- 1Department of Medical Oncology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Center for Dermato-oncology, Department of Dermatology, Eberhard Karls University of Tübingen, Tübingen, Germany
- 3Clinical Epidemiology and Applied Biostatistics, Eberhard Karls University of Tübingen, Tübingen, Germany
- 4Oral and Maxillofacial Surgery, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 5Second Department of Dermatology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 6Cluster of Excellence iFIT (EXC 2180) “Image-Guided and Functionally Instructed Tumor Therapies”, Tübingen, Germany
- 7First Department of Dermatology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Background: Despite durable responses achieved with Immune Checkpoint Inhibitors (ICIs), data about optimal duration of treatment, especially in the context of adverse events, remain scarce.
Objective: To systematically review the evidence concerning the impact of treatment discontinuation with ICIs for reasons other than progressive disease (PD) on relapse rates and survival of melanoma patients.
Methods: A systematic literature search was conducted in three electronic databases until July 2024. Studies referring to melanoma patients who ceased ICIs electively (i.e. due to complete response (CR), protocol completion or patient/physician’s wish) or due to treatment-limiting toxicities (TLTs) were selected. Relapse rates (RRs) post cessation, time to PD, rechallenge and disease control rate (DCR) after 2nd course were the main outcomes. Random-effects models were preferred, and subgroup and sensitivity analyses were conducted to investigate possible sources of heterogeneity.
Results: 38 and 35 studies were included in qualitative and quantitative synthesis, respectively. From 2542 patients discontinued treatment with ICIs electively or due to TLTs, 495 experienced progression [number of studies (n)=34, RR 20.9%, 95%CI 17.1 – 24.7%, I2 85%) and higher rates were detected in patients with TLTs compared to elective discontinuation. Mean time to PD was 14.26 months (n=18, mean time 14.26, 95%CI 11.54 – 16.98, I2 93%) and was numerically higher in patients who ceased for CR compared to patients with TLTs. Treatment duration before cessation was not associated with risk and time to relapse, while mucosal melanomas and non-CR as BOR during treatment led to increased risk for relapse and shorter time to PD compared to other histologic subtypes or CR. Rechallenge with ICI resulted in 57.3% DCR and 28.6% pooled CR rates (n=22, CR rate 28.6%, 95%CI 17.1 – 40.2, I2 68%). Heterogeneity among studies was high, but subgroup analysis based on type of ICI used (anti-CTL4 and anti-PD1 inhibitor or anti-PD1 monotherapy) and type of study (RCTs or observational studies), along with sensitivity analyses did not reveal significant alterations in results.
Conclusion: Discontinuation of ICIs in patients without progression is possible. Outcomes to rechallenge with ICIs may differ depending on the reason for discontinuation, but remains a considerable option.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024547792.
1 Introduction
Immune checkpoint inhibitors (ICIs) revolutionized the treatment of metastatic melanoma patients, contributing to significantly improved overall survival (OS) rates compared to other treatment modalities (1, 2). Importantly, responses on ICIs are considered durable and patients achieving complete or partial responses seem to remain on response, even after treatment cessation (3, 4). However, the optimal treatment duration for patients with melanoma without progression remains unknown (5), while the total duration of therapy with ICIs are determined arbitrary in treatment protocols, ranging from 2 years in some studies to treatment until progressive disease (PD) in others (1, 2, 6).
Careful consideration of treatment duration with ICIs lies in balancing response preservation after ICI cessation and toxicity avoidance, while reducing costs. Immune related adverse events (irAEs), and especially late-onset irAEs, remain a major issue, affecting quality of life of patients receiving ICI, which is particularly important in the context of complete response (CR) (7, 8). To answer this question, observational studies analyzed the impact of treatment discontinuation electively or due to AEs on relapse, but their results remain inconsistent (9–12). In addition, meta-analyses examining optimal duration of ICIs in solid tumors, including melanoma, did not report either a survival benefit of patients treated with fixed duration compared to treatment until disease progression, or explored factors influencing relapse risk comprehensively (13, 14).
Consequently, the main aim of our review was to systematically review all available evidence on disease relapse following ICI discontinuation for reasons other than progressive disease (PD), to evaluate the role of various factors, such as type of ICI or reason for discontinuation, in an extensive way, and to report post-relapse management of patients who experienced PD after ICI cessation.
2 Materials and methods
2.1 Guidelines followed
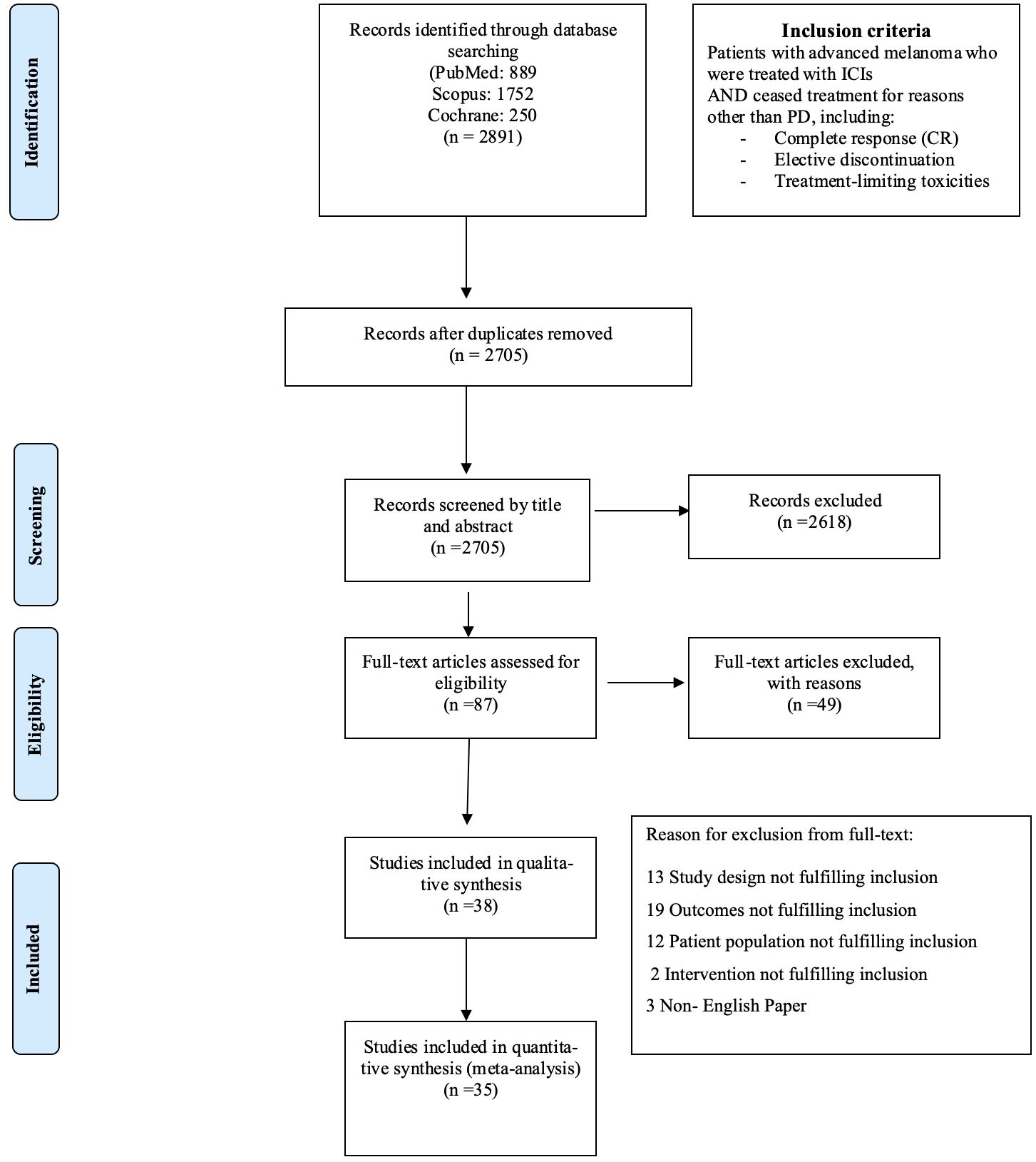
This systematic review followed the guidelines outlined in MOOSE (15) and PRISMA guidelines (16), where feasible. Figure 1 displays the flow chart diagram. Study has been registered in PROSPERO (International Prospective Register of Systematic Reviews, PROSPERO ID: CRD42024547792).
2.2 Search strategy
To identify eligible studies, 4 independent investigators (KL, DD, GF, MLR) conducted thorough literature search in the following electronic databases: MEDLINE (PubMed), Scopus and Cochrane (CENTRAL) until July 2024 and arising discrepancies were resolved by a fifth investigator (TA). In addition, conferences and grey literature were screened and manual search of references of included studies was conducted to search for relevant studies. To obtain missing data for included studies, authors were contacted via email. A representative example of search string in PubMed is: (“immunotherapy”[MeSH Terms] OR “immunotherapy”[Title/Abstract]
OR “immune checkpoint inhibit*”[Title/Abstract] OR “ICI”[Title/Abstract] OR “ICB”[Title/Abstract] OR “anti-PD1”[Title/Abstract] OR “anti-PDL1”[Title/Abstract] OR “anti-CTL4”[Title/Abstract]) AND (“discontinue*”[Title/Abstract] OR “disconti*”[Text Word] OR “cessa*”[Title/Abstract] OR “stop*”[Title/Abstract] OR “premature disconti*”[Title/Abstract] OR “early disconti*”[Title/Abstract] OR “interrupt*”[Title/Abstract] OR “break”[Title/Abstract]) AND (“melanoma”[MeSH Terms] OR “melanoma”[Title/Abstract] OR “skin cancer”[Title/Abstract] OR “cutaneous melanoma”[Title/Abstract] OR (“melanoma”[MeSH Terms] OR “melanoma”[All Fields] OR “melanomas”[All Fields] OR “melanoma s”[All Fields]) and further analysis can be found in Supplementary Marerial.
