- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Chengdu, China
Objectives: Traditional methods of treating allergies primarily revolve around avoiding allergens and promptly using rescue medications when allergic symptoms occur. However, this approach is known for its inefficiency and limited success in achieving long-term relief. Our aim was to conduct a comprehensive analysis of previously published randomized controlled trials (RCTs) that explore the effectiveness and safety of epicutaneous immunotherapy (EPIT) as a means to manage food allergies in children.
Methods: We conducted a comprehensive search across multiple databases, including PubMed, Web of Science, Embase, and Cochrane Library, to identify RCTs comparing EPIT versus placebo for the management of allergen-triggered allergic reactions in children. Only RCTs published in English that evaluated the efficacy and safety of EPIT in pediatric patients with allergic diseases were considered eligible for inclusion. The quality assessment of the included studies was performed using the Cochrane risk-of-bias tool. The analysis comprised of seven RCTs involving a total of 1141 participants. The meta-analysis demonstrated that EPIT significantly facilitated desensitization in patients with food allergy (RR: 2.12, 95% CI: 1.74-2.59, P = 0.296, I² = 17.5%), particularly in individuals with peanut allergy (RR: 2.29, 95% CI: 1.83-2.86, P = 0.463, I² = 0%). However, it is important to note that EPIT was associated with an increased occurrence of treatment-related adverse events (TRAEs; RR: 1.24, 95% CI: 1.14-1.34, P < 0.01, I² = 99.2%). Notably, there were no notable disparities in the frequency of serious adverse events or utilization of rescue medications between the EPIT and placebo groups. EPIT may potentially induce desensitization of peanut allergy in children, but also carries an elevated risk of TRAEs.
1 Introduction
Food allergy is a significant concern for public health and can potentially be life-threatening, affecting approximately 5% of adults and up to 8% of children in Western countries (1, 2). The prevalence rate of cow’s milk allergy is estimated to be around 2% among children aged five years or younger in the United States (3), making it a crucial issue contributing to fatal allergic reactions among young children in both the United States (4, 5) and European nations (6). Peanut allergy also represents the most prevalent life-threatening food allergy, affecting more than 2% of children in the United States (7). Milk and peanuts are crucial components of a balanced diet, and successfully avoiding these allergens can be extremely difficult. Even the slightest exposure carries the risk of accidental allergies, leading to frequent visits to the emergency room. Severe cases can even be life-threatening (4, 8), resulting in illness and significantly impacting an individual’s quality of life (9). The current standard approach for managing food allergies involves strict avoidance of allergens (10) and promptly using rescue medications when allergic symptoms occur.
The immunotherapy for food allergies involves modifying the immune response through repeated exposure to increasing amounts of allergenic foods (11). The goal is to reduce the risk associated with accidental ingestion by raising the threshold at which a patient experiences an allergic reaction (12). Presently employed immunotherapy methods, including oral immunotherapy (OIT), sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT), aim to induce immune tolerance towards the allergen by administering a specific dose repeatedly. However, each technique utilizes a distinct administration route and exhibits varying levels of effectiveness and safety. The efficacy of OIT in enhancing the tolerance level of individuals with persistent food allergies has been demonstrated through the administration of higher doses of sensitized food orally (13). This innovative approach has received FDA breakthrough-therapy designation (14); however, its widespread adoption is impeded by the occurrence of severe systemic reactions (15). Notably, gastrointestinal symptoms (such as nausea, vomiting, stomach discomfort, and acid reflux) are the primary reasons for discontinuing OIT. In severe cases, immediate measures such as administering epinephrine may be necessary (16), leading to its near abandonment. SLIT relies on the utilization of Langerhans cells (LCs), which are immune cells that foster tolerance and can be found in the oral mucosa (17). However, due to limitations in available extracts and the ability for fluid absorption beneath the tongue, SLIT is administered at a dosage that is 1000 times lower than OIT (18). Although SLIT treatment demonstrates improved safety, it shows diminished efficacy in desensitizing individuals to peanut and milk allergens compared to OIT (19, 20).
EPIT is a non-invasive technique that delivers small amounts of allergens to the outermost layer of the skin using a patch. This approach specifically targets regions of the skin with a high density of antigen-presenting cells (APCs) (21, 22). Subsequently, these APCs migrate to adjacent lymph nodes where they initiate an immune response and stimulate production of regulatory cytokines, ultimately leading to modified reactions from allergen-specific T-cells (23, 24). The delivery of allergens through EPIT is restricted to active immune cells located in the epidermis, thereby minimizing the likelihood of significant allergen release into the bloodstream due to the absence of blood vessels within this layer (25). Rendering it an attractive method for augmenting efficacy and shortening treatment duration. Through repeated exposures facilitated by this skin patch, the immune system is modulated, resulting in a reduction of symptoms and severity of food allergies, an increase in individual tolerance, and a decrease in sensitivity over time (26, 27). Xiong et al. conducted a systematic review, which revealed that EPIT may induce desensitization in peanut allergy and increase the likelihood of treatment-related adverse events (28). This finding is consistent with the systematic review and meta-analysis performed by Banatwala et al. (29) However, none of these studies specifically focused on pediatric populations. It is imperative to conduct an up-to-date systematic review of EPIT in children.
This systematic review and meta-analysis aim to comprehensively synthesize published randomized controlled trials (RCTs) investigating the efficacy and safety of EPIT as a therapeutic intervention for pediatric food allergy.
2 Methods
2.1 Protocol and registration
This investigation strictly adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (30). Furthermore, the research plan was officially registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD 42024538489) on February 5, 2024.
2.2 Search strategy
We conducted a comprehensive search across renowned databases, including PubMed, EMBASE, Cochrane Library, and Web of Science, to identify English-language studies published from the inception until May 5, 2024. Our search terms included “epicutaneous immunotherapy,” “immunotherapy,” “EPIT,” “peanut allergy,” “milk allergy,” “nut allergy,” “egg allergy,” “food allergy”, “children” “young” and “kids”. The search strategy was developed based on the MeSH Database journals of PubMed and subsequently applied to other databases. Emphasizing human studies exclusively, we limited our inclusion criteria to those written solely in English. Furthermore, we meticulously scrutinized the reference lists of all primary studies available to ensure no pertinent citations were overlooked.
2.3 Eligibility criteria
We have developed a “PICOS” (Patient, Intervention, Comparison, Outcome, and Study design) strategy to determine eligibility. The specific criteria are as follows: (1) Participants: Individuals below the age of 18, who have been diagnosed with food allergies or present compelling clinical evidence of food allergies such as peanut, cow’s milk, nuts, and other allergens based on their clinical history and laboratory tests; (2) Treatment: EPIT administered to the intervention group; (3) Control: placebo or allergen avoidance; (4) Outcome: evaluation of food allergy desensitization efficacy and intervention safety; and (5) Study design: limited to RCTs. Exclusion criteria include non-randomized controlled trials, systematic reviews, meta-analyses, narrative reviews, editorials, abstract reports, and case series.
2.4 Data collection
The titles, abstracts, and full-texts were meticulously reviewed by two reviewers. Data extraction and risk assessment were independently conducted by the two reviewers. In case of any discrepancies, a third reviewer was consulted for resolution through discussion. A customized data collection form was utilized for the extraction process. If the necessary information was not provided in the original article, efforts were made to contact the authors for its acquisition.
