- 1Neuroimmunology Unit, Department of Neurosciences, Hospital Aleman, Buenos Aires, Argentina
- 2Centro de Enfermedades Neuroinmunológicas de Rosario (CENRos), Neuroimmunology Clinic, Instituto de Neurologia Cognitiva (INECO) Neurociencias Oroño, Rosario, Argentina
- 3Department of Neurology, Hospital Universitario Centro de Educación Médica e Investigaciones Clínicas (CEMIC), Buenos Aires, Argentina
- 4Department of Neurology, Hospital Ramos Mejia, Buenos Aires, Argentina
- 5Department of Medicine, Divisions of Molecular Medicine and Infectious Diseases, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
- 6Department of Medicine Lundquist Institute for Biomedical Innovation at Harbor-University of California Los Angeles Medical Center, Los Angeles, CA, United States
- 7Department of Neurology, University of Virginia, Charlottesville, VA, United States
Background: We evaluated comprehension and application of the 2015 neuromyelitis optica spectrum disorder (NMOSD) criteria core elements by neurologists in Latin America (LATAM) who routinely diagnose and care for NMOSD patients by (i) identifying typical/suggestive NMOSD syndromes, (ii) detecting typical MRI NMOSD lesions and meeting MRI dissemination in space (DIS) criteria, and (iii) evaluating historical symptoms suggestive of NMOSD.
Methods: We conducted an anonymous, voluntary, self-administered web- and case-based survey cross-sectional study from October 2023 to January 2024 of neurologists identified through the LACTRIMS database. Questions were presented first through iterative clinical cases or imaging, followed by questions directly evaluating comprehension of definitions. “Correct” responses were based on the 2015 criteria and adjudicated by the consensus of the experts leading the project.
Results: A total of 106 neurologists (60.3% female; mean age: 46.6 ± 12.5 years) were included. Between 10.4% and 49.1% of neurologists inaccurately identified clinical or paraclinical aspects for DIS and 32.1% accurately identified the three non-cardinal (brainstem, diencephalic, and cerebral) syndromes for seronegative patients. Between 35.8% and 64.1% of neurologists identified the “optimal timing” of AQP4-IgG testing (e.g., during an attack or before receiving immunosuppressant treatments, among others); 56.6% considered live cell-based assay as the gold standard method for serological testing. Most neurologists accurately identified typical NMOSD MRI lesions, but periventricular, juxtacortical/cortical, fluffy infratentorial, corticospinal tract, and hypothalamic lesions were frequently misidentified.
Conclusion: Clinical scenarios were identified where the 2015 NMOSD criteria were susceptible to misinterpretation and misapplication by expert neurologists in LATAM. Implementing collaborative educational initiatives could improve NMOSD diagnosis and raise patient care standards.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare but debilitating inflammatory and immune-mediated disease of the central nervous system (CNS) linked to the presence of disease-specific, pathogenic aquaporin 4-antibodies (AQP4-IgG) in the majority of patients (80%) (1, 2). Differentiating NMOSD from its mimics is critical to reduce misdiagnosis, particularly in patients with negative or unknown AQP4-IgG testing (3). Multiple sclerosis (MS) and other immune-mediated conditions such as myelin oligodendrocyte glycoprotein antibody (MOG-IgG)-associated disease (MOGAD) are important differential diagnoses in clinical practice (4–6). Other rare or different conditions including infectious, metabolic, and vascular diseases can also present with similar symptoms at disease onset or during follow-up, such as transverse myelitis (TM), optic neuritis (ON), and attacks on the brainstem and/or the brain (6, 7). There may also be overlapping paraclinical and neuroradiological features observed, especially with MOGAD (7, 8). However, the 2015 NMOSD criteria did not explicitly address the differentiation of MOGAD from AQP4-IgG-positive NMOSD or MS. Overall, the diagnosis of NMOSD depends on successive clinical assessments (1, 2). An early diagnosis of NMOSD is crucial to improve long-term patient outcomes. There has been a concerted effort over the past 25 years to update and improve diagnostic criteria to facilitate earlier and more precise diagnosis (1, 2). Certain magnetic resonance imaging (MRI) lesions have been reported to be typical or suggestive of NMOSD, such as those occurring in the dorsal medulla and hypothalamus (1, 2). Among others, 37% of NMOSD patients may present with lesions that are characteristic of MS, fulfilling the 2017 criteria for dissemination in space (DIS) (9). Making a rapid and accurate diagnosis of NMOSD is crucial, as delays in acute or long-term therapeutic strategies may result in worsened prognosis and disability in NMOSD (10). AQP4-IgG and MOG-IgG tests are important for diagnosing antibody-mediated disorders, but they are not easily accessible in all countries (11). Thus, results are often delayed, limiting the contribution of this test to the differential diagnosis process (12). Unlike NMOSD, no specific biomarker for MS diagnosis has been identified in clinical practice (4). In this context, patients without MS (e.g., NMOSD) can be misdiagnosed with MS, despite following validated international diagnostic criteria (13). The NMOSD diagnostic criteria have evolved over time, including better characterization of serum tests, neuroimaging lesions, and well-defined clinical core characteristics (1, 2). NMOSD diagnosis is mainly based on accurately interpreting symptoms, disease history, neurologic examination, laboratory tests, and neuroradiological information (2). In addition, the 2015 NMOSD diagnostic criteria emphasized on the need to rule out any “other better explanation or alternative diagnoses” for the clinical scenario before making a definitive diagnosis of NMOSD (2). Application of these criteria in the Latin America (LATAM) population has resulted in a 62.5% increase in incidence of NMOSD diagnosis as compared to the 2006 NMO criteria, with a shorter median time to diagnosis (14). However, a recently published study found that 12% (56 out of 469 with an initial diagnosis other than NMOSD) of LATAM patients who had been referred for care with another previously established diagnosis had been misdiagnosed (i.e., the incorrect diagnosis of patients who truly have NMOSD) (13). This relatively low rate of misdiagnosis is a considerable improvement from historical rates of misdiagnosis, which were 50% or greater (15–19). Nonetheless, in that large LATAM cohort, misinterpretation and misapplication of core elements (clinical and neuroradiological aspects) of the 2015 NMOSD diagnostic criteria led to misdiagnosis of NMOSD (13). Consequently, in many cases, NMOSD misdiagnosis was associated with inappropriate treatment, leading to suboptimal patient benefit that potentially worsened disability and enabled relapses, along with promoting the unnecessary use of health resources in the region. However, there are no studies evaluating knowledge gaps for application of core elements of the 2015 NMOSD criteria by attending clinicians in LATAM countries. In this study, we evaluated comprehension and application of the 2015 NMOSD criteria core elements by neurologists in LATAM who routinely diagnose and care for NMOSD patients.
