
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 29 January 2025
Sec. Alloimmunity and Transplantation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1521895
 Yusuke Uchibori1†
Yusuke Uchibori1† Shuhei Kurosawa2†
Shuhei Kurosawa2† Yuho Najima1*
Yuho Najima1* Kyoko Haraguchi2
Kyoko Haraguchi2 Daichi Sadato3
Daichi Sadato3 Chizuko Hirama3
Chizuko Hirama3 Yasutaka Sadaga1
Yasutaka Sadaga1 Kaori Kondo1
Kaori Kondo1 Chika Kato1
Chika Kato1 Satoshi Sakai1
Satoshi Sakai1 Yasuhiro Kambara1
Yasuhiro Kambara1 Fumihiko Ouchi1
Fumihiko Ouchi1 Masashi Shimabukuro1
Masashi Shimabukuro1 Atsushi Jinguji1
Atsushi Jinguji1 Naoki Shingai1
Naoki Shingai1 Takashi Toya1
Takashi Toya1 Hiroaki Shimizu1
Hiroaki Shimizu1 Takeshi Kobayashi1
Takeshi Kobayashi1 Hironori Harada1,4
Hironori Harada1,4 Yuka Harada3
Yuka Harada3 Yoshiki Okuyama2
Yoshiki Okuyama2 Noriko Doki1
Noriko Doki1Introduction: Donor lymphocyte infusion (DLI) is a therapeutic approach for relapse after hematopoietic stem cell transplantation (HSCT). Despite their reported efficacy, the evolution of DLI practices over time remains underexplored.
Methods: This study provided a comprehensive analysis of DLI strategies and outcomes over 30 years at a single institution. A retrospective analysis was conducted on 75 patients who underwent DLI for disease relapse between April 1994 and March 2024. The primary endpoint was the 3-year overall survival (OS) rate after DLI. Secondary endpoints included the 100-day complete remission (CR) rate and incidence of acute graft-versus-host disease (GVHD).
Results: The median age at the first DLI was 49 years (range, 20–69 years). The most common underlying diseases in all 75 cases were acute myeloid leukemia (AML, n = 46) and myelodysplastic syndromes (MDS, n = 12). Until 2014, DLI was only performed in patients with AML (n = 14), MDS (n = 2), or chronic myeloid leukemia (n = 5). However, since 2015, patients with various diseases, including lymphoid malignancies, have also undergone DLI. Azacitidine was the most frequently used combination therapy with DLI (n = 34). Regimens including venetoclax and FLT3 inhibitors have been commonly used since 2019 (n = 18). The 3-year OS rate was 29.1% (95% CI, 18.8–40.2%). Factors negatively influencing OS included age ≥50 years and a high or very high refined disease risk index. The 100-day CR rate was 52.1%, and acute GVHD occurred in 25.3% of the patients, with no strong correlation between GVHD incidence and CR achievement. Among 18 patients who underwent three or more DLIs since 2018, 88.9% achieved remission following DLI or second HSCT, with a median follow-up of 949.5 days for survivors.
Conclusion: This study highlighted the evolving trends in DLI practices and the diversification of combination therapies. Future research should focus on further validating these findings and optimizing DLI protocols to improve patient outcomes.
Hematopoietic stem cell transplantation (HSCT) remains the cornerstone of treatment for high-risk hematological disorders and offers a potential cure. Despite improvements in non-relapse mortality rates, relapse remains a significant challenge to be addressed (1, 2). Donor lymphocyte infusion (DLI) is a promising therapeutic approach for post-transplant relapse (3–8). Historically, DLI has shown efficacy in treating chronic myeloid leukemia (CML) (9–11). However, the advent of tyrosine kinase inhibitors (TKIs) has led to a decline in the use of HSCT for CML (12, 13). In cases of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), achieving a graft-versus-leukemia (GVL) effect is more challenging because of the lower expression of costimulatory and adhesion molecules than that in CML (14, 15). The rapid progression of these diseases often necessitates therapies beyond DLI (16). In addition, the use of DLI in lymphoid malignancies is less frequently reported than in myeloid malignancies (17, 18).
Over the past decade, the diversity of donor sources has expanded, with an increasing number of transplants performed using haploidentical donors in addition to human leukocyte antigen (HLA)-matched donors (19, 20). Furthermore, the advent of targeted molecular therapies, such as BCL2 and FLT3 inhibitors, has broadened the treatment options for refractory AML cases (21–23). Similarly, the emergence of bispecific antibodies and chimeric antigen receptor T-cell therapy has significantly transformed the therapeutic approach to lymphoid malignancies (24–26). In this era of complex therapeutic regimens, the effect of DLI on contemporary HSCT practices and outcomes remains unclear. Thus, this study aimed to provide a comprehensive analysis of post-transplant DLI cases over the past 30 years at a single institution, evaluate the evolution of DLI strategies, and identify the prognostic factors influencing outcomes in the current therapeutic context.
We retrospectively analyzed patients with hematological malignancies who underwent DLI for disease relapse at our center between April 1994 and March 2024. The final day of observation was July 21, 2024. This study was approved by the Institutional Research Ethics Board of Tokyo Metropolitan Komagome Hospital (approval number: 2741) and was performed according to the tenets of the Declaration of Helsinki. Informed consent was obtained from the website in the form of opt-out.
The classification of myeloablative and reduced-intensity conditioning regimens was predicated on a prior publication (27). For HLA-matched or single-locus mismatched HSCT, myeloablative conditioning predominantly encompassed a total body irradiation (TBI) protocol (12 Gy), incorporating cyclophosphamide (CY; 60 mg/kg for 2 days) or a non-TBI regimen comprising intravenous busulfan (ivBU; 3.2 mg/kg for 4 days), and either CY (60 mg/kg for 2 days) or fludarabine (FLU; 180 mg/m2). Reduced-intensity conditioning primarily consisted of FLU (30 mg/m2 for 6 days), either ivBU (3.2 mg/kg for 2 days) or melphalan (40 or 70 mg/m2 for 2 days), and TBI (4 Gy). We implemented a calcineurin inhibitor (cyclosporine or tacrolimus) augmented with short-term methotrexate for graft-versus-host disease (GVHD) prophylaxis. Rabbit anti-thymocyte globulin (rATG) was added for GVHD prophylaxis at the discretion of the attending physician (28).
Haploidentical donors were defined as related donors exhibiting a 4/8 to 6/8 match at the allele level for HLA-A, HLA-B, HLA-C, and HLA-DRB1. The conditioning regimens and GVHD prophylaxis protocols for haploidentical HSCT encompassed a regimen incorporating low-dose rATG and an alternative protocol utilizing post-transplant CY, with the selection guided by the attending physician’s discretion. These methodologies have been elucidated in previous studies (29, 30).
The primary endpoint was the 3-year overall survival (OS) rate after DLI. The secondary outcomes were the 100-day complete remission (CR) rate and incidence of acute GVHD after the first DLI. We defined CR as the complete disappearance of all clinical, radiological, and histological/immunophenotypic evidence as described in a previous study (6). Disease risk classification was divided into “low,” “intermediate,” “high,” and “very high” using the refined disease risk index (R-DRI) at the time of HSCT (31). Previously established criteria were used to diagnose and grade acute and chronic GVHD (32, 33).
