- 1The First Affiliated Hospital of Guangxi University of Science and Technology, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
- 2Medicine College, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
Introduction: The combination of PD-1/PD-L1 inhibitor with CTLA-4 inhibitor for advanced non-small cell lung cancer(NSCLC) is presently a significant area of research, however its clinical application remains contentious. This meta-analysis aimed to assess the efficacy and safety of first-line PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor (CP) in the treatment of patients with advanced NSCLC.
Methods: A systemic search was conducted in four databases (PubMed, Cochrane library, Embase, and Web of Science) from their establishment until January 17, 2024, for randomized controlled trials that investigated the use of the first-line PD-1/PD-L1 inhibitor plus CTLA-4 inhibitor in patients with advanced NSCLC. Progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs) were subjected to meta-analyses.
Results: Totally 7 eligible randomized controlled trials including 4682 people were included. Two comparative analyses were performed: CP versus chemotherapy, CP versus PD-1/PD-L1 inhibitor (P). Compared with the chemotherapy group, CP improved OS (HR: 0.84, 95% CI: 0.75-0.94, p<0.05) but not PFS (HR: 0.94, 95%CI: 0.73-1.20, p = 0.63) or ORR (OR: 1.16, 95% CI: 0.79-1.71, p = 0.45). In terms of toxicity, CP had slightly fewer any AEs compared to chemotherapy (RR: 0.94, 95% CI: 0.91-0.97; p<0.05). Compared to the P group, there was no significant difference in OS (MD: -0,25, 95% CI: -2.47-1.98, p = 0.83), PFS (MD: -0.91, 95% CI: -3.19-1.36, p = 0.43), and ORR (OR:1.05, 95% CI. 0.80-1.36, p = 0.73). Subgroup analysis revealed that CP provided superior OS compared with P in patients with PD-L1 expression < 1%.
Conclusion: CP was a feasible and safe first-line therapy for patients with advanced NSCLC. Specifically, CP may function as a therapeutic alternative for individuals with low or negative PD-L1 expression, resulting in enhanced long-term outcomes compared to chemotherapy or P. Further randomized controlled trials with prolonged follow-up periods are necessary to validate these results, particularly focusing on efficacy in patients with differing PD-L1 expression levels, to improve the stratified implementation of immunotherapy.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024621116, identifier CRD42024621116.
1 Introduction
A recent publication from the World Health Organization’s International Agency for Research on Cancer indicates that lung cancer is the predominant malignant tumor globally regarding incidence and fatality rates (1). According to Cancer Statistics 2024 (2), although the incidence of lung cancer has declined in the United States, with lung cancer deaths in men down 59% from the 1990 peak and in women down 36% from the 2002 peak, lung cancer still causes far more deaths each year than colorectal, breast, and prostate cancers combined. Lung cancer is histologically categorized into small cell lung cancer and non-small cell lung cancer (NSCLC), the latter representing over 85 percent of all instances. The predominant histological subtype of NSCLC worldwide is adenocarcinoma (40%), succeeded by squamous carcinoma (25%). Due to the subtlety of early lung cancer symptoms, approximately one-third of patients are diagnosed at an advanced stage, so missing the optimal window for drastic surgical intervention. Age-standardized 5-year relative/net survival rate of lung cancer was typically low, with 10%-20% for most regions (3).
The advent of immunotherapy has introduced a novel approach for the treatment of advanced NSCLC, marking the onset of the immunotherapy era in lung cancer management. Immunotherapy for NSCLC often employs various antibodies to inhibit the identification of antigens by immune cells and ligands in tumor cells. Immune checkpoint inhibitors (ICIs) have emerged as the primary treatment for advanced and metastatic NSCLC. The predominant targets in NSCLC are cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed death receptor 1 (PD-1), and programmed death ligand 1 (PD-L1) (4). PD-1 and its ligands, PD-L1 and PD-L2, are essential regulators of immunosuppression within the local tumor microenvironment (TME). The PD-1 receptor is prominently expressed in fatigued T cells, particularly tumor-infiltrating lymphocytes. The PD-L1 receptor is abundantly expressed in all tumor cell types, while the PD-L2 receptor is only expressed on activated dendritic cells and some macrophages. PD-1/PD-L1 inhibitors can rejuvenate T lymphocytes from a dysfunctional condition by obstructing the interaction between PD-1 and PD-L1, so effectively eliminating tumor cells (5). CTLA-4 is an immunological checkpoint molecule mostly expressed on activated T cells and regulatory T (Treg) cells, which prevents T cell activation and maintains immune homeostasis. The interaction of CTLA-4 with the B7 molecule induces T cell anergy and contributes to the negative control of the immunological response. CTLA-4 inhibitor impedes T cell proliferation by obstructing the binding to B7 and disrupting the interaction between B7 and CD28. The monoclonal antibody reduces T cell proliferation by obstructing the binding to B7 and disrupting the interaction between B7 and CD28 (6).
The advancement of immunotherapy reveals that dual immunotherapy offers novel approaches for lung cancer treatment. Considering that PD-1 and CTLA-4 modulate effector T-cell activation, proliferation, and function through distinct yet complementary pathways, the application of dual immune checkpoint inhibitors (PD-1/PD-L1 and CTLA-4) is a rational approach to augment antitumor immunity (7, 8). The CheckMate227 (9) trial reported 5-year follow-up data, regardless of the patient’s PD-L1 expression level, compared with chemotherapy, nivolumab combined with ipilimumab could bring durable and long-term survival benefits. In patients with PD-L1 ≥ 1%, the 5-year OS rate of dual antibody was 24%, while it was 19% in patients with PD-L1 < 1%. It seems that the efficacy is different in patients with varying levels of PD-L1 expression. Besides, the combination of PD-1/PD-L1 and CTLA-4 for advanced non-small cell lung cancer is currently a prominent research focus, although its clinical applicability has not achieved consensus.
This meta-analysis aimed to assess the efficacy and safety of first-line PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor in the treatment of patients with advanced NSCLC. To be more specific, this meta-analysis specified the particular advantages of dual immune therapy compared to monotherapy or chemotherapy, and conducted stratified analyses on patients with different PD-L1 expression levels to explore the potential for personalized treatment.
2 Materials and methods
The present meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis standards (PRISMA). This meta-analysis has been formally registered at PROSPERO with the registration number CRD42024506196.
2.1 Search strategy
A comprehensive search was conducted in four databases: PubMed, Cochrane library, Embase, and Web of Science, covering all pertinent literature published from the library’s inception until January 17, 2024. The search was conducted using “subject + free word”, and the search terms included: “Non-Small Cell Lung Cancer”, “PD-1/L1 inhibitors” and “randomized controlled trial”. Supplementary Table S1 provided a comprehensive listing of the search results.
2.2 Selection criteria
The criteria for inclusion were as follows: (1) patients diagnosed with advanced non-small cell lung cancer (NSCLC) verified by pathology or imaging, and classified as NSCLC stage III-IV according to the TNM staging of lung cancer; (2) patients in the intervention group received PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor as their first-line treatment; (3) patients in the controlled group received other therapy as their first-line treatment; (4) at least one of the following outcomes were reported: ORR, PFS, OS, AEs, and irAEs (10). (5)Study design: randomized controlled trials (RCTs).
The criteria for exclusion were as follows: (1) other types of articles, such as case reports, publications, letters, comments, reviews, meta-analyses, editorials, animal studies, protocols, conference, etc.; (2) other types of diseases; (3) patients with EGFR or ALK genetic mutation, since tyrosine kinase inhibitors have become the first-line standard choice for these patients (11–16); (4) phase I trials; (5) single arm experiment; (6) data cannot be extracted; (7) duplicate patient cohort.
