- 1Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine, Lu’an, China
- 2Department of Orthopedics Department, Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine, Lu’an, China
- 3Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine, Lu’an, China
Immune checkpoint inhibitors (ICIs) are extensively utilized in the treatment of oncological patients, and the immune-related adverse reactions they induce merit close monitoring. This report describes a case of a lung cancer patient who, after receiving chemotherapy in combination with the programmed death-1 (PD-1) inhibitor sintilimab, presented with systemic fatigue, alterations in mental and behavioral patterns, somnolence, and symptoms of diabetes insipidus, leading to a diagnosis of grade 4 immune-related hypophysitis. The patient experienced symptomatic relief following pulse therapy with dexamethasone sodium phosphate injections (30mg every 12 hours), and was subsequently treated with prednisone acetate tablets (30 mg/day), which were gradually reduced to a physiological replacement dose. The treatment with sintilimab was discontinued, and the patient’s symptoms gradually improved, with normalization of urine output.
1 Introduction
In recent years, immune checkpoint inhibitors (ICIs) have made significant therapeutic advancements in the treatment of non-small cell lung cancer (NSCLC), effectively prolonging patient survival and improving their quality of life. However, while ICIs activate the immune system to combat tumors, they can also potentially cause damage to multiple organ systems in patients. Among these, endocrine system side effects are particularly common, and the endocrine glands’ susceptibility may be related to the high vascular distribution characteristics of glands such as the pituitary, thyroid, and adrenal glands (1). Immune-related hypophysitis (irH), as a relatively difficult-to-diagnose endocrine system adverse reaction, can present with a variety of clinical manifestations, including headaches, visual impairments, and symptoms of pituitary dysfunction (2). Literature reports indicate that the incidence of immune-related hypophysitis varies widely, ranging from 1.8% to 18.3%, and may be related to factors such as patient age, gender, drug dosage, and type (3).
Sintilimab, as a novel PD-1 inhibitor, has shown good efficacy and safety in the treatment of lung adenocarcinoma. However, with the increase in clinical application, cases of hypophysitis induced by sintilimab have also been increasingly reported (4), presenting new challenges for clinicians in terms of diagnosis and treatment. The purpose of this article is to detail, through a case report, the clinical manifestations, diagnostic process, treatment strategies, and prognosis of a lung adenocarcinoma patient who developed hypophysitis following treatment with sintilimab, with the aim of enhancing clinicians’ awareness and management capabilities regarding such adverse reactions.
2 Case report
2.1 Patient’s chief complaint, past medical history, personal history, physical examination, and auxiliary examinations
A 61-year-old male patient was admitted to the hospital with the complaint of “having been diagnosed with lung cancer for over six months, and experiencing facial palsy for three days.” The patient has a history of smoking for over 30 years, consuming 2 packs daily. He denies a history of hypertension, diabetes, coronary heart disease, surgery, trauma, and exposure to epidemic water or areas. He is married with children, all in good health, except for his second son who passed away from lung cancer. Upon admission, physical examination revealed: T 36.5℃, P 83 beats per minute, R 19 breaths per minute, BP 110/87 mmHg, height 170 cm, weight 70 kg, body surface area 1.82 m², Karnofsky Performance Status (KPS) 70, Numerical Rating Scale (NRS) 5. The patient is conscious, appears lethargic, cooperates with the examination, and responds appropriately. There is no jaundice of the skin or mucous membranes, no petechiae or ecchymosis. No superficial lymph node enlargement is palpable. The head is normocephalic, lips without cyanosis, no abnormalities in the eyes, ears, or nose, no jugular venous distension, the neck is supple, trachea is midline, and both thyroid lobes are not enlarged. Breath sounds in both lungs are clear, with no crackles or wheezes. The heart rhythm is regular, with no murmurs. The abdomen is soft, with no abnormalities palpable, no lower limb edema, and no pathological reflexes in the nervous system. Laboratory tests revealed the following levels: D-dimer at 8.81 μg·ml–1 (normal range: <0.5 μg·ml–1), fibrin degradation product at 16.11 μg·ml–1 (normal range: <5 μg·ml–1). Tumor marker tests for squamous cell carcinoma indicated elevated levels: neuron-specific enolase at 55.83 ng·ml–1 (normal range: <16.3 ng·ml–1), cytokeratin 19 fragment at 187.81 ng·ml–1 (normal range: <3.3 ng·ml–1), carcinoembryonic antigen at 8184.54 ng·ml–1 (normal range: <5 ng·ml–1), and carbohydrate antigen at 6421.68 U·ml–1 (normal range: <35 U·ml–1). Liver function tests showed: alanine aminotransferase at 48.58 U·L–1 (normal range: 5-40 U·L–1), aspartate aminotransferase at 43.28 U·L–1 (normal range: 8-40 U·L–1), albumin-to-globulin ratio at 1.41 (normal range: 1.5-2.5), alkaline phosphatase at 155.44 U·L–1 (normal range: 40-150 U·L–1), and gamma-glutamyl transferase at 54.62 U·L–1 (normal range: 11-50 U·L–1). Magnetic resonance imaging (MRI) revealed: multiple nodules of varying sizes in both cerebellar hemispheres, both cerebral hemispheres, brainstem, basal ganglia, thalamus, and outside the brain, some with ring-like signals and unclear boundaries, with the largest one located in the left posterior parietal area outside the brain, measuring approximately 24mm x 21mm x 15mm, showing significant uneven enhancement, and the adjacent meninges are markedly thickened and enhanced, some arterial-enhancing small nodules are seen in the skull, the midline structures are centered, the ventricular system is not significantly dilated, and the brain sulci and fissures are widened and deepened. The diagnosis is multiple metastatic tumors in both the brain, outside the brain, and the skull, with focal meningeal involvement.
