
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 06 February 2025
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1513599
This article is part of the Research Topic Intestinal microenvironment and autoimmune diseases View all articles
Studies suggest that gut dysbiosis occurs in autoimmune neurological diseases, but a comprehensive synthesis of the evidence is lacking. Our aim was to systematically review and meta-analyze the correlation between the gut microbiota and autoimmune neurological disorders to inform clinical diagnosis and therapeutic intervention. We searched the databases of PubMed, Embase, Web of Science, and the Cochrane Library until 1 March 2024 for research on the correlation between gut microbiota and autoimmune neurological disorders. A total of 62 studies provided data and were included in the analysis (n = 3,126 patients, n = 2,843 healthy individuals). Among the included studies, 42 studies provided data on α-diversity. Regarding α-diversity, except for Chao1, which showed a consistent small decrease (SMD = −0.26, 95% CI = −0.45 to −0.07, p < 0.01), other indices demonstrated no significant changes. While most studies reported significant differences in β-diversity, consistent differences were only observed in neuromyelitis optica spectrum disorders. A decrease in short-chain fatty acid (SCFA)-producing bacteria, including Faecalibacterium and Roseburia, was observed in individuals with autoimmune encephalitis, neuromyelitis optica spectrum disorders, myasthenia gravis, and multiple sclerosis. Conversely, an increase in pathogenic or opportunistic pathogens, including Streptococcus and Escherichia-Shigella, was observed in these patients. Subgroup analyses assessed the confounding effects of geography and immunotherapy use. These findings suggest that disturbances of the gut flora are associated with autoimmune neurological diseases, primarily manifesting as non-specific and shared microbial alterations, including a reduction in SCFA-producing bacteria and an increase in pathogenic or opportunistic pathogens.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023410215.
The intestinal microecological community is intimately associated with human neurodevelopment, and disruptions in gut flora are increasingly recognized as contributors to various diseases through immunomodulatory and metabolic pathways. Both preclinical and clinical research have emphasized the importance of the gut flora dysbiosis in conditions like depression, rheumatoid arthritis, and multiple sclerosis (MS) (1–3). Growing evidence suggests that disruptions in composition of gut microbiota and their immunomodulatory functions are implicated in various autoimmune neurological diseases, including MS, optic neuromyelitis optica spectrum disorders (NMOSD), myasthenia gravis (MG), and autoimmune encephalitis (AIE) (4–7). Moreover, recent research has demonstrated that interventions such as fecal transplantation or probiotic supplementation hold promise in mitigating disease progression (4, 8–11). Therefore, regulating gut microbiota homeostasis has the potential to serve as a diagnostic tool or therapeutic approach.
However, current research on gut microbiota in autoimmune neurological diseases yields varied findings, lacking a comprehensive synthesis. There is consensus that elevated α-diversity indicates a healthy gut microecology. However, diseases such as AIE, MS, and MG showed different patterns in gut microbiota α-diversity. Some studies reported reduced α-diversity, while others showed no significant change (6, 12–14). This variability in findings suggests that the relationship between gut flora α-diversity and autoimmune neurological disorders is complex and may be influenced by various factors, including measurement methods, environmental influences, and drug use. Moreover, identifying specific alterations in gut microbiota, whether increased or decreased, holds promise for enhancing disease diagnostic accuracy, deepening disease understanding, and informing treatment strategies. Identifying non-specific and common gastrointestinal microbes in different autoimmune neurological diseases is also important. Such insights could enhance our understanding of the common pathogenesis underlying these disorders. Hence, distinguishing between specific and shared gut microbial changes across a broader spectrum of autoimmune neurological diseases is imperative.
To date, no comprehensive investigation has systematically examined changes in gut microbiota across a spectrum of autoimmune neurological diseases. To address this gap, we performed an updated systematic review and meta-analysis to integrate mounting evidence regarding the connection between gut microbiota and autoimmune neurological disorders.
Pre-registration of this meta-analysis was undertaken through PROSPERO (CRD42023410215). We followed the reporting guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), along with the Cochrane Guidelines for Comprehensive and Updated Reviews (15, 16).
We performed a comprehensive literature search of the PubMed, Cochrane, Embase, and Web of Science databases up to 1 March 2024. Autoimmune neurological diseases involve the immune system’s erroneous assault on the nervous system, sparking inflammation and neural damage. These conditions can affect the brain, spinal cord, and peripheral nerves, resulting in various symptoms depending on the affected region (17). The study focused on a range of such conditions, including MS, AIE, MG, NMOSD, neuro-Behcet’s disease (NBD), acute disseminated encephalomyelitis, and Guillain–Barré syndrome. Comprehensive search details are provided in Supplementary Table S1.
Two independent investigators, XL Deng and X Gong, used Endnote to screen studies for inclusion in this evaluation. Initially, studies were screened by title and abstract to exclude those not meeting the eligibility criteria, followed by a full-text review for further evaluation. Any discrepancies between investigators regarding study inclusion were discussed to establish consensus. We included studies that fulfilled the following criteria: (1) implementation of observational designs including case–control studies, cross-sectional studies, cohort studies, or baseline data of intervention studies; (2) analysis of gut microbiota using available diversity or abundance data; (3) inclusion of populations diagnosed with autoimmune neurological diseases; and (4) publication of full-text articles in English in peer-reviewed journals. In cases where data were duplicated or shared between studies, the most recent and comprehensive dataset was used for analysis.
XL Deng and X Gong extracted data from full-text articles using a pre-designed template, which was then cross-checked for accuracy. The primary outcomes and metrics were α-diversity, β-diversity, and the relative abundance of intestinal microbes. α-diversity provides an overview of the microbial community within a single sample and is used to compare across different groups assessing richness (number of taxa) and evenness (the degree to which each taxon is represented). β-diversity serves as a metric for assessing the diversity among samples and examining the phylogenetic composition of communities relative to other analyzed samples (1, 18). Additionally, other pertinent information such as publication details, participant demographics, and methodologies was extracted.
The quality of the included research was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS) (19). Scores of 5 or lower were considered low quality, scores of 6 or 7 signified moderate quality, and scores of 8 or 9 indicated high quality. XL Deng and X Gong independently conducted assessments to determine the quality of the research involved in the analysis. In cases of score disagreement, the two reviewers collaboratively re-evaluated the articles to reach a consensus (2).
A meta-analysis was conducted to compare the α-diversity between individuals with autoimmune neurological diseases and healthy controls. The analysis included indices containing data from at least 10 research studies. The pooled standardized mean difference (SMD) and related 95% confidence interval (CI) were computed via inverse-variance random-effects meta-analysis for each index. The effect sizes were classified based on magnitude: trivial (SMD ≤ 0.2), small (0.2 < SMD < 0.5), moderate (0.5 ≤ SMD < 0.8), or large (SMD ≥ 0.8). Means and standard deviations were derived from medians and interquartile ranges. If numerical data were only presented graphically, they were extracted using WebPlotDigitizer V.4.6 (2). The DerSimonian-Laird estimator was utilized to assess heterogeneity, with significant heterogeneity defined as I2 > 50%. Publication bias was assessed using funnel plots and the Egger’s test.
Subgroup analysis was conducted to investigate the heterogeneity among studies, examining various factors such as the type of autoimmune neurological diseases, the geographical distribution of study populations (comparing countries in the East and West), and the use of immunotherapy (including immunosuppressants, immunomodulators, and immune-reconstitution therapy), distinguishing between patients receiving treatment and those without it. Eastern countries comprise East and South Asian nations, while Western countries encompass North America, Europe, and the Middle East. Studies where at least 80% of patients received immunotherapy were classified as involving treated patients. To assess the reliability of the results, two sensitivity analyses were carried out. Studies rated as low quality (with NOS scores of 5 or less) and those without matching characteristics (including age, sex, and body mass index) were excluded due to their susceptibility to confounding bias (1, 2). All analyses were conducted using the STATA/MP 17.0, with statistical significance defined as p-values less than 0.05.
When significant variations in β-diversity between patients with autoimmune neurological diseases and healthy individuals were observed across all studies focusing on a particular condition, these differences were considered consistent. A qualitative synthesis was undertaken due to the extensive findings and low overlap in the relative abundance of microbial species. Intra-diagnostic and inter-diagnostic comparisons were undertaken to delineate disease-specific and common alterations. Disease-related findings reported by at least two studies per taxon were summarized, with taxa categorized as exhibiting increased, decreased, or inconsistent patterns. Inconsistency was defined as less than 75% concordance between studies. Concordant findings from two studies were deemed notable for future validation, while those from three or more studies conducted by at least two independent research teams were deemed possibly relevant to the disease. A taxon exhibiting consistent change in only one disease was identified as a potential disorder-specific response, whereas changes observed in at least three disorders were categorized as common changes (2).
