- 1Department of Oncology, The First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, China
- 2Jiangxi "Flagship" Oncology Department of Synergy for Chinese and Western Medicine, The First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, China
- 3Department of Oncology, Jiangxi Clinical Medical Research Center for Cancer, The First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, China
- 4Department of Gastroenterology, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, Jiangxi, China
- 5Department of Otolaryngology, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, Jiangxi, China
Oral cancer is a highly malignant disease characterized by recurrence, metastasis, and poor prognosis. Autophagy, a catabolic process induced under stress conditions, has been shown to play a dual role in oral cancer development and therapy. Recent studies have identified that autophagy activation in oral epithelial cells suppresses cancer cell survival by inhibiting key pathways such as the mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK), while activating the adenosine monophosphate-activated protein kinase (AMPK) pathway. Inducing autophagy promotes degradation of eukaryotic initiation factor 4E, thus reducing metastasis and enhancing the efficacy of chemotherapy, radiotherapy, and immunotherapy. Furthermore, autophagy induction can modulate the tumor immune microenvironment and enhance antitumor immunity. This review comprehensively summarizes the relationship between autophagy and oral cancer, focusing on its mechanisms and therapeutic potential when combined with conventional treatments. While promising, the precise mechanisms and clinical applications of autophagy inducers in oral cancer therapy remain to be elucidated, offering new directions for future research to improve treatment outcomes and reduce recurrence.
1 Introduction
Oral cancer is one of the most prevalent cancers of the head and neck region, commonly affecting the lips, buccal mucosa, and tongue. Its high malignancy, recurrence, and metastasis significantly impair patients’ quality of life and impose substantial economic burdens. Key risk factors include alcohol consumption, tobacco use, betel quid chewing, and human papillomavirus (HPV) infection. Despite advancements in surgical, radiotherapeutic, and chemotherapeutic strategies, the overall 5-year survival rate of oral cancer patients remains approximately 50%. However, early detection can improve survival rates to nearly 80%, highlighting the urgent need for innovative therapeutic approaches (1).
Autophagy, a highly conserved cellular process, plays a critical role in maintaining cellular homeostasis and responding to stressors such as hypoxia, nutrient deprivation, and DNA damage (2). It facilitates the degradation and recycling of damaged organelles, misfolded proteins, and other cytoplasmic components. Dysregulation of autophagy has been implicated in cancer, where it exerts dual roles as a tumor suppressor in early stages and as a tumor promoter in advanced cancers (3).
In oral cancer, autophagy regulates a range of processes, including carcinogenesis, metastasis, and response to therapy. Recent studies have identified potential therapeutic applications of autophagy modulation, particularly in enhancing the efficacy of chemotherapy, radiotherapy, and immunotherapy. Although autophagy inducers show potential in enhancing therapeutic effects, their potential side effects and safety concerns in clinical applications need to be carefully considered in treatment strategies. This review aims to elucidate the relationship between autophagy and oral cancer progression, discuss the underlying molecular mechanisms, and explore its potential as a therapeutic target.
2 Autophagy and oral cancer progression
2.1 Role of autophagy in oncogenesis
Autophagy has emerged as a critical regulator in the progression of oral cancer, influencing both the initiation and advancement of the disease. Understanding its dual roles in tumor suppression and promotion provides valuable insights into its potential as a therapeutic target. Autophagy maintains genomic stability in normal oral epithelial cells by removing damaged DNA and organelles (4). Dysregulation of autophagy-related genes (ATGs), such as ATG5 and ATG7, leads to oxidative stress, the accumulation of dysfunctional proteins, and genomic instability, promoting tumorigenesis. For example, loss of ATG5 in oral epithelial cells has been associated with increased reactive oxygen species (ROS) production, contributing to DNA damage and malignant transformation (5). The tumor suppressor gene TP53, which encodes P53, is a pivotal regulator of autophagy. Under conditions of stress, such as nutrient deprivation or hypoxia, P53 activates autophagy by inhibiting the mTOR pathway and modulating the Bcl2/beclin-1 complex (6). This process prevents DNA damage and delays carcinogenesis. However, mutations in TP53, which occur in up to 80% of oral squamous cell carcinoma (OSCC) cases, impair autophagy activation, promoting tumor progression (6). Liu et al. (7) demonstrated that in a hamster oral cancer model, autophagy activation in precancerous lesions was associated with lower levels of DNA damage markers, suggesting a protective role in early carcinogenesis. Conversely, reduced autophagy activity in malignant lesions correlated with increased DNA damage, highlighting the importance of autophagy in preventing tumor initiation.
In the early stages of oral cancer, autophagy primarily functions as a tumor suppressor. It maintains genomic stability by facilitating the clearance of damaged organelles and misfolded proteins, which prevents oxidative stress and DNA damage. Studies have shown that autophagy activation in precancerous lesions leads to lower levels of DNA damage markers and protects against malignant transformation (5–7). In these early stages, autophagy acts as a defense mechanism, delaying carcinogenesis. However, in advanced stages of oral cancer, autophagy may contribute to tumor progression by promoting cancer cell survival under adverse conditions such as nutrient deprivation, hypoxia, and chemotherapy. It helps maintain cellular homeostasis by degrading cellular components, enabling tumor cells to resist apoptosis and promoting metastasis (8–10). Additionally, autophagy in advanced tumors has been shown to enhance the epithelial-mesenchymal transition (EMT) process and facilitate immune evasion, further driving cancer progression (8, 9).