2.5 Quality assessment
To evaluate the certainty of evidence utilized in this systematic review and meta-analysis, two reviewers employed a tool provided by the Cochrane Collaboration (31) to examine potential biases present in the included RCTs. The assessment domains encompassed various aspects such as “randomization procedure,” “deviations from intended interventions,” “unavailable outcome data,” “outcome measurement,” “selection of reported outcomes,” and “overall bias.” Each domain was categorized as either “high risk,” “some concerns,” or “low risk.” Discrepancies were resolved through discussion or consultation with the corresponding author.
2.6 Data synthesis and analysis
To analyze continuous outcomes, we aggregated the data by calculating the mean difference or standardized mean difference (SMD) if measurements were on different scales. For dichotomous outcomes, we employed the risk ratio (RR). The selection of a random-effects model or fixed-effects model was contingent upon the observed level of variation among studies. We computed a 95% confidence interval (CI) for each estimate of effect size and determined statistical significance based on a P-value below 0.05. To evaluate heterogeneity in the meta-analysis, we utilized the I2 statistic and considered it as low if I2 < 50% (32). If sufficient data were available, subgroup analyses would be conducted to examine primary outcomes based on factors such as allergen types and intervention dosages. Sensitivity analyses would be performed to assess the robustness of our findings by excluding studies with a “high” or “unclear” risk of bias in terms of selection, performance, detection, and attrition. Begg’s test and Egger’s test would be utilized to evaluate potential publication bias in primary outcomes. Meta-analyses would be carried out using the STATA software (Stata-Corp LLC, CollegeStation, TX, United States). Additionally, the quality of evidence would be assessed using the GRADE approach which categorizes certainty into high, moderate, low, and very low levels.
3 Results
3.1 Search results and study characteristics
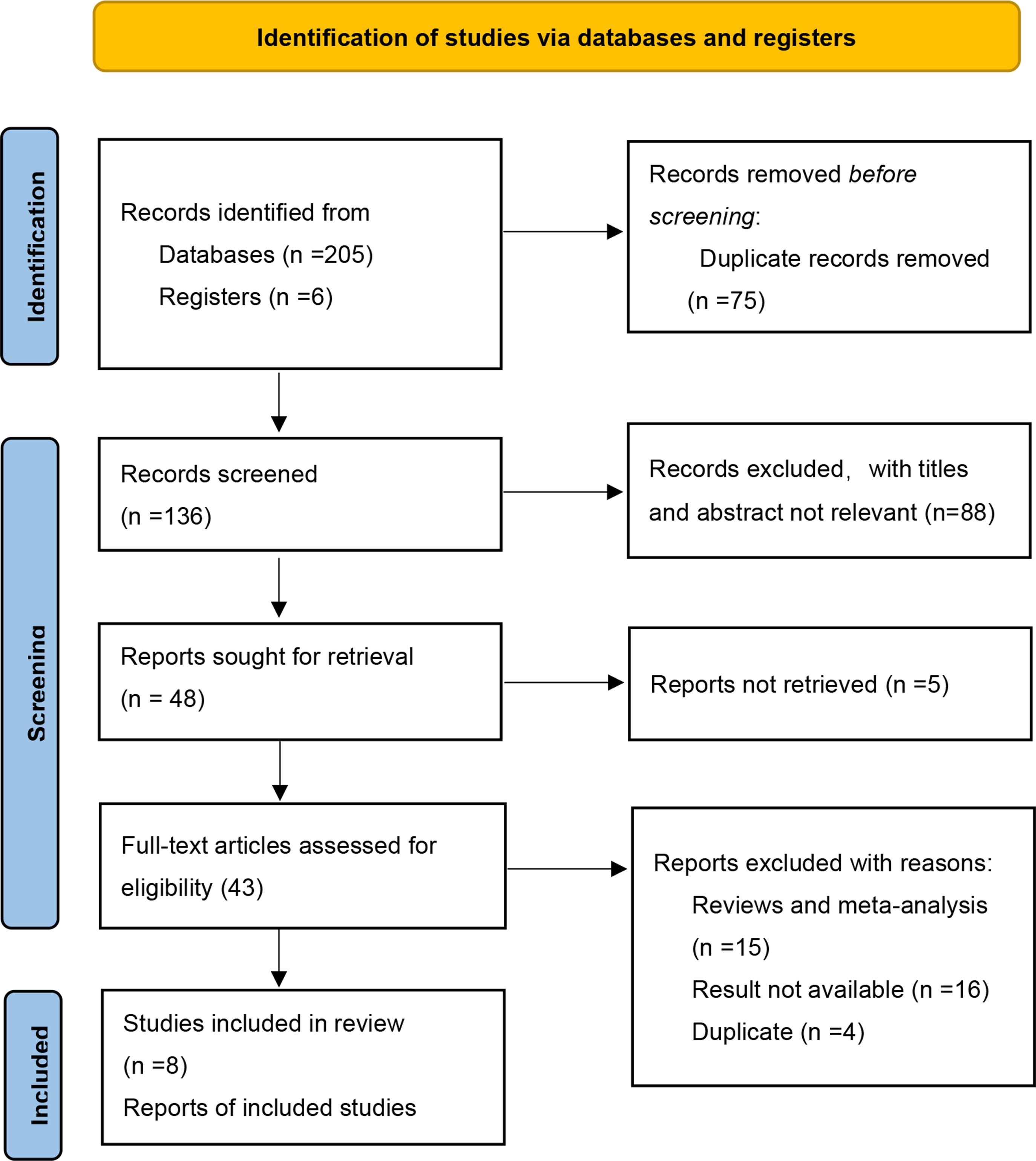
The initial search identified a total of 211 potentially relevant records. Subsequently, a comprehensive full-text screening was conducted on forty-three records, resulting in the inclusion of seven RCTs (33–39) for the final quantitative and qualitative analysis. Figure 1 presents a flow diagram illustrating the process of study selection.
