
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 10 February 2025
Sec. Cytokines and Soluble Mediators in Immunity
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1528763
This article is part of the Research Topic Cytokine Network Dynamics: Influence on Autoimmune Disorders and Cancer Therapy View all 8 articles
 Peipei Huang
Peipei Huang Fan Yang
Fan Yang Ruirui Dong
Ruirui Dong Lijun Wen
Lijun Wen Qiuling Zang
Qiuling Zang Dandan Song
Dandan Song Junshuang Guo
Junshuang Guo Yating Wang
Yating Wang Ruike Zhang
Ruike Zhang Zhiping Ren
Zhiping Ren Jinjin Qin
Jinjin Qin Junfang Teng
Junfang Teng Wang Miao*
Wang Miao*Background: To identify new intervention targets, we explored the correlation between cytokines and the development of refractory status epilepticus (RSE) in patients with severe viral encephalitis (SVE).
Methods: We examined the characteristics of 14 cytokines in the cerebrospinal fluid (CSF) and serum, analyzing their correlation with acute symptomatic seizures and prognosis. Furthermore, we conducted a dynamic analysis of differences and correlations in the expression of cytokines among patients with SVE without seizures, those with controlled seizures, and those with RSE.
Results: We included 161 patients with SVE; the incidence of seizures was 55.2%, and the mortality rate was 5.5%. Notably, 18.9% of these patients developed RSE, with a mortality rate of 20%. During the early stage of SVE, CSF interleukin (IL)-6 and IL-8 levels were significantly higher, declining over time and affecting the prognosis. CSF IL-6 and IL-8 levels were significantly elevated in the RSE group compared to patients without seizures and with controlled seizures, decreasing gradually and independently of serum cytokine levels. CSF IL-8 and age were independent risk factors for RSE, with clinical utility.
Conclusions: Patients with SVE exhibit intrathecal cytokine storms, primarily characterized by elevated levels of IL-6 and IL-8, which influence prognosis. The strong and persistent hyperinflammation underscored by CSF IL-6 and IL-8 is associated with the occurrence and development of RSE; thus, CSF IL-8 and age are independent risk factors for SVE with RSE, indicating potential anti-inflammatory intervention targets.
Severe viral encephalitis (SVE) is usually characterized by increased intracranial pressure, delirium, coma, status epilepticus (SE), and respiratory failure. Patients with this condition are prone to complications, requiring treatment in the intensive care unit (ICU). Refractory status epilepticus (RSE) or super-refractory status epilepticus (SRSE) is a major factor affecting the prognosis and mortality of SVE (1). RSE occurs when sufficient doses of first-line antiseizure medications (ASMs), such as benzodiazepines, are administered, but the addition of another ASM fails to terminate the convulsive seizures and electroencephalogram epileptiform discharges (2). SRSE is a condition where treatment of SE with anesthetics exceeds 24 h, including the time during which the maintenance or tapering of the anesthetic and clinical convulsive seizures and electroencephalogram epileptiform discharges remain uncontrollable or recur (2, 3). Previous studies revealed 1-year mortality rates of 25.4% and 40% for patients with RSE and SRSE, respectively, in the intensive care unit (ICU) (4, 5). Therefore, identifying the factors associated with SVE complicated by RSE is urgent.
The inflammation induced by cytokines is closely associated with epileptogenesis. Previous studies have indicated that microglia, astrocytes, and infiltrating macrophages are potential cellular dynamics of new-onset RSE (6). Higher concentrations of cytokines can activate astrocytes or microglia in the central nervous system, leading to the abnormal release of excitatory neurotransmitters and triggering seizures (7).These high concentrations are also associated with a persistent state of seizures, often leading to uncontrolled and repeated epileptic seizures (8, 9). Studies have demonstrated elevated cerebrospinal fluid (CSF) cytokines (CSF-CKs) and serum cytokines (S-CKs) levels in viral encephalitis (10, 11). However, their relevance and whether elevated cytokine levels are associated with SVE complicated by acute symptomatic seizures or RSE remain unclear. Therefore, monitoring peripheral and central cytokine levels and analyzing their correlation with acute symptomatic seizures and RSE may help to elaborate the mechanisms underlying epileptogenesis and the development of seizures in patients with SVE.
Additionally, the factors influencing epilepsy are complex and diverse, and current research suggests that multiple ion channels are involved in epileptogenesis (12).T lymphocytes play a key role in the early onset of seizures in autoimmune epilepsy (13). Thyroid hormones play essential roles in the pathology of epilepsy through excitatory/inhibitory imbalance and influence several genes involved in its development (14). Studies on factors playing crucial roles in development of SVE complicated by RSE are limited; therefore, identifying risk factors for this condition is the basis for the clinical realization of active interventions.
This study aimed to analyze the clinical data, CSF-CKs, and S-CKs of patients with SVE to explore the correlation between cytokines, acute symptomatic seizures, and RSE. Additionally, the risk factors for RSE were analyzed to identify possible anti-inflammatory intervention targets for patients with SVE complicated by RSE.
We searched for “viral encephalitis,” “viral meningitis,” and “viral meningoencephalitis” in the patient database of the First Affiliated Hospital of Zhengzhou University from January 2020 to August 2023 and identified 1,050 patients. Among these, 229 met the criteria for SVE. First, we evaluated all patients for satisfaction of the 2013 diagnostic criteria for encephalitis (15), and ensured that their medical history, examination findings, CSF cytology, and protein and glucose assessments aligned with the changes recommended by the 2010 European Federation of Neurological Societies (EFNS) diagnostic methods for viral meningoencephalitis (16). Then, patients with a combination of one of the following conditions and an ICU admission for >48 h (17) were diagnosed with SVE: (i) respiratory failure requiring mechanical ventilation, (ii) mental behavioral abnormalities requiring sedatives, (iii) loss of alertness and. (iv)severe intracranial hypertension. The inclusion criteria were as follows: (i) age ≥14 and <80 years, (ii) S-CKs or CSF-CKs testing conducted after admission, and (iii) early stage of onset (onset time <2 weeks). The exclusion criteria were as follows: (i) patients with bacterial, tuberculous, or fungal encephalitis, meningitis, encephalomyelitis, or autoimmune encephalitis; (ii) patients with a combination of intracranial hemorrhage, cerebral infarction, or intracranial tumor; or (iii) patients with incomplete clinical data. Ultimately, 161 patients with SVE were enrolled.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Approval No. 2023-KY-0375-001). The requirement for informed consent was waived according to the national legislation and institutional requirements.