2.3 Data extraction
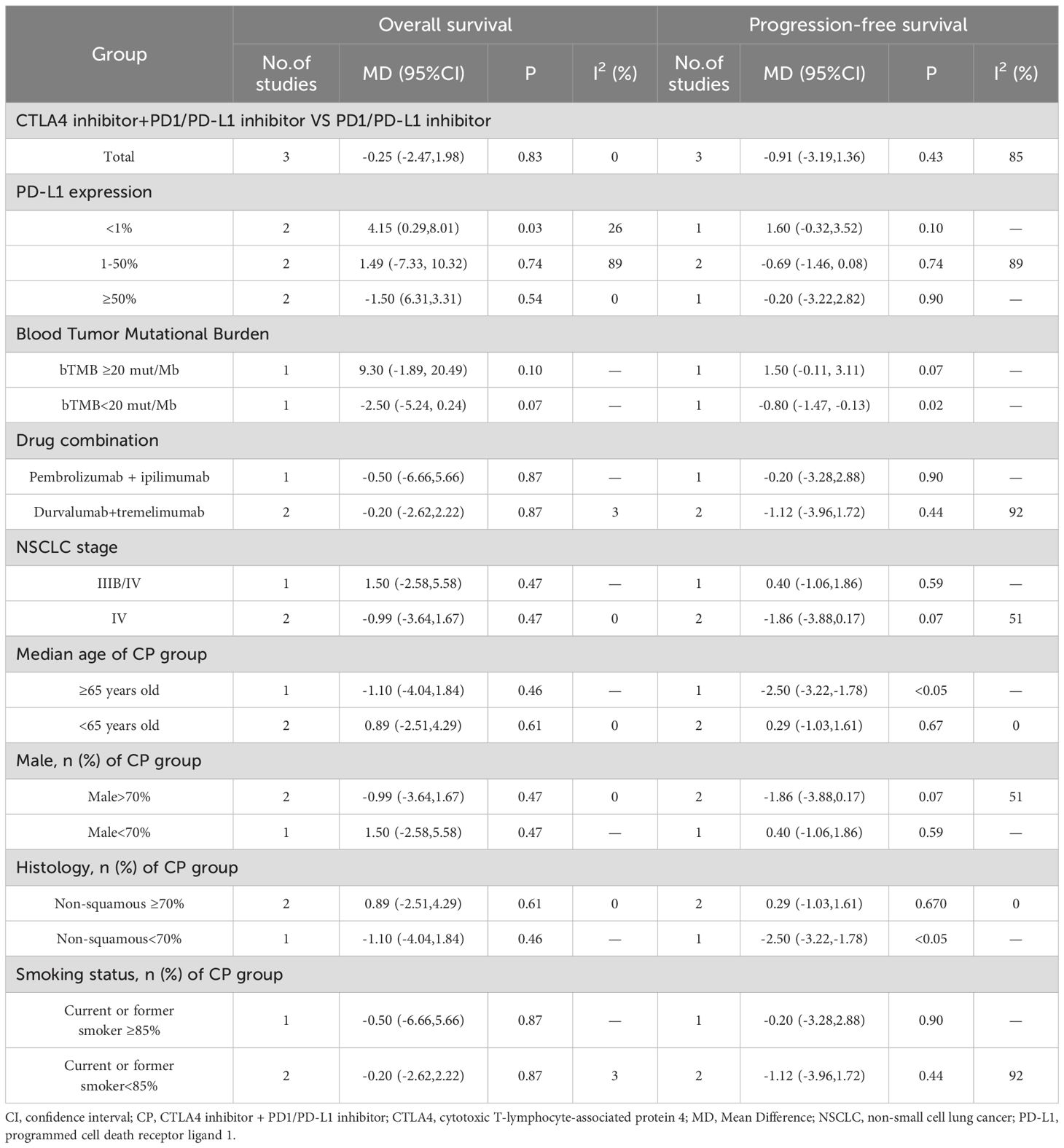
Two reviewers independently extracted the following pertinent data: first author, publication year, country, participant count, participant characteristics (median age, gender, stage of NSCLC, and performance status), treatment arm. The measures of antitumor effectiveness (OS, PFS and ORR) and toxicity (adverse events) were obtained. Any disagreements were settled by another reviewer. A subgroup analysis based on PD-L1 expression was performed. Patients were categorized into three groups: negative (<1%), low-positive (1-50%), and high-positive (>50%). Besides, analyses were performed based on blood tumor mutational burden (TMB), medication combinations, non-small cell lung cancer stage, median age, gender, histology and smoking status.
2.4 Quality assessment
The quality of the 7 included trials was evaluated using the risk of bias assessment tool implemented by the Cochrane Collaboration (17). The evaluation included the following seven crucial areas: (1) technique of randomization; (2) concealment of the allocation scheme; (3) blinding of participants and administrators of the treatment protocol; (4) blinding of outcome measurements; (5) completeness of outcome data; (6) selective reporting of research outcomes; and (7) additional sources of bias. All studies were assessed to be at risk in each of these seven domains. Upon examination according to the aforementioned criteria, each study was classified into high-quality, medium-quality, and low-quality categories based on the evaluation results.
2.5 Statistical analysis
The meta-analysis was conducted using Review Manager (Revman5.3) and Stata (version 17.0) for data analysis. The effect size for OS and PFS was identified as HR (18) or MD, the effect size for ORR was identified as OR, while the effect size for incidence of adverse events, and AEs was identified as RR. The effect sizes were visualized using forest plots. The heterogeneity test was evaluated using the Q test (19) (Q test, Chi square test), and quantitatively determined using I2. For I² values less than 50%, a fixed effect model was employed, whereas for I² values more than 50%, a random effect model was included in the study. When there is heterogeneity in the results, in order to analyze its source, subgroup analysis can be carried out, divided into two subgroups according to the type of study, when the two groups are homogeneous within the group, and there is heterogeneity in the results after combining them, it indicates that the type of study may be the source of the heterogeneity; or sensitivity analysis is carried out, adopting article-by-article elimination method, which is carried out by deleting the studies one by one to observe whether the heterogeneity is changed or not, and if the results are consistent before and after, it indicates that the results of performing if the results are consistent, it indicates that the results of the random effects calculation are stable and reliable. Discrepancies in the results of the sensitivity analysis suggest that the findings lack robustness and should be interpreted with caution. To evaluate the existence of publication bias, a funnel plot was constructed. The presence of symmetrical two sides in the funnel plot suggests the absence of evident publishing bias. Conversely, asymmetrical two sides of the funnel plot hint the potential existence of publication bias. Statistical significance was declared when the p-value was less than 0.05.
3 Results
3.1 Search results and study quality assessment
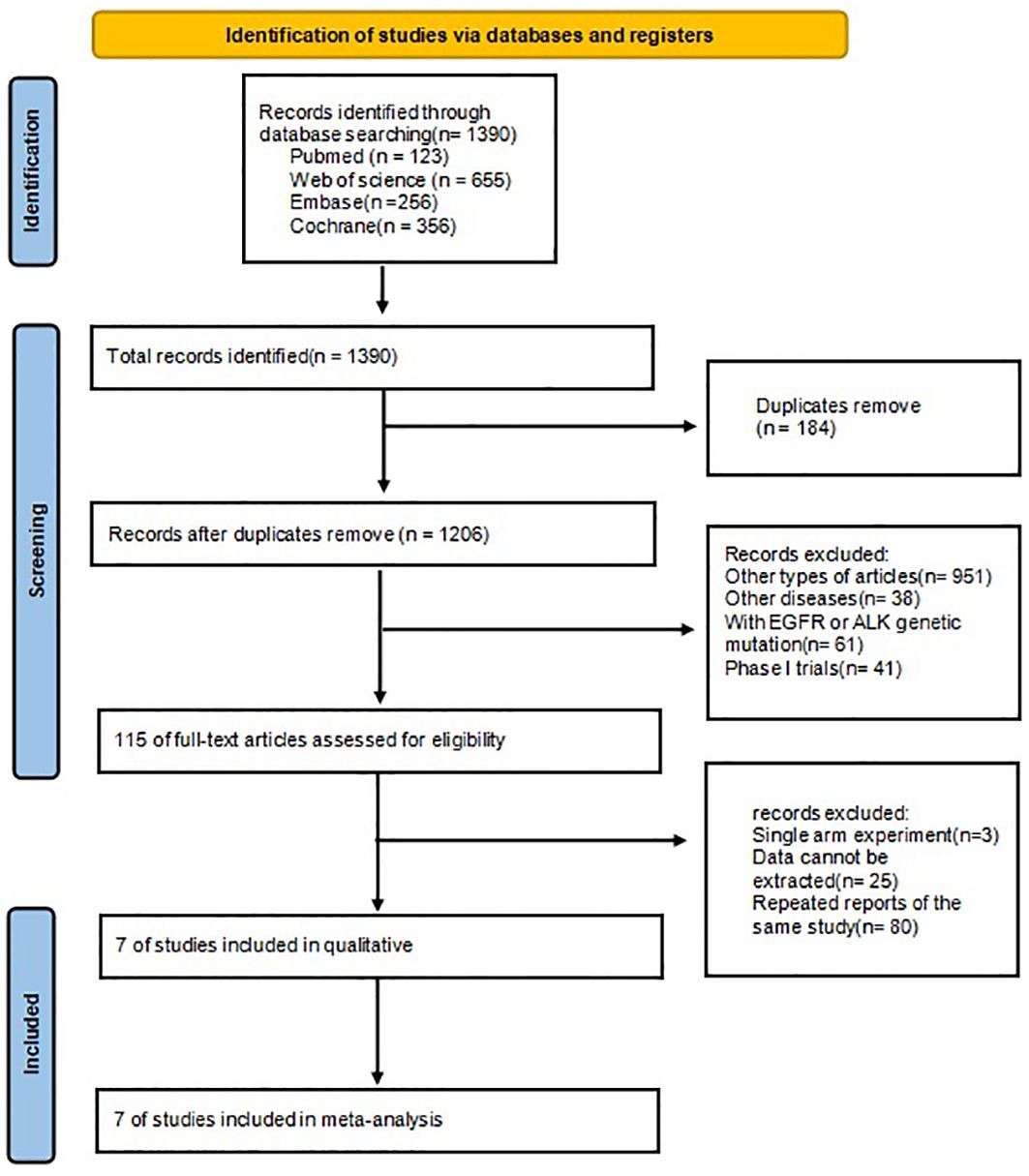
The procedure for selecting and integrating the literature is shown in Figure 1. A total of 1390 records were initially identified. After eliminating duplicate studies, a total of 1206 papers remained. Based on the assessment of titles and abstracts, a total of 115 papers were eliminated. After a thorough review of the full text, 7 studies were selected for inclusion in this meta-analysis.
