- Department of Entomology, Cornell University, Cornell AgriTech, Geneva, NY, United States
Allium leafminer (Phytomyza gymnostoma Loew) is an emerging invasive pest of Allium crops and has been threatening Allium crop production in the eastern United States since its introduction in 2015. Phytomyza gymnostoma can cause substantial economic loss in leek crops when late instars tunnel into the lower portion of the plant, which often renders the crop unmarketable. With limited management tools that are cost-effective and practical, especially for leeks produced in organic systems, we examined the attractiveness of other Allium crop species that might be considered in a trap cropping strategy. In 2021 and 2022, controlled environment choice tests and field trials were conducted to evaluate host preference of P. gymnostoma among Allium crop species including chives, scallion, an onion and scallion hybrid, and leek. We also assessed preference of P. gymnostoma for scallions that varied in size/age. Results from field trials indicated that only chives had more oviposition marks, cumulative numbers of eggs, and a higher density of P. gymnostoma larvae and pupae than leeks. Larger/older scallions had more oviposition marks and higher P. gymnostoma densities than smaller ones in controlled environment choice tests, but this size/age preference was not evident in field trials. Based on our findings, chives could be considered as a potential trap crop for minimizing P. gymnostoma damage in leek crops.
1 Introduction
Allium leafminer (Phytomyza gymnostoma Loew) (Diptera: Agromyzidae) originated from Europe and is now an invasive species in North America (1). Since the first report of P. gymnostoma in Pennsylvania, United States, in 2015, P. gymnostoma has spread to nearby states on the East coast from New York to Virginia (B. Nault, personal communication). As its common name implies, P. gymnostoma is a specialist with a host range limited to the genus Allium (family Liliaceae), which includes many important high-value crops grown in the United States, such as onion, garlic, scallion, leek, and chives (1–3). Aesthetic damage occurs when females puncture leaves with its ovipositor, leaving a series of linear white marks (blemishes) that can cause some Allium crops to become unmarketable. Eggs are not always laid during this process, rather the oviposition punctures produce plant exudates that are ingested by adults of both sexes. Larval feeding can cause damage to leaves such as mining, distortion and wilting. The most severe damage occurs when late instars feed and pupate at the base of plants. Larval feeding creates entry points for bacterial and/or fungal pathogens to infect the crop, and this can cause the crop to rot. Furthermore, the presence of larvae and pupae within leek plants and garlic cloves at harvest contaminates the crop and can make it unmarketable (1, 2, 4–6).
Phytomyza gymnostoma has two generations per year in North America as it does in Europe (1, 3, 4). In the northeastern United States, the spring generation is active from April to May. Phytomyza gymnostoma aestivates in the pupal stage during the summer months. Flies from the fall generation emerge in September and remain active through November. Afterward, P. gymnostoma overwinters in the pupal stage at the base of plants or in surrounding soil (3). While both generations can cause significant damage to Allium crops, the fall generation has a tendency to be more damaging than the spring generation on most farms in New York (B. Nault, personal observation). Phytomyza gymnostoma also has been more problematic on organic farms than on conventional farms, which could reflect differences in insecticide use. In addition, some growers stagger plantings of Allium crops throughout the year, and this provides access to hosts for both generations of P. gymnostoma, which could facilitate population growth on those farms (1). Damage to leek crops on some organic farms in the northeastern United States has been so prevalent and difficult to manage that leeks are no longer grown on these farms. The common name of P. gymnostoma in Europe is the leek mining fly, and leek has mostly been reported as the major crop that has suffered from severe damage by P. gymnostoma in many European countries including Belgium, Hungary, Italy, and Poland (1, 7–10).
Insecticide use is the most effective tool for managing P. gymnostoma infestations. Some of the best conventional products include cyantraniliprole, dinotefuran, and spinetoram, while spinosad is the best product for organic production (11). In addition, Nault et al. (12) demonstrated that P. gymnostoma could be managed with only two applications of spinosyn insecticides as long as the applications were made after the first week of initial P. gymnostoma detection. While P. gymnostoma infestations can be managed effectively with insecticides, growers are seeking alternative non-chemical management strategies that are less expensive and less time-consuming.
Crop rotation has been recommended for home gardens and organic production systems as P. gymnostoma overwinters within host plants and adjacent soil and is not known to disperse long distances. However, crop rotation might only be effective if no other Allium crops or wild Allium hosts are present nearby (1, 13). In some instances, delayed planting of Allium crops in the spring after peak adult flight has been successful (10, 13). Nevertheless, delayed planting is not feasible in the fall because infestations occur from September through December. Parasitism from two parasitoid wasps, Halticoptera circulus (Walker) and Chrysocharis oscinidis Ashmead, has been documented in Pennsylvania, but current parasitism rates have been too low to prevent P. gymnostoma from causing economic loss (3).
Trap cropping has not been explored as a management tactic for P. gymnostoma. This management tactic involves planting attractive host plants in proximity to the main cash crop in order to lure and concentrate populations of the target pest away from the main crop (14, 15). The foundation of trap cropping considers that insects have a strong preference for one host over another for feeding and reproduction (14). A preferred host could be a particular plant species, cultivar, as well as a particular phenological host stage (14, 16). Hence, one of the first steps for developing a trap cropping approach for P. gymnostoma management is to identify an Allium crop and a particular phenological stage that are highly attractive to females.
The objective of this study was to identify a highly preferred host for P. gymnostoma that could be considered in the future as a potential trap crop, especially for leek production in the fall. Therefore, Allium crops commonly grown in the fall such as scallion, an onion and scallion hybrid, and chives were selected as trap crop candidates. In addition, we tested whether plant size/age impacted host preference using small (young) and large (old) scallions. We hypothesized that P. gymnostoma would have a distinct preference for one or more of these Allium crops and for larger/older plants. Dependent variables used to determine host preference were number of oviposition punctures (for adult feeding and egg deposition) and densities of larvae and pupae. An ideal host for a future trap crop to minimize P. gymnostoma damage in leeks would be one that was highly preferred for both egg deposition and larval and pupal establishment.
2 Materials and methods
2.1 Choice tests in controlled environments
Choice tests were conducted in a glass greenhouse and a walk-in environmental chamber to evaluate P. gymnostoma adult host preference among selected Allium species and plant sizes/ages at Cornell AgriTech in Geneva, NY in April and May 2022. The greenhouse and the environmental chamber were set at 21°C during the day and 16°C at night with a 14:10 h day/night cycle throughout the study.
2.1.1 Phytomyza gymnostoma and plant material preparation
Phytomyza gymnostoma used in choice tests were reared from pupae that were extracted from field-collected leek crops the previous fall. Leeks infested by P. gymnostoma pupae were collected from the field in November 2021 and stored in a cold room (4.5°C) until April 2022. Pupae then were dipped in a sterilization solution (1% methyl paraben and 2% sodium hypochlorite in deionized water) for 15 seconds and then rinsed with deionized water. Pupae were air-dried on paper towels and then placed in plastic boxes containing moist, autoclaved sand. Pupae were kept in BugDorm cages (60 x 60 x 60 cm; MegaView Science Co., Ltd., Taichung, Taiwan) and incubated in the walk-in environmental chamber. BugDorm cages were supplied with moist cotton balls and greenhouse-grown scallion plants as the food source for newly emerged adult flies. All adults were allowed to mate for at least two days after emergence, and up to five-day old flies were used in the experiments. All plants used in the choice tests were grown from seeds in multi-cell plug flats (Griffin Greenhouse Supplies, Inc., Auburn, NY, USA) in organic potting mix (LM-111 Organic All Purpose Mix, Lambert Peat Moss, Quebec, Canada), and plants were propagated in the glass greenhouse. Plants were fertilized with fish emulsion (2-3-1 N-P-K, Liquid #3, The Fertrell Company, Bainbridge, PA, USA) at a rate of 9.7 ml/L once a week after gemination. Plants were transplanted in plastic pots (10.2 cm in diameter) four weeks after seeding. Plants were at least four weeks old when adult choice tests were initiated.
2.1.2 Experimental design
Four Allium crop species were used in the host species preference experiments, which included chives (Allium schoenoprasum var. ‘Staro’; Johnny’s seeds, Winslow, ME, USA), leek (Allium porrum var. ‘Lancelot’; High Mowing Organic Seeds, Wolcott, VT, USA), scallion (Allium fistulosum L., var.’Nabechan F1’; Johnny’s seeds, Winslow, ME, USA), and an onion and scallion hybrid (Allium fistulosum x A. cepa, var. ‘Guardsman’; Johnny’s seeds, Winslow, ME, USA). Choice experiments were conducted in BugDorm cages with one plant of each Allium species of the same age randomly arranged in each cage. Two mated females were released into each cage. Plant ages varied from seven to nine weeks old across experiments depending on the availability of adult flies and when the experiments were initiated, but plants within each cage were always the same age. Three and four experiments were set up in the greenhouse and the environmental chamber, respectively, from 12-27 April 2022 resulting in a total of seven experiments.
Host size/age choice tests were also conducted in the same controlled environments as the host species preference experiments. Scallions were selected for this study because they are an attractive host and highly susceptible to P. gymnostoma (11). Three sizes/ages of scallions were used for the experiments (i.e., four (small), six (medium), eight (large) week-old scallions; the height of the tallest leaf and width of the base of leaves were measured during each experiment and results are shown in Supplementary Figure 1). Three experiments were conducted in the greenhouse and another three experiments in the environmental chamber from 18-23 April 2022 resulting in a total of six experiments.
2.1.3 Data collection and statistical analyses
Oviposition marks per plant were recorded every two to four days for three weeks starting one to two days after flies were released into cages. All plants were dissected under a stereo microscope (Zeiss Stemi 2000, 6.5-10X) to obtain numbers of P. gymnostoma eggs, larvae, and pupae in each plant at the end of the experiments (~21 days after flies were released). Final numbers of oviposition marks per cm2 leaf area and final densities of P. gymnostoma (i.e., total number of eggs, larvae, and pupae per plant) were the response variables analyzed for host species and size/age preference. Data from all experiments were pooled within each preference choice test (i.e., host species and size/age preference). Both response variables were analyzed using generalized linear mixed models with the negative binomial distribution and the log link function under PROC GLIMMIX procedure in SAS Studio version 3.81 (Enterprise edition 2022, SAS Institute Inc., Cary, NC, USA). Allium species and plant size/age were the fixed factor in two separate analyses, while the controlled environment type (i.e., greenhouse or environmental chamber) was used as a blocking factor that served as a random factor in both analyses. Least squares means (LS-means) were used for post hoc comparisons with Tukey Studentized Range (HSD) Test when the fixed factor was significant at α = 0.05 for mean separation.
