
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 14 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1517495
This article is part of the Research Topic Cancer Metastases: Mechanisms of Tumor Dissemination, Formation of Metastatic Niche and Anti-metastatic Therapy View all 10 articles
Lung cancer, characterized by its high morbidity and mortality rates, has the capability to metastasize to various organs, thereby amplifying its detrimental impact and fatality. The metastasis of lung cancer is a complex biological phenomenon involving numerous physiological transformations. Exosomes, small membranous vesicles enriched with biologically active components, are pivotal in mediating intercellular communication and regulating physiological functions due to their specificity and stability. Extensive research has elucidated the production and functions of exosomes in cancer contexts. Multitude of evidence demonstrates a strong association between lung cancer metastasis and exosomes. Additionally, the concept of the pre-metastatic niche is crucial in the metastatic process facilitated by exosomes. This review emphasizes the role of exosomes in mediating lung cancer metastasis and their impact on the disease’s development and the progression to other tissues. Furthermore, it explores the potential of exosomes as biomarkers for lung cancer metastasis, offering significant insights for future clinical advancements.
Based on the estimates of the International Agency for Research on Cancer in 2022, Lung cancer remains one of the most prevalent malignancies globally, causing approximately 1.8 million deaths each year and thus imposing a significant burden on public health (1). It primarily manifests as small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC) (2), with the latter accounting for roughly 80% of all lung cancer diagnoses (3, 4). An expanding range of therapeutic options now exists for both SCLC and NSCLC, encompassing surgery, radiotherapy, chemotherapy, immunotherapy, and, specifically for NSCLC, treatments such as targeted therapies, antibody-drug conjugates, and bispecific antibodies (5, 6). Treatment strategies are typically tailored and adjusted in response to the patient’s disease progression; for instance, early-stage NSCLC (stage I or II) is primarily managed through tumor resection and adjuvant therapy, whereas advanced stages (III or IV) necessitate chemotherapy or radiotherapy (7).
Despite notable advancements in cancer research, significant improvements in overall survival and quality of life for patients with lung cancer remain elusive (8). The most detrimental aspect of lung cancer is its propensity for metastasis, where malignant cells spread from the lungs to distant organs (9–11).
Metastasis constitutes a defining characteristic of cancer progression (12). Extensive research underscores the pivotal role of the tumor microenvironment (TME) in facilitating metastasis, impacting both the invasive expansion at the primary site and the colonization of distant tissues (13, 14). Paget’s widely endorsed “seed and soil” theory suggests that specific tumor cells adapt to and thrive within the microenvironments of particular organs, facilitating their migration and growth (15). Unlike primary tumors, which often respond well to localized treatments such as surgery or radiotherapy, metastatic cancer is a systemic condition involving multiple organs, making it considerably more challenging to treat (16). Despite advancements in NSCLC management, particularly through chemotherapy, radiotherapy, and surgery, the prognosis for metastatic NSCLC remains dire, especially in cases of metastasis (11). Metastases are implicated in over 70% of NSCLC-related deaths, with the median survival for advanced-stage patients being approximately 18 months post-diagnosis (17).
The concept of the pre-metastatic niche (PMN) refers to the dynamic interaction between metastatic tumor cells and their microenvironment, especially the crosstalk between tumor cells and stromal cells within the target organ (18). The PMN fosters metastasis by promoting neovascularization, enhancing vascular permeability, suppressing immune responses, triggering inflammation, inducing lymphangiogenesis, increasing organ aggregation, and reprogramming cellular functions (10, 19, 20). For instance, miR-29a-3p, embedded in tumor-derived exosomes or liposomal nanopreparations, reduces collagen I levels in lung fibroblasts, thereby disrupting the metastasis-promoting PMN and inhibiting lung cancer metastasis (21). Similarly, Lin28B expression in primary tumors facilitates neutrophil recruitment and polarization towards the N2 phenotype, thereby impairing T-cell function and shaping the immune microenvironment in metastatic settings (22). Moreover, exosomal miR-3157-3p influences the expression of vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMP)-2, MMP9, and occludin in endothelial cells via the TIMP/KLF2 axis, driving angiogenesis, increasing vascular permeability, and promoting NSCLC metastasis through the establishment of pre-metastatic ecological sites (23). Additionally, breast cancer-derived exosomes containing Caveolin-1 (Cav-1) modulate inflammatory gene expression in lung epithelial cells, regulating matrix deposition in lung fibroblasts. These exosomes inhibit the PTEN/CCL2/VEGF-A signaling cascade in lung macrophages, thereby fostering M2 macrophage polarization, neovascularization, and PMN formation, ultimately contributing to lung metastasis (24). In summary, the PMN plays an integral role in facilitating metastasis.
Exosomes represent a subtype of extracellular vesicles (EVs), with exosomes being the smaller class and microvesicles constituting the larger variant (25, 26). These vesicles, typically ranging from 30 to 150 nanometers in size, are enclosed by a single lipid bilayer (27, 28). Exosomes are secreted by numerous cell types and are found abundantly in body fluids such as blood, saliva, urine, and milk (27, 29). Extensive research has established exosomes as critical mediators of intercellular communication, facilitating essential biological processes such as angiogenesis, cell proliferation, and metastasis (27, 30–32). They carry a diverse range of bioactive molecules, including proteins, lipids, and nucleic acids, which enable signal transduction or intercellular crosstalk, particularly within the TME (29–31). Notably, exosomal transfer of microRNAs (miRNAs) has emerged as a key mechanism for gene regulation between cells (33). For instance, circular RNAs (circRNAs) act as “sponges” for miRNAs, thereby influencing post-transcriptional gene regulation (34). Furthermore, long non-coding RNAs (lncRNAs) have been identified as key modulators in various cancers (35).
PMN, characterized by enhanced angiogenesis and increased vascular permeability, creates a favorable environment for tumor cell colonization and subsequent metastasis (10, 36). Tumor cells actively modulate distant organs to prepare potential metastatic sites, initiating microenvironmental changes even before metastasis occurs (10, 20). Evidence indicates that both carcinogenesis and PMN formation are heavily influenced by tumor cell-derived exosomes (37). These vesicles are integral to all stages of tumorigenesis and cancer progression, including tumor initiation, growth, metastasis, resistance to therapy, and immune system evasion (37). Additionally, exosomes enhance vascular permeability and trigger inflammatory responses in target tissues, which collectively contribute to the establishment of a conducive pre-metastatic microenvironment (38). These interlinked mechanisms facilitate the formation of metastatic tumors within the target organs (38, 39).
Lung cancer metastasis is a complex, multi-phase process encompassing tumor growth, cell migration, invasion, and neovascularization, all of which ultimately contribute to the development of distant metastases (40). This metastatic cascade can be significantly influenced by exosome-mediated signaling pathways (41). As illustrated in Figures 1, 2, exosomes can be produced by donor cells and transferred and diffused to recipient cells through various pathways to accomplish intercellular communication. lung cancer metastasis can be broadly categorized into two processes: outward and inward. Outward metastasis refers to the dissemination of lung cancer cells to distant organs via exosomes, whereas inward metastasis denotes the spread of cancerous cells to the lungs from other primary sites, facilitated by various exosome-driven mechanisms (42). Figures 3, 4 highlight that the brain and bones are the predominant targets of outward lung cancer metastasis, while inward metastasis to the lungs commonly originates from breast, bone, colon, and other cancer types (43–47). This review delves into the diverse pathways through which lung cancer metastasizes to distant organs and how other cancers metastasize to the lungs.
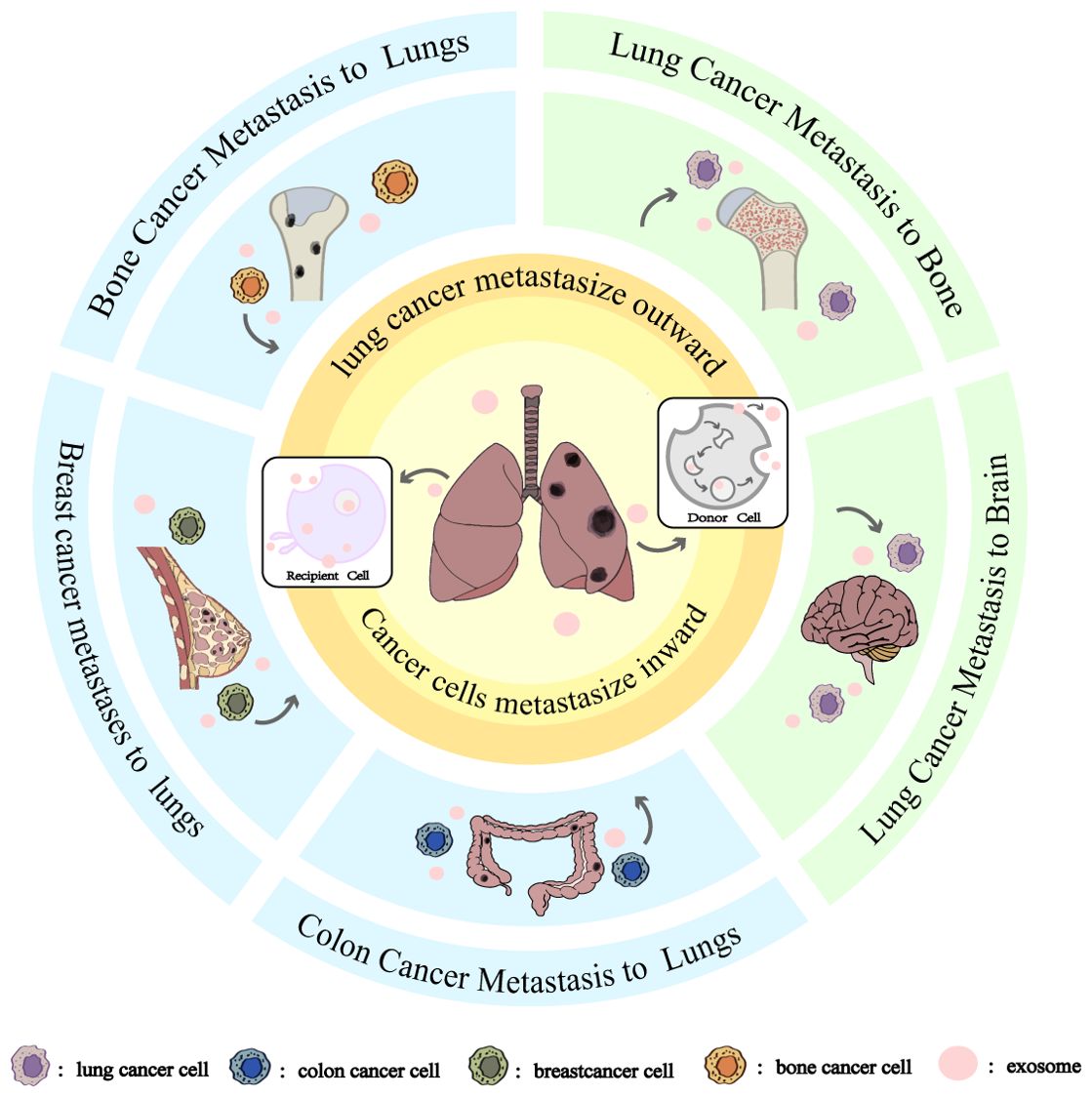
Figure 1. Exosomes and Directional Tissue Orientation in Lung Cancer Metastasis. The blue areas is cancer cells metastasize inward, From top to bottom are bone cancer metastasize to lungs, breast cancer metastasize to lungs, and colon cancer metastasize to lungs. The green area is cancer cells metastasize outward, From top to bottom are lung cancer metastasis to bone and lung cancer metastasis to brain. Whether it is inward or outward metastasis of cancer cells, the metastasis is accomplished through the secretion of exosomes by the donor cells and then diffusion to the recipient cells.
