- 1Department of Pharmaceutical Microbiology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- 2Department of Epidemiology and Biostatistics, School of Public Health and Social Sciences, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- 3Tanzania Medicines and Medical Devices Authority, Dar es Salaam, Tanzania
- 4Regulation and Prequalification Department, World Health Organization, Geneva, Switzerland
- 5Department of Pharmacy, Kampala International University in Tanzania, Dar es Salaam, Tanzania
- 6Department of Biochemistry, School of Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
Background: With increased livestock keeping, multiple prevailing infections, antimicrobial agents’ use and pattern in Tanzania, the development of antimicrobial resistance (AMR) becomes inevitable. Antibiotic-resistant pathogens have increasingly become a major challenge in human and animal medicine. Although inappropriate use of antibiotics in humans is the principal cause of resistance, antibiotic-resistant bacteria originating from animals contribute to the emergence and spread of these bacteria. Antibiotics help control a multitude of bacterial infections that are major causes of diseases in both animals and humans. Rational use in animals is crucial to control any development and transfer of AMR to humans. This study aimed to create quantitative evidence of animal antimicrobial usage patterns in Tanzania to serve as a baseline for surveillance of antimicrobial use and antimicrobial resistance control.
Methodology: This descriptive longitudinal retrospective study was conducted to explore the trend of veterinary-antibiotics consumed in the eight years, from 1st January 2010 to 31st December 2017 in Tanzania mainland. The data source was records of all antibiotics imported for veterinary use into Tanzania’s mainland according to the Tanzania Medicines and Medical Devices Authority (TMDA) records. The analysis employed the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology using Anatomical Therapeutic and Chemical (ATC) classification. Regression and time series analysis was used to establish trends in antibiotics consumption.
Results: A total of 12,147,491 kg of antibiotics were consumed in Tanzania from 2010 to 2017. Tetracycline, sulfonamides and trimethoprim, quinolones, aminoglycosides, beta-lactams and antibacterial combinations were the most commonly used antibacterial agents in Tanzania. Tetracycline class topped the list with about 8,090,798 kg (66.6%) out of 12,147,491 kg total quantity of antimicrobials consumed. Non-significant, linear curve estimations and time series analysis indicate a decline in the quantities of veterinary antibiotics used in the eight years from 2010 to 2017.
Conclusions: This study suggests that tetracycline is the most used antibiotic class for veterinary medicine in Tanzania. The trend of antimicrobial use is generally decreasing compared to other countries in Africa. Even though some antibiotics have the lowest consumption rate, they are also prone to AMR, prompting follow-up by the relevant regulatory authorities.
Introduction
Tanzania is ranked third among sub-Saharan African countries in having the largest livestock population in the continent with contribution by category as follows: chickens (86% of the households), goats (48%), cattle (35%), pigs (9%) and other animals accounting (10%) form which meat production accounts for an estimated 662,931 metric tonnes (1). The increased keeping of and demand for food of animal origin, particularly eggs, meat and milk, has led to intensification and commercial production systems where excessive use and misuse of antimicrobials may be inevitable (2). The National Action Plan 2017-2022 addresses actions needed to combat antimicrobial resistance (AMR) in the country where both humans and animals are taken on board (1). The action plan emphasizes raising awareness of antimicrobial resistance and promoting behavioral change through public communication programs targeting human, animal, and plant health.
The regulation pharmaceutical sector in Tanzania is the responsibility of the Tanzania Medicines and Medical Devices Authority (TMDA) (3), enacted by the Food, Drugs and Cosmetics Act, 2003 and later The Tanzania Medicines and Medical Devices Act, Cap 219 of 2019. On the one hand, TMDA regulates the quality, safety and effectiveness of medicines, medical devices and diagnostics. On the other hand, the regulation of private pharmacy practice in Tanzania is regulated by the Pharmacy Council (PC) of Tanzania (4) enacted by the Pharmacy Act, 2011. However, it is common in Tanzania to find an overwhelming proportion of medicines sold in pharmacies are dispensed without a prescription (4, 5), increasing the chance of unregulated sales of antimicrobials both for human and veterinary use.
In practice, antibiotics are used therapeutically or off-labeled to treat animal bacterial infections, promote animal growth or prophylaxis in intensive animal rearing (6). Antimicrobial treatment is pivotal to ensure animal welfare and to control infectious diseases, including zoonotic conditions. Antimicrobials prevent disease spread among animal lots, which is most important for poultry, fish (7) and other livestock. Specific mass treatment methods are designed to medicate unhealthy animals while treating the rest of the flock to avoid disease spread (7–9). Unlike most human diseases, where decisions are generally made about the need for individual therapy, the veterinary practice uses empiric treatment for the entire population (10).
Antimicrobial resistance (AMR) is a major global threat to human and animal health, endangering human and veterinary medicine, thus hindering their success prospects. Unfortunately, we can hardly spate resource-limited settings, including Tanzania, from the high burdens of infectious diseases where antibiotics are widely used to save lives (11). The wide use of antimicrobial substances coupled with inappropriate antibiotic use, spurious, falsified, and counterfeit antimicrobial often slipping into the medicine supply chain in animals contribute to heighten the magnitude of the AMR problem (12). A recent study was conducted along Msimbazi River, Dar es Salaam, Tanzania, on the drivers of antimicrobial use and resistance in poultry and domestic pig farming. The study revealed uncontrolled use of antibiotics both in animals coupled with environmental contamination that may exacerbate the development and spread of antimicrobial resistance (13). This study found a higher proportion of usage of veterinary antimicrobials for prophylactic than for therapeutic purposes in animal farming. Studies in fish farming in Tanzania have indicated the risk of antibiotic use to both human and aquatic animals’ health, including the development of resistance (2, 14–17) and resistance genes (17), particularly the heavy and uncontrolled use of antimicrobials (18) and recommended for policy change in use of antibiotics in aquaculture (19).
The recent widespread use of antibiotics in the food chain poses a significant public health risk in humans. Antibiotic resistance in humans hikes human medical care expenses reduces antibiotics’ efficacy and subsequently increases human morbidity and mortality. This is explained by the fact that exposing the bacteria to sub-optimal antibiotic levels may facilitate the emergence and spreading of resistant strains in humans and animals by exerting a selective pressure on bacteria. The selective pressure will kill the susceptible bacteria and leave resistant strains to replicate (20). Many of the antibiotics used in food-producing animals are the same as or surrogates of those used in human medicine (21). Therefore, the antimicrobial resistance that originated from the application of antimicrobials in the food chain may consequently transmit resistant strains to animals, animal products and the environment (7, 22, 23). Antibiotic-resistant bacteria are normally transferred to the general public through food consumption, the environment and farm workers (21, 24–26). In a study involving seven countries in Europe, a direct relationship between antimicrobial use and antimicrobial resistance in Escherichia coli isolates from pigs, poultry and cattle (27) was demonstrated. Infections such as non-typhoidal salmonellosis, campylobacteriosis, and methicillin-resistant Staphylococcus aureus (MRSA) can spread to animals and humans at the same time. This brings more challenges in treating antibiotic-resistant strains originating from food-producing animals (21, 25).