The patients with SVE were categorized into three groups as follows: encephalitis without seizures (E-WS; this included patients with viral encephalitis but no seizures), encephalitis with controlled seizures (E-CS; patients with acute symptomatic seizures that quickly terminated without recurrence and did not meet the diagnostic criteria for RSE), and encephalitis with RSE (E-RSE; patients clinically diagnosed with RSE [including SRSE]). All types met their respective defined criteria (2).
Patients’ clinical data were collected, including age, sex, severity at admission (assessed using the Glasgow Coma Scale score and Acute Physiology and Chronic Health Evaluation-II), inducement, clinical symptoms, imaging results, duration of ICU hospitalization, mechanical ventilation, and ASM administration. CSF data were collected, including CSF pressure, CSF cytology, CSF biochemistry, albumin quotient, CSF-CKs levels, and etiology. Additionally, S-CKs, complete blood count, procalcitonin, electrolytes, thyroid function, lymphocyte subsets, and other data were collected. Patients received outpatient visits or telephone follow-ups at 30 days and 3 months, and modified Rankin Scale assessment was performed, with a score of 0–2 suggesting a favorable prognosis and 3–6 indicating a poor prognosis.
On the second morning after the patients’ admission, 3 mL of whole blood was collected using a procoagulant tube. The samples were left to clot for 30 min at room temperature and then centrifuged at 1,000–1,200 ×g for 15 min to obtain the serum for testing. Lumbar punctures were performed within 24 h of admission to collect 2 mL of CSF samples. Dynamic analysis was performed based on the results of regular re-examinations of CSF-CKs and S-CKs. We utilized AimPlex Multiple Immunoassays for Flow (Tianjin Kuangbo Tongsheng Biotechnology Co., Ltd.) to detect the levels of 14 cytokines in the serum and CSF. These cytokines included interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12P70, IL-17A, IL-17F, IL-22, tumor necrosis factor (TNF)-α, TNF-β, and IFN-γ. The concentrations of all cytokines are expressed in pg/mL.
Normality of clinical sign variables and cytokines distribution in patients with SVE was assessed using the Shapiro–Wilk test (n<;50 samples) and Kolmogorov-Smirnov test (n≥50 samples). Normally expressed data are shown as mean ± standard deviation; otherwise, they are expressed as median (interquartile range [IQR]). The Mann–Whitney U test was used for comparison of non-normally distributed data. A heatmap of the Spearman’s correlation coefficient (r value) related to prognosis was created.
Among the three groups (E-WS, E-CS, and E-RSE), the Hartley test was used to assess the homogeneity of variance. As the variances were identified to be heterogeneous, the Kruskal–Wallis test was used to analyze differences, followed by post-hoc comparisons among the groups with significant differences.
Univariate logistic regression analyses were used to screen for risk variables associated with SVE complicated by RSE. Variables significantly associated with RSE in the univariate analysis were included in the multivariate logistic regression analysis. The relationship between risk variables and RSE were visualized using receiver operating characteristic (ROC) curves. In addition, we developed a nomogram to illustrate the risk factors for RSE and evaluated the clinical value of the model using the Hosmer–Lemeshow test and decision curve analysis. Data analysis was performed using SPSS 25.0 (IBM, Armonk, NY), and the graphs were generated with GraphPad Prism 8.0 (GraphPad Software, San Diego, CA) and R software4.2.2 (R Foundation, Vienna, Austria).