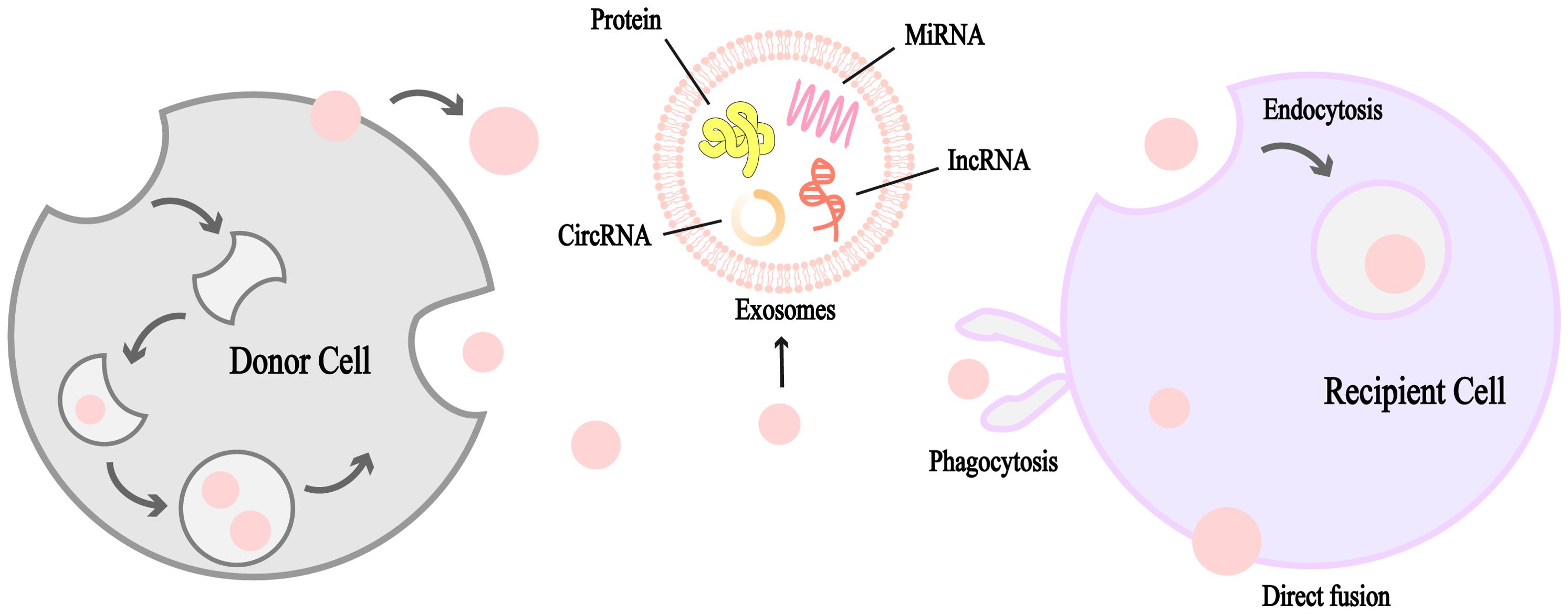
Figure 2. Schematic diagram of the formation and structure of exosomes. Exosomes are unilamellar lipid bilayer vesicles ranging from 30 to 150 nm in diameter containing a variety of biologically active molecules such as miRNAs, circRNAs, IncRNAs, proteins, and other vectors, which mediate intercellular communication, induce signaling, or mediate inter-cellular crosstalk between the cell and the microenvironment in a wide variety of malignant tumors. Exosomes are produced by donor cells and diffuse through various channels to recipient cells, which can receive them by phagocytosis, direct fusion and endocytosis. It is an important medium of intercellular communication.
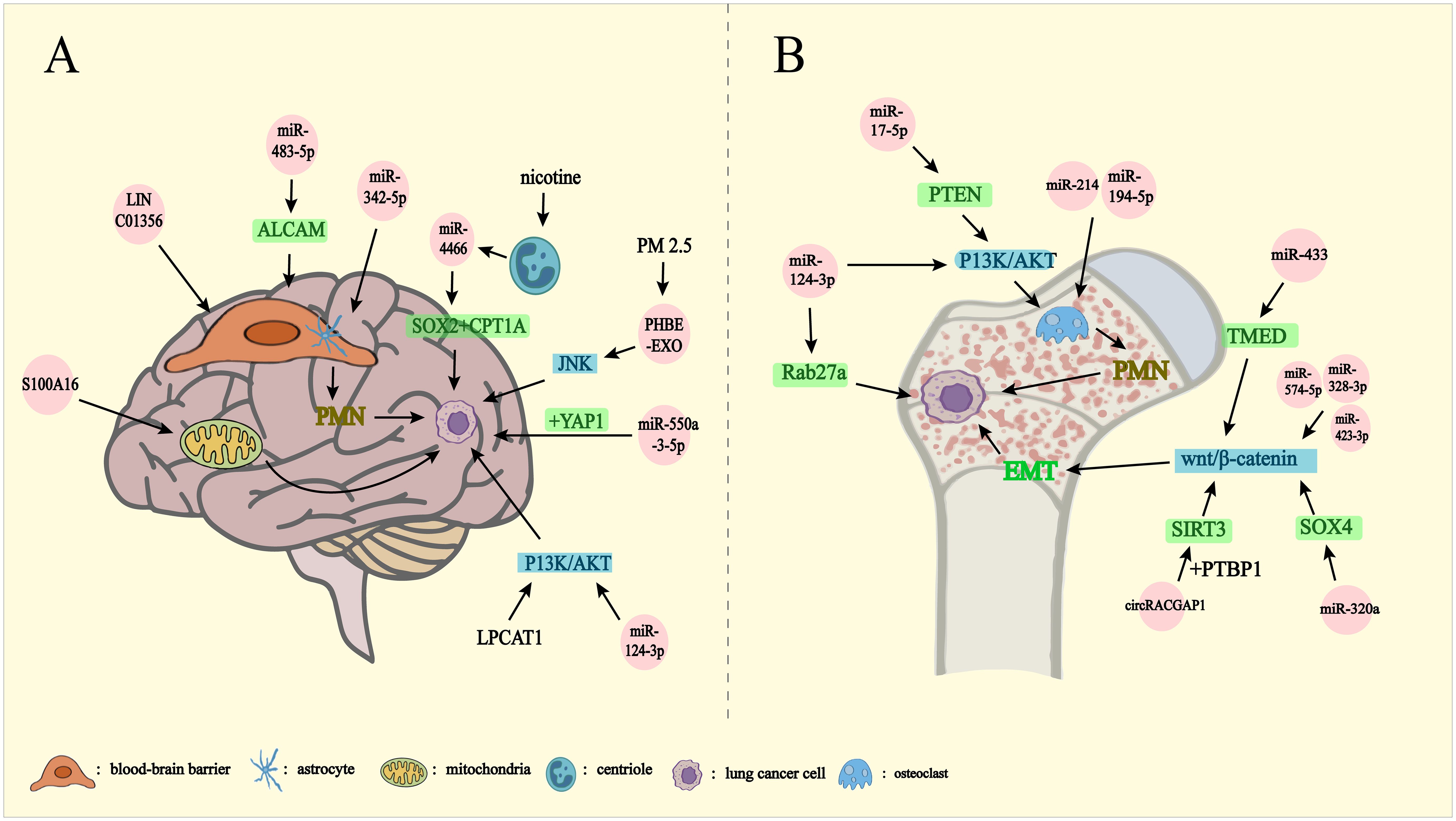
Figure 3. Lung cancer metastasizes outward through exosomes. PMN: pre-metastatic niche EMT: epithelial-mesenchymal transition. Exosomes mediate lung cancer metastasis to brain (A), bone (B). (A) miRNA-342-5p, miRNA-483-5p, and LINC01356 affect the BBB on the formation of PMN in the brain by regulating the differentiation of astrocytes, inhibiting the expression of ALCAM, and mediating the remodeling of the blood-brain barrier, respectively. S100A16 controls brain metastasis by up-regulating mitochondrial function and miRNA-550-3-5p binding to YAP1. Chronic nicotine leads to the release of miRNA-4466 from N2-neutrophils thereby activating SOX2, CPT1A genes. PM2.5-treated human bronchial epithelial cells activate the JNK signaling pathway, and miRNA-124-3p, LPCAT1, affects brain metastasis of lung cancer by influencing the P13K/AKT signaling pathway. (B) miRNA-214, miRNA-194-5p promote osteoclast formation affecting PMN formation. miRNA-17-5p activates the P13K/AKT signaling pathway via PTEN thereby driving osteoclast formation to promote PMN formation. miRNA-124-3p affects lung cancer bone metastasis by affecting the P13K/AKT signaling pathway and blocking Rab27a protein synthesis. miRNA-433 affects the Wnt/β-catenin signaling pathway through TMED5. miRNA-320a affects the Wnt/β-catenin signaling pathway via SOX4. CircRACGAP1 binding to PTBP1 enhances SIRT3 stabilization affecting the Wnt/β-catenin signaling pathway. miRNA-574-5p, miRNA-328-3p, and miRNA-423-3p affect the Wnt/β-catenin signaling pathway and thus drive the EMT, which affects lung cancer bone metastasis.
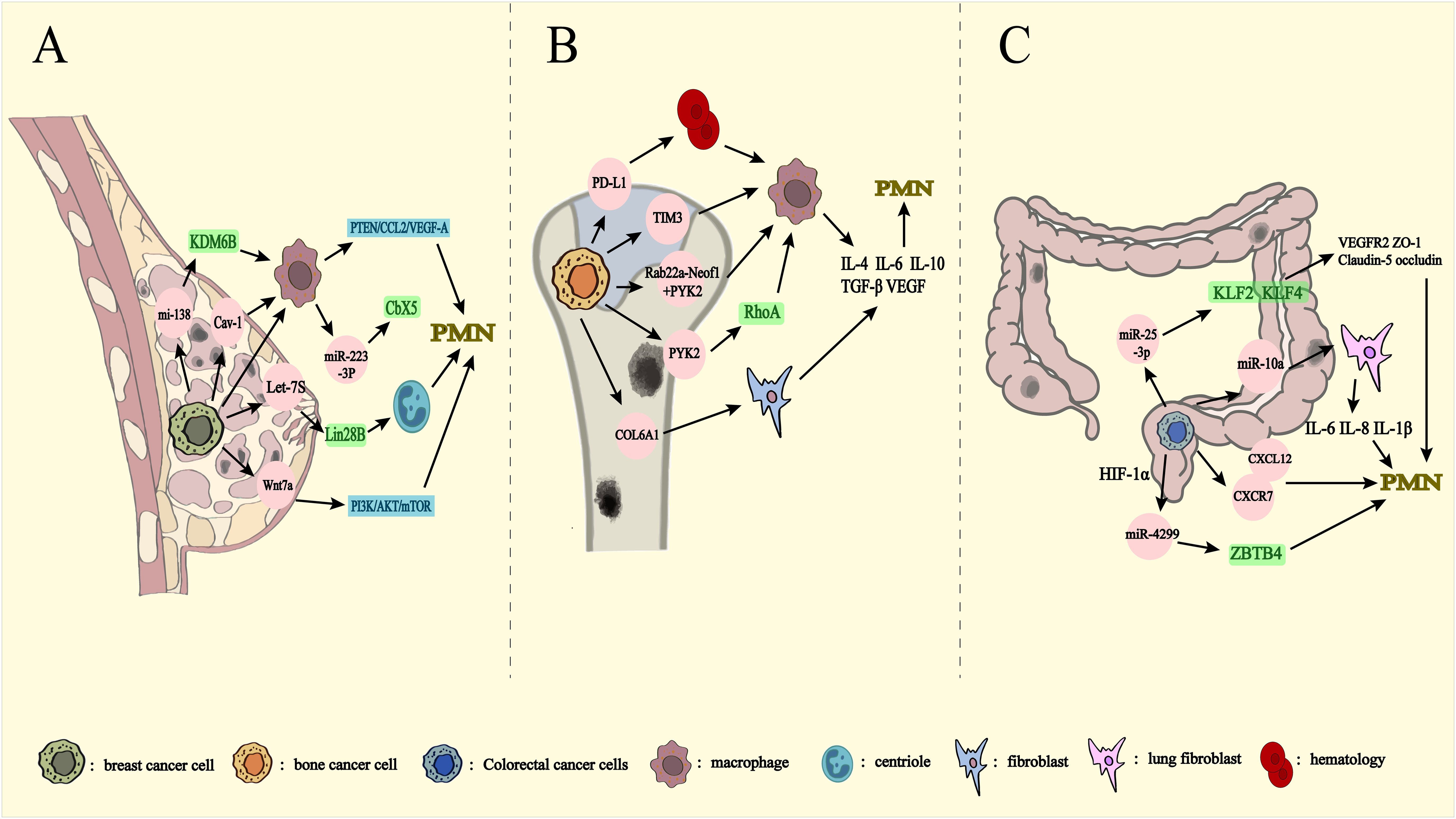
Figure 4. Metastasis from other organs to lungs via exosomes. PMN: pre-metastatic niche. Breast (A), bone (B), and colorectal (C) mediate exosome transfer to the lungs. (A) miRNA-138-5p decreases the expression level of KDM6B affecting macrophage polarization. Cav-1 inhibition of the PTEN/CCL2/VEGF-A signaling pathway affects macrophage polarization and PMN formation. Let-7s drive the transformation of N2 neutrophils through Lin28B Wnt7a has an impact on PMN formation by activating PI3K/Akt/mTOR, leading to changes in the cytokine milieu of the lung prior to metastasis. Release of miRNA-223-3p by breast cancer cells via macrophage exosomes affects breast cancer lung metastasis by inhibiting Cbx5. (B) PD-L1 is released into the bloodstream as exosomes to induce macrophage polarization. Exosome-mediated Tim3 induces macrophage polarization. Rab22a-NeoF1 binds to PYK2, while PYK2 activates the RhoA signaling pathway to promote macrophage polarization. COL6A1 activates fibroblasts. They promotes bone cancer lung metastasis by secreting cytokines such as IL-4, IL-6, IL-10, TGF-β and VEGF. (C) miRNA-10a inhibits human lung fibroblasts and reduces the expression of IL-6, IL-8, and IL-1β, affecting PMN production. miRNA-25-3p reduces the expression of VEGFR2, ZO-1, occludin and Claudin5 through KLF2 and KLF4, and affects PMN generation. CXCL12 and CXCR7 are important components of PMN. miRNA-4299 increases expression levels through HIF-1α and promotes colorectal cancer lung metastasis through ZBTB4.
Brain metastasis (BM) is a frequent and detrimental outcome of lung cancer, significantly impairing treatment efficacy and reducing overall survival (48). Notably, SCLC is recognized as the most aggressive histological subtype, with a pronounced tendency for early BM development (43). BM also constitutes a critical cause of mortality in patients with NSCLC (49). In advanced lung adenocarcinoma (LUAD), the incidence of BM has been reported to reach up to 45% (50). Exosomes are deeply implicated in this process through various mechanisms (Figure 3A).
