- 1Department of Orthopedic Surgery, Orthopedic Institute, The First Affiliated Hospital of Soochow University, Soochow, Jiangsu, China
- 2Department of Pathology, The First Affiliated Hospital of Soochow University, Soochow, Jiangsu, China
- 3Research Institute of Clinical Medicine, Jeonbuk National University Medical School, Jeonju, Republic of Korea
Objectives: The association between the neutrophil percentage to albumin ratio (NPAR) and the risk of osteoarthritis (OA) and rheumatoid arthritis (RA) remains unclear. This study aims to investigate the association between NPAR and the risk of OA and RA.
Methods: This cross-sectional study analyzed data from 92,062 American adults in the NHANES database between 1999 and 2016. Various statistical analyses were conducted to investigate the associations between NPAR and the risks of OA and RA, including multivariable logistic regression, subgroup analysis, smooth curve fitting, and threshold effect analysis.
Results: After screening, the final study population included 36,147 participants, with 3,881 individuals diagnosed with OA and 2,178 with RA. After adjusting for confounding factors, higher NPAR levels were associated with an increased risk of RA (OR=1.05; 95% CI: 1.03-1.07; P <0.0001), but not with OA (OR=1.01; 95% CI: 0.99-1.02; P =0.755). This association was remarkably consistent across subgroups by age, sex, body mass index (BMI), alcohol consumption, hypertension, diabetes, and smoking status. Further analyses using curve fitting and threshold effect models revealed a nonlinear association between NPAR and RA, with an inflection point identified at 15.56.
Conclusion: High levels of NPAR is positively associated with the prevalence of RA. This provides us with new insights for the management and treatment of RA patients.
1 Introduction
Osteoarthritis (OA) and rheumatoid arthritis (RA) are the two most common types of arthritis in the United States, contributing significantly to disability. Reports indicate that approximately 22.7% of individuals in the United States are affected by arthritis (1–3). OA primarily degrades the articular cartilage, leading to joint pain, stiffness, crepitus during movement, effusion, and reduced mobility. OA most commonly affects weight-bearing joints, particularly the knees and hips (4). A large-scale cohort study found that the prevalence of radiographically diagnosed knee OA was 11.4% in women and 6.8% in men (5). Risk factors for OA encompass age, gender, obesity, genetic predisposition, joint deformity, and injury (6). RA is an immune-mediated inflammatory disease that typically presents with pain, swelling, and stiffness in synovial joints. Women account for 75% of RA patients (7, 8). Without prompt treatment, RA can result in joint destruction, disability, and systemic complications (9, 10). In the United States, the annual direct medical costs associated with arthritis are estimated to be as high as $81 billion (11). Arthritis imposes a significant medical burden on both individuals and society. Consequently, early screening and identification of high-risk populations are crucial to facilitate timely interventions and enhance the effectiveness of arthritis management strategies (12–14).
Biomarkers play a crucial role in the assessment of arthritis. Traditional biomarkers such as Anti-citrullinated peptide/protein antibodies (ACPA) have demonstrated high specificity in identifying RA patients and have proven to be cost-effective (15, 16). In recent years, researchers have discovered several novel biomarkers that show considerable potential in arthritis patients. A study has shown that integrating plasma/serum biomarkers, such as citrullinated protein, hydroxyproline, and anti-cyclic citrullinated peptide, with a diagnostic algorithm can facilitate early diagnosis and subtyping of various arthritis forms (17). Despite their promise, the widespread implementation of these novel biomarkers in routine clinical practice remains limited due to challenges in accessibility and availability. Growing evidence suggests that chronic low-grade inflammation plays a pivotal role in the onset and progression of OA (18, 19). RA is primarily attributed to autoimmune responses, with CD4+ T lymphocytes, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) identified as key mediators in the initiation and perpetuation of inflammation (20). Studies have demonstrated that nutrition and dietary habits can modulate metabolic processes and immune responses in both OA and RA (21, 22). Considering the crucial roles of inflammation and nutrition in arthritis, identifying novel biomarkers based on these factors is essential for assessing disease risk in clinical settings and guiding targeted interventions.
Neutrophils, essential components of the innate immune system, serve as cost-effective and easily obtainable markers for assessing inflammatory processes. The critical role of neutrophils in the pathogenesis of OA and RA has been well documented (23, 24). Albumin, a protein with a molecular weight of 66-69 kDa, is essential in various physiological processes and serves as a crucial marker of nutritional status. The functions of albumin encompass the maintenance of plasma osmotic pressure, facilitation of transport, and antioxidant activity (25). The neutrophil percentage to albumin ratio (NPAR), a composite biomarker derived from neutrophil and albumin levels, has demonstrated efficacy in predicting inflammation in various conditions, such as acute kidney injury, septic shock, and rectal cancer (26). However, the association between NPAR and arthritis remains unexplored.
Therefore, this study primarily aimed to investigate the relationship between NPAR and OA and RA risk by performing a large population-based analysis of the comprehensive National Health and Nutrition Examination Survey (NHANES) database.
