- 1Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 2Translational Transplant Research Center and Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3Rheumatology and Autoinflammatory Diseases, IRCCS Istituto Giannina Gaslini, Genova, Italy
- 4Dipartimento di Neuroscienze, Riabilitazione, Oftalmologia, Genetica e Scienze Materno-Infantili (DiNOGMI), Università degli Studi di Genova, Genova, Italy
- 5Pathology Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy
Lupus nephritis (LN), present in 30%–50% of systemic lupus erythematosus (SLE) patients, often necessitates standard immunosuppressive therapy (glucocorticoids, MMF, CYC) as suggested by the European League Against Rheumatism/European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) and Kidney Disease Improving Global Outcomes (KDIGO) guidelines. However, a subset of subjects remains refractory. Recent findings suggested the efficacy of targeting CD38-long-lived plasma cells in LN and SLE refractory to standard treatment. However, previous experiences were limited to adult patients and described different therapeutical schemes based on daratumumab, with the addition or absence of belimumab. Moreover, the minimal effective dose of daratumumab has yet to be fully defined. In this report, we describe two cases of juvenile-onset refractory LN/SLE successfully managed with a combination of a single infusion of rituximab (targeting CD20 on B cells) and daratumumab (targeting CD38 on long-lived plasma cells), unlike prior regimens requiring prolonged daratumumab infusions. Our approach was safe and effective and may potentially reduce adverse effects and costs, providing a novel therapeutic option for juvenile refractory LN.
Introduction
Lupus nephritis (LN) is the most common complication in systemic lupus erythematosus (SLE), affecting 30%–50% of patients. Both the European League Against Rheumatism/European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) (1) and the Kidney Disease Improving Global Outcomes (KDIGO) guidelines (2) are mostly based on non-specific immunosuppressive agents, including glucocorticoids, mycophenolate mofetil (MMF), or cyclophosphamide (CYC), which represent the standard of care (SOC). However, the SOC may be only partially effective in more complicated cases. Indeed, a consistent minority of patients do not achieve a complete response and are classified as refractory. Refractory SLE/LN is defined by the failure to achieve a complete response within 3-4 months, partial remission within 6-12 months, or complete remission after 48 months of the SOC (3).
SLE is largely considered a typical autoimmune disease, sustained by different circulating autoantibodies targeting several cellular components (4, 5). Therefore, the imbalance of the B cell tolerance may represent a key point in the pathogenesis of SLE (6) and provides a rationale for B cell-targeted therapy, as previously reported. More recently, non-dividing CD38-long-lived plasma cells that, unlike short-lived plasmablasts, reside in dedicated niches in the bone marrow and do not express CD20 on the surface, have been proposed as a relevant target in refractory cases of SLE and LN. In line with this, previous studies on mouse models of SLE reported that antiproliferative immunosuppressive therapy, such as CYC, depleted short-lived plasmablasts, but not long-lived plasma cells, which may continue to produce autoantibodies (7).
Daratumumab is a fully human monoclonal antibody targeting CD38 and represents the cornerstone of treatment in multiple myeloma. In two different case series, Ostendorf et al. (8) and Roccatello et al. (9) have recently described the efficacy of targeting CD38-long-lived plasma cells in LN and SLE refractory to standard treatment. However, previous experience described different therapeutical schemes based on daratumumab, such as the addition or absence of maintaining treatment with belimumab (8). Moreover, the minimal effective dose of daratumumab has yet to be fully defined.
Therefore, herein, we describe two cases of juvenile refractory LN/SLE treated with a combined single infusion of the monoclonal chimeric anti-CD20 antibody rituximab and daratumumab.