2.2 Impact of autophagy on tumor metastasis
Metastasis, primarily involving cervical lymph nodes in oral cancer, is a major cause of treatment failure and mortality (11). Autophagy plays a complex role in modulating tumor metastasis by influencing EMT, matrix remodeling, and invasion (8) MTA2, a key driver of tumor metastasis, has been shown to suppress autophagy-related protein LC3-II expression in oral cancer cells, facilitating metastasis. Silencing MTA2 using shRNA restored autophagy and inhibited tumor migration and invasion. Autophagy also regulates EMT, a process critical for cancer cell migration and invasion (9). Liang et al. (10) reported that in rapamycin-treated OSCC cells, high expression of METTL14 enhanced autophagy, inhibited EMT, and suppressed metastatic potential. METTL14-mediated autophagy degraded eIF4E, reducing cancer cell invasion. Additionally, Autophagy significantly impacts the tumor microenvironment (TME) by modulating immune cell infiltration, cytokine secretion, and extracellular matrix (ECM) remodeling (12). It enhances antigen presentation by facilitating the processing and presentation of tumor antigens on major histocompatibility complex (MHC) molecules, thereby improving T cell activation and antitumor immune responses (13). Additionally, autophagy maintains the homeostasis and effector functions of T cells, NK cells, and macrophages, which are crucial for effective immune surveillance and response against tumor cells (14). Autophagy influences the secretion of cytokines and chemokines that modulate immune cell recruitment and activity within the TME. For instance, autophagy enhances the secretion of CXCL10, a chemokine that attracts effector T cells and NK cells to the tumor site, thereby strengthening the immune response (15). Furthermore, autophagy regulates the production of pro-inflammatory cytokines that contribute to a more favorable antitumor environment. Autophagy affects ECM remodeling by regulating the degradation of ECM components and the activity of MMPs. This modulation of the ECM can influence cancer cell migration and invasion, thereby affecting tumor progression and metastatic dissemination. By altering the ECM, autophagy can create a microenvironment that either supports or inhibits tumor growth and spread (16).
3 Molecular mechanisms underlying autophagy regulation
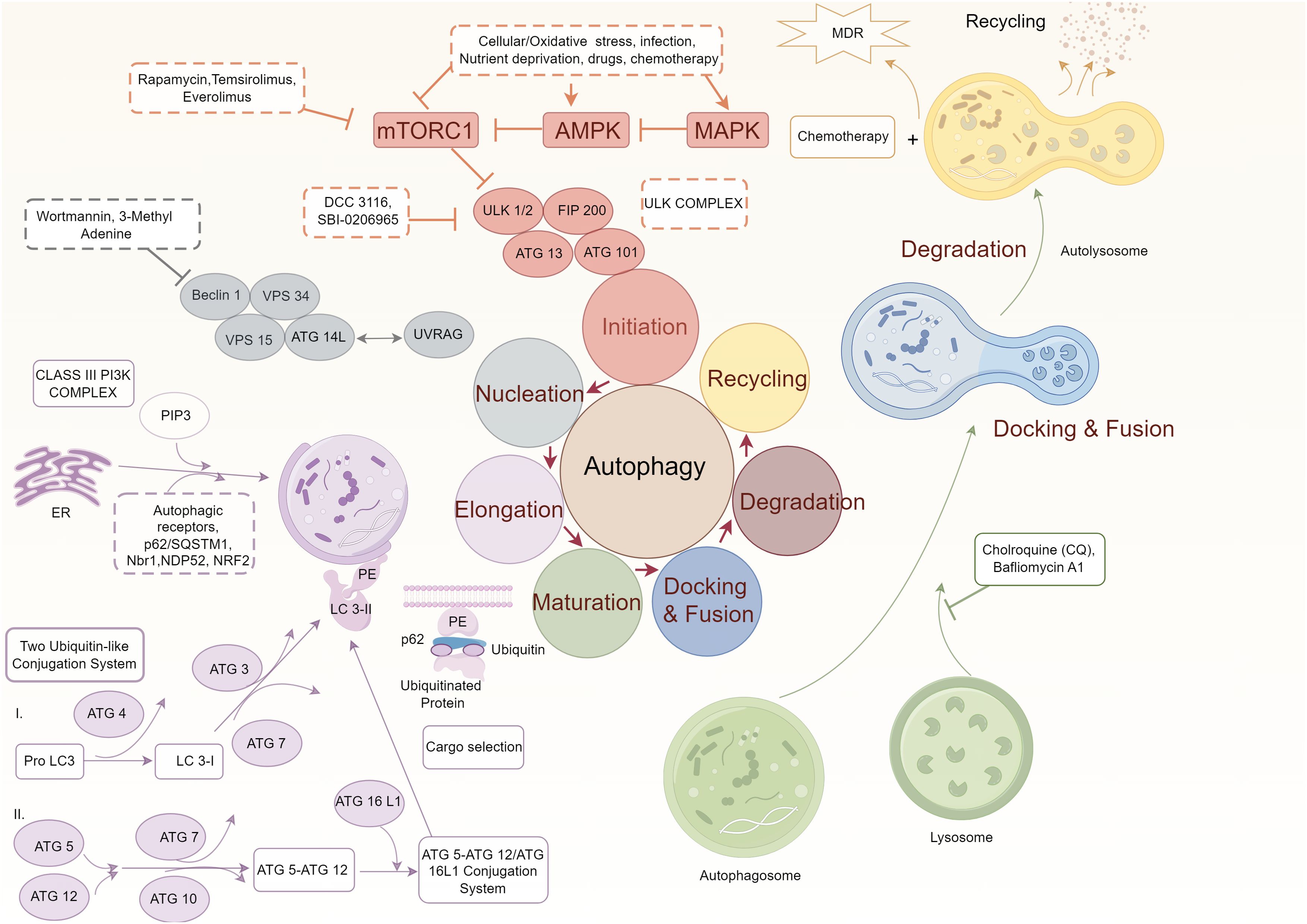
Autophagy is a tightly regulated process that plays a critical role in maintaining cellular homeostasis and responding to various stressors. In OSCC, several signaling pathways influence the regulation of autophagy, dictating its dual roles in tumor suppression and progression. This section explores three key pathways—mTOR, AMPK, and Beclin-1/Bcl2 interactions—and their therapeutic implications in oral cancer (Figure 1).
