allowed to take blood on a consented microfilaridermic volunteer (50 mf/mL) were captured using Simulium rearing tubes. and transported to the laboratory (58). Upon arrival in the laboratory, Simulium flies were maintained in captivity under controlled experimental conditions in the insectarium for a period of 10 days to allow ingested microfilariae to mature into infective stage larvae (L3). During this period, flies were fed with sterile 15% sucrose solution and maintained at a temperature of 23–28°C and 79–80% relative humidity (58). Four flies were frozen at -20°C on day 3, 4, 5, 6, 7, 8, 9 and 10 post infection. At the end of the experiment, the flies were distributed into pools of 2 flies/pool for each day post infection and DNA was extracted from each pool and subjected to the O-150 LAMP assay for detection of O. volvulus infection.
After providing informed consent, microfilaremic volunteers allowed Chrysops flies to take blood which were caught using 50 mL Falcon tubes (Corning, USA) (59). To produce experimental infections in flies, two batches of 18 flies were each fed on either a microfilaremic volunteer with low (<10 mf/mL) microfilaremia (Lot 1) or with high (>30,000 mf/mL of blood) microfilaremia (Lot 2). Flies were maintained in the insectarium (23–28°C; relative humidity between 79–80% (60)) for up to 14 days to monitor larval development to L3 stage. Flies were fed daily with sterile 15% sucrose solution. From each lot, two flies were frozen at -20°C on day 0 (<7 hours post infection), 1, 4, 6, 7, 10, 11, 12 and 14 post infection. At the end of the experiment, the flies were dissected into head, thorax and abdomen sections; DNA was extracted from each body part for analysis by RF4 LAMP assay.
Collections were done each working day from 6pm-6am using rectangular cage traps (2x2x2m). M. perstans microfilaremic volunteers were seated with their legs exposed to the knee level under a rectangular netting cage trap. The cage was raised for 10-15 minutes to allow contact between host and midges and then lowered to trap the attracted midges. After 15 minutes, the Culicoides spp. within the cage were aspirated and blown into labeled 50 mL Falcon tubes filled 3/4 with plaster of Paris (POP) which served as an absorbent layer at the bottom of the tubes to retain moisture. Once transported to the insectarium [23-28°C; relative humidity 79-80% (60)], they were monitored daily for 12 days (time for the mf to develop to L3 stage). The flies were maintained singly in 50 mL Falcon tubes and fed daily with sterile 15% sucrose solution soaked in cotton wool. At intervals of 24 hours, a drop or two of distilled water was added using a 10 mL syringe to the POP to replenish moisture content in the tube. Flies were pooled (max. 10 flies) into 0-2, 3-5, 6-8 and 9-12 days post infection groups. The flies were identified, and DNA was extracted from each pool for detection with the Mp419 LAMP assay.
Black flies were collected in June 2016 by the human landing collection method. The catch was done daily on an hourly basis between 7:00 to 18:00 for a period of 4 days. Female Simulium flies coming for a blood meal were caught just as they landed using an aspirator as described in other studies (58).
Captured female blackflies were anaesthetized with chloroform and identified for species and counted. Then the flies were dissected in physiological saline under a dissecting microscope by entomology experts who distinguished parous (very reduced or clear malpighian tubules and less or no fat bodies) from nulliparous (large or opec malpighian tubules and more fat bodies) flies as described (61). The abdomen, thorax and head of parous flies were further dissected and examined for the presence of Onchocerca. Larvae were identified by species, counted and recorded by larval stage (L1, L2, L3). Undissected flies were pooled in batches of 100 into 80% ethanol for DNA extraction and LAMP pool screening.
Each pool of whole flies, fly heads or bodies was weighed (average weight for head and body pools was 17 mg and 100 mg, respectively) and placed in a 1.5 mL Eppendorf tube. Total DNA was extracted using the Zymo Research Genomic DNA Tissue MiniPrep Kit (Epigenetics Company, USA) following manufacturer’s protocol; for pools of bodies (tissue mass >25 mg), the volumes of digestion buffer and proteinase K were doubled. All samples were eluted with 200 μL and the DNA stored at -20°C until use.