Case presentations
Patient 1
Patient 1 is a 17-year-old girl who presented at 14 years of age with low-grade fever, asthenia, malar rash, headache, oral ulcers, arthralgias/arthritis, and pericarditis. Laboratory tests showed leukopenia [3.280/mm3, normal values (n.v.) 4,200 - 9,800], hypocomplementemia (C3 89 mg/dL, n.v. 95-180, C4 4 mg/dL, n.v. 15 - 53), high inflammatory markers (VES 68 mm/h, n.v. 0-19), hypergammaglobulinemia, anti-nuclear antibodies (ANAs) (titer 1:320 - positivity >1:40) and anti-dsDNA antibodies (68 IU/mL - positivity>10UI/mL) as well as non-nephrotic proteinuria (1g/day) and microscopic hematuria. Therefore, she was admitted to our Nephrology Unit with a diagnosis of SLE and a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score (10) of 38 (Figures 1A, B). The kidney biopsy revealed LN class IV+V, characterized by diffuse mesangial expansion and endocapillary hypercellularity, and early signs of focal and segmental sclerosis (Figure 1C). Immunofluorescence (IF) staining was positive for a “full house” pattern (IgA ++, IgG +++, IgM ++, C3 +++, C4 +, C1q ++, glomerular C4d +++). The activity and the chronicity index score were 14 and 2, respectively. Therefore, she was treated with the SOC based on high-dose intravenous (IV) glucocorticoid pulses (methylprednisolone 1g/day for 3 consecutive days) and cyclophosphamide according to the Eurolupus regimen (11), consisting of 500 mg of IV cyclophosphamide every 2 weeks for a total of six administrations. After that, maintenance treatment with MMF, oral glucocorticoids, and hydroxychloroquine was started, leading to complete clinical and serological remission.
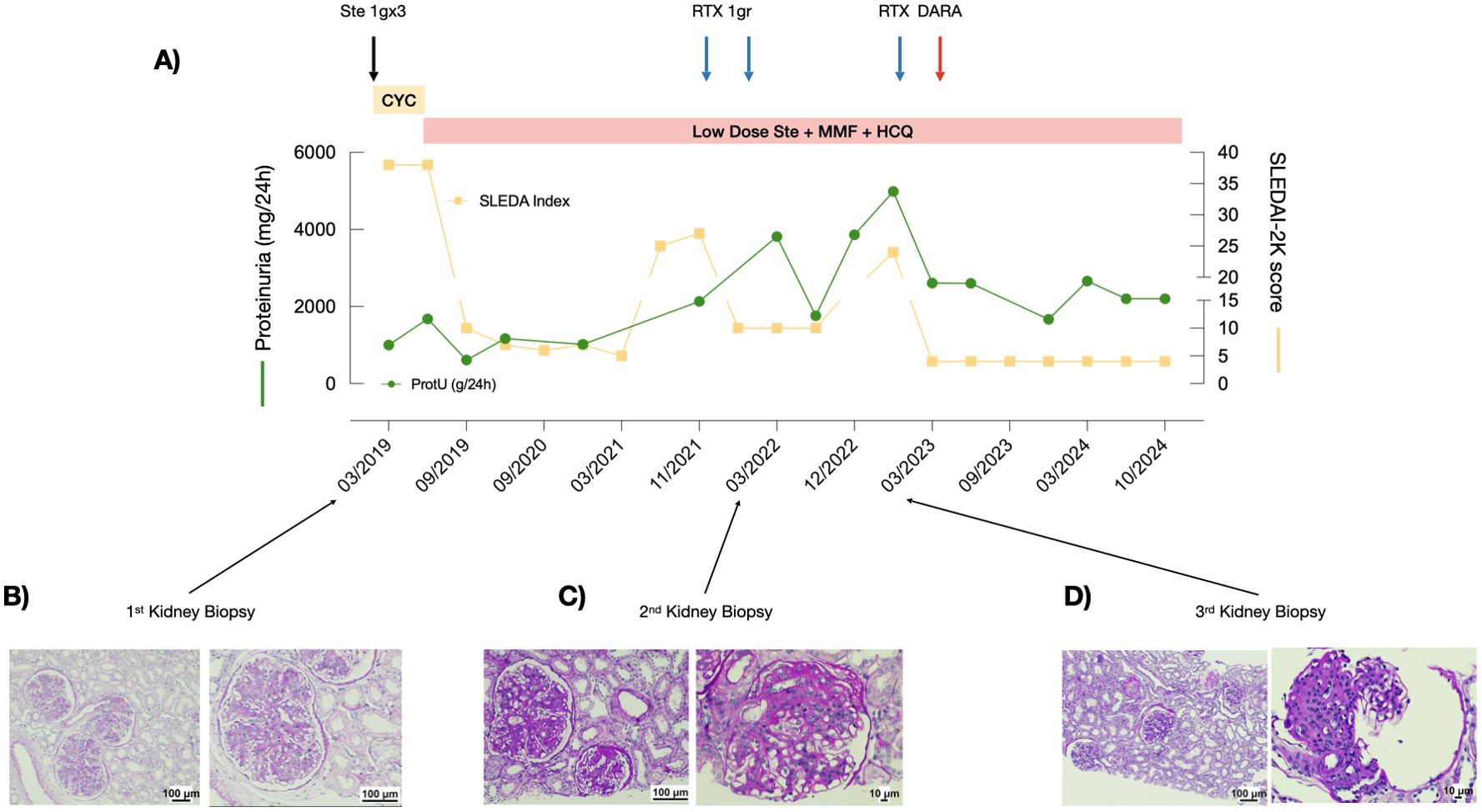
Figure 1. Response to treatment with combined rituximab and daratumumab in Patient 1. (A) Proteinuria levels (green line) and SLEDAI scores (yellow line) during the observation period. (B-D) PAS staining of the three kidney biopsies performed at recurrence.