2.3 Study selection
The following parameters were set as inclusion criteria: studies including patients with melanoma who were treated with immune checkpoint inhibitors (either combination anti-CTL4 and anti-PD1, or anti-PD1 monotherapy) for advanced disease, and who ceased treatment for reasons other than PD. Discontinuation after CR (verified by biopsy or per RECIST criteria), protocol completion and patient/physician choice (both defined as “elective discontinuation”), or due to the development of treatment-limiting toxicities (TLTs) were the main reasons for treatment cessation. Both randomized controlled trials (RCTs) and observational studies (including prospective or retrospective studies, cohorts, case-control studies and case-series) were included. Exclusion criteria encompassed studies that referred to treatment discontinuation for PD, or not providing the reason for discontinuation in a clear way, studies including patients treated in the adjuvant setting or receive treatment other than ICIs, or patients with conjunctival or uveal melanomas, studies not providing extractable data, reviews, meta-analyses, case reports and non-English papers.
2.4 Data extraction
Four independent researchers (KL, DD, GF and MLR) extracted data from the eligible studies that met the inclusion criteria. A standardized form was used to record the following parameters: (i) first author, (ii) year of publication, (iii) country in which the study was conducted, (iv) study design, (v) duration of follow up, (vi) number of patients analyzed and number of patients who discontinued treatment, (vii) reasons for treatment cessation (i.e. elective or due to toxicities), (viii) outcomes reported in every study [i.e. relapse rates, time to relapse after treatment cessation, rechallenge (type of treatment used for rechallenge and disease control rate (DCR), PFS and OS).
The primary outcome was relapse rate after treatment discontinuation for any reason other than PD. Time to PD after treatment discontinuation, rechallenge and DCR after rechallenge, progression-free survival (PFS) (defined as the time from randomization for RCTs or start of treatment for observational studies to PD), OS (defined as the time from randomization for RCTs or start of treatment for observational studies to death or censoring) were set as secondary outcomes.
Different analyses based on reason for discontinuation [(i) elective cessation after disease control (including patients with CR, partial response or stable disease)) and without TLTs, (ii) elective cessation on CR and (iii) discontinuation due to TLTs without progression], along with subgroup analysis regarding type of ICI used (anti-CTL4 and anti-PD1 or anti-PD1 monotherapy) and type of studies (RCTs or observational studies) were also conducted.
2.5 Risk of bias and study quality assessment
For RCTs, the Cochrane Risk of Bias tool (RoB) (version 5.1.0) was selected to evaluate the quality of included studies (17). For observational studies, quality of selected studies was assessed by 4 independent reviewers (KL, DD, GF and MLR), using Newcastle-Ottawa scale (NOS) to evaluate risk of bias (18). This scale consists of the following categories: (i) participant selection, with a maximum rating of four stars, (ii) comparability of study groups, with a maximum of two stars, and (iii) assessment of outcome or exposure, with the highest rating reaching three stars. NOS and RoB results are demonstrated in Supplementary Tables 1 and Supplementary Tables 2, respectively.
2.6 Statistical analysis
Relapse rates (RRs) were summarized using raw proportions and the generic inverse variance method. Risk of relapse after elective discontinuation or due to AE was expressed with Odds Ratios and 95% Confidence Intervals (CIs). Also, regarding time to PD after discontinuation, pseudo-individualized patient – data (IPDs) were extracted from Kaplan – Meier curves using methodology described by Tierney et al. (19), or from swimmer plots, according to Pala et al (13). Median times were converted into mean time to PD and standard errors based on methodology described by McGrath et al (20). Random effect models were used for data synthesis, in cases of high heterogeneity. I2 index was preferred for determining heterogeneity extent among studies, with values lower than 30% being considered as low heterogeneity, values ranging from 30% to 60% as moderate and values >60% as considerable heterogeneity. Subgroup analyses and sensitivity analyses were conducted to investigate possible sources of heterogeneity. Risk of publication bias was examined with Funnel plots and Egger’s test for small-study effects. All statistical tests were two-sided, p-value<0.05 was considered statistically significant and the analysis was conducted with Review Manager v.5.4.1 and IBM SPSS v29.
3 Results
3.1 Description of results from literature search
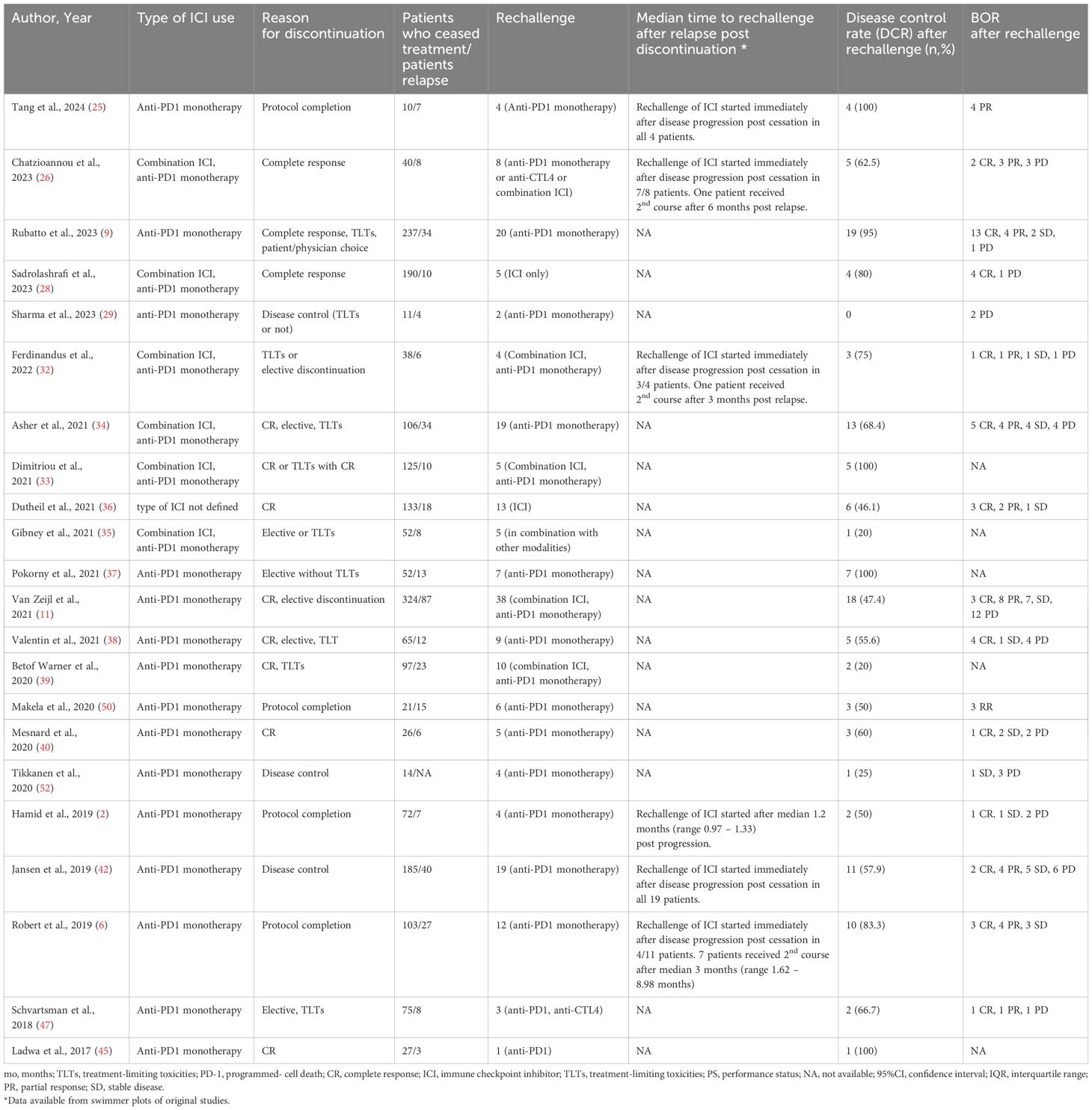
Until July 2024, 2,705 eligible studies were identified after duplicates removal, 87 of which were full-text assessed for eligibility. From those, 49 were excluded (for detailed reasons for exclusion please see Supplementary Table 3) and 38 and 35 were included in the qualitive and quantitative synthesis, respectively (Figure 1). Three studies were included in systematic review only, due to lack of extractable data available in the other publications (21–23). Overall, 34 studies investigated the relapse rates after discontinuation of ICI for reasons other than PD, 22 reported time to relapse after discontinuation, while 22 had available data concerning ICI rechallenge and DCR thereafter (Table 1).
