
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 15 April 2025
Sec. Inflammation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1554787
 Ancuta Lupu1
Ancuta Lupu1 Cristina Gavrilovici1
Cristina Gavrilovici1 Cristina Maria Mihai2*
Cristina Maria Mihai2* Denisa Claudia Tonco1*
Denisa Claudia Tonco1* Alin Horatiu Nedelcu3
Alin Horatiu Nedelcu3 Leonard Pertea1
Leonard Pertea1 Tatiana Chisnoiu2
Tatiana Chisnoiu2 Ginel Baciu4
Ginel Baciu4 Ramona Mihaela Stoicescu2
Ramona Mihaela Stoicescu2 Delia Lidia Salaru3
Delia Lidia Salaru3 Minerva Codruta Badescu3
Minerva Codruta Badescu3 Magdalena Cuciureanu3
Magdalena Cuciureanu3 Olga Cirstea5
Olga Cirstea5 Vasile Valeriu Lupu1
Vasile Valeriu Lupu1This narrative review aims to analyze and compare the current literature on multisystem inflammatory syndrome in children (MIS-C) and Kawasaki disease (KD), with a focus on case definitions, clinical features, diagnostic approaches, treatment strategies, and outcomes. Through a comprehensive review of relevant studies, including screening titles, abstracts, and full-text articles, key similarities and differences were identified. Both MIS-C and KD involve immune system dysregulation and share clinical manifestations such as rash, gastrointestinal symptoms, and cardiovascular involvement, with treatments often centered around immunomodulatory therapies. However, significant differences were observed, particularly in terms of age distribution, demographic prevalence, clinical presentation, and diagnostic criteria, with KD primarily affecting younger children and being associated more prominently with coronary artery abnormalities. While both diseases raise concerns about severe cardiac involvement and the need for intensive care, their pathogenic mechanisms have not been fully understood. Ongoing research is critical to elucidating these mechanisms, refining diagnostic criteria, and optimizing therapeutic approaches to improve outcomes for affected children. This comparative analysis is essential for advancing the understanding of both conditions, as accurately distinguishing between MIS-C and KD has significant implications for clinical decision-making and patient management. Given their overlapping yet distinct clinical features, precise differentiation is critical for ensuring timely diagnosis, optimizing therapeutic strategies, and improving patient outcomes. The concern among pediatric patients stems from the potential for severe complications, particularly cardiac involvement, which underscores the need for heightened awareness, early recognition, and evidence-based treatment strategies to minimize long-term morbidity and mortality.
Multisystem inflammatory syndrome in children (MIS-C) is a hyperinflammatory condition that affects children and adolescents 2–6 weeks after SARS-CoV-2 infection. It primarily affects children aged 6–12 years and shows a predominance in male individuals (1). The first cases of this syndrome were reported in the United Kingdom, Italy, and the United States. Verdoni and colleagues first described it in 2020, detailing seven boys and three girls aged 5–7 years in Bergamo, Italy, who were suffering from a Kawasaki-like disease (2). This condition had a higher incidence from February to April 2020 than Kawasaki disease (KD) registered in the previous 5 years (3). In 2019, following the spread of coronavirus globally, there were reported increases in cases of children presenting with a Kawasaki-like disease that could also be accompanied by prolonged fever, rash, and conjunctivitis (4).
The first case definition was issued by the Centers for Disease Control and Prevention (CDC) in 2020. Using this case definition, the incidence in the United States was estimated at 5.1 cases per million people per month from April to June. Most of the cases were among non-Hispanic Black, Hispanic, or non-Hispanic Asians compared to non-Hispanic white individuals (5). In 2022, the CDC and the Council of State and Territorial Epidemiologists (CSTE) created a new surveillance case definition that went into effect on January 1, 2023. Approximately 87% of cases reported met the 2023 case definition (6). The condition was initially termed pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) (5, 7). This definition was formulated by experts in the United Kingdom and published by the Royal College of Paediatrics and Child Health, with a corresponding article in The Lancet on May 7, 2020. The publication detailed nine children in south London with PIMS-TS requiring critical care, highlighting the severe nature of the disease. Prior to this, it was known that children infected with SARS-CoV-2 typically experienced minimal symptoms (8). The emergence of this novel syndrome has raised significant concern among healthcare professionals and parents, as it shares clinical features with toxic shock syndrome (TSS), secondary hemophagocytic lymphohistiocytosis (SHLH), macrophage activation syndrome (MAS), and KD. KD, also called mucocutaneous lymph node syndrome, is an acute and usually self-limiting vasculitis of the small and medium caliber arteries, which almost exclusively affects children below 5 years of age (9, 10). It is a leading cause of acquired heart disease in children (11). This condition is characterized by an acute, self-limiting vasculitis that particularly targets the coronary arteries in previously healthy young infants and children. Although it has been over 50 years since Dr. Tomisaku Kawasaki first identified this disease in Japan in 1967, its exact cause remains unclear. The prevailing hypothesis suggests that it may stem from an abnormal immune response to one or more unknown pathogens in genetically predisposed individuals. In the acute phase of the disease, patients with KD may have a condition known as Kawasaki disease shock syndrome (KDSS), which includes hemodynamic instability. Other patients with KD may fulfill the criteria of MAS, resembling secondary hemophagocytic lymphohistiocytosis (12).
We conducted an extensive narrative review comparing MIS-C and KD, analyzing data from clinical studies and reviews. Completed over a 6-month period, this review encompasses various aspects of both conditions, from etiology to treatment methods. We searched major international databases for relevant articles using the keywords “MIS-C and Kawasaki disease”, “Kawasaki-like disease”, and “multisystemic inflammatory syndrome due to SARS-CoV-2” combined with “clinical comparison” and “pediatric”. Furthermore, we conducted a manual review of eligible original articles by assessing the references from the initial search results, reviews, and other relevant literature.
MIS-C is a condition that appears 2–6 weeks after the SARS-CoV-2 infection and affects children aged 6–12 years, with a prevalence for the male gender. In 2023, the median age for MIS-C was 7 years, a shift to a younger age group compared to that in 2022 when the median age was reported to be 9 years. Race may be a potential risk factor, as a higher incidence of MIS-C has been observed in children of African, Hispanic, or South Asian origin (13). Studies from Japan and South Korea have reported a lower incidence of MIS-C, as well as fewer cases requiring intensive care and no deaths. Of these cases, 80% were encountered during the Omicron period (14, 15). The CDC from the United States reports that the incidence is declining from a peak of 6.79 cases per million persons in 2021 to 0.11 cases per million persons in 2023, with a high incidence during the fall when the overall number of COVID-19 cases was rising (6). In 2023, 80% of the US-reported cases were vaccine-eligible but were unvaccinated children (16). Many children with MIS-C have had fewer or no symptoms of COVID-19. In the pediatric population, the risk of severe COVID-19 was elevated in neonates, preterm infants, and children with underlying health conditions (17). Among these, obesity was the most commonly encountered comorbidity (18), along with diabetes, heart and neurological diseases, chronic lung conditions, asthma, and compromised immune systems (17). Recent findings suggest that SARS-CoV-2 infection may trigger the onset of type 1 and type 2 diabetes in children, which is a common chronic metabolic disorder in this age group. Some studies have shown that in patients with SARS-CoV-2 infection, those with diabetes had a higher likelihood of hospitalization. Furthermore, the data indicated that diabetes and other cardiovascular conditions are significant risk factors for adverse outcomes and mortality in COVID-19 patients (19, 20). The lower expression of the angiotensin-converting enzyme (ACE)-2 receptor gene, which is the target of SARS-CoV-2, may partly explain why children tend to experience milder COVID-19 symptoms compared to adults. This, along with fewer comorbidities and a trained innate immune system, likely contributes to their reduced disease severity (21). While multisystemic inflammatory syndrome is linked to COVID-19, the cause of KD remains unknown (22, 23). Experts suggest that a prior viral infection may be responsible. This self-limiting condition shows higher prevalence during winter and spring. Occurring more frequently in male individuals, KD mainly affects children under 5 years old, with the highest incidence between 18 and 24 months, particularly in predominantly White communities (3, 22, 23). Female individuals are affected approximately one-third as often as male individuals, and infants younger than 4 months are rarely impacted. The condition is most prevalent among children of Japanese and Asian descent compared to other regions globally (24). In East Asian countries, MIS-C remains relatively rare, whereas KD has the highest incidence globally (14). Studies from Japan have also indicated a higher occurrence in children with a family history of KD compared to the general population (8). While the etiology remains unknown, many case reports have linked KD with many viral agents like cytomegalovirus, adenovirus, rhinovirus, enterovirus, and bocavirus (25, 26). However, there are studies that have compared the X-ray findings in Mycoplasma pneumoniae infection with other types of pneumonia developed in KD, which show results that dispute the viral etiology theory (10, 23). While some studies have suggested a possible link between KD and environmental factors such as fungal toxins and pollution (27), no research has definitively confirmed this association (28–30).