After 2 years, she experienced a recurrence of clinical signs, including asthenia, arthritis and headache, accompanied by laboratory findings indicative of LN relapse: lymphopenia (890/mm³), reduced complement component C4 (5 mg/dL), elevated anti-dsDNA antibodies (17 IU/mL), and increased proteinuria (2.5 g/day), with a SLEDAI-2K score of 25. A kidney biopsy largely confirmed the previous histological findings, in accordance with LN class IV+V (Figure 1B). To minimize overall exposure to cyclophosphamide, two infusions of rituximab (375 mg/m2 6 weeks apart) were administered, resulting in clinical improvement but with persistence of significant proteinuria (1.7 g/day), low C4 levels (4 mg/dL), and positive anti-dsDNA (15 IU/ml). Fourteen months after rituximab infusions, she presented with a significative worsening of proteinuria (4 g/day) with a SLEDAI-2K score of 18. A kidney biopsy was repeated, demonstrating 3/8 sclerotic glomeruli, while the remaining 5/8 presented with mesangioproliferative and membranoproliferative lesions, with a thickening of the basement membrane, 25%-50% of the glomeruli exhibited fibrosis of Bowman’s capsule, and one glomerulus showed neutrophils within the tuft. Segmental sclerosis characterized 25%-50% of the glomeruli with segmental glomerular microthrombosis. The interstitium and large-size vessels did not present with significant lesions. IF staining showed a partial remission of the “full-house” pattern (IgA ++, IgG +, IgM -, C3 -, C4 -, C1q +), consistent with an activity index score of 5 and chronicity index score of 4 (Figure 1D).
In light of the histological findings, despite the overall moderate disease activity, the patient was treated with a combination of a single infusion of rituximab (375 mg/m2) and daratumumab (1.2g), with a consequent significant reduction in proteinuria (from 4.9 to 2.6 g/day), improvement of activity disease index, such as complement component C4 (17 mg/dl) and anti-dsDNA antibodies (3.6 UI/ml), and a reduction in SLEDAI-2K score to 4. At 2 years of follow-up, she is receiving standard therapies with low-dose glucocorticoids (prednisone 5 mg/day), MMF, and hydroxychloroquine, and proteinuria is stable at 2.6 g/day, with a SLEDAI-2K score of 4, marking the longest remission period since disease onset (Figure 1A). The residual significant proteinuria is consistent with chronic damage (chronicity index score of 4).
All the treatments were well tolerated, with no short- or long-term complications, including hypogammaglobulinemia. Renal function was consistently stable within normal values (0.5 mg/dl, n.v. 0.4-1.2). In Table 1, we provide the main circulating lymphocyte subtype analysis before the administration of the combined treatment (t0) and at 6 (t6) and 12 (t12) months of follow-up.