2 Methods
2.1 NHANES study population
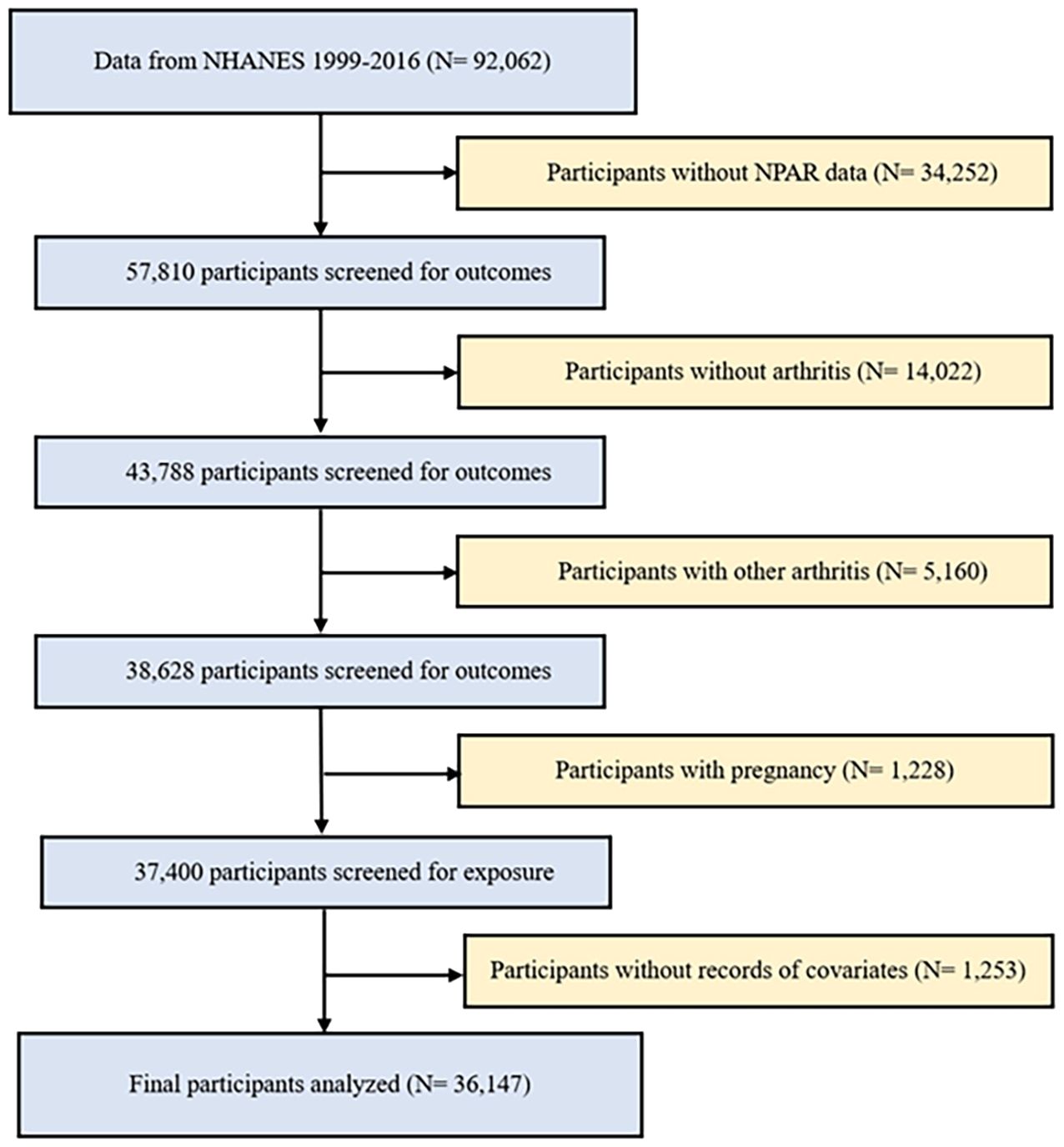
This study utilized data from nine biennial cycles of the NHANES spanning from 1999 to 2016. All measurements and tests were meticulously carried out at on-site mobile testing facilities, guaranteeing adherence to the standardized procedures and protocols of data collection. Exclusion criteria were applied to study participants to ensure the authenticity and reliability of the results. This study employed the following exclusion criteria: (1) individuals with missing or incomplete data on arthritis and NPAR; (2) individuals diagnosed with other types of arthritis, as the control group consisted of individuals without any form of arthritis; (3) pregnant women, due to potential alterations in their metabolic profiles during pregnancy; and (4) participants with missing or unknown covariable data (Figure 1). After applying the exclusion criteria, a total of 36,147 participants were included in the study, comprising 3,881 patients with OA, 2,178 patients with RA, and 30,088 control participants without any form of arthritis.
2.2 Assessment of the NPAR and arthritis
The NPAR was calculated as: Neutrophil percentage (% of total WBC count) × 100/Albumin (g/dL) (27, 28).
Consistent with our previous study (29), the arthritis diagnosis of participants was confirmed using two self-reported questions. The first question enquired whether the participants had arthritis or not. Then, “Which type of arthritis?” Participants who responded with either “Osteoarthritis” or “Rheumatoid Arthritis” were classified as having OA or RA, respectively. A study found a strong correlation between self-reported arthritis and clinically diagnosed arthritis (30).
2.3 Assessment of covariables of interest
Numerous covariates were utilized to consider any confounding effects, including age; sex; five race categories; marital status; education level; body mass index (BMI, underweight: <18.5 kg/m2, normal weight: ≥18.5 kg/m2 and <25 kg/m2, overweight: ≥25 kg/m2 and <30 kg/m2, and obese: ≥30 kg/m2); and poverty-to-income ratio (PIR).
Participants who consumed more than 12 drinks in any given year were defined as drinkers. Participants were considered to have hypertension if the answer was “yes” on the self-report questionnaire for high blood pressure. Diabetes mellitus status was determined using the following criteria: self-reported diagnosis, glycosylated hemoglobin level of ≥6.5%, or fasting glucose level of ≥126 mg/dL. Participants were categorized as current, former and never smoker depending on whether they had smoked 100 or more cigarettes during their life and current smoking status.
Red blood cell count (RBC), hemoglobin (Hb), alanine aminotransferases (ALT), aspartate aminotransferase (AST) and Alkaline phosphatase (ALP) were measured from blood samples collected during the study visit.
2.4 Statistical analyses
Continuous variables were presented as mean± standard deviation (SD) and median and interquartile range (IQR), If a variable followed a normal distribution, a two-sample t-test was used for comparisons between groups; otherwise (if it did not follow a normal distribution), the rank sum test was employed. Categorical variables were presented as frequency and percentage, and Pearson’s chi-squared test was used to evaluate differences between groups. Multivariate logistic regression analysis was conducted to investigate the relationship between the NPAR and OA and RA. NPAR values were then categorized into quartiles, with the lowest quartile (Q1) serving as the reference group. Three models were constructed to assess the associations: Model 1, a crude model; Model 2, adjusted for age, sex, and race; and Model 3, further adjusted for marital status, education level, BMI, PIR, RBC, Hb, ALT, AST, alcohol consumption, hypertension, diabetes and smoking status. Subgroup analyses were conducted using multivariate logistic regression, adjusting for the confounding variables in Model 3. Interaction analyses was assessed using the likelihood ratio test to explore the heterogeneity of associations between different groups based on age, sex, BMI, alcohol consumption, hypertension, diabetes, and smoking status. To further investigate the association between NPAR and RA, we utilized a generalized additive model along with smooth curve fitting, which identified a non-linear relationship. Then we used a recursive technique to calculate the inflection point in the NPAR and RA. Finally, two-segment piecewise regression models were employed to perform a threshold effect analysis on both sides of the inflection point. All statistical analyses were conducted using R software (version 4.1.3) and EmpowerStats (version 6.0).
3 Results
3.1 Individual socio-demographic and health characteristics of the OA, RA and non-arthritis groups
The study participants had a mean age of 45.36 ± 17.07 years, with males accounting for 52.75% of the sample. 42.24% of the participants were non-Hispanic white, and more than half had education beyond high school. The self-reported prevalence of RA was 6.03%, and the self-reported prevalence of OA was 10.74%. OA and RA patients were older and more likely to be female compared to non-arthritis participants. Furthermore, significant differences were observed in race, marital status, education level, BMI, PIR, alcohol consumption, hypertension, status, smoking status, RBC, Hb, ALT, ALP between the non-arthritis, OA, and RA groups. Patients with OA and RA exhibited higher neutrophil percentages, lower albumin levels, and markedly elevated NPAR compared to individuals without arthritis (Table 1).