OS was estimated using the Kaplan–Meier method, and the stratified comparisons between groups were conducted using the log-rank test. The incidence of acute GVHD after DLI was evaluated using Gray’s method, with death and receiving subsequent HSCT considered as competing risk factors. To elucidate prognostic factors influencing OS, both univariate and multivariate analyses were conducted using the Cox proportional hazards regression model. We introduced factors with a P-value < 0.20 in the univariate analysis into the multivariate analysis. Hazard ratios (HRs) and 95% confidence intervals (CI) were estimated using the Cox regression model. All statistical tests were 2-sided, with P values less than.05 considered statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 4.4.1). More precisely, it is a modified version of R commander (version 1.68) designed to add statistical functions frequently used in biostatistics (34).
The patient characteristics are summarized in Table 1 and Figure 1. At our institution, a total of 2,018 allogeneic or syngeneic HSCTs were performed between 1992 and 2023, with 510 patients experiencing relapse. Among these, 75 patients (14.7%) underwent DLI. The median age of the patients at the first DLI was 49 years (range: 20–69 years). The median age gradually increased over the study period, with older patients receiving DLI in recent years (Figure 1A). Until 2014, Among the underlying diseases, AML (n = 14) and CML (n = 5) were predominant until 2014. However, after 2015, patients with various diseases, including lymphoid malignancies, began to undergo DLI (Figure 1B). Thirty patients had low or intermediate R-DRI values. The numbers of HSCT before DLI were 1 (n = 59, 78.7%), 2 (n = 15, 20%), and 3 (n = 1, 1.3%). Among the graft sources, 24 patients (32.0%) received HLA-matched related donors (MRD), 35 (46.7%) received unrelated donors (UD), and 16 (21.3%) were administered HLA-haploidentical donors (Haplo). Since 2013, DLI has been performed on haploidentical donors and, since 2021, on unrelated peripheral blood stem cell donors (Figure 1C).
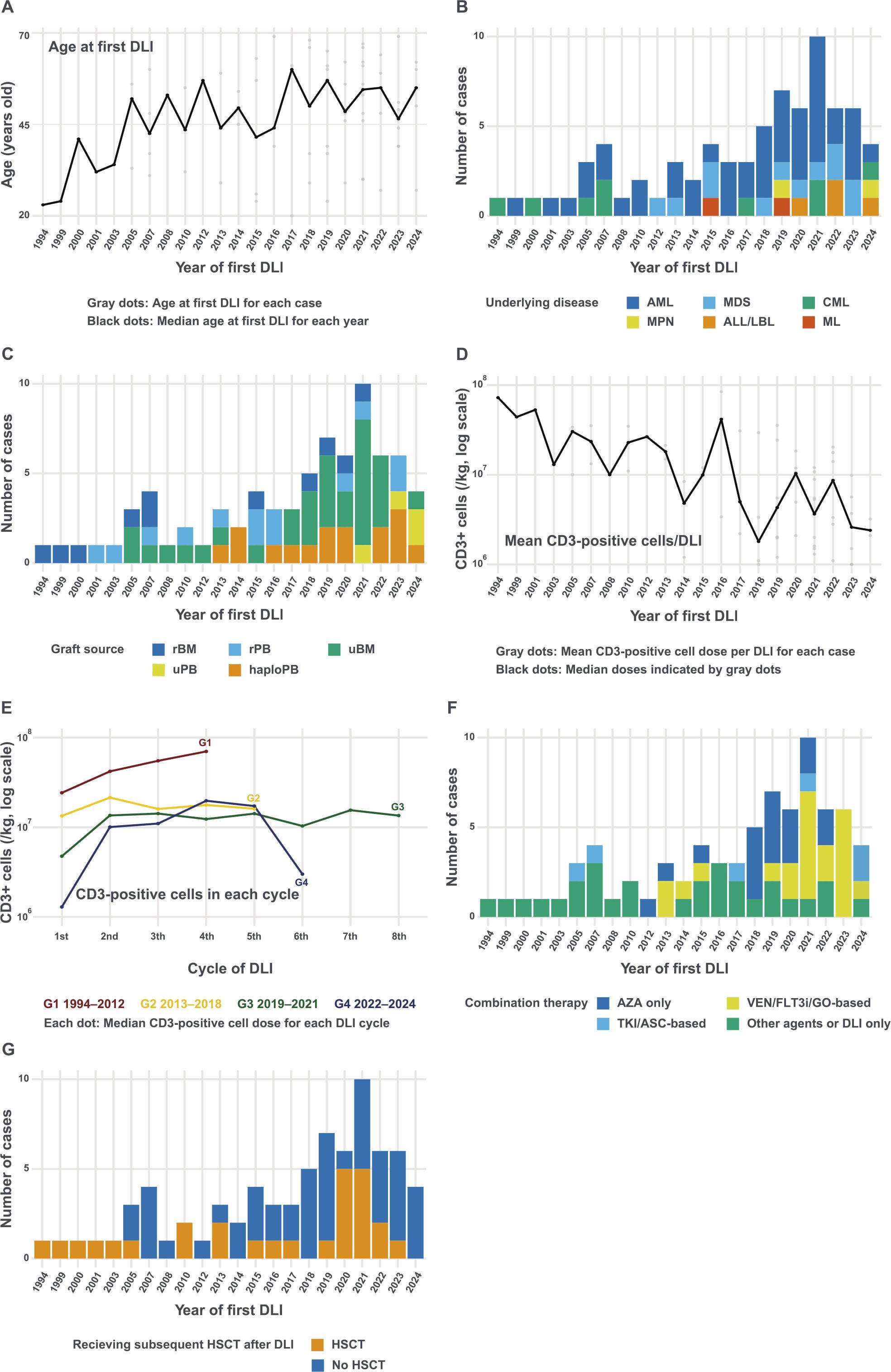
Figure 1. Changes in donor lymphocyte infusion at our institution (A) Median age at the first DLI by year. The plot shows the median age of patients receiving DLI each year, with individual ages represented by gray dots and the median age connected by a black line. (B) Number of DLI cases by year and underlying disease. (C) Number of DLI cases by year and graft source. (D) Temporal trend of mean CD3-positive cell doses (log scale). Gray dots indicate the mean CD3-positive cell dose per DLI for individual cases, while black dots represent the median of these means for each year. (E) Pattern of CD3-positive cell doses across DLI cycles by year of first DLI (log scale). Lines represent different time periods (Group [G] 1: 1994–2012, G2: 2013–2018, G3: 2019–2021, G4: 2022–2024), showing the median CD3-positive cell dose for each DLI cycle within each period. (F) Number of DLI cases by year and combination treatment. Regimens including venetoclax, FLT3 inhibitors, and gemtuzumab ozogamicin were classified into VEN/FLT3i/GO-based. Regimens including tyrosine kinase inhibitors and asciminib were classified into TKI/ASC-based. (G) Number of DLI cases by year and subsequent HSCT. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ASC, asciminib; AZA, azacitidine; CML, chronic myeloid leukemia; DLI, donor lymphocyte infusion; FLT3i, FLT3 inhibitor; GO, gemtuzumab ozogamicin; haploPB, haploidentical peripheral blood; HSCT, hematopoietic stem cell transplantation; LBL, lymphoblastic lymphoma; MDS, myelodysplastic syndromes; ML, malignant lymphoma; rBM, related bone marrow; rPB, related peripheral blood; TKI, tyrosine kinase inhibitor; uBM, unrelated bone marrow; uPB, unrelated peripheral blood.
