- 1Department of Emergency, The First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, Kunming, China
- 2Department of Hematology, The First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, Kunming, China
- 3Yunnan Province Clinical Research Center for Hematologic Disease, The First People’s Hospital of Yunnan Province, Kunming, China
- 4Yunnan Provincial Clinical Medical Center for Blood Diseases and Thrombosis Prevention and Treatment, The First People’s Hospital of Yunnan Province, Kunming, China
- 5Yunnan Atherosclerosis Cooperation Base of Chinese and Western Medicine, The First People’s Hospital of Yunnan Province, Kunming, China
- 6Department of Infectious Diseases, The First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, Kunming, China
- 7Department of integrated Chinese and Western medicine, The First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, Kunming, China
- 8Hospital Office, First Affiliated Hospital of Kunming Medical College, Kunming, China
Introduction: Is there a difference in pathogen infection among sepsis patients with different organ dysfunction and Post-sepsis persistent organ dysfunction? Is this related to survival? It is currently unclear.
Methods: This study reviewed 1982 sepsis patients between December 2019 and September 2023, and included 619 patients after removing patients with missing data. Of these, 332 were tested for metagenomic next-generation sequencing (mNGS). First, the pathogens distribution was assessed in all NGS-positive patients, followed by patients with different organ dysfunction (excluding those who died during hospitalization). Lastly, the survival analysis was conducted on patients infected with different pathogens.
Results: The results showed that the mortality rate in our cohort was 27.63% in patients with sepsis, and patients with Respiratory, Liver, Circulatory, Hematologic, Neurological, and Renal dysfunction had poor survival. And patients with post-sepsis persistent organ dysfunction after sepsis have worse survival rates. In addition, we found the infection rates of Legionella and Betapapillomavirus were higher in patients with liver dysfunction. The infection rates of Mastadenovirus, Enterococcus, and Candida were higher in patients with neurological dysfunction. The infection rates of Candida were higher in patients with renal dysfunction and hematologic dysfunction. The infection rates of Moraxella were higher in patients with circulatory dysfunction. The infection rates of Enterococcus, Pneumocystis, and Acinetobacter were higher in patients with Post-sepsis cardiac dysfunction.The infection rates of Enterococcus, Acinetobacter, and Morganella were higher in patients with Post-sepsis liver dysfunction. The infection rates of Enterococcus, Acinetobacter, and Staphylococcus were higher in patients with Post-sepsis respiratory dysfunction. The infection rates of Enterococcus, Candida, Pneumocystis, Staphylococcus, and Listeria were higher in patients with Post-sepsis renal dysfunction. In addition, we found that patients with Escherichia infection in sepsis had the lowest survival rate. The survival rate of patients with Enterococcus infection combined with post-sepsis persistent respiratory dysfunction is also worse.
Discussion: In conclusion, there are differences in the types and proportions of pathogens infected in patients with different organ dysfunction and Post-sepsis persistent organ dysfunction. The combination of Escherichia infection and Enterococcus infection with post-sepsis persistent respiratory dysfunction can affect the survival of patients. We should strengthen the management of sepsis patients, especially those with Post-sepsis persistent organ dysfunction.
Introduction
Sepsis is caused by infections and may result in life-threatening organ dysfunction because of host response disorder (1). The 2016 definition of sepsis (3.0) pays more attention to identifying organ dysfunction in the context of infection (2). Sepsis is the main cause of death in ICU patients globally with increasing incidence rates in developed and developing countries and is related to age, race, comorbidity, and host genetics (3). The complex mechanism of sepsis and its high incidence and mortality rates have increased human health and societal burdens (3). According to statistics, there are more than 18 million cases of severe sepsis worldwide every year, increasing at a rate of 1.5% per year. Furthermore, the fatality rate of sepsis is 28% per annum, with about 14,000 deaths per day globally (4). The most common sources of infection in sepsis patients are Escherichia coli, Staphylococcus aureus, Pseudomonas, etc. (5, 6). Timely and accurate identification of pathogens is crucial for improved clinical management and prognosis of these patients (7). Currently, metagenomic next-generation sequencing (mNGS) has the most accurate and sensitive detection methods than other techniques such as blood and sputum cultures (8, 9).
In recent years, with the high attention and standardized diagnosis and treatment of sepsis, its acute mortality rate in the hospital has decreased. It has been estimated that in 2016, 14 million patients suffering from sepsis were hospitalized (10). However, these patients further develop new physical disabilities and cognitive dysfunction, which may further deteriorate their health after discharge. It has been indicated that there is an increased risk of subsequent infection, cardiovascular events, acute renal failure, and inhalation after hospitalization (10). Moreover, Post-sepsis persistent organ dysfunction is related to persistent immune disorders. However, whether pathogens are related to these complications remains undetermined. Therefore, this study employed mNGS to detect pathogens in patients with sepsis to explore whether there is a relationship between infected pathogens and accompanying organ damage and Post-sepsis persistent organ dysfunction. And whether it has an impact on patient survival.
Methods
Standards and ethics
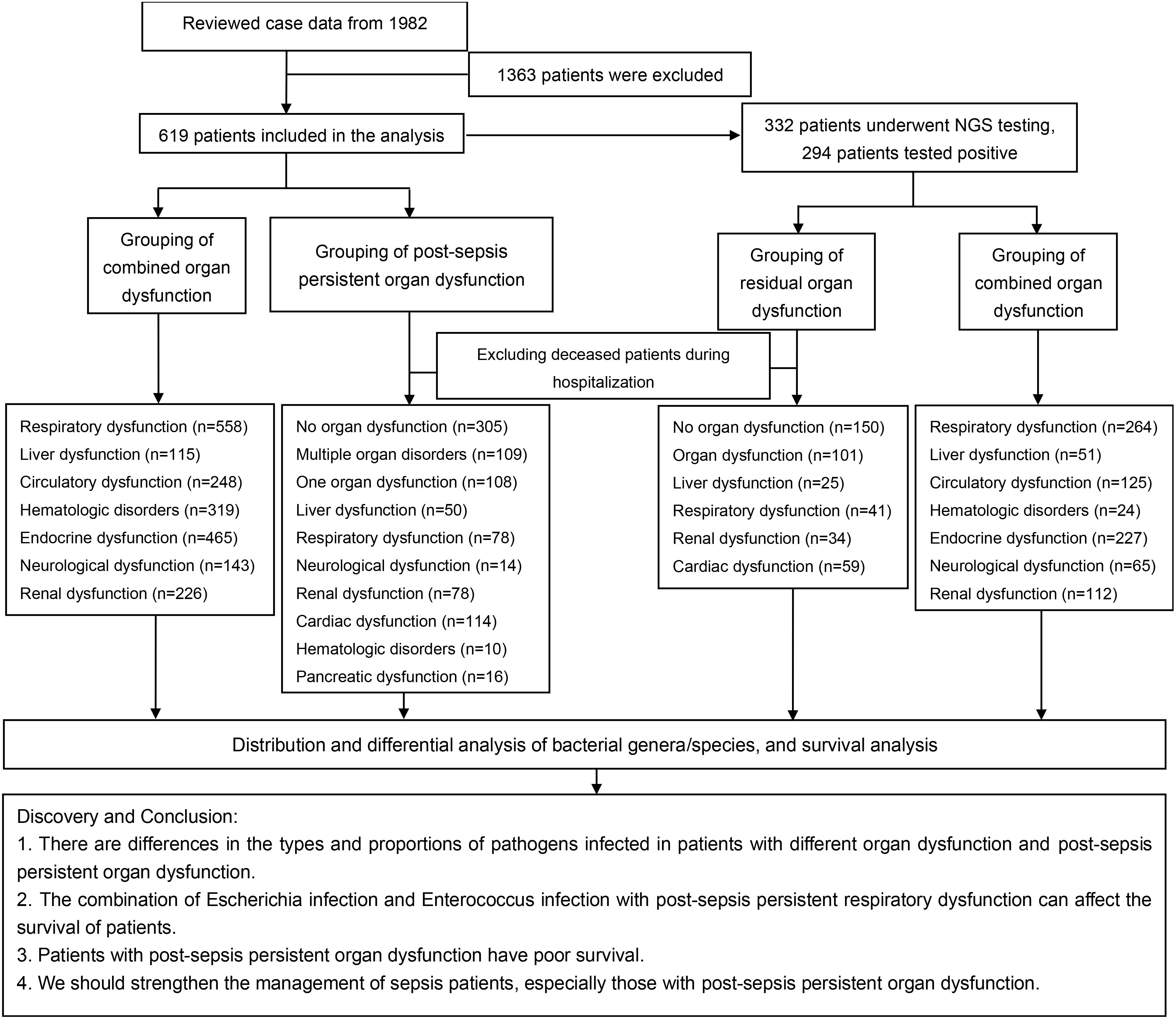
A total of 1982 cases of sepsis/septic shock patients diagnosed at the First People’s Hospital of Yunnan Province between December 2019 and September 2023 were reviewed. Patients who lost contact or had missing data were excluded. The definition and diagnostic criteria for sepsis 3.0 jointly released by the American Society for Critical Care Medicine (SCCM) and the European Society for Critical Care Medicine (ESICM) in 2016. The definitions, diagnostic criteria, and requirements formulated by the Poison Conference, that is, sepsis 3.0 = infection + Sequential Organ Failure Assessment (SOFA) ≥ 2 (17, 18). Furthermore, patients who did not meet the criteria for admission or had tumors were excluded, and finally retained 619 patients. The study was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province (No. KHLL2023-KY184) and strictly followed the requirements of the Helsinki Declaration, the International Ethical Code for Human Biomedical Research, the World Health Organization (WHO), and the Council of International Medical Science Organizations in the process of the research.