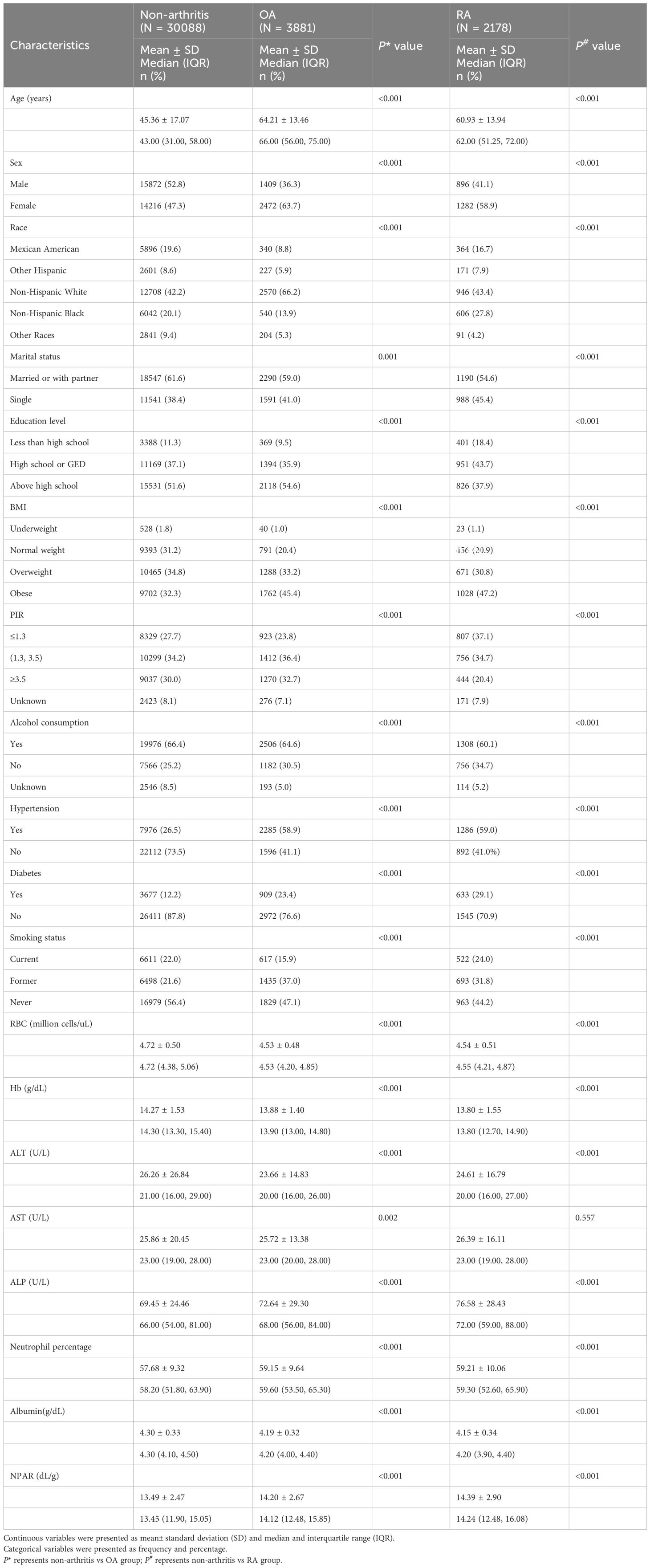
Table 1. Individual socio-demographic and health characteristics of the OA, RA and non-arthritis groups.
3.2 Associations between the NPAR and RA and OA
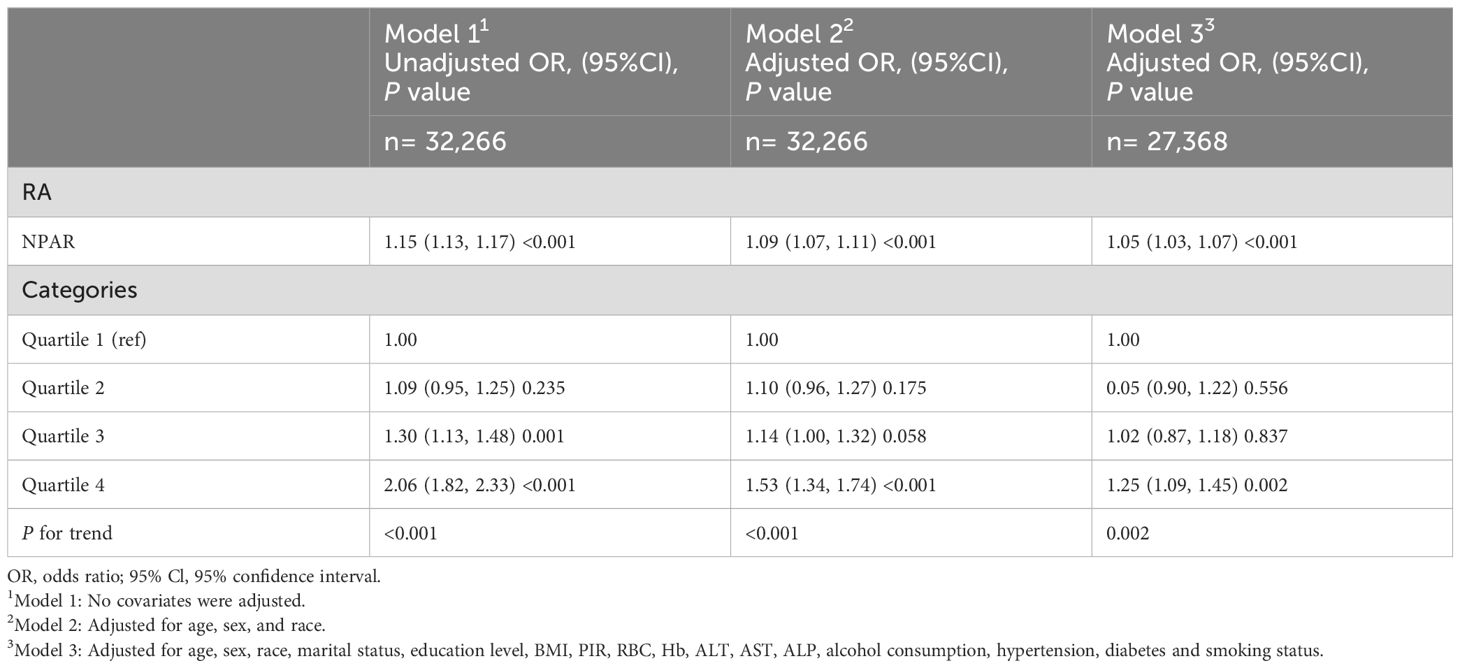
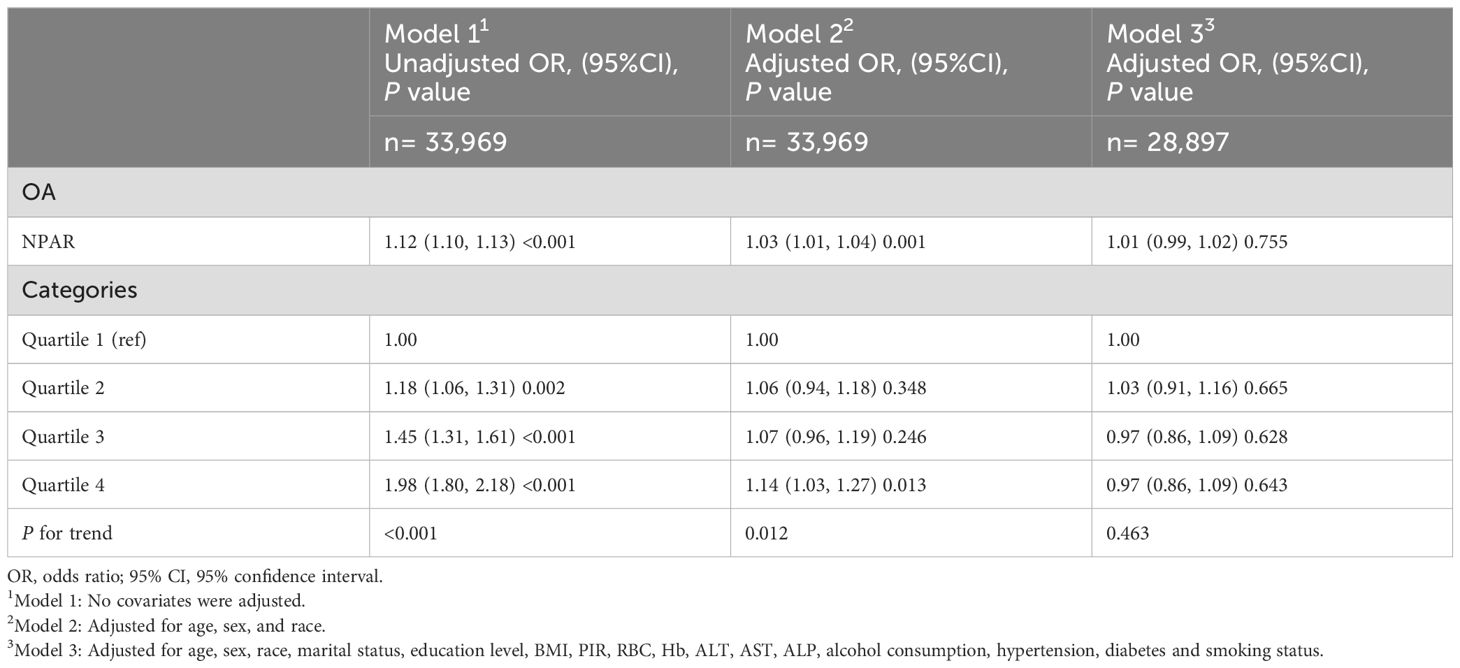
Table 2 illustrates the results of a multivariate logistic regression analysis examining the correlation between NPAR and self-reported RA. After adjusting for all covariates, the association between NPAR and self-reported RA remained statistically significant and positive (OR = 1.05; 95% CI: 1.03-1.07; P < 0.001). Moreover, individuals in the highest quartile (Q4) of NPAR exhibited a significantly increased risk of RA compared to those in the lowest quartile (Q1) as the reference group (OR = 1.25; 95% CI: 1.09-1.45; P = 0.002). Table 3 displays that among OA patients, there were no significant associations observed between NPAR and the risk of OA (P = 0.755).
