
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 25 February 2025
Sec. Multiple Sclerosis and Neuroimmunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1551379
Background: Myelin oligodendrocyte glycoprotein (MOG) antibody-associated diseases (MOGAD), which has been recognized as a distinct entity in patients with neuromyelitis optica spectrum disorders, often presents with acute disseminated encephalomyelitis (ADEM) symptoms in pediatric patients. Appropriate treatment based on accurate diagnosis is challenging in relapsing pediatric patients with MOGAD.
Case Presentation: An 11-year-old girl experienced relapses four times, exhibiting brainstem symptoms, an ADEM episode, seizures, and optic neuritis (ON). She was initially diagnosed with multiple sclerosis and received interferon beta-1a therapy with a mild effect on relapse suppression. She was then transferred from the pediatric department to the department of neurology of our hospital. Two months before her referral visit, she experienced left optic neuritis, and her annualized relapse rate reached 0.6. She desired to switch from the injectable treatment to oral dimethyl fumarate (DMF) administration. At that time, she was found to be seropositive for MOG antibody, but after that had no relapses for more than five years. Moreover, her seropositivity for serum MOG-antibody turned out to be seronegative.
Conclusions: DMF showed long-term effects on suppressing relapses in a pediatric patient with MOGAD, revealing its potential as a treatment option for such patients.
In patients with neuromyelitis optica spectrum disorders (NMOSD) who were seronegative for aquaporin-4 antibody (AQP4-IgG), a new disease entity known as myelin oligodendrocyte glycoprotein (MOG) antibody-associated diseases (MOGAD), was proposed in 2023 (1). The clinical phenotype in MOGAD, including optic neuritis and myelitis, is consistent with that of AQP4-IgG-seropositive NMOSD; however, some differences in clinical characteristics, such as frequent pediatric onset, fewer relapses, and favorable recovery, have been noted.
Recently, the concept of MOGAD has been further broadened. Pediatric patients with MOGAD have an acute disseminated encephalomyelitis (ADEM) presentation, which often raises concerns about its differential diagnosis with multiple sclerosis (MS) (2), which is particularly problematic for determining the choice of treatment.
Herein, we report the case of a relapsing pediatric patient with MOG antibody who responded well to dimethyl-fumarate (DMF), a drug indicated for MS, for more than five years. She experienced four relapses, which included brainstem symptoms, an ADEM episode, seizure, and unilateral optic neuritis. Prior to DMF treatment, she experienced two relapses before the induction of IFN beta-1a treatment, one relapse during self-interruption of IFN beta-1a, and the last relapse during IFN beta-1a treatment. Although she had a partial response to IFNbeta-1a, she had a better clinical course with DMF (Figure 1).
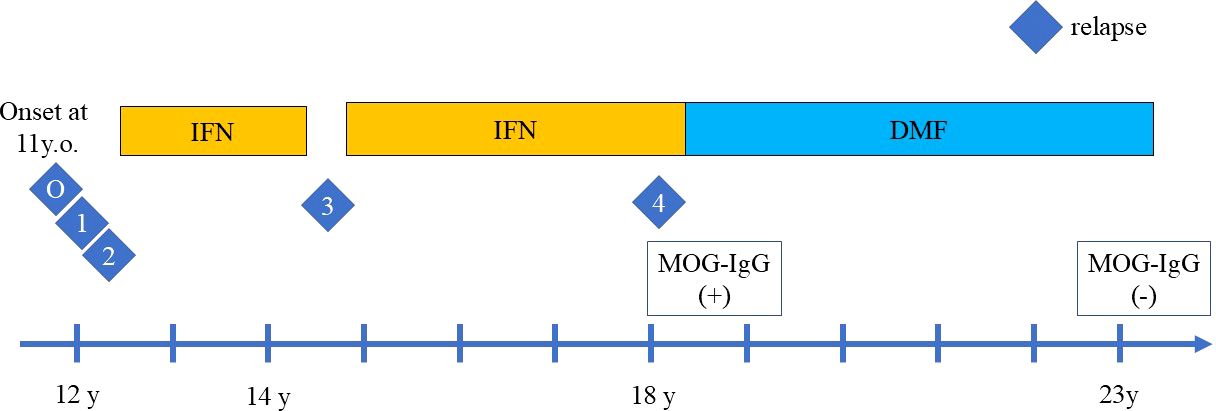
Figure 1. The clinical course of relapses during treatment with interferon beta-1a (IFN) injection and oral dimethyl fumarate (DMF). The relapses are indicated by the number. O indicates the disease onset, which manifested as ADEM with headache, vomiting, and hemiplegia followed by diplopia. One indicates the first relapse of ADEM episode with diplopia, right visual impairment, cerebellar symptoms, 2 indicates the second relapse with visual field impairment and left optic neuritis. IFN was then introduced, but the third relapse of focal seizure occurred during self-interruption of IFN, indicated by 3. Despite IFN treatment, the fourth relapse of left optic neuritis occurred, indicated by 4. After DMF induction, no relapses occurred.