Studies of both KD and MIS-C have shown increased interleukin (IL)-1, IL-6, and IL-18 secretion as well as signaling in tumor necrosis factor (TNF) and interferon-gamma (IFN-γ) pathways that cause the release of various cytokines that can further lead to cytokine storms (31, 32).
In MIS-C, residual COVID-19 viral particles persist in infected tissues, including myocardial cells, even after the virus has been cleared from the body. Viral RNA was detected in 35% of myocardial infarctions in individuals with another SARS-CoV. The direct viral impact is attributed to the interaction between the virus and ACE-2 receptors, which are found on the surface of endothelial and endocardial cells (21). These viral particles trigger a form of molecular mimicry, causing the immune system to mount an exaggerated inflammatory response (32) (Figure 1). The activation of monocytes and macrophages leads to a predominance of neutrophils. Carter et al. reported increased neutrophil and monocyte activation in the acute phase of MIS-C, indicated by high CD64 expression. This aligns with the findings of upregulated CD54 and CD64 in these cells. Additionally, antigen-presenting cells, including monocytes, dendritic cells, and B cells, exhibit low CD86 and HLA-DR expression, suggesting impaired antigen presentation. Monocyte profiling may help distinguish MIS-C from severe COVID-19, as patrolling monocytes dominate in MIS-C, whereas HLA-DRlo classical monocytes are more prevalent in severe COVID-19 (33). Complement activation, likely through the lectin pathway, has been observed in some MIS-C patients, as evidenced by elevated soluble C5b-9 levels. In MIS-C, CD8+ T-cell counts are significantly lower compared to those in children with mild SARS-CoV-2 infection. However, many of the remaining T cells exhibit activation markers, such as CD38 and HLA-DR. This is the main difference between MIS-C and TSS, which is typically triggered by bacterial superantigens like staphylococcal enterotoxin B and streptococcal mitogenic exotoxin Z. Although clinically they share similarities, in TSS, superantigen exposure leads to widespread T-cell activation and excessive proinflammatory cytokine release, resulting in multiorgan damage—paralleling the immune dysregulation seen in MIS-C (34). Regarding the B-cell response, patients with MIS-C exhibit reduced levels of total, effector, and memory B cells compared to healthy individuals, along with an increase in circulating plasmablasts. These short-lived plasmablasts may produce autoantibodies targeting self-antigens. Also, Gruber et al. identified anti-La autoantibodies in MIS-C patients, a finding commonly associated with systemic lupus erythematosus (SLE) and Sjogren’s disease. These findings suggest a potential autoimmune component in MIS-C pathogenesis (34, 35). The resulting inflammation and cytokine release cause cardiac damage as the immune system tries to eliminate the remaining viral particles. The exaggerated T-cell response to the SARS-CoV-2 spike glycoprotein leads to the formation of autoantibodies against cardiovascular, gastrointestinal, and endothelial antigens (33). The activation of IFN-γ increases human leukocyte antigen presentation in tissues, creating a more “sensitized” immune response. This, along with the activation of the IL-1 and IFN-γ pathways, triggers a response from CD8 cytotoxic T cells. These T cells migrate to the heart and other organs, damaging the tissues where the virus is present. Studies have shown that total T-cell frequencies were lower in both MIS-C and KD compared to healthy children, but MIS-C has a higher subpopulation of central memory and effector memory CD4+ T cells and a lower subpopulation of naïve CD4+ T cells when compared to KD. The levels of follicular helper T cells, which support B-cell responses, and CD8+ T cells were decreased in patients with multisystem inflammatory syndrome (36).
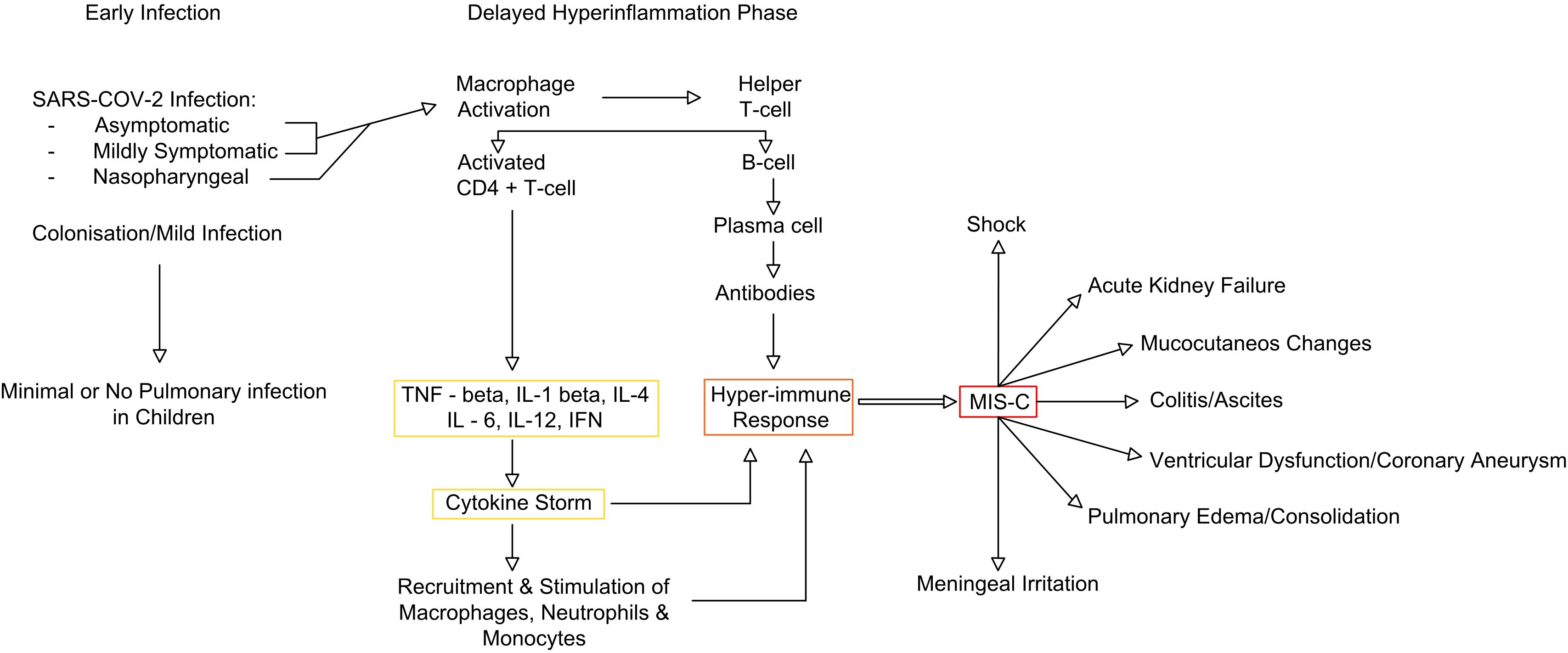
Figure 1. MIS-C immune pathway (adapted from 35). MIS-C, multisystem inflammatory syndrome in children.
KD is an acute vasculitis that affects medium-sized arteries including the coronary arteries (35). Although vascular inflammation is most pronounced in the coronary vessels, vasculitis can also occur in veins, capillaries, small arterioles, and larger arteries (37, 38). The underlying pathogenic mechanism is unknown. An exaggerated immune reaction is implicated, including both innate and adaptive systems, following a possible viral infection (39). Immune complexes are formed, which signal the proliferation of monocytes and macrophages, resulting in neutrophilia, a CD8+ lymphocyte proliferation, and an increased level of immunoglobulin A (IgA)-producing plasma cells. Apart from the immune complex reactions, IgA plays a role in KD, and studies have suggested that elevated IgA levels correlate with coronary artery involvement (40). Current research indicates that patients with MIS-C exhibit elevated levels of IgA and immunoglobulin G (IgG). The IgA elevated levels are linked with the immune responses of the gastrointestinal tract. Children with MIS-C develop a stronger IgA response in comparison to children with COVID-19, while IgG and immunoglobulin M responses are similar in both groups. The presence of several IgG and IgA autoantigens—some of which are associated with autoimmune diseases like SLE, Sjogren’s disease, polymyositis, and dermatomyositis—were also reported (41). A link cannot yet be established between lower or higher levels of IgA and the severity or frequency of complications in this pathology at pediatric age (42, 43).
