
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Insect Sci. , 21 October 2021
Sec. Insect Physiology
Volume 1 - 2021 | https://doi.org/10.3389/finsc.2021.744847
Stored grains used in artificial diets are often treated with insecticides to control infestation by pests. In recent years, insect growth regulators (IGRs) have become an increasingly popular form of insect pest control in agricultural settings. Most IGRs specifically target insects by either disrupting their endocrine system or their chitin synthesis. One type of IGRs comprises of chemical analogs of juvenile hormone (JH), a major hormone involved in growth and development of insects. Here we demonstrate that conventional wheat germ contains JH activity and impacts growth and development of the tobacco hornworm, Manduca sexta. Feeding diet containing conventional wheat germ delayed the timing of metamorphosis in wildtype larvae by extending the duration of the final instar. Diet with conventional wheat germ also inhibited melanization of the black mutant larvae and induced the expression of the JH response gene, Krüppel homolog 1. We demonstrate that the black mutant bioassay is a sensitive assay that can determine the amount of JH activity in stored grains and suggest that this assay may offer a quick and reliable assay to determine the amount of environmental juvenoids. Researchers are urged to use caution when purchasing stored grains for mass-rearing of research insects.
Stored grains are often treated with insecticides to control infestation by pests. Insect growth regulators (IGRs) have become a popular replacement for organophosphorus, pyrethroid and carbamate insecticides that often impact human health (1, 2). The major benefit of using IGRs stems from their specificity to insects (2). IGRs mainly target insects by interfering with their development, growth and chitin synthesis (2, 3). Many IGRs act as chemical analogs of two major insect hormones, the sesquiterpenoid juvenile hormone (JH) and the molting hormone, ecdysteroids (3). Application of such hormonal IGRs can disrupt major developmental events, such as metamorphosis, molting and reproduction (3).
JH plays several major roles in insect development and growth. During the early larval instars, the presence of JH prevents larvae from molting into a pupa (4). Thus, high levels of JH ensures that a larva molts into another larva. In the final larval instar, JH titers drop dramatically, signaling the larvae to undergo metamorphosis. JH is also involved in regulating the growth of many insects. In the final instar of the tobacco hornworm, Manduca sexta, prothoracicotropic hormone (PTTH) stimulates the prothoracic glands (5), which in turn release ecdysteroids that cause the larva to cease feeding, clear its gut and initiate the wandering behavior in search of a pupation site (6). JH must be cleared for the brain to become competent to secrete PTTH (7). Topical application of JH in the final instar therefore prevents metamorphosis and allows larvae to continue feeding and grow to a larger size (8, 9).
Despite its importance, detection of JH is notoriously challenging due to its low titers and chemical structure. Although several different assays have been developed [e.g., (10, 11)], here we focus on two sensitive and relatively easy methods to assay JH levels: the determination of the expression of a JH response gene and the black mutant bioassay. JH binds to the JH receptor, a basic helix-loop-helix-Per-Arnt-Sim domain protein receptor called Methoprene-tolerant (12, 13). Once bound by JH, Methoprene-tolerant binds to the promoter of the JH-response gene encoding a transcription factor called Krüppel homolog 1 (Kr-h1) (14). Kr-h1 mRNA expression has been used as a readout of JH titers in a number of species (14–19), including M. sexta (20). Thus, levels of JH in an insect can be assessed by examining the expression of Kr-h1.
In addition to examining the Kr-h1 expression, a useful bioassay to quantify hemolymph JH levels was developed over 40 years ago using the black mutant Manduca sexta larvae (21). The black mutant appears black due to excess deposition of melanin on its cuticle (22). This happens because melanin synthesis enzyme, dopa decarboxylase, is inhibited by JH (23), and the black mutant has a mutation that prevents production of typical amounts of JH (24). Since topical application of JH causes larvae to turn green, the color change of black mutant larvae can be used as a bioassay to determine the amount of JH in isolated hemolymph (21). Another potential application of the black mutant assay might be to determine the amount of JH-like activity (juvenoid activity) in the environment or food.
In the United States, methoprene, a JH mimic has been used commercially under the trademark name Diacon. Methoprene treatment of grains has been shown to effectively control several species of insect pests (25–28). Although studies have demonstrated the efficacy of IGR treatment on pest control, the extent to which commercially available conventional grains can impact the growth and development of laboratory insects has not been conducted.