An 11-year-old girl who was born healthy presented with headache, vomiting, and left hemiplegia. She had not been vaccinated before the onset of symptoms, but had a preceding infection with subtle rhinorrhea for one week. She was initially diagnosed with hemiplegic migraine. After half a month, she developed right eye abduction disorder and diplopia. Therefore, ADEM was diagnosed based on these symptoms of meningeal irritation followed by diplopia due to brainstem involvement. She was then referred to a nearby hospital, where brain magnetic resonance imaging (MRI) showed small lesions in the anterior horn of the right ventricle. A plain spinal MRI showed no apparent abnormalities. The examination of cerebrospinal fluid (CSF) showed pleocytosis, an elevation of myelin basic protein, but oligoclonal bands were not examined. The microbiological test of CSF was negative. The serological results showed no AQP4-IgG, but no measurement of MOG-IgG. She was treated with steroid pulse therapy, followed by oral steroid therapy for eight weeks.
Four months after the first episode, she developed diplopia with the restriction of her left eye abduction, followed by visual impairment in her right eye and cerebellar symptoms. Brain MRI revealed multiple white matter lesions (Figures 2A–C) and the examination of CSF was positive for oligoclonal band. Indicating multiple sclerosis (MS), she was treated with steroid pulse therapy for five days, which gradually improved her condition.
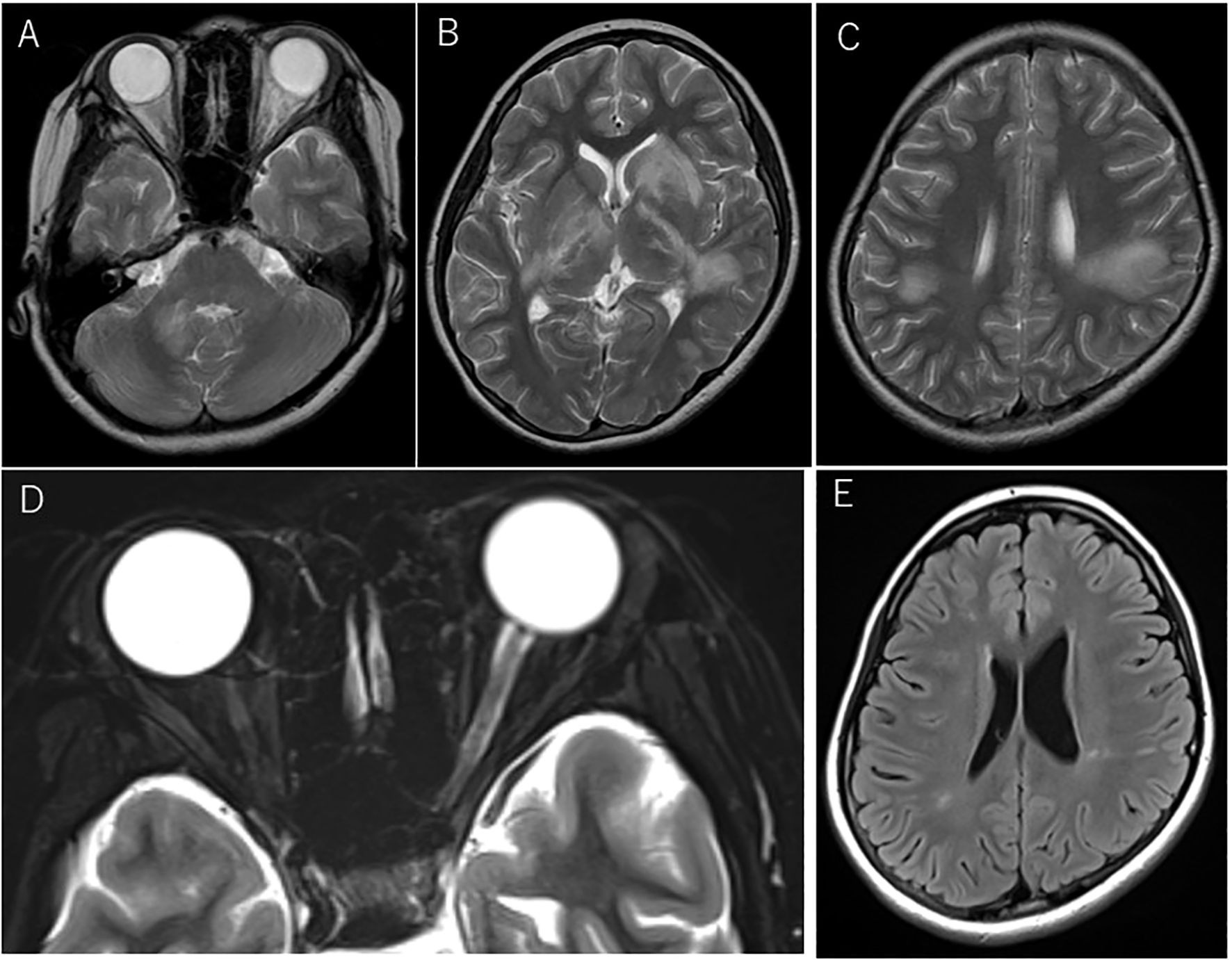
Figure 2. Magnetic resonance imaging (MRI) at acute disseminated encephalomyelitis (ADEM) and left optic neuritis relapses before dimethyl fumarate (DMF) administration. Axial T2-weighted MRI at the first relapse manifesting as ADEM (A–C). Lesions involved the cerebellum(A) and a part of the basal ganglia(B), including bilateral confluent lesions in the white matter (C). At the fourth relapse, swelling of the left optic nerve and the intensity change of the optic nerve sheath were seen on axial T2-weighted images with fat suppression (D). Shrinkage of multiple ADEM lesions was seen on FLAIR imaging at the referral visit to our hospital before the induction of oral DMF (E).