Insect collections were performed between 7:00 and 18:00 from the August to October 2014 for a period of 5 days/community (62). Trained collectors dressed in protective clothing to prevent insect bites and stationed near a wood fire caught blood-seeking female flies using sweep nets; fly numbers per hour were recorded. At the end of each session, wild-caught flies were randomly separated into three groups: 1) control group dissected to check for parity, retaining 138 nulliparous flies for the RF4 LAMP assay; 2) group for dissection and microscopy; 3) stored in 80% alcohol for DNA extraction and LAMP analysis.
In the field laboratory, wild Chrysops were dissected in 0.9% NaCl under a dissecting microscope. The head, thorax and abdomen of each fly were separated and the abdomens were teased gently to pull out the ovarioles to determine parous status of the flies. Parous flies were further dissected to find L. loa larvae, counted and the infection rates calculated as described (63, 64).
Individual Chrysops flies were crushed using micro pestlein Eppendorf tubes containing 95 μL of water, 95 μL 2 X digestion buffer (Zymo Research Genomic DNA Tissue™ MiniPrep Kit) and 10 μL Proteinase K, incubated at 55°C for 1-3 hours followed by DNA extraction according to manufacturer’s protocol. DNA was eluted with 200 µL of elution buffer and stored at -20°C until use.
Collections of midges were carried out using Centers for Disease Control miniature black UV-light traps (Model 512, John W. Hock Company, Gainesville, USA). UV-light traps set at strategic positions around human dwellings at each of the seven sites. Midges were collected hourly between 6:00 and 18:00. The contents of each trap were emptied hourly using aspirators into labelled plastic cups containing 80% alcohol and then transported to the laboratory for Culicoides identification.
Morphological identification of Culicoides species was done based on keys through the examination of the wing pigmentation pattern under a dissecting microscope (65, 66). After identification, the flies were pooled (10 or 50 flies/pool) according to species for DNA extraction.
DNA from Culicoides flies was extracted using the QIAGEN QIAmp DNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instruction. Flies were placed into 2 mL Eppendorf tubes containing 80 μL of 1X Phosphate Buffered Saline (SIGMA-ALDRICH, USA) and 18–20 (1.0–1.3 mm), glass beads (VWR International, Darmstadt, Germany) and homogenized at 4000 rpm for 90 seconds using a MagNA Lyser Instrument (Roche Diagnostics GmbH). After homogenization, 180 μL of ATL buffer and 20 μL of proteinase K were added into the homogenate and incubated at 56°C overnight. Following the overnight incubation, samples were processed per the protocol and the DNA was eluted with 200 μL of elution buffer. The eluted DNA was stored at -20°C until use for LAMP assays.
To prevent cross-contamination, all sufaces were cleaned with 10% bleach solution and filter tips were used. All tubes are tightly closed and never opened after amplification to avoid contaminating the work area. The O. volvulus tandem repeat region O-150 was targeted in the study, the colorimetric O-150 LAMP assay was performed with some modifications (39). The L. loa LAMP targeting the RF4 family repeat was performed as previously published (43). The M. perstans LAMP targeting the Mp419 repeat was performed as published (40), with some modifications. Primer sequences and reaction components are detailed in Tables S1 and S2, respectively. Reactions were performed in a GeneAmp®, PCR System 9700 Thermal Cycler (Applied Biosystems, Foster city, USA). Samples were considered positive for the indicated filarial nematode if a color change from pink to yellow was observed (Figure S1), while non-template controls (molecular biology grade H2O) remained pink.
Data collected and compiled on paper record sheets were entered into Microsoft Excel 2010 and then exported to GraphPad Prism version 9.0 for subsequent analyses. Contingency tables were used to express the relationship between variables. Fischer’s Exact test was used to compare proportions with P ≤ 0.05 considered significant.