2.2 Patient’s current medical history
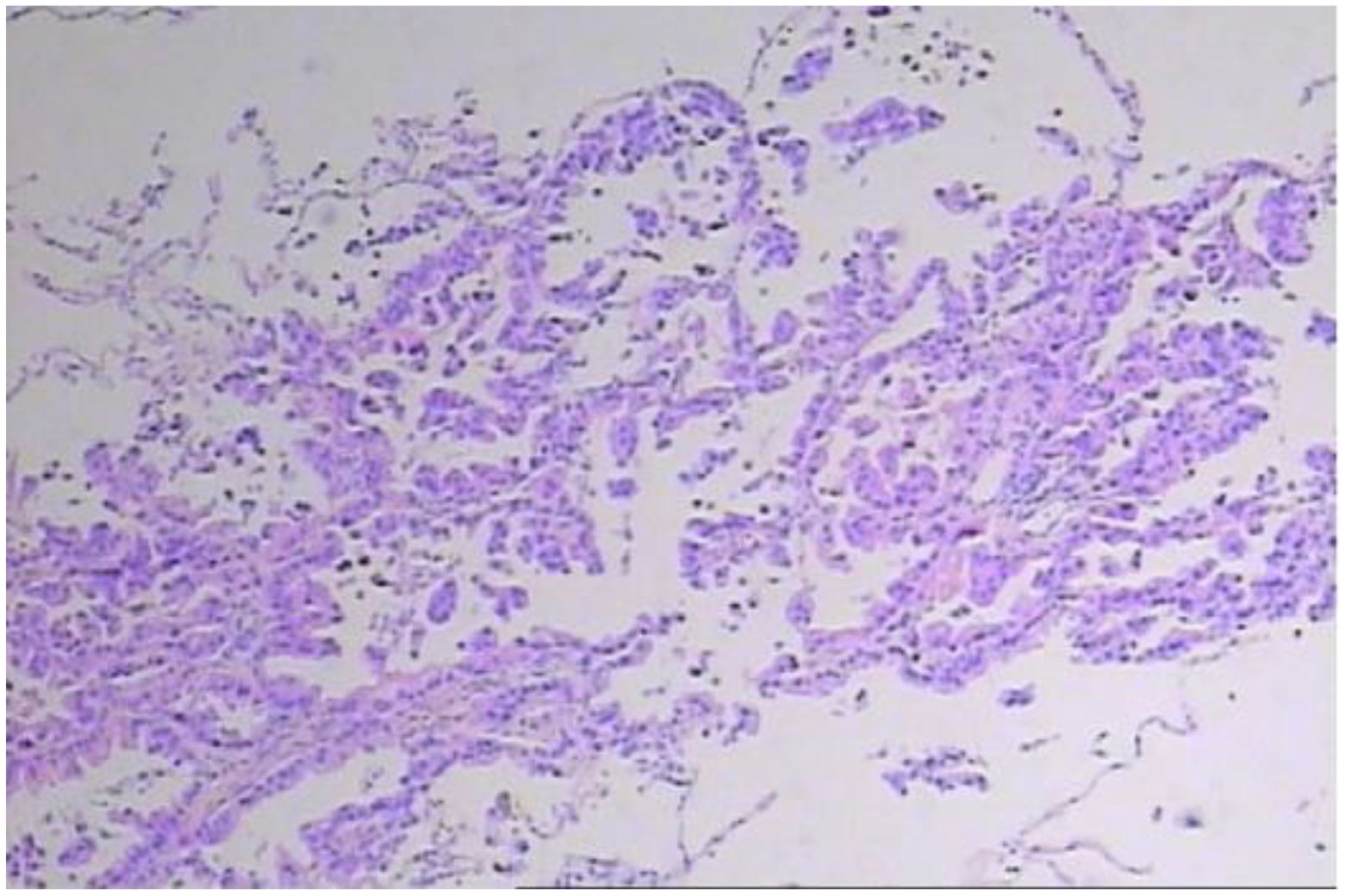
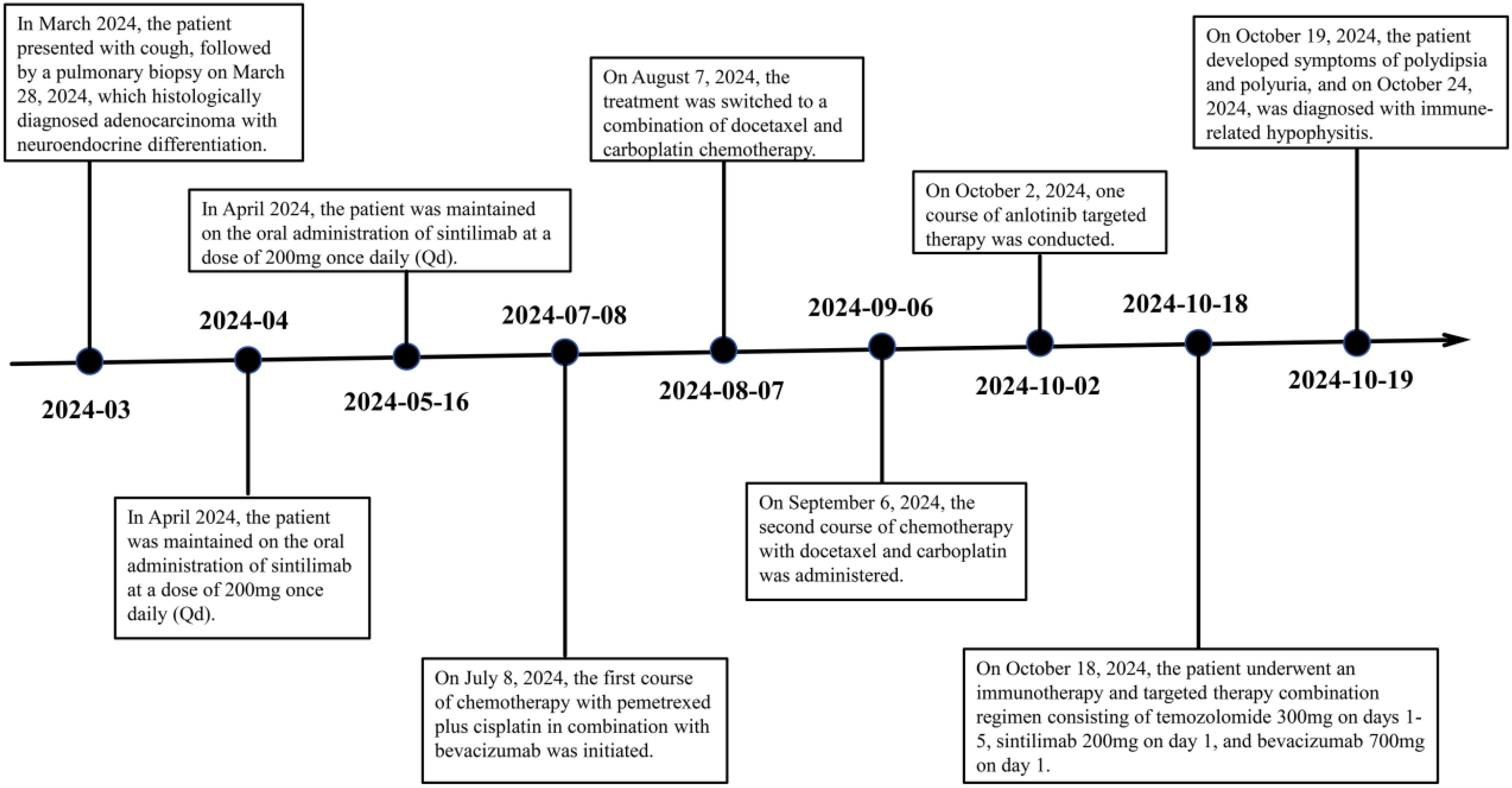
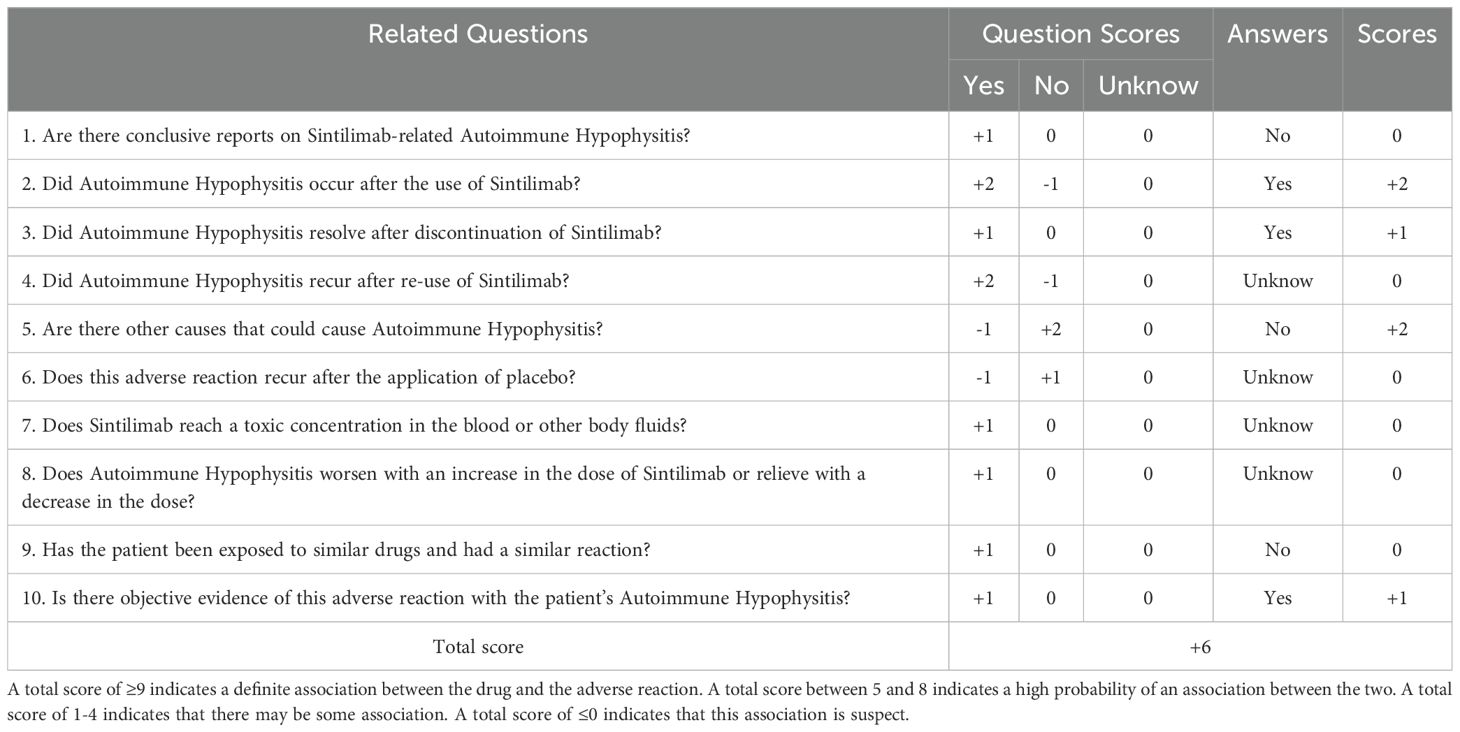
In early March 2024, a 61-year-old male patient was admitted for a persistent cough with white sputum, which had been ongoing for three months without any identifiable cause. He denied experiencing hemoptysis, chest pain, or tightness. Despite only slight symptomatic relief from anti-inflammatory drugs at a community hospital, a CT scan on March 22, 2024, revealed diffuse nodules in both lungs and enlarged lymph nodes, suggesting a malignant tumor (Figure 1). A lung biopsy on March 28, 2024, confirmed non-small cell lung cancer, specifically adenocarcinoma with neuroendocrine differentiation, based on Immunohistochemistry (IHC) (Figure 2). The results were as follows: IHC: p40 (–), CK5/6 (–), TTF-1 (+), NapsinA (+), Syn (–), CD56 (+), CK7 (+), Ki-67 (+, 65%). Genetic testing on the biopsy specimen (ID: M01240405469) on April 22, 2024, showed no mutations in PD-L1, EGFR, ALK, ROS-1, RET, C-MET, or HER-2. Given the neuroendocrine features, the patient was initiated on 200 mg Sulfatinib daily. A follow-up CT on May 14, 2024, showed lung lesion improvement, but an ECT revealed multiple bone destructions, leading to a second course of Sulfatinib with phosphates to prevent further bone damage. By July 2024, after two Sulfatinib courses, tumor markers and lung lesions had progressed. The patient then received pemetrexed combined with cisplatin and bevacizumab on July 8, followed by two courses of docetaxel with carboplatin due to lack of significant improvement in tumor markers and symptoms. On October 2, 2024, the patient began anlotinib targeted therapy. However, on October 16, 2024, he was readmitted with facial palsy and severe headache for three days.
