
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 28 February 2025
Sec. Immunological Tolerance and Regulation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1487296
This article is part of the Research Topic Exploring Immunomodulation to Balance Maladaptive Inflammation and Restore Tissue Homeostasis View all 3 articles
The emergence of immunotherapies has revolutionized cancer treatment by leveraging the immune system to target malignancies, offering new hope where traditional therapies often fall short. Within this context, hyperthermia (HT) has re-emerged as a promising adjunctive treatment, capable of enhancing the effectiveness of radiotherapy, chemotherapy, and immunotherapy. HT influences both the innate and adaptive immune systems, enhancing the activity of immune cells such as neutrophils, NK cells, and dendritic cells, while also modulating the tumor microenvironment (TME) to promote immunogenic cell death (ICD) and reduce immunosuppressive conditions. These effects contribute to the transformation of immunologically “cold” tumors into “hot” tumors, making them more susceptible to immune-mediated destruction. Furthermore, HT can amplify the efficacy of immune checkpoint inhibitors (ICIs) by improving immune cell infiltration, inducing damage-associated molecular pattern (DAMP) release, and enhancing antigen presentation. Preclinical and clinical studies support the combination of HT with ICIs, demonstrating improved outcomes in otherwise resistant tumors. However, the full therapeutic potential of the different technologies allowing to apply HT remains to be fully understood, and further research is needed to optimize treatment protocols, explore the differential impacts of local versus whole-body hyperthermia, and identify biomarkers for patient stratification. This review underscores the multifaceted role of HT in immunity and its potential to significantly enhance the efficacy of immunotherapy.
Cancer treatment has been revolutionized by immunotherapies, shifting the focus from traditional methods like chemotherapy and radiotherapy to harnessing the immune system to combat cancer (1). These immunotherapies, including immune checkpoint inhibitors (2), adoptive cell therapies (3), and cancer vaccines (4), have shown remarkable efficacy in various cancers, offering new hope where traditional treatments have often failed. The success of immunotherapies highlights the immune system’s vital role in cancer control, targeting cancer’s evasion strategies by enhancing immune cell activity and recognition of tumor antigens. This focus on the interplay between cancer and immunity drives innovative treatments aimed at reactivating immune responses within the tumor microenvironment (TME) (5–7). Within this framework, several tumor classification systems have been developed to more accurately categorize patients based on the distinct properties of their TME (8, 9). Grasping the complexity of TME heterogeneity is vital for crafting effective therapeutic combinations in immunotherapy protocols and for incorporating personalized treatment strategies tailored to individual patient needs.
Thermal therapy, commonly referred to as hyperthermia (HT), involves deliberately raising tissue temperatures to between 39°C and 42°C for a sustained period, typically around one hour (10–13). The therapeutic benefits of heat have been recognized since ancient times. The earliest documented use of heat treatment can be found in the Edwin Smith Surgical Papyrus, an Egyptian text dating back approximately 5,000 years (14), where a patient with breast cancer was treated using heat. As far back as the fifth century BC, Hippocrates (460–377 BC) observed that malarial fever could alleviate symptoms in epileptics (15, 16). About 150 years ago, physician W. Busch was the first to report the potential benefits of HT in cancer treatment. He observed the regression of a sarcoma following a high fever induced by accidental erysipelas infection (17). A key hypothesis in Thermal Medicine, is that HT induced by externally heating the body or specific tissues without the presence of pyrogenic agents, may provoke a strong thermoregulatory response, which could significantly impact the physiology of the TME and modify the immune response accordingly (18). This approach has been extensively studied, particularly in cancer treatment, where it is frequently combined with radiotherapy, chemotherapy, or immunotherapy to improve treatment efficacy. More recently, HT has emerged as a potential option for managing depression, offering a complementary or alternative method for mood stabilization (19). It is important to differentiate therapeutic HT from other heat-related conditions or treatments. For instance, malignant HT represents a severe, potentially fatal reaction triggered by specific medications (20). Meanwhile, thermal ablation involves heating tissues to temperatures above 44°C with the intent of destroying cancerous cells (21). These distinctions emphasize the unique purpose and temperature parameters of therapeutic HT. Additionally, HT treatments can be further classified based on their method of heat delivery and scope. Heating depth distinguishes the main types: superficial HT targets tissues near the skin’s surface (22), deep HT is designed to treat tissues or organs located further within the body (23), and interstitial HT involves placing heating devices directly into tissues for precise temperature regulation (24). The scale of heating also defines its application: local HT focuses on small, specific areas; loco-regional HT addresses larger zones, such as an entire organ or surrounding tissues; and whole-body hyperthermia (WBH) elevates the body’s overall temperature to induce systemic effects (25). Additionally, HT is categorized based on temperature intensity. Mild HT gently raises tissue temperatures, while moderate HT involves slightly more substantial heating. Fever-range HT mimics the natural temperature increase during fever, potentially stimulating immune activity and providing specific therapeutic benefits. Each of these classifications underscores the versatility of HT as a treatment strategy across various medical technology (Figure 1) and applications. HT exerts multiple biological effects that enhance its therapeutic potential, particularly when combined with radiotherapy or chemotherapy. One of the key mechanisms of HT is its ability to induce cell cycle inhibition, primarily by halting cells in the S-phase and G2/M-phase, making them more sensitive to radiation and other treatments (26, 27). HT also disrupts nuclear proteins involved in DNA repair processes, such as BRCA2, which impairs homologous recombination, thereby increasing DNA damage caused by radiotherapy or chemotherapy (28). Furthermore, HT can destabilize cell membranes by altering lipid composition and membrane permeability, leading to increased drug uptake and cytotoxic effects (29). Additionally, HT inactivates proteins responsible for DNA repair, including heat-sensitive enzymes such as PARP1, resulting in an accumulation of DNA damage (30). These effects collectively amplify the efficacy of conventional cancer treatments, highlighting the critical role of HT in enhancing therapeutic outcomes. Thus, alongside these advances in immunotherapy, HT has re-emerged as a promising adjunctive treatment in cancer treatment and may have the potential to amplify the effectiveness of radiotherapy, chemotherapy, and immunotherapy (13). This amplification occurs through various mechanisms, such as boosting blood flow within the TME, which supports lymphocyte infiltration and increases oxygen levels, as well as improving drug delivery (18). The induction of heat shock proteins (HSPs) can also trigger apoptosis or necrosis in cancer cells, leading to alterations in surface marker expression and the release of cellular debris, which act as antigens and stimulate an antitumor immune response (31, 32). These heat-induced cellular stress responses not only promote the direct killing of cancer cells but also enhance the visibility of these cells to the immune system, thereby facilitating the activation of dendritic cells and subsequent T-cell-mediated immunity (31, 32). Moreover, there is growing interest in the potential of HT to modulate the immune system itself (33, 34). These immunomodulatory effects indicate that HT may enhance the efficacy of radiotherapy (RT), chemotherapy (CT), and immunotherapies, as outlined in Table 1, which provides a non-exhaustive list of relevant preclinical studies (35–44), potentially enhancing their efficacy or overcoming resistance mechanisms (45, 46). Growing interest in hyperthermia has led to numerous randomized clinical trials (47–52) investigating diverse approaches to its application. These efforts provide a robust foundation for proposing a detailed exploration of its potential mechanisms and therapeutic strategies. In this context of immune transformation after HT, the TME is altered and can shift from “hot,” with high immune infiltration and pro-inflammatory activity, to an even more immunostimulatory state by enhancing T-cell function, trafficking, and heat shock protein expression (53). In contrast, “cold” tumors are characterized by minimal immune cell infiltration and an immunosuppressive environment, which makes them less responsive to immunotherapies. HT can transform these “cold” tumors by increasing immunogenicity, promoting dendritic cell activation, and remodeling the stroma to allow immune cell infiltration, thereby enhancing their susceptibility to immune-based treatments (34, 54).
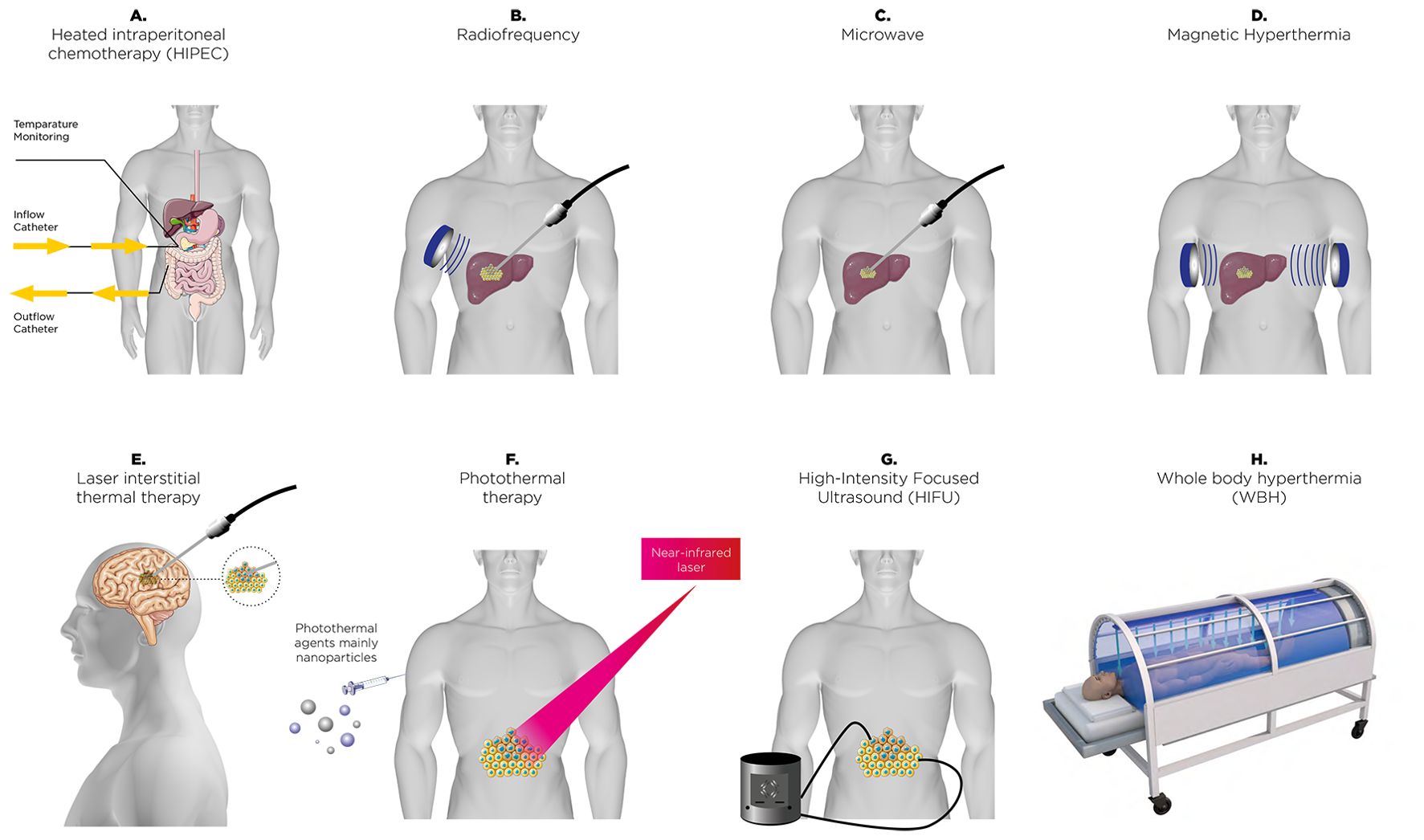
Figure 1. A range of thermal therapies employed in clinical and research contexts to enhance cancer treatment. These approaches include heated intraperitoneal chemotherapy (HIPEC) (A), radiofrequency energy-based ablation (B), microwave-based (C), magnetic field-induced hyperthermia (D), laser interstitial thermal therapy (E), nanoparticle-driven photothermal therapy (F), high-intensity focused ultrasound (HIFU) ablation (G), and systemic whole-body hyperthermia (WBH) (H). Each method uniquely leverages thermal energy for precise or widespread tumor targeting.