3.3 Subgroup analyses
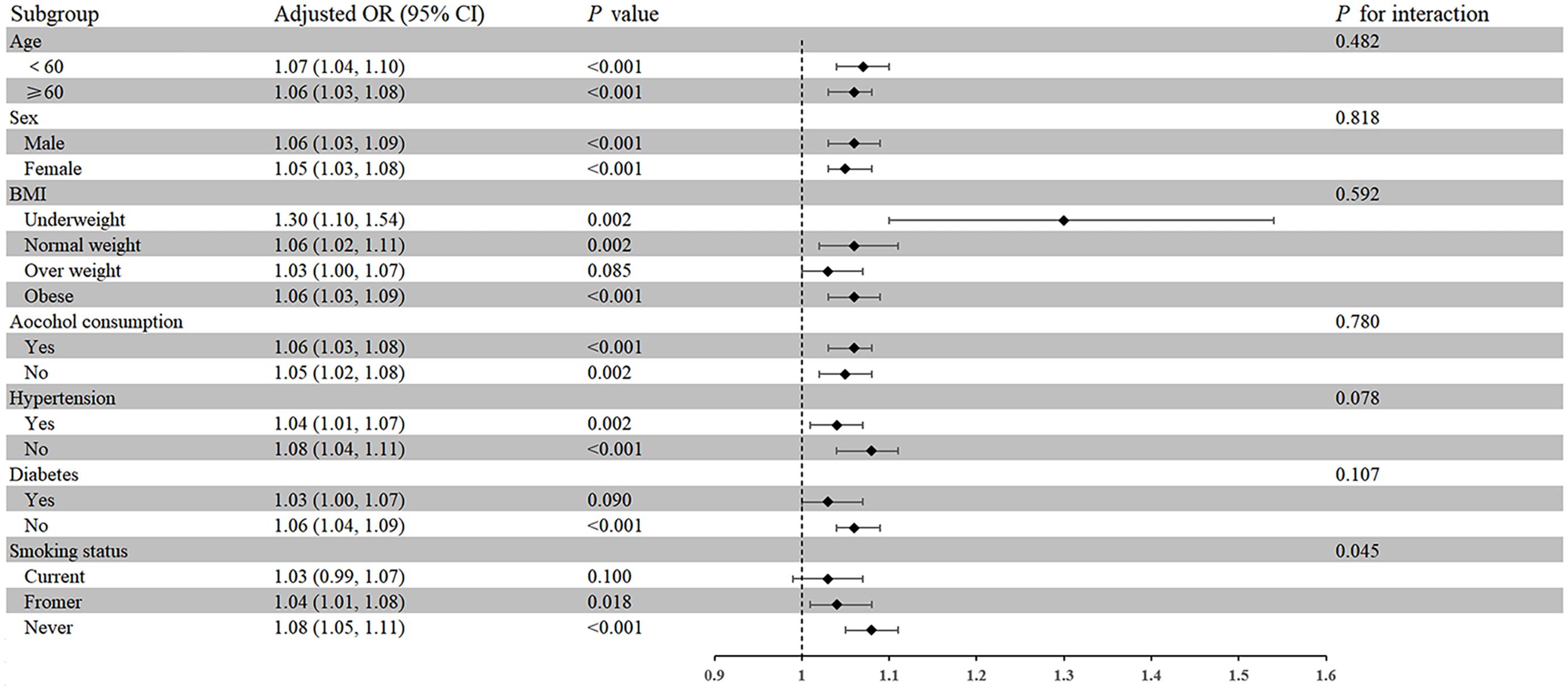
A subgroup analysis of seven factors (age, sex, BMI, alcohol consumption, hypertension, diabetes, and smoking status) was conducted to evaluate the strength and potential variations of the association between NPAR and self-reported RA among different populations (Figure 2). Moreover, except for subgroups with overweight, diabetes, and current smoking, the association between NPAR and RA was positive in all other subgroups (P < 0.05). Interaction tests showed that the association between NPAR and RA was more significant in never smokers than in current smokers and former smokers (OR = 1.08; 95% CI: 1.05-1.1, P for interaction < 0.05).
