
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Insect Sci., 09 May 2023
Sec. Insect Molecular Genetics
Volume 3 - 2023 | https://doi.org/10.3389/finsc.2023.1188343
Unveiling the proximate mechanism of caste differentiation is crucial for understanding insect social evolution, and gene function analysis is an important tool in this endeavor. The RNA interference (RNAi) technique is useful in termites, but its knockdown effects may differ among species. One of the most important model species in the field of termite sociogenomics is Reticulitermes speratus Kolbe (Isoptera: Rhinotermitidae). Presoldier and worker differentiation of this species can be artificially induced by juvenile hormone and 20-hydroxyecdysone application, respectively. However, appropriate RNAi technique of genes expressed during caste differentiation has never been considered. To clarify this issue, first, we injected nine different volumes of nuclease-free water (NFW, 0–404.8 nL) into workers and found that survival and caste differentiation rates were strongly reduced by the application of the top three largest volumes. Second, we injected double-stranded (ds) RNA of ecdysone receptor homolog (RsEcR) (2.0 µg/151.8 nL NFW) into workers with hormone treatments. The expression levels of RsEcR were significantly reduced at 9 days after dsRNA injection. RsEcR RNAi strongly affected both molting events during presoldier and worker differentiation induced by hormone treatments. The present results highlight the need for caution regarding injection volumes for RNAi experiments using hormone treatments. We suggest that the injection of dsRNA solution (2 µg; approximately 100–200 nL) is suitable for RNAi experiments during caste differentiation induced by hormone application in R. speratus.
The complex society of eusocial insects, such as bees, ants, and termites, is maintained by the division of labor among castes (1). Because the acquisition of castes may be an important event for social evolution in these insects, molecular developmental analyses have been performed to reveal the genetic determinants underlying caste differentiation and/or the regulatory mechanism for caste-specific phenotypic formation, especially in the Hymenoptera (ants, bees, and wasps) (2–4). Molecular evidence in other social insects, including termites, has been accumulated (5–8), and insect sociogenomics is receiving increased attention in related fields of research (9).
Termites are hemimetabolous eusocial insects that are phylogenetically distantly related to holometabolous hymenopteran taxa. Because termite caste differentiation is a molting process with some specific morphological changes, to reveal the regulatory mechanism of caste differentiation, we should focus on the developmental processes during a molt (10, 11). Gene function analysis using RNA interference (RNAi) have been shown to be useful in termites, and the RNAi technique has been applied in some species (reviewed by 12). However, RNAi-based knockdown analyses during caste differentiation have been conducted mostly for soldier differentiation (13–18) because soldier differentiation can be easily induced by juvenile hormone (JH) treatment. To understand the commonality and diversity of caste differentiation mechanisms in termites, however, we should prepare an appropriate technique for gene function analysis during other molts induced by hormone treatment. Moreover, to reveal the specific phenomenon during differentiation of each caste (e.g., unique hormonal regulation; 11), it is necessary to compare the functions of genes among each molt.
Reticulitermes speratus Kolbe (Isoptera: Rhinotermitidae) is an important species for understanding the regulatory mechanism of caste differentiation because the differentiation of all castes, including soldiers, workers, and reproductives, can be induced artificially only in this species. Namely, presoldier (intermediate stage of soldier) differentiation can be induced by JH application to workers (19). Meanwhile, worker molt can be induced by 20-hydroxyecdysone (20E) application to workers (20). Replacement reproductives (neotenics) can be induced by the isolation of nymphs from natal nests (21). Moreover, in this species, genome sequencing was completed, and the comprehensive transcriptome analysis among all castes and during all caste differentiations were performed (8, 22). Thus, the appropriate method of gene functional analysis should be established in R. speratus. However, RNAi-based functional analysis with hormone treatment has not been performed in R. speratus, although the presoldier differentiation and worker molts can be induced from the same developmental stage (old-age workers). Generally, in insects, RNAi efficiencies are suggested to be different among species, even in the same order (23). Consequently, based on the previous analyses performed in termites, an effective RNAi method during caste differentiation with hormone treatment should be verified in R. speratus. RNAi analysis with no hormone treatment [using 2 μg of double-stranded RNA (about 260 bp) of the JH receptor gene (Methoprene-tolerant)] was previously succeeded in this species (24), and thus we especially focused on the effects of the volumes of injection.
In this study, we intended to confirm the RNAi effects on caste differentiation induced by two hormones. Both JH and 20E hormones are crucial intrinsic factors for termite caste differentiation, always accompanied by the molting event. We therefore focused on the ecdysone receptor (EcR) gene, which is important for the molting event, including termite caste differentiation (25). Knockdown of this gene caused molting failure during soldier differentiation in Zootermopsis nevadensis (18). The effects of RNAi were confirmed based on the quantification of gene expression levels and phenotypic observations during caste differentiation. Based on these results, we propose a method for gene function analysis during caste differentiation via the treatment of R. speratus with both hormones.
Mature R. speratus colonies were collected in Toyama Prefecture, Japan, in November 2018 and 2019. Pieces of logs housing the termites were brought to the laboratory and kept in plastic cases in constant darkness. Three colonies collected in 2018 (colonies A–C) were used to validate the injection volume. A colony collected in 2019 (colony D) was used for the RNAi analysis.
Total RNA for double-stranded RNA (dsRNA) synthesis was extracted from whole bodies of the 5th-6th stage workers (old-age workers) (five individuals in each sample) using ISOGEN II (Nippon Gene, Tokyo, Japan). Old-age workers were discriminated from other developmental stages based on the body size and antennal segments (26, 27). The extracted total RNA was purified using DNase treatment to remove genomic DNA. RNA purity and quantity were measured using a NanoVue spectrophotometer (GE Healthcare BioSciences, Tokyo, Japan). cDNA was synthesized from the purified RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster, CA, USA).