The median interval from HSCT to relapse was 178 days (range, 21–2,688 days). The relapse types were hematological (n = 32, 42.7%), extramedullary (n = 8, 10.7%), or molecular/cytogenetic (n = 35, 46.7%). Figure 1D displays the mean CD3-positive cell dose infused per DLI for each case, with the doses showing a decreasing trend over the years. Figure 1E demonstrates the CD3-positive cell doses across each DLI cycle, stratified by the year of the first DLI. The initial doses show a decreasing trend in more recent periods, particularly in patients receiving from Haplo (Supplementary Figure 1). Azacitidine was the most frequently used combination therapy with DLI (n = 34, 45.3%). Regimens including venetoclax and FLT3 inhibitors have been commonly used since 2019 (n = 18, 24.0%; Figure 1F). Twenty-seven (36.0%) patients underwent HSCT after DLI (Figure 1G).
Supplementary Table 1 shows baseline characteristics stratified by year of DLI. The first group consisted of 36 cases from 1994 to 2018, and the second group included 39 cases from 2019 to 2024. Patients in the 2019–2024 cohort tended to be older than those in the 1994–2018 cohort (P = 0.12), had a greater proportion of HSCT from unrelated or Haplo donors (P = 0.042), received a lower CD3-positive cell dose per DLI (P < 0.001), and had a higher frequency of concurrent chemotherapy with DLI (P = 0.006).
The median follow-up period from first DLI for survivors was 1,157 days (range, 104–10,869 days). Regarding the study endpoints, the 3-year OS rate after DLI was 29.1% (95% confidence interval [CI], 18.8–40.2%; Figure 2A). The 3-year OS rate was significantly higher in patients aged <50 years than that in those aged ≥50 years (38.0% [95% CI, 22.2–53.6%] versus 20.3% [95% CI, 8.8–35.1%]; Figure 2B). Patients who had undergone a single HSCT prior to DLI had better outcomes than those who had undergone two or three HSCTs (31.3% [95% CI, 19.4–43.9%] versus 20.8% [95% CI, 5.2–43.6%]; Figure 2C). The relapse interval of ≥180 days post-HSCT was associated with improved OS compared to the relapse interval of <180 days (39.1% [95% CI, 22.9–55.0%] versus 19.6% [95% CI, 8.5–34.2%]; Figure 2D). The 3-year OS rate for patients with CML was 62.5% (95% CI, 22.9–86.1%), which was higher than that of other subtypes, including AML (25.4% [95% CI, 13.5–39.2%]), MDS (24.4% [95% CI, 4.5–52.8%]), and lymphoid malignancies (22.2% [95% CI, 1.0–61.5%]) (Figure 2E). Patients with low or intermediate R-DRI demonstrated better 3-year OS than those with high or very high R-DRI (49.7% [95% CI, 30.1–66.5%] versus 14.1% [95% CI, 5.1–27.5%]; Figure 2F). Relapse type, subsequent HSCT, and year of DLI did not significantly affect the OS (Figures 2G–I).
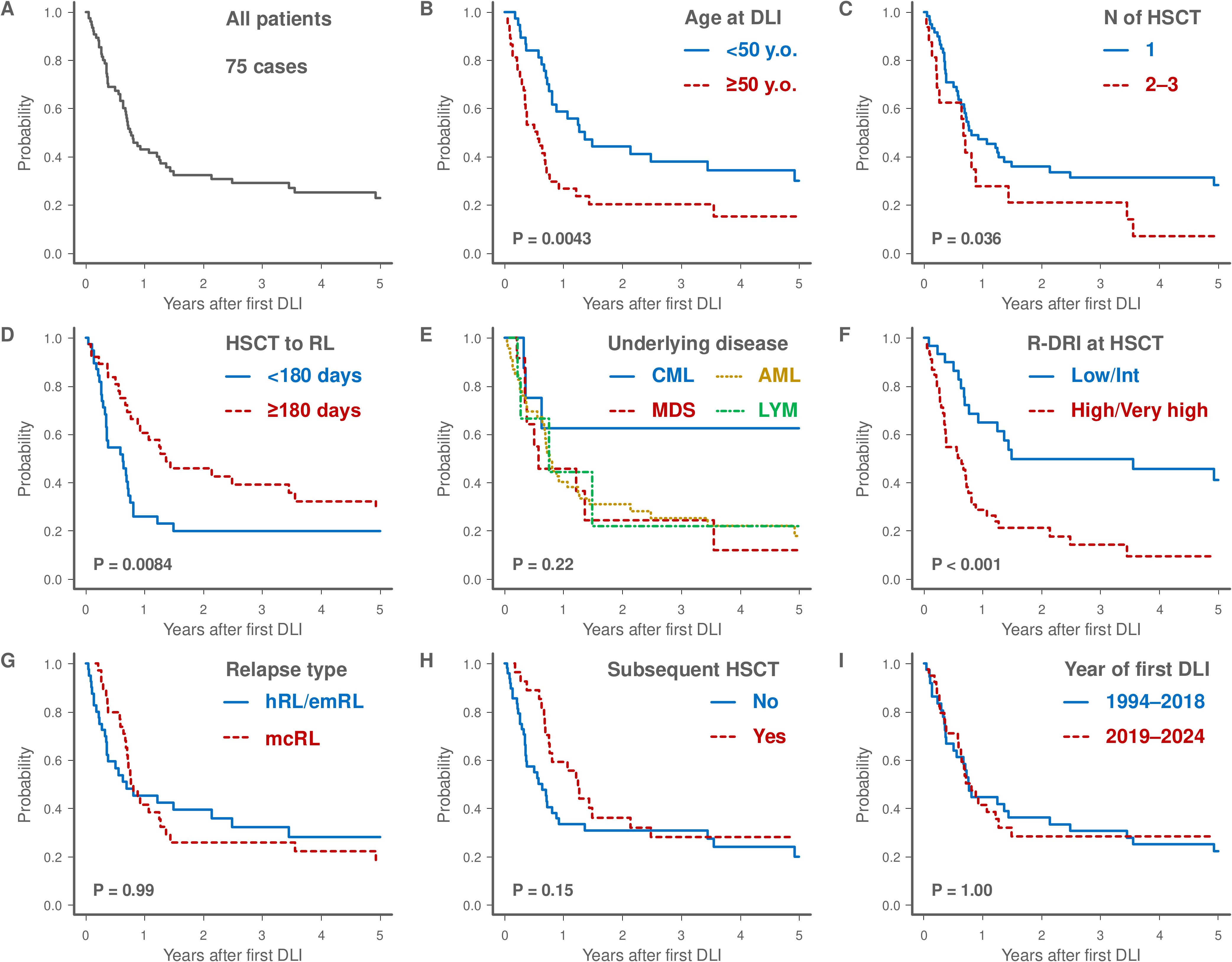
Figure 2. Kaplan-Meier survival curves of the 75 patients who underwent DLI (A). The plots show overall survival stratified by various factors and the log-rank test was used to assess any significant differences: age at first DLI (<50 versus ≥50 years), (B), number of HSCT before DLI (1 versus 2–3), (C), interval from HSCT to relapse (<180 days versus ≥180 days), (D), underlying disease (CML versus MDS versus AML versus LYM), (E), R-DRI at HSCT (Low/Intermediate versus High/Very high), (F), relapse type (hRL/emRL versus mcRL), (G), receiving subsequent HSCT (Yes versus No, H), and year of first DLI (1994–2018 versus 2019–2024), (I). AML, acute myeloid leukemia; CML, chronic myeloid leukemia; DLI, donor lymphocyte infusion; emRL, extramedullary relapse; hRL, hematological relapse; HSCT, hematopoietic stem cell transplantation; LYM, lymphoid malignancies; mcRL, molecular or cytogenetic relapse; MDS, myelodysplastic syndromes; N, number; R-DRI, refined disease risk index; RL, relapse.