2.2 Field trials
In 2021, field trials were conducted on commercial organic farms in eastern New York near Hurley (41°55'47.6"N 74°03'58.8"W) and Red Hook (42°01'37.3"N 73°52'15.8"W). In 2022, one trial was conducted on a commercial organic farm in central NY near Fenner (42°58'48.4"N 75°48'48.6"W), while the other trial was on the same farm in Hurley, NY (41°55'50.6"N 74°03'58.4"W). This study was conducted in the fall of both years to take advantage of high P. gymnostoma pressure. All field trials were conducted on white plastic mulch with drip irrigation, except in Hurly in 2022 where the trial was conducted on silver reflective mulch with drip irrigation. Organic fertilizer was applied by the cooperating grower prior to transplanting at rates typically used on that farm. No pesticides were applied in our field trials. Infestation by other insect pests and infection by foliar pathogens were minimal both years. Ground cloth (Sunbelt woven ground cover; DeWitt Company, Sikeston, MO, USA) was placed between beds for weed management, and weeds in beds were removed by hand as needed.
2.2.1 Experimental design
Four field trials were conducted in 2021 and 2022 to determine host species preference. The same four Allium crop species used in the controlled environment experiments were included in the field trials. The four Allium species were arranged in a randomized complete block design with five replications. Each experimental unit (plot) was 7.6 m long and contained three rows of plants in which rows were spaced 0.15 to 0.3 m apart. Plant density was two plants per 0.3 m. Plot beds were spaced 2 m apart and plots were separated within beds by 1-1.5 m wide buffer zones.
Two field trials were conducted in 2022 to determine host size/age preference. Two sizes/ages of scallions were evaluated in which large scallions were eight weeks older and four weeks older than small scallions at Hurley and Fenner, respectively (size measurements in Supplementary Figure 2). Large (old) and small (young) plants were arranged in a randomized complete block design with four replications. Each experimental unit (plot) was 3 m long with the same number of rows, plant spacing and bed spacing as described for the host species preference trials. Plots were spaced 0.75 m apart within beds.
2.2.2 Plant material preparation
Seeds for all species were planted in multi-cell plug flats with five seeds per cell in organic potting mix on 21 June for both trials in 2021. In 2022, seeds of all species were planted with three seeds per cell on 16 June and 14 July for trials at Fenner and Hurley, respectively, except that a subset of scallions were planted earlier on 16 May for large size plants in the size preference study. All plants were propagated in glass greenhouses maintained at the same settings as mentioned in the controlled environment experiments. All flats were thinned to one plant per cell two weeks after seeding. Plants were fertilized with fish emulsion once a week after germination as described previously. One to three weeks before transplanting in the field, seedlings were removed from the greenhouse and placed in a shaded area with open air for hardening-off, which prevented plants from going through “transplant shock” by sudden exposure to direct sunlight. Seedlings were transplanted by hand on 20 and 25 August 2021 at Hurley and Red Hook, respectively. In 2022, plants were transplanted on 26 August and 1 September at Fenner and Hurley, respectively.
2.2.3 Data collection
Data collection started within seven days of first detecting either P. gymnostoma adults or their oviposition marks on leaves. Twenty-five plants in each plot were randomly selected and visually examined for P. gymnostoma oviposition marks. Plants with at least one oviposition mark were considered damaged. Up to ten damaged plants per plot were pulled, and whole plant samples were placed in brown paper grocery bags (1/6 BL; Uline, Pleasant Prairie, WI). Samples were brought back to the laboratory and stored in a cold room (4.5 °C) before further evaluation. All plant samples were processed no more than 14 days after collection from the field. Number of P. gymnostoma oviposition marks were counted on each plant. The whole plant was then dissected under a stereo microscope, and the numbers of P. gymnostoma eggs, larvae, and pupae were recorded. Plant samples were collected once every two weeks for a total of five sampling events. In 2022, when P. gymnostoma damage became extremely high later in the season on chives (i.e., the last two and final sampling dates for Hurley and Fenner, respectively), count data were obtained from only half the leaves from each plant, and data were extrapolated to a per plant basis.
Degree of infestation over time at a plant population level was determined by proportions of damaged plants (i.e., plants with P. gymnostoma oviposition marks); the number of plants with P. gymnostoma damage was divided by 25 (total number sampled) to obtain the proportion of plants with oviposition marks. Number of oviposition marks per cm2 leaf area over time, cumulative number of eggs per plant throughout the season, number of larvae per plant over time, and number of pupae per plant on the last sampling date of the season were used to compare possible feeding and reproduction host preferences among Allium species and plant sizes/ages. Number of larvae plus pupae per plant on the sampling date with the highest overall P. gymnostoma numbers was an extra response variable used to represent overall P. gymnostoma damage on individual plants. In addition, the frequency of eggs deposited by P. gymnostoma, determined by percentages of oviposition marks with eggs, was calculated to serve as another response variable for comparing reproduction preference. For simplicity, only data collected on the sampling date with the overall highest number of eggs deposited was used for statistical analysis.
2.2.4 Statistical analyses
The effect of host species and sizes/ages on the numbers of P. gymnostoma oviposition marks, eggs, larvae, and pupae were analyzed using generalized linear mixed models with PROC GLIMMIX procedure in SAS Studio version 3.81. Data were analyzed separately by location and year. Repeated measures and the binomial distribution with the logit link function were used to analyze the proportions of plants with oviposition marks; however, when the analysis was not able to converge with the binomial distribution, the Poisson distribution with the log link function was used instead. The beta distribution with the logit link function was used for the frequency of eggs deposited (percentage of oviposition marks with eggs). For count data including oviposition marks, eggs, larvae, pupae, and larvae plus pupae, when normality assumption was met, the Gaussian distribution with the identity link function was used; otherwise, either the negative binomial or the Poisson distribution with the log link function with a lower Akaike information criterion (AIC) value (i.e., a better fit) was used. Across response variables, host plant species/sizes and sampling dates (when applicable) were the fixed factors, while replication was the random factor in the analyses. LS-means were used for post hoc comparisons with Tukey Studentized Range (HSD) Test when the fixed factor was significant at α = 0.05 for mean separation. In the analysis for the effect of host plant species on number of pupae per plant on the last sampling date at Fenner in 2022, T-grouping was used for mean separation as Tukey Studentized Range (HSD) Test was too conservative to separate LS-means.
3 Results
3.1 Host plant species preference in controlled environment choice tests
In host species choice experiments, numbers of P. gymnostoma oviposition marks per plant varied among Allium species (F3, 18 = 3.61, P = 0.034). The onion and scallion hybrid had more oviposition marks than leeks, while numbers of marks in chives and scallions were not statistically different from leeks, but all were numerically higher than leeks (Figure 1). However, total number of P. gymnostoma (eggs, larvae, and pupae combined) per plant at 21 days after flies were released were not different among Allium species (P = 0.244; Supplementary Figure 3).
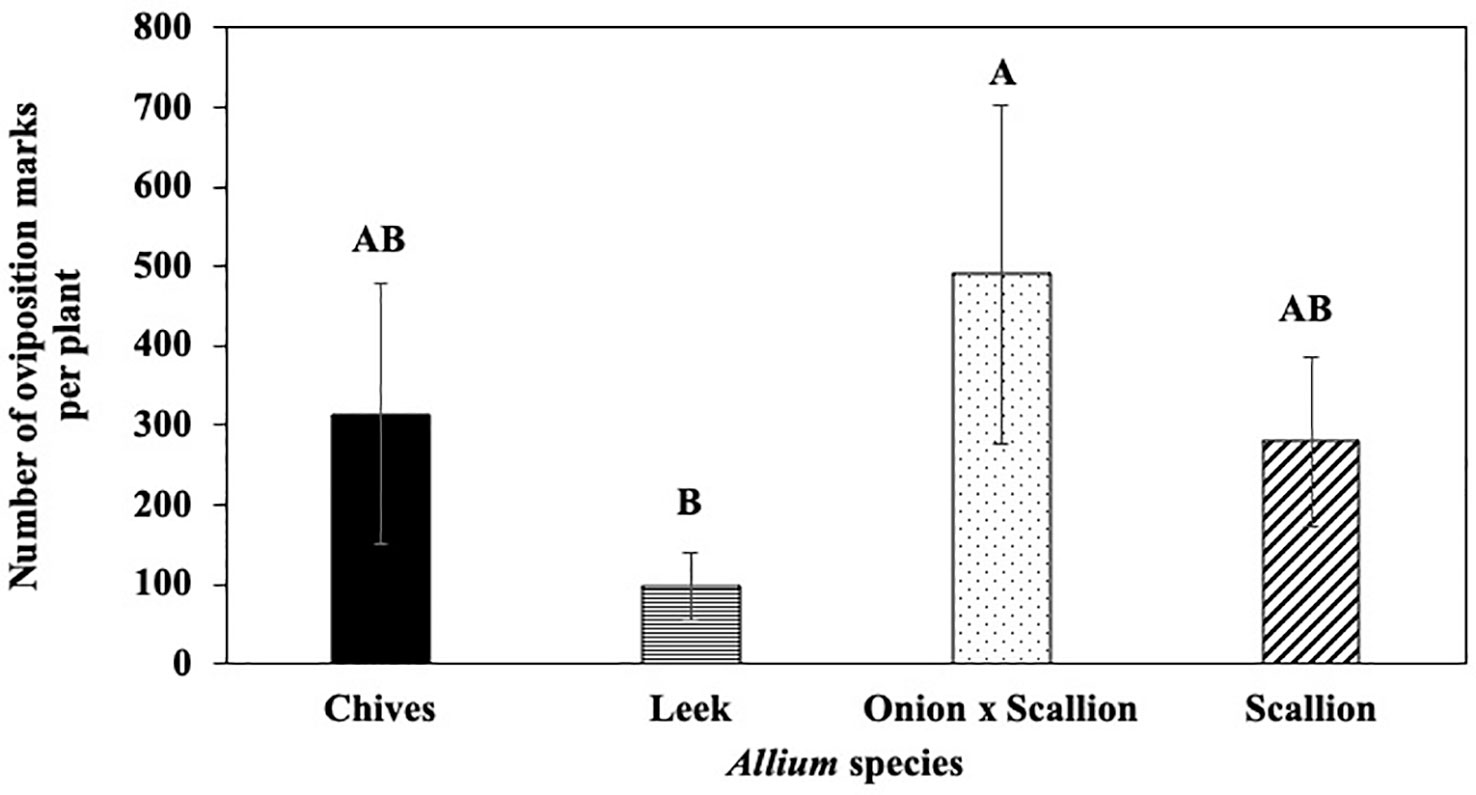
Figure 1 Mean (± SE) number of Phytomyza gymnostoma oviposition marks per plant at 21 days after flies were released in controlled environment choice tests. Different letters indicate significant differences in least squares means of number of oviposition marks among Allium species (Tukey-HSD; P < 0.05; n = 7).