In total, 161 patients with SVE were included, with an average age of 42 ± 18 years. Males accounted for 59% of the study population. The median duration of ICU stay was 10 days (IQR: 6–17). Moreover, 55.2% (89/161) had seizures, 40.3% (65/161) required mechanical ventilation, and the mortality rate was 5.5% (9/161). Additionally, 18.6% (30/161) of patients had comorbid RSE, 40% (12/30) of whom progressed to SRSE and 66.6% (20/30) of whom required mechanical ventilation. The median ICU stay for patients with RSE was 17 days (IQR: 8–28), and the mortality rate was 20% (6/30) (Supplementary Table 1).
A comparative analysis between S-CKs (n = 158) and CSF-CKs (n = 95) revealed significantly elevated levels of IL-6 with a median of 28.55 mg/dL (IQR: 5.71–326.1) and IL-8 with a median of 125.35 mg/dL (IQR: 59.00–748.90) in the CSF, contrary to the corresponding S-CKs, with significant differences (P < 0.001). The remaining 12 CSF-CKs were lower than their serum counterparts (P < 0.001) (Figure 1A, Supplementary Table 2).
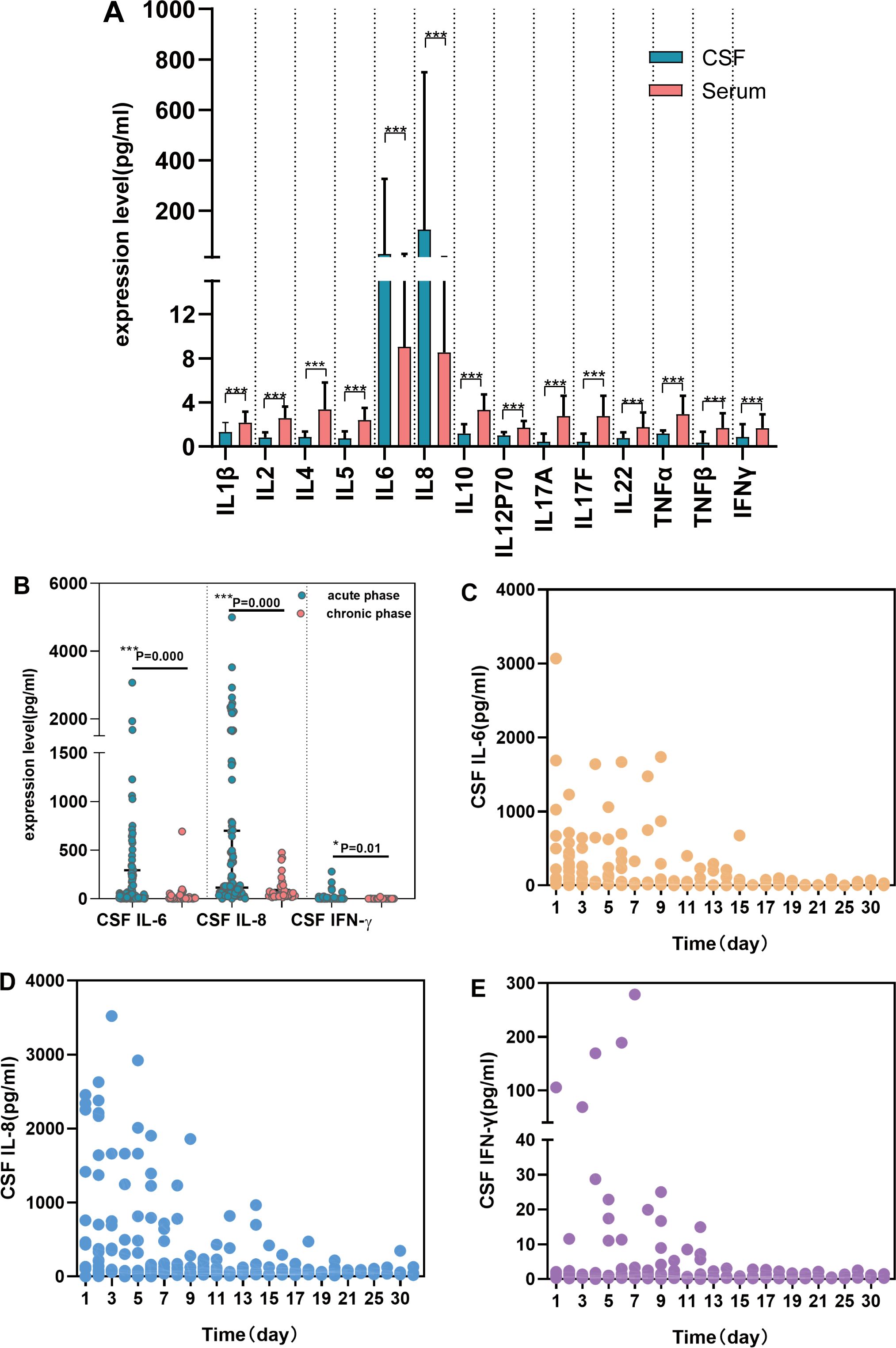
Figure 1. Comparative analysis of CSF and serum cytokines and changes over time in SVE. Comparison of cytokines’ expression in CSF (n = 95) and serum (n = 158) in SVE (A)(P<;0.001). Expression characteristics of CSF cytokines over time in SVE (n = 95) (B-E). CSF IL-6, CSF IL-8, and CSF IFN-γ were elevated during the acute phase (B) (P<;0.05). The trend of CSF IL-6 (C), CSF IL-8 (D) and CSF IFN-γ (E) over time respectively (***P < 0.001, *P < 0.05). CSF, cerebrospinal fluid; SVE, severe viral encephalitis; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin.
CSF and serum cytokine analyses in patients with SVE within 24 h of admission showed markedly elevated CSF IL-6 and IL-8 levels (P < 0.001) as well as increased IFN-γ levels (P = 0.015, Figure 1B). The trend chart of CSF IL-6, IL-8, and IFN-γ levels over time showed an overall downward trend with time, decreasing to the baseline level after 2 weeks (Figures 1C-E). No significant changes in S-CKs levels were observed over time.
We performed Spearman’s correlation analysis between 14 CSF and serum cytokines, as well as the presence of concomitant seizures and prognosis, and constructed a heatmap of Spearman’s r values. Among the 14 cytokines, CSF IL-6 (r = 0.368, P < 0.001) and CSF IL-8 (r = 0.272, P = 0.008) were associated with the 30-day outcome (Figure 2). CSF IL-6 (r = 0.314, P = 0.002), CSF IL-8 (r =0.211, P = 0.040), and CSF IFN-γ (r=0.219, P = 0.033) were associated with the 3-month prognosis, while S-CKs showed no association with prognosis. CSF IL-6 (r = 0.369, P < 0.001) and IL-8 (r = 0.421, P < 0.001) levels were also associated with RSE, which was positively correlated with the outcomes (P = 0.016).
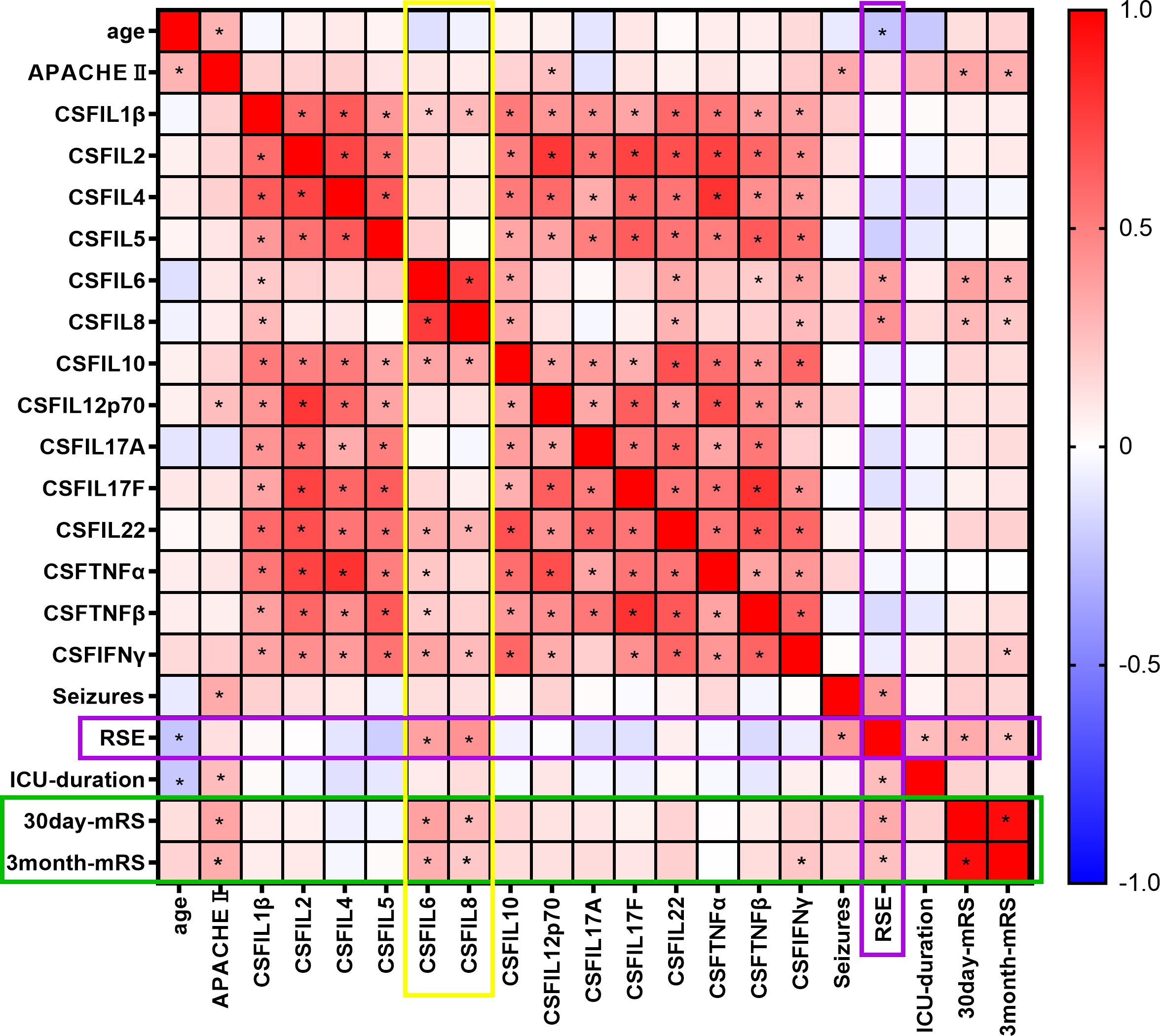
Figure 2. Spearman’s correlation heat map of cytokine levels, epilepsy, and outcomes. Spearman’s correlation coefficient, r value heat map. * indicates P < 0.05. The colors range from -1 (negative correlation, blue) to 0 (no correlation, white), and then to 1 (positive correlation, red).