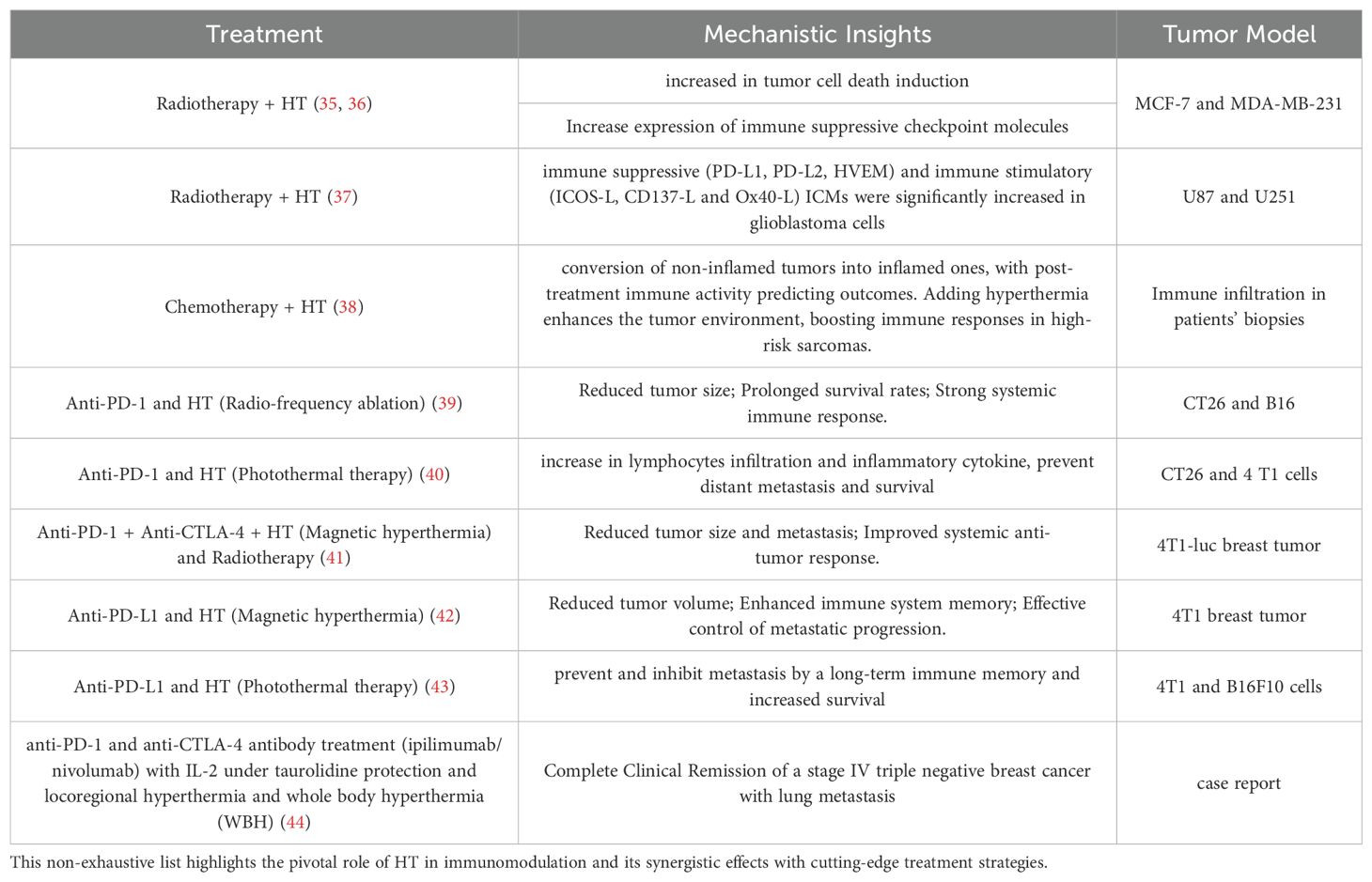
Table 1. A curated selection of preclinical studies showcasing the transformative potential of hyperthermia (HT) in reshaping immune responses and advancing cancer therapy.
This review explores the intricate interactions between HT and the immune system, a promising and emerging field with the potential to reshape cancer therapy. By providing a comprehensive overview of current knowledge, we aim to highlight how HT influences immune responses and its implications for advancing cancer treatment strategies.
HT, along with various other stress conditions, triggers the production of heat shock proteins (HSPs), which are essential for cellular protection and survival (55). These proteins are grouped into different families based on their molecular weight, and they function as molecular chaperones. The heat shock response (HSR) is structured as a sequential process, where information is transmitted through the localized activity of molecular chaperones (8). This means they assist in stabilizing and repairing damaged proteins, preventing harmful interactions between misfolded proteins, and aiding in the removal of defective proteins from the cell. The primary families of heat shock proteins (HSPs) encompass both small and large molecular weight groups. These include small HSPs like HSP27, as well as larger HSPs such as HSP47 (56, 57), HSP70 and HSP90 (58). Additionally, the human chaperonin families, including HSP60/HSP10, are also part of these main categories. Among the various HSPs, HSP27 and HSP70 are particularly notable for their ability to protect cells from potentially lethal stimuli by enhancing resistance to apoptosis and promoting cellular homeostasis. The synthesis of HSPs is generally upregulated in response to HT, a process that enables cells to cope with elevated temperatures. However, at extremely high temperatures, the production of HSPs is inhibited, which can lead to cell death. Under normal physiological conditions, HSPs play a critical role in maintaining cellular integrity, particularly by protecting cells from damage induced by stress and by enhancing their survival capabilities (32). While the intracellular functions of HSPs are well-documented, their roles in the extracellular environment are equally important, particularly in the context of cancer and the immune response (32, 59, 60). In cancer, HSPs are often overexpressed, which contributes to tumor development, progression, resistance to therapy and angiogenesis (61–64). This overexpression is associated with several processes, including the inhibition of apoptosis, the promotion of cell proliferation, and the enhancement of metastatic potential. For example, HSP70 and HSP90 are frequently upregulated in tumors, where they help stabilize oncogenic proteins, thereby supporting cancer cell survival and growth (63, 64). Extracellular HSPs have garnered significant attention for their involvement in the immune response, particularly in the context of cancer (65, 66). When released into the extracellular space, these proteins can function as danger signals, signaling the immune system to recognize the existence of damaged or stressed cells., including cancer cells (Figure 2). This alert system is crucial for initiating and coordinating an immune response against these aberrant cells. One of the primary mechanisms through which extracellular HSPs influence the immune system is by facilitating the cross-presentation of tumor antigens. HSPs such as gp96, HSP70 and HSP90 can chaperone tumor-derived peptides and deliver them to antigen-presenting cells (APCs), including dendritic cells and macrophages (Figure 2). This process is essential for the activation of cytotoxic T lymphocytes (CTLs/CD8+ T cells), which are the immune cells responsible for targeting and destroying cancer cells. The interaction between HSPs and APCs is often mediated by specific receptors, such as CD91, which plays a crucial role in the uptake of HSP-peptide complexes and the subsequent presentation of these peptides on MHC class I molecules (67–71). This pathway of antigen presentation is particularly important in the context of tumors, where the immune system’s ability to recognize and respond to cancer cells is often impaired (32, 65). By enhancing the visibility of tumor antigens to the immune system, extracellular HSPs can help restore immune surveillance and promote the destruction of cancer cells. This process is vital in cancer therapy, where the goal is to elicit a robust immune response against the tumor. The dual role of HSPs in cancer—acting both as protectors of tumor cells and as modulators of immune responses—presents a unique challenge but also offers promising opportunities for therapeutic intervention.
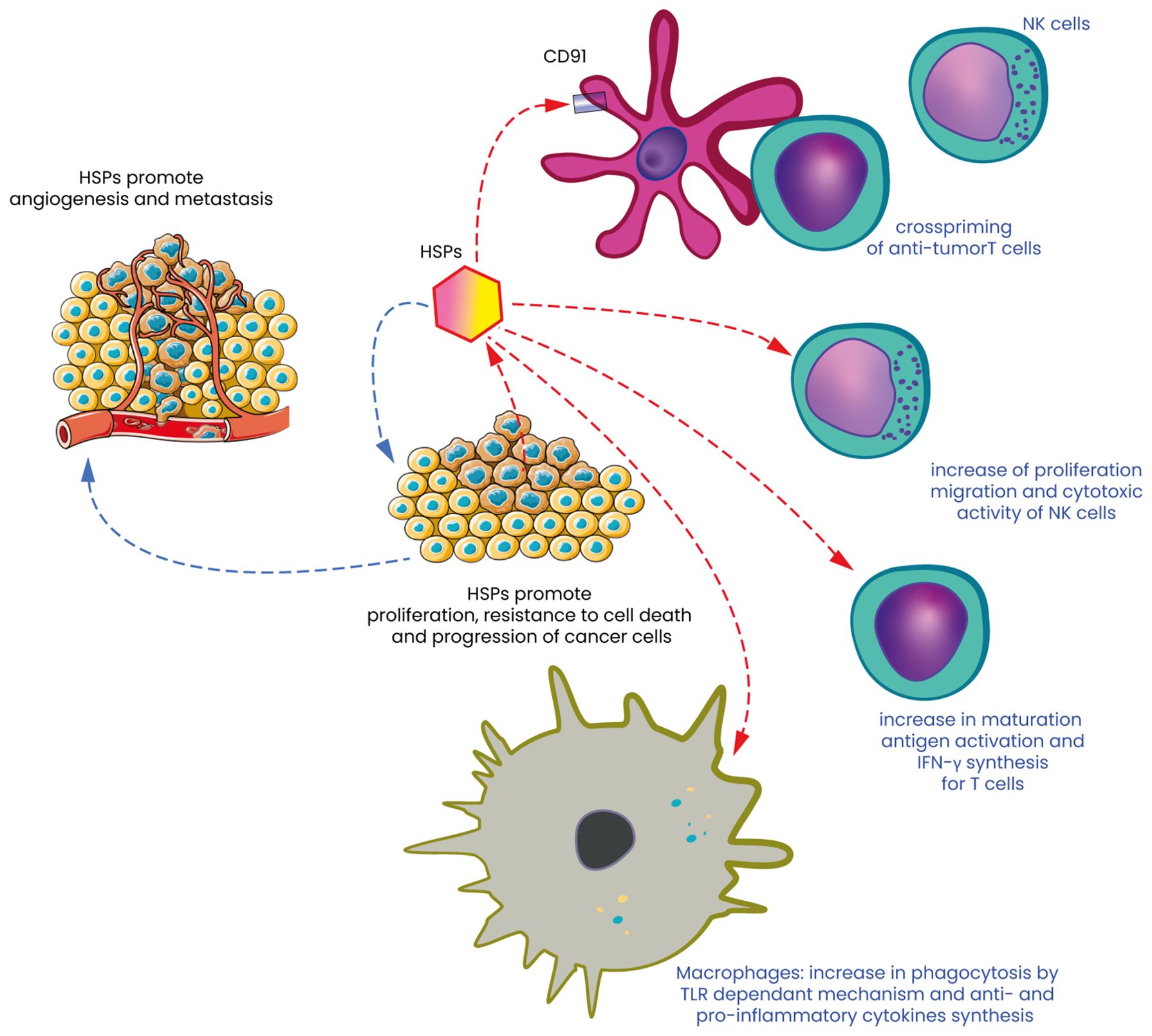
Figure 2. Heat shock proteins: the double-edged sword of cancer cell survival and immune response. Heat Shock Proteins (HSPs) are molecular chaperones that play a crucial role in maintaining cellular homeostasis by assisting in the proper folding of proteins and preventing aggregation under stress conditions. In the context of cancer, HSPs act as a double-edged sword. On one hand, they contribute to cancer cell survival, promoting tumor progression and angiogenesis (left part of Figure 1) and on the other hand, HSPs can also potentiate the immune response against tumors and destroy these same cells. They serve as danger signals that are recognized by the immune system, leading to the activation of dendritic cells (DC) in particular by activating CD91 and the subsequent presentation of tumor antigens to T cells. HSPs can also act as immunogenic molecules, enhancing the recognition and destruction of cancer cells by NK cells. HSPs are often expressed on the surface of stressed or damaged cancer cells, where they can bind to receptors on NK cells, such as NKG2D. This binding triggers the activation of NK cells, leading to the release of cytotoxic granules and the destruction of the target cancer cells. Moreover, extracellular HSPs can act as danger signals, promoting the recruitment and activation of NK cells within the TME, thereby contributing to the anti-tumor immune response. Finally, macrophages, another crucial component of the innate immune system, are influenced by HSPs by interacting with macrophages by binding to Toll-like receptors (TLRs) and other pattern recognition receptors on the surface of these immune cells. This interaction can lead to phagocytosis but also to the activation of macrophages in a pro-inflammatory (M1) phenotype, which supports anti-tumor immunity.
HT significantly enhances the activity of innate immune cells, such as neutrophils, natural killer (NK) cells, monocytes/macrophages, and dendritic cells.
Fever-range HT have been associated to activation and bactericidal function of neutrophils (72, 73). Thermal stress also promotes increased neutrophil recruitment to tumors (72). This thermal stress-induced neutrophil migration is partly driven by heat-induced elevations in circulating neutrophils, which are dependent on granulocyte colony-stimulating factor (G-CSF). G-CSF plays a pivotal role in a model of radiation-induced neutropenia, where fever-range WBH significantly accelerates neutrophil recovery in the bloodstream and increases the number of hematopoietic stem cells and neutrophil progenitors in the bone marrow (74). However, the effects of HT are highly dependent on the specific heating protocols and the localization of recruited cells (75).
