- 1Laboratory Center, School of Preventive Medicine and Public Health, Hanoi Medical University, Hanoi, Vietnam
- 2Department of Biochemistry, Hanoi Medical University, Hanoi, Vietnam
- 3Department of Nutrition and Dietetics, School of Preventive Medicine and Public Health, Hanoi, Vietnam
- 4Department of Occupational Health, School of Preventive Medicine and Public Health, Hanoi, Vietnam
- 5Quality Control Center for Medical Laboratory, Hanoi Medical University, Hanoi, Vietnam
The COVID-19 global pandemic has been going on for more than two years, and the evolution of SARS-CoV-2 with many variants of concern still poses a risk to public health. Sufficient access to qualified and validated testing plays an important role in detecting and alerting trends of the pandemic and provides evidence for making decisions in preventive strategies and policies. Depending on the method of testing and laboratory conditions, validation parameters (i.e., analytical sensitivity, limit of detection, diagnostic sensitivity, analytical specificity, diagnostic specificity, repeatability, reproducibility, robustness, positive predictive value, negative predictive value, applicability, practicability, and time to results) can be very different. With three main types of COVID-19 detection kits available, comprising nucleic acid, serological, and antigen detection, the kind of validation parameters that should be used becomes a complicated consideration and takes time to assess. Our review provides valuable and comprehensive information for laboratories in the assessment and selection of the optimal parameters to validate new COVID-19 test kits.
Introduction
The SARS-CoV-2 virus, having been discovered and publicized in the last months of 2019, quickly gained momentum and became a global pandemic affecting health, lives, and economy in most countries around the world (1). As of May 2022, globally there were more than 529 million people who had contracted COVID-19, over 6.2 million deaths, and the number of new daily cases was high at about 275,326 cases (2). In Vietnam, after four stages of the COVID-19 pandemic, 10.7 million had been infected with SARS-CoV-2, and the number of deaths was 43,086 people (2). In addition to the success of vaccines, early detection, screening, and diagnosing cases of the infection remain some of the best ways to minimize the spread of COVID-19, controlling the consequences of the pandemic and supporting timely health care.
There are many different methods to detect the SARS-CoV-2 virus and COVID-19 cases. These methods can generally be classified into three main groups based on technical principles, namely: (1) determination of viral nucleic acids, (2) determination of specific IgG/IgM/IgG-IgM-class antibodies to viral antigens, and (3) direct determination of viral antigens (3). To detect the virus, the molecular biology method of determining the presence of viral nucleic acid by real-time RT-PCR technique is considered the gold standard and is the recommended method to identify SARS-CoV-2 (3). This method provides quick and accurate results, but it has some limitations such as the requirements for expensive equipment, advanced facilities, a laboratory management system, and certification of proficient technicians, as well as having a long test turnaround time (4). To eliminate these drawbacks, a number of other tests have been researched and developed that are based on the principle of identifying proteins from the SARS-CoV-2 virus or determining viral antigens or antibodies of an infected person formed during the immune response to SARS-CoV-2; these tests analyze the serum component of blood, or use nasopharyngeal swabs or saliva to detect the presence of the virus (4).
The three testing methods used to screen for the SARS-CoV-2 virus require common guidelines to control the quality and validate the results of biological test kits. While there are regulations on the criteria for the validation of SARS-CoV-2 testing methods, the parameters for validation are still inconsistent between the different documents and complicated questions that each laboratory use to apply the new methods. In this article, we review the most common parameters from various documents and guidelines for the validation of SARS CoV-2 testing methods to bring comprehensive information to laboratories in the assessment and selection of appropriate parameters to validate new COVID-19 test kits.
Validation of the biological methods for COVID 19 detection
Detection of SARS-CoV-2 by nucleic acid determination
Principles and influential factors of nucleic acid determination methods
There are many test techniques involved in the determination of viral nucleic acid, including PCR-based viral nucleic acid determination (real-time RT-PCR, real-time RT-PCR digital), viral nucleic acid isothermal amplification method (LAMP, RPA, etc.), and non-routine viral nucleic acid determination (viral genome sequencing, CRISPR-based COVID-19 identification) (5–8). Among the groups of techniques related to the determination of viral nucleic acids, PCR-based identification is the most commonly used and considered the “gold standard” for diagnosis of infection. The SARS-CoV-2 virus identification kits using the real-time RT-PCR technique have the principle of identifying the SARS-CoV-2 virus genome as that containing single-stranded RNA, which is efficiently performed by real-time amplification reaction of the gene sequence. When laboratories perform real-time RT-PCR for diagnosis of cases of SARS-COV-2 infection, there are some requirements which need to be considered as follows:
(1) The quality of specimens: The real-time RT-PCR technique is used to identify SARS-CoV-2 virus based on clinical samples, including upper respiratory tract specimens (saliva, throat swabs, nasopharyngeal swab), lower respiratory tract specimens (sputum, endotracheal suction fluid, broncho-alveolar lavage (BAL), bronchial biopsy specimens), blood (serum, plasma), urine, feces, and corneal secretions (9). However, upper and lower respiratory tract specimens are the most commonly used in clinical sample testing. Laboratories need to ensure the quality of clinical specimens collected from different sources. To do this, laboratories should develop an optimized procedure for RNA extraction to obtain high-quality RNA from sample types that are then analyzed by spectrophotometer and electrophoresis on agarose gel (10).
(2) Evaluating the effectiveness of a PCR reaction: The data analysis process can have an important influence on the PCR results. The process of analyzing PCR data is based on a calibration curve or PCR-efficiency assessment. An estimate of the efficiency of a real-time RT-PCR reaction using a calibration curve is performed using a series of dilutions from the initial stock solution concentration. After this, the standard sample is analyzed by calculating (Cq) using standard operating procedure (SOP). The most commonly used Cq value is the threshold cycle (Ct) value at which the signal for the expression of the target gene overcomes the background threshold of the fluorescence signal. For example, when small amounts of SARS-CoV-2 RNA have been determined, laboratories need to perform tests with a series of diluted template RNA concentrations and then determine the Ct value, thereby providing a standard curve for calculating the reaction efficiency. If laboratories do not have a calibration curve established against the reference material, the Ct value itself cannot be interpreted as viral load. When laboratories interpret the results of a real-time RT-PCR SARS-CoV-2 assay, the accuracy of the calibration curve should be considered against the evidence of the reference material used to be able to interpret the Ct value as a viral load (11, 12). The equation for calculation of % efficiency is as follows (13–15):
Where s is slope for the standard curve
(3) Viral load: There is a strong link between viral load and successful virus isolation. Many investigations showed that viral loads in sputum and throat swabs were high when collected at seven days after symptom onset, ranging from 104 to 107 copies/Ml. This pattern was broken and the viral load was reduced after day eight. Usually sputum has a higher viral load than nasopharyngeal swab specimens, while viral RNA load is low in urine and stool samples (16, 17). The two main factors affecting the quantitative measurement of viral load are that the Cq value has a repeatability with acceptable uncertainty and is reliably significant for conversion from Ct value to viral load. In qPCR, the Ct or Cq value is established from the amplification cycle, where the fluorescence curve exhibits the greatest curvature and exceeds the background fluorescence threshold (18). With biomolecule diagnostic tests, the limit of detection (LOD) and limit of quantitation (LOQ) are also defined by the lowest concentration of target RNA that can be detected/quantified by real-time RT-PCR (13). The LOD is considered the minimum concentration of RNA virus that can be detected in > 95% of repeat replicates. The LOD for real-time RT-PCR (qPCR) can be measured based on a replicate standard curve under error-free conditions. From the definition of LOD, it follows that the value of LOD is found when 95% of replicates are positive. In general, the LOD measures assay sensitivity in comparison to clinical sensitivity, which assesses a specimen from a case of infection detected by another test. At present, almost all commercial testing kits for detecting the virus publish the LOD parameters in the manufacturer’s instructions. LOQ is defined as the amount of RNA virus in the sample that can be quantitatively measured with acceptable precision and accuracy in simple experimental conditions. This can be done by assessing the amount of RNA virus and the corresponding Cq value, where the sum of sensitivity and specificity of the assay is at maximum. The point found is known as the optimal cutoff point. The LOQ is then determined as the amount of RNA corresponding to the optimal cutoff point on a replicate standard curve (19, 20).