LAMP assay performance characteristic values (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and Kappa index) standard methods employing 2x2 contingency tables for evaluation of diagnostic tests were used. Microscopy was used as the “gold standard”. The 95% confidence intervals for all values were calculated as previously described (67).
Infection and infective rates for Simulium flies using microscopy were computed as previously described (68). The infection and infective rates from pool screening of Simulium and Culicoides flies were computed using the algorithm described by Katholi and colleagues (69).
Where m = number of pools, n = size of pool, k = number of negative pools and P = prevalence of infection.
The infection rate for Chrysops was determined as the proportion of infected flies to the total number of flies dissected as previously described (59).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Cameroon National Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Ethical approval for both parasitological and entomological evaluations was obtained from the National Ethics Committee for Human Health Research (Ref: N°2019/03/1150/CE/CNERSH/SP; Ref: N°2015/09/641/CE/CNERSH/SP) (26, 70).
SW conceived the work and designed the protocol with assistance of CC, ZL, AH, KP, GA and RA. GA, RA, RE, CK, MR, BL, NI, RE performed the experimental section supervised by SW, MH, KP, CC and ZL. GA, NI, RA, AN, AB, FF, PE, ME, SW and KD performed data curation and analysis. GA, RA, KP, and SW drafted the manuscript that was reviewed and edited and approved by all authors.
This study received funding from the German Center for Infection Research (DZIF) to AH (Grant No. TTU 03.815) from the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft) within the German-African Cooperation Projects in Infectiology scheme to MR and SW (RI 3036/1-1), and to MH (HU 2144/3-1). Reagents and consumables used for the LAMP assays were graciously donated by colleagues from the New England Biolabs (USA). KD was supported by the Wellcome Trust (201900/Z/16/Z) as part of his international intermediate Fellowship. CC and ZL gratefully acknowledge financial support from New England Biolabs USA. The funders had no involvement in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
We are very grateful to the inhabitants of the Loum, Melong, Yabassi, Manjo and Nkondjock health districts who willingly accepted to participate in this study. Special thanks also to the volunteers who, after informed consent, accepted to collect vector flies fed and unfed. Many thanks to the Chief of the health centers, District Medical Officer and Chief of Bureau Health, the Regional Delegates of Public Health for facilitating the work, and the Regional and National Coordinators of filariasis control programme for their administrative assistance.
ZL and CC are employees of New England Biolabs, manufacturer of LAMP reagents described in the manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.1016176/full#supplementary-material
1. World Health Organization. Ending the neglect to attain the sustainable development goals – a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization (2020).
2. Brattig NW, Cheke RA, Garms R. Onchocerciasis (river blindness) - more than a century of research and control. Acta Trop (2021) 218:105677. doi: 10.1016/j.actatropica.2020.105677
3. Zoure HG, Wanji S, Noma M, Amazigo UV, Diggle PJ, Tekle AH, et al. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the rapid assessment procedure for loiasis (RAPLOA). PloS Negl Trop Dis (2011) 5:e1210. doi: 10.1371/journal.pntd.0001210
4. Hawking F. The distribution of human filariasis throughout the world. part III. Africa. Trop Dis Bull (1977) 74:649–79.