Recent findings confirm that hyperthermia enhances the cytotoxic activity of natural killer (NK) cells and their mobilization, improving their ability to recognize and eliminate tumor cells, addressing previous uncertainties surrounding this effect (76). Indeed, HT at varying temperatures might indicate that NK cell activity and cytotoxic T-cell function are enhanced at moderate temperatures like 40°C (77). Studies have shown that therapeutic WBH leads to reversible changes in lymphocyte subpopulations, such as an increase in NK cells, NKT cells, and γδ-T cells, all of which are associated with innate immune functions (78).
Moreover, the myeloid-derived suppressor cells (MDSCs) are often recruited to the TME, where they support tumor progression by suppressing anti-tumor immune responses (79, 80). MDSCs are often recruited to the TME, where they support tumor progression by suppressing anti-tumor immune responses. Given their central role in promoting immune suppression in cancer and other diseases, MDSCs have become an important target for therapeutic intervention. HT, applied through various in vitro or in vivo methodologies, has been demonstrated to affect MDSCs in multiple ways, including decreasing their recruitment to the TME (81–86).
HT induces the release of damage-associated molecular patterns (DAMPs), such as extracellular HSP70, which not only diminish the immunosuppressive activity of MDSCs but also enhance CD4+ T cell-mediated anti-tumor responses. This effect has been highlighted in studies by Zhu et al. (84, 87). Additionally, the use of low-dose β-adrenergic receptor blocker therapy, such as propranolol, has been identified as a complementary approach to reducing MDSC accumulation (85). Propranolol mitigates physiological stress responses by blocking β-adrenergic signaling, thereby decreasing stress-induced recruitment of MDSCs. This finding underscores the potential for integrating stress-reducing interventions with thermal treatments to optimize anti-tumor immunity. Moreover, HT drives the secretion of pro-inflammatory cytokines such as CXCL10 and IL-6, which create an immune-supportive environment that counters MDSC-mediated suppression (83). Finally, Extracellular vesicles (EVs) released during hyperthermia (HT) have been demonstrated to significantly impact the immune landscape by decreasing MDSC recruitment and promoting anti-tumor immunity. The study by Cen et al. (88) delves into the role of serum-derived extracellular vesicles (sEVs) released following cryo-thermal therapy in reducing MDSC-mediated immunosuppression and enhancing therapeutic outcomes (88).
HT can influence the expression and function of proinflammatory cytokines (89), which play a critical role in regulating antitumor immune responses. WBH has been demonstrated to stimulate the release of various pro-inflammatory cytokines (78, 90, 91). HT has been shown to enhance IL-1-induced T-cell proliferation, demonstrating that IL-1’s function is highly responsive to thermal changes. In addition to its sensitivity to increased temperature, IL-1 also plays a crucial role in raising body temperature during fever (92). Another study demonstrated that a slight increase in body temperature to 39.5°C in mice exposed to total body irradiation led to improved recovery from neutropenia (74, 93). This recovery was propelled by a heat-induced cytokine cascade that significantly increase neutrophil production (93). Moreover, tumor samples from a group of 22 pet dogs with naturally occurring soft tissue sarcomas who underwent thermoradiotherapy demonstrated that before and 24 hours after the initial HT session alterations in the water diffusion coefficient, a marker for inflammation, were associated with changes in various inflammation-related genes (94). Additionally, systemic HT has been shown to influence the activity of the proinflammatory cytokine IL-6 within the TME, potentially diminishing its role in tumor progression (95). Indeed, HT has been propose to counteract the protumorigenic effects of IL-6 by promoting the trafficking of effector T lymphocytes to the TME (96). They found that neutralizing IL-6 prevented the selectin and Intercellular adhesion molecule-1 (ICAM-1) – dependent migration of adoptively transferred CD8+ T cells through the tumor vasculature (96). Furthermore, in IL-6-deficient mice, HT increased ICAM-1 expression on tumor vessels and induce CD8+ T-cell infiltration into the tumor (96). Temperatures around 42°C, appear to induce a temporary shift in lymphocyte function towards an anti-inflammatory state. This is evidenced by a significant rise in plasma IL-10 levels, coupled with a decrease in IL-12 and IFN-γ during and shortly after treatment (97). Moreover a marked increase in serum levels of sIL2-R has been observed, indicating significant T-cell activation (78). More recently, studies suggest that fever-range WBH could have significant impacts on the immune system, enhancing responses such as antigen-specific T-cell responses. Indeed, in 5 healthy volunteers, exposure to fever-range HT (38.5°Celsius for 60 minutes) has been shown to stimulate immune responses, specifically in T-cells. Kobayashi et al. demonstrated that exposure to physiologically relevant thermal stress can significantly boost cytokine production in human peripheral T cells, enhancing their sensitivity and response to specific antigens (98). In the study, volunteers underwent WBH, during which their rectal temperature was elevated and maintained above 38.5°C for over an hour. Peripheral blood mononuclear cells (PBMCs) were sampled both before and after this thermal treatment. The induced thermal stress appeared to increase membrane fluidity in T cells, potentially accelerating and optimizing the clustering of molecules essential for antigen recognition and signal transduction, thereby amplifying T-cell activation and immune efficiency (98).
Sulyok et al. conducted a randomized trial evaluating the impact of preoperative fever-range (FR) whole-body hyperthermia (WBH) on immune markers in colorectal cancer surgery patients (99). Their findings revealed that the FR-WBH group exhibited a significant increase in heat shock proteins (HSPs), particularly HSP60 and HSP90, compared to the control group, while HSP70 levels remained unchanged. Interestingly, tumor necrosis factor-alpha (TNF-α) levels surged post-surgery in the control group but remained near baseline in the FR-WBH group, suggesting a protective immune-modulatory effect of hyperthermia (99). These findings align with earlier studies emphasizing the immunostimulatory properties of fever-range hyperthermia. Additionally, Yu et al. reported that HT-induced immune activation might influence long-term survival in rectal cancer patients, reinforcing the potential of HT as an adjunctive immunotherapeutic strategy (100).
HT has been described to activate lymphocytes. Indeed, initial studies suggested that applying WBH at 41.8-42.2°C in patients with advance cancer, led to a reduction in CD4+ T-cells, accompanied by an increase in NK cells and γδ-T cells, which resulted in a decrease in the CD4+/CD8+ ratio (97). Additionally, a decrease in T-lymphocyte counts was also noted in cancer patients receiving therapeutic WBH at temperatures ranging from 39° to 40°C (101). Moreover, lymphocyte apoptosis could also play a crucial role in immune regulation, and HT appears to influence this process through multiple pathways beyond direct thermal damage. First, HT could induce a stress-related apoptosis of lymphocytes (102, 103) at least in part through a Fas dependent mechanism (104). During WBH, increased lymphocyte apoptosis has been reported, mainly involving CD4+ T cell (105). This is likely due to direct heat damage, suggesting that, at least in part, this phenomenon is stress-related. Second, HT can induce immunologically mediated apoptosis (106). Apoptosis is not only a response to stress but also a crucial immune regulatory mechanism. During immune development, T and B lymphocytes undergo programmed cell death if they fail to meet certain functional criteria, such as lacking a functional antigen receptor at various stages of their development, maintenance, and activation (107, 108). Later in their maturation, lymphocytes that exhibit overly strong or insufficiently weak interactions with antigens are also eliminated through apoptosis, a process that helps prevent autoimmune responses and its regulation in the immune microenvironment (109). This form of apoptosis is regulated by several factors, including nuclear steroid hormone receptors like NUR77, which influence both transcriptional programs and mitochondrial function by releasing cytochrome c (107). Heat has been demonstrated as a influencer of lymphocyte trafficking since a transient reductions in circulating T cells has been observed in mice or cancer patients FR-WBH (75, 96, 101). Subsequent research demonstrated that applying direct heat to T or B cells enhanced their adhesion to high endothelial venules (HEVs) and improved their homing capabilities to lymph nodes. This increased homing of lymphocytes from the bloodstream to tissues has been associated to upregulated expression of L-selectin and α4β7 integrin (96). The heat-induced upregulation of these molecules suggest that FR-HT can effectively mimic inflammatory conditions to support stable lymphocyte adhesion and migration (110). Once inside lymphoid organs, lymphocytes exposed to febrile temperatures exhibit an enhanced capacity to respond to stimulatory signals. Exposing T cells directly to heat significantly boosts their proliferation when stimulated by mitogens (111). Additionally, heat activated CD8+ T cells demonstrate greater differentiation towards an effector phenotype, including reduced L-selectin expression, increased cytotoxic activity, and higher IFNγ production (112, 113). Heat-induced alterations in membrane fluidity and molecular organization are also seen in CD4+ T cells, effectively reducing the dependency on CD28 co-stimulation for IL-2 production (114). These results suggest that HT could potentiate T cell activation by lowering the activation threshold and speeding up effector T cell differentiation. The question now is whether the enhanced immune response, along with broader effects on the TME, plays a significant role during HT treatment. Mechanisms of HT on innate and adaptative immunity are shown and outlined in Figure 3.
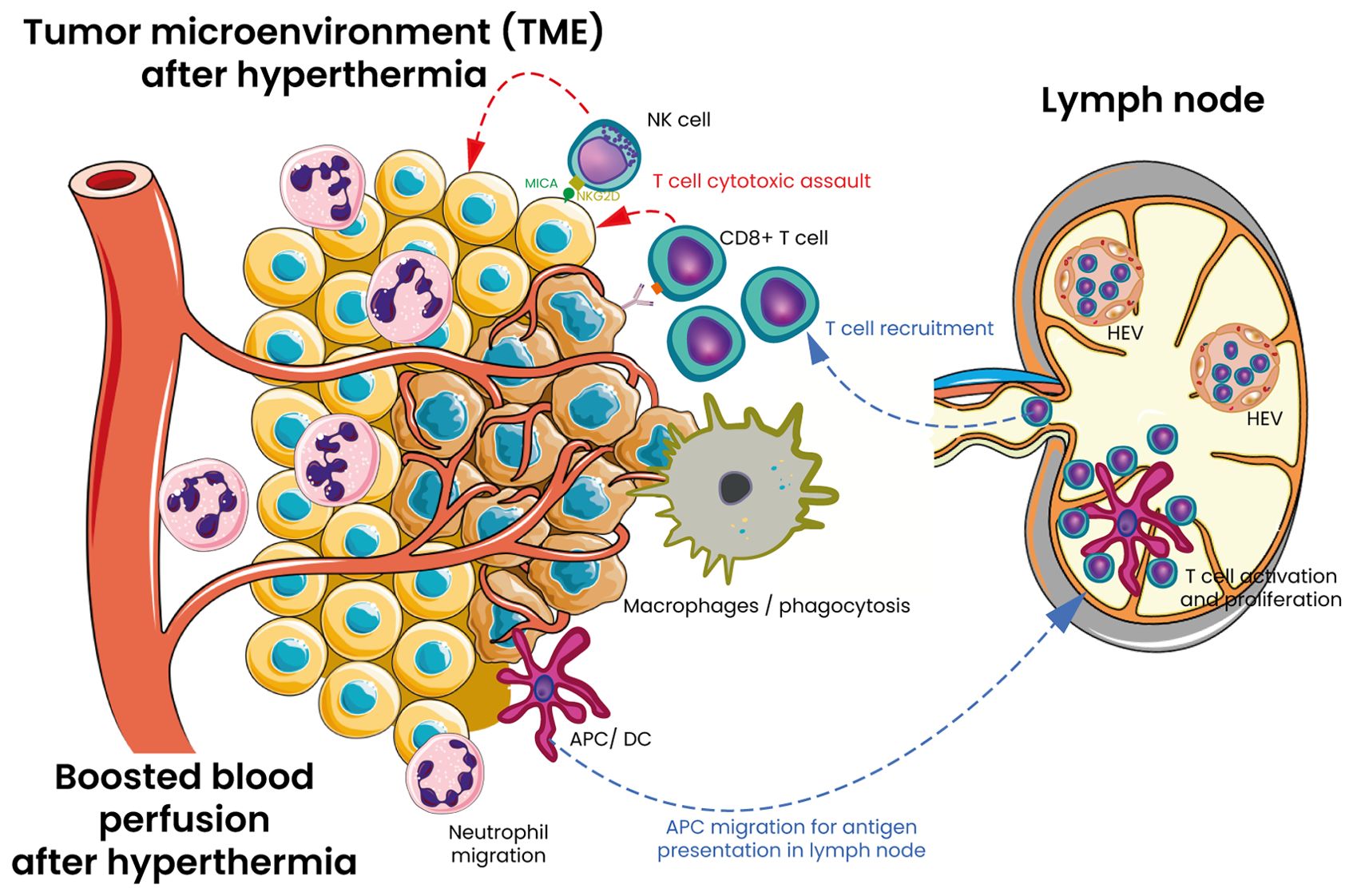
Figure 3. Mechanisms of HT on innate and adaptative immunity in tumor TME. HT serves as a versatile adjuvant that modifies the TME through several pathways. It enhances vascular perfusion and blood circulation within the tumor. Additionally, HT promotes the trafficking of CD8+ T cells by inducing the expression of E/P selectin and intercellular adhesion molecule 1 (ICAM-1) on tumor-associated blood vessels. It also boosts T-cell receptor (TCR) signaling and facilitates the differentiation of naïve T cells into effector cells and at the end cytotoxic attack of tumor cells. Furthermore, HT enhances the expression of MHC class I ligand (MICA/B) on tumor cells and upregulates the NKG2D ligand on NK cells, thereby increasing the cytotoxic potential of NK cells. The functional activity of macrophages and dendritic cells is also elevated which stimulates further immune responses and enhances antigen presentation. HT enhances neutrophil degranulation, phagocytic activity, and antigen presentation by M1 macrophages and dendritic cells. Following antigen uptake, dendritic cells travel to lymph nodes, where they display tumor antigens to T cells, initiating the proliferation of cytotoxic T cells. These cytotoxic T cells (CD8+ T cells), along with natural killer (NK) cells, then infiltrate tumors and target cancer cells for destruction by releasing cytotoxic granules and activating the Fas-FasL pathway. HT also enhances adaptive immune responses by accelerating lymphocyte movement through high endothelial venules (HEVs) in peripheral lymph nodes by modulating different stages of the adhesion process. Moreover, HT directly affects HEVs, promoting the shift from transient rolling to firm arrest of lymphocytes by increasing the density of several adhesion molecules on the vascular surface.