Two months later, she developed visual field impairment suggestive of inferior quadrantanopia and new white matter lesions involving right basal ganglia and right parietal lobe were found on brain MRI. The left relative afferent pupillary defect (RAPD) was positive. She then experienced decreased visual acuity on the left side, and the optic neuritis was confirmed with temporal pallor of the optic disc on the fundus examination and an enlarged left optic nerve on MRI, which prompted the administration of steroid pulse therapy. The interferon beta-1a (IFN) injection was initiated to suppress the relapse, but the treatment was discontinued after two years and two months at the patient’s request. At three months after discontinuation, she exhibited bilateral tonic clonic seizure that started from focal seizures in the left facial area. Long-term video-EEG monitoring was performed the day after the seizure, but no epileptogenic findings were seen. The test for NMDA receptor antibodies was not performed. She was then started on levetiracetam. The brain MRI showed no new lesions, but a follow-up brain MRI after one month indicated a new lesion, which prompted us to restart the IFN injection. The oral antiepileptic medication was discontinued within several months, with the patient remaining relapse-free for about three years and 7months. However, she suddenly developed visual impairment in her left eye decreased to 20/200 visual acuity, and her left RAPD was positive. Fundus examination revealed a slightly pallor of the optic disc and the left optic nerve on MRI showed the swelling except optic disc and intensity change of the optic nerve sheath in the orbit (Figure 2D) and faint gadolinium-enhancement in the optic canal, which was diagnosed as left optic neuritis. She was recovered rapidly in several days by steroid pulse therapy, followed by oral post-steroid therapy (tapering from a maximum dose of 1 mg/kg) for three months.