(4) Sample processing method: Real-time RT-PCR tests for the identification of SARS-CoV-2 show large differences between false negative rate and false positive rate. Laboratories strive to improve diagnostic methods for SARS-CoV-2 with the aim of improving sensitivity, specificity, and safety, and reducing result time. For example, to create an experiment for checking the effectiveness and sensitivity of SARS-CoV-2 detection on clinical specimens collected directly in a lysis buffer and nucleic acid stabilization obtained after lysis, a mixture of lysis buffer and RNA preservative are used, instead of a virus transport solution capable of inactivating the virus immediately after sampling (21, 22).
(5) Origin of specimens: To improve the performance of SARS-CoV-2 testing, there are many approaches to initial sampling, including obtaining nasal fluid, pooled nasal fluid, throat fluid, and saliva. Differences in clinical sampling methods, such as their sensitivity and specificity, can affect the performance of real-time RT-PCR diagnostic testing for SARS-CoV-2 infection and should therefore be carefully considered. Synthetic cotton swabs are widely recommended as suitable specimen collection materials for real-time RT-PCR diagnostics (23). Several other studies have shown various levels of stability of SARS-CoV-2 virus, depending on the type of material that the specimen is taken from (plastic, stainless steel, copper, printed paper, cardboard, wool or cloth, glass) or the laboratory specimen (feces, urine, diarrhea, cell culture supernatant, throat swabs, nasal swabs, sputum samples). Thus, in addition to the procedures involved in RT-PCR testing for the detection of SARS-CoV-2, when taking and analyzing samples, close attention should be paid to sample origin, sample type, and sample stabilization time for best results (17, 24–26).
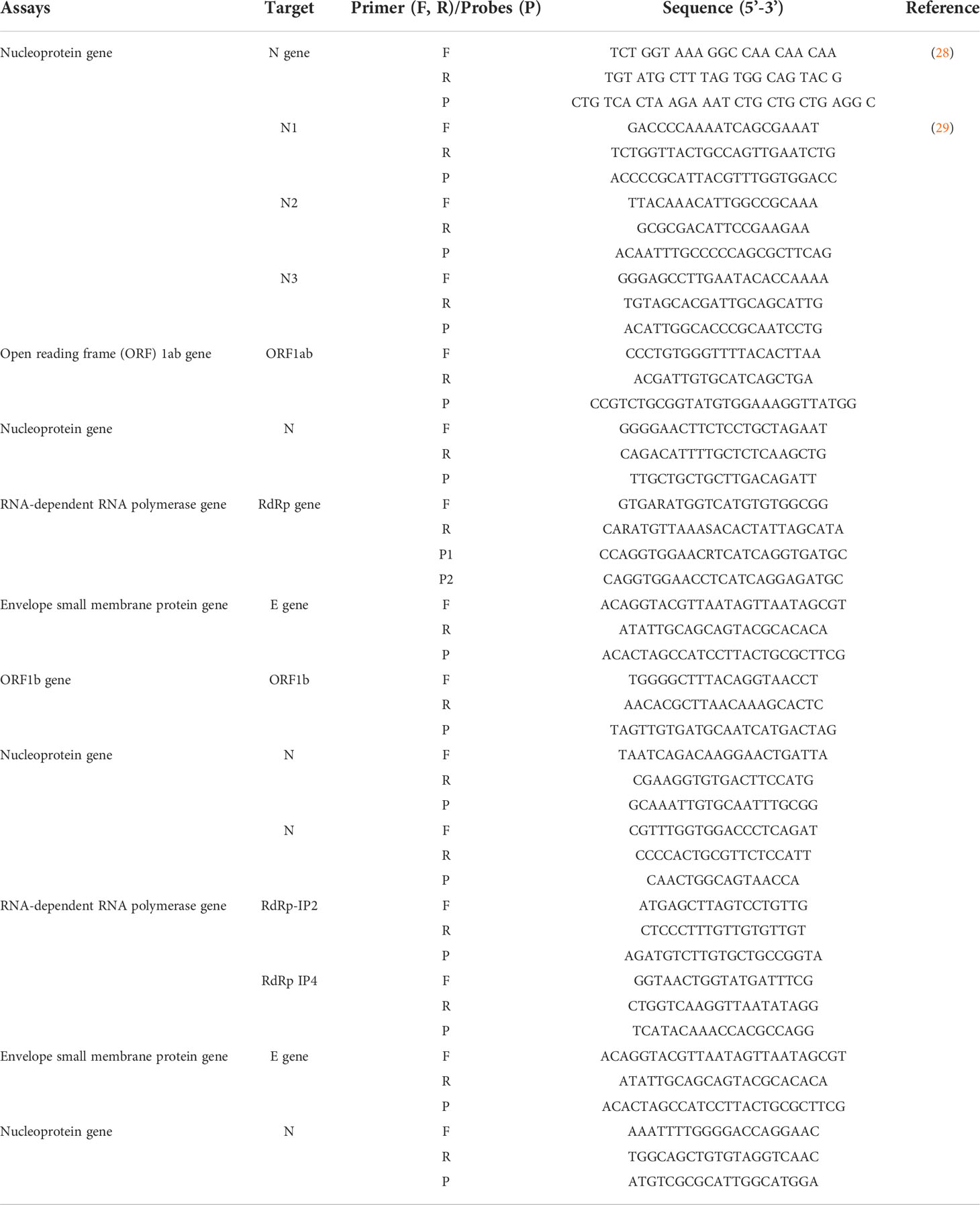
(6) Target design of real-time RT-PCR reaction: Conserved regions in the genome of the SARS-CoV-2 virus are targeted as standard genes for primer and probe design, including replicase (ORF 1a/ORF1b), RNA-dependent polymerase (RdRp), envelope (E), membrane (M), and nucleocapsid (N). However, initial reports of SARS-CoV-2 and other coronavirus gene sequences that produced bases incompatible with the SARS-CoV-2 RNA sequence were found. In addition, there were reports related to reduced sensitivity in designs using RdRp as the target gene for real-time RT-PCR assays (27). As the pandemic continued, many laboratories around the world continued to use these methods, and primer pairs and probes became routine methods of testing. Some examples are shown in Table 1. Indeed, additional tests could increase the sensitivity of detecting SARS-CoV-2 and help minimize the spread of the virus (30, 31).