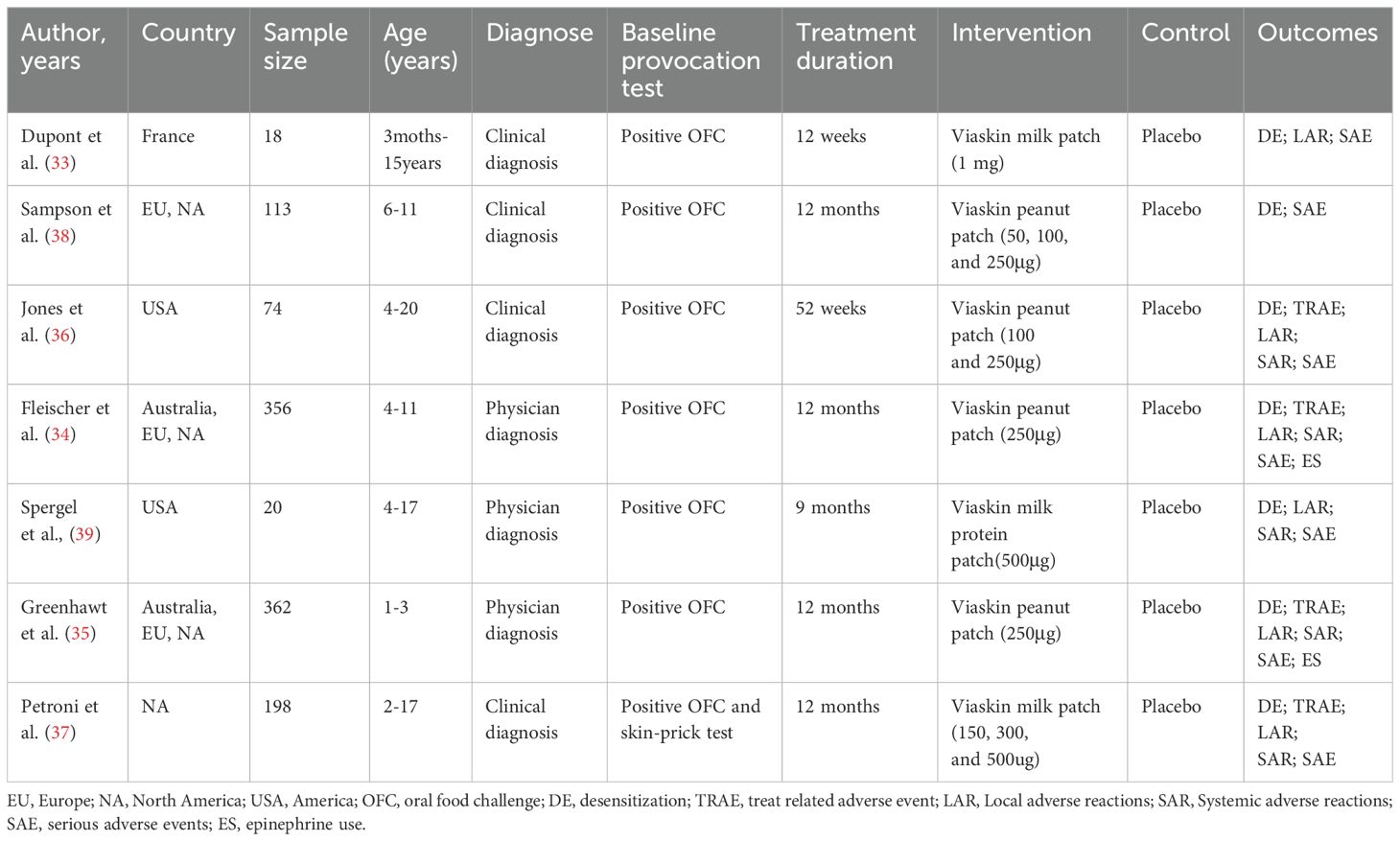
The characteristics of the studies included in our analysis are summarized in Table 1. A total of 1141 participants were enrolled, with 783 assigned to the EPIT group and 358 to the placebo group. Simultaneous multicenter trials were conducted across all continents, including two trials in the USA, one trial in France, and another trial in North America. Most trials (5 out of 7) had an intervention duration of 52 weeks, while the remaining two trials lasted for 9 months and 12 weeks respectively. Three studies focused on cow’s milk allergy and utilized EPIT doses ranging from 150 µg to 1 mg, whereas the other four studies concentrated on peanut allergy with EPIT doses ranging from 50 to 250 µg.
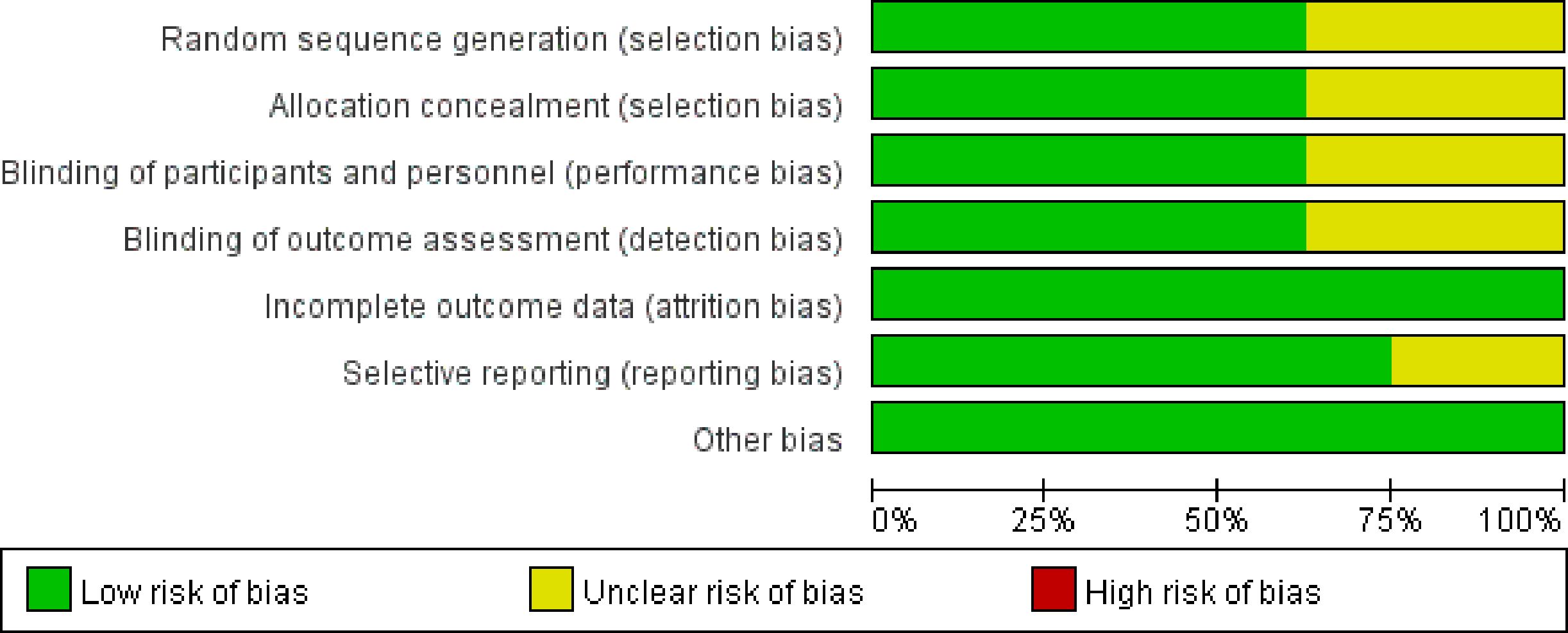
The risk of bias in the included studies is depicted in Figures 2. Following the guidelines outlined in the Cochrane Handbook, four studies demonstrated a low risk of bias (34, 35, 37, 39). Concerns regarding incomplete data on random sequence generation, allocation concealment, blinding of participants and personnel, as well as blinding of outcome assessment were raised for two studies (33, 36). Furthermore, one study was flagged for potential issues related to selective reporting (38).
