
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Immunol. , 03 December 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1514836
This article is a correction to:
Anti-cancer immune effect of human colorectal cancer neoantigen peptide based on MHC class I molecular affinity screening
 Siyu Zhang1
Siyu Zhang1 Changxin Huang1*
Changxin Huang1* Yongqiang Li1
Yongqiang Li1 Zhaoyang Li1
Zhaoyang Li1 Ying Zhu2
Ying Zhu2 Lili Yang1
Lili Yang1 Haokun Hu1
Haokun Hu1 Quan Sun1
Quan Sun1 Mengmeng Liu3
Mengmeng Liu3 Songqiang Cao4
Songqiang Cao4A Corrigendum on
Anti-cancer immune effect of human colorectal cancer neoantigen peptide based on MHC class I molecular affinity screening
By Zhang S, Huang C, Li Y, Li Z, Zhu Y, Yang L, Hu H, Sun Q, Liu M and Cao S (2024) Front. Immunol. 15:1473145. doi: 10.3389/fimmu.2024.1473145
In the published article, there was two error in affiliation(s) 2.
1. Instead of “3Department of Psychiatry and Psychology, 155 Hospital of Kaifeng City, Hangzhou, China”, it should be “3Department of Psychiatry and Psychology, 155 Hospital of Kaifeng City, Kaifeng, China”.
2. Instead of “4Department of Urology, Huaihe Hospital, Henan University, Kaifeng City, China”, it should be “4Department of Urology, Huaihe Hospital of Henan University, Kaifeng, China”.
In the published article, there was an error in the legend for Figure 5 as published. “The percentages of T, B and NK cells in the mixed cells were identified by flow cytometry in control group shows the experimental group after adding peptides. The proportions of CD3+CD4+ double positive (CD4+ T cells), CD3+CD8+ double positive (CD8+ T cells), CD3-CD56 + (NK cells) and CD3-CD19 + (B cells) were 7.8%, 14.63%, 7.81% and 1.40%, respectively” The corrected legend appears below.
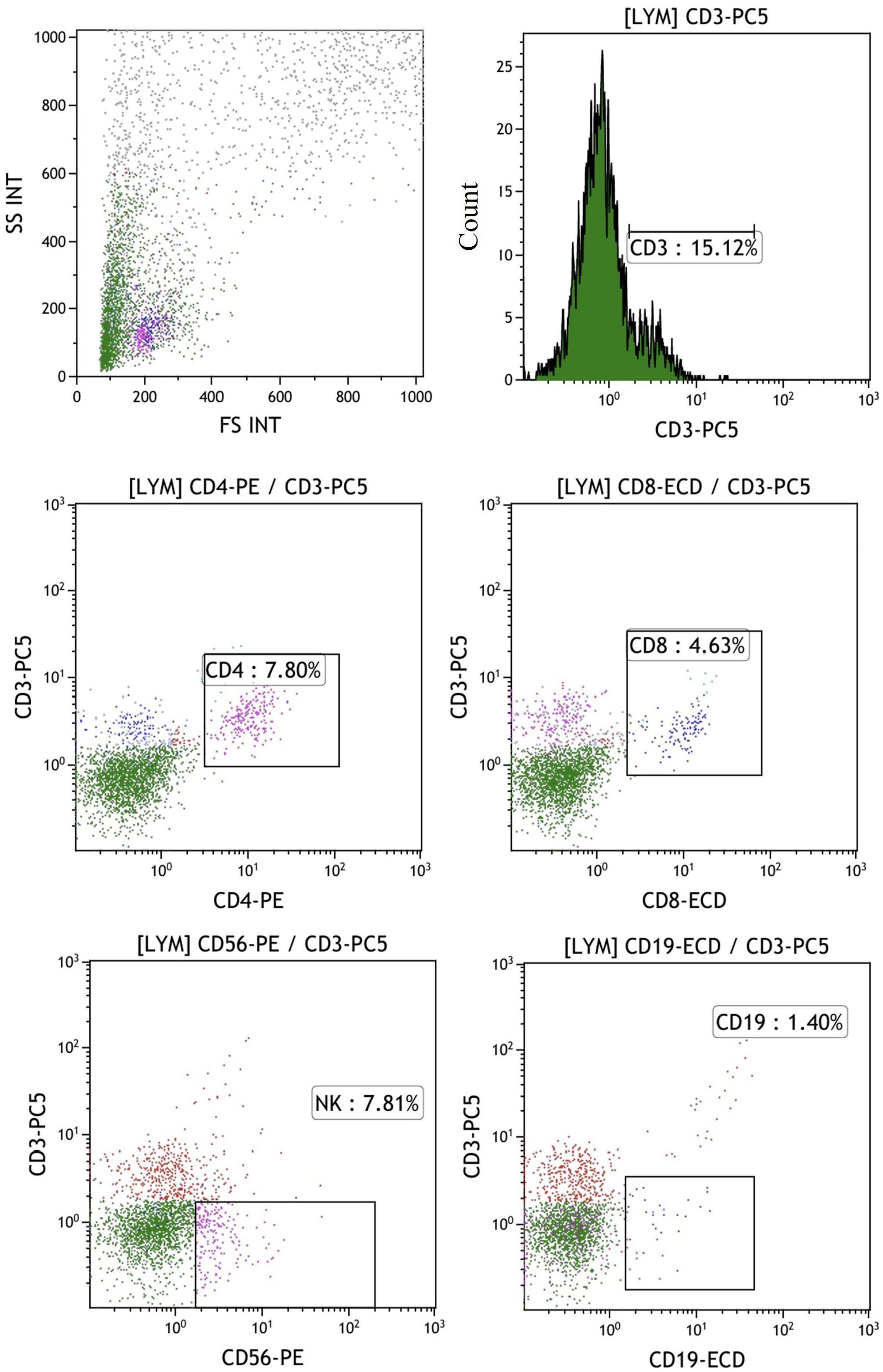
Figure 5. The percentages of T, B, and NK cells in the mixed cell population were analyzed by flow cytometry in the control group, which did not include the addition of peptides. The proportions of CD3+CD4+ double positive (CD4+ T cells), CD3+CD8+ double positive (CD8+ T cells), CD3-CD56 + (NK cells) and CD3-CD19 + (B cells) were 7.8%, 4.63%, 7.81% and 1.40%, respectively.
