
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 19 February 2025
Sec. Vaccines and Molecular Therapeutics
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1501609
 Hing Wai Tsang1
Hing Wai Tsang1 Gilbert T. Chua1
Gilbert T. Chua1 Keith Tsz Suen Tung1
Keith Tsz Suen Tung1 Rosa Sze Man Wong2
Rosa Sze Man Wong2 Sabrina Siu Ling Tsao1
Sabrina Siu Ling Tsao1 Joshua Sung Chih Wong3
Joshua Sung Chih Wong3 Joanna Yuet Ling Tung4
Joanna Yuet Ling Tung4 Janette Siu Yin Kwok5
Janette Siu Yin Kwok5 Jason Cheuk Sing Yam6
Jason Cheuk Sing Yam6 Godfrey Chi Fung Chan7
Godfrey Chi Fung Chan7 Kelvin Kai Wang To8
Kelvin Kai Wang To8 Ian Chi Kei Wong9,10,11
Ian Chi Kei Wong9,10,11 Wing Hang Leung1
Wing Hang Leung1 Mike Yat Wah Kwan1
Mike Yat Wah Kwan1 Patrick Ip1,4*
Patrick Ip1,4*Introduction: Vaccine-related myocarditis is recognized as a rare but important complication, especially after mass-scale mRNA COVID-19 vaccination. Knowledge regarding how to minimize the risk is limited. As NK cells can mediate acute myocarditis after mRNA COVID-19 vaccination and vitamin D may inhibit NK cells via cytokine modulation, we hypothesize that the myocarditis side effect is related to a hypovitaminosis D – mRNA vaccine – hypercytokinemia – NK cell axis, which is amendable to clinical intervention.
Methods: Biochemical, immunophenotypic and genotyping assays were performed to examine vitamin D status and immune profiles in 60 patients who had BNT162b2 vaccine-related acute myocarditis.
Results: A high incidence of hypovitaminosis D (73.3%) was observed in these individuals with vaccine-related myocarditis, particularly in those presented with chest pain or intensive care unit (ICU) admission. Moreover, vitamin D level was negatively associated with peak serum cardiac troponin T level during vaccine-related myocarditis. Genotypically, the GC (vitamin D binding protein) rs4588T allele which encoded the GC2 isoform of vitamin D binding protein was a risk allele, whereas the GC1S isoform was protective. Mechanistically, hypovitaminosis D was associated with higher levels of cytokines pivotal for natural killer (NK) cells (particularly interleukin-1β (IL-1β), IL-12, Interferon-γ (IFN-γ), and IL-8) and higher percentage of CD69+ NK cells in blood, which in turn correlated with chest pain presentation.
Conclusion: These data support the hypothesis that vitamin D plays a crucial role in mitigating mRNA vaccine-related myocarditis by modulating proinflammatory cytokine milieu and subsequent unfavorable NK cell activation, laying a groundwork for preventive and treatment strategies.
COVID-19 vaccines have been shown to be highly effective against SARS-CoV-2 infection in real-world settings (1). COVID-19 vaccination programs have now been implemented worldwide for several years, with more than 13.6 billion vaccine doses administered (2).
Following the mass administration of COVID-19 vaccinations worldwide, different adverse side effects were observed and reported after the first, second, or booster doses of the different COVID-19 vaccines. In particular, acute myocarditis has been recognized as a rare but important specific complication following mRNA COVID-19 vaccinations (3–5). The immune-mediated adverse events following mRNA vaccinations are generally accepted to be associated with an exaggerated inflammatory response to the molecular mimicry of the antigen present in the mRNA vaccines (6, 7). Susceptible individuals with underlying factors may present with a rapid, amplified, and prolonged inflammatory response after vaccination, with elevated levels of pro-inflammatory cytokines, such as, interleukin-18 (IL-18), IL-1β and IL-15 (8, 9). Our group was the first to show that NK cells were involved in the pathogenic mechanism of BNT162b2 vaccine-related myocarditis. We demonstrated that KIR genes and NK cell cytotoxic genes contributed to the observed expansion of CD57+ NK subsets in a BNT162b2 vaccine-related myocarditis patients cohort. Moreover, we found a prominent expansion of the identified NK cell subset in male patients or in those receiving the second dose of mRNA COVID-19 vaccine. However, an underlying explanation for these vulnerability factors and how NK cells were selectively activated have not been elucidated (10).
As vitamin D level has been linked to COVID-19 severity via its regulation of pro-inflammatory cytokines and 1,25(OH)D3 has been shown to have a direct inhibitory effect on NK cytotoxicity in a dose-dependent manner, we hypothesized that sufficient vitamin D levels play a critical protective role in minimizing the exaggerated hyperinflammatory response and the undesirable NK cell reactions after mRNA vaccines, thereby reducing the risk of vaccine-related myocarditis (11–13). At present, very few published studies have investigated the role of vitamin D in minimizing the risk and progression of vaccine-related myocarditis.
This study was conducted in a patient cohort that aimed at identifying all suspected cases of acute myocarditis in adolescents who received the BNT2162b2 vaccine, and the findings was reported earlier in our published studies (10, 14). Individuals who received the mRNA COVID-19 vaccine consented to link their electronic health records from the Hong Kong Hospital Authority (HA), the major publicly funded healthcare provider, to their vaccination records through the COVID-19 vaccines Adverse events Response and Evaluation (CARE) program. Between July 2021 to June 2022, individuals who aged between 12-17 years with suspected post-vaccine related acute myocarditis who had received the 1st, 2nd and 3rd dose of mRNA COVID-19 vaccine within 14 days prior to admission to one of the HA hospitals were reported to the Advanced Incident Reporting System (AIRS) on admission, a system for HA to report adverse drug events to the Department of Health, HKSAR Government. The subjects were recruited at the time of hospital admission and participants’ information on age, sex and ethnicity was self-reported. The suspected cases were managed by clinicians according to the Hong Kong Paediatrics Investigation Protocol for Comirnaty-related Myocarditis/Pericarditis. At the time of admission, all patients were serially monitored for potential cardiac abnormalities by electrocardiogram (ECG), echocardiogram, and serum cardiac troponin T levels. The ECGs were interpreted by a single investigator (S.S.T.), whereas echocardiograms were performed and analyzed by the cardiologists at the admitting hospital. Cardiac magnetic resonance imaging (cMRI) was conducted to confirm cases upon admission to hospital or at the Hong Kong Children’s Hospital within two weeks of symptoms onset. Radiologists at the MRI unit interpreted the images according to Lake Louise Myocarditis Criteria 2018 (15). All cases of BNT162b2 vaccine-related myocarditis reported in the study were later confirmed by the study team following the guidelines listed in the Brighton Collaboration Case Definition of Myocarditis and Pericarditis (16). Moreover, 9 convalescent patient samples were collected during their follow-up clinics after recovery from myocarditis. (Supplementary Table 1). Patients were excluded if they subsequently tested positive for SARS-CoV-2, influenza A/B/C, parainfluenza virus 1/2/3/4, adenovirus, human metapneumovirus, respiratory syncytial virus, or enterovirus. Those with a past history of COVID-19 confirmed by SARS-CoV-2 receptor binding domain (RBD) antibody and nucleocapsid protein (NP) antibody testing were also excluded from this study.
A representative local cohort, composed of 378 Hong Kong healthy infants in a previously published study, were included to estimate the population frequencies of vitamin D-binding protein genetic polymorphisms (17). Infants aged between 2 – 12 months were recruited in 5 main districts Maternal and Child Health Centers in Hong Kong using stratified sampling from July 2019 to May 2021 and the obtained allelic and genotypic frequency results aligned with other published studies among Han Chinese population (18–21). Infants with major congenital malformations, conditions including low birth weight and premature birth, with known genetic diseases, chronic medical problems were excluded.
Whole blood samples were collected from patients during hospitalization for vaccine-related myocarditis and during the recovery period. Serum samples were recovered by centrifugation, aliquoted, and stored at -80°C until analysis. PBMCs were isolated from heparinized blood by the gradient density centrifugation method and cryopreserved. The samples were recovered in batches for immunophenotyping.