In this study, we compared the growth of wildtype larvae, coloration of black mutant larvae and gene expression differences between larvae fed an artificial diet containing conventional wheat germ and one containing organic wheat germ. We also demonstrated the use of the black mutant larvae to determine the relative amount of juvenoid activity in conventional wheat germ. We found that conventional wheat germ extended the final larval instar. Moreover, we found that conventional wheat germ causes black mutant larvae to turn green with concomitant increases in the expression of the JH response gene, Kr-h1.
Wildtype M. sexta were purchased from Carolina Biological Supply. The black mutant larvae arose spontaneously in a colony of wildtype M. sexta in the early 1970s and have been maintained by several labs. All larvae were raised on a long day photoperiod (16 h light: 8 h dark) at 26.5° C.
Approximately 100 M. sexta were raised individually on four different diets, with ~25 larvae on each diet. The ingredients and their proportions were kept constant for each diet except for the type of wheat germ used. Two conventional brands of wheat germ were sourced from two animal diet companies, Frontier (Conventional wheat germ A) and BioServ (Conventional wheat germ B). Organic wheat germ (Lekithos), and a cornmeal (Palmetto Farms) and soy flour (NutriSoy) mix were also used as controls. The diet ingredients are listed in Supplementary Table 1.
To assess the growth trajectory of larvae, wildtype larvae were weighed daily after they reached the third instar. Measurements were terminated once they initiated the wandering stage. The wandering stage is identified by weight loss from gut purge and dorsal vessel exposure. Once gut purge initiates, larvae stop feeding and clear their guts which results in weight loss. Dorsal vessel exposure is visible as a pulsating dark line appears along the dorsal side. Larval growth rates were compared amongst the different diets. The black mutant larvae were reared on different diets. For controls, artificial diet containing organic wheat germ was supplemented with methoprene diluted in DMSO. All diets contained 1% DMSO. One day after the molt to the fifth instar, the color of the larvae was scored as this is the time when the larvae are darkest in color. A color scoring system (29) was used for this purpose (Figure 3A). JMP (SAS Institute) was used to perform one-way ANOVA and Tukey HSD post-hoc tests.
The expression of Kr-h1 in black mutants was assayed using quantitative real-time PCR. The epidermis of the first and second abdominal sections of larvae at the onset of a molt, just as the old cuticle of the head capsule begins to detach from the underlying new head capsule. This corresponds to the JH-sensitive period for black or green coloration (4). The epidermis was placed in Trizol (ThermoFisher), and mRNA was isolated using chloroform extraction. Subsequently, the sample was treated with DNAse (Promega) to remove any trace amount of genomic DNA. One μg of RNA was then converted to cDNA using the RevertAid First Strand cDNA Synthesis Kit (ThermoFisher). Real-time PCR was used to determine the amount of Kr-h1 with RpL17 serving as an internal control. For each gene, primers for each gene was mixed with SYBR Green Supermix (Bio-Rad). For Kr-h1, the following primers were used: 5′-GCATCGTTCACAACCTACACC-3′ (forward primer) and 5′-TCCGAGTGGAAAGCGTCAA-3′ (reverse primer) (20). For RpL17, the following primers were used: 5′-TCCGCATCTCACTGGGTCT-3′ (forward primer) and 5′-CACGGCAATCACATACAGGTT-3′ (reverse primer) (30). A standard curve method was used to determine the relative expression of Kr-h1. Four biological replicates were used with three technical replicates each. JMP (SAS Institute) was used to perform one-way ANOVA and Tukey HSD post-hoc tests.
Wildtype Manduca larvae were fed diets containing conventional wheat germ, organic wheat germ or cornmeal/soy flour in order to compare their growth trajectories. The cornmeal/soy flour diet serves as another control and was used to demonstrate its efficacy as a potential alternative to wheat germ. Larvae fed diets containing conventional wheat germ continued to grow past the period when the gut purge and wandering behavior normally begins in larvae fed organic and cornmeal diets (Figure 1A). This resulted in an increase in peak mass when caterpillars were reared on conventional diets [Figure 1B; One-way ANOVA: F(3, 85) = 17.403, p < 0.0001]. The extended growing period caused by conventional wheat germ occurred primarily during the fifth instar [Figure 1C; One-way ANOVA: F(3, 85) = 111.297, p < 0.0001], while the length of time from hatching to the fourth instar similar between diets [One-way ANOVA: F(3, 85) = 1.993, p = 0.1212].