Experts suggest that antibiotic consumption surveillance can help countries identify antibiotic use problems and, thus, device interventions to tackle antimicrobial resistance (28). Surveillance of antimicrobials uses helps in comparisons of consumption patterns between countries to set targets and spot antibiotic use changes (28). The goal is to control the amount of antimicrobials used in animals to generate objective quantitative information to evaluate antimicrobial class used in different animal species (10). Data obtained are essential for risk analysis and planning. Also, it helps interpret resistance surveillance data and evaluate the success of prudent use and mitigation strategies (10) when used in conjunction with hospitals’ utilization and resistance data. Surveillance of antimicrobial use can also be useful for a specific country evaluating the trends of antibiotic consumption per the number of animals.
Antimicrobial resistance is a prevailing global challenge rather than a future concern that affects Tanzania too. However, antimicrobials consumption data is scanty and rarely reported in low- and middle-income countries. In Tanzania, national data on antimicrobial consumption for veterinary usage is not yet well documented. Recent data shows that antimicrobial use (17) in humans is on the increasing trend. However, data on antimicrobial resistance in animals had been reported even in the 1990s, where resistance to antimicrobials in common use, such as ampicillin, tetracycline and trimethoprim-sulfamethoxazole, were significant (29, 30).
The objective of this study was to generate quantitative information for monitoring antimicrobial consumption in Tanzania using WHO Collaborating Centre for Drug Statistics Methodology. The results can be used as baseline information for future monitoring of antimicrobial consumption and resistance in food-producing and non-food-producing animals in Tanzania and beyond.
Materials and Methods
Study Design
This descriptive longitudinal retrospective study was conducted to document the trend of veterinary-antibiotics imported in the eight years from 1st January 2010 to 31st December 2017 in Tanzania mainland, Eastern Africa.
Study Setting
Tanzania, officially known as the United Republic of Tanzania, is located at a latitude of 6.3690° S. The longitude is 34.8888° E, bordered by eight countries with the Indian Ocean along the eastern border. Uganda is to the north, Malawi and Mozambique are found to the south. Zambia is on the southwest border and Kenya is northeast. The Democratic Republic of Congo, Rwanda, and Burundi are three countries on the western border. These borders are the point of entry of imported medicines into the country in addition to the sea harbor and airports. The ports of entries of medicines imported to Tanzania include Dar es Salaam airports and sea harbor (6.7924° S, 39.2083° E), Kilimanjaro airport (3.4245° S, 37.0651° E) border checkpoints at Sirari at 1.2512° S, 34.4763° E, Horohoro ay 6.369° S and 34.8888° E, Namanga (Kenya border), Tunduma at 9.3096° S, 32.7689° E (Zambia border) and Mutukula at 1.0007° S, 31.4156° E (Uganda border).
Data Sources
The study used the data of all antibiotics imported for veterinary use into Tanzania’s mainland according to importation records of the Tanzania Medicines and Medical Devices Authority (TMDA), which was called Tanzania Food and Drug Authority (TFDA) before 2019. Collected data were captured from different entry ports, including Tanzania airports, border checkpoints at Sirari, Horohoro, Namanga, Tunduma, Mutukula and the seaport in Dar es Salaam.
Antibiotics Studied
All antibiotics imported into Tanzania mainland for veterinary use during the study period were included in the study. Data for veterinary antibiotics whose records were not completed were excluded from the study.
Data Collection
Data were extracted from the TMDA importation database. TMDA is a medical products regulatory authority that regulates and approves the importation of medicines into the Tanzania mainland market. The importation procedure set up by TMDA compels importers to apply for an importation permit at TMDA. Successful applications are issued with import permits and recorded in the TMDA’s Management Information System (MIS).
Records of antibiotics imported in eight years, between 2010 and 2017, were extracted from the TMDA’s MIS by research assistants with the help of the TMDA’s import and export unit staff. The WHO Anatomical Therapeutic and Chemical (ATC) classification (31) system promulgated by the WHO Collaborating Centre for Drug Statistics Methodology (32) was used to filter antimicrobial data for each product from the database. The list of all antibiotics by generic name with their veterinary ATC codes was navigated under its section of the vetATC system to classify veterinary medicines, similar to the system used for human medicines. The list includes “antibacterial for systemic use” (vetATC level 1 = QJ01). The exclusion criteria was a medicine not belonging to the vetATC code QJ01.
Each antibiotic in the list was searched in the TMDA’s MIS, and data was queried, retrieved and each antibiotic recorded as a separate Microsoft Excel file. The importation record included the information about TMDA registration number, the year the permit was approved, the dosage, the drug dosage form, the generic name of the drug, strength, pack size, the vetATC classification of the drug, the quantity imported, country of origin, and the date of importation.
Data Cleaning and Analysis
Data cleaning was done and data were checked for accuracy, completeness and reliability before analysis. Data files were combined, pivoted and aggregated using Microsoft Excel 2013 (Microsoft Corporation, Redmond, Washington, USA). We converted the strength, pack size and the quantity of the antibiotic product in mg, g and kg. Generic names were harmonized to match the names in the vetATC mapping file. We assigned each product a corresponding vetATC code by matching it with the imported product’s generic name. This mapping allowed the matching of the ATC category and the pharmaceutical category. The amount in a kilogram of an antibiotic agent’s active ingredient was calculated and aggregated for each class collected. Tables, charts and graphs were plotted to present the trends in antibiotic consumption. Annual consumption data, aggregated per overall and ATC classes consumption, were entered into the Statistical Package for the Social Sciences (SPSS) version 20 (IBM Corp., Armonk, NY, USA). Time series and regression analysis were performed to ascertain the annual trend of antibiotic consumption. An autoregressive integrated moving average (ARIMA) model was established to predict the trends of antibiotics. A P-value of less than 0.05 was considered statistically significant.
Ethical Considerations
Ethical clearance for this study was sought and granted by the Muhimbili University of Health and Allied Sciences Institutional Review Board with reference number DA.25/111/01/03/2018. Permission to access the data was sought at the TMDA. The names and identifiers of the importer were not recorded.
Results
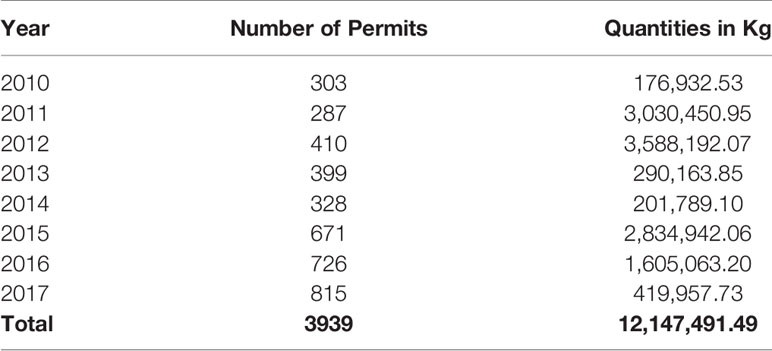
A total of 3,939 import permits were issued by TMDA between 2010 and 2017. The quantities issued were equal to 12,147,491.49kg of veterinary antibiotics, annual mean (SD) 1,518,436.44 (± 1,442,184.33) kg with maximum importation occurring in 2012, where a total of 3,588,192.07 kg of veterinary antibiotics were imported (Table 1).