5. Ratmanov P, Mediannikov O, Raoult D. Vectorborne diseases in West Africa: geographic distribution and geospatial characteristics. Trans R Soc Trop Med Hyg (2013) 107:273–84. doi: 10.1093/trstmh/trt020
6. Kelly-Hope LA, Bockarie MJ, Molyneux DH. Loa loa ecology in central Africa: role of the Congo river system. PloS Negl Trop Dis (2012) 6:e1605. doi: 10.1371/journal.pntd.0001605
7. Fernández-Soto P, Mvoulouga PO, Akue JP, Aban JL, Santiago BV, Sanchez MC, et al. Development of a highly sensitive loop-mediated isothermal amplification (LAMP) method for the detection of Loa loa. PloS One (2014) 9:e94664. doi: 10.1371/journal.pone.0094664
8. Akue JP, Nkoghe D, Padilla C, Moussavou G, Moukana H, Mbou RA, et al. Epidemiology of concomitant infection due to Loa loa and Mansonella perstans in Gabon. PloS Negl Trop Dis (2011) 5:e1329. doi: 10.1371/journal.pntd.0001329
9. Boussinesq M, Gardon J. Prevalences of Loa loa microfilaraemia throughout the area endemic for the infection. Ann Trop Med Parasitol (1997) 91:573–89. doi: 10.1080/00034989760671
10. Boussinesq M, Gardon J, Gardon-Wendel N, Chippaux JP. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J (2003) 2 Suppl 1:S4. doi: 10.1186/1475-2883-2-S1-S4
11. Anonymous. Report of a scientific working group on serious adverse events following Mectizan(R) treatment of onchocerciasis in Loa loa endemic areas. Filaria J (2004) 2 Suppl 1:S2. doi: 10.1186/1475-2883-2-S1-S2
12. Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet (1997) 350:18–22. doi: 10.1016/S0140-6736(96)11094-1
13. Hoerauf A, Pfarr K, Mand S, Debrah AY, Specht S. Filariasis in Africa-treatment challenges and prospects. Clin Microbiol Infect (2011) 17:977–85. doi: 10.1111/j.1469-0691.2011.03586.x
14. Vinkeles Melchers NVS, Coffeng LE, Boussinesq M, Pedrique B, Pion SDS, Tekle AH, et al. Projected number of people with onchocerciasis-loiasis coinfection in Africa 1995 to 2025. Clin Infect Dis (2020) 70:2281–9. doi: 10.1093/cid/ciz647
15. Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop (2011) 120 Suppl 1:S109–120. doi: 10.1016/j.actatropica.2010.01.014
16. Mourembou G, Fenollar F, Lekana-Douki JB, Ndjoyi Mbiguino A, Maghendji Nzondo S, Matsiegui PB, et al. Mansonella, including a potential new species, as common parasites in children in Gabon. PloS Negl Trop Dis (2015) 9:e0004155. doi: 10.1371/journal.pntd.0004155
17. Ta-Tang TH, Crainey JL, Post RJ, Luz SL, Rubio JM. Mansonellosis: Current perspectives. Res Rep Trop Med (2018) 9:9–24. doi: 10.2147/RRTM.S125750
18. Wanji S, Tayong DB, Layland LE, Datchoua Poutcheu FR, Ndongmo WP, Kengne-Ouafo JA, et al. Update on the distribution of Mansonella perstans in the southern part of Cameroon: influence of ecological factors and mass drug administration with ivermectin. Parasit Vectors (2016) 9:311. doi: 10.1186/s13071-016-1595-1
19. Bregani ER, Ceraldi T, Rovellini A, Ghiringhelli C. Case report: Intraocular localization of Mansonella perstans in a patient from south Chad. Trans R Soc Trop Med Hyg (2002) 96:654. doi: 10.1016/S0035-9203(02)90343-3
20. Ritter M, Ndongmo WPC, Njouendou AJ, Nghochuzie NN, Nchang LC, Tayong DB, et al. Mansonella perstans microfilaremic individuals are characterized by enhanced type 2 helper T and regulatory T and b cell subsets and dampened systemic innate and adaptive immune responses. PloS Negl Trop Dis (2018) 12:e0006184. doi: 10.1371/journal.pntd.0006184
21. Metenou S, Babu S, Nutman TB. Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS (2012) 7:231–8. doi: 10.1097/COH.0b013e3283522c3d
22. Dieki R, Nsi-Emvo E, Akue JP. The human filaria Loa loa: Update on diagnostics and immune response. Res Rep Trop Med (2022) 13:41–54. doi: 10.2147/RRTM.S355104
23. Gounoue-Kamkumo R, Nana-Djeunga HC, Bopda J, Akame J, Tarini A, Kamgno J. Loss of sensitivity of immunochromatographic test (ICT) for lymphatic filariasis diagnosis in low prevalence settings: Consequence in the monitoring and evaluation procedures. BMC Infect Dis (2015) 15:579. doi: 10.1186/s12879-015-1317-x
24. Bakajika DK, Nigo MM, Lotsima JP, Masikini GA, Fischer K, Lloyd MM, et al. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the democratic republic of Congo. Am J Trop Med Hyg (2014) 91:1142–8. doi: 10.4269/ajtmh.14-0358
25. Pion SD, Montavon C, Chesnais CB, Kamgno J, Wanji S, Klion AD, et al. Positivity of antigen tests Used for diagnosis of lymphatic filariasis in Individuals without Wuchereria bancrofti infection but with high Loa loa microfilaremia. Am J Trop Med Hyg (2016) 95, 1417–23. doi: 10.4269/ajtmh.16-0547.