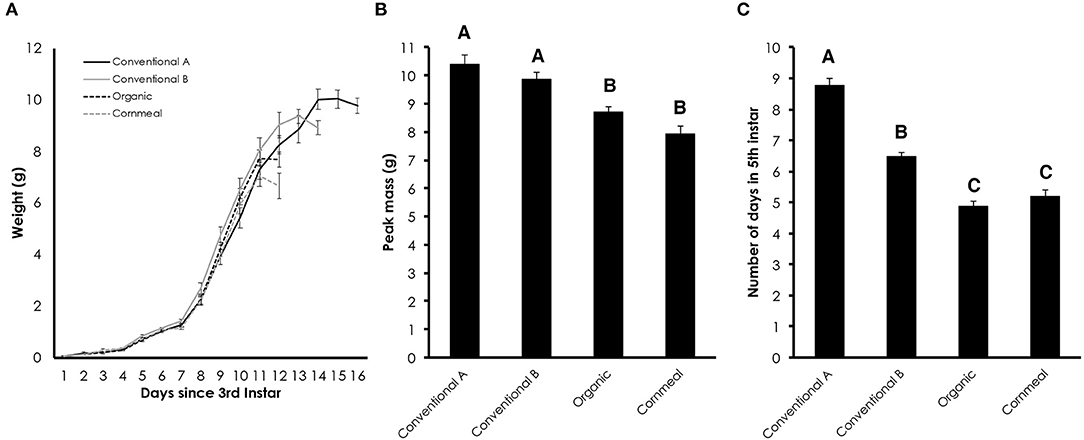
Figure 1. Diet containing conventional wheat germ prolongs the fifth instar duration of M. sexta. (A) Growth trajectories of larvae fed diets containing different grains. The time since the molt to the third instar is plotted. (B) Average peak mass of larvae fed diets containing different grains. (C) Number of days in the fifth instar from the first day in the fifth instar to the date of gut purge. Error bars represent standard error. Different letters represent statistically significant differences (p < 0.05; One-way ANOVA with post hoc Tukey HSD test).
While rearing the wildtype larvae, we noticed that one of the diets containing conventional wheat germ (Conventional wheat germ A) inhibited the presence of the melanic marks on the dorsal side (Figure 2). Because JH is known to inhibit melanization, we further characterized this effect by rearing black mutant larvae on different diets. As a control, we fed larvae on diets containing different amount of methoprene and the colors were scored (Figure 3A). Control diet containing 0.1 ppm methoprene was sufficient to cause the black mutant larvae to turn green (Figure 3B). In black mutant larvae fed the experimental diets, both diets containing the conventional wheat germ caused the larva to turn green whereas most of the larvae fed diets with organic wheat germ or cornmeal remained black [Figures 3C,D; One-way ANOVA: F(3, 61) = 132.019, p < 0.0001]. These results indicate that juvenoids are present in conventional wheat germ in quantities sufficient to suppress melanin deposition.
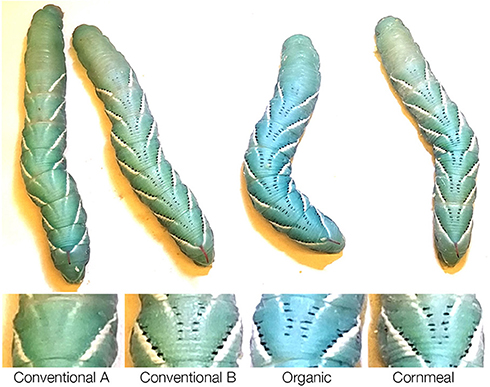
Figure 2. Diet containing conventional wheat germ sourced from company A eliminates black markings on the wildtype M. sexta larvae. (Top row) Whole body phenotype of representative fifth instar larvae reared on different diets weighing ~6 g. (Bottom row) Close-up of dorsal view of one of the segments.
