
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 February 2025
Sec. T Cell Biology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1507800
Background: Neutrophil acts as a double-edged sword in the immune system. We hypothesized that an elevated neutrophil granule protein level is associated with sepsis-associated lymphopenia (SAL).
Methods: We enrolled 61 patients with sepsis admitted to the Department of Critical Care Medicine of Peking Union Medical College Hospital between May 2022 and October 2023 in this study. Clinical and immunological parameters were recorded. Levels of neutrophil granule proteins, including myeloperoxidase (MPO) and neutrophil elastase (NE), and pyroptosis factors were examined.
Results: Levels of neutrophil granule proteins (MPO, 82.9 vs. 175.3, p < 0 <.0001; NE, 56.3 vs. 144.2, p < 0.0001) were significantly higher in patients with sepsis with lymphopenia. Neutrophil granule protein levels were independently associated with SAL risk (MPO: OR = 1.0841, 95% CI, 1.0020–1.1730; NE: OR = 1.0540, 95% CI, 1.0040–1.1065). The area under the curve of MPO levels predicting SAL occurrence was 0.939 (95% CI, 0.846–0.984), and that of NE was 0.950 (95% CI, 0.862–0.989). Furthermore, neutrophil granule proteins were significantly correlated with CD4+ T cell and its pyroptosis [MPO and CD4+ T cells (r = −0.4039, p < 0.0001), CD4+NLRP3 (r = 0.4868, p < 0.0001), NE and CD4+ T cells (r = −0.5140, p < 0.0001), and CD4+NLRP3 (r = 0.6513, p < 0.0001)].
Conclusion: Increased levels of neutrophil granule proteins were significantly associated with SAL incidence, and a significant relationship between neutrophil granule proteins and the pyroptosis pathway of CD4+ T cells was revealed.
Clinical trial registration: chictr.org.cn identifier ChiCTR-ROC-17010750.
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (1). In this dysregulated response, many immune mechanisms that are initially activated to protect host are harmful, on account of both excessive inflammation and immune suppression (2). The mechanistic underpinnings of concurrent immune suppression and relevant immune system changes in patients with sepsis still need further exploration.
Polymorphonuclear neutrophils are traditionally regarded as professional phagocytic and acute inflammatory cells that engulf the microbial pathogens (3). As an innate immune system response, antigen presentation triggers the adaptive immune system to activate cytotoxic T lymphocytes and induce B lymphocytes to secrete specific antibodies. Upon activation, neutrophils extruded their nuclear DNA and histones in association with the attached granule proteins to form neutrophil extracellular traps (NETs) to trap the environmental foreign invaders (4). However, granule proteins not only contribute to killing bacteria within the phagosome, but also are capable of inflicting tissue damage (5), which prompted us to explore the interaction of neutrophil granule proteins with adaptive immune cells and their contribution to sepsis-associated immunosuppression.
Pyroptosis is a type of programmed cell death mediated by caspase-dependent activation of members of the gasdermin family and inflammasome formation (6). In patients with sepsis, pyroptosis is observed in multiple immune cells (7). Our previous study revealed that a reduction in CD4+ T cells is central to the immunosuppression phase of sepsis and that the canonical pyroptosis pathway obliterates CD4+ T cells (8). NETs have already been verified to interact with the NLRP3 inflammasome in septic lung injury (9, 10). In this research, we aimed to explore the relationship between neutrophil granule proteins and sepsis-associated lymphopenia (SAL) and its interaction with CD4+ T-cell pyroptosis. We hypothesized that the level of neutrophil granule proteins may reflect the occurrence of SAL through the pyroptosis pathway of CD4+ T cells.
A prospective study was performed from May 2022 to October 2023 in the Department of Critical Care Medicine of Peking Union Medical College Hospital (PUMCH). The PUMCH institutional Ethic Committee approved this study (approval number JS-1170), and all patients enrolled in this study signed informed consent. The study was registered at the Chinese Clinical Trial Registry (ChiCTR-ROC-17010750).
The inclusion criteria were as follows: (1) ≥ 18 years old, (2) ICU (intensive care unit) stay time over 1 day, and (3) diagnosis of sepsis (see below). Patients had basic immunological diseases or malignant tumors were excluded. Sepsis was diagnosed according to the definition of the Third International Consensus (1); lymphopenia was diagnosed if the total lymphocyte count was < 1.1 × 109/L according to the 2022 SAI expert consensus (11).
Basic clinical evaluation and laboratory characteristics were collected on the first day of ICU admission. Basic clinical data, including age, sex, and basic information on immunological diseases and malignant tumors, were collected. Laboratory characteristics such as routine blood biochemistry, immunological parameters such as the peripheral blood lymphocyte count and lymphocyte subset counts, the SOFA (Sequential Organ Failure Assessment) score, and the APACHE (Acute Physiology and Chronic Health Evaluation) II score were recorded. Information on life-sustaining treatment, including the need for mechanical ventilation and renal replacement, was recorded. The follow-up data were 28-day mortality rate and ICU stay time.
Neutrophil granules can be divided into four groups according to the different maturation stages (12), and the levels of myeloperoxidase (MPO) and neutrophil elastase (NE) were measured as primary or azurophil granules. The NOD-like receptor family pyrin domain containing 3 (NLRP3) is a type of cytoplasmic pattern recognition receptor that may trigger inflammasome formation. NLRP3 was measured to evaluate the inflammasome and quantify pyroptosis in cells (13, 14). As the upstream canonical activation pathway of pyroptosis, the levels of NLRP3, Caspase-1 and gasdermin D (GSDMD) were measured gasdermin D (GSDMD) were measured (15).
Within 24 h of ICU admission, peripheral blood samples were collected. Blood sample was centrifuged immediately, and plasma was collected and stored at −80°C. Peripheral blood mononuclear cells (PBMCs) were purified by Ficoll density-gradient separation. Plasma and PBMCs were stored in the Clinical Biobank (ISO 20387: 2018) of Peking Union Medical College Hospital. The levels of MPO and NE were quantified by enzyme-linked immunosorbent assay (ELISA) (MPO: JL11580-96T Beijing China; NE: JL12352-96T Beijing China). For phenotypic staining, antibodies specific to CD4 (BioLegend, 980802, San Diego, CA), NLRP3 (Invitrogen, PA5-79740, Thermo Fisher Scientific, Inc.), Caspase-1 (Invitrogen, PA5-140994, Thermo Fisher Scientific, Inc.), and GSDMD (Invitrogen, PA5-119680, Thermo Fisher Scientific, Inc.) were used for surface staining of cells. Thawed PBMCs were resuspended and incubated with antibody under absolute dark conditions in room temperature. Having been washed by staining buffer, cells were fixed and permeabilized according to the instructions. The gating strategy of flow cytometry experiments is shown in Figure 1. Lymphocytes were gated on side scatter (SSC) and forward scatter (FSC). T cells (CD3+), CD4+, and CD8+ T-cell subsets were quantitated. Then, CD4+ lymphocytes were gated and immunoassayed with combinations of fluorochrome-conjugated antibodies, including NLRP3, Caspase-1, and GSDMD. The percentages of CD4+ lymphocytes expressing NLRP3, Caspase-1, and GSDMD and the MFI of these markers on CD4+ lymphocytes were further quantitated. A total of 10,000 cells were analyzed from each sample. The samples were analyzed through a BDCalibur flow cytometer (BD Biosciences) with FlowJo software (BD Biosciences).