Serum 25(OH)D levels were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). An AB Sciex Triple Quad QTRAP 5500+ LC-MS/MS system (AB Sciex Pte. Ltd., Framingham, MA) was used to simultaneously detect levels of cholecalciferol (25(OH)D3), ergocalciferol (25(OH)D2), and 3-epi-25 hydroxyvitamin D (3-epi-25(OH)D3). Total serum 25(OH)D level, defined as the sum of 25(OH)D3 and 25(OH)D2 adjusted by 3-epi-25(OH)D3, was determined by the standard curve method using accredited standard solutions (MilliporeSigma, St. Louis, MO). Data obtained through the LC/MS-MS method in this study have been certified by the external quality assurance program conducted by the vitamin D External Quality Assessment Scheme (DEQAS, Endocrine Laboratory, Charing Cross Hospital, London, UK) (22). Vitamin D sufficiency was defined as serum 25(OH)D > 50 nmol/L; vitamin D insufficiency was defined as 50 nmol/L ≥ serum 25(OH)D ≥ 25 nmol/L; and vitamin D deficiency was defined as serum 25(OH)D <25 nmol/L (23).
Serum cardiac troponin T concentration was measured using the Human Cardiac Troponin T ELISA Kit (Abcam, Cambridge, United Kingdom) using the standard curve method according to the manufacturer’s protocol.
Levels of serum cytokines were measured using pre-designed LEGENDplex™ cytokine panels (Biolegend, San Diego, CA). This is a bead-based immunoassay targeting pro-inflammatory cytokines (IL-1β, IFN-α2, IFN-γ, TNF-α, MCP-1, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33) and selected T helper cytokines (IL-2, IL-4, IL-5, IL-9, IL-13, and IL-22). Fluorescence intensity was captured by a BD LSR-II flow cytometer (BD Bioscience, San Jose, CA) and analyte concentration was estimated by the standard curved method.
The recovered PBMCs were stained with viability dye (VioGreen™, Miltenyi Biotechnology, Germany) before fixation for further analysis. Cells were cold-fixed, permeabilized, and labeled with an antibody cocktail containing Pacific Blue anti-human CD3 (Clone no.: HIT3a, Biolegend), APC anti-human CD56 (Clone no.:5.1H11, Biolegend), and APC/Cyanine 7 anti-human CD14 (Clone no.:M5E2, Biolegend) for the identification of NK cells and monocytes, and exclusion of T cells; and FITC anti-human CD69 (Clone no.: FN50, Biolegend) and PE anti-human HLA-DR (Clone no.: LN3, Biolegend) for the corresponding activation analyses. The stained cells were analyzed by a BD LSR-II flow cytometer (BD Biosciences) and 100,000 representative events were captured. Flow gating was applied to select immune cells as illustrated in Supplementary Figure 1.
Leukocytic DNA was extracted from heparinized blood samples by QIAamp® DNA Mini Kit (QIAGEN, Venlo, Netherlands). Four common genetic variants, rs7041, rs4588, rs2282679, and rs2228570, which are significantly associated with circulating vitamin D levels, were genotyped to study their association with the risk of BNT162b2 vaccine-related myocarditis (24–27). Genotyping was performed by Taqman-based SNP assay (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s protocol.
Restriction Fragment Length Polymorphism (RFLP) was performed to identify the rs7041-rs4588 haplotype or different GC isoforms in patients and controls. Primer pairs (forward primer: 5′-CTGGACTTCCAATTCAGCAG-3′; reverse primer: 5′-AATGGCATCTCAATAACAGG-3′) were designed to generate an amplicon from the subject’s genomic DNA including rs7041 and rs4588 loci by PCR, followed by double restriction digestion by 1U StyI and HaeIII (New England Biolabs Inc., Ipswich, MA, USA). The GC isoform was detected by 2% agarose gel electrophoresis (Supplementary Figure 2).
Statistical analyses were performed using SPSS for Windows (version 27.0, SPSS Inc., Chicago, IL) and Prism for Windows (version 8.0.1, GraphPad Software, San Diego, CA). Comparative analysis and correlation analysis with p<0.05 were considered statistically significant. Immunophenotyping data analysis was performed using FlowJo (version 10.1, BD Biosciences). LEGENDplex™ data analysis software suite (version 2023-02-15, Biolegend) was used to estimate cytokine concentrations. Cohen’s d was calculated to quantify differences in cTnT levels between risk and non-risk haplotype carriers, as well as cytokine levels between vitamin D deficiency/insufficiency and sufficiency groups (28). Odds ratio was used to assess the strength of the association to the risk of BNT162b2 vaccine-related myocarditis. Spearman’s correlation analysis was performed to analyze the relationship between 25(OH)D level, risk of symptoms onset, and cTnT levels in patients with BNT162b2 vaccine-related myocarditis. In addition, the relationship between the selected NK cell and monocyte subsets to the presented symptoms and vitamin D levels was analyzed by Spearman’s test and expressed as Spearman’s Rank correlation coefficient (ρ) and p-value in the association analysis.
The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Reference: UW21-149 and UW21-138) and the Department of Health Ethics Committee (LM21/2021). Written consent was obtained from the parents or legal guardians of the subjects.
Between 1st July 2021 to 30th June 2022, 50 males and 10 female patients who were diagnosed with BNT162b2 vaccine-related myocarditis were recruited from public hospitals in Hong Kong SAR. Demographics and presenting clinical characteristics of subjects during the acute myocarditis period are shown in Table 1. All the recruited subjects were Han Chinese. Fifty (83.3%) patients were male with a median age of 15 years and 10 (16.7%) patients were female with a median age of 13 years. Patients showed symptoms on a median of 3 days after receiving mRNA COVID-19 vaccination. The majority (70%) of patients showed symptoms after receiving the second dose of the vaccine. Most patients presented with chest pain (88.3%), followed by fever and palpitation (~30%), shortness of breath (SOB) (23.3%), and dizziness and headache (~20%). Eighteen patients (30.0%) needed to be transferred to the intensive care unit (ICU) at the time of hospitalization. Cardiac monitoring showed 48 (80.0%) patients had abnormal ECG, 42 (70.0%) had abnormal cMRI, and 22 (36.7%) had abnormal echocardiogram and all patients also had elevated serum cardiac troponin T (cTnT) level. All patients presented mild symptoms that either required no treatment or were alleviated through the use of non-steroidal anti-inflammatory drugs. Spontaneous recovery occurred without the necessity for systemic steroids, intravenous immunoglobulins, intubation, inotropic support, or ventricular assist devices.