Two months later, she entered the university and was transferred from the pediatric department to our neurology department. At her first visit, no neurological abnormalities were observed, and a brain MRI showed mildly abnormal findings with shrinkage of the previous ADEM lesions involving the right cerebellar peduncle, bilateral basal ganglia, and multiple deep white matter lesions in the right temporal lobe and bilateral parietal lobes (Figure 2E), but no swelling of the bilateral optic nerves or laterality of signal intensity on MRI was observed. The annualized relapse rate until the referral visit was 0.6. Her neurological conditions and the MRI findings prompted us to switch her current treatment from IFN injection to oral dimethyl fumarate (DMF) administration, in line with the patient’s request. After starting DMF, blood tests revealed a high titer of anti-MOG antibody by cell-based assay, for which she was closely monitored by regular brain MRI at least every 6 months. The follow-up brain MRI showed no new lesions. Since then, however, no relapses have occurred for more than five years, with the disappearance of the anti-MOG antibody.
Biological drugs for treating AQP4-IgG-seropositive NMOSD have been extensively developed and widely used in recent years, with the primary targets being complement 5, IL-6, and B cells (3–7). On the other hand, the treatment of MOGAD, which was detected in patients with AQP4-IgG-seronegative NMOSD, has not yet been fully established.
DMF was indicated initially for psoriasis but was subsequently developed as a new drug for multiple sclerosis (8). Currently, DMF is commonly prescribed for patients with relapsing-remitting MS with mild disease activity and for pediatric patients with MS. However, there are only a few reports of patients with MOGAD treated with DMF. A case report suggested that DMF was ineffective in suppressing relapses in patients with MOGAD (9). In contrast, another case series showed that DMF was a potential trigger for the production of MOG antibodies in patients with MOGAD (10).
In a nationwide survey in Japan, only 44% of patients with MOGAD experienced relapses, and only some patients required treatment for relapse prevention (11, 12). However, sufficient therapy with rituximab or intravenous immunoglobulin (IVIG) upon disease onset (13) and treatment with prednisolone (12.5mg) for at least three months (14) could reduce the risk of subsequent relapses. In the current case, the patient received standard treatment, such as methylprednisolone steroid pulse therapy for the first attack, with no additional treatment.
Although MOG antibody titers at disease onset could not predict the disease course, seroconversion of MOG antibodies in relapsing patients has been associated with a decreased risk of relapse. Hence, conversion to seronegative during long-term treatment with DMF may support the patient’s relapse-free status in the future (15).
The clinical course of MOGAD mainly includes ADEM episodes, relapses of optic neuritis or myelitis, and infrequent brain symptoms, which may differ depending on whether the patient is aged over and under 18 years (16). Each clinical subtype may have varying pathophysiologies in patients with MOGAD. If the first symptom is an ADEM episode, a different diagnosis of MS may be complicated, requiring close monitoring. The patient in our case was indeed diagnosed with MS and was treated with IFN, which showed partial efficacy. However, she desired to discontinue the injections and instead receive oral treatment for MS.
Although our case showed a relapsing pattern and severe attacks, each attack displayed good recovery. In our case, optic neuritis occurred three times, once in the right eye and twice in the left eye. Although she did not show typical findings of MOGAD, such as bilateral optic neuritis or anterior involvement associated with optic disc edema, MRI showed not only swelling of the affected optic nerve but also involvement of the optic nerve sheath, which is known to be a unique finding in patients with MOGAD, distinct from multiple sclerosis and neuromyelitis optica spectrum disorder.