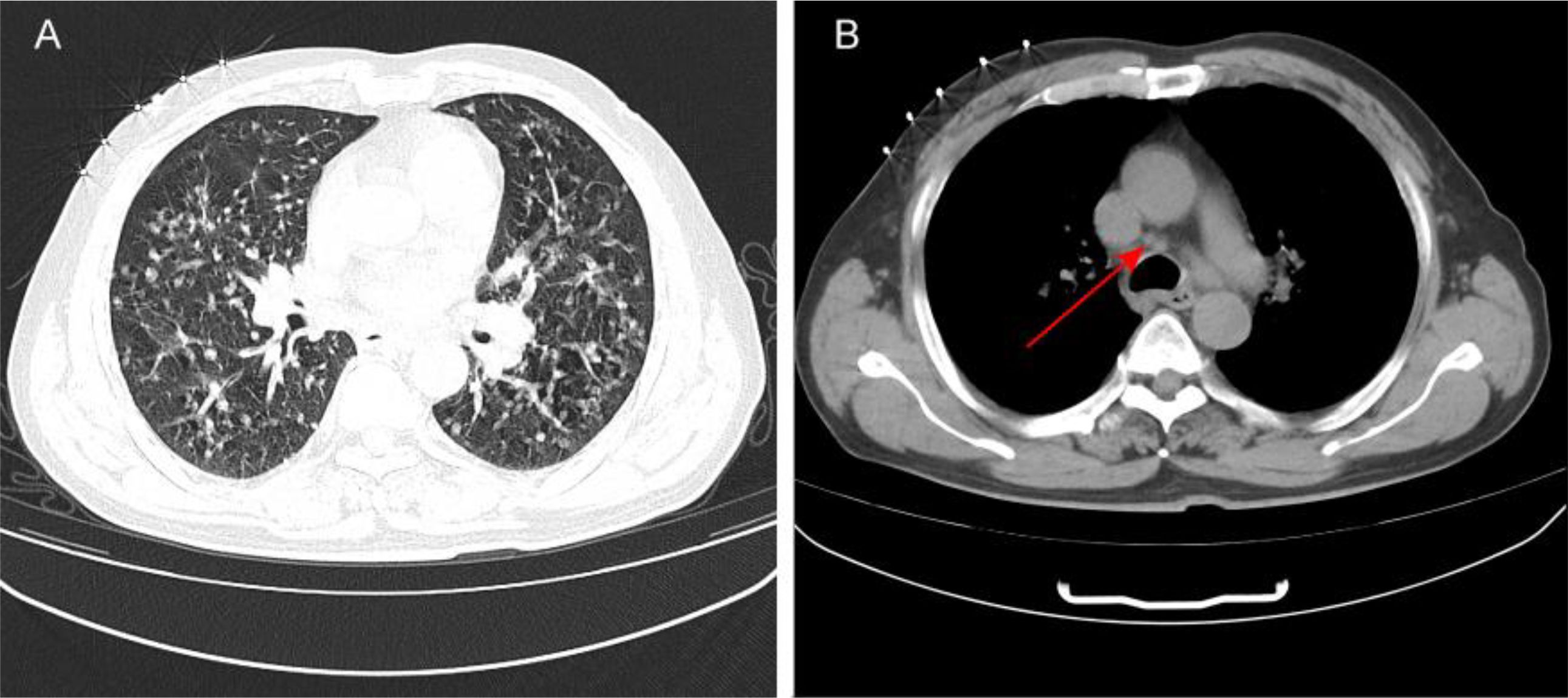
Figure 1. (A) The patient’s initial CT scan shows diffuse nodules in the lungs. (B): The patient’s initial CT scan indicates enlarged mediastinal lymph nodes.
2.3 The patient’s treatment process
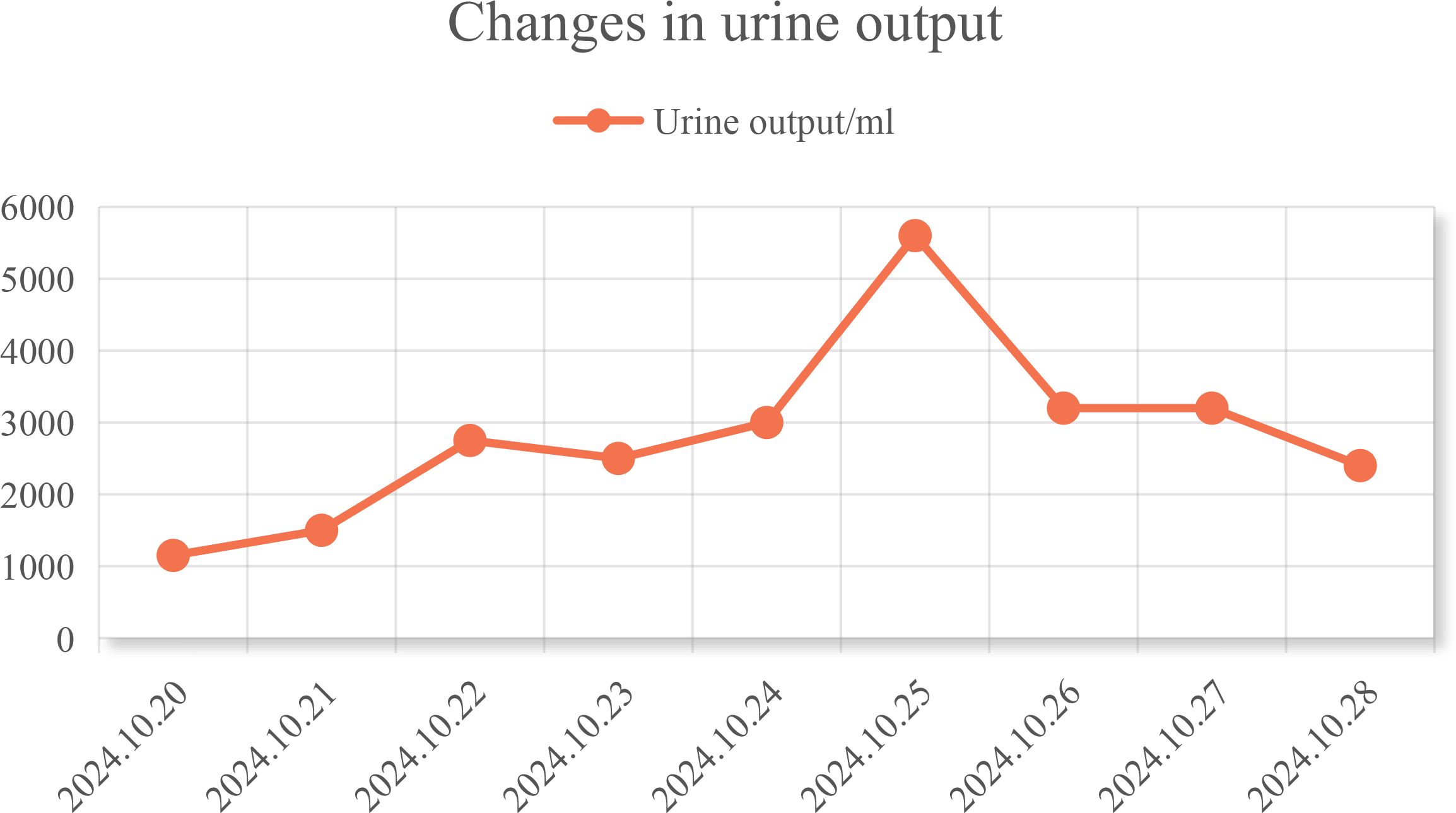
In conjunction with the patient’s symptoms and auxiliary examination data, multiple intracranial metastases were confirmed. Considering the patient’s physical condition, on October 18, 2024, a combined immunotherapy and targeted treatment regimen of “Temozolomide 300mg D1-5 + Sintilimab 200mg D1 + Bevacizumab 700mg D1” was administered. On October 19, 2024, the patient reported severe thirst after the treatment on October 18, drinking 3000ml of water in half a day. The patient was instructed to record the 24-hour urine volume, and on October 22, the patient’s urine volume was 2750ml. A review of the inpatient biochemistry revealed: Alanine aminotransferase (ALT) was 47.97 U·L–1 (normal range: 5-40 U·L–1), Aspartate aminotransferase was 166.82 U·L–1 (normal range: 8-40 U·L–1), Urea was 1.51 mmol·L–1 (normal range: 3.1-8.2 mmol·L–1), Sodium was 146.34 mmol·L–1 (normal range: 136-145 mmol·L–1), Chloride was 110.45 mmol·L–1 (normal range: 96-108 mmol·L–1). Pituitary prolactin was 24.67 ng·ml–1 (normal range: 3.5-19.5 ng·ml–1), blood cortisol was 181.03 nmol·L–1 (normal range: 138-635 nmol·L–1), adrenocorticotropic hormone (ACTH) was 111.66 ng·L–1 (normal range: 7.2-63.3 ng·L–1), and urine specific gravity was 1.005 (normal range: 1.01-1.03). A magnetic resonance scan with plain and enhanced imaging of the pituitary gland indicated abnormal signal on the left side of the pituitary, diagnosing pituitary metastasis (see Figure 3). At this time, it was the 5th day after the use of Sintilimab, and based on the auxiliary examinations and radiological data, a diagnosis of secondary immune-related hypophysitis was made. On October 24, 2024, the patient was treated with Dexamethasone sodium phosphate injection 30mg every 12 hours. After 2 days of use, the urine volume decreased from 5600ml to 3200ml (see Figure 4), and the patient’s thirst symptoms and facial palsy showed slight improvement. On October 27, 2024, Prednisone acetate tablets 140mg were taken once daily, and on October 29, ACTH was 3.29 ng·L–1 (normal range: 7.2-63.3 ng·L–1). The patient’s vital signs were stable, and discharge was arranged with instructions to halve the medication dose every week. A follow-up phone call after half a month, the family reported that the urine volume had returned to normal (see Figure 5 for changes in the patient’s face before and after treatment, and Figure 6 for the treatment course).