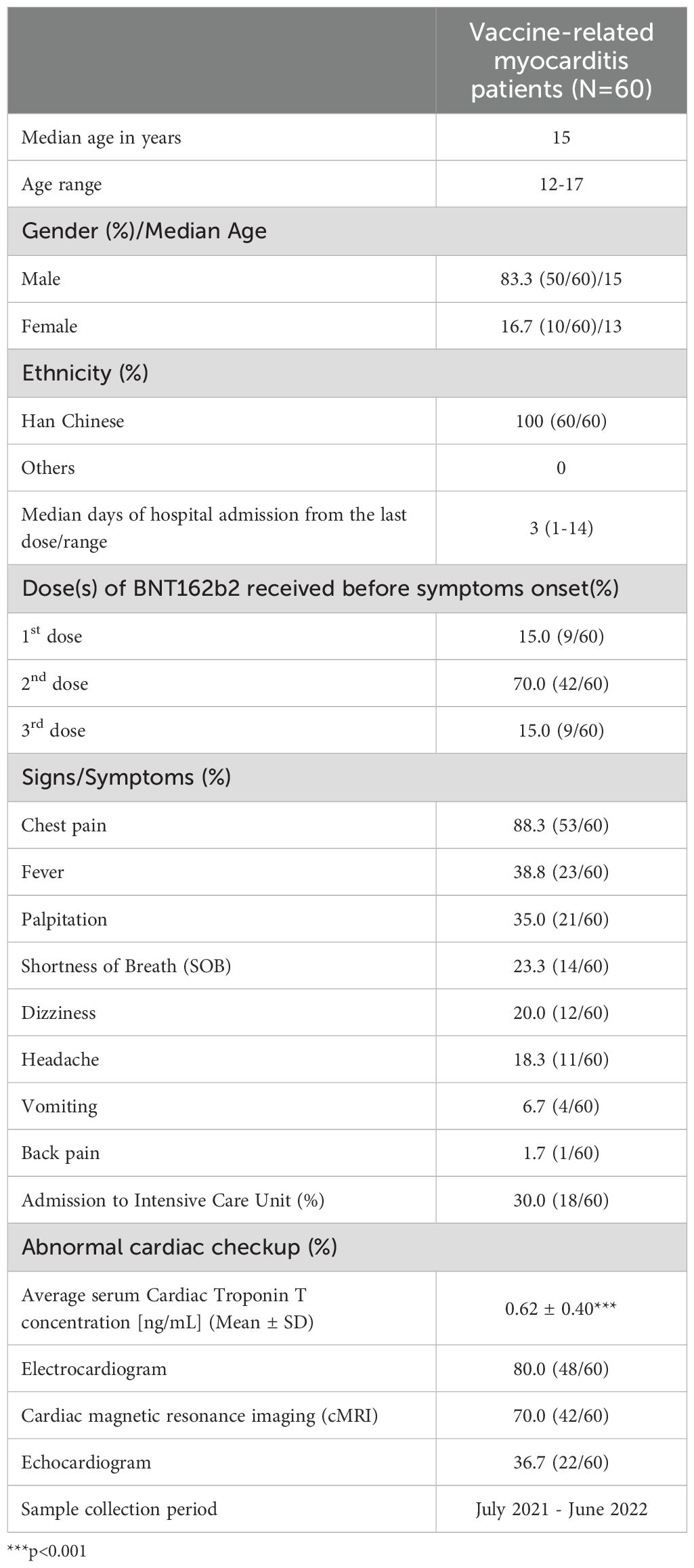
Table 1. Demographics and clinical characteristics of Comirnaty-related acute myocarditis/percarditis patients.
A high prevalence of vitamin D deficiency or insufficiency (73.3%) was observed in the patients with BNT162b2 vaccine-related myocarditis (Figure 1A). In 9 patients with hypovitaminosis D and paired convalescent samples available (collected at a median of 36 days after myocarditis onset) (Supplementary Table 1), the 25(OH)D level remained low during recovery period, similar to that at the acute phase. (Figure 1B). Further analysis revealed the serum 25(OH)D level correlated with the presenting symptoms. Specifically, vitamin D level was found to be significantly associated with the risk of chest pain (ρ=0.336, p=0.015), with those presenting without chest pain having significantly higher average 25(OH)D levels (p=0.0215). A similar trend was observed for ICU admission (ρ=0.242, p=0.084) (Figure 1C), with non-ICU admitted patients having numerically higher 25(OH)D levels (Figure 1D ii), although this did not reach statistical significance (p=0.062). There was a negative association between 25(OH)D level and cTnT levels (ρ=-0.3053, p=0.0221) (Figure 1Eii), with significantly lower cTnT levels observed in patients with sufficient vitamin D levels compared to patients with insufficient/deficient vitamin D levels (p=0.0099) (Figure 1Ei). Moreover, all four patients with the highest cTnT levels had insufficient/deficient vitamin D levels (<50 nmol/L) (Figure 1Eii, green box). Overall, our findings support the hypothesis that sufficient vitamin D levels may reduce the risk and severity of BNT162b2 vaccine-related myocarditis, in line with the known function of vitamin D in maintaining normal immune function and modulating hyperinflammatory responses (29–32).
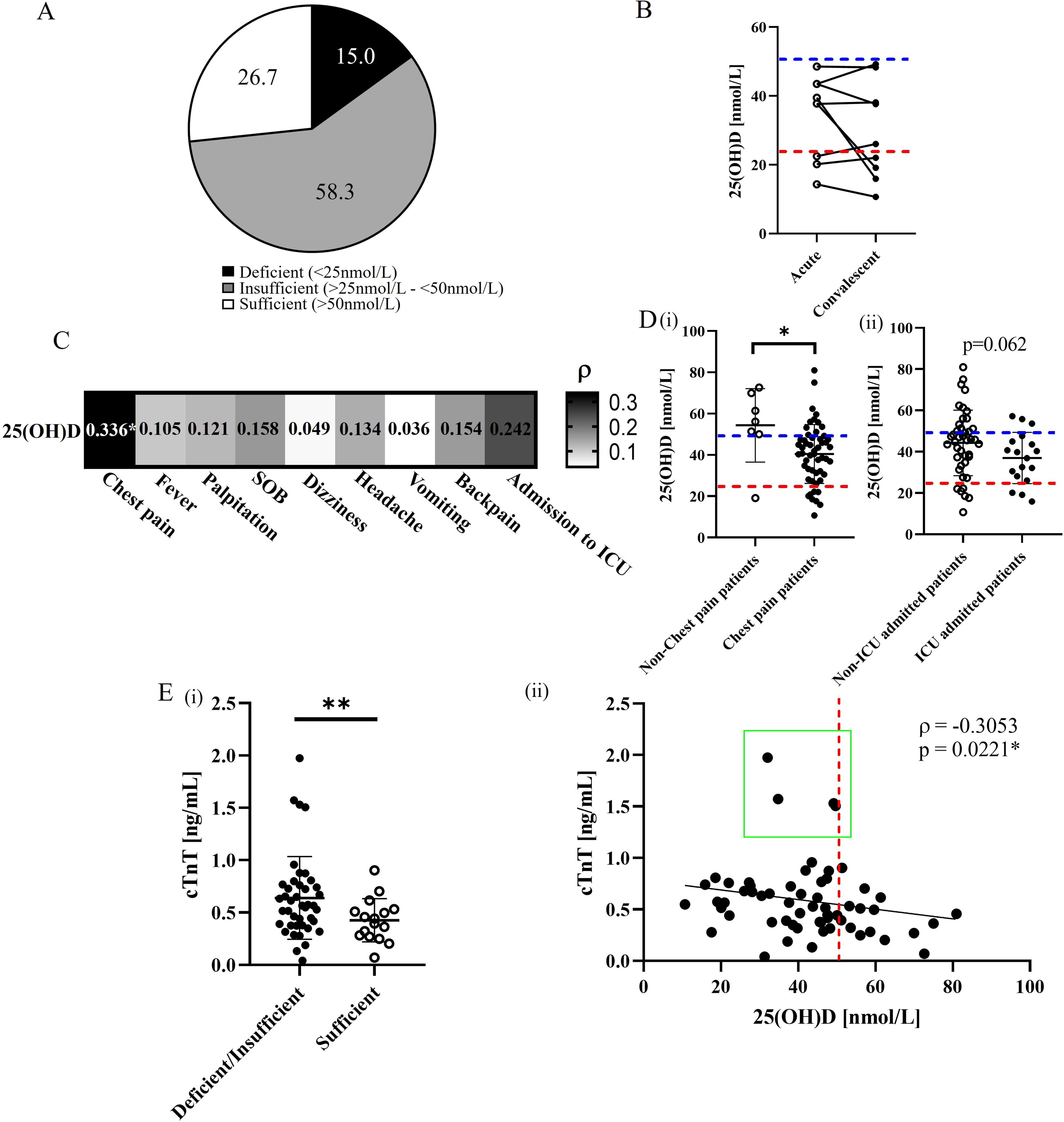
Figure 1. Assessment of vitamin D status and serum levels related to cardiac complications in BNT162b2 vaccine-related myocarditis. (A) Prevalence (%) of vitamin D deficiency, insufficiency, and sufficiency 60 BNT162b2 vaccine-related myocarditis patients. (B) Comparative analysis of circulating 25(OH)D levels between 9 acute and convalescent paired patient samples. Blue and red dotted lines represent the cutoffs for vitamin D insufficiency and deficiency, respectively. *p<0.05. (C) Spearman’s correlation analysis between 25(OH)D levels and presented symptoms in vaccinated myocarditis patients. *p<0.05. (D) Comparative analysis of 25(OH)D levels between (i) patients presenting with or without chest pain and (ii) those admitted to the ICU or not. *p<0.05. (E)(i) Comparative analysis of serum cTnT levels between vitamin D deficient/insufficient and sufficient patients. **p<0.01. (ii) Spearman’s analysis between cTnT levels and 25(OH)D levels among patients. The red dotted line represents the cutoff between vitamin D deficiency/insufficiency and sufficiency (50 nmol/L). The green box highlights patients with relatively higher cTnT levels. *p<0.05.