3.2 Host plant size/age preference in controlled environment choice tests
In host size/age choice tests, numbers of P. gymnostoma oviposition marks per cm2 leaf area differed among host sizes/ages (F2, 10 = 4.93, P = 0.032). Large scallions had more oviposition marks per cm2 leaf area than small scallions, and numbers of marks per cm2 leaf area in medium scallions were not different from large or small scallions (Figure 2A). In addition, densities of P. gymnostoma also differed among host sizes/ages at 21 days after flies were released (F2, 10 = 11.39, P = 0.003). Large scallions had more P. gymnostoma (eggs, larvae, and pupae combined) per plant than small scallions, while P. gymnostoma densities in medium scallions were not different from large or small scallions (Figure 2B).
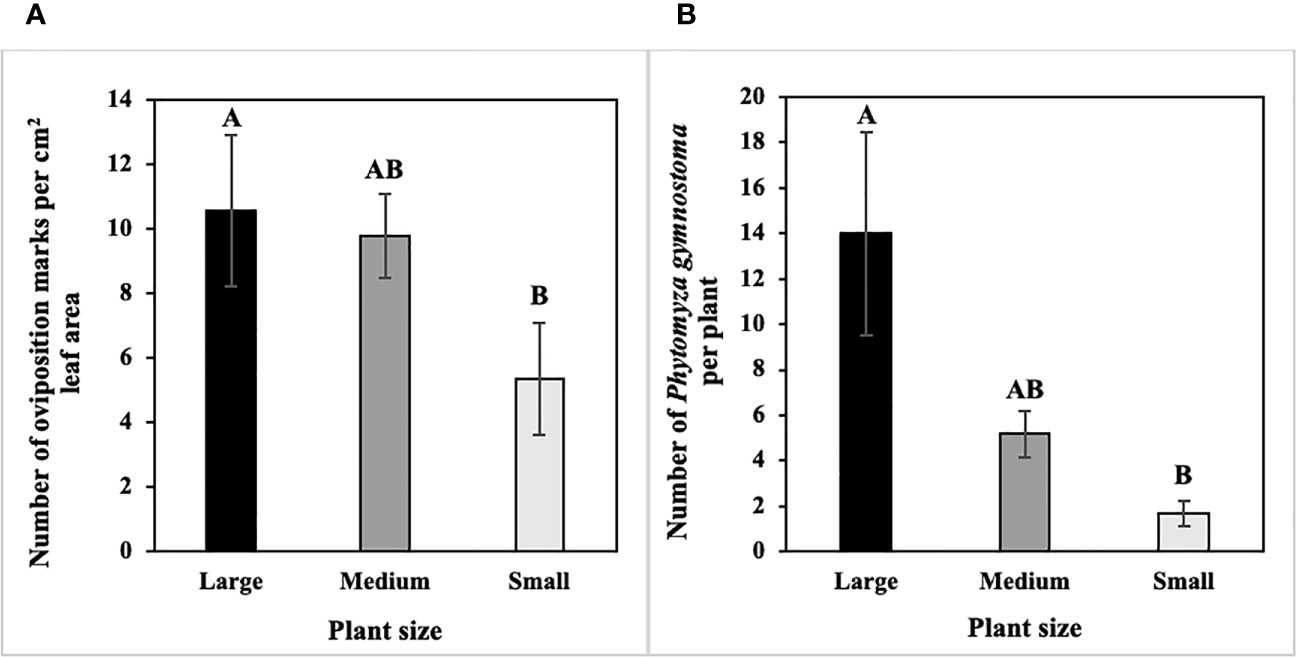
Figure 2 Mean (± SE) number of Phytomyza gymnostoma (A) oviposition marks per cm2 leaf area and (B) eggs, larvae, and pupae combined per plant at 21 days after flies were released in controlled environment choice tests. Different letters within each graph indicate significant differences in least squares means of number of oviposition marks or total number of P. gymnostoma among plant sizes (Tukey-HSD; P < 0.05; n = 6).
3.3 Host plant species preference in field trials
In the two-year field study, extremely high populations of P. gymnostoma were observed in field sites in eastern NY in 2021 and 2022, whereas moderate population pressure was observed in central NY in 2022. In 2021 at Hurley and Red Hook, 91% and 98% of plants were considered damaged (i.e., presence of oviposition marks) by the end of the season across all Allium species (Figures 3A, B). In 2022 at Hurley, the overall plant damage was nearly 100% averaged across all Allium species, whereas at Fenner the overall plant damage was 65% averaged across all Allium species (Figures 3C, D).
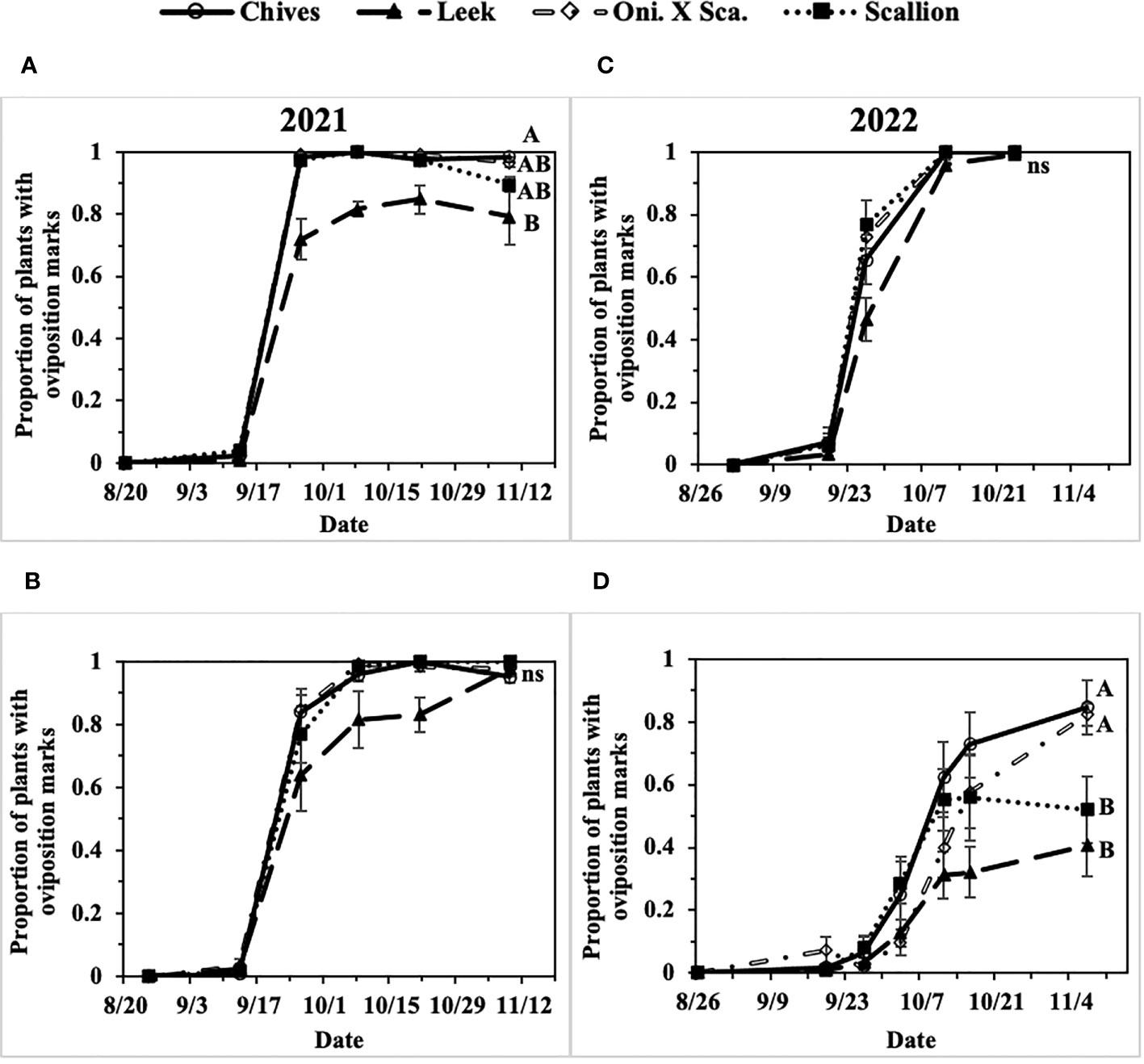
Figure 3 Mean (± SE) proportion of plants with Phytomyza gymnostoma oviposition marks over time in (A) Hurley, NY, 2021, (B) Red Hook, NY, 2021, (C) Hurley, NY, 2022, and (D) Fenner, NY, 2022. Different letters within each graph indicate significant differences in least squares means of proportion of plants with oviposition marks among Allium species on the final sampling date (Tukey-HSD; P < 0.05; n = 5); an ‘ns’ indicate no significant difference (P > 0.05) among Allium species on the final sampling date. “Oni. X Sca.” in the legend denotes the onion and scallion hybrid.
3.3.1 Progression of P. gymnostoma infestations in Allium crops
The first activity of fall-generation P. gymnostoma in eastern New York (Hurley, NY) was reported on 7 and 14 September of 2021 and 2022, respectively. Progression of P. gymnostoma infestations at a plant population level, represented as proportions of plants with oviposition marks, followed sigmoid curves with less than 10% of plants with oviposition marks within the first week after initial detection of activity. In general, infestations of P. gymnostoma progressed more slowly in leeks than in chives, scallions, and the onion and scallion hybrid (Figure 3).
In 2021 at both locations, infestation levels increased to over 50% within three weeks after initial detection in all Allium species (Figures 3A, B). At the end of the season at Hurley, there were significantly lower proportions of leeks with oviposition marks than chives (F3, 16 = 4.62, P = 0.016; Figure 3A). While at Red Hook, almost all plants had P. gymnostoma oviposition marks regardless of Allium species (P = 0.787; Figure 3B).
In 2022 at Hurley, infestation levels increased over 50% within two weeks in chives, scallions, and the onion and scallion hybrid, but not in leeks. At the end of the season, almost all plants had P. gymnostoma oviposition marks regardless of Allium species (P = 0.999; Figure 3C). In 2022 at Fenner, P. gymnostoma infestation levels progressed more slowly and did not exceed 50% infestation until four to five weeks after initial detection of activity in chives, scallions, and the onion and scallion hybrid, while the level of infestation in leeks never exceeded 50%. At the end of the season, there were significantly lower proportions of leeks and scallions with oviposition marks than chives and the onion and scallion hybrid (F3, 10.13 = 11.99, P = 0.001; Figure 3D).