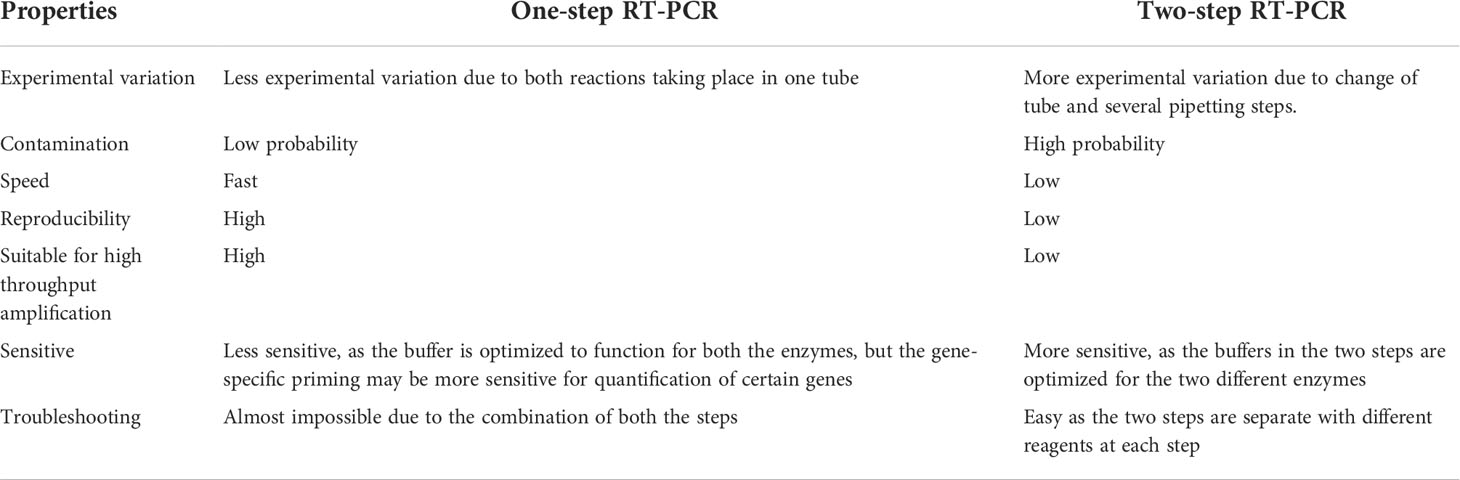
Considering all the practical factors affecting the real-time RT-PCR test for SARS-CoV-2 detection that will be mentioned in the section “Practicability”, there are two ways to perform this test. The details for each method are provided as follows. Method 1: performing the reaction in two steps by creating complementary DNA from the viral template RNA with reverse transcriptase, then amplifying the target gene segment by real-time PCR reaction to identify the virus. Method 2: one-step real-time RT-PCR method that combines reverse transcriptase and PCR in a single tube and uses a reactive enzyme that is both reverse transcriptase and DNA polymerase (13, 32, 33). Table 2 shows the characteristics that laboratory personnel should be aware of when performing one-step RT-PCR and two-step RT-PCR tests (34, 35).
Overview of validating real-time RT-PCR method for determination of SARS-CoV-2
When developing or deploying test kits related to the detection of SARS-CoV-2 by real-time RT-PCR, most laboratories use protocols developed using common guidelines or existing protocols for quantitative analysis of real-time RT-PCR test results. However, these procedures may not be adequate or meet the requirements of a qualitative analysis of real-time RT-PCR SARS-CoV-2 testing that may require theoretical information (i.e., parameters to be evaluated and their definition, evaluation criteria) or the specific experimental setup (material, copy number, DNA count, etc.) to be included (32). In this article, we analyze and evaluate the existing literature to develop guidelines for the qualitative analysis of real-time RT-PCR. The parameters are discussed and evaluated based on the feasibility of primary sample treatments, the use of different chemicals, time, and cost (36, 37).
There are many parameters used in quantitative methods, but they need to be selected, adjusted, and supplemented. The parameters can be divided into two main groups: method acceptance parameters (checked with test kit development organizations during internal validation) and practicability of parameters (assessed through collaborative interlaboratory trials) (37, 38). Discussed in detail, the following parameters need to be evaluated for a method to be accepted during method deployment and internal laboratory validation.
Applicability
When making a claim about whether a method is applicable or not, a complete record of the scope of the method’s application, the target of the method, and the amount of RNA tested by the method developer are required. Laboratories need to evaluate each method when using specimens of different substrates – nasal swab, throat swab, lower respiratory tract specimens (sputum, broncho alveolar lavage, endotracheal aspiration, bronchial biopsy samples), blood samples, stool samples, or urine samples. Moreover, the amount of template RNA at different concentrations should be tested, allowing the identification of RT-PCR inhibitors that may be present in the patient’s sample. The results of these tests should be reported and consistent among tests. Recommendations should be made for substrates, and the types of patient samples and sample conditions that are not suitable for SARS-CoV-2 testing with the evaluated kits should be made clear (36, 39).
Practicability
Feasibility refers to the fact that the method can be performed under laboratory conditions. In addition, there is an assessment of the cost and the requirement to train employees in new methods of testing. Furthermore, the feasibility assessment can be additionally evaluated by transferring the method under evaluation to one or more other laboratories (within the same test field) and repeating the experiments under the same controlled conditions as the original laboratory. A method can be considered viable when it is ultimately low cost, easy to train staff, and the laboratory facilities meet the requirements of the method (39, 40).
Specificity
It is necessary to distinguish between specificity (analytical specificity) and relative specificity (diagnostic specificity) (13, 39–43). The analytical specificity of the SARS-CoV-2 detection method by real-time RT-PCR is the competence to amplify only viral target genes while not amplifying non-target genes of the virus or other related viruses and bacteria closely related to it (39). During the design of primers and probes for target genes for the detection of SARS-CoV-2, laboratories need to check for analytical specificity to ensure that the method is only responsive to the target sequence. Firstly, this check needs to be done on a computer (in silico), i.e., based on finding viral RNA sequences publicly available on the GenBank database (13, 40). Based on requirements, primers and probes specific for the desired target gene segment can be designed and sequenced. Alternatively, the published primer sequence of the test kit deployed in the laboratory should be checked to ensure that the position on the reference sequence corresponds to the published test kit information. Then conduct a test setup to evaluate the specificity of the analysis by selecting 20 standard material samples that do not have the target gene of the SARS-CoV-2 virus and 20 standard material samples with the target gene region of the SARS-CoV-2 virus confirmed (40). The following calculations should be undertaken to assess specificity for the real-time RT-PCR assay (39, 40):
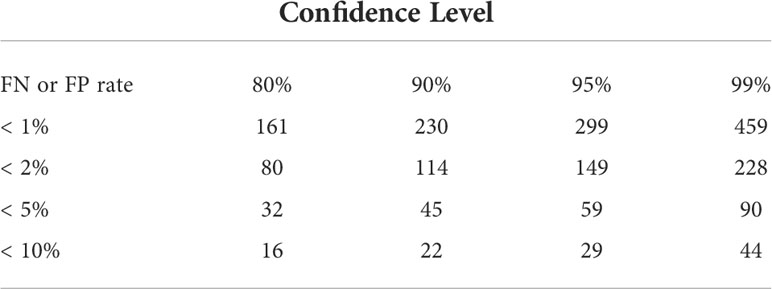
In addition, the false positive (FP%) is also expressed as 1- TN%. % FP should be 0. Ideally, the value of false positives and false negatives should be zero. When signals that are not suitable for the method are present, all factors that may affect the analytical specificity should be considered (39, 40). To achieve high confidence levels, some research suggests choosing a specific number of samples for the evaluation of FN or FP (41), i.e., to achieve a method with 99% FN or FP values< 5%, test 90 samples with a target nucleic acid present at the required concentration, usually LOD or a related area of interest. The criterion is met if all 90 test results are positive, as required by the method presented in Table 3 (41).