Based on the presence of concomitant seizures, patients were categorized into three groups: E-WS (S-CKs, n = 69; CSF-CKs, n = 35), E-CS (S-CKs, n = 59; CSF-CKs, n = 40), and E-RSE (S-CKs, n = 30; CSF-CKs, n = 20). Analysis of the differences in 14 serum and CSF cytokines among the three groups revealed significant differences in CSF IL-6 (P = 0.002) and IL-8 (P < 0.001) levels. Post-hoc intergroup comparisons of CSF IL-6 and IL-8 levels showed no difference between the E-WS and E-CS groups (P = 1.0); however, significant increases were observed in the E-RSE group compared with the levels in the E-WS (CSF IL-6, P = 0.005; CSF IL-8, P = 0.002) and E-CS (CSF IL-6, P = 0.002; CSF IL-8, P < 0.001) groups (Table 1, Figures 3A-C). Comparison of S-CKs levels among the E-WS, E-CS, and E-RSE groups showed no significant difference (P > 0.05, Table 1).
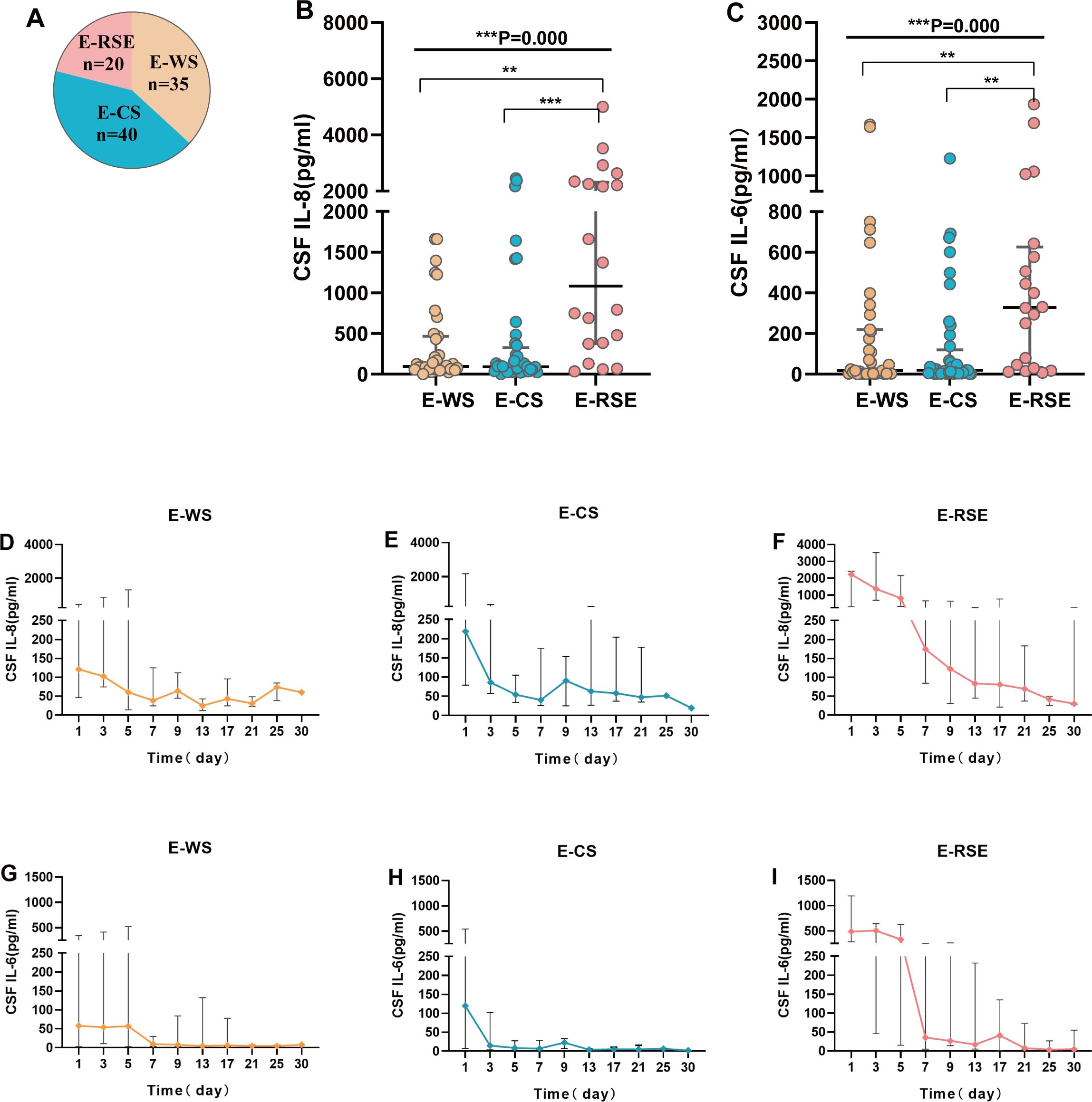
Figure 3. Correlation analysis of cytokines with seizures and the trends in CSF IL-6 and IL-8 over time among the three groups. Correlation analysis of CSF cytokines with acute symptom seizures (A-C). Pie chart of population distribution in SVE with CSF cytokines (A). CSF IL-8 (B) and CSF IL-6 (C) were compared among the three groups and post-hoc intergroup comparisons(P<;0.01). (D-I) displays the trends in CSF IL-8 (D-F) and CSF IL-6 (G-I) levels in patients in the E-WS, E-CS, and E-RSE groups over time respectively (***P < 0.001, **P < 0.01). E-WS, encephalitis without seizures; E-CS, encephalitis with controlled seizures (included patients with seizure or status epilepticus that quickly terminate without recurrence); E-RSE, encephalitis with refractory status epilepticus; CSF, cerebrospinal fluid; SVE, severe viral encephalitis; IL, interleukin; RSE, refractory status epilepticus.