26. Wanji S, Amvongo-Adjia N, Koudou B, Njouendou AJ, Chounna Ndongmo PW, Kengne-Ouafo JA, et al. Cross-reactivity of filariais ICT cards in areas of contrasting endemicity of Loa loa and Mansonella perstans in Cameroon: Implications for shrinking of the lymphatic filariasis map in the central African region. PloS Negl Trop Dis (2015) 9:e0004184. doi: 10.1371/journal.pntd.0004184
27. Okorie PN, De Souza DK. Prospects, drawbacks and future needs of xenomonitoring for the endpoint evaluation of lymphatic filariasis elimination programs in Africa. Trans R Soc Trop Med Hyg (2016) 110:90–7. doi: 10.1093/trstmh/trv104
28. Fischer P, Büttner DW, Bamuhiiga J, Williams SA. Detection of the filarial parasite Mansonella streptocerca in skin biopsies by a nested polymerase chain reaction-based assay. Am J Trop Med Hygiene (1998) 58:816–20. doi: 10.4269/ajtmh.1998.58.816
29. Ta-Tang TH, Lopez-Velez R, Lanza M, Shelley AJ, Rubio JM, Luz SL. Nested PCR to detect and distinguish the sympatric filarial species Onchocerca volvulus, mansonella ozzardi and Mansonella perstans in the Amazon region. Mem Inst Oswaldo Cruz (2010) 105:823–8. doi: 10.1590/S0074-02762010000600016
30. Korbmacher F, Komlan K, Gantin RG, Poutouli WP, Padjoudoum K, Karabou P, et al. Mansonella perstans, Onchocerca volvulus and Strongyloides stercoralis infections in rural populations in central and southern Togo. Parasite Epidemiol Control (2018) 3:77–87. doi: 10.1016/j.parepi.2018.03.001
31. Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PloS Negl Trop Dis (2011) 5:e1299. doi: 10.1371/journal.pntd.0001299
32. Gilbert J, Nfon CK, Makepeace BL, Njongmeta LM, Hastings IM, Pfarr KM, et al. Antibiotic chemotherapy of onchocerciasis: In a bovine model, killing of adult parasites requires a sustained depletion of endosymbiotic bacteria (Wolbachia species). J Infect Dis (2005) 192:1483–93. doi: 10.1086/462426
33. Colebunders R, Mandro M, Mokili JL, Mucinya G, Mambandu G, Pfarr K, et al. Risk factors for epilepsy in bas-uele province, democratic republic of the Congo: A case-control study. Int J Infect Dis (2016) 49:1–8. doi: 10.1016/j.ijid.2016.05.018
34. Hamill L, Trotignon G, Mackenzie C, Hill B, Pavluck A, Yumba D, et al. Navigating the way to onchocerciasis elimination: The feasibility and affordability of onchocerciasis elimination mapping. Int Health (2022) 14:i17–23. doi: 10.1093/inthealth/ihab083
35. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res (2000) 28:E63. doi: 10.1093/nar/28.12.e63
36. Poole CB, Li Z, Alhassan A, Guelig D, Diesburg S, Tanner NA, et al. Colorimetric tests for diagnosis of filarial infection and vector surveillance using non-instrumented nucleic acid loop-mediated isothermal amplification (NINA-LAMP). PloS One (2017) 12:e0169011. doi: 10.1371/journal.pone.0169011
37. Avendaño C, Patarroyo MA. Loop-mediated isothermal amplification as point-of-Care diagnosis for neglected parasitic infections. Int J Mol Sci (2020) 21:7981. doi: 10.3390/ijms21217981