3.2 Patient characteristics
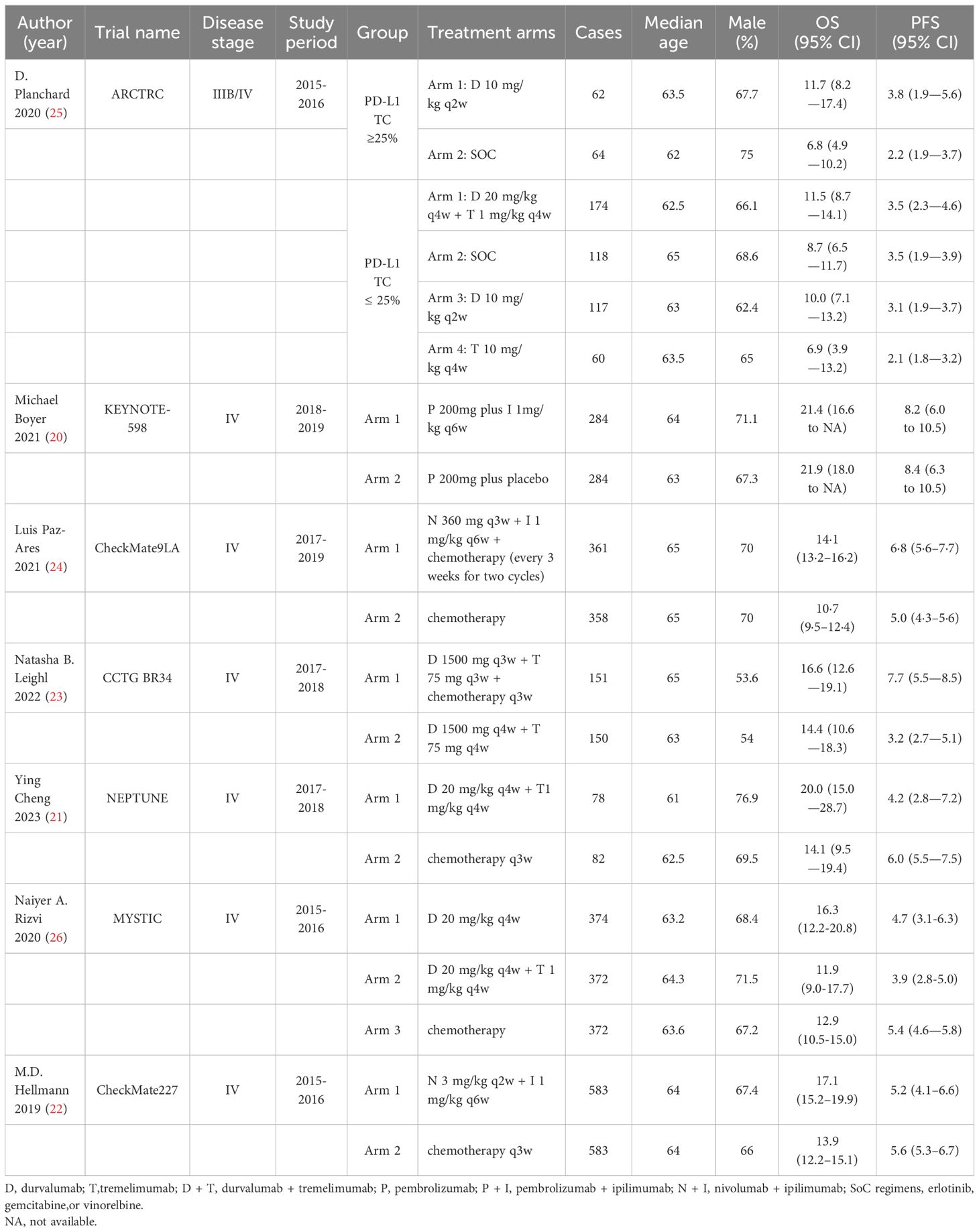
This meta-analysis comprised 7 RCTs (20–26) published between 2020 and 2023. These trials included a total of 4682 patients who were diagnosed with advanced non-small cell cancer. The authors, year, trial name, disease stage, study period, group, treatment arms, cases, median age, male, OS and PFS of the included literature are shown in Table 1.
3.3 Risk of bias
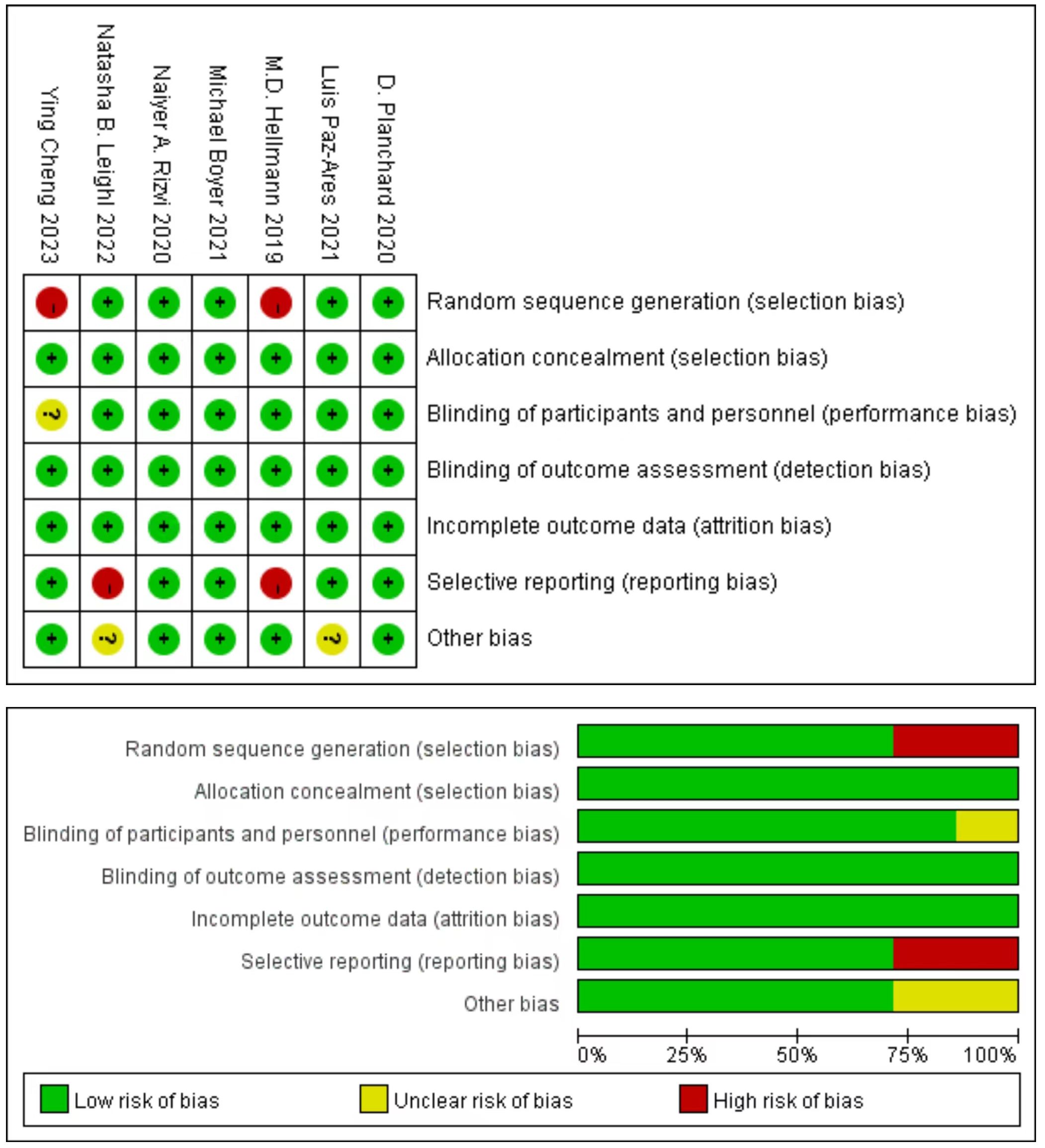
Cochrane Risk of Bias Tool evaluation indicated that the included literature consisted of studies of medium to high quality, five studies produced a sufficient random sequence, seven studies reported appropriate allocation concealment, six studies clearly implemented participant blinding, seven studies reported outcome assessor blinding, seven studies provided complete outcome data, five studies did not engage in selective reporting, and five studies did not exhibit any other bias (Figure 2).
3.4 CP versus chemotherapy
Four studies (21, 22, 25, 26) compared the OS between the CP group and the chemotherapy group (heterogeneity: p = 0.38, I2 = 2%). The results indicated that the CP provided significantly better OS compared with chemotherapy(HR: 0.84, 95% CI: 0.75-0.94, p <0.05) (Figure 3).