The ecdysone receptor homolog of R. speratus (RsEcR) was obtained from genome sequence data [RsEcR (gene ID: RS006194; 8)]. Using gene-specific primers (Supplementary Table 1; Supplementary Figure 1), the dsRNA of RsEcR was amplified. RsEcR-specific primers were adapted to the T7 promoter sequences. Gene-specific primers were designed using the Primer3 Plus software (28). To obtain template dsRNA, amplified RsEcR dsRNA was purified using a QIAquick gel extraction kit (Qiagen, Tokyo, Japan). In accordance with previous studies (14, 16, 24, 29), the GFP sequence for the control experiment was amplified using the GFP vector pQBI-poll I (Wako, Osaka, Japan). GFP-specific primers with T7 promoter sequences were newly designed using the Primer3 Plus (Supplementary Table 1; Supplementary Figure 2), because we intended to synthesize a shorter dsRNA (about 300 bp) (see Results). RsEcR and GFP dsRNA were synthesized using the MEGA script T7 Transcription Kit (Invitrogen, Carlsbad, CA, USA).
Nuclease-free water (NFW: solvent of dsRNA; 50.6, 101.2, 151.8, 202.4, 253.0, 303.6 354.2, and 404.8 nL, respectively) was injected into the lateral thorax of old-age workers of R. speratus (n = 60 per treatment) using a Nanoliter 2000 microinjector (World Precision Instruments, Sarasota, FL, USA) attached to a glass capillary. These individuals were kept in 65 mm petri dishes with 55 mm filter paper treated with 80 μg juvenile hormone III (JH III; Santa Cruz Biotechnology, Dallas, TX, USA) or 40 µg 20-hydroxyecdysone (20E; Sigma Aldrich, St. Louis, MO, USA) dissolved in 200 μL of acetone (20 individuals in each dish), in accordance with previous studies (19, 20, 30). All dishes were maintained in constant darkness at 25°C for 2 weeks and checked every 24 h to monitor the individuals. We calculated the rates of presoldier molt, worker molt and mortality. The rates of presoldier and worker molting individuals were percentages of those in survived individuals. The mortality were percentages of those in all the treated individuals. Statistical analysis was performed using two-way ANOVA followed by Tukey’s test with Mac statistical analysis ver. 3.0 (Esumi, Tokyo, Japan). Prior to the use of the ANOVA, we performed the Levene’s test on the variance equality using Mac statistical analysis ver. 3.0 (Esumi).
RNAi was performed according to methods described in the previous study (24). RsEcR and GFP dsRNA (2 μg/151.8 nL) were injected into the side of the thorax of old-age workers of R. speratus (n = 50 per treatment) following the method described above. RNAi-treated individuals were kept in 65-mm petri dishes with 55-mm filter paper treated with 80 μg JH III (Santa Cruz Biotechnology) or 20 µg 20E (Sigma Aldrich) dissolved in 200 μL acetone (10 individuals in each dish). Before the RNAi experiment, workers collected from colony D were treated with different 20E concentrations (40, 20, 10, and 5 µg), because high mortality were observed when workers were treated with 40 µg 20E (see Results). These petri dishes were kept in an incubator at 25°C for 2 weeks to monitor the rates of molted presoldiers and workers. Induced and dead individuals were immediately removed from dishes and preserved in FAA solution (ethanol:formalin:acetic acid = 16:6:1) for 24 h and stored in 70% ethanol.
To check for the knockdown of gene expression, RNAi-treated individuals of R. speratus (n = 6 per treatment) were collected 6 and 9 days after RNAi treatment. Total RNA was extracted from the whole body of each individual using ISOGEN II (Nippon Gene, Tokyo, Japan). As described above, extracted RNA was used for cDNA synthesis, which was prepared for gene expression analysis of RsEcR. Relative quantification of transcripts was performed using PowerUp SYBR Green Master Mix (Applied Biosystems, Foster, CA, USA) and the QuantStudio 3 Real-Time PCR System (Applied Biosystems, Foster, CA, USA). Gene-specific primers for qPCR were designed using Primer3 Plus (Supplementary Table 1). According to the previous study (31), the suitability of six reference genes was evaluated using GeNorm (32) and NormFinder (33) software [EF1-alpha (accession No. AB602838; 34), NADH-dh (No. AB602837; 34), beta-actin (No. AB520714; 35), glutathione S-transferase 1 (GstD1, gene ID: RS001168; 8), ribosomal protein S18 (RPS18, ID: RS015150; 8), and eukaryotic initiation factor 1A (eIF-1A, ID: RS005199; 8)]. These were used as candidate reference genes in the respective termite species (24) and other insects, including Drosophila melanogaster and Apis mellifera (36, 37). Expression levels were calculated using biological replications (number of replications: n = 6). Statistical analysis was performed using the Mann-Whitney U test with Mac statistical analysis ver. 3.0 (Esumi).
To evaluate the effects of RsEcR RNAi, we calculated the rates of individuals with gut purging (elimination of gut contents before the molt), presoldier induction and worker molting. The rates of gut-purged individuals were percentages of those in all the treated individuals. The rates of presoldier induction and worker molting individuals were percentages of those in gut-purged individuals. Statistical analysis was performed using Fisher’s test with Mac statistical analysis ver. 3.0 (Esumi).