3.3.2 Number of P. gymnostoma oviposition marks and eggs (Allium species)
In 2021 at both locations, numbers of P. gymnostoma oviposition marks per plant differed among Allium species and sampling dates with a significant interaction effect (Hurley: F12, 76 = 5.14, P < 0.001; Red Hook: F12, 76 = 3.62, P < 0.001; Figure 4). At Hurley, scallions had the highest number of oviposition marks on the first sampling date. Numbers of oviposition marks on chives and scallions were higher than those on leeks on three of the four sampling dates after the first sampling date, while number of marks on the onion and scallion hybrid was consistently higher than those on leeks after the first sampling (Figure 4A). At Red Hook, numbers of oviposition marks were not different among Allium species on three of five sampling dates; however, the number of oviposition marks on chives and the onion and scallion hybrid was significantly greater than on leek on 8 October (the middle sampling date), and there were more oviposition marks on chives, scallions, and the hybrid than on leek on the last sampling date. (Figure 4B).
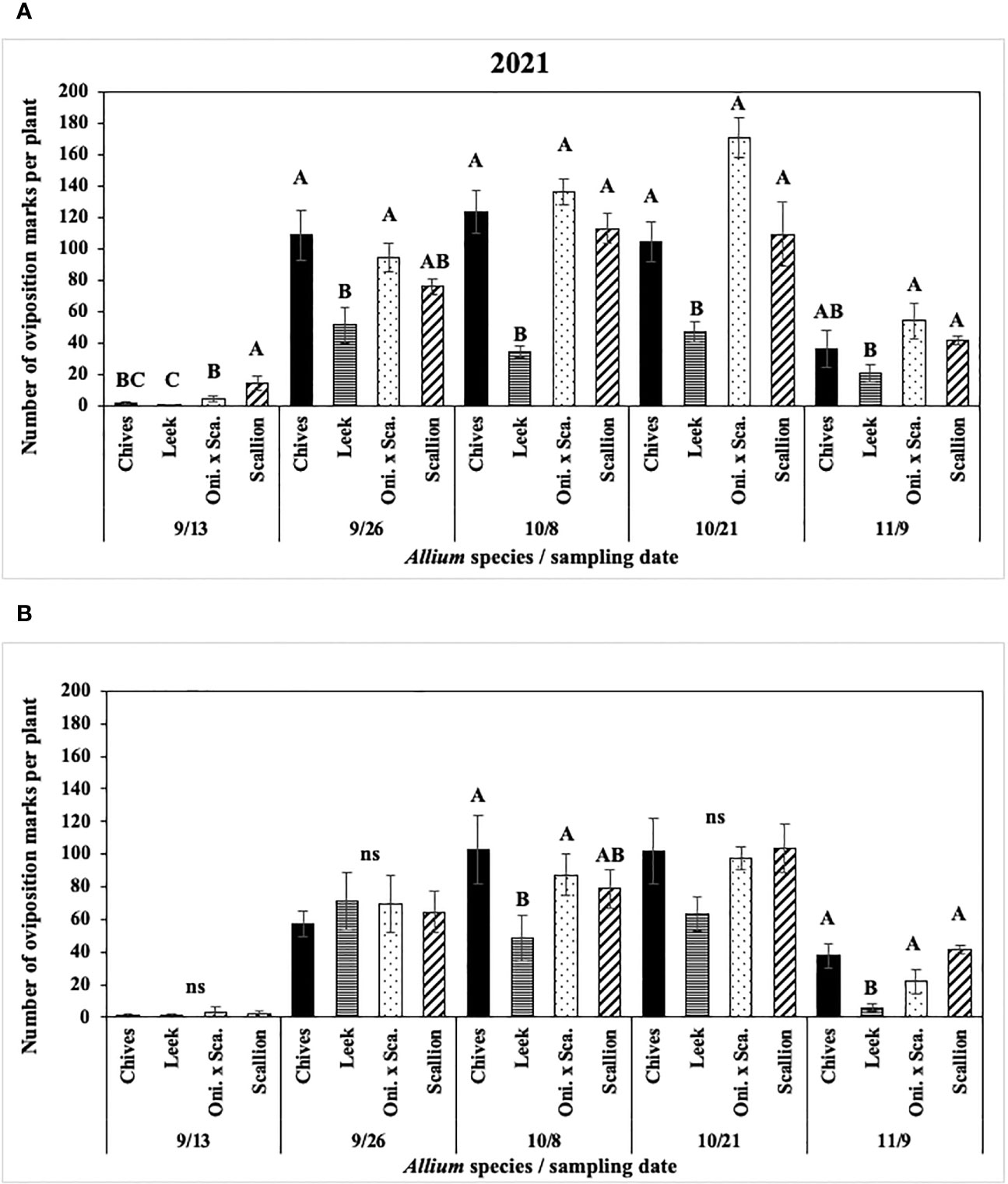
Figure 4 Mean (± SE) number of Phytomyza gymnostoma oviposition marks per plant on each of five sampling dates in (A) Hurley, NY and (B) Red Hook, NY in 2021. Different letters within each sampling date of each graph indicate significant differences in least squares means of number of oviposition marks among Allium species on each of the sampling date (Tukey-HSD; P < 0.05; n = 5), while ‘ns’ indicates no significant differences (P > 0.05) among Allium species. “Oni. x Sca.” on the x-axes denotes the onion and scallion hybrid.
In 2022 at both locations, the interaction effect between Allium species and sampling dates on numbers of P. gymnostoma oviposition marks per plant was not significant (Hurley: P = 0.10; Fenner: P = 0.11). Mean numbers of oviposition marks across all sampling dates differed among Allium species. At Hurley, leeks had significantly lower numbers of oviposition marks than chives and the onion and scallion hybrid (F3, 60 = 7.19, P < 0.001; Figure 5A). At Fenner, scallions had significantly lower numbers of oviposition marks than chives, while number of marks on leeks and the onion and scallion hybrid was not different from those on scallions or chives (F3, 60 = 3.25, P = 0.028; Figure 5B).
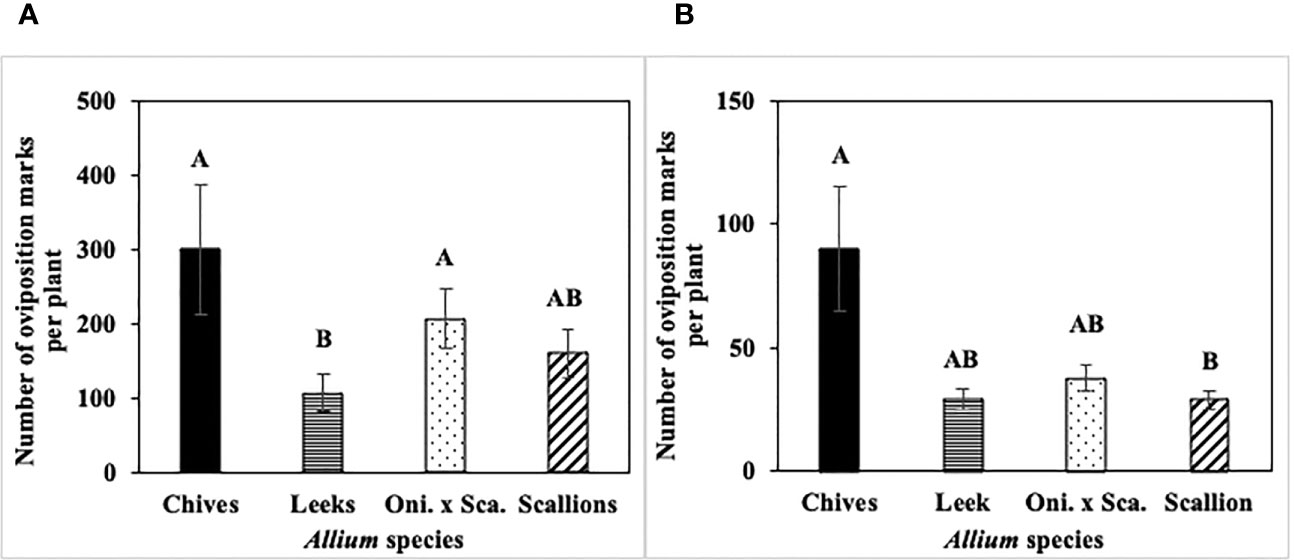
Figure 5 Mean (± SE) number of Phytomyza gymnostoma oviposition marks per plant across the season in (A) Hurley, NY and (B) Fenner, NY in 2022. Different letters within each graph indicate significant differences (P < 0.05) in least squares means of number of oviposition marks among Allium species (Tukey-HSD; P < 0.05; n = 5). “Oni. x Sca.” on the x-axes denotes the onion and scallion hybrid. Note that y-axis scales differ between locations.
Cumulative number of P. gymnostoma eggs per plant across the season differed among Allium species in three of four trials (Figure 6). In both 2021 and 2022 at Hurley, chives had significantly higher numbers of eggs than leeks, scallions, and the onion and scallion hybrid (2021: F3, 12 = 9.67, P = 0.002, Figure 6A; 2022: F3, 16 = 20.31; P < 0.001, Figure 6B). In 2021 at Red Hook, no difference was found in egg numbers among species (P = 0.08; Figure 6C). In 2022 at Fenner, chives had significantly higher numbers of eggs than leeks (F3, 16 = 4.83; P = 0.014; Figure 6D).