The establishment of a brain PMN in lung cancer individuals with BM largely depends on the disruption of the blood-brain barrier (BBB). Astrocytes, as a critical component of the BBB, play a pivotal role in maintaining the homeostasis of the brain microenvironment (51). Exosomes are capable of crossing the BBB, thereby participating in intercellular communication within the central nervous system (CNS), supporting neuronal development, and modulating inflammatory responses (52). For example, exosomes secreted by the lung cancer cell line H1299, containing MAP2K1, TUBA1C, RELA, and CASP6, induce astrocyte apoptosis and trigger the release of pro-inflammatory cytokines, thereby fostering an immune-evading microenvironment (51).
Exosomal miRNAs play diverse and critical roles in the regulation of BM. In particular, serum-derived exosomal miRNAs from lung cancer individuals with BM are involved in several regulatory pathways. miRNA-342-5p, for instance, has been identified as a downstream target of Notch signaling, promoting the differentiation of neural stem cells into neural progenitors and astrocytes. Meanwhile, the overexpression of miRNA-483-5p has been shown to inhibit ALCAM, a key protein in maintaining BBB integrity (53). Furthermore, miR-550a-3-5p, enriched in exosomes isolated from the plasma of lung cancer individuals with BM, inhibits cell viability and migration while inducing apoptosis in human brain microvascular endothelial cells through YAP1 targeting (54). Additional studies on plasma-derived tumor exosomes from patients with cerebellar metastases have identified six upregulated miRNAs, including has-miRNA-331-5p, -542-5p, -26a-2-3p, -99a-3p, -184, and -3065-5p, which may serve as potential biomarkers for BM (48). Interestingly, miR-124-3p targets the 3’UTR of Rab27a, reducing exosome secretion and inhibiting the PI3K/AKT signaling pathway, thereby curtailing NSCLC metastasis (55). Moreover, lysophosphatidylcholine acyltransferase 1 (LPCAT1), an enzyme involved in phospholipid metabolism, has been found to regulate the PI3K/AKT pathway by modulating the MYC transcription factor, thus enhancing cancer cell growth and metastasis (56). Single-cell RNA sequencing of LUAD brain metastases confirmed significantly elevated LPCAT1 expression in lung cancer cells at BM sites compared to primary lung tumor cells (49). Upregulation of LPCAT1 in NSCLC has been associated with an increased incidence of BM, while LPCAT1 inhibition effectively curbs lung cancer cell proliferation and metastasis (49, 56).
LncRNAs and proteins in exosomes are equally pivotal in the development of brain metastasis (BM). For example, exosomes secreted by the NSCLC cell line H1299 that carry LINC01356 play a pivotal role in remodeling the BBB and facilitating BM in lung cancer (57). Additionally, calcium-dependent proteins and S100 proteins are strongly linked to BM formation (58). Notably, exosome-mediated delivery of S100A16 from brain microvascular endothelial cells enhances SCLC cell survival and promotes BM by boosting mitochondrial function (43). Moreover, plasma exosomal MUC5B, a cell surface glycoprotein that stimulates brain metastasis, has shown potential as a biomarker for diagnosing BM in patients with lung cancer (58).
The influence of external environmental factors on lung cancer brain metastasis is also profound. Studies indicate that patients with lung cancer with a history of smoking are more susceptible to BM compared to non-smokers. Chronic nicotine exposure leads to the accumulation of N2-neutrophils around brain metastases, which release exosomes carrying miRNA-4466. These exosomes activate tumor stem cell-associated genes and metabolic regulators, such as SOX2 and CPT1A, thereby promoting brain tumor metastasis and solid tumor growth (59). Similarly, exosomes derived from human bronchial epithelial cells exposed to PM2.5 have been shown to enhance the migration, invasion, and epithelial-mesenchymal transition (EMT) of lung cancer cells, activating the JNK signaling pathway to accelerate lung cancer metastasis in vivo (60).
In summary, the progression of brain metastasis during lung cancer metastasis to the brain, mediated by exosomes, is shaped by both intrinsic and extrinsic environmental factors. The establishment of the PMN within the intrinsic environment further contributes to BM development. However, safeguarding the BBB is paramount in preventing tumor metastasis to the brain. Therefore, future research into strategies aimed at enhancing the protective function of the BBB during tumor cell metastasis holds significant promise.
The skeletal environment plays a pivotal role in supporting lung cancer cell proliferation (61). Bone is one of the most frequent sites for lung cancer metastasis, with an incidence rate of 30-40% among patients with lung cancer (44). The presence of bone metastases drastically reduces the three-year survival rate from 71.6% to 46.8% (62). Bone metastases in lung cancer are classified as either osteoblast-associated or osteoclast-associated. In metastatic NSCLC, osteolytic bone destruction driven by heightened osteoclast activity is the predominant mechanism (63). Exosomes are deeply involved in this pathological process (Figure 3B), delivering bioactive molecules—such as proteins, lipids, and non-coding RNAs (ncRNAs)—that play a critical role in bone remodeling by modulating osteoblast function (64).
The interaction between osteoclasts and cancer cells is reciprocal. Exosomes carrying miR-214 and miR-194-5p secreted by lung cancer cells promote osteoclast formation and enhance bone resorption, creating a bone marrow microenvironment conducive to cancer cell survival and metastasis (65, 66). Exosomal miRNAs play a pivotal role in facilitating lung cancer bone metastasis by modulating key signaling pathways. Research suggests that the dysregulation of miRNAs, particularly those involved in the Wnt/β-catenin pathway, significantly drives cancer progression, metastatic spread, and resistance to therapy (67). This pathway is integral to the EMT, a critical process in tumor development and metastasis, and is especially important in CNS maturation (68, 69). Key miRNAs, such as miR-574-5p, miR-328-3p, miR-423-3p, miR-770, miR-433, and miR-1260b, actively participate in the Wnt/β-catenin pathway, influencing EMT. Notably, inhibiting miR-574-5p, miR-328-3p, and miR-423-3p may synergistically disrupt the Wnt/β-catenin signaling pathway, potentially playing a critical role in preventing lung cancer bone metastasis. Further analysis has identified 43 miRNAs, predominantly located on chromosome 14, that are upregulated in bone metastases and linked to EMT and bone metastasis (69). For instance, miR-433 targets the 3′-UTR of TMED5, thereby inhibiting the Wnt/β-catenin pathway (70), while miR-320a from human umbilical cord MSC-derived exosomes modulates this pathway by targeting SOX4 (71). Moreover, circRACGAP1 enhances the stemness and metastatic potential of NSCLC cells by interacting with PTBP1, stabilizing SIRT3, and activating the RIF1-mediated Wnt/β-catenin pathway (72).
The PI3K/AKT signaling pathway also plays a central role in lung cancer bone metastasis. miR-17-5p promotes osteoclast formation by targeting the tumor suppressor PTEN and activating the PI3K/AKT signaling cascade in lung cancer bone metastasis (73). Conversely, miR-124-3p inhibits PI3K/AKT signaling by targeting the 3’UTR of Rab27a, reducing exosome secretion and thereby limiting NSCLC metastasis (55).
Bone marrow mesenchymal stem cells (BMSCs) also contribute to the proliferation and further metastasis of lung cancer cells in the bone marrow microenvironment. Quiescent lung cancer cells release exosomes that are internalized by BMSCs, enhancing the glycolytic capacity of BMSCs via the insulin-like growth factor 1 receptor (IGF-1R) signaling pathway. Blocking IGF-1R signaling or glycolytic pathways effectively slows the proliferation of lung cancer cells within the bone marrow (61).
Both the Wnt/β-catenin and PI3K/AKT signaling pathways play integral roles in lung cancer metastasis to bone via exosomes. By modulating the bone marrow microenvironment, these pathways provide cancer cells with enhanced survival and proliferation capacities, ultimately promoting metastasis. The mutual reinforcement between osteoclasts and cancer cells underscores the importance of osteoclasts in the metastatic process. Future research should focus on understanding osteoclast formation and function, as altering their properties could potentially disrupt the metastatic cycle.
In conclusion, exosomes play a critical role in driving the metastasis of lung cancer to the brain and bone through various molecular mechanisms, making them a promising target for future diagnostic and therapeutic interventions in the management of this lethal disease.
Breast cancer (BC) metastasis accounts for approximately 90% of fatalities associated with the disease, with lung metastasis being a predominant route (45). The 5-year survival rate for patients with metastatic breast cancer is a mere 20% (74), and it is estimated that 60-70% of those with lung metastases eventually succumb to recurrence (75). Exosomes play a pivotal role in facilitating these metastatic processes (Figure 4A).
Exosome-mediated PMN formation is essential for the metastasis of breast cancer cells to the lungs. Exosomes derived from metastatic cells are integral in creating a microenvironment conducive to metastasis, particularly through the involvement of specific proteins (76). For instance, exosomes from BC cells containing Cav-1 upregulate inflammatory gene expression in lung epithelial cells, thereby modulating matrix deposition in lung fibroblasts. These exosomes disrupt the PTEN/CCL2/VEGF-A signaling pathway in lung macrophages, promoting M2-type polarization, neovascularization, and PMN formation, all of which facilitate lung metastasis in breast cancer (24). Moreover, exosomes containing Wnt7a protein from low-metastatic cell lines, such as LM.4T1, can enhance the metastatic potential of high-metastatic cells by activating the PI3K/AKT/mTOR signaling pathway. These low-metastatic cells also stimulate angiogenesis within the TME, providing a more favorable setting for highly metastatic cells to evade immune surveillance. Notably, Wnt7a increases the risk of lung metastasis in triple-negative breast cancer, highlighting its potential as a novel therapeutic target (77).
High levels of the lncRNA Lin28B, coupled with low levels of let-7s, are prognostic indicators for poor outcomes and lung metastasis in patients with breast cancer. Lin28B alters the cytokine environment in the lungs before metastasis occurs, facilitating neutrophil recruitment and N2 polarization. Even in the absence of tumor cell migration, Lin28B triggers changes in the PMN, leading to the production of IL-6 and IL-10, which drive the N2-type conversion (22). Comparative analysis of primary tumors, non-tumor tissues, and lung metastases has shown that tumor suppressors miR-200c and let-7a are downregulated in both tumors and metastatic tissues, but their levels in exosomes are elevated, particularly in lung metastases. Exosomal miR-200c may suppress the immune response of F4/80+ macrophages, negating the tumor-suppressive effects on recipient cells. Rab1A facilitates the packaging of miR-200c into exosomes, circumventing its inhibitory effect on tumor proliferation. Thus, targeting anti-Rab1A proteins to enhance miR-200c expression could impede breast cancer metastasis to the lungs (78).
miRNAs within exosomes secreted by breast cancer cells also influence macrophage remodeling and programming. For example, miR-138-5p translocates into macrophages and downregulates KDM6B expression, regulating macrophage polarization (79). M2-type macrophages, in particular, play a tumor-supportive role, aiding in tumor invasion and metastasis (80, 81). Exosomes derived from tumor-associated macrophages (TAMs), containing miR-223-3p, promote lung metastasis by targeting and inhibiting Cbx5, thus facilitating breast cancer cell metastasis to the lungs (82).
In conclusion, breast cancer utilizes exosomes to influence target genes and proteins, modify multiple signaling pathways, and reshape macrophage polarization. These changes ultimately contribute to the formation of the PMN, which drives the initiation of lung metastasis in breast cancer cells.
Osteosarcoma (OS) predominantly arises in regions of rapid bone growth, such as the femur, tibia, and humerus. This malignancy is characterized by cancer cells that produce immature bone or bone-like tissue (83). OS is one of the most common malignant tumors originating in the skeletal system, particularly affecting children and adolescents, and is notorious for its high metastatic potential (84). The 5-year overall survival rate remains relatively low, around 20%, largely due to the presence of lung metastases in 15-30% of patients at the time of diagnosis (85). Exosomes play a critical role in the lung metastasis of bone cancer (Figure 4B).
The complex genetic makeup of OS suggests that immune suppression is a key factor in its progression (86). In patients with OS, immunosuppressive cells, supportive cells within the TME, and tumor cells can release programmed death ligand 1 (PD-L1) via exosomes, which collectively contribute to systemic immunosuppression (87). Studies have shown that PD-L1 expression in the TME, both in mouse models and humans, promotes systemic immune suppression, thereby increasing tumor burden and reducing patient survival across various cancers (88). In contrast, lung metastasis was significantly reduced in mice treated with exosomes from PD-L1 knockdown cells, suggesting that exosomal PD-L1 is crucial in promoting lung metastasis in OS (89). Furthermore, tumor-derived exosomes enhance glucose uptake via the TLR2 and NF-κB pathways, leading to increased nitric oxide synthase 2 (NOS2) levels, which inhibit mitochondrial oxidative phosphorylation. This metabolic shift increases PD-L1 expression by converting more pyruvate to lactate, which in turn stimulates NF-κB activity and polarizes macrophages towards an immunosuppressive phenotype (90).