Patient 2
Patient 2 is a 22-year-old man who was diagnosed with SLE at 15 years of age after presenting with fever, arthritis, malar rash, chilblains, proteinuria (0.5-0.7 g/day), microscopic hematuria, consumption of serum complement components C3 and C4 (C3 42 mg/dL, C4 6 mg/dL), ANA positivity (1:160), and anti-dsDNA antibodies >240 IU/mL (SLEDAI-2K=19)(Figure 2A). A kidney biopsy was performed, revealing mesangial expansion and proliferation (Figure 2B). IF staining showed IgA +, IgG +++, IgM -, C3 ++, C4 +, C1q ++, and glomerular C4d ++. The activity and the chronicity index scores were 10 and 2, respectively. He received the SOC including MMF, glucocorticoids, and hydroxychloroquine, with consequent complete clinical and serological remission. After 3 years, he presented with a disease flare, with proteinuria 2.7 g/day and consumption of the complement components (C3 61mg/dL, C4 7 mg/dL) (SLEDAI-2K score was 12). A second kidney biopsy was performed, which showed mesangial and endocapillary hypercellularity in approximately 70%-80% of the glomeruli, with cellular crescents (Figure 2C). IF revealed IgA +, IgG +++, IgM +, and C3 +++. The activity and the chronicity index scores were 14 and 2, respectively. Therefore, IV glucocorticoid pulses (1 g/day for 3 consecutive days) and CYC according to the EULAR/ERA recommendations (11) were administered, with remission of the proteinuria. Low-dose glucocorticoids, MMF, and hydroxychloroquine were maintained. However, over the following 2 years, the patient continued to exhibit signs and symptoms consistent with refractory SLE, including headache, photophobia, low complement levels (C3 49-70 mg/dL, C4 6-15 mg/dL), and elevated anti-dsDNA antibodies (169-240 IU/mL) with SLEDAI-2K scores between 10 and 12 (Figure 2A), leading to a poor quality of life. Despite this, proteinuria remained negative.
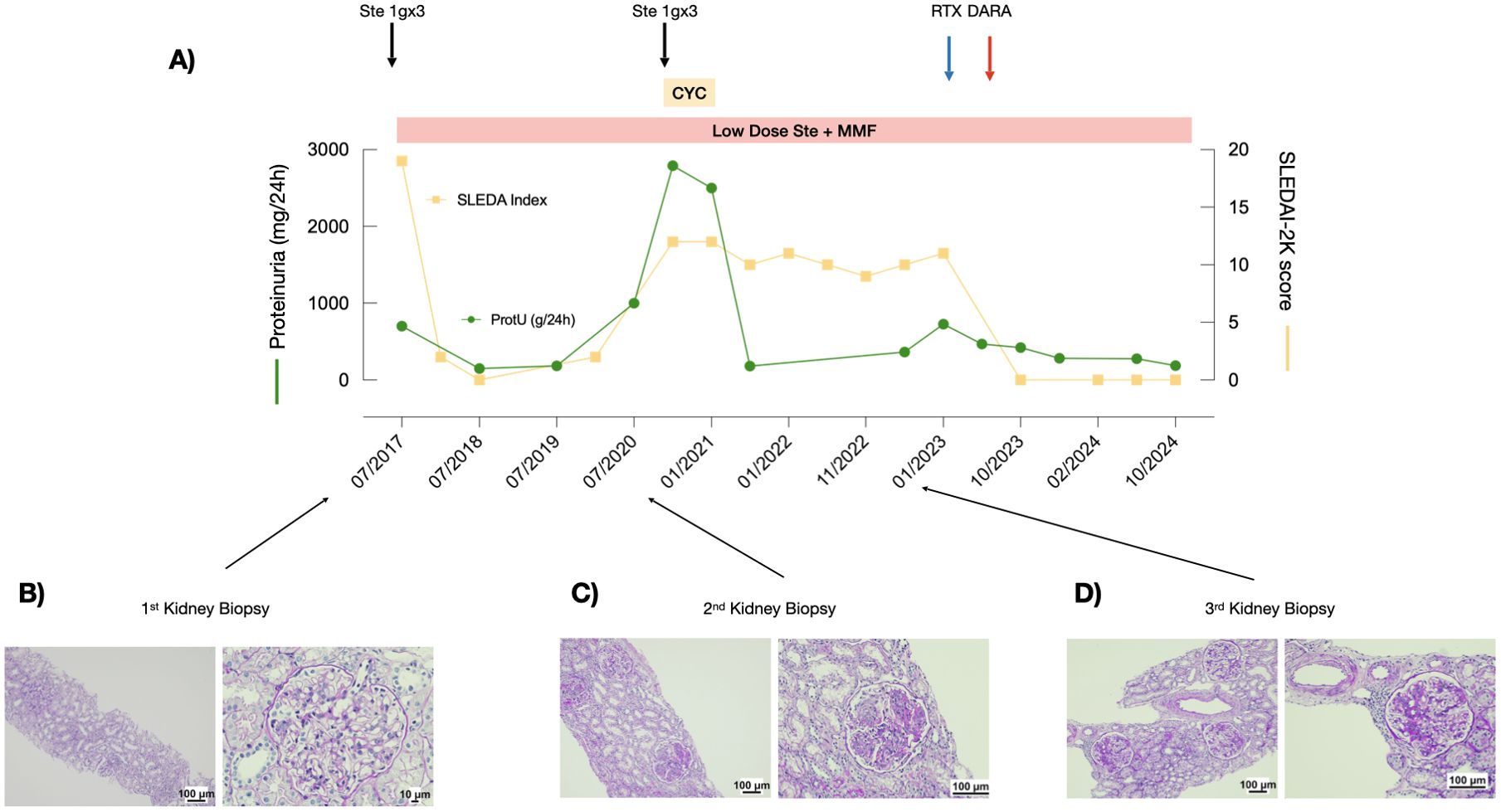
Figure 2. Response to treatment with combined rituximab and daratumumab in Patient 2. (A) Proteinuria levels (green line) and SLEDAI scores (yellow line) during the observation period. (B–D) PAS staining of the three kidney biopsies performed at recurrence.
At the 2 year-follow-up after the Eurolupus regimen administration, a third kidney biopsy was performed due to a significant worsening of proteinuria (0.7 g/day): all the glomeruli showed mesangioproliferative and membranoproliferative lesions with a thickening of the basement membrane, which displayed cribriform patterns, double contours, and spikes. Glomerulosclerosis was present in approximately half of the glomeruli (Figure 2D). IF revealed deposits of only C3 and C1q (C3 +, C1q ++). His SLEDAI-2K score was 11. The activity and the chronicity index scores were 9 and 2, respectively. Therefore, the patient was treated with a combination of a single infusion of rituximab (375 mg/m2) and daratumumab (1g). After the infusions, proteinuria rapidly normalized (0.28 g/day) and laboratory findings improved (C3 112 mg/dl; C4 24 mg/dl). The anti-dsDNA antibody test was negative 1 month after the last infusion.