Initially, a total of 3,042 studies were identified (Figure 1). Following the removal of 1,403 duplicates, 1,550 studies that failed to fulfill the inclusion criteria were screened based on their titles and abstracts. Subsequently, 89 studies underwent full-text review, of which 27 were excluded (Supplementary Table S2). Finally, 62 studies focusing on six autoimmune neurological disorders (including AIE, MG, NMOSD, MS, and DON) were included (4–7, 9, 11–13, 20–73). MS was the most extensively studied disease, followed by MG, NMOSD, and AIE (Supplementary Table S3). The total number of participants was 3,126, ranging from 13 for NBD to 2,393 for MS. The mean age of participants varied from 32.93 years for AIE to 42.10 years for NBD (Table 1).
More than half of the studies [39 (62.90%)] were conducted in Western countries (e.g., USA, Germany, UK, Italy, and Israel), 21 (33.87%) were conducted in Eastern countries (China and Japan), and only 2 (3.23%) were conducted in Africa (Egypt). Most studies on MS were conducted in Western countries [37 (84.09%)], while the majority of studies on other diseases were conducted in Eastern countries (Supplementary Table S3). Sample sizes for most studies were small to moderate, ranging from 7 to 500 participants. Although the exclusion criteria were generally consistent, the use of immunotherapy varied widely. Out of the studies analyzed, 22 studies (35.48%) were conducted without immunotherapy. In contrast, six studies (9.68%) included only patients who had received immunotherapy. The remaining studies did not control for this, resulting in a wide variation in the proportion of patients on immunotherapy, ranging from 9% to 95.45%. Stool processing methods and compositional analyses also varied significantly. Sequencing of 16S ribosomal RNA (16S rRNA) was the most widely applied method, used in 54 studies, followed by shotgun metagenomics in 12 studies (5 of which used metagenomics exclusively), real-time quantitative polymerase chain reaction in 2 studies, and fluorescence in situ hybridization in 1 study (Supplementary Table S5). Based on the NOS score, two studies were rated as high quality, 43 studies were rated as moderate quality, and 17 studies were rated as low quality (Supplementary Table S4).
Data on α-diversity were contributed by 42 studies, encompassing metrics of richness [observed species, abundance-based coverage estimator (ACE), Chao1, richness, and Good’s coverage), evenness (Shannon index, Simpson index, Pielou’s evenness, and evenness), and biodiversity (Faith’s phylogenetic diversity). The most frequently used measures were the observed species, ACE, Chao1, Shannon index, and Simpson index. Inspection of funnel plots revealed some asymmetry (Supplementary Figure S1), but no statistically significant results were observed using Egger’s test (Supplementary Table S10).
In terms of richness, data on observed species in patients (n = 644) compared to healthy individuals (n = 773) were provided by 16 studies (11, 12, 21, 23, 25–27, 29–33, 35, 44, 49, 50). The aggregated assessment indicated no significant differences between the groups (SMD = −0.16, 95% CI = −0.40 to 0.08, p = 0.20), with considerable heterogeneity (I2 = 76%). When analyzing individual diseases, a significant reduction in gut microbiota richness was found exclusively in MG patients (SMD = −0.74, 95% CI = −1.38 to −0.09, p = 0.025) (Figure 2A). ACE data were available from 11 studies (4, 5, 11, 21, 25, 29, 31, 32, 41, 49, 55), comparing patients with autoimmune neurological diseases (n = 390) to controls (n = 378). The overall estimate for combined autoimmune neurological diseases showed a decline in ACE compared to controls, albeit with a small effect size (SMD = −0.26, 95% CI = −0.58 to 0.07, p = 0.12; I2 = 80%), which did not reach statistical significance. However, a moderate significant decrease was observed in MG patients individually (SMD = −0.79, 95% CI = −1.53 to −0.06, p = 0.03; I2 = 74%) (Figure 2B). Chao1 data were reported in 22 studies (4–6, 11, 12, 21, 23, 25, 27, 29–33, 41, 44, 49, 55, 61, 63, 72, 73), encompassing patients with autoimmune neurological diseases (n = 942) and controls (n = 866). Overall, there was a significant reduction in Chao1, though the effect size was small (SMD = −0.26, 95% CI = −0.45 to −0.07, p < 0.01), with high heterogeneity (I2 = 73%). When focusing on specific diseases, a significant reduction was also only found in MG (SMD = −0.73, 95% CI = −1.19 to −0.27, p < 0.01; I2 = 69%) (Figure 2C). Regarding evenness, the Shannon index was reported in 38 studies (4–6, 11–13, 20, 21, 23–27, 29–33, 35, 41, 42, 44, 49, 51–53, 55, 60, 61, 63–69, 72, 73), comparing patients (n = 2,101) to controls (n = 1,754). The combined assessment revealed no significant difference (SMD = −0.09, 95% CI = −0.23 to 0.05, p = 0.21). When examining specific diseases, a significant decrease was only observed in the AIE group (SMD = −0.39, 95% CI = −0.74 to −0.04, p = 0.03; I2 = 57%) (Figure 3A). Data on the Simpson index were supplied by 22 studies (4, 5, 11, 20, 21, 23, 25–27, 29–31, 33, 41, 44, 49, 51, 52, 55, 62, 63, 73), comparing patients (n = 712) to controls (n = 664). No significant change between the groups was observed (SMD = 0.07, 95% CI = −0.15 to 0.28, p = 0.48; I2 = 75%) (Figure 3B).
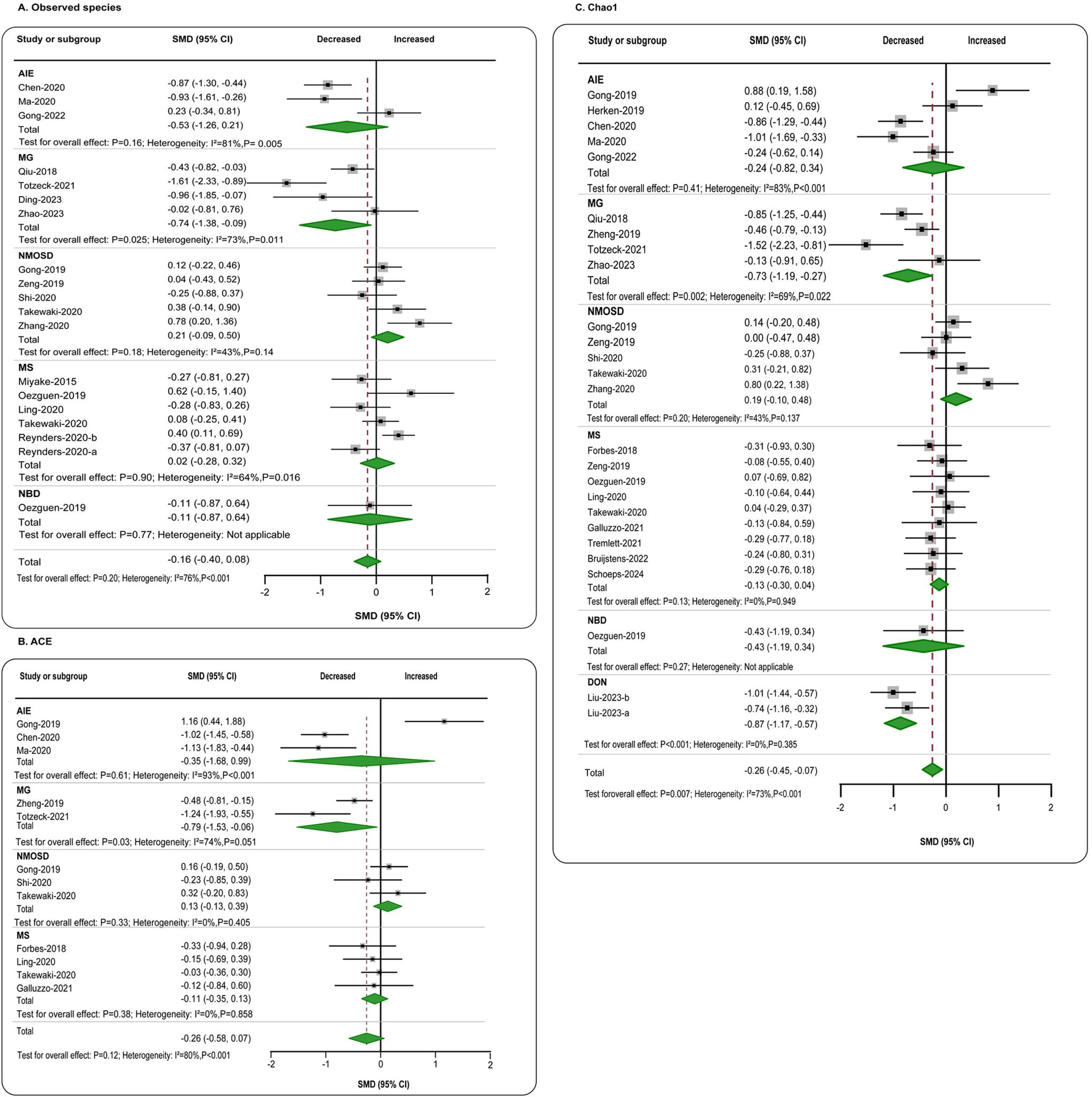
Figure 2. Forest plots of α-diversity richness estimators in the gut microbiota of patients with autoimmune neurological diseases compared with healthy controls. (A) Shannon index; (B) Simpson index. AIE, autoimmune encephalitis; MG, myasthenia gravis; NMOSD, optic neuromyelitis optica spectrum disorders; MS, multiple sclerosis; NBD, neuro-Behcet’s disease; DON, demyelinating optic neuritis; CI, confidence interval; NA, not applicable.