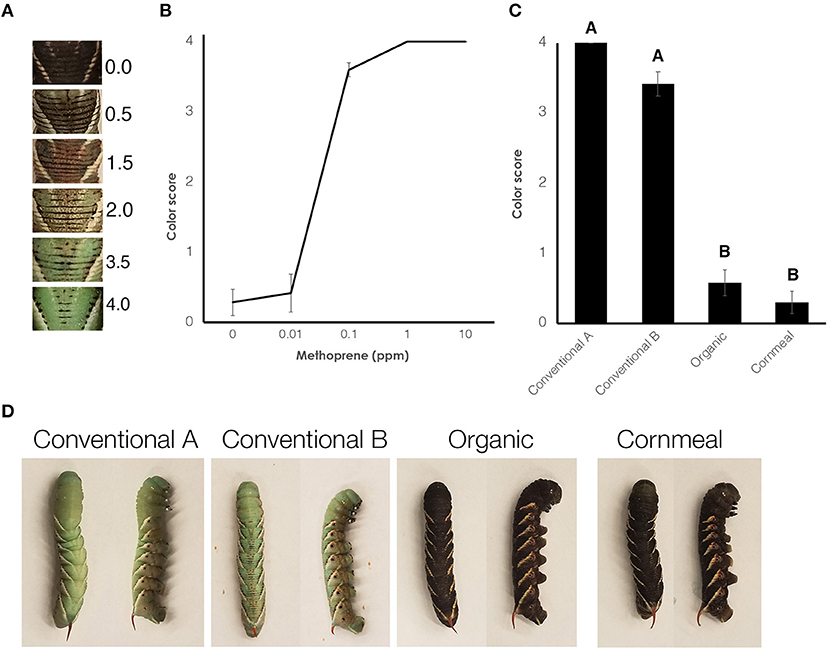
Figure 3. Diet containing conventional wheat germ causes black mutant larvae to turn green. (A) Scale used to score the larvae. (B) A dose response curve showing the average color of black mutants fed on diet containing organic wheat germ that has been treated with methoprene. Error bars represent standard error. (C) Average color of black mutants fed on different diets. Error bars represent standard error. Different letters represent statistically significant differences (p < 0.05; One-way ANOVA with post hoc Tukey HSD test). (D) Typical black mutant larvae fed different diets. On the left is a dorsal view; on the right is a lateral view.
We next sought to molecularly characterize whether the change in coloration was due to the presence of juvenoids in the diet. The expression of the JH response gene, Kr-h1, was significantly higher in larvae fed conventional wheat germ A compared to larvae on diets containing organic wheat germ or cornmeal/soy flour [Figure 4; One-way ANOVA: F(3, 12) = 7.219, p < 0.005]. Larvae fed conventional wheat germ B had intermediate expression of Kr-h1. Thus, the diets which caused delays in metamorphosis and inhibited melanization also led to increased Kr-h1 expression, indicating that the effects are likely due to the presence of juvenoids in conventional wheat germ (Figure 4).
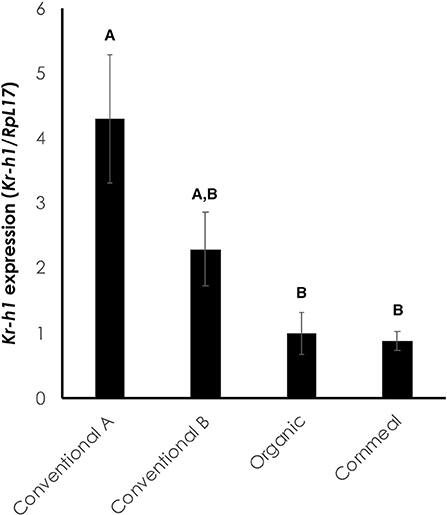
Figure 4. Kr-h1 expression is elevated in the epidermis of black mutant larvae fed on diet containing conventional wheat germ. The epidermis was isolated at the onset of the molt to the fifth instar during the sensitive period for color determination (4). RpL17 was used as an internal control. Each bar represents an average of four biological replicates, each with three technical replicates. Error bars represent standard error. Different letters represent statistically significant differences (p < 0.05; One-way ANOVA with post hoc Tukey HSD test).
In this study, we compared the impact of conventional and organic wheat germ on the growth and development of M. sexta. We found that conventional wheat germ caused larvae to delay metamorphosis and exhibit minor developmental changes. Using the black mutant larvae, we demonstrated that the conventional wheat germ contains sufficient juvenoid activity to change the color and induce the expression of the JH response gene, Kr-h1. The amount of juvenoid activity differed between the two conventional batches of wheat germ.