Grouping of patients with Post-sepsis persistent organ dysfunction
The cohort of patients who died during the exclusionary hospitalization were followed up by phone on January 30, 2024. Most of the patients went to the hospital for a laboratory examination related to sepsis after discharge. The NGS samples were collected within 1-6 hours of the patient’s admission (before antibiotics treatment) and the results were completed within 24 hours. The samples include (Pleural fluid: 2/332, ascites: 2/332, cerebrospinal fluid: 4/332, alveolar lavage: 22/332, peripheral blood: 302/332). Furthermore, Genskey (China) completed the high-throughput sequencing of infectious pathogens. Patients with Post-sepsis persistent organ dysfunction were grouped according to their examination results and description. That is (10): neurological dysfunction: patients have cognitive dysfunction, limited limb movement, CT examination of brain infarction or ischemic hypoxic encephalopathy, etc., while patients with acute cerebrovascular lesions and other diseases were excluded. Cardiac dysfunction: NT-proBNP ≥ 3000 pg/mL or cTnI ≥ 0.2 ng/mL or cardiac ultrasound EF < 0.5, oral drugs are needed to improve cardiac function. However, individuals with acute myocardial infarction, acute pericarditis, myocarditis, pulmonary embolism, and other diseases were excluded. Liver dysfunction: AST ≥ 200 U/L, ALT ≥ 200 U/L or TBIL ≥ 35 umol/L, and required oral liver-protecting drugs. Additionally, individuals with diseases such as biliary stone obstruction, acute hepatitis, and liver damage caused by poisoning or drugs were excluded. Kidney dysfunction: Cr ≥ 300 umol/L, requiring oral kidney protection drugs or hemodialysis treatment, while excluding patients with drug-induced renal function injury, urinary obstruction, urinary stones, poisoning, and other diseases. Blood system dysfunction: PLT ≤ 50×109/L, requires a dynamic review of blood routine, DIC, and platelet therapy. Moreover, patients with blood systems, immune systems, and hemorrhagic diseases were removed. Figure 1 shows our research process.
Statistical method
After exclusion, 619 patients with different organ damage and Post-sepsis persistent organ dysfunction (excluding those who died during hospitalization) were included. Of the 619 patients, 332 were tested for NGS, and 294 were NGS positive. First, the pathogens distribution was assessed in all NGS-positive patients, followed by patients with different organ dysfunction. Lastly, the survival analysis was conducted on patients infected with different pathogens. The SOFA score in this study was expressed by the mean ± standard deviation. Moreover, the mortality rate, the detection frequency of pathogens, and the infection rate of the population were presented by percentage. A chi-square test (IBM SPSS Statistics 21.0) was performed to assess the differences between pathogens, while a Log-rank (Mantel-Cox) test was conducted to evaluate the survival difference. All statistical analyses were carried out and graphs were drawn using GraphPad Prism 9.5.1. p < 0.05 indicated statistical differences.
Results
Patient’s characteristics
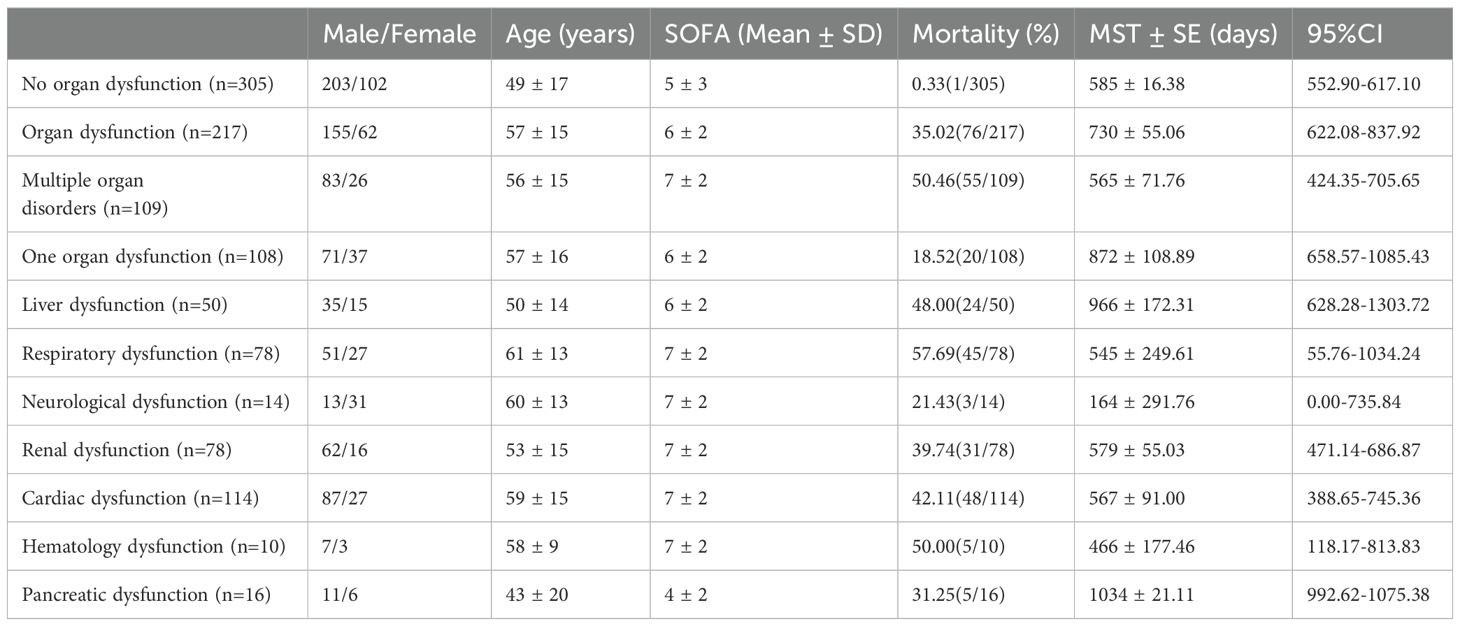
Figure 1 shows a brief process.362 patients were excluded from our cohort, and a total of 619 sepsis patients were included, with a mortality rate of 27.63% (Figure 2A). Based on the SOFA scores, patients were grouped according to their dysfunction, including liver dysfunction, respiratory dysfunction, endocrine dysfunction, neurological dysfunction, renal dysfunction, Hematologic dysfunction, and Circulatory dysfunction. Supplementary Table S4 shows the basic characteristics of patients. Furthermore, the survival analysis of these patients was conducted, which revealed that patients with respiratory dysfunction (Figure 2B) (P=0.0037), circulatory dysfunction (P<0.0001) (Figure 2C), hematologic dysfunction(P=0.0072) (Figure 2D), renal dysfunction(P <0.0001) (Figure 2E), and neurological dysfunction (Figure 2F) (P<0.0001) had poor survival. However, patients with liver dysfunction survived better(P=0.0354) (Figure 2G).
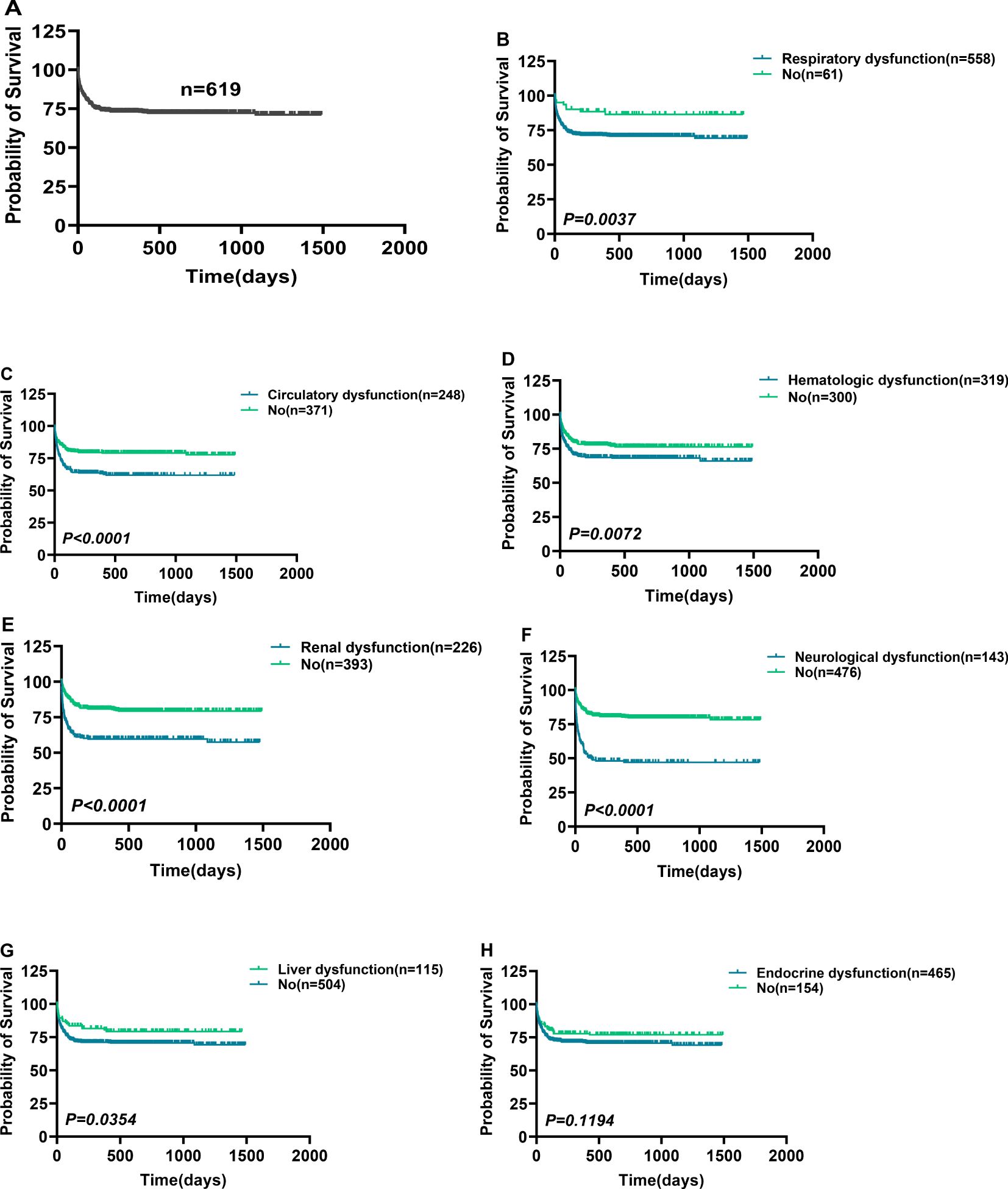
Figure 2. Comparison of survival in patients with different organ dysfunction. Comparison of survival in patients with different organ dysfunction. (A) A total of 619 patients were included in this study. (B–F) Sepsis-associated respiratory (B), circulatory (C), hematologic (D), renal (E), neurological (F), liver (G), and endocrine dysfunction (H) Comparison of survival with non-corresponding dysfunction. p < 0.0500 = statistically difference, and p > 0.0500 = no statistical difference.