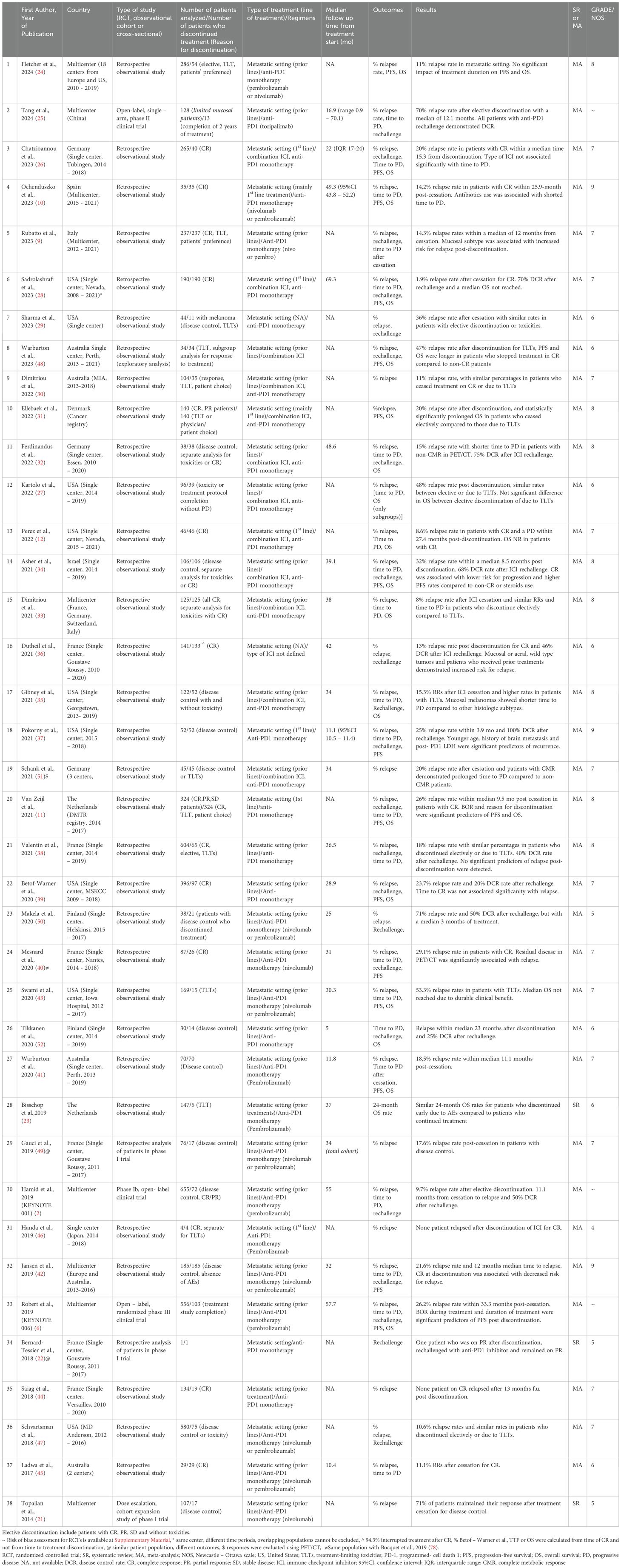
Table 1. Baseline characteristics of studies included in systematic review/meta-analysis, reasons for ICI discontinuation, outcomes analyzed and significant results of each study.
3.2 Relapse rates after ICI discontinuation
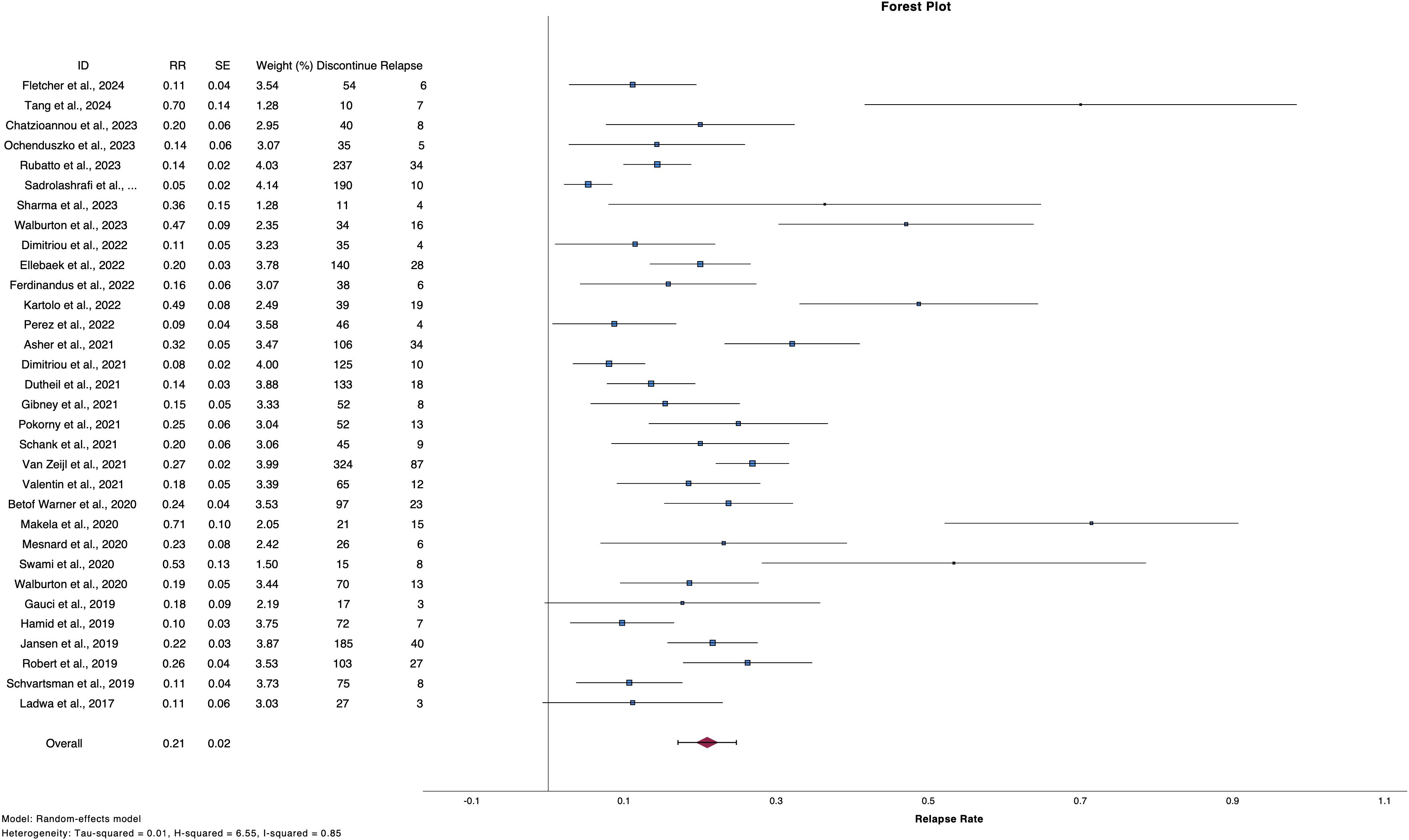
2,542 patients discontinued treatment with ICIs electively or due to TLTs and from them, 495 patients experienced progression [number of studies (n)=34, RR 20.9%, 95%CI 17.1 – 24.7%, I2 85%) (Table 2, Figure 2) (2, 6, 9–12, 24–51). Regarding reason for discontinuation, 28 studies reported data for elective cessation after disease control, leading to a pooled relapse rate of 15.9% (n=27, RR 15.8%, 95%CI 12.4-19.4%, I2 72%), while for patients who discontinued after CR, relapse rate was smaller (n=22, RR 13.2%, 95%CI 10.5 – 16.0, I2 43%). Nine studies provided data about patients who discontinue electively with BOR of PR/SD and concluded to a 27.7% relapse rate after ICI cessation (n=9, RR 27.7%, 95%CI 19.2 – 36.3, I2 53%) (Supplementary Table 4, Supplementary Figure 1). Finally, patients with TLTs demonstrated higher rate of relapse post-cessation (n=14, RR 25.9%, 95%CI 18.3 – 33.4, I2 70%) (Supplementary Figure 2).
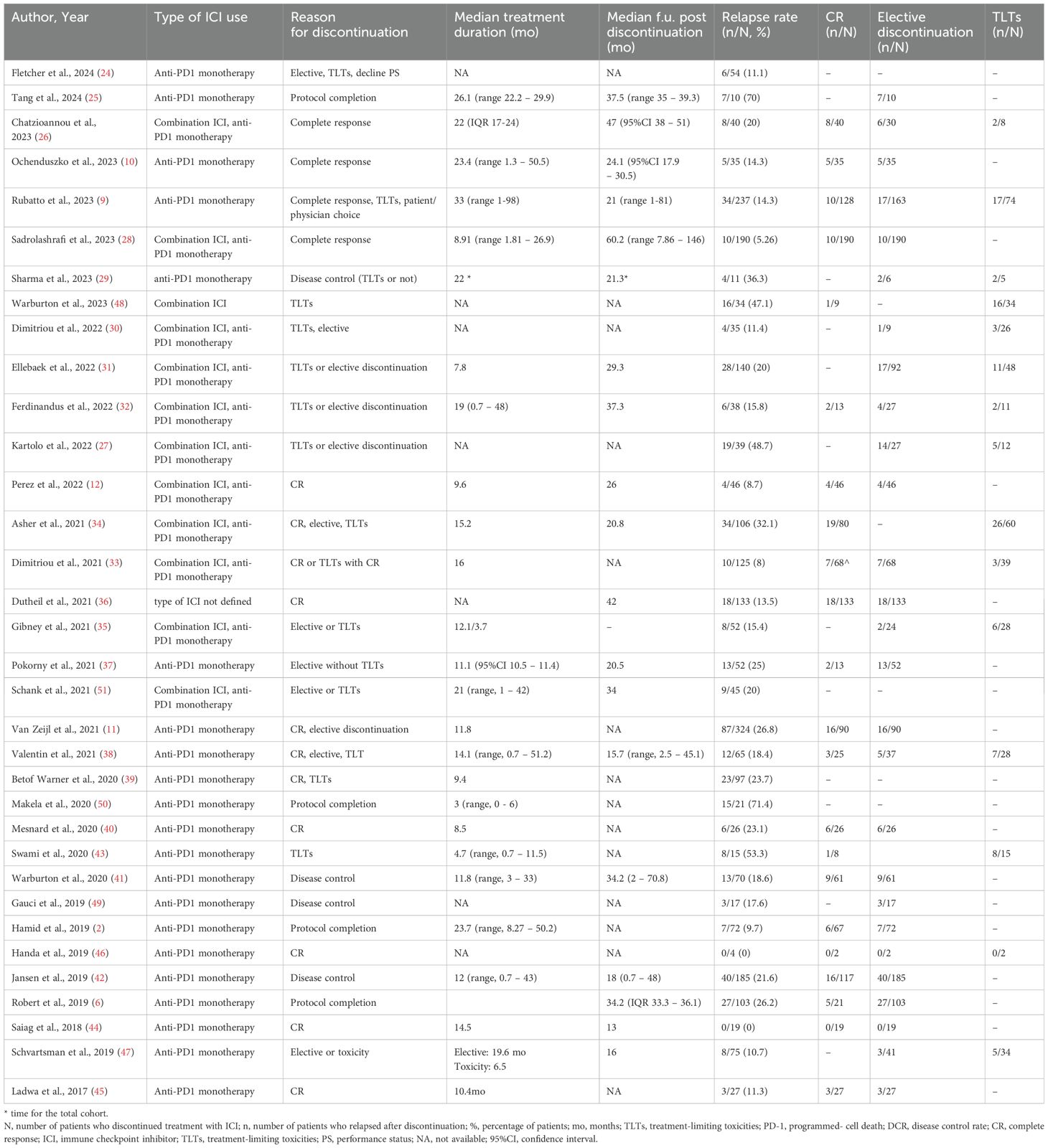
Table 2. Studies referring to discontinuation of ICIs, reason for discontinuation, relapse rates and subgroup analysis based on reason for cessation.