Several proteins are involved in the pathogenesis of KD; N-terminal prohormone of brain natriuretic peptide (BNP) can be a potential biomarker for myocardial injury. Tenascin-C, an extracellular glycoprotein, can be used as a predictor of coronary artery aneurysm (CAA) development (44, 45). The inflammatory cells secrete various cytokines, ILs, and matrix metalloproteinases, which lead to the damage of internal elastic lamina and necrosis of smooth muscle cells. IL-1 appears to be the key factor in the pathogenesis of the disease. During the acute phase, high concentrations of IL-1 have been identified in the peripheral blood. The severe inflammation caused by immune complexes triggers a cytokine release that results in organ injury (46). High concentrations of TNF-α had also been identified in the peripheral blood of patients diagnosed in the acute phase (44).
As a conclusion to this subchapter, MIS-C and KD exhibit distinct immunological profiles despite some overlapping features. MIS-C is thought to involve a superantigen-driven mechanism, whereas KD likely results from an immune response to a conventional antigen (47). Additionally, MIS-C is characterized by differences in T-cell subsets, including lower levels of naïve CD4+ and follicular helper T cells but higher levels of central and effector memory CD4+ T cells compared to those in KD. Whether these syndromes are distinct or part of a shared immunopathogenic spectrum remains under investigation (33).
Multisystem clinical findings common to MIS-C and KD—such as rash, fever, gastrointestinal tract abnormalities, swollen lymph nodes, headaches, and fatigue—also overlap with clinical manifestations triggered by other pediatric viral illnesses (48).
For patients diagnosed with multisystemic inflammatory syndrome, the most common symptom is a fever that lasts for more than 24 hours. It is usually associated with at least one of the following: abdominal pain, vomiting, diarrhea, skin rash, and mucocutaneous lesions (i.e., conjunctivitis). In severe cases, children can present with hypotension and shock (49). The signs and symptoms of MIS-C vary among children, and some may exhibit symptoms not mentioned here. MIS-C can develop weeks after a child has been infected with SARS-CoV-2, even if the infection was asymptomatic or unknown to the child and caregivers. Gastrointestinal inflammation in MIS-C can present with abdominal pain, vomiting, and diarrhea, sometimes leading to a misdiagnosis of acute appendicitis. Neck pain is also reported and may be accompanied by phlegmon visible on radiographic imaging. Neurologic involvement in children with MIS-C is usually transient, manifesting as headaches, myalgia, or fatigue (50, 51). Although rare, severe neurologic symptoms can include encephalopathy, stroke, demyelination, fulminant cerebral edema, Guillain–Barre syndrome, benign intracranial hypertension, meningoencephalitis, and acute disseminated encephalomyelitis (20, 50, 52). Cerebral venous thrombosis, in particular, is closely associated with COVID-19, and its underlying mechanisms include immune system dysregulation, cytokine storms, increased blood viscosity, thrombogenesis, hypercoagulability, and inflammation (49, 53).
Fever is also the most common sign in KD. It is usually higher than 39°C, and it can be persistent for approximately 3 weeks without treatment. Conjunctival injection can be present, bilateral, and non-purulent. A careful history may indicate the presence of signs and symptoms like conjunctival injection before the hospital presentation. Other important signs include cracking of the lips, erythema, a strawberry-colored tongue, and oral ulceration, none of which can definitively diagnose KD. The lymphadenopathy, if present, is usually unilateral. The rash onset occurs 5 days after the fever, and it can be polymorphous, usually being maculopapular or scarlatiniform. It is usually expressed on the trunk and extremities and can undergo desquamation in its evolution (54).
Patients with MIS-C commonly present with prominent cardiac involvement manifested by left ventricular (LV) systolic and diastolic dysfunction, myocardial inflammation, and coronary artery dilations. LV dysfunction is more severe in patients with MIS-C than in patients with KD (48). Although ventricular dysfunction and CAAs are the predominant risk factors for morbidity in patients with multisystemic inflammatory syndrome, the mechanism is not completely known (33). Some clinical studies have shown the presence of the SARS-CoV-2 virus in the heart, lungs, and kidneys of patients with severe cardiac involvement with or without underlying conditions prior to the viral infection (55, 56). In contrast with MIS-C patients, who generally experience fewer coronary artery abnormalities that are usually transient and resolve quickly, KD primarily affects the coronary arteries, resulting in dilation and aneurysms (as seen in Table 1). While small to moderate CAAs in KD may take up to 2 years to normalize, the rapid resolution of cardiovascular complications in MIS-C after the acute phase suggests that these issues are likely due to hyperinflammation causing capillary leakage and vasodilation, rather than direct immune cell damage to the myocardium (57, 58). KD can also lead to myocarditis, which is associated with arrhythmias and potential long-term fibrosis (59).
The differences from the 2020 CDC case definition for MIS-C include the removal of the fever duration requirement and the specification of systemic inflammation as indicated by a C-reactive protein level of ≥3.0 mg/dL. Additionally, respiratory, renal, and neurologic systems are no longer included in the organ involvement criteria, while shock has been added as a distinct organ system manifestation. SARS-CoV-2 testing criteria now include time parameters, such as viral testing within 60 days of MIS-C hospitalization or serologic testing during the illness (32). The diagnosis criteria encompass clinical signs and symptoms, laboratory tests, and epidemiologic linkage, as detailed in Tables 2–4.
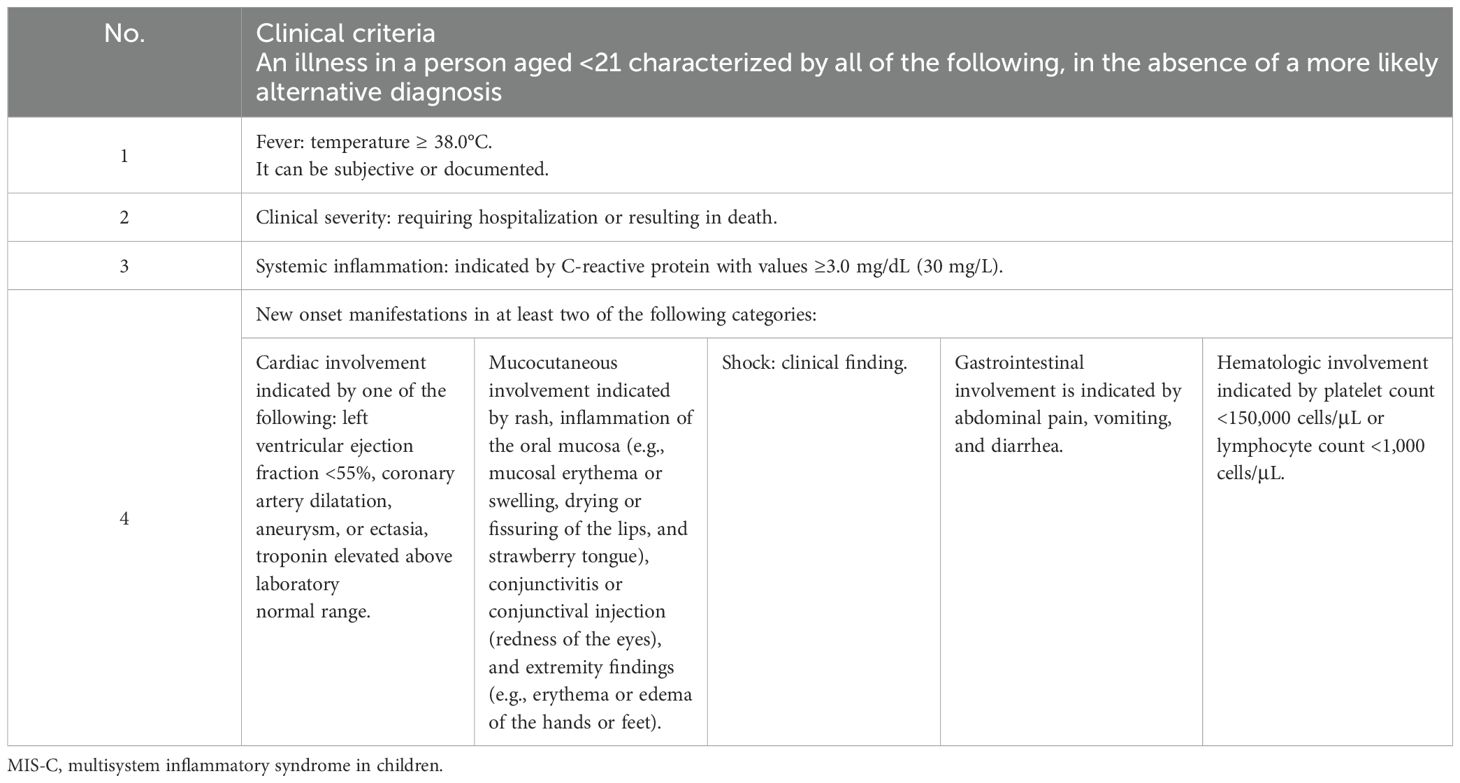
Table 2. MIS-C—clinical diagnosis criteria [adapted from (60)].