To test our hypothesis that the protective effect of vitamin D was mediated mechanistically via modulation of cytokines pivotal for NK cell activation, we further measured serum cytokine levels and correlated with vitamin D status. (Table 2). Compared to the vitamin D sufficient group, general pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, IL-10, IL-12 were found to be significantly elevated in vitamin D deficient/sufficient groups (all Cohen’s d >0.22). Particularly, the four most significantly elevated cytokines in the deficient/insufficient group were IL-1β, IL-8, IL-12, and IFN-γ (all Cohen’s d>0.25), all of which have been shown in our prior study to be specifically associated with NK-cell mediated vaccine-induced myocarditis (10). In contrast, the pleiotropic cytokine IL-4, which is a potent direct inhibitor of NK cells, showed higher levels in the sufficient group than in deficient/insufficient group (Cohen’s d=0.3847) (33, 34). Notably, cytokines that are crucial for monocyte-mediated tissue inflammation such as monocyte chemoattractant protein-1 (MCP-1) and IL-22 did not correlate with vitamin D status (35). Taken together, these results suggest that sufficient vitamin D levels could regulate vaccine-associated exaggerated inflammation via a specific inhibitory cytokine profile on NK cells but not on monocytes.
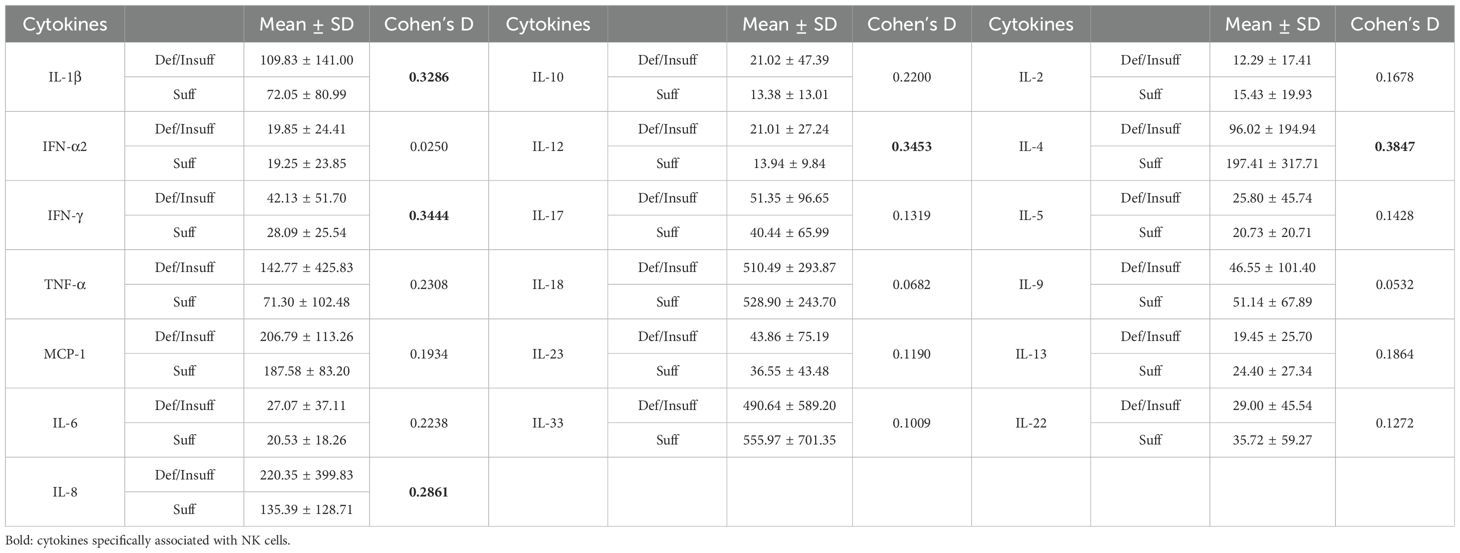
Table 2. Selected inflammatory cytokine levels among vitamin D deficient/insufficient or vitamin D sufficient BNT162b2 vaccine-related myocarditis patients.
As we observed significant differences in myocarditis-associated NK cell-specific cytokine profile in patients with different vitamin D status, we subsequently measured the early activation antigen CD69 expression on NK cells from patients with BNT162b2 vaccine-related myocarditis to confirm its correlation with clinical features and vitamin D status, in contrast to the non-specific activated HLA-DR+ monocyte subset (Figure 2). While the frequency of CD69+ NK cells in blood correlated with myocarditis-specific symptoms of chest pain (ρ=0.269; Figure 2A, upper panel), the frequency of HLA-DR+ monocytes correlated with non-specific febrile reaction instead (ρ=0.442; p=0.001, lower panel). There was significantly higher frequency of CD69+ NK cell subsets in patients with chest pain compared to non-chest pain subjects (p=0.0041) (Figure 2Bi), with subset frequency the highest in deficient/insufficient vitamin D groups (p<0.0001) compared to the control group (Figure 2Ci). The frequency of CD69+ NK cells was inversely correlated with serum 25(OH)D level generally (ρ=-0.2756; p=0.036, Figure 2Di), and was low in subjects with adequate 25(OH)D level >50 nmol/L uniformly. In contrast, there was no significant difference in the frequency of monocyte HLA-DR+ subsets in patient groups with or without chest pain, ICU admission (Figures 2Biii, Biv) or with different vitamin D status (Figure 2Cii) and 25(OH)D levels (Figure 2Dii). Overall, these results confirm the central role of NK cells in mediating the specific symptoms of BNT162b2 vaccine-related myocarditis and highlight the immunoregulatory role of vitamin D in modulating the specific CD69+ NK cell subset but not the non-specific monocyte-mediated febrile reaction.