Methods
A cross-sectional study was conducted from October 2023 to January 2024. An anonymous, voluntary, self-administered web- and case-based survey was conducted by coordinating investigators of the study (the survey is displayed in the Supplementary Materials).
The survey was available online for 4 months and was developed in both Spanish and English. Before distribution via email, the pilot English survey was reviewed by an international expert in NMOSD (B.W.) to ensure that the items accurately addressed the research questions. Additionally, five neurologists from LATAM tested the survey, who were not involved in designing the survey and did not participate in the study. Participants were identified through the Latin American Committee for Treatment and Research in MS (LACTRIMS) database, including several countries’ working groups. Brazilian neurologists received the survey in English, and the rest of the participants received the survey in Spanish. Responses were securely collected online, via Google forms. Reminder emails were sent every 2 weeks via LACTRIMS mailing.
Participants were instructed not to review the NMOSD criteria while performing the survey. “Correct or accuracy” responses were based on the 2015 diagnostic criteria and adjudication by the consensus of the experts leading the project. To minimize learning effect, questions designed to evaluate criteria interpretation and application were presented first through iterative hypothetical and fictional clinical case examples for the survey using multiple choice or imaging, followed by questions directly evaluating comprehension of definitions. Except for questions with ordinal responses, potential responses were randomly ordered for each participant.
The purpose of this survey was to assess the understanding, interpretation, and application of the key elements of the 2015 NMOSD diagnostic criteria by (i) identifying typical or suggestive NMOSD syndromes, (ii) detecting typical MRI NMOSD lesions and meeting MRI DIS criteria, and (iii) evaluating historical symptoms suggestive of NMOSD.
This study was approved by the Independent Ethics Committee of the “Hospital Alemán de Buenos Aires”. All participants signed an electronic informed consent form before data collection.
For this study, we followed the Guidelines from Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), as shown in the Supplementary Materials.
Statistical analysis
The data analysis was carried out using the STATA program. Descriptive statistics were presented as mean ± standard deviation (SD), median value, and percentages to evaluate the diversity of the cohort study. Because of the exploratory nature of the study, no sample size calculations were performed.
Results
We surveyed 123 participants, including 3 ophthalmologists. Fourteen surveys were incomplete in more than 60% of their content. A total of 106 responses from neurologists were included and analyzed. Incomplete surveys and those from ophthalmologists were excluded (N = 17). The survey was sent to approximately 450 LATAM neurologists; however, we were unable to calculate a response rate due to both design and diffusion of the survey (total number of recipients of the study survey email invitation was unknown).