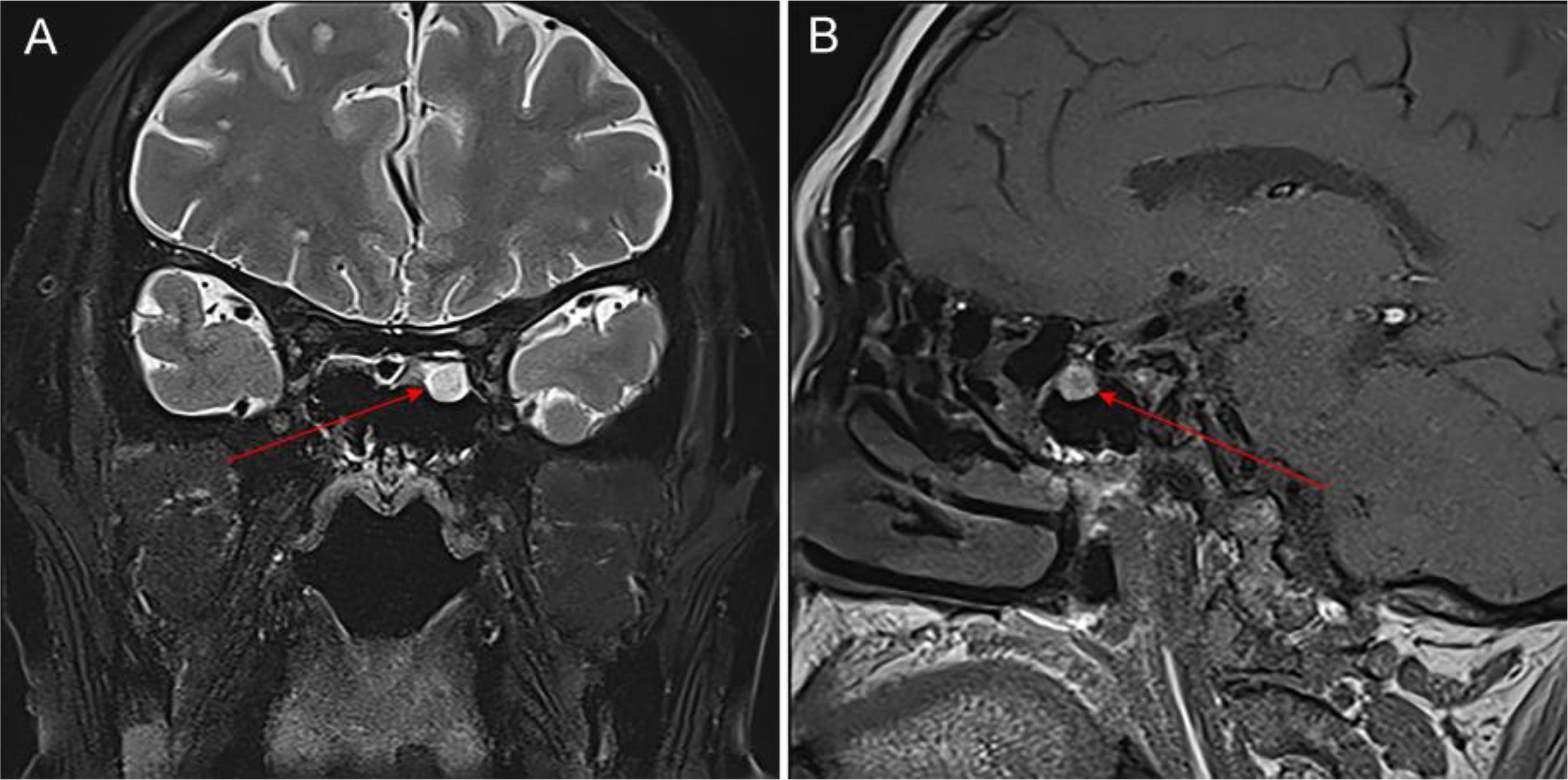
Figure 3. Imaging changes of the pituitary gland in the patient after using Sintilimab. (A) shows the coronal view of the pituitary T2 signal, and (B) shows the sagittal view of the pituitary T1 signal. Description of the pituitary MRI with plain and contrast: The sella turcica has a regular shape with no depression of the sellar floor, and no significant abnormal signal in the bone of the sella, there is a slight exudate-like change around the pituitary, about 7mm in height, and a nodule-like isointense slightly long T1 and slightly long T2 signal can be seen on the left side of the pituitary, which shows mild enhancement (lower than the adjacent normal pituitary tissue), with a long diameter of about 4mm, the pituitary stalk is slightly deviated to the left, with no significant abnormal signal. The bilateral cavernous sinus structure is clear, and the signal is not significantly abnormal, there is no obvious sign of compression and upward displacement of the optic chiasm, a circular short T1 and slightly long T2 signal can be seen in the left sphenoid sinus, which shows high signal on enhancement, with a clear edge; multiple isointense slightly long T1 and slightly long T2 signals can be seen in the brain tissue, with ring-like and nodular enhancement.
Figure 5. Changes in the patient’s facial appearance before and after steroid treatment (left image is before treatment, right image is after treatment).
3 Discussion
Recent literature reports indicate that sintilimab has a good antitumor effect (5), and it has shown similar antitumor effects and better safety profiles compared to nivolumab and pembrolizumab in Hodgkin’s lymphoma, NKT-cell lymphoma, and advanced non-small cell lung cancer (6). Sintilimab can block the interaction between PD-1 and its ligands, helping T cells to restore their antitumor function. However, due to its lack of tumor tissue specificity (7), it can damage T cells’ immune tolerance to their antigens, leading to a series of immune-related adverse events (iAEs) (8). In this case, the patient developed hypophysitis and diabetes insipidus after receiving chemotherapy and sintilimab immunotherapy for lung adenocarcinoma with brain metastasis. These conditions were promptly corrected with hormone replacement therapy, stabilizing pituitary function.
3.1 Definition, clinical manifestations, and differential diagnosis of immune-related hypophysitis