The results of univariate analysis evaluating the pre-DLI prognostic factors for OS are presented in Supplementary Table 2. In the multivariate analysis, patients aged ≥50 years (HR 3.10; 95% CI, 1.74–5.53; P < 0.001) and those with high or very high R-DRI (HR 3.45; 95% CI, 1.72–6.92; P < 0.001) were identified as adverse prognostic factors for OS (Table 2).
A subgroup analysis focusing on AML (n = 46) and MDS (n = 12) was performed, and the patient characteristics are summarized in Supplementary Table 3. In the AML subgroup, the median age at the first DLI was 47 years (range: 20–69 years). Seventeen patients (37.0%) received subsequent HSCT after DLI. The median OS was 273 days (range: 18–9281 days). Stratified analysis using the log-rank test revealed that 3-year OS was significantly inferior in patients aged ≥50 years compared to those aged <50 years (not calculable vs. 40.9% [95% CI, 21.8–59.1%]) and in those with high or very high R-DRI compared to those with low or intermediate R-DRI (18.2% [95% CI, 6.6–34.5%] vs. 41.7% [95% CI, 15.2–66.5%]; Supplementary Figure 2). Univariate Cox proportional hazards analysis identified age ≥50 years (HR 4.37; 95% CI, 2.09–9.12; P < 0.001) and high or very high R-DRI (HR 2.26; 95% CI, 1.01–5.07; P = 0.049) as adverse prognostic factors for OS (Supplementary Table 4).
In the MDS subgroup, the median age at the first DLI was 58 years (range: 26–69 years), and all but one patient received azacitidine in combination with DLI (Supplementary Table 2). Only two patients (16.7%) received subsequent HSCT after DLI. The median OS was 198 days (range: 78–2,736 days). Stratified analysis using the log-rank test demonstrated significantly worse 3-year OS in patients with relapse intervals <180 days compared to those with relapse intervals ≥180 days (not calculable vs. 44.4% [95% CI, 6.6–78.5%]) and in those with high or very high R-DRI compared to those with low or intermediate R-DRI (not calculable vs. 53.3% [95% CI, 6.8–86.3%]; Supplementary Figure 3). Univariate Cox analysis identified a relapse interval ≥180 days (HR 0.14; 95% CI, 0.03–0.77; P = 0.024) as a favorable prognostic factor and high or very high R-DRI (HR 10.54; 95% CI, 1.22–91.24; P = 0.033) as an adverse prognostic factor for OS (Supplementary Table 5).
The 100-day CR rate and incidence of acute GVHD were 52.1% and 25.3%, respectively. Figure 3A illustrates the severity of acute GVHD at 100 days post-DLI, stratified by graft source. This figure also includes patients who died within 100 days and those who underwent subsequent HSCT. Acute GVHD was observed in 6 patients with MRD, 7 with UD, and 7 with Haplo. No GVHD-related deaths occurred following DLI.
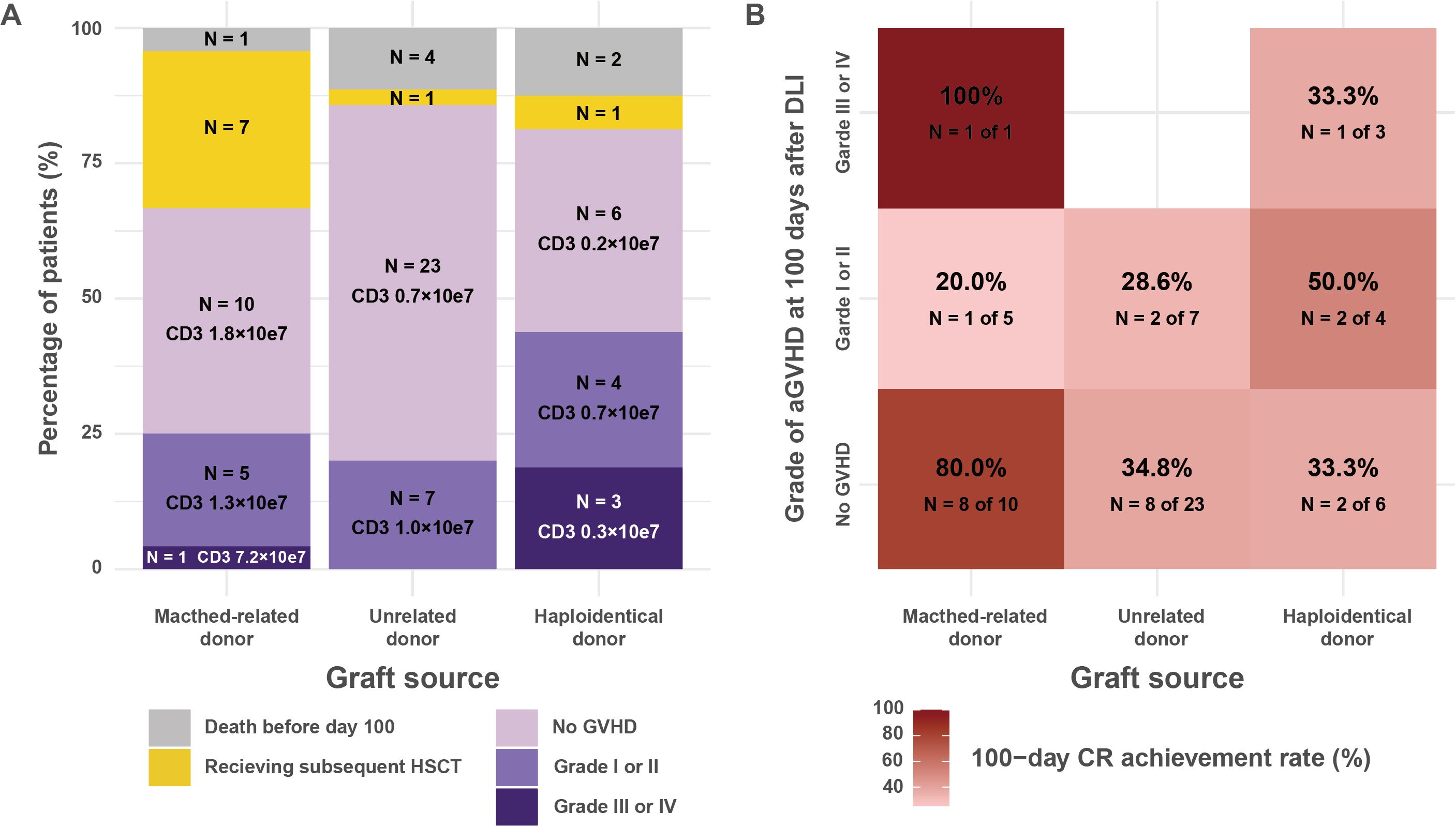
Figure 3. Acute GVHD incidence and CR rates following donor lymphocyte infusion stratified by graft source. (A) Maximum grade of acute GVHD at 100 days post-DLI, categorized by donor source. The bar graph displays the percentage of patients with varying severities of acute GVHD (death before day 100, subsequent HSCT, no GVHD, grades I or II, and grades III or IV) after DLI. Death before day 100 and subsequent HSCT were treated as competing events for the development of acute GVHD. The data are stratified by donor source into matched-related, unrelated, and haploidentical donors. Each bar indicates the number and percentage of patients, along with the median CD3-positive cell dose (average dose per DLI). (B) Heatmap depicting the 100-day CR rates, stratified by donor source and acute GVHD severity. The color intensity corresponds to the CR rate, with darker shades indicating higher rates. Each cell contains the CR rate as a percentage, along with the number of patients achieving CR out of the total in that category (N). The analysis excludes patients who died before day 100. CR rates are shown for patients without GVHD, with grade I or II GVHD, and with grade III or IV GVHD, across the three donor sources. CR, complete remission; DLI, donor lymphocyte infusion; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation.