In many cases, non-specific late amplification curves were observed from materials that did not contain the SARS-CoV-2 target region, probably due to primer-dimer formation, and could not be avoided by the real-time RT-PCR technique. In these cases, it is necessary to establish a threshold Cq value lower than the Cq value of the non-target gene products. This threshold Cq should be included in the method acceptability assessment prior to specificity and sensitivity assessment because it affects sensitivity and specificity (39). The analytical specificity of the method for the detection of SARS-CoV-2 and other qualitative biological methods by real-time RT-PCR can also be verified by other appropriate techniques such as amplicon sequencing, gel electrophoresis, restriction enzyme analysis, or hybridization technique (40, 44, 45).
The relative specificity (diagnostic specificity) is the ability of the alternative method to not detect the analyte when it is not detected by the reference method (43). It is shown in the equation below (13, 43):
Where, DSp = Diagnostic specificity, TN = True negative, FP = False positive
The data for the calculation of diagnostic specificity consists of components calculated from the diagnostic specificity formula above. Experimental designs should use sufficient sample numbers that were confirmed negative by real-time RT-PCR. Preferably, depending on sample availability and laboratory conditions, approximately 250 patient specimens with confirmed real-time RT-PCR results can be used, according to the most recent guidelines of the United Kingdom Health Security Agency (46). These guidelines also request the provision of both the clinical specificity (95% CI) and the negative predictive value (NPV) of the diagnostic specificity study and to compare these with a reference method that is marked as CE (Conformité Européenne). The CT values or equivalent of diagnostic specificity for both the validated and reference methods must be included in the validation report (42).
Sensitivity
There are many documents describing the concept and method of determining sensitivity in real-time RT-PCR method validation, but it is necessary to distinguish between sensitivity (analytical sensitivity, LOD) and relative sensitivity (diagnostic sensitivity) (13, 39–43).
The sensitivity or LOD of a test refers to the minimum amount of a substance that can be detected by a method developed by a laboratory (39). In the SARS-CoV-2 detection method by real-time RT-PCR, LOD is defined as the minimum amount of viral RNA that can be successfully detected, yielding a positive result in 95% of the replicates (13, 39). It is essential when assessing the sensitivity of an assay to determine its ability to detect the minimum amount of the target gene that may exist in the sample. If the test’s sensitivity is low (LOD is high), the test will be limited in its ability to detect targets and may not be suitable for diagnostic use. To determine the sensitivity of the SARS-CoV-2 detection method by real-time RT-PCR, it is recommended to use standard materials containing known recombinant target gene regions of known concentrations and 10-fold dilution from the two of initial different concentrations, with six dilution points and repeated testing six times for each concentration point. The calibration curve can be established, and the ideal amplification efficiency is in the range of 80%–120% (but best amplification efficiency as close to 100% as possible) (13–15, 39), thereby giving the method detection limit; however this is not required for singleplex assays (39, 47).
The relative sensitivity (diagnostic sensitivity) is the ability of the alternative method to detect the analyte when it is detected by the reference method (43), as shown in the equation below (13, 43):
Where, DSe = Diagnostic sensitivity, TP= True positive, FN = False negative
The data for the calculation of diagnostic sensitivity consists of components calculated from the diagnostic sensitivity formula above. Experimental designs should use sufficient sample numbers that were confirmed positive by real-time RT-PCR. Preferably, depending on sample availability and laboratory conditions, approximately 150 patient specimens with confirmed real-time RT-PCR results should be used, according to the most recent guidelines of the United Kingdom Health Security Agency (46). Both the clinical sensitivity (95% CI) and the positive predictive value (PPV) of the diagnostic sensitivity study should be provided and compared against a reference method that is CE (Conformité Européenne) marked. The CT values or equivalent of diagnostic sensitivity for both the validated and reference methods must be included in the validation report (42).
Repeatability
Repeatability of measurement or precision of results is achieved when a test is repeated under the same conditions. Repeatability is measured by repeated testing with identical but independent samples in the same running time, in the same laboratory, by the same operator, and on the same machine, within the same short intervals of time (39).
Determination of repeatability is usually performed by quantitative assessment in real-time RT-PCR (43). Not many guidelines require repeatability calculations for real-time RT PCR detection of the target agent (39, 42). However, some laboratories have designed experiments and calculated the repeatability of the method based on the standard deviation and the mean of the results per run (42). For the assessment of the repeatability of a qualitative RT-PCR real-time test for SARS-CoV-2 detection, a design can be implemented that uses at least seven samples containing the target gene region of the test kit and three samples containing no target regions, and run at least three times; an alternative method that the laboratory has developed could also be used. In addition, the concentration of samples selected for the repeatability test should be carried out from at least four different RNA concentrations, including a concentration close to the LOD (39).
Reproducibility
Reproducibility is the agreement between results obtained from an experiment when repeated under different laboratory conditions. Reproducibility is measured by repeating the test on the same sample with the same method in different laboratories, with different operators, and on different systems (39). Similar to repeatability, determination of reproducibility is usually performed by quantitative assessment of real-time RT-PCR (43). Not many guidelines require reproducibility calculations for real-time RT-PCR detection of the target agent (39, 42). However, some laboratories have designed experiments and calculated the reproducibility of the method also based on the standard deviation and the mean of the results per run (42). For example, reproducibility assessment is carried out on at least seven RNA samples containing the target gene region, with the same test kit that the laboratory used to perform the test, and three RNA samples that do not carry the target gene are tested at least three times. Reproducibility was also tested on at least four different dilution concentrations, including dilutions close to the LOD (39).
Repeatability and reproducibility should only be tested, and the results reported as the number of samples giving the same results (positive/negative), when the test is repeated under the same conditions. False-positive and false-negative results should always be zero if the test has achieved confirmation of sensitivity and specificity (39).
Robustness
The robustness of a method is a measure of its capacity to remain unaffected by small, accidental changes in the environment or in the conditions in which the measurement was performed. In order to determine robustness, measurement results corresponding to small, deliberate changes in the measurement conditions are collected (15, 40, 41, 48).