3.1 mTOR pathway
The mechanistic target of rapamycin (mTOR) is a serine/threonine kinase and a central regulator of autophagy. It integrates signals from nutrients, growth factors, and cellular energy levels to suppress autophagy under favorable conditions (17–20). In OSCC, mTOR is frequently hyperactivated due to mutations or dysregulation of upstream signaling pathways, leading to autophagy inhibition and promoting uncontrolled tumor growth. mTOR inhibits autophagy by ULK1 at Ser757, disrupting its interaction with AMPK and preventing autophagosome formation (21). Pharmacological inhibition of mTOR, using drugs such as rapamycin and its analogs, restores autophagic activity. Rapamycin binds to FKBP12, forming a complex that specifically inhibits mTORC1, thereby inducing autophagy. Additionally, natural compounds like curcumin inhibit the mTOR pathway by downregulating upstream activators such as PI3K/Akt, further promoting autophagy. Pre-clinical studies in OSCC have demonstrated that rapamycin enhances autophagosome formation, reduces cancer cell proliferation, and increases the sensitivity of cancer cells to chemotherapeutic agents (22–24). Additionally, mTOR inhibitors improve the tumor microenvironment by increasing immune cell infiltration, further enhancing antitumor immune responses.
Natural compounds targeting the mTOR pathway have also shown promise in inducing autophagy in OSCC. For instance, curcumin and its derivative MTH-3 inhibit the EGFR/AKT/mTOR signaling pathway, upregulating autophagy-related markers such as LC3B-II and p62 while downregulating p38 MAPK expression, thereby reducing OSCC cell viability (25). The molecular mechanism of curcumin involves the inhibition of the PI3K/Akt pathway, leading to decreased phosphorylation of mTOR and its downstream targets, which promotes autophagosome formation. Pterostilbene restores autophagic activity in cisplatin-resistant OSCC cells by activating autophagy-related genes, including ATG5, ATG7, and Beclin-1, through modulation of the AKT pathway, leading to enhanced cancer cell death. Similarly, pterostilbene restores autophagic activity in cisplatin-resistant OSCC cells by activating autophagy-related genes, including ATG5, ATG7, and Beclin-1, through modulation of the AKT pathway, leading to enhanced cancer cell death. Potential antitumor drugs like chlorpromazine have also been found to induce autophagy in gingival cancer cells by upregulating autophagy-related proteins (Atg5, Atg7, Atg12, Beclin-1, and LC3A/B-II) while downregulating negative regulators (mTOR, PI3K, Akt, and p70S6K) (26–28). This mechanism promotes apoptosis and suppresses cancer cell growth. Other compounds, such as ursolic acid, enhance autophagy by modulating multiple pathways, including mTOR, AKT, and p38 MAPK, while inducing apoptotic cell death in OSCC cells.
3.2 AMPK pathway
AMPK is a cellular energy sensor that responds to low ATP levels by promoting catabolic pathways, including autophagy, to restore energy homeostasis. AMPK activates autophagy through two primary mechanisms (29). Direct Activation of ULK1: AMPK phosphorylates ULK1 at Ser317 and Ser777, directly stimulating its kinase activity and initiating autophagosome formation. This phosphorylation occurs at a different site than mTOR, enabling AMPK to bypass mTOR inhibition under energy-deprived conditions. Inhibition of mTOR: AMPK phosphorylates TSC2 and Raptor, components of the mTORC1 complex, leading to mTOR inhibition (30). This dual action ensures robust activation of autophagy even in the presence of conflicting upstream signals.
In oral cancer, AMPK agonists like metformin and AICAR have shown promise in preclinical studies. Metformin induces autophagy by activating AMPK and reducing cancer cell viability. In OSCC xenograft models, metformin-mediated autophagy has been associated with decreased tumor growth and enhanced sensitivity to cisplatin (31). Moreover, AMPK activation modulates metabolic reprogramming in cancer cells, reducing glycolytic flux and oxidative stress, which indirectly promotes autophagic cell death (32).
3.3 Beclin-1/Bcl2 interaction
Beclin-1 is a central regulator of autophagy that forms complexes with VPS34 to initiate autophagosome formation (33). However, its activity is negatively regulated by anti-apoptotic Bcl2 family proteins, including Bcl2 and Bcl-XL, which bind to the Bcl2 homology 3 (BH3) domain of Beclin-1, preventing its interaction with VPS34 (34).