Figure 3B presents a heatmap depicting the 100-day CR achievement rates, categorized by graft source and acute GVHD severity. Among patients without acute GVHD, the 100-day CR rates were 80.0% (8 of 10 patients) for MRD, 34.8% (8 of 23) for UD, and 33.3% (2 of 6) for Haplo. In cases of grade I to II acute GVHD, the CR rates were 20.0% (1 of 5) for MRD, 28.6% (2 of 7) for UD, and 50.0% (2 of 4) for Haplo. In patients with grade III to IV acute GVHD, the CR rates were 100.0% (1 of 1) for MRD and 33.3% (1 of 3) for Haplo.
The 3-year OS for patients who received three or more DLIs was 68.0% (95% CI, 44.3–83.3%). Their median survival duration was 893 days (range, 114–10,869 days) (Supplementary Figure 1). Figure 4 elucidates recent successful strategies for DLI use through a swimmer plot of 18 patients who underwent three or more DLIs since 2018. The median age at the time of the first DLI was 48 years (range, 27–66 years). The underlying diseases included AML in 11 patients (61.1%), CML in 3 patients (16.7%), lymphoid malignancies in 3 patients (16.7%), and MDS in 1 patient (5.6%). Eight patients (44.4%) underwent a second HSCT following DLI. One patient (5.6%) received prophylactic DLI after the second HSCT and maintained cytogenetic CR (case 3). Regarding combination therapies, 10 patients (55.6%) were treated with azacitidine, 5 (27.8%) with venetoclax, 3 (16.7%) with TKI, and 2 (11.1%) with FLT3 inhibitors. Case 4 treated with blinatumomab and case 12 treated with quizartinib were previously documented (35, 36). Sixteen patients (88.9%) achieved remission after either DLI or a second HSCT. Of the 10 patients who did not undergo a second HSCT, six (60.0%) were alive with a median follow-up of 949.5 days (range, 193–2,219 days) from relapse and five (50.0%) maintained remission.
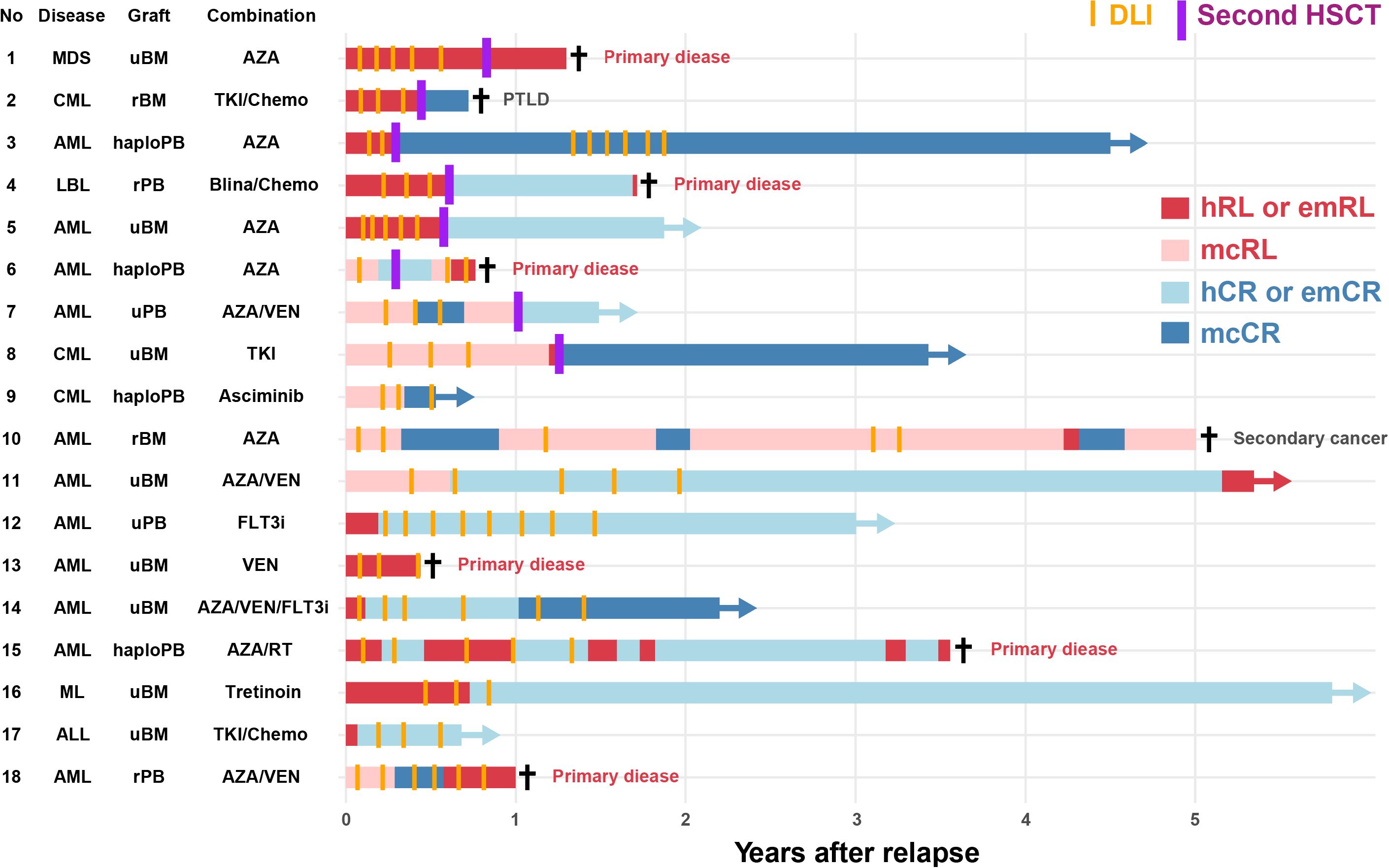
Figure 4. Swimmer plot of recent cases receiving donor lymphocyte infusion This swimmer plot illustrates the clinical course of 18 patients who received donor lymphocyte infusions three or more times since 2018. Horizontal bars represent the duration from relapse to subsequent clinical events, such as DLI and second HSCT. Colors indicate different clinical states and therapies, with markers denoting significant events, such as survival and death. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ASC, asciminib; AZA, azacitidine; Blina, blinatumomab; Chemo, chemotherapy; CML, chronic myeloid leukemia; DLI, donor lymphocyte infusion; emCR, complete remission; emRL, extramedullary relapse; FLT3i, FLT3 inhibitor; haploPB, haploidentical peripheral blood; hCR, hematological complete remission; hRL, hematological relapse; HSCT, hematopoietic stem cell transplantation; LBL, lymphoblastic lymphoma; mcCR, molecular or cytogenetic complete remission; mcRL, molecular or cytogenetic relapse; MDS, myelodysplastic syndromes; ML, malignant lymphoma; PB, peripheral blood; PTLD, post-transplant lymphoproliferative disorder; rBM, related bone marrow; rPB, related peripheral blood; RT, radiotherapy; TKI, tyrosine kinase inhibitor; uBM, unrelated bone marrow; uPB, unrelated peripheral blood; VEN, venetoclax.