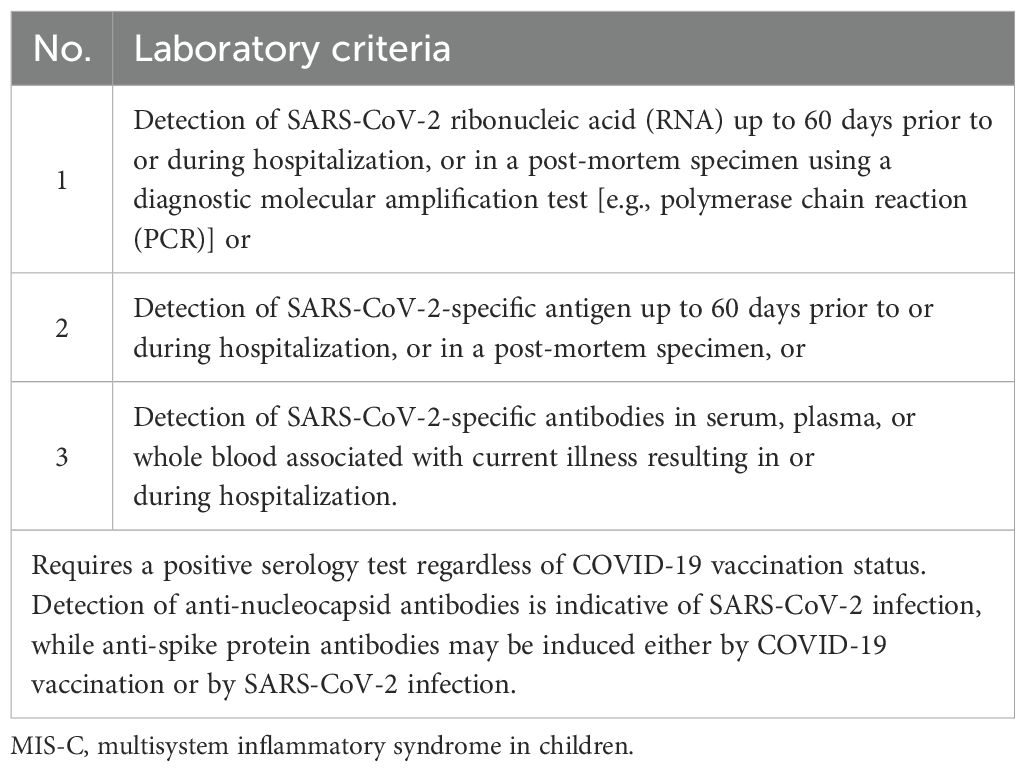
Table 3. MIS-C—laboratory diagnosis criteria (adapted from (60)).

Table 4. MIS-C—epidemiologic linkage [adapted from (60)].
A person should be aged <21 years whose death certificate lists MIS-C or multisystem inflammatory syndrome as an underlying cause of death or a significant condition contributing to death.
A case should be recorded as new if the person has never been previously recorded as a case, or if the most recent case was recorded with an illness onset date (if available) or a hospital admission date more than 90 days prior. A patient is suspected of having MIS-C when the vital criteria are present. A case is confirmed if it meets both the clinical and confirmatory laboratory criteria. A diagnosis of MIS-C is considered probable if the clinical criteria and epidemiologic linkage are positive (60).
As for KD, there are two types of disease, known as complete KD and incomplete KD, further discussed in Tables 5, 6.
Lymphopenia is a characteristic of COVID-19 and is more pronounced in MIS-C cases compared to children with mild SARS-CoV-2 infection or KD (34). Another significant observation is the low platelet count, which is a feature of MIS-C, in contrast to KD where thrombocytosis is more common. However, a decline in platelet count is a frequent element of TSS, which was described in 5% of the cases diagnosed with KD (61).
Regarding MIS-C, C-reactive protein, erythrocyte sedimentation rate, and ferritin levels may also be elevated. Abnormal coagulation parameters, including increased D-dimer, fibrinogen, and international normalized ratio, have been observed (62). Additionally, elevated levels of N-terminal pro-BNP and troponin have been reported to suggest myocardial damage (62–64). Since patients with KD typically exhibit lower inflammatory markers, it is suggested that inflammation plays a crucial role in the more severe cardiovascular complications seen in MIS-C (61).
In KD, the leukocyte count is characterized by neutrophilia and lymphopenia with elevated levels of C-reactive protein and erythrocyte sedimentation rate, thrombocytosis, and elevated levels of alanine aminotransferase and aspartate aminotransferase. Decreased levels of albumin, hemoglobin, sodium, potassium, and cholesterol have also been detected (64).
In comparison, studies have shown that MIS-C patients tend to have lower levels of leukocytes, lymphocytes, and platelets but higher levels of C-reactive protein, D-dimer, and ferritin. Meanwhile, patients with MIS-C appear to have similar levels of procalcitonin and erythrocyte sedimentation rate compared to KD. Concerning the cardiac markers, MIS-C patients had higher levels of creatine phosphokinase (CPK), and no significant differences were found as regards N-terminal pro-BNP levels or troponin levels. MIS-C patients can have lower levels of albumin, sodium, and potassium, while KD patients can have higher levels of creatinine (65, 66). A detailed list of investigations conducted for the diagnosis of MIS-C and KD is presented in Table 7.
The initial treatment includes both immunomodulatory and antithrombotic therapy. Immunomodulatory medications intravenous immunoglobulin (IVIG) and glucocorticoids are the most common immunomodulatory medications used for children diagnosed with MIS-C. It is postulated that the association of these two therapeutic methods decreases the treatment failure rate and results in enhanced cardiac function recovery with shorter intensive care unit stays (2).
Anakinra, a recombinant IL-1 receptor antagonist, is a well-established treatment for MAS and is increasingly used in children with MIS-C who have refractory disease unresponsive to glucocorticoid therapy or when steroids are contraindicated. Its main advantages include a short half-life and a rapid onset of action (71, 72).
Infliximab is a chimeric monoclonal antibody that binds to TNF-α and inhibits its downstream pro-inflammatory effects. It is used to treat IVIG refractory MIS-C and KD (57). Tocilizumab is a recombinant humanized anti-IL6R monoclonal antibody, which is not recommended for all pediatric patients due to the lack of benefit in randomized studies, the risk of infections, and the long half-life (73, 74).
Antithrombotic therapy includes the use of antiplatelets such as aspirin in low doses for all patients without risk factors for bleeding. In addition, anticoagulants are used for patients with large coronary aneurysms and also for those who have moderate to severe LV dysfunction but no risk for bleeding. For all MIS-C patients without aneurysms or cardiac dysfunction, prophylactic or therapeutic anticoagulation should be considered on an individual basis. This decision should take into account risk factors for thrombosis and bleeding, including malignancy, critical illness, obesity, pre-existing inflammatory disease, history of thrombosis, inherited thrombophilia, immobility, and indwelling central lines (75).
The recommended aspirin dosage is 3–5 mg/kg/day orally, once daily, with a maximum dose of 81 mg/day. This dosage should be avoided in cases when the platelet count is <80,000, fibrinogen is <100 mg/dL, or if there is active bleeding or a high risk of bleeding (76–78). Aspirin may be combined with a prophylactic dose of enoxaparin for coronary artery protection (79, 80).
Anticoagulant therapy should be considered in patients presenting with any of the following: acute thrombosis, moderate to severe ventricular dysfunction, coronary dilatation/aneurysm with a Z-score > 10, or D-dimer levels 10 times the upper limit of normal. Unfractionated heparin, direct thrombin inhibitors, and low-molecular-weight heparin can be used, with anti-factor Xa activity monitored. Prophylactic anticoagulant therapy should also be considered alongside aspirin in patients with one of the following conditions: venous thromboembolism, mild to moderate ventricular dysfunction, coronary dilatation/aneurysm with a Z-score of 2.5–10, or D-dimer levels 5–10 times the upper normal limit. The recommended enoxaparin dose is 0.5 mg/kg every 12 hours, with a maximum dose of 30 mg for patients aged >2 months to <18 years (81).
Post-discharge anticoagulation: Patients with MIS-C and documented thrombosis or an ejection fraction >35% should receive therapeutic anticoagulation for at least 2 weeks after discharge from the hospital (71).
In conclusion, enoxaparin is widely used for thromboprophylaxis in MIS-C due to its role in inhibiting thrombin and factor Xa, thereby reducing fibrin clot formation and preventing thromboembolic events, which are often seen in MIS-C due to endothelial dysfunction and hypercoagulability (82). However, cytokine inhibitors such as anakinra, an IL-1 receptor antagonist, and tocilizumab, an IL-6 receptor antagonist, specifically target the pro-inflammatory cytokines, driving the hyperinflammatory response in MIS-C (72). By blocking these inflammatory signals, these inhibitors help modulate the immune system and mitigate the tissue damage and organ dysfunction associated with the condition.