In OSCC, overexpression of Bcl2 and Bcl-XL leads to reduced autophagic activity and increased resistance to apoptosis. Disrupting the Beclin-1/Bcl2 complex using BH3 mimetics, such as ABT-737, restores autophagic activity and induces cancer cell death (35). Additionally, small molecule inhibitors that disrupt the Beclin-1/Bcl2 interaction have been shown to effectively induce autophagy. These inhibitors bind to the BH3 domain of Bcl2, preventing its interaction with Beclin-1 and thereby freeing Beclin-1 to initiate autophagy. This strategy not only promotes autophagy but also sensitizes cancer cells to apoptosis, offering a dual therapeutic benefit. Studies have demonstrated that reactivation of Beclin-1 in OSCC cells significantly reduces tumor proliferation and migration (31). Additionally, Beclin-1-mediated autophagy sensitizes OSCC cells to radiotherapy by increasing DNA damage and impairing repair mechanisms.
3.4 Autophagy in enhancing chemotherapy and radiotherapy for oral cancer
Autophagy enhances the efficacy of chemotherapy and radiotherapy in oral cancer by modulating signaling pathways and overcoming resistance (36). Curcumin and MTH-3 regulate transcription factor EB and the EGFR/AKT/mTOR pathway, increasing LC3B-II and p62 levels while reducing P38, thereby inducing autophagy and inhibiting OSCC cell activity (37). Pterostilbene promotes autophagy and cell death in cisplatin-resistant OSCC by activating Atg5, Atg7, Atg12, Beclin-1, and LC3-II via the AKT pathway (27). Chlorpromazine increases autophagy-related proteins and inhibits mTOR, PI3K, Akt, and p70S6K, inducing autophagy and apoptosis in gingival cancer cells (38). Furthermore, AMPK activators enhance LC3 and p62 accumulation, inducing apoptosis in SCC2095 cells (39). Ursolic acid modulates AKT/mTOR, NF-κB, ERK, and P38 MAPK, regulating LC3B-II and p62 and enhancing autophagy and apoptosis in OSCC (40).
Combining autophagy inducers with radiotherapy or chemotherapy also shows synergistic effects (18, 41, 42). Tanshinone IIA enhances radiosensitivity by upregulating Beclin-1, ATG5, and LC3-II in SCC090 cells (43). Cordycepin plus radiotherapy elevates ATG5, BECN1, LC3-II, and p62, inducing autophagy, G2/M arrest, and cell death in tongue squamous cell carcinoma (44). Saikosaponin increases radiosensitivity and apoptosis via autophagosome accumulation (30). Rapamycin facilitates autophagosome formation, enhancing radiosensitivity in OSCC (45). USP14 inhibition increases autophagy-related proteins, reducing radio-resistance (46). In chemotherapy, Paris saponin G promotes autophagy through ERK and JNK, increasing Beclin-1, LC3-II, and lowering p62, thereby enhancing chemotherapeutic efficacy (47). Overall, autophagy-induced type II programmed cell death has shown promise across various cancers, including glioblastoma, endometrial, lung, and breast cancers (48, 49). In OSCC, activating AMPK or inhibiting MAPK and mTOR, combined with autophagy inducers, may further improve treatment outcomes and reduce proliferation and metastasis.
4 Autophagy and immunotherapy in oral cancer
4.1 The relationship between autophagy and tumor immunity
Autophagy plays a dual role in tumor immunity, functioning as both a facilitator of immune surveillance and an enabler of immune evasion (50–53). It significantly influences tumor immunoregulation by modulating antigen presentation, immune cell activity, and the tumor microenvironment. Autophagy is crucial for antigen presentation, particularly in delivering tumor antigens to major histocompatibility complex (MHC) class I and II molecules (54). By promoting the degradation and processing of tumor antigens within antigen-presenting cells (APCs) such as dendritic cells and macrophages, autophagy enhances cross-epitope presentation and subsequently improves T cell activation. Studies have demonstrated that autophagy in tumor cells increases MHC class I-dependent cross-presentation, enhancing CD8+ cytotoxic T lymphocyte-mediated tumor cell killing (55). Similarly, autophagy-mediated MHC class II antigen presentation activates CD4+ helper T cells, contributing to sustained antitumor immune responses (56).
Autophagy also impacts immune cell homeostasis and function within the tumor microenvironment (54). In T cells, autophagy is critical for maintaining metabolic balance and effector function. The loss of autophagy-related gene ATG5 in T cells leads to metabolic dysfunction and impaired proliferation, weakening antitumor immunity (57). Additionally, autophagy clears damaged mitochondria and oxidative stress products, protecting T cells from damage and preserving their effector capabilities. In NK cells, autophagy enhances tumor infiltration and direct cytotoxicity (58). In macrophages (59), autophagy regulates polarization, influencing the immune microenvironment’s pro-tumor (M2) or antitumor (M1) characteristics. By promoting M1 polarization, autophagy may enhance macrophage-mediated antitumor activity (60).