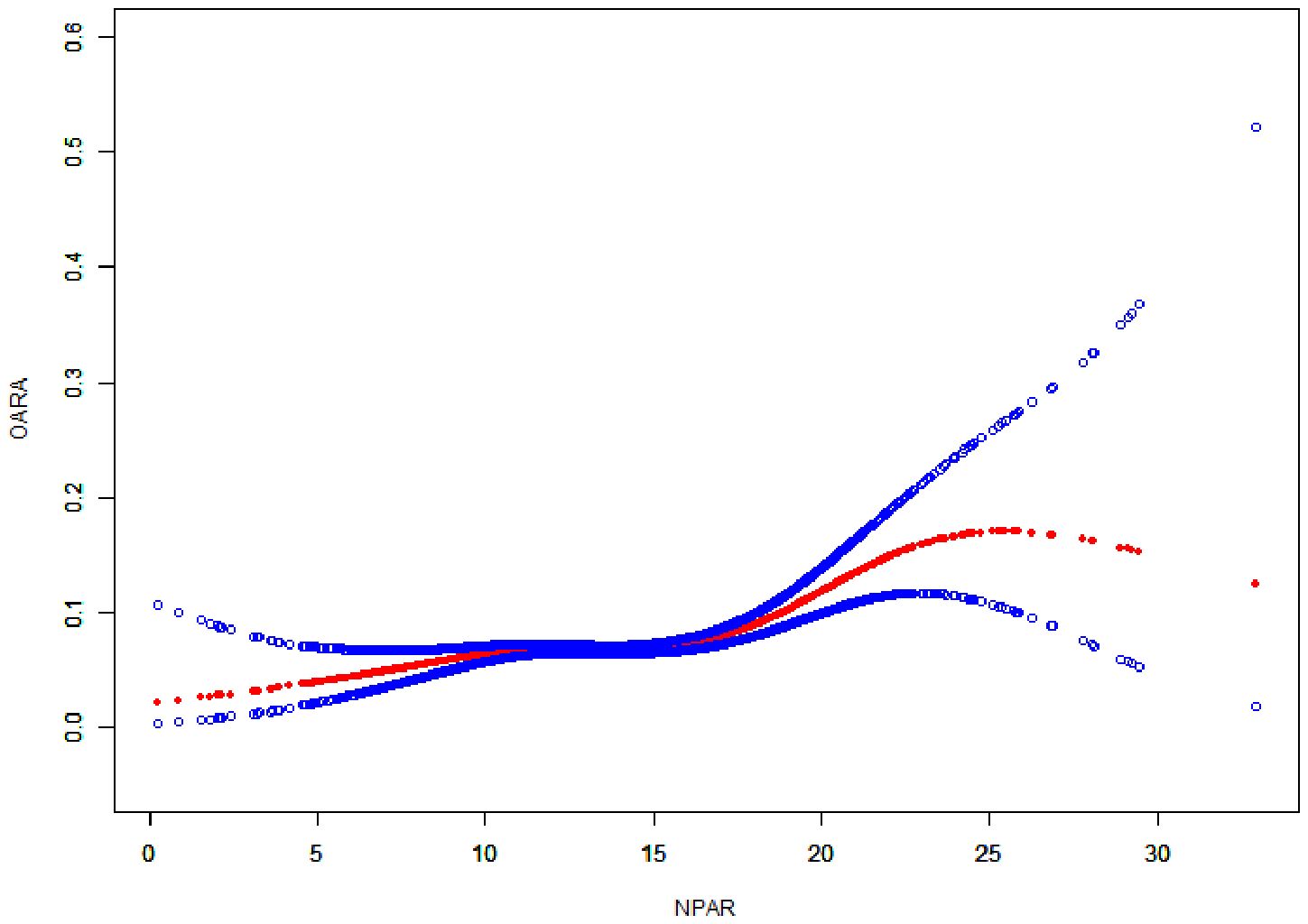
3.4 Nonlinear association between the NPAR and RA
The smoothing curve (Figure 3) revealed a nonlinear relationship between NPAR and self-reported RA. Through threshold effect analysis, we identified an inflection point at 15.56 dL/g (Table 4). When NPAR was below 15.56 dL/g, no statistically significant association was observed between NPAR and RA (OR = 1.02, 95% CI: 0.99-1.05; P = 0.207). However, when NPAR exceeded 15.56 dL/g, a significant positive correlation was found between NPAR and RA (OR = 1.12, 95% CI: 1.08-1.17; P < 0.001).
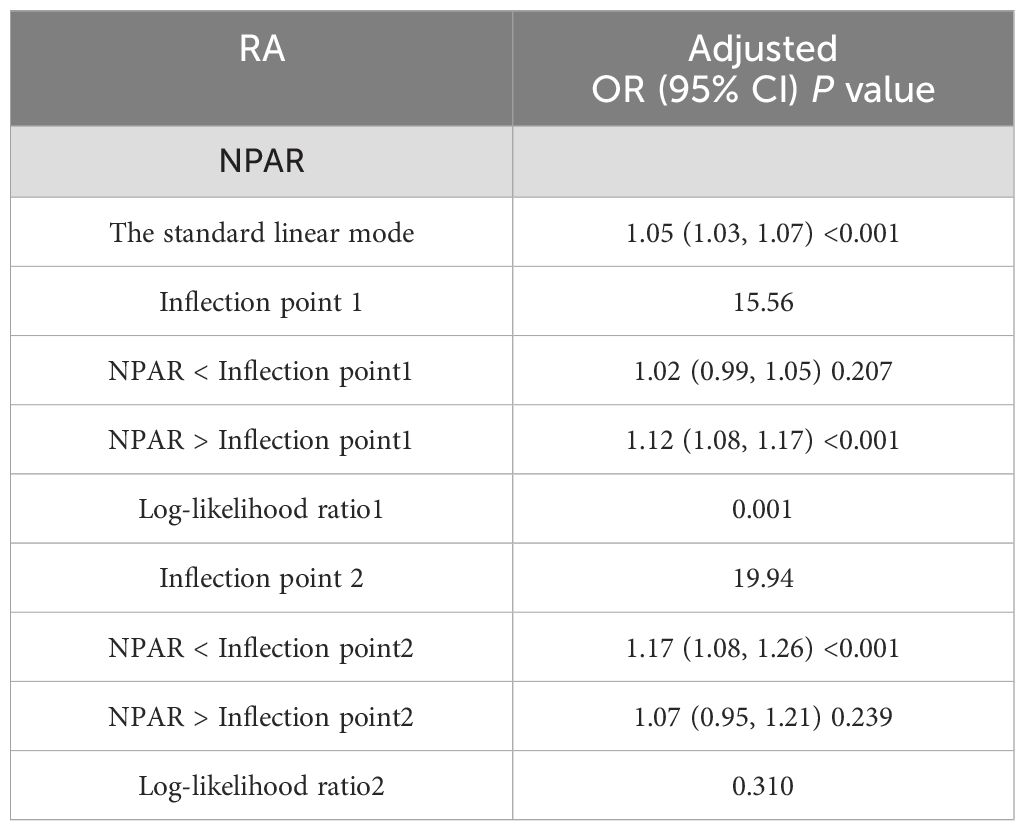
Table 4. Analysis of the threshold effect of NPAR and RA measured by the two-segment piecewise regression model.
4 Discussion
This study investigated the association between NPAR and the prevalence of self-reported OA and self-reported RA in a large sample of U.S. adults, including 3,881 OA patients, 2,178 RA patients, and 30,088 non-arthritis individuals. Multivariable logistic regression analysis revealed a positive association between NPAR and RA, which remained significant after adjusting for confounding factors. Additionally, subgroup analyses and interaction tests demonstrated that the association between NPAR and RA was consistent across various demographic and clinical subgroups, such as age, sex, BMI, alcohol consumption, hypertension, and diabetes. However, a significant interaction effect was observed for smoking status, where the association was stronger among never smokers compared to current and former smokers. Smooth curve fitting and threshold effect analysis revealed a nonlinear relationship between NPAR and RA, with an inflection point at 15.56 dL/g. In contrast, multivariable logistic regression analysis did not identify a statistically significant association between NPAR and OA. In conclusion, NPAR may be a useful tool for monitoring the prevalence of RA among U.S. adults, potentially aiding in risk stratification and early intervention strategies for susceptible populations.