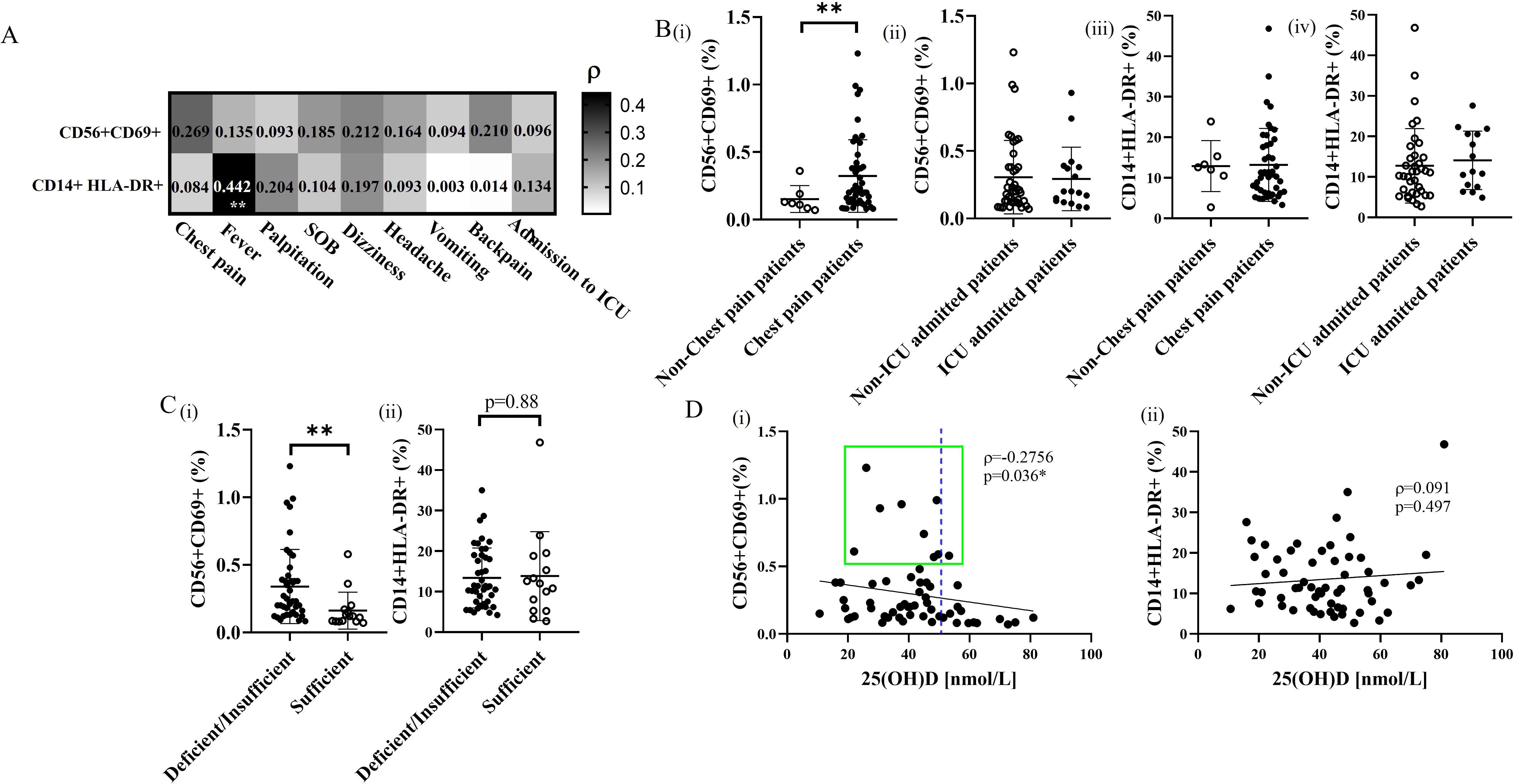
Figure 2. Correlation and qualitative comparison of activation subsets of NK cells and monocytes in BNT162b2 vaccine-related myocarditis patients with different vitamin D status. (A) Association of measured frequencies of activation subsets of NK cells and monocytes with the occurrence of the initial presented symptoms in BNT162b2 vaccine-related myocarditis patients. **p<0.01. (B) Comparative analysis of CD69+ NK cell and HLA-DR+ monocyte subsets between (i,iii) patients presenting with or without chest pain and (ii,iv) those admitted to the ICU or not. **p<0.01. (C) Qualitative comparison of activation frequency of (i) NK cell and (ii) monocyte subsets in vitamin D deficient/insufficient patients, and vitamin D sufficient patients. Data are presented as mean ± SD and analyzed using a two-sided Student’s t-test. **p<0.01. (D) Spearman’s correlation analysis of activated subset frequencies of (i) NK cells and (ii) monocytes plotted against 25(OH)D levels in BNT162b2 vaccine-related myocarditis patients. The best-fit trendline is shown. *p<0.05.
Vitamin D metabolites are transported by vitamin D binding protein (DBP) which is encoded by the GC gene, whereas vitamin D receptor is encoded by the VDR gene. Previous studies have identified that genetic polymorphism of GC and VDR were associated with circulating 25(OH)D levels and responsiveness of vitamin D receptor (36). We next associated the selected genetic loci in patients with BNT162b2 vaccine-related myocarditis to a Han Chinese representative cohort for prediction of vitamin D-associated genetic risk of vaccine side effects (Table 3). In the 52 genotyped patients, increased risk of BNT162b2 vaccine-related myocarditis was observed for rs4588T carriers (OR=1.6340; 95%Cl=1.0559 – 2.5288; p=0.0275) and potentially for rs7041A (OR=1.4068; 95%Cl=0.8585-2.2790); [Table 2(i)]. Its genetic effects were found to be the most prominent in the homozygous risk alleles carriers (OR>1.33) of the 3 identified genetic loci whereas the rs4588G alleles (OR=0.6120; 95%Cl=0.3954 – 0.9471; p=0.0275) [Table 3(i)] and the homozygous rs4588GG (OR=0.5560; 95%Cl=0.3092 – 0.9996; p=0.0498) carriers was observed as protective. [Table 3(ii)] Considering the rs4588T haplotypes determines the GC2 isoform (rs7041A-rs4588T), BNT162b2 vaccine-related myocarditis risk was significantly associated with GC2 (OR=1.6340; 95%Cl=1.0559 – 2.5288; p=0.0275), whereas the GC1S (encoded by the non-risk rs7041C-rs4588G) appeared to be protective (OR=0.6847; 95%Cl=0.4216 – 1.1208; p=0.1329) [Table 2(i)]. The combined genetic effects were estimated by comparing patients carrying the identified risk haplotypes (GC2-rs2282679G-rs2228570C) to those with non-risk haplotypes (GC1S-rs2282679T-rs2228570T) [Table 3(iii)]. Risk haplotype carriers exhibited lower average 25(OH)D levels (Cohen’s d=0.837) and higher average cTnT levels (Cohen’s d=0.859), whereas the opposite was observed in non-risk haplotype carriers, suggesting that vitamin D genetic polymorphisms may predispose individuals to BNT162b2 vaccine-related myocarditis.
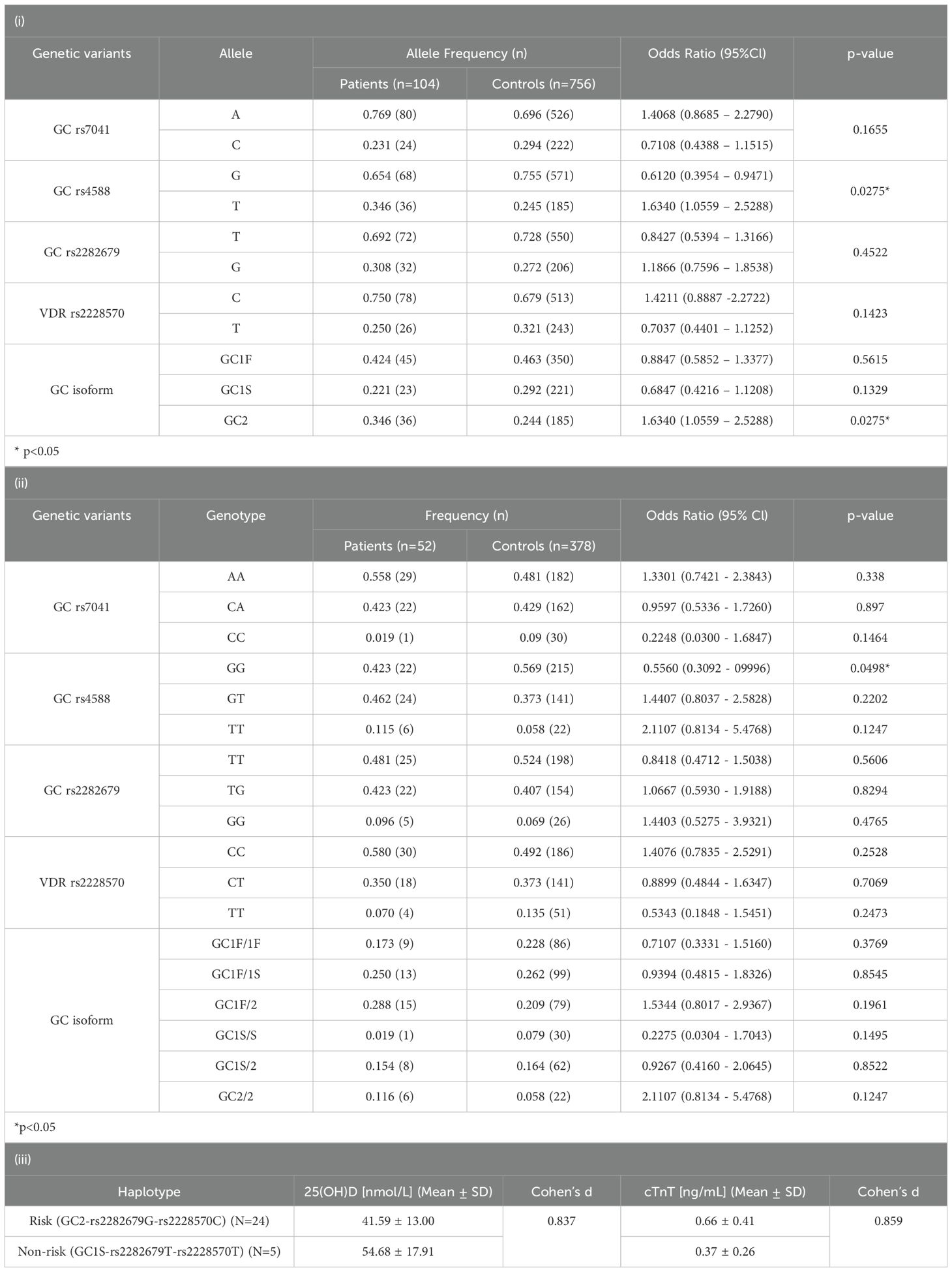
Table 3. The associated vitamin D genetic risk in selected variants’ (i) alleles, (ii) genotypes and, (iii) the cumulative genetic effects in BNT162b2 vaccine-related myocarditis.