3.2 Primary outcome
3.2.1 Desensitization
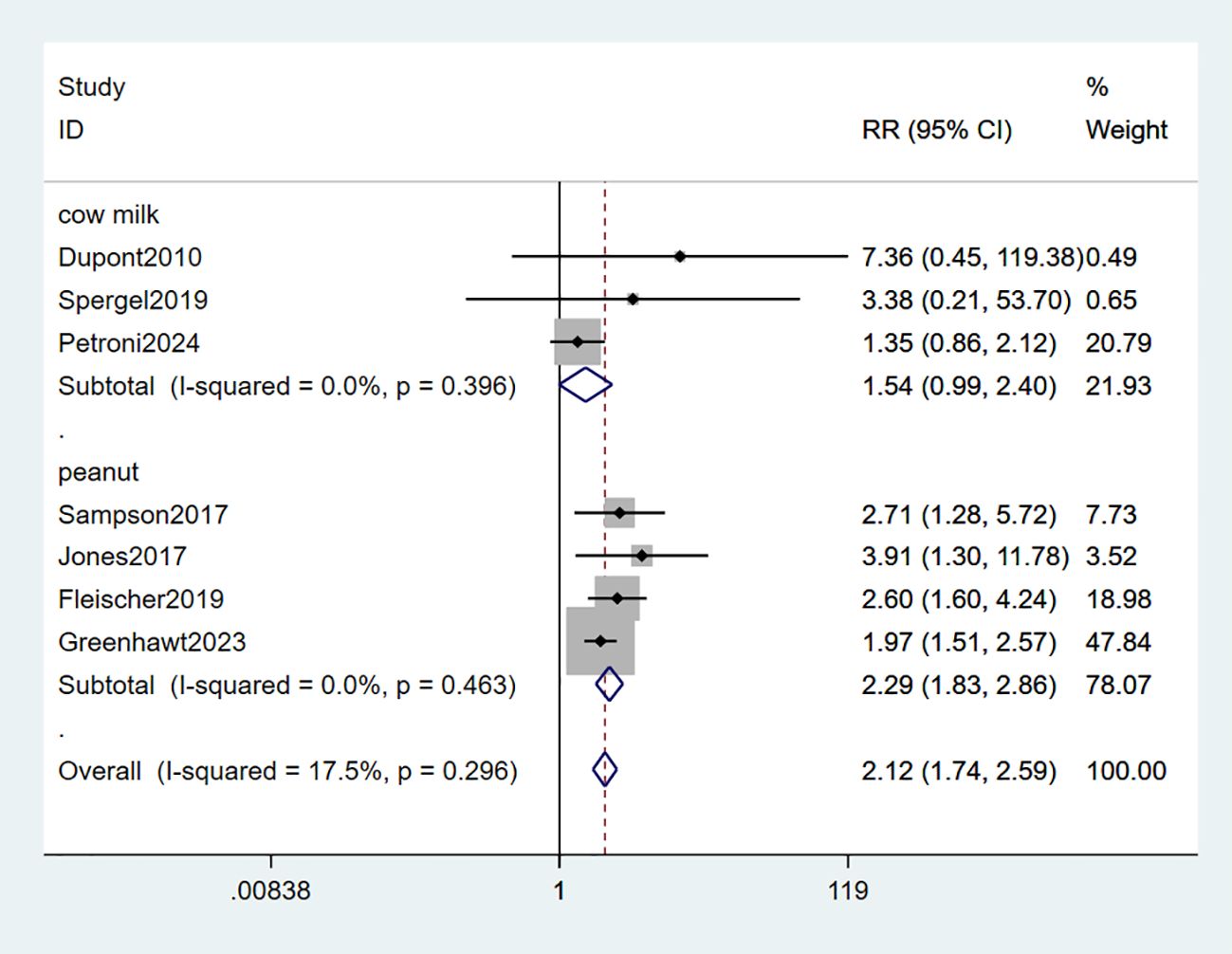
All seven studies included in the analysis provided data on desensitization. Among these, four studies demonstrated an increased ability to tolerate peanut allergies (34–36, 38), while two studies reported an improved tolerance towards milk allergies (33, 37). Only one study specifically assessed the efficacy of EPIT in reducing eosinophil counts to less than 15/hpf from biopsy for milk-induced eosinophilic esophagitis (EOE) (39). The combined data showed a statistically significant tolerance of food allergy in the EPIT group compared with placebo (RR: 2.12, 95% CI: 1.74-2.59, P = 0.296, I² = 17.5%) with minimal heterogeneity observed. Interestingly, all four studies involving peanut patches demonstrated favorable outcomes in terms of desensitization (RR: 2.29, 95% CI: 1.83-2.86, P = 0.463, I² = 0%); however, no study involving milk patches reported any significant improvement in this regard (RR: 1.54, 95% CI: 0.99-2.40, P = 0.396, I² = 0%) (high certainty evidence; Figure 3).
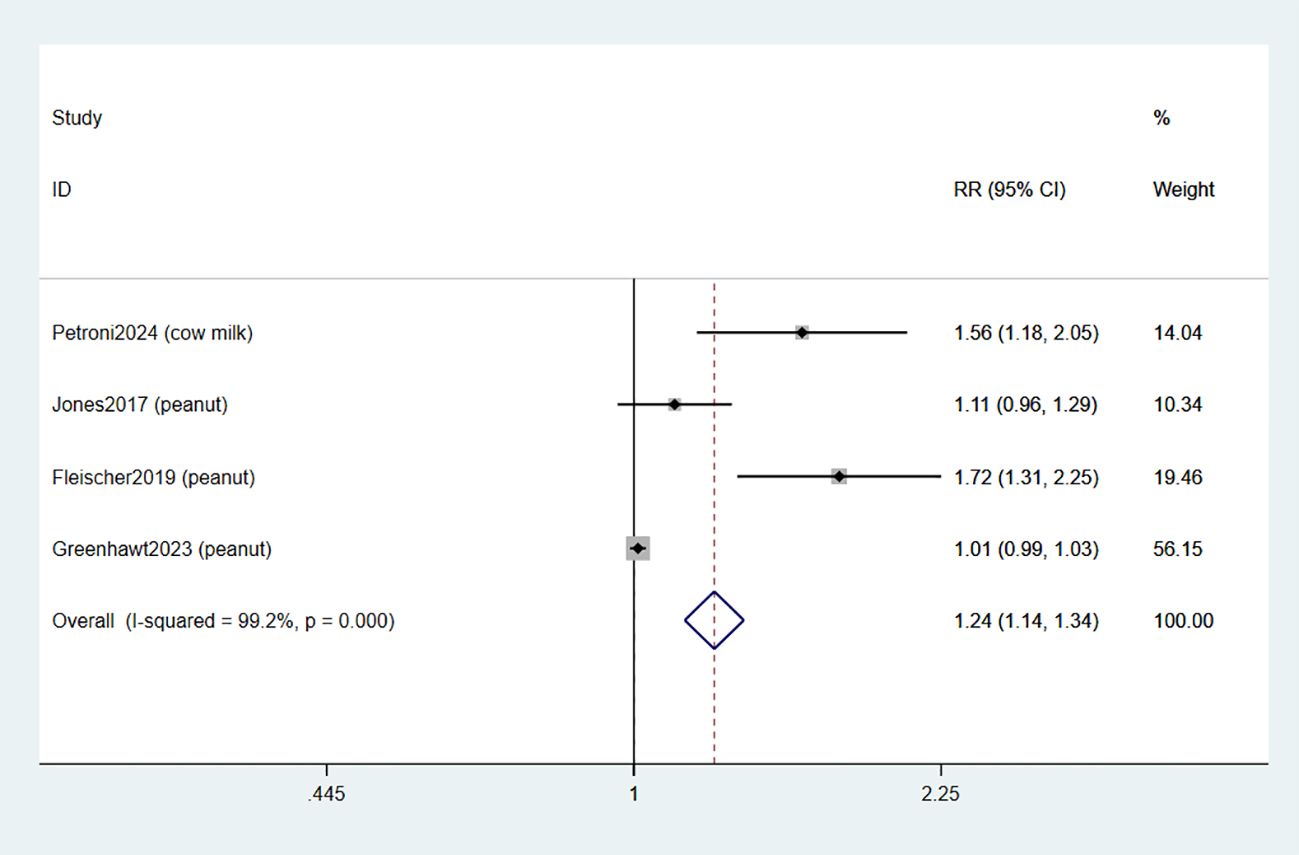
3.2.2 Treatment-related adverse events
Four studies documented the occurrence of adverse events related to treatment (TRAE) (34–37). The combined data indicated that EPIT was associated with an increased risk of any TRAE compared to placebo (RR: 1.24, 95% CI: 1.14-1.34, P < 0.01, I2 = 99.2%) with a significant level of heterogeneity when compared to placebo (low certainty evidence; Figure 4).