To determine the optimal injection volume, we injected various volumes of NFW into the hormone-treated individuals. Presoldiers were strongly induced from workers (non-injection) by JH III treatment [73.3% ± 10.4% (colony A, mean ± SD) and 80.0 ± 0% (colony B)], and no presoldiers were observed in the control (acetone) treatment of both colonies (0%) (Figure 1A; Supplementary Table 2). The rates of presoldier molt in NFW-injected individuals varied among treatments with different injection volumes [two-way ANOVA, colony: p = 0.96, injection volume: p = 1.31E-17, interaction (colony vs injection volume): p = 0.86] (Figure 1A; Supplementary Table 2). In the treatments with 50.6–253.0 nL NFW injection, rates of presoldier molt were essentially similar to those of non-injection in both colonies (about 60–80%). However, presoldier molting rates were drastically dropped in the treatments with 303.6–404.8 nL injection (below 30%). Mortality were significantly different among treatments with NFW injection volumes after feeding JH III [two-way ANOVA, colony: p = 0.8, injection volume: p = 2.43E-7, interaction (colony vs injection volume): p = 0.44] (Figure 1B; Supplementary Table 2). High mortality was observed in treatments with large injection volumes.
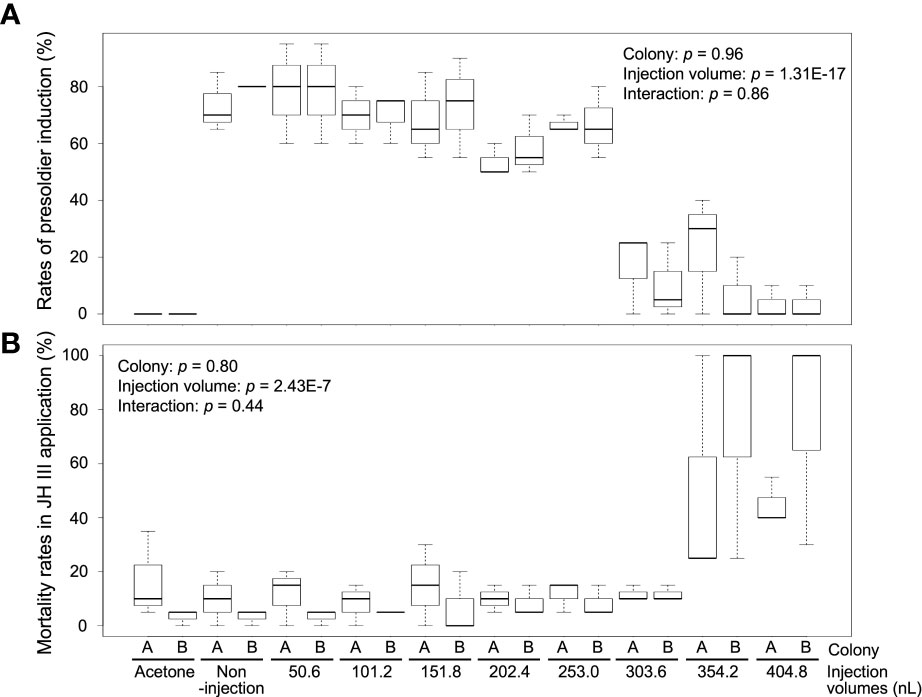
Figure 1 The rates of presoldier molt (A) and mortality (B) within 2 weeks after NFW (nuclease-free water) injection. The rates are calculated in each petri dish including 60 individuals (n = 3 dishes in each colony, Supplementary Table 2). NFW-injected workers were treated by juvenile hormone (JH) III application (80 µg per dish). Boxes and whiskers indicate the median, quartiles, and range. Statistical results of two-way ANOVA are shown in each graph (two-way ANOVA, p < 0.05). Both data are consistent with the use of parametric statistics by the Levene’s test [p = 0.8939 (A) and 0.8742 (B)].
Worker molt was strongly induced in workers (non-injection) by 20E treatment [83.3 ± 10.4% (colony A) and 75.0 ± 13.2% (colony C)], but almost never occurred in the control treatment [5.0% ± 5.0 (colony A) and 3.3 ± 2.9% (colony C)] (Figure 2A; Supplementary Table 3). The rates of worker molt were also affected by the different NFW injection volumes [two-way ANOVA, colony: p = 7.87E-5, injection volume: p = 3.37E-19, interaction (colony vs injection volume): p = 4.2E-3]. In the treatments with 50.6–253.0 nL injection, rates of worker molt were similar to those of non-injection in both colonies (about 60–80%). However, molting rates were significantly lower than those of non-injected individuals in the treatments with 303.6–404.8 nL injection in both colonies (below 40%). Mortality was significantly different among treatments with NFW injection volumes after feeding 20E [colony: p = 2.64E-3, injection volume: p = 1.58E-11, interaction (colony vs injection volume): p = 0.43] (Figure 2B; Supplementary Table 3). High mortality were observed in the treatments with large injection volumes, similar to JH III application.