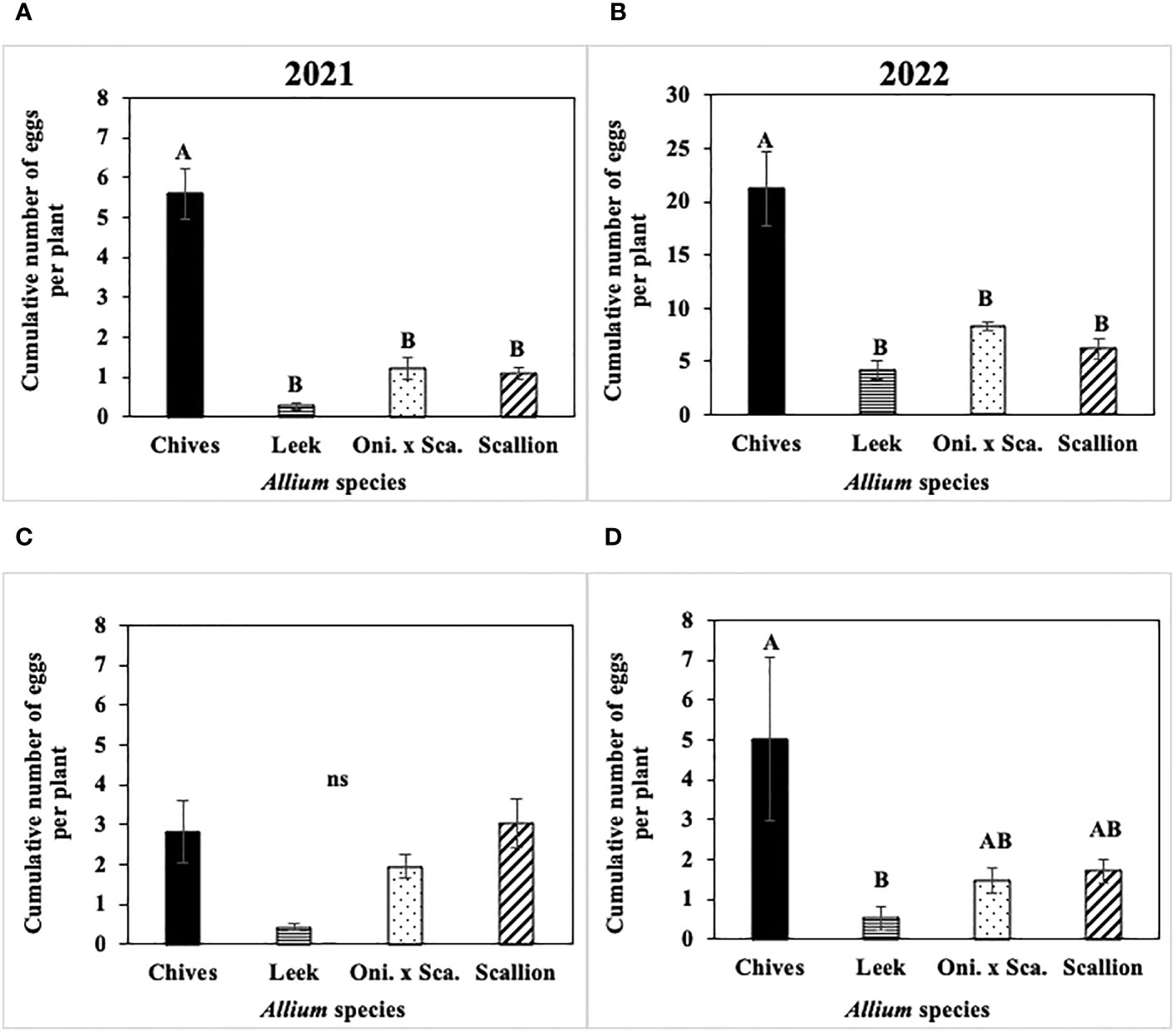
Figure 6 Mean (± SE) cumulative number of Phytomyza gymnostoma eggs per plant across the season in (A) Hurley 2021, NY, (B) Hurley 2022, NY, (C) Red Hook 2021, NY, and (D) Fenner 2022, NY. Different letters within each graph indicate significant differences in least squares means of cumulative number of eggs per plant among Allium species (Tukey-HSD; P < 0.05; n = 5), while ‘ns’ indicates no significant differences (P > 0.05) among Allium species. “Oni. x Sca.” on the x-axes denotes the onion and scallion hybrid. Note that the y-axis scale in (B) differs from those in (A, C, D).
The frequency of P. gymnostoma eggs deposited while making the same number of oviposition marks differed among Allium species (Figure 7). In 2021 at Hurley, the percentage of oviposition with eggs was higher in chives than in leeks, scallions, and the onion and scallion hybrid (F3, 11 = 8.74; P = 0.003; Figure 7A); while in 2021 at Red Hook, the percentage of oviposition with eggs was higher in chives than in leeks and the hybrid (F3, 12 = 7.27; P = 0.005; Figure 7B). In 2022 at Hurley, the percentage of oviposition with eggs in chives was higher than in leeks (F3, 12 = 4.21; P = 0.023; Figure 7C); while in 2022 at Fenner, the percentage of oviposition with eggs in chives and scallions were higher than in leeks and the onion and scallion hybrid (F3, 9 = 5.26; P = 0.023; Figure 7D). Across Allium species and trials, P. gymnostoma averaged laying eggs 4% of the time when they made oviposition marks.
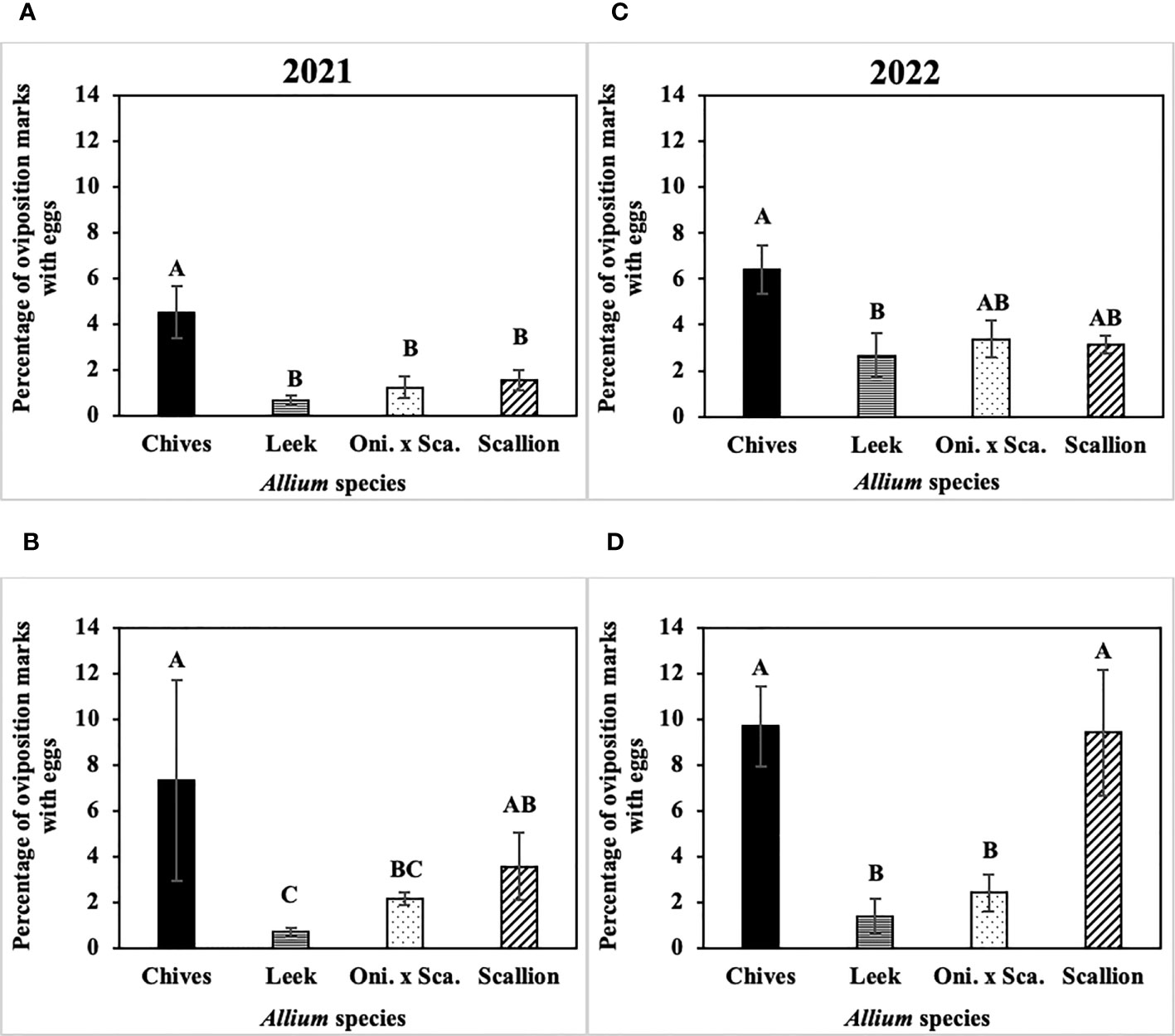
Figure 7 Mean (± SE) percentage of Phytomyza gymnostoma oviposition marks with eggs on the sampling date with the highest overall number of eggs during the season in (A) Hurley, NY on 26 September 2021, (B) Red Hook, NY on 26 September 2021, (C) Hurley, NY on 26 September 2022, and (D) Fenner, NY on 3 October 2022. Different letters within each graph indicate significant differences in least squares means of percentage of oviposition marks with eggs among Allium species (Tukey-HSD; P < 0.05; n = 5). “Oni. x Sca.” on the x-axes denotes the onion and scallion hybrid.
Overall, chives had more P. gymnostoma oviposition marks and eggs than the other Allium species, while leeks had the fewest.
3.3.3 Number of P. gymnostoma larvae and pupae (Allium species)
Numbers of P. gymnostoma larvae per plant were affected by Allium species in three of four trials (Figures 8, 9A). In both years at Hurley, numbers of larvae per plant differed among Allium species and sampling dates with a significant interaction effect (2021: F9, 60 = 2.44, P = 0.019; 2022: F9, 60 = 3.86, P < 0.001; Figure 8). In 2021 at Hurley, while the number of larvae did not differ among species on 26 September, the number of larvae in leek was significantly lower than the numbers in the other three species on 8 October; numbers of larvae in leeks were significantly lower than those in scallions, but were similar to those in chives and the onion and scallion hybrid on the latter two sampling dates (Figure 8A). In 2022 at Hurley, numbers of larvae were not different among species on 26 September and 18 November; however, the number of larvae in leeks was lower than those in chives and the onion and scallion hybrid, but not different from scallions on 11 October; numbers of of larvae were lower in leek, scallions and the onion and scallion hybrid than in chives on 24 October. (Figure 8B).