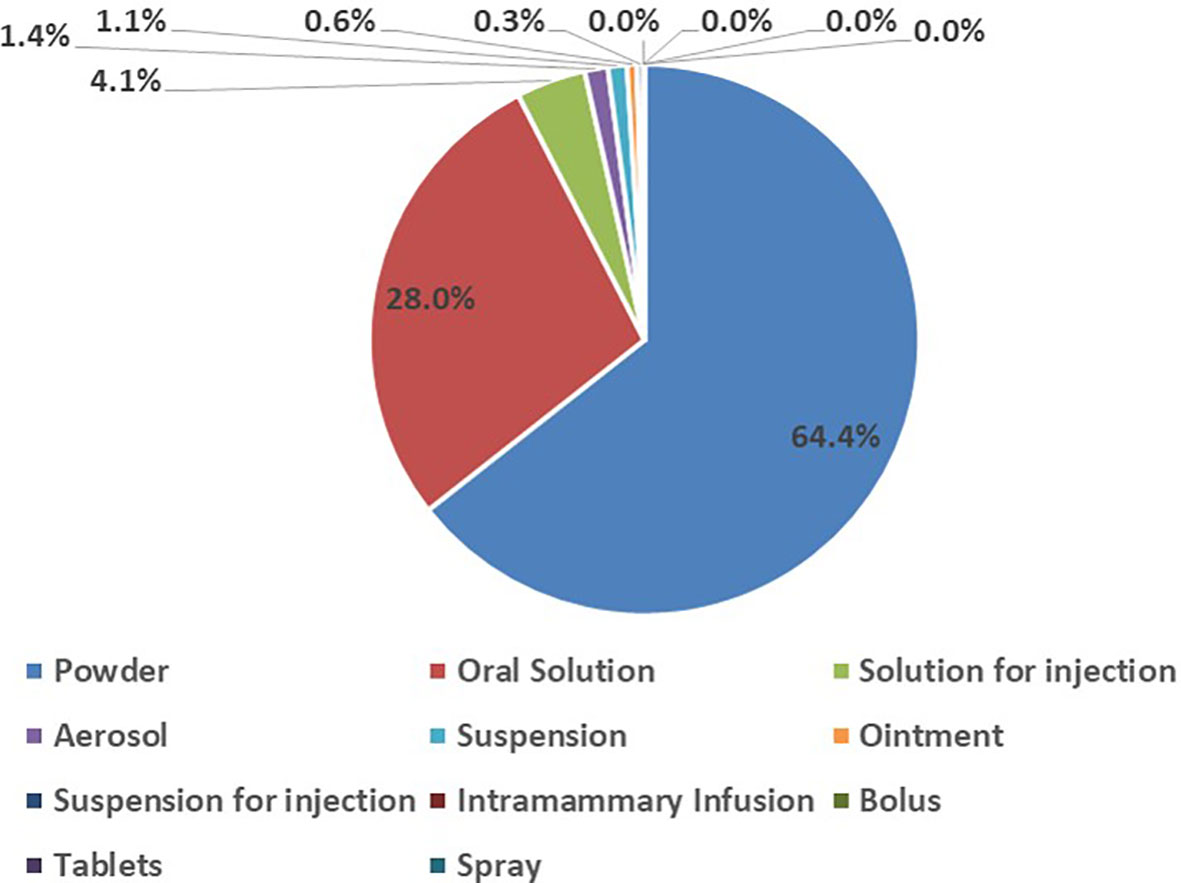
In the study, various dosage forms of antibiotics for veterinary use were consumed during the eight years. The majority of the antibiotics consumed were in powder form (64.4%), followed by oral solutions (28.0%). Solutions for injections, bolus, tablets, intramammary infusions and spray were the least consumed forms of antibiotics for veterinary use in the eight years (Figure 1).
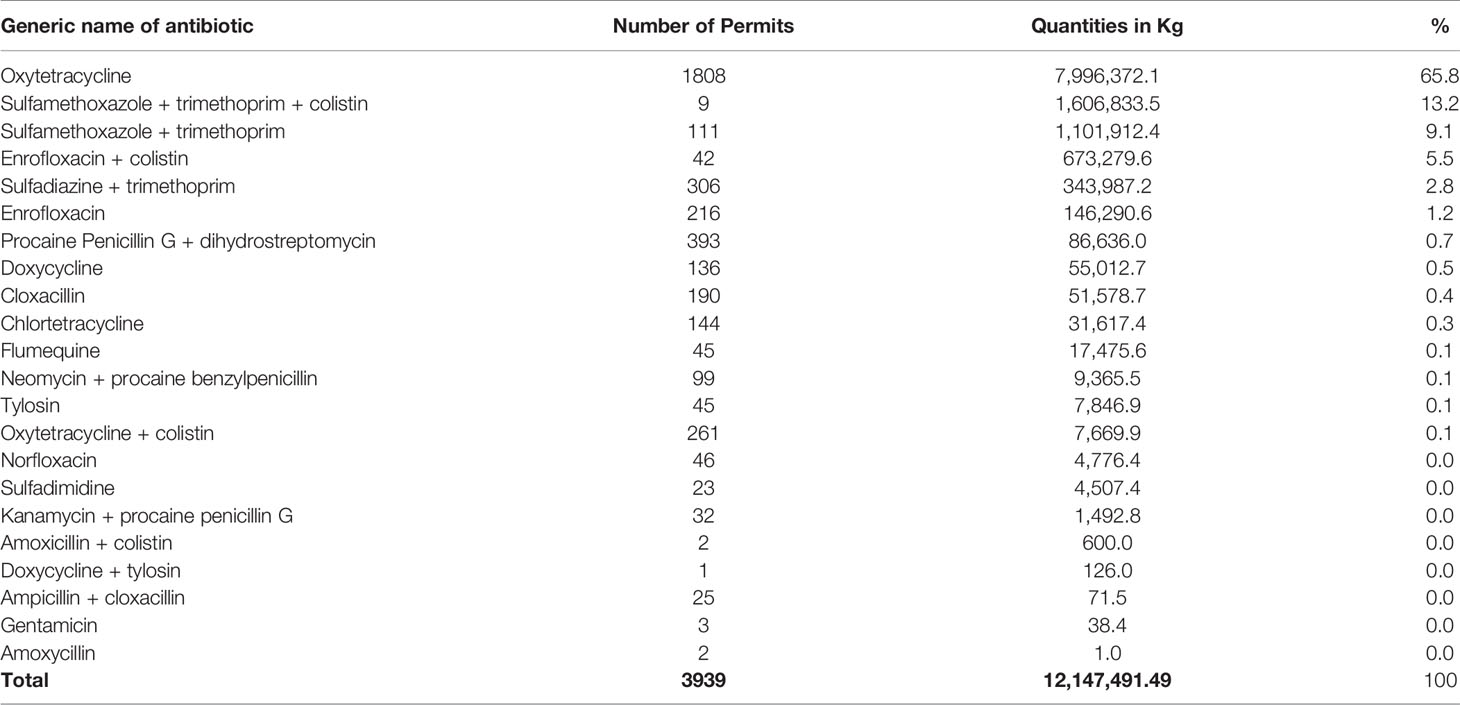
This study shows the total quantities of each antibiotic consumed in eight years (Table 2). Oxytetracycline and sulfamethoxazole-trimethoprim combination, with or without colistin, contributed 88.1% of all consumed antibiotics for veterinary use in Tanzania mainland. Oxytetracycline contributed over two-thirds of all the consumed antibiotics for veterinary use in the study period. Doxycycline + tylosin, ampicillin + cloxacillin and gentamicin were the least consumed antibiotics in the eight years under study (Table 2).