4.2 Autophagy and immune evasion
Tumor cells exploit autophagy to evade immune surveillance through various mechanisms (61, 62). Autophagy suppresses apoptosis and the release of antitumor immune factors, such as interferon-γ and tumor necrosis factor-α, allowing tumor cells to evade immune detection (63) Moreover, autophagy regulates immune checkpoint molecules, such as PD-L1, on the tumor cell surface, weakening T cell-mediated immune responses. For instance, autophagy inducers can degrade PD-L1 protein, reversing immune suppression and enhancing antitumor immunity (64). The tumor microenvironment plays a crucial role in determining immune therapy efficacy. Autophagy influences immune cell infiltration and activity by regulating key components of the tumor microenvironment, including cytokines, stromal cells, and angiogenesis. Studies have shown that autophagy enhances the secretion of chemokines, such as CXCL10, by tumor cells, attracting effector T cells and NK cells to tumor sites (65). Additionally, autophagy regulates tumor metabolism, such as lactate and glucose consumption, improving the acidic microenvironment and indirectly boosting immune cell functionality.
4.3 Synergistic effects of autophagy and immunotherapy
During cancer progression, tumor cells often escape immune surveillance, contributing to their survival. Cancer immunotherapy aims to enhance various immune response steps to eliminate tumor cells effectively. Autophagy contributes to immunotherapy by enhancing antigen presentation, maintaining lymphocyte homeostasis, promoting T cell activation, and facilitating NK cell infiltration into tumor tissues (66, 67). Autophagy inducers, such as spermidine and trehalose, have shown potential in boosting immune function (68). Current strategies for activating tumor immunity often involve blocking immune checkpoints, such as PD-1/PD-L1. Studies indicate that autophagy induction can reduce PD-L1 expression, promoting tumor cell death (69). For example, anti-PD-1 therapy activates autophagy and enhances tumor cell death in lung cancer (70). In OSCC, combining anti-PD-1 therapy with autophagy inducers, such as rapamycin or metformin, improves antitumor efficacy, prolongs survival, and strengthens immune responses in preclinical models (13). Furthermore, autophagy plays a promising role in tumor vaccine development by enhancing antigen presentation and T cell activation, thereby improving vaccine-mediated antitumor effects (14).
5 Conclusion
Autophagy plays a critical role in the pathogenesis and progression of oral cancer. Numerous studies have revealed aberrant expression and dysregulation of autophagy-related genes in oral cancer tissues. In the early stages of oral cancer, inducing autophagy in hyperplastic epithelial cells can inhibit tumor progression. Moreover, promoting autophagy in oral cancer cells has been shown to suppress metastasis and enhance the efficacy of chemotherapy, radiotherapy, and immunotherapy.
However, the dual role of autophagy in tumor promotion and suppression remains a topic of debate. To ensure the safety and efficacy of autophagy inducers in clinical applications, it is essential to thoroughly investigate their potential side effects and safety concerns. The specific targets and optimal timing for autophagy induction in oral cancer therapy require further investigation through comprehensive basic and clinical research. Additionally, the development of targeted drug delivery systems and monitoring biomarkers are key strategies for managing the risks associated with autophagy inducers. It is also essential to consider that tumor cell death is not solely a direct result of drug action but may also stem from changes in the tumor microenvironment. Clarifying the precise mechanisms by which autophagy modulation enhances the synergistic effects of chemotherapy, radiotherapy, and immunotherapy in oral cancer will pave the way for innovative therapeutic strategies. Precise induction of autophagy offers a promising avenue to improve treatment outcomes and reduce the recurrence of oral cancer, potentially representing a significant breakthrough in the field.
Author contributions
XZ: Writing – original draft. YC: Writing – original draft. JW: Writing – original draft. MH: Writing – original draft. JQ: Writing – original draft. YH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the “Jiangxi Provincial Collaborative Flagship Department of Chinese and Western Medicine Construction Project.”
Acknowledgments
The figure in this manuscript was created using the Figdraw platform (https://www.figdraw.com/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang W, Adeoye J, Thomson P, Choi SW. Statistical profiling of oral cancer and the prediction of outcome. J Oral Pathol Med. (2021) 50:39–46. doi: 10.1111/jop.13110
2. Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: A comprehensive review. BioMed Pharmacother. (2018) 104:485–95. doi: 10.1016/j.biopha.2018.05.007
3. Jacquin E, Apetoh L. Cell-intrinsic roles for autophagy in modulating CD4 T cell functions. Front Immunol. (2018) 9:1023. doi: 10.3389/fimmu.2018.01023
4. Han Y, Fan S, Qin T, Yang J, Sun Y, Lu Y, et al. Role of autophagy in breast cancer and breast cancer stem cells (Review). Int J Oncol. (2018) 52:1057–70. doi: 10.3892/ijo.2018.4270
5. Ionescu C, Kamal FZ, Ciobica A, Halitchi G, Burlui V, Petroaie AD. Oxidative stress in the pathogenesis of oral cancer. Biomedicines. (2024) 12:1150. doi: 10.3390/biomedicines12061150
6. Hu W, Chen S, Thorne RF, Wu M. TP53, TP53 target genes (DRAM, TIGAR), and autophagy. Adv Exp Med Biol. (2019) 1206:127–49. doi: 10.1007/978-981-15-0602-4_6
7. Liu Q, Liu Y, Li SE, Geng JH. Bcl-2 interacts with beclin 1 and regulates autophagy in 7, 12-dimethylbenz[a]anthracene-induced hamster buccal-pouch squamous-cell tumorigenesis. Curr Med Sci. (2021) 41:1198–204. doi: 10.1007/s11596-021-2472-5
8. Strippoli R, Niayesh-Mehr R, Adelipour M, Khosravi A, Cordani M, Zarrabi A, et al. Contribution of autophagy to epithelial mesenchymal transition induction during cancer progression. Cancers (Basel). (2024) 16:807. doi: 10.3390/cancers16040807
9. Garg M. Epithelial plasticity, autophagy and metastasis: potential modifiers of the crosstalk to overcome therapeutic resistance. Stem Cell Rev Rep. (2020) 16:503–10. doi: 10.1007/s12015-019-09945-9
10. Liang J, Cai H, Hou C, Song F, Jiang Y, Wang Z, et al. METTL14 inhibits Malignant progression of oral squamous cell carcinoma by targeting the autophagy-related gene RB1CC1 in an m6A-IGF2BP2-dependent manner. Clin Sci (Lond). (2023) 137:1373–89. doi: 10.1042/CS20230219
11. Pang ZQ, Wang JS, Wang JF, Wang YX, Ji B, Xu YD, et al. JAM3: A prognostic biomarker for bladder cancer via epithelial-mesenchymal transition regulation. Biomol BioMed. (2024) 24:897–911. doi: 10.17305/bb.2024.9979
12. Tian W, Liu Y, Cao C, Zeng Y, Pan Y, Liu X, et al. Chronic stress: impacts on tumor microenvironment and implications for anti-cancer treatments. Front Cell Dev Biol. (2021) 9:777018. doi: 10.3389/fcell.2021.777018
13. Munz C. The macroautophagy machinery in MHC restricted antigen presentation. Front Immunol. (2021) 12:628429. doi: 10.3389/fimmu.2021.628429
14. de Souza ASC, Goncalves LB, Lepique AP, de Araujo-Souza PS. The role of autophagy in tumor immunology-complex mechanisms that may be explored therapeutically. Front Oncol. (2020) 10:603661. doi: 10.3389/fonc.2020.603661
15. Brandt EF, Baues M, Wirtz TH, May JN, Fischer P, Beckers A, et al. Chemokine CXCL10 modulates the tumor microenvironment of fibrosis-associated hepatocellular carcinoma. Int J Mol Sci. (2022) 23:8112. doi: 10.3390/ijms23158112
16. Zhang Z, Yu Y, Zhang Z, Li D, Liang Z, Wang L, et al. Cancer-associated fibroblasts-derived CXCL12 enhances immune escape of bladder cancer through inhibiting P62-mediated autophagic degradation of PDL1. J Exp Clin Cancer Res. (2023) 42:316. doi: 10.1186/s13046-023-02900-0
17. Xiao J, Lin H, Liu B, Xia Z, Zhang J, Jin J. Decreased S1P and SPHK2 are involved in pancreatic acinar cell injury. biomark Med. (2019) 13:627–37. doi: 10.2217/bmm-2018-0404
18. Zhang X, Zhang P, Cong A, Feng Y, Chi H, Xia Z, et al. Unraveling molecular networks in thymic epithelial tumors: deciphering the unique signatures. Front Immunol. (2023) 14:1264325. doi: 10.3389/fimmu.2023.1264325
19. Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang W, et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell Mol Life Sci. (2024) 81:96. doi: 10.1007/s00018-024-05114-5
20. Li Z, Zhou H, Xia Z, Xia T, Du G, Franziska SD, et al. HMGA1 augments palbociclib efficacy via PI3K/mTOR signaling in intrahepatic cholangiocarcinoma. biomark Res. (2023) 11:33. doi: 10.1186/s40364-023-00473-w
21. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. (2007) 12:9–22. doi: 10.1016/j.ccr.2007.05.008
22. Huang KJ, Kuo CH, Chen SH, Lin CY, Lee YR. Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J Cell Mol Med. (2018) 22:1894–908. doi: 10.1111/jcmm.2018.22.issue-3
23. Chan EY. Regulation and function of uncoordinated-51 like kinase proteins. Antioxid Redox Signal. (2012) 17:775–85. doi: 10.1089/ars.2011.4396
24. Wang Y, Zhang Y, Feng X, Tian H, Fu X, Gu W, et al. Metformin inhibits mTOR and c-Myc by decreasing YAP protein expression in OSCC cells. Oncol Rep. (2021) 45:1249–60. doi: 10.3892/or.2020.7909