Figures 3D-I display the trends in CSF IL-6 and IL-8 levels in the E-WS, E-CS, and E-RSE groups over time. In the E-RSE group, CSF IL-8 levels increased significantly after onset, with a median increase of more than 2000 pg/mL on the first day (Figure 3F). The median level of CSF IL-6 also increased to approximately 500 pg/mL on the first day and gradually decreased over time, reaching baseline levels approximately 14 days later (Figure 3I). The increases in CSF IL-8 and CSF IL-6 levels in the early stage in the E-CS and E-WS groups were significantly smaller than those in the E-RSE group, and both returned to baseline levels within approximately 1 week (Figures 3D, E, G, H).
Overall, 95 patients (with complete CSF-CKs data) were included in the analysis of risk factors associated with RSE. Patients were categorized into two groups based on whether RSE occurred during hospitalization: the non-RSE (NRSE) group (including the E-WS and E-CS groups, n = 75) and the RSE group (including the E-RSE group [n = 20]).
The age at onset in the RSE group was lower than that in the NRSE group (P = 0.015). The median CSF IL-6 level in the RSE group was 365.08 mg/dL (IQR: 54.43–632.13), and the median CSF IL-8 level was 1082.74 mg/dL (IQR: 3.35–178.96), which was significantly higher than that in the NRSE group. Furthermore, the median CSF IL-6 level in the NRSE group was 18.17 mg/dL (IQR: 3.35–178.96), and the median CSF IL-8 level was 86.01 mg/dL (IQR: 47.14–360.34) (P < 0.001 for both). Additionally, CD4+CD8+T-lymphocyte count (P = 0.008), serum IL-2 (P = 0.009), and serum IL-6 levels (P = 0.042) were higher in the RSE group than in the NRSE group (Table 2, Supplementary Tables 3A, B).
The multivariate logistic regression model included significant indicators from the univariate analysis of risk factors associated with RSE. The results revealed that CSF IL-8 levels (P= 0.005) and age (P = 0.032) were independent risk factors for RSE in patients with SVE (Table 2, Figures 4A, B, Supplementary Table 4).
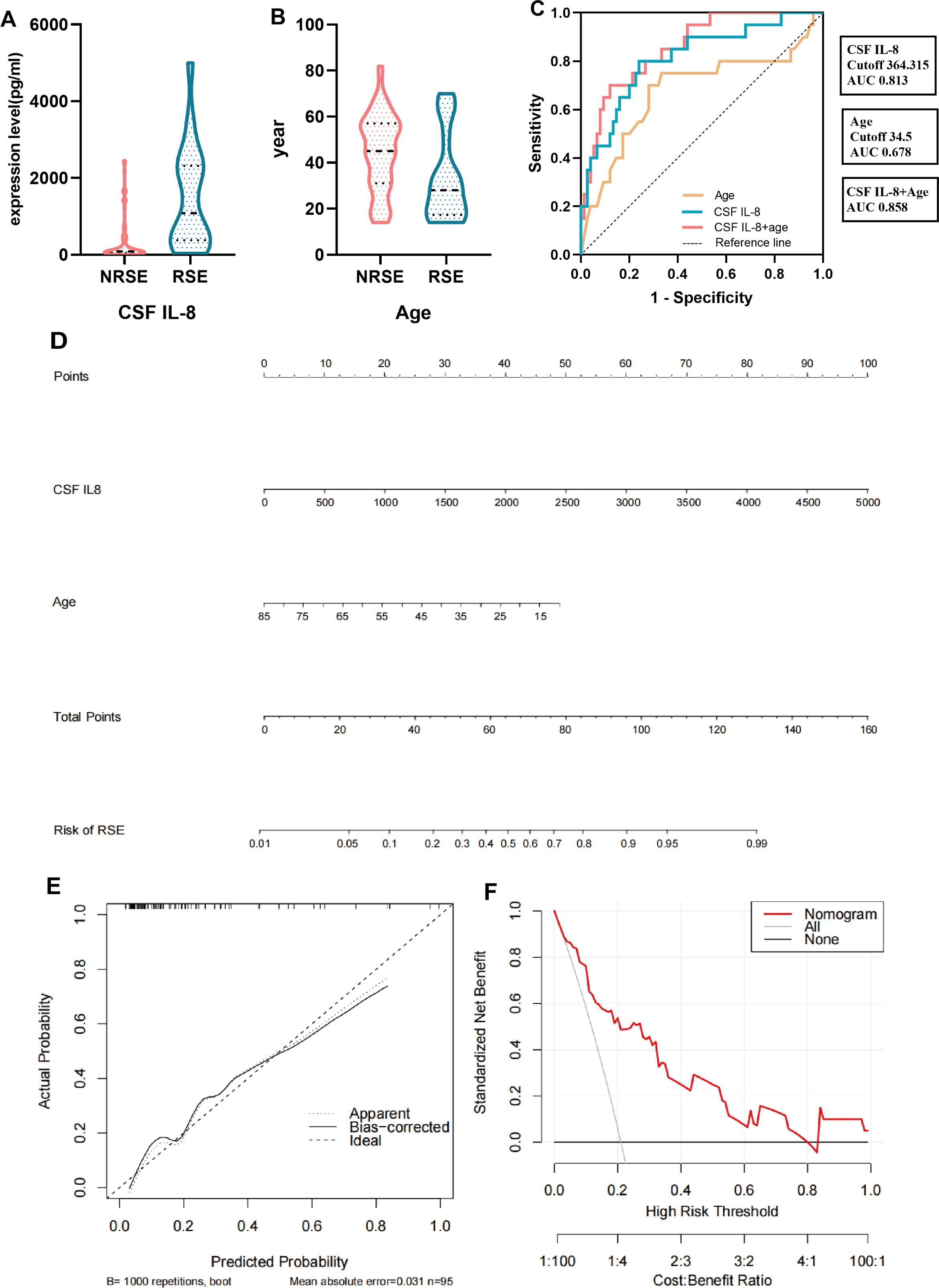
Figure 4. Modeling of relevant factors for patients with SVE complicated by RSE. (A, B) Multivariate logistic analysis of relevant factors associated with RSE. (C) ROC curves of CSF IL-8, age and their combination. (D) Nomogram constructed by CSF IL-8 combined with age. (E) Calibration curve corresponding to the nomogram. (F) Clinical decision curve analysis for SVE patients with concurrent RSE. RSE, refractory status epilepticus group (including the E-RSE group, n = 20); NRSE, non-refractory status epilepticus group (including the E-WS and E-CS groups, n = 75); CSF, cerebrospinal fluid; SVE, severe viral encephalitis; IL, interleukin; ROC, receiver operating characteristic.