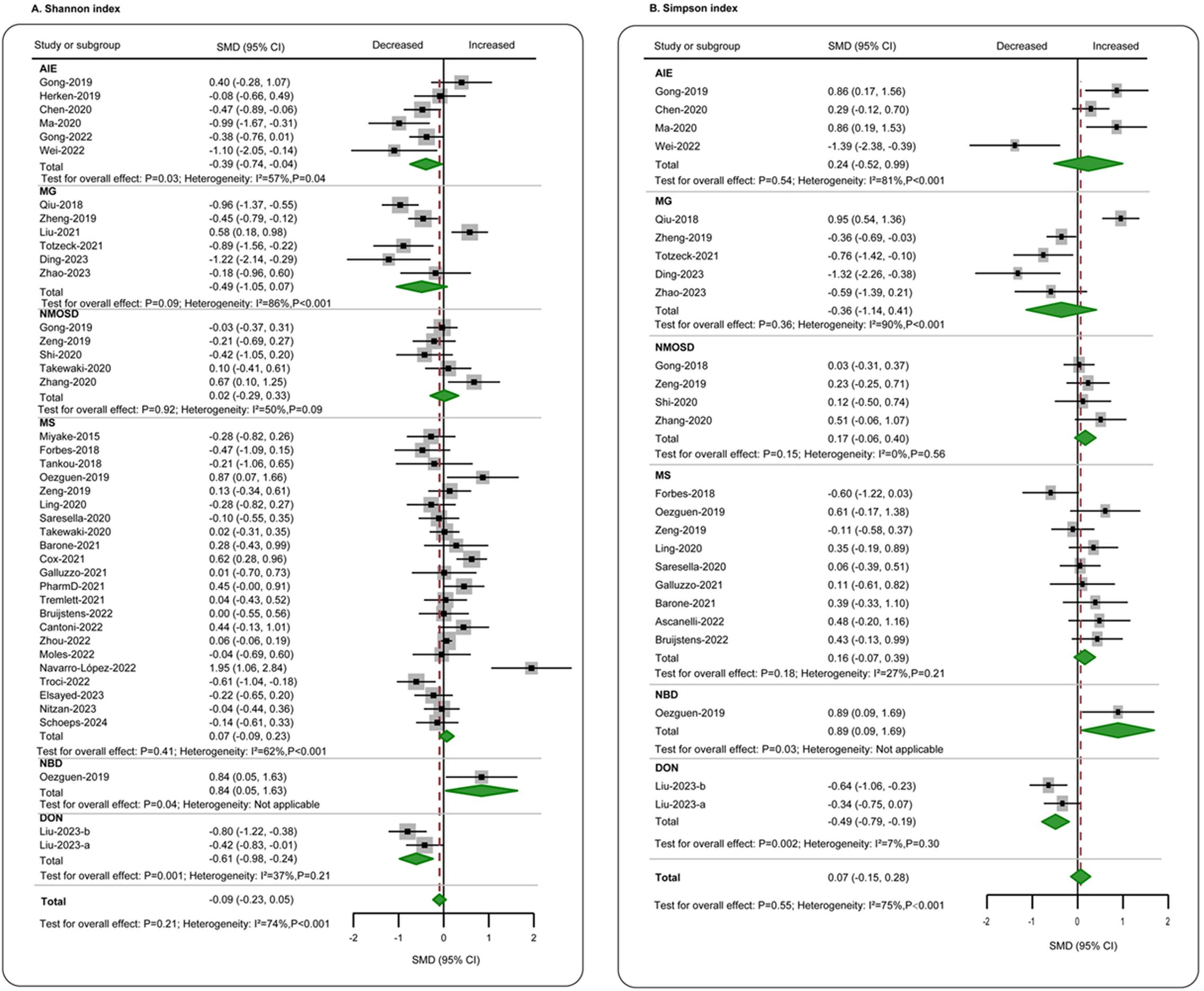
Figure 3. Forest plots of α-diversity evenness estimators in the gut microbiota of patients with autoimmune neurological diseases compared with healthy controls. (A) Shannon index; (B) Simpson index. AIE, autoimmune encephalitis; MG, myasthenia gravis; NMOSD, optic neuromyelitis optica spectrum disorders; MS, multiple sclerosis; NBD, neuro-Behcet’s disease; DON, demyelinating optic neuritis; CI, confidence interval; NA, not applicable.
To explore the reasons for the higher heterogeneity, subgroup analyses were conducted. In the subgroup analyses of Eastern and Western studies, a minor difference in the Shannon index was noted solely in the Eastern population (SMD = −0.27, 95% CI = −0.47 to −0.07, p < 0.01; I2 = 72%) (Supplementary Table S6). Most α-diversity indices exhibited no significant difference, and substantial heterogeneity persisted (I2 ranged from 46% to 85%). In the subgroup analyses of treatment-naive patients versus those on treatment, a decrease in most α-diversity indices was evident only in treatment-naive patients. Nevertheless, substantial heterogeneity remained (I2 ranged from 44% to 82%) (Supplementary Table S7). Additionally, sensitivity analyses were conducted by excluding low-quality studies (Supplementary Table S8) and studies without variable matching (Supplementary Table S9). Consistent with the overall analysis, only Chao1 remained significantly decreased in individuals with autoimmune neurological diseases, indicating that the data are robust.
A comparison of β-diversity between patients with autoimmune neurological disorders and healthy individuals was conducted across 53 studies encompassing six distinct diseases (AIE, MG, NMOSD, MS, NBD, and DON) using varied methodologies (Figure 4). Significant changes in β-diversity between patients with autoimmune neurological disorders and healthy individuals were reported in 4 out of 5 studies on anti-NMDAR encephalitis, 1 study on anti-LGI1 encephalitis, 5 out of 7 studies on MG, all 7 studies on NMOSD, 21 out of 34 studies on MS, 1 study on NBD, and 1 study on DON. Notably, among these disorders, only NMOSD showed consistently distinct β-diversity.