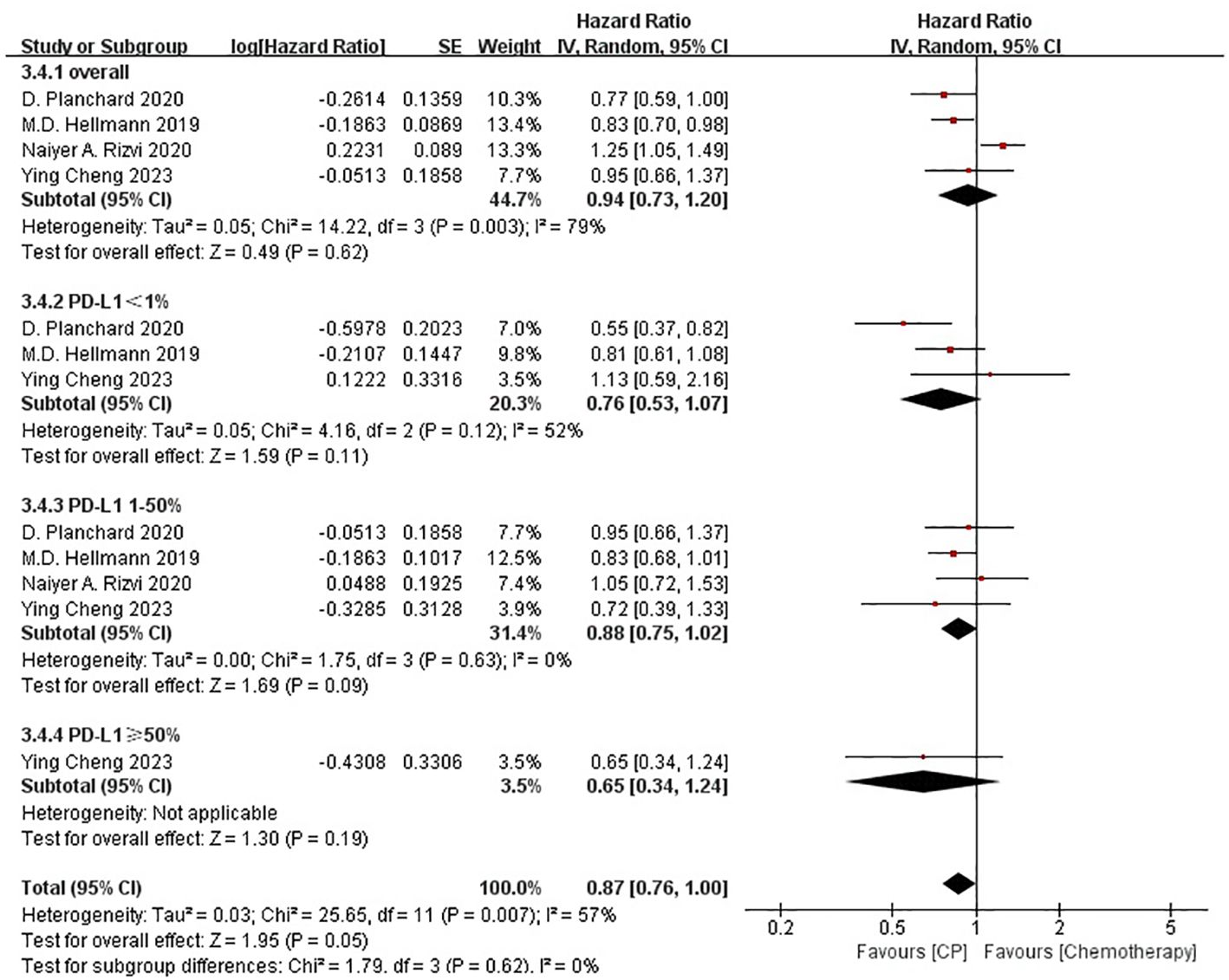
Four studies (21, 22, 25, 26) compared the PFS between the CP group and the chemotherapy group (heterogeneity: p = 0.003, I2 = 79%). The results indicated no significant difference in PFS between the CP group and the chemotherapy group(HR: 0.94, 95% CI: 0.73-1.20, p = 0.62) (Figure 4).
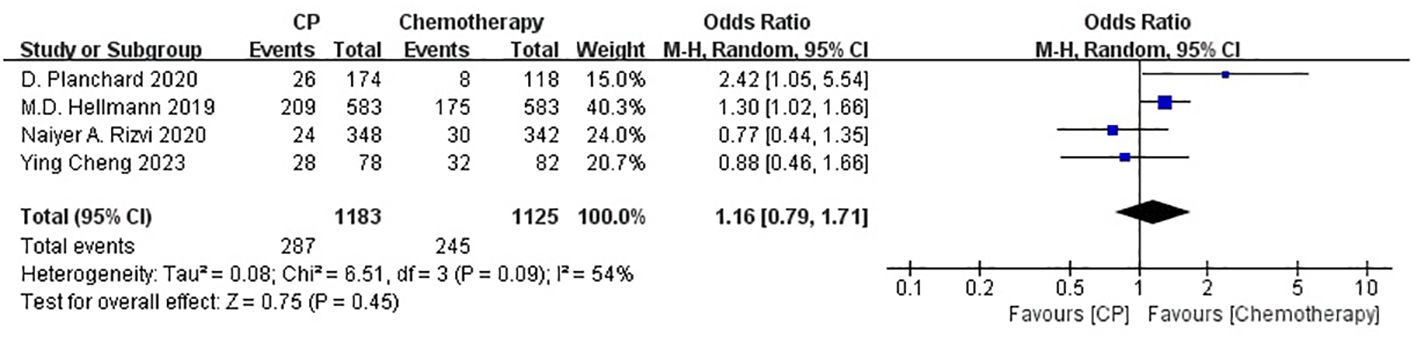
Four studies (21, 22, 25, 26) compared the ORR between the CP group and the chemotherapy group(heterogeneity: p = 0.09, I2 = 54%). The results indicated no significant difference in ORR between the CP group and the chemotherapy group(OR: 1.16, 95% CI: 0.79-1.71, p = 0.45) (Figure 5).
The non-significant results regarding PFS and ORR may be affected by sample size or heterogeneity. Therefore, subgroup analyses were conducted regarding PD-L1 expression, blood TMB, medication combinations, tumor stage, median age, gender, histology and smoking status (Table 2). It was worth noting that, CP provided significantly better OS compared with chemotherapy while PD-L1 expression was less than 1% or greater than 50%, or bTMB over 20 mut/Mb.
3.5 CP versus P
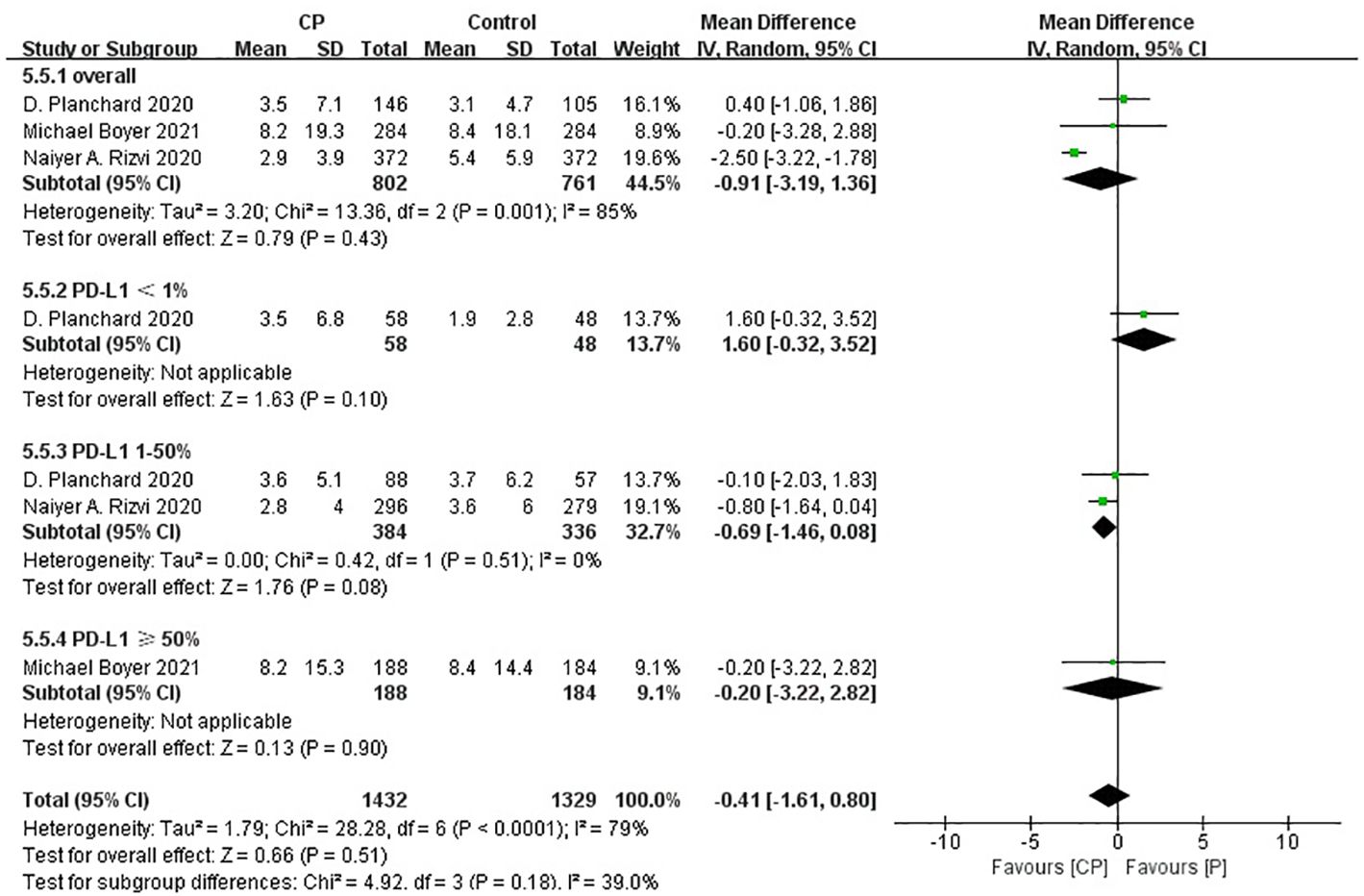
Three studies (20, 25, 26) compared the OS between the CP group and the P group (heterogeneity: p = 0.60, I2 = 0%). The results indicated no significant difference in OS between the CP group and the P group (MD: -0.25, 95%CI: -2.47-1.98, p = 0.83) (Figure 6).