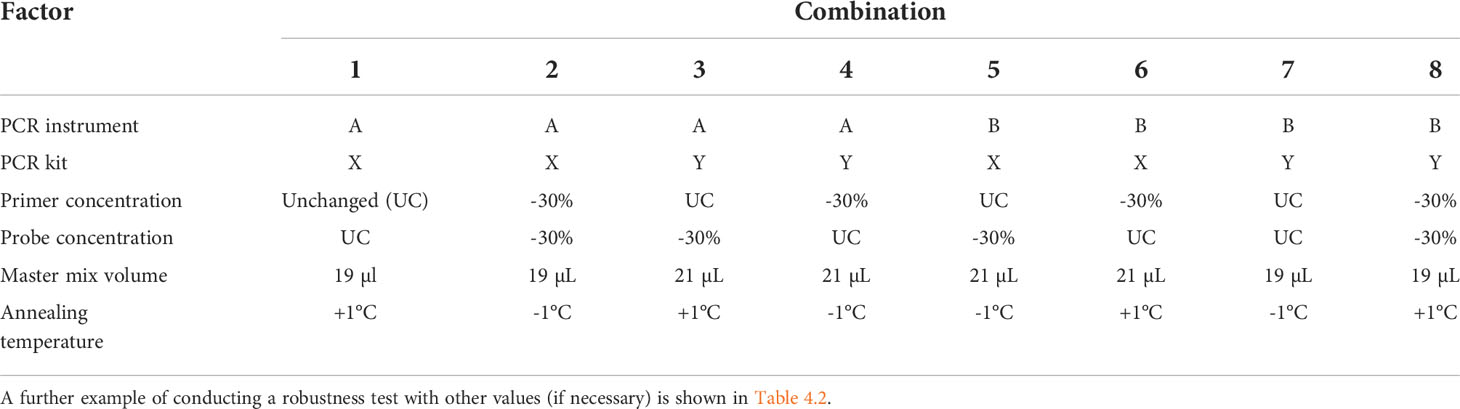
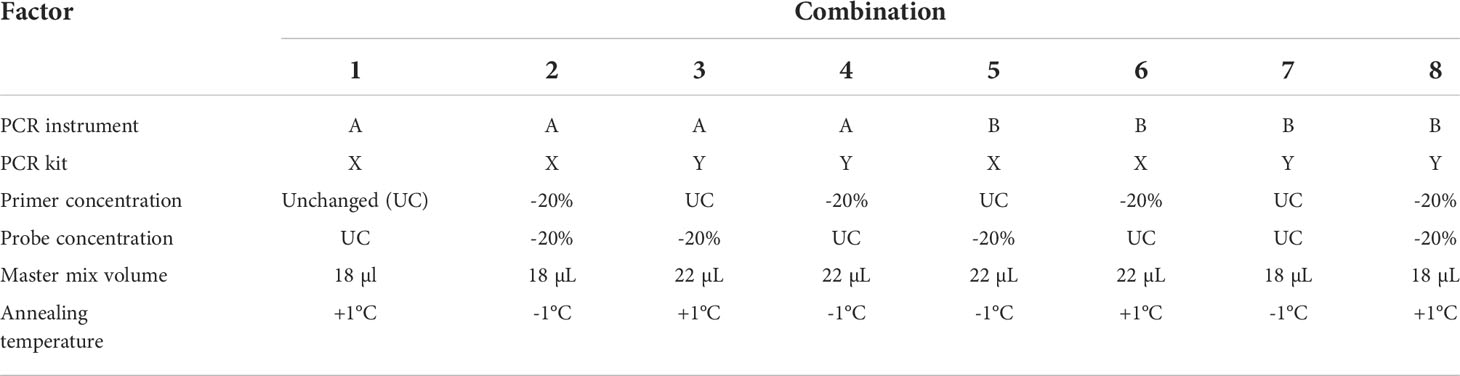
A multifactorial experimental design is implemented. For every combination of the factor levels, target DNA is added in a concentration around the LOQ. Dilute the target DNA in non-target DNA (“background” DNA, e.g., 20 ng/µl. For each factor-level combination, PCR tests should be performed in triplicate. An example for conducting a robustness test is shown in Table 4.1 (15, 41).
The method should yield positive results for all mixes despite the modified conditions. In the case of negative results, the PCR test for the corresponding mixes should be repeated. In the case of repeated negative results, the method is not sufficiently robust and needs to be optimized. Considerable deviations between Ct values could be an indication that the robustness of the method is insufficient (15).
Detection of antibodies to SARS-CoV-2
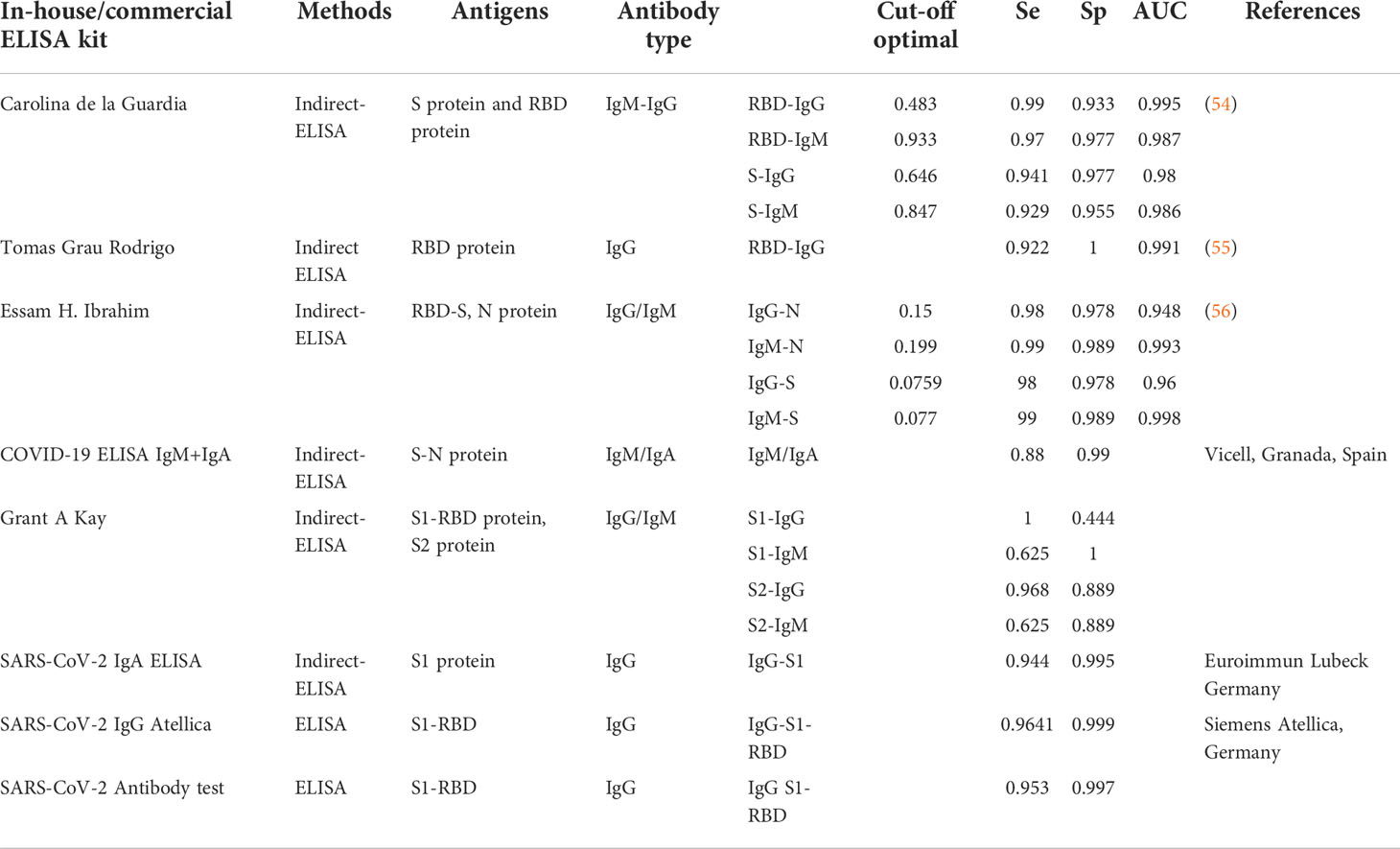
When a virus enters the human body, it reaches a certain concentration, and as a consequence, a certain amount of antigen also enters the body’s circulatory system. A healthy person’s immune system activates when this occurs and produces a large number of antibodies corresponding to the type of antigen it has received (IgM, IgG). The pool of antibodies persists in greater concentrations than antigens for a long time. When testing for viral antigens, researchers prefer to use serological antibodies, which are easier to detect and more responsive. Based on the principle of immunology, the detection of antibodies produced by the specific region of the virus will give more specific and accurate results than the detection of the whole antibody molecule (Ig). Several studies and clinical trials with different kits have selected antibodies specific to S or N proteins (IgM/IgA/IgG) as diagnostic targets for SARS-CoV-2 infection (49–52). In addition, several serum immunoassay kits have been developed by in-vitro-diagnostic (IVD) companies to detect SARS-CoV-2 virus proteins and antibodies in human serum or plasma. Current methods of detecting antibodies in serum include the enzyme-linked immunosorbent assays, chemiluminescence immunoassays, lateral flow immunoassays, and immune fluorescence assays (3, 51). Furthermore, several ELISA test kits have been developed and are available on the market for the detection of proteins N and S, and are also used for research purposes (53). In addition, some diagnostic kits use S1 subunits or receptor-binding domains (RBDs) as antigens to improve test specificity (See Table 5) (6, 51, 57, 58). In general, IgM and IgA antibodies appear in the early stages of infection, while IgG antibodies sometimes appear later (6). According to reports from the research of (51), serum IgA is positive five days after symptom onset, serum IgM is positive around days 10 to 30, and serum IgG is positive around days 20 to 90 when the body is infected with SARS-CoV-2. (51). An important point affecting the diagnostic yield of serological tests is the time of sampling after onset of symptoms because the composition, quantity, and structure of antibodies can change throughout the duration of the infection (59). The effectiveness of the COVID-19 serology test in the early stages of viral infection is unsatisfactory, but the serum antibody level will gradually increase until an antibody plateau is reached despite a decrease in antigens. As such, it makes more sense to use serological testing from the middle and late stages of COVID-19 infection (51). Serological tests to detect protective antibodies against pathogens have always received great interest, especially during the SARS CoV-2 pandemic. While IgA appears at the first week of symptom onset, high IgM and IgG can be detected from the second week of illness (51, 60, 61). If IgM and IgG SARS-CoV-2 antibodies are detected in the blood sample, this indicates that the individual has likely been infected recently. If only IgG is detected, this indicates that the individual has likely been infected in the past or in a later stage of infection. Therefore, it is recommended to use a kit for the simultaneous detection of both IgG and IgM antibodies (2-in-1) to increase the sensitivity of the method (51, 61).