Macrophages, as primary phagocytes involved in the innate immune response, play a critical role in combating OS and other cancers, significantly influencing survival outcomes (91). M1 macrophages exert tumor-suppressive effects, while M2 macrophages promote tumor growth and metastasis. OS cells enhance migration, invasion, EMT, and lung metastasis primarily through exosome-mediated immunomodulatory factors, such as Tim3, which induce M2 macrophage polarization and the secretion of cytokines like IL-4, IL-6, IL-10, TGF-β, and VEGF (81, 91–94). For example, visfatin treatment of chondrosarcoma cells has been shown to increase exosome production, which in turn stimulates M2 macrophage polarization, enhancing chondrosarcoma motility and contributing to lung metastasis (95). However, interactions between tumor cells and macrophages may vary depending on the metastatic potential of the tumor. For instance, EVs from highly metastatic OS interact differently with macrophages compared to those from less metastatic cells. In highly metastatic K7M2 OS cells, EVs decrease the levels of pro-inflammatory cytokines such as INFγ, IL-2, IL-6, and TNFα in macrophages, suggesting that these EVs further promote M2 macrophage differentiation, reinforcing the metastatic process. This represents the first evidence of differential interactions between OS-EVs and macrophages based on their polarization states (96).
Exosomal proteins are pivotal in facilitating the metastasis of OS to the lungs. One key player in this process is the upregulation of the COL6A1 gene in OS tissues, which has been strongly correlated with an increased likelihood of lung metastasis and reduced survival rates in patients with OS. This upregulation occurs through the binding of the transcription factor c-Jun to p300, which enhances the acetylation modification (H3K27ac) of histone H3 lysine 27 in the promoter region of the COL6A1 gene. Moreover, COL6A1 facilitates OS metastasis by downregulating STAT1 expression through its degradation via the ubiquitin-proteasome pathway. OS cells package COL6A1 into exosomes and transfer it to fibroblasts, inducing their activation and stimulating TGF-β secretion, which in turn enhances the invasive and migratory abilities of OS cells (92). Additionally, exosomes derived from OS cells carrying Rab22a-NeoF1 fusion proteins and PYK2 further exacerbate lung metastasis by recruiting bone marrow-derived macrophages to establish a favorable metastatic microenvironment. PYK2 in exosomes activates the RhoA signaling pathway within OS cells and promotes the generation of M2-type macrophages, which are known to support tumor metastasis, thereby accelerating OS metastasis to the lungs (97). Furthermore, Rab27a, a gene critical for exosome-specific protein transport, is essential for OS cell metastasis. Knocking out Rab27a in OS cells reduces exosome secretion, significantly lowering their metastatic potential to the lungs (89).
As discussed, OS cells exploit exosomes to influence several pathways, including macrophage polarization and fibroblast activation, by releasing various cytokines. These exosomal interactions create an immunosuppressive microenvironment that favors metastasis, supporting the migration of OS cells and the development of lung metastasis. Thus, identifying strategies to detect, disrupt, or prevent the formation of this immunosuppressive environment represents a promising avenue for future research. Such approaches may offer new therapeutic insights into mitigating the progression and metastasis of OS to the lungs.
Colorectal cancer (CRC) is the third most common malignancy globally and ranks second in cancer-related mortality (4). Approximately 20-25% of patients with CRC present with synchronous metastatic disease at diagnosis (98), with the liver and lungs being the most frequent sites of metastasis. Approximately 10-25% of patients with CRC eventually develop lung metastases (47). Exosomes play a significant role in mediating CRC lung metastasis through various mechanisms (Figure 4C).
The formation of PMN is a critical step in CRC lung metastasis, where exosomal miRNAs facilitate communication between cancer cells and stromal cells (99). Notably, miR-10a expression in CRC-derived exosomes is inversely correlated with the depth of tumor invasion. Exosomal miR-10a from CRC cells inhibits the proliferation and metastatic potential of normal human lung fibroblasts (NHLFs) and reduces the expression of inflammatory cytokines, such as IL-6, IL-8, and IL-1β in NHLFs (100). Additionally, miR-25-3p secreted by CRC cells targets KLF2 and KLF4 in vascular endothelial cells, downregulating the expression of vascular endothelial growth factor receptor 2 (VEGFR2), ZO-1, occludin, and Claudin5, thereby affecting neovascularization and PMN formation, promoting CRC metastasis (36).
CXCL12 is another key component of the PMN that is often upregulated before metastasis occurs. In noncancerous lung tissues of patients with CRC, CXCL12 expression is significantly higher compared to patients with benign lung disease. Immunohistochemistry analysis reveals that CXCR7 and CXCL12, both indicators of colon cancer lung metastasis, are more highly expressed in lung metastatic tissues than in primary tumor sites (101). Moreover, circLONP2 interacts with the DGCR8/Drosha complex in a DDX1-dependent manner, enhancing the maturation of primary microRNA-17. The resulting elevated levels of miR-17-5p can be encapsulated in exosomes and transferred to neighboring cells, thereby promoting migration and spread of CRC cells (102).
Hypoxic conditions in tumors also enhance exosome production in CRC. In a low-oxygen environment, tumor cells alter their metabolic pathways and migrate toward oxygen-rich areas, promoting metastasis (103). For instance, macrophages exposed to exosomes under hypoxic conditions undergo M2-type macrophage differentiation through activation of the AMPK/p38 signaling pathway, which in turn supports lung tumor proliferation and metastasis (104). Hypoxia also elevates miR-4299 levels in CRC cells and hypoxia-derived exosomes in a hypoxia-inducible factor 1 alpha (HIF1α) dependent manner, which directly targets ZBTB4, thereby promoting CRC proliferation and metastasis (103).
Thus, CRC cells utilize mechanisms such as hypoxia and cytokine production to increase exosome release and promote PMN formation, contributing to lung metastasis.
In summary, exosomes play a central role in the metastasis of breast, bone, and colorectal cancers to the lungs by influencing various signaling pathways and facilitating PMN formation. This underscores the importance of exosome-mediated PMNs in metastasis and highlights potential diagnostic and therapeutic targets for mitigating lung metastasis from primary tumors.
In the TME, angiogenesis is a critical driver of cancer progression and metastasis (20, 105). VEGFA and its receptor VEGFR2, are central to the regulation of tumor-induced angiogenesis (106). Exosome-transported miRNAs produced by cancer cells play a vital role in facilitating neovascularization, reshaping the TME, and establishing a PMN (36, 105, 107).
One example is exosome-transported miR-197-3p, which has been shown to downregulate the expression of TIMP3 in human umbilical vein endothelial cells (HUVECs). This downregulation activates VEGFR2, stimulating downstream ERK signaling and further promoting angiogenesis. Simultaneously, miR-197-3p targets TIMP2, reducing the expression of MMP2 and MT1-MMP, thus enhancing neovascularization (108). Similarly, exosomal miR-3157-3p from lung cancer cells downregulates KLF2, ZO-1, occludin, and Claudin5, while simultaneously elevating levels of VEGF, MMP2, and MMP9, significantly increasing the permeability of HUVECs and promoting angiogenesis (23). Additionally, pedunculated vesicles serve as key structural elements in neoangiogenesis, with angiogenesis playing a fundamental role in their formation within the TME (20). For instance, phosphoglycerate translocase 1 (PGAM1) in HUVEC-derived exosomes interacts with γ-actinin (ACTG1) to stimulate the development of pedunculated vesicles, thereby contributing to the neovascularization process (109).
Thus, exosomes significantly influence angiogenesis by modulating VEGF and associated pathways, further promoting cancer metastasis through enhanced blood vessel formation.
In lung cancer, macrophages are the most abundant type of white blood cell within the TME and play a pivotal role in all stages of cancer development (110). The complex interactions between tumor cells and TAMs significantly impact tumor progression. Tumor cells drive macrophage polarization towards the immunosuppressive M2 phenotype through various mechanistic pathways, which exacerbate tumor malignancy and are closely associated with poor prognosis (111). Tumor cells achieve this by releasing inflammatory cytokines, chemokines, extracellular vesicles, and tumor metabolites, which induce TAMs to adopt the M2 phenotype. In turn, M2-type TAMs enhance tumor cell proliferation and EMT (112) and promote tumor angiogenesis, PMN formation, immune escape, and ultimately tumor growth and metastasis (80, 112, 113). Recent studies have identified key pathways involved in tumor-derived exosome-mediated macrophage transformation, including the NF-κB and STAT3 signaling pathways, as well as hypoxic conditions (90).
Exosomes promote TAMs polarization toward the M2 phenotype by activating the NLRP6/NF-κB signaling pathway (114, 115). Tumor cells also transfer TRIM59 via exosomes to macrophages, where it interacts with ABHD5 to activate the NLRP3 signaling pathway. This promotes the release of pro-inflammatory cytokines such as IL-18 and IL-1β, further contributing to an immunosuppressive environment (114, 115). Additionally, tumor cells undergoing enhanced glycolysis, which supports their proliferation, angiogenesis, and metastasis (116), release exosomes that boost glucose uptake through TLR2 and NF-κB pathways. These exosomes increase NOS2 levels, inhibiting mitochondrial oxidative phosphorylation and shifting energy production towards lactate. This metabolic change amplifies NF-κB activity, which upregulates PD-L1 expression and further drives macrophages towards the M2 phenotype, thereby enhancing lung cancer’s metastatic potential (90).
The STAT3 signaling pathway is another critical factor in macrophage polarization. Activation of this pathway leads to M2 macrophage differentiation. Exosomal MFG-E8, a significant component of small cellular debris exosomes, mitigates inflammatory responses by activating SOCS3; however, this also interferes with the regulation of STAT3 phosphorylation, facilitating M2 polarization (117). PYK2 in tumor exosomes can also trigger STAT3 activation in macrophages by activating the RhoA pathway in OS cells (97). Furthermore, exosomal miR-19b-3p from LUAD cells induces M2 macrophage polarization by targeting PTPRD proteins to activate STAT3 signaling, supporting lung metastasis of tumor cells (118).
Exosomes released under hypoxic conditions further promote M2 macrophage polarization. In NSCLC, circPLEKHM1 in exosomes, regulated by HIF1α, activates the OSMR/JAK/STAT3 signaling pathway, promoting M2 polarization in the TME by enhancing the interaction between PABPC1 and eIF4G (46). Additionally, the AMPK/p38 signaling pathway is upregulated under hypoxia, driving the differentiation of macrophages into the M2 phenotype (104).
Exosomes play a pivotal role in driving macrophage polarization toward the M2 phenotype by transmitting various bioactive molecules. For instance, lung cancer cell-derived exosomes can facilitate this transition by delivering lncRNAs such as PCAT6 and LINC00963, which promote M2 macrophage polarization (119, 120). Additionally, exosomal circ_0001715 internalizes and inhibits miR-205-5p, leading to the upregulation of TREM2, which further stimulates M2 macrophage polarization and creates a feedback loop that accelerates lung cancer growth, metastasis, and EMT (120–122). In contrast, lung cancer cells can also release miR-770-containing exosomes, which suppress M2 macrophage transformation by targeting the MAP3K1 gene, thereby reducing immunosuppression (121). Tumor-derived exosomes additionally enhance I-IFN secretion via the circPIK3R3/miR-872-3p/IRF7 axis, boosting the anti-tumor immune response of CD8+ T cells (123).
Beyond tumor-secreted exosomes, M2-type TAMs contribute to NSCLC metastasis by releasing exosomes carrying miRNAs such as miR-155 and miR-196a-5p, which promote metastasis by directly inhibiting the RASSF4 gene through interaction with its 3’UTR (124). Tumor cells induce M2 macrophage polarization via multiple pathways, including NF-κB, STAT3, and hypoxia-related mechanisms, thereby intensifying the malignant characteristics of the tumor. M2 macrophage polarization not only enhances metastasis of lung cancer to distant sites through exosomes but also facilitates metastasis from other regions, particularly bone cancer, to the lungs. This expands diagnostic options by broadening the range of potential indicators. Moreover, macrophages can interact with other immunosuppressive cells, tumor cells, and various components within the microenvironment, fostering an immunosuppressive setting during M2 polarization. Targeting this process through artificial interventions aimed at engaging macrophages and disrupting the immunosuppressive milieu during the M2 transition could significantly mitigate cancer metastasis.
miRNAs play a significant role in influencing tumor progression and prognosis through their regulatory effects on the TME. The release of exosomes is closely linked to the expression levels of various miRNAs (125). As indicated in Table 1, exosomal miRNAs exhibit dual roles in lung cancer metastasis, with certain miRNAs promoting cancer progression through multiple pathways. Among the identified promoters of lung cancer metastasis are miR-133a-3p, miR-665, miR-486-5p, miR-375-3p, miR-1260b, miR-3180-3p, miR-210-3p, and miR-582-3p.
For example, miR-133a-3p, found in lung cancer serum exosomes, silences specific proteins such as Sirtuin 1 (SIRT1) through targeted mechanisms. Overexpression of miR-133a-3p has been shown to significantly enhance the biological properties of A549 lung cancer cells, promoting cell viability, migration, invasion, angiogenesis, and proliferation (126). Similarly, miR-582-3p in exosomes derived from hypoxic NSCLC cell lines (A549 and H1299) enhances proliferation, metastasis, and invasion of normoxic NSCLC cell lines by inhibiting the expression of secreted frizzled-related protein 1 (SFRP1), thus contributing to tumorigenesis through the modulation of various signaling pathways (127). In another case, exosomes derived from malignant pleural effusion (MPE) in lung cancer, enriched with miR-665, are taken up by NSCLC (H1975, H1650) and SCLC (H446) cells. This uptake stimulates their invasive and metastatic capabilities by modulating the transcription factor HEYL, which acts downstream of the Notch signaling pathway (128). Moreover, miR-486-5p present in serum and tumor tissues of patients with NSCLC promotes metastasis by targeting the CADM1/TJs pathway in vascular endothelial cells, impairing endothelial barrier function and increasing the concentration of miR-486-5p in the bloodstream (129). Similarly, miR-375-3p, released by SCLC cells (H446 and H1048), acts on vascular endothelial cells by downregulating claudin-1, a key tight junction protein, further impairing vascular barrier function and promoting metastasis (130). In another instance, exosomes from H1299 and A549 cells have been observed to deliver miR-1260b to surrounding tumor cells, reducing the expression of SFRP1 and Smad4. This activates the Wnt/β-catenin signaling pathway, enhancing the invasive ability of LAC cells and promoting metastatic progression (131).
miRNAs also target specific proteins or genes to drive tumor progression. miR-1260b enhances neovascularization, migration, and chemotherapy resistance by inhibiting HIPK2 and regulating the Wnt/β-catenin signaling pathway in lung cancer cells (105). Additionally, exosomal miR-210-3p released from lung stem cells derived from A549 cells enhances the migration and invasion of A549 and NCI-H1703 lung cancer cells. This effect is associated with increased expression of N-cadherin, vimentin, MMP-9, and MMP-1, alongside reduced expression of E-cadherin. miR-210-3p further inhibits FGFRL1, promoting EMT and enhancing tumor migration and invasion (132).
