
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 13 March 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1560280
This article is part of the Research TopicPrecision Medicine and Targeted Therapies in Gastrointestinal and Genitourinary Solid TumorsView all 8 articles
Gastric cancer (GC) ranks as the fifth most prevalent cancer on a global scale, with HER2-positive GC representing a distinct subtype that exhibits more intricate biological characteristics. Conventional chemotherapy typically exhibits restricted efficacy in the management of HER2-positive GC. In light of the incessant advancement in molecular targeted therapies, targeting HER2 has emerged as a promising therapeutic approach for this subtype. The advent of antibody-drug conjugates (ADCs) and chimeric antigen receptor T-cell therapy (CAR-T) has furnished novel treatment alternatives for HER2-positive GC. Nevertheless, owing to the pronounced heterogeneity of GC and the complex tumor microenvironment, drug resistance frequently emerges, thereby substantially influencing the effectiveness of HER2-targeted therapy. This article comprehensively summarizes and deliberates upon the strategies of HER2-targeted therapy as well as the underlying resistance mechanisms.
In accordance with the global cancer statistics published by GLOBOCAN in 2022 (1), GC ranks as the fifth leading cause of cancer-related mortality globally and the fifth most prevalent malignant neoplasm. Significant geographical disparities in GC incidence are evident, with the highest rates observed in East Asian countries (1). Despite the incidence of GC in China is declining, it remains among the top three cancers in terms of incidence and mortality nationwide (2).In the pathogenesis and advancement of GC, chronic Helicobacter pylori infection, smoking, alcohol consumption, a high-salt diet, elevated body mass index, and Epstein-Barr virus infection assume significant roles (3, 4). The overexpression of human epidermal growth factor receptor 2 (HER2) in GC is intimately correlated with tumor progression and poor prognosis (5). Approximately 10-20% of GC patients display HER-2 overexpression, rendering it a valuable prognostic and predictive biomarker (6). HER2 expression is divided into different states. Immunohistochemistry (IHC) is used to detect HER2 protein, fluorescent in situ hybridization (FISH) or chromogenic in situ hybridization (CISH) is used to detect gene amplification. HER2 overexpression can be classified as IHC0 (Negative), IHC1+ (negative), IHC2+ (suspicious), or IHC3+ (positive), and samples with IHC2+ values should undergo another FISH or CISH test(Figure 1) (7, 8).
HER2, encoded by ErbB2 gene located on human chromosome17 (9), is a transmembrane receptor with intrinsic tyrosine kinase activity. As a member of the epidermal growth factor receptor (EGFR) family, HER2 belongs to a group that encompasses HER1 or EGFR, HER2, HER3, and HER4. Each receptor in this family comprises three key components: an extracellular ligand-binding domain, a transmembrane domain, and an intracellular activation domain (10, 11). The binding of various ligands to the extracellular domain triggers a series of signal transduction pathways (Figure 2), which play pivotal roles in regulating tumor cell growth, apoptosis, adhesion, migration, and differentiation (12).
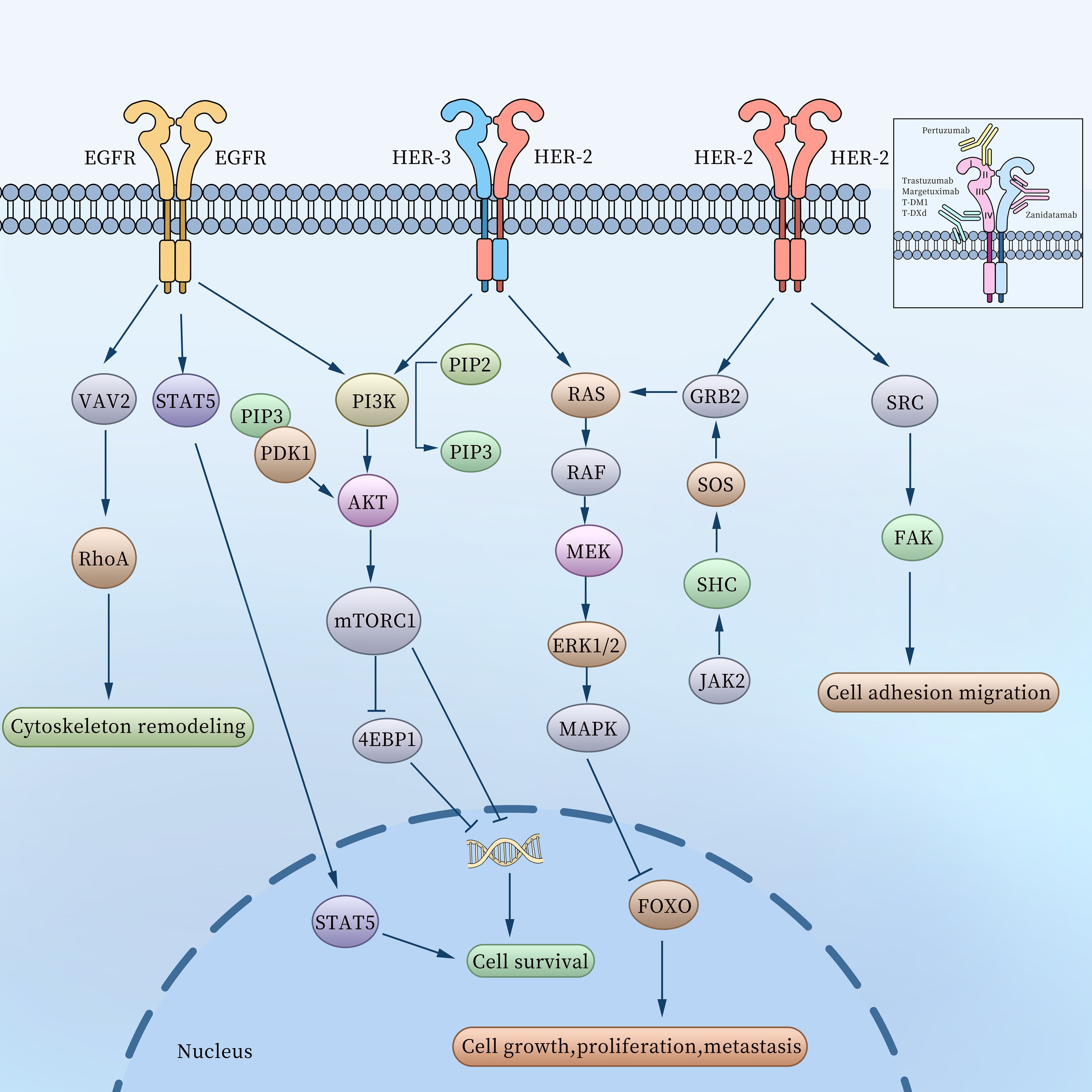
Figure 2. The related molecular mechanisms of the EGFR family in gastric cancer and binding sites of HER2-targeting antibodies.
The HER2 monomer lacks ligand-binding activity and requires dimerization to become functionally active (13), thus forming HER2-HER2 homodimers, HER2-HER3 and EGFR-HER2 heterodimers. Among these, the HER2-HER3 heterodimer is the most potent HER signaling receptor and plays a vital role in the initiation and progression of cancer (14). The HER2-HER2 homodimer promotes cell adhesion, migration, growth, proliferation, and metastasis by activating signaling pathways such as SRC-FAK (15), GRB2-SOS-JAK2 (16), and RAS-MEK-MAPK (17) within GC cells. Meanwhile, the HER2-HER3 heterodimer transmits signals through the RAS-MEK-MAPK and PI3K-AKT pathways, further driving tumor progression (18). After EGFR binds to HER2 to form EGFR-HER2 heterodimers, it triggers receptor conformational changes, activates intracellular tyrosine kinase domains, significantly enhances downstream signals (such as MAPK, PI3K-AKT pathways), and promotes tumor cell proliferation, survival and metastasis (19). In addition, it has been found that elevated EGFR expression levels can promote the formation of EGFR-HER2 heterodimers and inhibit the internalization and efficacy of antibody-drug conjugates (ADCs), and the internalization and efficacy of ADCs can be restored after knockout or drug-targeted EGFR (20).
Over the past three decades, within the realm of solid tumors exemplified by gastric cancer and breast cancer, HER2 has emerged as a key target for novel drugs, including tyrosine-kinase inhibitors (TKIs), antibody-drug conjugates (ADCs), and dual-antibody agents. HER2 also occupies key target positions in gastric cancer(Figure 3). However, looking at the development history of HER2 and its targeted drugs, there are only a few successful drugs. Trastuzumab is the first proven first-line targeted therapeutic drug for gastric cancer, with good results in the treatment of advanced GC. Clinical efficacy is the first-line standard therapy for advanced HER-2-positive GC (21). Numerous novel HER2-targeted drugs are currently under development, including Pertuzumab, Margetuximab, Zanidatamab (ZW25), KN026 (a HER2 bispecific antibody),Tyrosine kinase inhibitors (TKIs),as well as Antibody Drug Conjugates like Trastuzumab Emtansine (T-DM1), Trastuzumab Deruxtecan (T-DXd), and Disitamab Vedotin (RC48).These drugs all exhibit certain therapeutic efficacies in the treatment of gastric cancer (Table 1).
These HER2-targeting antibodies exhibit unique binding specificities. For instance, Trastuzumab, Margetuximab, T-DM1, and T-DXd bind to extracellular domain IV of HER2, while Pertuzumab binds to extracellular domain II. Zanidatamab binds to both extracellular domains II and IV of HER2. The molecular activation pathways and binding sites of HER2-targeting antibodies are illustrated in Figure 2.
Monoclonal antibodies targeting HER2 recognize HER2 antigens via their Fab segment and attach to immune cells through their Fc segment, thereby performing anti-tumor functions by means of antibody-dependent cell cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) (22, 23). In comparison with other targeted drugs, monoclonal antibodies targeting HER2 possess greater specificity in anti-tumor activities. Nevertheless, due to its relatively large molecular weight, the antibody is incapable of traversing the blood-brain barrier, thus exhibiting limitations in the treatment of patients with brain metastases.
Trastuzumab is a recombinant humanized monoclonal IgG antibody that specifically targets the HER2 receptor, binds to the HER2 extracellular domain IV, impedes cell proliferation signal transduction, and exhibits an ADCC effect (23). The TOGA trial demonstrated that in comparison to chemotherapy alone, the overall survival (OS) of patients receiving chemotherapy in combination with trastuzumab was remarkably enhanced (11.1 months vs 13.8 months) (24). This finding was further supported by a Japanese clinical trial (25). In addition to effectively augmenting OS, the combination of chemotherapy and trastuzumab treatment can also ameliorate the quality of life of patients, and the recovery and adjustment time of toxic side effects is also shorter than that of chemotherapy alone (26). In a clinical study evaluating trastuzumab combined with capecitabine and oxaliplatin for advanced gastric cancer (GC), the combination treatment group showed notable efficacy, with an OS of 13.8 months compared to 7.1 months for chemotherapy alone (27, 28). Several other studies have also substantiated the efficacy and safety of trastuzumab in the treatment of HER-2 positive GC (29, 30).
With the advancement of tumor immunotherapy, the combination of trastuzumab and immune checkpoint inhibitors has emerged as a highly promising treatment modality. A phase II trial indicated that the combination of trastuzumab, nivolumab (a PD-1 inhibitor), and FOLFOX chemotherapy significantly improved overall survival (OS) in HER2-positive gastric cancer (GC) patients (21.8 months vs. 16.4 months) (31). Simultaneously, pembrolizumab (PD-1 inhibitor) can also be safely utilized in combination with trastuzumab and chemotherapy (32). In a phase III trial of trastuzumab and chemotherapy, the objective response rate (ORR) of patients was increased by 22.7% (77.4% vs 51.9%) and the complete response rate (CRR) was increased by 8.2% (11.3% vs 3.1%) after pembrolizumab was combined with trastuzumab and chemotherapy. These interim results uniformly suggest that the efficacy of this combination regimen, with further results on overall survival (OS) and progression-free survival (PFS) eagerly awaited (33).
Pertuzumab is a second-generation anti-HER2 drug with a distinct mechanism of action compared to trastuzumab. Pertuzumab targets the extracellular domain II of HER2, effectively inhibiting the formation of HER2 dimers, particularly HER2-HER3 heterodimers, and blocking downstream signal transduction pathways (34).Studies have revealed that, in comparison with the combination of trastuzumab and chemotherapy, the addition of pertuzumab can significantly enhance the prognosis of patients with advanced HER2-positive breast cancer (BC) (35). Another study demonstrated that pertuzumab significantly prolonged the PFS of patients with HER2-positive BC (36). However, in the treatment of HER2-positive GC, the combination of pertuzumab with trastuzumab and chemotherapy did not result in a significant improvement in patient survival (37). This might be associated with the tumor heterogeneity between BC and GC. Consequently, additional studies are requisite to further ascertain the efficacy of pertuzumab in HER2-positive GC.