The ROC curve of CSF IL-8 and age, and their combined predictive probabilities were plotted to construct a risk factor model for patients with SVE complicated by RSE. The area under the curve of CSF IL-8 was 0.813, with an optimal cutoff value of 364.315 pg/mL, predictive sensitivity of 0.8, and specificity of 0.76. The area under the curve for age was 0.678, with a cutoff value of 34.5 years, sensitivity of 0.7, and specificity of 0.72. The area under the curve for the combined predictive probability of both was 0.858, indicating good clinical performance when combined (Figure 4C, Supplementary Table 5). We generated a nomogram based on CSF IL-8 levels and age and validated it using bootstrapping. Internal validation revealed good calibration curves, and decision curve analysis was used to evaluate the effectiveness and applicability of the nomogram plots. The results revealed that our regression model showed good net clinical benefits (Figures 4D-F).
This study revealed that 55.2% of patients with SVE had seizures, and 18.6% had RSE. RSE prolongs ICU stay and increases patient mortality rate. In this study, CSF IL-6 and IL-8 levels were significantly elevated in the early stage of SVE, were associated with RSE, and influenced outcomes. The elevation of CSF IL-6 and IL-8 levels in the RSE group was more pronounced and exhibited a slower decline. CSF IL-8 levels and age were identified as independent risk factors for RSE in patients with SVE.
The incidence of seizures in encephalitis is 2–67% (18). More than half of the patients in our study experienced acute symptomatic seizures, with approximately one-third of them experiencing RSE, possibly because our study population consisted entirely of patients with severe illness. The rate of mechanical ventilation in patients with concurrent RSE was approximately 70%, leading to prolonged hospital stays and a mortality rate as high as 20%, consistent with previous research findings (1). Therefore, identifying the factors that correlate with RSE is important to improve the prognosis of patients with SVE. Considering the low positivity rate of pathogen detection (19–21), identifying common epileptogenic factors aligns with current clinical needs.
Virus-induced encephalitis is accompanied by the activation of pro- and anti-inflammatory responses, and patients with severe encephalitis may have stronger inflammation than those with mild or moderate encephalitis. Furthermore, patients with viral encephalitis exhibit high levels of S-CKs (22). Elevated S-CKs levels can serve as diagnostic indicators for acute viral encephalitis in children (23); there are reports of high levels of TNF-α and IL-6 in the CSF (24, 25). This study compared CSF-CKs and S-CKs in patients with SVE showing early-stage onset to demonstrate the significance of peripheral and central inflammatory responses in SVE. The results revealed a significant increase in IL-6 and IL-8 levels in the CSF during the early stage, which gradually decreased throughout the disease course. In contrast, no significant increase in S-CKs or clear changes were observed over time. These findings suggest that the cytokine-related inflammation caused by SVE occurs primarily within the central nervous system, rather than in the periphery. Therefore, the use of CSF-CKs as a clinical assessment indicator for patients with SVE may be more valuable than the use of S-CKs.
Here, analysis of the correlation between cytokines and RSE revealed that CSF IL-6 and IL-8 were associated with RSE in SVE, but not in cases of absent seizures or easily controllable seizures. S-CKs did not correlate with any of the three groups. Dynamic analysis of CSF IL-6 and IL-8 in the three groups indicated a significant increase and slow decline in the early stage of RSE, suggesting a stronger and more persistent intrathecal cytokine storm in patients with SVE complicated by RSE. Therefore, markedly elevated CSF IL-6 and IL-8 levels in patients with SVE followed by a gradual decline to maintain a certain level may play a crucial role in the development and maintenance of RSE. Prolonged neuroinflammation may be a molecular mechanism underlying refractory seizures, with pro-inflammatory cytokines being the main effector molecules (26–28). An imbalance of pro- and anti-inflammatory cytokines, or an imbalance in cytokine production or receptor expression, leads to various pathological conditions (29). Moreover, neuroinflammation caused by pro-inflammatory cytokines, such as IL-1β, TNF-α, and HMGB1, plays a role in the generation and recurrence of seizures through mechanisms such as impairing the homeostatic function of astrocytes, disrupting the blood–brain barrier, and causing an imbalance of synaptic excitatory/inhibitory transmission (30). Notably, IL-6 promotes seizures by increasing microglial proliferation and reducing hippocampus neurogenesis (31). IL-6 receptor antagonists have been reported to block seizures in rats (32) and treat new-onset RSE (33, 34). However, studies on the correlation between IL-8 levels and RSE are lacking (35).
Through univariate screening and multivariate analysis, we identified CSF IL-8 levels and age as independent risk factors for patients with SVE complicated by RSE. CSF IL-8 plays a more prominent role than CSF IL-6, which may be related to CSF IL-8 acting as a chemoattractant for peripheral monocytes entering the central nervous system, leading to amplified cascade inflammation. IL-8 belongs to the chemokine family and is primarily produced peripherally by monocytes and macrophages, whereas IL-6 is primarily produced by activated astrocytes or microglia in the central nervous system. IL-8 is mainly a neutrophil chemokine; in addition to neutrophils, it affects various cells, including lymphocytes and monocytes (36). Notably, elevated levels of chemokine receptors are a distinctive feature of monocytes (37, 38). In various disease models, these cells are specifically directed to areas of inflammation and infection (39), as observed in rheumatoid arthritis (40), atherosclerosis (41), and central nervous system infections (42, 43). Recent reports have indicated the use of monocyte and macrophage regulation for the treatment of rheumatoid arthritis (44) and atherosclerosis (45). Recent research has demonstrated the recruitment of monocytes to the brains of animals infected with viruses, where they differentiate into dendritic cells, macrophages, and clusters of microglial cells (46, 47). Once differentiation is complete, these cells participate in a range of effective functional activities, such as antigen presentation, activation of T-cells, and the synthesis and secretion of pro-inflammatory mediators, mediating the generation of immunopathology (48). Evidence suggests that these migrating microglia exhibit stronger pro-inflammatory functions than resident microglia (47), and that RSE is closely related to microglia and inflammatory mediators. Therefore, intrathecal hyperinflammation caused by CSF IL-8 may be a potential mechanism underlying RSE in patients with SVE. Using CSF IL-8 as an early biological indicator and potential therapeutic target for SVE combined with RSE, early monitoring of elevated CSF IL-8 levels and attempts to regulate the overexpression of CSF IL-8 may help control RSE episodes and improve patient outcomes.