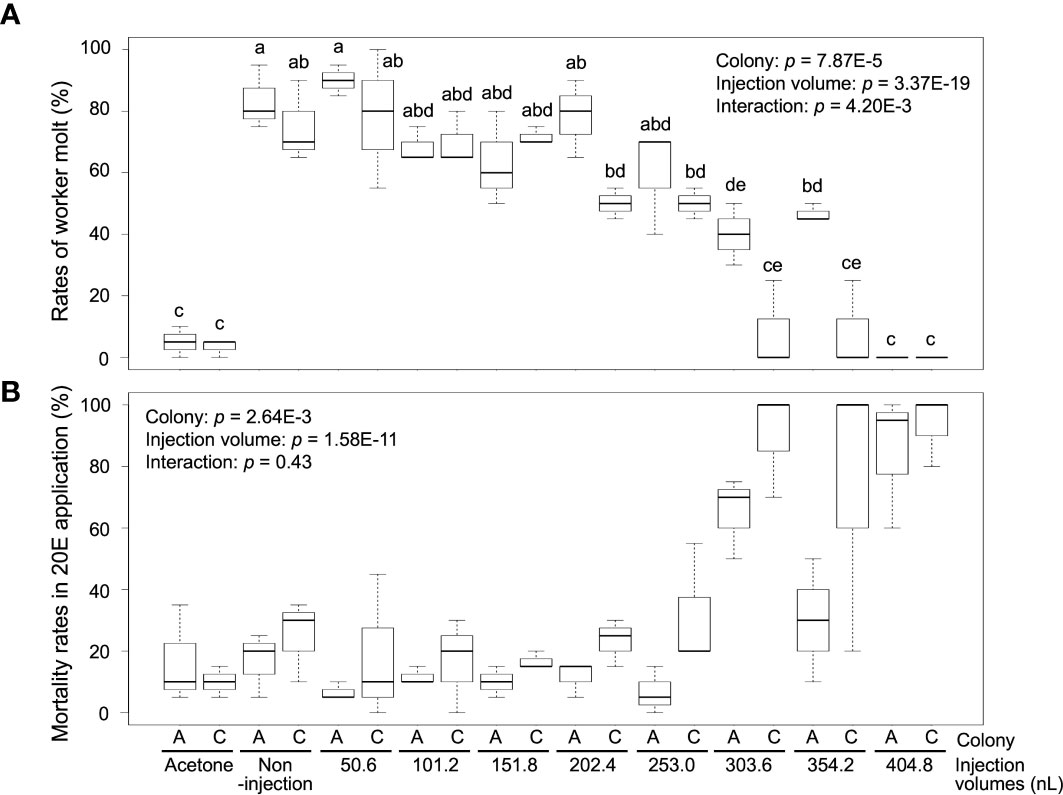
Figure 2 The rates of worker molt (A) and mortality (B) within 2 weeks after NFW (nuclease-free water) injection. The rates are calculated in each petri dish including 60 individuals (n = 3 dishes in each colony, Supplementary Table 3). NFW-injected workers were treated by 20-hydroxyecdysone (20E) application (40 µg per dish). Boxes and whiskers indicate the median, quartiles, and range. Statistical results of two-way ANOVA are shown in each graph. Different letters above the boxes indicate a significant difference (two-way ANOVA followed by Tukey’s test, p < 0.05). Both data are consistent with the use of parametric statistics by the Levene’s test [p = 0.8534 (A) and 0.9539 (B)].
Termite soldier differentiation requires high JH titers in the worker body (38, 39). Moreover, in insects, 20E titer levels generally increase before the larval molt (40). There is a possibility that the reduction in the molting rates is due to the dilution of hormone titer levels with the injected NFW (303.6–404.8 nL). Alternatively, the high mortality observed with large volumes of NFW injections may be due to some mechanical effects using pure water. To clarify this possibility, further injection analysis using a physiological saline solution should be performed. In the damp-wood termite Hodotermopsis sjostedti, RNAi-based knockdown was effectively induced by the injection of 1 µL dsRNA solution to the 7th instar treated with JH analog (15). We suggest that the proper (non-lethal) volumes of injection should be determined, especially considering the body size of the target individuals, because H. sjostedti 7th instars are much larger than the R. speratus workers used in this study. For RNAi-based knockdown with hormone treatment in R. speratus, injection volumes should be below 253 nL.
A previous study showed that RNAi-based knockdown was effectively caused by injection of 2 µg dsRNA (about 260 bp) in R. speratus nymphs without hormone treatment (24). In Drosophila S2 cells, the length of dsRNA for effective RNAi was shown to be >211 bp (41). In this study, we prepared 2 µg dsRNA (about 300 bp) dissolved in 151.8 nL. According to the results described above, injection volumes should be reduced as small as possible (50.6 nL in this study). However, when we used injection volumes of 50.6 nL, a glass capillary was immediately clogged probably due to high viscosity. We then selected injection volumes of 151.8 nL, and injected GFP or RsEcR dsRNA solution (2.0 µg/151.8 nL) into R. speratus workers. EF1-alpha was selected as the most appropriate reference gene for real-time qPCR analyses (Supplementary Table 4). Gene expression levels of RsEcR were not affected by RsEcR RNAi 6 days after injection (day 6, Figure 3A; Supplementary Table 5). However, the expression levels of RsEcR were significantly decreased by RsEcR RNAi on day 9 (more than 50%) compared to the GFP control (Figure 3B; Supplementary Table 5). We did not observe any gross morphological and phenotypic changes in the RNAi-treated individuals without hormone treatments.
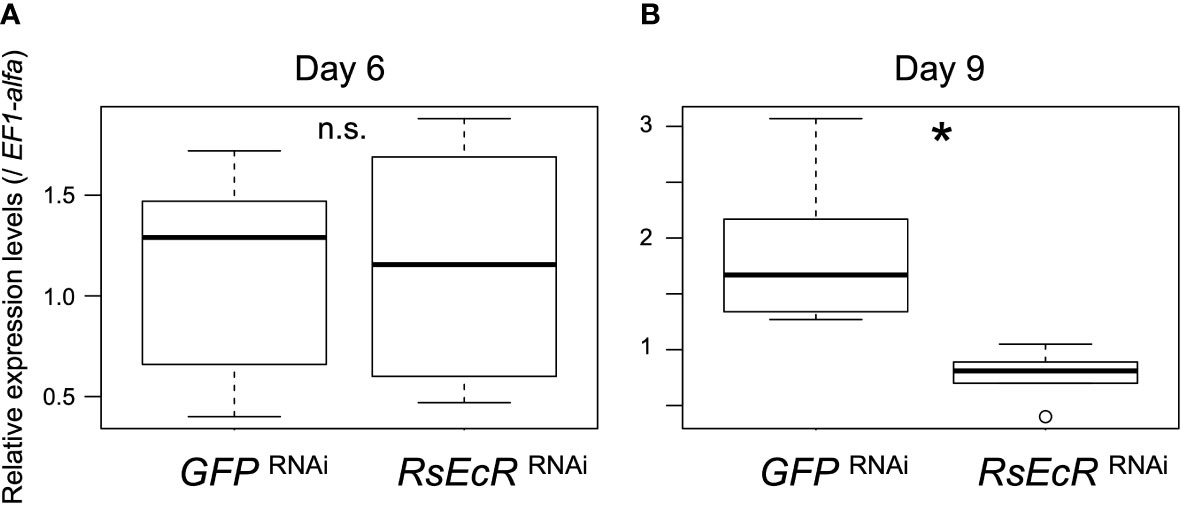
Figure 3 Expression levels of RsEcR (n = 6) 6 days (A) and 9 days (B) after RNAi treatment. Total RNA was extracted from the whole body of each individual, and six different individuals were used for each treatment. Boxes and whiskers indicate the median, quartiles, and range. An asterisk over the boxes indicates a significant difference (Mann-Whitney U test, p < 0.05). The term n.s. means not significant by statistical test.