Immune-related hypophysitis is a rare form of hypophysitis and is the most common iAE associated with ICIs, typically occurring around the 8th to 10th week after starting ICIs (9). Clinical manifestations are mainly characterized by pituitary endocrine abnormalities or inflammatory compression, with typical symptoms including headache, nausea, fatigue, vision changes, and hypotension. Other less common clinical manifestations include mental confusion, memory loss, anorexia, hyponatremia, decreased sexual function, amenorrhea, and diabetes insipidus (10, 11). Due to the complex clinical presentation of hypophysitis patients and the similarity to symptoms caused by tumor progression, it is important to differentiate it from hypophysitis caused by other reasons such as infection or tumor (12). Additionally, it should be distinguished from other space-occupying lesions like pituitary adenomas, craniopharyngiomas, and Rathke’s cleft cysts. Complications arising from pituitary dysfunction may include secondary hypothyroidism, hypogonadotropic hypogonadism, and secondary adrenal insufficiency, with incidence rates of 7.6%, 7.5%, and 6.1%, respectively (13). Furthermore, there are various changes in adrenocorticotropic hormone (ACTH), TSH, growth hormone, and prolactin (14). These can be differentiated in conjunction with the patient’s gender, age, pituitary imaging, and laboratory tests.
3.2 Mechanism of action and common adverse reactions of sintilimab
Sintilimab, an immune checkpoint inhibitor and a recombinant fully human IgG monoclonal antibody against PD-1, binds to PD-1, blocks its interaction with PD-L1 and PD-L2, restores endogenous antitumor T-cell responses, and exerts antitumor effects (15). Approved by China National Medical Products Administration in 2018 for relapsed or refractory classical Hodgkin’s lymphoma after at least two lines of systemic chemotherapy. In 2021, sintilimab was granted additional indications for use in combination with other drugs for the first-line treatment of unresectable advanced or recurrent squamous cell carcinoma. Studies have reported that immune-related hypophysitis is due to excessive immune activation and represents an autoimmune reaction. CTLA-4 and PD-1 are immune checkpoint molecules found on T lymphocytes that inhibit T-cell activity (16). Anti-PD-1/PD-L1 therapy can enhance reactive T-cell activity in the pituitary, thereby mediating immune-related hypophysitis. The instructions for Sintilimab list common adverse reactions such as immune-related pneumonia, colitis, hepatitis, nephritis, and endocrine disorders, which require clinical vigilance (17).