Consumption Trends of Antibiotics for Veterinary Use
The highest consumption noted was in 2012, and 2011 where a total of 3,588,191 kg and 3,030,450 kg of antibiotics were consumed in those years, respectively. Sulfonamides and trimethoprim contributed the highest proportion in 2012 with 2,705,481 kg, while tetracycline contributed the highest in 2011 by consumption of 3,009,223 kg. The least consumption was noted in 2010, when only 176,932 kg were consumed, out of which tetracyclines contributed 122,140 kg of total consumption in that year (Table 3).
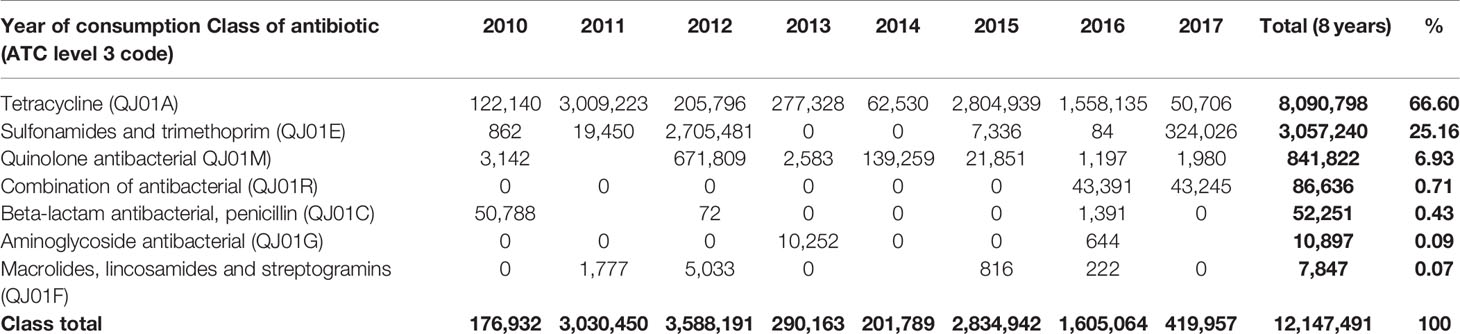
Table 3 Quantities in kg of the most consumed class of antibiotics by vetATC level 3 from 2010-2017.
Major classes of antibiotics consumed during eight years were tetracycline (8,090,798), Sulfonamides, and trimethoprim (3,057,240 kg). Other classes include quinolones, aminoglycosides, beta-lactams and penicillin antibiotics (Table 3). Tetracycline is the leading consumed antibiotic, with maximum consumption in 2015 and the only class consumed each year from 2010-2017. The consumption of combination antibiotics began in 2016.
A linear curve estimation shows that, over the eight years, from 2010 to 2017, consumption of antibiotics for veterinary use generally decreased with fluctuation noted between the years (Figure 2). However, the trend of the linear model was not statistically significant. Although not statistically significant, the consumption of the trend of tetracycline was constant over eight years, while the decline in the consumption of quinolones and sulfonamides and trimethoprim were responsible for the overall decline (Figure 2). Time series analysis, the data could not significantly fit the ARIMA model to predict the trends of antibiotics.
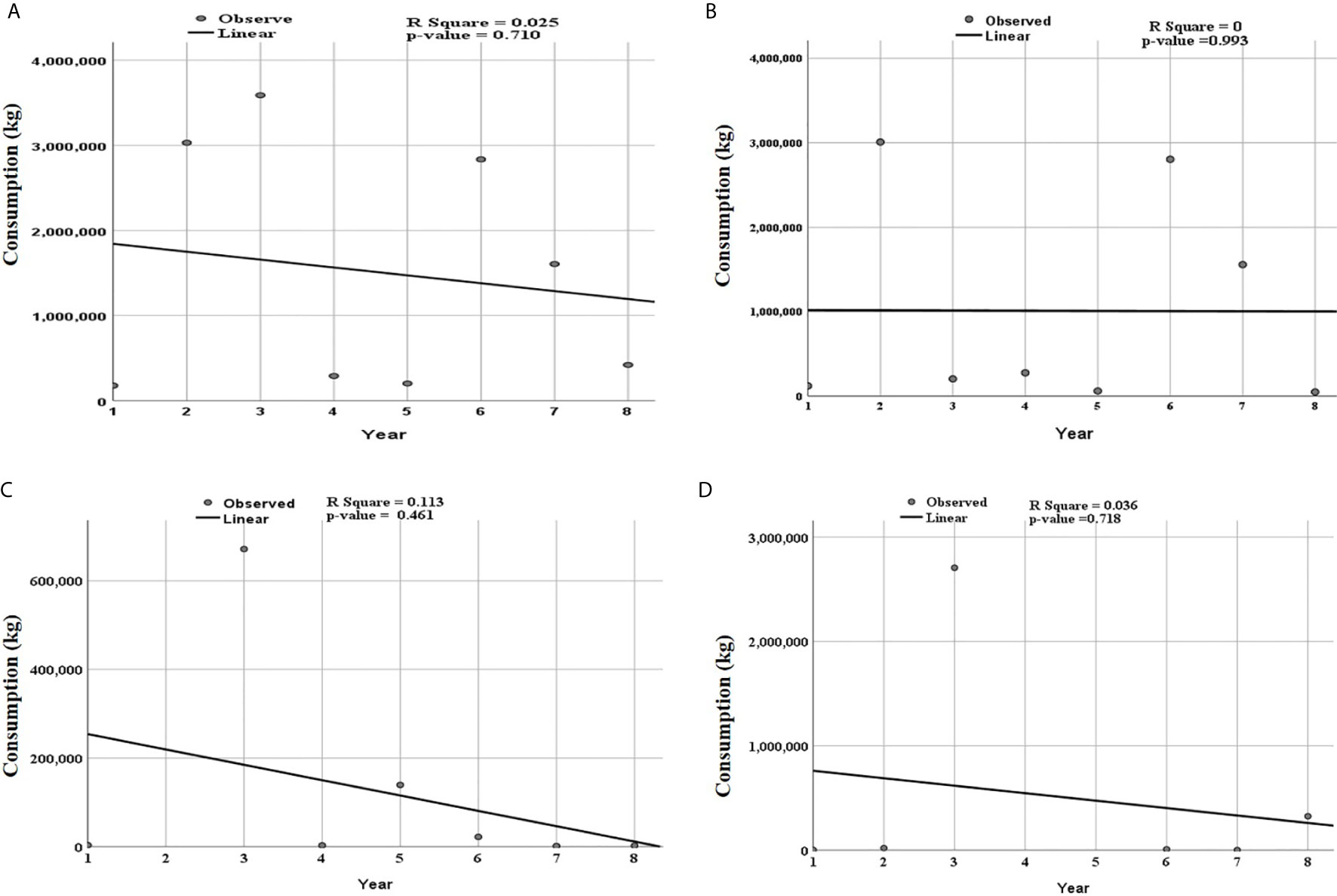
Figure 2 Trends of total consumed antibiotics for the individual classes of antibiotics according to ATC level three over eight years from 2010 to 2017. Period 1 corresponds to 2010 and year 8 to 2017. The linear curve estimation for (A) overall consumption of antibiotics, (B) tetracycline, (C) quinolones and, (D) sulfonamide trimethoprim show a non-significant decreasing trend. R square value and p-value for each class of antibiotics is shown in the respective panel.