Neurologist demographic profile
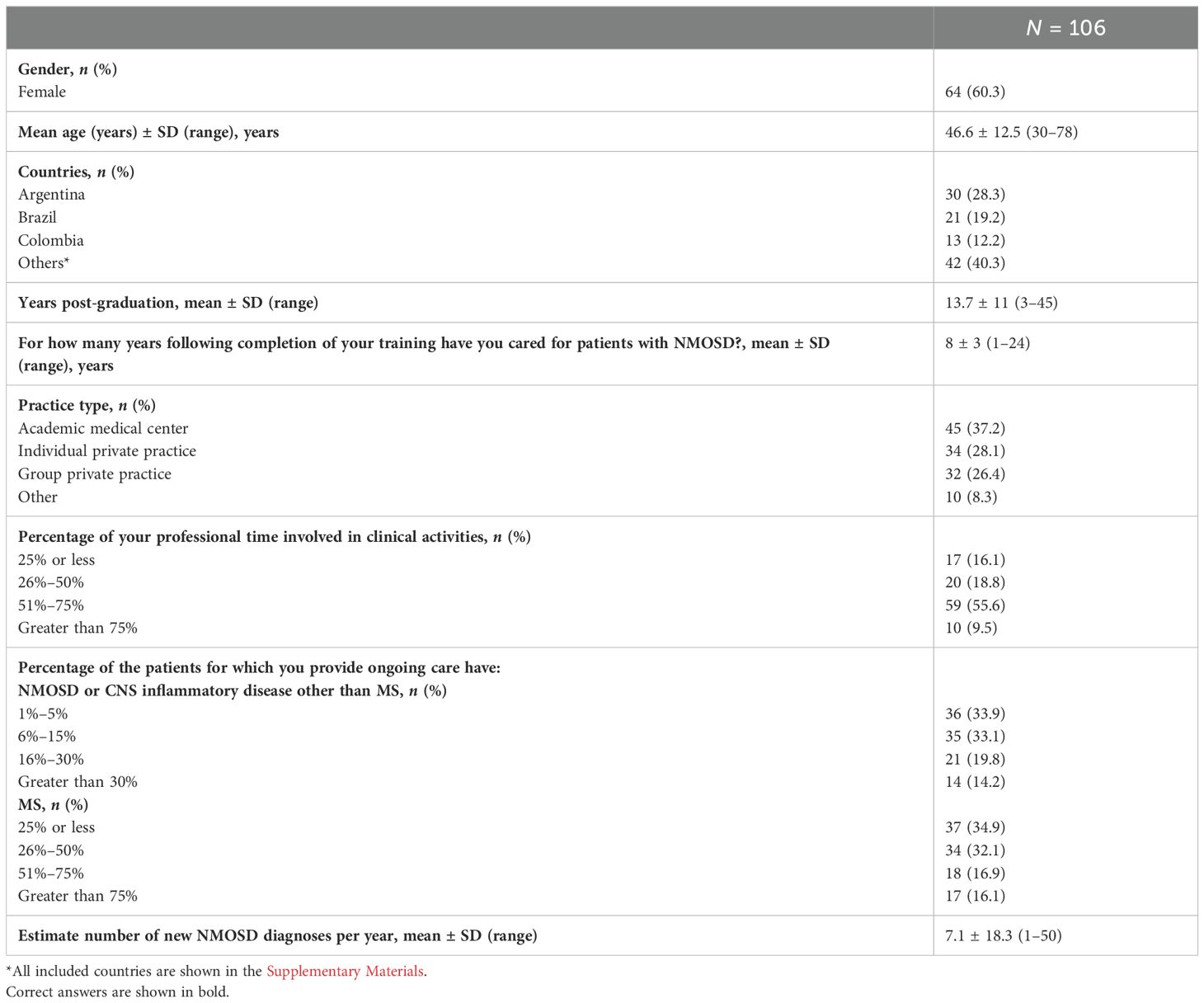
As indicated in Table 1, 60.3% of participants were women, with a mean age of 46.6 ± 12.5 years. Respondents were from Argentina (28.3%), Brazil (19.2%), Colombia (12.2%), and 11 additional countries (40.3%) (Supplementary Table 1). Respondents were 13.7 ± 11 years post-graduation, of which 8 ± 3 years were dedicated to NMOSD healthcare after completing their training. On average, neurologists diagnose 7.1 ± 18.3 NMOSD new cases per year (ranging from 1 to 50), with most of them (37.2%) practicing in an academic medical center.
Access to educational meeting or training from LATAM neurologists
As shown in Supplementary Table 2, 64 (60.3%) participants underwent training focused on neuroimmunology, of whom 46.9% received training in referral centers from LATAM. The majority of LATAM neurologists expressed a desire to access international educational meetings/training (78.3%), but a significant portion of them (70.8%) are unable to do so for financial reasons. Most neurologists (91.5%) find the 2015 NMOSD criteria easy to comprehend and apply in clinical practice, with most of them (87.7%) carefully reviewing the manuscript for the 2015 IPND NMOSD criteria more than twice, at least 1 month apart.
Comprehension and application of the 2015 NMOSD criteria
The initial case vignette (Case #1) featured a patient with a history of neuromyelitis (ON+TM). She was currently experiencing myelopathy associated with LETM on spinal MRI, illustrating a typical case of NMOSD (Table 2). Brain MRI did not reveal any lesions. Most (98.1%) neurologists accurately recognized that this patient exhibited findings commonly observed in NMOSD patients seropositive for AQP4-IgG. Additionally, 89.6% of neurologists accurately identified the concept of DIS in a patient with a history of confirmed ON and TM with poor recovery, and 95.5% correctly identified the application of the 2015 NMOSD criteria for seronegative patients. Nonetheless, participants were asked if a history of ON would not have been present, but VEP was prolonged (despite the absence of clinical symptoms in that eye), which would have satisfied DIT in this patient, and 49.1% incorrectly responded that VEP prolongation in the absence of objective evidence of an attack of ON would satisfy the criteria for diagnosis.
As shown in Table 3, the majority (86.8%) of LATAM neurologists accurately noted that the radiological presentation was uncommon and atypical [Case #2; history of ON with current short transverse myelitis (STM) on MRI]. However, they accurately mentioned that this does not exclude the diagnosis of NMOSD. In the other case, most participants accurately pointed out that the clinical presentation was typical for NMOSD [Case #3, patients presenting with area postrema syndrome (APS), which is a core clinical characteristic]. Neurologists accurately identified that both patients should be tested for AQP4-IgG to reach NMOSD diagnosis.