25. Beevers CS, Li F, Liu L, Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer. (2006) 119:757–64. doi: 10.1002/ijc.v119:4
26. Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, et al. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett. (2009) 285:127–33. doi: 10.1016/j.canlet.2009.04.037
27. Chang HP, Lu CC, Chiang JH, Tsai FJ, Juan YN, Tsao JW, et al. Pterostilbene modulates the suppression of multidrug resistance protein 1 and triggers autophagic and apoptotic mechanisms in cisplatin-resistant human oral cancer CAR cells via AKT signaling. Int J Oncol. (2018) 52:1504–14. doi: 10.3892/ijo.2018.4298
28. Weng JR, Dokla EME, Bai LY, Chen CS, Chiu SJ, Shieh TM. A 5’ AMP-activated protein kinase enzyme activator, compound 59, induces autophagy and apoptosis in human oral squamous cell carcinoma. Basic Clin Pharmacol Toxicol. (2018) 123:21–9. doi: 10.1111/bcpt.2018.123.issue-1
29. Lin CW, Chin HK, Lee SL, Chiu CF, Chung JG, Lin ZY, et al. Ursolic acid induces apoptosis and autophagy in oral cancer cells. Environ Toxicol. (2019) 34:983–91. doi: 10.1002/tox.22769
30. Tian YD, Lin S, Yang PT, Bai MH, Jin YY, Min WL, et al. Saikosaponin-d increases the radiosensitivity of hepatoma cells by adjusting cell autophagy. J Cancer. (2019) 10:4947–53. doi: 10.7150/jca.30286
31. Zhao W, Chen C, Zhou J, Chen X, Cai K, Shen M, et al. Inhibition of autophagy promotes the anti-tumor effect of metformin in oral squamous cell carcinoma. Cancers (Basel). (2022) 14:4185. doi: 10.3390/cancers14174185
32. Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. (2012) 485:661–5. doi: 10.1038/nature11066
33. Li X, Yang KB, Chen W, Mai J, Wu XQ, Sun T, et al. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy. (2021) 17:4323–40. doi: 10.1080/15548627.2021.1912270
34. Decuypere JP, Parys JB, Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells. (2012) 1:284–312. doi: 10.3390/cells1030284
35. Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. (2009) 15:1126–32. doi: 10.1158/1078-0432.CCR-08-0144
36. Abd El-Aziz YS, Leck LYW, Jansson PJ, Sahni S. Emerging role of autophagy in the development and progression of oral squamous cell carcinoma. Cancers (Basel). (2021) 13:6152. doi: 10.3390/cancers13246152
37. Tsai SC, Yang JS, Lu CC, Tsai FJ, Chiu YJ, Kuo SC. MTH-3 sensitizes oral cancer cells to cisplatin via regulating TFEB. J Pharm Pharmacol. (2022) 74:1261–73. doi: 10.1093/jpp/rgac056
38. Matteoni S, Matarrese P, Ascione B, Ricci-Vitiani L, Pallini R, Villani V, et al. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J Exp Clin Cancer Res. (2021) 40:347. doi: 10.1186/s13046-021-02144-w
39. Bonnet LV, Palandri A, Flores-Martin JB, Hallak ME. Arginyltransferase 1 modulates p62-driven autophagy via mTORC1/AMPk signaling. Cell Commun Signal. (2024) 22:87. doi: 10.1186/s12964-024-01499-9
40. Wang YJ, Lu J, Wu DM, Zheng ZH, Zheng YL, Wang XH, et al. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-kappaB mediated inflammatory pathways. Neurobiol Learn Mem. (2011) 96:156–65. doi: 10.1016/j.nlm.2011.03.010
41. Jiang S, Yang X, Lin Y, Liu Y, Tran LJ, Zhang J, et al. Unveiling Anoikis-related genes: A breakthrough in the prognosis of bladder cancer. J Gene Med. (2024) 26:e3651. doi: 10.1002/jgm.v26.1
42. Wang J, Zuo Z, Yu Z, Chen Z, Tran LJ, Zhang J, et al. Collaborating single-cell and bulk RNA sequencing for comprehensive characterization of the intratumor heterogeneity and prognostic model development for bladder cancer. Aging (Albany NY). (2023) 15:12104–19. doi: 10.18632/aging.205166
43. Ding L, Wang S, Qu X, Wang J. Tanshinone IIA sensitizes oral squamous cell carcinoma to radiation due to an enhanced autophagy. Environ Toxicol Pharmacol. (2016) 46:264–9. doi: 10.1016/j.etap.2016.07.021
44. Ho SY, Wu WS, Lin LC, Wu YH, Chiu HW, Yeh YL, et al. Cordycepin enhances radiosensitivity in oral squamous carcinoma cells by inducing autophagy and apoptosis through cell cycle arrest. Int J Mol Sci. (2019) 20:5366. doi: 10.3390/ijms20215366
45. Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol. (2019) 59:125–32. doi: 10.1016/j.semcancer.2019.07.009
46. Xie W, Xu L. Ubiquitin-specific protease 14 promotes radio-resistance and suppresses autophagy in oral squamous cell carcinoma. Exp Cell Res. (2021) 398:112385. doi: 10.1016/j.yexcr.2020.112385
47. Xiang YC, Peng P, Liu XW, Jin X, Shen J, Zhang T, et al. Paris saponin VII, a Hippo pathway activator, induces autophagy and exhibits therapeutic potential against human breast cancer cells. Acta Pharmacol Sin. (2022) 43:1568–80. doi: 10.1038/s41401-021-00755-9
48. Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. (2008) 9:1004–10. doi: 10.1038/nrm2529
49. Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer. (2018) 124:3307–18. doi: 10.1002/cncr.31335