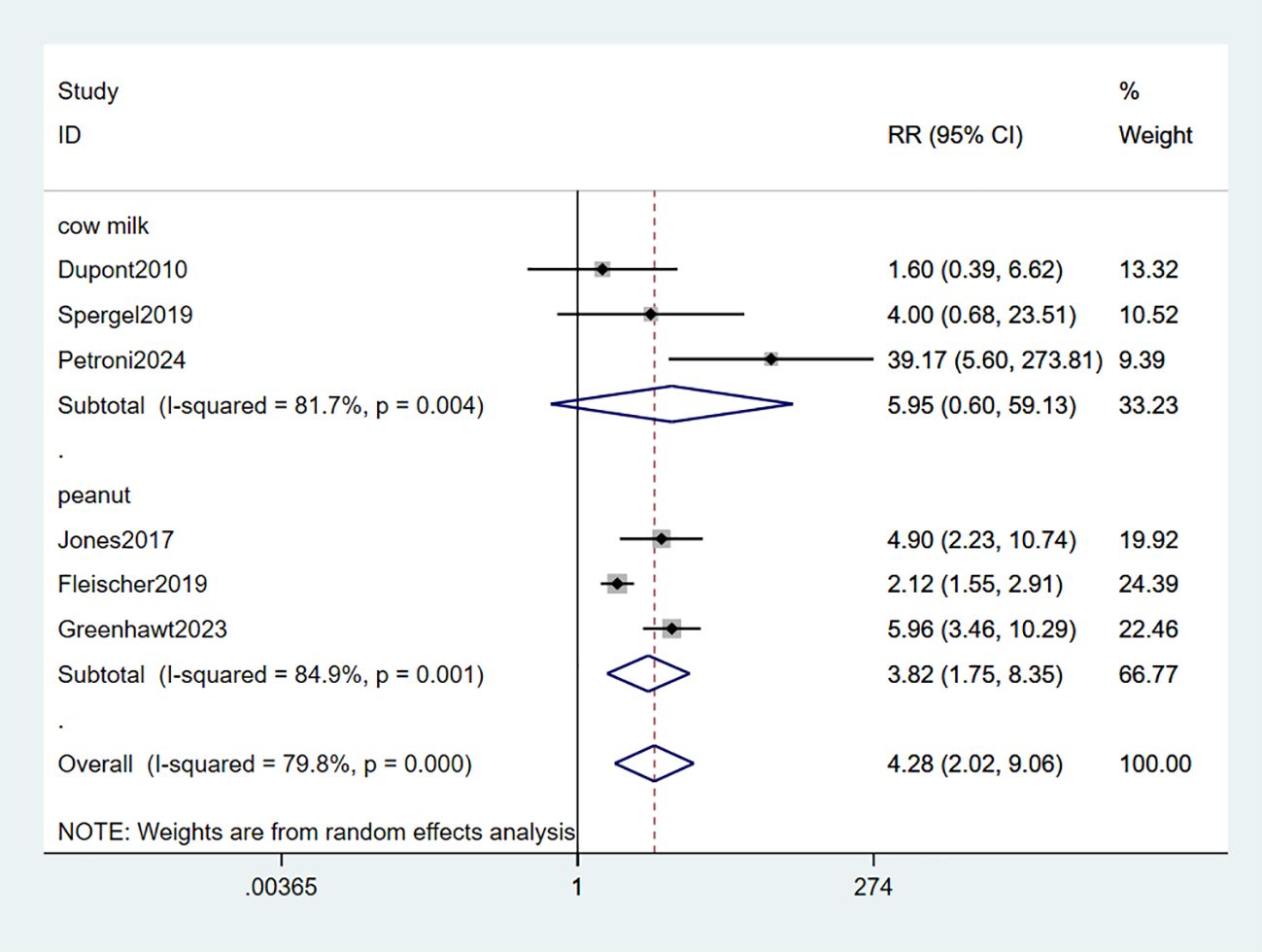
3.2.3 Local adverse reactions
Six studies observed adverse reactions in the local area (LARs) (33–37, 39). The combined data indicated that EPIT significantly increased the risk of local reactions (RR: 4.28, 95% CI: 2.02-9.06, P < 0.01, I2 = 79.8%) with substantial heterogeneity compared to placebo. Among these studies, three studies involving milk did not demonstrate statistical significance (RR: 5.95, 95% CI: 0.6-59.13, P < 0.01, I2 = 81.7%). However, the subgroup analysis focusing on peanuts revealed a significant association with LARs risk (RR: 3.83, 95% CI: 1.75-8.35, P < 0.01, I2 = 84.9%) (low certainty evidence; Figure 5).
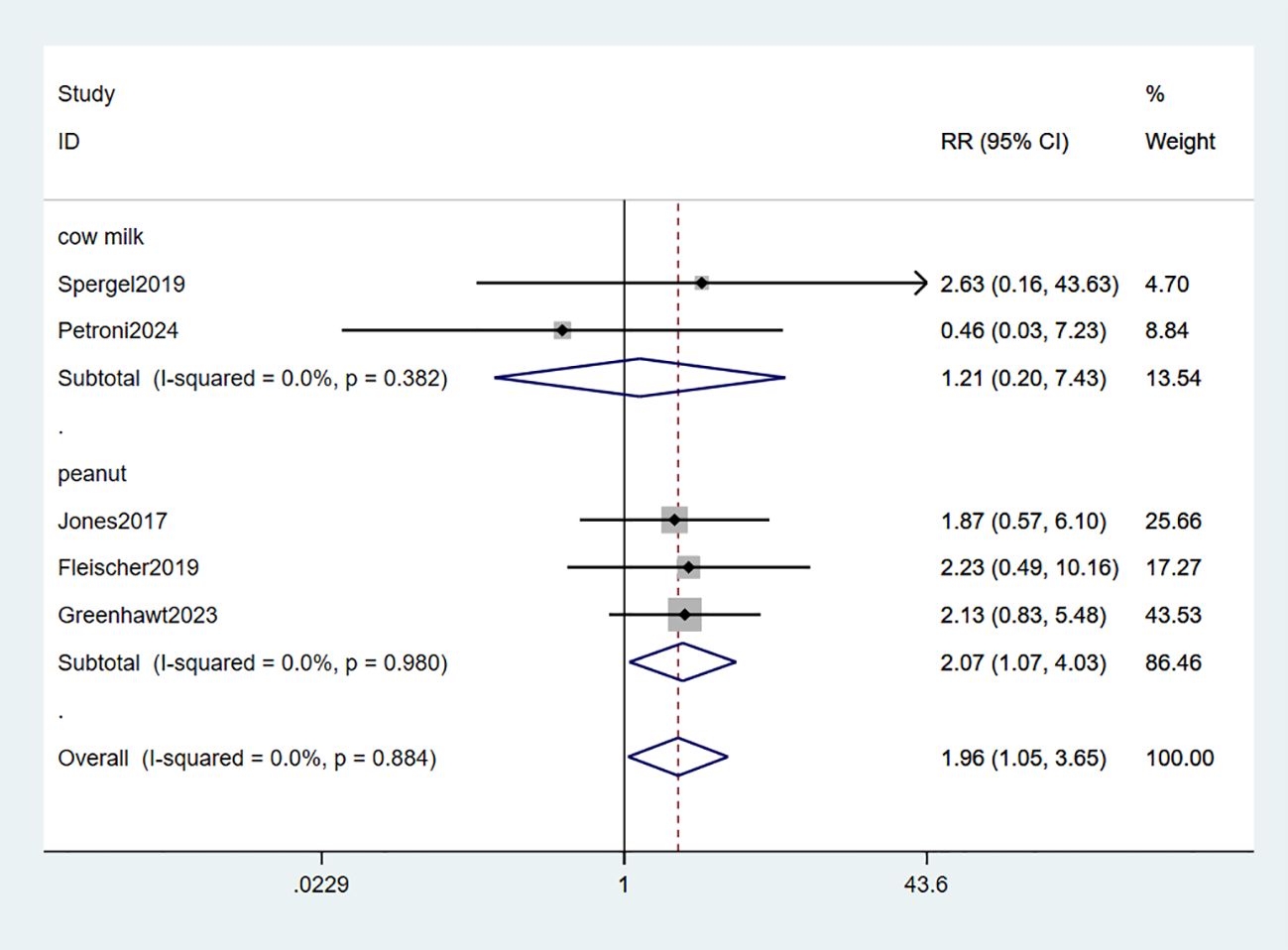
3.2.4 Systemic adverse reactions
Systemic adverse reactions (SARs) were reported in five studies (34–37, 39). The meta-analysis revealed a significant increase in the risk of systemic reactions with EPIT compared to placebo, exhibiting a relative risk of 1.96 and a 95% confidence interval ranging from 1.05 to 3.65 (P = 0.884, I2 = 0%). Notably, three studies focusing on peanuts demonstrated a statistically significant association with SARs risk (RR: 2.07, 95% CI: 1.07-4.03, P = 0.980, I2 = 0%), while two other milk-related studies did not reach statistical significance (RR: 1.21, 95% CI: 0.20-7.43, P = 0.382, I2 = 0%). Importantly, minimal observed heterogeneity ensures robust evidence (Figure 6).