This study provides a detailed account of the 30-year evolution of DLI practices at a single institution. Recently, the patient population has aged, and the underlying diseases and combination therapies have diversified. Although limited in number, some patients achieved long-term survival without undergoing subsequent HSCT. Table 3 presents a comparison between the results of this study and previous large-scale retrospective studies conducted in Japan (5–8). The previous studies were multi-center retrospective analyses based on registry data. Unlike HSCT, DLI lacks standardized protocols, leading to potential inter-center procedural variability. Our single-center study leveraged its strengths to provide detailed insights into DLI strategies, including CD3-positive cell dose, infusion cycles, and combination therapies, all aspects that are difficult to elucidate from registry data.
Studies focusing on AML have identified the interval from HSCT to relapse and disease status at the time of DLI as significant prognostic factors. In this study, subgroup analysis of AML identified age and R-DRI as significant prognostic factors. For patients with these factors, DLI may be a viable treatment option. However, these factors have also been reported as prognostic indicators in post-transplant relapse cases without DLI (2, 37, 38). Thus, further exploration is warranted to identify populations that could benefit from DLI. Additionally, with the advent of FLT3 and BCL2 inhibitors (21–23), AML treatment options have diversified, necessitating discussions on the role of DLI in the context of these emerging therapies.
In the study focusing on DLI for patients with MDS, older age and a higher prevalence of azacitidine use were frequently observed (8), trends that were also reflected in our study. For patients with MDS, post-HSCT relapse is associated with particularly poor outcomes (8, 39). Compared to those with AML, patients with MDS are often older and face greater limitations in eligibility for second or third HSCT. Moreover, treatment options beyond hypomethylating agents remain scarce. The role of DLI in patients with MDS may differ from its role in AML. To reduce the toxicity associated with both chemotherapy and cellular therapy, combining hypomethylating agents with DLI warrants further exploration as a potential treatment strategy for post-HSCT relapse in MDS (40, 41). It is crucial to build a stronger evidence base for DLI tailored specifically to patients with MDS.
In our cohort, several patients achieved long-term survival without subsequent HSCT in recent years. Whether HSCT after DLI improves patient prognosis remains inconclusive (42, 43). With the expanding array of treatment options for refractory cases, combination therapies incorporating DLI may offer a viable alternative for patients who are ineligible for a second or third HSCT. Although the efficacy and safety of combining azacitidine and/or venetoclax with DLI have been explored in several studies (40, 41, 44, 45), the present study underscores the potential for establishing the safety and efficacy of DLI in conjunction with other novel agents (35, 36, 46, 47).
This study evaluated the impact of GVHD on diverse DLI settings. Although this study included a limited number of cases, no strong correlation was observed between the incidence of GVHD and CR achievement rates. Several studies have reported that GVHD after DLI contributes to CR achievement and prolongs survival (6, 48). However, the present study included a significant percentage of patients who were administered agents other than DLI. Independent of the GVL effect, pharmacological antitumor effects may modify the incidence of GVHD and the CR achievement rates. Notably, an important finding of this study was the increasing trend in DLIs from haploidentical donors over time. In a study on DLI from haploidentical donors, the number of CD3-positive cells was associated with GVHD incidence, and severe GVHD was linked to treatment-related mortality (7). At our institution, the number of CD3-positive cells infused has shown a decreasing trend with an increase in haploidentical HSCT, and there have been no treatment-related deaths due to GVHD. With increasing treatment options for refractory cases, a safer approach may be more beneficial for DLI than the stronger GVL effect. In particular, DLI from haploidentical donors lacks sufficient evidence (7, 49, 50, 51), necessitating further investigation.
This retrospective study has several limitations. Firstly, the criteria for DLI administration, timing, CD3+ cell dose, and number of infusions were determined by each attending physician. This heterogeneity in DLI applications may have influenced our results. Second, the study only included patients who underwent DLI, necessitating cautious interpretation of the findings when generalizing to other patients. Third, the analysis combined cases with and without subsequent HSCT. The concurrent use of various medications further complicates the evaluation of the direct impact of DLI on prognosis. Fourth, this study spans a 30-year period to focus on temporal trends. This long timeframe may have introduced unaddressed factors that changed over time and influenced the outcomes. Fifth, this study comprises patients with various diseases, each receiving distinct regimens other than DLI. The heterogeneity in treatment approaches may have influenced the results. However, conducting prospective studies on post-transplant relapse remains challenging. We believe that this retrospective study offers valuable insights for both patients and healthcare providers in managing post-transplant relapse.
In conclusion, this study highlights the refined DLI strategies developed over 30 years, alongside the increasing diversity of combination therapies. Meticulous case-by-case assessments are crucial for advancing treatment, especially for patients who achieve long-term survival after DLI. Future efforts should validate these findings and optimize DLI protocols to improve outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Institutional Research Ethics Board of Tokyo Metropolitan Komagome Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YU: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. SK: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing, Methodology. YN: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Methodology. KH: Formal analysis, Methodology, Resources, Writing – review & editing, Investigation. DS: Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. CH: Investigation, Methodology, Resources, Writing – review & editing. YS: Investigation, Methodology, Resources, Writing – review & editing. KK: Investigation, Methodology, Resources, Writing – review & editing. CK: Investigation, Methodology, Resources, Writing – review & editing. SS: Investigation, Methodology, Resources, Writing – review & editing. YK: Investigation, Methodology, Resources, Writing – review & editing. FO: Investigation, Methodology, Resources, Writing – review & editing. MS: Investigation, Methodology, Resources, Writing – review & editing. AJ: Investigation, Methodology, Resources, Writing – review & editing. NS: Investigation, Methodology, Resources, Writing – review & editing. TT: Data curation, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. HS: Investigation, Methodology, Resources, Writing – review & editing. TK: Investigation, Methodology, Resources, Writing – review & editing. HH: Investigation, Methodology, Resources, Writing – review & editing. YH: Investigation, Methodology, Resources, Writing – review & editing. YO: Investigation, Methodology, Resources, Writing – review & editing. ND: Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This Research was supported by the Clinical Research Fund of the Tokyo Metropolitan Hospital Organization R050401001/2023-2024 (YN), and by JSPS KAKENHI Grant Numbers JP22K15615 (YN) and JP23K06709 (ND).