Several miRNAs also act as negative regulators of lung cancer progression through diverse mechanisms. For instance, miR-124-3p, miR-326, miR-15a-5p, and miR-338-3p have been identified as inhibitors of lung cancer metastasis. These miRNAs typically bind to the 3’UTR of target mRNAs, leading to either mRNA degradation or inhibition of translation (133). For example, serum exosomal miR-124-3p in NSCLC inhibits exosome secretion and activation of the PI3K/AKT signaling pathway in A549 and NCI-H1299 cells by targeting the 3’UTR of Rab27a. Conversely, LINC00511 functions as a competing endogenous RNA for miR-124-3p, counteracting its inhibitory effects and promoting NSCLC metastasis (55). Similarly, miR-130b-3p, found in the serum exosomes of patients with NSCLC, reduces DEPDC1 expression by directly binding to its 3’UTR, which promotes apoptosis through the TGF-β signaling pathway and inhibits EMT and metastasis in NSCLC cells (134).
In another example, exosomal miR-3180-3p derived from A549 cells suppresses proliferation and metastasis by targeting the oncogene FOXP4 in NSCLC cells (135). Additionally, studies have shown that apelin, a natural ligand for G protein-coupled receptors, can promote tumor growth as an oncogenic factor in various cancers (136). However, miR-15a-5p, which is underexpressed in lung cancer tissues, negatively regulates the proliferation and migration of A549 lung cancer cells by targeting CDCA4. It also modulates EMT by increasing E-cadherin expression and regulating vimentin and N-cadherin levels (137). Furthermore, exosomal miR-338-3p has been shown to inhibit the growth and metastasis of A549 and SK-MES-1 cells by targeting CHL1 and influencing the MAPK signaling pathway (138).
lncRNAs, circRNAs, and proteins in exosomes also contribute to lung cancer metastasis and invasion through various pathways and signaling axes. For example, upregulation of exosomal lncRNA ZBED5-AS1 in LUAD-derived HCC827 cells increases the growth, migration, and invasiveness of H1299 and A549 cells by activating the ZNF146/ATR/CHK1 signaling axis and promoting EMT (139). On the other hand, exosomal lncRNA FGD5-AS1 from A549 cells decreases the viability, migration, and invasion of A549 and H1299 cells. In NSCLC tissues, lncRNA FGD5-AS1 targets miR-944, and its knockdown reduces the viability, migration, and invasion of NSCLC cells, thereby attenuating metastasis (140). Additionally, exosomal HOTAIR, derived from NCI-H1975 cells, has been closely linked to lymph node metastasis and TNM clinical staging, enhancing the proliferation and migration of A549 cells. Similarly, LINC00963 in A549 cell exosomes stabilizes the Zeb1 protein by interacting with HNRNPA2B1, facilitating EMT and promoting proliferation, migration, and invasion in A549 and NCI-H1650 cells (141).
Finally, human serum exosomal circSATB2, which is highly expressed in A549, H460, and H1299 cells, upregulates fascin homologous protein 1 (FSCN1) in lung tumor cells by targeting miR-326. Exosomal delivery of FSCN1 enhances NSCLC cell growth, migration, and invasion (142).
Research has demonstrated that circ_0008717 is highly expressed in NSCLC cells, such as A549 and H1299, and functions as an oncogenic factor within exosomes by binding to miR-1287-5p, leading to increased PAK2 expression and accelerated NSCLC tumor formation (143). Additionally, exosomes derived from A549 and H1299 cells containing circRAPGEF5 were found to enhance proliferation, migration, invasion, and EMT in lung adenocarcinoma cells (HCC827, A549, and H1299). In lung adenocarcinoma, ZEB1, negatively regulated by miR-1236-3p, has been identified as a critical target for circRAPGEF5. The inhibition of miR-1236-3p reversed the decrease in ZEB1 expression caused by circRAPGEF5 knockdown, highlighting circRAPGEF5’s significant role in cell proliferation and metastasis through modulation of the miR-1236-3p/ZEB1 signaling pathway (144).
Moreover, Leucine-rich α2-glycoprotein-1 (LRG1), which is highly expressed in A549 and PC-9 cells, has been shown to enhance proliferation, migration, invasion, and EMT of NSCLC cells. Research indicates that exosomal LRG1, abundant in NSCLC cells, promotes tumor metastasis in animal models (145).
The biologically active substances within exosomes—miRNAs, lncRNAs, circRNAs, and proteins—derived from various cells, play diverse roles in regulating the metastatic and invasive potential of lung cancer. These molecules target specific proteins and genes and modulate key signaling pathways. They can promote lung cancer progression by stimulating proliferation, migration, invasion, and angiogenesis, while others exert a suppressive effect on metastasis. This dual role suggests that targeting the pro-metastatic proteins and pathways while enhancing those that inhibit metastasis may be a viable strategy to reduce lung cancer spread, ultimately improving patient survival rates and outcomes.
Interest in exosomes as biomarkers and therapeutic targets is rapidly increasing, particularly in oncology (146). Exosomes serve as versatile carriers, capable of being engineered to improve sampling and targeting precision. Their key benefits include low immunogenicity, minimal toxicity, and high stability (147). The miRNAs, mRNAs, and proteins encapsulated in exosomes are highly stable, making them promising candidates for predicting the health trajectory of patients with malignant tumors (148–151). Additionally, elevated levels of lncRNA have been closely associated with the regulation of tumor behavior across various cancers, including NSCLC (152).
Liquid biopsy has emerged as a highly promising, non-invasive approach for lung cancer screening (153). Among the techniques used, exosome testing has gained popularity within liquid biopsy (29, 154) in recent years due to its substantial advantages in predicting cancer progression and metastasis (29). The poor prognosis of patients with patients with NSCLCs, largely driven by high recurrence rates, underscores the critical need for early identification of novel, sensitive, and specific biomarkers (155). Conventional lung cancer markers, such as carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cytokeratin 19 fragment (CYFRA21-1), and squamous cell carcinoma antigen (SCCA), are limited by their low sensitivity and specificity (156). In contrast, exosomes offer a more reliable platform for diagnosing lung cancer, monitoring patient survival, evaluating metastasis, and improving diagnostic accuracy.
Distinguishing between healthy individuals and patients can be effectively achieved by analyzing differences in exosome expression levels in vivo. For instance, both patients with NSCLC and those with early-stage NSCLC exhibited significantly reduced miR-620 levels in their exosomes compared to healthy individuals. These differences yielded area under the curve (AUC) values of 0.728 and 0.707, respectively, demonstrating relatively high diagnostic accuracy (157). Moreover, exosome levels in bodily fluids, such as blood and urine, offer additional diagnostic insights. Patients with NSCLC, for example, show significantly elevated expression of DLX6-AS1 in their blood (158). Serum exosomal lncRNAs TBILA and AGAP2-AS1 were also highly expressed in patients with NSCLC, with critical diagnostic values of 0.923 and 1.12, respectively, distinguishing patients from healthy cohorts (159). Additionally, urinary exosomal markers, such as WASL, which is downregulated in patients with lung cancer, and STK10 and WNK1, which are upregulated, can aid in diagnosis. These markers, when detected together in urinary exosomes, yield an AUC value of 0.760 for lung cancer detection (160).
Exosome expression also provides insights into patient survival outcomes. For instance, a combination of biomarkers, including the protein levels of fibrinogen beta chain, fibrinogen gamma chain, and von Willebrand factor, has shown strong potential for diagnosing early-stage NSCLC and correlates directly with patient survival (161). Lower levels of BTG-1 in plasma exosomes were associated with shorter disease-free and overall survival times compared to higher levels (162). Similarly, high miR-125b-5p expression in lung cancer tissues was linked to better overall survival, while low expression correlated with worse outcomes (155). Exosomal levels of miR-5684 and miR-125b-5p were lower in patients with NSCLC than in healthy individuals, with an AUC value of 0.793. Additionally, lncRNA-SOX2OT expression in exosomes from patients with NSCLC showed a negative correlation with overall survival, making it a valuable prognostic marker (65). These findings highlight the utility of these exosome-derived biomarkers as diagnostic and prognostic tools in NSCLC.
The metastatic potential of lung cancer can also be evaluated based on exosome expression and AUC values. For instance, miR-1290 expression in exosomes can differentiate between patients with LUAD at various stages (163). LUCAT1 promotes metastasis in LUAD cells by adsorbing miR-4316, thereby releasing VEGFA (155). Biomarkers such as CFHR5, C9, and MBL2, particularly CFHR5 alone, are significantly associated with overall survival in patients with NSCLC (161). Furthermore, lipopolysaccharide-binding protein has demonstrated the ability to distinguish between metastatic and non-metastatic NSCLC cases, with an AUC value of 0.803, further underscoring its efficacy as a marker of metastatic potential (164).
The reliability of commonly tested tumor markers, such as CEA, CYFRA21-1, NSE, and SCCA, is limited due to issues with sensitivity and specificity (156). To improve diagnostic accuracy, alternative markers and combination approaches are often utilized. Serum-derived exosomes, secreted by tumor cells, offer a more precise and dynamic reflection of tumor cell activity compared to tissue-based assessments. Detecting miRNAs in exosomes allows for real-time monitoring through blood samples, eliminating the spatial heterogeneity found in tissue biopsies and providing a more continuous assessment of treatment response. Furthermore, the dual membrane structure of exosomes ensures the stability of internal miRNAs in the bloodstream, offering higher reliability for detection (157). For example, serum exosomal miR-216b has been shown to effectively differentiate between patients with NSCLC and healthy controls (165).
It is well established that versican is notably upregulated in the plasma and plasma exosomes of patients with NSCLC. Receiver operating characteristic analysis indicated that the AUC values of versican in both plasma and exosomes exceeded those of traditional markers, such as NSE, CYFRA21-1, and SCCA, indicating superior diagnostic efficacy in NSCLC (166). Similarly, plasma exosomal BTG-1 more accurately predicted three-year disease-free and overall survival in patients with NSCLC, demonstrating stronger discrimination and specificity (162). The combined use of miRNAs has also been shown to enhance diagnostic accuracy for SCLC, with a 3-miRNA linear combination model (miR-200b-3p, miR-3124-5p, and miR-92b-5p) demonstrating a significant association with lung disease presence, including lung cancer (167). Moreover, integrating exosomal markers with traditional tumor markers further improved diagnostic performance. While combining lncRNAs did not outperform individual ones, pairing exosomal TBILA and AGAP2-AS1 with Cyfra21-1 resulted in improved accuracy for NSCLC diagnosis (159). Likewise, serum exosomal miR-216b significantly enhanced diagnostic performance when combined with standard tumor markers (165). Evaluating exosomal miR-1290 and lncRNA DLX6-AS1 alongside conventional markers further improved sensitivity and specificity in comparison to standard markers alone (158, 163).
Thus, lung cancer diagnosis, prognosis, metastasis evaluation, and test precision can be significantly enhanced by examining exosome expression in vivo. The levels of exosomal biomarkers, their AUC values, and their inherent stability play pivotal roles in diagnostic accuracy. Notably, the integration of exosomes with traditional tumor markers has shown promise in enhancing both the precision and specificity of lung cancer diagnostics and treatment strategies. This combined approach not only offers innovative methodologies for tumor detection but also opens up new avenues for improving the sensitivity and accuracy of cancer diagnosis.
The role of exosomes in lung cancer metastasis has been extensively explored, highlighting their critical involvement in communication between tumor cells. Through the regulation of exosomal content, cancer cells can manipulate the microenvironment of distant organs, facilitating metastasis and influencing organ-specific preferences, thus accelerating the spread of cancer.
This review presents a comprehensive overview of the multiple mechanisms by which exosomes contribute to the dissemination of lung cancer to the brain and bone, offering potential targets for intervention in both diagnosis and treatment of lung cancer metastasis, a life-threatening condition. Moreover, cancers such as breast, bone, and colon utilize exosomes to influence the formation of PMNs before metastasizing to the lungs, pointing to a possible strategy for preventing lung metastasis. This underscores the critical role of PMNs in both lung cancer metastasis to other organs and the metastasis of cancers from other sites to the lungs, all facilitated by exosomes. These processes involve the establishment of a favorable microenvironment for tumor metastasis, achieved through various mechanisms that are difficult to detect in clinical settings. Therefore, monitoring the establishment of such environments by tumor cells and identifying indicators that signal a shift toward metastasis are key to deepening our understanding of exosome-mediated metastasis.