Margetuximab represents a novel Fc segment-optimized anti-HER2 monoclonal antibody (38). Unlike trastuzumab, margetuximab has modified five amino acid residues (L235V/F243L/R292P/Y300L/P396L) within the Fc region. This modification not only augments the binding with the activating Fc receptor FcγRIIIA-CD16A but also diminishes the binding with the inhibitory Fc receptor FcγRIIB-CD32B, thereby significantly enhancing the response rate of margetuximab (39). Additionally, CD16A is a crucial receptor that mediates ADCC. Consequently, margetuximab is more efficacious than trastuzumab with a wild-type Fc domain and exhibits a more potent ADCC effect, thus manifesting a stronger cytotoxic effect on tumor cells (40, 41). In a phase Ib/II clinical trial, the combination of margetuximab and pembrolizumab demonstrated synergistic anti-tumor activity, especially in tumors with low HER2 expression or in patients with low CD16A binding alleles (42). Another phase II trial of margetuximab and pembrolizumab as second-line treatment for HER2-positive gastric cancer (GC) demonstrated notable efficacy, with an objective response rate (ORR) of 28.2%, a disease control rate (DCR) of 63.4%, a median progression-free survival (mPFS) of 4.3 months, and a median overall survival (mOS) of 13.9 months, accompanied by good safety and tolerability (43). The aforementioned studies have indicated that the combination of margetuximab and pembrolizumab can cooperatively enhance the activity of anti-tumor drugs. The CMAHOGANY trial combined retifanlimab (PD-1 inhibitor) with margetuximab for the first-line treatment of HER2-positive GC, and the results exhibited an ORR of 53% and a DCR of 73% (44). Currently, a phase II/III randomized trial is underway to evaluate the efficacy of margetuximab combined with retifanlimab (PD-1 antibody) (with or without chemotherapy) and margetuximab combined with tebotelimab (PD-1 antibody) in patients with HER2-positive GC or gastroesophageal junction cancer (45).
ZW25 is a bispecific antibody capable of simultaneously binding to the extracellular domains II and IV of HER2. In comparison to trastuzumab or pertuzumab, ZW25 exhibits stronger anti-tumor activity, effectively suppresses HER2 signaling, and enhances immune system activation and antibody-dependent cellular cytotoxicity (ADCC) effects (46, 47). In a clinical study of HER2-positive GC, the combination of ZW25 and chemotherapy yielded favorable outcomes (ORR: 54%, DCR: 79%) (48). Another clinical trial determined that ZW25 has a significant anti-tumor effect in the treatment of HER2-expressing or amplified cancers (49). A phase II study evaluating ZW25 in combination with chemotherapy demonstrated notable improvements in patient survival, achieving an ORR of 79%, a duration of response (DOR) of 20.4 months, and progression-free survival (PFS) and overall survival (OS) of 12.5 months and not yet reached, respectively (50). The most common treatment-related adverse event associated with ZW25 is diarrhea, necessitating prophylactic use of antidiarrheal medications during clinical trials (51). A phase III trial comparing ZW25 in combination with chemotherapy, ZW25 in combination with tislelizumab and chemotherapy, and trastuzumab in combination with chemotherapy is currently in progress, and promising research results are anticipated.
KN026 is a novel bispecific antibody targeting HER2. It binds to both extracellular domains II and IV of HER2. KN026 has demonstrated high affinity and potent tumor-suppressing capabilities in HER2-positive tumor cell lines. Additionally, it exerts inhibitory effects on tumors with low HER2 expression and trastuzumab-resistant cell lines (52).In a study involving patients with previously treated advanced HER2-positive gastric cancer, the ORR in the HER2 high-expression cohort was 56%, with a remission duration of 9.7 months. In the HER2 low-expression cohort, the ORR was 14%. The most prevalent adverse events were related to gastrointestinal disorders, and no drug-related fatalities occurred. This indicates that KN026 has a favorable safety profile along with potent anti-tumor activity (53).Clinical investigations have shown that the anti-tumor activity of KN026 surpasses that of trastuzumab or pertuzumab used alone. Moreover, its anti-tumor activity is comparable to or better than the combination of trastuzumab and pertuzumab (54).The results of a phase II study on the combination of KN026 and docetaxel for the treatment of HER2-positive breast cancer demonstrated good efficacy and a manageable safety profile in the first-line treatment of HER2-positive recurrent/metastatic breast cancer (55).
TKIs are a class of medications that impede cell signaling by suppressing the activity of tyrosine kinases. They are predominantly utilized for treating a diverse range of cancers. TKIs inhibit tyrosine kinase activity via multiple mechanisms, such as obstructing ATP-binding sites, inhibiting phosphorylation reactions, and blocking signaling pathways (56). These inhibitors can be categorized into various types, including those targeting EGFR, VEGFR,HER2, ALK,RET, MET, MEK,FGFR (57). Lapatinib was initially approved in 2007 as a small-molecule tyrosine-kinase inhibitor targeting both EGFR and HER2 for the treatment of HER2-positive breast cancer (58). In the treatment of HER2-positive gastric cancer, a comparison of the combination of lapatinib with oxaliplatin and capecitabine against capecitabine and oxaliplatin alone revealed OS values of 10.5 months and 12.2 months, and PFS values of 6.0 months and 5.4 months in the respective groups (59). In another study, when comparing lapatinib plus paclitaxel to paclitaxel alone, the OS was 11.0 months and 8.9 months, and the PFS was 5.4 months and 4.4 months, respectively (60). These two studies indicated that the combination of lapatinib with chemotherapy drugs did not significantly enhance the OS of HER2-positive gastric cancer patients. This could potentially be ascribed to lapatinib-related toxicity and patient-specific demographic factors, including age and geographical region. Although afatinib and tucatinib have demonstrated some antitumor activity in HER2-positive gastric cancer patients (61, 62), additional research is necessary to precisely determine their efficacy.
Antibody-drug conjugates (ADCs) represent a class of targeted therapies composed of monoclonal antibodies directed against specific antigens, small molecule cytotoxic drugs, and chemical linkers(Table 2) (63). By leveraging blood circulation, ADCs precisely deliver their therapeutic payload to the target site, where they exert dual functions: the potent cytotoxic effects of small-molecule chemotherapy drugs and the tumor-targeting specificity of antibody-based therapies (64), as depicted in Figure 4.
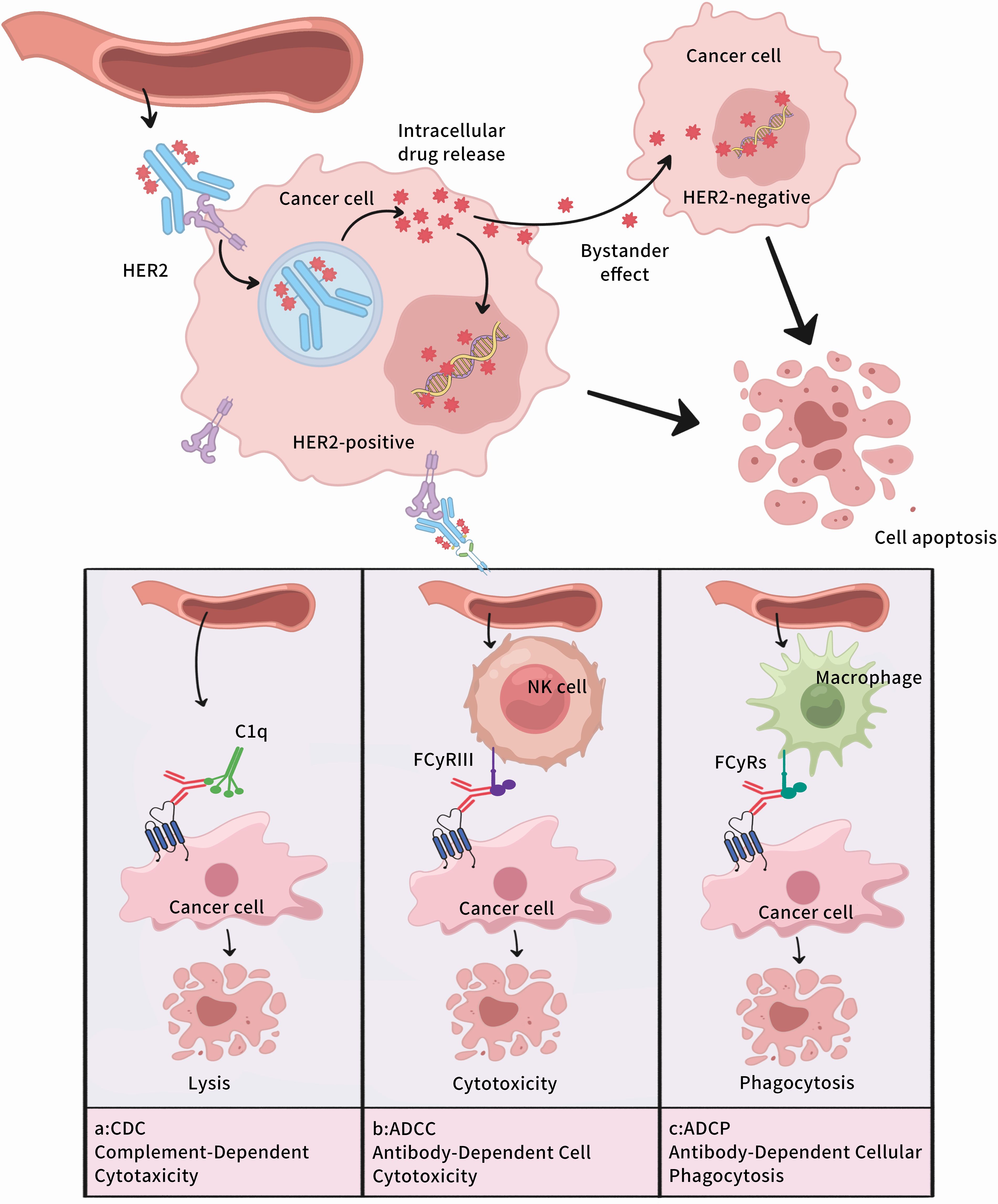
Figure 4. Mechanism of Action of Antibody-Drug Conjugates. (A)Complement Dependent Cytotoxicity,CDC;(B) Antibody dependent cell mediated cytotoxicity,ADCC;(C) Antibody-Dependent Cellular Phagocytosis,ADCP.
T-DM1 is an antibody-drug conjugate (ADC) comprising trastuzumab, a maytansine derivative (DM1, a tubulin inhibitor), and a thioether linker, with a drug-antibody ratio (DAR) of 3.5 (65). Serving as a targeted delivery system, T-DM1 transports DM1 to tumor cells with high HER2 expression through cell membrane receptor-mediated endocytosis. It then binds to tubulin, prevents microtubule polymerization and formation, and halts the cell cycle at the metaphase of mitosis. Meanwhile, trastuzumab binds to the HER2 extracellular domain IV, blocks the PI3K/AKT pathway, inhibits tumor cell proliferation, and the Fc segment of trastuzumab can also activate ADCC and induce apoptosis of tumor cells (66).
T-DM1 is the first ADC for solid tumors and was approved by the US Food and Drug Administration (FDA) in 2013 for the treatment of HER2-positive advanced breast cancer (67). In the KATHERINE-PHASE III STUDY, T-DM1 reduced the risk of disease recurrence or death by 50% in patients with residual HER-2-positive breast cancer after neoadjuvant therapy (68). In patients with HER2-positive advanced breast cancer who had previously been treated with paclitaxel and trastuzumab, T-DM1 demonstrated PFS of 9.6 months and OS of 29.9 months (69). Given its favorable therapeutic effect on breast cancer, its efficacy in GC has also attracted significant attention. Although T-DM1 demonstrated potent suppression of GC cell proliferation in preclinical trials, it did not yield a significant survival benefit in subsequent clinical trials (70). The GATSBY trial, which assessed the efficacy of T-DM1 in patients with HER2-positive GC, reported a median overall survival of 11.8 months in the T-DM1 group compared to 10.0 months in the paclitaxel chemotherapy group. The difference in survival between the two groups was not statistically significant, indicating that T-DM1 did not offer a clear survival advantage (71). Consequently, large-scale clinical trials are required to comprehensively explore the potential of T-DM1 as a viable treatment option for advanced GC.
T-DXd is an antibody-drug conjugate (ADC) composed of trastuzumab, topoisomerase I inhibitor, and cleavable peptide linker (72).This design enables the precise delivery of its cytotoxic payload into HER2-positive tumor cells, minimizing damage to normal cells (73). In contrast to T-DM1, T-DXd has DAR of 8, exhibits a robust bystander effect, and also demonstrates favorable antitumor activity in HER2-low and negative GC patients (74).
T-DXd significantly enhanced the RR (51% vs 14%) and prolonged OS(12.5 months vs 8.4 months) in patients with HER2-positive GC when compared with conventional chemotherapy (75). In a phase II trial, the outcomes of T-DXd were as follows: ORR:25%, DCR:42%, and mOS:7.6 months in HER2-positive patients who had received two or more systemic chemotherapy treatments (76). The DESTINY-Gastric02 results indicated that T-DXd alone was effective as a second-line treatment for patients with HER2-positive advanced GC (ORR: 42.0% and CRR: 5%) (77). A study demonstrated that T-DXd has significant efficacy and safety in the treatment of HER2-positive GC (78). Another Phase II clinical study evaluated T-DXd in HER2-positive solid tumors(mDOR: 11.3 months, mPFS: 6.9 months, mOS: 13.4 months) (79). Although T-DXd has exhibited good therapeutic efficacy, a substantial number of studies are still necessary to confirm the safety and efficacy of T-DXd monotherapy or its combination with chemotherapy and immunotherapy.