COVID-19 mRNA vaccine-related myocarditis is a rare and potentially fatal side effect, of which the biological mechanisms and vulnerable populations have not been fully elucidated. To the best of our knowledge, this is the first study examining the role of vitamin D in protection against mRNA COVID-19 vaccine-related acute myocarditis. Specifically, we found high prevalence of vitamin D deficiency or insufficiency (>70%) in vaccine-related myocarditis patients, particularly those presented with chest pain and need for ICU admission, and the serum vitamin D level was found to be negatively correlated with cardiac troponin T level. Herein, we further explored the underlying immune mechanisms and observed lower levels of proinflammatory cytokines, including those pivotal for NK cells, in sufficient vitamin D patients when compared to the vitamin D insufficiency/deficiency groups. The abundance of CD69+ NK subsets, which correlated with the chest pain risk, was also negatively correlated with serum vitamin D level. Vitamin D binding protein rs4588T and the encoded GC2 isoform related to vitamin D transportation were identified as genetic risk factors for vaccine-related myocarditis, whereas rs4588GG and GC1S appear to be protective. Collectively, these data strongly support the hypothesis that the fundamental immunomodulatory role of vitamin D in mRNA vaccine-related myocarditis is via suppression of pro-NK cell cytokine milieu.
Numerous studies have highlighted the critical role of Vitamin D in immune regulation. Importantly, individuals with Vitamin D deficiency are more prone to inflammatory conditions, which can lead to unregulated inflammation and extensive cellular damage (31, 37, 38). In this study, consistent Vitamin D levels seen in patients during both the disease and recovery phases suggest that Vitamin D insufficiency was pre-existing, rather than being a temporary result of COVID-19 vaccination or associated cardiac events. Our earlier studies noted an elevation of a wide variety of proinflammatory cytokines in patients with myocarditis following mRNA vaccination (10). This hypercytokinemia environment in vaccine-related myocarditis may stem from the diminished anti-inflammatory effects of Vitamin D, leading to an increased inflammatory response seen in patients with vaccine-related myocarditis. Such pre-existing susceptibility risk may be primarily driven by innate immunity, as myocarditis related to the mRNA COVID-19 vaccine typically develops rapidly within 3 to 4 days post-vaccination (14, 39). With insufficient Vitamin D levels, innate immune cells expressing VDR may lose the immunomodulatory effects of Vitamin D, such as acting through the NF-κB signaling pathway, a well-known inflammation mediator typically suppressed by VDR (40). The reduced Vitamin D-mediated NF-κB suppression may lead to the stronger expression of pro-inflammatory cytokines in various innate immune cells, such as IL-1β, IL-6, IL-8, TNF-α in macrophages, IL-12 in dendritic cells, and IFN-γ in NK cells, as seen in the Vitamin D insufficient/deficient patients in this study. Overall, these data support the hypothesis that Vitamin D insufficiency is a significant risk factor for general hypercytokinemia after mRNA vaccination. However, whether certain innate immunity subsets were responsible specifically for the myocarditis side effect remained uncertain.
Recently, we were the first group to identify NK cells as the primary mediator of BNT162b2 vaccine-related myocarditis. In patients with myocarditis, an unique cytokine signature was observed, consisting high levels of IL-1β, IL-8, IL-12, and IFN-γ. In this study, we found that the four highest ranking cytokines that were most associated with hypovitaminosis D were exactly these 4 cytokines, strongly supporting the hypothesis that vitamin D plays role in this myocarditis-specific cytokine profile pivotal for early activation of CD69+ NK cells. The non-specific febrile reaction and expansion of HLA-DR+ monocytes after mRNA vaccines, however, was distinct and did not correlate with vitamin D levels or myocarditis-associated chest pain. Notably, vitamin insufficiency did not result in a higher level of IL-22 or MCP-1, which are crucial for migration and infiltration of monocytes and macrophages in heart tissue. In contrast, myocarditis patients with adequate vitamin D had high serum levels of IL-4, which is known to be a potent inhibitor of NK cells directly in a concentration-dependent manner and may suppress the binding and cytotoxicity of NK cells in the vascular endothelium specifically, leading to lower cTnT levels and fewer patients with chest pain (33, 34, 41). Notably, CD69+ NK cells are not only an early activation subset, but also a tissue-resident NK cell subset (42). The observed prevalence of the CD69+ NK cell subset in the blood of BNT162b2 vaccine-related myocarditis patients with vitamin insufficiency might in part be related to the increase in myocardium-resident NK cells during acute myocarditis, which may in turn lead to increased circulation of CD69+ NK cells.
Our study provides evidence of genetic predisposition contributing to hypovitaminosis D, which escalates the risk and severity of vaccine-related myocarditis. We identified the GC rs4588T allele and GC2 isoform as risk factors, while the GC rs4588GG genotype and GC1S isoform appeared to be protective. Notably, the GC2 haplotype, encoded by rs7041A-rs4588T, is negatively associated with serum vitamin D levels consistently in the Han Chinese population, thus increasing susceptibility to vitamin D deficiency or insufficiency (18, 19). A potential correlation was found between lower circulatory concentrations of DBP and lower affinity to its metabolites, which might explain the reduced circulatory vitamin D in GC2 carriers compared to those carrying GC1F or GC1S haplotypes (43). Serum DBP is an important reservoir for vitamin D metabolites (44, 45). Furthermore, our cumulative genetic analysis supported the idea that the bioavailability of vitamin D could influence the severity of this adverse side effect, particularly with respect to the serum cardiac damage marker cTnT. Therefore, the identified negative vitamin D genetics may enhance the risk of hypovitaminosis D, thereby increasing the likelihood of vaccine-related myocarditis. Besides genetic factors, environmental aspects such as oral supplementation practices, sunlight exposure, diet, seasons, and non-modifiable factors like sex, age, and racial differences can also affect serum vitamin D levels in patients. This leads to variability in the risk and severity of vaccine-related myocarditis, necessitating further investigation.