Other therapies, including extracorporeal membrane oxygenation (ECMO), should be considered in cases of cardiorespiratory failure refractory to treatment (83). Therapeutic plasma exchange has been used in critically ill patients who have not responded to third-line immunomodulatory treatment. It is not routinely used for patients with MIS-C (84).
The acute phase treatment starts with IVIG (85, 86). The mechanism of action of IVIG in the treatment of KD is unknown, but it may include the modulation of cytokine production, neutralization of toxins, augmentation of regulatory T-cell activity, suppression of antibody synthesis, and provision of anti-idiotypic antibodies (87). IVIG is administered at a dosage of 2 g/kg, given over 12 hours, along with oral aspirin. Aspirin, during the acute phase of illness, should be administered at high or moderate doses every 6 to 8 hours (88, 89). Low-molecular-weight heparin should be given to patients with large aneurysms and with a high risk for thrombosis and rupture. It is recommended to be associated with aspirin. Warfarin is preferred in the acute phase due to its anti-inflammatory effect and remodeling action (89, 90).
Several studies have suggested trying a second IVIG infusion for patients with fever, but no treatment guidelines are available (91). Infliximab is noted to reduce fever and both the duration of hospitalization and the incidence of hospital admissions, although no effect has been observed on coronary artery outcomes (92). Etanercept, a biological TNF inhibitor, acts as a soluble TNF receptor that binds TNF-α. It has been shown to reduce fever and inflammation (93). Atorvastatin, known for its antioxidant effects, has been concluded to be safe for use in young children with periodic monitoring (94). Cyclosporine has been studied in patients with refractory KD, and a recent study in Japan has completed enrollment (95, 96).
Scoring systems have been developed to predict resistance to IVIG treatment. One such system assigns 1 point for infants younger than 6 months, before 4 days of illness, platelet counts higher than 300,000 per mm3, and C-reactive protein levels higher than 8 mg/dL. An additional 2 points are assigned for Alanine Aminotransferase (ALT) levels equal to or higher than 80 IU/L. This scoring system demonstrated a sensitivity of 78% and a specificity of 76%, with a cutoff score of 3 or more to indicate IVIG resistance (97).
The treatment doses are outlined in Table 8.
Although most cases of MIS-C require intensive care unit admission, studies have shown that only one-third of these cases require mechanical ventilation, and an even smaller number need ECMO (98, 99). The majority of patients recover, but cases of death are also reported. In 2024, 117 cases of MIS-C with illness onset in 2023 were reported in the United States. Among these cases, the median age was 7 years, and 68% had no underlying medical conditions. Of these patients, 50% needed intensive care, 34% experienced shock, and 29% had cardiac dysfunction. The mortality rate was 3%. Although 96% of patients were eligible for vaccination, only 18% had documented vaccination, and 60% of them had received their last dose more than 12 months before MIS-C onset (66). A 2021 study conducted over 6 months in the United States examined 102 MIS-C cases in patients aged 12–18 years. It found that only 5% of patients were fully vaccinated with two doses at least 28 days before hospitalization, while 95% were unvaccinated. Notably, all 38 MIS-C patients who required life support were unvaccinated. No deaths were reported in the study, and the length of hospital stay was similar between vaccinated and unvaccinated patients (100).
As for KD, early detection and treatment usually lead to complete recovery approximately 6 weeks after the onset of symptoms (101). In the acute stage, immunoglobulins administered at high doses may decrease coronary artery injury, but 15%–20% of such cases will develop IVIG resistance (90). According to the literature, the incidence of CAA is nine times higher in cases of KD that are resistant to IVIG treatment compared to those that are IVIG-sensitive (91, 102).
While both MIS-C and KD share several clinical features, they are distinct entities with important differences in their origins and overall impact. MIS-C, a post-infectious hyperinflammatory syndrome, has been closely linked to SARS-CoV-2 infection and typically affects older children and adolescents (1). KD, by contrast, has a longer-established history, generally affecting younger children under the age of 5, with its precise cause still unclear (9, 12). It is believed to arise from a combination of genetic and environmental factors, yet no specific infectious agent has been identified, unlike the clear viral association seen with MIS-C during the COVID-19 pandemic (25, 26, 103).
The inflammatory mechanisms behind each condition, while similar in triggering systemic inflammation, lead to different patterns of organ involvement. MIS-C is characterized by a heightened and more severe immune response, often involving multiple organs, including the heart, gastrointestinal tract, and central nervous system (32, 33). This extensive involvement leads to more severe presentations, including shock and myocardial dysfunction. KD, while also inflammatory in nature, tends to focus more narrowly on the coronary arteries, resulting in the development of CAAs if left untreated. While both conditions are driven by an overactive immune system, the severity of multisystem involvement in MIS-C often presents a more critical situation compared to KD, where coronary artery complications are the primary concern (37).
In terms of clinical presentation, both syndromes can present with common symptoms such as persistent fever, rash, conjunctivitis, and mucosal inflammation, which can make differentiation challenging, especially in the early stages. However, MIS-C is more likely to involve significant gastrointestinal symptoms, such as abdominal pain, vomiting, and diarrhea, as well as neurological signs like confusion and headaches (48). Cardiovascular involvement is also more pronounced in MIS-C, with many patients developing hypotension or shock (49). KD, however, typically follows a more predictable course, presenting with prolonged fever, mucocutaneous signs, and swelling of the extremities. Coronary artery changes are specific to KD and serve as a critical differentiating factor, although they tend to develop later in the disease process (57, 59). Laboratory findings provide further clues that help distinguish between these two conditions. In MIS-C, inflammatory markers such as C-reactive protein, ferritin, and D-dimer levels are often markedly elevated, and there is frequent evidence of cardiac injury, such as elevated troponin or BNP. Lymphopenia and thrombocytopenia are also more common in MIS-C. In contrast, KD is often characterized by elevated inflammatory markers as well, although thrombocytosis may be seen in later stages (65, 66). Evidence of SARS-CoV-2 exposure or infection is a key diagnostic factor for MIS-C, while the diagnosis of KD relies on clinical criteria, with no identifiable infectious trigger.
Despite these differences, treatment approaches overlap, with IVIG serving as a primary therapy for both conditions (76). In MIS-C, however, the more severe inflammatory response often necessitates the use of additional therapies such as corticosteroids or biologics like anakinra or infliximab (57, 71). Patients with MIS-C may require intensive care due to cardiovascular involvement and shock, whereas KD patients generally respond well to IVIG and aspirin, which helps prevent coronary artery complications (76). The aggressive nature of MIS-C requires close monitoring and more intensive treatment to prevent serious complications such as multiorgan failure. Ultimately, both conditions, when recognized and treated early, have favorable outcomes. However, the potential for severe complications is higher in MIS-C due to its rapid progression and widespread organ involvement. Ongoing research will continue to clarify the pathophysiological mechanisms and treatment strategies for these complex syndromes, ensuring better care for affected children and improving long-term outcomes (99).
MIS-C and KD are distinct inflammatory conditions with overlapping features but different causes and treatment approaches. MIS-C, a post-viral syndrome associated with SARS-CoV-2, triggers widespread systemic inflammation and can lead to severe complications, particularly affecting the cardiovascular system. It is thought to result from an exaggerated immune response, requiring timely intervention with IVIG, corticosteroids, and sometimes biological therapies to manage inflammation and prevent organ failure.
KD, primarily affecting young children, remains a leading cause of coronary artery abnormalities. Although its exact cause is unknown, genetic and environmental factors are believed to play roles. Left untreated, it can result in CAAs, making IVIG and aspirin essential in reducing inflammation and preventing long-term cardiovascular damage.
Both conditions show elevated inflammatory markers and share some treatment strategies, but MIS-C tends to cause more extensive organ involvement, requiring prompt medical intervention. Accurate diagnosis and early treatment are crucial in preventing complications. As research progresses, a deeper understanding of these syndromes will improve patient outcomes and refine treatment strategies tailored to their distinct pathophysiological mechanisms.