RC48 is a first-in-class ADC drug in China. It is composed of Hertuzumab coupled to monomethyl auristatin E (MMAE) through a cleavable linker and exerts anti-tumor effects by modulating the proliferation, differentiation, apoptosis, and migration of tumor cells (80). Compared with Trastuzumab, RC48 has a higher affinity for HER2-positive cells and a stronger ADCC effect (81). RC48 has demonstrated a favorable safety profile and notable antitumor activity in studies involving HER2-positive gastric cancer (GC) (82). Additionally, when co-cultured with HER2-positive and HER2-negative GC cells, RC48 exhibited cytotoxic effects on HER2-negative cells as well, highlighting its strong bystander effect (83).
RC48 was capable of significantly inhibiting the proliferation of HER2-positive GC cells in a dose-dependent manner and exhibited potent antitumor activity in a trastuzumab-resistant xenograft tumor model. This might be related to the RC48 bystander effect (84). Wang applied RC48 in combination with toripalimab (PD-1 inhibitor) for the treatment of GC patients (85). The results showed that the ORR was 43%, the mPFS was 6.2 months, and the mOS was 16.8 months. Clinical benefits were observed in both HER2-positive and HER2-negative populations. The combination of RC48 and toripalimab had a manageable safety profile and good clinical efficacy. A phase II study in advanced GC yielded similar results (86), with an ORR of 24.8%, mPFS of 4.1 months, and mOS of 7.9 months. The study found that RC48 had significant anti-tumor activity and a good safety profile in HER2-positive GC patients, and its effect was not entirely dependent on HER2 expression (82). Meanwhile, the combination of RC48 with other targeted drugs or chemotherapeutic agents might be a promising therapeutic strategy for the treatment of advanced HER2-positive GC and is expected to achieve more favorable results in the future.
In recent years, gastric cancer treatment has entered the era of precision medicine, and the advent of ADCs has revolutionized the treatment paradigm of solid tumors. Nevertheless, the majority of patients fail to achieve sustained disease control following ADC administration and develop resistance to ADCs. Consequently, combination therapy has emerged as the primary approach to optimizing the therapeutic efficacy of ADCs. Such combination therapies encompass combinations with chemotherapy, targeted therapy, immunotherapy, among others.
The combination of ADCs and chemotherapy has been demonstrated to be a widely-accepted strategy for overcoming drug resistance and enhancing therapeutic efficacy (87). The selection of chemotherapeutic agents can influence the levels of surface antigens targeted by ADCs. For instance, gemcitabine can upregulate the expression of HER2 on pancreatic cancer cells by 14.81-fold. This implies that the combination of T-DM1 and gemcitabine may enhance the effective binding of T-DM1 to HER2, thereby augmenting the combined treatment effect (88). However, the combination of ADCs with chemotherapy may exacerbate adverse reactions. Research has indicated that when T-DM1 is combined with docetaxel in HER2-positive breast cancer patients, dose-related toxicity and adverse events occur in 80% of patients, including leukopenia, epistaxis, nausea, and diarrhea (89). Additionally, the combination of T-DM and capecitabine has led to an increased drug discontinuation rate without a significant improvement in clinical efficacy (90). In metastatic HER2-positive gastric cancer patients, the combination of T-DXd with 5-fluorouracil or capecitabine is associated with dose-related oral mucositis and a higher incidence of adverse events (91). Overall, these study results suggest that the toxicity significantly increases when ADCs are combined with conventional chemotherapy. This may be attributed to the elevated toxicity resulting from the off-target effects and extratumoral actions of the ADC payload.
Targeted therapies have demonstrated clinical safety and efficacy. However, the efficacy of their combination with ADCs remains relatively poorly understood. The combined use of T-DM1 and pertuzumab has shown synergistic activity in cell culture models and was well-tolerated in phase Ib and phase II studies (92, 93). Bordeau et al. discovered that in a mouse model of NCI-N87 subcutaneous xenograft tumors, the co-administration of an anti-trastuzumab single-domain antibody (1HE) and T-DM1 significantly enhanced the efficacy of T-DM1 and prolonged the median survival period (94). The results of several phase III clinical trials indicate that the T-DM1 plus pertuzumab regimen reduces the incidence of adverse events. Compared with the chemotherapy plus trastuzumab and pertuzumab regimens, this regimen exhibits superior clinical efficacy (95–97). As a small-molecule tyrosine kinase inhibitor targeting HER2, tucatinib significantly enhances its efficacy when combined with trastuzumab or docetaxel, effectively improving the partial and complete tumor regression rates (98). Several other studies have also shown that the combination of T-DM1 and tucatinib can enhance the efficacy of T-DM1 (99, 100). The co-administration of osimertinib (an EGFR inhibitor) and T-DM1 facilitates the enhancement of antitumor effects, and T-DM1 can overcome osimertinib resistance in an EGFR-mutated non-small-cell lung cancer model (101).
The combination of ADCs with immunotherapy can synergistically enhance efficacy, and the selection of low-toxic and well-tolerated ADCs can lead to better therapeutic outcomes. There is a growing body of evidence suggesting that the combination of immunotherapy with ADCs has improved antitumor effects (102). Some researchers have utilized low-dose ADCs as immune stimulators in animal experiments and found that ADCs exhibit a more potent effect in animal models with normal immune function compared to immunodeficient models, indicating that ADCs possess significant immunomodulatory capabilities and can enhance the efficacy of immunotherapy (103, 104). A prospective clinical trial (KATE2) in advanced HER2-positive breast cancer evaluated the efficacy of the combined treatment of ADC and immune checkpoint inhibitors (105). The efficacy of T-DM1 combined with atezolizumab was compared to that of T-DM1 placebo. The study revealed that the mPFS of patients in the atezolizumab group was 8.2 months, while that in the placebo group was 6.8 months. In terms of safety, serious adverse events occurred in 43 out of 132 patients treated with atezolizumab and 13 out of 68 patients treated with placebo (19%). Another single-arm study employed T-DM1 in combination with pembrolizumab for the treatment of HER2-positive metastatic breast cancer. Twenty patients received the combination therapy, with an mPFS of 9.6 months and an ORR of 20%, without dose-related toxicity (106).
In summary, ADCs have demonstrated significant efficacy as monotherapy in many cancers. Their combination with chemotherapy or targeted therapy has shown certain advantages, yet issues such as adverse reactions and drug resistance still persist. Although the combination of ADCs with ICIs holds promising prospects, further research is required to elucidate the survival improvement and biological mechanisms in solid tumors.
The aforementioned HER2-targeted drugs have demonstrated favorable therapeutic effects in clinical applications. Compared to traditional chemotherapy, targeted drugs exhibit high specificity, fewer side effects, and better efficacy. Moreover, targeted drugs can be combined with chemotherapy or immunotherapy to further enhance their efficacy. However, targeted therapy drugs are restricted to HER2-positive gastric cancer patients and are ineffective in HER2-negative patients. Simultaneously, during the course of targeted drug therapy, the emergence of primary or secondary drug resistance is a key factor influencing patient efficacy. Addressing the issue of drug resistance and improving drug sensitivity are the focal points in the development of HER2-targeted therapy drugs.
Chimeric antigen receptor T (CAR-T) represents a novel type of precision-targeted immunotherapy for tumor treatment. In this approach, CAR-T cells are genetically engineered either from patients or donors to acquire the capacity to recognize and efficiently eliminate tumor cells. Subsequently, these engineered cells are infused back into the patient’s body to initiate an effective anti-tumor immune response, thereby achieving the objective of treating malignant tumors (107). Unlike conventional T cells, CAR-T cells function independently of major histocompatibility complexes (MHCs), enabling them to recognize a wide range of T cell tumor-associated antigens (108). CAR-T cells are composed of four components: the antigen recognition region, the hinge chain, the transmembrane region, and the intracellular signaling region. The antigen recognition region is typically constituted by a signal peptide and a single-chain antibody (scFv) fragment that recognizes tumor-specific antigens. This region can identify and bind to antigens on the surface of tumor cells and connect the antigen recognition region to the transmembrane region via a hinge, thus anchoring the entire CAR molecule to the cell membrane. Intracellularly, there exists an intracellular domain containing activation signals, which consists of a costimulatory molecular domain and a CD3ζ signaling domain (109, 110).
Currently, CAR-T cell therapy has achieved remarkable success in treating hematologic malignancies such as B-lymphocytic leukemia and B-cell non-Hodgkin lymphoma (111). In recent years, its potential efficacy in treating solid tumors has also been recognized (112). When applied in the treatment of HER2-positive GC (Figure 5), the HER2 antigen-specific activation of expanded CAR-T cells can effectively eradicate HER2-positive GC cells from patients, enhancing treatment efficacy and prognosis (11). The study revealed that CAR-T cell therapy effectively targeted the HER2 antigen on the surface of GC cells in an MHC-independent manner, leading to the apoptosis of tumor cells (113). Compared with controls, CAR-T cell therapy demonstrated potent tumor suppression and cytotoxic abilities in HER2-positive xenograft tumors (113). Additionally, study also demonstrated that CAR-T cells exhibited significant tumor suppression and precise targeting in comparison to untransduced T cells (114). Another study evaluated the safety, feasibility, and clinical efficacy of CAR-T cell therapy in patients with HER2-positive advanced tumors and found that one patient achieved a partial response lasting 4.5 months, and five patients had stable disease with a median progression-free survival of 4.8 months (115). This indicates that CAR-T cell therapy has favorable clinical efficacy. However, during a treatment, a patient with HER2-positive advanced GC developed severe upper gastrointestinal bleeding, suggesting that HER2-targeted CAR-T cell therapy may carry certain risks during the anti-tumor process. Early studies targeting Claudin18.2 and Claudin6 in CAR-T cell therapy for gastrointestinal tumors also show substantial potential (116–118).
CAR-T therapy stands as a revolutionary gene-therapeutic technology, widely employed in cancer treatment, particularly for hematological neoplasms. Although its current application in solid tumors is somewhat restricted, CAR-T cell therapy remains a highly promising immunotherapeutic strategy for solid tumor ablation. CAR-T cells are cells endowed with specific recognition capabilities, generated by modifying autologous T cells. These cells can endure and proliferate within the body over an extended duration, precisely identify, and eradicate cancer cells. By minimizing the risk of immune rejection, they also confer a long-term anti-cancer effect, thereby eliminating the requirement for repeated treatments.Notwithstanding, the clinical utilization of CAR-T cell therapy remains relatively limited. This could be ascribed to its high cost, substantial technical complexity, and the potential onset of severe adverse reactions, such as cytokine release syndrome (CRS) and neurotoxic responses.
As a novel cancer treatment modality, CAR-T cell therapy demonstrates remarkable efficacy and broad application prospects. Nevertheless, issues such as its side-effects and treatment costs still demand further research and resolution. It is expected that in the near future, with the continuous progress of genetic engineering technology, through the optimized design of CAR-T cell therapy, as well as the regulation of tumor burden, antigen expression, and distribution, CAR-T cell therapy will evolve into an economical, safe, and effective treatment option for solid tumors, including gastric cancer.
A cancer vaccine represents a form of active immunotherapy. It functions by introducing tumor antigens into the patient’s body, thereby activating the body’s immune system. This activation enhances the immune response against the tumor, enabling the body to recognize and eliminate cancer cells (119). Gastric cancer vaccines are a subset of cancer vaccines. Common types of gastric cancer vaccines include cellular vaccines (such as tumor cell vaccines or immune cells), protein/peptide vaccines, and nucleic acid vaccines (DNA vaccines, RNA vaccines, or viral vector vaccines).
HER2 serves as a tumor-associated antigen in gastric cancer and is an excellent target for targeted therapy. The HER2 dendritic cell vaccine can be utilized for the treatment of HER2-positive gastric cancer. One study revealed that 9 patients with unresectable or recurrent HER2-positive gastric cancer achieved partial clinical remission, accompanied by a decrease in tumor markers (CA19-9, CEA) after treatment with the HER2 dendritic cell vaccine. Additionally, 1 patient had a 3-month stable disease period (120). A phase I trial demonstrated that the HER2 dendritic cell vaccine exhibited favorable clinical efficacy in patients with HER2-positive gastric cancer, along with an outstanding safety profile (121). Jung discovered that the BVAC-B vaccine (an autologous B-cell-and monocyte-based immunotherapy vaccine) activated immune cells in patients with HER2-positive gastric cancer, although its clinical efficacy was limited, in the treatment of HER2-positive advanced gastric cancer (122). HER-Vaxx is a B-cell vaccine targeting HER2. In clinical practice, the overall survival (OS) was significantly longer in patients who received HER-Vaxx in combination with chemotherapy compared to those who received chemotherapy alone (13.9 months vs 8.3 months). Moreover, it was found that the levels of HER2-specific antibody IgG induced by HER-Vaxx were significantly correlated with a reduction in tumor volume. HER-Vaxx also inhibited the phosphorylation of the HER2 signaling pathway and promoted ADCC effects (123).