3.3 Time to relapse
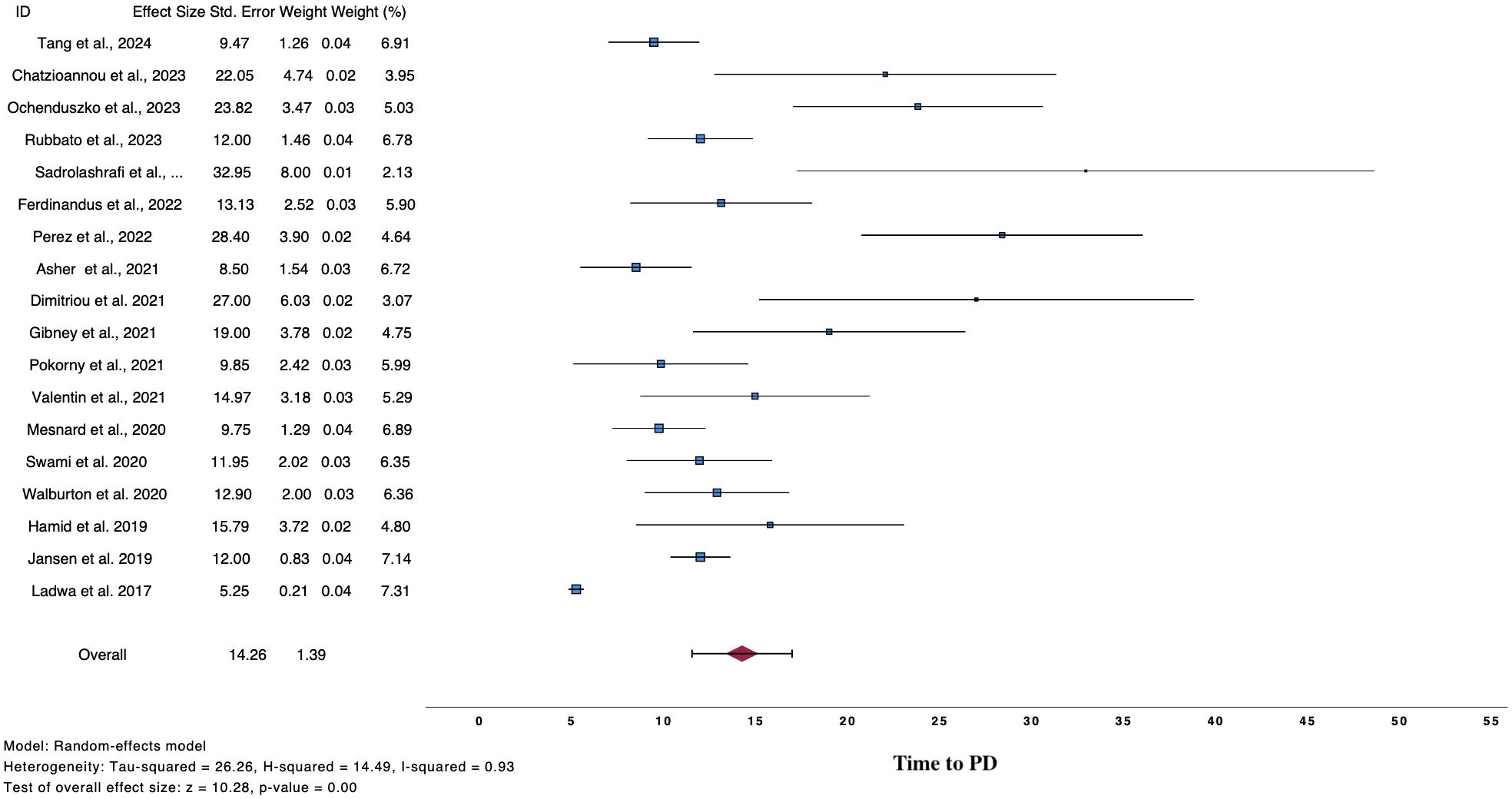
Twenty-two studies analyzed time to PD post discontinuation (Table 3), 18 provided extractable data and the pooled mean time to PD was 14.26 months (n=18, mean time 14.26, 95%CI 11.54 – 16.98, I2 93%) (Figure 3) (2, 9, 10, 12, 25, 26, 28, 32–35, 37, 38, 40–43, 45). Similar results were drawn, when the analysis was restricted to 140 patients, whose data were extracted from swimmer plots only (n=14, mean time 14.61 months, 95%CI 12.31 – 16.91) (2, 10, 12, 25, 26, 28, 32, 34, 35, 37, 38, 41, 42, 45). In addition, patients who ceased treatment for CR demonstrated numerically higher mean time to PD (n=13, mean time 15.88, 95%CI 12.29 – 19.47) compared to overall population and patients with TLTs (Supplementary Figure 3).
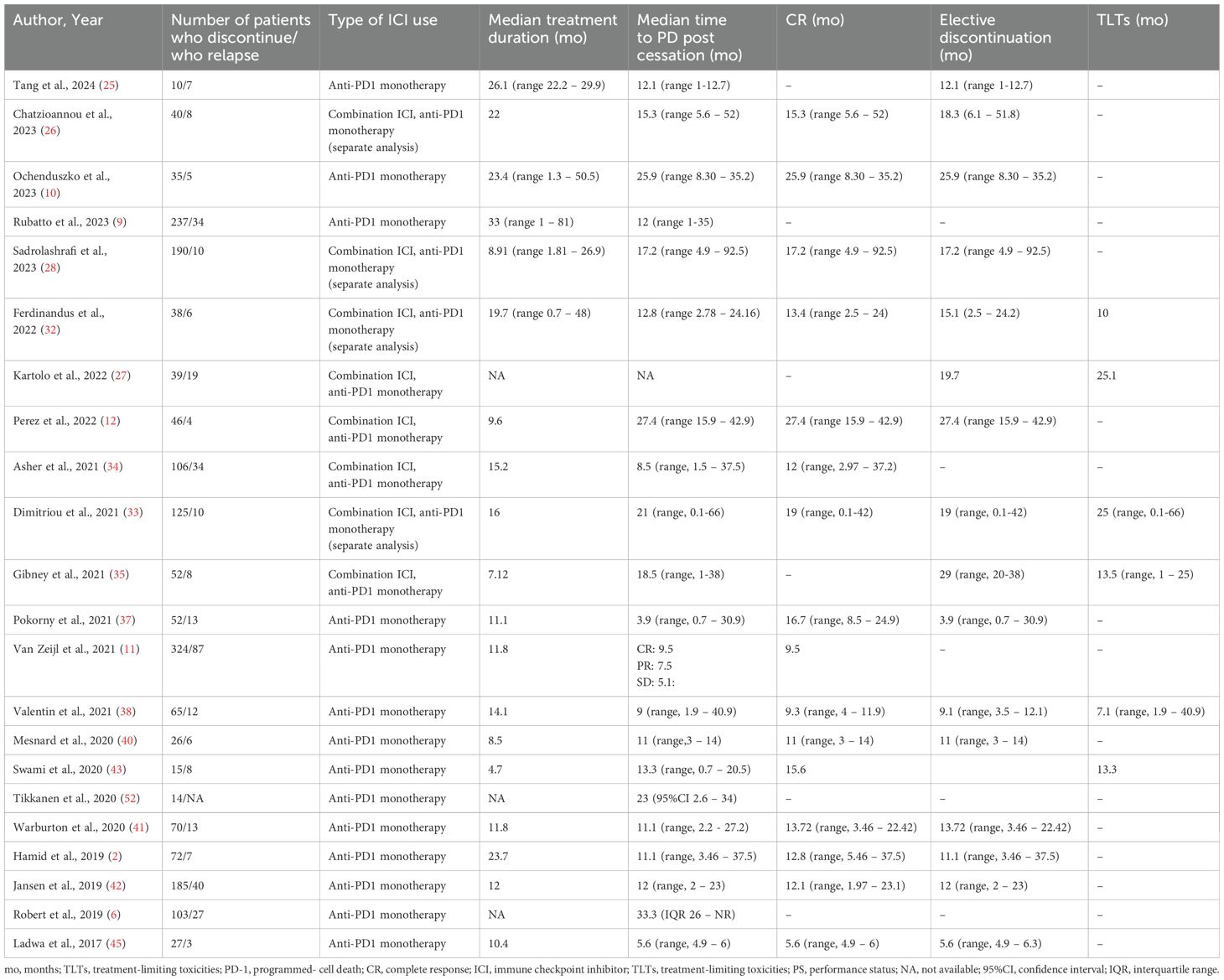
Table 3. Time to progressive disease post cessation in overall population and according to reason for discontinuation.