Identification of typical clinical syndromes and MRI features for NMOSD
Most neurologists inaccurately identified all three non-cardinal/common core clinical characteristics (brainstem + diencephalic + cerebral syndromes) for NMOSD to accurately evaluate the main clinical manifestation supporting a diagnosis of NMOSD in AQP4-IgG-seronegative cases. Over 77% of LATAM neurologists correctly identified typical clinical presentations of NMOSD or highly suggestive for NMOSD (5 out of 14), while atypical presentations were chosen by at least 33% (Table 4). Many participants missed typical/suggestive NMOSD MRI lesions, which are also additional MRI requirements for NMOSD with negative AQP4-IgG and NMOSD with unknown AQP4-IgG status, except for ON, myelitis, and APS lesions. Simultaneously, we assessed the same concepts regarding AQP4-IgG, revealing that between 35.8% and 64.1% of neurologists correctly identified the optimal moment to request AQP4-IgG (e.g., during an attack or before receiving immunosuppressant treatments, among others) and 56.6% considered the live cell-based assay as the gold standard method to improve the sensitivity and to avoid false-negative cases. However, tissue-based indirect immunofluorescence (IIF) (4.7%) and enzyme-linked immunosorbent assay (ELISA) (4.7%) were incorrectly selected as the gold standard method. Regarding the clinical practice aspects of treating neurologists before making a new diagnosis of NMOSD, most participants request for general lab tests and spinal cord MRI, while approximately half undergo CSF testing and orbital MRI (Supplementary Table 3). The proportion of responses for AQP4-IgG, typical clinical syndromes, and MRI features for NMOSD are summarized in Table 4.
Identification and comprehension of MRI features for NMOSD
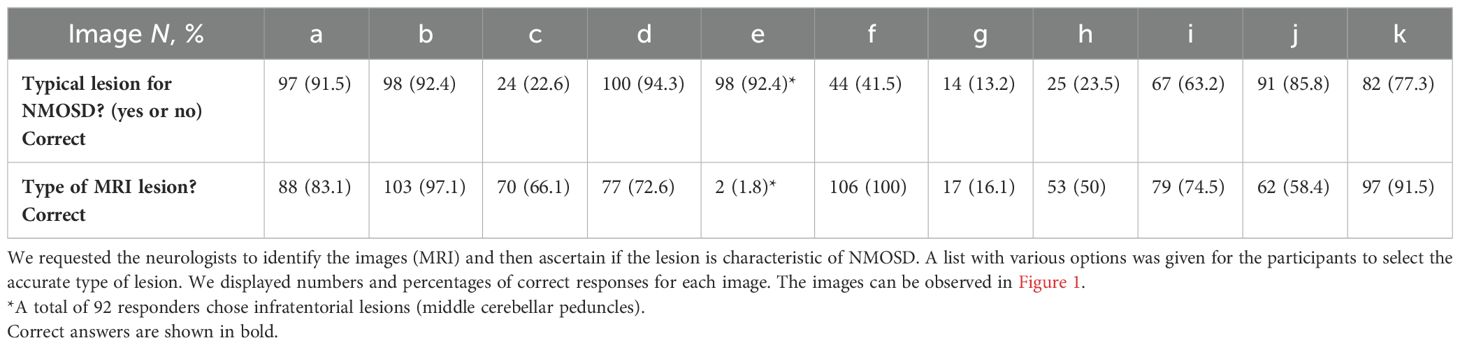
Neurologists were asked to identify typical lesions for NMOSD and type of lesions on 11 T2/FLAIR and T1 with and without contrast conventional MRI based on real cases. As shown in Table 5 and Figure 1, most neurologists accurately identified typical NMOSD MRI lesions, but periventricular, juxtacortical/cortical, fluffy infratentorial, corticospinal tract, and hypothalamic lesions were frequently misidentified and misclassified as typical or atypical for NMOSD (Table 5). Based on the lesion type and its classification as typical or atypical, we observed adequate recognition of lesions associated with the three cardinal syndromes (ON, TM, and APS). However, while all neurologists correctly identified LETM, only 41.5% classified it as typical of NMOSD. Similarly, 63.2% classified the lesion in the APS as typical of NMOSD.