In RsEcR RNAi with JH treatment, the rates of gut-purged individuals (26%) were significantly lower than those in the GFP control (64%) (Fisher’s test, p < 0.05; Figure 4A; Supplementary Table 6). Furthermore, presoldiers emerged from most gut-purged individuals in the GFP control (87.5%; Figures 4B, C), but never from those in RsEcR RNAi treatment (0%; Figure 4B; Supplementary Table 6). All gut-purged individuals in the latter failed to molt (Figure 4D), as shown in Z. nevadensis (18).
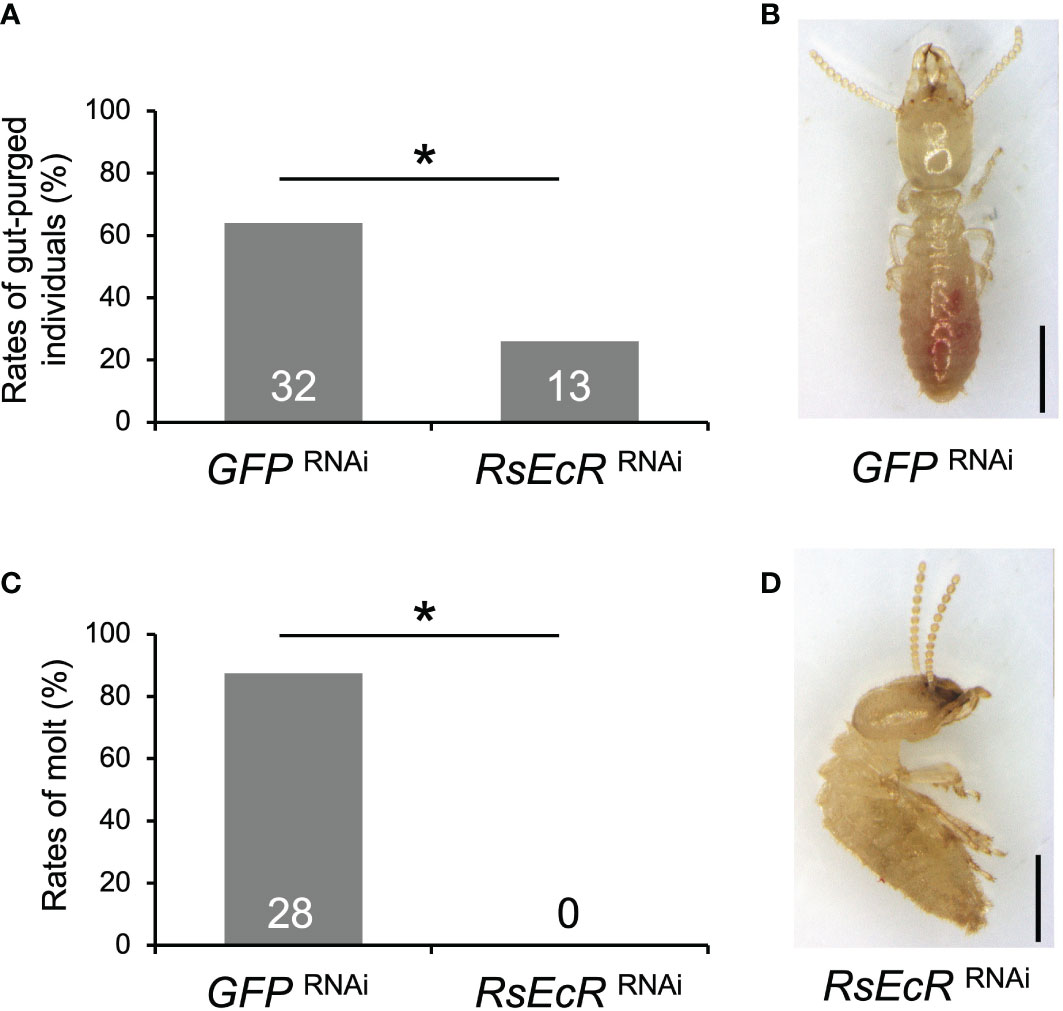
Figure 4 Phenotypic effects of RsEcR RNAi in workers treated by JH III application (80 μg per dish). Only one colony collected in 2019 (colony D) was used for the RNAi analysis. The rates of gut-purged (A) and molted individuals (B) within 2 weeks after RNAi treatment. The rates are calculated by the numbers of gut-purged individuals per 50 individuals examined (A), and by the numbers of molted individuals per gut-purged individuals (B). The numbers of individuals examined are indicated in each bar (Supplementary Table 6). An asterisk over the bars indicates a significant difference (Fisher’s test, p < 0.05). The typical phenotypes of the GFP RNAi-treated individual after the molt (C, normal presoldier) and the RsEcR RNAi-treated dead individual before the molt (D, dead old-age worker). Scale bar indicates 1 mm.