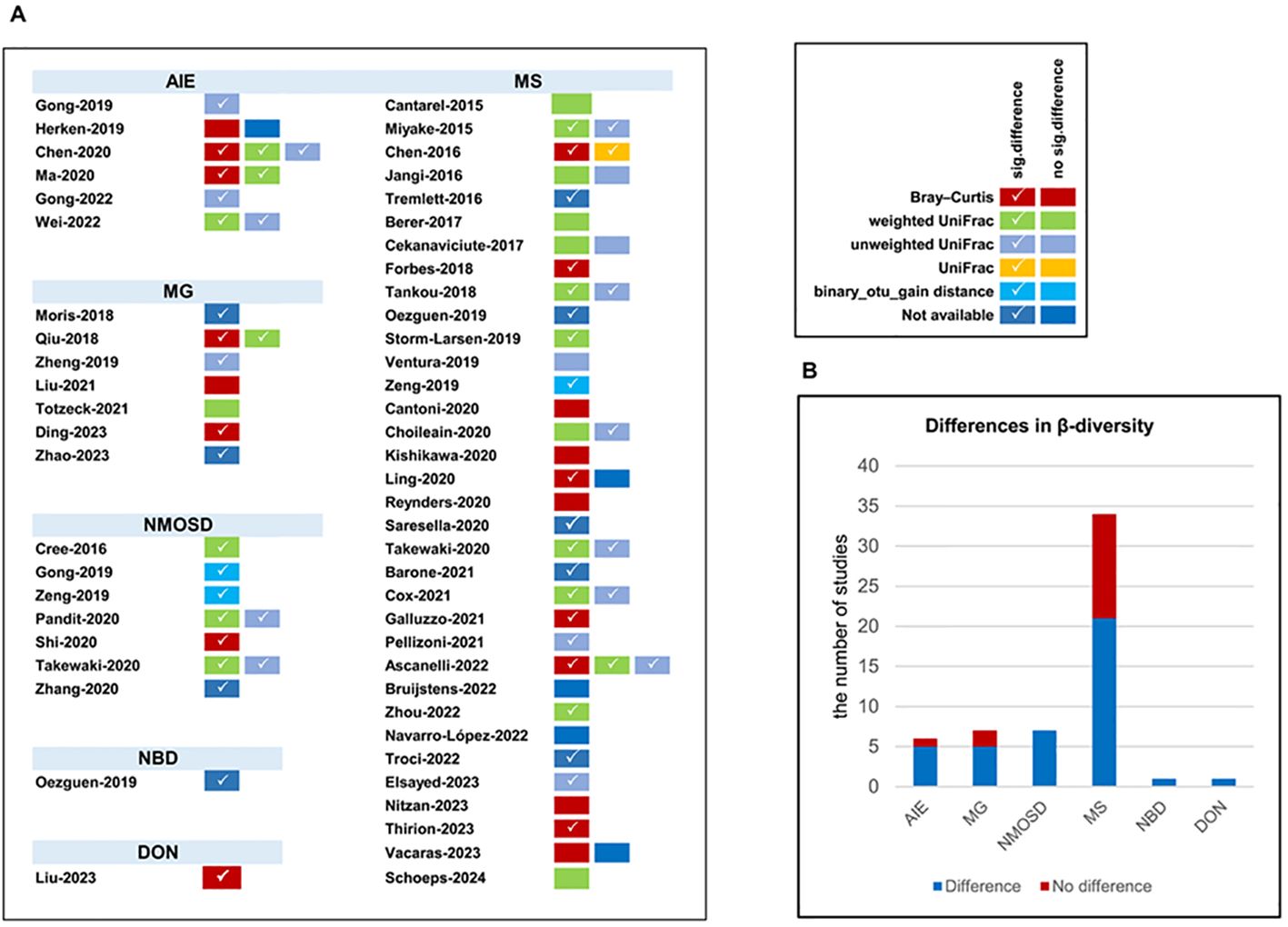
Figure 4. The β-diversity comparison between patients with autoimmune neurological diseases compared with healthy controls. (A) β-diversity comparison in included studies; (B) distribution of β-diversity in neurological autoimmune diseases. AIE, autoimmune encephalitis; MG, myasthenia gravis; NMOSD, optic neuromyelitis optica spectrum disorders; MS, multiple sclerosis; NBD, neuro-Behcet’s disease; DON, demyelinating optic neuritis. Number of studies: autoimmune encephalitis (AIE), 5/6; myasthenia gravis (MG), 5/7; optic neuromyelitis optica spectrum disorders (NMOSD), 7/7; multiple sclerosis (MS), 21/34; neuro-Behcet’s disease (NBD), 1/1; demyelinating optic neuritis (DON), 1/1.
A total of 55 studies compared the relative abundance of gut microbiota at the genus, family, or phylum levels between patients with autoimmune neurological disorders and healthy individuals. These alterations encompassed 11 phyla, 32 families, and 88 genera after excluding non-replicated findings (Supplementary Figure S2). Within-disease and between-disease comparisons were summarized for diseases with sufficient research (AIE, MG, NNMOSD, and MS reported by two or more studies). The findings suggest that specific microorganisms undergo common changes across multiple autoimmune neurological diseases, although most consistent disease-related changes were replicated by only two studies. At the phylum level, the most consistent alterations were an increase in Proteobacteria (8 of 8 studies) and a decrease in Firmicutes (8 of 8 studies) in AIE, MG, and NMOSD, and an increase in Verrucomicrobia (9 of 10 studies) in AIE, NMOSD, and MS. At the family level, Ruminococcaceae (11 of 13 studies) and Lachnospiraceae (14 of 16 studies) consistently showed decreases, while Streptococcaceae showed an increase (10 of 11 studies) in AIE, MG, NMOSD, and MS. At the genus level, the most consistent alterations were an increase in Streptococcus (16 of 18 studies) and Escherichia-Shigella (12 of 13 studies), and a decrease in Roseburia (17 of 18 studies) and Faecalibacterium (23 of 24 studies) (Figure 5) in AIE, MG, NMOSD, and MS.
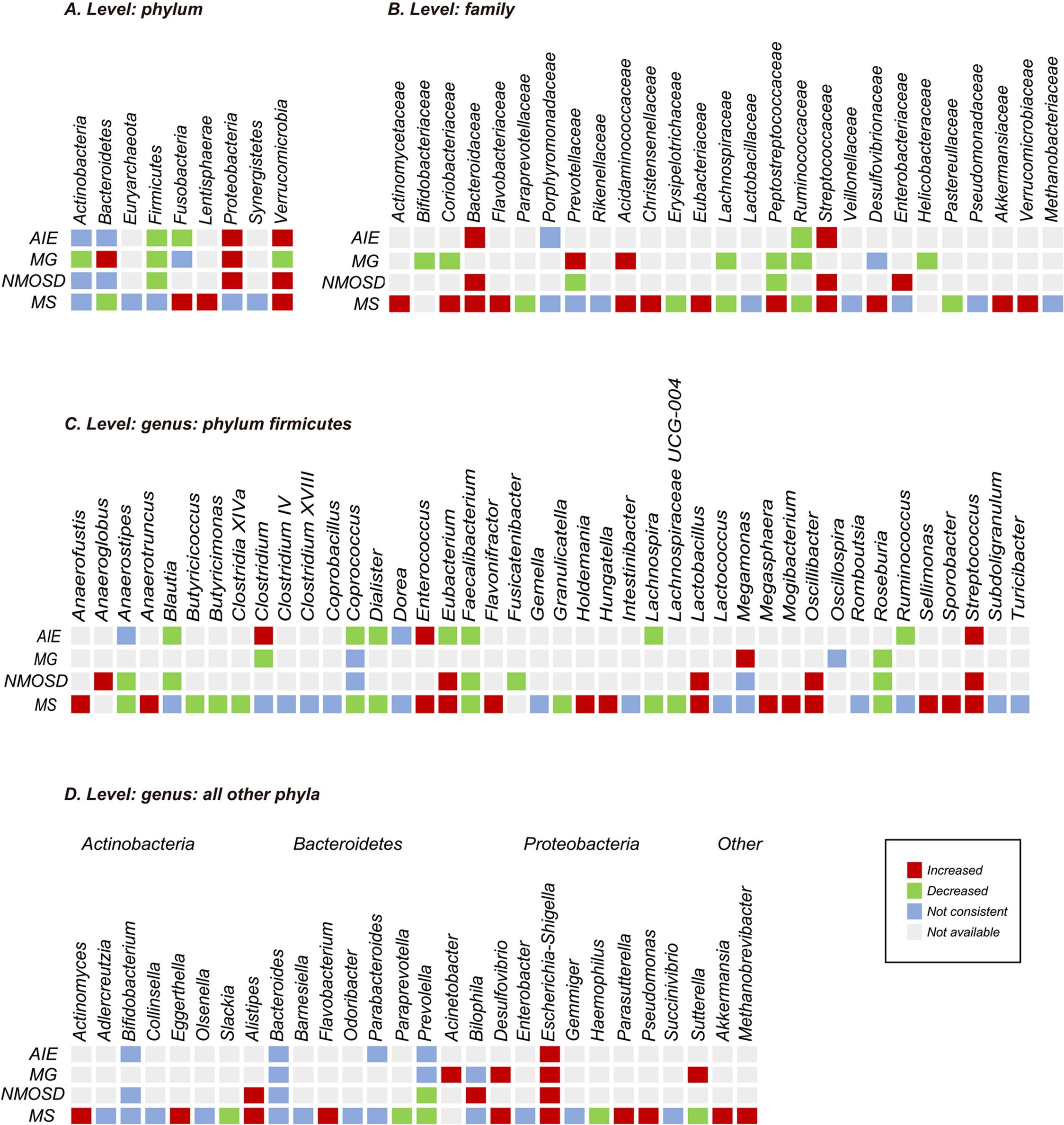
Figure 5. Changes in relative abundance of microbial taxa reported by at least two studies from a diagnostic category. (A) Phylum level; (B) family level; (C) genus level: phylum firmicutes; (D) genus level: all other phyla. All have been reported by more than one research group. The red and green grids indicate increase and decrease, respectively. Purple grids indicate inconsistency that was defined as less than 75% agreement between studies. Gray grids indicate not examined, not reported, or not replicated. Number of studies: autoimmune encephalitis (AIE), 6; myasthenia gravis (MG), 6; optic neuromyelitis optica spectrum disorders (NMOSD), 7; multiple sclerosis (MS), 35.