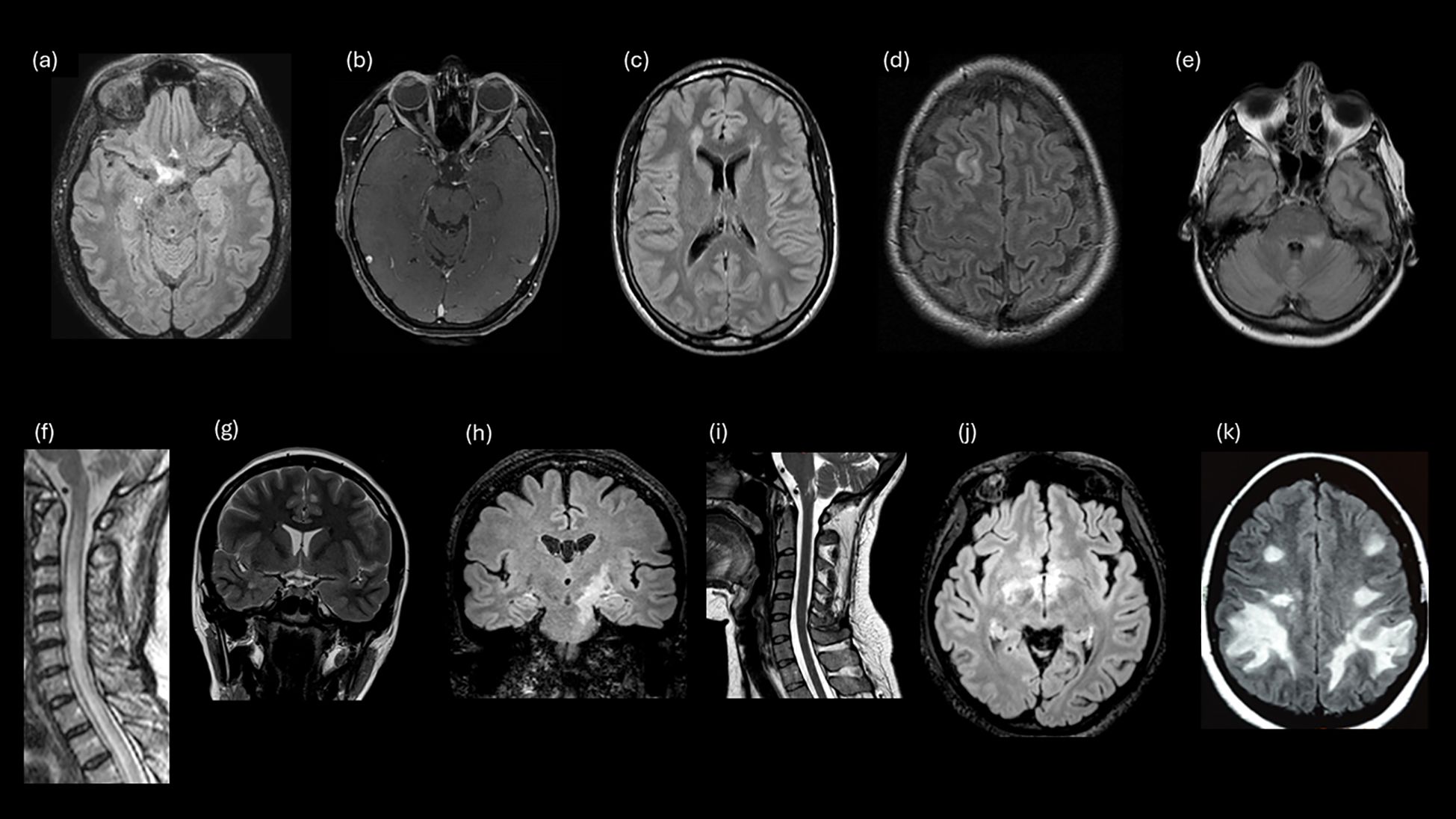
Figure 1. Examples of study survey images that evaluated participant knowledge for MRI lesions. (A) Lesion involving optic chiasm and posterior ON. (B) LEON (longitudinally extensive left optic neuritis). (C) Periventricular (MS). (D) Juxtacortical (MS). (E) Fluffy lesion and poorly demarcated lesion (MOGAD). (F) LETM. (G) Cortical lesion lob temporal (MS). (H) Corticospinal tract lesion. (I) Lesion involving the area postrema. (J) Hypothalamic lesion. (K) Large, confluent, bilateral subcortical or deep white matter lesions.
Discussion
Over the past decade, there has been a profound improvement in clinical diagnosis and therapy for patients with NMOSD. However, room for improvement remains, particularly with respect to rapid and accurate diagnosis. In this study, we evaluated comprehension and application of the 2015 NMOSD criteria core elements by neurologists in LATAM who routinely diagnose and care for NMOSD patients. The findings revealed clinical scenarios with propensity for misunderstanding and/or misapplication of these 2015 NMOSD criteria. Areas of particular vulnerability to misdiagnosis of NMOSD syndromes were recognition of prototypical MRI NMOSD lesions, meeting MRI DIS criteria, and evaluating historical symptoms suggestive of NMOSD. In addition, most neurologists were less accurate in identifying all three non-cardinal core clinical characteristics for NMOSD (i.e., brainstem, diencephalic, and cerebral syndromes). Supporting the view that interpretation of imaging is a particularly challenging aspect of diagnosis, many of the study neurologists did not accurately identify some typical/suggestive NMOSD MRI lesions in AQP4-IgG-seronegative and unknown status, except for ON, myelitis, or APS lesions (2). For example, periventricular, juxtacortical/cortical, fluffy infratentorial, corticospinal tract, and hypothalamic lesions were frequently misidentified and misclassified in MRI. Recognizing clinical and radiologic requirements is important, particularly in AQP4-IgG-seronegative or in those with unknown results, because the 2015 diagnostic criteria require at least two core clinical characteristics supported by specific MRI findings to establish a diagnosis (2–6). One of these characteristics must be a cardinal manifestation such as ON, TM (with LETM MRI lesion), or APS (with associated medullary MRI lesion). Therefore, the 2015 criteria can be satisfied by a single clinical event even in an AQP4-IgG-seronegative patient with the requisite areas of neuroanatomic involvement (2).