We speculate that an appropriate level of sunlight exposure or taking vitamin D supplements before vaccination would provide substantial benefits to minimize the side effects of the mRNA COVID-19 vaccine, as vitamin D deficiency or insufficiency can be modified in a simple and safe approach. However, given the limited number of patients, the cross-sectional study design, and the individual variability in vitamin D levels affected by various factors (46–50), a larger patient cohort is necessary for further investigation into the likelihood of modifiable factors and health conditions in reducing the risk and severity of vaccine-related myocarditis. A longitudinal follow-up study could also be conducted to associate initial and subsequent vitamin D levels with long-term cardiac outcomes in patients, highlighting the significance of vitamin D in managing this adverse side effect. Previous research has suggested vitamin D as an adjunctive therapeutic agent for COVID-19 patients, due to its ability to decrease pro-inflammatory cytokine production (51). This same anti-inflammatory strategy could also be applied in treating patients presenting this side effect. This study paves the way for future clinical trials to explore the potential use of vitamin D in treating vaccine-related myocarditis patients.
The findings of this study need to be interpreted with the following caveats. First, the number of patients in this study was relatively small, as BNT162b2 vaccine-related myocarditis is uncommon. However, our extensive network and efficient reporting system allowed us to recruit sufficient cases within the study period. This is by far one of the largest adolescent cohorts of mRNA COVID-19 vaccine-related myocarditis, and also provides a Han Chinese cohort for further investigation and exploration. Second, this study was conducted exclusively on Han Chinese individuals, and the data obtained, particularly the genetic data, may not be applicable to other ethnic groups. Significant variations in the GC haplotypes have been observed across different populations, which could influence the measured genetic susceptibility risk related to vitamin D genetics (52–55). Third, environmental factors such as physical activity and sunlight exposure level that can influence serum 25(OH)D levels were not measured, which may affect the interpretations of the risk to vaccine-related myocarditis in this study. Furthermore, the vitamin D status of the included participants before vaccination would represent an important measurement for predicting the risk of this side effect. Nevertheless, measuring serum 25(OH)D level during the rapid-onset cardiac inflammatory period may still represent the baseline vitamin D status of patients prior to vaccination, as circulating 25(OH)D levels have a half-life of 15 days, hence, environmental factors affecting vitamin D level before and soon after the COVID-19 vaccination should be minimal (56).
In conclusion, our study provides the first evidence supporting the immunomodulatory role of vitamin D in reducing the risk and influencing the inflammatory cytokine response associated with unfavorable NK cell activation in BNT162b2 vaccine-related myocarditis. Our findings emphasize the importance of maintaining sufficient vitamin D levels, especially in those genetically susceptible to hypovitaminosis D, before receiving mRNA-based vaccines, which is likely to be the dominant vaccine platform in the future. Overall, the novel protective role of vitamin D in vaccine-related myocarditis uncovered in this study may pave the way for a new paradigm in understanding the biology of this unique side effect and devising new prevention and intervention strategies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by University of Hong Kong/Hospital Authority Hong Kong West Cluster and the Department of Health Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
HT: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. GC: Conceptualization, Methodology, Supervision, Writing – review & editing. KTST: Funding acquisition, Validation, Writing – review & editing. RW: Validation, Writing – review & editing. ST: Conceptualization, Writing – review & editing. JW: Writing – review & editing. JT: Writing – review & editing. JK: Writing – review & editing. JY: Writing – review & editing. GC-FC: Writing – review & editing. KKWT: Writing – review & editing. IW: Writing – review & editing. WL: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. MK: Writing – review & editing. PI: Conceptualization, Formal analysis, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hong Kong Collaborative Research Fund (CRF) 2020/21 and CRF Coronavirus and Novel Infectious Diseases Research Exercises (Reference Number: C7149-20G), Health and Medical Research Fund (HMRF) (Reference Number: 18192311), and URC Seed Funding for Basic Research for New Staff (Reference Number: 103034016). The funding sources were not involved in the study design, data collection, analysis and interpretation, writing of the manuscripts, and the decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1501609/full#supplementary-material
1. Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. (2022) 114:252–60. doi: 10.1016/j.ijid.2021.11.009
2. WHO coronavirus (COVID-19) dashboard. (2020). Available online at: https://covid19.who.int/ (Accessed November 13, 2023).
3. Salah HM, Mehta JL. COVID-19 vaccine and myocarditis. Am J Cardiol. (2021) 157:146–8. doi: 10.1016/j.amjcard.2021.07.009
4. Li X, Lai FTT, Chua GT, Kwan MYW, Lau YL, Ip P, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in hong kong. JAMA Pediatr. (2022) 176:612–4. doi: 10.1001/jamapediatrics.2022.0101
5. Carleton BC, Salmon DA, Ip P, Wong ICK, Lai FTT. Benefits v. risks of COVID-19 vaccination: an examination of vaccination policy impact on the occurrence of myocarditis and pericarditis. Lancet Reg Health West Pac. (2023) 37:100797. doi: 10.1016/j.lanwpc.2023.100797
6. Heo JY, Seo YB, Kim EJ, Lee J, Kim YR, Yoon JG, et al. COVID-19 vaccine type-dependent differences in immunogenicity and inflammatory response: BNT162b2 and ChAdOx1 nCoV-19. Front Immunol. (2022) 13:975363. doi: 10.3389/fimmu.2022.975363
7. Jara LJ, Vera-Lastra O, Mahroum N, Pineda C, Shoenfeld Y. Autoimmune post-COVID vaccine syndromes: does the. Spectr autoimmune/inflammatory syndrome expand? Clin Rheumatol. (2022) 41:1603–9. doi: 10.1007/s10067-022-06149-4
8. Barmada A, Klein J, Ramaswamy A, Brodsky NN, Jaycox JR, Sheikha H, et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis. Sci Immunol. (2023) 8:eadh3455. doi: 10.1126/sciimmunol.adh3455
9. Won T, Gilotra NA, Wood MK, Hughes DM, Talor MV, Lovell J, et al. Increased interleukin 18-dependent immune responses are associated with myopericarditis after COVID-19 mRNA vaccination. Front Immunol. (2022) 13:851620. doi: 10.3389/fimmu.2022.851620
10. Tsang HW, Kwan MYW, Chua GT, Tsao SSL, Wong JSC, Tung KTS, et al. The central role of natural killer cells in mediating acute myocarditis after mRNA COVID-19 vaccination. Med. (2024) 5(4):335–47.E3. doi: 10.1016/j.medj.2024.02.008
11. Bayraktar N, Turan H, Bayraktar M, Ozturk A, Erdogdu H. Analysis of serum cytokine and protective vitamin D levels in severe cases of COVID-19. J Med Virol. (2022) 94:154–60. doi: 10.1002/jmv.27294
12. Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. (2013) 88:720–55. doi: 10.1016/j.mayocp.2013.05.011
13. Leung KH. Inhibition of human natural killer cell and lymphokine-activated killer cell cytotoxicity and differentiation by vitamin D3. Scand J Immunol. (1989) 30:199–208. doi: 10.1111/j.1365-3083.1989.tb01202.x
14. Chua GT, Kwan MYW, Chui CSL, Smith RD, Cheung ECL, Ma T, et al. Epidemiology of acute myocarditis/pericarditis in hong kong adolescents following comirnaty vaccination. Clin Infect Dis. (2022) 75:673–81. doi: 10.1093/cid/ciab989
15. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. (2018) 72:3158–76. doi: 10.1016/j.jacc.2018.09.072
16. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, Savorgnan F, Guggilla RK, Khuri-Bulos N, et al. Myocarditis and pericarditis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2022) 40:1499–511. doi: 10.1016/j.vaccine.2021.11.074
17. Tsang HW, Tung KTS, Wong RS, Wong SY, Tung JYL, Chua GT, et al. Association of vitamin D-binding protein polymorphisms and serum 25(OH)D concentration varies among Chinese healthy infants of different VDR-FokI genotypes: A multi-centre cross-sectional study. Nutr Bull. (2023) 49(1):63–72. doi: 10.1111/nbu.12656
18. Shao B, Jiang S, Muyiduli X, Wang S, Mo M, Li M, et al. Vitamin D pathway gene polymorphisms influenced vitamin D level among pregnant women. Clin Nutr. (2018) 37:2230–7. doi: 10.1016/j.clnu.2017.10.024
19. Zhou JC, Zhu Y, Gong C, Liang X, Zhou X, Xu Y, et al. The GC2 haplotype of the vitamin D binding protein is a risk factor for a low plasma 25-hydroxyvitamin D concentration in a Han Chinese population. Nutr Metab (Lond). (2019) 16:5. doi: 10.1186/s12986-019-0332-0
20. Cheung CL, Lau KS, Sham PC, Tan KC, Kung AW. Genetic variant in vitamin D binding protein is associated with serum 25-hydroxyvitamin D and vitamin D insufficiency in southern Chinese. J Hum Genet. (2013) 58:749–51. doi: 10.1038/jhg.2013.84
21. McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. (2010) 121:471–7. doi: 10.1016/j.jsbmb.2010.03.073
22. Carter GD, Berry J, Durazo-Arvizu R, Gunter E, Jones G, Jones J, et al. Quality assessment of vitamin D metabolite assays used by clinical and research laboratories. J Steroid Biochem Mol Biol. (2017) 173:100–4. doi: 10.1016/j.jsbmb.2017.03.010
24. Cooper JD, Smyth DJ, Walker NM, Stevens H, Burren OS, Wallace C, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. (2011) 60:1624–31. doi: 10.2337/db10-1656
25. Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. (2008) 93:3381–8. doi: 10.1210/jc.2007-2702
26. Kurylowicz A, Ramos-Lopez E, Bednarczuk T, Badenhoop K. Vitamin D-binding protein (DBP) gene polymorphism is associated with Graves' disease and the vitamin D status in a Polish population study. Exp Clin Endocrinol Diabetes. (2006) 114:329–35. doi: 10.1055/s-2006-924256
27. Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, Chao MJ, et al. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr. (2008) 88:441–7. doi: 10.1093/ajcn/88.2.441
28. Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. (2012) 4:279–82. doi: 10.4300/JGME-D-12-00156.1
29. Baeke F, Gysemans C, Korf H, Mathieu C. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol. (2010) 25:1597–606. doi: 10.1007/s00467-010-1452-y
30. Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. (2015) 7:3011–21. doi: 10.3390/nu7043011
31. Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. (2017) 85:78–97. doi: 10.1016/j.jaut.2017.07.007
32. Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. (2009) 183:5458–67. doi: 10.4049/jimmunol.0803217
33. Nagler A, Lanier LL, Phillips JH. The effects of IL-4 on human natural killer cells. A potent regulator of IL-2 activation and proliferation. J Immunol. (1988) 141:2349–51. doi: 10.4049/jimmunol.141.7.2349
34. Paganin C, Matteucci C, Cenzuales S, Mantovani A, Allavena P. IL-4 inhibits binding and cytotoxicity of NK cells to vascular endothelium. Cytokine. (1994) 6:135–40. doi: 10.1016/1043-4666(94)90034-5
35. Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. (2005) 52:1037–46. doi: 10.1002/art.20965
36. Fu L, Wong BYL, Li Z, Horst RL, Williams R, Lee B, et al. Genetic variants in the vitamin D pathway and their association with vitamin D metabolite levels: Detailed studies of an inner-city pediatric population suggest a modest but significant effect in early childhood. J Steroid Biochem Mol Biol. (2023) 233:106369. doi: 10.1016/j.jsbmb.2023.106369
37. Herold K, Mrowka R. Inflammation-Dysregulated inflammatory response and strategies for treatment. Acta Physiol (Oxf). (2019) 226:e13284. doi: 10.1111/apha.2019.226.issue-3
38. Zhang H, Sun SC. NF-kappaB in inflammation and renal diseases. Cell Biosci. (2015) 5:63. doi: 10.1186/s13578-015-0056-4
39. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. (2021) 326:1210–2. doi: 10.1001/jama.2021.13443
40. Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J Biol Chem. (2013) 288:19450–8. doi: 10.1074/jbc.M113.467670
41. Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. (1998) 160:5314–9. doi: 10.4049/jimmunol.160.11.5314
42. Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, et al. Tissue determinants of human NK cell development, function, and residence. Cell. (2020) 180:749–763 e13. doi: 10.1016/j.cell.2020.01.022
43. Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. (2005) 77:15–22. doi: 10.1007/s00223-004-0227-5
44. Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. (1999) 96:507–15. doi: 10.1016/S0092-8674(00)80655-8
45. Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. (1999) 103:239–51. doi: 10.1172/JCI5244
47. Hansen JG, Tang W, Hootman KC, Brannon PM, Houston DK, Kritchevsky SB, et al. Genetic and environmental factors are associated with serum 25-hydroxyvitamin D concentrations in older African Americans. J Nutr. (2015) 145:799–805. doi: 10.3945/jn.114.202093
48. Khatiwada A, Wolf BJ, Mulligan JK, Shary JR, Hewison M, Baatz JE, et al. Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy. Pediatr Res. (2021) 89:554–62. doi: 10.1038/s41390-020-0885-7
49. Tran V, Janda M, Lucas RM, McLeod DSA, Thompson BS, Waterhouse M, et al. Vitamin D and sun exposure: A community survey in Australia. Curr Oncol. (2023) 30:2465–81. doi: 10.3390/curroncol30020188
50. Wang X, Lu K, Shen J, Xu S, Wang Q, Gong Y, et al. Correlation between meteorological factors and vitamin D status under different season. Sci Rep. (2023) 13:4762. doi: 10.1038/s41598-023-31698-2
51. Ohaegbulam KC, Swalih M, Patel P, Smith MA, Perrin R. Vitamin D supplementation in COVID-19 patients: A clinical case series. Am J Ther. (2020) 27:e485–90. doi: 10.1097/MJT.0000000000001222
52. Baca KM, Govil M, Zmuda JM, Simhan HN, Marazita ML, Bodnar LM. Vitamin D metabolic loci and vitamin D status in Black and White pregnant women. Eur J Obstet Gynecol Reprod Biol. (2018) 220:61–8. doi: 10.1016/j.ejogrb.2017.11.013
53. Braithwaite VS, Jones KS, Schoenmakers I, Silver M, Prentice A, Hennig BJ. Vitamin D binding protein genotype is associated with plasma 25OHD concentration in West African children. Bone. (2015) 74:166–70. doi: 10.1016/j.bone.2014.12.068
54. Hart MD, Girma M, Strong MD, Tadesse BT, Taddesse BM, Alemayehu FR, et al. Vitamin D binding protein gene polymorphisms are associated with lower plasma 25-hydroxy-cholecalciferol concentrations in Ethiopian lactating women. Nutr Res. (2022) 107:86–95. doi: 10.1016/j.nutres.2022.09.003
55. Li F, et al. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese Han population. BMC Med Genet. (2011) 12:103. doi: 10.1186/1471-2350-12-103
Keywords: vitamin D, BNT162b2 vaccine-related myocarditis, mRNA COVID-19 vaccines, natural killer cell, vitamin D deficiency, hyperinflammation, hypercytokinemia, vitamin D genetics
Citation: Tsang HW, Chua GT, Tung KTS, Wong RSM, Tsao SSL, Wong JSC, Tung JYL, Kwok JSY, Yam JCS, Chan GCF, To KKW, Wong ICK, Leung WH, Kwan MYW and Ip P (2025) The protective role of vitamin D in BNT162b2 vaccine-related acute myocarditis. Front. Immunol. 16:1501609. doi: 10.3389/fimmu.2025.1501609
Received: 25 September 2024; Accepted: 27 January 2025;
Published: 19 February 2025.
Edited by:
Pedro A. Reche, Complutense University of Madrid, SpainReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaCopyright © 2025 Tsang, Chua, Tung, Wong, Tsao, Wong, Tung, Kwok, Yam, Chan, To, Wong, Leung, Kwan and Ip. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick Ip, cGF0cmljaXBAaGt1Lmhr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.