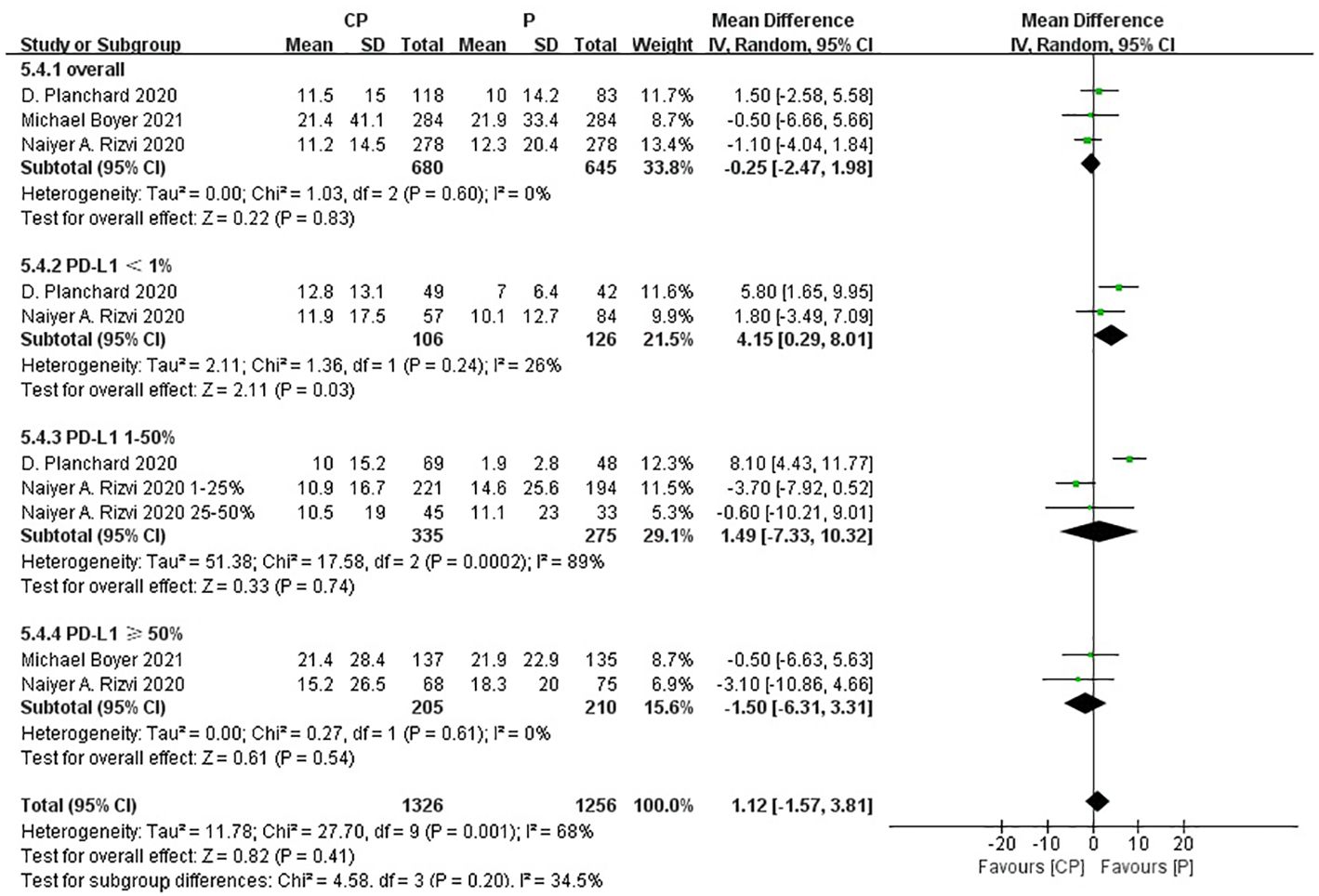
Three studies (20, 25, 26) compared the PFS between the CP group and the P group (heterogeneity: p = 0.001, I2 = 85%). The results indicated no significant difference in PFS between the CP group and the P group (MD: -0.91, 95%CI: -3.19–1.36, p = 0.43) (Figure 7).
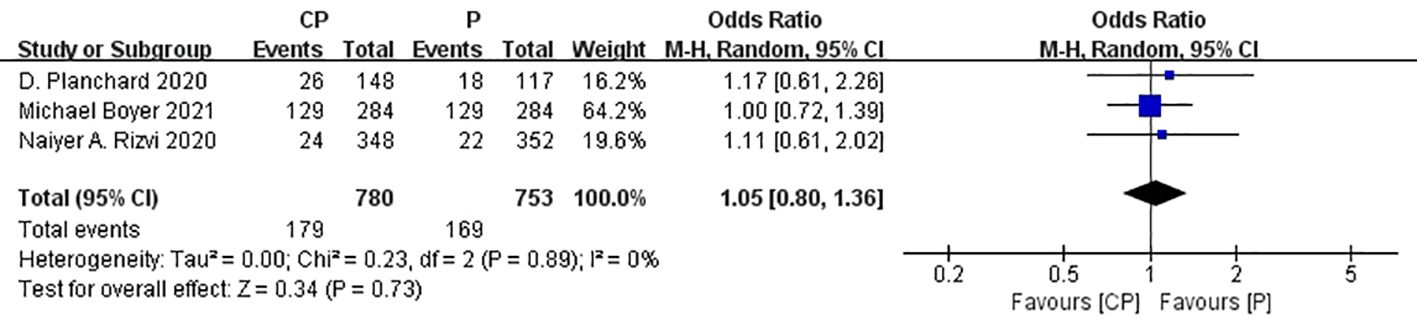
Three studies (20, 25, 26) that compared the ORR between the CP group and the P group(heterogeneity: p = 0.89, I2 = 0%). The results indicated no significant difference in ORR between the CP group and the P group (OR: 1.05, 95%CI: 0.80–1.36, p = 0.73) (Figure 8).
The non-significant results regarding OS, PFS and ORR may be affected by sample size or heterogeneity. Therefore, subgroup analyses were conducted regarding PD-L1 expression, blood TMB, medication combinations, tumor stage, median age, gender, histology and smoking status (Table 3). It was worth noting that, CP provided significantly better OS compared with P while PD-L1 expression was less than 1%.
3.6 CP plus chemotherapy
Within the literature reviewed, two trials assessed the regimen of CP combined with chemotherapy: nivolumab + ipilimumab + chemotherapy (27) and durvalumab + tremelimumab + chemotherapy (28). However, we failed to performed a meta-analysis due to the different control groups in the two trials. Therefore, only a concise overview of the two clinical trials involving different control groups is presented here. Nivolumab + ipilimumab + chemotherapy compared with chemotherapy in CheckMate9LA (24) found that double-exempt combination chemotherapy with ICIs improved OS compared with chemotherapy alone (HR: 0.66, 95% Cl: 0.55-0.80), PFS(HR: 0.68, 95% CI: 0.57-0.82), but the incidence of AEs(91% versus 87%) and 3-5 AEs(47% versus 38%) was also significantly higher and toxicity was increased; in CCTG BR34 (23) durvalumab + tremelimumab + chemotherapy was compared with durvalumab + tremelimumab, and it was found that ICIs double-exempt combination chemotherapy improved PFS (HR: 0.67, 95% CI: 0.52-0.88), but OS was not prolonged (HR: 0.88, 90% CI: 0.67-1.16), and chemotherapy plus within the immunotherapy group, 82% of patients experienced grade 3 or higher adverse events, while in the control group, 70% experienced such events. These adverse events were associated with a heightened toxic response to the combination of ICIs double-elimination and chemotherapy.