Age was an independent risk factor for SVE complicated by RSE. Younger age was correlated with a higher risk of developing RSE; this may be related to the tendency of younger patients to experience excessive inflammation (49). A clinical model of CSF IL-8 levels combined with age was constructed, and the combined area under the curve reached 0.858, indicating an excellent predictive value for RSE. Further analysis of the calibration and decision curves showed that the combined model has an excellent clinical net benefit, which could guide physicians in the early diagnosis and assessment of RSE.
This study has some limitations. First, since this was a single-center investigation with a limited sample size, external validation was not conducted. Second, this was a preliminary study on only the potential mechanisms of action of SVE complicated by RSE related to cytokines. Therefore, prospective clinical trials and animal, cellular, and molecular biology experiments are required to confirm these findings.
In summary, patients with SVE exhibited strong and persistent intrathecal hyperinflammation (underscored by IL-6 and IL-8), which influenced prognosis and was associated with the occurrence and development of RSE. CSF IL-8 levels and age were independent risk factors for SVE complicated by RSE, indicating potential anti-inflammatory interventional targets to control RSE and improve outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Approval No. 2023-KY-0375-001). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
PH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FY: Data curation, Software, Writing – review & editing. RD: Data curation, Writing – review & editing. LW: Data curation, Writing – review & editing. QZ: Data curation, Writing – review & editing. DS: Data curation, Writing – review & editing. JG: Data curation, Writing – review & editing. YW: Data curation, Writing – review & editing. RZ: Data curation, Writing – review & editing. ZR: Data curation, Writing – review & editing. JQ: Data curation, Writing – review & editing. JT: Resources, Supervision, Writing – review & editing. WM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Henan Provincial Key Scientific Research Project of Higher Education Institutions (23A320022) and the 2022 “Four New” Teaching Reform Research and Practice Projects of Zhengzhou University (2022zzusx021).
We would like to acknowledge the physicians and nurses at the First Affiliated Hospital of Zhengzhou University Neurological Intensive Care Unit.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1528763/full#supplementary-material
1. Thakur KT, Motta M, Asemota AO, Kirsch HL, Benavides DR, Schneider EB, et al. Predictors of outcome in acute encephalitis. Neurology. (2013) 81:793–800. doi: 10.1212/WNL.0b013e3182a2cc6d
2. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. (2015) 56:1515–23. doi: 10.1111/epi.13121
3. Ochoa JG, Dougherty M, Papanastassiou A, Gidal B, Mohamed I, Vossler DG. Treatment of super-refractory status epilepticus: A review. Epilepsy Curr. (2021) 21:15357597219 99670. doi: 10.1177/1535759721999670
4. Kantanen AM, Kälviäinen R, Parviainen I, Ala-Peijari M, Bäcklund T, Koskenkari J, et al. Predictors of hospital and one-year mortality in intensive care patients with refractory status epilepticus: a population-based study. Crit Care. (2017) 21:71. doi: 10.1186/s13054-017-1661-x
5. Rossetti AO, Claassen J, Gaspard N. Status epilepticus in the ICU. Intensive Care Med. (2024) 50:1–16. doi: 10.1007/s00134-023-07263-w
6. Hanin A, Zhang L, Huttner AJ, Plu I, Mathon B, Bielle F, et al. Single-cell transcriptomic analyses of brain parenchyma in patients with new-onset refractory status epilepticus (NORSE). Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200259. doi: 10.1212/nxi.0000000000200259
7. Barker-Haliski ML, Löscher W, White HS, Galanopoulou AS. Neuroinflammation in epileptogenesis: insights and translational perspectives from new models of epilepsy. Epilepsia. (2017) 58; Suppl 3; Suppl 3:39–47. doi: 10.1111/epi.13785
8. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. (2011) 7:31–40. doi: 10.1038/nrneurol.2010.178
9. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. (2018) 15:144. doi: 10.1186/s12974-018-1192-7
10. Zhou Y, Bian P, Du H, Wang T, Li M, Hu H, et al. The comparison of inflammatory cytokines (IL-6 and IL-18) and immune cells in Japanese encephalitis patients with different progression. Front Cell Infect Microbiol. (2022) 12:826603. doi: 10.3389/fcimb.2022.826603
11. Soltani Khaboushan A, Pahlevan-Fallahy MT, Shobeiri P, Teixeira AL, Rezaei N. Cytokines and chemokines profile in encephalitis patients: A meta-analysis. PloS One. (2022) 17:e0273920. doi: 10.1371/journal.pone.0273920
12. Chen TS, Lai MC, Huang HI, Wu SN, Huang CW. Immunity, ion channels and epilepsy. Int J Mol Sci. (2022) 23(12):6446. doi: 10.3390/ijms23126446
13. Thaler FS, Meinl E. Cytotoxic T cells and plasma cells dominate early in temporal lobe epilepsy with GAD antibodies. Brain. (2023) 146:1231–3. doi: 10.1093/brain/awad066
14. Tamijani SM, Karimi B, Amini E, Golpich M, Dargahi L, Ali RA, et al. Thyroid hormones: possible roles in epilepsy pathology. Seizure. (2015) 31:155–64. doi: 10.1016/j.seizure.2015.07.021
15. Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Cli Infect Dis. (2013) 57:1114–28. doi: 10.1093/cid/cit458
16. Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, et al. Viral meningoencephalitis: a review of diagnostic methods and guidelines for management. Eur J Neurol. (2010) 17:999–e57-e957. doi: 10.1111/j.1468-1331.2010.02970.x
17. Zhao JL, Wang ZY, Li SJ, Ma HK, Liu X, Zhan XW, et al. The efficacy of haemoperfusion combined with continuous venovenous haemodiafiltration in the treatment of severe viral encephalitis in children. Ital J Pediatr. (2023) 49:21. doi: 10.1186/s13052-023-01411-0
18. Michael BD, Solomon T. Seizures and encephalitis: clinical features, management, and potential pathophysiologic mechanisms. Epilepsia. (2012) 53; Suppl 4:63–71. doi: 10.1111/j.1528-1167.2012.03615.x
19. Bloch KC, Glaser C, Gaston D, Venkatesan A. State of the art: acute encephalitis. Clin Infect Dis. (2023) 77:e14–33. doi: 10.1093/cid/ciad306