In subgroup analyses, microbial changes were found only in studies from Eastern countries, including an increase in genus Acinetobacter and decreases in genera Clostridium_XIV, Granulicatella, and Megasphaera. In contrast, decreases in the genus Slackia and increases in the genera Holdemania, Megasphaera, and Olsenella were observed in studies from Western countries. In subgroup analyses of immunotherapy use, comparisons were made between studies without treatment (n = 22) and those with 80% or more of patients on medication (n = 13). Increases in the genera Acinetobacter, Collinsella, Enterococcus, Escherichia, Fusicatenibacter, Succinivibrio, and Sutterella, along with decreases in the family Rikenellaceae and the genera Gemella and Haemophilus, were exclusively reported in treatment-naive studies. However, these findings should be regarded as preliminary due to the uneven distribution of studies by region (e.g., AIE, MG, and NMOSD were predominantly studied in Eastern populations) and immunotherapy use (e.g., AIE and MG were predominantly studied in treatment-naive patients, while NMOSD was predominantly studied in patients receiving immunotherapy).
Our review undertook a comprehensive assessment of the alterations observed in the intestinal microbiota associated with autoimmune neurological diseases. We included 62 studies covering six neurological autoimmune illnesses, indicating a correlation between gut microbiota dysbiosis and autoimmune neurological disorders in general. The findings predominantly reflect non-specific, shared microbial changes rather than disease-specific patterns. We found that (1) there were no significant alterations in overall α-diversity indices across autoimmune neurological diseases, though a reduction in α-diversity was noted in treatment-naive patients; (2) although most studies reported significant variations in β-diversity, only NMOSD consistently exhibited differences; and (3) a reduction in SCFA-producing bacteria, including Faecalibacterium and Roseburia, was observed in patients with AIE, MG, NMOSD, and MS, alongside an increase in pathogenic or opportunistic pathogens, including Streptococcus and Escherichia-Shigella. Disease-specific microbial changes were not detected, and even when specific microbial changes were observed, they were rarely replicated across studies.
A decrease in α-diversity is commonly observed in various diseases and is believed to contribute to their development. Higher α-diversity is generally seen as an indicator of better gut health. However, no significant reduction in α-diversity was detected in autoimmune neurological diseases as a whole, with the exception of MG. A meta-analysis by PLASSAIS et al. also noted that gut microbiota α-diversity does not serve as a biomarker for MS and Parkinson’s disease (14). It appears that α-diversity is an unreliable biomarker for autoimmune neurological diseases. However, as noted by Ashley Shade, the oversimplified view that “higher diversity is better” overlooks the intricate mechanisms underlying microbiota and host interactions (74). The α-diversity is affected by numerous factors, including measurement methods, environment, and medication use (75). In subgroup analyses of studies focusing on untreated patients, slight decreases in α-diversity were observed, suggesting that patients with autoimmune neurological diseases experience gut dysbiosis, and immunotherapy may recover the microbial diversity. However, these findings should be viewed with caution given the considerable heterogeneity and potential publication bias among the included studies. The β-diversity analyses consistently demonstrated distinct patterns in each autoimmune neurological disease compared to healthy controls. However, it remains unclear whether autoimmune neurological diseases differ significantly among themselves or from other diseases. Only five studies conducted cross-diagnostic comparisons of β-diversity, revealing no significant differences (25, 28, 30, 32, 41, 44). Furthermore, these analyses did not specify the nature or extent of the differences. Further research is required to effectively utilize diversity as a diagnostic tool.
The brain–gut axis facilitates bidirectional gut–brain regulation through a variety of processes such as chemical signaling, neuronal pathways, and immunological modulation (76). Among these, immunomodulatory effects play a crucial role. Gut microbiota not only regulates the peripheral immune response by disrupting gut barrier integrity and altering metabolite synthesis but also participates in the immune response of the central nervous system. It influences the development, maturation, and activation of microglia and increases the permeability of the blood–brain barrier (BBB). Disruption of the intestinal barrier can cause systemic inflammation by disrupting T- and B-cell homeostasis. Additionally, microbial metabolites, such as short-chain fatty acids (SCFAs), exhibit direct immunomodulatory effects (77). Our findings indicate that similar microbial changes may be shared across autoimmune neurological diseases. Roseburia and Faecalibacterium were reduced, whereas Streptococcus and Escherichia-Shigella were elevated in patients with AIE, MG, NMOSD, and MS. Faecalibacterium and Roseburia are known producers of SCFAs, notably butyrate. Faecalibacterium, a Gram-positive bacterium with potent anti-inflammatory properties, has been shown to suppress TNF-α expression and alleviate inflammation in various immunological and neurological diseases (78, 79). Roseburia generates SCFAs, mainly butyrate, by the fermentation of dietary fibers, and has been observed to decrease in various disorders (80). SCFAs play multifaceted roles in affecting gastrointestinal physiology, peripheral immunity, activation of microglia, and BBB integrity, thereby indirectly influencing brain function. Research has shown that SCFAs can initiate metabolic and epigenetic reprogramming, potentially attenuating inflammatory immune responses (81). In the included studies, patients with autoimmune neurological diseases exhibited significantly lower levels of SCFAs in both their blood and feces compared to healthy individuals (13, 22–24, 29–31, 51, 64, 65). SCFAs can alleviate clinical symptoms by increasing IL-10 production and inhibiting T helper 17 (Th17) cells (82). In contrast, Streptococcus, known for its association with inflammation, is positively correlated with Th17 cell and negatively with regulatory T cells. It also produces neurotoxins, such as streptokinase and streptomycin, which may cause irreversible neuronal damage (83). Streptococcus level was reported to be increased in immunotherapy-naive studies, but decreased in treatment studies (59, 62, 78). Similarly, Escherichia-Shigella has been associated with proinflammatory processes in various disorders, including acute intestinal damage, increased BBB permeability, and neuroinflammation. The findings indicate a decrease in SCFA-producing bacteria, including Roseburia and Faecalibacterium, alongside an increase in pathogenic or opportunistic pathogens including Streptococcus and Escherichia-Shigella in patients with autoimmune neurological diseases. These consistent inflammation-associated changes in gut flora across autoimmune neurological diseases may elucidate shared pathophysiological mechanisms and potentially exacerbate persistent inflammation within the nervous system.
Interestingly, Akkermansia has shown potential in alleviating various diseases such as obesity, diabetes, and epilepsy, and has been considered a potential next-generation probiotic (84–86). However, its role in autoimmune neurological disorders remains ambiguous. Elevated Akkermansia level has been reported in patients with MS and NMOSD, with several studies indicating a positive correlation between its abundance with disease severity (51, 62, 87). Extracts of Akkermansia muciniphila have been shown to promote T-cell differentiation into Th1 cells, contributing to proinflammatory responses (9). Additionally, Vallino et al. reported that the cerebrospinal fluid of patients with MS contained higher levels of anti-A. muciniphila IgG than those of healthy individuals (88). Conversely, Cox et al. reported a negative correlation between Akkermansia abundance and disability, as well as T2 lesion volume, while observing a positive correlation with brain volume in MS patients. Furthermore, MS-derived Akkermansia was found to improve experimental autoimmune encephalomyelitis via lowering RORγt+ and IL-17-producing γδ T cells, indicating a potentially beneficial, compensatory response of the microbiome in MS (53). Liu and colleagues further demonstrated that administering miR-30d orally in mice augmented Treg counts and alleviated symptoms of experimental autoimmune encephalomyelitis by enhancing Akkermansia level in the gastrointestinal tract (89). Akkermansia’s anti-inflammatory properties may be strain-specific, as suggested by recent findings (90). With advancements in technology, its role in autoimmune neurological diseases may become better understood.