3.7 Toxicity
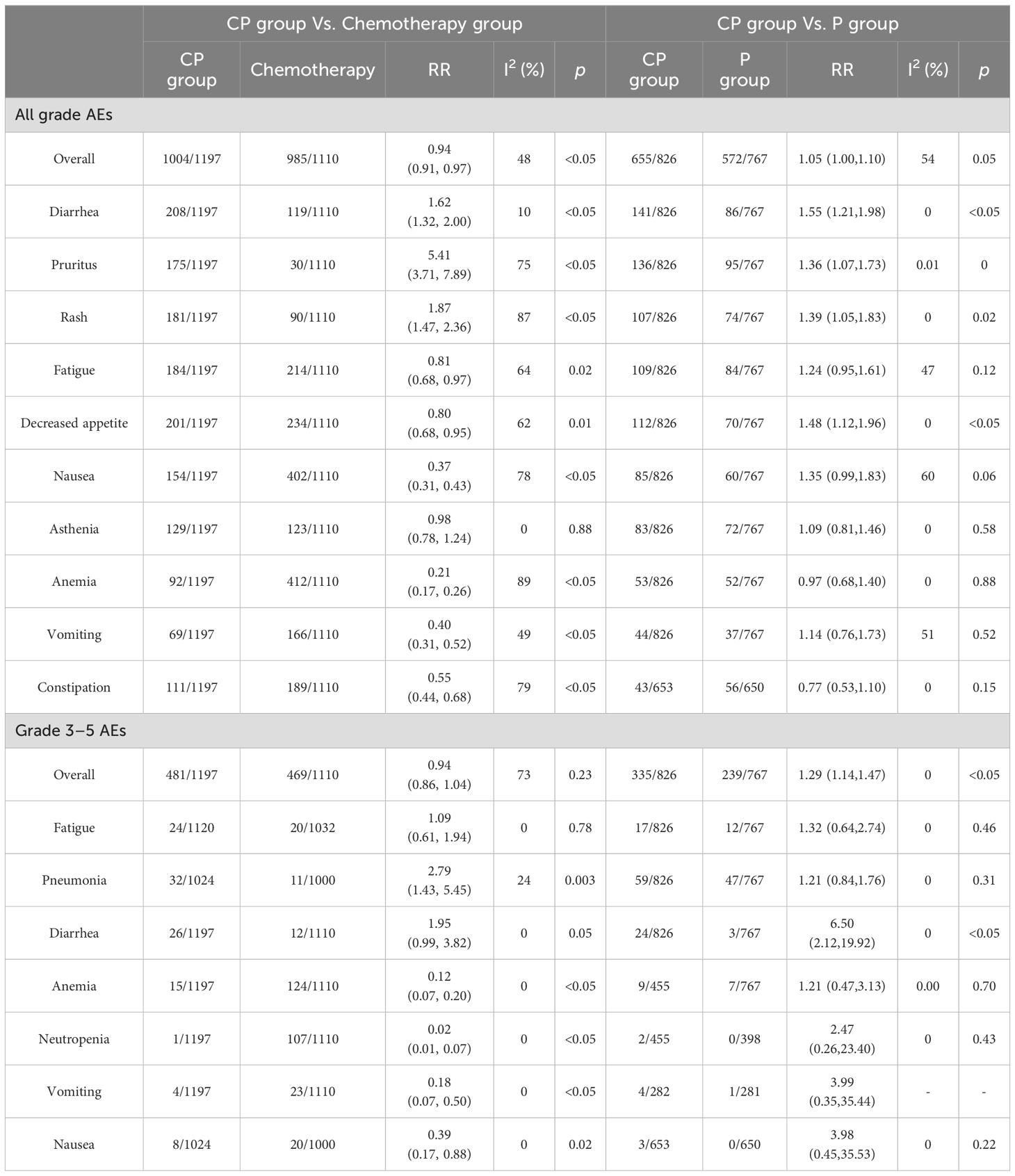
The occurrence of all grade AEs in all included studies were summarized (Table 4, Supplementary Table S2); the most common AEs in the CP group were diarrhea (17.38%), decreased appetite (16.79%), fatigue (15.37%), rash (15.12%), pruritus (14.12%), nausea (12.87%), weight decreased (10.94%), asthenia (10.78%), back pain (10.24%), aspartate aminotransferase increased (9.60%), CP had lower incidence of all grade AEs (RR: 0.94, 95% CI: 0.91-0.97, p<0.05) compared to chemotherapy (heterogeneity: p = 0.12, I2 = 48%). In comparison to P (heterogeneity: p = 0.11, I2 = 54%), CP had a higher occurrence of all grade AEs (RR: 1.05, 95% CI: 1.00-1.10, p = 0.05).
A summary of all grade 3–5 AEs was compiled (Table 2, Supplementary Table S3); the most prevalent grade 3–5 AEs in the CP group were pneumonia (3.13%), febrile neutropenia (3.07%), diarrhea(2.17%), fatigue (2.14%), rash(1.74%), autoimmune hepatitis (1.73%), colitis (1.65%), pancreatitis (1,56%), dehydration(1.37%), anemia (1.25%). CP had comparable incidence of grade 3–5 AEs (RR: 0.94, 95% CI: 0.86-1.04, p = 0.23) compared with chemotherapy, but higher incidence of grade 3–5 AE in comparison to P (RR:1.29, 95% CI: 1.14-1.47, p<0.05) (Table 4, Supplementary Table S3).
3.8 Publication bias
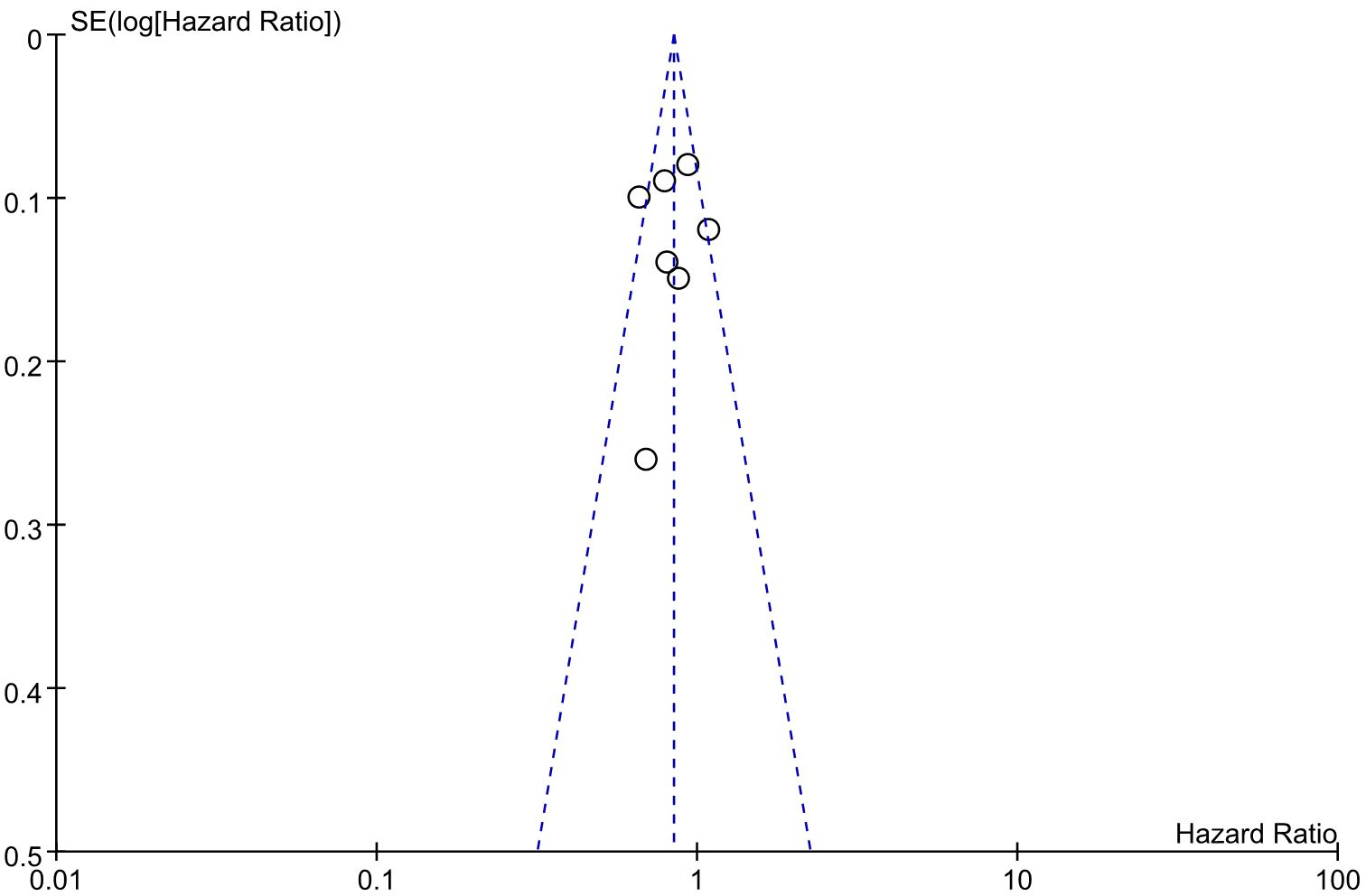
An evaluation of publication bias in relation to the OS was conducted using a funnel plot (Figure 9). The bilateral symmetric funnel plot of the OS did not reveal any significant evidence of publication bias.
4 Discussion
Non-small cell lung cancer constitutes 80-85% of all lung cancers and is among the most prevalent malignant neoplasms detected globally (29). Approximately 70% of patients are diagnosed in intermediate to advanced stages of the disease (30), thereby resulting in a dismal 5-year survival rate. The contemporary approach to treating NSCLC mostly involves the use of immunosuppressants and chemotherapy. However, there is ongoing debate about the comparative effectiveness of combining two major immune checkpoint inhibitors (CTLA-4 inhibitors and PD-1/PD-L1 inhibitors) compared to using either an immunosuppressant or chemotherapy alone. This meta-analysis assessed the efficacy and safety of first-line CTLA-4 inhibitor in combination with PD-1/PD-L1 inhibitor in the treatment of patients with advanced NSCLC. Our results revealed that CP demonstrated superior efficacy, longer OS, and comparable and controllable toxicity in comparison to chemotherapy. However, there were no significant differences in total OS, PFS, and ORR compared to P, with a slightly higher toxicity.