Regarding factors affecting the time to PD after cessation, 18 studies investigated the role of clinical characteristics of patients and the tumor (i.e. age, gender, anatomic location, histologic subtype), type of ICI used and response to treatment on relapse, and were synthesized qualitatively (6, 9–11, 26, 27, 32, 33, 35–42, 47, 51). Patients with mucosal melanoma had shorter time to PD compared to patients with other histologic subtypes, while CR at discontinuation was associated with decreased risk and prolonged time to PD compared to TLTs (Supplementary Table 5).
3.4 Rechallenge after relapse
Post relapse management of patients who progressed after discontinuation is summarized in Table 4. From 380 patients with PD, 208 received a second course of ICIs, including both anti-CTL4 and anti-PD1 or anti-PD1 monotherapy (2, 6, 9, 11, 25, 26, 28, 29, 32–40, 42, 45, 47, 50, 52). Pooled CR rate after rechallenge was 28.6% (n=16, CR rate 28.6%, 95%CI 17.1 – 40.2, I2 68%), while 57.3% of patients exhibited disease control (n=22, DCR rate 57.3%, 95%CI 43.9 – 70.6, I2 73%) (Figure 4).
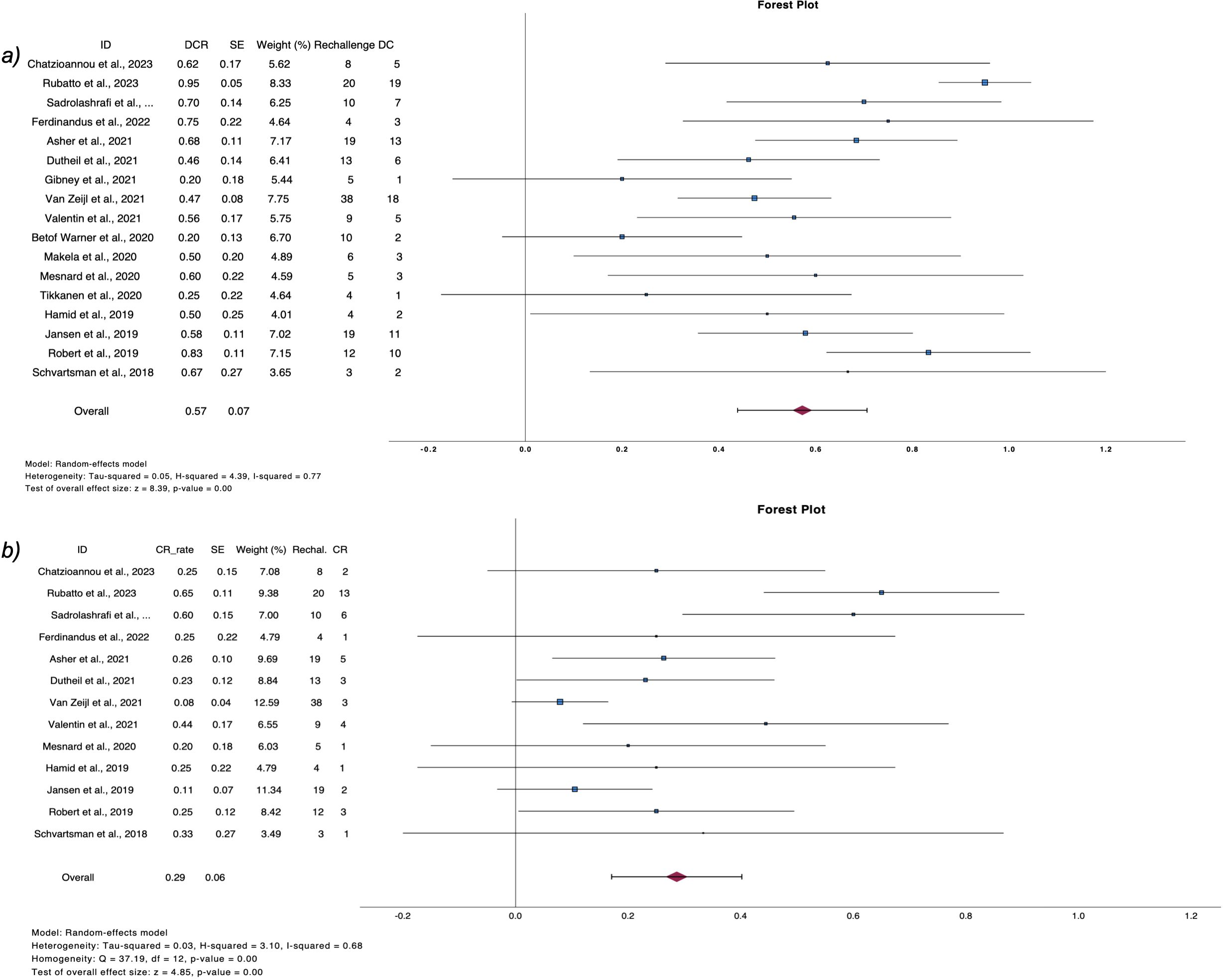
Figure 4. Forest plots demonstrate (A) disease control rate and (B) complete response rate after ICI rechallenge following PD.
3.5 PFS – OS
PFS and OS for patients who ceased treatment were analyzed qualitatively, along with factors affecting time to PD and the results were presented in Supplementary Table 6. Elective treatment discontinuation or due to CR, led to higher OS rates compared to treatment cessation due to TLTs.
3.6 Subgroup analysis
3.6.1Type of ICI used
Patients were treated either with combination ICI or anti-PD1 monotherapy in 12 studies and from them, 7 provided extractable data (12, 26, 32–35, 48), concluding to 18.7% pooled RR (n=7, RR 18.7%, 95%CI 7.4 – 30.3). Contrary to, 1,792 patients were treated with anti-PD1 monotherapy, and 20.8% relapsed post-cessation (n=7, RR 20.8%, 95%CI 16.5 – 24.9). Risk of relapse did not reach statistical significance when anti-CTL4+anti-PD1 treatment was compared to anti-PD1 monotherapy (n=7, OR 1.29, 95%CI 0.69 – 2.41).
3.6.2 Type of studies
Higher pooled RR was detected in RCTs (n=3, RR 30.0%, 95%CI 9.0 – 50.4%) (2, 6, 25) compared to observational studies (n=31, RR 20.4%, 95%CI 16.5 – 24.4%) in the overall population. On the other hand, similar RRs were found between type of study when the analysis was restricted to patients with CR, or who had an elective discontinuation.
CR rates after rechallenge with ICI did not differ among various study types, while a second course of ICI led to higher DCR in RCTs (n=3, DCR 70.4%, 95%CI 45.3 – 98.6%) (2, 6, 25) compared to observational studies.
3.7 Sensitivity analysis
Through leave one-out sensitivity analysis and exclusion of low-quality studies (as assessed by NOS scale and GRADE checklist), the primary and secondary outcomes remained unaltered.
3.8 Meta-regression analysis
Duration of treatment before cessation was investigated as a predictor of relapse rate or time to PD in meta-regression analysis, concluding to a non-statistically significant association in both outcomes (Supplementary Figures 4-6).
3.9 Publication bias
Funnel plots regarding relapse rate and time to PD did not demonstrate a uniform distribution, implying possible small studies effect. This was not the case for DCR (Egger’s test, p>0.20) (Supplementary Figure 7).
4 Discussion
Survival outcomes after ICI treatment cessation for reasons other than disease progression or relapse in patients with advanced melanoma remains an unanswered question in medical literature (53–55). Our analysis shows a 20.9% relapse rate after a mean of 14.26 months post cessation. In addition, lower rates were found in patients who stopped treatment electively compared to patients who stopped therapy due to TLTs. When patients had PD after therapy cessation, reintroducing ICI led to a substantial DCR, including CRs in approximately one third of cases.
The optimal duration of treatment with ICI remains a matter of great debate in the literature (5, 56). As far as we know, there is no clinical, biological or scientific rationale to continue therapy until PD or to stop after 2 years. Still, although RCTs employed different schedules for total treatment duration, a substantial number of patients who ceased treatment for reasons other than PD will not recur during follow-up (1, 2, 6, 57). According to our analysis, approximately four out of 5 patients who discontinue treatment will not recur and this rate is line with relapse rates reported in observational studies and RCTs. Patients included in KEYNOTE-001 and KEYNOTE-006 trials, ceased treatment for CR or after completing 2 years of pembrolizumab, resulting in a 9.2% and 26.2% relapse rates respectively (2, 4, 6). Similar results were drawn from multicenter retrospective registry-based studies (9, 11, 42). Those findings hold significant implications, especially in CR patients, as treatment cessation could be associated with lower rates of chronic or delayed irAEs and reduced financial toxicity from prolonged ICI treatment.
Several factors influencing relapse rates after therapy discontinuation have been studied, particularly in the lack of reliable predictive biomarkers for safe ICI cessation. Dose-response and exposure-response curves of anti-CTL4 anti-PD1 inhibitors provided useful insights regarding the effect of treatment duration on outcomes. Recently published long-term survival data from randomized phase III trials, highlighted that patient who completed ≥94 weeks of treatment in KEYNOTE-006 or remained progression free at 3 years in CheckMate-067, maintained durable clinical benefit from ICI treatment (3, 4). In concordance, pharmacokinetic studies of nivolumab have demonstrated that receptor occupancy can be achieved with low doses, which persist for a significantly longer duration than the antibody’s half-life at given (58, 59). This suggest that efficacy is maintained independently of dose and treatment duration, which was verified also by our results, where duration of ICI treatment was not a significant predictor of relapse or time to PD post cessation in meta-regression analysis. However, real world data of patients with CR or PR from the EUMelaReg registry proved that prolonged ICI treatment led to longer PFS, compared to early cessation (<6 months), and patients with PR were mainly benefited (60). On the contrary, Lodde et al., reported no difference in outcomes of patients who achieved early responses to ICI compared to late, and these were not associated with the duration on treatment (61).