Various clinical and demographic factors may account for the inconsistencies observed across studies. Based on current evidence, we concentrated on two key aspects: geographical region and immunotherapy. Geographical region, along with diet as a related factor, play a significant role in shaping the composition of the microbiota (91). Our analysis revealed that some microbiota changes appear to be population-specific, such as higher levels of Megasphaera and Olsenella in Western populations and Acinetobacter in Eastern populations. We also recognize significant regional variations in disease incidence rates. MS, which has a higher global incidence, is more prevalent in Western countries, leading to greater representation in the studies analyzed. Conversely, NMOSD, AIE, and MG are less common, with NMOSD showing a higher incidence among non-White populations, driving focused research efforts in regions like China (92–94). These disparities may influence the analysis and limit the generalizability of the findings. Furthermore, we observed microbiota alterations associated with immunotherapy, such as decreased Streptococcus and increased Prevotella. To better differentiate between the effects of confounders and genuine disease-related changes, it is imperative that forthcoming studies provide exhaustive data encompassing all predominant microbial taxa, as well as variations across diverse countries and regions.
Our findings suggest that disturbances in the gut microbiota are related to autoimmune neurological diseases, with shared microbiota changes observed across different conditions. However, some limitations should be acknowledged. Firstly, our review may be subject to language bias, as only English-language studies were included, potentially excluding significant research from non-English-speaking regions. Secondly, this review focused solely on gut microbiota composition without establishing causality. Geographical differences were observed in the studies, with MS predominantly studied in Western countries and AIE, MG, and NMOSD predominantly studied in China. While the categorization of countries into Eastern and Western regions serves as a basic method to address differences in diet and genetics, this approach remains crude. Additionally, most studies had relatively small to medium sample sizes, suggesting that analysis may still be preliminary and inconclusive. At the same time, differences in the methods of analysis can also lead to variations in results. Furthermore, given that the majority of research encompassed individuals receiving diverse immunotherapies, as well as those not on any treatment, inconsistency in data collection on covariates among studies and the lack of control for possible confounders in some analyses also pose limitations (95). As research is conducted on larger samples, encompassing a wider range of regions and population groups, and with increasing availability of metadata, there will be a corresponding enhancement in the reliability of findings. Concurrently, it is recommended that further studies should include longitudinal studies to elucidate the chronological interactions among gut microbiota, disease evolution, and therapeutic strategies. Finally, it is noteworthy that most included studies utilized 16s rRNA sequencing to analyze intestinal flora. As the field evolves, employing alternative histologic techniques will facilitate a deeper exploration of the gut microbiota beyond compositional analysis. Notwithstanding the aforementioned limitations, the findings from the majority of studies provide a critical foundation for understanding the role of gut microbiota in autoimmune neurological disorders.
This study provides the first comprehensive assessment of gut microbiota changes in autoimmune neurological disorders. The existing evidence suggests that gut microbiota dysbiosis is associated with these conditions, primarily due to potential non-specific changes in microorganisms, including decreased SCFA-producing bacteria with anti-inflammatory capacity, and increased pathogenic or opportunistic pathogens. These insights provide a promising foundation for diagnosing these diseases and developing novel therapeutic strategies targeting the gut microbiota to manage or even prevent these conditions.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
XD: Conceptualization, Data curation, Formal analysis, Writing – original draft. XG: Data curation, Formal Analysis, Conceptualization, Writing – review & editing. DZ: Writing – review & editing. ZH: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by China’s National Key R&D Program (Grant No. 2022YFC2503800), the National Natural Science Foundation of China (Grant No. 82471388), the Clinical Research Incubation Project of West China Hospital of Sichuan University (Grant No. 22HXFH022), the Sichuan Science and Technology Program (Grant No. 2024YFHZ0056), the Postdoctoral Fellowship Program of CPSF (Grant No. GZC20241130), and the China Postdoctoral Science Foundation (Grant No. 2024M752236).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1513599/full#supplementary-material
MS, multiple sclerosis; NMOSD, optic neuromyelitis optica spectrum disorders; MG, myasthenia gravis; AIE, autoimmune encephalitis; NBD, neuro-Behcet’s disease; NOS, Newcastle–Ottawa Quality Assessment Scale; SMD, standardized mean difference; CI, confidence interval; 16S rRNA, 16S ribosomal RNA; ACE, abundance-based coverage estimator; BBB, blood–brain barrier; SCFAs, short-chain fatty acids; Th17, T helper 17 cells.
1. Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. (2021) 78:1343–54. doi: 10.1001/jamapsychiatry.2021.2573
2. Wang Y, Wei J, Zhang W, Doherty M, Zhang Y, Xie H, et al. Gut dysbiosis in rheumatic diseases: A systematic review and meta-analysis of 92 observational studies. eBioMedicine. (2022) 80:104055. doi: 10.1016/j.ebiom.2022.104055
3. Noto D, Miyake S. Gut dysbiosis and multiple sclerosis. Clin Immunol. (2022) 235:108380. doi: 10.1016/j.clim.2020.108380
4. Zheng P, Li Y, Wu J, Zhang H, Huang Y, Tan X, et al. Perturbed microbial ecology in myasthenia gravis: evidence from the gut microbiome and fecal metabolome. Adv Sci (Weinh). (2019) 6:1901441. doi: 10.1002/advs.201901441
5. Gong X, Liu X, Li C, Chen C, Lin J, Li A, et al. Alterations in the human gut microbiome in anti-N-methyl-D-aspartate receptor encephalitis. Ann Clin Transl Neurol. (2019) 6:1771–81. doi: 10.1002/acn3.50874
6. Herken J, Bang C, Rühlemann MC, Finke C, Klag J, Franke A, et al. Normal gut microbiome in NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e632. doi: 10.1212/nxi.0000000000000632
7. Pandit L, Cox LM, Malli C, D’Cunha A, Rooney T, Lokhande H, et al. Clostridium bolteae is elevated in neuromyelitis optica spectrum disorder in India and shares sequence similarity with AQP4. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e907. doi: 10.1212/nxi.0000000000000907
8. Gong X, Ma Y, Deng X, Li A, Li X, Kong X, et al. Intestinal dysbiosis exacerbates susceptibility to the anti-NMDA receptor encephalitis-like phenotype by changing blood brain barrier permea bility and immune homeostasis. Brain Behav Immun. (2024) 116:34–51. doi: 10.1016/j.bbi.2023.11.030
9. Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria frommultiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. (2017) 114:10713–8. doi: 10.1073/pnas.1711235114
10. Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. (2022) 18:544–58. doi: 10.1038/s41582-022-00697-8
11. Chen H, Chen Z, Shen L, Wu X, Ma X, Lin D, et al. Fecal microbiota transplantation from patients with autoimmune encephalitis modulates Th17 response and relevant behaviors in mice. Cell Death Discovery. (2020) 6:75. doi: 10.1038/s41420-020-00309-8
12. Gong X, Liu Y, Liu X, Li A, Guo K, Zhou D, et al. Disturbance of gut bacteria and metabolites are associated with disease severity and predict outcome of NMDAR encephalitis: a prospective case-control study. Front Immunol. (2022) 12:791780. doi: 10.3389/fimmu.2021.791780
13. Zhou X, Baumann R, Gao X, Mendoza M, Singh S, Sand IK, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. (2022) 185:3467–86.e16. doi: 10.1016/j.cell.2022.08.021
14. Plassais J, Gbikpi-Benissan G, Figarol M, Scheperjans F, Gorochov G, Derkinderen P, et al. Gut microbiome alpha-diversity is not a marker of Parkinson’s disease and multiple sclerosis. Brain Commun. (2021) 3:fcab113. doi: 10.1093/braincomms/fcab113
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Cochrane. (2024). www.training.cochrane.org/handbook.
17. Liewluck T, Miravalle A. Immune-mediated neurological disorders. Curr Neurol Neurosci Rep. (2015) 15:61. doi: 10.1007/s11910-015-0581-x
18. Stanislawski MA, Dabelea D, Lange LA, Wagner BD, Lozupone CA. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes. (2019) 5:18. doi: 10.1038/s41522-019-0091-8