38. Kaneko H, Kawana T, Fukushima E, Suzutani T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J Biochem Biophys Methods (2007) 70:499–501. doi: 10.1016/j.jbbm.2006.08.008
39. Alhassan A, Osei-Atweneboana MY, Kyeremeh KF, Poole CB, Li Z, Tettevi E, et al. Comparison of a new visual isothermal nucleic acid amplification test with PCR and skin snip analysis for diagnosis of onchocerciasis in humans. Mol Biochem Parasitol (2016) 210:10–2. doi: 10.1016/j.molbiopara.2016.07.006
40. Poole CB, Sinha A, Ettwiller L, Apone L, Mckay K, Panchapakesa V, et al. In silico identification of novel biomarkers and development of new rapid diagnostic tests for the filarial parasites Mansonella perstans and Mansonella ozzardi. Sci Rep (2019) 9:10275. doi: 10.1038/s41598-019-46550-9
41. Van Smeden M, Naaktgeboren CA, Reitsma JB, Moons KG, De Groot JA. Latent class models in diagnostic studies when there is no reference standard–a systematic review. Am J Epidemiol (2014) 179:423–31. doi: 10.1093/aje/kwt286
42. World Health Organization. Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis: Criteria and procedures. Geneva: World Health Organization (2016).
43. Poole CB, Ettwiller L, Tanner NA, Evans TC Jr., Wanji S, Carlow CK. Genome filtering for new DNA biomarkers of Loa loa infection suitable for loop-mediated isothermal amplification. PloS One (2015) 10:e0139286. doi: 10.1371/journal.pone.0139286
44. Abong RA, Amambo GN, Chounna Ndongmo PW, Njouendou AJ, Manuel R, Beng AA, et al. Differential susceptibility of Onchocerca volvulus microfilaria to ivermectin in two areas of contrasting history of mass drug administration in Cameroon: relevance of microscopy and molecular techniques for the monitoring of skin microfilarial repopulation within six months of direct observed treatment. BMC Infect Dis (2020) 20:726. doi: 10.1186/s12879-020-05444-2
45. Bottomley C, Isham V, Vivas-Martinez S, Kuesel AC, Attah SK, Opoku NO, et al. Modelling neglected tropical diseases diagnostics: the sensitivity of skin snips for Onchocerca volvulus in near elimination and surveillance settings. Parasit Vectors (2016) 9:343. doi: 10.1186/s13071-016-1605-3
46. Turner HC, Walker M, Churcher TS, Basanez MG. Modelling the impact of ivermectin on river blindness and its burden of morbidity and mortality in African savannah: EpiOncho projections. Parasit Vectors (2014) 7:241. doi: 10.1186/1756-3305-7-241
47. Stolk WA, Walker M, Coffeng LE, Basanez MG, De Vlas SJ. Required duration of mass ivermectin treatment for onchocerciasis elimination in Africa: A comparative modelling analysis. Parasit Vectors (2015) 8:552. doi: 10.1186/s13071-015-1159-9
48. Alhassan A, Makepeace BL, Lacourse EJ, Osei-Atweneboana MY, Carlow CK. A simple isothermal DNA amplification method to screen black flies for Onchocerca volvulus infection. PloS One (2014) 9:e108927. doi: 10.1371/journal.pone.0108927
49. Connal A, Connal SLM. The development of Loa loa (Guyot) in Chrysops silacea (Austen) and in Chrysops dimidiata (van der wulp). Trans R Soc Trop Med Hygiene (1922) 16:64–89. doi: 10.1016/S0035-9203(22)90984-8