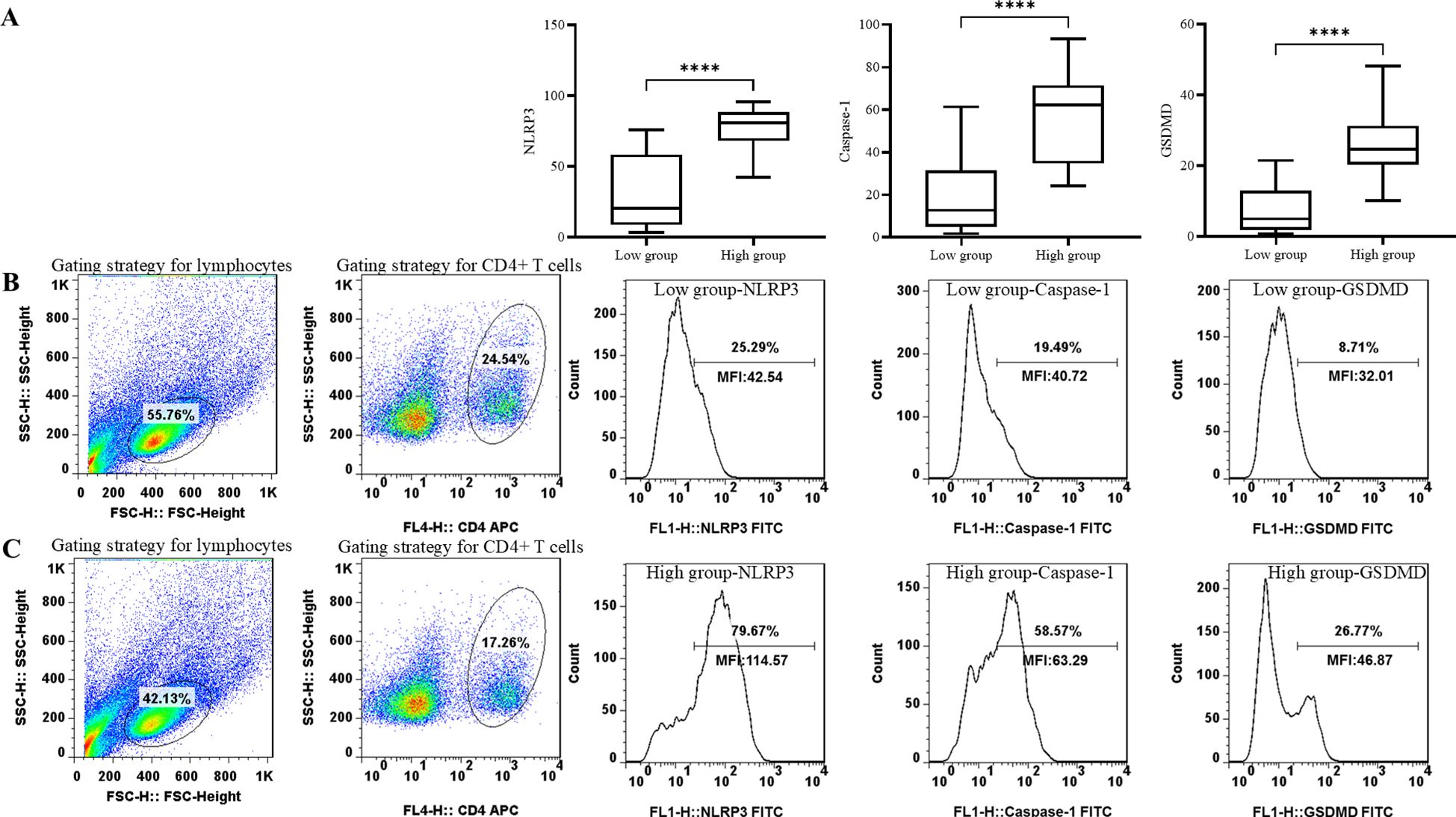
Figure 1. Comparison of pyroptosis biomarker levels, including NLRP3, Caspase-1, and GSDMD in groups with different neutrophil granule protein levels. ****p < 0.0001 (A) Comparison of percentage of NLRP3+ CD4+ T cells, percentage of Caspase-1+ CD4+ T cells, and percentage of GSDMD+ CD4+ T cells between patients with low neutrophil granule protein level and high neutrophil granule protein level. (B) Representative flow dot plots of the lymphocyte gating strategy of the lymphocyte, CD4+ T cell, NLRP3 MFI on CD4+ T cells, Caspase-1 MFI on CD4+ T cells, and GSDMD MFI on CD4+ T cells in patients with low neutrophil granule protein level. (C) Representative flow dot plots of the lymphocyte gating strategy of the lymphocyte, CD4+ T cell, NLRP3 MFI on CD4+ T cells, Caspase-1 MFI on CD4+ T cells, and GSDMD MFI on CD4+ T cells in patients with high neutrophil granule protein level.
Normally distributed data are shown as the mean with standard deviation and were analyzed with Student’s t-test. Nonnormally distributed data are expressed as the median and interquartile range and were compared through the Mann–Whitney U test. Categorical variables are recorded as proportions and were analyzed by the chi-square test. Logistic regression was performed to identify parameters with predictive value for SAL risk. Statistically significant variables were subsequently analyzed using a multi-logistic regression, and the results were expressed as P and odds ratio (OR) with 95% confidence interval (CI). The value of neutrophil granule proteins for SAL risk prediction was calculated via ROC (receiver operating characteristic) curve analysis with the Hanley–McNeil test. The area under the curves (AUCs) were examined to determine a cutoff level to predict SAL occurrence. Based on the cutoff level, all the patients were divided into a low neutrophil granule protein group and a high neutrophil granule protein group. Pearson’s correlation analysis was performed for the detection of correlation between neutrophil granule proteins and expression levels of pyroptosis markers on CD4+ T cells. Statistical analyses were performed with the SPSS 13.0 software package (SPSS, Chicago, USA).