Integration of clinical, serological, and radiological findings is essential in diagnosing NMOSD and related diseases. Alternative diagnoses and red flags that require extensive investigation should be considered before a conclusive diagnosis of NMOSD (6, 15) to avoid misdiagnoses (12). The primary consideration for differential diagnosis in NMOSD patients seronegative for both AQP4-IgG and MOG-IgG measured by CBA, termed “double-seronegative”, is MS (16). False-negative results can be observed depending on the used methodology (e.g., live CBA vs. others) and the timing of when the sample was obtained for serological analysis (e.g., before or after PLEX, corticosteroids, or receiving immunotherapy) (2, 15). In this context, many neurologists have correctly identified the best assay for AQP4-IgG (i.e., live CBA) and the optimal timing to obtain the serum sample (e.g., during an attack). However, a study in LATAM has reported that the AQP4-IgG test was accessible only in 54% of countries and the MOG-IgG test was accessible only in 42% of countries (11). Such limitation in access to testing for AQP4-IgG and MOG-IgG poses challenges to the diagnosis of NMOSD in clinical settings (17). A recent study evaluated the frequency and factors associated with misdiagnosis of NMOSD in a cohort of patients from LATAM (13). The most frequent alternative diagnoses were MS (66.1%), clinically isolated syndrome (17.9%), and cerebrovascular disease (3.6%). NMOSD misdiagnosis was determined by MS/NMOSD specialists in 33.9% of cases. In addition, 86% of misdiagnosed patients were found to have an atypical MS syndrome; 50% of misdiagnosed patients had red flags (13). A study conducted in Poland, involving 1,112 patients with a suspected or confirmed diagnosis of acute or subacute onset of neurological deficits, examined the factors influencing the underdiagnosis of NMOSD (18); 18 patients had an established diagnosis of NMOSD, but 15 patients (83%) were initially misdiagnosed. The most common misdiagnosis was associated with idiotypic, monophasic, or non-specific demyelinating disease and MS. Atypical presentation, prolonged time to symptom development, overlap with other conditions, especially MS, and incorrect application or interpretation of diagnostic criteria were the main factors contributing to these findings. Additionally, variables such as gender, age of onset, and age of diagnosis may also play a significant role in the misdiagnosis process. In another study involving 199 NMOSD patients from the US, 71 were initially misdiagnosed (19). Factors associated with misdiagnosis included prolonged APS without other neurological symptoms, longer time to see a specialist, and delays in MRI acquisition. A greater proportion of misdiagnosed patients were identified with a negative live-CBA AQP4-IgG serum test result, 13/13 (100%) compared with 22/114 (19.3%). The time between first negative and subsequent positive AQP4-IgG tests was longer for misdiagnosed patients (19). These results are in line with surveys of MS specialists and non-specialist neurologists in the UK (20) and the US (21, 22) regarding the application of McDonald diagnostic criteria for MS that revealed numerous challenges related to understanding typical MS syndromes and applying “objective evidence” of a CNS MS-typical lesion, along with a low level of implementation in clinical practice.
Recently, a systematic review was conducted to identify reports of patients with non-demyelinating disorders that mimicked or were misdiagnosed with NMOSD. A total of 68 patients were included and 56 (82%) patients did not fulfill the 2015 NMOSD diagnostic criteria (7). The clinical syndromes misinterpreted for NMOSD were myelopathy (41%), myelopathy + optic neuropathy (41%), optic neuropathy (6%), or other (12%). Alternative etiologies included genetic/metabolic disorders, neoplasms, infections, vascular disorders, spondylosis, and other immune-mediated disorders. Common red flags associated with misdiagnosis were lack of cerebrospinal fluid (CSF) pleocytosis (57%), lack of response to immunotherapy (55%), progressive disease course (54%), and lack of MRI gadolinium enhancement (31%) (7). The most common conditions leading to misdiagnosis such as MS lack specific diagnostic biomarkers. The ultimate diagnosis relies on accurately recognizing and interpreting clinical symptoms, MRI results, and clinical expertise. As previously mentioned, misdiagnosis of NMOSD has not only been observed in LATAM; international data have highlighted that missed diagnosis or misdiagnosis of NMOSD also occur in other regions of the world.
The N-MOmentum trial (23) included both AQP4-IgG-seropositive and -seronegative patients to encompass a wide range of patient phenotypes diagnosed with NMO or NMOSD. AQP4-IgG-seronegative participants were required to meet the NMO clinical threshold according to the 2006 criteria. The independent eligibility committee (EC) assessed 50 AQP4-IgG-seronegative cases and determined that only 18 (36.0%) met the 2006 criteria; 10 patients were unanimously confirmed by members of the committee. Nearly two-thirds of potential AQP4 participants reviewed by the EC were deemed not eligible for randomization, despite all participants having an existing diagnosis of NMOSD at screening. Key reasons for exclusion included inadequate history of ON or myelitis, unclear MRI images, absence of LETM evidence, and inaccuracies in AQP4-IgG tests. Alternative diagnoses such as MS or sarcoidosis also led to exclusion. The primary reason for exclusion was the lack of LETM on MRI in 75% of cases (24 out of 50 AQP4-IgG-seronegative participants). Seven of 18 participants meeting the 2006 criteria and included in the clinical trial were later diagnosed with MOGAD (24); the 2015 NMOSD diagnostic criteria were developed before MOGAD was established as a disease distinct from AQP4-IgG-associated NMOSD. These observations underscore diagnostic challenges in seronegative NMOSD even when considered by an expert selection committee.
In the present study, we considered lack of awareness and education about the disease as a primary explanation for misunderstanding and misapplication of the diagnostic criteria for NMOSD. Approximately 60% of responses in this study were those of neurologists with subspeciality training in neuroimmunology. NMOSD is a rare disease, and repeated training and experience are required to appropriately identify clinical symptoms, imaging, and laboratory results to adequately diagnose this condition. A potential area for improvement in this respect is collaboration between neuroradiologists and neurologists for interpretation of imaging results in the differential diagnosis of NMOSD. Another explanation is overlap with other diseases that results in mistakes in the diagnosis of the disease. NMOSD can present symptoms and radiologic findings that overlap with other conditions such as MS and other neuroinflammatory disorders, thus explaining part of the findings (8). An international study aimed to evaluate if expert clinicians approach the diagnosis and treatment of overlapping NMO/MS patients similarly. Twelve carefully chosen AQP4-IgG-negative patients represented various clinical presentations seen in an NMO clinic. A total of 27 experts in NMO and MS scrutinized detailed clinical vignettes, along with pertinent imaging and lab results. Diagnoses fell into four groups (NMO, MS, indeterminate, and other). Overall clinician agreement on diagnosis was moderate (po = 0.51), with individual patient consensus varying from 0.25 to 0.73. For nine cases, opinions swayed between NMOSD and MS diagnoses, while others were labeled as monophasic LETM, acute disseminated encephalomyelitis (ADEM), or recurrent isolated ON (RION). Key NMO features, like LETM, held more sway in diagnosis than those akin to MS, such as STM (25). Additionally, a limitation in the access to diagnostic tests and procedures in some areas of LATAM may explain the frequency of misdiagnosis as well (26). Considering the previous findings, a need for awareness and continuous education in NMOSD among healthcare professionals in LATAM is crucial for improving diagnoses. This could involve training programs, public awareness campaigns, and international collaborations to share knowledge and resources (27, 28).