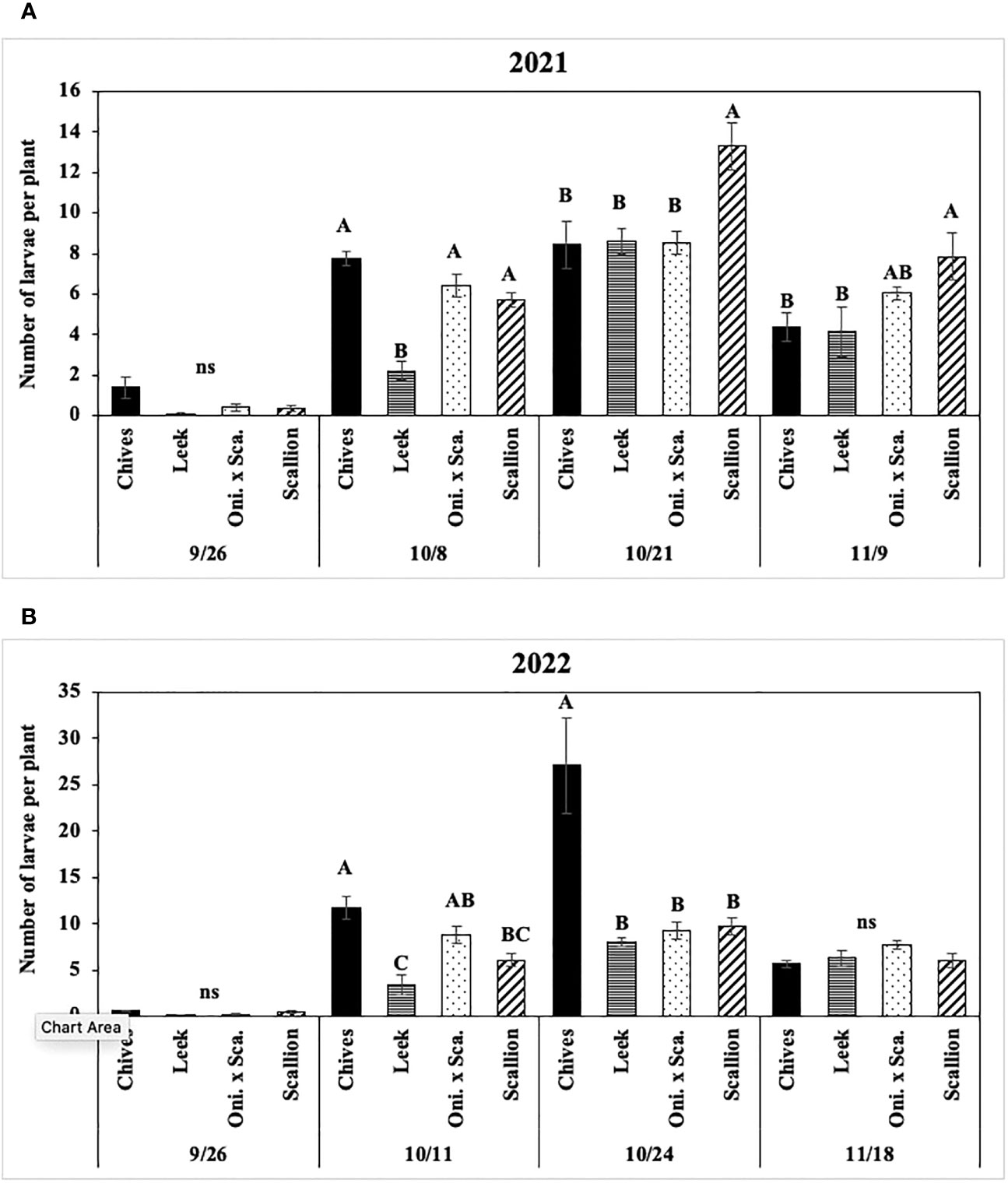
Figure 8 Mean (± SE) number of Phytomyza gymnostoma larvae per plant on each of four sampling dates in Hurley, NY in (A) 2021 and (B) 2022. Different letters within each sampling date of each graph indicate significant differences in least squares means of number of larvae per plant among Allium species (Tukey-HSD; P < 0.05; n = 5), while ‘ns’ indicates no significant differences (P > 0.05) among Allium species. “Oni. x Sca.” on the x-axes denotes the onion and scallion hybrid. Note that the y-axis scales differ between two years.
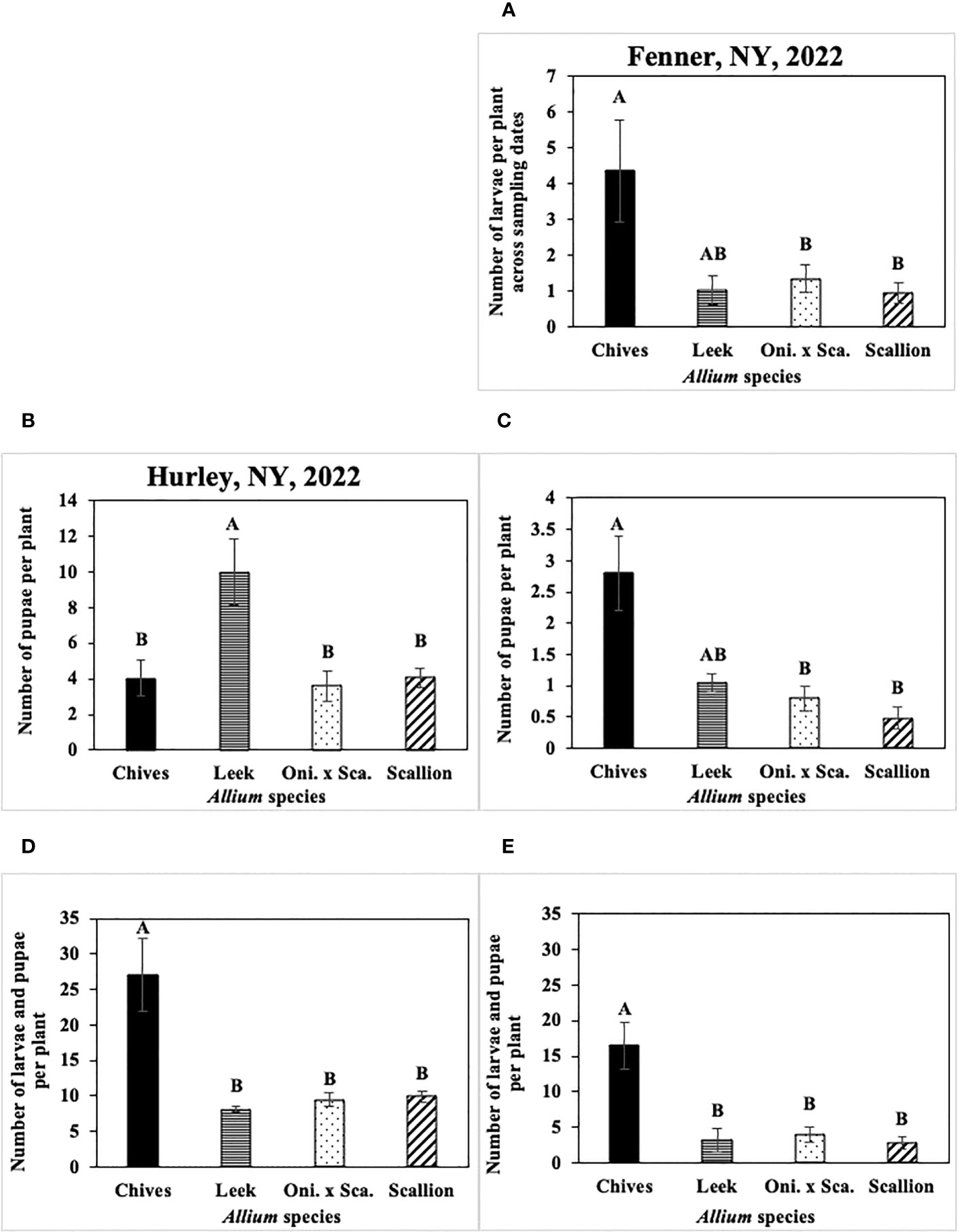
Figure 9 (A) Mean (± SE) number of Phytomyza gymnostoma larvae per plant across sampling dates in Fenner, NY in 2022. Mean (± SE) number of P. gymnostoma pupae per plant on the last sampling date in (B) Hurley, NY on 09 November 2022 and (C) Fenner, NY on 18 November 2022. Mean (± SE) number of P. gymnostoma larvae and pupae per plant on the sampling date with the highest overall number in (D) Hurley, NY on 24 October 2022 and (E) Fenner, NY on 7 November 2022. Different letters within each graph indicate significant differences in least squares means of number of P. gymnostoma per plant among Allium species (Tukey-HSD; P < 0.05; n = 5). “Oni. x Sca.” on the x-axes denotes the onion and scallion hybrid. Note that the y-axis scales differ among parameters and locations.
In 2021 at Red Hook, the effect of Allium species on numbers of larvae was not significant (P = 0.767). In 2022 at Fenner, numbers of larvae per plant differed among Allium species without a significant interaction effect between species and sampling date. Chives had significantly higher numbers of larvae than scallions and the onion and scallion hybrid across all sampling dates, while numbers of larvae in leeks were similar to those in the other species (F3, 76 = 48.36, P < 0.001; Figure 9A).
Numbers of P. gymnostoma pupae per plant on the last sampling date differed among Allium species in two of four trials. In 2021 at Hurley and Red Hook, the effect of Allium species on numbers of pupae was not significant (Hurley: P = 0.316; Red Hook: P = 0.199). In 2022 at Hurley, leeks had significantly higher numbers of pupae than chives, scallions, and the onion and scallion hybrid (F3, 12 = 7.96, P = 0.004; Figure 9B). In 2022 at Fenner, chives had significantly higher numbers of pupae than scallions and the onion and scallion hybrid, while numbers of pupae in leeks were similar to those in the other species (F3, 12 = 3.58, P = 0.047; Figure 9C).
Numbers of P. gymnostoma larvae plus pupae per plant on the sampling date with the highest overall numbers also differed among Allium species in two of four trials. In 2021 at Hurley and Red Hook, the effect of Allium species on numbers of larvae and pupae was not significant (Hurley: P = 0.083; Red Hook: P = 0.269). In 2022 at Hurley and Fenner, chives had significantly higher numbers of larvae plus pupae than those in leeks, scallions, and the onion and scallion hybrid (Hurley: F3, 12 = 26.96, P < 0.001, Figure 9D; Fenner: F3, 12 = 26.56, P < 0.001, Figure 9E).
Overall, numbers of P. gymnostoma in plants throughout the season largely varied among Allium species and trials. However, chives had higher numbers of larvae plus pupae than the other species late in the season, suggesting that chives is a preferred reproductive host among the species evaluated. Alternatively, there was a higher probability of P. gymnostoma survival to pupation in chives compared with the other species.
3.4 Host plant size/age preference in field trials
3.4.1 Progression of P. gymnostoma infestations (size/age)
Progression of the P. gymnostoma infestations at a plant population level, represented as proportions of plants with oviposition marks, followed sigmoid curves with no apparent difference in progression trend between large (older) and small (younger) scallions. In 2022 at Hurley and Fenner, the final proportions of plants with oviposition marks were not different between large and small scallions (Hurley: P = 0.991, Fenner: P = 0.494; Figure 10).