From May 2022 to October 2023, 107 patients admitted to the Critical Care Medicine Department of PUMCH were diagnosed with sepsis. Among these patients, 25 who did not meet the inclusion criteria (4 were < 18 years old and 21 were discharged within 24 h) were excluded, 12 patients had basic immunological diseases or malignant tumors, 6 refused to sign the informed consent, and 3 had withdrawn. A total of 61 patients were enrolled in this research, 42 of whom met the criteria for lymphopenia (Figure 2).
Basic clinical characteristics after ICU admission were compared between patients with or without SAL (non-SAL group vs. SAL group), as shown in Table 1. Age of patients between the different groups was accordant, and the majority of patients were male. Infection sources were similar in patients with SAL and those without SAL. The level of C-reactive protein in the SAL group (111.1 vs. 172.3 mg/L, p = 0.0371), but not the level of procalcitonin, was significantly greater. The mortality (0 vs. 19%, p = 0.0488), ICU stay time (2 vs. 7 days, p = 0.0176), SOFA score (6.3 vs. 9.0, p = 0.0201), APACHE II score (11.9 vs. 17.5, p = 0.0176), and need for mechanical ventilation (68.4 vs. 92.3%, p = 0.0207) and renal replacement (5.3 vs. 28.6%, p = 0.0474) were significantly greater in the SAL group than in the control group. No significant difference in white blood cell count was shown between the groups.
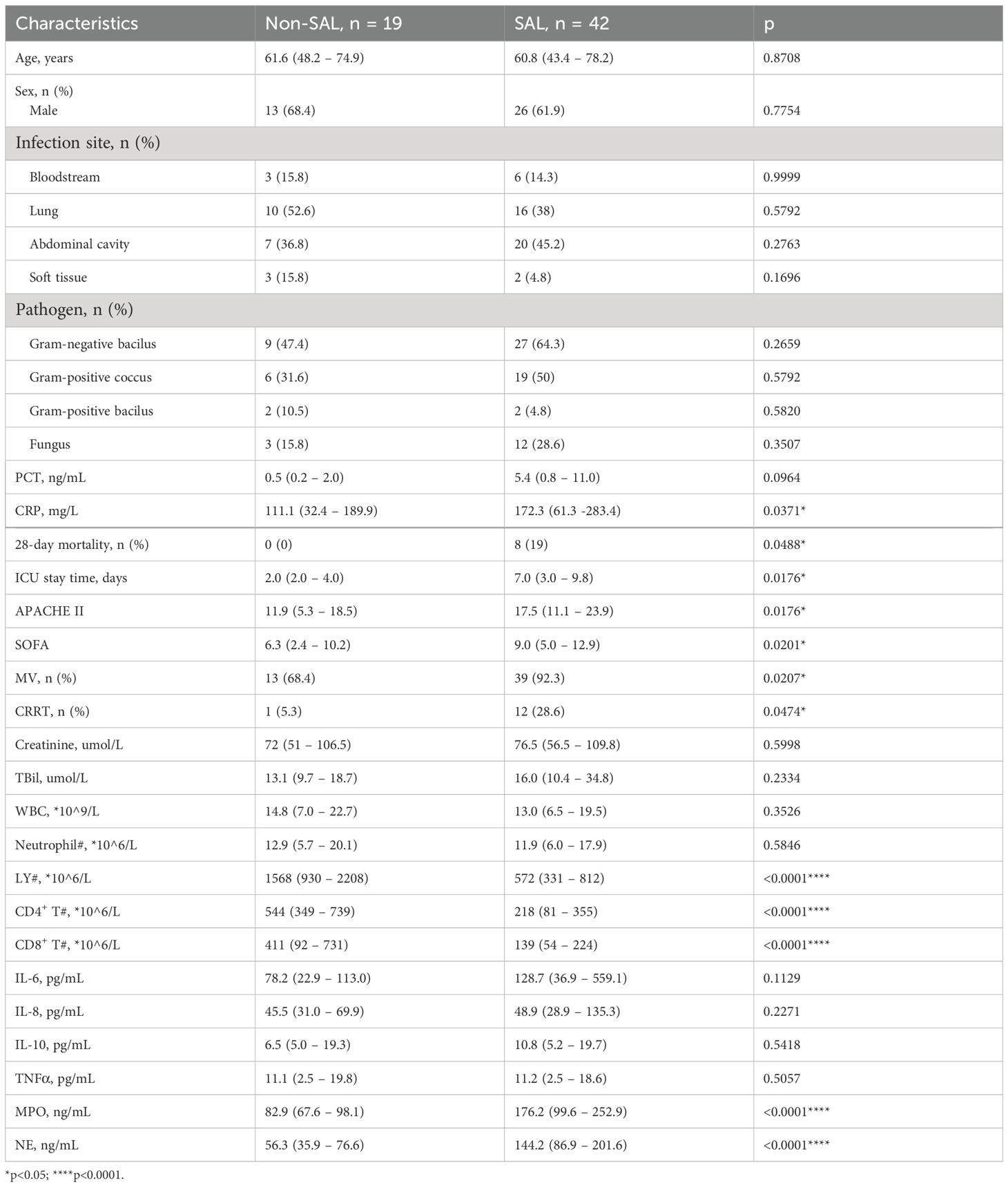
Table 1. Basic characteristics of Study Population in SAL and Non-SAL Groups included in this study.
In terms of the lymphocyte counts and their subsets, the lymphocyte count (1,568 vs. 572 × 106/L, p < 0.0001) and CD4+ T-cell (544 vs. 218 × 106/L, p < 0.0001) and CD8+ T-cell counts (411 vs. 139 × 106/L, p < 0.0001) were significantly lower in the SAL group. The levels of neutrophil granule proteins, including MPO (82.9 vs. 175.3, p < 0 <.0001) and NE (56.3 vs. 144.2, p < 0 <.0001), were significantly higher in the SAL group, as shown in Figure 3. In summary, patients with SAL showed significantly higher levels of neutrophil granule proteins.