3.3 Drug adverse reaction association assessment
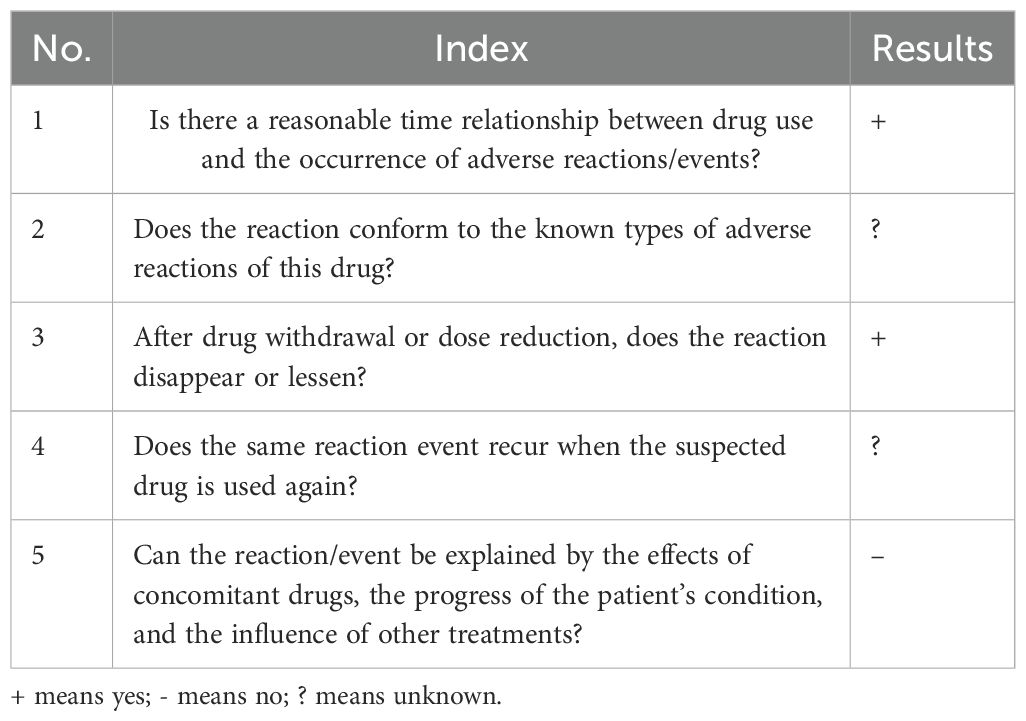
After admission, the patient was diagnosed with lung adenocarcinoma and brain metastasis. Given the patient’s condition, a chemotherapy regimen of sintilimab + bevacizumab + temozolomide was selected. The patient had severe thirst on the second day of treatment, drinking 3000ml of water in half a day, and on the eighth day after chemotherapy, the urine volume reached 5600ml, leading to a diagnosis of diabetes insipidus. Concurrent lab tests showed abnormal pituitary prolactin and blood cortisol levels, and MRI showed abnormal signals in the pituitary area with exudate-like changes. Based on symptoms and test data, the patient was diagnosed with immune-related hypophysitis. The association between sintilimab and the patient’s immune-related hypophysitis was evaluated (Table 1) (1): The patient had no history of hypophysitis or pre-existing immune system-related diseases, and no other immunomodulatory drugs were used, excluding the immune system itself and past medical history as causes. The patient developed thirst and excessive drinking on the second day of chemotherapy with the sintilimab + bevacizumab + temozolomide regimen, showing a strong temporal association (2). The patient had facial palsy on admission, and MRI excluded cerebral infarction while diagnosing multiple intracranial metastatic tumors. Sintilimab can activate the immune system to attack tumor cells but may also cause immune cells to attack normal cells, triggering an autoimmune response. The pituitary, with rich blood supply, is vulnerable. Previous studies have observed changes in adrenocorticotropic hormone and cortisol in patients treated with sintilimab, further confirming the diagnosis of immune-related hypophysitis in this case (18, 19) (3). After discontinuing sintilimab and undergoing hormone therapy, the patient’s thirst decreased, vision improved, diabetes insipidus was relieved, and adrenocorticotropic hormone returned to normal (4). The concurrent medications, temozolomide and bevacizumab, have mechanisms that do not seem to trigger immune-related hypophysitis, and no relevant reports were found, excluding their influence (20, 21). In summary, the association rating between sintilimab and immune-related hypophysitis is “likely,” with a score of 6 points according to the Naranjo evaluation principle, indicating a strong association (22) (Table 2).
3.4 Treatment measures for sintilimab-associated immune-related hypophysitis
The occurrence of immune-related hypophysitis is associated with excessive immune activation and is an autoimmune reaction. According to literature searches, the main treatment measures include (1): Glucocorticoid therapy: For patients with headache, visual impairment, or pituitary dysfunction, moderate-dose glucocorticoid therapy is typically used, such as prednisone. Initially, a higher dose is usually adopted, which is then gradually tapered based on the patient’s response and discontinued after symptom improvement (2). Hormone replacement therapy: For patients with pituitary insufficiency, corresponding hormone replacement therapy is necessary to maintain normal endocrine function (3). Immunosuppressants: In some refractory or recurrent cases, immunosuppressants such as cyclophosphamide or azathioprine may be required (4). Monitoring and follow-up: During treatment, regular monitoring of the patient’s pituitary hormone levels is necessary, and changes in pituitary morphology should be assessed through magnetic resonance imaging (MRI) to guide treatment decisions.
In summary, as an effective immunotherapy drug for cancer, sintilimab-induced immune-related hypophysitis, although rare, requires close attention from clinicians due to its insidious nature and association with immunotherapy. For non-small cell carcinoma patients with pituitary metastasis, special attention should be paid to the patient’s urine volume and visual changes during diagnosis and treatment to detect hypophysitis promptly and intervene early. Additionally, patients should have regular follow-ups after treatment to monitor symptom changes and pituitary imaging, and when necessary, undergo MRI and hematological examinations to ensure timely identification and management of potential complications, thereby improving treatment outcomes and quality of life.
4 Conclusion
When using sintilimab to treat non-small cell lung cancer, close attention should be paid to patient symptoms, and early intervention with glucocorticoids should be considered if immune-related hypophysitis occurs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
M-XW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. A-XL: Data curation, Funding acquisition, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. Q-MS: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft. W-HD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Scientific Research Project of Higher Education Department of Anhui Province (No. 2023AH050834).