In recent years, numerous studies have identified critical biomarkers in patients with arthritis. Zhao et al. found a significant inverse correlation between the systemic immune-inflammation index and serum Klotho levels, indicating that Klotho may have a protective role in the inflammatory response of RA patients and could be a potential therapeutic target for RA (3). Huang et al. demonstrated an inverse U-shaped nonlinear relationship between lipid accumulation product (LAP) level and OA, suggesting that LAP is a more reliable predictor of OA than traditional obesity measures like BMI, which could aid in OA prevention and treatment (31). Furthermore, using data from the NHANES database, Zhou et al. verified that an elevated neutrophil-to-lymphocyte ratio independently predicts a higher risk of all-cause and cardiovascular mortality in adults with RA, which could improve risk stratification and prognosis in RA patients (32). Wang et al. demonstrated that elevated NPAR levels were significantly associated with increased cardiovascular disease prevalence (33). Similarly, Li et al. found that NPAR exhibited a J-shaped association with all-cause mortality and a positive linear association with cardiovascular disease mortality among individuals with diabetes (34). These studies underscore the potential usefulness of novel serum biomarkers derived from routine blood tests in improving the diagnosis, risk stratification, and prognostic assessment of disease. However, the association between a novel inflammatory index, the NPAR, and arthritis has not been investigated to date.
These studies underscore the potential usefulness of novel serum biomarkers derived from routine blood tests in improving the diagnosis, risk stratification, and prognostic assessment of arthritis. However, the association between a novel inflammatory index, the NPAR, and arthritis has not been investigated to date.
Our study observed a significant positive association between elevated NPAR and the presence of self-reported RA, but no significant association with self-reported OA. NPAR can serve as an additional serum biomarker to complement the evaluation of RA. Recent studies have revealed associations between NPAR and various diseases, indicating its potential as a research target. For example, He et al. demonstrated a positive correlation between NPAR and the incidence of diabetic retinopathy, suggesting that NPAR could help assess the risk of retinopathy in diabetic patients and guide personalized treatment strategies (35). Similarly, Jiao et al. demonstrated that NPAR exhibited good sensitivity and specificity in assessing the one-year postoperative prognosis of elderly hip fracture patients, helping to identify individuals at high risk of post-operative mortality (36). Furthermore, a cohort study using NHANES data by Lan et al. found that that elevated NPAR was significantly associated with increased all-cause mortality, cardiovascular disease mortality, and mortality from chronic lower respiratory disease in patients with chronic obstructive pulmonary disease (COPD), suggesting that NPAR may be a better predictor of mortality than other hematological indices in COPD patients (37). In another study utilizing NHANES database, researchers found that NPAR exhibited excellent discriminatory ability for nonalcoholic fatty liver disease (NAFLD) in non-diabetic patients, with an area under the receiver operating characteristic curve of 0.810 (95% CI: 0.794–0.825), a sensitivity of 0.761, and a specificity of 0.715. Furthermore, the findings suggested that a higher NPAR is significantly associated with an increased risk of NAFLD, and may be a more effective biomarker for predicting NAFLD than albumin and neutrophil percentage alone (28). NPAR has unexpected potential in diagnosing, monitoring, and prognosticating various diseases. Given its accessibility and cost-effectiveness in routine medical practice, NPAR may provide additional benefits in the diagnosis and treatment of RA patients.
Elevated NPAR levels in RA patients may be attributed to increased neutrophil counts or decreased albumin levels. Excessive neutrophils release various proinflammatory mediators, such as reactive oxygen species, proteolytic enzymes, and cytokines (23). These mediators directly damage synovial tissue and stimulate the activation of other immune cells, including macrophages and T lymphocytes, which further amplifies the inflammatory response (38). In RA patients, the blood and synovial fluid show elevated levels of cytokines, such as granulocyte-macrophage colony-stimulating factor, TNF-α, IL-1β, and interferon. These cytokines delay neutrophil apoptosis through the upregulation of myeloid cell leukemia-1, phosphorylation of nuclear factor-kappa B, and increased caspase-9 activity, prolonging the lifespan of neutrophils and potentially increasing the absolute peripheral blood neutrophil count (39). Moreover, the increased tendency of neutrophils to form neutrophil extracellular traps in RA patients leads to the release of anti-citrullinated protein antibody and the induction of inflammatory cytokines, such as IL-6 and IL-8. These cytokines may then promote neutrophil production and survival, creating a vicious cycle that perpetuates the inflammatory process (40–42). Concurrently, the persistent inflammatory state may also impair the liver’s ability to synthesize albumin. Previous studies had demonstrated that inflammatory cytokines, such as IL-6 and TNF-α, can suppress hepatocyte albumin production, likely due to a reduction in albumin mRNA concentrations caused by decreased gene transcription, resulting in a decline in albumin synthesis (43). During the acute phase of inflammation, C-reactive protein (CRP) levels increase while albumin levels decrease, possibly due to the liver’s altered synthesis priorities (44, 45). The decrease in albumin levels in RA may also be related to other factors, such as inflammation-induced increased capillary permeability and increased protein loss due to RA-associated kidney involvement (44, 46, 47).
CRP and erythrocyte sedimentation Rate (ESR) are widely used and valuable indicators of systemic inflammation in RA and NPAR offers a unique perspective by virtue of its composite nature, integrating both inflammatory activity and nutritional status. The inclusion of albumin in the NPAR calculation provides valuable information regarding the patient’s nutritional status, a critical factor in RA. Chronic inflammation in RA patients often induces a catabolic state, leading to malnutrition and hypoalbuminemia, both of which have been associated with increased disease activity and poorer clinical outcomes (48, 49). While CRP primarily reflects the systemic acute-phase response and ESR can be influenced by factors beyond inflammation, NPAR may offer a more direct reflection of neutrophil-driven inflammation within the synovial joints, a central process in RA pathogenesis (42). Furthermore, NPAR’s reliance on readily available laboratory values, obtained from routine blood tests, renders it an accessible and cost-effective tool for routine clinical practice. Therefore, we propose that NPAR offers unique and complementary insights into the complex interplay of inflammation and nutritional status in RA, warranting further investigation as a potential biomarker for disease activity, prognosis, and treatment response.
These findings align with the well-established differences in the pathogenic mechanisms of RA and OA. While RA is characterized by systemic autoimmune-mediated inflammation, OA is primarily driven by mechanical joint injury, age-related degeneration, and localized inflammation (11, 50). These distinctions underscore the need for personalized diagnostic and therapeutic strategies for arthritis and further research to elucidate the complex mechanisms underlying these conditions.
This study provided robust evidence supporting a positive correlation between NPAR and RA, while demonstrating no association with OA, contributing to the arthritis literature. By leveraging the extensive sample size of the NHANES database, the findings are more comprehensive, representative, and generalizable. Furthermore, NPAR is inexpensive and easily accessible in clinical practice. When neutrophil percentage and albumin levels do not substantially deviate from normal ranges, NPAR may aid in identifying individuals with unrecognized RA risk.