Characteristics of patients with Post-sepsis persistent organ dysfunction
This research included 305 patients that indicated no Post-sepsis persistent organ dysfunction and had a mortality rate of 0.33% (1/305), whereas 217 patients had Post-sepsis persistent organ dysfunction (excluding accidental death), 109 indicated post-sepsis multi-organ dysfunction, and 108 patients had Post-sepsis persistent organ dysfunction with a mortality rate of 35.02% (76/217), 50.46% (55/109), and 18.52% (20/108), respectively. The patients were further subdivided based on different organ dysfunction, and Table 1 shows the basic information of these patients. The survival analysis revealed that the survival rate of patients with one or multiple organ dysfunction was significantly lower than that of patients without Post-sepsis persistent organ dysfunction and those with different organ dysfunction (P<0.0001) (Figure 3A, Supplementary Figures S1A–I). Furthermore, the survival of patients with Post-sepsis persistent multi-organ dysfunction was also substantially reduced than those with Post-sepsis persistent organ dysfunction (P<0.0001) (Figure 3B). Moreover, the survival of patients with Post-sepsis persistent Cardiac dysfunction (P=0.0098) (Figure 3C), liver dysfunction (P=0.0254) (Figure 3D), and respiratory dysfunctions(P<0.0001) (Figure 3E) was worse than other patients with Post-sepsis persistent organ dysfunction. In addition, there was no statistical difference in the survival rate of patients with post-sepsis renal dysfunction (P=0.3664) (Figure 3F), pancreatic dysfunction (P=0.6218) (Figure 3G), neurological dysfunction (P=0.3620) (Figure 3H), hematologic dysfunction (P=0.1920) (Figure 3I) than other patients with Post-sepsis persistent organ dysfunction. Overall, it was revealed that patients with Post-sepsis persistent cardiac, liver, and respiratory dysfunctions have poor survival.
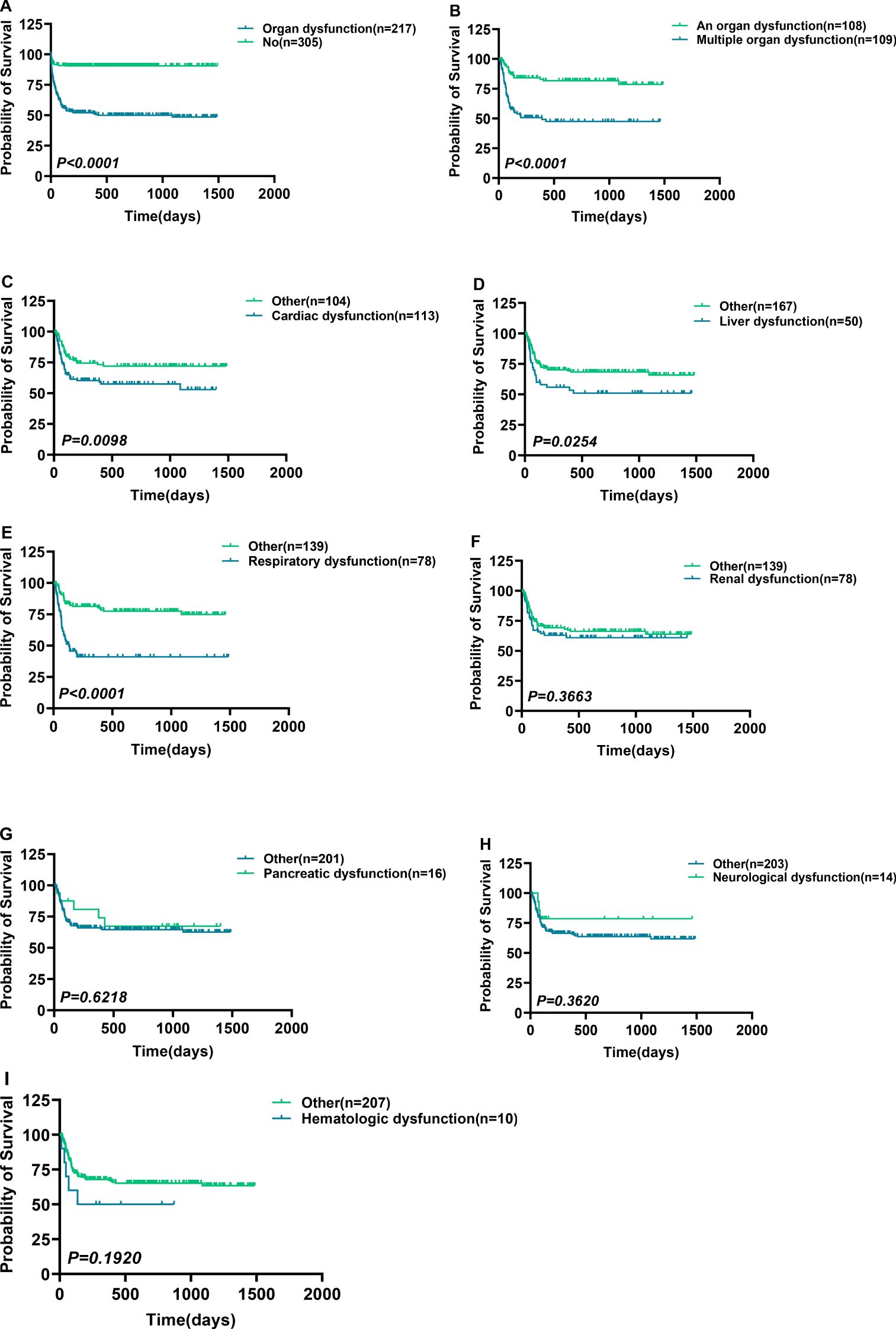
Figure 3. Survival differences in patients with post-sepsis persistent organ dysfunction. Survival differences in patients with post-sepsis persistent organ dysfunction. (A, B) Survival comparison of Post-sepsis persistent organ dysfunction patients with patients without Post-sepsis persistent organ dysfunction (A), and patients with post-sepsis persistent multiple organ dysfunction (B). (C–I) Ppost-sepsis persistent cardiac (C), liver (D), respiratory (E), renal (F), pancreatic (G), neurological (H), and hematologic (I) dysfunction comparison of survival without remaining corresponding dysfunction. p < 0.05 = statistically difference, and p > 0.05 = no statistical difference.