The TME is a complex ecosystem comprising immune cells, stromal cells, blood vessels, extracellular matrix, and signaling molecules, all of which influence tumor progression and therapy response (5, 115). A key feature of the TME is hypoxia, resulting from rapid tumor growth that outpaces oxygen supply (116). Hypoxia fosters an immunosuppressive environment by impairing cytotoxic T-cell activity and recruiting suppressive cells like regulatory T cells and myeloid-derived suppressor cells (34, 116–118). It also promotes angiogenesis via VEGF-A upregulation and drives tumor survival through hypoxia-inducible factors (119–121). The TME is also crucial in defining tumors as “hot” or “cold” (Figure 4) (122–125). Hot tumors are highly infiltrated by CD8+ T cells and express checkpoint molecules (e.g., PD-L1), making them more responsive to immunotherapy (9). Cold tumors, in contrast, have low immune infiltration and are dominated by suppressive cells such as regulatory T cells and MDSCs, leading to immune evasion and reduced immunotherapy efficacy (9). Cold stress can further modulate immune activity in the TME (126). It drives macrophages toward alternative activation via IL-4 and IL-13, leading to norepinephrine production (127). Cold exposure also reduces dendritic cell-mediated T-cell activation and promotes an immunosuppressive TME characterized by increased MDSCs and regulatory T cells, while decreasing CD8+ effector T cells, ultimately accelerating tumor progression (126, 128–130).
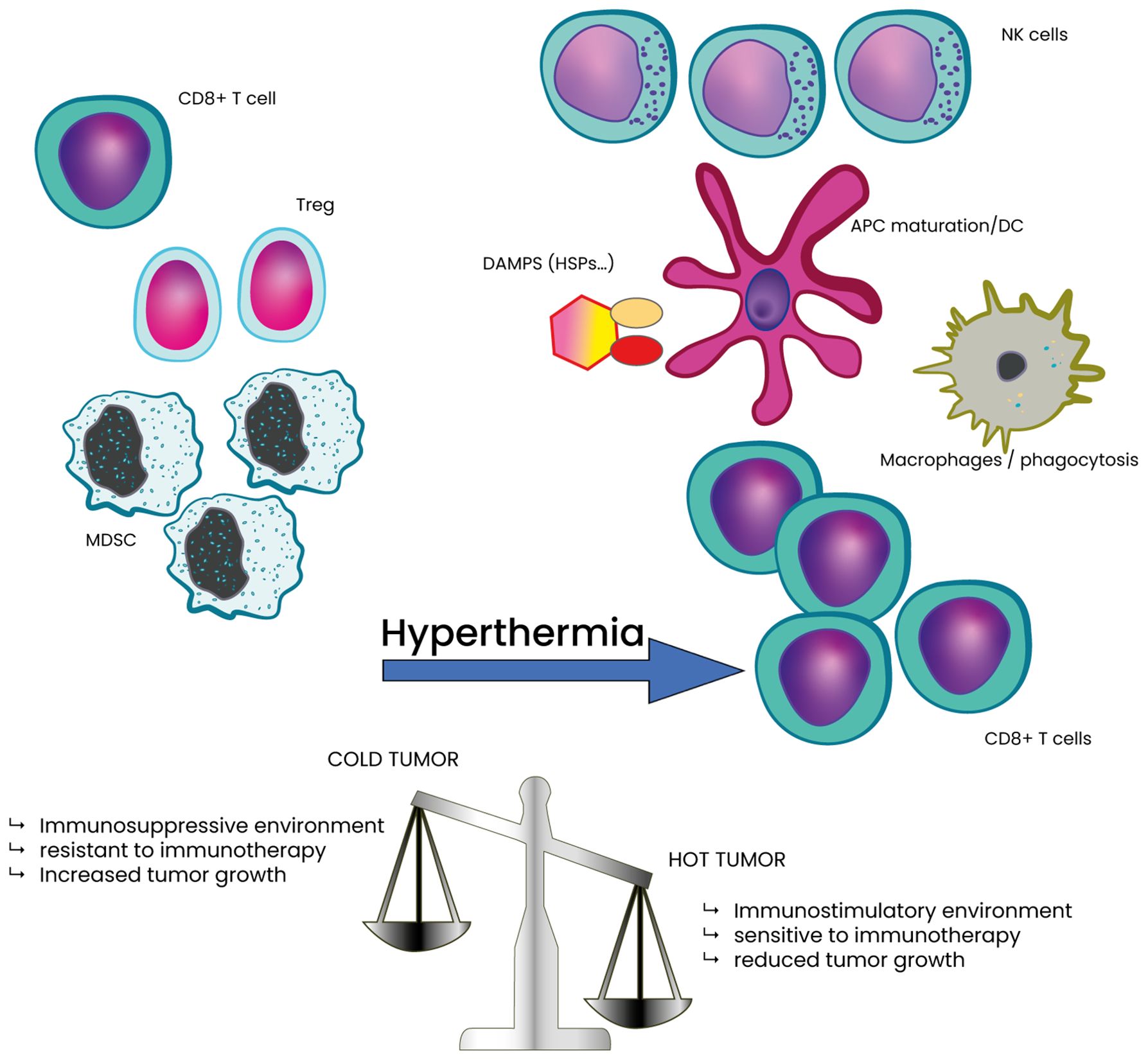
Figure 4. Classification of tumor as cold or hot tumor based on the TME composition in immune cells and potential effect of HT. HT stress shifts the TME towards a less immunosuppressive state. This shift is marked by a significant decrease in intra-tumoral myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells, along with a corresponding increase in CD8+ T cells, compared Hot tumor also contributes to lower tumor growth and decreased tumor cell survival.
HT has the potential to significantly influence both hypoxia in the TME and the cold/hot tumor classification. It enhances oxygenation through improved blood flow and vascular perfusion while simultaneously modulating immune responses by increasing immune cell infiltration and activity. These effects can convert cold tumors into hot, immunologically active tumors, making them more responsive to therapies.
HT modifies the tumor vasculature by improving blood flow and oxygenation, which helps reduce hypoxia. When tumors are exposed to heat, oxygen levels increase and this reoxygenation is associated with enhanced tumor sensitivity to radiation (131, 132). In canine studies, combining HT with radiotherapy led to a prolonged enhancement in oxygenation of hypoxic tumors, which in turn improved their response to radiation (133). The ability of mild HT to reoxygenate tumors is not only critical for enhancing the effectiveness of radiation therapy but also plays a key role in tumor immunology. Sen and colleagues proposed that heating large areas of normal tissue surrounding a tumor triggers thermoregulatory responses controlled by the nervous system, which increase blood flow to dissipate excess heat (134).
HT also targets tumor cell metabolism, influencing hypoxia. For instance, heat-induced activation of hypoxia-inducible factor 1 (HIF-1), a key transcription factor in oxygen homeostasis, along with its downstream targets, such as vascular endothelial growth factor A (VEGF-A) and 3-phosphoinositide-dependent protein kinase 1 (PDK1), leads to improved tumor vascularization and oxygenation (135, 136). Finally, improved oxygenation of the tumor following HT may enhance the eradication of cancer stem cells and increase their sensitivity to both radiotherapy and chemotherapy (137).
Apart from improving oxygenation, HT enhances immune responses by increasing the infiltration and activation of immune cells, such as T and NK cells. Strategies to convert “cold” tumors into “hot” ones are an area of active research to improve the effectiveness of immunotherapies in these types of tumors. HT has the potential to transform non-immunogenic “cold” tumors into immunogenic “hot” tumors by inducing immunogenic cell death (ICD), thereby enhancing the antitumor immune response (138). By increasing the temperature within the TME, HT can initiate the release of danger-associated molecular patterns (DAMPs) from dying tumor cells, which subsequently attract and activate immune cells (138). This thermal intervention not only enhances immune cell recruitment but also re-polarizes immunosuppressive M2 macrophages into pro-inflammatory, anti-tumor M1 macrophages (139). This re-polarization process plays a crucial role in inducing ICD, which in turn activates dendritic cells (DC), T lymphocytes, and natural killer (NK) cells, leading to a robust antitumor immune response and contributing to the inhibition of tumor growth. Consequently, HT can convert previously immune-silent tumors into immunogenic ones, making them more recognizable and susceptible to immune system attacks.
HT, in combination with nanomedicines that generate localized heat and reactive oxygen species (ROS), presents a promising strategy for improving cancer immunotherapy efficacy (138, 140, 141). These nanomedicines can precisely target tumor sites, creating optimal conditions for ICD and enhancing immune-mediated tumor destruction. Consequently, HT and nanomedicine-based approaches hold great potential for transforming cold tumors into hot tumors, making them more susceptible to immune system attacks.
Radiotherapy (RT) remains a cornerstone in cancer treatment, effectively delivering ionizing radiation to destroy tumor cells and shrink tumors. For a long time, radiotherapy (RT) was considered immunosuppressive due to the radiosensitivity of immune cells (142). However, recent evidence shows that RT can activate the immune system through ICD leading to the release of tumor antigens and DAMPs, stimulating immune responses and enabling bystander and abscopal effects (143). ICD promotes the release of key DAMPs like calreticulin (CRT), which facilitates phagocytosis by dendritic cells (DCs), ATP, which recruits and primes immune cells, and HMGB1, which interacts with TLR4 to activate antigen presentation (144). These signals polarize DCs and macrophages, enhancing the uptake and presentation of tumor-associated antigens (TAAs) to CD8+ T-cells in lymph nodes (145). RT also activates the cGAS-STING pathway, where irradiation-induced DNA damage causes cytosolic DNA accumulation (146–149). This triggers cGAS to produce cGAMP, which activates STING and drives type-I interferon (IFN-α/β) production (150). Type-I IFNs recruit DCs, enhance T-cell priming, and improve immune trafficking to the TME (151). Additionally, IFN-γ from activated T-cells upregulates MHC-I on tumor cells, enhancing immune recognition (152). RT further remodels the TME by increasing inflammatory chemokines like CXCL9, CXCL10, and adhesion molecules such as ICAM-1 and VCAM-1, which promote T-cell infiltration and leukocyte migration into tumors (153). However, excessively high doses can activate DNA-degrading enzymes like TREX1, limiting cGAS-STING activation and systemic immune responses (143, 151). Various RT subtypes have been developed to improve precision, dose delivery, and immunological effects. Among these, Stereotactic Body Radiotherapy (SBRT) and lattice radiotherapy are particularly notable due to their ability to enhance immune responses and modulate the TME. SBRT delivers high-dose radiation in 1–5 fractions with sub-millimeter precision to extracranial tumors, such as those found in the lung, liver, pancreas, and spine, while sparing adjacent normal tissues (154). This high-dose, hypofractionated approach induces ICD, releasing tumor-specific neoantigens and DAMPs, such as HMGB1 and ATP. These molecules enhance dendritic cell maturation and antigen presentation to T-cells, ultimately activating the adaptive immune system (155). Through this mechanism, SBRT has demonstrated its potential to trigger abscopal effects, where local irradiation leads to systemic tumor regression at distant, untreated sites (156). When combined with immune checkpoint inhibitors, such as anti-PD-1 or anti-CTLA-4 antibodies, SBRT creates a favorable TME that enhances the efficacy of immunotherapy (157). Lattice radiotherapy is a novel approach that delivers non-uniform radiation doses, creating high-dose “hotspots” (vertices) interspersed with lower-dose regions within large, bulky tumors. The high-dose vertices induce localized ICD releasing neoantigens and pro-inflammatory signals such as HSPs and cytokines (158). Meanwhile, the low-dose regions preserve tumor perfusion, reducing hypoxia and enabling better infiltration of immune cells into the TME (159). Lattice RT also triggers a bystander effect, where immune responses initiated in the high-dose regions spread to the lower-dose areas, amplifying the overall immune response and tumor control (160). This approach can also prime systemic immune activation by increasing the release of neoantigens and inflammatory cytokines, effects that are further enhanced when combined with HT or immunotherapy (161). The combined use of HT and RT has shown clinical benefits across various cancers, including head and neck, melanoma, breast, cervical, and rectal cancers (47–49, 151, 162). Both treatments exhibit complementary and synergistic effects on the immune system, enhancing anti-tumor responses through shared pathways. Together, they stimulate the release of DAMPs, which activate CD8+ T-cells and promote leukocyte trafficking by upregulating cell adhesion molecules. RT and HT upregulate together leukocyte adhesion and improved lymphocyte infiltration into tumors. HT complements RT by causing sublethal damage, enhancing blood flow, and creating a favorable TME that supports immune cell recruitment even at distant metastatic sites (151). Both modalities also upregulate extracellular HSP70, a critical DAMP that boosts macrophage and dendritic cell (DC) recognition and enhances tumor antigen presentation in lymph nodes, leading to more robust cytotoxic T-cell activation. Additionally, the increased tumor cell death observed with combined HT and RT amplifies DAMP release, further stimulating immune responses. The combination of HT and RT holds significant potential to generate stronger systemic anti-tumor immunity compared to either treatment alone, owing to their complementary mechanisms and synergistic effects on immune activation, cell death, and lymphocyte trafficking.