“The percentages of T, B, and NK cells in the mixed cell population were analyzed by flow cytometry in the control group, which did not include the addition of peptides. The proportions of CD3+CD4+ double positive (CD4+ T cells), CD3+CD8+ double positive (CD8+ T cells), CD3-CD56 + (NK cells) and CD3-CD19 + (B cells) were 7.8%, 4.63%, 7.81% and 1.40%, respectively.”
In the published article, there was an error in the Funding statement. “This work was supported by grants from major science and technology project of Zhejiang Province, China (Project number: 4125C4011724448), the Hangzhou Science and Technology Development Program Project (Project number: 202004A21), Hangzhou Medical and Health Technology Project (Project number: A20220868), Hangzhou Biomedicine and Health Industry Development Support Project (Project number: 2022WJC030), and Henan Province Medical Science and Technology Research Program Project (Project number: LHGI20230440).” The correct Funding statement appears below.
“This work was supported by grants from major science and technology project of Zhejiang Province, China (Project number: 2017C03053), the Hangzhou Science and Technology Development Program Project (Project number: 202004A21), Hangzhou Medical and Health Technology Project (Project number: A20220868), Hangzhou Biomedicine and Health Industry Development Support Project (Project number: 2022WJC030), and Henan Province Medical Science and Technology Research Program Project (Project number: LHGI20230440).”
In the published article, there were three errors.
1. A correction has been made to Abstract, Results, 1. This sentence previously stated:
“3. Neoantigen Peptides Promote CD4+, CD8+ T, and NK Cell Proliferation: After 14 days, flow cytometry showed higher percentages of CD4+ T (37.41% vs 7.8%), CD8+ T (16.67% vs 14.63%), and NK cells (33.09% vs 7.81%) in the experimental group, indicating that the neoantigen peptides induced proliferation of CD4+, CD8+ T cells, and NK cells.”
The corrected sentence appears below:
“3. Neoantigen Peptides Promote CD4+, CD8+ T, and NK Cell Proliferation: After 14 days, flow cytometry showed higher percentages of CD4+ T (37.41% vs 7.8%), CD8+ T (16.67% vs 4.63%), and NK cells (33.09% vs 7.81%) in the experimental group, indicating that the neoantigen peptides induced proliferation of CD4+, CD8+ T cells, and NK cells.”
2. A correction has been made to 3.3 The cellular immune effect induced by neoantigen peptides, 3.3.2 Investigating the percentages of T, B and NK cells in the final activated immune cells, 1. This sentence previously stated:
“The proportions of CD3+CD4+ double positive (CD4+ T cells), CD3+CD8+ double positive (CD8+ T cells), CD3-CD56 + (NK cells) and CD3-CD19 + (B cells) were 7.8%, 14.63%, 7.81% and 1.40%, respectively.”
The corrected sentence appears below:
“The proportions of CD3+CD4+ double positive (CD4+ T cells), CD3+CD8+ double positive (CD8+ T cells), CD3-CD56 + (NK cells) and CD3-CD19 + (B cells) were 7.8%, 4.63%, 7.81% and 1.40%, respectively.”
3. A correction has been made to 3 Results, 3.4 Neoantigen peptide ELISpot results, 4. This sentence previously stated:
“Immunogenicity quantification enabled determination of the relationship between a neoantigen’s immune effects and its HLA molecular affinity changes, as depicted in Chart 1.”
The corrected sentence appears below:
“Immunogenicity quantification enabled determination of the relationship between a neoantigen’s immune effects and its HLA molecular affinity changes, as depicted in Supplementary Material.”
The authors apologize for these errors and state that these do not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: tumor immunotherapy, tumor vaccine, neoantigen, MHC molecular affinity, colorectal cancer
Citation: Zhang S, Huang C, Li Y, Li Z, Zhu Y, Yang L, Hu H, Sun Q, Liu M and Cao S (2024) Corrigendum: Anti-cancer immune effect of human colorectal cancer neoantigen peptide based on MHC class I molecular affinity screening. Front. Immunol. 15:1514836. doi: 10.3389/fimmu.2024.1514836
Received: 21 October 2024; Accepted: 20 November 2024;
Published: 03 December 2024.
Edited by:
Wenxue Ma, University of California, San Diego, United StatesReviewed by:
Rong-Hua Tao, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2024 Zhang, Huang, Li, Li, Zhu, Yang, Hu, Sun, Liu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changxin Huang, aGN4NTg4QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.