Although there is currently no FDA-approved cancer vaccine for the treatment of gastric cancer available on the market, this field holds substantial potential for development. We eagerly anticipate breakthroughs in combination trials involving multiple vaccines.
Over the past few decades, the landscape of cancer treatment for patients has undergone a remarkable transformation with the emergence of monoclonal antibodies, immune-checkpoint inhibitors, bispecific antibodies, and innovative T-cell therapies. ADC drugs have also brought about a revolutionary change in cancer treatment. Nevertheless, their efficacy remains constrained by drug resistance stemming from diverse mechanisms. These include antigen-related drug resistance, aberrant signaling pathways, incorrect internalization pathways, lysosomal dysfunction, and overexpression of efflux pumps. If these drug-resistance issues can be effectively resolved, it will undoubtedly be a great blessing for cancer patients.
Heterogeneity in HER2 expression implies that, within GC cells, the expression of the HER2 protein varies among different cells or different regions of the same tumor, as well as between primary and metastatic lesions. Heterogeneity of HER2 expression in GC tissues is prevalent, with the incidence of heterogeneity ranging from 30.0% to 75.4% across different studies (124). HER2 expression levels vary significantly across different regions of GC tissues, influenced by microenvironmental factors such as vascular distribution, nutrient supply, and cell-cell interactions. This regional heterogeneity presents challenges in detecting HER2 and other biomarkers for targeted therapy. HER2 heterogeneity is regarded as a poor prognostic indicator in GC, as patients with heterogeneous HER2-positive expression have significantly shorter PFS and OS than those without heterogeneous expression (125). Additionally, it has been demonstrated that HER2 expression heterogeneity is associated with the amplification of other receptor tyrosine kinases (RTKs), such as EGFR, MET, and FGFR2, thereby facilitating resistance to HER2-targeted therapies.
Furthermore, the heterogeneity of HER2 expression between primary and metastatic lesions should not be overlooked. The GASTHER1 study reported that 5.7% of patients with HER2-negative advanced GC had HER2-positive metastases (126). During GC metastasis, GC cells undergo a series of genetic and epigenetic alterations. HER2 expression in metastatic cancer cells can differ from that in primary lesions. In some patients, HER2 expression is low in primary tumors but elevated in metastatic sites. As a result, targeted therapy regimens based solely on HER2 detection in primary lesions may fail to effectively target metastases, potentially compromising treatment efficacy and patient prognosis. Therefore, the European Society of Oncology (ESMO) recommends HER2 testing for metastatic GC, and the American Society of Clinical Oncology (ASCO) recommends HER2 testing for all primary or metastatic GC lesions in patients who can tolerate combination therapy (127).
The loss of HER2 expression represents a targeted mechanism of acquired resistance to HER2-targeted therapy. The expression of HER2 on the cell surface of resistant cancer cells is significantly decreased compared to that of cancer cells sensitive to targeted therapy (128). Studies have shown that 69% of GC patients with HER2-positive tumors lose HER2 expression after receiving targeted HER2 therapy, and this secondary loss of HER2 expression is for a major contributor to drug resistance (129). Another study confirmed that29.1% of advanced GC patients treated with HER2-targeted agents experienced a change from HER2-positive to negative status (130). This change diminished the efficacy of targeted HER2 therapy and contributed to drug resistance. It was also found that HER2 heterogeneity increased from 2.9% to 21.9% in metastases, and most patients who became HER2-negative after targeted HER2 therapy also exhibited new heterogeneity. The loss of HER2 expression and changes in heterogeneity are associated with adverse effects of targeted therapy and a shortening of PFS. The factors driving the overall change in HER2 expression remain unclear, and further research on the mechanism is required. It is also recommended that patients who have progressed after first-line targeted HER2 therapy should consider HER2 testing to determine the current HER2 expression status and guide subsequent treatments.
The binding of monoclonal antibodies to HER2 is influenced by multiple factors. Among them, deletion of the extracellular segment of the HER2 receptor and molecular masking are potential resistance mechanisms. The p95HER2 receptor, which is a soluble truncated form resulting from the shedding of the full-length p185HER2 receptor, serves as a poor prognostic marker and increases the risk of lymph node metastasis (131). Tumors expressing the full-length receptor have been observed to have a high response to trastuzumab. In contrast, the truncated P95HER2 shows resistance and is also anticipated to lead to T-DM1 resistance because the p95HER2 receptor lacks trastuzumab binding sites (132).Molecular masking may be another contributing factor to resistance. Cells in the tumor or the microenvironment express membrane-associated mucin 4 (MUC4). The interaction between MUC4 and HER2 exerts a steric hindrance effect, inhibiting ADCC. MUC4 masks the binding site on the extracellular segment of HER2, preventing the monoclonal antibody from binding to HER2, which leads to resistance to both trastuzumab and T-DM1. However, silencing MUC4 can restore the sensitivity of tumor cells to T-DM1 (133–135).Moreover, neuromodulins can also impact the sensitivity of ADC drugs. They impair the efficacy of T-DM1 by promoting the heterodimerization of HER2 with HER3 and HER4 (92).
The PI3K/AKT signaling pathway is activated in a variety of tumors and regulates multiple mechanisms, including cell survival, proliferation, growth, metabolism, angiogenesis, and metastasis (136). A The PI3K/AKT pathway, a critical downstream signaling pathway of HER2, plays a pivotal role in the development of drug resistance. This pathway is regulated by multiple factors, such as mesenchymal-epithelial transition factor (MET) amplification, phosphatidylinositol 4,5-diphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutation, and phosphatase and tensin homolog (PTEN) deletions.
Recent studies have shown that MET activation is associated with increased resistance to multiple targeted therapies (137). MET is a tyrosine-kinase receptor present on cell membranes that is often overexpressed or co-amplified in HER2-positive GC and is associated with a lower ORR (138). Previous studies have also revealed that the co-amplification of MET and HER2 results in a significant reduction in OS in GC compared to the overexpression of MET and HER2 alone (139). Further studies have revealed that increased levels of MET and its ligand, hepatocyte growth factor (HGF), contribute to resistance to HER2-targeted therapy by re-stimulating the PI3K/AKT signaling pathway. This resistance can be effectively reversed through the inhibition of MET activity (140). Similarly, the inhibition of MET has been shown to help overcome resistance in afatinib-resistant GC cell lines (141).
PIK3CA is a proto-oncogene located on human chromosome 3 (3q26.3), and mutations have been identified in a variety of tumors, including breast, endometrial, colorectal, and GC tumors (142–145). PIK3CA mutations can enhance its kinase activity, leading to the sustained activation of the PI3K/AKT signaling pathway, which in turn is associated with the development of resistance to anti-HER2 therapy (142). PIK3CA mutations occur in approximately 4% to 25% of GC patients (146). In addition, there is a significant correlation between PIK3CA mutations and HER2 gene amplification (147), which may provide an explanation for the development of resistance to anti-HER2 therapy.
PTEN is a tumor-suppressor gene that inhibits tumor invasion and metastasis (148). Genetic mutations are often the main cause of PTEN activity loss, which is closely related to the shortening of PFS and OS and the efficacy of targeted therapy. Studies have shown that PTEN loss occurs in a significant proportion of patients with HER2-positive gastric cancer (GC), with one study reporting a prevalence of 34.5% (149) and another reporting 47.9% (150). At the same time, PTEN is also associated with HER2 overexpression (151), and the deletion of PTEN activity leads to the over-activation of the PI3K/AKT signaling pathway. In PTEN-deficient GC cells, the effect of anti-HER2 therapy on tumor growth inhibition and apoptosis is significantly reduced because PTEN deletion can activate the PI3K/AKT signaling pathway (152), thereby affecting the sensitivity of HER2-targeted therapy. In addition, the down-regulation of PTEN is associated with miR-21, which can negatively regulate PTEN and up-regulate the PI3K/AKT signaling pathway, leading to the emergence of drug resistance (153). This finding is beneficial for addressing the drug-resistance problem of HER2-targeted therapy in some patients and provides a reference for formulating personalized treatment plans.
Aberrant activation of the MEK/ERK signaling pathway is closely related to the occurrence and development of various cancers, and this pathway is involved in regulating cell proliferation, differentiation, and apoptosis. Steroid-receptor coactivator (SRC) is a non-receptor tyrosine kinase that can activate multiple downstream signaling pathways, such as MEK/ERK, PI3K/AKT, JAK/STAT, and other signaling pathways (154). SRC expression in HER2-positive tumor cells has been found to be consistent with HER2 expression (155), and evidence suggests that SRC may act as a link between EGFR and MET (156). These findings imply that SRC-mediated activation of downstream signaling pathways could play a role in the development of resistance to HER2 therapy. One study demonstrated that in HER2-positive breast cancer, SRC activation drives trastuzumab resistance, and treatment with SRC inhibitors can effectively reverse this resistance (157). Additionally, the use of Bosulif, an SRC tyrosine-kinase inhibitor, was able to block downstream signaling, thereby restoring sensitivity in gastric and biliary cancer cell lines that were previously resistant to Trastuzumab (158).
The Wnt/β-catenin signaling pathway is essential for regulating cell proliferation, differentiation, and survival. It is influenced by a variety of factors, such as Wnt protein family members, precursor cytokines, genetic variations, microRNAs and other regulatory elements (159). Dysregulation of this pathway is associated with numerous diseases, such as cardiovascular and lung diseases, and its abnormal activation is also closely related to tumorigenesis, progression, and poor prognosis (160). β-catenin can directly bind to HER2 and promote the phosphorylation of HER2 at Y877 and Y1248 and is also capable of inducing resistance to Trastuzumab treatment by promoting the HER2-SRC interaction (161).The study found that Wnt3A, FZD6, and CTNNB1 were up-regulated in trastuzumab-resistant cells, while GSK-3β was down-regulated (162). By modulating CTNNB1, this signaling pathway can achieve a reversal of the resistance phenotype. Another study also demonstrated that the Wnt/β-catenin signaling pathway promotes the development of drug resistance during targeted therapy by regulating the stemness and EMT phenotype of GC cells (163). These findings suggest that targeting the Wnt/β-catenin signaling pathway may overcome drug resistance in HER2-positive GC-targeted therapy.
SHCBP1 is a SHC1-binding protein that is a downstream effector protein of HER2 and plays an important role in cell signaling mechanisms. HER2 interacts with SHCBP1 to activate PLK1 and regulate the cell cycle and mitotic progression. Numerous studies have shown that SHCBP1 is up-regulated in various types of tumor tissues (164), and the expression level of SHCBP1 in breast cancer is closely related to the expression levels of GC and HER2 (165, 166). In addition, resistance to anti-HER2 therapy has been linked to the downstream signaling pathway of PLK1, and targeting PLK1 can enhance drug sensitivity (167). However, research on the role of the PLK1 signaling pathway in GC treatment remains limited. HER2 can activate the SHCBP1-PLK1-MISP signaling pathway, promote cell mitosis, reduce the sensitivity of HER2-positive GC cells to Trastuzumab, and TFBG sensitizes GC to Trastuzumab treatment by blocking the SHCBP1-PLK1-MISP axis (168).
When an ADC drug binds to the target antigen, it enters cancer cells via endocytosis. Endocytosis mechanisms include clathrin-mediated endocytosis, caveolin-mediated endocytosis, and clathrin-caveolin-independent endocytosis. Among them, clathrin-mediated endocytosis is the most frequently adopted pathway by ADCs (169). Once endocytosis is impaired, ADC drugs cannot function properly. Thus, impaired endocytosis is regarded as a mechanism of ADC resistance. EndophilinA2 (encoded by SH3GL1) is a scaffold protein involved in endocytosis. In HER2-positive breast cancer models, EndophilinA2 promotes HER2 internalization and enhances the sensitivity of breast cancer cells to trastuzumab treatment. Targeting the SH3GL1 gene to inhibit EndophilinA2 expression results in reduced internalization and significantly inhibits T-DM1-mediated cytotoxicity (170). In T-DM1-resistant gastric cancer N87 cell lines, caveolin-mediated endocytosis was found to be significantly enhanced. This might be because caveolin is not conducive to drug transport to lysosomes. However, knocking down caveolin did not restore T-DM1 sensitivity (171). Another study indicated that upregulating the expression of caveolin improved the activity of T-DM1 in different cell lines (172). Additionally, hypoxia-induced translocation of caveolin from vesicles to plasma membranes has been proposed as a possible mechanism for the reduced internalization of trastuzumab in the hypoxic microenvironment of HER2-positive breast cancer (173). In summary, the role of caveolae-mediated endocytosis in T-DM1 resistance remains controversial and may be related to the dominant endocytic pathway of the target antigen in certain cell lines.