Hyperthermia (HT) has emerged as a valuable adjunct in cancer therapy, with increasing evidence supporting its role in enhancing anti-tumor immune responses. When combined with immune checkpoint inhibitors (ICIs) such as PD-1/PD-L1 and CTLA-4 blockers (161, 162), HT has the potential to overcome resistance in tumors that are poorly infiltrated by immune cells, also known as “cold” tumors, as described in the previous chapter of this paper (9). The synergy between HT and ICIs is driven by multiple mechanisms (163, 164), including modulation of the TME, enhancement of immune cell infiltration, and induction of immunogenic cell death (ICD) (45, 46, 165).
One of the key mechanisms by which HT enhances the efficacy of ICIs is through its ability to alter the TME. HT improves blood flow and oxygenation, reducing tumor hypoxia, a condition that often supports tumor growth and immune evasion (166). This reoxygenation diminishes immunosuppressive signals within the TME, making tumors more susceptible to immune attack. Additionally, HT promotes the release of danger-associated molecular patterns (DAMPs) from dying tumor cells (Figure 4). These signals attract dendritic cells, which are crucial for antigen presentation and help prime the immune system for a stronger response to ICIs.
HT facilitates the infiltration of immune cells into tumors, a critical step in effective immune-mediated tumor destruction (Figure 4). Studies have demonstrated that HT increases vascular permeability, allowing CD8+ T cells and NK cells to enter tumors more efficiently. This effect is further supported by HT-induced upregulation of adhesion molecules on endothelial cells (167, 168), which enhances immune cell recruitment and retention at the tumor site. By promoting sustained immune activity, HT improves the long-term effectiveness of ICIs in tumor control.
A crucial aspect of HT’s synergy with ICIs is its ability to induce ICD. Unlike apoptotic cell death, which is typically non-immunogenic, ICD triggers the release of immune-stimulatory molecules that alert the immune system to the presence of dying tumor cells. HT-induced protein denaturation exposes tumor antigens, making them more recognizable to immune cells. This not only enhances HT’s direct cytotoxic effects but also amplifies the efficacy of ICIs by providing a continuous source of tumor antigens for immune recognition and attack (136, 137).
Preclinical studies provide strong evidence supporting the combination of HT and ICIs in cancer treatment (45, 46). In murine models of colorectal cancer, HT has been shown to increase the infiltration of CD8+ T cells into tumors, thereby enhancing the efficacy of anti-PD-1 therapy (169). Similarly, in models of pancreatic cancer, a notoriously “cold” tumor (170–172), HT has been demonstrated to improve the effectiveness of ICIs by altering the dense stromal environment that typically hinders immune cell infiltration. Combining HT with anti-PD-1 or anti-CTLA-4 therapy in breast cancer models has resulted in significant tumor regression and improved survival compared to either treatment alone (41, 173–176). In glioblastoma (GBM), also considered an immune-cold tumor like pancreatic cancer (37, 177–179), HT combined with ICIs may shift the immunosuppressive environment into a more immune-responsive state. Gold nanoparticles amplify photothermal ablation, and when paired with anti-PD-L1 therapy, they reduce tumor size, improve survival, and induce long-term immunity (180). Overcoming the blood-brain barrier (BBB) remains a crucial challenge, but magnetic HT therapy (MHT) shows promise in enhancing antibody delivery (181, 182). Additionally, studies suggest that combining Prussian blue nanoparticles with photothermal therapy and anti-CTLA-4 therapy may effectively treat neuroblastoma, improving survival and preventing recurrence (183). These findings highlight HT’s immunomodulatory potential, particularly in tumors that are otherwise resistant to ICI therapy.
Several clinical trials are currently investigating the combination of HT and ICIs, with promising early results. Lyu et al. explored combining anti-PD-1 therapies (nivolumab and pembrolizumab) with thermal ablation in hepatocellular carcinoma (HCC) patients who had failed sorafenib treatment, leading to a significant improvement in the objective response rate (184). In biliary tract cancer, Xie’s application of tremelimumab combined with HT and ICIs achieved better progression-free survival (PFS) than second-line chemotherapy (185). Kleef’s research on stage IV triple-negative breast cancer with lung metastases, combining HT with immunotherapy, improved patient outcomes (44). In patients with advanced melanoma, the addition of localized HT to ICI therapy resulted in higher response rates and longer progression-free survival (PFS) compared to ICI therapy alone (186). Wei’s study on non-small cell lung cancer (NSCLC) patients using camrelizumab with microwave ablation also demonstrated promising outcomes (187). These findings suggest that the combination of HT and immunotherapy holds significant potential in clinical practice, though larger trials are necessary to refine treatment protocols and optimize patient selection.
Despite these encouraging findings, several challenges remain in the clinical application of HT combined with ICIs. One of the main concerns is optimizing the timing and dosage of HT to maximize its immunomodulatory effects while minimizing toxicity. Another challenge is identifying biomarkers that can predict which patients are most likely to benefit from this combination therapy. Ongoing research is focused on refining HT techniques, including the use of nanotechnology to deliver targeted heat to tumors. Researchers are also exploring strategies for both whole-body and localized HT, as well as the potential of combining HT with other immunotherapeutic approaches, such as cancer vaccines and adoptive cell therapies. HT in combination with ICIs represents a promising approach to enhancing anti-tumor immune responses, particularly in tumors resistant to immunotherapy alone. As clinical evidence continues to accumulate, this combined approach may become an integral component of cancer treatment, offering new hope for patients with difficult-to-treat tumors.
Hyperthermia (HT) has emerged as a promising adjunct in cancer treatment, particularly in combination with immune checkpoint inhibitors (ICIs) and radiotherapy (RT). By modulating the tumor microenvironment, increasing immune cell infiltration, and promoting immunogenic cell death, HT can transform immunologically “cold” tumors into “hot” ones, enhancing their responsiveness to immunotherapy.
Future research should refine sequencing and dosing strategies for RT, HT, and immunotherapy to maximize synergy. Identifying predictive biomarkers such as HSP70, HSP90, and HMGB1 is crucial for improving patient selection and monitoring treatment efficacy (151, 188). Cytokines like IL-6 and TNF-α, as well as tumor-infiltrating lymphocytes (TILs), also serve as key indicators of immune activation (143). The TNFα-TNFR2 pathway, which regulates immunosuppressive and pro-angiogenic processes, warrants further investigation for its potential role in optimizing HT-ICI therapy (189).
A key research direction is comparing local HT and whole-body HT (WBH). Local HT improves tumor oxygenation and immune cell infiltration, while WBH enhances systemic immune responses and may amplify the abscopal effect. Future studies should evaluate their differential impacts on cytokine release, immune activation, and clinical outcomes to optimize treatment approaches.
All in all, integrating HT into multimodal cancer therapy requires further research into biomarker-driven patient selection and personalized treatment strategies. By enhancing immune checkpoint blockade efficacy and reshaping the tumor microenvironment, HT offers new therapeutic opportunities for patients with resistant tumors.
MA: Conceptualization, Writing – original draft, Writing – review & editing. AC: Writing – original draft, Writing – review & editing. DS: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank Dr Mohammad Hosseine-Farid for helpful discussions.
MA is the founder of BTT Medical Institute.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C, et al. Immunotherapy in head and neck cancers: A new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev. (2018) 65:54–64. doi: 10.1016/j.ctrv.2018.02.008
2. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
3. Espie D, Donnadieu E. New insights into CAR T cell-mediated killing of tumor cells. Front Immunol. (2022) 13:1016208. doi: 10.3389/fimmu.2022.1016208
4. Tran T, Blanc C, Granier C, Saldmann A, Tanchot C, Tartour E. Therapeutic cancer vaccine: building the future from lessons of the past. Semin Immunopathol. (2019) 41:69–85. doi: 10.1007/s00281-018-0691-z
5. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. (2018) 24:541–50. doi: 10.1038/s41591-018-0014-x
6. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. (2012) 21:309–22. doi: 10.1016/j.ccr.2012.02.022
7. Edwardson DW, Parissenti AM, Kovala AT. Chemotherapy and inflammatory cytokine signalling in cancer cells and the tumour microenvironment. Adv Exp Med Biol. (2019) 1152:173–215. doi: 10.1007/978-3-030-20301-6_9
8. Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: A therapeutic challenge for immunotherapy. Front Immunol. (2019) 10:168. doi: 10.3389/fimmu.2019.00168
9. De Guillebon E, Dardenne A, Saldmann A, Séguier S, Tran T, Paolini L, et al. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int J Cancer. (2020) 147:1509–18. doi: 10.1002/ijc.32889
10. Conte E, Psihogios A, Seely D. Hyperthermia in cancer care: A literature review. CAND J. (2021) 28:14–30. doi: 10.54434/candj.92
11. Elming P, Sørensen B, Oei A, Franken N, Crezee J, Overgaard J, et al. Hyperthermia: the optimal treatment to overcome radiation resistant hypoxia. Cancers. (2019) 11:60. doi: 10.3390/cancers11010060
12. Mallory M, Gogineni E, Jones GC, Greer L, Simone CB. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit Rev Oncol Hematol. (2016) 97:56–64. doi: 10.1016/j.critrevonc.2015.08.003
13. Smadja DM. Hyperthermia for targeting cancer and cancer stem cells: insights from novel cellular and clinical approaches. Stem Cell Rev Rep. (2024) 20(6):1532–9. doi: 10.1007/s12015-024-10736-0
14. Van Middendorp JJ, Sanchez GM, Burridge AL. The Edwin Smith papyrus: a clinical reappraisal of the oldest known document on spinal injuries. Eur Spine J. (2010) 19:1815–23. doi: 10.1007/s00586-010-1523-6
16. Whitrow M. Wagner-Jauregg and fever therapy. Med Hist. (1990) 34:294–310. doi: 10.1017/s0025727300052431
17. Busch W. Aus der Sitzung der medicinischen Section vom 13 November 1867. Berl Klin Wochenschr. (1868) 5:137.
18. Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. (2002) 43:33–56. doi: 10.1016/s1040-8428(01)00179-2
19. Janssen CW, Lowry CA, Mehl MR, Allen JJB, Kelly KL, Gartner DE, et al. Whole-body hyperthermia for the treatment of major depressive disorder: A randomized clinical trial. JAMA Psychiatry. (2016) 73:789–95. doi: 10.1001/jamapsychiatry.2016.1031
20. Kollmann-Camaiora A, Alsina E, Domínguez A, Del Blanco B, Yepes MJ, Guerrero JL, et al. Clinical protocol for the management of Malignant hyperthermia. Rev Esp Anestesiol Reanim. (2017) 64:32–40. doi: 10.1016/j.redar.2016.06.004
21. Wu F. Heat-based tumor ablation: role of the immune response. Adv Exp Med Biol. (2016) 880:131–53. doi: 10.1007/978-3-319-22536-4_8
22. Dobšíček Trefná H, Crezee J, Schmidt M, Marder D, Lamprecht U, Ehmann M, et al. Quality assurance guidelines for superficial hyperthermia clinical trials : II. Technical requirements for heating devices. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. (2017) 193:351–66. doi: 10.1007/s00066-017-1106-0
23. Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. (2011) 187:605–10. doi: 10.1007/s00066-011-1145-x
24. Dobšíček Trefná H, Schmidt M, van Rhoon GC, Kok HP, Gordeyev SS, Lamprecht U, et al. Quality assurance guidelines for interstitial hyperthermia. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2019) 36:277–94. doi: 10.1080/02656736.2018.1564155
25. Heckel-Reusser S. Whole-body hyperthermia (WBH): historical aspects, current use, and future perspectives, in: Water-filtered Infrared A (wIRA) Irradiation: From Research to Clinical Settings (2022). Cham (CH: Springer. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK593451/ (Accessed May 18, 2024).