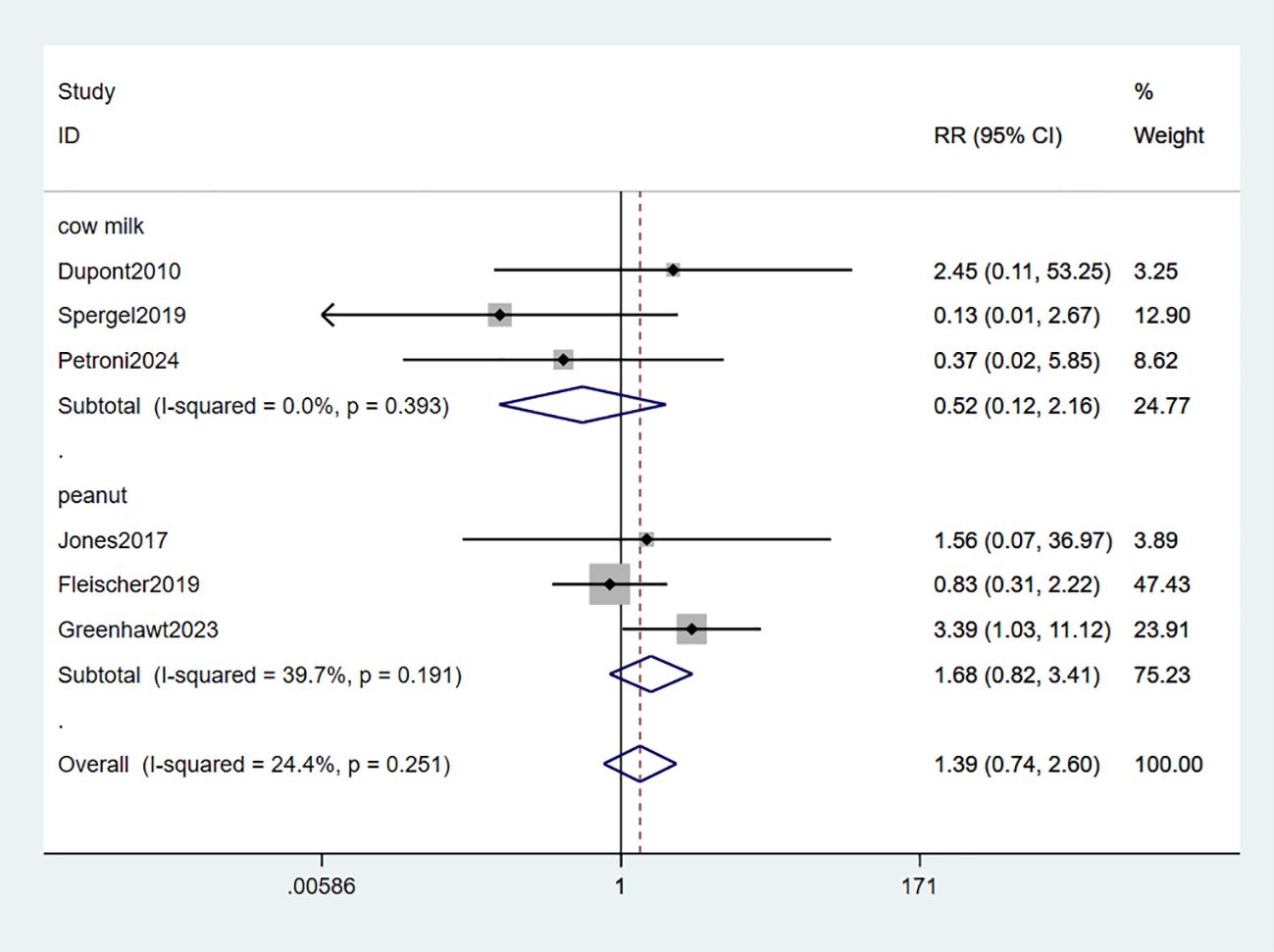
3.2.5 Serious adverse events
Six studies reported serious adverse events (SAEs) (33–37, 39). The meta-analysis findings revealed no statistically significant differences in the risk of SAEs between EPIT and placebo (RR: 1.39, 95% CI: 0.74-2.60, P = 0.251, I2 = 24.4%). Neither the peanut group (RR: 1.68, 95% CI: 0.82-3.41, P = 0.191, I2 = 39.7%) nor the milk group (RR: 0.52, 95% CI: 0.12-2.16, P = 0.393, I2 = 0%) exhibited any risk of experiencing SAEs (high certainty evidence; Figure 7).