Detection of SARS-CoV-2 antigens
There are several methods for antigen detection. Besides nucleic acid amplification assays (NAATs) used to detect active replicating viruses in the early stages of SARS-CoV-2 infection through RNA, detection of antigens based on SARS-CoV-2 proteins is another method. The structures of nucleocapsid protein (nucleoprotein), spike protein, membrane protein, and envelope protein are focused on by research institutes and commercial companies to detect SARS-CoV-2. Among them, the antigen detection kit applying nucleoprotein and spike protein is the most commonly used for SARS-CoV-2 detection (3, 59). The lateral flow immunoassay test, used to detect the viral nucleoprotein N and the most common rapid antigen test, has also been studied for its sensitivity with relatively large numbers (62). Samples used in serological detection contain an analyte (i.e., the antibody of interest) found in serum, plasma, and blood samples. In contrast, the analyte used in the protein antigen detection method is viral antigen. The viral antigen is extracted from synthetic swabs used to collect oropharyngeal and nasopharynx samples. Antigen detection kits used in the acute phase of SARS-CoV-2 infection include a protein extraction buffer (6). The detection of SARS-CoV-2 can also take place using specialized instruments and specific tools like mass spectrometry. This method takes a long time to perform, is difficult to conduct, and requires intensive training for staff (3), so it has become unpopular in diagnosis. Other methods for antigen detection use devices with the same operating principles as serological methods. Testing for viral antigens with large numbers of samples is also performed on enzyme immunoassay devices such as enzyme-linked immunosorbent assays for antibody detection. However, manufacturers and users prefer simple and rapid-result antigen detection that can be easily performed onsite such as lateral flow immunochromatographic assays or lateral flow assays (59). Instead of conducting RT-PCR, the detection of viral particles and antigens is a feasible solution for the screening of SARS-COV-2, due to its low cost, convenience, rapidity, and that it can be used to diagnose and screen patients in the early stages of viral infection. Several methods are commercialized and available as point-of-care tests. These techniques are simple and can be performed by patients and the general public as long as they follow the steps issued by the manufacturer (3).
The following parameters should be evaluated during method development and in-laboratory validation for antigen and antibody detection in SARS-CoV-2 infected/suspected specimens (61, 63, 64).
Conclusions
The validation method is used at the onset of SARS-CoV-2 infection. The testing method and conditions (facility, human resources, equipment, etc.) of each laboratory will decide what kind of parameters should be used for COVID-19 testing validation. The basic parameters for validation of the real-time RT-PCR method are sensitivity (analytical sensitivity, limit of detection, LOD), diagnostic sensitivity (relative sensitivity), specificity (analytical specificity), diagnostic specificity (relative specificity), repeatability, reproducibility, and robustness (if necessary). The basic parameters for the validation of the detection method of antigens/antibodies to SARS-CoV-2 are sensitivity (relative sensitivity), specificity (relative specificity), positive predictive value, and negative predictive value. The reference method should be marked CE (Conformité Européenne). In addition to the main parameters for COVID-19 testing validation above, the applicability, practicability, time to results, and cost per test should not be ignored.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, et al. A new coronavirus associated with human respiratory disease in China. Nature (2020) 579:265–9. doi: 10.1038/s41586-020-2008-3