Frequency of pathogens detection in patients with different organ dysfunction
To explore the distribution of pathogens in sepsis patients with different organ dysfunctions, the genus of 332 mNGS-tested patients was analyzed. Of these, 294 (88.55%) patients were positive and 38 patients were negative for pathogens infection. Among these patients, 58 different pathogens genera were detected, with a total of 669 pathogens species identified across these genera. The first three pathogens with the highest abundance were Alphatorquevirus (10.61%), Klebsiella (10.16%), and Cytomegalovirus (8.22%) (Figure 4A, Supplementary Table S1). Then the patients who had the NGS test were grouped according to the SOFA 3.0 score table, which revealed that the abundance of Klebsiella (10.35%), Alphatorquevirus (10.18%), and Escherichia (8.35%) was the highest in patients with respiratory dysfunction (Figure 4B, Supplementary Table S1), while that of Alphatorquevirus (11.25%), Lymphocryptovirus (7.90%), Klebsiella (7.90%), Cytomegalovirus (7.90%) and Escherichia (7.60%) was highest in patients with Hematologic dysfunction (Figure 4C, Supplementary Table S1). Furthermore, the detection frequency of Alphatorquevirus (9.92%), Klebsiella (9.09%), Cytomegalovirus (9.09%), and Lymphocryptovirus (8.26%) was greatest in patients with liver dysfunction (Figure 4D, Supplementary Table S1). Moreover, Escherichia (9.34%), Lymphocryptovirus (9.00%), Klebsiella (9.00%), and Alphatorquevirus (7.96%) were detected most in patients with circulatory dysfunction (Figure 4E, Supplementary Table S1). Among patients with renal dysfunction, Alphatorquevirus (10.34%), Escherichia (9.58%) and Klebsiella (9.20%) had the highest detection frequency (Figure 4F, Supplementary Table S1). Whereas in neurological dysfunction patients, Cytomegalovirus (9.74%), Alphatorquevirus (9.74%), Roseolovirus (7.79%), Escherichia (7.79%), Klebsiella (7.14%) and Enterococcus (7.14%) had the highest abundance (Figure 4G, Supplementary Table S1). In addition, Alphatorquevirus (10.27%), Klebsiella (9.89%) and Cytomegalovirus (8.94%) had the highest detection frequency among patients with endocrine system dysfunction (Figure 4H, Supplementary Table S1). These results indicated that infectious pathogens distribution in sepsis patients with different organ dysfunctions is different.
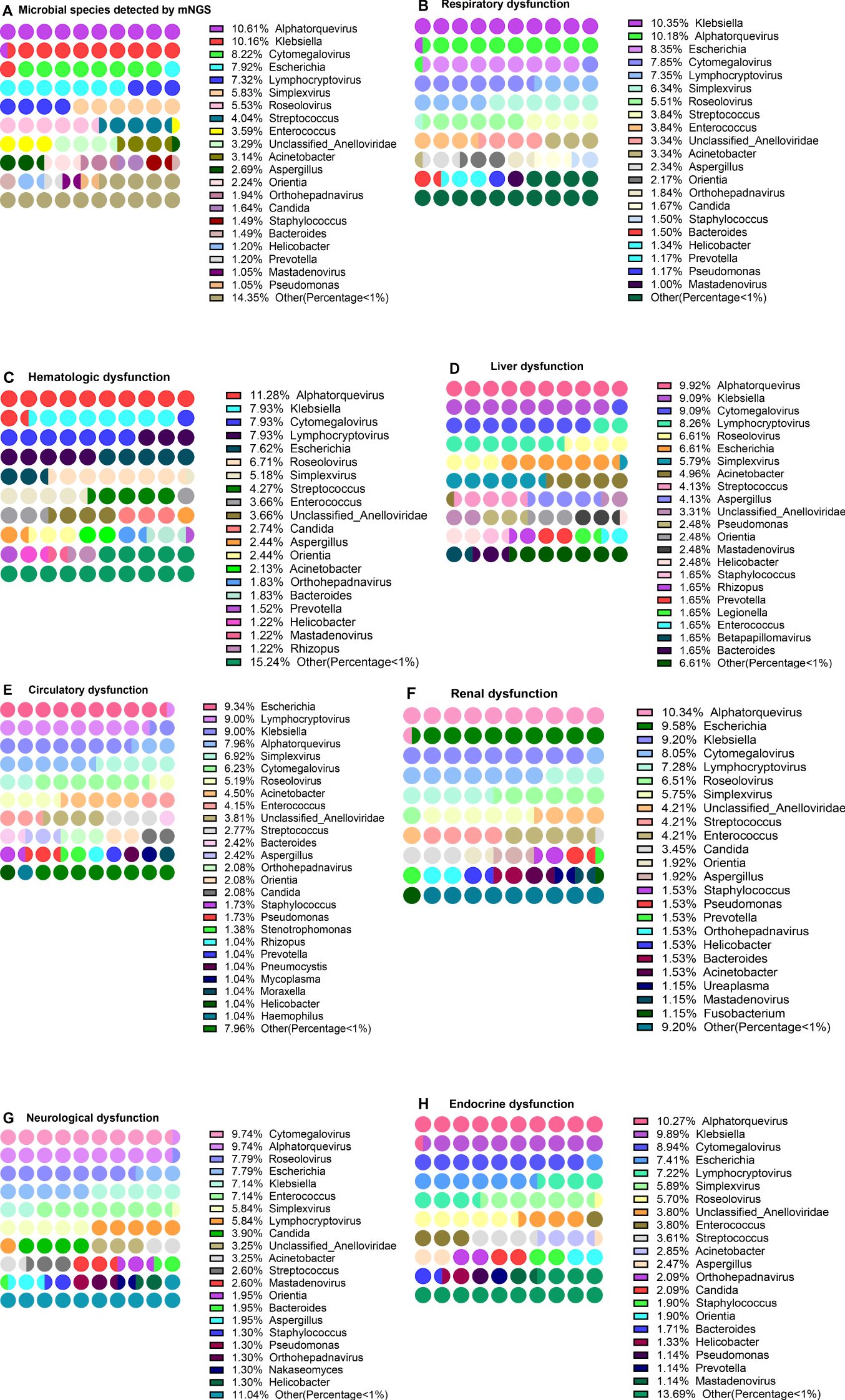
Figure 4. Distribution of pathogen in patients with different organ dysfunction. Distribution of pathogen in patients with different organ dysfunction. (A) Pathogen detected by mNGS, (B–H) respiratory dysfunction (B), hematologic dysfunction (C), liver dysfunction (D), circulatory dysfunction (E), renal dysfunction (F), neurological dysfunction (G), and Endocrine dysfunction (H).
Frequency of detection of pathogens in patients with Post-sepsis persistent organ dysfunction
In addition to different organ dysfunction caused by sepsis, patients often suffer from certain organ dysfunction even after sepsis treatment (here referred to as Post-sepsis persistent organ dysfunction). To explore the distribution of pathogens in patients with Post-sepsis persistent organ dysfunction, 43 patients who died during hospitalization were removed, and the remaining 251 were analyzed. Of these, 101 patients were analyzed for the detection frequency of pathogens. Among the patients with Post-sepsis persistent organ dysfunction, 258 of 44 pathogens were detected. The first three genera with the highest detection frequency were Alphatorquevirus (11.63), Klebsiella (8.91%), Escherichia (8.14%), and Cytomegalovirus (8.14%) (Figure 5A, Supplementary Table S2). There were 150 patients without Post-sepsis persistent organ dysfunction, and indicated 53 of 328 pathogens. The first three pathogens with the highest detection frequency are Alphatorquevirus (11.28%), Klebsiella (10.67%), and Cytomegalovirus (8.23%) (Figure 5B, Supplementary Table S2).
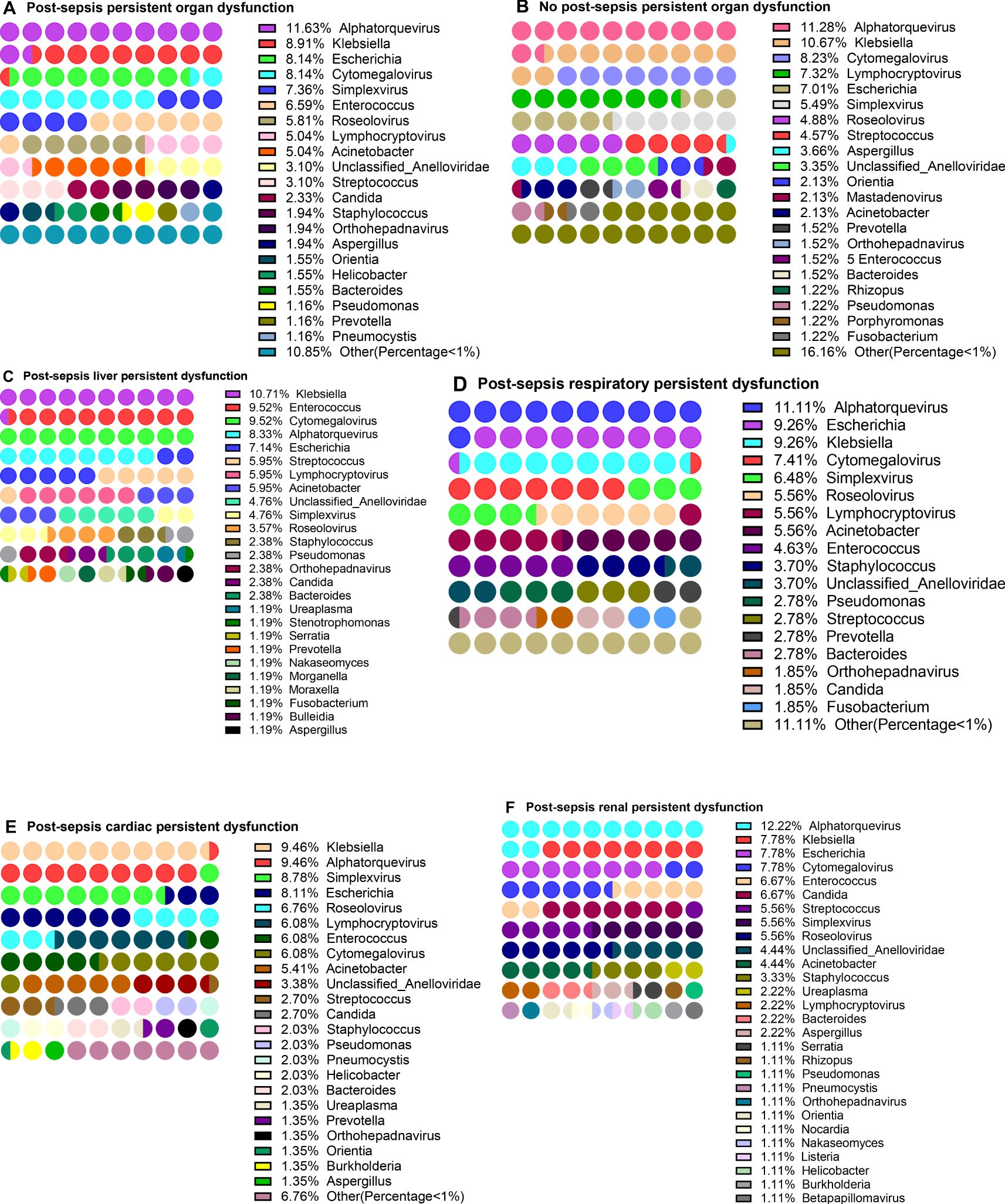
Figure 5. Distribution of pathogens in patients with post-sepsis persistent organ function. Distribution of pathogens in patients with post-sepsis persistent organ function. (A–F) Pathogensl distribution of patients with Post-sepsis persistent organ dysfunction (A), no Post-sepsis persistent organ dysfunction (B), post-sepsis persistent liver dysfunction (C), post-sepsis persistent respiratory dysfunction (D), post-sepsis persistent Cardiac dysfunction (E), and post-sepsis persistent renal dysfunction (F).