In addition, the process of lung metastasis is heavily influenced by angiogenesis and macrophage polarization toward the M2 phenotype, both of which are mediated by bioactive substances within exosomes. These substances affect several tumor progression factors, including proliferation, migration, invasion, and ultimately metastasis. Investigating how bioactive molecules from exosomes influence lung cancer metastasis can help identify pathways to enhance anti-metastatic effects or inhibit pro-metastatic signaling. It is essential to recognize that different cell types release exosomes containing distinct bioactive substances, which may act through varying mechanistic pathways. A key consideration in clinical applications is the selection of optimal bioactive substances within exosomes, informed by current research.
Despite the progress in understanding lung cancer metastasis and exosomes, several areas require further investigation. Specifically, the exact mechanisms governing exosome secretion by tumor or immune cells and their role in lung cancer metastasis remain unclear. Additionally, the therapeutic potential of exosomes—including their capacity to enhance cancer treatment by serving as carriers for anticancer agents—requires further elucidation. Another important aspect to explore is whether the diagnostic accuracy of exosomes changes before and after cancer treatment, particularly concerning the use of therapeutic agents. These unresolved questions emphasize the need for more in-depth research and discussion. In conclusion, exosomes hold significant promise in advancing cancer therapy, and future comprehensive studies are both essential and urgent.
HC: Writing – original draft. LL: Writing – original draft. DZ: Writing – review & editing. GX: Writing – review & editing. NA: Writing – review & editing. JH: Writing – review & editing. YL: Writing – review & editing. GZ: Supervision, Visualization, Writing – review & editing. ML: Supervision, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Sichuan Science and Technology Program (2022YSF0625); Natural Science Foundation of Sichuan Province (2023NSFSC0666); Joint Project from Luzhou city and Southwest Medical University (2024LZXNYDJ023); the Luzhou Science and Technology Project (2024SYF186) and the provincial college students’ innovation and entrepreneurship training programme in Sichuan Province (S202210632233).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AUC, area under the curve; BM, Brain metastasis; BBB, blood-brain barrier; BMSCs, Bone marrow mesenchymal stem cells; BC, Breast cancer; Cav-1, Caveolin-1; CircRNAs, Circular RNAs; CRC, Colorectal cancer; CYFRA21-1, cytokeratin 19 fragment; EVs, extracellular vesicles; EMT, epithelial-mesenchymal transition; FSCN1, fascin homologous protein 1; IncRNAs, long non-coding RNAs; LUAD, lung adenocarcinoma; LPCAT1, lysophosphatidylcholine acyltransferase 1; miRNAs, microRNAs; MPE, Malignant pleural effusion; MMP, matrix metalloproteinases; NSCLC, non-small cell lung cancer; NSE, neuron-specific enolase; OS, Osteosarcoma; PD-L1, programmed death ligand 1; PGAM1, phosphoglycerate translocase 1; PMN, pre-metastatic niche; SIRT1, Sirtuin 1; SFRP1, secreted frizzled-related protein 1; SCCA, squamous cell carcinoma antigen; SCLC, small cell lung cancer; TAMs, tumor-associated macrophages; TME, tumor microenvironment; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Abedi Gaballu F, Abbaspour-Ravasjani S, Mansoori B, Yekta R, Hamishehkar H, Mohammadi A, et al. Comparative of in-vitro Evaluation between Erlotinib Loaded Nanostructured Lipid Carriers and Liposomes against A549 Lung Cancer Cell Line. Iran J Pharm Res. (2019) 18:1168–79. doi: 10.22037/ijpr.2019.1100775
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
5. Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond). (2022) 42:937–70. doi: 10.1002/cac2.v42.10
6. Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin. (2023) 73:620–52. doi: 10.3322/caac.21785
7. Wathoni N, Puluhulawa LE, Joni IM, Muchtaridi M, Mohammed AFA, Elamin KM, et al. Monoclonal antibody as a targeting mediator for nanoparticle targeted delivery system for lung cancer. Drug Deliv. (2022) 29:2959–70. doi: 10.1080/10717544.2022.2120566
8. Sharma R. Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int J Clin Oncol. (2022) 27:665–75. doi: 10.1007/s10147-021-02108-2
9. Bai S, Wang Z, Wang M, Li J, Wei Y, Xu R, et al. Tumor-derived exosomes modulate primary site tumor metastasis. Front Cell Dev Biol. (2022) 10:752818. doi: 10.3389/fcell.2022.752818
10. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. (2017) 17:302–17. doi: 10.1038/nrc.2017.6
11. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553:446–54. doi: 10.1038/nature25183
12. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
13. Kfoury Y, Baryawno N, Severe N, Mei S, Gustafsson K, Hirz T, et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell. (2021) 39:1464–78.e8. doi: 10.1016/j.ccell.2021.09.005
14. Dai J, Cimino PJ, Gouin KH 3rd, Grzelak CA, Barrett A, Lim AR, et al. Astrocytic laminin-211 drives disseminated breast tumor cell dormancy in brain. Nat Cancer. (2022) 3:25–42. doi: 10.1038/s43018-021-00297-3
15. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. (1989) 8:98–101.
16. Blasco MT, Espuny I, Gomis RR. Ecology and evolution of dormant metastasis. Trends Cancer. (2022) 8:570–82. doi: 10.1016/j.trecan.2022.03.002
17. Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol. (2010) 28:5311–20. doi: 10.1200/JCO.2010.28.8126
18. Gu Y, Liu Y, Fu L, Zhai L, Zhu J, Han Y, et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med. (2019) 25:312–22. doi: 10.1038/s41591-018-0309-y
19. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. (2005) 438:820–7. doi: 10.1038/nature04186
20. Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. (2016) 30:668–81. doi: 10.1016/j.ccell.2016.09.011
21. Yan Y, Du C, Duan X, Yao X, Wan J, Jiang Z, et al. Inhibiting collagen I production and tumor cell colonization in the lung via miR-29a-3p loading of exosome-/liposome-based nanovesicles. Acta Pharm Sin B. (2022) 12:939–51. doi: 10.1016/j.apsb.2021.08.011
22. Qi M, Xia Y, Wu Y, Zhang Z, Wang X, Lu L, et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat Commun. (2022) 13:897. doi: 10.1038/s41467-022-28438-x
23. Ma Z, Wei K, Yang F, Guo Z, Pan C, He Y, et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis. (2021) 12:840. doi: 10.1038/s41419-021-04037-4
24. Wang Y, Li Y, Zhong J, Li M, Zhou Y, Lin Q, et al. Tumor-derived Cav-1 promotes pre-metastatic niche formation and lung metastasis in breast cancer. Theranostics. (2023) 13:1684–97. doi: 10.7150/thno.79250
25. Zhou L, Lv T, Zhang Q, Zhu Q, Zhan P, Zhu S, et al. The biology, function and clinical implications of exosomes in lung cancer. Cancer Lett. (2017) 407:84–92. doi: 10.1016/j.canlet.2017.08.003
26. Lobb RJ, Lima LG, Möller A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Semin Cell Dev Biol. (2017) 67:3–10. doi: 10.1016/j.semcdb.2017.01.004
27. Liu C, Liu D, Wang S, Gan L, Yang X, Ma C. Identification of the SNARE complex that mediates the fusion of multivesicular bodies with the plasma membrane in exosome secretion. J Extracell Vesicles. (2023) 12:e12356. doi: 10.1002/jev2.12356
28. Roy S, Lin HY, Chou CY, Huang CH, Small J, Sadik N, et al. Navigating the landscape of tumor extracellular vesicle heterogeneity. Int J Mol Sci. (2019) 20:1349. doi: 10.3390/ijms20061349
29. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. (2013) 200:373–83. doi: 10.1083/jcb.201211138
30. Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. (2010) 9:197–208. doi: 10.1074/mcp.M900152-MCP200
31. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
32. Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. (2012) 14:1036–45. doi: 10.1038/ncb2574
33. Cheng W, Wang K, Zhao Z, Mao Q, Wang G, Li Q, et al. Exosomes-mediated transfer of miR-125a/b in cell-to-cell communication: A novel mechanism of genetic exchange in the intestinal microenvironment. Theranostics. (2020) 10:7561–80. doi: 10.7150/thno.41802
34. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. (2013) 495:333–8. doi: 10.1038/nature11928
35. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. (2011) 43:904–14. doi: 10.1016/j.molcel.2011.08.018
36. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. (2018) 9:5395. doi: 10.1038/s41467-018-07810-w
37. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. (2019) 1871:455–68. doi: 10.1016/j.bbcan.2019.04.004
38. Grigoryeva ES, Tashireva LA, Savelieva OE, Zavyalova MV, Popova NO, Kuznetsov GA, et al. The association of integrins β3, β4, and αVβ5 on exosomes, CTCs and tumor cells with localization of distant metastasis in breast cancer patients. Int J Mol Sci. (2023) 24:2929. doi: 10.3390/ijms24032929
39. Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. (2011) 21:139–46. doi: 10.1016/j.semcancer.2011.01.002
40. Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. (2016) 35:75–91. doi: 10.1007/s10555-016-9618-0
41. Jiang C, Zhang N, Hu X, Wang H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol Cancer. (2021) 20:117. doi: 10.1186/s12943-021-01411-w
42. Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. (2010) 70:5649–69. doi: 10.1158/0008-5472.CAN-10-1040
43. Xu ZH, Miao ZW, Jiang QZ, Gan DX, Wei XG, Xue XZ, et al. Brain microvascular endothelial cell exosome-mediated S100A16 up-regulation confers small-cell lung cancer cell survival in brain. FASEB J. (2019) 33:1742–57. doi: 10.1096/fj.201800428R
44. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. (2014) 25 Suppl 3:iii124–37. doi: 10.1093/annonc/mdu103
45. Huang QY, Liu GF, Qian XL, Tang LB, Huang QY, Xiong LX. Long non-coding RNA: dual effects on breast cancer metastasis and clinical applications. Cancers (Basel). (2019) 11:1802. doi: 10.3390/cancers11111802
46. Wang D, Wang S, Jin M, Zuo Y, Wang J, Niu Y, et al. Hypoxic exosomal circPLEKHM1-mediated crosstalk between tumor cells and macrophages drives lung cancer metastasis. Adv Sci (Weinh). (2024) 11:e2309857. doi: 10.1002/advs.202309857
47. Rama N, Monteiro A, Bernardo JE, Eugénio L, Antunes MJ. Lung metastases from colorectal cancer: surgical resection and prognostic factors. Eur J Cardiothorac Surg. (2009) 35:444–9. doi: 10.1016/j.ejcts.2008.10.047
48. Zhou J, Wu Y, Xie M, Fang Y, Zhao J, Lee SY, et al. The clinical outcome and risk factors analysis of immune checkpoint inhibitor-based treatment in lung adenocarcinoma patients with brain metastases. Transl Lung Cancer Res. (2022) 11:656–69. doi: 10.21037/tlcr-22-260
49. Jiang J, Lu Y, Chu J, Zhang X, Xu C, Liu S, et al. Anti-EGFR ScFv functionalized exosomes delivering LPCAT1 specific siRNAs for inhibition of lung cancer brain metastases. J Nanobiotechnol. (2024) 22:159. doi: 10.1186/s12951-024-02414-7
50. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. (2005) 23:6207–19. doi: 10.1200/JCO.2005.03.145
51. Ye L, Wu Y, Zhou J, Xie M, Zhang Z, Su C. Influence of exosomes on astrocytes in the pre-metastatic niche of lung cancer brain metastases. Biol Proced Online. (2023) 25:5. doi: 10.1186/s12575-023-00192-4
52. Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discovery. (2021) 20:362–83. doi: 10.1038/s41573-021-00139-y
53. Xu Q, Ye L, Huang L, Zhou L, Chen X, Ye M, et al. Serum exosomal miRNA might be a novel liquid biopsy to identify leptomeningeal metastasis in non-small cell lung cancer. Onco Targets Ther. (2021) 14:2327–35. doi: 10.2147/OTT.S291611
54. Wei L, Wang G, Yang C, Zhang Y, Chen Y, Zhong C, et al. MicroRNA-550a-3-5p controls the brain metastasis of lung cancer by directly targeting YAP1. Cancer Cell Int. (2021) 21:491. doi: 10.1186/s12935-021-02197-z