It is important to note the limitations of this study that may have impacted the validity and generalizability of the research, including its cross-sectional design, which precluded evaluation of changes over time; causality could not be evaluated. Nonetheless, we utilized methods similar to those previously employed in many studies (20–22). Sampling bias, stemming from the recruitment of participants from the LACTRIMS database and specific working groups from different neurological associations, may have skewed the study’s findings by favoring individuals already immersed in NMOSD research. This approach may not fully represent the broader population of neurologists across LATAM, potentially underestimating the true understanding and application of NMOSD diagnostic criteria among practitioners who are less actively engaged in research initiatives. We recognize that interpretation of imaging results can be challenging and is often shaped by subjective experience, particularly among non-radiologists. Thus, in the absence of objective assessment such as artificial intelligence methods, correctness of imaging interpretation is inherently subjective. Additionally, self-selection bias, inherent in the voluntary nature of participation, may have influenced the study’s results by potentially attracting neurologists with a higher level of experience or interest in NMOSD. Such individuals may have been more inclined to participate due to their familiarity with the topic or their desire to contribute to research efforts in the field. Taking these limitations into account provides a comprehensive perspective on the potential constraints and implications of the study findings. Despite these limitations, we consider that this is a valuable first study examining comprehension and application of the 2015 NMOSD criteria core elements by neurologists in LATAM.
In conclusion, our research found discrepancies among specialists in LATAM regarding the interpretation of clinical and radiological features related to the 2015 NMOSD criteria. Along with refining diagnostic criteria and their interpretation, there is a need to implement targeted continuing medical education campaigns and training initiatives focused specifically on NMOSD for healthcare professionals, including physicians, nurses, and allied health professionals. Additionally, it is important to consider improving education for other frontline clinicians most likely to encounter patients presenting with NMOSD, including emergency room or urgent care, ophthalmology, optometry, gastroenterology, or family practice healthcare providers. A multidisciplinary approach that facilitates collaboration across various specialties, including neurology, ophthalmology, immunology, and radiology, is important. Our study underscores the challenges LATAM specialists face in accessing training programs. Improving educational opportunities for reducing diagnostic errors and enhancing patient care standards is also important not only in LATAM but also worldwide.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Independent Ethics Committee of the “Hospital Alemán de Buenos Aires”. All participants signed an electronic informed consent form before data collection.
Author contributions
EC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JR: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. RA: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. MY: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. BW: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guthy-Jackson Charitable Foundation.
Acknowledgments
We would like to thank all colleagues who participated in the survey (please see the Supplementary Materials).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1515481/full#supplementary-material
References
1. Carnero Contentti E, Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflamm. (2021) 18:208. doi: 10.1186/s12974-021-02249-1
2. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
3. Carnero Contentti E, Okuda DT, Rojas JI, Chien C, Paul F, Alonso R. MRI to differentiate multiple sclerosis, neuromyelitis optica, and myelin oligodendrocyte glycoprotein antibody disease. J Neuroimaging. (2023) 33:688–702. doi: 10.1111/jon.13137
4. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
5. Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. (2023) 22:268–82. doi: 10.1016/S1474-4422(22)00431-8
6. Solomon AJ, Arrambide G, Brownlee WJ, Flanagan EP, Amato MP, Amezcua L, et al. Differential diagnosis of suspected multiple sclerosis: an updated consensus approach. Lancet Neurol. (2023) 22:750–68. doi: 10.1016/S1474-4422(23)00148-5
7. Zara P, Dinoto A, Carta S, Floris V, Turilli D, Budhram A, et al. Non-demyelinating disorders mimicking and misdiagnosed as NMOSD: a literature review. Eur J Neurol. (2023) 30:3367–76. doi: 10.1111/ene.15983
8. Juryńczyk M, Craner M, Palace J. Overlapping CNS inflammatory diseases: differentiating features of NMO and MS. J Neurol Neurosurg Psychiatry. (2015) 86:20–5. doi: 10.1136/jnnp-2014-308984