Before the experiment with RsEcR RNAi with 20E treatment, mortality of workers with 40, 20, 10, and 5 µg 20E were compared, because high mortality was observed in workers (colony D) treated with 40 µg 20E dissolved in 200 µL acetone. Although there were no statistical differences among treatments, the 20 µg 20E treatment tended to be higher rates of worker molt and lower levels of mortality (Supplementary Figure 3; Supplementary Table 7). Consequently, we decided to perform RNAi using 20 µg of 20E treatment. The rate of gut-purged individuals (60%) was significantly lower than that in the GFP control (76%) (Fisher’s test, p < 0.05; Figure 5A; Supplementary Table 8). RsEcR RNAi decreased the rates of worker molt (33.3%) compared to the control (55.3%) (Figure 5B; Supplementary Table 8), and most RNAi-treated individuals failed to molt (Figures 5C, D). These results indicate that the RsEcR RNAi assay performed here (injection of 2 µg dsRNA dissolved in 151.8 nL NFW) was successful in R. speratus workers with hormone treatments. Although the proper amount of dsRNA should be recognized for each gene, we suggest that the concentration obtained here can be used as the starting point for RNAi method during caste differentiation using hormone treatment in this species. According to the present study and previous works (15–18, 24), proper amount of dsRNA may be around 0.5–2 µg for the RNAi experiments in termites.
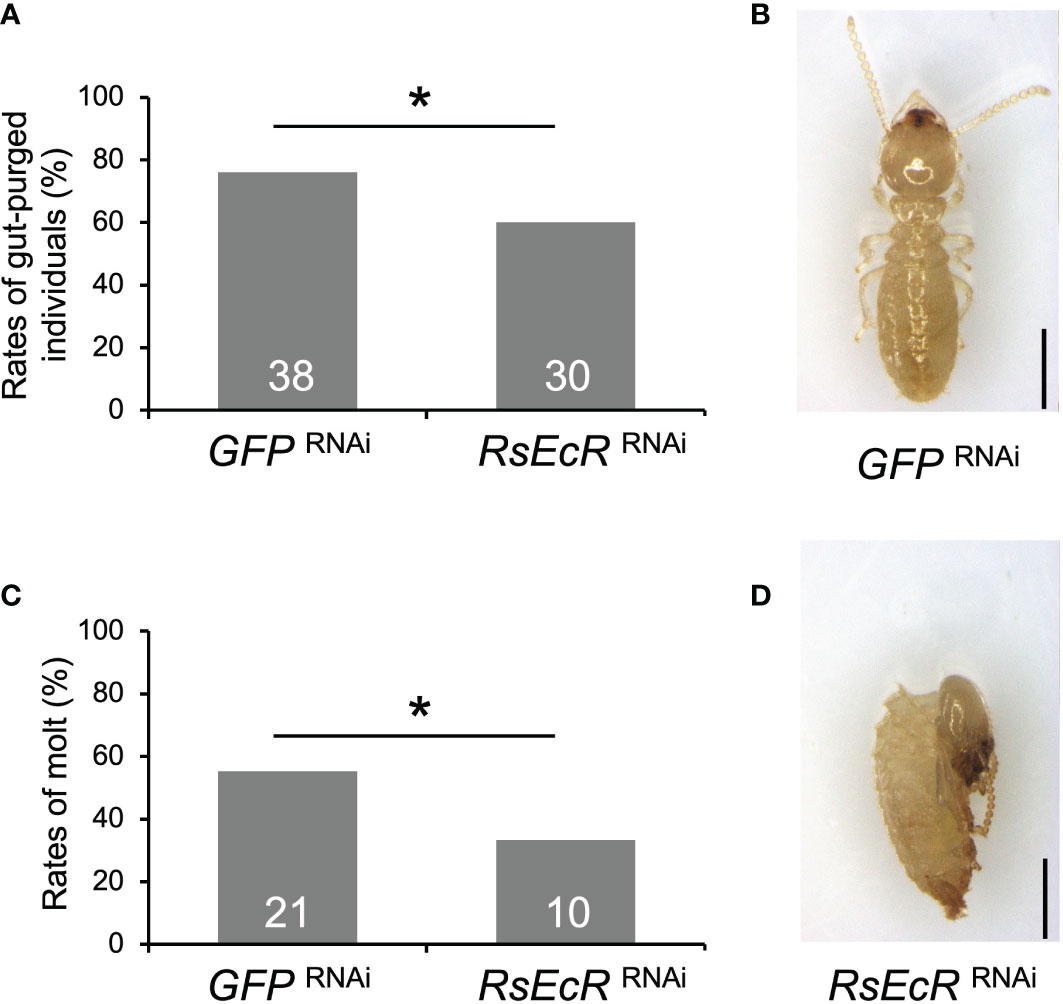
Figure 5 Phenotypic effects of RsEcR RNAi in workers treated by 20E application (20 μg per dish). Only one colony collected in 2019 (colony D) was used for the RNAi analysis. The rates of gut-purged (A) and molted individuals (B) within 2 weeks after RNAi treatment. The rates are calculated by the numbers of gut-purged individuals per 50 individuals examined (A), and by the numbers of molted individuals per gut-purged individuals (B). The numbers of individuals examined are indicated in each bar (Supplementary Table 8). An asterisk over the bars indicates a significant difference (Fisher’s test, p < 0.05). The typical phenotype of the GFP RNAi-treated individual after the molt (C, normal old-age worker), and the RsEcR RNAi-treated dead individual before the molt (D, dead old-age worker). Scale bar indicates 1 mm.
The relatively weaker effects of RsEcR RNAi treated with 20E (Figure 5), compared to those with JH III (Figure 4), may be due to the timing of knockdown effects caused by RNAi. Knockdown effects of RNAi could not be observed 6 days, but observed 9 days after dsRNA injection (Figure 3). Since the JH application induces the molting event to presoldiers, 20E-EcR action may be promoted after the increase of JH titer in workers treated with JH III. In contrast, 20E-EcR action may be immediately promoted by the 20E treatment in workers. Indeed, the initiation of molting event is occurred early in the 20E treatment, compared to the JH treatment (approximately 10-11 or 13-14 days after the treatment, respectively; 20). To clarify the possibility of the different timings of physiological action after each treatment, further expression analysis of hormone-related genes should be performed during caste differentiation.