Those findings underscore the importance of the depth of response to treatment rather than the duration of treatment, as a biomarker of long-term preservation of response post-cessation. As demonstrated in the final analysis of CheckMate 067, patients who achieved at least 80% reduction in tumor size experienced long-term survival regardless of treatment with anti-CTL4 and anti-PD1 or anti-PD1 monotherapy (3). Similar findings were evident from our meta-analysis, where patients with CR demonstrated the lowest relapse rates, while type of treatment used did not influence significantly the risk of relapse, in line with findings from Pala et al. (13) and observational studies (26). In addition, retrospective multicenter observational studies concluded to a lower risk of relapse in patients who achieved CR on primary treatment compared to patients who ceased therapy with BOR of PR/SD or due to TLTs (9, 10, 42). The above are in line with our results, and highlights, especially for patients on PR/SD, the importance of careful co-estimation of disease-related factors along with the response to treatment, when ICI cessation is considered.
In contrast to better survival outcomes in patients who ceased ICI due to CR, our analysis found a survival disadvantage in patients with TLTs, characterized by higher relapse rates and shorter time to PD. Results about the effect of irAEs on outcomes of melanoma patients remain contradictory (62–65). While observational studies reported a prolonged PFS in patients who developed irAEs, implicating the presence of a strong immunogenic reaction against the tumor, our meta-analysis, along with other studies, did not verify that benefit after discontinuation (34, 62, 65). From a pathophysiologic perspective, the durability of ICI response is based on the generation of tissue-resident memory (TRM) CD8-T- cells (13). However, the possible use of high dose of corticosteroids and immunosuppressants for the management of high grade irAEs, especially in the early phase of ICI administration (66), an cause inhibitory effect on TCR affinity and decrease the production of effective memory cells, which may justify the increased relapse rate post-cessation (67–69).
In our analysis the mean time to PD after ICI cessation was estimated to be 14.26 months, with studies in the literature reporting similar times from 3.9 (37) to 33.3 months (6). The estimation of that time is considered clinically relevant for two reasons. First, a numerically longer time to PD in patients who ceased for CR compared to TLTs, possibly corroborate the aforementioned pathophysiologic mechanisms associated with relapse post cessation. In concordance, disease-related factors, such as histologic subtype, could influence the time to PD, as shown by our quantitative synthesis, where mucosal melanomas demonstrated shorter time to PD compared to other histologies. This could be explained by the distinct biologic characteristics between subtypes, with mucosal lesions exhibiting more aggressive biologic course, absence of UV signature and lower mutational burden, which could explain the lower efficacy of ICIs in this subtype and the shorter time to PD compared to cutaneous lesions (70, 71). Moreover, accurate estimation of the interval between ICI cessation and PD is crucial for implementing effective surveillance strategies in these patients, especially considering the importance of early detection of recurrence in patients where consensus on the follow-up scheme has not been reached. Simultaneously, the emergence of imaging- (i.e. FDG PET/CT) and blood-based biomarkers (i.e. ctDNA), combined with the knowledge of time to relapse, could provide valuable tools not only for patient monitoring, but also for decision making regarding treatment continuation or intensification in high-risk patients (31, 32, 48).
Following progression after cessation, a second course of ICI could be considered a reasonable option, especially in the context of limited therapeutic choices in patients with BRAF-wild type melanomas. Our analysis showed a 57.9% DCR and 28.9% after ICI (of any type) rechallenge, while in the literature, re-introducing ICI after PD resulted in a various ORRs, ranged from 20% (39) to 40% (72) and more than 60% (73, 74) and influenced by factors, such as the time of rechallenge after PD or the concomitant use of other treatments, such as radiotherapy. Our analysis included only patients who ceased treatment without progression, which means that, by definition, patients with primary resistance to treatment were excluded. Gang et al. summarized the evidence regarding rechallenge strategies in patients with NSCLC, pinpointing differences in pathophysiologic mechanisms according to reason for discontinuation (75). Consistent results were evident for melanoma also, reinforcing the concept of T-cell revitalization after rechallenge. This could potentially improve the ability of immune cells to recognize and eliminate tumor cells that escape from memory-T-cells surveillance (76). Although results from our analyses are promising, supporting rechallenge with ICI after PD, more sophisticated analyses on factors affecting ORRs, such as reason for cessation (including further analyses for patients who discontinue treatment on CR or for TLTs), type and sequence of ICI in rechallenge and BOR at first course are important, but were out of the scope of our analysis.
Our meta-analysis has several strengths. We reported the pooled RRs after ICI cessation, DCR after ICI rechallenge and the estimation of time to PD post discontinuation, which have not been reported before. Some limitations need to be mentioned. There was a high heterogeneity among studies both in primary and secondary outcomes, which is mainly attributed to different definitions of reason for discontinuation, the type of ICI investigated in each study and the different treatment duration. To minimize this heterogeneity, we conducted several subgroup and sensitivity analyses. However, we didn’t see significant changes in our primary results. Another limitation of our study was that uveal melanoma was an exclusion criterion. There are distinct biologic characteristics between histologic subtypes and, because of that, most of clinical trials and observational studies published in the literature included only patients with cutaneous melanoma, with uveal melanoma being actively excluded, especially in clinical trials. For those reasons, we decided to focus on cutaneous melanoma only, aiming to synthesize the evidence for the most reported subtype and simultaneously aiming to avoid higher heterogeneity in our results.
5 Conclusions
Discontinuation of immunotherapy for reasons other than PD could be a reasonable option for patients diagnosed with stage IV melanoma, possibly mitigating the risk of chronic irAEs and the economic burden of prolonged ICI therapy. Time and risk of PD after therapy discontinuation seem to be affected mostly by disease-related factors. A second course of ICI at time of PD remains considerable. Future clinical trials supported by more real-world evidence may help to answer the question about the optimal duration of ICI treatment in this setting (77).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. EC: Conceptualization, Methodology, Supervision, Writing – review & editing, Formal analysis. DD: Conceptualization, Methodology, Writing – review & editing, Formal analysis. GF: Conceptualization, Formal analysis, Methodology, Writing – review & editing. LS-H: Conceptualization, Data curation, Formal analysis, Writing – review & editing, Methodology. M-LR: Conceptualization, Methodology, Writing – review & editing, Formal analysis. AK: Conceptualization, Data curation, Formal analysis, Software, Writing – review & editing, Methodology. ET: Conceptualization, Methodology, Writing – review & editing, Formal analysis. ZA: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing, Formal analysis. UL: Conceptualization, Formal analysis, Methodology, Writing – review & editing. LF: Conceptualization, Formal analysis, Methodology, Writing – review & editing. AL: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. TA: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors acknowledge support from the Open Access Publication Fund of the University of Tübingen.
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: TA- personal fees for advisory board membership from Delcath and Philogen; personal fees as an invited speaker from BMS, Neracare, Novartis and Pierre Fabre; personal fees for a writing engagement from CeCaVa and Medtrix; institutional fees as local principal investigator PI from Agenus Inc., AstraZeneca, BioNTech, BMS, HUYA Bioscience, Immunocore, IO Biotech, MSD, Pfizer, Philogen, Re-generon, Roche and University Hospital Essen; institutional fees as coordinating PI from Unicancer; institutional research grants from iFIT and Novartis; institutional funding from MNI - Naturwissen-schaftliches und Medizinisches Institut, Neracare, Novartis, Pascoe, Sanofi and Skyline-Dx; non-remunerated membership of the American Society of Clinical Oncology ASCO and the Portuguese Society for Medical Oncology; and a role as clinical expert in the area of medical oncology for In-farmed. UE- personal fees from Regeneron, Sanofi, personal fees from MSD, personal fees from Novartis, Almirall Hermal, personal fees from Sun Pharma, outside the submitted work. LF - grants or contracts from Hookipa Pharma, SAKK/Immunophotonics, DFG Grant Deutsche Forschungsgemeinschaft, Philogen and Mundipharma; consulting fees from Philogen, Sanofi, Novartis, BMS; participation on Data Safety Board University of Basel and stocks or stock options from Hookipa Pharma,outside the submitted work. AL- personal fees from Avene, personal fees from MSD, personal fees from Regeneron, personal fees from BMS, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1524945/full#supplementary-material
References
1. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. (2022) 40:127–37. doi: 10.1200/JCO.21.02229
2. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. (2019) 30:582–8. doi: 10.1093/annonc/mdz011
3. Wolchok JD, Chiarion-Sileni V, Rutkowski P, Cowey CL, SChadendorf D, Wagstaff J, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. (2024) 392(1):11–22. doi: 10.1056/NEJMoa2407417
4. Long GV, Carlino MS, McNeil C, Ribas A, Gaudy-Marqueste C, Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: 10-year follow-up of the phase III KEYNOTE-006 study. Ann Oncol. (2024). doi: 10.1016/j.annonc.2024.08.2330
5. Robert C, Marabelle A, Herrscher H, Caramella C, Rouby P, Fizazi K, et al. Immunotherapy discontinuation - how, and when? Data from melanoma as a paradigm. Nat Rev Clin Oncol. (2020) 17:707–15. doi: 10.1038/s41571-020-0399-6
6. Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicenter, randomized, controlled, phase 3 study. Lancet Oncol. (2019) 20:1239–51. doi: 10.1016/S1470-2045(19)30388-2
7. Goodman RS, Lawless A, Woodford R, Fa’ak F, Tipirneni A, Patrinely JR, et al. Extended follow-up of chronic immune-related adverse events following adjuvant anti-PD-1 therapy for high-risk resected melanoma. JAMA Netw Open. (2023) 6:e2327145. doi: 10.1001/jamanetworkopen.2023.27145
8. Ghisoni E, Wicky A, Bouchaab H, Imbimbo M, Delyon J, Gautron Moura B, et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy. Eur J Cancer. (2021) 149:153–64. doi: 10.1016/j.ejca.2021.03.010
9. Rubatto M, Fava P, Stanganelli I, Ribero S, Pigozzo J, Di Giacomo AM, et al. Discontinuation of anti-PD1 in advanced melanoma: an observational retrospective study from the Italian Melanoma Intergroup. Eur J Cancer. (2023) 187:25–35. doi: 10.1016/j.ejca.2023.03.020
10. Ochenduszko S, García Sanchez J, Fita MJJ, González-Barrallo I, Herrero Colomina J, Mujika K, et al. Characteristics and outcomes of advanced melanoma patients with complete response and elective discontinuation of first-line anti-programmed death-1 monotherapy: A real-world multicenter observational cohort study. Pigment Cell Melanoma Res. (2023) 36:388–98. doi: 10.1111/pcmr.13093
11. van Zeijl MCT, van den Eertwegh AJM, Wouters M, de Wreede LC, Aarts MJB, van den Berkmortel F, et al. Discontinuation of anti-PD-1 monotherapy in advanced melanoma-Outcomes of daily clinical practice. Int J Cancer. (2022) 150:317–26. doi: 10.1002/ijc.v150.2
12. Perez L, Samlowski W, Lopez-Flores R. Outcome of elective checkpoint inhibitor discontinuation in patients with metastatic melanoma who achieved a complete remission: real-world data. Biomedicines. (2022) 10(5):1144. doi: 10.3390/biomedicines10051144
13. Pala L, Pagan E, Sala I, Oriecuia C, Oliari M, De Pas T, et al. Outcomes of patients with advanced solid tumors who discontinued immune-checkpoint inhibitors: a systematic review and meta-analysis. EClinicalMedicine. (2024) 73:102681. doi: 10.1016/j.eclinm.2024.102681
14. Bogani G, Cinquini M, Signorelli D, Pizzutilo EG, Romanò R, Bersanelli M, et al. A systematic review and meta-analysis on the optimal treatment duration of checkpoint inhibitors in solid tumors: The OTHERS study. Crit Rev Oncol Hematol. (2023) 187:104016. doi: 10.1016/j.critrevonc.2023.104016
15. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Stud Epidemiol (MOOSE) Group Jama. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021), 372:n71. doi: 10.1136/bmj.n71
17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Wells GA, Wells G, Shea B, Shea B, O’Connell D, Peterson J, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. (2014).
19. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
20. McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J. (2020) 62:69–98. doi: 10.1002/bimj.201900036
21. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. (2014) 32:1020–30. doi: 10.1200/JCO.2013.53.0105
22. Bernard-Tessier A, Baldini C, Martin P, Champiat S, Hollebecque A, Postel-Vinay S, et al. Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer. (2018) 101:160–4. doi: 10.1016/j.ejca.2018.06.005
23. Bisschop C, Wind TT, Blank CU, Koornstra RHT, Kapiteijn E, Van den Eertwegh AJM, et al. Association between pembrolizumab-related adverse events and treatment outcome in advanced melanoma: results from the dutch expanded access program. J Immunother. (2019) 42:208–14. doi: 10.1097/CJI.0000000000000271
24. Fletcher K, Cortellini A, Ganta T, Kankaria R, Song H, Ye F, et al. Safety and efficacy outcomes of early cessation of anti-PD1 therapy in patients 80 years or older: A retrospective cohort study. Cancer Lett. (2024) 596:217001. doi: 10.1016/j.canlet.2024.217001
25. Tang B, Duan R, Zhang X, Qin S, Wu D, Chen J, et al. Five-year follow-up of POLARIS-01 phase II trial: toripalimab as salvage monotherapy in chinese patients with advanced melanoma. Oncologist. (2024) 29:e822–e7. doi: 10.1093/oncolo/oyae045
26. Chatziioannou E, Leiter U, Thomas I, Keim U, Seeber O, Meiwes A, et al. Features and long-term outcomes of stage IV melanoma patients achieving complete response under anti-PD-1-based immunotherapy. Am J Clin Dermatol. (2023) 24:453–67. doi: 10.1007/s40257-023-00775-7
27. Kartolo A, Tong J, Yeung C, Kuksis M, Hopman W, Baetz T. Survivals following discontinuation of PD-1 inhibitor treatment in advanced melanoma patients. Melanoma Res. (2023) 33:50–7. doi: 10.1097/CMR.0000000000000858
28. Sadrolashrafi K, Samlowski W. Retreatment of patients with metastatic cutaneous melanoma who relapse after elective checkpoint inhibitor discontinuation after a complete remission. Oncologist. (2023) 28:e270–e5. doi: 10.1093/oncolo/oyad016
29. Sharma H, Moturi KR, Pankratz VS, Yilmaz E, Gbolahan OB, Kumar A, et al. Outcomes of responders to PD-1/PD-L1 inhibitors who discontinue therapy after sustained disease control. J Cancer Res Clin Oncol. (2023) 149:8673–80. doi: 10.1007/s00432-023-04812-0
30. Dimitriou F, Lo SN, Tan AC, Emmett L, Kapoor R, Carlino MS, et al. FDG-PET to predict long-term outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol. (2022) 33:99–106. doi: 10.1016/j.annonc.2021.10.003
31. Ellebaek E, SChina A, Andersen R, Hendel HW, Svane IM, Donia M. Clinical value of routine [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography scans as a decision tool for early immunotherapy discontinuation in advanced melanoma. Int J Cancer. (2022) 150:1870–8. doi: 10.1002/ijc.v150.11
32. Ferdinandus J, Zaremba A, Zimmer L, Umutlu L, Seifert R, Barbato F, et al. Metabolic imaging with FDG-PET and time to progression in patients discontinuing immune-checkpoint inhibition for metastatic melanoma. Cancer Imaging. (2022) 22:11. doi: 10.1186/s40644-022-00449-3
33. Dimitriou F, Zaremba A, Allayous C, Kähler KC, Gerard CL, Festino L, et al. Sustainable responses in metastatic melanoma patients with and without brain metastases after elective discontinuation of anti-PD1-based immunotherapy due to complete response. Eur J Cancer. (2021) 149:37–48. doi: 10.1016/j.ejca.2021.02.037
34. Asher N, Israeli-Weller N, Shapira-Frommer R, Ben-Betzalel G, Schachter J, Meirson T, et al. Immunotherapy discontinuation in metastatic melanoma: lessons from real-life clinical experience. Cancers (Basel). (2021) 13(12):3074. doi: 10.3390/cancers13123074
35. Gibney GT, Zaemes J, Shand S, Shah NJ, Swoboda D, Gardner K, et al. PET/CT scan and biopsy-driven approach for safe anti-PD-1 therapy discontinuation in patients with advanced melanoma. J Immunother Cancer. (2021) 9(10):e002955. doi: 10.1136/jitc-2021-002955
36. Dutheil A, Belkadi D, Antigny M, Roy S, Lupu J, Vallet A, et al. Complete responders to checkpoint inhibitors in advanced melanoma: Relapse risk factors, and patients’ outcomes. J Clin Oncol. (2021) 39:9550. doi: 10.1200/JCO.2021.39.15_suppl.9550
37. Pokorny R, McPherson JP, Haaland B, Grossmann KF, Luckett C, Voorhies BN, et al. Real-world experience with elective discontinuation of PD-1 inhibitors at 1 year in patients with metastatic melanoma. J Immunother Cancer. (2021) 9(1):e001781. doi: 10.1136/jitc-2020-001781
38. Valentin J, Ferté T, Dorizy-Vuong V, Dousset L, Prey S, Dutriaux C, et al. Real-world survival in patients with metastatic melanoma after discontinuation of anti-PD-1 immunotherapy for objective response or adverse effects: A retrospective study. J Oncol. (2021) 2021:5524685. doi: 10.1155/2021/5524685
39. Betof Warner A, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol. (2020) 38:1655–63. doi: 10.1200/JCO.19.01464
40. Mesnard C, Bodet-Milin C, Eugène T, Nguyen JM, Khammari A, Dréno B. Predictive value of FDG-PET imaging for relapse in metastatic melanoma patients treated with immunotherapy. J Eur Acad Dermatol Venereol. (2020) 34:2261–7. doi: 10.1111/jdv.v34.10
41. Warburton L, Calapre L, Pereira MR, Reid A, Robinson C, Amanuel B, et al. Circulating tumor DNA in advanced melanoma patients ceasing PD1 inhibition in the absence of disease progression. Cancers (Basel). (2020) 12(11):3486. doi: 10.3390/cancers12113486
42. Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. (2019) 30:1154–61. doi: 10.1093/annonc/mdz110
43. Swami U, Monga V, Bossler AD, Zakharia Y, Milhem M. Durable clinical benefit in patients with advanced cutaneous melanoma after discontinuation of anti-PD-1 therapies due to immune-related adverse events. J Oncol. (2019) 2019:1856594. doi: 10.1155/2019/1856594
44. Saiag P, AitMehdi R, Blom A, Longvert C, Emile J-F, Boru B, et al. Discontinuation of anti-PD-1 mAb after complete response in advanced melanoma pts. J Clin Oncol. (2018) 36:e21549–e. doi: 10.1200/JCO.2018.36.15_suppl.e21549
45. Ladwa R, Atkinson V. The cessation of anti-PD-1 antibodies of complete responders in metastatic melanoma. Melanoma Res. (2017) 27:168–70. doi: 10.1097/CMR.0000000000000336
46. Handa T, Kato J, Sumikawa Y, Hida T, Horimoto K, Sato S, et al. Durable response after cessation of anti-programmed death 1 therapy in four melanoma patients. J Dermatol. (2019) 46:e461–e2. doi: 10.1111/1346-8138.15095
47. Schvartsman G, Ma J, Bassett RL, Haydu LE, Amaria RN, Hwu P, et al. Outcomes of metastatic melanoma (MM) patients (pts) after discontinuation of anti-Programmed-Death 1 (PD1) therapy without disease progression. J Clin Oncol. (2018) 36:9549. doi: 10.1200/JCO.2018.36.15_suppl.9549
48. Warburton L, Reid A, Amanuel B, Calapre L, Millward M, Gray E. Detectabl ctDNA at the time of treatment cessation of ipilimumab and nivolumab for toxicity predicts disease progression in advanced melanoma patients. Front Oncol. (2023) 13:1280730. doi: 10.3389/fonc.2023.1280730
49. Gauci ML, Lanoy E, Champiat S, Caramella C, Ammari S, Aspeslagh S, et al. Long-term survival in patients responding to anti-PD-1/PD-L1 therapy and disease outcome upon treatment discontinuation. Clin Cancer Res. (2019) 25:946–56. doi: 10.1158/1078-0432.CCR-18-0793
50. Mäkelä S, Kohtamäki L, Laukka M, Juteau S, Hernberg M. Limited-duration anti-PD-1 therapy for patients with metastatic melanoma. Acta Oncol. (2020) 59:438–43. doi: 10.1080/0284186X.2020.1716388