The ADC drug target molecule binds and enters the cell through endocytosis. Once it reaches the lysosome, the cytotoxic agent in the ADC drug is released by chemical or enzymatic cleavage. The degradation of ADCs depends on the acidic lysosomal environment and active lysosomal enzymes. It has been discovered that T-DM1 accumulates in lysosomes in cells that develop resistance to T-DM1. The proteolytic activity in these drug-resistant cells is lower than that in sensitive cells. This is due to an increase in lysosomal pH, which inhibits proteolytic enzyme activity, resulting in T-DM1 not being adequately cleaved to release cytotoxic agents (174). Moreover, the acidic environment required for ADC degradation in lysosomes is maintained by V-ATPase, a proton pump that regulates lysosomal acidity (175). Low V-ATPase activity was detected in T-DM1-resistant N87 cell lines. Inhibition of V-ATPase activity in T-DM1-sensitive N87 cells with bafilomycin A1 induced resistance to T-DM1, suggesting that abnormal V-ATPase activity reduces T-DM1 metabolism, leading to T-DM1 resistance (176). Therefore, V-ATPase activity in lysosomes can be used as a novel biomarker to predict T-DM1 resistance. The cytotoxic agents in ADC drugs exert cytotoxic effects during their transport from lysosomes to the cytoplasm through lysosomal transporters. SLC46A3 belongs to the solute carrier (SLC) transporter family, which is a transporter on the lysosomal membrane. Silencing of this protein leads to the accumulation of catabolites in lysosomes, ultimately resulting in drug failure (177).
ATP-binding cassette transporters (ABCs) are transporters located on cell membranes and play a crucial role in drug absorption, distribution, metabolism, and excretion (178). Their overexpression causes the extracellular pumping of cytotoxic drugs from the cell, inhibiting the cytotoxic effects of anticancer drugs. This is one of the main causes of multidrug resistance in human malignancies (179). Since many payloads are substrates for ABC, upregulation of ABC can lead to an increase in the active efflux of payloads (180), thereby protecting tumor cells from cytotoxic damage and causing ADC resistance. The ABC transporter family consists of 48 proteins, including ABCB1 (also known as MDR1/P-glycoprotein), ABCG2, ABCC1, ABCC2, and others (181). ABCB1-mediated efflux is the most common in clinical resistance, and ABCB1 has been shown to be a major driver of resistance to vc-MMAE-based conjugates (vc: valine-citrulline, MMAE: tubulin inhibitors) (182). Another study demonstrated that the expression of the ABC transporter ABCG2 or ABCB1 leads to DXd/SN-38 efflux, causing the drug-resistance problem in DXd/SN-38 ADCs (183). The use of ABCG2 inhibitors and ABCB1 inhibitors, respectively, on ASPC-1 and H2170 cells with high ABCG2 expression and HCT15 cells with high ABCB1 expression can enhance the sensitivity of cancer cells to DXd/SN-38.
Metabolic reprogramming of tumor cells is an important manifestation of their adaptation to the external environment and plays a crucial role in tumor growth, proliferation, metastasis, and drug resistance. Numerous studies have shown that metabolic reprogramming has been observed in trastuzumab-resistant HER2-positive GC, including glycolysis, amino acid metabolism, and lipid metabolism (184).
Glycolysis plays a critical role in tumor metabolism, with tumor cells exhibit higher glycolytic activity compared to normal cells. In HER2-positive GC, fructose-6-phospho-kinase 2 activates the glycolytic pathway, contributing to the development of trastuzumab resistance Targeting PFKFB3 (a key enzyme for glycolysis) can increase sensitivity to anti-HER2 therapy (185). It was found that the related transporters and metabolic enzymes involved in glutamine catabolism were significantly increased in drug-resistant cells, and glutaminase 1 (GLS1) was the most elevated (186). Lipid metabolism is also involved in the anti-HER2 therapy process, and fatty acid synthase is significantly expressed in drug-resistant cells. The combined treatment of HER2 and fatty acid synthase has achieved a favorable anti-tumor effect (187).
The tumor microenvironment (TME) is composed of a variety of immune cells and proteins, and its main function is to promote tumor progression (188). The immune cells within the TME have a dual role, both inhibiting tumors and participating in tumor angiogenesis, immunosuppression, and cell proliferation through cytokine secretion (189). These complex and multiple roles of immune cells in the TME tend to promote tumor progression and enhance resistance to drug therapy. Studies suggest that HER2 signaling may be involved in the regulation of immune cell activation in the TME, which inhibits immune cell activation in the TME by inhibiting interferon-gene-stimulating factor signaling in GC cells, resulting in drug resistance (190). Macrophages in the TME are closely related to tumor progression. Studies have shown that GC cells promote GLS1 expression and the release of GLS1-containing microvesicles into the TME through the CDC42/NF-κB signaling pathway (186). These microvesicles promote macrophage polarization to the M2 phenotype and pro-angiogenic functions, leading to acquired trastuzumab resistance in HER2-positive GC cells.
The mechanism of drug resistance in HER2-targeted therapy has been relatively well-elucidated. However, in clinical practice, it remains infeasible to prevent the emergence of drug resistance through technical detection methods. This could be attributed to several reasons. First, the development of drug resistance may be driven by multiple mechanisms, such as the activation of drug-efflux pumps, target mutations, and bypass activation. As a result, it is arduous for a single biomarker or mechanism to comprehensively account for the overall drug-resistance phenotype. Second, it may be associated with treatment-related stress. The mechanism of drug resistance can change dynamically in response to treatment-induced stress, such as tumor evolution or adaptive mutations. Moreover, clinical samples are typically collected at a single time-point, which makes it challenging to capture this dynamic process. Third, it may also be related to factors such as the patient’s immune status, gut microbiota, or metabolites. Regrettably, these factors are often neglected in current clinical practice.
GC ranks among the most prevalent malignancies worldwide, being characterized by high morbidity and mortality rates. A substantial number of GC patients are often diagnosed at the middle-and advanced-stages. This makes treatment far more challenging, leads to a poor prognosis, and results in a low survival rate. The role of human epidermal growth factor receptor 2 (HER2) in GC has been well-established. Targeting HER2 has significantly improved the outcomes of both early-and late-stage HER2-positive GC patients. Nevertheless, drug resistance is a common issue in current GC treatments, whether involving traditional chemotherapy drugs or some of the targeted-therapy drugs already in use.
ADCs are characterized by more precise targeting and stronger cytotoxicity, enabling them to more effectively recognize and eliminate GC cells. Combination-therapy regimens can exert drug synergy through different mechanisms of action, interfering with the growth, proliferation, and metastasis of tumor cells at multiple levels, thereby enhancing the prognosis of patients. However, problems such as adverse reactions and drug resistance still persist. Thus, it is crucial to re-optimize the design strategy, explore the development of bispecific-antibody ADCs to enhance efficacy and safety, identify new predictive biomarkers, and achieve precise and individualized treatment. For first-line ADC combination-therapy regimens, if the tumor can be effectively controlled, it can reduce the occurrence of tumor-related symptoms, thereby improving the patient’s quality of life. Additionally, patients are in relatively better physical condition and more tolerant of new treatment options during first-line treatment compared to second-line or subsequent treatments. The research and development of new ADCs and combination regimens as first-line therapies can drive innovation and development in the field of GC therapeutics. Through continuous exploration of new drug combinations and treatment models, more treatment options can be provided for GC patients, and references and ideas can also be offered for the treatment of other malignant tumors.
As an innovative and promising ablative immunotherapy for solid tumors, CAR-T therapy is gradually emerging, attracting the attention of numerous researchers and clinicians. CAR-T cell therapy has achieved remarkable results in the treatment of hematologic tumors, significantly improving the survival of some patients. Many experts and scholars predict that in the near future, CAR-T cell therapy is expected to overcome many difficulties and become a safe and effective treatment modality for various solid tumors, including GC. However, the emergence of drug resistance has gradually become a key factor impeding its further development and affecting patient prognosis. Tumor cells are highly heterogeneous and can evade CAR-T cell attack through a variety of complex mechanisms. For example, tumor cells may downregulate or alter the antigen expression on their surface, making it difficult for CAR-T cells to recognize them; they may also interfere with the activity and function of CAR-T cells by secreting some inhibitory cytokines. The emergence of these drug-resistance phenomena greatly reduces the long-term effectiveness of CAR-T cell therapy, leaving some patients still at risk of tumor recurrence and disease progression after treatment.
Looking ahead, the treatment of HER2-positive GC patients holds great potential. In terms of exploring new targets, researchers will focus on the molecules on the surface of tumor cells that have not been fully studied and the key regulators in the tumor microenvironment, aiming to identify new and more specific targets to lay the foundation for the development of more efficient therapeutic drugs. Besides traditional small-molecule targeted drugs and antibody drugs, drugs based on new mechanisms of action, such as bispecific antibodies, improved ADCs and antibody neodegrader conjugate (AnDC), are expected to exhibit unique advantages in the treatment of HER2-positive GC. In the exploration of new models, in addition to the combined application of various treatment methods carried out so far, personalized treatment models will also be considered based on the individual characteristics of patients, such as genetic background, lifestyle habits, and underlying diseases. This can realize the transition from “one-size-fits-all” treatment to precise and individualized treatment, bringing unprecedented breakthroughs to the treatment of HER2-positive GC patients.
QS: Writing – original draft. JD: Funding acquisition, Writing – review & editing. HW: Writing – review & editing. ZH: Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82203267), Gansu Province Health Industry Science and Technology Innovation Major Projects (No. GSWSKY2024-06), and The Cuiying Science and Technology Innovation Project of Lanzhou University Second Hospital(Nos. CY2024-MS-B18,CY2022-MS-A12)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
3. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. (2023) 20:338–49. doi: 10.1038/s41571-023-00747-0
4. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. (2020) 21:4012. doi: 10.3390/ijms21114012
5. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. (2001) 2:127–37. doi: 10.1038/35052073
6. Haffner I, Schierle K, Raimúndez E, Geier B, Maier D, Hasenauer J, et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J Clin Oncol. (2021) 39:1468–78. doi: 10.1200/JCO.20.02761
7. Hanna WM, Kwok K. Chromogenic in-situ hybridization: a viable alternative to fluorescence in-situ hybridization in the HER2 testing algorithm. Mod Pathol. (2006) 19:481–7. doi: 10.1038/modpathol.3800555
8. Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. (2008) 52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x
9. Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. (2013) 13:663–73. doi: 10.1038/nrc3559
10. Drago JZ, Ferraro E, Abuhadra N, Modi S. Beyond HER2: Targeting the ErbB receptor family in breast cancer. Cancer Treat Rev. (2022) 109:102436. doi: 10.1016/j.ctrv.2022.102436
11. Sun J, Li X, Chen P, Gao Y. From anti-HER-2 to anti-HER-2-CAR-T cells: an evolutionary immunotherapy approach for gastric cancer. J Inflammation Res. (2022) 15:4061–85. doi: 10.2147/JIR.S368138
12. Ma C, Wang X, Guo J, Yang B, Li Y. Challenges and future of HER2-positive gastric cancer therapy. Front Oncol. (2023) 13:1080990. doi: 10.3389/fonc.2023.1080990
13. Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. (2007) 26:6469–87. doi: 10.1038/sj.onc.1210477
14. Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. (2012) 8:961–87. doi: 10.2217/fon.12.95
15. Nam HJ, Im SA, Oh DY, Elvin P, Kim HP, Yoon YK, et al. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. (2013) 12:16–26. doi: 10.1158/1535-7163.MCT-12-0109
16. Liu Y, Shi Y, Han R, Liu C, Qin X, Li P, et al. Signaling pathways of oxidative stress response: the potential therapeutic targets in gastric cancer. Front Immunol. (2023) 14:1139589. doi: 10.3389/fimmu.2023.1139589
17. Su CC. Tanshinone IIA inhibits gastric carcinoma AGS cells by decreasing the protein expression of VEGFR and blocking Ras/Raf/MEK/ERK pathway. Int J Mol Med. (2018) 41:2389–96. doi: 10.3892/ijmm.2018.3407
18. Pan L, Li J, Xu Q, Gao Z, Yang M, Wu X. HER2/PI3K/AKT pathway in HER2-positive breast cancer: A review. Med (Baltimore). (2024) 103:e38508. doi: 10.1097/MD.0000000000038508
19. Bai X, Sun P, Wang X, Long C, Liao S, Dang S, et al. Structure and dynamics of the EGFR/HER2 heterodimer. Cell Discovery. (2023) 9:18. doi: 10.1038/s41421-023-00523-5
20. Gupta A, Michelini F, Shao H, Yeh C, Drago JZ, Liu D, et al. EGFR-directed antibodies promote HER2 ADC internalization and efficacy. Cell Rep Med. (2024) 5:101792. doi: 10.1016/j.xcrm.2024.101792
21. Smyth EC, Sundar R. Combining chemotherapy, trastuzumab, and immune-checkpoint inhibitors in HER2-positive gastro-oesophageal cancer. Lancet. (2023) 402:2168–70. doi: 10.1016/S0140-6736(23)02296-1
22. Guti E, Regdon Z, Sturniolo I, Kiss A, Kovács K, Demény M, et al. The multitargeted receptor tyrosine kinase inhibitor sunitinib induces resistance of HER2 positive breast cancer cells to trastuzumab-mediated ADCC. Cancer Immunol Immunother. (2022) 71:2151–68. doi: 10.1007/s00262-022-03146-z
23. Gao Y, Li L, Zheng Y, Zhang W, Niu B, Li Y. Monoclonal antibody Daratumumab promotes macrophage-mediated anti-myeloma phagocytic activity via engaging FC gamma receptor and activation of macrophages. Mol Cell Biochem. (2022) 477:2015–24. doi: 10.1007/s11010-022-04390-8
24. Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. (2012) 15:313–22. doi: 10.1007/s10120-011-0118-1
25. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial [published correction appears in Lancet. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X. 2010 Oct 16;376(9749):1302].