Furthermore, the patients with post-sepsis persistent different organ dysfunction were further grouped and revealed that in the post-sepsis persistent liver dysfunction patients, Klebsiella (10.71%), Enterococcus (9.52%), Cytomegalovirus (9.52%) and Alphatorquevirus (8.33%) had the highest detection frequency (Figure 5C, Supplementary Table S2). In patients with post-sepsis persistent respiratory dysfunction, the highest detection frequency was of Alphatorquevirus (11.11%), Escherichia (9.26%), Klebsiella (9.26%) Cytomegalovirus (7.41%) (Figure 5D, Supplementary Table S2).In patients with Post-sepsis persistent cardiac dysfunction, Klebsiella (9.46%), Alphatorquevirus (9.46%), Simplexvirus (8.78%), and Escherichia (8.11%) had the highest detection frequency (Figure 5E, Supplementary Table S2). Moreover, Alphatorquevirus (12.22%), Escherichia (7.78%), Klebsiella (7.78%), Cytomegalovirus (7.78%), Enterococcus (6.67%) and Candida (6.67%) had the highest the detection frequency in patients with Post-sepsis persistent renal dysfunction (Figure 4F, Supplementary Table S2). These results suggest that the distribution of pathogens is not the same in patients with Post-sepsis persistent organ dysfunction.
Differences in pathogens in patients with organ dysfunction
The aforementioned data indicated that the detection frequency of pathogens in patients with different organ dysfunction is different from the infection rate of the population, and there were also differences in survival rate (Supplementary Table S1, Figure 2). Therefore, it was suspected that different pathogens infections are related to differential organ dysfunction and survival rates. To confirm this, A chi-square test was performed on patients with different organ dysfunction, the results showed (Supplementary Table S3) that the frequency of detection and population infection of Legionella(P=0.0234) and Betapapillomavirus(P=0.0234) in patients with liver dysfunction was higher than in patients without liver dysfunction. In addition, compared with patients without respiratory dysfunction, patients with respiratory dysfunction have lower detection rates of Haemophilus influenzae (P=0.0303) and population infection rates (P=0.0264). Among patients with neurological dysfunction, the detection frequency and population infection of Mastadenovirus, Enterococcus, and Candida were significantly higher than in non-neurological disorder patients (P<0.0500). However, infection with Enterococcus(P=0.3200) and Candida(P=0.9189) in patients with neurological dysfunction does not seem to affect survival (Supplementary Figures S2A, B). The detection frequency of Candida and the incidence of infection was significantly higher in kidney dysfunction and Hematologic dysfunction patients than those without these dysfunction (P<0.0500), but whether infection with Candida affects the survival of patients with renal dysfunction does not seem to affect survival (P=0.3115) (Supplementary Figure S2C). Furthermore, the detection frequency of Burkholderia in Hematological dysfunction patients was lower than that in patients without Hematological dysfunction (P=0.0485), and the infection rate in the population differed significantly (P=0.0500). Among patients with circulatory dysfunction, Moraxella’s detection frequency and infection incidence were significantly higher than those without circulatory dysfunction (P<0.0500). In addition, the Alphatorque virus infection incidence rate in patients without circulatory system dysfunction was markedly higher than in patients with circulatory system dysfunction (P=0.0476), but the Alphatorquevirus infection effect on the survival of patients with circulatory dysfunction does not seem to affect survival (P=0.2636) (Supplementary Figure S2D). The genus without survival analysis is due to the sample size of < 5 (Supplementary Table S1). Overall, sepsis patients with different organ dysfunction may be infected with different pathogens.
Differences in pathogens in patients with Post-sepsis persistent organ dysfunction
As mentioned above, in addition to different organ dysfunction caused by sepsis, patients often suffer from certain organ dysfunction even after sepsis treatment. Patients with Post-sepsis persistent organ dysfunction often have poor survival (Figures 3A–I). The analysis of post-sepsis dysfunction of different organs revealed that the detection frequency of different pathogens is different in post-sepsis different organ dysfunction patients (Figure 5, Supplementary Table S2). To explore whether the infection of different pathogens is related to Post-sepsis persistent persistent organ dysfunction, A Chi-square test (Table 2) was performed to compare the pathogens between Post-sepsis persistent organ dysfunction patients and those without Post-sepsis persistent organ dysfunction patients. It was found that among patients with Post-sepsis persistent organ dysfunction, the detection frequency and population infection rate of Enterococcus was significantly higher than that of patients without Post-sepsis persistent organ dysfunction, but the detection frequency and population infection rate of Mastadenovirus were higher in patients without Post-sepsis persistent organ dysfunction. In addition, Pneumocystis(P=0.0337) and Acinetobacter(P=0.0186) had a higher population infection rate in patients with Post-sepsis persistent organ dysfunction, but there is no statistical difference in detection frequency (P>0.0500). Moreover, no statistical difference was observed in the survival of Enterococcus(P=0.9646) and Acinetobacter-infected(P=0.1184) patients with Post-sepsis persistent organ dysfunction (P>0.0500) (Supplementary Figures S3A, B). Among patients with post-sepsis persistent liver dysfunction, the detection frequency and infection rate of Enterococcus and Morganella were much higher than that of patients without post-sepsis persistent liver dysfunction (P<0.0500). The Population infection rate of Acinetobacter was much higher than the patients without post-sepsis persistent liver dysfunction (P = 0.0050), but there is no statistical difference in the detection frequency of Acinetobacter in the two groups (P=0.0633). There seems to be no impact on the survival of patients with post-sepsis persistent liver dysfunction infected with Enterococcus(P=0.6112) or Acinetobacter(P=0.5613)(Supplementary Figure S3C, D). Furthermore, the detection frequency and population infection rate of Staphylococcus were higher in patients with post-sepsis persistent respiratory dysfunction (P<0.0500), but there was no statistical difference in the detection frequency of Enterococcus(P=0.0615) and Acinetobacter(P=0.0698). In addition, Enterococcus-infected patients with Post-sepsis persistent respiratory dysfunction had worse survival than patients who were not infected with Enterococcus (P = 0.0041) (Supplementary Figure S3E), Infection with Acinetobacter also had no statistical significance (P=0.1162) (Supplementary Figure S3F). Moreover, Enterococcus and Candida have higher detection frequency and population infection rates in patients with post-sepsis persistent Renal dysfunction(P<0.0500). In addition, the infection rate of Pneumocystis(P=0.0352), Staphylococcus(P=0.0431), and Listeria(P=0.0352) was significantly higher in post-sepsis persistent renal dysfunction patients than those without post-sepsis persistent renal dysfunction; however, there was no statistical difference in detection frequency (Pneumocystis, P=0.0560; Staphylococcus, P=0.0875; Listeria,P=0.0560). Furthermore, there was also no statistical difference in the survival rate of Enterococcus(P=0.2684) and Candida-infected(P=0.3659) patients with post-sepsis persistent renal dysfunction (Supplementary Figures S3G, H). Enterococcus and Pneumocystis also had higher detection frequency and population infection rates in patients with post-sepsis persistent cardiac dysfunction (P<0.0500). Additionally, compared with patients with post-sepsis persistent cardiac dysfunction,the infection rate of Acinetobacter was markedly higher than that of patients without post-sepsis persistent cardiac dysfunction (P=0.0250), but there was no statistical difference in detection frequency (P=0.0586). No statistical difference was observed in the survival of Enterococcus(P=0.7821) and Acinetobacter-infected(P=0.4701) patients with post-sepsis persistent cardiac dysfunction(Supplementary Figures S3I-J). The pathogens without survival analysis are due to the sample size of < 5 (Supplementary Table S2).In summary, these results indicate that the types of pathogen infections in patients with different post-sepsis persistent organ dysfunction are different.In addition, patients with post-sepsis persistent respiratory dysfunction infected with Enterococcus have worse survival.