The clinical course of patients with MOGAD may not always be benign with good recovery (17). In relapsing patients with MOGAD who present with an aggressive and refractory course, another treatment option should be considered, and regular infusion of IVIG (18) might be one of the best choices, especially in steroid-dependent cases (19). IL-6 has also been identified as a potential target for MOGAD (20), and a clinical trial of IL-6 receptor blockade is currently ongoing.
In conclusion, our case suggests that DMF showed favorable long-term effects in suppressing relapses in a relapsing pediatric patient, highlighting its potential as a treatment option for patients with MOGAD.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
The requirement of ethical approval was waived by Institutional Review Board of Utano Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. TA: Data curation, Investigation, Resources, Writing – review & editing. KS: Conceptualization, Writing – review & editing. HS: Conceptualization, Data curation, Formal Analysis, Investigation, Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was partly supported by the Health and Labour Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labour and Welfare of Japan (20FC1009).
The MOG antibody assay was conducted by the Department of Neurology at Tohoku University. The authors thank Toshiyuki Takahashi and Tatsuro Misu.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. (2023) 22:268–82. doi: 10.1016/S1474-4422(22)00431-8
2. Bruijstens AL, Lechner C, Flet-Berliac L, Deiva K, Neuteboom RF, Hemingway C, et al. E.U. paediatric MOG consortium consensus: Part 1 - Classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. (2020) 29:2–13. doi: 10.1016/j.ejpn.2020.10.006
3. Tahara M, Oeda T, Okada K, Kiriyama T, Ochi K, Maruyama H, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2020) 19:298–306. doi: 10.1016/S1474-4422(20)30066-1
4. Tahara M, Oeda T, Okada K, Ochi K, Maruyama H, Fukaura H, et al. Compassionate open-label use of rituximab following a randomised clinical trial against neuromyelitis optica (RIN-2 study): B cell monitoring-based administration. Mult Scler Relat Disord. (2022) 60:103730. doi: 10.1016/j.msard.2022.103730
5. Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. (2019) 394:1352–63. doi: 10.1016/S0140-6736(19)31817-3
6. Pittock SJ, Fujihara K, Palace J, Berthele A, Kim HJ, Oreja-Guevara C, et al. Eculizumab monotherapy for NMOSD: Data from PREVENT and its open-label extension. Mult Scler. (2022) 28:480–6. doi: 10.1177/13524585211038291
7. Kleiter I, Traboulsee A, Palace J, Yamamura T, Fujihara K, Saiz A, et al. Long-term efficacy of satralizumab in AQP4-igG-seropositive neuromyelitis optica spectrum disorder from SAkuraSky and SAkuraStar. Neurol Neuroimmunol Neuroinflamm. (2022) 10:e200071. doi: 10.1212/NXI.0000000000200071
8. Schimrigk S, Brune N, Hellwig K, Lukas C, Bellenberg B, Rieks M, et al. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol. (2006) 13:604–10. doi: 10.1111/j.1468-1331.2006.01292.x
9. Warabi Y, Takahashi T, Isozaki E. Dimethyl fumarate was ineffective but not harmful for a patient with myelin oligodendrocyte glycoprotein antibody disease. Cureus. (2019) 11:e6040. doi: 10.7759/cureus.6040
10. Takahashi K, Takeuchi H, Fukai R, Nakamura H, Morihara K, Higashiyama Y, et al. Case report: anti-MOG antibody seroconversion accompanied by dimethyl fumarate treatment. Front Immunol. (2021) 12:625465. doi: 10.3389/fimmu.2021.625465