19. Wells G, Shea B, O’Connell J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Res Institute Web Site. (2014) 7.
20. Wei J, Zhang X, Yang F, Shi X, Wang X, Chen R, et al. Gut microbiome changes in anti-N-methyl-D-aspartate receptor encephalitis patients. BMC Neurol. (2022) 22:276. doi: 10.1186/s12883-022-02804-0
21. Ma X, Ma L, Wang Z, Liu Y, Long L, Ma X, et al. Clinical features and gut microbial alterations in Anti-leucine-rich glioma-Inactivated 1 encephalitis-A pilot study. Front Neurol. (2020) 11:585977. doi: 10.3389/fneur.2020.585977
22. Moris G, Arboleya S, Mancabelli L, Milani C, Ventura M, de-Los-Reyes-Gavilán CG, et al. Fecal microbiota profile in a group of myasthenia gravis patients. Sci Rep. (2018) 8:14384. doi: 10.1038/s41598-018-32700-y
23. Qiu D, Xia Z, Jiao X, Deng J, Zhang L, Li J. Altered gut microbiota in myasthenia gravis. Front Microbiol. (2018) 9:2627. doi: 10.3389/fmicb.2018.02627
24. Liu P, Jiang Y, Gu S, Xue Y, Yang H, Li Y, et al. Metagenome-wide association study of gut microbiome revealed potential microbial marker set for diagnosis of pediatric myasthenia gravis. BMC Med. (2021) 19:159. doi: 10.1186/s12916-021-02034-0
25. Totzeck A, Ramakrishnan E, Schlag M, Stolte B, Kizina K, Bolz S, et al. Gut bacterial microbiota in patients with myasthenia gravis: results from the MYBIOM study. Ther Adv Neurol Disord. (2021) 14:17562864211035657. doi: 10.1177/17562864211035657
26. Ding XJ, Li HY, Wang H, Zhang XH, Song M, Jiang XH, et al. Altered gut microbiota and metabolites in untreated myasthenia gravis patients. Front Neurol. (2023) 14:1248336. doi: 10.3389/fneur.2023.1248336
27. Zhao M, Liu L, Liu F, Liu L, Liu Z, Gao Y, et al. Traditional Chinese medicine improves myasthenia gravis by regulating the symbiotic homeostasis of the intestinal microbiota and host. Front Microbiol. (2023) 13:1082565. doi: 10.3389/fmicb.2022.1082565
28. Cree BA, Spencer CM, Varrin-Doyer M, Baranzini SE, Zamvil SS. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann Neurol. (2016) 80:443–7. doi: 10.1002/ana.24718
29. Gong J, Qiu W, Zeng Q, Liu X, Sun X, Li H, et al. Lack of short-chain fatty acids and overgrowth of opportunistic pathogens define dysbiosis of neuromyelitis optica spectrum disorders: A Chinese pilot study. Mult Scler. (2019) 25:1316–25. doi: 10.1177/1352458518790396
30. Zeng Q, Junli G, Liu X, Chen C, Sun X, Li H, et al. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem Int. (2019) 129:104468. doi: 10.1016/j.neuint.2019.104468
31. Shi Z, Qiu Y, Wang J, Fang Y, Zhang Y, Chen H, et al. Dysbiosis of gut microbiota in patients with neuromyelitis optica spectrum disorders: A cross sectional study. J Neuroimmunol. (2020) 339:577126. doi: 10.1016/j.jneuroim.2019.577126
32. Takewaki D, Suda W, Sato W, Takayasu L, Kumar N, Kimura K, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci U S A. (2020) 117:22402–12. doi: 10.1073/pnas.2011703117
33. Zhang J, Xu YF, Wu L, Li HF, Wu ZY. Characteristic of gut microbiota in southeastern Chinese patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. (2020) 44:102217. doi: 10.1016/j.msard.2020.102217
34. Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. (2015) 63:729–34. doi: 10.1097/jim.0000000000000192
35. Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV Clusters. PloS One. (2015) 10:e0137429. doi: 10.1371/journal.pone.0137429
36. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. (2016) 6:28484. doi: 10.1038/srep28484
37. Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. (2016) 7:12015. doi: 10.1038/ncomms12015
38. Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. (2016) 23:1308–21. doi: 10.1111/ene.13026
39. Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. (2017) 114:10719–24. doi: 10.1073/pnas.1711233114
40. Swidsinski A, Doerffel Y, Loening-Baucke V, Gille C, Goektas O, Reisshauer A, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front Microbiol. (2017), 8:1141. doi: 10.3389/fmicb.2017.01141
41. Forbes JD, Chen CY, Knox NC, Marrie RA, El-Gabalawy H, de Kievit T, et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome. (2018) 6:221. doi: 10.1186/s40168-018-0603-4
42. Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. (2018) 83:1147–61. doi: 10.1002/ana.25244
43. Kozhieva M, Naumova N, Alikina T, Boyko A, Vlassov V, Kabilov MR. Primary progressive multiple sclerosis in a Russian cohort: relationship with gut bacterial diversity. BMC Microbiol. (2019) 19:309. doi: 10.1186/s12866-019-1685-2
44. Oezguen N, Yalcinkaya N, Kücükali CI, Dahdouli M, Hollister EB, Luna RA, et al. Microbiota stratification identifies disease-specific alterations in neuro-Behçet’s disease and multiple sclerosis. Clin Exp Rheumatol. (2019) 37 Suppl 121:58–66.
45. Storm-Larsen C, Myhr KM, Farbu E, Midgard R, Nyquist K, Broch L, et al. Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis - a pilot trial. Mult Scler J Exp Transl Clin. (2019) 5:2055217319888767. doi: 10.1177/2055217319888767
46. Ventura RE, Iizumi T, Battaglia T, Liu M, Perez-Perez GI, Herbert J, et al. Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci Rep. (2019) 9:16396. doi: 10.1038/s41598-019-52894-z
47. Choileáin SN, Kleinewietfeld M, Raddassi K, Hafler DA, Ruff WE, Longbrake EE. CXCR3+ T cells in multiple sclerosis correlate with reduced diversity of the gut microbiome. J Transl Autoimmun. (2020) 3:100032. doi: 10.1016/j.jtauto.2019.100032
48. Kishikawa T, Ogawa K, Motooka D, Hosokawa A, Kinoshita M, Suzuki K, et al. A metagenome-wide association study of gut microbiome in patients with multiple sclerosis revealed novel disease pathology. Front Cell Infect Microbiol. (2020) 10:585973. doi: 10.3389/fcimb.2020.585973
49. Ling Z, Cheng Y, Yan X, Shao L, Liu X, Zhou D, et al. Alterations of the fecal microbiota in Chinese patients with multiple sclerosis. Front Immunol. (2020) 11:590783. doi: 10.3389/fimmu.2020.590783
50. Reynders T, Devolder L, Valles-Colomer M, Van Remoortel A, Joossens M, De Keyser J, et al. Gut microbiome variation is associated to Multiple Sclerosis phenotypic subtypes. Ann Clin Transl Neurol. (2020) 7:406–19. doi: 10.1002/acn3.51004
51. Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol. (2020) 11:1390. doi: 10.3389/fimmu.2020.01390
52. Barone M, Mendozzi L, D’Amico F, Saresella M, Rampelli S, Piancone F, et al. Influence of a high-impact multidimensional rehabilitation program on the gut microbiota of patients with multiple sclerosis. Int J Mol Sci. (2021) 22:7173. doi: 10.3390/ijms22137173
53. Cox LM, Maghzi AH, Liu S, Tankou SK, Dhang FH, Willocq V, et al. Gut microbiome in progressive multiple sclerosis. Ann Neurol. (2021) 89:1195–211. doi: 10.1002/ana.26084
54. Elgendy SG, Abd-Elhameed R, Daef E, Mohammed SM, Hassan HM, El-Mokhtar MA, et al. Gut microbiota in forty cases of Egyptian relapsing remitting multiple sclerosis. Iran J Microbiol. (2021) 13:632–41. doi: 10.18502/ijm.v13i5.7428
55. Galluzzo P, Capri FC, Vecchioni L, Realmuto S, Scalisi L, Cottone S, et al. Comparison of the intestinal microbiome of Italian patients with multiple sclerosis and their household relatives. Life (Basel). (2021) 11:620. doi: 10.3390/life11070620
56. Levi I, Gurevich M, Perlman G, Magalashvili D, Menascu S, Bar N, et al. Potential role of indolelactate and butyrate in multiple sclerosis revealed by integrated microbiome-metabolome analysis. Cell Rep Med. (2021) 2:100246. doi: 10.1016/j.xcrm.2021.100246
57. Mekky J, Wani R, Said SM, Ashry M, Ibrahim AE, Ahmed SM. Molecular characterization of the gut microbiome in Egyptian patients with remitting relapsing multiple sclerosis. Mult Scler Relat Disord. (2022) 57:103354. doi: 10.1016/j.msard.2021.103354
58. Mirza AI, Zhu F, Knox N, Forbes JD, Van Domselaar G, Bernstein CN, et al. Metagenomic analysis of the pediatric-onset multiple sclerosis gut microbiome. Neurology. (2022) 98:e1050–e63. doi: 10.1212/wnl.0000000000013245