50. Li Z, Zhang Y, Lei J, Wu Y. Autophagy in oral cancer: Promises and challenges (Review). Int J Mol Med. (2024) 54:116. doi: 10.3892/ijmm.2024.5446
51. Wang Y, Ji B, Zhang L, Wang J, He J, Ding B, et al. Identification of metastasis-related genes for predicting prostate cancer diagnosis, metastasis and immunotherapy drug candidates using machine learning approaches. Biol Direct. (2024) 19:50. doi: 10.1186/s13062-024-00494-x
52. Wang Y, Li C, He J, Zhao Q, Zhou Y, Sun H, et al. Multi-omics analysis and experimental validation of the value of monocyte-associated features in prostate cancer prognosis and immunotherapy. Front Immunol. (2024) 15:1426474. doi: 10.3389/fimmu.2024.1426474
53. Wang Y, Zhu H, Zhang L, He J, Bo J, Wang J, et al. Common immunological and prognostic features of lung and bladder cancer via smoking-related genes: PRR11 gene as potential immunotherapeutic target. J Cell Mol Med. (2024) 28:e18384. doi: 10.1111/jcmm.v28.10
54. Oynebraten I. Involvement of autophagy in MHC class I antigen presentation. Scand J Immunol. (2020) 92:e12978. doi: 10.1111/sji.12978
55. Harsha C, Banik K, Ang HL, Girisa S, Vikkurthi R, Parama D, et al. Targeting AKT/mTOR in oral cancer: mechanisms and advances in clinical trials. Int J Mol Sci. (2020) 21:3285. doi: 10.3390/ijms21093285
56. Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. (2013) 13:722–37. doi: 10.1038/nri3532
57. Panahi Meymandi AR, Akbari B, Soltantoyeh T, Hadjati J, Klionsky DJ, Badie B, et al. Crosstalk between autophagy and metabolic regulation of (CAR) T cells: therapeutic implications. Front Immunol. (2023) 14:1212695. doi: 10.3389/fimmu.2023.1212695
58. Oravecz-Wilson K, Rossi C, Zajac C, Sun Y, Li L, Decoville T, et al. ATG5-dependent autophagy uncouples T-cell proliferative and effector functions and separates graft-versus-host disease from graft-versus-leukemia. Cancer Res. (2021) 81:1063–75. doi: 10.1158/0008-5472.CAN-20-1346
59. Zhai X, Zhang H, Xia Z, Liu M, Du G, Jiang Z, et al. Oxytocin alleviates liver fibrosis via hepatic macrophages. JHEP Rep. (2024) 6:101032. doi: 10.1016/j.jhepr.2024.101032
60. Wu MY, Lu JH. Autophagy and macrophage functions: inflammatory response and phagocytosis. Cells. (2019) 9:70. doi: 10.3390/cells9010070
61. Xia Z, Chen S, He M, Li B, Deng Y, Yi L, et al. Editorial: Targeting metabolism to activate T cells and enhance the efficacy of checkpoint blockade immunotherapy in solid tumors. Front Immunol. (2023) 14:1247178. doi: 10.3389/fimmu.2023.1247178
62. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
63. Xia H, Green DR, Zou W. Autophagy in tumour immunity and therapy. Nat Rev Cancer. (2021) 21:281–97. doi: 10.1038/s41568-021-00344-2
64. Limagne E, Nuttin L, Thibaudin M, Jacquin E, Aucagne R, Bon M, et al. MEK inhibition overcomes chemoimmunotherapy resistance by inducing CXCL10 in cancer cells. Cancer Cell. (2022) 40:136–52.e112. doi: 10.1016/j.ccell.2021.12.009
65. Singh UP, Singh R, Singh S, Karls RK, Quinn FD, Taub DD, et al. CXCL10+ T cells and NK cells assist in the recruitment and activation of CXCR3+ and CXCL11+ leukocytes during Mycobacteria-enhanced colitis. BMC Immunol. (2008) 9:25. doi: 10.1186/1471-2172-9-25
66. Merkley SD, Chock CJ, Yang XO, Harris J, Castillo EF. Modulating T cell responses via autophagy: the intrinsic influence controlling the function of both antigen-presenting cells and T cells. Front Immunol. (2018) 9:2914. doi: 10.3389/fimmu.2018.02914
67. Germic N, Frangez Z, Yousefi S, Simon HU. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. (2019) 26:715–27. doi: 10.1038/s41418-019-0297-6
68. Zheng N, Fang J, Xue G, Wang Z, Li X, Zhou M, et al. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance. Cancer Cell. (2022) 40:973–85.e977. doi: 10.1016/j.ccell.2022.08.001
69. Liu X, Yin M, Dong J, Mao G, Min W, Kuang Z, et al. Tubeimoside-1 induces TFEB-dependent lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting mTOR. Acta Pharm Sin B. (2021) 11:3134–49. doi: 10.1016/j.apsb.2021.03.039
Keywords: autophagy, oral cancer, apoptosis, chemotherapy, radiotherapy, immunotherapy
Citation: Zeng X, Chen Y, Wang J, He M, Qiu J and Huang Y (2025) Targeting autophagy to enhance chemotherapy and immunotherapy in oral cancer. Front. Immunol. 15:1535649. doi: 10.3389/fimmu.2024.1535649
Received: 27 November 2024; Accepted: 17 December 2024;
Published: 07 January 2025.
Edited by:
Minghua Ren, First Affiliated Hospital of Harbin Medical University, ChinaReviewed by:
Min Miao, Ningbo University, ChinaCopyright © 2025 Zeng, Chen, Wang, He, Qiu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Huang, Ymx1ZWh1YW5nMjAyNEAxNjMuY29t
 Xiaoli Zeng
Xiaoli Zeng Yue Chen
Yue Chen Jing Wang1,3
Jing Wang1,3