26. Kaur P, Hurwitz MD, Krishnan S, Asea A. Combined hyperthermia and radiotherapy for the treatment of cancer. Cancers. (2011) 3:3799–823. doi: 10.3390/cancers3043799
27. Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. (2001) 77:399–408. doi: 10.1080/09553000010024687
28. Oei AL, Vriend LEM, Crezee J, Franken NAP, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. (2015) 10:165. doi: 10.1186/s13014-015-0462-0
29. Roti Roti JL. Cellular responses to hyperthermia (40–46°C): Cell killing and molecular events. Int J Hyperthermia. (2008) 24:3–15. doi: 10.1080/02656730701769841
30. Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia. (2012) 28:509–17. doi: 10.3109/02656736.2012.695427
31. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. (2002) 2:185–94. doi: 10.1038/nri749
32. Mazurakova A, Solarova Z, Koklesova L, Caprnda M, Prosecky R, Khakymov A, et al. Heat shock proteins in cancer - Known but always being rediscovered: Their perspectives in cancer immunotherapy. Adv Med Sci. (2023) 68:464–73. doi: 10.1016/j.advms.2023.10.005
33. Dieing A, Ahlers O, Hildebrandt B, Kerner T, Tamm I, Possinger K, et al. The effect of induced hyperthermia on the immune system. Prog Brain Res. (2007) 162:137–52. doi: 10.1016/S0079-6123(06)62008-6
34. Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. (2013) 1:210–6. doi: 10.1158/2326-6066.CIR-13-0118
35. Hader M, Savcigil DP, Rosin A, Ponfick P, Gekle S, Wadepohl M, et al. Differences of the immune phenotype of breast cancer cells after ex vivo hyperthermia by warm-water or microwave radiation in a closed-loop system alone or in combination with radiotherapy. Cancers. (2020) 12:1082. doi: 10.3390/cancers12051082
36. Sengedorj A, Hader M, Heger L, Frey B, Dudziak D, Fietkau R, et al. The effect of hyperthermia and radiotherapy sequence on cancer cell death and the immune phenotype of breast cancer cells. Cancers. (2022) 14:2050. doi: 10.3390/cancers14092050
37. Stoll E, Hader M, Rückert M, Weissmann T, Lettmaier S, Putz F, et al. Detailed in vitro analyses of the impact of multimodal cancer therapy with hyperthermia and radiotherapy on the immune phenotype of human glioblastoma cells. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2022) 39:796–805. doi: 10.1080/02656736.2022.2080873
38. Issels RD, Noessner E, Lindner LH, Schmidt M, Albertsmeier M, Blay J-Y, et al. Immune infiltrates in patients with localised high-risk soft tissue sarcoma treated with neoadjuvant chemotherapy without or with regional hyperthermia: A translational research program of the EORTC 62961-ESHO 95 randomised clinical trial. Eur J Cancer Oxf Engl 1990. (2021) 158:123–32. doi: 10.1016/j.ejca.2021.09.015
39. Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, et al. PD-1 blockade boosts radiofrequency ablation–elicited adaptive immune responses against tumor. Clin Cancer Res. (2016) 22:1173–84. doi: 10.1158/1078-0432.CCR-15-1352
40. Luo L, Yang J, Zhu C, Jiang M, Guo X, Li W, et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Controlled Release. (2018) 278:87–99. doi: 10.1016/j.jconrel.2018.04.002
41. Oei AL, Korangath P, Mulka K, Helenius M, Coulter JB, Stewart J, et al. Enhancing the abscopal effect of radiation and immune checkpoint inhibitor therapies with magnetic nanoparticle hyperthermia in a model of metastatic breast cancer. Int J Hyperthermia. (2019) 36:47–63. doi: 10.1080/02656736.2019.1685686
42. Pan J, Hu P, Guo Y, Hao J, Ni D, Xu Y, et al. Combined magnetic hyperthermia and immune therapy for primary and metastatic tumor treatments. ACS Nano. (2020) 14:1033–44. doi: 10.1021/acsnano.9b08550
43. Huang L, Li Y, Du Y, Zhang Y, Wang X, Ding Y, et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat Commun. (2019) 10:4871. doi: 10.1038/s41467-019-12771-9
44. Kleef R, Moss R, Szasz AM, Bohdjalian A, Bojar H, Bakacs T. Complete clinical remission of stage IV triple-negative breast cancer lung metastasis administering low-dose immune checkpoint blockade in combination with hyperthermia and interleukin-2. Integr Cancer Ther. (2018) 17:1297–303. doi: 10.1177/1534735418794867
45. Yang X, Gao M, Xu R, Tao Y, Luo W, Wang B, et al. Hyperthermia combined with immune checkpoint inhibitor therapy in the treatment of primary and metastatic tumors. Front Immunol. (2022) 13:969447. doi: 10.3389/fimmu.2022.969447
46. Liu P, Ye M, Wu Y, Wu L, Lan K, Wu Z. Hyperthermia combined with immune checkpoint inhibitor therapy: Synergistic sensitization and clinical outcomes. Cancer Med. (2023) 12:3201–21. doi: 10.1002/cam4.5085
47. Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic Malignant melanoma. Eur Soc Hyperthermic Oncol Lancet Lond Engl. (1995) 345:540–3. doi: 10.1016/s0140-6736(95)90463-8
48. van der Zee J, González González D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group Lancet Lond Engl. (2000) 355:1119–25. doi: 10.1016/s0140-6736(00)02059-6
49. Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol Off J Am Soc Clin Oncol. (2005) 23:3079–85. doi: 10.1200/JCO.2005.05.520
50. Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem B-C, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. (2010) 11:561–70. doi: 10.1016/S1470-2045(10)70071-1
51. Wessalowski R, Schneider DT, Mils O, Friemann V, Kyrillopoulou O, Schaper J, et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular Malignant germ-cell tumours: an open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. (2013) 14:843–52. doi: 10.1016/S1470-2045(13)70271-7
52. Aronson SL, Lopez-Yurda M, Koole SN, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): final survival analysis of a randomised, controlled, phase 3 trial. Lancet Oncol. (2023) 24:1109–18. doi: 10.1016/S1470-2045(23)00396-0
53. Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol. (2005) 35:2518–27. doi: 10.1002/eji.200535002
54. Frey B, Weiss E-M, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2012) 28:528–42. doi: 10.3109/02656736.2012.677933
55. Chatterjee S, Burns T. Targeting heat shock proteins in cancer: A promising therapeutic approach. Int J Mol Sci. (2017) 18:1978. doi: 10.3390/ijms18091978
56. Thienel M, Müller-Reif JB, Zhang Z, Ehreiser V, Huth J, Shchurovska K, et al. Immobility-associated thromboprotection is conserved across mammalian species from bear to human. Science. (2023) 380:178–87. doi: 10.1126/science.abo5044
57. Khan ES, Däinghaus T. HSP47 in human diseases: Navigating pathophysiology, diagnosis and therapy. Clin Transl Med. (2024) 14:e1755. doi: 10.1002/ctm2.1755
58. Tsan M-F, Gao B. Heat shock proteins and immune system. J Leukoc Biol. (2009) 85:905–10. doi: 10.1189/jlb.0109005
59. Singh MK, Shin Y, Ju S, Han S, Choe W, Yoon K-S, et al. Heat shock response and heat shock proteins: current understanding and future opportunities in human diseases. Int J Mol Sci. (2024) 25:4209. doi: 10.3390/ijms25084209
60. Caruso Bavisotto C, Marino Gammazza A, Campanella C, Bucchieri F, Cappello F. Extracellular heat shock proteins in cancer: From early diagnosis to new therapeutic approach. Semin Cancer Biol. (2022) 86:36–45. doi: 10.1016/j.semcancer.2021.09.010
61. Thuringer D, Jego G, Wettstein G, Terrier O, Cronier L, Yousfi N, et al. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J Off Publ Fed Am Soc Exp Biol. (2013) 27:4169–83. doi: 10.1096/fj.12-226977
62. Calderwood SK, Gong J. Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem Sci. (2016) 41:311–23. doi: 10.1016/j.tibs.2016.01.003
63. Kunachowicz D, Król-Kulikowska M, Raczycka W, Sleziak J, Błażejewska M, Kulbacka J. Heat shock proteins, a double-edged sword: significance in cancer progression, chemotherapy resistance and novel therapeutic perspectives. Cancers. (2024) 16:1500. doi: 10.3390/cancers16081500
64. Somu P, Mohanty S, Basavegowda N, Yadav AK, Paul S, Baek K-H. The interplay between heat shock proteins and cancer pathogenesis: A novel strategy for cancer therapeutics. Cancers. (2024) 16:638. doi: 10.3390/cancers16030638
65. Binder RJ. Immunosurveillance of cancer and the heat shock protein-CD91 pathway. Cell Immunol. (2019) 343:103814. doi: 10.1016/j.cellimm.2018.05.007
66. Zhou YJ, Binder RJ. The heat shock protein-CD91 pathway mediates tumor immunosurveillance. Oncoimmunology. (2014) 3:e28222. doi: 10.4161/onci.28222
67. Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. (2001) 14:303–13. doi: 10.1016/s1074-7613(01)00111-x
68. Dai J, Liu B, Caudill MM, Zheng H, Qiao Y, Podack ER, et al. Cell surface expression of heat shock protein gp96 enhances cross-presentation of cellular antigens and the generation of tumor-specific T cell memory. Cancer Immun. (2003) 3:1.
69. Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. (2014) 3:e955691. doi: 10.4161/21624011.2014.955691
70. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. (2013) 31:51–72. doi: 10.1146/annurev-immunol-032712-100008
71. Zhu H, Fang X, Zhang D, Wu W, Shao M, Wang L, et al. Membrane-bound heat shock proteins facilitate the uptake of dying cells and cross-presentation of cellular antigen. Apoptosis Int J Program Cell Death. (2016) 21:96–109. doi: 10.1007/s10495-015-1187-0
72. Ostberg JR, Ertel BR, Lanphere JA. An important role for granulocytes in the thermal regulation of colon tumor growth. Immunol Invest. (2005) 34:259–72. doi: 10.1081/imm-200064477
73. Takada Y, Sato EF, Nakajima T, Hosono M, Tsumura M, Inoue M, et al. Granulocyte-colony stimulating factor enhances anti-tumour effect of hyperthermia. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2000) 16:275–86. doi: 10.1080/026567300285286
74. Capitano ML, Nemeth MJ, Mace TA, Salisbury-Ruf C, Segal BH, McCarthy PL, et al. Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner. Blood. (2012) 120:2600–9. doi: 10.1182/blood-2012-02-409805
75. Ostberg JR, Repasky EA. Comparison of the effects of two different whole body hyperthermia protocols on the distribution of murine leukocyte populations. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2000) 16:29–43. doi: 10.1080/026567300285402
76. Chen K-C, Yang S-J, Yang S-H, Pai J-A, Shieh M-J. Hyaluronan-coated gold nanoshells for enhanced synergistic effect and immunogenic cell response of chemo-photothermal therapy on lung cancer. Int J Biol Macromol. (2025) 300:140114. doi: 10.1016/j.ijbiomac.2025.140114
77. Shen RN, Lu L, Young P, Shidnia H, Hornback NB, Broxmeyer HE. Influence of elevated temperature on natural killer cell activity, lymphokine-activated killer cell activity and lectin-dependent cytotoxicity of human umbilical cord blood and adult blood cells. Int J Radiat Oncol Biol Phys. (1994) 29:821–6. doi: 10.1016/0360-3016(94)90571-1
78. Atanackovic D, Nierhaus A, Neumeier M, Hossfeld DK, Hegewisch-Becker S. 41.8 degrees C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various Malignant diseases. Cancer Immunol Immunother CII. (2002) 51:603–13. doi: 10.1007/s00262-002-0327-x
79. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. (2016) 37:208–20. doi: 10.1016/j.it.2016.01.004
80. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. (2015) 125:3356–64. doi: 10.1172/JCI80005
81. Xie Y, Liu P, Xu LX. A novel thermal treatment modality for controlling breast tumor growth and progression. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf. (2012) 2012:5703–6. doi: 10.1109/EMBC.2012.6347290
82. Werthmöller N, Frey B, Rückert M, Lotter M, Fietkau R, Gaipl US. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2016) 32:23–30. doi: 10.3109/02656736.2015.1106011
83. Liu P, Jia S, Lou Y, He K, Xu LX. Cryo-thermal therapy inducing MI macrophage polarization created CXCL10 and IL-6-rich pro-inflammatory environment for CD4+ T cell-mediated anti-tumor immunity. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2019) 36:408–20. doi: 10.1080/02656736.2019.1579373
84. Zhu J, Lou Y, Liu P, Xu LX. Tumor-related HSP70 released after cryo-thermal therapy targeted innate immune initiation in the antitumor immune response. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2020) 37:843–53. doi: 10.1080/02656736.2020.1788173
85. MacDonald C, Ministero S, Pandey M, Robinson D, Forti Hong E, Hylander B, et al. Comparing thermal stress reduction strategies that influence MDSC accumulation in tumor bearing mice. Cell Immunol. (2021) 361:104285. doi: 10.1016/j.cellimm.2021.104285
86. Multhoff G, Repasky EA, Vaupel P. Mild hyperthermia induced by water-filtered infrared A irradiation: A potent strategy to foster immune recognition and anti-tumor immune responses in superficial cancers?, in: Water-filtered Infrared A (wIRA) Irradiation: From Research to Clinical Settings. (2022). Cham (CH: Springer. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK593463/ (Accessed January 25, 2025).