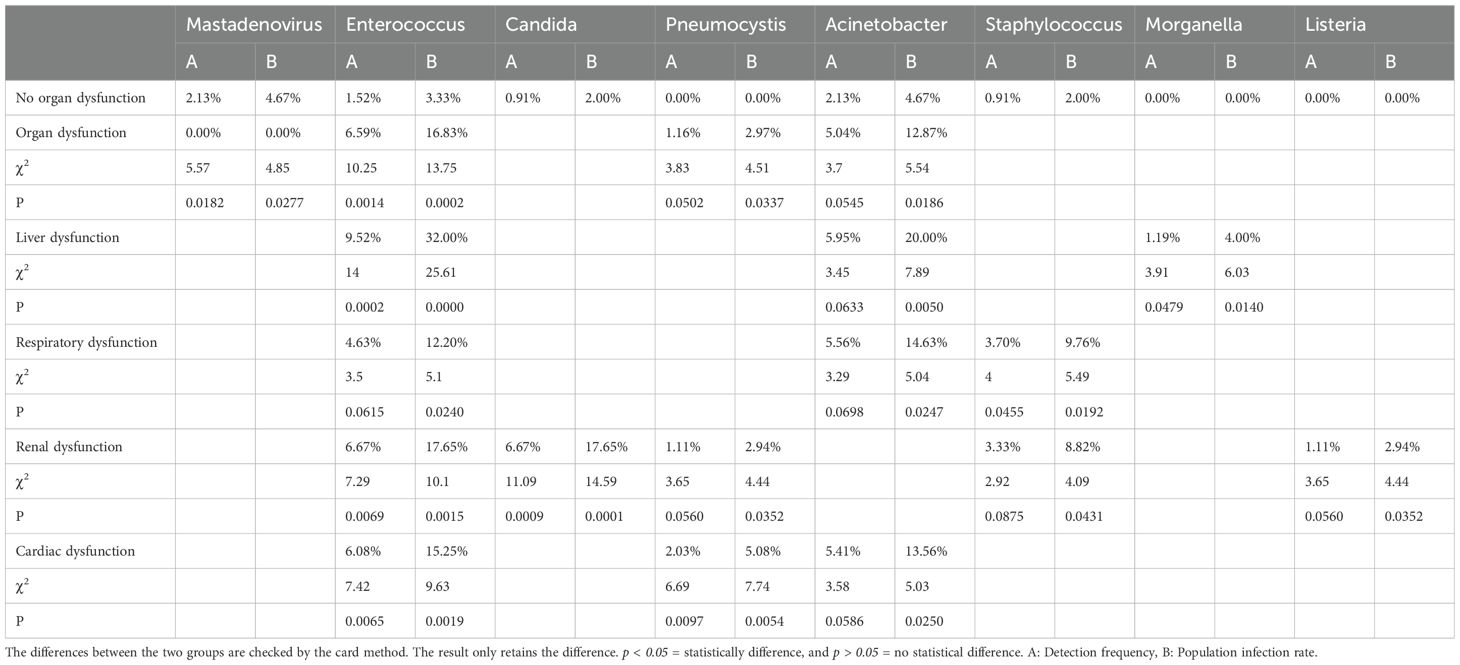
Table 2. Differences between pathogens and population infections in patients with Post-sepsis persistent organ dysfunction.
Differences in pathogens of surviving and dead patients
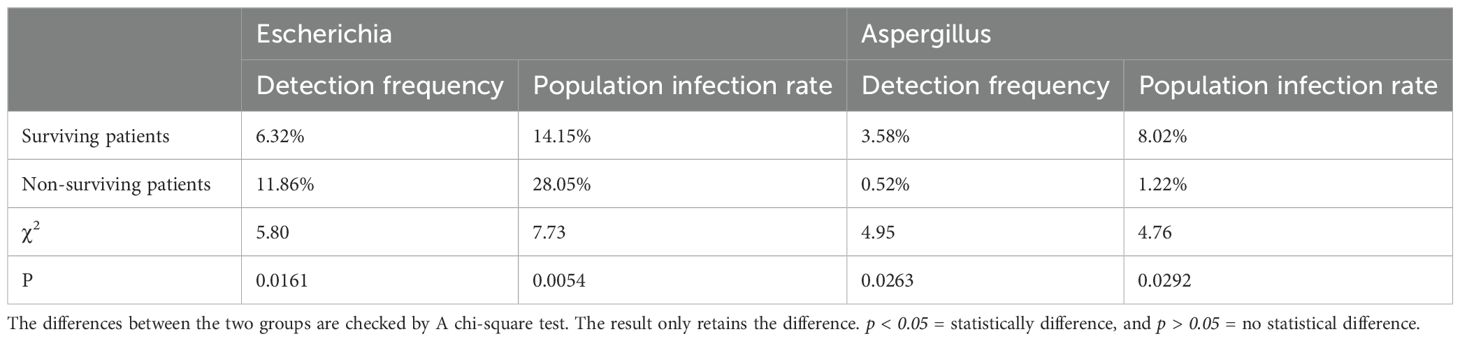
The above data proved that patients with multiple organ dysfunction and post-sepsis dysfunction of different organs are related to different pathogens genera. Whether the pathogens genus was related to patient survival was also assessed. Of 294 patients, 82 died and 212 survived. A total of 475 pathogens types were detected in surviving patients. The first three pathogens with the highest detection frequency were Alphatorquevirus (11.79%), Klebsiella (10.32%), and Cytomegalovirus (8.63%) (Figure 6A), the infection rate in the population was 26.42%, 23.11% and 19.34% respectively. Furthermore, 36 of 194 pathogens types were detected in the dead patients, where Escherichia (11.86%), Klebsiella (9.79%), Lymphocryptovirus (7.73%) and Alphatorquevirus (7.73%) (Figure 6B) were the most abundant pathogens with the infection rate of 28.05%, 23.17%, 18.29%, and 18.29%, respectively. It was found that Escherichia had a higher detection frequency(P=0.0161) and population infection rate(P=0.0054) among dead patients (Table 3), and alive patients infected with Escherichia had a higher mortality rate (P= 0.0029) (Figure 6C). Overall, sepsis patients should be carefully monitored for Escherichia infection. And take proactive measures against infected patients.
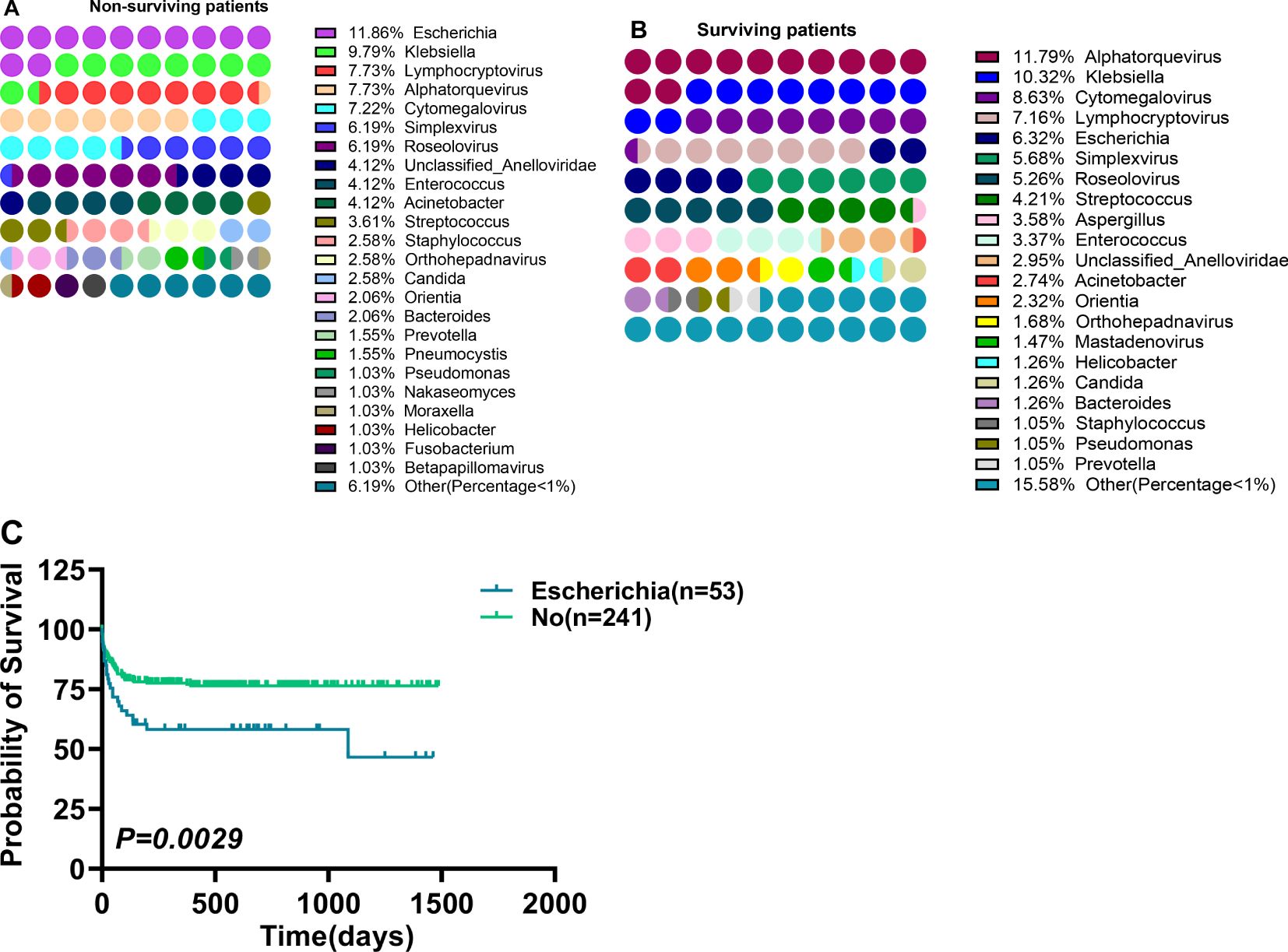
Figure 6. Differences between pathogens distribution in surviving and non-surviving patients, and the survival of patients infected with different pathogens. (A, B) Differences between pathogens distribution in surviving (A) and non-surviving patients (B), (C) Survival differences in patients infected with Escherichia. p < 0.05 = statistically difference, and p > 0.05 = no statistical difference.