50. Kershaw WE, Duke BO. Studies on the intake of microfilariae by their insect vectors, their survival, and their effect on the survival of their vectors. v. the survival of Loa loa in Chrysops silacea under laboratory conditions. Ann Trop Med Parasitol (1954) 48:340–4. doi: 10.1080/00034983.1954.11685632
51. Wanji S, Kengne-Ouafo JA, Esum ME, Chounna PW, Adzemye BF, Eyong JE, et al. Relationship between oral declaration on adherence to ivermectin treatment and parasitological indicators of onchocerciasis in an area of persistent transmission despite a decade of mass drug administration in Cameroon. Parasit Vectors (2015) 8:667. doi: 10.1186/s13071-015-1283-6
52. El-Setouhy M, Abd Elaziz KM, Helmy H, Farid HA, Kamal HA, Ramzy RM, et al. The effect of compliance on the impact of mass drug administration for elimination of lymphatic filariasis in Egypt. Am J Trop Med Hyg (2007) 77:1069–73. doi: 10.4269/ajtmh.2007.77.1069
53. Hussain MA, Sitha AK, Swain S, Kadam S, Pati S. Mass drug administration for lymphatic filariasis elimination in a coastal state of India: a study on barriers to coverage and compliance. Infect Dis Poverty (2014) 3:31. doi: 10.1186/2049-9957-3-31
54. Kamga GR, Dissak-Delon FN, Nana-Djeunga HC, Biholong BD, Mbigha-Ghogomu S, Souopgui J, et al. Still mesoendemic onchocerciasis in two cameroonian community-directed treatment with ivermectin projects despite more than 15 years of mass treatment. Parasit Vectors (2016) 9:581. doi: 10.1186/s13071-016-1868-8
55. Esum M, Wanji S, Tendongfor N, Enyong P. Co-Endemicity of loiasis and onchocerciasis in the south West province of Cameroon: implications for mass treatment with ivermectin. Trans R Soc Trop Med Hyg (2001) 95:673–6. doi: 10.1016/S0035-9203(01)90112-9
56. Takougang I, Meli J, Lamlenn S, Tatah PN, Ntep M. Loiasis–a neglected and under-estimated affliction: endemicity, morbidity and perceptions in eastern Cameroon. Ann Trop Med Parasitol (2007) 101:151–60. doi: 10.1179/136485907X154511
57. Orihel TC, Lowrie RC Jr. Loa loa: development to the infective stage in an American deerfly, Chrysops atlanticus. Am J Trop Med Hyg (1975) 24:610–5. doi: 10.4269/ajtmh.1975.24.610
58. Albers A, Esum ME, Tendongfor N, Enyong P, Klarmann U, Wanji S, et al. Retarded Onchocerca volvulus L1 to L3 larval development in the Simulium damnosum vector after anti-wolbachial treatment of the human host. Parasit Vectors (2012) 5:12. doi: 10.1186/1756-3305-5-12
59. Wanji S, Tendongfor N, Esum ME, Enyong P. Chrysops silacea biting densities and transmission potential in an endemic area of human loiasis in south-west Cameroon. Trop Med Int Health (2002) 7:371–7. doi: 10.1046/j.1365-3156.2002.00845.x
60. Tendongfor N, Wanji S, Ngwa JC, Esum ME, Specht S, Enyong P, et al. The human parasite Loa loa in cytokine and cytokine receptor gene knock out BALB/c mice: Survival, development and localization. Parasit Vectors (2012) 5:43. doi: 10.1186/1756-3305-5-43
61. Duke BO. The differential dispersal of nulliparous and parous Simulium damnosum. Tropenmed Parasitol (1975) 26:88–97.