The top supplier country for these antibiotics was China, followed by Spain and United Kingdom. China alone contributed 6,994,665.55 kg (Table 4), equivalent to 57.6% of all consumed antibiotics.
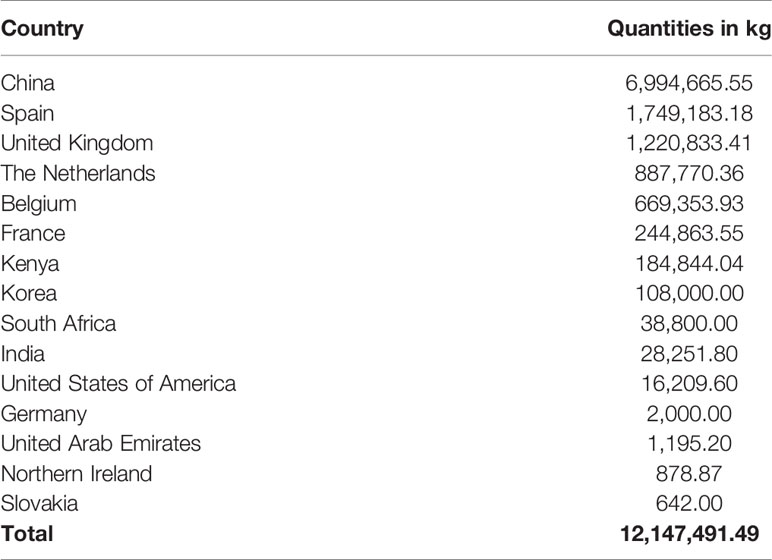
Table 4 Contribution of supplier countries to the antibiotics consumed in Tanzania between 2010 and 2017.
Discussion
This study reports a longitudinal survey of veterinary antibiotics consumption using information from the TMDA’s importation database between 2010 to 2017 for Tanzania mainland. The main assumption is that the imported veterinary antibiotics were released onto the Tanzanian market for immediate use. Tetracycline was the most common class of antibiotics consumed for veterinary use in Tanzania, followed by sulfonamides and trimethoprim and quinolones. The tetracyclines group included antibiotics such as oxytetracycline, chlortetracycline and combined oxytetracycline and colistin. The sulfonamides and trimethoprim group included antibiotic combination such as sulfamethoxazole, trimethoprim and colistin, sulfamethoxazole, trimethoprim and sulfadiazine and trimethoprim. Quinolones included enrofloxacin and norfloxacin. In this study, antibiotic consumption trends suggest a generally decreasing pattern of antibiotics use. This decrease might be due to prevailing legislative measures and campaigns to reduce inappropriate use and overuse of antibiotics in animals (33, 34). Tetracycline topped the list of quantities consumed annually, with the highest consumption in 2015, followed by a declining trend.
In this study, tetracyclines, sulfonamides, trimethoprim, and quinolones were the leading consumed antibiotics in Tanzania’s mainland. Tetracycline is quite popular among many veterinarians and importers because of its broad spectrum of antibacterial activity and affordability (35). Tetracycline is also widely and extensively used in treating diseases like anaplasmosis. It is also used in, early stages of theileriosis, and several supportive therapies in viral infection in animals like cattle (36). Soluble tetracyclines are preferred as growth promoters (8), which may partly explain why tetracycline was the most consumed class of antibiotics in the study period in Tanzania. The sulfonamides and trimethoprim are also popular in poultry farming in controlling coccidiosis and poultry colibacillosis. Sulfonamides and trimethoprim are extensively used in calf scours and pneumonia (36). The therapeutic usages in animals like these may have accounted for the relatively high quantities in this study.
This study’s findings are in line with findings from other studies done in the Tanzanian communities that highlighted how these antibiotics are used and the pattern of the consequences in terms of their resistance profiles in animals and humans (13, 29, 37). These same antibiotics were commonly found at the pastoralist’s homesteads in Northern Tanzania communities (37). Consistently, tetracyclines were the most common antibiotic used by these farmers. However, other antibiotics such as penicillin and aminoglycosides, which were seen to be commonly used by these farmers, did not emerge at or near the top in this study. Similar trends of antibiotic use among food-producing animals were found in the neighboring country of Kenya (26).
The selection pressure to bacteria depends on the way antibiotics are administered. Some studies suggested increased levels of resistance with systemic administration of antibiotics than with non-systemic (38). This study has revealed a greater proportion of systemic usage of antibiotics compared to the non-systemic, a situation that deserves attention in the antibiotic resistance surveillance data. Studies that have looked at the consequences of antibiotic use have yielded a similar resistance pattern to these antibiotics in Tanzania. A study by Katakweba et al. in Tanzania reported that over 70% of isolates sampled from food animals had resistance to tetracyclines, sulfamethoxazole and ampicillin (39). Subbiah et al., using enteric bacteria as indicators, reported similar findings where resistance was found in over half of the samples from the animals, both domestic, wild, and for the water sources. Tetracyclines, ampicillin, sulfamethoxazole, streptomycin, and trimethoprim had higher levels compared to other antibiotics (40). Apart from agreeing largely with our findings, these findings expose the gap in our findings where penicillin seems to be one of the highly used antibiotics that was not reflected in our study. The difference may be due to divergent data sources. We used the central national data while the other studies surveyed farmers in selected regions.
In this study, quinolones were the third most used veterinary antibiotics. The use of quinolones in veterinary medicine has been studied to develop resistance, transferring from food animals to humans. According to the WHO guidelines, quinolones can only be used for food-producing animals if culture and sensitivity tests are done (36, 41). Apart from WHO restriction, quinolones are relatively expensive and this could account for low consumption data. Quinolones are used in the treatment of diseases like mycoplasmosis, colibacilliosis in poultry (36).
The highest trend of tetracycline consumption, sulfonamides and trimethoprim, and combinations of antibiotics in some years might suggest the increasing need to use last-resort antibiotics. This trend has been seen in other regions in Africa. For instance, in a 3-year survey of antimicrobial use in animals, in south-western Nigeria, the majority of antimicrobials used were tetracyclines, fluoroquinolones, and β-lactams at the proportion of 33.6%, 26.5% and 20.4%, respectively (21, 42). In the Nigeria study, there was an increasing trend as opposed to these results (42). These usage statistics are consistent with the increasing evidence of tetracycline residues in chicken meat in African countries, including Tanzania (43). Quinolones and aminoglycosides are very important pharmaco-therapeutic armaments in human medicine. However, in this study, these were very low compared to other antibiotic classes such as sulfonamides, trimethoprim and tetracycline. This is good news because these agents’ overuse in animals may impact antibiotic resistance in human pathogens. However, this information may not allow us to comment conclusively on the increasing or decreasing consumption trend. Still, both quantitative and qualitative records of antibiotic use are crucial to enable the impact of antibiotic policy to be evaluated and determine possible associations between using certain groups of antibacterial drugs and the emergence of resistance.