Discussion
The literature has indicated that the tissue and organ damage of sepsis patients is mainly caused by either pathogens, a series of inflammatory reactions, or metabolic abnormalities. Whether other sources of infection cause damage to the function of different organs remains to be determined. This research revealed that the distribution of pathogens in survivors and sepsis patients who died is different, and the survival rate of patients with Enterococcus infection combined with post-sepsis persistent respiratory dysfunction is also worse.And patients with Respiratory, Liver, Circulatory, Hematologic, Neurological, and Renal dysfunction had poor survival. And patients with post-sepsis persistent organ dysfunction after sepsis have worse survival rates. In addition, we found the infection rates of Legionella and Betapapillomavirus were higher in patients with liver dysfunction. The infection rates of Mastadenovirus, Enterococcus, and Candida were higher in patients with neurological dysfunction. The infection rates of Candida were higher in patients with renal dysfunction and hematologic dysfunction. The infection rates of Moraxella were higher in patients with circulatory dysfunction.The infection rates of Enterococcus, Pneumocystis, and Acinetobacter were higher in patients with Post-sepsis cardiac dysfunction.The infection rates of Enterococcus, Acinetobacter, and Morganella were higher in patients with Post-sepsis liver dysfunction. The infection rates of Enterococcus, Acinetobacter, and Staphylococcus were higher in patients with Post-sepsis respiratory dysfunction. The infection rates of Enterococcus, Candida, Pneumocystis, Staphylococcus, and Listeria were higher in patients with Post-sepsis renal dysfunction.In addition, we found that patients with Escherichia infection in sepsis had the lowest survival rate.
With advancing research, the mechanism of organ function damage caused by various pathogens is becoming clearer. The interaction of alpha-hydroxyprotein protein and its cell receptor ADAM10 (inducing rapid platelet aggregation) after Staphylococcus infection impures endothelial repair function, and promotes neutrophil inflammation signal transmission to form harmful platelet-neutrophil aggregation in organs. Dynamic microthrombus forms in microcirculation cause multi-organ dysfunction of the liver, kidney, and lungs (11–13). Moreover, it has been observed that in Enterococcus fecal infection, the determined cluster disulfide bond-forming protein A (DsbA) is necessary for its virulence, inhibits cardiomyocyte’s inflammatory immune response, and promotes apoptosis as well as necrosis, thus forming cardiac micropathy (14). The fecal Enterococcus synthesizes peptidoglycan in the liver cell, indicating that fecal Enterococcus replicates in the liver cells. After the liver’s innate immune induction, macrophages and neutrophils almost disappear, eventually leading to the formation of Enterococcus lesions in the liver. Moreover, this process is not limited to specific cell types and may also occur in other organs such as it has been observed that the intracellular division of fecal Enterococcus can be extended to the kidneys (15). In addition, Enterococcus is related to the enzymatic ability to convert tyrosine or levodopamine into dopamine, which affects the dopamine content of the body and the function of the nervous system (16, 17). This study also found an increased rate of Enterococcus infection in neurological dysfunction (Supplementary Table S3), and post-sepsis persistent liver, respiratory, renal, and cardiac dysfunctions (Table 2).
This research identified that the mortality rate of patients infected with Escherichia is higher (Figure 5C). This may be related to Escherichia’s ability to acquire virulence factors that cause diseases (adhesiverin, pod membrane, synthesis and secretion of toxins, etc.) and antibiotic resistance, which reduces their sensitivity to certain anti-infective molecules (16, 17). In addition, some studies have shown that Escherichia has a highly diverse genetic background and VF gene profile in sepsis patients. Furthermore, the expression of Escherichia cnf and blaTEM genes in sepsis patients is related to the disease severity, while the expression of the yuA gene is related to mortality (18, 19). Dispersive Candida infection mainly occurs in patients with neutropenia, and human neutrophils are the only immune cells that can prevent yeast’s transformation into filamentous growth. Furthermore, increased levels of Candida albicans’ transcription response in the whole blood (20–22) and the complement system defects enhance the susceptibility to invasive Candida albicans infection (23, 24). Patients with neutropenia invasive candidiasis may need corticosteroid treatment after neutrophil reconstruction to avoid adverse reactions and remnants of excessive inflammation because Candida raises neutrophils in the kidneys and produces IL-17 to damage the kidneys (25). Here, Candida was observed to be associated with the incidence of nervous system, renal, and respiratory system dysfunctions (Supplementary Table S3), as well as post-sepsis renal dysfunction (Table 2).
Although the mechanism from infection to organ dysfunction comprises the interaction of complex factors, the different pathways are all related to inflammatory reactions, immune disorders, metabolic abnormalities, etc. Currently, the sepsis treatment is primarily focused on antipathogensl treatment and organ protection support. However, the high mortality rate of sepsis patients has been constant for the past 10 years. Furthermore, the increased mortality rate of sepsis might be due to the lack of treatment for host immunity that can reverse the disorder (26). Severe inflammation can benefit from anti-inflammatory treatment; however, for immunosuppression, immunostimulant treatment should be considered. A suitable reference for appropriate immunotherapy strategies can be selected from biomarkers such as absolute count of lymphocytes and HLA-DR expression on monocytes can stimulate recombinant glycosylated human IL-7, recombinant interferon-γ (INF-γ), anti-TNF-α, recombinant granulocyte-macrophage colony (GM-CSF) and anti-PD-1 antibodies stimulation for suitable patient’s treatment (26–29). For example, according to the serum ferritin concentration and the expression of HLA-DR on circulating CD14+ monocytes, some patients with macrophage activation-like syndrome (MALS) (high serum ferritin) receive IL-1Ra treatment (30). Since sepsis often involves multiple pathogen infections, organ injuries, and complex immune responses, a single drug cannot reverse these effects throughout the disease range. Therefore, subgroup classification of patients with similar pathophysiology and clinical stratification is more significant for improving the efficacy of immunomodulatory therapy (26, 31, 32).
Overall, we found differences in the types and proportions of pathogens infected in patients with different organ dysfunction and Post-sepsis persistent organ dysfunction. The combination of Escherichia infection and Enterococcus infection with post-sepsis persistent respiratory dysfunction can affect the survival of patients. We should strengthen the management of sepsis patients, especially those with Post-sepsis persistent organ dysfunction. However, this study has certain limitations: 1) Because there are often multiple pathogens and organ dysfunctions in sepsis patients, a single pathogen or organ dysfunction cannot be studied. This research only indicated their possible relation. 2) This is a single-center data analysis, which may not represent all sepsis patients. These factors are universal and objective and also affect the research of other institutions. The mechanism of organ function damage and legacy organ dysfunction is complex crosstalk, which has not been fully analyzed. The early reaction of sepsis patients is the activation of the body’s self-protective mechanism for survival, and with the disease progression, this self-protection mechanism causes serious damage to the body. Therefore, some treatment strategies should be altered and in addition to the removal of pathogens and supportive treatment of organ function, sepsis management should also be considered. For instance, initially, active empirical antibiotic treatment should be given and a plan should be quickly formulated to identify pathogens for accurate treatment and maximum eradication of pathogens. Furthermore, biomarkers should be monitored in real-time during treatment, and suitable immune stimulation (GM-CSF, IFNγ, IL-7, etc.), inflammatory response suppression (corticosteroids, TNF-α antagonists, PD-1 antagonists, IL-1 receptor antagonists) according to biomarkers and the patient’s clinical characteristics (Akinra), metabolic regulation (cholesterol supplementation, iron removal therapy, antioxidant therapy, etc.), and nutrient supplementation treatment should be considered to achieve precision. Moreover, after patients are discharged from the hospital, especially those with Post-sepsis persistent organ dysfunction, should receive more attention. If the patient has PICS, it may lead to long-term chronic diseases, re-infection, and other diseases. For such patients, it is recommended to formulate a strategy to promote the elimination of inflammation, antioxidants, immune balance, and metabolic recovery according to the patient’s situation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First People’s Hospital of Yunnan Province. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QG: Writing – original draft, Conceptualization, Validation. ZL: Conceptualization, Funding acquisition, Writing – original draft. JG: Data curation, Investigation, Writing – original draft. BH: Formal Analysis, Project administration, Writing – original draft. TY: Validation, Writing – review & editing. SF: Resources, Writing – review & editing. LZ: Resources, Writing – review & editing. YT: Data curation, Writing – review & editing. YL: Visualization, Writing – review & editing. RZ: Software, Writing – review & editing. WZ: Conceptualization, Methodology, Writing – review & editing. YW: Conceptualization, Investigation, Writing – review & editing. YJW: Conceptualization, Funding acquisition, Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from The Open Project of Yunnan Blood Clinical Medical Center (2022LCZXKF-XY01). Yunnan Provincial Department of Science and Technology—Kunming Medical University Joint Special Project on Applied Basic Research (202301AY070001-064). The Medical Leading Talent Program of Yunnan Province (L-201618). Yunnan Fundamental Research Projects (202501AS070009, and 202501AT070046). Thousand Talents Plan for Yunnan Province (KH-SQR-QNRC-2020-001). Hu Yu expert workstation (202305AF150149). Yunnan Province major difficult diseases of Chinese and Western clinical cooperation pilot project – leukemia.