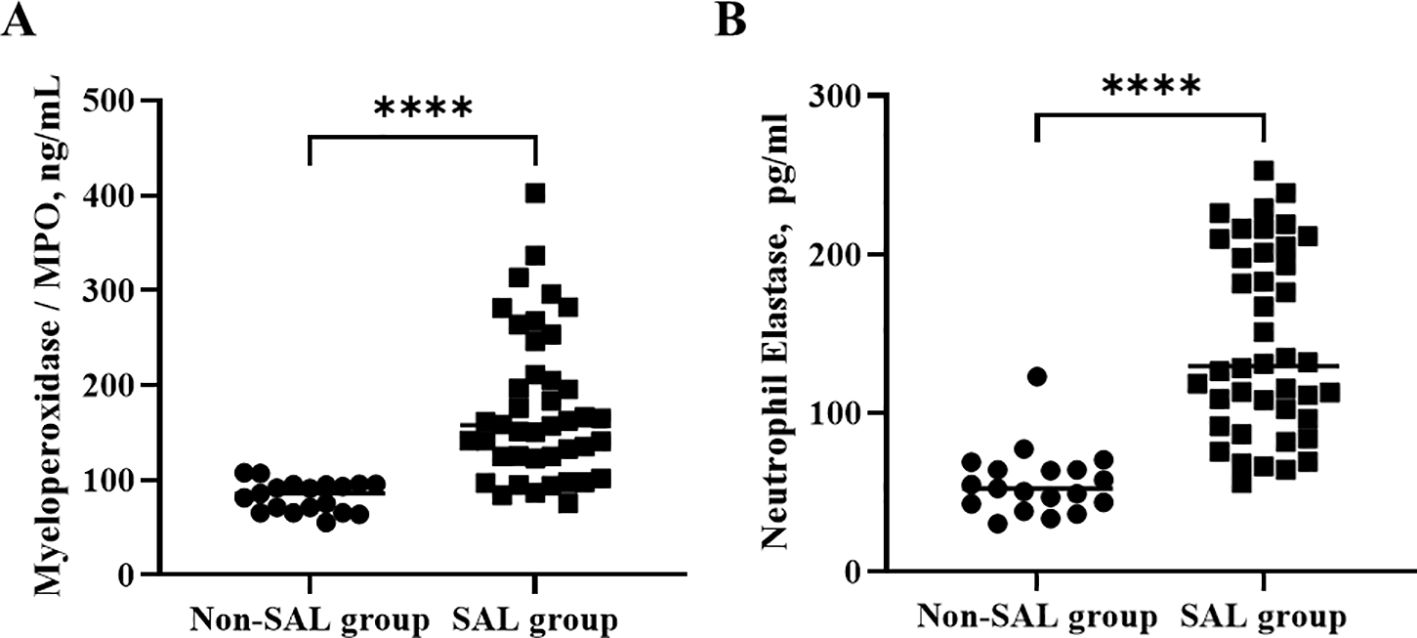
Figure 3. Comparison of neutrophil granule protein levels, including MPO (A) and NE (B) in groups of patients with sepsis with or without lymphopenia. ****p < 0.0001. SAL, sepsis-associated lymphopenia.
Lymphopenia risk in patients with sepsis was examined by univariate logistic regression analysis (Table 2). Among them, the variables SOFA score, APACHE II score, MPO, and NE were associated with lymphopenia risk in patients with sepsis. After further adjustment by multivariate logistic regression analysis, the OR of MPO after adjustment was 1.0841 (95% CI, 1.0020–1.1730, p = 0.0446), and the OR of NE after adjustment was 1.0540 (95% CI, 1.0040–1.1065, p = 0.0339) (Table 3). To further determine the value of neutrophil granule proteins for SAL risk prediction in patients with sepsis, ROC curve analysis was generated. The area under the ROC curve for the prediction of SAL occurrence of MPO was 0.939 (95% CI, 0.846–0.984; p < 0.0001), and that of NE was 0.950 (95% CI, 0.862–0.989; p < 0.0001) (Figure 4).
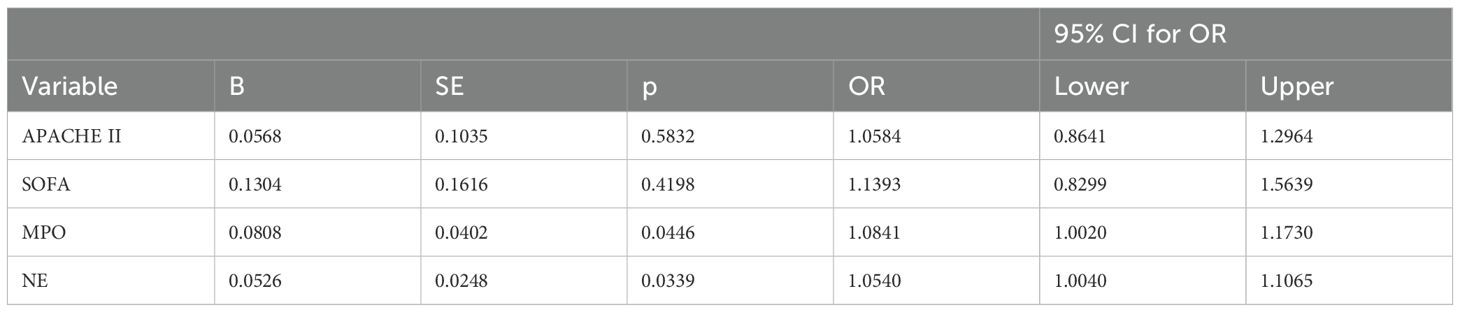
Table 3. Multivariate logistic regression analysis of possible risk factors significantly in predicting SAL in Sepsis Patients.