20. Granerod J, Tam CC, Crowcroft NS, Davies NW, Borchert M, Thomas SL. Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology. (2010) 75:924–32. doi: 10.1212/WNL.0b013e318f11d65
21. Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. (2019) 380:2327–40. doi: 10.1056/NEJMoa1803396
22. Palus M, Formanová P, Salát J, Žampachová E, Elsterová J, Růžek D. Analysis of serum levels of cytokines, chemokines, growth factors, and monoamine neurotransmitters in patients with tick-borne encephalitis: identification of novel inflammatory markers with implications for pathogenesis. J Med Virol. (2015) 87:885–92. doi: 10.1002/jmv.24140
23. Imaging CM. Retracted: detection and diagnostic value of cytokines in serum of children with acute viral encephalitis. Contrast Media Mol Imaging. (2023) 2023:9782381. doi: 10.1155/2023/9782381
24. Guasp M, Muñoz-Sánchez G, Martínez-Hernández E, Santana D, Carbayo Á, Naranjo L, et al. CSF biomarkers in COVID-19 associated encephalopathy and encephalitis predict long-term outcome. Front Immunol. (2022) 13:866153. doi: 10.3389/fimmu.2022.866153
25. Winter PM, Dung NM, Loan HT, Kneen R, Wills B, Thu le T, et al. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. (2004) 190:1618–26. doi: 10.1086/423328
26. Dupuis N, Mazarati A, Desnous B, Chhor V, Fleiss B, Le Charpentier T, et al. Pro-epileptogenic effects of viral-like inflammation in both mature and immature brains. J Neuroinflammation. (2016) 13:307. doi: 10.1186/s12974-016-0773-6
27. Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain. (2009) 132:2478–86. doi: 10.1093/brain/awp177
28. Soltani Khaboushan A, Yazdanpanah N, Rezaei N. Neuroinflammation and proinflammatory cytokines in epileptogenesis. Mol Neurobiol. (2022) 59:1724–43. doi: 10.1007/s12035-022-02725-6
29. Tayal V, Kalra BS. Cytokines and anti-cytokines as therapeutics–an update. Eur J Pharmacol. (2008) 579:1–12. doi: 10.1016/j.ejphar.2007.10.049
30. Villasana-Salazar B, Vezzani A. Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol Dis. (2023) 178:106027. doi: 10.1016/j.nbd.2023.106027
31. Levin SG, Godukhin OV. Modulating effect of cytokines on mechanisms of synaptic plasticity in the brain. Biochemistry(Mosc). (2017) 82:264–74. doi: 10.1134/S000629791703004X
32. Leo A, Nesci V, Tallarico M, Amodio N, Gallo Cantafio EM, De Sarro G, et al. IL-6 receptor blockade by tocilizumab has anti-absence and anti-epileptogenic effects in the WAG/Rij rat model of absence epilepsy. Neurotherapeutics. (2020) 17:2004–14. doi: 10.1007/s13311-020-00893-8
33. Jun JS, Lee ST, Kim R, Chu K, Lee SK. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol. (2018) 84:940–5. doi: 10.1002/ana.25374
34. Wadayama T, Shimizu M, Yata T, Ishikura T, Kajiyama Y, Hirozawa D, et al. Cryptogenic new-onset refractory status epilepticus responded to anti-interleukin-6 treatment. J Neuroimmunol. (2022) 363:577789. doi: 10.1016/j.jneuroim.2021.577789
35. Hanin A, Cespedes J, Dorgham K, Pulluru Y, Gopaul M, Gorochov G, et al. Cytokines in new-onset refractory status epilepticus predict outcomes. Ann Neurol. (2023) 94:75–90. doi: 10.1002/ana.26627
36. Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. American journal of physiology. Am J Physiol Lung Cell Mol Physiol. (2003) 284:L566–77. doi: 10.1152/ajplung.00233.2002
37. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. (2003) 19:71–82. doi: 10.1016/s1074-7613(03)00174-2
38. Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. (2009) 206:595–606. doi: 10.1084/jem.20081385
39. Coillard A, Segura E. In vivo differentiation of human monocytes. Front Immunol. (2019) 10:1907. doi: 10.3389/fimmu.2019.01907
40. Misharin AV, Cuda CM, Saber R, Turner JD, Gierut AK, Haines GK 3rd, et al. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. (2014) 9:591–604. doi: 10.1016/j.celrep.2014.09.032
41. Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. (2014) 63:1–11. doi: 10.1016/j.jacc.2013.09.019
42. Mildner A, Mack M, Schmidt H, Brück W, Djukic M, Zabel MD, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. (2009) 132:2487–500. doi: 10.1093/brain/awp144
43. Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. (2012) 122:3063–87. doi: 10.1172/62636
44. Roszkowski L, Ciechomska M. Tuning monocytes and macrophages for personalized therapy and diagnostic challenge in rheumatoid arthritis. Cells. (2021) 10(8):1860. doi: 10.3390/cells10081860
45. Guo Z, Wang L, Liu H, Xie Y. Innate immune memory in monocytes and macrophages: the potential therapeutic strategies for atherosclerosis. Cells. (2022) 11(24):4072. doi: 10.3390/cells11244072
46. Getts DR, Terry RL, Getts MT, Müller M, Rana S, Shrestha B, et al. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. (2008) 205:2319–37. doi: 10.1084/jem.20080421
47. Getts DR, Terry RL, Getts MT, Müller M, Rana S, Deffrasnes C, et al. Targeted blockade in lethal West Nile virus encephalitis indicates a crucial role for very late antigen (VLA)-4-dependent recruitment of nitric oxide-producing macrophages. J Neuroinflammation. (2012) 9:246. doi: 10.1186/1742-2094-9-246
48. Terry RL, Getts DR, Deffrasnes C, van Vreden C, Campbell IL, King NJ. Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflammation. (2012) 9:270. doi: 10.1186/1742-2094-9-270
Keywords: cerebrospinal fluid, cytokines, serum, severe viral encephalitis, refractory status epilepticus
Citation: Huang P, Yang F, Dong R, Wen L, Zang Q, Song D, Guo J, Wang Y, Zhang R, Ren Z, Qin J, Teng J and Miao W (2025) Cerebrospinal fluid and serum cytokine profiles in severe viral encephalitis with implications for refractory status epilepticus: a retrospective observational study. Front. Immunol. 16:1528763. doi: 10.3389/fimmu.2025.1528763
Received: 15 November 2024; Accepted: 22 January 2025;
Published: 10 February 2025.
Edited by:
Ming Yu Lien, China Medical University Hospital, TaiwanReviewed by:
Alejandro Ortega-Martínez, Instituto Nacional de Enfermedades Respiratorias (INER), MexicoCopyright © 2025 Huang, Yang, Dong, Wen, Zang, Song, Guo, Wang, Zhang, Ren, Qin, Teng and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Miao, bWlhb3dhbmc3MjExQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.