62. Duke BO. Studies on the biting habits of Chrysops. II. the effect of wood fires on the biting density of Chrysops silacea in the rain-forest at kumba, British cameroons. Ann Trop Med Parasitol (1955) 49:260–72. doi: 10.1080/00034983.1955.11685674
63. Duke BO. Studies on factors influencing the transmisson of onchocerciasis. IV. the biting-cycles, infective biting density and transmission potential of "forest" Stimulium dannosum. Ann Trop Med Parasitol (1968) 62:95–106. doi: 10.1080/00034983.1968.11686535
64. Noireau F, Nzoulani A, Sinda D, Itoua A. Transmission indices of Loa loa in the chaillu mountains, Congo. Am J Trop Med Hyg (1990) 43:282–8. doi: 10.4269/ajtmh.1990.43.282
65. Glick JI. Culicoides biting midges (Diptera: Ceratopogonidae) of Kenya. J Med Entomol (1990) 27:85–195. doi: 10.1093/jmedent/27.2.85
66. Boorman J. Biting midges (Ceratopogonidae). In: Lane RP, Crosskey RW, editors. Medical insects and arachnids. Dordrecht: Springer Netherlands (1993). p. 288–309.
67. Hess AS, Shardell M, Johnson JK, Thom KA, Strassle P, Netzer G, et al. Methods and recommendations for evaluating and reporting a new diagnostic test. Eur J Clin Microbiol Infect Dis (2012) 31:2111–6. doi: 10.1007/s10096-012-1602-1
68. Walsh JF. Light trap studies on simulium damnosum s.l. in northern Ghana. Tropenmed Parasitol (1978) 29:492–6.
69. Katholi CR, Toe L, Merriweather A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis (1995) 172:1414–7. doi: 10.1093/infdis/172.5.1414
70. Wanji S, Chounna Ndongmo WP, Fombad FF, Kengne-Ouafo JA, Njouendou AJ, Longang Tchounkeu YF, et al. Impact of repeated annual community directed treatment with ivermectin on loiasis parasitological indicators in Cameroon: Implications for onchocerciasis and lymphatic filariasis elimination in areas co-endemic with Loa loa in Africa. PloS Negl Trop Dis (2018) 12:e0006750. doi: 10.1371/journal.pntd.0006750
Keywords: Onchocerca volvulus, Loa loa, Mansonella perstans, LAMP (loop mediated isothermal amplification), diagnostic test
Citation: Amambo GN, Innocentia N, Abong RA, Fombad FF, Njouendou AJ, Nietcho F, Ekanya R, Kien CA, Ebai R, Lenz B, Ritter M, Esum ME, Deribe K, Cho JF, Beng AA, Enyong PI, Li Z, Hübner MP, Pfarr K, Hoerauf A, Carlow C and Wanji S (2023) Application of loop mediated isothermal amplification (LAMP) assays for the detection of Onchocerca volvulus, Loa loa and Mansonella perstans in humans and vectors. Front. Trop. Dis 3:1016176. doi: 10.3389/fitd.2022.1016176
Received: 11 August 2022; Accepted: 07 December 2022;
Published: 09 January 2023.
Edited by:
Catherine A. Gordon, QIMR Berghofer Medical Research Institute, The University of Queensland, AustraliaReviewed by:
Donato Antonio Raele, Experimental Zooprophylactic Institute of Puglia and Basilicata (IZSPB), ItalyCopyright © 2023 Amambo, Innocentia, Abong, Fombad, Njouendou, Nietcho, Ekanya, Kien, Ebai, Lenz, Ritter, Esum, Deribe, Cho, Beng, Enyong, Li, Hübner, Pfarr, Hoerauf, Carlow and Wanji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Wanji, c3dhbmppQHlhaG9vLmZy; Kenneth Pfarr, S2VubmV0aC5wZmFyckB1Ymtvbm4uZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.