11. Niino M, Isobe N, Araki M, Ohashi T, Okamoto T, Ogino M, et al. Clinical practice guidelines for multiple sclerosis, neuromyelitis optica spectrum disorder, and myelin oligodendrocyte glycoprotein antibody-associated disease 2023 in Japan. Mult Scler Relat Disord. (2024) 90:105829. doi: 10.1016/j.msard.2024.105829
12. Azumagawa K, Nakashima I, Kaneko K, Torisu H, Sakai Y, Kira R, et al. A nation-wide survey of Japanese pediatric MOG antibody-associated diseases. Brain Dev. (2021) 43:705–13. doi: 10.1016/j.braindev.2021.01.008
13. Virupakshaiah A, Schoeps VA, Race J, Waltz M, Sharayah S, Nasr Z, et al. Predictors of a relapsing course in myelin oligodendrocyte glycoprotein antibody-associated disease. J Neurol Neurosurg Psychiatry. (2024) 96(1):68–75. doi: 10.1212/WNL.0000000000208303
14. Trewin BP, Dale RC, Qiu J, Chu M, Jeyakumar N, Dela Cruz F, et al. Oral corticosteroid dosage and taper duration at onset in myelin oligodendrocyte glycoprotein antibody-associated disease influences time to first relapse. J Neurol Neurosurg Psychiatry. (2024) 95(11):1054–63. doi: 10.1136/jnnp-2024-333463
15. Wendel EM, Thonke HS, Bertolini A, Baumann M, Blaschek A, Merkenschlager A, et al. Temporal dynamics of MOG antibodies in children with acquired demyelinating syndrome. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e200035. doi: 10.1212/NXI.0000000000200035
16. Sechi E, Cacciaguerra L, Chen JJ, Mariotto S, Fadda G, Dinoto A, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A review of clinical and MRI features, diagnosis, and management. Front Neurol. (2022) 13:885218. doi: 10.3389/fneur.2022.885218
17. Misu T, Matsumoto Y, Kaneko K, Takahashi T, Takai Y, Ono H, et al. Myelin oligodendrocyte glycoprotein antibody-associated disorders: An overview. Clin Exp Neuroimmunol. (2024) 15:6–15. doi: 10.1111/cen3.12771
18. Chen JJ, Huda S, Hacohen Y, Levy M, Lotan I, Wilf-Yarkoni A, et al. Association of maintenance intravenous immunoglobulin with prevention of relapse in adult myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. (2022) 79:518–25. doi: 10.1001/jamaneurol.2022.0489
19. Kamijo Y, Usuda M, Matsuno A, Katoh N, Morita Y, Tamaru F, et al. Successful maintenance therapy with intravenous immunoglobulin to reduce relapse attacks and steroid dose in a patient with refractory myelin oligodendrocyte glycoprotein antibody-positive optic neuritis. Intern Med. (2024). doi: 10.2169/internalmedicine.3593-24
20. Kaneko K, Sato DK, Nakashima I, Ogawa R, Akaishi T, Takai Y, et al. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: a cross-sectional study and potential therapeutic implications. J Neurol Neurosurg Psychiatry. (2018) 89:927–36. doi: 10.1136/jnnp-2018-317969
Keywords: myelin oligodendrocyte glycoprotein antibody-associated disease, dimethyl fumarate, acute disseminated encephalomyelitis, optic neuritis, seizure
Citation: Tahara M, Awaya T, Saito K and Sawada H (2025) Case Report: Long-term suppression of relapses by dimethyl fumarate in a relapsing pediatric patient with myelin oligodendrocyte glycoprotein antibody–associated disease manifesting as acute disseminated encephalomyelitis, unilateral optic neuritis, and seizure episodes. Front. Immunol. 16:1551379. doi: 10.3389/fimmu.2025.1551379
Received: 25 December 2024; Accepted: 05 February 2025;
Published: 25 February 2025.
Edited by:
Tatjana Pekmezovic, University of Belgrade, SerbiaReviewed by:
Ivan Adamec, University Hospital Centre Zagreb, CroatiaCopyright © 2025 Tahara, Awaya, Saito and Sawada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masayuki Tahara, dGFoYXJhLm1hc2F5dWtpLm5lQG1haWwuaG9zcC5nby5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.