The authors thank the nursing staff at Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, for their excellent patient care. We also thank Editage (https://www.editage.com/) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1521895/full#supplementary-material
1. McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng G-S, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med. (2020) 172:229–39. doi: 10.7326/m19-2936
2. Yanada M, Konuma T, Yamasaki S, Kondo T, Fukuda T, Shingai N, et al. Relapse of acute myeloid leukemia after allogeneic hematopoietic cell transplantation: clinical features and outcomes. Bone Marrow Transplant. (2021) 56:1126–33. doi: 10.1038/s41409-020-01163-z
3. Castagna L, Sarina B, Bramanti S, Perseghin P, Mariotti J, Morabito L. Donor lymphocyte infusion after allogeneic stem cell transplantation. Transfusion Apheresis Sci. (2016) 54:345–55. doi: 10.1016/j.transci.2016.05.011
4. Harada K. Pre-emptive and prophylactic donor lymphocyte infusion following allogeneic stem cell transplantation. Int J Hematol. (2023) 118:158–68. doi: 10.1007/s12185-023-03595-x
5. Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, Kanda Y, et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: a retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. (2014) 20:1785–90. doi: 10.1016/j.bbmt.2014.07.010
6. Miyamoto T, Fukuda T, Nakashima M, Henzan T, Kusakabe S, Kobayashi N, et al. Donor lymphocyte infusion for relapsed hematological Malignancies after unrelated allogeneic bone marrow transplantation facilitated by the Japan marrow donor program. Biol Blood Marrow Transplant. (2017) 23:938–44. doi: 10.1016/j.bbmt.2017.02.012
7. Harada K, Mizuno S, Yano S, Takami A, Ishii H, Ikegame K, et al. Donor lymphocyte infusion after haploidentical hematopoietic stem cell transplantation for acute myeloid leukemia. Ann Hematol. (2022) 101:643–53. doi: 10.1007/s00277-021-04731-5
8. Marumo A, Nagata Y, Fujioka M, Kurosawa S, Najima Y, Sakaida E, et al. Outcome of donor lymphocyte infusion after allogeneic hematopoietic stem cell transplantation in relapsed myelodysplastic syndrome. Cytotherapy. (2024). doi: 10.1016/j.jcyt.2024.09.006
9. Drobyski WR, CA K, MS R, Koethe S, Hanson G, McFadden P, et al. Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood. (1993) 82:2310–8. doi: 10.1182/blood.V82.8.2310.2310
10. Mackinnon S, EB P, MH C, Reich L, NH C, Boulad F, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. (1995) 86:1261–8. doi: 10.1182/blood.V86.4.1261.bloodjournal8641261
11. Bär BM, Schattenberg A, EJ M, Geurts Van Kessel A, TF S, GH K, et al. Donor leukocyte infusions for chronic myeloid leukemia relapsed after allogeneic bone marrow transplantation. J Clin Oncol. (1993) 11:513–9. doi: 10.1200/jco.1993.11.3.513
12. Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. (2022) 107:1045–53. doi: 10.3324/haematol.2021.279189
13. Iida M, Liu K, XJ H, Depei W, Kuwatsuka Y, JH M, et al. Trends in disease indications for hematopoietic stem cell transplantation in the Asia-Pacific region: A report of the Activity Survey 2017 from APBMT. Blood Cell Ther. (2022) 5:87–98. doi: 10.31547/bct-2022-002
14. Dermime S, Mavroudis D, YZ J, Hensel N, Molldrem J, Barrett AJ. Immune escape from a graft-versus-leukemia effect may play a role in the relapse of myeloid leukemias following allogeneic bone marrow transplantation. Bone Marrow Transplant. (1997) 19:989–99. doi: 10.1038/sj.bmt.1700778
15. Brouwer RE, Hoefnagel J, BB vdB, Jedema I, Zwinderman Koos H, Starrenburg ICW, et al. Expression of co-stimulatory and adhesion molecules and chemokine or apoptosis receptors on acute myeloid leukaemia: high CD40 and CD11a expression correlates with poor prognosis. Br J Haematol. (2001) 115:298–308. doi: 10.1046/j.1365-2141.2001.03085.x
16. Levine JE, Braun T, SL P, Beatty P, Cornetta K, Martino R, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid Malignancies after allogeneic stem-cell transplantation. J Clin Oncol. (2002) 20:405–12. doi: 10.1200/jco.2002.20.2.405
17. Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. (2012) 26:1211–7. doi: 10.1038/leu.2011.351
18. Eefting M, Halkes CJM, LC DEW, CM VP, Kersting S, Marijt EWA, et al. Myeloablative T cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transplant. (2013) 49:287–91. doi: 10.1038/bmt.2013.111
19. Inoue T, Koyama M, Kaida K, Ikegame K, KS E, Samson L, et al. Peritransplant glucocorticoids redistribute donor T cells to the bone marrow and prevent relapse after haploidentical SCT. JCI Insight. (2021) 6:e153551. doi: 10.1172/jci.insight.153551
20. Sugita J, Kamimura T, Ishikawa T, Ota S, Eto T, Kuroha T, et al. Reduced dose of posttransplant cyclophosphamide in HLA-haploidentical peripheral blood stem cell transplantation. Bone Marrow Transplant. (2021) 56:596–604. doi: 10.1038/s41409-020-01065-0
21. DiNardo CD, BA J, Pullarkat V, MJ T, JS G, AH W, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
22. Perl AE, Martinelli G, JE C, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. (2019) 381:1728–40. doi: 10.1056/NEJMoa1902688
23. Cortes JE, Khaled S, Martinelli G, AE P, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. (2019) 20:984–97. doi: 10.1016/s1470-2045(19)30150-0
24. Kantarjian HM, DJ D, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. (2016) 375:740–53. doi: 10.1056/NEJMoa1509277
25. Kantarjian H, Stein A, Gokbuget N, AK F, AC S, JM R, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. (2017) 376:836–47. doi: 10.1056/NEJMoa1609783
26. Maude SL, TW L, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
27. Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. (2009) 15:367–9. doi: 10.1016/j.bbmt.2008.12.497
28. Kuriyama K, Fuji S, Inamoto Y, Tajima K, Tanaka T, Inoue Y, et al. Impact of low-dose rabbit anti-thymocyte globulin in unrelated hematopoietic stem cell transplantation. Int J Hematol. (2016) 103:453–60. doi: 10.1007/s12185-016-1947-9
29. Konishi T, Doki N, Nagata A, Yamada Y, Takezaki T, Kaito S, et al. Unmanipulated haploidentical hematopoietic stem cell transplantation using very low-dose antithymocyte globulin and methylprednisolone in adults with relapsed/refractory acute leukemia. Ann Hematol. (2020) 99:147–55. doi: 10.1007/s00277-019-03865-x
30. Sugita J, Kagaya Y, Miyamoto T, Shibasaki Y, Nagafuji K, Ota S, et al. Myeloablative and reduced-intensity conditioning in HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant. (2019) 54:432–41. doi: 10.1038/s41409-018-0279-1
31. Armand P, HT K, BR L, Wang Z, EP A, ME K, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. (2014) 123:3664–71. doi: 10.1182/blood-2014-01-552984
32. Przepiorka D, Weisdorf D, Martin P, HG K, Beatty P, Hows J, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant. (1994) 15:825–8.