AL: Conceptualization, Investigation, Writing – original draft. CG: Validation, Visualization, Writing – review & editing. DT: Conceptualization, Investigation, Writing – original draft. AN: Investigation, Software, Writing – original draft. LP: Investigation, Software, Writing – original draft. CM: Methodology, Validation, Writing – review & editing. TC: Investigation, Software, Writing – original draft. GB: Investigation, Software, Validation, Visualization, Writing – original draft. RS: Writing – review & editing. DS: Writing – review & editing, Visualization, Validation. MB: Validation, Writing – review & editing, Visualization. MC: Software, Writing – original draft, Investigation. OC: Writing – review & editing, Visualization, Validation. VL: Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. New Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
2. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicenter of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 6:395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X
3. Waseem M, Shariff MA, Lim CA, Nunez J, Narayanan N, Patel K, et al. Multisystem inflammatory syndrome in children. West J Emergency Med. (2022) 23:505–13. doi: 10.5811/westjem.2022.3.55325
4. Belay ED, Abrams J, Oster ME, Giovanni J, Pierce T, Meng L, et al. Trends in geographic and temporal distribution of U.S. children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. (2021) 175(8):837–45. doi: 10.1001/jamapediatrics.2021.0630
5. Payne AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM, et al. Incidence of multisystem inflammatory syndrome in children among U.S. persons infected with SARS-CoV-2. JAMA Netw Open. (2021) 4(6):e2116420. doi: 10.1001/jamanetworkopen.2021.16420
6. Yousaf AR, Lindsey KN, Wu MJ, Shah AB, Free RJ, Simeone RM, et al. Notes from the field: Surveillance for multisystem inflammatory syndrome in children — United States, 2023. MMWR Morbid Mortal Week Rep. (2024) 73(10):225–8. doi: 10.15585/mmwr.mm7310a2
7. Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, Harwood R, et al. A national consensus management pathway for pediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): Results of a national Delphi process. Lancet Child Adolesc Health. (2021) 5:133–41. doi: 10.1016/S2352-4642(20)30304-7
8. Viner RM, Whittaker E. Kawasaki-like disease: Emerging complication during the COVID-19 pandemic. Lancet. (2020) 395:1741–3. doi: 10.1016/S0140-6736(20)31129-6
9. Takahashi K, Oharaseki T, Yokouchi Y, Hiruta N, Naoe S. Kawasaki disease as a systemic vasculitis in childhood. Ann Vasc Dis. (2010) 3:173–81. doi: 10.3400/avd.sasvp01003
10. Agarwal S, Agrawal DK. Kawasaki disease: Etiopathogenesis and novel treatment strategies. Expert Rev Clin Immunol. (2017) 13:247–58. doi: 10.1080/1744666X.2017.1232165
11. Elakabawi K, Lin J, Jiao F, Guo N, Yuan Z. Kawasaki disease: Global burden and genetic background. Cardiol Res. (2020) 11:9–14. doi: 10.14740/cr993
12. Mărginean CO, Meliţ LE, Mărginean MO. The peculiarities of Kawasaki disease at the extremes of age. Med (Baltimore). (2019) 98:e17595. doi: 10.1097/MD.0000000000017595
13. Nadua KD, Chong CY, Kam KQ, Mok YH, Choo JTL, Lam JCM, et al. Multisystem inflammatory syndrome in children in Singapore. Singapore Med J. (2022) 51:669–76. doi: 10.47102/annals-acadmedsg.202283
14. Matsubara D, Matsubara Y, Ayusawa M, Hamada H, Seki M, Yamagishi H, et al. Nationwide survey of multisystem inflammatory syndrome in children associated with coronavirus disease 2019 in Japan. J Clin Immunol. (2024) 45:51. doi: 10.1007/s10875-024-01845-z
15. Choe YJ, Choi EH, Choi JW, Eun BW, Eun LY, Kim YJ. Change in severity and clinical manifestation of MIS-C over SARS-coV-2 variant outbreaks in Korea. J Korean Med Sci. (2023) 38:e225. doi: 10.3346/jkms.2023.38.e225
16. Yousaf AR, Miller AD, Lindsey K, Shah AB, Wu MJ, Melgar M, et al. Multisystem inflammatory syndrome in children (MIS-C) among persons who completed a two-dose COVID-19 vaccine primary series compared with those reporting no COVID-19 vaccination, U.S. National MIS-C surveillance. Pediatr Infect Dis J. (2023) 42(12):e476–e478. doi: 10.1097/INF.0000000000004103
17. Lupu A, Miron IC, Gavrilovici C, Raileanu AA, Starcea IM, Ioniuc I, et al. Pediatric systemic lupus erythematosus in COVID-19 era. Viruses. (2023) 15:272. doi: 10.3390/v15020272
18. Rhedin S, Lundholm C, Horne AC, Smew AI, Osvald EC, Haddadi A, et al. Risk factors for multisystem inflammatory syndrome in children — A population-based cohort study of over 2 million children. Lancet Region Health - Europe. (2022) 19:100443. doi: 10.1016/j.lanepe.2022.100443
19. Fotea S, Ghiciuc CM, Stefanescu G, Cianga AL, Mihai CM, Lupu A, et al. Pediatric COVID-19 and diabetes: an investigation into the intersection of two pandemics. Diagnostics. (2023) 13:2436. doi: 10.3390/diagnostics13142436
20. Ghimire LV, Chou F-S, Aljohani OA, Moon-Grady AJ. Impact of congenital heart disease on outcomes among pediatric patients hospitalized for COVID-19 infection. BMC Pediatr. (2023) 23:240. doi: 10.1186/s12887-023-04058-2
21. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. (2020) 323:2427–9. doi: 10.1001/jama.2020.8707
22. Marek-Iannucci S, Junarta J, Vishnevsky A, Rajapreyar IN, Fradin JJ, Arditi M, et al. Severe late cardiovascular sequelae of Kawasaki disease in a young adult. J Paediatr Child Health. (2024) 60:147–50. doi: 10.1111/jpc.16533
23. Lee W, Cheah CS, Suhaini SA, Azidin AH, Khoo MS, Ismail NAS, et al. Clinical manifestations and laboratory findings of Kawasaki disease: Beyond the classic diagnostic features. Medicina. (2022) 58:734. doi: 10.3390/medicina58060734
24. Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. (2012) 22:79–85. doi: 10.2188/jea.je20110131
25. Chang LY, Lu CY, Shao PL, Lee PI, Lin MT, Fan TY, et al. Viral infections associated with Kawasaki disease. J Formosan Med Assoc. (2014) 113:148–54. doi: 10.1016/j.jfma.2013.12.008
26. Dean AG, Melish ME, Seaver LH. Global epidemiology of Kawasaki disease: A comprehensive systematic review and meta-analysis. Kawasaki Dis Res J. (2022) 25:1–7. doi: 10.1007/s1180
27. Manlhiot C, Mueller B, O’Shea S, Majeed H, Bernknopf B, Labelle M, et al. Environmental epidemiology of Kawasaki disease: Linking disease etiology, pathogenesis and global distribution. PloS One. (2018) 13(2):e0191087. doi: 10.1371/journal.pone.0191087
28. Rodó X, Curcoll R, Robinson M, Ballester J, Burns JC, Cayan DR, et al. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc Natl Acad Sci U S A. (2014) 111:7952–7. doi: 10.1073/pnas.1400380111
29. Rowley AH. Is Kawasaki disease an infectious disorder? Int J Rheum Dis. (2018) 21:20–5. doi: 10.1111/1756-185X.13213
30. Burns JC, Cayan DR, Tong G, Bainto EV, Turner CL, Shike H, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. (2005) 16:220–5. doi: 10.1097/01.ede.0000152901.06689.d4
31. Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev Med Virol. (2020) 30:1–9. doi: 10.1002/rmv.2141
32. Shankaralingappa A, Thirunavukkarasu AB. Pathogenesis of COVID-19 and multi-system inflammatory syndrome in children. Int J Contemp Pediatr. (2021) 8(4):777–81. doi: 10.18203/2349-3291.ijcp20211096
33. Lin J, Harahsheh AS, Raghuveer G, Jain S, Choueiter NF, Garrido-Garcia LM, et al. Emerging insights into the pathophysiology of multisystem inflammatory syndrome associated with COVID-19 in children. Can J Cardiol. (2023) 39:793–802. doi: 10.1016/j.cjca.2023.01.002