59. Pellizoni FP, Leite AZ, Rodrigues NC, Ubaiz MJ, Gonzaga MI, Takaoka NNC, et al. Detection of dysbiosis and increased intestinal permeability in Brazilian patients with relapsing–remitting multiple sclerosis. Int J Environ Res Public Health. (2021) 18:4621. doi: 10.3390/ijerph18094621
60. Sterlin D, Larsen M, Fadlallah J, Parizot C, Vignes M, Autaa G, et al. Perturbed microbiota/immune homeostasis in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e997. doi: 10.1212/nxi.0000000000000997
61. Tremlett H, Zhu F, Arnold D, Bar-Or A, Bernstein CN, Bonner C, et al. The gut microbiota in pediatric multiple sclerosis and demyelinating syndromes. Ann Clin Transl Neurol. (2021) 8:2252–69. doi: 10.1002/acn3.51476
62. Ascanelli S, Bombardini C, Chimisso L, Carcoforo P, Turroni S. D’Amico F et al. Trans-anal irrigation in patients with multiple sclerosis: Efficacy in treating disease-related bowel dysfunctions and impact on the gut microbiota: A monocentric prospective study. Mult Scler J Exp Transl Clin. (2022) 8:20552173221109771. doi: 10.1177/20552173221109771
63. Bruijstens AL, Molenaar S, Wong YYM, Kraaij R, Neuteboom RF. Gut microbiota analysis in pediatric-onset multiple sclerosis compared to pediatric monophasic demyelinating syndromes and pediatric controls. Eur J Neurol. (2022) 30:3507–15. doi: 10.1111/ene.15594
64. Cantoni C, Lin Q, Dorsett Y, Ghezzi L, Liu Z, Pan Y, et al. Alterations of host-gut microbiome interactions in multiple sclerosis. eBioMedicine. (2022) 76:103798. doi: 10.1016/j.ebiom.2021.103798
65. Moles L, Delgado S, Gorostidi-Aicua M, Sepúlveda L, Alberro A, Iparraguirre L, et al. Microbial dysbiosis and lack of SCFA production in a Spanish cohort of patients with multiple sclerosis. Front Immunol. (2022) 13:960761. doi: 10.3389/fimmu.2022.960761
66. Navarro-López V, Méndez-Miralles M, Vela-Yebra R, Fríes-Ramos A, Sánchez-Pellicer P, Ruzafa-Costas B, et al. Gut Microbiota as a potential predictive biomarker in relapsing-remitting multiple sclerosis. Genes (Basel). (2022) 13:930. doi: 10.3390/genes13050930
67. Troci A, Zimmermann O, Esser D, Krampitz P, May S, Franke A, et al. B-cell-depletion reverses dysbiosis of the microbiome in multiple sclerosis patients. Sci Rep. (2022) 12:3728. doi: 10.1038/s41598-022-07336-8
68. Elsayed NS, Valenzuela RK, Kitchner T, Le T, Mayer J, Tang ZZ, et al. Genetic risk score in multiple sclerosis is associated with unique gut microbiome. Sci Rep. (2023) 13:16269. doi: 10.1038/s41598-023-43217-4
69. Nitzan Z, Staun-Ram E, Volkowich A, Miller A. Multiple sclerosis-associated gut microbiome in the Israeli diverse populations: associations with ethnicity, gender, disability status, vitamin D levels, and mediterranean diet. Int J Mol Sci. (2023) 24:15024. doi: 10.3390/ijms241915024
70. Thirion F, Sellebjerg F, Fan Y, Lyu L, Hansen TH, Pons N, et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. (2023) 15:1. doi: 10.1186/s13073-022-01148-1
71. Vacaras V, Muresanu DF, Buzoianu AD, Nistor C, Vesa SC, Paraschiv AC, et al. The role of multiple sclerosis therapies on the dynamic of human gut microbiota. J Neuroimmunol. (2023) 378:578087. doi: 10.1016/j.jneuroim.2023.578087
72. Schoeps VA, Zhou X, Horton MK, Zhu F, McCauley KE, Nasr Z, et al. Short-chain fatty acid producers in the gut are associated with pediatric multiple sclerosis onset. Ann Clin Transl Neurol. (2024) 11:169–84. doi: 10.1002/acn3.51944
73. Liu Y, Fan H, Shao Y, Zhang J, Zuo Z, Wang J, et al. Gut microbiota dysbiosis associated with different types of demyelinating optic neuritis in patients. Mult Scler Relat Disord. (2023) 72:104619. doi: 10.1016/j.msard.2023.104619
74. Shade A. Diversity is the question, not the answer. ISME J. (2016) 11:1–6. doi: 10.1038/ismej.2016.118
75. Nagata N, Nishijima S, Miyoshi-Akiyama T, Kojima Y, Kimura M, Aoki R, et al. Population-level metagenomics uncovers distinct effects of multiple medications on the human gut microbiome. Gastroenterology. (2022) 163:1038–52. doi: 10.1053/j.gastro.2022.06.070
76. Morais LH, Schreiber HLT, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. (2021) 19:241–55. doi: 10.1038/s41579-020-00460-0
77. Aburto MR, Cryan JF. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat Rev Gastroenterol Hepatol. (2024) 21:222–47. doi: 10.1038/s41575-023-00890-0
78. Shao L, Fu J, Xie L, Cai G, Cheng Y, Zheng N, et al. Fecal microbiota underlying the coexistence of schizophrenia and multiple sclerosis in Chinese patients. Can J Infect Dis Med Microbiol. (2023) 2023:5602401. doi: 10.1155/2023/5602401
79. Martín R, Rios-Covian D, Huillet E, Auger S, Khazaal S, Bermúdez-Humarán LG, et al. Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol Rev. (2023) 47:fuad039. doi: 10.1093/femsre/fuad039
80. Zhao C, Bao L, Qiu M, Wu K, Zhao Y, Feng L, et al. Commensal cow Roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice. Cell Rep. (2022) 41:111681. doi: 10.1016/j.celrep.2022.111681
81. Kim CH. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol Immunol. (2023) 20:341–50. doi: 10.1038/s41423-023-00987-1
82. Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. (2019) 10:760. doi: 10.1038/s41467-019-08711-2
83. Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev. (2014) 27:264–301. doi: 10.1128/cmr.00101-13
84. Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. (2017) 23:107–13. doi: 10.1038/nm.4236
85. Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. (2018) 173:1728–41.e13. doi: 10.1016/j.cell.2018.04.027
86. Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. (2022) 19:625–37. doi: 10.1038/s41575-022-00631-9
87. Cekanaviciute E, Pröbstel AK, Thomann A, Runia TF, Casaccia P, Katz Sand I, et al. Multiple sclerosis-associated changes in the composition and immune functions of spore-forming bacteria. mSystems. (2018) 3:e00083-18. doi: 10.1128/mSystems.00083-18
88. Vallino A, Dos Santos A, Mathé CV, Garcia A, Morille J, Dugast E, et al. Gut bacteria Akkermansia elicit a specific IgG response in CSF of patients with MS. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e688. doi: 10.1212/nxi.0000000000000688
89. Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, et al. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host Microbe. (2019) 26:779–94.e8. doi: 10.1016/j.chom.2019.10.008
90. Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in Mice. Front Cell Infect Microbiol. (2019) 9:239. doi: 10.3389/fcimb.2019.00239
91. Ross FC, Patangia D, Grimaud G, Lavelle A, Dempsey EM, Ross RP, et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol. (2024) 22:671–86. doi: 10.1038/s41579-024-01068-4
92. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. (2020) 26:1816–21. doi: 10.1177/1352458520970841
93. Papp V, Magyari M, Aktas O, Berger T, Broadley SA, Cabre P, et al. Worldwide incidence and prevalence of neuromyelitis optica: a systematic review. Neurology. (2021) 96:59–77. doi: 10.1212/wnl.0000000000011153
94. Ramanathan S, Brilot F, Irani SR, Dale RC. Origins and immunopathogenesis of autoimmune central nervous system disorders. Nat Rev Neurol. (2023) 19:172–90. doi: 10.1038/s41582-023-00776-4
Keywords: gut microbiota, gut dysbiosis, autoimmune neurological diseases, systematic review, meta-analysis
Citation: Deng X, Gong X, Zhou D and Hong Z (2025) Perturbations in gut microbiota composition in patients with autoimmune neurological diseases: a systematic review and meta-analysis. Front. Immunol. 16:1513599. doi: 10.3389/fimmu.2025.1513599
Received: 18 October 2024; Accepted: 16 January 2025;
Published: 06 February 2025.
Edited by:
Wenrui Qu, Jilin University, ChinaReviewed by:
Simona Rolla, University of Turin, ItalyCopyright © 2025 Deng, Gong, Zhou and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Hong, aG9uZ3poZW5nb29nQGFsaXl1bi5jb20=
†ORCID: Dong Zhou, orcid.org/0000-0001-7101-4125
Zhen Hong, orcid.org/0000-0002-0014-6873
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.