By comparing dual immune therapy to monotherapy or chemotherapy, this meta-analysis highlighted its unique benefits. To further investigate the possibility of personalized treatment, subgroup analyses were performed on patients with varying PD-L1 expression levels. For patients with PD-L1 expression <1%, CP provided significantly longer OS in comparison to chemotherapy or P, as well as longer PFS (though no statistical difference); For patients with PD-L1 expression 1-50%, CP provided similar OS and PFS compared with chemotherapy or P; For patients with PD-L1 expression ≥50%, CP provided similar OS and PFS compared with P, but significantly longer OS in comparison to chemotherapy. In numerous trials, anti-PD-1/PD-L1 medicines have shown clinical efficacy in patients with diverse PD-L1 expression levels on tumor cells (31–35). Enhanced efficacy outcomes with PD-1/PD-L1 inhibitors have been observed in patients exhibiting higher PD-L1expression levels (31, 32, 34, 36, 37). However, low PD-L1 expression was associated with poor efficacy outcomes (36, 38, 39). Low PD-L1 expression is associated with low T-cell infiltration, resulting in a primary resistance to PD-1 inhibitors (40–42). Despite promising results from extensive clinical trials, no definitive optimal treatment existed for people with PD-L1-negative NSCLC. The findings of this meta-analysis revealed that CP could serve as a therapeutic option for patients exhibiting low or negative PD-L1 expression, which provided better long-term outcomes compared with chemotherapy or P. The fundamental principle of combining PD-1/PD-L1 inhibitors and CTLA-4 inhibitors is that they operate through distinct modes of action. Anti-CTLA-4 primarily targets the lymph node region, facilitating the generation and multiplication of activated T cells, while PD-1 antagonists predominantly function at the tumor periphery, inhibiting tumor progression.-infiltrating tumor PD-L1-expressing tumors and plasma-like dendritic cells from neutralizing cytotoxic T cells (40). Given that PD-1 and CTLA-4 regulate effector T-cell activation, proliferation, and function via independent yet complimentary pathways, the utilization of dual immune checkpoint inhibitors (PD-1/PD-L1 and CTLA-4) is a logical strategy to enhance antitumor immunity (7, 8). The concurrent inhibition of the PD-1/PD-L1 and CTLA-4 pathways has demonstrated additive or synergistic antitumor efficacy in prior research (42–44).
The heterogeneity was considerable in this meta-analysis, which might be caused by differences in baseline patient characteristics or study design. In order to identify potential sources of heterogeneity, subgroup analyses were performed based on PD-L1 expression, TMB, medication combinations, non-small cell lung cancer stage, median age, gender, histology and smoking status (Table 2, Table 3). The PD-L1 expression seemed to be an important sources of heterogeneity. However, since the poor sample size, the non-significant results regarding most subgroup analyses made it difficult to further analyze the source of heterogeneity.
Regarding safety, the findings revealed that the occurrence of adverse events in the CP group was lower than that in the chemotherapy group, but higher than P. The increased occurrence of AEs in the CP group, may be attributed to the expression of CTLA-4 after T-cell activation, which has the ability to down-regulate or prevent T-cell activation (45). Additionally, the cytoplasmic region of CTLA-4 contains immunoreceptor tyrosine inhibitory sequences that transmit inhibitory signals and thereby reduce the immune response (46). The most common AEs with an incidence greater than 10% included diarrhea, decreased appetite, fatigue, rash, pruritus, nausea, weight decreased, asthenia and back pain. The most prevalent grade 3–5 AEs with an incidence greater than 1% included pneumonia, febrile neutropenia, diarrhea, fatigue, rash, autoimmune hepatitis, colitis, pancreatitis, dehydration, anemia. It is advisable to formulate clinical response strategies for these high-frequency or severe AEs to offer more thorough guidance for clinical applications. Unfortunately, due to the limitations of the raw data, we failed to analyze adverse events by patient characteristics to explore if certain subgroups were more prone to specific adverse events, thereby providing personalized treatment recommendations.
The analysis of two clinical trials in the CP combination chemotherapy group was limited due to inadequate data sets. Both studies showed that while ICIs double-exempt combination chemotherapy improved OS and PFS, it also was associated with a notable rise in toxicity. Nevertheless, the limited sample size and preexisting experimental data may introduce inaccuracies, thereby necessitating further studies to substantiate the comparison of its therapeutic efficacy with CP, chemotherapy, or P. Nevertheless, it has been demonstrated that both have a coordinated impact on the enhancement of immunological sensitivity in cancer cells and the activation of effector immune cells (47). Furthermore, the inhibitory impact of chemotherapy on the impairment of the immune system establishes advantageous circumstances for the use of combination therapy with CP, so enhancing the reactive immune response against malignancies. The distinct and mutually exclusive toxicity profile of CP and chemotherapy renders them highly suitable for combination approaches (48, 49).
This meta-analysis was conducted on two experimental groups comparing CP versus chemotherapy, CP versus P, and three treatment regimens for patients with advanced non-small cell lung cancer. These regimens can be selected based on tumor characteristics to determine the most clinically individualized treatment. As a first-line treatment option for advanced non-small cell lung cancer, the combination of a PD-1/PD-L1 inhibitor plus a CTLA-4 inhibitor is both effective and well tolerated. PD-L1 expression has been summarized as negative (<1%), low-positive (1-50%), or high-positive (≥50%). Stratification of patients according to PD-L1 expression level as well as bTMB status revealed that patients who were negative for PD-L1 expression and treated with PD-1/PD-L1 inhibitors in combination with CTLA-4 inhibitors had improved OS. When PD-L1 expression was negative, PD-L1 inhibitor monotherapy improved OS but not PFS. Meanwhile, the results of the study showed that for patients with advanced non-small cell lung cancer with strongly positive PD-L1 expression, undergoing a dual-immunity combination therapy was more effective than chemotherapy in terms of both survival benefit and toxicity. In terms of AEs, PD-1/PD-L1 inhibitor monotherapy had the highest acceptable toxicity, followed by CP (dual-immunity combination therapy with ICIs), and finally chemotherapy. Taking PD-L1 expression and bTMB into account, treatment options can be divided into the following three categories: Patients with negative PD-L1 expression or low tumor mutation (bTMB <20 mut/Mb) can be considered to be preferred to ICIs combination therapy due to the susceptibility to ICI monotherapy resistance; patients with strong positive PD-L1 expression or high tumor mutation (bTMB ≥20 mut/Mb) can be considered as preferred to ICIs combination therapy in view of their susceptibility to ICI treatment and their susceptibility to ICI monotherapy. ICI therapy is sensitive, and ICI monotherapy has a low toxicity response. ICI monotherapy can be preferred, followed by ICIs combination therapy; patients with low positive PD-L1 expression, because there is no study directly showing which drug is more efficacious, can be considered first, followed by ICIs combination therapy. Because of the lack of prospective direct comparisons, the choice of treatment options should be considered on a patient-by-patient basis, with transparent communication with the patient about the advantages, costs, and risks of each option.
This meta-analysis has included the highest number of trials studying the efficacy and safety of first-line PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor in the treatment of patients with advanced non-small cell lung cancer, to the best of our knowledge. Compared with previous meta-analyses (50), this meta-analysis included more updated trials with detailed subgroup analyses and exhaustive discussions of the results. The findings of our study provide valuable insights into the clinical outcomes of CP that contribute to both clinical practice and research in the field of advanced non-small cell lung cancer. However, there were some possible limitations in the meta-analysis. To begin with, while the meta-analysis have included the latest trials, the results may be unstable because of the limited sample size of. For example, only four trials reported outcomes of CP versus chemotherapy, and only three trials reported outcomes of CP versus P. The limited sample sizes could lead to poor statistical power and instability results. In addition, the heterogeneity was considerable in this meta-analysis, which might be caused by differences in baseline patient characteristics or study design. For example, the inclusion criteria was different among these trials. Only two trials included patients with PD-L1 expression <1%, while other trials did not. The treatment regimens in some trials were Nivolumab plus ipilimumab while Durvalumab plus tremelimuma in other trials. Though subgroup analyses were performed to identify potential sources of heterogeneity, it was still difficult to further analyze the source of heterogeneity due to the poor sample size and the non-significant results.
In conclusion, the findings of this meta-analysis revealed that CP was feasible and safe as first-line treatment for patients with advanced NSCLC. Specially, CP could serve as a therapeutic option for patients exhibiting low or negative PD-L1 expression, yielding superior long-term outcomes relative to chemotherapy or P. Additional RCTs with extended follow-up durations are required to substantiate these findings, particularly emphasizing efficacy among patients with varying PD-L1 expression levels, to enhance the stratified application of immunotherapy.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
HMZ: Conceptualization, Data curation, Formal analysis, Project administration, Writing – original draft. SH: Conceptualization, Data curation, Formal analysis, Writing – original draft. JWu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – review & editing. YL: Data curation, Formal analysis, Funding acquisition, Resources, Writing – review & editing. YZ: Investigation, Methodology, Writing – original draft. HJZ: Investigation, Methodology, Writing – original draft. CL: Investigation, Methodology, Writing – original draft. JWa: Software, Supervision, Writing – original draft. XZ: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing. SD: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing. WL: Funding acquisition, Software, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Scientific Foundation of China (No. 82460594), Guangxi Natural Science Foundation Program (No. 2024GXNSFAA010167), the Guangxi Medical and Healthcare Appropriate Technology Development and Popularization and Application Project (S2023125), Key Laboratory Construction Project of Guangxi Health Commission (ZPZH2020007), the Scientific Research Foundation of Guangxi University of Science and Technology(20Z13), the Scientific Research Foundation of Guangxi Health Commission (Z-B20220927,Z-B20220930).