26. Satoh T, Bang YJ, Gotovkin EA, Hamamoto Y, Kang YK, Moiseyenko VM, et al. Quality of life in the trastuzumab for gastric cancer trial. Oncologist. (2014) 19:712–9. doi: 10.1634/theoncologist.2014-0058
27. Rivera F, Romero C, Jimenez-Fonseca P, Izquierdo-Manuel M, Salud A, Martínez E, et al. Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial [published correction appears in Cancer Chemother Pharmacol. Cancer Chemother Pharmacol. (2019) 83:1175–81. doi: 10.1007/s00280-019-03820-7
28. Rivera F, Izquierdo-Manuel M, García-Alfonso P, Martínez de Castro E, Gallego J, Limón ML, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectabl gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. (2021) 145:158–67. doi: 10.1016/j.ejca.2020.12.005
29. An E, Ock CY, Kim TY, Lee KH, Han SW, Im SA, et al. Quantitative proteomic analysis of HER2 expression in the selection of gastric cancer patients for trastuzumab treatment. Ann Oncol. (2017) 28:110–5. doi: 10.1093/annonc/mdw442
30. Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng ZL, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. (2019) 68:1152–61. doi: 10.1136/gutjnl-2018-316522
31. Stein A, Paschold L, Tintelnot J, Goekkurt E, Henkes SS, Simnica D, et al. Efficacy of ipilimumab vs FOLFOX in combination with nivolumab and trastuzumab in patients with previously untreated ERBB2-positive esophagogastric adenocarcinoma: the AIO INTEGA randomized clinical trial. JAMA Oncol. (2022) 8:1150–8. doi: 10.1001/jamaoncol.2022.2228
32. Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. (2020) 21:821–31. doi: 10.1016/S1470-2045(20)30169-8
33. Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. (2021) 600:727–30. doi: 10.1038/s41586-021-04161-3
34. Metzger-Filho O, Winer EP, Krop I. Pertuzumab: optimizing HER2 blockade. Clin Cancer Res. (2013) 19:5552–6. doi: 10.1158/1078-0432.CCR-13-0518
35. Gleeson JP, Keegan NM, Morris PG. Adding Pertuzumab to Trastuzumab and Taxanes in HER2 positive breast cancer. Expert Opin Biol Ther. (2018) 18:251–62. doi: 10.1080/14712598.2018.1410132
36. Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. (2020) 21:519–30. doi: 10.1016/S1470-2045(19)30863-0
37. Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. (2018) 19:1372–84. doi: 10.1016/S1470-2045(18)30481-9
38. Markham A. Margetuximab: first approval. Drugs. (2021) 81:599–604. doi: 10.1007/s40265-021-01485-2
39. Musolino A, Gradishar WJ, Rugo HS, Nordstrom JL, Rock EP, Arnaldez F, et al. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J Immunother Cancer. (2022) 10:e003171. doi: 10.1136/jitc-2021-003171
40. Nur Husna SM, Wong KK. Margetuximab and trastuzumab deruxtecan: New generation of anti-HER2 immunotherapeutic agents for breast cancer. Mol Immunol. (2022) 152:45–54. doi: 10.1016/j.molimm.2022.10.005
41. Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. (2017) 28:855–61. doi: 10.1093/annonc/mdx002
42. Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. (2011) 13:R123. doi: 10.1186/bcr3069
43. Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. (2020) 21:1066–76. doi: 10.1016/S1470-2045(20)30326-0
44. Catenacci DVT, Kang YK, Yoon HH, Shim BY, Kim ST, Oh DY, et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectabl or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open. (2022) 7:100563. doi: 10.1016/j.esmoop.2022.100563
45. Catenacci DV, Rosales M, Chung HC, Yoon H, Shen L, Moehler M, et al. MAHOGANY: margetuximab combination in HER2+ unresectabl/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. (2021) 17:1155–64. doi: 10.2217/fon-2020-1007
46. Weisser NE, Sanches M, Escobar-Cabrera E, O'Toole J, Whalen E, Chan PWY, et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat Commun. (2023) 14:1394. doi: 10.1038/s41467-023-37029-3
47. Tabernero J, Shen L, Elimova E, Ku G, Liu T, Shitara K, et al. HERIZON-GEA-01: Zanidatamab+chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. (2022) 18:3255–66. doi: 10.2217/fon-2022-0595
48. Meric-Bernstam F, Ep H, Beeram M, Hanna DL, El-Khoueiry A, Kang YK, et al. Zanidatamab (ZW25) in HER2-expressing gastroesophageal adenocarcinoma (GEA): Results from a phase I study. J Clin Oncol. (2021) 39:164. doi: 10.1200/JCO.2021.39.3_suppl.164
49. Meric-Bernstam F, Beeram M, Hamilton E, Oh DY, Hanna DL, Kang YK, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. (2022) 23:1558–70. doi: 10.1016/S1470-2045(22)00621-0
50. Elimova E, Ajani JA, Burris HA, Denlinger CS, Iqbal S, Kang K-K, et al. Zanidatamab + chemotherapy as first-line treatment for HER2-expressing metastatic gastroesophageal adenocarcinoma (mGEA). J Clin Oncol. (2023) 41(4_suppl):347. doi: 10.1200/jco.2023.41.4_suppl.347
51. Ku GY, Elimova E, Denlinger CS, Mehta R, Lee K, Syma I, et al. 1380P Phase (Ph) II study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). Ann Oncol. (2021) 32:S1044–5. doi: 10.1016/j.annonc.2021.08.1489
52. Ariga S. History and future of HER2-targeted therapy for advanced gastric cancer. JClinMed. (2023) 12:3391. doi: 10.3390/jcm12103391
53. Xu J, Ying J, Liu R, Wu J, Ye F, Xu N, et al. KN026 (anti-HER2 bispecific antibody) in patients with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancer. Eur J Cancer. (2023) 178:1–12. doi: 10.1016/j.ejca.2022.10.004
54. Zhang J, Ji D, Cai L, Yao H, Yan M, Wang X, et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I study. Clin Cancer Res. (2022) 28:618–28. doi: 10.1158/1078-0432.CCR-21-2827
55. Ma J, Wang J, Xu T, Ouyang Q, Wang X, Wang J, et al. Efficacy and safety of KN026 and docetaxel for HER2-positive breast cancer: a phase II clinical trial. Cancer Commun (Lond). (2025) 1–10. doi: 10.1002/cac2.12662
56. Levitzki A. Tyrosine kinase inhibitors: views of selectivity, sensitivity, and clinical performance. Annu Rev Pharmacol Toxicol. (2013) 53:161–85. doi: 10.1146/annurev-pharmtox-011112-140341
57. Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang YS. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. (2018) 17:36. doi: 10.1186/s12943-018-0801-5
58. Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling [published correction appears in Mol Cancer Ther. Mol Cancer Ther. (2007) 6:667–74. doi: 10.1158/1535-7163.MCT-06-0423. 2008 Nov;7(11):3654].
59. Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–A randomized phase III trial. J Clin Oncol. (2016) 34:443–51. doi: 10.1200/JCO.2015.62.6598
60. Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J Clin Oncol. (2014) 32:2039–49. doi: 10.1200/JCO.2013.53.6136
61. Janjigian YY, Ku GY, Ilson DH, Boyar MS, Capanu M, Chou JF, et al. A phase II study of afatinib in patients (pts) with metastatic human epidermal growth factor receptor (HER2)-positive trastuzumab refractory esophagogastric (EG) cancer. J Clin Oncol. (2015) 33:59–9. doi: 10.1200/jco.2015.33.3_suppl.59
62. Tabernero JM, Strickler JH, Nakamura Y, Shitara K, Janjigian YY, Barzi A, et al. P-156 MOUNTAINEER-02: Phase 2/3 study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma: Trial in progress. Ann Oncol. (2022) 33:S305–6. doi: 10.1016/j.annonc.2022.04.246
63. Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Exploring the next generation of antibody-drug conjugates. Nat Rev Clin Oncol. (2024) 21:203–23. doi: 10.1038/s41571-023-00850-2
64. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. (2022) 7:93. doi: 10.1038/s41392-022-00947-7
65. Liang Y, Zhang P, Li F, Lai H, Qi T, Wang Y. Advances in the study of marketed antibody-drug Conjugates (ADCs) for the treatment of breast cancer. Front Pharmacol. (2024) 14:1332539. doi: 10.3389/fphar.2023.1332539
66. Zhu Y, Zhou M, Kong W, Li C. Antibody-drug conjugates: the clinical development in gastric cancer. Front Oncol. (2023) 13:1211947. doi: 10.3389/fonc.2023.1211947
67. Von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. (2019) 380:617–28. doi: 10.1056/NEJMoa1814017
68. Denkert C, Lambertini C, Fasching PA, Pogue-Geile KL, Mano MS, Untch M, et al. Biomarker Data from the Phase III KATHERINE Study of Adjuvant T-DM1 versus Trastuzumab for Residual Invasive Disease after Neoadjuvant Therapy for HER2-Positive Breast Cancer. Clin Cancer Res. (2023) 29:1569–81. doi: 10.1158/1078-0432.CCR-22-1989
69. Conte B, Fabi A, Poggio F, Blondeaux E, Dellepiane C, D'Alonzo A, et al. Effectiveness of trastuzumab emtansine (TDM1) in patients with HER2-positive advanced breast cancer (ABC) progressing after taxane plus pertuzumab plus trastuzumab. Ann Oncol. (2018) 29:viii105. doi: 10.1093/annonc/mdy272.316
70. Zhang J, Fan J, Zeng X, Nie M, Chen W, Wang Y, et al. Targeting the autophagy promoted antitumor effect of T-DM1 on HER2-positive gastric cancer. Cell Death Disease. (2021) 12:0–0. doi: 10.1038/s41419-020-03349-1
71. Shitara K, Honma Y, Omuro Y, Yamaguchi K, Chin K, Muro K, et al. Efficacy of trastuzumab emtansine in Japanese patients with previously treated HER2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma: A subgroup analysis of the GATSBY study. Asia Pac J Clin Oncol. (2020) 16:5–13. doi: 10.1111/ajco.13243
72. Lee J, Park YH. Trastuzumab deruxtecan for HER2+ advanced breast cancer. Future Oncol. (2022) 18:7–19. doi: 10.2217/fon-2021-0550
73. Von Arx C, De Placido P, Caltavituro A, Di Rienzo R, Buonaiuto R, De Laurentiis M, et al. The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer. Cancer Treat Rev. (2023) 113:102500. doi: 10.1016/j.ctrv.2022.102500
74. Martín M, Pandiella A, Vargas-Castrillón E, Díaz-Rodríguez E, Iglesias-Hernangómez T, Martínez Cano C, et al. Trastuzumab deruxtecan in breast cancer. Crit Rev Oncol Hematol. (2024) 198:104355. doi: 10.1016/j.critrevonc.2024.104355
75. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. (2020) 382:2419–30. doi: 10.1056/NEJMoa2004413
76. Ku GY, Di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, et al. 1205MO Updated analysis of DESTINY-Gastric02: A phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectabl/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Ann Oncol. (2022) 33:S1100. doi: 10.1016/j.annonc.2022.07.1323
77. Van Cutsem E, di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. (2023) 24:744–56. doi: 10.1016/S1470-2045(23)00215-2
78. Yamaguchi K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J Clin Oncol. (2023) 41:816–25. doi: 10.1200/JCO.22.00575
79. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
80. Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M, et al. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. (2022) 29:1335–44. doi: 10.1080/10717544.2022.2069883
81. Yu J, Fang T, Yun C, Liu X, Cai X. Antibody-drug conjugates targeting the human epidermal growth factor receptor family in cancers. Front Mol Biosci. (2022) 9:847835. doi: 10.3389/fmolb.2022.847835
82. Xu Y, Wang Y, Gong J, Zhang X, Peng Z, Sheng X, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. (2021) 24:913–25. doi: 10.1007/s10120-021-01168-7
83. Ha SYY, Anami Y, Yamazaki CM, Xiong W, Haase CM, Olson SD, et al. An enzymatically cleavable tripeptide linker for maximizing the therapeutic index of antibody-drug conjugates. Mol Cancer Ther. (2022) 21:1449–61. doi: 10.1158/1535-7163.MCT-22-0362