51. Schank TE, Forschner A, Sachse MM, Dimitrakopoulou-Strauss A, Sachpekidis C, Stenzinger A, et al. Complete metabolic response in FDG-PET-CT scan before discontinuation of immune checkpoint inhibitors correlates with long progression-free survival. Cancers (Basel). (2021) 13(11):2616. doi: 10.3390/cancers13112616
52. Tikkanen A, Iivanainen S, Koivunen JP. Treatment discontinuation and re-initiation of anti-PD-(L)1 agents in metastatic cancers. J Cancer Res Clin Oncol. (2020) 146:2153–60. doi: 10.1007/s00432-020-03217-7
53. Banks LB, Sullivan RJ. When is it OK to stop anti-programmed death 1 receptor (PD-1) therapy in metastatic melanoma? Am J Clin Dermatol. (2020) 21:313–21. doi: 10.1007/s40257-020-00506-2
54. Davies MA. Is it safe to stop anti-PD-1 immunotherapy in patients with metastatic melanoma who achieve a complete response? J Clin Oncol. (2020) 38:1645–7. doi: 10.1200/JCO.20.00136
55. Boutros C, Belkadi-Sadou D, Marchand A, Roy S, Routier E, Robert C. Cured or not? Long-term outcomes of immunotherapy responders. Focus Melanoma. Curr Oncol Rep. (2023) 25:989–96. doi: 10.1007/s11912-023-01429-x
56. Lorigan P, Eggermont AMM. Anti-PD1 treatment of advanced melanoma: development of criteria for a safe stop. Ann Oncol. (2019) 30:1038–40. doi: 10.1093/annonc/mdz182
57. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. (2018) 36:1668–74. doi: 10.1200/JCO.2017.75.6270
58. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. (2010) 28:3167–75. doi: 10.1200/JCO.2009.26.7609
59. Centanni M, Moes D, Trocóniz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. (2019) 58:835–57. doi: 10.1007/s40262-019-00748-2
60. Asher N, Mohr P, Ellebaek E, Bender M, Mangana J, Kandolf L, et al. Length of treatment after partial or complete remission in immunotherapy for metastatic melanoma: An EUMelaReg real world study. J Clin Oncol. (2024) 42:9531. doi: 10.1200/JCO.2024.42.16_suppl.9531
61. Lodde GC, Zhao F, Herbst R, Terheyden P, Utikal J, Pföhler C, et al. Early versus late response to PD-1-based immunotherapy in metastatic melanoma. Eur J Cancer. (2024) 210:114295. doi: 10.1016/j.ejca.2024.114295
62. Van Buren I, Madison C, Kohn A, Berry E, Kulkarni RP, Thompson RF. Survival among veterans receiving steroids for immune-related adverse events after immune checkpoint inhibitor therapy. JAMA Netw Open. (2023) 6:e2340695. doi: 10.1001/jamanetworkopen.2023.40695
63. Lev-Ari S, Serzan M, Wu T, Ip A, Pascual L, Sinclaire B, et al. The impact of immunosuppressive agents on immune checkpoint inhibitor efficacy in patients with advanced melanoma: A real-world, multicenter, retrospective study. Cancer. (2023) 129:1885–94. doi: 10.1002/cncr.34742
64. Watson AS, Goutam S, Stukalin I, Ewanchuk BW, Sander M, Meyers DE, et al. Association of immune-related adverse events, hospitalization, and therapy resumption with survival among patients with metastatic melanoma receiving single-agent or combination immunotherapy. JAMA Netw Open. (2022) 5:e2245596. doi: 10.1001/jamanetworkopen.2022.45596
65. van Not OJ, Verheijden RJ, van den Eertwegh AJM, Haanen J, Aarts MJB, van den Berkmortel F, et al. Association of immune-related adverse event management with survival in patients with advanced melanoma. JAMA Oncol. (2022) 8:1794–801. doi: 10.1001/jamaoncol.2022.5041
66. Kochanek C, Gilde C, Zimmer L, Ugurel S, Meier F, Utikal J, et al. Effects of an immunosuppressive therapy on the efficacy of immune checkpoint inhibition in metastatic melanoma - An analysis of the prospective skin cancer registry ADOREG. Eur J Cancer. (2024) 198:113508. doi: 10.1016/j.ejca.2023.113508
67. Verheijden RJ, van Eijs MJM, May AM, van Wijk F, Suijkerbuijk KPM. Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance. NPJ Precis Oncol. (2023) 7:41. doi: 10.1038/s41698-023-00380-1
68. Goodman RS, Johnson DB, Balko JM. Corticosteroids and cancer immunotherapy. Clin Cancer Res. (2023) 29:2580–7. doi: 10.1158/1078-0432.CCR-22-3181
69. Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, et al. Selective inhibition of low-affinity memory CD8(+) T cells by corticosteroids. J Exp Med. (2019) 216:2701–13. doi: 10.1084/jem.20190738
70. Tyrrell H, Payne M. Combatting mucosal melanoma: recent advances and future perspectives. Melanoma Manag. (2018) 5:Mmt11. doi: 10.2217/mmt-2018-0003
71. D’Angelo SP, Larkin J, Sosman JA, Lebbé C, Brady B, Neyns B, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: A pooled analysis. J Clin Oncol. (2017) 35:226–35. doi: 10.1200/JCO.2016.67.9258
72. Inno A, Roviello G, Ghidini A, Luciani A, Catalano M, Gori S, et al. Rechallenge of immune checkpoint inhibitors: A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2021) 165:103434. doi: 10.1016/j.critrevonc.2021.103434
73. Nardin C, Hennemann A, Diallo K, Funck-Brentano E, Puzenat E, Heidelberger V, et al. Efficacy of immune checkpoint inhibitor (ICI) rechallenge in advanced melanoma patients’ Responders to a first course of ICI: A multicenter national retrospective study of the french group of skin cancers (Groupe de cancérologie cutanée, GCC). Cancers (Basel). (2023) 15(14):3564. doi: 10.3390/cancers15143564
74. Reschke R, Ziemer M. Rechallenge with checkpoint inhibitors in metastatic melanoma. J Dtsch Dermatol Ges. (2020) 18:429–36. doi: 10.1111/ddg.14091
75. Gang X, Yan J, Li X, Shi S, Xu L, Liu R, et al. Immune checkpoint inhibitors rechallenge in non-small cell lung cancer: Current evidence and future directions. Cancer Lett. (2024) 604:217241. doi: 10.1016/j.canlet.2024.217241
76. Zaremba A, Eggermont AMM, Robert C, Dummer R, Ugurel S, Livingstone E, et al. The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur J Cancer. (2021) 155:268–80. doi: 10.1016/j.ejca.2021.07.002
77. Mulder E, de Joode K, Litière S, Ten Tije AJ, Suijkerbuijk KPM, Boers-Sonderen MJ, et al. Early discontinuation of PD-1 blockade upon achieving a complete or partial response in patients with advanced melanoma: the multicenter prospective Safe Stop trial. BMC Cancer. (2021) 21:323. doi: 10.1186/s12885-021-08018-w
Keywords: immunotherapy, immune checkpoint inhibitors, therapy discontinuation, stage IV, overall survival, melanoma
Citation: Lallas K, Chatziioannou E, Durak D, Frey G, Serna-Higuita LM, Rasch M-L, Kyrgidis A, Timotheadou E, Apalla Z, Leiter U, Flatz L, Lallas A and Amaral T (2025) Discontinuation of immune checkpoint inhibitors for reasons other than disease progression and the impact on relapse and survival of advanced melanoma patients. A systematic review and meta-analysis. Front. Immunol. 16:1524945. doi: 10.3389/fimmu.2025.1524945
Received: 08 November 2024; Accepted: 13 January 2025;
Published: 31 January 2025.
Edited by:
Selma Ugurel, Essen University Hospital, GermanyReviewed by:
Kevinn Eddy, Calder Biosciences, Inc, United StatesLuisa Piccin, Veneto Institute of Oncology (IRCCS), Italy
Copyright © 2025 Lallas, Chatziioannou, Durak, Frey, Serna-Higuita, Rasch, Kyrgidis, Timotheadou, Apalla, Leiter, Flatz, Lallas and Amaral. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teresa Amaral, VGVyZXNhLmFtYXJhbEBtZWQudW5pLXR1ZWJpbmdlbi5kZQ==
 Konstantinos Lallas
Konstantinos Lallas Eftychia Chatziioannou
Eftychia Chatziioannou Derya Durak
Derya Durak Georg Frey2
Georg Frey2 Lina Maria Serna-Higuita
Lina Maria Serna-Higuita Athanassios Kyrgidis
Athanassios Kyrgidis Zoe Apalla
Zoe Apalla Aimilios Lallas
Aimilios Lallas Teresa Amaral
Teresa Amaral