33. Jagasia MH, HT G, Arora M, KM W, Wolff D, EW C, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401 e1. doi: 10.1016/j.bbmt.2014.12.001
34. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
35. Mori J, Shingai N, Kobayashi T, Doki N. Combination of donor lymphocyte infusion and blinatumomab for B-cell lymphoblastic lymphoma relapse after allogeneic stem-cell transplantation. Case Rep Oncol. (2023) 16:640–4. doi: 10.1159/000531834
36. Ouchi F, Shingai N, Najima Y, Sadato D, Hirama C, Wakita S, et al. Quizartinib with donor lymphocyte infusion for post-transplant relapse of FLT3-ITD-positive acute myeloid leukemia. Int J Hematol. (2024). doi: 10.1007/s12185-024-03863-4
37. Choi EJ, JH L, JH L, HS P, SH KO, Seol M, et al. Treatment and clinical outcomes of patients relapsing after allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. Blood Res. (2018) 53:288–93. doi: 10.5045/br.2018.53.4.288
38. Gao Y, Wu H, Shi Z, Gao F, Shi J, Luo Y, et al. Prognostic factors and clinical outcomes in patients with relapsed acute leukemia after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2023) 58:863–73. doi: 10.1038/s41409-023-01989-3
39. Shimomura Y, Hara M, Tachibana T, Ohashi K, Sakura T, Fukuda T, et al. Outcomes of second allogeneic haematopoietic stem cell transplantation in patients with relapse of myelodysplastic syndrome. Br J Haematol. (2019) 186:86–90. doi: 10.1111/bjh.15898
40. Li X, Wang W, Zhang X, Wu Y. Azacitidine and donor lymphocyte infusion for patients with relapsed acute myeloid leukemia and myelodysplastic syndromes after allogeneic hematopoietic stem cell transplantation: A meta-analysis. Front Oncol. (2022) 12:949534. doi: 10.3389/fonc.2022.949534
41. Guillaume T, Thépot S, Peterlin P, Ceballos P, AL B, Garnier A, et al. Prophylactic or preemptive low-dose azacitidine and donor lymphocyte infusion to prevent disease relapse following allogeneic transplantation in patients with high-risk acute myelogenous leukemia or myelodysplastic syndrome. Transpl Cell Ther. (2021) 27:839.e1–.e6. doi: 10.1016/j.jtct.2021.06.029
42. Schmid C, LC DEW, van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica. (2018) 103:237–45. doi: 10.3324/haematol.2017.168716
43. Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol. (2018) 4. doi: 10.1001/jamaoncol.2018.2091
44. Zhao P, Ni M, Ma D, Fang Q, Zhang Y, Li Y, et al. Venetoclax plus azacitidine and donor lymphocyte infusion in treating acute myeloid leukemia patients who relapse after allogeneic hematopoietic stem cell transplantation. Ann Hematol. (2022) 101:119–30. doi: 10.1007/s00277-021-04674-x
45. Amit O, YB ON, Perez G, Shargian-Alon L, Yeshurun M, Ram R. Venetoclax and donor lymphocyte infusion for early relapsed acute myeloid leukemia after allogeneic hematopoietic cell transplantation. A retrospective multicenter trial. Ann Hematol. (2021) 100:817–24. doi: 10.1007/s00277-021-04398-y
46. Othman J, Potter N, Mokretar K, Taussig D, Khan A, Krishnamurthy P, et al. FLT3 inhibitors as MRD-guided salvage treatment for molecular failure in FLT3 mutated AML. Leukemia. (2023) 37:2066–72. doi: 10.1038/s41375-023-01994-x
47. Fernando F, AJ I, Claudiani S, Pryce A, Hayden C, Byrne J, et al. The outcome of post-transplant asciminib in patients with chronic myeloid leukaemia. Bone Marrow Transplant. (2023) 58:826–8. doi: 10.1038/s41409-023-01975-9
48. Eefting M, PA vdB, LC DEW, CJ H, Kersting S, EW M, et al. Intentional donor lymphocyte-induced limited acute graft-versus-host disease is essential for long-term survival of relapsed acute myeloid leukemia after allogeneic stem cell transplantation. Haematologica. (2013) 99:751–8. doi: 10.3324/haematol.2013.089565
49. Ghiso A, AM R, Gualandi F, Dominietto A, Varaldo R, MT VL, et al. DLI after haploidentical BMT with post-transplant CY. Bone Marrow Transplant. (2015) 50:56–61. doi: 10.1038/bmt.2014.217
50. Goldsmith SR, Slade M, JF D, Westervelt P, MA S, Gao F, et al. Donor-lymphocyte infusion following haploidentical hematopoietic cell transplantation with peripheral blood stem cell grafts and PTCy. Bone Marrow Transplant. (2017) 52:1623–8. doi: 10.1038/bmt.2017.193
Keywords: donor lymphocyte infusion, hematopoietic stem cell transplantation, refined disease risk index, acute graft-versus-host disease, acute myeloid leukemia, myelodysplastic syndromes, azacitidine, venetoclax
Citation: Uchibori Y, Kurosawa S, Najima Y, Haraguchi K, Sadato D, Hirama C, Sadaga Y, Kondo K, Kato C, Sakai S, Kambara Y, Ouchi F, Shimabukuro M, Jinguji A, Shingai N, Toya T, Shimizu H, Kobayashi T, Harada H, Harada Y, Okuyama Y and Doki N (2025) Changes in donor lymphocyte infusion for relapsed patients post-hematopoietic stem cell transplantation: a 30-year single-center experience. Front. Immunol. 16:1521895. doi: 10.3389/fimmu.2025.1521895
Received: 03 November 2024; Accepted: 02 January 2025;
Published: 29 January 2025.
Edited by:
Aurore Saudemont, Xap Therapeutics, United KingdomReviewed by:
Wenyi Shen, Nanjing Medical University, ChinaCopyright © 2025 Uchibori, Kurosawa, Najima, Haraguchi, Sadato, Hirama, Sadaga, Kondo, Kato, Sakai, Kambara, Ouchi, Shimabukuro, Jinguji, Shingai, Toya, Shimizu, Kobayashi, Harada, Harada, Okuyama and Doki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuho Najima, eXVob25hamltYUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.