84. Chen Z, Yuan J, Xu Y, Zhang C, Li Z, Gong J, et al. From AVATAR mice to patients: RC48-ADC exerted promising efficacy in advanced gastric cancer with HER2 expression. Front Pharmacol. (2022) 12:757994. doi: 10.3389/fphar.2021.757994
85. Wang Y, Gong J, Wang A, Wei J, Peng Z, Wang X, et al. Disitamab vedotin (RC48) plus toripalimab for HER2-expressing advanced gastric or gastroesophageal junction and other solid tumours: a multicentre, open label, dose escalation and expansion phase 1 trial. EClinicalMedicine. (2024) 68:102415. doi: 10.1016/j.eclinm.2023.102415
86. Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). (2021) 41:1173–82. doi: 10.1002/cac2.12214
87. Yardley DA. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int J Breast Cancer. (2013) 2013:137414. doi: 10.1155/2013/137414
88. Kan S, Koido S, Okamoto M, Hayashi K, Ito M, Kamata Y, et al. Up-regulation of HER2 by gemcitabine enhances the antitumor effect of combined gemcitabine and trastuzumab emtansine treatment on pancreatic ductal adenocarcinoma cells. BMC Cancer. (2015) 15:726. doi: 10.1186/s12885-015-1772-1
89. Martin M, Fumoleau P, Dewar JA, Albanell J, Limentani SA, Campone M, et al. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: results from a phase Ib/IIa study. Ann Oncol. (2016) 27:1249–56. doi: 10.1093/annonc/mdw157
90. Cortés J, Diéras V, Lorenzen S, Montemurro F, Riera-Knorrenschild J, Thuss-Patience P, et al. Efficacy and safety of trastuzumab emtansine plus capecitabine vs trastuzumab emtansine alone in patients with previously treated ERBB2 (HER2)-positive metastatic breast cancer: A phase 1 and randomized phase 2 trial. JAMA Oncol. (2020) 6:1203–9. doi: 10.1001/jamaoncol.2020.1796
91. Janjigian YY, Oh D-Y, Rha SY, Lee MH, Steeghs N, Chao Y, et al. Abstract 295: Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. J Clin Oncol. (2022) 40:295–5. doi: 10.1200/JCO.2022.40.4_suppl.295
92. Phillips GD, Fields CT, Li G, Dowbenko D, Schaefer G, Miller K, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. (2014) 20:456–68. doi: 10.1158/1078-0432.CCR-13-0358
93. Miller KD, Diéras V, Harbeck N, Andre F, Mahtani RL, Gianni L, et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J Clin Oncol. (2014) 32:1437–44. doi: 10.1200/JCO.2013.52.6590
94. Bordeau BM, Yang Y, Balthasar JP. Transient competitive inhibition bypasses the binding site barrier to improve tumor penetration of trastuzumab and enhance T-DM1 efficacy. Cancer Res. (2021) 81:4145–54. doi: 10.1158/0008-5472.CAN-20-3822
95. Krop IE, Im SA, Barrios C, Bonnefoi H, Gralow J, Toi M, et al. Trastuzumab emtansine plus pertuzumab versus taxane plus trastuzumab plus pertuzumab after anthracycline for high-risk human epidermal growth factor receptor 2-positive early breast cancer: the phase III KAITLIN study. J Clin Oncol. (2022) 40:438–48. doi: 10.1200/JCO.21.00896
96. Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. (2018) 19:115–26. doi: 10.1016/S1470-2045(17)30716-7
97. Hurvitz SA, Martin M, Jung KH, Huang CS, Harbeck N, Valero V, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. (2019) 37:2206–16. doi: 10.1200/JCO.19.00882
98. Kulukian A, Lee P, Taylor J, Rosler R, de Vries P, Watson D, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. (2020) 19:976–87. doi: 10.1158/1535-7163.MCT-19-0873
99. O’Brien NA, Huang HKT, McDermott MSJ, Madrid AM, Luo T, Ayala R, et al. Tucatinib has selective activity in HER2-positive cancers and significant combined activity with approved and novel breast cancer-targeted therapies. Mol Cancer Ther. (2022) 21:751–61. doi: 10.1158/1535-7163.MCT-21-0847
100. Olson D, Taylor J, Willis K, Hensley K, Allred S, Zaval M, et al. HER2-selective and reversible tyrosine kinase inhibitor tucatinib potentiates the activity of T-DM1 in preclinical models of HER2-positive breast cancer. Cancer Res Commun. (2023) 3:1927–39. doi: 10.1158/2767-9764.CRC-23-0302
101. La Monica S, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Digiacomo G, et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J Exp Clin Cancer Res. (2017) 36:174. doi: 10.1186/s13046-017-0653-7
102. Nicolò E, Giugliano F, Ascione L, Tarantino P, Corti C, Tolaney SM, et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat Rev. (2022) 106:102395. doi: 10.1016/j.ctrv.2022.102395
103. Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. (2011) 128:347–56. doi: 10.1007/s10549-010-1090-x
104. Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, et al. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods. (2014) 65:114–26. doi: 10.1016/j.ymeth.2013.06.035
105. Emens LA, Esteva FJ, Beresford M, Saura C, De Laurentiis M, Kim SB, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. (2020) 21:1283–95. doi: 10.1016/S1470-2045(20)30465-4
106. Waks AG, Keenan TE, Li T, Tayob N, Wulf GM, Richardson ET 3rd, et al. Phase Ib study of pembrolizumab in combination with trastuzumab emtansine for metastatic HER2-positive breast cancer. J Immunother Cancer. (2022) 10:e005119. doi: 10.1136/jitc-2022-005119
107. Guzman G, Reed MR, Bielamowicz K, Koss B, Rodriguez A. CAR-T therapies in solid tumors: opportunities and challenges. Curr Oncol Rep. (2023) 25:479–89. doi: 10.1007/s11912-023-01380-x
108. Korell F, Berger TR, Maus MV. Understanding CAR T cell-tumor interactions: Paving the way for successful clinical outcomes. Med. (2022) 3:538–64. doi: 10.1016/j.medj.2022.05.001
109. Lin YJ, Mashouf LA, Lim M. CAR T cell therapy in primary brain tumors: current investigations and the future. Front Immunol. (2022) 13:817296. doi: 10.3389/fimmu.2022.817296
110. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. (2020) 17:147–67. doi: 10.1038/s41571-019-0297-y
111. Sengsayadeth S, Savani BN, Oluwole O, Dholaria B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. EJHaem. (2021) 3:6–10. doi: 10.1002/jha2.338
112. Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discovery. (2021) 20:531–50. doi: 10.1038/s41573-021-00189-2
113. Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. (2018) 9:867–78. doi: 10.1007/s13238-017-0384-8
114. Forsberg EMV, Lindberg MF, Jespersen H, Alsén S, Bagge RO, Donia M, et al. HER2 CAR-T cells eradicate uveal melanoma and T-cell therapy-resistant human melanoma in IL2 transgenic NOD/SCID IL2 receptor knockout mice. Cancer Res. (2019) 79:899–904. doi: 10.1158/0008-5472.CAN-18-3158
115. Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. (2018) 9:838–47. doi: 10.1007/s13238-017-0440-4
116. Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. (2022) 28:1189–98. doi: 10.1038/s41591-022-01800-8
117. Simon AG, Lyu SI, Laible M, Wöll S, Türeci Ö, Şahin U, et al. The tight junction protein claudin 6 is a potential target for patient-individualized treatment in esophageal and gastric adenocarcinoma and is associated with poor prognosis. J Transl Med. (2023) 21:552. doi: 10.1186/s12967-023-04433-8
118. Entezam M, Sanaei MJ, Mirzaei Y, Mer AH, Abdollahpour-Alitappeh M, Azadegan-Dehkordi F, et al. Current progress and challenges of immunotherapy in gastric cancer: A focus on CAR-T cells therapeutic approach. Life Sci. (2023) 318:121459. doi: 10.1016/j.lfs.2023.121459
119. Igarashi Y, Sasada T. Cancer vaccines: toward the next breakthrough in cancer immunotherapy. J Immunol Res. (2020) 2020:5825401. doi: 10.1155/2020/5825401
120. Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. (2002) 8:3394–400.
121. Maeng HM, Moore BN, Bagheri H, Steinberg SM, Inglefield J, Dunham K, et al. Phase I clinical trial of an autologous dendritic cell vaccine against HER2 shows safety and preliminary clinical efficacy. Front Oncol. (2021) 11:789078. doi: 10.3389/fonc.2021.789078
122. Jung M, Lee JB, Kim HS, Kwon WS, Kim HO, Kim S, et al. First-in-human phase 1 study of a B cell- and monocyte-based immunotherapeutic vaccine against HER2-positive advanced gastric cancer. Cancer Res Treat. (2024) 56:208–18. doi: 10.4143/crt.2022.1328
123. Tobias J, Maglakelidze M, Andrić Z, Ryspayeva D, Bulat I, Nikolić I, et al. Phase II trial of HER-vaxx, a B-cell peptide-based vaccine, in HER2-overexpressing advanced gastric cancer patients under platinum-based chemotherapy (HERIZON). Clin Cancer Res. (2024) 30:4044–54. doi: 10.1158/1078-0432.CCR-24-0742
124. Zhang H, Wang Y, Wang Y, Wu D, Lin E, Xia Q. Intratumoral and intertumoral heterogeneity of HER2 immunohistochemical expression in gastric cancer. Pathol Res Pract. (2020) 216:153229. doi: 10.1016/j.prp.2020.153229
125. Wakatsuki T, Yamamoto N, Sano T, Chin K, Kawachi H, Takahari D, et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J Gastroenterol. (2018) 53:1186–95. doi: 10.1007/s00535-018-1464-0
126. Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. (2016) 53:42–50. doi: 10.1016/j.ejca.2015.09.018
127. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1005–20. doi: 10.1016/j.annonc.2022.07.00
128. Yoon J, Oh DY. HER2-targeted therapies beyond breast cancer-an update. Nat Rev Clin Oncol. (2024) 21:675–700. doi: 10.1038/s41571-024-00924-9
129. Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y, et al. Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT study). J Clin Oncol. (2020) 38:1919–27. doi: 10.1200/JCO.19.03077
130. Seo S, Ryu MH, Park YS, Ahn JY, Park Y, Park SR, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. (2019) 22:527–35. doi: 10.1007/s10120-018-0891-1
131. Nami B, Wang Z. HER2 in breast cancer stemness: A negative feedback loop towards trastuzumab resistance. Cancers (Basel). (2017) 9:40. doi: 10.3390/cancers9050040
132. Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. (2007) 99:628–38. doi: 10.1093/jnci/djk134
133. Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. (2009) 15:7479–91. doi: 10.1158/1078-0432.CCR-09-0636
134. Mercogliano MF, De Martino M, Venturutti L, Rivas MA, Proietti CJ, Inurrigarro G, et al. TNFα-induced mucin 4 expression elicits trastuzumab resistance in HER2-positive breast cancer. Clin Cancer Res. (2017) 23:636–48. doi: 10.1158/1078-0432.CCR-16-0970
135. Nagy P, Friedländer E, Tanner M, Kapanen AI, Carraway KL, Isola J, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. (2005) 65:473–82. doi: 10.1158/0008-5472.473.65.2
136. Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. (2023) 22:138. doi: 10.1186/s12943-023-01827-6
137. Fernandes M, Jamme P, Cortot AB, Kherrouche Z, Tulasne D. When the MET receptor kicks in to resist targeted therapies. Oncogene. (2021) 40:4061–78. doi: 10.1038/s41388-021-01835-0
138. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in HER2-positive advanced gastric cancer: exploratory biomarker analysis of the randomized, phase 2 DESTINY-Gastric01 trial. Nat Med. (2024) 30:1933–42. doi: 10.1038/s41591-024-02992-x
139. Ha SY, Lee J, Jang J, Hong JY, Do IG, Park SH, et al. HER2-positive gastric cancer with concomitant MET and/or EGFR overexpression: a distinct subset of patients for dual inhibition therapy. Int J Cancer. (2015) 136:1629–35. doi: 10.1002/ijc.29159
140. Takahashi N, Furuta K, Taniguchi H, Sasaki Y, Shoji H, Honma Y, et al. Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget. (2016) 7:4925–38. doi: 10.18632/oncotarget.6753
141. Ebert K, Mattes J, Kunzke T, Zwingenberger G, Luber B. MET as resistance factor for afatinib therapy and motility driver in gastric cancer cells. PloS One. (2019) 14:e0223225. doi: 10.1371/journal.pone.0223225