55. Zhu Q, Zhang Y, Li M, Zhang Y, Zhang H, Chen J, et al. MiR-124-3p impedes the metastasis of non-small cell lung cancer via extracellular exosome transport and intracellular PI3K/AKT signaling. biomark Res. (2023) 11:1. doi: 10.1186/s40364-022-00441-w
56. Wei C, Dong X, Lu H, Tong F, Chen L, Zhang R, et al. LPCAT1 promotes brain metastasis of lung adenocarcinoma by up-regulating PI3K/AKT/MYC pathway. J Exp Clin Cancer Res. (2019) 38:95. doi: 10.1186/s13046-019-1092-4
57. Geng S, Tu S, Bai Z, Geng Y. Exosomal lncRNA LINC01356 derived from brain metastatic nonsmall-cell lung cancer cells remodels the blood-brain barrier. Front Oncol. (2022) 12:825899. doi: 10.3389/fonc.2022.825899
58. Li S, Qu Y, Liu L, Zhang X, He Y, Wang C, et al. Comparative proteomic profiling of plasma exosomes in lung cancer cases of liver and brain metastasis. Cell Biosci. (2023) 13:180. doi: 10.1186/s13578-023-01112-5
59. Tyagi A, Wu SY, Sharma S, Wu K, Zhao D, Deshpande R, et al. Exosomal miR-4466 from nicotine-activated neutrophils promotes tumor cell stemness and metabolism in lung cancer metastasis. Oncogene. (2022) 41:3079–92. doi: 10.1038/s41388-022-02322-w
60. Yu HY, Guo HQ, Feng Y, Cheng W, Wang Y. Exosomes from PM (2.5)-treated human bronchial epithelial cells increase lung cancer metastatic potential. BioMed Environ Sci. (2022) 35:473–84. doi: 10.3967/bes2022.066
61. Xu J, Feng X, Yin N, Wang L, Xie Y, Gao Y, et al. Exosomes from cisplatin-induced dormant cancer cells facilitate the formation of premetastatic niche in bone marrow through activating glycolysis of BMSCs. Front Oncol. (2022) 12:922465. doi: 10.3389/fonc.2022.922465
62. Wang H, Zhang Y, Zhu H, Yu J. Risk factors for bone metastasis in completely resected non-small-cell lung cancer. Future Oncol. (2017) 13:695–704. doi: 10.2217/fon-2016-0237
63. Miller RE, Jones JC, Tometsko M, Blake ML, Dougall WC. RANKL inhibition blocks osteolytic lesions and reduces skeletal tumor burden in models of non-small-cell lung cancer bone metastases. J Thorac Oncol. (2014) 9:345–54. doi: 10.1097/JTO.0000000000000070
64. Yang Q, Wang W, Cheng D, Wang Y, Han Y, Huang J, et al. Non-coding RNA in exosomes: Regulating bone metastasis of lung cancer and its clinical application prospect. Transl Oncol. (2024) 46:102002. doi: 10.1016/j.tranon.2024.102002
65. Ni J, Zhang X, Li J, Zheng Z, Zhang J, Zhao W, et al. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis. (2021) 12:662. doi: 10.1038/s41419-021-03928-w
66. Zhang J, Wu J. The potential roles of exosomal miR-214 in bone metastasis of lung adenocarcinoma. Front Oncol. (2020) 10:611054. doi: 10.3389/fonc.2020.611054
67. Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. (2014) 106:djt356. doi: 10.1093/jnci/djt356
68. Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol. (2018) 69:185-96. doi: 10.26402/jpp.2018.2.07
69. Yang XR, Pi C, Yu R, Fan XJ, Peng XX, Zhang XC, et al. Correlation of exosomal microRNA clusters with bone metastasis in non-small cell lung cancer. Clin Exp Metastasis. (2021) 38:109–17. doi: 10.1007/s10585-020-10062-y
70. Liu B, Zhang R, Zhu Y, Hao R. Exosome-derived microRNA-433 inhibits tumorigenesis through incremental infiltration of CD4 and CD8 cells in non-small cell lung cancer. Oncol Lett. (2021) 22:607. doi: 10.3892/ol.2021.12868
71. Xie H, Wang J. MicroRNA-320a-containing exosomes from human umbilical cord mesenchymal stem cells curtail proliferation and metastasis in lung cancer by binding to SOX4. J Recept Signal Transduct Res. (2022) 42:268–78. doi: 10.1080/10799893.2021.1918166
72. Xiong H, Liu B, Liu XY, Xia ZK, Lu M, Hu CH, et al. circ_rac GTPase-activating protein 1 facilitates stemness and metastasis of non-small cell lung cancer via polypyrimidine tract-binding protein 1 recruitment to promote sirtuin-3-mediated replication timing regulatory factor 1 deacetylation. Lab Invest. (2023) 103:100010. doi: 10.1016/j.labinv.2022.100010
73. Wang M, Zhao M, Guo Q, Lou J, Wang L. Non-small cell lung cancer cell-derived exosomal miR-17-5p promotes osteoclast differentiation by targeting PTEN. Exp Cell Res. (2021) 408:112834. doi: 10.1016/j.yexcr.2021.112834
74. Ullah I, Karthik GM, Alkodsi A, Kjällquist U, Stålhammar G, Lövrot J, et al. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J Clin Invest. (2018) 128:1355–70. doi: 10.1172/JCI96149
75. Gao D, Du J, Cong L, Liu Q. Risk factors for initial lung metastasis from breast invasive ductal carcinoma in stages I-III of operable patients. Jpn J Clin Oncol. (2009) 39:97–104. doi: 10.1093/jjco/hyn133
76. Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, et al. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. (2017) 36:639–51. doi: 10.1038/onc.2016.229
77. Li C, Yoshimura T, Tian M, Wang Y, Kondo T, Yamamoto KI, et al. Exosomal Wnt7a from a low metastatic subclone promotes lung metastasis of a highly metastatic subclone in the murine 4t1 breast cancer. Breast Cancer Res. (2022) 24:60. doi: 10.1186/s13058-022-01557-5
78. Liu Y, Tang J, Qiu X, Teng LA, Sriwastva MK, Han X, et al. Rab1A-mediated exosomal sorting of miR-200c enhances breast cancer lung metastasis. Breast Cancer (Dove Med Press). (2023) 15:403–19. doi: 10.2147/BCTT.S400974
79. Feng T, Zhang P, Sun Y, Wang Y, Tong J, Dai H, et al. High throughput sequencing identifies breast cancer-secreted exosomal LncRNAs initiating pulmonary pre-metastatic niche formation. Gene. (2019) 710:258–64. doi: 10.1016/j.gene.2019.06.004
80. Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes Malignant growth. Nat Cell Biol. (2015) 17:170–82. doi: 10.1038/ncb3090
81. Cheng Z, Wang L, Wu C, Huang L, Ruan Y, Xue W. Tumor-derived exosomes induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through tim-3. Arch Med Res. (2021) 52:200–10. doi: 10.1016/j.arcmed.2020.10.018
82. Wang Z, Zhang C, Guo J, Wang W, Si Q, Chen C, et al. Exosomal miRNA-223-3p derived from tumor associated macrophages promotes pulmonary metastasis of breast cancer 4T1 cells. Transl Oncol. (2023) 35:101715. doi: 10.1016/j.tranon.2023.101715
83. Isaoff MS, Beilack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. (2015) 33:3029–35. doi: 10.1200/JCO.2014.59.4895
84. Whelan JS, Davis LE. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol. (2018) 36:188–93. doi: 10.1200/JCO.2017.75.1743
85. Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. (2006) 125:555–81. doi: 10.1309/UC6KQHLD9LV2KENN
86. Zheng B, Ren T, Huang Y, Sun K, Wang S, Bao X, et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J Hematol Oncol. (2018) 11:16. doi: 10.1186/s13045-018-0560-1
87. Wang J, Guo W, Wang X, Tang X, Sun X, Ren T. Circulating exosomal PD-L1 at initial diagnosis predicts outcome and survival of patients with osteosarcoma. Clin Cancer Res. (2023) 29:659–66. doi: 10.1158/1078-0432.CCR-22-2682
88. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. (2018) 560:382–6. doi: 10.1038/s41586-018-0392-8
89. Wang J, Zhang H, Sun X, Wang X, Ren T, Huang Y, et al. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J Nanobiotechnol. (2020) 18:151. doi: 10.1186/s12951-020-00710-6
90. Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. (2021) 33:2040–58.e10. doi: 10.1016/j.cmet.2021.09.002
91. Dumars C, Ngyuen JM, Gaultier A, Lanel R, Corradini N, Gouin F, et al. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget. (2016) 7:78343–54. doi: 10.18632/oncotarget.13055
92. Zhang Y, Liu Z, Yang X, Lu W, Chen Y, Lin Y, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. (2021) 11:1473–92. doi: 10.7150/thno.51245
93. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. doi: 10.1038/nri2448
94. Feng W, Dean DC, Hornicek FJ, Shi H, Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol Cancer. (2019) 18:124. doi: 10.1186/s12943-019-1049-4
95. Song CY, Wu CY, Lin CY, Tsai CH, Chen HT, Fong YC, et al. The stimulation of exosome generation by visfatin polarizes M2 macrophages and enhances the motility of chondrosarcoma. Environ Toxicol. (2024) 39:3790–8. doi: 10.1002/tox.24236
96. Griffin KH, Mizenko RR, Arun V, Carney RP, Leach JK. Extracellular vesicles from highly metastatic osteosarcoma cells induce pro-tumorigenic macrophage phenotypes. Adv Biol (Weinh). (2024) 8:e2300577. doi: 10.1002/adbi.202300577
97. Zhong L, Liao D, Li J, Liu W, Wang J, Zeng C, et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. (2021) 6:59. doi: 10.1038/s41392-020-00414-1
98. van de Velde CJ. Surgery: palliative primary tumour resection in mCRC-debate continues. Nat Rev Clin Oncol. (2015) 12:129–30. doi: 10.1038/nrclinonc.2015.7
99. Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. (2014) 26:707–21. doi: 10.1016/j.ccell.2014.09.005
100. Wang J, Liu Y, Li Y, Zheng X, Gan J, Wan Z, et al. Exosomal−miR−10a derived from colorectal cancer cells suppresses migration of human lung fibroblasts, and expression of IL−6, IL−8 and IL−1β. Mol Med Rep. (2021) 23:84. doi: 10.3892/mmr.2020.11723
101. Wang M, Yang X, Wei M, Wang Z. The role of CXCL12 axis in lung metastasis of colorectal cancer. J Cancer. (2018) 9:3898–903. doi: 10.7150/jca.26383
102. Han K, Wang FW, Cao CH, Ling H, Chen JW, Chen RX, et al. CircLONP2 enhances colorectal carcinoma invasion and metastasis through modulating the maturation and exosomal dissemination of microRNA-17. Mol Cancer. (2020) 19:60. doi: 10.1186/s12943-020-01184-8
103. Wu L, Xue M, Lai S, Chen J, Lin Y, Ding N, et al. Hypoxia derived exosomes promote the proliferation and metastasis of colorectal cancer through the regulation of HIF-1α/miR-4299/ZBTB4. Life Sci. (2023) 329:121872. doi: 10.1016/j.lfs.2023.121872
104. Zhou S, Lan Y, Li Y, Li Z, Pu J, Wei L. Hypoxic tumor-derived exosomes induce M2 macrophage polarization via PKM2/AMPK to promote lung cancer progression. Cell Transplant. (2022) 31:9636897221106998. doi: 10.1177/09636897221106998
105. Kim DH, Park H, Choi YJ, Kang MH, Kim TK, Pack CG, et al. Exosomal miR-1260b derived from non-small cell lung cancer promotes tumor metastasis through the inhibition of HIPK2. Cell Death Dis. (2021) 12:747. doi: 10.1038/s41419-021-04024-9
106. Janning M, Loges S. Anti-angiogenics: their value in lung cancer therapy. Oncol Res Treat. (2018) 41:172–80. doi: 10.1159/000488119
107. Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. (2018) 20:597–609. doi: 10.1038/s41556-018-0083-6
108. Chang RM, Fu Y, Zeng J, Zhu XY, Gao Y. Cancer-derived exosomal miR-197-3p confers angiogenesis via targeting TIMP2/3 in lung adenocarcinoma metastasis. Cell Death Dis. (2022) 13:1032. doi: 10.1038/s41419-022-05420-5
109. Luo JQ, Yang TW, Wu J, Lai HH, Zou LB, Chen WB, et al. Exosomal PGAM1 promotes prostate cancer angiogenesis and metastasis by interacting with ACTG1. Cell Death Dis. (2023) 14:502. doi: 10.1038/s41419-023-06007-4
110. Conway EM, Pikor LA, Kung SH, Hamilton MJ, Lam S, Lam WL, et al. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. (2016) 193:116–30. doi: 10.1164/rccm.201508-1545CI
111. Ge Z, Ding S. The crosstalk between tumor-associated macrophages (TAMs) and tumor cells and the corresponding targeted therapy. Front Oncol. (2020) 10:590941. doi: 10.3389/fonc.2020.590941
112. Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. (2017) 10:58. doi: 10.1186/s13045-017-0430-2
113. Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. (2016) 74:103–41. doi: 10.1016/bs.acc.2015.12.005
114. Liang M, Chen X, Wang L, Qin L, Wang H, Sun Z, et al. Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res. (2020) 39:176. doi: 10.1186/s13046-020-01688-7