At the last follow-up, 2 years after daratumumab administration, the patient is receiving standard therapies with low-dose glucocorticoids, hydroxychloroquine, and MMF. Proteinuria remains negative and renal function normal (0.8 mg/dl) and the patient’s SLEDAI-2K score is 0 (Figure 2A).
Neither infusion-related reactions, nor short-term adverse events were noted after rituximab and/or daratumumab administration, including hypogammaglobulinemia (Table 1).
Discussion
We recently described the efficacy and the safety of combining treatment with rituximab and daratumumab for complicated forms of podocytopathies both in native kidneys (12) and in post-transplant recurrence in pediatric and young adult subjects (13). Given the favorable safety profile of the therapeutic regimen in pediatric populations, this study reports on the efficacy and safety of combined rituximab and daratumumab treatment in two young adults with refractory class IV and V LN, initially diagnosed during childhood. The administration of daratumumab in SLE/LN was recently reported in two different case series. Ostendorf et al. (8) described two patients affected by life-threatening and refractory SLE with severe multisystemic involvement. The first subject had LN class III-V with nephrotic syndrome, pericarditis, arthritis, and skin rash, despite treatment with MMF, cyclosporine A, and glucocorticoids. The second subject presented with hemolytic anemia, immune thrombocytopenia, cutaneous vasculitis, arthritis, alopecia, and mucosal ulcers, despite previous treatments with cyclophosphamide, MMF, belimumab, rituximab, azathioprine, methotrexate, hydroxychloroquine, plasmapheresis, immunoglobulin, and bortezomib. Both patients were treated with daratumumab (16 mg/kg once a week for 4 weeks), in addition to maintenance treatment consisting of belimumab, low-dose glucocorticoids, and MMF. The four administrations of daratumumab resulted in a significant reduction in anti-dsDNA antibodies and SLEDAI-2K score (8). More recently, Roccatello et al. (9) reported six adult patients with refractory LN treated with daratumumab, according to the multiple myeloma protocol (16 mg/kg once a week for 8 weeks followed by eight biweekly infusions and up to eight monthly infusions). In 5/6 patients, their SLEDAI-2K score significantly decreased, with improvement in kidney function and remission of proteinuria at the 12-month follow-up.