87. Zhu J, Zhang Y, Zhang A, He K, Liu P, Xu LX. Cryo-thermal therapy elicits potent anti-tumor immunity by inducing extracellular Hsp70-dependent MDSC differentiation. Sci Rep. (2016) 6:27136. doi: 10.1038/srep27136
88. Cen Y, Lou Y, Wang J, Wang S, Peng P, Zhang A, et al. Supplementation with serum-derived extracellular vesicles reinforces antitumor immunity induced by cryo-thermal therapy. Int J Mol Sci. (2021) 22:11021. doi: 10.3390/ijms222011021
89. Kozłowski HM, Sobocińska J, Jędrzejewski T, Maciejewski B, Dzialuk A, Wrotek S. Fever-range whole body hyperthermia leads to changes in immune-related genes and miRNA machinery in Wistar rats. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2023) 40:2216899. doi: 10.1080/02656736.2023.2216899
90. Robins HI, Kutz M, Wiedemann GJ, Katschinski DM, Paul D, Grosen E, et al. Cytokine induction by 41.8 degrees C whole body hyperthermia. Cancer Lett. (1995) 97:195–201. doi: 10.1016/0304-3835(95)03976-4
91. Haveman J, Geerdink AG, Rodermond HM. Cytokine production after whole body and localized hyperthermia. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (1996) 12:791–800. doi: 10.3109/02656739609027685
92. Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discovery. (2012) 11:633–52. doi: 10.1038/nrd3800
93. Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med. (2012) 209:1069–74. doi: 10.1084/jem.20120988
94. Chi J-T, Thrall DE, Jiang C, Snyder S, Fels D, Landon C, et al. Comparison of genomics and functional imaging from canine sarcomas treated with thermoradiotherapy predicts therapeutic response and identifies combination therapeutics. Clin Cancer Res Off J Am Assoc Cancer Res. (2011) 17:2549–60. doi: 10.1158/1078-0432.CCR-10-2583
95. Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. (2011) 121:3846–59. doi: 10.1172/JCI44952
96. Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. (2001) 97:2727–33. doi: 10.1182/blood.v97.9.2727
97. Ahlers O, Hildebrandt B, Dieing A, Deja M, Böhnke T, Wust P, et al. Stress induced changes in lymphocyte subpopulations and associated cytokines during whole body hyperthermia of 41.8-42.2 degrees C. Eur J Appl Physiol. (2005) 95:298–306. doi: 10.1007/s00421-005-0009-4
98. Kobayashi Y, Ito Y, Ostapenko VV, Sakai M, Matsushita N, Imai K, et al. Fever-range whole-body heat treatment stimulates antigen-specific T-cell responses in humans. Immunol Lett. (2014) 162:256–61. doi: 10.1016/j.imlet.2014.09.014
99. Sulyok I, Fleischmann E, Stift A, Roth G, Lebherz-Eichinger D, Kasper D, et al. Effect of preoperative fever-range whole-body hyperthermia on immunological markers in patients undergoing colorectal cancer surgery. Br J Anaesth. (2012) 109:754–61. doi: 10.1093/bja/aes248
100. Yu H, Luo Y, Peng H, Kang L, Huang M, Luo S, et al. The predicting value of postoperative body temperature on long-term survival in patients with rectal cancer. Tumor Biol. (2015) 36:8055–63. doi: 10.1007/s13277-015-3535-7
101. Kraybill WG, Olenki T, Evans SS, Ostberg JR, O’Leary KA, Gibbs JF, et al. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumours: correlation with mouse models. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2002) 18:253–66. doi: 10.1080/02656730110116704
102. Zastko L, Petrovičová P, Račková A, Jakl L, Jakušová V, Marková E, et al. DNA damage response and apoptosis induced by hyperthermia in human umbilical cord blood lymphocytes. Toxicol Vitro Int J Publ Assoc BIBRA. (2021) 73:105127. doi: 10.1016/j.tiv.2021.105127
103. Meinander A, Söderström TS, Kaunisto A, Poukkula M, Sistonen L, Eriksson JE. Fever-like hyperthermia controls T Lymphocyte persistence by inducing degradation of cellular FLIPshort. J Immunol Baltim Md 1950. (2007) 178:3944–53. doi: 10.4049/jimmunol.178.6.3944
104. Cippitelli M, Fionda C, Di Bona D, Piccoli M, Frati L, Santoni A. Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J Immunol Baltim Md 1950. (2005) 174:223–32. doi: 10.4049/jimmunol.174.1.223
105. Dieing A, Ahlers O, Kerner T, Wust P, Felix R, Löffel J, et al. Whole body hyperthermia induces apoptosis in subpopulations of blood lymphocytes. Immunobiology. (2003) 207:265–73. doi: 10.1078/0171-2985-00236
106. Shirvalilou S, Khoei S, Afzalipour R, Ghaznavi H, Shirvaliloo M, Derakhti Z, et al. Targeting the undruggable in glioblastoma using nano-based intracellular drug delivery. Med Oncol. (2024) 41:303. doi: 10.1007/s12032-024-02546-8
107. Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. (2002) 109 Suppl:S97–107. doi: 10.1016/s0092-8674(02)00704-3
108. Kennedy BE, Noftall EB, Dean C, Roth A, Clark KN, Rowles D, et al. Targeted intra-tumoral hyperthermia using uniquely biocompatible gold nanorods induces strong immunogenic cell death in two immunogenically ‘cold’ tumor models. Front Immunol. (2025) 15:1512543. doi: 10.3389/fimmu.2024.1512543
109. Wang X, Yan B, Li H, Yuan J, Guo J, Wang S, et al. Reprogrammed IDO-induced immunosuppressive microenvironment synergizes with immunogenic magnetothermodynamics for improved cancer therapy. ACS Appl Mater Interfaces. (2024) 16:30671–84. doi: 10.1021/acsami.4c02740
110. Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. (2015) 15:335–49. doi: 10.1038/nri3843
111. Smith JB, Knowlton RP, Agarwal SS. Human lymphocyte responses are enhanced by culture at 40 degrees C. J Immunol Baltim Md 1950. (1978) 121:691–4.
112. Mace TA, Zhong L, Kilpatrick C, Zynda E, Lee C-T, Capitano M, et al. Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. J Leukoc Biol. (2011) 90:951–62. doi: 10.1189/jlb.0511229
113. Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2012) 28:9–18. doi: 10.3109/02656736.2011.616182
114. Zynda ER, Grimm MJ, Yuan M, Zhong L, Mace TA, Capitano M, et al. A role for the thermal environment in defining co-stimulation requirements for CD4(+) T cell activation. Cell Cycle Georget Tex. (2015) 14:2340–54. doi: 10.1080/15384101.2015.1049782
115. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. (2012) 12:298–306. doi: 10.1038/nrc3245
116. Li Y, Zhao L, Li X-F. Hypoxia and the tumor microenvironment. Technol Cancer Res Treat. (2021) 20:15330338211036304. doi: 10.1177/15330338211036304
117. Mortezaee K, Majidpoor J. The impact of hypoxia on immune state in cancer. Life Sci. (2021) 286:120057. doi: 10.1016/j.lfs.2021.120057
118. Krzywinska E, Stockmann C. Hypoxia, metabolism and immune cell function. Biomedicines. (2018) 6:56. doi: 10.3390/biomedicines6020056
119. You L, Wu W, Wang X, Fang L, Adam V, Nepovimova E, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. (2021) 41:1622–43. doi: 10.1002/med.21771
120. Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol OncolJ Hematol Oncol. (2022) 15:77. doi: 10.1186/s13045-022-01292-6
121. Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: perspectives for therapeutic implications. Med Oncol. (2020) 37:2. doi: 10.1007/s12032-019-1329-2
122. Khosravi G, Mostafavi S, Bastan S, Ebrahimi N, Gharibvand RS, Eskandari N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. (2024) 44:521–53. doi: 10.1002/cac2.12539
123. Duan Q, Zhang H, Zheng J, Zhang L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer. (2020) 6:605–18. doi: 10.1016/j.trecan.2020.02.022
124. Yi Y, Yu M, Feng C, Hao H, Zeng W, Lin C, et al. Transforming “cold” tumors into “hot” ones via tumor-microenvironment-responsive siRNA micelleplexes for enhanced immunotherapy. Matter. (2022) 5:2285–305. doi: 10.1016/j.matt.2022.04.032
125. Benoit A, Vogin G, Duhem C, Berchem G, Janji B. Lighting up the fire in the microenvironment of cold tumors: A major challenge to improve cancer immunotherapy. Cells. (2023) 12:1787. doi: 10.3390/cells12131787
126. James CM, Olejniczak SH, Repasky EA. How murine models of human disease and immunity are influenced by housing temperature and mild thermal stress. Temp Austin Tex. (2023) 10:166–78. doi: 10.1080/23328940.2022.2093561
127. Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. (2011) 480:104–8. doi: 10.1038/nature10653
128. Kokolus KM, Capitano ML, Lee C-T, Eng JW-L, Waight JD, Hylander BL, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U.S.A. (2013) 110:20176–81. doi: 10.1073/pnas.1304291110
129. Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, Lee KP, Repasky EA. Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naïve and tumor-bearing mice. Front Immunol. (2014) 5:23. doi: 10.3389/fimmu.2014.00023
130. Eng JW-L, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat Commun. (2015) 6:6426. doi: 10.1038/ncomms7426
131. Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. (2000) 46:179–85. doi: 10.1016/s0360-3016(99)00362-4
132. Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. (2004) 10:4287–93. doi: 10.1158/1078-0432.CCR-04-0133
133. Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2006) 22:365–73. doi: 10.1080/02656730600836386
134. Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. (2011) 71:3872–80. doi: 10.1158/0008-5472.CAN-10-4482
135. Kim W, Kim M-S, Kim H-J, Lee E, Jeong J-H, Park I, et al. Role of HIF-1α in response of tumors to a combination of hyperthermia and radiation in vivo. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2018) 34:276–83. doi: 10.1080/02656736.2017.1335440
136. Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci U.S.A. (2010) 107:20477–82. doi: 10.1073/pnas.1006646107
137. Huang H, Yu K, Mohammadi A, Karanthanasis E, Godley A, Yu JS. It’s getting hot in here: targeting cancer stem-like cells with hyperthermia. J Stem Cell Transplant Biol. (2017) 2:113.