2. WORLDOMETER. 2022. COVID-19 CORONAVIRUS PANDEMIC [Online]. Worldometer.info/coronavirus/. [Accessed].
3. Filchakova O, Dossym D, Ilyas A, Kuanysheva T, Abdizhamil A, Bukasov R. Review of COVID-19 testing and diagnostic methods. Talanta (2022) 244:123409. doi: 10.1016/j.talanta.2022.123409
4. Schildgen V, Demuth S, Lüsebrink J, Schildgen O. Limits and opportunities of SARS-CoV-2 antigen rapid tests: An experienced-based perspective. Pathogens (2021) 10(1):38. doi: 10.3390/pathogens10010038
5. Abbott TR, Dhamdhere G, Liu Y, Lin X, Goudy L, Zeng L, et al. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell (2020) 181:865–876.e12. doi: 10.1016/j.cell.2020.04.020
6. Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat Biotechnol (2020) 38:870–4. doi: 10.1038/s41587-020-0513-4
7. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus, (2019-nCoV) by real-time RT-PCR. Euro. surveill. (2020) 25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045
8. Chen L, Liu W, Zhang Q. RNA Based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 wuhan outbreak. Emerg Microbes Infect (2020) 9:313–9. doi: 10.1080/22221751.2020.1725399
9. Sharma K, Aggarwala P, Gandhi D, Mathias A, Singh P, Sharma S, et al. Comparative analysis of various clinical specimens in detection of SARS-CoV-2 using rRT-PCR in new and follow up cases of COVID-19 infection. Quest Best choice (2021) 16:e0249408. doi: 10.1371/journal.pone.0249408
10. Udvardi MK, Czechowski T, Scheible W-RD. Eleven golden rules of quantitative RT-PCR. Plant Cell (2008) 20:1736–7. doi: 10.1105/tpc.108.061143
11. Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinf (2005) 62. doi:10.1186/1471-2105-6-62
12. Sherina V, Mcmurray H, Powers W, Land H, Love T, Mccall M. Statistical approaches to decreasing the discrepancy of non-detects in qPCR data. bioRxiv 231621 (2017). 1–18 doi: 10.1101/231621
13. Arnaout R, Lee RA, Lee GR, Callahan C, Cheng A, Yen CF, et al. The limit of detection matters: The case for benchmarking severe acute respiratory syndrome coronavirus 2 testing. Clin Infect Dis (2021) 73:e3042–6. doi: 10.1093/cid/ciaa1382
14. Charles-Delobel C, Burns M, Charels D, Ciabatti I. Verification of analytical methods for GMO testing when implementing interlaboratory validated methods. JRC Sci Tech Rep (2011) 1:1–22. doi: 10.2788/87679.
15. Lutz Grohmann ED, Hermann Broll. Guidelines for the single-laboratory validation of qualitative real-time PCR methods. ResearchGate (2016).
16. Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z, et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med (2020) 201:1435–8. doi: 10.1164/rccm.202003-0572LE
17. Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis (2020) 20:411–2. doi: 10.1016/S1473-3099(20)30113-4
18. Meagan N, Esbin ONW, Chong S, Maurer A, Darzacq X, Tjian R. Overcoming the bottleneck to widespread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA (2020) 26:771–83.
19. Forootan A, Sjöback R, Björkman J, Sjögreen B, Linz L, Kubista M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol. Detect. Quantif. (2017) 12:1–6. doi: 10.1016/j.bdq.2017.04.001
20. Nutz S, Döll K, Karlovsky P. Determination of the LOQ in real-time PCR by receiver operating characteristic curve analysis: application to qPCR assays for fusarium verticillioides and f. proliferatum. Analyt. Bioanalyt. Chem (2011) 401:717–26. doi: 10.1007/s00216-011-5089-x
21. Wikramaratna PS, Paton RS, Ghafari M, Lourenço J. Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR. Euro. Surveill. (2020) 25(50). doi: 10.2807/1560-7917.ES.2020.25.50.2000568
22. Xiao AT, Tong YX, Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. (2020) 92:1755–6.
23. Tsang NNY, So HC, Ng KY, Cowling BJ, Leung GM, Ip DKM. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. Lancet Infect Dis (2021) 21:1233–45. doi: 10.1016/S1473-3099(21)00146-8
24. WHO. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network (2020) (Accessed June 09, 2021).
25. Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen HL, Chan MCW, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe (2020) 1:e10. doi: 10.1016/S2666-5247(20)30003-3
26. Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson N, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med (2020) 382:1564–7.
27. Vogels CBF, Brito AF, Wyllie AL, Fauver JR, Ott IM, Kalinich CC, et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat Microbiol (2020) 5:1299–305. doi: 10.1038/s41564-020-0761-6
28. Brown JR, O'sullivan DM, Shah D, Atkinson L, Pereira RPA, Whale AS, et al. Comparison of SARS-CoV-2 n gene real-time RT-PCR targets and commercially available mastermixes. J Virol Methods (2021) 295:114215. doi: 10.1016/j.jviromet.2021.114215
29. Nayar G, Seabolt EE, Kunitomi M, Agarwal A, Beck KL, Mukherjee V, et al. Analysis and forecasting of global real time RT-PCR primers and probes for SARS-CoV-2. Sci Rep (2021) 118988.
30. Hu B, Guo H, Zhou P, Shi ZL. Author correction: Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol (2022) 20:315. doi: 10.1038/s41579-022-00711-2
31. Jung Y, Park G-S, Moon JH, Ku K, Beak S-H, Lee C-S, et al. Comparative analysis of primer-probe sets for RT-qPCR of COVID-19 causative virus (SARS-CoV-2). ACS Infect Dis (2020) 6:2513–23. doi: 10.1021/acsinfecdis.0c00464
32. Dharavath B, Yadav N, Desai S, Sunder R, Mishra R, Ketkar M, et al. A one-step, one-tube real-time RT-PCR based assay with an automated analysis for detection of SARS-CoV-2. Heliyon (2020) 6:e04405. doi: 10.1016/j.heliyon.2020.e04405
33. Malekshahi A, Khanizadeh S, Fallahi S, Talei G, Birjandi M, Hajizadeh F. Diagnostic power of one-step and two-step RT-qPCR methods to SARS−CoV−2 detection. BMC Infect Dis (2022) 22:505. doi: 10.1186/s12879-022-07478-0
34. Clementi M, Menzo S, Bagnarelli P, Manzin A, Valenza A, Varaldo PE. Quantitative PCR and RT-PCR in virology. PCR Methods Appl (1993) 2:191–6. doi: 10.1101/gr.2.3.191
35. Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun (2005) 6:279–84. doi: 10.1038/sj.gene.6364190
36. COMMISSION. Current performance of COVID-19 test methods and devices and proposed performance criteria. DocsRoom - European Commission (2020), 13–20.
37. COMMISSION. Guidelines on performance criteria and validation of methods for detection, identification and quantification of specific DNA sequences and specific protiens in foods. CAC/GL (2010), 74–2010: 1–22
38. Dinon AZ, Prins TW, Van Dijk JP, Arisi ACM, Scholtens IMJ, Kok EJ. Development and validation of real-time PCR screening methods for detection of cry1A.105 and cry2Ab2 genes in genetically modified organisms. Analyt. Bioanalyt. Chem (2011) 400:1433–42. doi: 10.1007/s00216-011-4875-9
39. Shehata HR, Ragupathy S, Shanmughanandhan D, Kesanakurti P, Ehlinger TM, Newmaster SG. Guidelines for validation of qualitative real-time PCR methods for molecular diagnostic identification of probiotics. J AOAC Int (2019) 102:1774–8. doi: 10.5740/jaoacint.18-0320
40. Broeders SIH, Rohmann L, Berben G, Taverniers I, Mazzara M, Roosens N, et al. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci Technol (2014) 37:12.