34. Porritt RA, Paschold L, Rivas MN, Cheng MH, Yonker LM, Chandnani H, et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. (2021). J Clin Invest. (2021) 131(10):e146614. doi: 10.1172/JCI146614
35. Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell. (2020) 183:982–995.e914. doi: 10.1016/j.cell.2020.09.034
36. Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. (2020) 183:968–981.e7. doi: 10.1016/j.cell.2020.09.016
37. Sosa TK. Kawasaki disease. Cincinnati Children’s Hosp Med Center. (2023) 173(3):278–9. doi: 10.1001/jamapediatrics.2018.3307
38. Cherqaoui B, Koné-Paut I, Yager H, Le Bourgeois F, Piram M. Delineating phenotypes of kawasaki disease and SARS-coV-2-related inflammatory multisystem syndrome: A french study and literature review. Rheumatol (Oxford). (2021) 60(10):4530–8. doi: 10.1093/rheumatology/keab026
39. Duignan S, Doyle SL, McMahon CJ. Refractory Kawasaki disease: diagnostic and management challenges. Pediatr Health Med Ther. (2019) 10:131–9. doi: 10.2147/PHMT.S165935
40. Kim JJ, Kim HJ, Yu JJ, Yun SW, Lee KY, Yoon KL, et al. IgA levels are associated with coronary artery lesions in Kawasaki disease. Korean Circ J. (2021) 51:267–78. doi: 10.4070/kcj.2020.0345
41. Isaza-Correa J, Ryan L, Kelly L, Aleen J, Melo A, Jones J, et al. Innate immune dysregulation in multisystem inflammatory syndrome in children (MIS-C). Sci Rep. (2023) 13:16463. doi: 10.1038/s41598-023-43390-6
42. Thiriard A, Meyer B, Eberhardt CS, Loevy N, Grazioli S, Adouan W, et al. Antibody response in children with multisystem inflammatory syndrome related to COVID-19 (MIS-C) compared to children with uncomplicated COVID-19. Front Immunol. (2003) 14:1107156. doi: 10.3389/fimmu.2023.1107156
43. Filippatos F, Tats EB, Michos A. Immunology of multisystem inflammatory syndrome after COVID-19 in children: A review of the current evidence. Int J Mol Sci. (2023) 24:5711. doi: 10.3390/ijms24065711
44. Suzuki H, Uemura S, Tone S, Iizuka T, Koike M, Hirayama K, et al. Effects of immunoglobulin and gamma-interferon on the production of tumor necrosis factor-alpha and interleukin-1 beta by peripheral blood monocytes in the acute phase of Kawasaki disease. Eur J Pediatr. (1996) 155:291–6. doi: 10.1007/BF02002715
45. Yokouchi Y, Oharaseki T, Enomoto Y, Sato W, Imanaka-Yoshida K, Takahashi K. Expression of tenascin C in cardiovascular lesions of Kawasaki disease. Cardiovasc Pathol. (2019) 38:25–30. doi: 10.1016/j.carpath.2018.10.005
46. Atici AE, Rivas MN, Arditi M. The central role of interleukin-1 signaling in the pathogenesis of Kawasaki disease vasculitis: path to translation. Can J Cardiol. (2024) 40(12):2305–20. doi: 10.1016/j.carpath.2018.10.005
47. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Carcillo JA, Chopra A, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
48. Noval Rivas M, Arditi M. Kawasaki disease and multisystem inflammatory syndrome in children. Rheum Dis Clinics North America. (2023) 49:647–59. doi: 10.1016/j.rdc.2023.03.002
49. Kwak JH, Lee SY, Choi JW, The Korean Society of Kawasaki Disease. Clinical features, diagnosis, and outcomes of multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Clin Exp Pediatr. (2021) 64:68–75. doi: 10.3345/cep.2020.01900
50. Maaloul I, Jallouli L, Gargouri R, Chabchoub I, Abid L, Aloulou H, et al. Guillain-Barré syndrome in a child with multisystem inflammatory syndrome related to COVID-19. Pediatr Infect Dis J. (2022) 41:e324–5. doi: 10.1097/INF.0000000000003577
51. Mihai CM, Chisnoiu T, Cambrea CS, Frecus CE, Mihai L, Balasa AL, et al. Neurological manifestations found in children with multisystem inflammatory syndrome. Exp Ther Med. (2022) 23:261. doi: 10.3892/etm.2022.11187
52. Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: A literature review. J Clin Neurosci. (2020) 77:8–12. doi: 10.1016/j.jocn.2020.05.017
53. Axelerad A, Muja LF, Mihai CM, Stuparu AZ, Gogu AE, Jianu DC, et al. SARS-CoV-2 infection and associated risk factors for clinical cases of cerebral venous thrombosis: A case series. Exp Ther Med. (2024) 27:13. doi: 10.3892/etm.2023.12300
54. de Magalhães CRM, de Almeida FC, Gandolfi L, Pratesi R, Ribeiro de M. Alves N, Selleski N, et al. Clinical manifestations of Kawasaki disease at different age spectrum: A ten-year study. Med (Kaunas). (2020) 56:145. doi: 10.3390/medicina56040145
55. Duarte-Neto AN, Caldini EG, Gomes-Gouvêa MS, Kanamura CT, Monteiro RAA, Ferranti JF, et al. An autopsy study of the spectrum of severe COVID-19 in children: From SARS to different phenotypes of MIS-C. EClinicalMedicine. (2021) 35:100850. doi: 10.1016/j.eclinm.2021.100850
56. Dolhnikoff M, Ferranti JF, Monteiro RAA, Duarte-Neto AN, Gomes-Gouvêa MS, Degaspare NV, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. (2020) 4:790–4. doi: 10.1016/S2352-4642(20)30257-1
57. Cattalini M, Della Paolera S, Zunica F, Bracaglia C, Giangreco M, Verdoni L, et al. Defining Kawasaki disease and pediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2 infection during SARS-CoV-2 epidemic in Italy: Results from a national, multicenter survey. Pediatr Rheumatol Online J. (2021) 19:29. doi: 10.1186/s12969-021-00511-7
58. Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. (2020) 76:1947–61. doi: 10.1016/j.jacc.2020.08.056
59. Soni PR, Noval Rivas M, Arditi M. A comprehensive update on Kawasaki disease vasculitis and myocarditis. Curr Rheumatol Rep. (2020) 22:6. doi: 10.1007/s11926-020-0882-1
60. Melgar M, Lee EH, Miller AD, Lim S, Brown CM, Yousaf AR, et al. Council of State and Territorial Epidemiologists/CDC surveillance case definition for multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection — United States. MMWR Recommend Rep. (2022) 71:1–14. doi: 10.15585/mmwr.rr7104a1
61. Ravelli A. MIS-C and Kawasaki disease: Different illnesses or part of the same spectrum? Global Pediatr. (2024) 8:100150. doi: 10.1016/j.gpeds.2024.100150
62. Mihai CM, Chisnoiu T, Balasa AL, Frecus CE, Mihai L, Pantazi AC, et al. Clinical characteristics and laboratory findings in children with multisystem inflammatory syndrome (MIS-C)—A retrospective study of a tertiary care center from Constanta, Romania. Healthcare. (2023) 11:544. doi: 10.3390/healthcare11040544
63. Abbas Q, Ali H, Amjad F, Hussain MZH, Rahman AR, Khan MH, et al. Clinical presentation, diagnosis, and management of multisystem inflammatory syndrome in children (MIS-C): A systematic review. BMJ Paedia Open. (2024) 8(1):e002344. doi: 10.1136/bmjpo-2023-002344
64. da Costa MA, de Almeida RPA, Pondé RA. Clinical-laboratory profile of children and adolescents with multisystem inflammatory syndrome temporarily associated with COVID-19 in Goiás, Brazil. Arch Virol. (2023) 168:142. doi: 10.1007/s00705-023-05748-z
65. Zhou C, Zhao Y, Wang X, Huang Y, Tang X, Tang L. Laboratory parameters between multisystem inflammatory syndrome in children and Kawasaki disease. Pediatr Pulmonol. (2021) 56:3688–98. doi: 10.1002/ppul.25687
66. Chuang GT, Tsai I-J, Lin M-T, Chang L-Y. Acute kidney injury in patients with Kawasaki disease. Pediatr Res. (2016) 80:224–7. doi: 10.1038/pr.2016.81
67. Tritt A, Abda I-N, Dahdah N. Review of MIS-C clinical protocols and diagnostic pathways: Towards a consensus algorithm. CJC Pediatr Congenital Heart Dis. (2022) 1:86–93. doi: 10.1016/j.cjcpc.2022.01.003
68. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. (2015) 91(6):365–71.