However, this study has several limitations. First, the ascertainment of OA and RA diagnoses was based on self-reported data, without further confirmation from medical records, which is susceptible to recall and information bias. This cross-sectional design precludes the establishment of a temporal and causal relationship between NPAR and RA. While this study included numerous covariates to reduce the influence of confounding factors, it did not account for additional confounders that are more challenging to extract from the NHANES database, such as occupational exposure, genetic factors, and medication use. Moreover, a limitation of this study is the challenge in distinguishing between RA-specific processes and general inflammatory activity, which could potentially influence our findings. To address this, we have outlined several directions for future research, including prospective, real-world cohort studies, and investigations that account for or stratify by infections and other comorbidities. Furthermore, it is necessary to evaluate the performance of NPAR against established RA biomarkers, such as CRP, ESR, rheumatoid factor and ACPA. Finally, we emphasize the need for mechanistic studies, including in vivo investigations, to better understand the biological basis of our findings. These future studies are crucial for validating our findings and further elucidating the clinical utility of NPAR in RA.
5 Conclusion
In conclusion, this study reveals a positive correlation between NPAR and the risk of RA, while no association was observed with OA. The findings enhance our understanding of the roles of systemic inflammation and albumin levels in the pathogenesis of RA, potentially offering valuable insights for early detection, prevention, and targeted therapeutic approaches for RA management. Nevertheless, future research with larger sample sizes and longitudinal study designs is warranted to validate these findings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics review board of the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
Author contributions
WD: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. RL: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. SW: Conceptualization, Data curation, Methodology, Validation, Writing – original draft. ZH: Conceptualization, Data curation, Methodology, Writing – original draft. DJ: Data curation, Validation, Writing – original draft. ZZ: Conceptualization, Data curation, Methodology, Writing – original draft. HN: Data curation, Validation, Writing – original draft. WX: Conceptualization, Data curation, Methodology, Validation, Writing – review & editing. LH: Conceptualization, Methodology, Validation, Writing – review & editing. QW: Conceptualization, Data curation, Funding acquisition, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Jiangsu Provincial Traditional Chinese Medicine Science and Technology Development Project (no. ZD202232).
Acknowledgments
The authors thank all the staff for their help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACPA, Anti-citrullinated peptide/protein antibodies; ALP, Alkaline phosphatase; ALT, Alanine aminotransferases; AST, Aspartate aminotransferase; BMI, Body mass index; CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; ESR, Erythrocyte sedimentation Rate; Hb, Hemoglobin; IL, Interleukin; IQR, Interquartile range; LAP, Lipid accumulation product; NAFLD, Nonalcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey; NPAR, Neutrophil percentage to albumin ratio; OR, Odds Ratio; OA, Osteoarthritis; RA, Rheumatoid arthritis; SD, Standard deviation; RBC, Red blood cell count; PIR, Poverty Income Ratio; TNF, Tumor necrosis factor.
References
1. Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. (2018) 108:256–8. doi: 10.2105/AJPH.2017.304179
2. MacDonald IJ, Huang CC, Liu SC, Lin YY, Tang CH. Targeting CCN proteins in rheumatoid arthritis and osteoarthritis. Int J Mol Sci. (2021) 22(9):4340. doi: 10.3390/ijms22094340
3. Zhao J, Jia Y, Zeng L, Huang H, Liang G, Hong K, et al. Interplay of systemic immune-inflammation index and serum klotho levels: unveiling a new dimension in rheumatoid arthritis pathology. Int J Med Sci. (2024) 21:396–403. doi: 10.7150/ijms.89569
4. Bruno MC, Cristiano MC, Celia C, d’Avanzo N, Mancuso A, Paolino D, et al. Injectable drug delivery systems for osteoarthritis and rheumatoid arthritis. ACS Nano. (2022) 16:19665–90. doi: 10.1021/acsnano.2c06393
5. Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. (2007) 34:172–80.
6. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheumatol. (2012) 64:1697–707. doi: 10.1002/art.34453
7. Brown P, Pratt AG, Hyrich KL. Therapeutic advances in rheumatoid arthritis. BMJ. (2024) 384:e070856. doi: 10.1136/bmj-2022-070856
8. Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis. (2002) 61:290–7. doi: 10.1136/ard.61.4.290
9. Ait Eldjoudi D, Cordero Barreal A, Gonzalez-Rodriguez M, Ruiz-Fernandez C, Farrag Y, Farrag M, et al. Leptin in osteoarthritis and rheumatoid arthritis: player or bystander? Int J Mol Sci. (2022) 23(5):2859. doi: 10.3390/ijms23052859
10. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: A review. JAMA. (2018) 320:1360–72. doi: 10.1001/jama.2018.13103
11. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis)! Osteoarthritis Cartilage. (2013) 21:16–21. doi: 10.1016/j.joca.2012.11.012
12. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. (2010) 62:2569–81. doi: 10.1002/art.27584
13. Demoruelle MK, Deane KD. Treatment strategies in early rheumatoid arthritis and prevention of rheumatoid arthritis. Curr Rheumatol Rep. (2012) 14:472–80. doi: 10.1007/s11926-012-0275-1
14. Felson DT, Hodgson R. Identifying and treating preclinical and early osteoarthritis. Rheum Dis Clin North Am. (2014) 40:699–710. doi: 10.1016/j.rdc.2014.07.012
15. Aggarwal R, Liao K, Nair R, Ringold S, Costenbader KH. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheumatol. (2009) 61:1472–83. doi: 10.1002/art.v61:11
16. Wu CY, Yang HY, Luo SF, Lai JH. From rheumatoid factor to anti-citrullinated protein antibodies and anti-carbamylated protein antibodies for diagnosis and prognosis prediction in patients with rheumatoid arthritis. Int J Mol Sci. (2021) 22(2):686. doi: 10.3390/ijms22020686
17. Ahmed U, Anwar A, Savage RS, Costa ML, Mackay N, Filer A, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep. (2015) 5:9259. doi: 10.1038/srep09259
18. Motta F, Barone E, Sica A, Selmi C. Inflammaging and osteoarthritis. Clin Rev Allergy Immunol. (2023) 64:222–38. doi: 10.1007/s12016-022-08941-1
19. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. (2013) 5:77–94. doi: 10.1177/1759720X12467868
20. Jang S, Kwon EJ, Lee JJ. Rheumatoid arthritis: pathogenic roles of diverse immune cells. Int J Mol Sci. (2022) 23(2):905. doi: 10.3390/ijms23020905
21. Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, Di Franco M. Dietary habits and nutrition in rheumatoid arthritis: can diet influence disease development and clinical manifestations? Nutrients. (2020) 12(5):1456. doi: 10.3390/nu12051456
22. Messina OD, Vidal Wilman M, Vidal Neira LF. Nutrition, osteoarthritis and cartilage metabolism. Aging Clin Exp Res. (2019) 31:807–13. doi: 10.1007/s40520-019-01191-w
23. Cascao R, Rosario HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun Rev. (2010) 9:531–5. doi: 10.1016/j.autrev.2009.12.013
24. Hsueh MF, Zhang X, Wellman SS, Bolognesi MP, Kraus VB. Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol. (2021) 73:89–99. doi: 10.1002/art.41486
25. Yu Y, Liu Y, Ling X, Huang R, Wang S, Min J, et al. The neutrophil percentage-to-albumin ratio as a new predictor of all-cause mortality in patients with cardiogenic shock. BioMed Res Int. (2020) 2020:7458451. doi: 10.1155/2020/7458451
26. Hu Z, Wang J, Xue Y, Zhang Q, Xu Q, Ji K, et al. The neutrophil-to-albumin ratio as a new predictor of all-cause mortality in patients with heart failure. J Inflammation Res. (2022) 15:701–13. doi: 10.2147/JIR.S349996
27. Lv XN, Shen YQ, Li ZQ, Deng L, Wang ZJ, Cheng J, et al. Neutrophil percentage to albumin ratio is associated with stroke-associated pneumonia and poor outcome in patients with spontaneous intracerebral hemorrhage. Front Immunol. (2023) 14:1173718. doi: 10.3389/fimmu.2023.1173718
28. Liu CF, Chien LW. Predictive role of neutrophil-percentage-to-albumin ratio (NPAR) in nonalcoholic fatty liver disease and advanced liver fibrosis in nondiabetic US adults: evidence from NHANES 2017-2018. Nutrients. (2023) 15(8):1892. doi: 10.3390/nu15081892
29. Yan Y, Zhou L, La R, Jiang M, Jiang D, Huang L, et al. The association between triglyceride glucose index and arthritis: a population-based study. Lipids Health Dis. (2023) 22:132. doi: 10.1186/s12944-023-01899-9
30. Peeters GM, Alshurafa M, Schaap L, de Vet HC. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. (2015) 68:452–9. doi: 10.1016/j.jclinepi.2014.09.019
31. Huang J, Han J, Rozi R, Fu B, Lu Z, Liu J, et al. Association between lipid accumulation products and osteoarthritis among adults in the United States: A cross-sectional study, NHANES 2017-2020. Prev Med. (2024) 180:107861. doi: 10.1016/j.ypmed.2024.107861
32. Zhou E, Wu J, Zhou X, Yin Y. The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among U.S. adults with rheumatoid arthritis: results from NHANES 1999-2020. Front Immunol. (2023) 14:1309835. doi: 10.3389/fimmu.2023.1309835
33. Wang R, Tao W, Chen H, Ma T, Cheng X. Investigating nonlinear associations between neutrophil percentage to albumin ratio and cardiovascular disease: a nationally representative cross-sectional study. Sci Rep. (2024) 14:23632. doi: 10.1038/s41598-024-75111-y
34. Li X, Gu Z, Gao J. Elevated neutrophil percentage-to-albumin ratio predicts increased all-cause and cardiovascular mortality among individuals with diabetes. Sci Rep. (2024) 14:27870. doi: 10.1038/s41598-024-79355-6
35. He X, Dai F, Zhang X, Pan J. The neutrophil percentage-to-albumin ratio is related to the occurrence of diabetic retinopathy. J Clin Lab Anal. (2022) 36:e24334. doi: 10.1002/jcla.24334
36. Jiao S, Zhou J, Feng Z, Huang J, Chen L, Li Z, et al. The role of neutrophil percentage to albumin ratio in predicting 1-year mortality in elderly patients with hip fracture and external validation. Front Immunol. (2023) 14:1223464. doi: 10.3389/fimmu.2023.1223464
37. Lan CC, Su WL, Yang MC, Chen SY, Wu YK. Predictive role of neutrophil-percentage-to-albumin, neutrophil-to-lymphocyte and eosinophil-to-lymphocyte ratios for mortality in patients with COPD: Evidence from NHANES 2011-2018. Respirology. (2023) 28:1136–46. doi: 10.1111/resp.v28.12
38. Cecchi I, Arias de la Rosa I, Menegatti E, Roccatello D, Collantes-Estevez E, Lopez-Pedrera C, et al. Neutrophils: Novel key players in Rheumatoid Arthritis. Current and future therapeutic targets. Autoimmun Rev. (2018) 17:1138–49. doi: 10.1016/j.autrev.2018.06.006
39. Fresneda Alarcon M, McLaren Z, Wright HL. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: same foe different M.O. Front Immunol. (2021) 12:649693. doi: 10.3389/fimmu.2021.649693
40. Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. (2014) 16:R122. doi: 10.1186/ar4579
41. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. (2013) 5:178ra40. doi: 10.1126/scitranslmed.3005580
42. Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. (2014) 10:593–601. doi: 10.1038/nrrheum.2014.80
43. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. (2005) 39:S143–6. doi: 10.1097/01.mcg.0000155514.17715.39
44. Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. (2000) 85:599–610. doi: 10.1093/bja/85.4.599
45. Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O. Serum albumin levels and inflammation. Int J Biol Macromol. (2021) 184:857–62. doi: 10.1016/j.ijbiomac.2021.06.140
46. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43:181–93. doi: 10.1002/jpen.2019.43.issue-2
47. Becetti K, Oeser A, Ormseth MJ, Solus JF, Raggi P, Stein CM, et al. Urinary albumin excretion is increased in patients with rheumatoid arthritis and associated with arterial stiffness. J Rheumatol. (2015) 42:593–8. doi: 10.3899/jrheum.141295
48. Tian P, Xiong J, Wu W, Shi S, Chen A, Chen K, et al. Impact of the malnutrition on mortality in Rheumatoid arthritis patients: A cohort study from NHANES 1999-2014. Front Nutr. (2022) 9:993061. doi: 10.3389/fnut.2022.993061
49. Oz N, Gezer HH, Cilli Hayiroglu S, Duruoz MT. Evaluation of the prognostic nutritional index (PNI) as a tool for assessing disease activity in rheumatoid arthritis patients. Clin Rheumatol. (2024) 43:1461–7. doi: 10.1007/s10067-024-06927-2
Keywords: rheumatoid arthritis, osteoarthritis, neutrophil percentage to albumin ratio, inflammation, National Health and Nutrition Examination Survey, cross-sectional study
Citation: Ding W, La R, Wang S, He Z, Jiang D, Zhang Z, Ni H, Xu W, Huang L and Wu Q (2025) Associations between neutrophil percentage to albumin ratio and rheumatoid arthritis versus osteoarthritis: a comprehensive analysis utilizing the NHANES database. Front. Immunol. 16:1436311. doi: 10.3389/fimmu.2025.1436311
Received: 21 May 2024; Accepted: 06 January 2025;
Published: 23 January 2025.
Edited by:
Alenka Gagro, Children’s Hospital Zagreb, CroatiaReviewed by:
Mary Deeb, Lebanese American University, LebanonAthanasios Vassilopoulos, Rhode Island Hospital, United States
Copyright © 2025 Ding, La, Wang, He, Jiang, Zhang, Ni, Xu, Huang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Wu, cWlhbnd1am9pbnRAMTYzLmNvbQ==; Lixin Huang, c3podWFuZ2x4QHllYWgubmV0; Wu Xu, eHV3dUBzdWRhLmVkdS5jbg==
†These authors have contributed equally to this work
 Wenquan Ding1†
Wenquan Ding1† Lixin Huang
Lixin Huang Qian Wu
Qian Wu