The amount of antimicrobials consumed by animals is much higher than in humans. Resistance to these antibiotics used in animals is not only a veterinary issue but also human since these classes used in food animal production serve as essential drugs in human medicine (21, 44). Livestock and poultry serve as major bacterial infection sources to man, hence a source of transmission of resistant bacterial strains. A study done in Tanzania involving ducks indicates that ducks are carriers of Campylobacter spp. and serve as major infection sources to humans (29). The authors tested 50 C. jejuni isolates and found that they were susceptible to streptomycin, nitrofurantoin, and amikacin, while they showed some resistance to cefuroxime (48%) tetracycline (74%), and ampicillin (82%) (29).
A recent survey investigated the frequently used antibiotics for veterinary use among commercial chicken farmers and pharmaceutical outlets. It was observed that the most frequently used antibiotics were tetracycline (32.2%) and sulfonamides (20.8%). Others were fluoroquinolones (9.85%), macrolides (9.4%), polypeptides (8.0%), amprolium (6.5%), aminoglycosides (6.4%), trimethoprim (5.5%), furazolidone (0.8%) and quinoxalines (0.4%) (45).
In Tanzania, antibiotics are commonly used to treat tick-borne diseases, particularly anaplasmosis and heartwater in cattle; fowl typhoid in poultry; and salmonellosis coccidiosis and other causes of diarrheal conditions in different animal species (46). Despite high usage, no studies have been conducted to establish antibiotic resistance in correlation with the consumption patterns. In both our study and other studies (40, 45, 46) in different regions of Tanzania, it was observed that the most frequently imported antibiotics are the ones that are highly consumed and are the ones that are more prone to resistance.
Even though antibiotics use may be the right management decision when bacteria in animals are susceptible to the antibiotics used (47), it is worth considering that the practice can be damaging due to the build-up of resistance to the currently available antibiotics. This is because resistance may be already well established in bacterial populations in humans, animals and the environment. It is worth considering antibiotic withdrawal, which can lead to a temporary decline in antibiotic resistance. Noting that resistance levels may rapidly rebound if the antibiotic is reintroduced.
To emphasize the control of the use of antibiotics, a One-Health systematic approach of surveillance across the public and animal health sectors has been recommended despite the missing link between the increasing trend in antibiotic resistance in humans and the use of antibiotics in animals, either as feed additives or veterinary prescription (48). One health approach (49) can help devise multi-sectoral interventions, review and formulate policies that promote rational use and reduce antimicrobial consumption and resistance in humans and animals (13, 50). To complement the one health approach, diagnostics and detection of antibiotic susceptibility testing capacity will support the rational use of antibiotics, tracking and monitoring antimicrobial resistance in the country (51). The efforts will oversee establishing a well-coordinated national surveillance system for antimicrobial resistance. It will also build capacity for a national reference laboratory and designated laboratories for AMR surveillance, data management and sharing systems in human, animal and plant health settings (1, 2) to strengthen the prevailing efforts to limit the antimicrobial use and spread of AMR.
Limitations
The main limitation is that the study utilizes national regulatory authority importation data, assuming that all the antibiotics imported are consumed in the country for veterinary use. However, there is a possibility of expiry and losses; thus, not all antibiotics imported are used. Further, these data only reflect total national imports and estimate the quantities of veterinary drugs in circulation for defined periods. The study assumed that most antibiotics for veterinary use are all imported from abroad. In addition, the regulatory approval data cannot differentiate the consumed antibiotics into the therapeutic, prophylactic and growth promotion groups.
Nevertheless, this method includes only imported drugs through the normal legal port of entries regulated by the drug regulatory authority. The reported data may have excluded the counterfeit and substandard medicines which are prevalent in pharmacies and drugstores in Tanzania, as reported by other studies (52, 53). These medicines may not be in TMDA’s MIS since they enter the market via the unofficial, porous ports of entry. In addition, a recent study showed that farmers had easy access to antibiotics from veterinary stores and that sellers encouraged farmers to use combined antimicrobials to treat their animals (13), indicating easy accessibility of uncontrolled veterinary medicines (54).
Conclusions
This study suggests that the tetracycline class of antibiotics is the one that animals highly consume in Tanzania. Oxytetracycline topped the list of tetracycline antibiotics consumed for eight years.
Even though some antibiotics have the lowest consumption rate, they are also prone to resistance. Therefore, competent authorities should take appropriate measures to control antibiotic consumption, enforce prudent use of antibiotics and counteract antibiotic resistance.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical clearance for this study was sought and granted by the Muhimbili University of Health and Allied Sciences Institutional Review Board with reference number DA.25/111/01/. Permission to access the data was sought at the TMDA. The names and identifiers of the importer were not recorded.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Tanzania Medicines and Medical Devices Authority staff for their assistance in retrieving the antibiotics’ importation data.
References
1. Government of Tanzania (GoT). Tanzania Livestock Master Plan (2017/2018–2021/2022). Available at: www.mifugouvuvi.go.tz/uploads/projects/1553601793-TANZANIALIVESTOCKMASTERPLAN.pdf (Accessed May 18, 2021).
2. Mdegela RH, Mwakapeje ER, Rubegwa B, Gebeyehu DT, Niyigena S, Msambichaka V, et al. Antimicrobial Use, Residues, Resistance and Governance in the Food and Agriculture Sectors, Tanzania. Antibiotics (2021) 10(4):454. doi: 10.3390/antibiotics10040454
3. Goodman C, Kachur SP, Abdulla S, Bloland P, Mills A. Drug Shop Regulation and Malaria Treatment in Tanzania Why Do Shops Break the Rules, and Does It Matter? Health Policy Plan (2007) 22(6):393–403. doi: 10.1093/heapol/czm033
4. Poyongo BP, Sangeda RZ. Pharmacists’ Knowledge, Attitude and Practice Regarding the Dispensing of Antibiotics Without Prescription in Tanzania: An Explorative Cross-Sectional Study. Pharmacy (2020) 8(4):238. doi: 10.3390/pharmacy8040238
5. Kagashe GAB, Minzi O, Matowe L. An Assessment of Dispensing Practices in Private Pharmacies in Dar-Es-Salaam, Tanzania. Int J Pharm Pract (2011) 19(1):30–5. doi: 10.1111/j.2042-7174.2010.00075.x
6. Agyare C, Etsiapa Boamah V, Ngofi Zumbi C, Boateng Osei F. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. In: Antimicrobial Resistance - A Global Threat. IntechOpen (2019). doi: 10.5772/intechopen.79371
7. McEwen SA, Fedorka-Cray PJ. Antimicrobial Use and Resistance in Animals. Clin Infect Dis (2002) 34(s3):S93–S106. doi: 10.1086/340246
8. Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, et al. Antimicrobial Resistance in Developing Countries. Part I: Recent Trends and Current Status. Lancet Infect Dis (2005) 5(8):481–93. doi: 10.1016/S1473-3099(05)70189-4
9. Oliver SP, Murinda SE, Jayarao BM. Impact of Antibiotic Use in Adult Dairy Cows on Antimicrobial Resistance of Veterinary and Human Pathogens: A Comprehensive Review. Foodborne Pathog Dis (2011) 8(3):337–55. doi: 10.1089/fpd.2010.0730
10. World Health Organization. WHO Global Strategy for Containment of Antimicrobial Strategy for Containment of Antimicrobial Resistance. (2001). Available at: https://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf (Accessed April 12, 2021).
11. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and Regional Mortality From 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0
12. Kluytmans JAJW. Methicillin-Resistant Staphylococcus Aureus in Food Products: Cause for Concern or Case for Complacency? Clin Microbiol Infect (2010) 16(1):11–5. doi: 10.1111/j.1469-0691.2009.03110.x
13. Kimera ZI, Frumence G, Mboera LEG, Rweyemamu M, Mshana SE, Matee MIN. Assessment of Drivers of Antimicrobial Use and Resistance in Poultry and Domestic Pig Farming in the Msimbazi River Basin in Tanzania. Antibiotics (2020) 9(12):838. doi: 10.3390/antibiotics9120838
14. Limbu SM. Antibiotics Use in African Aquaculture: Their Potential Risks on Fish and Human Health. In: Current Microbiological Research in Africa. Cham: Springer International Publishing (2020). p. 203–21. doi: 10.1007/978-3-030-35296-7_8.
15. Mhongole OJ, Mdegela RH, Kusiluka LJM, Forslund A, Dalsgaard A. Characterization of Salmonella Spp. From Wastewater Used for Food Production in Morogoro, Tanzania. World J Microbiol Biotechnol (2017) 33(3):42. doi: 10.1007/s11274-017-2209-6
16. Moremi N, Manda EV, Falgenhauer L, Ghosh H, Imirzalioglu C, Matee M, et al. Predominance of CTX-M-15 Among ESBL Producers From Environment and Fish Gut From the Shores of Lake Victoria in Mwanza, Tanzania. Front Microbiol (2016) 7:1862. doi: 10.3389/fmicb.2016.01862
17. Shah SQA, Colquhoun DJ, Nikuli HL, Sørum H. Prevalence of Antibiotic Resistance Genes in the Bacterial Flora of Integrated Fish Farming Environments of Pakistan and Tanzania. Environ Sci Technol (2012) 46(16):8672–9. doi: 10.1021/es3018607
18. Lanka S, Manage PM. Heavy Use of Antibiotics in Aquaculture: Emerging Human and Animal Health Problems-A Review. J Aquat Sci (2018) 23(1):13–27. doi: 10.4038/sljas.v23i1.7543
19. Lulijwa R, Rupia EJ, Alfaro AC. Antibiotic Use in Aquaculture, Policies and Regulation, Health and Environmental Risks: A Review of the Top 15 Major Producers. Rev Aquac (2020) 12(2):640–63. doi: 10.1111/raq.12344
20. Tenover FC. Mechanisms of Antimicrobial Resistance in Bacteria. Am J Med (2006) 119(6):S3–S10. doi: 10.1016/j.amjmed.2006.03.011
21. Van TTH, Yidana Z, Smooker PM, Coloe PJ. Antibiotic Use in Food Animals Worldwide, With a Focus on Africa: Pluses and Minuses. J Glob Antimicrob Resist (2020) 20:170–7. doi: 10.1016/j.jgar.2019.07.031
22. Bywater RJ. Veterinary Use of Antimicrobials and Emergence of Resistance in Zoonotic and Sentinel Bacteria in the EU. J Vet Med Ser B (2004) 51(8–9):361–3. doi: 10.1111/j.1439-0450.2004.00791.x
23. Marshall BM, Levy SB. Food Animals and Antimicrobials: Impacts on Human Health. Clin Microbiol Rev (2011) 24(4):718–33. doi: 10.1128/CMR.00002-11
24. Heuer H, Schmitt H, Smalla K. Antibiotic Resistance Gene Spread Due to Manure Application on Agricultural Fields. Curr Opin Microbiol (2011) 14(3):236–43. doi: 10.1016/j.mib.2011.04.009
25. Bengtsson B, Greko C. Antibiotic Resistance—Consequences for Animal Health, Welfare, and Food Production. Ups J Med Sci (2014) 119(2):96–102. doi: 10.3109/03009734.2014.901445
26. Mitema ES, Kikuvi GM, Wegener HC, Stohr K. An Assessment of Antimicrobial Consumption in Food Producing Animals in Kenya. J Vet Pharmacol Ther (2001) 24(6):385–90. doi: 10.1046/j.1365-2885.2001.00360.x
27. Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation Between Veterinary Antimicrobial Use and Antimicrobial Resistance in Food-Producing Animals: A Report on Seven Countries. J Antimicrob Chemother (2014) 69(3):827–34. doi: 10.1093/jac/dkt443
28. Tao W, Ivanovska V, Schweickert B, Muller A. Proxy Indicators for Antibiotic Consumption; Surveillance Needed to Control Antimicrobial Resistance. Bull World Health Organ (2019) 97(1):3–3A. doi: 10.2471/BLT.18.227348
29. Kimera ZI, Mshana SE, Rweyemamu MM, Mboera LEG, Matee MIN. Antimicrobial Use and Resistance in Food-Producing Animals and the Environment: An African Perspective. Antimicrob Resist Infect Control (2020) 9(1):37. doi: 10.1186/s13756-020-0697-x
30. Kimera ZI, Mdegela RH, Mhaiki CJN, Karimuribo ED, Mabiki F, Nonga HE, et al. Determination of Oxytetracycline Residues in Cattle Meat Marketed in the Kilosa District, Tanzania. Onderstepoort J Vet Res (2015) 82(1):1–5. doi: 10.4102/ojvr.v82i1.911
31. Organization World Health. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation. Geneva: World Health Organization. (2018). Available at: https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf?ua=1 (Accessed April 12, 2021).
32. Sataloff RT, Johns MM, Kost KM. Methodology, W. C. C. for D. s. Guidelines for ATC Classification and DDD Assignment (2021). Available at: https://www.whocc.no/filearchive/publications/2021_guidelines_web.pdf (Accessed Jan 16, 2021).
33. Frumence G, Mboera LEG, Sindato C, Katale BZ, Kimera S, Metta E, et al. The Governance and Implementation of the National Action Plan on Antimicrobial Resistance in Tanzania: A Qualitative Study. Antibiotics (2021) 10(3):273. doi: 10.3390/antibiotics10030273
34. Sangeda RZ, Kibona J, Munishi C, Arabi F, Manyanga VP, Mwambete KD, et al. Assessment of Implementation of Antimicrobial Resistance Surveillance and Antimicrobial Stewardship Programs in Tanzanian Health Facilities a Year After Launch of the National Action Plan. Front Public Heal (2020) 8:454. doi: 10.3389/fpubh.2020.00454
35. Granados-Chinchilla F, Rodríguez C. Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J Anal Methods Chem (2017) 2017:1–24. doi: 10.1155/2017/1315497
36. Agunos A, Léger D, Carson C. Review of Antimicrobial Therapy of Selected Bacterial Diseases in Broiler Chickens in Canada. Can Vet J La Rev Vet Can (2012) 53(12):1289–300.