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1515027/full#supplementary-material
References
1. Sun S, Wang Y, Gao X, Wang H, Zhang L, Wang N, et al. Current perspectives and trends in nanoparticle drug delivery systems in breast cancer: bibliometric analysis and review. Front Bioengineering Biotechnol. (2023) 11. doi: 10.3389/fbioe.2023.1253048
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Bi J-H, Tuo JY, Xiao YX, Tang DD, Zhou XH, Jiang YF, et al. Observed and relative survival trends of lung cancer: A systematic review of population-based cancer registration data. Thorac Cancer. (2024) 15:142–51. doi: 10.1111/1759-7714.15170
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Guo Q, Liu L, Chen Z, Fan Y, Zhou Y, Yuan Z, et al. Current treatments for non-small cell lung cancer. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.945102
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Yan T, Yu L, Shangguan D, Li W, Liu N, Chen Y, et al. Advances in pharmacokinetics and pharmacodynamics of PD-1/PD-L1 inhibitors. Int Immunopharmacol. (2023) 115:1-16. doi: 10.1016/j.intimp.2022.109638
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Kim G-R, Choi J-M. Current understanding of cytotoxic T lymphocyte antigen-4 (CTLA-4) signaling in T-cell biology and disease therapy. Molecules Cells. (2022) 45:513–21. doi: 10.14348/molcells.2022.2056
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. (2013) 14:1212–8. doi: 10.1038/ni.2762
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 checkMate 227 part 1 trial. J Thorac Oncol. (2022) 17:289–308. doi: 10.1016/j.jtho.2021.09.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Hollen PJ, Gralla RJ, Kris MG, Potanovich LM. Quality-of-life assessment in individuals with lung-cancer - testing the lung-cancer symptom scale (Lcss). Eur J Cancer. (1993) 29A:S51–8. doi: 10.1016/S0959-8049(05)80262-X
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Lynch TJ, Bell RSordella DW, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New Engl J Med. (2004) 350:2129–39. doi: 10.1056/NEJMoa040938
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New Engl J Med. (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer:: Correlation with clinical response to gefitinib therapy. Science. (2004) 304:1497–500. doi: 10.1126/science.1099314
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New Engl J Med. (2013) 368:2385–94. doi: 10.1056/NEJMoa1214886
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. (2009) 27:4247–53. doi: 10.1200/JCO.2009.22.6993
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. (2007) 448:561–U3. doi: 10.1038/nature05945
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
17. Higgins JPT, Altman DG, Gotzsche PC, Jueni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj-British Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
18. Guyatt GH, Oxman AD, Schuenemann HJ, Tugwell P, Knottnerus A, et al. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj-British Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. (2021) 39:2327. doi: 10.1200/jco.20.03579
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Cheng Y, Zhou Q, Han B, Fan Y, Shan L, Chang J, et al. NEPTUNE China cohort: First-line durvalumab plus tremelimumab in Chinese patients with metastatic non-small-cell lung cancer. Lung Cancer. (2023) 178:87–95. doi: 10.1016/j.lungcan.2023.01.013
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Hellmann MD, Paz-Ares L, Caro Bernabe R, Zurawski B, Kim SW, Costa Carcereny E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. New Engl J Med. (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
23. Leighl NB, Laurie SA, Goss GD, Hughes BG, Stockler M, Tsao MS, et al. CCTG BR34: A randomized phase 2 trial of durvalumab and tremelimumab with or without platinum-based chemotherapy in patients with metastatic NSCLC. J Thorac Oncol. (2022) 17:434–45. doi: 10.1016/j.jtho.2021.10.023
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
24. Paz-Ares L, Ciuleanu MCobo TE, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
25. Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. (2020) 31:609–18. doi: 10.1016/j.annonc.2020.02.006
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
26. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:661–74. doi: 10.1001/jamaoncol.2020.0237
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Bian J, Shao R, Li J, Zhu JF, Shao AZ, Liu C, et al. Mechanism research of non-coding RNA in immune checkpoint inhibitors therapy. Cancer Sci. (2024) 115:3520–31. doi: 10.1111/cas.v115.11
28. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
29. Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun. (2022) 42:937–70. doi: 10.1002/cac2.v42.10
30. Goldstraw P, Ball D, Jett JR, Chevalier Le T, Lim E, Nicholson AG, et al. Non-small-cell lung cancer. Lancet. (2011) 378:1727–40. doi: 10.1016/S0140-6736(10)62101-0
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Crit Rev Oncol Hematol. (2016) 101:75–85. doi: 10.1016/j.critrevonc.2016.03.007
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
32. Brahmer J, Horn L, Hossein B, Ramalingam S, Pluzanski A, Burgio MA, et al. Long-term survival outcomes with nivolumab (NIVO) in pts with previously treated advanced non-small cell lung cancer (NSCLC): impact of early disease control and response. J Thorac Oncol. (2019) 14:S1152–3. doi: 10.1016/j.jtho.2019.09.089
33. Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Calio A, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PloS One. (2015) 10:1–16. doi: 10.1371/journal.pone.0130142
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
34. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. (2018) 19:521–36. doi: 10.1016/S1470-2045(18)30144-X
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
35. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
36. Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A, Li X, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol. (2014) 32:s8021. doi: 10.1200/jco.2014.32.15_suppl.8021
37. Messori A, Rivano M, Chiumente M, Mengato D, et al. Tislelizumab plus chemotherapy vs. pembrolizumab plus chemotherapy for the first-line treatment of advanced non-small cell lung cancer: systematic review and indirect comparison of randomized trials. Chin Clin Oncol. (2023) 12:1–11. doi: 10.21037/cco-23-26
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
38. Antonia SJ, Balmanoukian A, Brahmer J, Ou SHI, Hellmann MD, Kim SW, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol. (2019) 14:1794–806. doi: 10.1016/j.jtho.2019.06.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
39. Chaft J, Cho BC, Ahn MJ, Moulec Le S, Cho EK, Papadimitrakopoulou V, et al. Safety and activity of second-line durvalumab plus tremelimumab in non-squamous advanced NSCLC. Cancer Res. (2018) 78:1538–7445. doi: 10.1158/1538-7445.Am2018-ct113
40. Eggermont AMM, Crittenden M, Wargo J. Combination Immunotherapy Development in Melanoma. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annu Meeting. (2018) 38:197–207. doi: 10.1200/edbk_201131
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
41. Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, et al. Clinical importance of epstein-barr virus-associated gastric cancer. Cancers. (2018) 10:1–13. doi: 10.3390/cancers10060167
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
42. Rizvi NA, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol. (2018) 29:x40–1. doi: 10.1093/annonc/mdy511.005
43. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
44. Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. (2017) 18:31–41. doi: 10.1016/S1470-2045(16)30624-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
45. Tang S, Qin C, Hu H, Liu T, He Y, Guo H, et al. Immune checkpoint inhibitors in non-small cell lung cancer: progress, challenges, and prospects. Cells. (2022) 11:1–27. doi: 10.3390/cells11030320
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
46. Koustas E, Sarantis P, Papavassiliou AG, Karamouzis MV. The resistance mechanisms of checkpoint inhibitors in solid tumors. Biomolecules. (2020) 10:1–17. doi: 10.3390/biom10050666
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
47. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differentiation. (2014) 21:15–25. doi: 10.1038/cdd.2013.67
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
48. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. (2019) 37:992. doi: 10.1200/JCO.18.01042
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
49. Sereno M, Higuera O, Castellanos Cruz P, Falagan S, Mielgo-Rubio X, Trujillo-Reyes JC, et al. Immunotherapy combinations and chemotherapy sparing schemes in first line non-small cell lung cancer. World J Clin Oncol. (2021) 12:1182–92. doi: 10.5306/wjco.v12.i12.1182
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
50. Liu Q, Fang Z, Liu M, Xu R, Yi F, Wei Y, et al. The benefits and risks of CTLA4 inhibitor plus PD1/PDL1 inhibitor in stage IIIB/IV non-small cell lung cancer: A systematic analysis and meta-analysis based on randomized controlled trials. J Clin Pharm Ther. (2021) 46:1519–30. doi: 10.1111/jcpt.13465
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: PD-1 inhibitor, programmed cell death protein 1 inhibitor, CTLA-4 inhibitor, non-small cell lung cancer, overall survival, objective response rate, meta-analysis
Citation: Zhao H, Huang S, Wu J, Lu Y, Zou Y, Zeng H, Li C, Wang J, Zhang X, Duan S and Liang W (2025) Efficacy and safety of first-line PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor in the treatment of patients with advanced non-small cell lung cancer: a systemic review and meta-analysis. Front. Immunol. 16:1515027. doi: 10.3389/fimmu.2025.1515027
Received: 22 October 2024; Accepted: 20 January 2025;
Published: 06 February 2025.
Edited by:
Jianqiang Xu, Dalian University of Technology, ChinaReviewed by:
Daniele Mengato, University Hospital of Padua, ItalyLi Xiaoyang, Shanghai Jiao Tong University, China
Copyright © 2025 Zhao, Huang, Wu, Lu, Zou, Zeng, Li, Wang, Zhang, Duan and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochen Zhang, emhhbmd4aWFvY2hlbkBneHVzdC5lZHUuY24=; Siliang Duan, ZHVhbnNpbGlhbmdAZ3h1c3QuZWR1LmNu; Weiming Liang, d2VpbWluZ2xpYW5nQGd4dXN0LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Huimin Zhao
Huimin Zhao Shanshan Huang1†
Shanshan Huang1† Jin Wang
Jin Wang Weiming Liang
Weiming Liang