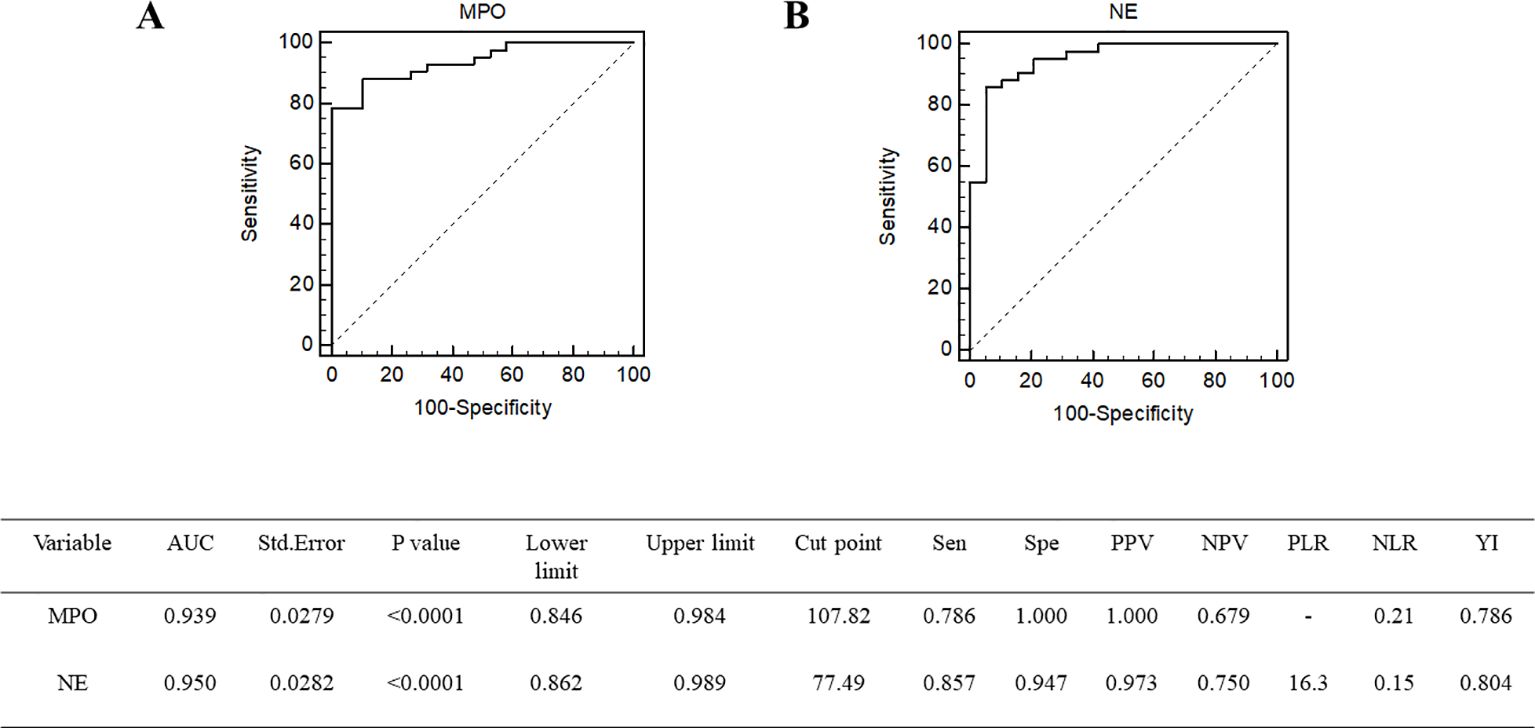
Figure 4. Receiver operating characteristic curve of MPO (A) and NE (B) for the prediction of SAL occurrence. MPO, myeloperoxidase; NE, neutrophil elastase.
Based on the cutoff level of neutrophil granule proteins in ROC curve analysis, all the patients were divided into a low neutrophil granule protein group and a high neutrophil granule protein group, with high levels of both MPO and NE. Table 4 shows the clinical characteristics of patients in the different neutrophil granule protein groups. Age, sex, and infection site were in agreement between the groups. Gram-negative bacilli (73.3% vs. 45.2%, p = 0.0374) and fungi (55.0% vs. 12.9%, p = 0.0402) were more prevalent in the high neutrophil granule protein level group. The mortality (23.3% vs. 3.2%, p = 0.0261), ICU stay time (6.0 vs. 3.0, p = 0.0451), APACHE II score (18.4 vs. 13.2, p = 0.0030), and SOFA score (9.8 vs. 6.5, p = 0.0018) were significantly greater in the high neutrophil granule protein level group than in the low neutrophil granule protein level group. Both the need for mechanical ventilation (96.7% vs. 74.2%, p = 0.0261) and the need for renal replacement (33.3% vs. 9.7%, p = 0.0311) were significantly greater in the high neutrophil granule protein level group. The levels of inflammatory biomarkers, such as CRP (186.4 vs. 121.2, p = 0.0160), IL-6 (276.0 vs. 46.9, p = 0.0094), and IL-8 (64.0 vs. 35.7, p = 0.0410) were significantly different in the groups with different neutrophil granule protein levels. Lower lymphocyte counts and subset cell counts were seen in patients with high neutrophil granule protein levels. Overall, patients with sepsis with high neutrophil granule protein levels had worse outcomes and experienced longer ICU stay times and more severe organ dysfunction.
Biomarkers of pyroptosis in CD4+ T cells were evaluated in groups with different neutrophil granule protein levels. The level of NLRP3 [78.4 (65.9–90.9) vs. 31.3 (6.2–56.3), p < 0.0001****] was significantly higher in the high neutrophil granule protein level group, and the levels of the components of its subsequent pathway, Caspase-1 [56.2 (36.0–76.4) vs. 19.4 (1.4–37.5), p < 0.0001****] and GSDMD [26.5 (17.4–35.6) vs. 7.9 (0.9–14.9), p < 0 <.0001****], were higher in the high neutrophil granule protein level group (shown in Figure 1, Table 5).
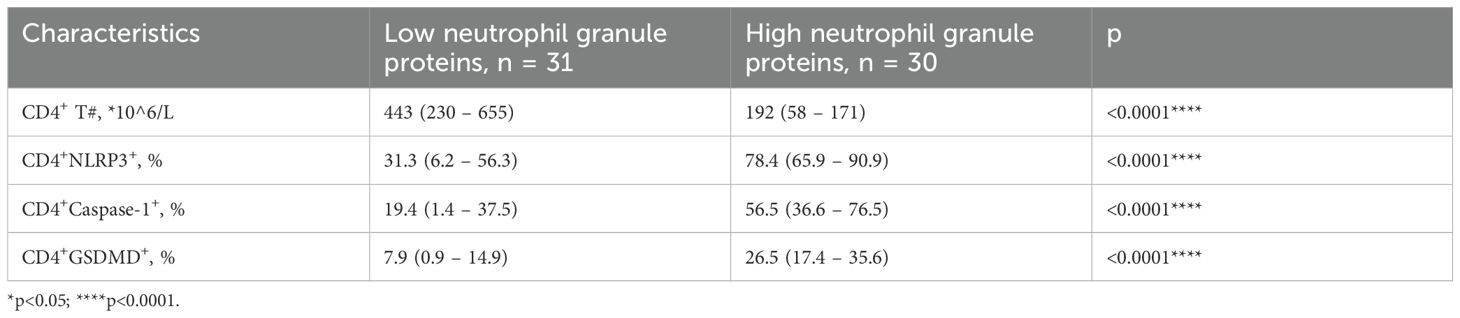
Table 5. Levels of biomarkers of pyroptosis expressed on CD4+ T cells in groups with different neutrophil granule proteins levels.
We hypothesized that neutrophil granule proteins, CD4+ T cells, and pyroptosis interact closely in the mechanism underlying SAL. Consistent with this hypothesis, Pearson’s correlation analysis revealed significant correlations between MPO levels and CD4+ T cells (r = −0.4039, p < 0.0001), CD4+NLRP3 levels (r = 0.4868, p < 0.0001), CD4+Caspase-1 levels (r = 0.5510, p < 0.0001), and CD4+GSDMD levels (r = 0.5416, p < 0.0001), as well as between NE levels and CD4+ T cells (r = −0.5140, p < 0.0001), CD4+NLRP3 levels (r = 0.6513, p < 0.0001), CD4+Caspase-1 levels (r = 0.6921, p = 0.0002), and CD4+GSDMD levels (r = 0.6084, p < 0.0001) (Figure 5). A significant relationship between neutrophil granule proteins and the pyroptosis pathway of CD4+ T cells was revealed.