115. Rao X, Zhou X, Wang G, Jie X, Xing B, Xu Y, et al. NLRP6 is required for cancer-derived exosome-modified macrophage M2 polarization and promotes metastasis in small cell lung cancer. Cell Death Dis. (2022) 13:891. doi: 10.1038/s41419-022-05336-0
116. Paul S, Ghosh S, Kumar S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin Cancer Biol. (2022) 86:1216–30. doi: 10.1016/j.semcancer.2022.09.007
117. Ren J, Zhu B, Gu G, Zhang W, Li J, Wang H, et al. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. (2023) 14:70. doi: 10.1038/s41419-023-05607-4
118. Chen J, Zhang K, Zhi Y, Wu Y, Chen B, Bai J, et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin Transl Med. (2021) 11:e478. doi: 10.1002/ctm2.v11.9
119. Chen Y, Hong C, Qu J, Chen J, Qin Z. Knockdown of lncRNA PCAT6 suppresses the growth of non-small cell lung cancer cells by inhibiting macrophages M2 polarization via miR-326/KLF1 axis. Bioengineered. (2022) 13:12834–46. doi: 10.1080/21655979.2022.2076388
120. Hu R, Xu B, Ma J, Li L, Zhang L, Wang L, et al. LINC00963 promotes the Malignancy and metastasis of lung adenocarcinoma by stabilizing Zeb1 and exosomes-induced M2 macrophage polarization. Mol Med. (2023) 29:1. doi: 10.1186/s10020-022-00598-y
121. Liu J, Luo R, Wang J, Luan X, Wu D, Chen H, et al. Tumor Cell-Derived Exosomal miR-770 Inhibits M2 Macrophage Polarization via Targeting MAP3K1 to Inhibit the Invasion of Non-small Cell Lung Cancer Cells. Front Cell Dev Biol. (2021) 9:679658. doi: 10.3389/fcell.2021.679658
122. Chen M, Cao C, Ma J. Tumor-related exosomal circ_0001715 promotes lung adenocarcinoma cell proliferation and metastasis via enhancing M2 macrophage polarization by regulating triggering receptor expressed on myeloid cells-2. Thorac Cancer. (2024) 15:227–38. doi: 10.1111/1759-7714.15182
123. Wang L, Shen K, Gao Z, Ren M, Wei C, Yang Y, et al. Melanoma derived exosomes amplify radiotherapy induced abscopal effect via IRF7/I-IFN axis in macrophages. Adv Sci (Weinh). (2024) 11:e2304991. doi: 10.1002/advs.202304991
124. Li X, Chen Z, Ni Y, Bian C, Huang J, Chen L, et al. Tumor-associated macrophages secret exosomal miR-155 and miR-196a-5p to promote metastasis of non-small-cell lung cancer. Transl Lung Cancer Res. (2021) 10:1338–54. doi: 10.21037/tlcr-20-1255
125. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. (2014) 20:460–9. doi: 10.1016/j.molmed.2014.06.005
126. Yang X, Yu F, Huang G, Ni Y, Zhang T, Zou Z, et al. Exosomal miR-133a-3p promotes the growth and metastasis of lung cancer cells following incomplete microwave ablation. Int J Hyperthermia. (2023) 40:2190065. doi: 10.1080/02656736.2023.2190065
127. Wang J, Zhao J, Zhu J, Zhang S. Hypoxic non-small-cell lung cancer cell-secreted exosomal microRNA-582-3p drives cancer cell Malignant phenotypes by targeting secreted frizzled-related protein 1. Cancer Manag Res. (2020) 12:10151–61. doi: 10.2147/CMAR.S263768
128. Wang Z, Lin M, He L, Qi H, Shen J, Ying K. Exosomal lncRNA SCIRT/miR-665 Transferring Promotes Lung Cancer Cell Metastasis through the Inhibition of HEYL. J Oncol. (2021) 2021:9813773. doi: 10.1155/2021/9813773
129. Sun B, Han Y, Shi M. Stromal-derived miR-486-5p promotes metastasis of non-small-cell lung cancer cells by targeting the CADM1/tight junctions axis in vascular endothelial cells. Cell Biol Int. (2021) 45:849–57. doi: 10.1002/cbin.11531
130. Mao S, Zheng S, Lu Z, Wang X, Wang Y, Zhang G, et al. Exosomal miR-375-3p breaks vascular barrier and promotes small cell lung cancer metastasis by targeting claudin-1. Transl Lung Cancer Res. (2021) 10:3155–72. doi: 10.21037/tlcr-21-356
131. Xia Y, Wei K, Hu LQ, Zhou CR, Lu ZB, Zhan GS, et al. Exosome-mediated transfer of miR-1260b promotes cell invasion through Wnt/β-catenin signaling pathway in lung adenocarcinoma. J Cell Physiol. (2020) 235:6843–53. doi: 10.1002/jcp.v235.10
132. Wang L, He J, Hu H, Tu L, Sun Z, Liu Y, et al. Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. J Cell Mol Med. (2020) 24:6324–39. doi: 10.1111/jcmm.v24.11
133. Liang Y, Xu X, Wang T, Li Y, You W, Fu J, et al. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. (2017) 8:e2928. doi: 10.1038/cddis.2017.325
134. Lv M, Li X, Zheng C, Tian W, Yang H, Yin Z, et al. Exosomal miR-130b-3p suppresses metastasis of non-small cell lung cancer cells by targeting DEPDC1 via TGF-β signaling pathway. Int J Biol Macromol. (2024) 275:133594. doi: 10.1016/j.ijbiomac.2024.133594
135. Chen T, Liu Y, Chen J, Zheng H, Chen Q, Zhao J. Exosomal miR-3180-3p inhibits proliferation and metastasis of non-small cell lung cancer by downregulating FOXP4. Thorac Cancer. (2021) 12:372–81. doi: 10.1111/1759-7714.13759
136. Wysocka MB, Pietraszek-Gremplewicz K, Nowak D. The role of apelin in cardiovascular diseases, obesity and cancer. Front Physiol. (2018) 9:557. doi: 10.3389/fphys.2018.00557
137. Ran J, Li Y, Liu L, Zhu Y, Ni Y, Huang H, et al. Apelin enhances biological functions in lung cancer A549 cells by downregulating exosomal miR-15a-5p. Carcinogenesis. (2021) 42:243–53. doi: 10.1093/carcin/bgaa089
138. Tian W, Yang X, Yang H, Lv M, Sun X, Zhou B. Exosomal miR-338-3p suppresses non-small-cell lung cancer cells metastasis by inhibiting CHL1 through the MAPK signaling pathway. Cell Death Dis. (2021) 12:1030. doi: 10.1038/s41419-021-04314-2
139. Jiang F, Huang X, Ling L, Tang S, Zhou H, Cai X, et al. Long noncoding RNA ZBED5-AS1 facilitates tumor progression and metastasis in lung adenocarcinoma via ZNF146/ATR/chk1 axis. Int J Mol Sci. (2023) 24:13925. doi: 10.3390/ijms241813925
140. Lv J, Li Q, Ma R, Wang Z, Yu Y, Liu H, et al. Long noncoding RNA FGD5-AS1 knockdown decrease viability, migration, and invasion of non-small cell lung cancer (NSCLC) cells by regulating the microRNA-944/MACC1 axis. Technol Cancer Res Treat. (2021) 20:1533033821990090. doi: 10.1177/1533033821990090
141. Chen L, Huang S, Huang J, Chen Q, Zhuang Q. Role and mechanism of exosome-derived long noncoding RNA HOTAIR in lung cancer. ACS Omega. (2021) 6:17217–27. doi: 10.1021/acsomega.1c00905
142. Zhang N, Nan A, Chen L, Li X, Jia Y, Qiu M, et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. (2020) 19:101. doi: 10.1186/s12943-020-01221-6
143. Wang H, Tang Z, Duan J, Zhou C, Xu K, Mu H. Cancer-released exosomal circular RNA circ_0008717 promotes cell tumorigenicity through microRNA-1287-5p/P21-activated kinase 2 (PAK2) axis in non-small cell lung cancer. Bioengineered. (2022) 13:8937–49. doi: 10.1080/21655979.2022.2056822
144. Zhou H, Huang X, Yang X, Jiang F, Shao F, Shi W, et al. CircRAPGEF5 Promotes the Proliferation and Metastasis of Lung Adenocarcinoma through the miR-1236-3p/ZEB1 Axis and Serves as a Potential Biomarker. Int J Biol Sci. (2022) 18:2116–31. doi: 10.7150/ijbs.66770
145. Wu H, Zeng C, Wu G, Fang F, Xiao C, Li L, et al. Exosomal LRG1 promotes non-small cell lung cancer proliferation and metastasis by binding FN1 protein. Exp Cell Res. (2024) 439:114097. doi: 10.1016/j.yexcr.2024.114097
146. An T, Qin S, Xu Y, Tang Y, Huang Y, Situ B, et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracell Vesicles. (2015) 4:27522. doi: 10.3402/jev.v4.27522
147. Pi YN, Xia BR, Jin MZ, Jin WL, Lou G. Exosomes: Powerful weapon for cancer nano-immunoengineering. Biochem Pharmacol. (2021) 186:114487. doi: 10.1016/j.bcp.2021.114487
148. Grimaldi A, Zarone MR, Irace C, Zappavigna S, Lombardi A, Kawasaki H, et al. Non-coding RNAs as a new dawn in tumor diagnosis. Semin Cell Dev Biol. (2018) 78:37–50. doi: 10.1016/j.semcdb.2017.07.035
149. Yang SJ, Wang DD, Li J, Xu HZ, Shen HY, Chen X, et al. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene. (2017) 623:5–14. doi: 10.1016/j.gene.2017.04.031
150. Sonbhadra S, Mehak, Pandey LM. Biogenesis, isolation, and detection of exosomes and their potential in therapeutics and diagnostics. Biosensors (Basel). (2023) 13:802. doi: 10.3390/bios13080802
151. Fortunato O, Gasparini P, Boeri M, Sozzi G. Exo-miRNAs as a new tool for liquid biopsy in lung cancer. Cancers (Basel). (2019) 11:888. doi: 10.3390/cancers11060888
152. Li J, Wang J, Chen Y, Li S, Jin M, Wang H, et al. LncRNA MALAT1 exerts oncogenic functions in lung adenocarcinoma by targeting miR-204. Am J Cancer Res. (2016) 6:1099–107.
153. Pich O, Bailey C, Watkins TBK, Zaccaria S, Jamal-Hanjani M, Swanton C. The translational challenges of precision oncology. Cancer Cell. (2022) 40:458–78. doi: 10.1016/j.ccell.2022.04.002
154. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:EAAU6977. doi: 10.1126/science.aau6977
155. Zhang Z, Tang Y, Song X, Xie L, Zhao S, Song X. Tumor-derived exosomal miRNAs as diagnostic biomarkers in non-small cell lung cancer. Front Oncol. (2020) 10:560025. doi: 10.3389/fonc.2020.560025
156. Duffy MJ, O’Byrne K. Tissue and blood biomarkers in lung cancer: A review. Adv Clin Chem. (2018) 86:1–21. doi: 10.1016/bs.acc.2018.05.001
157. Tang Y, Zhang Z, Song X, Yu M, Niu L, Zhao Y, et al. Tumor-derived exosomal miR-620 as a diagnostic biomarker in non-small-cell lung cancer. J Oncol. (2020) 2020:6691211. doi: 10.1155/2020/6691211
158. Zhang X, Guo H, Bao Y, Yu H, Xie D, Wang X. Exosomal long non-coding RNA DLX6-AS1 as a potential diagnostic biomarker for non-small cell lung cancer. Oncol Lett. (2019) 18:5197–204. doi: 10.3892/ol.2019.10892
159. Tao Y, Tang Y, Yang Z, Wu F, Wang L, Yang L, et al. Exploration of serum exosomal lncRNA TBILA and AGAP2-AS1 as promising biomarkers for diagnosis of non-small cell lung cancer. Int J Biol Sci. (2020) 16:471–82. doi: 10.7150/ijbs.39123
160. Jin S, Liu T, Wang W, Li T, Liu Z, Zhang M. Lymphocyte migration regulation related proteins in urine exosomes may serve as a potential biomarker for lung cancer diagnosis. BMC Cancer. (2023) 23:1125. doi: 10.1186/s12885-023-11567-x
161. Luo B, Que Z, Lu X, Qi D, Qiao Z, Yang Y, et al. Identification of exosome protein panels as predictive biomarkers for non-small cell lung cancer. Biol Proced Online. (2023) 25:29. doi: 10.1186/s12575-023-00223-0
162. Wan L, Chen X, Deng J, Zhang S, Tu F, Pei H, et al. Plasma exosome-derived B-cell translation gene 1: a predictive marker for the prognosis in patients with non-small cell lung cancer. J Cancer. (2021) 12:1538–47. doi: 10.7150/jca.52320
163. Wu Y, Wei J, Zhang W, Xie M, Wang X, Xu J. Serum exosomal miR-1290 is a potential biomarker for lung adenocarcinoma. Onco Targets Ther. (2020) 13:7809–18. doi: 10.2147/OTT.S263934
164. Wang N, Song X, Liu L, Niu L, Wang X, Song X, et al. Circulating exosomes contain protein biomarkers of metastatic non-small-cell lung cancer. Cancer Sci. (2018) 109:1701–9. doi: 10.1111/cas.2018.109.issue-5
165. Liu W, Liu J, Zhang Q, Wei L. Downregulation of serum exosomal miR-216b predicts unfavorable prognosis in patients with non-small cell lung cancer. Cancer biomark. (2020) 27:113–20. doi: 10.3233/CBM-190914
166. Chang W, Zhu J, Yang D, Shang A, Sun Z, Quan W, et al. Plasma versican and plasma exosomal versican as potential diagnostic markers for non-small cell lung cancer. Respir Res. (2023) 24:140. doi: 10.1186/s12931-023-02423-4
Keywords: lung cancer, exosomes, metastasis, biomarkers, pre-metastatic niche
Citation: Chen H, Liu L, Xing G, Zhang D, A. N, Huang J, Li Y, Zhao G and Liu M (2025) Exosome tropism and various pathways in lung cancer metastasis. Front. Immunol. 16:1517495. doi: 10.3389/fimmu.2025.1517495
Received: 26 October 2024; Accepted: 20 January 2025;
Published: 14 February 2025.
Edited by:
Julia Schueler, Charles River Discovery Research Services GmbH, GermanyReviewed by:
Prof Dwijendra k. Gupta, Allahabad University, IndiaCopyright © 2025 Chen, Liu, Xing, Zhang, A, Huang, Li, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghua Liu, bGl1bWh2aXBAMTYzLmNvbQ==; Ge Zhao, emhhb2dlOTlAc3dtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.