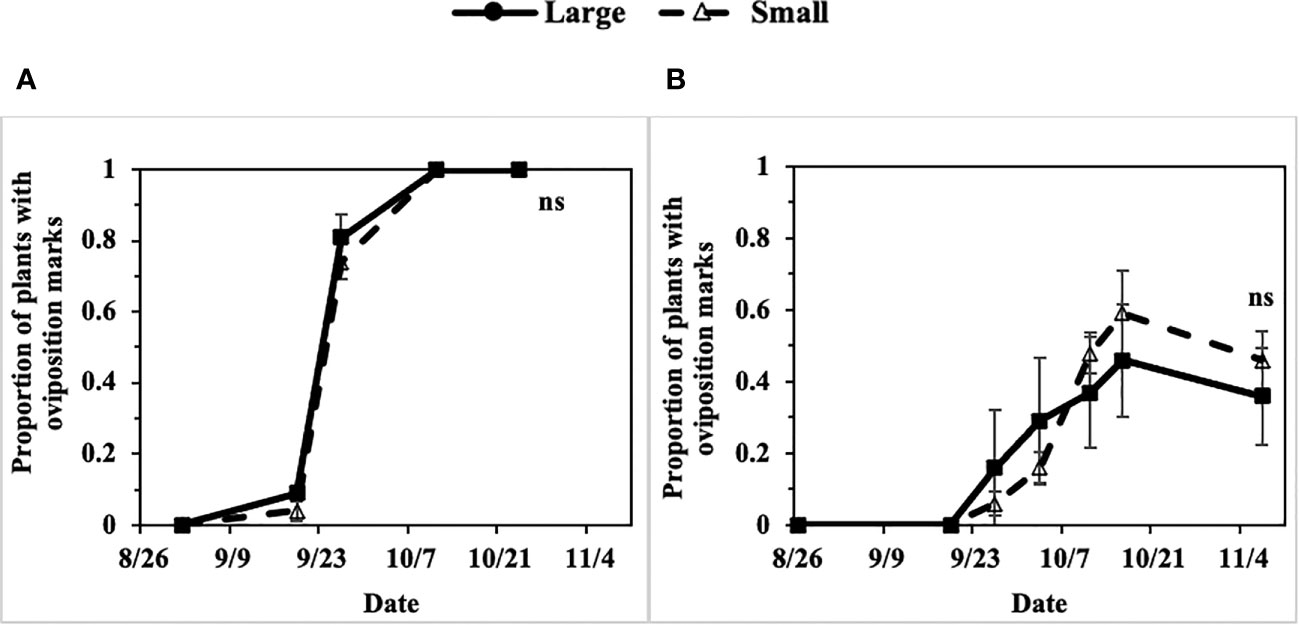
Figure 10 Mean (± SE) proportion of large and small scallions with Phytomyza gymnostoma oviposition marks over time in (A) Hurley, NY and (B) Fenner, NY in 2022. An ‘ns’ indicates no significant differences (Tukey-HSD; P > 0.05; n = 4) in least squares means of proportion of plants with oviposition marks between large and small scallions on the last sampling date.
3.4.2 Number of P. gymnostoma oviposition marks and eggs (size/age)
In 2022 at Hurley, the number of P. gymnostoma oviposition marks per cm2 leaf area differed by plant size and sampling dates with a significant interaction effect (F3, 21 = 6.02, P = 0.004; Figure 11A). Small scallions had significantly more oviposition marks per cm2 leaf area than large scallions on 11 October, while no differences existed between the two treatments on the other three sampling dates (Figure 11A). In 2022 at Fenner, where the overall pressure was lower than at Hurley, the number of P. gymnostoma oviposition marks per cm2 leaf area also differed by plant size, but without a significant interaction effect with sampling date (F1, 21 = 7.31, P = 0.013). The number of oviposition marks per cm2 leaf area in smaller scallions was significantly higher than those in larger scallions across sampling dates (Figure 11B).
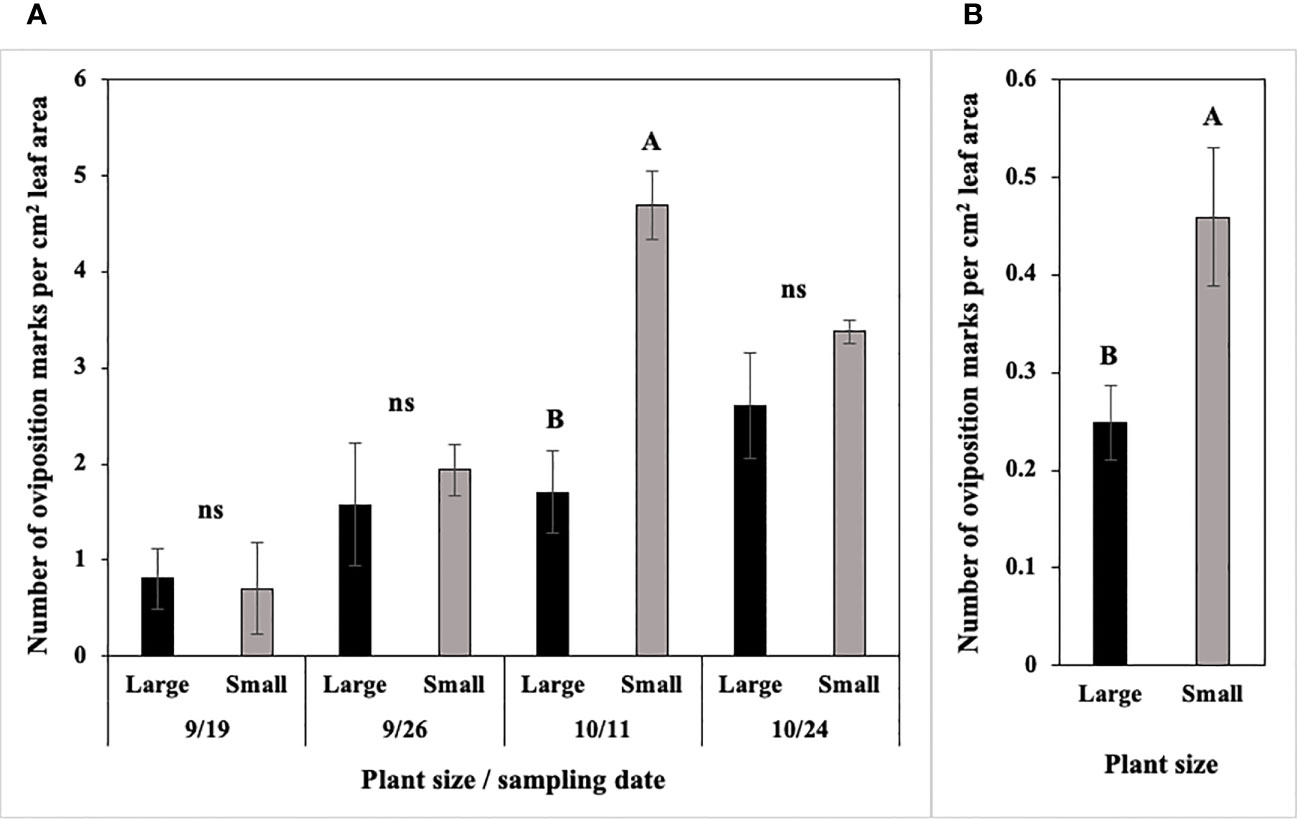
Figure 11 Mean (± SE) number of Phytomyza gymnostoma oviposition marks per cm2 leaf area (A) on four sampling dates at Hurley, NY and (B) across sampling dates at Fenner, NY. Different letters within each graph indicate significant differences in least squares means of number of oviposition marks between plant size (Tukey-HSD; P < 0.05; n = 4), while ‘ns’ indicates no significant differences. Note that the y-axis scales differ between two locations.
Cumulative number of P. gymnostoma eggs per plant was not statistically different between large and small scallions in either trial (Hurley: P = 0.417, Fenner: P = 0.904). However, small scallions tended to have a numerically higher cumulative numbers of eggs than large scallions in both trials (Supplementary Figures 4A, B).
Similarly, the frequency of P. gymnostoma eggs deposited did not differ by plant size/age in either trial (Hurley: P = 0.088, Fenner: P = 0.247). Small scallions tended to have numerically higher percentages of oviposition marks with eggs than large scallions in both trials (Supplementary Figures 4C, D).
Overall, smaller scallions had significantly higher oviposition marks per cm2 leaf area and numerically higher number of eggs per plant and frequency of egg deposition, indicating a preference for smaller plants over larger ones.
3.4.3 Number of P. gymnostoma larvae and pupae (size/age)
In 2022 at Hurley and Fenner, numbers of P. gymnostoma larvae across all sampling dates, numbers of pupae on the last sampling date, and numbers of larvae plus pupae on the sampling date with the highest total P. gymnostoma number did not differ by plant size/age [larvae: P = 0.124 (Hurley), P = 0.988 (Fenner); pupae: P = 0.267 (Hurley), P = 0.980 (Fenner); larvae plus pupae: P = 0.058 (Hurley), P = 0.935 (Fenner)]. However, a consistent numerical trend was observed that large scallions had numerically more larvae, pupae, and larvae plus pupae than small scallions (larvae: Supplementary Figures 4E, F; pupae: Supplementary Figures 4G, H; larvae plus pupae: Supplementary Figures 4I, J).
Overall, P. gymnostoma larval and pupal densities were statistically similar between large (old) and small (young) plants. However, there was a consistent numerical trend for larger (older) scallions to support more larvae and pupae than smaller (younger) ones. These results likely reflect the ability of more larvae to survive and complete development to the pupal stage on a larger plant compared with smaller ones.
4 Discussion
The most important component of a trap crop strategy for pest management is to identify a highly attractive host plant that the target pest distinctly prefers to feed and reproduce on compared with the main crop so that the main crop will avoid or suffer less damage. Our study suggested that P. gymnostoma prefers to feed and reproduce on chives more than leeks because chives had more oviposition marks, eggs, larvae and pupae than leeks. Therefore, chives could be considered as a potential trap crop for P. gymnostoma management in leek production. Chives were considered highly suitable host plants for P. gymnostoma in Austria because they remain in a vegetative stage during both spring and fall generations (17). In our study, we also observed that chives remained in a vegetative stage longer than the other Allium crop species as new leaves were continually being produced. Phytomyza gymnostoma made oviposition marks and laid eggs in chives more than they did in leeks and other Allium species examined, especially towards the end of season. New leaves are often softer and thinner than old leaves, which might have played a role in why P. gymnostoma made so many oviposition punctures.
Host feeding and oviposition preference of P. gymnostoma has not been investigated elsewhere, and preference can be affected by single or multiple physical and/or chemical factors. Host feeding selection of pea leafminer (Liriomyza huidobrensis), another Agromyzid, was positively correlated with moisture content of leaves and negatively correlated with the thickness of the epidermis wall; thickness of the epidermis wall can serve as a physical barrier to female oviposition (18). Chemical content of host leaves could also affect host preference of leaf mining flies. For example, the American serpentine leafminer (L. trifolii) preferred to feed and oviposit on tomato plants with a higher nitrogen content, and performance variables of the larvae such as development rate, survivorship, and pupal size were increased with increasing plant nitrogen levels (19). The physical and chemical characteristics of Allium species in this study were not investigated, except for visual observation of leaf texture and thickness. While the underlying mechanism of host species preference of P. gymnostoma is not known, this is an important topic for future studies to consider. Understanding such a mechanism could be useful for breeding programs seeking to develop resistant cultivars.