The excess duration of growth seen in the wildtype M. sexta that were fed conventional wheat germ stems primarily from lengthened duration of the 5th instar. This is consistent with elevated JH signaling, which prevents the production of PTTH from the brain (7). The black mutant assay clearly demonstrates the presence of juvenoids in the conventional wheat germ. The black mutant bioassay has been used to determine hemolymph titers of JH (21, 29, 31). In this study, we demonstrate the utility of this strain in determining the amount of environmental juvenoids. With the increasing use of IGRs, a sensitive indicator of environmental juvenoid activity will be increasingly important. The black mutant assay may be one such tool for assessing the amount of juvenoid activity in both food crops and other substrates.
IGRs have been linked to potential declines in honeybees (32). Here, we showed that laboratory insects can also be negatively impacted by IGRs used in agricultural settings. We find that physiology and gene expression can both be altered by IGRs applied to stored grains. Our work highlights the need for careful monitoring of IGRs in the environment and in dietingredients.
Our study demonstrates that the use of IGRs in agricultural settings can impact the development and physiology of insects in the laboratory. We urge researchers to take caution when using stored grains from animal feed companies for mass-rearing of insects. We also urge careful monitoring of IGR levels in nature and propose that bioassays may be an inexpensive and sensitive way to monitor environmental IGR levels.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JF, JT, and YS contributed to conception and design of the study, carried out the experiments, and wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was funded by the National Science Foundation grant IOS-2002354 to YS and funds provided by Wellesley College.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Dr. Mike Kanost (Kansas State University) who first alerted the Lepidoptera genetics community of the issues with the conventional wheat germ being treated with the IGRs. This work is a follow up to confirm his astute observations.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2021.744847/full#supplementary-material
1. Arthur FH. Grain protectants: current status and prospects for the future. J Stored Prod Res. (1996) 32:293–302. doi: 10.1016/S0022-474X(96)00033-1
2. Wijayaratne LKW, Arthur FH, Whyard S. Methoprene and control of stored-product insects. J Stored Prod Res. (2018) 76:161–9. doi: 10.1016/j.jspr.2016.09.001
3. Mondal KAMSH, Parween S. Insect growth regulators and their potential in the management of stored-product insect pests. Integrated Pest Manag Rev. (2000) 5:255–95. doi: 10.1023/A:1012901832162
5. Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. (2002) 47:883–916. doi: 10.1146/annurev.ento.47.091201.145302
6. Truman JW, Riddiford LM. Physiology of insect rhythms. 3. The temporal organization of the endocrine events underlying pupation of the tobacco hornworm. J Exp Biol. (1974) 60:371–82. doi: 10.1242/jeb.60.2.371
7. Nijhout HF, Williams CM. Control of molting and metamorphosis in tobacco hornworm, Manduca-sexta (L) - cessation of juvenile-hormone secretion as a trigger for pupation. J Exp Biol. (1974) 61:493–501. doi: 10.1242/jeb.61.2.493
8. Hatem NE, Wang Z, Nave KB, Koyama T, Suzuki Y. The role of juvenile hormone and insulin/TOR signaling in the growth of Manduca sexta. BMC Biol. (2015) 13:44. doi: 10.1186/s12915-015-0155-z
9. Nijhout HF, Davidowitz G, Roff DA. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J Biol. (2006) 5:16. doi: 10.1186/jbiol43
10. Bergot BJ, Ratcliff M, Schooley DA. Method for quantitative determination of the four known juvenile hormones in insect tissue using gas chromatography-mass spectroscopy. J Chromatogr. (1981) 204:231–44. doi: 10.1016/S0021-9673(00)81664-7
11. Rivera-Perez C, Nouzova M, Noriega FG. A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. PLoS ONE. (2012) 7:e43784. doi: 10.1371/journal.pone.0043784
12. Charles JP, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. (2011) 108:21128–33. doi: 10.1073/pnas.1116123109
13. Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. (2015) 11:e1005394. doi: 10.1371/journal.pgen.1005394
14. Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, Kamimura M, et al. Transcriptional regulation of juvenile hormone-mediated induction of Kruppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. (2012) 109:11729–34. doi: 10.1073/pnas.1204951109
15. Cheng C, Ko A, Chaieb L, Koyama T, Sarwar P, Mirth CK, et al. The POU factor ventral veins lacking/Drifter directs the timing of metamorphosis through ecdysteroid and juvenile hormone signaling. PLoS Genet. (2014) 10:e1004425. doi: 10.1371/journal.pgen.1004425
16. Konopova B, Smykal V, Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE. (2011) 6:e28728. doi: 10.1371/journal.pone.0028728
17. Lozano J, Belles X. Conserved repressive function of kruppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci Rep. (2011) 1:163. doi: 10.1038/srep00163
18. Minakuchi C, Namiki T, Shinoda T. Kruppel homolog 1, an early juvenile hormone-response gene downstream of methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle tribolium castaneum. Dev Biol. (2009) 325:341–50. doi: 10.1016/j.ydbio.2008.10.016
19. Minakuchi C, Namiki T, Yoshiyama M, Shinoda T. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle tribolium castaneum. Febs J. (2008) 275:2919–31. doi: 10.1111/j.1742-4658.2008.06428.x
20. Xu LC, Nunes C, Wang VR, Saito A, Chen T, Basak P, et al. Distinct nutritional and endocrine regulation of prothoracic gland activities underlies divergent life history strategies in manduca sexta and drosophila melanogaster. Insect Biochem Mol Biol. (2020) 119:103335. doi: 10.1016/j.ibmb.2020.103335
21. Fain MJ, Riddiford LM. Juvenile hormone titers in the hemolymph during late larval development of the tobacco hornworm, manduca sexta (L.). Biol Bull. (1975) 149:506–21. doi: 10.2307/1540383
22. Hori M, Hiruma K, Riddiford LM. Cuticular melanization in the tobacco hornworm larva. Insect Biochem. (1984) 14:267–74. doi: 10.1016/0020-1790(84)90059-3
23. Hiruma K, Riddiford LM. Hormonal regulation of dopa decarboxylase during a larval molt. Dev Biol. (1985) 110:509–13. doi: 10.1016/0012-1606(85)90109-5
24. Safranek L, Riddiford LM. The biology of the black larval mutant of the tobacco hornworm, manduca sexta. J Insect Physiol. (1975) 21:1931–8. doi: 10.1016/0022-1910(75)90225-5
25. Arthur FH. Evaluation of methoprene alone and in combination with diatomaceous earth to control rhyzopertha dominica (Coleoptera: Bostrichidae) on stored wheat. J Stored Prod Res. (2004) 40:485–98. doi: 10.1016/S0022-474X(03)00060-2
26. Arthur FH. Efficacy of methoprene for multi-year protection of stored wheat, brown rice, rough rice and corn. J Stored Prod Res. (2016) 68:85–92. doi: 10.1016/j.jspr.2016.04.005
27. Mian LS, Mulla MS. Biological activity of IGRs against four stored-product coleopterans. J Econ Entomol. (1982) 75:80–5. doi: 10.1093/jee/75.1.80
28. Oberlander H, Silhacek DL, Shaaya E, Ishaaya I. Current status and future perspectives of the use of insect growth regulators for the control of stored product insects. J Stored Prod Res. (1997) 33:1–6. doi: 10.1016/S0022-474X(96)00047-1
29. Suzuki Y, Nijhout HF. Evolution of a polyphenism by genetic accommodation. Science. (2006) 311:650–2. doi: 10.1126/science.1118888
30. Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. Identification, characterization and developmental expression of halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, manduca sexta. Insect Biochem Mol Biol. (2006) 36:188–99. doi: 10.1016/j.ibmb.2005.12.002
31. Rankin MA, Riddiford LM. Significance of haemolymph juvenile hormone titer changes in timing of migration and reproduction in adult oncopeltus fasciatus. J Insect Physiol. (1978) 24:31–8. doi: 10.1016/0022-1910(78)90008-2
Keywords: insect growth regulator (IGR), juvenile hormone (JH), bioassay, Manduca sexta (Insecta), growth
Citation: Ffrench J, Tracewell J and Suzuki Y (2021) Conventional and Organic Wheat Germ Have Distinct Physiological Effects in the Tobacco Hornworm, Manduca Sexta: Use of Black Mutant Assay to Detect Environmental Juvenoid Activity of Insect Growth Regulators. Front. Insect Sci. 1:744847. doi: 10.3389/finsc.2021.744847
Received: 21 July 2021; Accepted: 27 September 2021;
Published: 21 October 2021.
Edited by:
Christopher Weldon, University of Pretoria, South AfricaReviewed by:
Esther Elizabeth Du Rand, University of Pretoria, South AfricaCopyright © 2021 Ffrench, Tracewell and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuichiro Suzuki, eXN1enVraUB3ZWxsZXNsZXkuZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.