Overall, previous data suggest the potential effectiveness of multiple infusions of daratumumab in refractory LN and SLE (8, 9). We here described the effectiveness of combining a single infusion of monoclonal anti-CD20 with anti-CD38 antibodies in two cases of juvenile SLE, which are usually characterized by higher incidence of renal involvement than patients with adult-onset SLE (14). We did not administer daratumumab alone because rituximab, despite a previous negative clinical trial (15), is already suggested as possible treatment in refractory SLE (16). Therefore, from an ethical standpoint, we decided not to prevent patients from receiving a treatment already recognized as potentially effective.
Therefore, in contrast to previous experiences, we administered only single infusions of rituximab and daratumumab. Indeed, after the dual treatment with rituximab and daratumumab, the remission was maintained with standard therapies consisting with low-dose glucocorticoids, hydroxychloroquine, and MMF in both patients, without the chronic addition of belimumab as reported by Ostendorf et al. (8).
As expected, we report a significant reduction in total CD19+ B cells and circulating CD38+ plasma cells after the combined treatment that fully recovered 12 months after treatment. Different immune phenotyping did not reveal other major changes after treatment (Table 1). However, further studies are needed to assess whether daratumumab alone, by depleting CD38+ plasma cells, is sufficient in promoting disease remission.
Overall, this therapeutic approach led to a significant response in both cases with rapid and sustained remission, reducing the need for additional immunosuppressive agents or further infusion of daratumumab, along with their possible side effects and costs. Moreover, both patients confirmed that the stable disease remission led to a significant increase in their quality of life. Generally, the standard treatment for SLE and LN, involving high-dose glucocorticoids and immunosuppressants such as cyclophosphamide, may lead to severe complications such as infections, metabolic issues, and possible organ damage. Achieving sustained remission is crucial to reduce toxic therapies, prevent flares, and slow CKD progression in LN, improving long-term outcomes. Emerging targeted therapies offer the promise of greater efficacy with less toxicity, enhancing patient quality of life and reducing healthcare burdens.
In conclusion, we described the efficacy and the safety of a combined single infusion of rituximab and daratumumab in two young adult subjects with refractory juvenile-onset SLE SLE/LN. Currently, a Phase 2 Open-label trial, testing the efficacy and safety of daratumumab in adult subjects affected by LN, is in the enrolling phase (NCT04868838). However, given the safety profile reported here, and considering that juvenile-onset disease more frequently involves renal manifestations compared to adult-onset (17), future studies should also include pediatric patients with LN and explore whether this approach might also benefit different systemic involvements, such as neuropsychiatric SLE (NPSLE).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AA: Conceptualization, Data curation, Funding acquisition, Writing - review & editing. DC: Writing – review & editing. RM: Writing – review & editing. CB: Data curation, Writing – review & editing. GC: Funding acquisition, Investigation, Writing – review & editing. AC: Formal analysis, Visualization, Writing – review & editing. VN: Data curation, Validation, Visualization, Writing – review & editing. GM: Visualization, Writing – review & editing. XK: Methodology, Visualization, Writing – review & editing. FL: Visualization, Writing – review & editing. MG: Conceptualization, Visualization, Writing – review & editing. GMG: Visualization, Writing – review & editing. EP: Visualization, Writing – review & editing. GG: Data curation, Formal analysis, Visualization, Writing – review & editing. EV: Funding acquisition, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. AA was supported with public funds granted by the Italian Ministry of Health “Ricerca Corrente” and “5x1000 funds”. GC and AA were supported by the European Union - Next Generation EU - NRRP M6C2 - Investment 2.1 Enhancement and strengthening of biomedical research in the NHS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fanouriakis A, Kostopoulou M, Andersen J, Aringer M, Arnaud L, Bae SC, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. (2024) 83:15–29. doi: 10.1136/ard-2023-224762
2. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
3. Roccatello D, Sciascia S, Rossi D, Fenoglio R. Refractory systemic lupus erythematosus: identification and pharmacological management. Drugs. (2023) 83:117–34. doi: 10.1007/s40265-022-01824-x
4. Renaudineau Y, Pers JO, Bendaoud B, Jamin C, Youinou P. Dysfunctional B cells in systemic lupus erythematosus. Autoimmun Rev. (2004) 3:516–23. doi: 10.1016/j.autrev.2004.07.035
5. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheumatol. (2010) 62:222–33. doi: 10.1002/art.27233
6. Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. (2001) 2:764–6. doi: 10.1038/ni0901-764
7. Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. (2004) 199:1577–84. doi: 10.1084/jem.20040168
8. Ostendorf L, Burns M, Durek P, Heinz GA, Heinrich F, Garantziotis P, et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N Engl J Med. (2020) 383:1149–55. doi: 10.1056/NEJMoa2023325
9. Roccatello D, Fenoglio R, Caniggia I, Kamgaing J, Naretto C, Cecchi I, et al. Daratumumab monotherapy for refractory lupus nephritis. Nat Med. (2023) 29:2041–7. doi: 10.1038/s41591-023-02479-1
10. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:1400–12. doi: 10.1136/annrheumdis-2018-214819
11. Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. (2010) 69:61–4. doi: 10.1136/ard.2008.102533
12. Angeletti A, Bin S, Kajana X, Spinelli S, Bigatti C, Caridi G, et al. Combined rituximab and daratumumab treatment in difficult-to-treat nephrotic syndrome cases. Kidney Int Rep. (2024) 9:1892–6. doi: 10.1016/j.ekir.2024.04.006
13. Angeletti A, Bin S, Magnasco A, Bruschi M, Cravedi P, Ghiggeri GM. Efficacy of combined rituximab and daratumumab treatment in posttransplant recurrent focal segmental glomerulosclerosis. Am J Transplantation. (2024) 24:688–92. doi: 10.1016/j.ajt.2023.12.010
14. Hoffman IE, Lauwerys BR, De Keyser F, Huizinga TW, Isenberg D, Cebecauer L, et al. Juvenile-onset systemic lupus erythematosus: different clinical and serological pattern than adult-onset systemic lupus erythematosus. Ann Rheum Dis. (2009) 68:412–5. doi: 10.1136/ard.2008.094813
15. Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheumatol. (2012) 64:1215–26. doi: 10.1002/art.34359
16. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. (2021) 100:753–79. doi: 10.1016/j.kint.2021.05.015
Keywords: systemic lupus erythematous, lupus nephritis, rituximab, daratumumab, monoclonal antibodies, pediatric
Citation: Chiarenza DS, Mancini R, Bigatti C, Caridi G, Consolaro A, Natoli V, Mortari G, Kajana X, Lugani F, Gattorno M, Ghiggeri GM, La Porta E, Gaggero G, Verrina EE and Angeletti A (2025) Case report: Single infusion of combined anti-CD20 and anti-CD38 monoclonal antibodies in pediatric refractory lupus nephritis. Front. Immunol. 16:1525892. doi: 10.3389/fimmu.2025.1525892
Received: 10 November 2024; Accepted: 06 January 2025;
Published: 28 January 2025.
Edited by:
Li-Tung Huang, Kaohsiung Chang Gung Memorial Hospital, TaiwanReviewed by:
Chrysanthi Skalioti, Laiko General Hospital of Athens, GreeceThomas Schindler, Roche, Switzerland
Copyright © 2025 Chiarenza, Mancini, Bigatti, Caridi, Consolaro, Natoli, Mortari, Kajana, Lugani, Gattorno, Ghiggeri, La Porta, Gaggero, Verrina and Angeletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Angeletti, YW5kcmVhYW5nZWxldHRpQGdhc2xpbmkub3Jn
 Decimo Silvio Chiarenza1
Decimo Silvio Chiarenza1 Gianluca Caridi
Gianluca Caridi Valentina Natoli
Valentina Natoli Gabriele Mortari
Gabriele Mortari Xhuliana Kajana
Xhuliana Kajana Francesca Lugani
Francesca Lugani Marco Gattorno
Marco Gattorno Gian Marco Ghiggeri
Gian Marco Ghiggeri Edoardo La Porta
Edoardo La Porta Enrico E. Verrina
Enrico E. Verrina Andrea Angeletti
Andrea Angeletti