We considered appropriate RNAi method during caste differentiation using hormone treatment in R. speratus. We suggest that it is necessary to regard not only dsRNA mass but also injection volumes using RNAi methods with hormone treatments. Using the method shown here, gene function analysis during caste differentiation can be performed effectively in R. speratus.
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
RS, YM and KM designed this study. RS, RHS and KM collected samples. RS, YM and RHS performed experiments. RS, YM and KM analyzed the data. RS, YM and KM drafted the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported in part by Grants-in-Aid for JSPS Fellows (No. 18J15134 to RS) and Scientific Research (No. 21K19293 and 22H02672 to KM) from the Japan Society for the Promotion of Science.
We are grateful to Tsutomu Tsuchida, Keima Kai, Keigo Nuibe, Shogo Suzuki, Hiroki Matsutani, Makoto Matsushita, Moe Kimura, Akimi Karasawa, Kokuto Fujiwara, and Mizuna Usui (University of Toyama) for their help during both field and laboratory work.
RHS is employed by Earth Corporation, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2023.1188343/full#supplementary-material
JH, juvenile hormone; 20E, 20-hydroxyecdysone; NFW, nuclease-free water; EcR, ecdysone receptor; RNAi, RNA interference; dsRNA, double-stranded RNA.
2. Abouheif E, Wray GA. Evolution of the gene network underlying wing polyphenism in ants. Science (2002) 297:249–52. doi: 10.1126/science.1071468
3. Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science (2008) 319:1827–30. doi: 10.1126/science.1153069
4. Simola DF, Graham RJ, Brady CM, Enzmann BL, Desplan C, Ray A, et al. Epigenetic (re)programming of caste-specific behavior in the ant Camponotus floridanus. Science (2016) 351:6268. doi: 10.1126/science.aac6633
5. Terrapon N, Li C, Robertson HM, Ji L, Meng X, Booth W, et al. Molecular traces of alternative social organization in a termite genome. Nat Commun (2014) 5:3636. doi: 10.1038/ncomms4636
6. Harrison MC, Jongepier E, Robertson HM, Arning N, Bitard-Feildel T, Chao H, et al. Hemimetabolous genomes reveal molecular basis of termite eusociality. Nat Ecol Evol (2018) 2:557–66. doi: 10.1038/s41559-017-0459-1
7. Itakura S, Yoshikawa Y, Togami Y, Umezawa K. Draft genome sequence of the termite, Coptotermes formosanus: genetic insights into the pyruvate dehydrogenase complex of the termite. J Asia-Pacific Entomol (2020) 23:666–74. doi: 10.1016/j.aspen.2020.05.004
8. Shigenobu S, Hayashi Y, Watanabe D, Tokuda G, Hojo MY, Toga K, et al. Genomic and transcriptomic analyses of the subterranean termite Reticulitermes speratus: gene duplication facilitates social evolution. Proc Nat Acad Sci USA (2022) 119:e2110361119. doi: 10.1073/pnas.2110361119
9. Maekawa K, Hayashi Y, Lo N. Termite sociogenomics: evolution and regulation of caste-specific expressed genes. Curr Opin Insect Sci (2022) 50:100880. doi: 10.1016/j.cois.2022.100880
10. Miura T, Scharf ME. Molecular basis underlying caste differentiation in termites. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termite: a modern synthesis. Heidelberg: Springer (2011). p. 211–53.
11. Miura T, Maekawa K. The making of the defensive caste: physiology, development and evolution of the soldier differentiation in termites. Evol Dev (2020) 22:e12335. doi: 10.1111/ede.12335
12. Scharf ME. Termites as targets and models for biotechnology. Ann Rev Entomol (2015) 60:77–102. doi: 10.1146/annurev-ento-010814-020902
13. Zhou X, Oi FM, Scharf ME. Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc Nat Acad Sci USA (2006) 103:4499–504. doi: 10.1073/pnas.0508866103
14. Toga K, Hojo M, Miura T, Maekawa K. Expression and function of a limb-patterning gene distal-less in the soldier-specific morphogenesis in the nasute termite Nasutitermes takasagoensis. Evol Dev (2012) 4:286–95. doi: 10.1111/j.1525-142X.2012.00545.x
15. Hattori A, Sugime Y, Sasa C, Miyakawa H, Ishikawa Y, Miyazaki S, et al. Soldier morphogenesis in the damp-wood termite is regulated by the insulin signaling pathway. J Exp Zool B (2013) 320:295–306. doi: 10.1002/jez.b.22501
16. Masuoka Y, Yaguchi H, Suzuki R, Maekawa K. Knockdown of the juvenile hormone receptor gene inhibits soldier-specific morphogenesis in the damp-wood termite Zootermopsis nevadensis (Isoptera: Archotermopsidae). Insect Biochem Mol Biol (2015) 64:25–31. doi: 10.1016/j.ibmb.2015.07.013
17. Masuoka Y, Yaguchi H, Toga K, Shigenobu S, Maekawa K. TGFβ signaling related genes are involved in hormonal mediation during termite soldier differentiation. PloS Genet (2018) 14:e1007338. doi: 10.1371/journal.pgen.1007338
18. Masuoka Y, Toga K, Nalepa CA, Maekawa K. A crucial caste regulation gene detected by comparing termites and sister group cockroaches. Genetics (2018) 209:1225–34. doi: 10.1534/genetics.118.301038
19. Tsuchiya M, Watanabe D, Maekawa K. Effect on mandibular length of juvenile hormones and regulation of soldier differentiation in the termite Reticulitermes speratus (Isoptera: rhinotermitidae). Appl Entomol Zool (2008) 43:307–14. doi: 10.1303/aez.2008.307
20. Masuoka Y, Miyazaki S, Saiki R, Tsutomu T, Maekawa K. High Laccase2 expression is likely involved in the formation of specific cuticular structures during soldier differentiation of the termite Reticulitermes speratus. Arthropod Struct Dev (2013) 42:469–75. doi: 10.1016/j.asd.2013.08.003
21. Saiki R, Maekawa K. Imaginal organ development and vitellogenin gene expression changes during the differentiation of nymphoids of the termite Reticulitermes speratus. Sociobiology (2011) 58:499–511.