Acknowledgments
Thank you to all patients for their contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1541634/full#supplementary-material
References
1. Vincent J-L, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. (2013) 381:774–5. doi: 10.1016/S0140-6736(12)61815-7
2. Oczkowski S, Alshamsi F, Belley-Cote E, Centofanti JE, Hylander Møller M, Nunnaly ME, et al. Surviving Sepsis Campaign Guidelines 2021: highlights for the practicing clinician. Polish Arch Internal Med. (2022) 132. doi: 10.20452/pamw.16290
3. Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. Bmj. (2016) 353:i1585. doi: 10.1136/bmj.i1585
4. Rudd K, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. LANCET. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
5. Hollenberg S, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. (2021) 18:424–34. doi: 10.1038/s41569-020-00492-2
6. Okomo U, Senghore M, Darboe S, Bojang E, Zaman SMA, Hossain MJ, et al. Investigation of sequential outbreaks of Burkholderia cepacia and multidrug-resistant extended spectrum β-lactamase producing Klebsiella species in a West African tertiary hospital neonatal unit: a retrospective genomic analysis. Lancet Microbe. (2020) 1:e119–29. doi: 10.1016/S2666-5247(20)30061-6
7. Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. (2006) 34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9
8. Ren D, Ren C, Yao R, Zhang L, Liang X, Li G, et al. The microbiological diagnostic performance of metagenomic next-generation sequencing in patients with sepsis. BMC Infect Dis. (2021) 21:1257. doi: 10.1186/s12879-021-06934-7
9. Bragg L, Tyson GW. Metagenomics using next-generation sequencing. Environ Microbiol. (2014) 1096:183–201. doi: 10.1007/978-1-62703-712-9_15
10. Prescott HC, Angus DC. Enhancing recovery from sepsis. Jama. (2018) 319:62–75. doi: 10.1001/jama.2017.17687
11. Surewaard BGJ, Thanabalasuriar A, Zeng Z, Tkaczyk C, Cohen TS, Bardoel BW, et al. [amp]]alpha;-Toxin Induces Platelet Aggregation and Liver Injury during Staphylococcus aureus Sepsis. Cell Host Microbe. (2018) 24:271–284.e3. doi: 10.1016/j.chom.2018.06.017
12. Powers ME, Becker RE, Sailer A, Turner JR, Bubeck Wardenburg J. Synergistic action of staphylococcus aureus α-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell Host Microbe. (2015) 17:775–87. doi: 10.1016/j.chom.2015.05.011
13. Alfano DN, Miller MJ, Bubeck Wardenburg J. Endothelial ADAM10 utilization defines a molecular pathway of vascular injury in mice with bacterial sepsis. J Clin Invest. (2023) 133. doi: 10.1172/JCI168450
14. Zhou X, Tang G. Prevalence of infective endocarditis in enterococcus faecalis bacteremia, methodology issues. J Am Coll Cardiol. (2019) 74:2434. doi: 10.1016/j.jacc.2019.08.1040
15. Ciocan D, Cassard A-M. Des bactéries intestinales responsables des maladies nutritionnelles du foie éradiquées par phagothérapie. médecine/sciences. (2020) 36:310–2. doi: 10.1051/medsci/2020052
16. Lüthje P, Brauner A. Virulence factors of uropathogenic E. coli and Their Interaction with the Host. Adv Bacterial Pathogen Biol. (2014) 65:337–72. doi: 10.1016/bs.ampbs.2014.08.006
17. Gladstone RA, McNally A, Pöntinen AK, Tonkin-Hill G, Lees JA, Skytén K, et al. Emergence and dissemination of antimicrobial resistance in Escherichia coli causing bloodstream infections in Norway in 2002–17: a nationwide, longitudinal, microbial population genomic study. Lancet Microbe. (2021) 2:e331–41. doi: 10.1016/S2666-5247(21)00031-8
18. Fröding I, Hasan B, Sylvin I, Coorens M, Nauclér P, Giske CG. Extended-spectrum-β-lactamase- and plasmid ampC-producing escherichia coli causing community-onset bloodstream infection: association of bacterial clones and virulence genes with septic shock, source of infection, and recurrence. Antimicrobial Agents Chemother. (2020) 64. doi: 10.1128/AAC.02351-19
19. Mora-Rillo M, Fernández-Romero N, Navarro-San Francisco C, Díez-Sebastián J, Romero-Gómez MP, Fernández FA, et al. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence. (2015) 6:93–100. doi: 10.4161/21505594.2014.991234
20. Wozniok I, Hornbach A, Schmitt C, Frosch M, Einsele H, Hube B, et al. Induction of ERK-kinase signalling triggers morphotype-specific killing of Candida albicans filaments by human neutrophils. Cell Microbiol. (2008) 10:807–20. doi: 10.1111/j.1462-5822.2007.01086.x
21. Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, et al. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol. (2005) 56:397–415. doi: 10.1111/j.1365-2958.2005.04557.x
22. Ermert D, Niemiec MJ, Röhm M, Glenthøj A, Borregaard N, Urban CF. Candida albicans escapes from mouse neutrophils. J LEUKOCYTE Biol. (2013) 94:223–36. doi: 10.1189/jlb.0213063
23. Tsoni SV, Kerrigan AM, Marakalala MJ, Srinivasan N, Duffield M, Taylor PR, et al. Complement C3 plays an essential role in the control of opportunistic fungal infections. Infect Immun. (2009) 77:3679–85. doi: 10.1128/IAI.00233-09
24. Tuite A, Elias M, Picard S, Mullick A, Gros P. Genetic control of suceptibility to Candida albicans in susceptible A/J and resistant C57BL/6J mice. Genes Immun. (2005) 6:672–82. doi: 10.1038/sj.gene.6364254
25. Legrand F, Lecuit M, Dupont B, Bellaton E, Huerre M, Rohrlich PS, et al. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis. (2008) 46:696–702. doi: 10.1086/527390
26. Giamarellos-Bourboulis E, Aschenbrenner AC, Bauer M, Bock C, Calandra T, Gat-Viks I, et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat Immunol. (2024) 25:19–28. doi: 10.1038/s41590-023-01660-5
27. Karakike E, Scicluna BP, Roumpoutsou M, Mitrou I, Karampela N, Karageorgos A, et al. Effect of intravenous clarithromycin in patients with sepsis, respiratory and multiple organ dysfunction syndrome: a randomized clinical trial. Crit Care. (2022) 26:183. doi: 10.1186/s13054-022-04055-4
28. Sehgal IS, Basumatary NM, Dhooria S, Prasad KT, Muthu V, Aggarwal AN, et al. A randomized trial of mycobacterium w in severe presumed gram-negative sepsis. Chest. (2021) 160:1282–91. doi: 10.1016/j.chest.2021.03.062
29. Peters van Ton AM, Kox M, Abdo WF, Pickkers P. Precision immunotherapy for sepsis. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.01926
30. Leventogiannis K, Kyriazopoulou E, Antonakos N, Kotsaki A, Tsangaris I, Markopoulou D, et al. Toward personalized immunotherapy in sepsis: The PROVIDE randomized clinical trial. Cell Rep Med. (2022) 3:100817. doi: 10.1016/j.xcrm.2022.100817
31. van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. (2021) 54:2450–64. doi: 10.1016/j.immuni.2021.10.012
Keywords: sepsis, organ dysfunction, pathogenic pathogens, post-sepsis persistent organ dysfunction, survive
Citation: Gao Q, Li Z, Geng J, Han B, Yang T, Feng S, Zhao L, Teng Y, Li Y, Zhao R, Zhang W, Wang Y and Wang Y (2025) The type of pathogen is associated with organ failure and legacy dysfunction in patients with sepsis. Front. Immunol. 16:1541634. doi: 10.3389/fimmu.2025.1541634
Received: 08 December 2024; Accepted: 10 March 2025;
Published: 31 March 2025.
Edited by:
Kuldeep Gupta, University of Arizona, United StatesReviewed by:
Isha Pandey, Rutgers University, Newark, United StatesShivang Sharma, Johns Hopkins University, United States
Copyright © 2025 Gao, Li, Geng, Han, Yang, Feng, Zhao, Teng, Li, Zhao, Zhang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajie Wang, a2JiMTY1d3lqQHNpbmEuY29t; Yi Wang, cWtnaHZtMDE4QDE2My5jb20=; Wei Zhang, emhhbmd3ZWlAeWR5eS5jbg==
†These authors have contributed equally to this work
 Qinfen Gao1†
Qinfen Gao1† Zengzheng Li
Zengzheng Li