Attractiveness of host plants could come from other physiological characteristics such as plant sizes or ages. In our controlled environment experiments, P. gymnostoma preferred to make oviposition marks on larger/older scallions than on smaller/younger scallions. Larger/older plants have more leaf area and tissue than smaller plants, which likely makes larger/older plants easier to be visually and/or chemically detected by P. gymnostoma if visual and/or chemical cues are important for host-finding. However, P. gymnostoma preferred to make oviposition marks on smaller plants than on larger ones in the field. Leaves of scallions in our controlled environment studies were in a relatively young vegetative stage, whereas scallions in the field trials were much older and leaves likely became tougher and thicker over time. Ovipositional preference for smaller plants could be influenced by the softer leaves of smaller plants rather than plant size. In addition, plants grew faster in the field under suitable conditions than in controlled environments. As a consequence, smaller plants tended to catch up phenologically with the larger/older plants more quickly in the field. Large and small plants in our field trials might not have been different enough to trigger a host preference, especially in 2022 at Fenner where large and small scallions were seeded only four weeks apart. Overall, P. gymnostoma’s preference for larger/older plants as hosts was only supported by our laboratory studies, but not by our field studies. Therefore, host size/age alone should not be considered as an important factor when designing a trap crop strategy for this pest.
Trap cropping has been evaluated for management of Liriomyza spp. in shallot and onion crops. Trap cropping significantly reduced leafminer populations by 40%, and population reduction was up to 48% when trap cropping was integrated with the use of arbuscular mycorrhizae (20). Moreover, trap cropping also attracted and preserved parasitoid wasps as natural enemies in the cropping system in addition to reducing leafminer infestation (21). For developing a trap cropping strategy for P. gymnostoma management in leek production in the northeastern United States, our study showed that chives are the best candidate trap crop among the Allium species we evaluated. Future studies should focus on determination of a spatial pattern and configuration of planting a trap crop as well as planting distance from the main crop. We observed a relatively pronounced edge effect of P. gymnostoma infestation and damage where higher numbers of flies and levels of damage occurred on plants nearest to the previous crop that P. gymnostoma had infested. This pattern of crop colonization would lend itself nicely to placing a trap crop between the current season’s crop and the preceding one. Chives (trap crop) could be planted between the preceding Allium crop and the current season’s leek crop (main crop) in order to intercept flies in the chives before reaching the leeks (15). Future studies should also look into the impact of using chives as a trap crop in leek production on natural enemy population such as parasitoid wasps.
In addition to host preference of P. gymnostoma among common Allium crops species and between plant sizes, our study also provided knowledge of P. gymnostoma infestation progression in Allium crops grown in northeastern United States, which can be useful information for developing and optimizing P. gymnostoma management in the field. For example, physical control methods such as exclusion netting and vacuuming should take place soon after initial detection of P. gymnostoma because eggs were found as early as five days after initial detection, and infestation reached 100% in the field in two to three weeks under heavy fly pressure in our study.
In conclusion, identifying chives as a potential trap crop that can be explored for reducing P. gymnostoma damage in leeks was a promising initial step towards development of an alternative management approach to insecticide use. Our study demonstrated that P. gymnostoma preferred chives over leeks for feeding and reproduction. However, the level of preference is not likely strong enough for trap cropping to be effective on its own. Besides trap cropping, cultural practices such as reflective mulch, exclusion netting, and vacuuming are management tactics that have potential for P. gymnostoma management, but need to be evaluated. Integrating one or more cultural practices with trap cropping could potentially create synergism to provide an economically acceptable level of P. gymnostoma control in leek crops.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
BN, PL, and RS contributed to conception and design of the study. BN acquired the funding for this study. PL and RS executed experiments, collected data, and organized the database. PL performed the statistical analysis. PL wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Federal Formula Grants and was partially supported by USDA NIFA Crop Protection Pest Management Program award 2018-70006-28920.
Acknowledgments
The authors thank Matthew Garlick for the technical support as well as Ethan Grundberg and Teresa Rusinek for arranging commercial organic farms for this study. The authors are also grateful for assistance in transplanting and data collection by Erin Equinozzi, Nathan Hesler, Leonardo Salgado, Lidia Komondy, Miles Todaro, Dugan Doeblin, Enya Shen, Luke Weber, and Alisa Marks.
Conflict of interest
Author RS is currently employed by FMC Corporation. However, RS's involvement in this research occurred prior to her employment with FMC.
The author(s) BN declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2023.1233130/full#supplementary-material
References
1. Barringer LE, Fleischer SJ, Roberts D, Spichiger SE, Elkner T. The first North American record of the allium leafminer. J Integr Pest Manag (2018) 9:1–8. doi: 10.1093/JIPM/PMX034
2. CABI. Phytomyza gymnostoma (onion leaf miner). CABI Compendium (2022). doi: 10.1079/CABICOMPENDIUM.41008
3. Lingbeek B, Roberts D, Elkner T, Gates M, Fleischer SJ. Phenology, development, and parasitism of allium leafminer (Diptera: Agromyzidae), a recent invasive species in the United States. Environ Entomol (2021) 50:878–87. doi: 10.1093/EE/NVAB043
4. Coman M, Rosca I. Biology and life cycle of leafminer Napomyza (Phytomyza) gymnostoma Loew, a new pest of allium plants in Romania. South West J Horti Biol Environ (2011) 2:57–64.
5. Coman M, Rosca I. Allium crops protection plant management for Napomyza gymnostoma Loew pest, Scientific Paper Series - Management, Economic Engineering in Agriculture and Rural Development (2011). 11:26–9, PRINT ISSN 1844-5640. E-ISSN 2247-3527.
6. Laznik Ž, Bohinc T, Vidrih M, Trdan S. Testing the suitability of three herbs as intercrops against the allium leaf miner (Phytomyza gymnostoma Loew, Diptera, Agromyzidae) in onion production. J Food Agric Environ (2012) 10:751–5.
7. Zandigiacomo P, Montà LD. Occurrence in Northern Italy of the leek mining fly Napomyza gymnostoma (Loew) (Diptera, Agromyzidae). Bollettino di Zoologia Agraria e di Bachicoltura (2002) 34:265–8.
8. Darvas B, Szarukán I, Papp L. Napomyza gymnostoma (Loew) (Dipt.: Agromyzidae), an agromyzid pest on leek in Hungary. Novenyvedelem (Bp 1965) (1988) 24:450–5.
9. Ebrahimi N, Cap N, Spiessens K, De MJ, Temmerman F, Bonte J, et al. Seasonal occurrence and life cycle of the allium leaf miner (Phytomyza gymnostoma) in Belgium, in: 70th International Symposium on Crop Protection (2018) Conference proceeding, Conference Abstract.
10. Sionek R. Effect of leek planting dates on damage to plants by spring generation of the leek leaf miner (Napomyza gymnostoma Loew). Prog Plant Prot (1999) 39(2):500–2.
11. Nault BA, Iglesias LE, Harding RS, Grundberg EA, Rusinek T, Elkner TE, et al. Managing allium leafminer (Diptera: Agromyzidae): an emerging pest of Allium crops in North America. J Econ Entomol (2020) 113(5):2300–9. doi: 10.1093/JEE/TOAA128
12. Nault BA, Sandhi RK, Harding RS, Grundberg EA, Rusinek T. Optimizing spinosyn insecticide applications for allium leafminer (Diptera: Agromyzidae) management in Allium crops. J Econ Entomol (2022) 115(2):618–23. doi: 10.1093/JEE/TOAC016
13. MacLeod A. CSL Pest risk analysis for Phytomyza gymnostoma. Sand Hutton, York, United Kingdom (2007). European and Mediterranean Plant Protection Organization (EPPO) Secretariat 1–13 p.
14. Hokkanen HMT. Trap cropping in pest management. Annu Rev Entomol (1991) 36:119–38. doi: 10.1146/annurev.en.36.010191.001003
15. Shelton AM, Badenes-Perez FR. Concepts and applications of trap cropping in pest management. Annu Rev Entomol (2006) 51:285–308. doi: 10.1146/ANNUREV.ENTO.51.110104.150959
16. Javaid I, Joshi JM. Trap cropping in insect pest management. J Sustain Agric (1995) 5:117–36. doi: 10.1300/J064V05N01_09
17. Kahrer A. (1999). Biology and control of the leek mining fly, Napomyza gymnostoma, In: Integrated control in field vegetable crops, Proceedings of the meeting at Chania, Crete, 6-8 October 1997, Vol. 22. pp. No. 5 205–11.
18. Wei J, Zou L, Kuang R, He L. Influence of leaf tissue structure on host feeding selection by pea leafminer Liriomyza huidobrensis (Diptera: Agromyzidae). Zool Stud (2000) 39:295–300.
19. Minkenberg OPJM, Fredrix MJJ. Preference and performance of an herbivorous fly, Liriomyza trifolii (Diptera: Agromyzidae), on tomato plants differing in leaf nitrogen. Ann Entomol Soc Am (1989) 82:350–4. doi: 10.1093/AESA/82.3.350
20. Saleh S, Anshary A, Made U. Integrated management of leaf miners Liriomyza spp. (Diptera: Agromyzidae) on shallot crops by trap cropping system and arbuscular mycorrhizae. J Biopest (2018) 11:114–20.
Keywords: Phytomyza gymnostoma, leek, scallion, onion, chives, phenology, pest management
Citation: Lai P-C, Sandhi RK and Nault BA (2023) Allium leafminer (Diptera: Agromyzidae) host preference: implications for developing a trap cropping strategy. Front. Insect Sci. 3:1233130. doi: 10.3389/finsc.2023.1233130
Received: 01 June 2023; Accepted: 04 August 2023;
Published: 17 August 2023.
Edited by:
Michael Brewer, Texas A&M AgriLife Research, United StatesReviewed by:
Arthur Vieira Ribeiro, University of Minnesota Twin Cities, United StatesJian-Feng Liu, Guizhou University, China
Copyright © 2023 Lai, Sandhi and Nault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pin-Chu Lai, cGw0ODRAY29ybmVsbC5lZHU=
 Pin-Chu Lai
Pin-Chu Lai Ramandeep Kaur Sandhi
Ramandeep Kaur Sandhi Brian A. Nault
Brian A. Nault