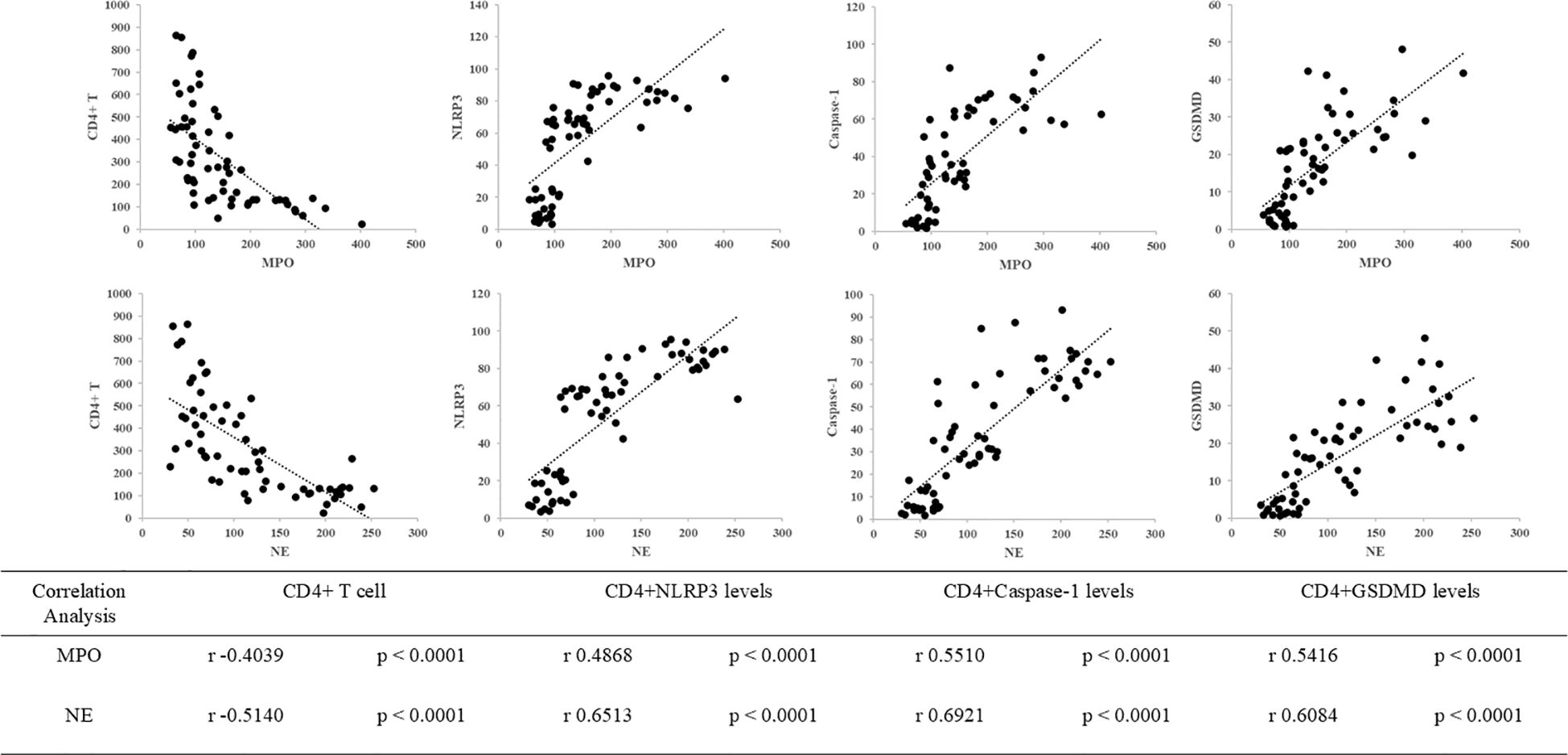
Figure 5. Correlations between neutrophil granule protein level (including MPO and NE), CD4+ T cells, and pyroptosis biomarkers (including NLRP3, Caspase-1, and GSDMD) in patients with sepsis. The correlation between MPO levels and CD4+ T cells (r = −0.4039, p < 0.0001), MPO levels and CD4+NLRP3 levels (r = 0.4868, p < 0.0001), MPO levels and CD4+Caspase-1 levels (r = 0.5510, p < 0.0001), and MPO levels and CD4+GSDMD levels (r = 0.5416, p < 0.0001). The correlation between NE levels and CD4+ T cells (r = −0.5140, p < 0.0001), NE levels and CD4+NLRP3 levels (r = 0.6513, p < 0.0001), NE levels and CD4+Caspase-1 levels (r = 0.6921, p = 0.0002), and NE levels and CD4+GSDMD levels (r = 0.6084, p < 0.0001).
Our study first evaluated the role of plasma neutrophil granule protein level in patients with SAL. To explore the relationship between neutrophil granule proteins and SAL, we evaluated the levels of plasma neutrophil granule proteins within 24 h after admission. We found that higher plasma neutrophil granule protein levels showed close relationship with the incidence of lymphopenia in patients with sepsis. Moreover, patients with sepsis with high neutrophil granule protein levels had lower CD4+ T lymphocyte counts and higher levels of biomarkers of pyroptosis. The significant correlation between neutrophil granule proteins and CD4+ T-cell counts and levels of biomarkers of pyroptosis revealed that plasma neutrophil granule proteins may play a role in SAL through the pyroptosis pathway of CD4+ T cells.
Immunosuppressive changes, often implicated in increased susceptibility to secondary infections (16, 17), are a main cause of overall mortality of patients with sepsis (18). The immune system is composed of the innate and the adaptive immune system. Research over the last decade has documented neutrophil heterogeneity and functional versatility far beyond their antimicrobial function (19). Emerging evidence indicates that neutrophils utilize granule proteins to interact with innate and adaptive immune cells and regulate the inflammatory response. Our study explored that patients with SAL had higher plasma neutrophil granule protein levels than controls did. Neutrophil granule proteins were independently associated with lymphopenia risk (MPO: OR = 1.0841, 95% CI, 1.0020–1.1730, p = 0.0446; NE: OR = 1.0540, 95% CI, 1.0040–1.1065, p = 0.0339). The area under the ROC curve in predicting SAL occurrence for MPO was 0.939 (95% CI, 0.846–0.984; p < 0.0001), whereas that for NE was 0.950 (95% CI, 0.862–0.989; p < 0.0001). Overall, patients with sepsis with high neutrophil granule protein levels had longer ICU stays and higher mortality, more severe organ dysfunction, and frequent need for organ support. Our study firstly explored the effects of neutrophil granule proteins in patients with sepsis with lymphopenia.