69. Watanabe T. Pyuria in patients with Kawasaki disease. World J Clin Pediatr. (2015) 4:25–9. doi: 10.5409/wjcp.v4.i2.25
70. Kentsis A, Shulman A, Ahmed S, Brennan E, Monuteaux MC, Lee Y-H, et al. Urine proteomics for discovery of improved diagnostic markers of Kawasaki disease. EMBO Mol Med. (2013) 5:210–20. doi: 10.1002/emmm.201201494
71. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–coV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. (2021) 73:e13–29. doi: 10.1007/s00540-021-02952-6
72. Çaǧlayan Ş, Sönmez HE, Otar Yener G, Baǧlan E, Öztürk K, Ulu K, et al. Anakinra treatment in multisystemic inflammatory syndrome in children (MIS-C) associated with COVID-19. Front Pediatr. (2022) 10:942455. doi: 10.3389/fped.2022.942455
73. Banday AZ, Vignesh P. Use of tocilizumab in multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2. J Pediatr. (2021) 228:315. doi: 10.1016/j.jpeds.2020.09.054
74. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. (2020) 20:565–74. doi: 10.1016/S1473-3099(20)30196-1
75. McElroy S, Cramer E, Amos L. COVID-19 venous thromboembolism prophylaxis guidelines in pediatrics. Thromb Res. (2024) 243:109169. doi: 10.1016/j.thromres.2024.109169
76. Mahmoud S, El-Kalliny M, Kotby A, El-Ganzoury M, Fouda E, Ibrahim H. Treatment of MIS-C in children and adolescents. Curr Pediatr Rep. (2022) 10:1–10. doi: 10.1007/s40124-021-00259-4
77. Sharathkumar AA, Faustino EVS, Takemoto CM. How we approach thrombosis risk in children with COVID-19 infection and MIS-C. Pediatr Blood Cancer. (2021) 68:e29049. doi: 10.1002/pbc.29049
78. Bansal N, Azeka E, Neunert C, Kim JS, Murray J, May L, et al. Multisystem inflammatory syndrome associated with COVID-19 anti-thrombosis guideline of care for children by action. Prog Pediatr Cardiol. (2021) 42:1635–9. doi: 10.1007/s00246-021-02651-9
79. Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, et al. Characteristics, Cardiac Involvement and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. J Pediatr. (2020) 224:141–5. doi: 10.1016/j.jpeds.2020.06.044
80. Aurora T, Joseph N, Bhoopalan SV, Caniza MA, Flerlage T, Ghafoor S, et al. The successful use of eculizumab for treatment of thrombotic microangiopathy in pediatric acute SARS-CoV-2 infection and multisystem inflammatory syndrome in children. Haematologica. (2022) 107:2517–22. doi: 10.3324/haematol.2022.280603
81. Sochet AA, Morrison JM, Jaffray J, Godiwala N, Wilson HP, Thornburg CD, et al. Enoxaparin thromboprophylaxis in children hospitalized for COVID-19: A phase 2 trial. Pediatrics. (2022) 150:e2022056726. doi: 10.1542/peds.2022-056726
82. Sönmez HE, Yener GO, Bağlan E, Öztürk K, Ulu K, Guliyeva V, et al. Anakinra treatment in multisystemic inflammatory syndrome in children (MIS-C) associated with COVID-19. Front Pediatr. (2022) 10:944743. doi: 10.3389/fped.2022.944743
83. Schlapbach LJ, Andre MC, Grazioli S, Schöbi N, Ritz N, Aebi C, et al. Best practice recommendations for the diagnosis and management of children with pediatric inflammatory multisystem syndrome temporally associated with SARS-coV-2 (PIMS-TS; MIS-C) in Switzerland. Front Pediatr. (2021) 9:667507. doi: 10.3389/fped.2021.667507
84. Emeksiz S, Özcan S, Perk O, Uyar E, Çelikel Acar B, Kibar Gül AE, et al. Therapeutic plasma exchange: A potential management strategy for critically ill MIS-C patients in the pediatric intensive care unit. Pediatr Hematol Oncol. (2021) 60:3103119. doi: 10.1016/j.transci.2021.103119
85. Mori M, Miyamae T, Imagawa T, Katakura S, Kimura K, Yokota S. Meta-analysis of the results of intravenous gamma globulin treatment of coronary artery lesions in Kawasaki disease. Modern Rheumatol. (2004) 14:361–6. doi: 10.1007/s10165-004-0324-3
86. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
87. Burns JC, Franco A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev Clin Immunol. (2015) 11:*819. doi: 10.1586/1744666X.2015.1044980
88. Dallaire F, Fortier-Morissette Z, Blais S, Dhanrajani A, Basodan D, Renaud C, et al. Aspirin dose and prevention of coronary abnormalities in Kawasaki disease. Pediatrics. (2017) 139:e20170098. doi: 10.1542/peds.2017-0098
89. Johnson PN, Kuhn RJ. Combination thrombolytic and anti-platelet therapies in an infant with incomplete Kawasaki disease and coronary aneurysms. J Pediatr Pharmacol Ther. (2008) 13:242–50. doi: 10.18632/jpp.13.4.242
90. Marchesi A, Rigante D, Cimaz R, Ravelli A, Tarissi de Jacobis I, Rimini A, et al. Revised recommendations of the Italian Society of Pediatrics about the general management of Kawasaki disease. Ital J Pediatr. (2021) 47:16. doi: 10.1186/s13052-021-00962-4
91. Pan Y, Fan Q, Hu L. Treatment of immunoglobulin-resistant Kawasaki disease: a Bayesian network meta-analysis of different regimens. Front Pediatr. (2023) 11:1149519. doi: 10.3389/fped.2023.1149519
92. Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, et al. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr. (2011) 158:644–649.e1. doi: 10.1016/j.jpeds.2010.10.012
93. Tremoulet AH. Adjunctive therapies in Kawasaki disease. Int J Rheum Dis. (2018) 21:76–9. doi: 10.1111/1756-185X.13208
94. Niedra E, Chahal N, Manlhiot C, Yeung RSM, McCrindle BW. Atorvastatin safety in Kawasaki disease patients with coronary artery aneurysms. Pediatr Cardiol. (2014) 35:89–92. doi: 10.1007/s00246-013-0746-9
95. Suzuki H, Suenaga T, Kakimoto N, Takeuchi T, Shibuta S. Cyclosporin A treatment for refractory Kawasaki disease. Nihon Rinsho. (2014) 72:1636–40. doi: 10.1097/INF.0b013e318220c3cf
96. Aoyagi R, Hamada H, Sato Y, Suzuki H, Onouchi Y, Ebata R, et al. Study protocol for a phase III multicentre, randomised, open-label, blinded-end point trial to evaluate the efficacy and safety of immunoglobulin plus cyclosporin A in patients with severe Kawasaki disease (KAICA Trial). BMJ Open. (2015) 5:e009562. doi: 10.1136/bmjopen-2015-009562
97. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. (2006) 149:237–40. doi: 10.1016/j.jpeds.2006.03.050
98. Varol F, Şahin E, Kılıç A, Şahin BS, Önalan MA, Uğur M, et al. Efficacy of extracorporeal membrane oxygenation in pediatric COVID-19 and MIS-C cases: A single-center experience. Turkish J Thorac Cardiovasc Surg. (2022) 30:363–71. doi: 10.5606/tgkdc.dergisi.2022.23392
99. Bembea MM, Loftis LL, Thiagarajan RR, Young CC, McCadden TP, Newhams MM, et al. Extracorporeal membrane oxygenation characteristics and outcomes in children and adolescents with COVID-19 or multisystem inflammatory syndrome admitted to U.S. ICUs. Pediatr Crit Care Med. (2023) 24:356–71. doi: 10.1097/PCC.0000000000003212
100. Zambrano LD, Newhams MM, Olson SM, Halasa NB, Price AM, Boom JA, et al. Effectiveness of BNT162b2 (Pfizer-bioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep. (2022) 71:52–8. doi: 10.15585/mmwr.mm7102e1
101. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease. Circulation. (2004) 110:2752–76. doi: 10.1161/01.CIR.0000145143.19711.78
102. Öztarhan K, Varlı YZ, Ayaz NA. Usefulness of Kawasaki disease risk scoring systems to the Turkish population. Anatolian J Cardiol. (2020) 24:97–106. doi: 10.14744/AnatolJCardiol.2020.37560
Keywords: multisystem inflammatory syndrome in children, Kawasaki disease, SARS-CoV-2, immune response, inflammatory response, pediatrics
Citation: Lupu A, Gavrilovici C, Mihai CM, Tonco DC, Nedelcu AH, Pertea L, Chisnoiu T, Baciu G, Stoicescu RM, Salaru DL, Badescu MC, Cuciureanu M, Cirstea O and Lupu VV (2025) Multisystem inflammatory syndrome in children and Kawasaki disease. Front. Immunol. 16:1554787. doi: 10.3389/fimmu.2025.1554787
Received: 02 January 2025; Accepted: 17 March 2025;
Published: 15 April 2025.
Edited by:
Soohyun Kim, Konkuk University, Republic of KoreaReviewed by:
Claudio Pignata, University of Naples Federico II, ItalyCopyright © 2025 Lupu, Gavrilovici, Mihai, Tonco, Nedelcu, Pertea, Chisnoiu, Baciu, Stoicescu, Salaru, Badescu, Cuciureanu, Cirstea and Lupu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Maria Mihai, Y3Jpc3RpbmFfbWloYWlAMzY1LnVuaXYtb3ZpZGl1cy5ybw==; Denisa Claudia Tonco, ZGVuaXNhY2xhdWRpYS5mcmFudEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.