138. Wang F, Pu K, Li J. Activating nanomedicines with electromagnetic energy for deep-tissue induction of immunogenic cell death in cancer immunotherapy. Small Methods. (2023) 7:e2201083. doi: 10.1002/smtd.202201083
139. Chen H, Liu L, Ma A, Yin T, Chen Z, Liang R, et al. Noninvasively immunogenic sonodynamic therapy with manganese protoporphyrin liposomes against triple-negative breast cancer. Biomaterials. (2021) 269:120639. doi: 10.1016/j.biomaterials.2020.120639
140. Covarrubias G, Lorkowski ME, Sims HM, Loutrianakis G, Rahmy A, Cha A, et al. Hyperthermia-mediated changes in the tumor immune microenvironment using iron oxide nanoparticles. Nanoscale Adv. (2021) 3:5890–9. doi: 10.1039/d1na00116g
141. Fang Y, He Y, Wu C, Zhang M, Gu Z, Zhang J, et al. Magnetism-mediated targeting hyperthermia-immunotherapy in “cold” tumor with CSF1R inhibitor. Theranostics. (2021) 11:6860–72. doi: 10.7150/thno.57511
142. Zheng Z, Su J, Bao X, Wang H, Bian C, Zhao Q, et al. Mechanisms and applications of radiation-induced oxidative stress in regulating cancer immunotherapy. Front Immunol. (2023) 14:1247268. doi: 10.3389/fimmu.2023.1247268
143. Zhao X, Shao C. Radiotherapy-mediated immunomodulation and anti-tumor abscopal effect combining immune checkpoint blockade. Cancers. (2020) 12:2762. doi: 10.3390/cancers12102762
144. Xi Y, Chen L, Tang J, Yu B, Shen W, Niu X. Amplifying “eat me signal” by immunogenic cell death for potentiating cancer immunotherapy. Immunol Rev. (2024) 321:94–114. doi: 10.1111/imr.13251
145. Liu S, Wang W, Hu S, Jia B, Tuo B, Sun H, et al. Radiotherapy remodels the tumor microenvironment for enhancing immunotherapeutic sensitivity. Cell Death Dis. (2023) 14:679. doi: 10.1038/s41419-023-06211-2
146. Du S-S, Chen G-W, Yang P, Chen Y-X, Hu Y, Zhao Q-Q, et al. Radiation Therapy Promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING Activation. Int J Radiat Oncol. (2022) 112:1243–55. doi: 10.1016/j.ijrobp.2021.12.162
147. Constanzo J, Faget J, Ursino C, Badie C, Pouget J-P. Radiation-induced immunity and toxicities: the versatility of the cGAS-STING pathway. Front Immunol. (2021) 12:680503. doi: 10.3389/fimmu.2021.680503
148. Zhang Y, Li Z, Hong W, Hsu S, Wang B, Zeng Z, et al. STING-dependent sensing of self-DNA driving pyroptosis contributes to radiation-induced lung injury. Int J Radiat Oncol. (2023) 117:928–41. doi: 10.1016/j.ijrobp.2023.05.029
149. Zheng Z, Jia S, Shao C, Shi Y. Irradiation induces cancer lung metastasis through activation of the cGAS–STING–CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. (2020) 11:326. doi: 10.1038/s41419-020-2546-5
150. Du S, Chen G, Yuan B, Hu Y, Yang P, Chen Y, et al. DNA sensing and associated type 1 interferon signaling contributes to progression of radiation-induced liver injury. Cell Mol Immunol. (2021) 18:1718–28. doi: 10.1038/s41423-020-0395-x
151. Van Dieren L, Quisenaerts T, Licata M, Beddok A, Lellouch AG, Ysebaert D, et al. Combined radiotherapy and hyperthermia: A systematic review of immunological synergies for amplifying radiation-induced abscopal effects. Cancers. (2024) 16:3656. doi: 10.3390/cancers16213656
152. Wang X, Wang Y, Zhang Y, Shi H, Liu K, Wang F, et al. Immune modulatory roles of radioimmunotherapy: biological principles and clinical prospects. Front Immunol. (2024) 15:1357101. doi: 10.3389/fimmu.2024.1357101
153. De Martino M, Daviaud C, Vanpouille-Box C. Radiotherapy: An immune response modifier for immuno-oncology. Semin Immunol. (2021) 52:101474. doi: 10.1016/j.smim.2021.101474
154. Swamy K. Therapeutic in situ cancer vaccine using pulsed stereotactic body radiotherapy—A translational model. Vaccines. (2023) 12:7. doi: 10.3390/vaccines12010007
155. Lu Q, Yan W, Zhu A, Tubin S, Mourad WF, Yang J. Combining spatially fractionated radiation therapy (SFRT) and immunotherapy opens new rays of hope for enhancing therapeutic ratio. Clin Transl Radiat Oncol. (2024) 44:100691. doi: 10.1016/j.ctro.2023.100691
156. McMillan MT, Khan AJ, Powell SN, Humm J, Deasy JO, Haimovitz-Friedman A. Spatially fractionated radiotherapy in the era of immunotherapy. Semin Radiat Oncol. (2024) 34:276–83. doi: 10.1016/j.semradonc.2024.04.002
157. Tubin S, Gupta S, Grusch M, Popper HH, Brcic L, Ashdown ML, et al. Shifting the immune-suppressive to predominant immune-stimulatory radiation effects by SBRT-PArtial tumor irradiation targeting HYpoxic segment (SBRT-PATHY). Cancers. (2020) 13:50. doi: 10.3390/cancers13010050
158. Pontoriero A, Critelli P, Chillari F, Ferrantelli G, Sciacca M, Brogna A, et al. Modulation of radiation doses and chimeric antigen receptor T cells: A promising new weapon in solid tumors—A narrative review. J Pers Med. (2023) 13:1261. doi: 10.3390/jpm13081261
159. Jiang L, Li X, Zhang J, Li W, Dong F, Chen C, et al. Combined high-dose LATTICE radiation therapy and immune checkpoint blockade for advanced bulky tumors: the concept and a case report. Front Oncol. (2021) 10:548132. doi: 10.3389/fonc.2020.548132
160. Bertho A, Iturri L, Prezado Y. Radiation-induced immune response in novel radiotherapy approaches FLASH and spatially fractionated radiotherapies. Int Rev Cell Mol Biol. (2023) 376:37–68. doi: 10.1016/bs.ircmb.2022.11.005
161. Lukas L, Zhang H, Cheng K, Epstein A. Immune priming with spatially fractionated radiation therapy. Curr Oncol Rep. (2023) 25:1483–96. doi: 10.1007/s11912-023-01473-7
162. Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: results of thermoradiotherapy versus radiotherapy. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (1990) 6:479–86. doi: 10.3109/02656739009140944
163. Su X, Li J, Xu X, Ye Y, Wang C, Pang G, et al. Strategies to enhance the therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody in cancer therapy. J Transl Med. (2024) 22:751. doi: 10.1186/s12967-024-05552-6
164. Cheng W, Kang K, Zhao A, Wu Y. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer. J Hematol OncolJ Hematol Oncol. (2024) 17:54. doi: 10.1186/s13045-024-01581-2
165. Balakrishnan PB, Sweeney EE, Ramanujam AS, Fernandes R. Photothermal therapies to improve immune checkpoint blockade for cancer. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (2020) 37:34–49. doi: 10.1080/02656736.2020.1797190
166. Van Den Tempel N, Horsman MR, Kanaar R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int J Hyperthermia. (2016) 32:446–54. doi: 10.3109/02656736.2016.1157216
167. Fajardo LF, Prionas SD. Endothelial cells and hyperthermia. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. (1994) 10:347–53. doi: 10.3109/02656739409010278
168. Waight-Sharma A, Grooby W, Betts WH, Russ GR. Effects of in vitro hyperthermia on the expression of adhesion molecules in cytokine-stimulated human umbilical vein endothelial cells. Transplant Proc. (1992) 24:2319–20.
169. Geva R, Alon G, Nathanson M, Bar-David S, Nevo N, Aizic A, et al. PD-1 blockade combined with heated intraperitoneal chemotherapy improves outcome in experimental peritoneal metastases from colonic origin in a murine model. Ann Surg Oncol. (2023) 30:2657–63. doi: 10.1245/s10434-022-13025-7
170. Tao J, Yang G, Zhou W, Qiu J, Chen G, Luo W, et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J Hematol OncolJ Hematol Oncol. (2021) 14:14. doi: 10.1186/s13045-020-01030-w
171. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: A review. JAMA. (2021) 326:851. doi: 10.1001/jama.2021.13027
172. Rubin SJS, Sojwal RS, Gubatan J, Rogalla S. The tumor immune microenvironment in pancreatic ductal adenocarcinoma: neither hot nor cold. Cancers. (2022) 14:4236. doi: 10.3390/cancers14174236
173. Zhao W, Hu X, Li W, Li R, Chen J, Zhou L, et al. M2-like TAMs function reversal contributes to breast cancer eradication by combination dual immune checkpoint blockade and photothermal therapy. Small. (2021) 17:2007051. doi: 10.1002/smll.202007051
174. Fite BZ, Wang J, Kare AJ, Ilovitsh A, Chavez M, Ilovitsh T, et al. Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci Rep. (2021) 11:927. doi: 10.1038/s41598-020-80135-1
175. Granja A, Pinheiro M, Sousa CT, Reis S. Gold nanostructures as mediators of hyperthermia therapies in breast cancer. Biochem Pharmacol. (2021) 190:114639. doi: 10.1016/j.bcp.2021.114639
176. Chen H, Luan X, Paholak HJ, Burnett JP, Stevers NO, Sansanaphongpricha K, et al. Depleting tumor-associated tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomed. (2020) 15:77–92. doi: 10.2217/nnm-2019-0190
177. Obrador E, Moreno-Murciano P, Oriol-Caballo M, López-Blanch R, Pineda B, Gutiérrez-Arroyo JL, et al. Glioblastoma therapy: past, present and future. Int J Mol Sci. (2024) 25:2529. doi: 10.3390/ijms25052529
178. Yang J, Shi Z, Liu R, Wu Y, Zhang X. Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics. (2020) 10:3223–39. doi: 10.7150/thno.40298
179. Persano S, Vicini F, Poggi A, Fernandez JLC, Rizzo GMR, Gavilán H, et al. Elucidating the innate immunological effects of mild magnetic hyperthermia on U87 human glioblastoma cells: an in vitro study. Pharmaceutics. (2021) 13:1668. doi: 10.3390/pharmaceutics13101668
180. Liu Y, Chongsathidkiet P, Crawford BM, Odion R, Dechant CA, Kemeny HR, et al. Plasmonic gold nanostar-mediated photothermal immunotherapy for brain tumor ablation and immunologic memory. Immunotherapy. (2019) 11:1293–302. doi: 10.2217/imt-2019-0023
181. Wiwatchaitawee K, Quarterman JC, Geary SM, Salem AK. Enhancement of therapies for glioblastoma (GBM) using nanoparticle-based delivery systems. AAPS PharmSciTech. (2021) 22:71. doi: 10.1208/s12249-021-01928-9
182. Chao Y, Chen G, Liang C, Xu J, Dong Z, Han X, et al. Iron nanoparticles for low-power local magnetic hyperthermia in combination with immune checkpoint blockade for systemic antitumor therapy. Nano Lett. (2019) 19:4287–96. doi: 10.1021/acs.nanolett.9b00579
183. Cano-Mejia J, Burga RA, Sweeney EE, Fisher JP, Bollard CM, Sandler AD, et al. PRussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine Nanotechnol Biol Med. (2017) 13:771–81. doi: 10.1016/j.nano.2016.10.015
184. Lyu N, Kong Y, Li X, Mu L, Deng H, Chen H, et al. Ablation reboots the response in advanced hepatocellular carcinoma with stable or atypical response during PD-1 therapy: A proof-of-concept study. Front Oncol. (2020) 10:580241. doi: 10.3389/fonc.2020.580241
185. Xie C, Duffy AG, Mabry-Hrones D, Wood B, Levy E, Krishnasamy V, et al. Tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer. Hepatology. (2019) 69:2048–60. doi: 10.1002/hep.30482
186. Kleef R, Nagy R, Baierl A, Bacher V, Bojar H, McKee DL, et al. Low-dose ipilimumab plus nivolumab combined with IL-2 and hyperthermia in cancer patients with advanced disease: exploratory findings of a case series of 131 stage IV cancers - a retrospective study of a single institution. Cancer Immunol Immunother CII. (2021) 70:1393–403. doi: 10.1007/s00262-020-02751-0
187. Wei Z, Yang X, Ye X, Huang G, Li W, Han X, et al. Camrelizumab combined with microwave ablation improves the objective response rate in advanced non-small cell lung cancer. J Cancer Res Ther. (2019) 15:1629. doi: 10.4103/jcrt.JCRT_990_19
188. Zhu M, Yang M, Zhang J, Yin Y, Fan X, Zhang Y, et al. Immunogenic cell death induction by ionizing radiation. Front Immunol. (2021) 12:705361. doi: 10.3389/fimmu.2021.705361
Keywords: hyperthermia, immunotherapy, immune checkpoint inhibitors (ICIs), heat shock, heat shock protein, CTLA 4, PD1, thermal stress
Citation: Abreu MM, Chocron AF and Smadja DM (2025) From cold to hot: mechanisms of hyperthermia in modulating tumor immunology for enhanced immunotherapy. Front. Immunol. 16:1487296. doi: 10.3389/fimmu.2025.1487296
Received: 27 August 2024; Accepted: 03 February 2025;
Published: 28 February 2025.
Edited by:
Petr O. Ilyinskii, Selecta Biosciences, United StatesReviewed by:
Mau-Shin Chi, Shin Kong Wu Ho-Su Memorial Hospital, TaiwanCopyright © 2025 Abreu, Chocron and Smadja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David M. Smadja, ZGF2aWQuc21hZGphQGFwaHAuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.