9. Cacciaguerra L, Meani A, Mesaros S, Radaelli M, Palace J, Dujmovic-Basuroski I, et al. Brain and cord imaging features in neuromyelitis optica spectrum disorders. Ann Neurol. (2019) 85:371–84. doi: 10.1002/ana.25411
10. Palace J, Lin DY, Zeng D, Majed M, Elsone L, Hamid S, et al. Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrum disorders. Brain. (2019) 142:1310–23. doi: 10.1093/brain/awz054
11. Rojas JI, Gracia F, Patrucco L, Alonso R, Carnero Contentti E, Cristiano E. Multiple sclerosis and neuromyelitis optica spectrum disorder testing and treatment availability in Latin America. Neurol Res. (2021) 43:1081–6. doi: 10.1080/01616412.2021.1949686
12. Carnero Contentti E, Rojas JI, Cristiano E, Marques VD, Flores-Rivera J, Lana-Peixoto M, et al. Latin American consensus recommendations for management and treatment of neuromyelitis optica spectrum disorders in clinical practice. Mult Scler Relat Disord. (2020) 45:102428. doi: 10.1016/j.msard.2020.102428
13. Carnero Contentti E, López PA, Criniti J, Alonso R, Silva B, Luetic G, et al. Frequency of NMOSD misdiagnosis in a cohort from Latin America: Impact and evaluation of different contributors. Mult Scler. (2023) 29:277–86. doi: 10.1177/13524585221136259
14. Carnero Contentti E, Soto de Castillo I, Daccach Marques V, López PA, Antunes Barreira A, Armas E, et al. Application of the 2015 diagnostic criteria for neuromyelitis optica spectrum disorders in a cohort of Latin American patients. Mult Scler Relat Disord. (2018) 20:109–14. doi: 10.1016/j.msard.2018.01.001
15. Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord. (2017) 10:265–89. doi: 10.1177/1756285617709723
16. Jarius S, Aktas O, Ayzenberg I, Bellmann-Strobl J, Berthele A, Giglhuber K, et al. Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis. J Neurol. (2023) 270:3341–68. doi: 10.1007/s00415-023-11634-0
17. Ricardo A, Carnero Contentti E, Anabel SB, Adrian LP, Orlando G, Fernando H, et al. Decision-making on management of ms and nmosd patients during the COVID-19 pandemic: A latin american survey. Mult Scler Relat Disord. (2020) :44:102310. doi: 10.1016/j.msard.2020.102310
18. Szewczyk AK, Papuć E, Mitosek-Szewczyk K, Woś M, Rejdak K. NMOSD-diagnostic dilemmas leading towards final diagnosis. Brain Sci. (2022) 12:885. doi: 10.3390/brainsci12070885
19. Smith AD, Moog TM, Burgess KW, McCreary M, Okuda DT. Factors associated with the misdiagnosis of neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. (2023) 70:104498. doi: 10.1016/j.msard.2023.104498
20. Lumley R, Davenport R, Williams A. Most Scottish neurologists do not apply the 2010 McDonald criteria when diagnosing multiple sclerosis. J R Coll Phys Edinb. (2015) 45:23–6. doi: 10.4997/JRCPE.2015.106
21. Solomon AJ, Kaisey M, Krieger SC, Chahin S, Naismith RT, Weinstein SM, et al. Multiple sclerosis diagnosis: Knowledge gaps and opportunities for educational intervention in neurologists in the United States. Mult Scler. (2022) 28:1248–56. doi: 10.1177/13524585211048401
22. Solomon AJ, Pettigrew R, Naismith RT, Chahin S, Krieger S, Weinshenker B. Challenges in multiple sclerosis diagnosis: Misunderstanding and misapplication of the McDonald criteria. Mult Scler. (2021) 27:250–8. doi: 10.1177/1352458520910496
23. Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. (2019) 394:1352–63. doi: 10.1016/S0140-6736(19)31817-3
24. Marignier R, Pittock SJ, Paul F, Kim HJ, Bennett JL, Weinshenker BG, et al. AQP4-IgG-seronegative patient outcomes in the N-MOmentum trial of inebilizumab in neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. (2022) 57:103356. doi: 10.1016/j.msard.2021.103356
25. Juryńczyk M, Weinshenker B, Akman-Demir G, Asgari N, Barnes D, Boggild M, et al. Status of diagnostic approaches to AQP4-IgG seronegative NMO and NMO/MS overlap syndromes. J Neurol. (2016) 263:140–9. doi: 10.1007/s00415-015-7952-8
26. Rivera VM, Hamuy F, Rivas V, Gracia F, Rojas JI, Bichuetti DB, et al. Status of the neuromyelitis optica spectrum disorder in Latin America. Mult Scler Relat Disord. (2021) 53:103083. doi: 10.1016/j.msard.2021.103083
27. Mateen FJ. Rectifying global inequities in neuromyelitis optica diagnosis and treatment. Mult Scler. (2023) 29:932–5. doi: 10.1177/13524585231179108
Keywords: neuromyelitis optica spectrum disorder, diagnosis, misdiagnosis, criteria, MRI
Citation: Carnero Contentti E, Rojas JI, Alonso R, Yeaman MR and Weinshenker BG (2024) Application and interpretation of core elements of the 2015 NMOSD diagnostic criteria in routine clinical practice. Front. Immunol. 15:1515481. doi: 10.3389/fimmu.2024.1515481
Received: 22 October 2024; Accepted: 21 November 2024;
Published: 13 December 2024.
Edited by:
Joachim Havla, Ludwig Maximilian University of Munich, GermanyReviewed by:
Paulus Stefan Rommer, Medical University of Vienna, AustriaAchim Berthele, Technical University of Munich, Germany
Copyright © 2024 Carnero Contentti, Rojas, Alonso, Yeaman and Weinshenker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edgar Carnero Contentti, anVuaW9yLmNhcm5lcm9AaG90bWFpbC5jb20=; ZWNhcm5lcm9jb250ZW50dGlAaG9zcGl0YWxhbGVtYW4uY29t
 Edgar Carnero Contentti
Edgar Carnero Contentti Juan I. Rojas
Juan I. Rojas Ricardo Alonso
Ricardo Alonso Michael R. Yeaman5,6
Michael R. Yeaman5,6