Different cell types and characteristics were involved in immunosuppression in patients with sepsis. A major reason for immunosuppression in sepsis is the depletion of immune cells (20, 21). Notably, the abnormal quantity and function of CD4+ T cells cannot be ignored in the development and progression of immunosuppression (22). Previous research has shown that NETs may interact with adaptive immune cells through different pathways (23, 24). In our study, patients with high neutrophil granule proteins had significantly fewer CD4+ T cells [192 (58–171) vs. 443 (230–655), p < 0 <.0001]. Neutrophil granule proteins were closely related to the CD4+ T-cell count (MPO vs. CD4+ T-cell count, r = −0.4039, p < 0.0001; NE vs. CD4+ T-cell count, r = −0.5140, p < 0.0001). In addition to traditional signal transduction between the innate and adaptive immune system (21, 25), the connections between neutrophil granule proteins and T lymphocytes deserve further exploration.
As a type of programmed cell death discovered in recent years, pyroptosis of immune cell in sepsis is complicated (26). A protective role in sepsis was initially thought with the downregulation of inflammation, but an increasing number of studies have demonstrated the disadvantages of excessive pyroptosis (27, 28). Observational study findings have suggested that the NLRP3 inflammasome may drive post-septic immunosuppression (29, 30). Our previous study verified that canonical pyroptosis is an important mechanism underlying CD4+ T-cell lymphocytopenia during sepsis (8, 31). In this study, biomarkers of CD4+ T-cell pyroptosis were evaluated in groups with different neutrophil granule protein levels. Levels of NLRP3 [78.4 (65.9–90.9) vs. 31.3 (6.2–56.3), p < 0.0001], Caspase-1 [56.2 (36.0–76.4) vs. 19.4 (1.4–37.5), p < 0.0001], and GSDMD [26.5 (17.4–35.6) vs. 7.9 (0.9–14.9), p < 0 <.0001] were significantly higher in the high neutrophil granule protein level group. Neutrophil granule proteins are closely related to CD4+ T-cell pyroptosis. We hypothesize that neutrophil granule proteins may participate in SAL through CD4+ T-cell pyroptosis.
The impact of neutrophil granule proteins on immune responses is largely implicated in APC T-cell immunity. MPO can modulate immune responses by either CD4+ T -cell activation or dendritic cell suppression, and elastase can potentially promote Th17 response but simultaneously induces DC production of TGF-β for suppressing T-cell proliferation (32, 33). Our study first explored crosstalk between neutrophil granule proteins and the pyroptosis pathway of CD4+ T cells. Neutrophil granule proteins have been identified as important modulators of neutrophil trafficking, reverse transendothelial migration, phagocytosis, neutrophil life span, NET formation, efferocytosis, cytokine activity, and autoimmunity (5). Based on recent studies, NETs may interact with pyroptosis signal pathway through different approaches. Increased NETs may induce expression of pyroptosis-related proteins and promote NLRP3 inflammasome activation (9, 10). The STING/IRE1α signaling pathway or the NF-κB/caspase 3/GSDME axis may contribute to the process (34, 35). However, the complex regulatory mechanisms in sepsis are still worth exploring.
There are still several limitations in our study. As a single-center study, it may limit the generalizability of the results. Relatively small sample and complex confounders may lower the credibility of the results. A larger sample size or larger multicenter studies should be considered. Second, although the correlation between neutrophil granule proteins and CD4+ T-cell pyroptosis was identified in this research, the causal relationship still requires further basic studies. More than that, immunosuppression is a complex status that may change during different courses of disease. In our study, we only collected blood samples within 24 h after being admitted, and a dynamic analysis of neutrophil granule proteins may be worthy of further study. Finally, neutrophil granule protein detections still have several limitations because plasma neutrophil granule proteins can be induced by different mechanisms like neutrophil degranulation, necroptosis, pyroptosis, or NETosis. Further animal experiments are still needed to explore detailed mechanisms.
Increased levels of neutrophil granule proteins were significantly associated with SAL incidence, and a significant relationship between neutrophil granule proteins and the pyroptosis pathway of CD4+ T cells was revealed.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving humans were approved by PUMCH institutional Ethic Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
NC: Writing – review & editing. JM: Writing – original draft. YX: Data curation, Writing – review & editing. XL: Data curation, Writing – review & editing. JZ: Conceptualization, Writing – review & editing. WC: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) 2023-I2M-2-002 from the Chinese Academy of Medical Sciences, National Key R&D Program of China 2022YFC2009803 from the Ministry of Science and Technology of the People’s Republic of China, the National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-B-126 and No. 2022-PUMCH-A-219), and the National Natural Science Foundation of China (No. 82072226).
We would like to thank Clinical Biobank, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, which has received accreditation to ISO 20387: 2018, for the proper preservation and handling of our samples. This manuscript has been edited and proofread by American Journal Experts.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NETs, Neutrophil extracellular traps; SAL, sepsis-associated lymphopenia; MPO, myeloperoxidase; NE, neutrophil elastase; PUMCH, Peking Union Medical College Hospital; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; NLRP3, NOD-like receptor family, pyrin domain containing 3; GSDMD, gasdermin D; PBMC, peripheral blood mononuclear cell; ELISA, enzyme-linked immunosorbent assay.