22. Saiki R, Hayashi Y, Toga K, Yaguchi H, Masuoka Y, Suzuki R, et al. Comparison of gene expression profiles among caste differentiations in the termite Reticulitermes speratus. Sci Rep (2022) 12:11947. doi: 10.1038/s41598-022-15984-z
23. Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, et al. RNA Interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol (2011) 57:231–45. doi: 10.1016/j.jinsphys.2010.11.006
24. Saiki R, Gotoh H, Toga K, Miura T, Maekawa K. High juvenile hormone titer and abdominal activation of the JH signaling may induce reproduction of termite neotenics. Insect Mol Biol (2015) 24:432–41. doi: 10.1111/imb.12169
25. Masuoka Y, Maekawa K. Ecdysone signaling regulates soldier-specific cuticular pigmentation in the termite Zootermopsis nevadensis. FEBS Lett (2016) 590:1694–703. doi: 10.1002/1873-3468.12219
26. Tsunoda K, Doki H, Nishimoto K. Effect of developmental stages of workers and nymphs of Reticulitermes speratus (Kolbe) (Isoptera: Rhinotermitidae) on caste differentiation induced by JHA treatment. Mater Org (1986) 21:47–61.
27. Takematsu Y. Biometrical study on the development of the castes in Reticulitermes speratus (Isoptera, Rhinotermitidae). Jap J Entomol (1992) 60:67–76.
28. Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM. Primer3Plus, an enhanced web interface to Primer3. Nuc Acid Res (2007) 35:W71–4. doi: 10.1093/nar/gkm306
29. Suzuki R, Yaguchi H, Maekawa K. Histone modifying genes are involved in the molting period during soldier differentiation in Zootermopsis nevadensis. J Insect Physiol (2019) 117:103892. doi: 10.1016/j.jinsphys.2019.103892
30. Watanabe D, Gotoh H, Miura T, Maekawa K. Soldier presence suppresses presoldier differentiation through a rapid decrease of JH in the termite Reticulitermes speratus. J Insect Physiol (2011) 57:791–5. doi: 10.1016/j.jinsphys.2011.03.005
31. Miyazaki S, Fujiwara K, Kai K, Masuoka Y, Gotoh H, Niimi T, et al. Evolutionary transition of doublesex regulation from sex-specific splicing to male-specific transcription in termites. Sci Rep (2021) 11:15992. doi: 10.1038/s41598-021-95423-7
32. Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol (2002) 3:research0034. doi: 10.1186/gb-2002-3-7-research0034
33. Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res (2004) 64:5245–50. doi: 10.1158/0008-5472.CAN-04-0496
34. Hojo M, Toga K, Watanabe D, Yamamoto T, Maekawa K. High-level expression of the geranylgeranyl diphosphate synthase gene in the frontal gland of soldiers in Reticulitermes speratus (Isoptera: Rhinotermitidae). Arch Insect Biochem Physiol (2011) 77:17–31. doi: 10.1002/arch.20415
35. Maekawa K, Ishitani K, Gotoh H, Cornette R, Miura T. Juvenile hormone titre and vitellogenin gene expression related to ovarian development in primary reproductives compared with nymphs and nymphoid reproductives of the termite Reticulitermes speratus. Physiol Entomol (2010) 35:52–8. doi: 10.1111/j.1365-3032.2009.00711.x
36. Scharlaken B, Graaf DC, Goossens K, Brunain M, Peelman LJ, Jacobs FJ. Reference gene selection for insect expression studies using quantitative real-time PCR: the head of the honeybee, Apis mellifera, after a bacterial challenge. J Insect Sci (2008) 8:1–10. doi: 10.1673/031.008.3301
37. Ling D, Salvaterra PM. Robust RT-qPCR data normalization: validation and selection of internal reference genes during post-experimental data analysis. PloS One (2011) 6:e17762. doi: 10.1371/journal.pone.0017762
38. Cornette R, Gotoh H, Koshikawa S, Miura T. Juvenile hormone titers and caste differentiation in the damp-wood termite Hodotermopsis sjostedti. J Insect Physiol (2008) 54:922–30. doi: 10.1016/j.jinsphys.2008.04.017
39. Watanabe D, Gotoh H, Miura T, Maekawa K. Social interactions affecting caste development through physiological actions in termites. Front Physiol (2014) 5:127. doi: 10.3389/fphys.2014.00127
40. Riddiford LM. Hormone action at the cellular level. In: Kerkut GA, Girbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology. Oxford: Pergamon press (1985). p. 37–84.
Keywords: caste differentiation, RNAi, juvenile hormone, 20-hydroxyecdysone, ecdysone receptor gene
Citation: Suzuki R, Masuoka Y, Suzuki RH and Maekawa K (2023) Efficient RNA interference method during caste differentiation with hormone treatment in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Front. Insect Sci. 3:1188343. doi: 10.3389/finsc.2023.1188343
Received: 17 March 2023; Accepted: 24 April 2023;
Published: 09 May 2023.
Edited by:
Luc Swevers, National Centre of Scientific Research Demokritos, GreeceReviewed by:
Charles Jean-Philippe, UMR6265 Centre des Sciences du Goût et de l’Alimentation (CSGA), FranceCopyright © 2023 Suzuki, Masuoka, Suzuki and Maekawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiyoto Maekawa, a21hZWthd2FAc2NpLnUtdG95YW1hLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.