3.3 Secondary outcome
3.3.1 Epinephrine use
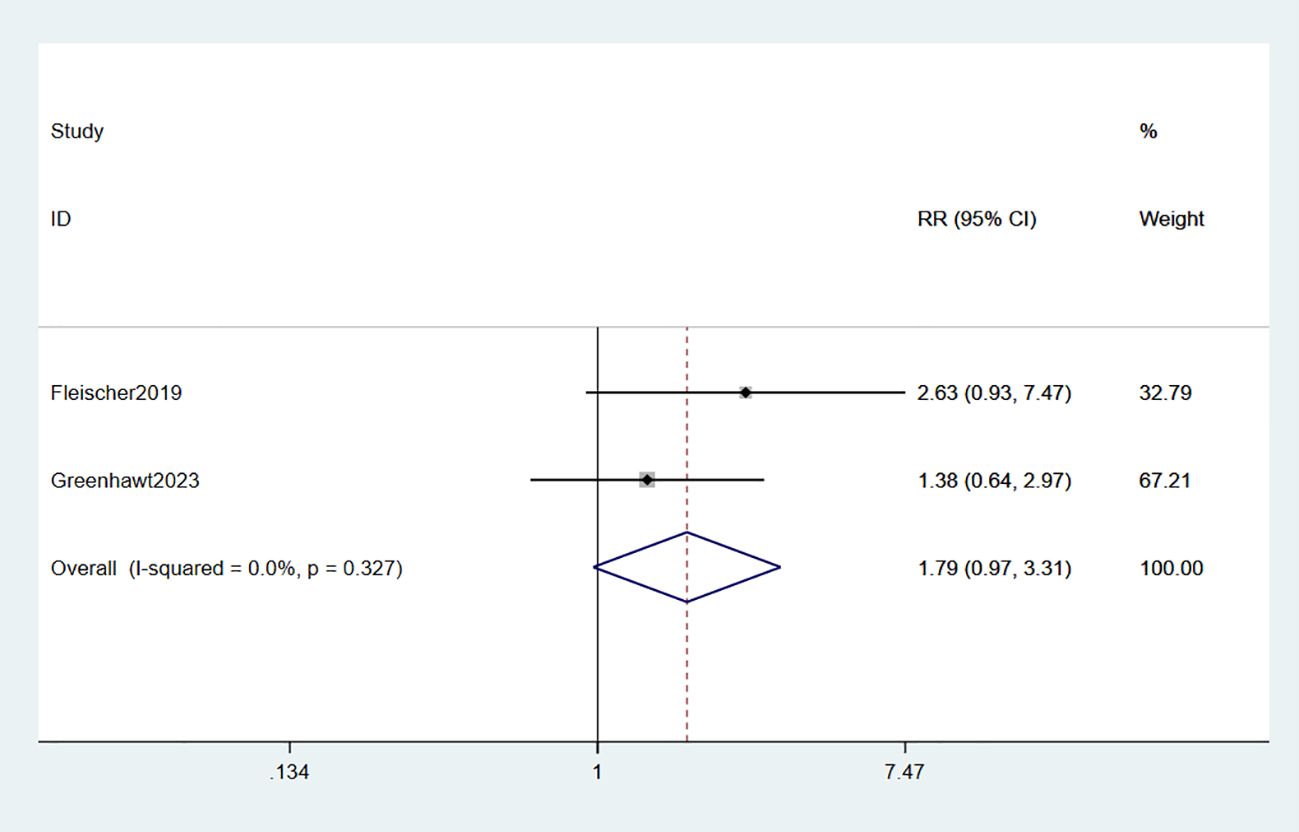
The administration of epinephrine was investigated in two studies involving a total of 718 participants (34, 35). Meta-analysis revealed that the use of epinephrine in EPIT did not show any significant evidence of increased risk compared to placebo (RR: 1.79, 95% CI: 0.97-3.31, p = 0.062, I2 = 0%) with minimal deviation from placebo (high certainty evidence; Figure 8).
3.3.2 Allergic reaction of organ systems
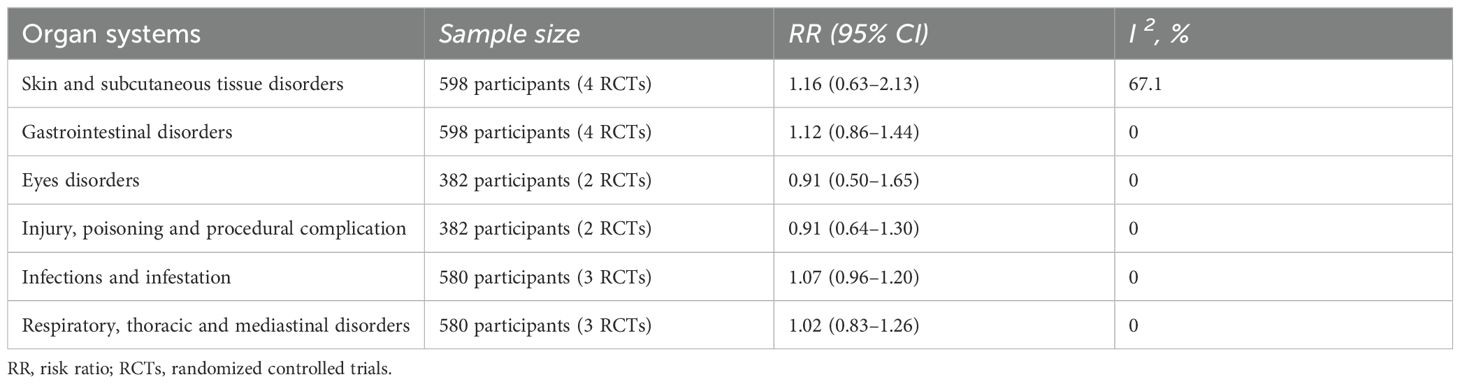
The results of allergic reactions induced by EPIT in various organ systems are presented in Table 2. No conclusive evidence has been found to indicate a higher likelihood of adverse reactions occurring in the organ system when compared to the placebo group.
3.4 Subgroup analysis
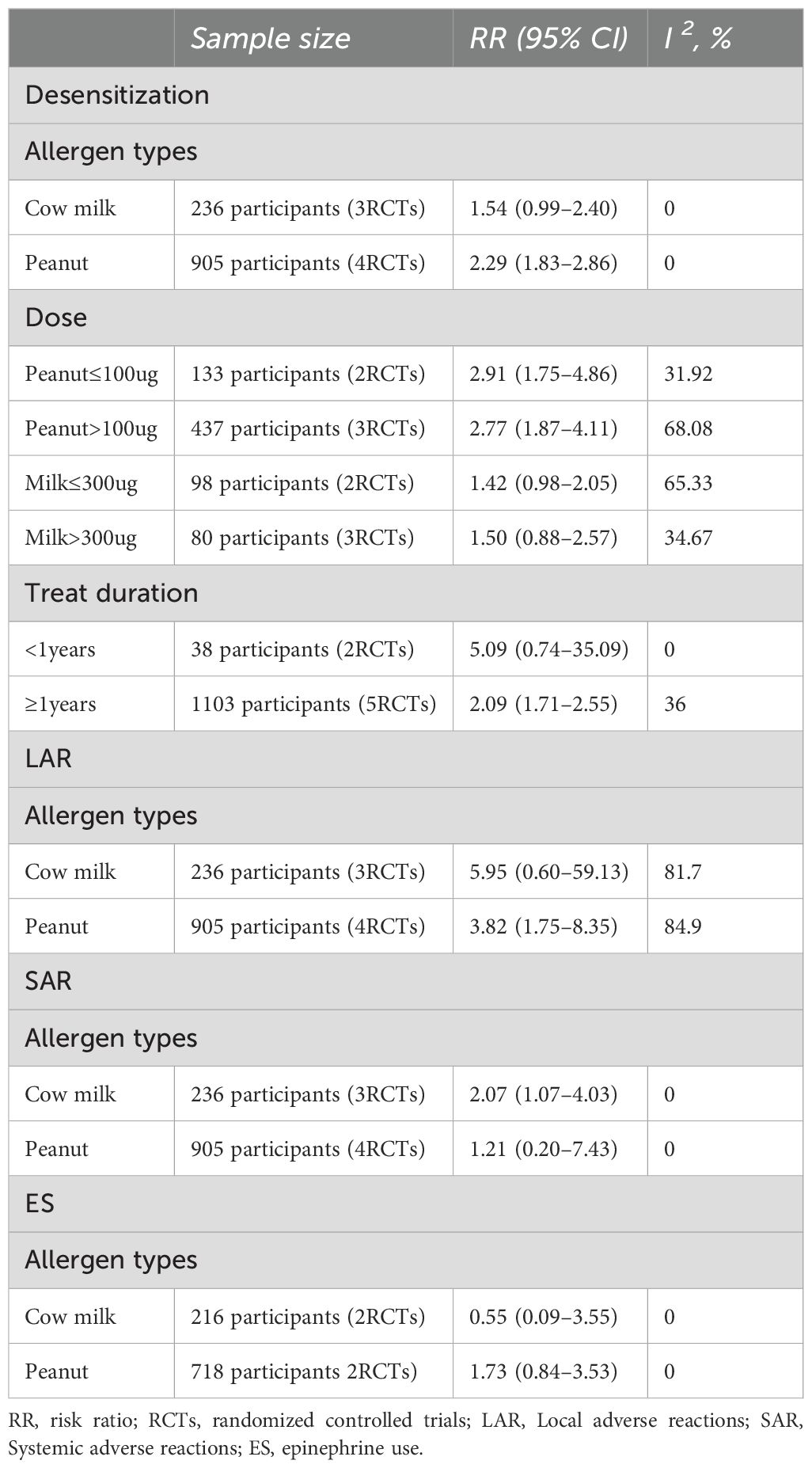
The study conducted subgroup analyses to investigate the impact of allergen type, therapeutic dosage, and treatment duration on the primary outcome. Additionally, desensitization was assessed for cow milk versus peanut allergens. Epidermal peanut immunotherapy demonstrated efficacy in both high-dose (> 100 µg) (RR: 2.77, 95% CI: 1.87-4.11, I² = 0%) and low-dose (≤ 100 µg) groups (RR: 2.91, 95% CI: 1.75-4.86, I² = 0%). However, no significant improvement in the efficacy of epidermal milk immunotherapy was observed in either the high-dose groups (> 300 µg) (RR: 1.50, 95% CI: 0.88-2.57, I² = 9.6%) or low-dose groups (≤ 300 µg) (RR: 1.42, 95% CI: 0.98-2.05, I² = 0%). Furthermore, subgroup analyses based on the durations (≥ 1 year versus < 1 year) indicated that only for durations ≥ 1 year did tolerance exhibit a statistically significant enhancement (RR: 2.12, 95% CI: 1.74-2.59, I² = 36%). Regarding LAR, subgroup analyses revealed that epidermal peanut immunotherapy alone had a significantly higher risk of LAR (RR: 3.82, 95% CI: 1.75-8.35, I² = 84.9%). The same trend was observed for SAR as well. Subgroup analyses were performed on the primary outcome and are presented in Table 3.
3.5 Sensitivity analysis and publication bias
Sensitivity analyses were conducted on the primary findings by excluding studies with significant or uncertain potential for bias in terms of selection, performance, detection, or attrition. The exclusion of all studies with a high or unclear risk of bias did not impact the outcome measures (Supplementary Material).
The results of Egger’s test did not reveal any statistically significant indication of publication bias in relation to desensitization (p = 0.957) or the primary outcomes TRAE (p = 0.643), LAR (p = 0.861), SAR (p = 0.425), and SAE (p = 0.073). Therefore, there is no substantial evidence suggesting the presence of significant publication bias concerning these outcomes.
4 Discussion
Our analysis suggests that EPIT holds promise for improving tolerance in pediatric patients with food allergies. Subgroup analysis indicates a potential benefit of EPIT in enhancing peanut allergy tolerance among children, while its impact on cow’s milk allergy remains inconclusive. Furthermore, our findings suggest a possible association between EPIT and adverse events such as TRAE, LAR, SAR, etc. However, no statistically significant increase was observed in the risk of SAEs or epinephrine usage associated with EPIT.
The effectiveness of EPIT in the treatment of food allergy observed in our study aligns with previous meta-analyses conducted on this topic. Xiong et al. (28) performed a meta-analysis that included ten RCTs investigating the efficacy of EPIT for food allergy, and their findings indicated significant benefits in terms of desensitization specifically for peanut allergy. Additionally, subgroup analyses based on different age groups confirmed notable improvements in tolerance among children aged below 12 years. The authors also noted an increased likelihood of local skin reactions associated with EPIT but did not identify any significant correlations with TRAE, SAR, SAEs, or the use of rescue medications. Banatwala et al. (29) conducted a meta-analysis to evaluate the efficacy and safety of EPIT in individuals with peanut allergies. Their results revealed that only the higher dosage group (250 µg) exhibited significantly greater desensitization compared to placebo, while no significant difference was observed in the lower dosage group (100 µg). However, they noted a notable increase in both local and systemic adverse events associated with EPIT treatment. Furthermore, individuals undergoing EPIT were more likely to require rescue medications such as epinephrine and topical corticosteroids. On the other hand, Alvarez-Florian et al. (40) performed a systematic review focusing on children and adolescents with milk allergy but found insufficient evidence to determine the efficacy of EPIT for cow’s milk allergy.