Acknowledgments
This study was conducted with the written informed consent of the patient, who agreed to the publication of this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fernandes S, Varlamov EV, McCartney S, Fleseriu M. A novel etiology of hypophysitis: immune checkpoint inhibitors. Endocrinol Metab Clinics North America. (2020) 49:387–99. doi: 10.1016/j.ecl.2020.05.002
2. Van der Leij S, Suijkerbuijk KPM, van den Broek MFM, Valk GD, Dankbaar JW, van Santen HM. Differences in checkpoint-inhibitor-induced hypophysitis: mono- versus combination therapy induced hypophysitis. Front endocrinology. (2024) 15:1400841. doi: 10.3389/fendo.2024.1400841
3. Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin endocrinology. (2016) 85:331–9. doi: 10.1111/cen.13063
4. Shen X, Yang M, Xu H, Zhou H, Wang L, Ma J. Immunotherapy-associated hypophysitis under anti-PD1: two case reports. Endocrine Metab Immune Disord Drug targets. (2023) 23:996–1004. doi: 10.2174/1871530323666221208111823
5. Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, et al. Circulating tumor DNA predicts response in Chinese patients with relapsed or refractory classical hodgkin lymphoma treated with sintilimab. EBioMedicine. (2020) 54:102731. doi: 10.1016/j.ebiom.2020.102731
6. Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: A promising anti-tumor PD-1 antibody. Front Oncol. (2020) 10:594558. doi: 10.3389/fonc.2020.594558
7. Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematology. (2019) 6:e12–9. doi: 10.1016/s2352-3026(18)30192-3
8. Tan MH, Iyengar R, Mizokami-Stout K, Yentz S, MacEachern MP, Shen LY, et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin Diabetes endocrinology. (2019) 5:1. doi: 10.1186/s40842-018-0073-4
9. Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma Y, et al. Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J immunotherapy cancer. (2018) 6:155. doi: 10.1186/s40425-018-0477-9
10. Chiloiro S, Capoluongo ED, Tartaglione T, Giampietro A, Bianchi A, Giustina A, et al. The changing clinical spectrum of hypophysitis. Trends Endocrinol metabolism: TEM. (2019) 30:590–602. doi: 10.1016/j.tem.2019.06.004
11. Agrawal L, Bacal A, Jain S, Singh V, Emanuele N, Emanuele M, et al. Immune checkpoint inhibitors and endocrine side effects, a narrative review. Postgraduate Med. (2020) 132:206–14. doi: 10.1080/00325481.2019.1709344
12. Caranci F, Leone G, Ponsiglione A, Muto M, Tortora F, Muto M, et al. Imaging findings in hypophysitis: a review. La Radiologia medica. (2020) 125:319–28. doi: 10.1007/s11547-019-01120-x
13. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinology. (2017) 13:195–207. doi: 10.1038/nrendo.2016.205
14. Mahzari M, Liu D, Arnaout A, Lochnan H. Immune checkpoint inhibitor therapy associated hypophysitis. Clin Med Insights Endocrinol diabetes. (2015) 8:21–8. doi: 10.4137/cmed.S22469
15. Hoy SM. Sintilimab: first global approval. Drugs. (2019) 79:341–6. doi: 10.1007/s40265-019-1066-z
16. Angelousi A, Alexandraki K, Tsoli M, Kaltsas G, Kassi E. Hypophysitis (Including igG4 and immunotherapy). Neuroendocrinology. (2020) 110:822–35. doi: 10.1159/000506903
17. Yin C, Hu B, Yang X, Kou L, Tian B, Wang C, et al. Neoadjuvant sintilimab combined with chemotherapy in resectable locally advanced non-small cell lung cancer: case series and literature review. World J Surg Oncol. (2023) 21:304. doi: 10.1186/s12957-023-03194-4
18. Lin SH, Zhang A, Li LZ, Zhao LC, Wu LX, Fang CT. Isolated adrenocorticotropic hormone deficiency associated with sintilimab therapy in a patient with advanced lung adenocarcinoma: a case report and literature review. BMC endocrine Disord. (2022) 22:239. doi: 10.1186/s12902-022-01151-y
19. Li R, Jiang B, Zhu Y, Gao L, Zhou Y, Yang S. Hypophysitis induced by sintilimab in the treatment of bladder cancer: A case report. Endocrine Metab Immune Disord Drug targets. (2024) 24:606–10. doi: 10.2174/0118715303257557231002064417
20. Tian J, Luo Y, Xiang J, Tang J. Combined treatment for non-small cell lung cancer and breast cancer patients with brain metastases with whole brain radiotherapy and temozolomide: a systematic review and meta-analysis. J neuro-oncology. (2017) 135:217–27. doi: 10.1007/s11060-017-2572-z
21. Seiwerth F, Bitar L, Samaržija M, Jakopović M. Long-term progression-free survival in non-small cell lung cancer patients: a spotlight on bevacizumab and its biosimilars. Expert Opin Biol Ther. (2024) 24:1017–24. doi: 10.1080/14712598.2024.2405562
Keywords: sintilimab, hypophysitis, diabetes insipidus, adverse reactions, immune checkpoint inhibitors
Citation: Wang M-x, Liu A-x, Sun Q-m and Dong W-h (2025) Sintilimab for the treatment of lung adenocarcinoma-induced immune-related hypophysitis: a case report. Front. Immunol. 16:1534179. doi: 10.3389/fimmu.2025.1534179
Received: 25 November 2024; Accepted: 06 January 2025;
Published: 23 January 2025.
Edited by:
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, BrazilReviewed by:
Xiao Jiang, Second Affiliated Hospital of Dalian Medical University, ChinaNiladri Das, Nil Ratan Sircar Medical College and Hospital, India
Copyright © 2025 Wang, Liu, Sun and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan-hui Dong, ZG9uZ3dhbmh1aUBsYXN6eXkuY24=
†These authors have contributed equally to this work
 Ming-xing Wang
Ming-xing Wang Ai-xin Liu2†
Ai-xin Liu2† Qing-ming Sun
Qing-ming Sun Wan-hui Dong
Wan-hui Dong