41. Administration. Guidelines for the validation of analytical methods for nucleic acid sequence-based analysis of food, feed, cosmetics and veterinary products. U.S Food and Drug Administratio Foods Program (2020).
42. Agency. Technical validation protocol for SARS-CoV-2 nucleic acid detection. GOV.UK (2022). Available at: https://www.gov.uk.
43. Standardization IOF. Microbiology of the food chain — method validation. In: Part 4: Protocol for method validation in a single laboratory. International Organization for Standardization ISO 16140-4:2020 (2020).
44. Mazzara M, Savini C, Delobel C, Broll H, Damant A, Paoletti C, et al. Definition of minimum performance requirements for analytical methods of GMO testing. Ispra.: Joint Res Centre. (2008). 1:1–8. doi: 10.2788/65827
45. Toohey-Kurth K, Reising MM, Tallmadge RL. Suggested guidelines for validation of real-time PCR assays in veterinary diagnostic laboratories. Inc (2020) 32(2020):802–14. doi: 10.1177/1040638720960829
46. UKHSA. Technical validation protocol for SARS-CoV-2 nucleic acid detection. GOV.UK (2022). Available at: https://www.gov.uk.
47. Birch L, Dawson C, Cornett J, Keer J. A comparison of nucleic acid amplification techniques for the assessment of bacterial viability. Lett Appl Microbiol (2001) 33:296–301. doi: 10.1046/j.1472-765X.2001.00999.x
48. UKHSA. Technical validation protocol for SARS-CoV-2 nucleic acid detection. GOV.UK (2022). Available at: https://www.gov.uk.
49. Chauhan N, Soni S, Jain U. Optimizing testing regimes for the detection of COVID-19 in children and older adults. Expert Rev Mol Diagn (2021) 21:999–1016. doi: 10.1080/14737159.2021.1962708
50. Shirazi S, Stanford CM, Cooper LF. Testing for COVID-19 in dental offices: Mechanism of action, application, and interpretation of laboratory and point-of-care screening tests. J Am Dent Assoc (2021) 152:514–525 e8. doi: 10.1016/j.adaj.2021.04.019
51. Gong F, Wei HX, Li Q, Liu L, Li B. Evaluation and comparison of serological methods for COVID-19 diagnosis. Front Mol Biosci (2021) 8:682405. doi: 10.3389/fmolb.2021.682405
52. Sharma A, Ahmad Farouk I, Lal SK. COVID-19: A review on the novel coronavirus disease evolution, transmission, detection, control and prevention. Viruses (2021) 13(2):202. doi: 10.3390/v13020202
53. Vashist SK. In vitro diagnostic assays for COVID-19: Recent advances and emerging trends. Diagn. (Basel) (2020) 10(4):202. doi: 10.3390/diagnostics10040202
54. de La Guardia C, Rangel G. Development of in-house, indirect ELISAs for the detection of SARS-CoV-2 spike protein-associated serology in COVID-19 patients in Panama. PloS one (2021) 16(9). doi: 10.1371/journal.pone.0257351
55. Tomasgrau R, Ploper D, Avila CL, Verapingitore E, Maldonado C, Chaves S, et al. An “In-House” ELISA for SARS-CoV-2 RBD uncovers elevated immune response at higher altitudes. (2021).
56. Ibrahim EH, Ghramh HA, Kilany M. Development of in-house ELISAs for the detection of anti-SARS−CoV−2 RBD and n IgG and IgM antibodies in biological samples. J King Saud Univ - Sci (2021) 33:101439. doi: 10.1016/j.jksus.2021.101439
57. Ma H, Zeng W, He H, Zhao D, Jiang D, Zhou P, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol (2020) 17:773–5. doi: 10.1038/s41423-020-0474-z
58. Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature (2021). doi: 10.1038/s41586-021-04385-3
59. Safiabadi Tali SH, Leblanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, et al. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev (2021) 34(3):e00228-20. doi: 10.1128/CMR.00228-20
60. Peeling RW, Heymann DL, Teo YY, Garcia PJ. Diagnostics for COVID-19: moving from pandemic response to control. Lancet (2021) 399(10326):757–68. doi: 10.1016/S1473-3099(21)00291-7
61. Mundodan J, Hasnain S, Khogali H, Al Bayat SS, Ali D, Alateeg S, et al. Validation of rapid antibody (IgG-IgM) test kit for SARS-CoV-2 infection in Qatar. J Public Health Res (2021) 11(1):2421. doi: 10.4081/jphr.2021.2421
62. Scheiblauer H, Filomena A, Nitsche A, Puyskens A, Corman VM, Drosten C, et al. Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV-2 antigen, Germany, September 2020 to April 2021. Euro. Surveill. (2021) 26(44):2100441. doi: 10.2807/1560-7917.ES.2021.26.44.2100441
63. Chaimayo C, Kaewnaphan B, Tanlieng N, Athipanyasilp N, Sirijatuphat R, Chayakulkeeree M, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J (2020) 17:177. doi: 10.1186/s12985-020-01452-5
64. Fernandez-Monteroa A, Argemi J, Rodríguez JA, Cariño AH, Moreno-Galarraga L. Validation of a rapid antigen test as a screening tool for SARS-CoV-2 infection in asymptomatic populations. sensitivity, specificity and predictive values. EClinicalMedicine (2021) 37:100954. doi: 10.1016/j.eclinm.2021.100954
Keywords: COVID-19, SARS-CoV-2, validation, verification method, detection
Citation: Chung LTK, Vung ND, Uyen NT, Hanh BTM, Huong LT, Hien PT, Xuan LTT, Ha NT and Dat DX (2022) A brief review on the validation of biology methods for COVID-19 detection. Front. Trop. Dis 3:1013130. doi: 10.3389/fitd.2022.1013130
Received: 06 August 2022; Accepted: 16 September 2022;
Published: 17 November 2022.
Edited by:
Suraj Bhattarai, Global Institute for Interdisciplinary Studies (GIIS), NepalReviewed by:
Zhen Qin, University of Toronto, CanadaCopyright © 2022 Chung, Vung, Uyen, Hanh, Huong, Hien, Xuan, Ha and Dat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Thi Kim Chung, bGVraW1jaHVuZ0BobXUuZWR1LnZu; Dao Xuan Dat, RGFveHVhbmRhdEBobXUuZWR1LnZu
†These authors have contributed equally to this work
 Le Thi Kim Chung
Le Thi Kim Chung Nguyen Dang Vung1
Nguyen Dang Vung1 Ngo Thi Uyen
Ngo Thi Uyen Bui Thi Minh Hanh
Bui Thi Minh Hanh Le Thi Huong
Le Thi Huong Le Thi Thanh Xuan
Le Thi Thanh Xuan Dao Xuan Dat
Dao Xuan Dat