142. Zhang H, Zhang L, He Y, Jiang D, Sun J, Luo Q, et al. PI3K PROTAC overcomes the lapatinib resistance in PIK3CA-mutant HER2 positive breast cancer. Cancer Lett. (2024) 598:217112. doi: 10.1016/j.canlet.2024.217112
143. Wu W, Chen X, Liu X, Bao HJ, Li QH, Xian JY, et al. SNORD60 promotes the tumorigenesis and progression of endometrial cancer through binding PIK3CA and regulating PI3K/AKT/mTOR signaling pathway. Mol Carcinog. (2023) 62:413–26. doi: 10.1002/mc.23495
144. Peng K, Liu Y, Liu S, Wang Z, Zhang H, He W, et al. Targeting MEK/COX-2 axis improve immunotherapy efficacy in dMMR colorectal cancer with PIK3CA overexpression. Cell Oncol (Dordr). (2024) 47:1043–58. doi: 10.1007/s13402-024-00916-y
145. Choi S, Kim H, Heo YJ, Kang SY, Ahn S, Lee J, et al. PIK3CA mutation subtype delineates distinct immune profiles in gastric carcinoma. J Pathol. (2023) 260:443–54. doi: 10.1002/path.6134
146. Stanland LJ, Ang HX, Hoj JP, Chu Y, Tan P, Wood KC, et al. CBF-beta mitigates PI3K-alpha-specific inhibitor killing through PIM1 in PIK3CA-mutant gastric cancer. Mol Cancer Res. (2023) 21:1148–62. doi: 10.1158/1541-7786.MCR-23-0034
147. Yao J, You Q, Zhang X, Zhang Y, Xu J, Zhao X, et al. PIK3CA somatic mutations as potential biomarker for immunotherapy in elder or TP53 mutated gastric cancer patients. Clin Genet. (2023) 103:200–8. doi: 10.1111/cge.14260
148. Zhang Y, Yang L, Ou Y, Hu R, Du G, Luo S, et al. Combination of AAV-delivered tumor suppressor PTEN with anti-PD-1 loaded depot gel for enhanced antitumor immunity. Acta Pharm Sin B. (2024) 14:350–64. doi: 10.1016/j.apsb.2023.06.006
149. Zhang X, Park JS, Park KH, Kim KH, Jung M, Chung HC, et al. PTEN deficiency as a predictive biomarker of resistance to HER2-targeted therapy in advanced gastric cancer. Oncology. (2015) 88:76–85. doi: 10.1159/000366426
150. Deguchi Y, Okabe H, Oshima N, Hisamori S, Minamiguchi S, Muto M, et al. PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer. (2017) 20:416–27. doi: 10.1007/s10120-016-0627-z
151. Acharya S, Thakur B, Mittal R. Immunoexpression of PTEN, HER2/neu, and Ki-67 in endoscopic gastric carcinoma biopsies; their correlation with histological subtypes and one-year survival. Indian J Pathol Microbiol. (2022) 65:29–34. doi: 10.4103/IJPM.IJPM_299_20
152. Zhu L, Li Z, Yu X, Ruan Y, Shen Y, Shao Y, et al. The tRNA-derived fragment 5026a inhibits the proliferation of gastric cancer cells by regulating the PTEN/PI3K/AKT signaling pathway. Stem Cell Res Ther. (2021) 12:418. doi: 10.1186/s13287-021-02497-1
153. Jasim SA, Ahmed AT, Kubaev A, Kyada A, Alshahrani MY, Sharma S, et al. Exosomal microRNA as a key regulator of PI3K/AKT pathways in human tumors. Med Oncol. (2024) 41:265. doi: 10.1007/s12032-024-02529-9
154. Raji L, Tetteh A, Amin ARMR. Role of c-src in carcinogenesis and drug resistance. Cancers (Basel). (2023) 16:32. doi: 10.3390/cancers16010032
155. Abousawan J, Orofiamma LA, Fairn GD, Antonescu CN. HER2 expression defines unique requirements for flotillin and c-Src in EGFR signaling. J Cell Sci. (2023) 136:jcs260133. doi: 10.1242/jcs.260133
156. Lee YY, Kim HP, Kang MJ, Cho BK, Han SW, Kim TY, et al. Phosphoproteomic analysis identifies activated MET-axis PI3K/AKT and MAPK/ERK in lapatinib-resistant cancer cell line. Exp Mol Med. (2013) 45:e64. doi: 10.1038/emm.2013.115
157. Peiró G, Ortiz-Martínez F, Gallardo A, Pérez-Balaguer A, Sánchez-Payá J, Ponce JJ, et al. Src, a potential target for overcoming trastuzumab resistance in HER2-positive breast carcinoma. Br J Cancer. (2014) 111:689–95. doi: 10.1038/bjc.2014.327
158. Jin MH, Nam AR, Park JE, Bang JH, Bang YJ, Oh DY, et al. Resistance mechanism against trastuzumab in HER2-positive cancer cells and its negation by src inhibition. Mol Cancer Ther. (2017) 16:1145–54. doi: 10.1158/1535-7163.MCT-16-0669
159. Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. (2021) 6:307. doi: 10.1038/s41392-021-00701-5
160. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. (2022) 7:3. doi: 10.1038/s41392-021-00762-6
161. Hao X, Zheng J, Yu X, Li Z, Ren G. [amp]]beta;-catenin promotes resistance to trastuzumab in breast cancer cells through enhancing interaction between HER2 and SRC. Acta Biochim Pol. (2023) 70:261–9. doi: 10.18388/abp.2020_6357
162. Liu W, Yuan J, Liu Z, Zhang J, Chang J. Label-free quantitative proteomics combined with biological validation reveals activation of wnt/β-catenin pathway contributing to trastuzumab resistance in gastric cancer. Int J Mol Sci. (2018) 19:1981. doi: 10.3390/ijms19071981
163. Kim Y, Bae YJ, Kim JH, Kim H, Shin SJ, Jung DH, et al. Wnt/β-catenin pathway is a key signaling pathway to trastuzumab resistance in gastric cancer cells. BMC Cancer. (2023) 23:922. doi: 10.1186/s12885-023-11447-4
164. Wang N, Zhu L, Wang L, Shen Z, Huang X. Identification of SHCBP1 as a potential biomarker involving diagnosis, prognosis, and tumor immune microenvironment across multiple cancers. Comput Struct Biotechnol J. (2022) 20:3106–19. doi: 10.1016/j.csbj.2022.06.039
165. Siraj AK, Poyil PK, Padmaja D, Parvathareddy SK, Alobaisi K, Thangavel S, et al. PLK1 and PARP positively correlate in Middle Eastern breast cancer and their combined inhibition overcomes PARP inhibitor resistance in triple negative breast cancer. Front Oncol. (2024) 13:1286585. doi: 10.3389/fonc.2023.1286585
166. Dong YD, Yuan YL, Yu HB, Tian GJ, Li DY. SHCBP1 is a novel target and exhibits tumor−promoting effects in gastric cancer. Oncol Rep. (2019) 41:1649–57. doi: 10.3892/or.2018.6952
167. Li Z, Zhao H, Hu H, Shang H, Ren Y, Qiu W, et al. Mechanisms of resistance to trastuzumab in HER2-positive gastric cancer. Chin J Cancer Res. (2024) 36:306–21. doi: 10.21147/j.issn.1000-9604.2024.03.07
168. Shi W, Zhang G, Ma Z, Li L, Liu M, Qin L, et al. Hyperactivation of HER2-SHCBP1-PLK1 axis promotes tumor cell mitosis and impairs trastuzumab sensitivity to gastric cancer. Nat Commun. (2021) 12:2812. doi: 10.1038/s41467-021-23053-8
169. Kalim M, Chen J, Wang S, Lin C, Ullah S, Liang K, et al. Intracellular trafficking of new anticancer therapeutics: antibody-drug conjugates. Drug Des Devel Ther. (2017) 11:2265–76. doi: 10.2147/DDDT.S135571
170. Baldassarre T, Truesdell P, Craig AW. Endophilin A2 promotes HER2 internalization and sensitivity to trastuzumab-based therapy in HER2-positive breast cancers. Breast Cancer Res. (2017) 19:110. doi: 10.1186/s13058-017-0900-z
171. Sung M, Tan X, Lu B, Golas J, Hosselet C, Wang F, et al. Caveolae-mediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1). Mol Cancer Ther. (2018) 17:243–53. doi: 10.1158/1535-7163.MCT-17-0403
172. Chung YC, Chang CM, Wei WC, Chang TW, Chang KJ, Chao WT. Metformin-induced caveolin-1 expression promotes T-DM1 drug efficacy in breast cancer cells. Sci Rep. (2018) 8:3930. doi: 10.1038/s41598-018-22250-8
173. Indira Chandran V, Månsson AS, Barbachowska M, Cerezo-Magaña M, Nodin B, Joshi B, et al. Hypoxia attenuates trastuzumab uptake and trastuzumab-emtansine (T-DM1) cytotoxicity through redistribution of phosphorylated caveolin-1. Mol Cancer Res. (2020) 18:644–56. doi: 10.1158/1541-7786.MCR-19-0856
174. Ríos-Luci C, García-Alonso S, Díaz-Rodríguez E, Nadal-Serrano M, Arribas J, Ocaña A, et al. Resistance to the antibody-drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res. (2017) 77:4639–51. doi: 10.1158/0008-5472.CAN-16-3127
175. Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol. (2015) 77:57–80. doi: 10.1146/annurev-physiol-021014-071649
176. Wang H, Wang W, Xu Y, Yang Y, Chen X, Quan H, et al. Aberrant intracellular metabolism of T-DM1 confers T-DM1 resistance in human epidermal growth factor receptor 2-positive gastric cancer cells. Cancer Sci. (2017) 108:1458–68. doi: 10.1111/cas.13253
177. Hamblett KJ, Jacob AP, Gurgel JL, Tometsko ME, Rock BM, Patel SK, et al. SLC46A3 is required to transport catabolites of noncleavable antibody maytansine conjugates from the lysosome to the cytoplasm. Cancer Res. (2015) 75:5329–40. doi: 10.1158/0008-5472.CAN-15-1610
178. Sajid A, Rahman H, Ambudkar SV. Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters. Nat Rev Cancer. (2023) 23:762–79. doi: 10.1038/s41568-023-00612-3
179. Yu M, Ocana A, Tannock IF. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer Metastasis Rev. (2013) 32:211–27. doi: 10.1007/s10555-012-9402-8
180. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. (2021) 18:327–44. doi: 10.1038/s41571-021-00470-8
181. Vig S, Srivastava P, Rahman I, Jaranson R, Dasgupta A, Perttilä R, et al. Screening of photosensitizers-ATP binding cassette (ABC) transporter interactions in vitro. Cancer Drug Resist. (2024) 7:35. doi: 10.20517/cdr.2024.50
182. Yu SF, Zheng B, Go M, Lau J, Spencer S, Raab H, et al. A novel anti-CD22 anthracycline-based antibody-drug conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. (2015) 21:3298–306. doi: 10.1158/1078-0432.CCR-14-2035
183. Weng W, Meng T, Zhao Q, Shen Y, Fu G, Shi J, et al. Antibody-exatecan conjugates with a novel self-immolative moiety overcome resistance in colon and lung cancer. Cancer Discovery. (2023) 13:950–73. doi: 10.1158/2159-8290.CD-22-1368
184. Chang J, Wang Q, Bhetuwal A, Liu W. Metabolic pathways underlying GATA6 regulating Trastuzumab resistance in Gastric Cancer cells based on untargeted metabolomics. Int J Med Sci. (2020) 17:3146–64. doi: 10.7150/ijms.50563
185. Yao X, He Z, Qin C, Zhang P, Sui C, Deng X, et al. Inhibition of PFKFB3 in HER2-positive gastric cancer improves sensitivity to trastuzumab by inducing tumour vessel normalisation. Br J Cancer. (2022) 127:811–23. doi: 10.1038/s41416-022-01834-2
186. Hu X, Ma Z, Xu B, Li S, Yao Z, Liang B, et al. Glutamine metabolic microenvironment drives M2 macrophage polarization to mediate trastuzumab resistance in HER2-positive gastric cancer. Cancer Commun (Lond). (2023) 43:909–37. doi: 10.1002/cac2.12459
187. Castagnoli L, Corso S, Franceschini A, Raimondi A, Bellomo SE, Dugo M, et al. Fatty acid synthase as a new therapeutic target for HER2-positive gastric cancer. Cell Oncol (Dordr). (2023) 46:661–76. doi: 10.1007/s13402-023-00769-x
188. Borlongan MC, Saha D, Wang H. Tumor microenvironment: A niche for cancer stem cell immunotherapy. Stem Cell Rev Rep. (2024) 20:3–24. doi: 10.1007/s12015-023-10639-6
189. Pu Q, Gao H. The role of the tumor microenvironment in triple-positive breast cancer progression and therapeutic resistance. Cancers (Basel). (2023) 15:5493. doi: 10.3390/cancers15225493
Keywords: gastric cancer, HER2, targeted therapy, cell therapy, drug resistance
Citation: Shao Q, Deng J, Wu H and Huang Z (2025) HER2-positive gastric cancer: from targeted therapy to CAR-T cell therapy. Front. Immunol. 16:1560280. doi: 10.3389/fimmu.2025.1560280
Received: 14 January 2025; Accepted: 27 February 2025;
Published: 13 March 2025.
Edited by:
Rahul Shivahare, The Ohio State University, United StatesReviewed by:
Dileep Nair, RWTH Aachen University, GermanyCopyright © 2025 Shao, Deng, Wu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeping Huang, TGR5eV9odWFuZ3pwQGx6dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.