37. Caudell MA, Quinlan MB, Subbiah M, Call DR, Roulette CJ, Roulette JW, et al. Antimicrobial Use and Veterinary Care Among Agro-Pastoralists in Northern Tanzania. PLoS One (2017) 12(1):e0170328. doi: 10.1371/journal.pone.0170328
38. Schwarz S, Chaslus-Dancla E. Use of Antimicrobials in Veterinary Medicine and Mechanisms of Resistance. Vet Res (2001) 32(3/4):201–25. doi: 10.1051/vetres:2001120
39. Katakweba AAS, Muhairwa AP, Lupindu AM, Damborg P, Rosenkrantz JT, Minga UM, et al. First Report on a Randomized Investigation of Antimicrobial Resistance in Fecal Indicator Bacteria From Livestock, Poultry, and Humans in Tanzania. Microb Drug Resist (2018) 24(3):260–8. doi: 10.1089/mdr.2016.0297
40. Subbiah M, Caudell MA, Mair C, Davis MA, Matthews L, Quinlan RJ, et al. Antimicrobial Resistant Enteric Bacteria are Widely Distributed Amongst People, Animals and the Environment in Tanzania. Nat Commun (2020) 11(1):228. doi: 10.1038/s41467-019-13995-5
41. World Health Organization. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals . Available at: https://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf?sequence=1 (Accessed Mar 28, 2021).
42. Adesokan HK, Akanbi IO, Akanbi IM, Obaweda RA. Pattern of Antimicrobial Usage in Livestock Animals in South-Western Nigeria: The Need for Alternative Plans. Onderstepoort J Vet Res (2015) 82:1–6. doi: 10.4102/ojvr.v82i1.816
43. Darwish WS, Eldaly EA, El-Abbasy MT, Ikenaka Y, Nakayama S, Ishizuka M. Antibiotic Residues in Food: The African Scenario. Jpn J Vet Res (2013) 61 Suppl:S13–22.
44. World Health Organization. Critically Important Antimicrobials for Human Medicine. Available at: https://apps.who.int/iris/bitstream/handle/10665/255027/9789241512220-eng.pdf (Accessed Mar 17, 2020).
45. Mubito EP, Shahada F, Kimanya ME, Buza JJ. Antimicrobial Use in the Poultry Industry in Dar-Es-Salaam, Tanzania and Public Health Implications. Am J Res Commun (2014) 2(4):51–63.
46. Nonga HE, Simon C, Karimuribo ED, Mdegela RH. Assessment of Antimicrobial Usage and Residues in Commercial Chicken Eggs From Smallholder Poultry Keepers in Morogoro Municipality, Tanzania. Zoonoses Public Health (2009) 57:339–44. doi: 10.1111/j.1863-2378.2008.01226.x
47. Barton MD. Antibiotic Use in Animal Feed and Its Impact on Human Healt. Nutr Res Rev (2000) 13(2):279–99. doi: 10.1079/095442200108729106
48. Mshana SE, Matee M, Rweyemamu M. Antimicrobial Resistance in Human and Animal Pathogens in Zambia, Democratic Republic of Congo, Mozambique and Tanzania: An Urgent Need of a Sustainable Surveillance System. Ann Clin Microbiol Antimicrob (2013) 12(1):28. doi: 10.1186/1476-0711-12-28
49. Aenishaenslin C, Häsler B, Ravel A, Parmley EJ, Mediouni S, Bennani H, et al. Evaluating the Integration of One Health in Surveillance Systems for Antimicrobial Use and Resistance: A Conceptual Framework. Front Vet Sci (2021) 8:611931. doi: 10.3389/fvets.2021.611931
50. Wall B, Mateus A, Marshall L, Pfeiffer D, Lubroth J, Ormel H, et al. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production Vol. 5. Rome: Food Agric Organ United Nations (2016). Available at: http://www.fao.org/3/i6209e/i6209e.pdf (Accessed April 12, 2021).
51. Mayenga EL, Ågren E, Balala A, Banda GK. Monitoring of Antimicrobial Resistance in Diagnostic Veterinary Laboratory in Dar Es Salaam, Tanzania. Tanzania Vet J (2020) 37:25–34. doi: 10.4314/tvj.v37i1.5s
52. Höllein L, Kaale E, Mwalwisi YH, Schulze MH, Holzgrabe U. Routine Quality Control of Medicines in Developing Countries: Analytical Challenges, Regulatory Infrastructures and the Prevalence of Counterfeit Medicines in Tanzania. TrAC Trends Anal Chem (2016) 76:60–70. doi: 10.1016/j.trac.2015.11.009
53. Almuzaini T, Choonara I, Sammons H. Substandard and Counterfeit Medicines: A Systematic Review of the Literature. BMJ Open (2013) 3(8):e002923. doi: 10.1136/bmjopen-2013-002923
54. Mitema E. The Role of Unregulated Sale and Dispensing of Antimicrobial Agents on the Development of Antimicrobial Resistance in Developing Countries. In: Sosa A, Byarugaba D, Amábile-Cuevas C, Hsueh PR, Kariuki S, Okeke I, editors. Antimicrobial Resistance in Developing Countries. New York, NY: Springer (2010). doi: 10.1007/978-0-387-89370-9_23
Keywords: antimicrobial, drug resistance, antibiotic consumption, Tanzania, veterinary antibiotics, defined daily doses, anatomical therapeutic and chemical classification, medicine utilization
Citation: Sangeda RZ, Baha A, Erick A, Mkumbwa S, Bitegeko A, Sillo HB, Fimbo AM, Chambuso M and Mbugi EV (2021) Consumption Trends of Antibiotic for Veterinary Use in Tanzania: A Longitudinal Retrospective Survey From 2010-2017. Front. Trop. Dis 2:694082. doi: 10.3389/fitd.2021.694082
Received: 12 April 2021; Accepted: 02 June 2021;
Published: 17 June 2021.
Edited by:
Eric Sampane-Donkor, University of Ghana, GhanaReviewed by:
Luther King Abia Akebe, University of KwaZulu-Natal, South AfricaTuhina Banerjee, Banaras Hindu University, India
Copyright © 2021 Sangeda, Baha, Erick, Mkumbwa, Bitegeko, Sillo, Fimbo, Chambuso and Mbugi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raphael Z. Sangeda, c2FuZ2VkYUBnbWFpbC5jb20=
 Raphael Z. Sangeda
Raphael Z. Sangeda Andrea Baha
Andrea Baha Alexander Erick
Alexander Erick Sonia Mkumbwa
Sonia Mkumbwa Adonis Bitegeko3
Adonis Bitegeko3 Hiiti B. Sillo
Hiiti B. Sillo Adam M. Fimbo
Adam M. Fimbo Erasto V. Mbugi
Erasto V. Mbugi