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. (2021) 54:2450–64. doi: 10.1016/j.immuni.2021.10.012
3. Tsai CY, Hsieh SC, Liu CW, Lu CS, Wu CH, Liao HT, et al. Cross-Talk among Polymorphonuclear Neutrophils, Immune, and Non-Immune Cells via Released Cytokines, Granule Proteins, Microvesicles, and Neutrophil Extracellular Trap Formation: A Novel Concept of Biology and Pathobiology for Neutrophils. Int J Mol Sci. (2021) 22(6):3119. doi: 10.3390/ijms22063119
4. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
5. Othman A, Sekheri M, Filep JG. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. (2022) 289:3932–53. doi: 10.1111/febs.v289.14
6. Vigneron C, Py BF, Monneret G, Venet F. The double sides of NLRP3 inflammasome activation in sepsis. Clin Sci (Lond). (2023) 137:333–51. doi: 10.1042/CS20220556
7. Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. (2022) 9:56. doi: 10.1186/s40779-022-00422-y
8. Guo R, Zhao G, Bai G, Chen J, Han W, Cui N, et al. Depletion of mTOR ameliorates CD(4)(+) T cell pyroptosis by promoting autophagy activity in septic mice. Int Immunopharmacol. (2023) 124:110964. doi: 10.1016/j.intimp.2023.110964
9. Cui Y, Yang Y, Tao W, Peng W, Luo D, Zhao N, et al. Neutrophil extracellular traps induce alveolar macrophage pyroptosis by regulating NLRP3 deubiquitination, aggravating the development of septic lung injury. J Inflammation Res. (2023) 16:861–77. doi: 10.2147/JIR.S366436
10. Liu C, Zhou Y, Tu Q, Yao L, Li J, Yang Z. Alpha-linolenic acid pretreatment alleviates NETs-induced alveolar macrophage pyroptosis by inhibiting pyrin inflammasome activation in a mouse model of sepsis-induced ALI/ARDS. Front Immunol. (2023) 14:1146612. doi: 10.3389/fimmu.2023.1146612
11. Pei F, Yao RQ, Ren C, Bahrami S, Billiar TR, Chaudry IH, et al. Expert consensus on the monitoring and treatment of sepsis-induced immunosuppression. Mil Med Res. (2022) 9:74. doi: 10.1186/s40779-022-00430-y
12. Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. (1997) 89:3503–21. doi: 10.1182/blood.V89.10.3503
13. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. (2016) 16:407–20. doi: 10.1038/nri.2016.58
14. Moltrasio C, Romagnuolo M, Marzano AV. NLRP3 inflammasome and NLRP3-related autoinflammatory diseases: From cryopyrin function to targeted therapies. Front Immunol. (2022) 13:1007705. doi: 10.3389/fimmu.2022.1007705
15. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. (2015) 526:660–5. doi: 10.1038/nature15514
16. Ong DSY, Bonten MJM, Spitoni C, Verduyn Lunel FM, Frencken JF, Horn J, et al. Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. (2017) 64:1204–10. doi: 10.1093/cid/cix120
17. Otto GP, Sossdorf M, Claus RA, Rödel J, Menge K, Reinhart K, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. (2011) 15:R183. doi: 10.1186/cc10332
18. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
19. Cassatella MA, Östberg NK, Tamassia N, Soehnlein O. Biological roles of neutrophil-derived granule proteins and cytokines. Trends Immunol. (2019) 40:648–64. doi: 10.1016/j.it.2019.05.003
20. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. (2013) 13:862–74. doi: 10.1038/nri3552
21. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. (2017) 17:407–20. doi: 10.1038/nri.2017.36
22. Martin MD, Badovinac VP, Griffith TS. CD4 T cell responses and the sepsis-induced immunoparalysis state. Front Immunol. (2020) 11:1364. doi: 10.3389/fimmu.2020.01364
23. Melero I, Villalba-Esparza M, Recalde-Zamacona B, Jiménez-Sánchez D, Teijeira Á, Argueta A, et al. Neutrophil extracellular traps, local IL-8 expression, and cytotoxic T-lymphocyte response in the lungs of patients with fatal COVID-19. Chest. (2022) 162:1006–16. doi: 10.1016/j.chest.2022.06.007
24. Wilson AS, Randall KL, Pettitt JA, Ellyard JI, Blumenthal A, Enders A, et al. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat Commun. (2022) 13:528. doi: 10.1038/s41467-022-28172-4
25. Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. (2012) 188:3150–9. doi: 10.4049/jimmunol.1103414
26. Zheng X, Chen W, Gong F, Chen Y, Chen E. The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: A review. Front Immunol. (2021) 12:711939. doi: 10.3389/fimmu.2021.711939
27. Gao YL, Zhai JH, Chai YF. Recent advances in the molecular mechanisms underlying pyroptosis in sepsis. Mediators Inflamm. (2018) 2018:5823823. doi: 10.1155/2018/5823823
28. Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. (2015) 265:130–42. doi: 10.1111/imr.2015.265.issue-1
29. Wang YC, Liu QX, Liu T, Xu XE, Gao W, Bai XJ, et al. Caspase-1-dependent pyroptosis of peripheral blood mononuclear cells predicts the development of sepsis in severe trauma patients: A prospective observational study. Med (Baltimore). (2018) 97:e9859. doi: 10.1097/MD.0000000000009859
30. Roth S, Cao J, Singh V, Tiedt S, Hundeshagen G, Li T, et al. Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade. Immunity. (2021) 54:648–59.e8. doi: 10.1016/j.immuni.2021.02.004
31. Zhao G, Xie Y, Lei X, Guo R, Cui N. mTOR aggravated CD4(+) T cell pyroptosis by regulating the PPARγ-Nrf2 pathway in sepsis. Int Immunopharmacol. (2024) 140:112822. doi: 10.1016/j.intimp.2024.112822
32. Gan PY, Holdsworth SR, Kitching AR, Ooi JD. Myeloperoxidase (MPO)-specific CD4+ T cells contribute to MPO-anti-neutrophil cytoplasmic antibody (ANCA) associated glomerulonephritis. Cell Immunol. (2013) 282:21–7. doi: 10.1016/j.cellimm.2013.04.007
33. Souwer Y, Groot Kormelink T, Taanman-Kueter EW, Muller FJ, van Capel TMM, Varga DV, et al. Human T(H)17 cell development requires processing of dendritic cell-derived CXCL8 by neutrophil elastase. J Allergy Clin Immunol. (2018) 141:2286–9.e5. doi: 10.1016/j.jaci.2018.01.003
34. Cao Y, Shi M, Liu L, Zuo Y, Jia H, Min X, et al. Inhibition of neutrophil extracellular trap formation attenuates NLRP1-dependent neuronal pyroptosis via STING/IRE1α pathway after traumatic brain injury in mice. Front Immunol. (2023) 14:1125759. doi: 10.3389/fimmu.2023.1125759
Keywords: sepsis, sepsis-associated lymphopenia, neutrophil granule proteins, CD4 lymphocytes, pyroptosis
Citation: Mao JY, Xie YW, Lei XL, Zhang JH, Cheng W and Cui N (2025) Effects of neutrophil granule proteins on sepsis-associated lymphopenia and their relationship with CD4+ T-cell pyroptosis. Front. Immunol. 16:1507800. doi: 10.3389/fimmu.2025.1507800
Received: 08 October 2024; Accepted: 20 January 2025;
Published: 07 February 2025.
Edited by:
Montserrat Marí, Spanish National Research Council (CSIC), SpainReviewed by:
Qinghe Meng, Upstate Medical University, United StatesCopyright © 2025 Mao, Xie, Lei, Zhang, Cheng and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Cui, cHVtY2hjbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.