The urgent requirement for safe and effective treatments is underscored by the detrimental impact of food allergy on patients’ physical, psychological, and social well-being. Our study found that EPIT treatment was associated with the incidence of TRAEs, LARs and SARs. However, it did not lead to an increased occurrence of SAEs or necessitate additional epinephrine use. Current clinical research focuses on food allergen-specific immunotherapy through OIT, SLIT, or EPIT routes. Compared to SLIT and EPIT, OIT necessitates a higher maintenance dose of food protein and is associated with an elevated incidence of systemic adverse events. While SLIT exhibits safety, its efficacy is constrained by the sublingual administration of low-dose allergen. In contrast, EPIT surpasses both SLIT and EPIT in terms of safety and tolerability while demonstrating moderate therapeutic effects (41). Consequently, EPIT demonstrates a favorable safety profile. In the context of food allergy immunotherapy, when considering the augmentation of immune tolerance, both the potential risks and benefits should be taken into account alongside patient expectations. Patients who possess high expectations for achieving enhanced immunization thresholds and are willing to accept the associated risk or cost of side effects may be suitable candidates for OIT. Conversely, patients with heightened safety expectations might find EPIT more appropriate (42).
The phenomenon of desensitization was primarily observed in younger children, which is consistent with the superior outcomes of OIT observed in this age group (16, 43). This observation can be attributed to the enhanced permeability of water-soluble allergens through the skin. In the Viaskin epidermal delivery system, water-soluble allergens are absorbed into both the epidermis and dermis layers (44), where they encounter dendritic cells. Subsequently, these cells migrate to nearby lymph nodes, thereby triggering immune responses (45, 46). Children have different skin characteristics compared to adults, including differences in structure, barrier function, and composition (47, 48). Furthermore, the outermost layer of children retains higher levels of moisture and exhibits a faster rate of water movement compared to that of adults (48, 49). Due to the presence of only about 15 layers in children’s outermost layer versus 25 layers in adults’, it can be inferred that water-soluble allergens are more likely to penetrate through less developed outermost layers in children when compared to adults. Additionally, the age disparity in immune mechanisms also serves as a significant factor contributing to the heightened desensitization observed in younger children. The correlation between the gradual postnatal increase of IgE levels and the persistence of food allergy in infants (50, 51) further underscores this relationship. Notably, a substantial expansion of IgE epitope recognition occurs after reaching two years of age (52, 53). Consequently, the immune response to food allergens remains immature during the early childhood. Moreover, the functional abilities of IgE-producing B cells are also immature in the first few years of life. Notably, age-dependent developmental changes in peripheral blood B cell pool composition exhibit their most pronounced effects within the initial five years of life, while the number of naive B cells gradually decreases to adult levels by 10-15 years of age (54). Therefore, altering the balance of food allergen tolerance becomes more challenging following the maturation of B-cell function, and this initial immaturity of the immune response to food allergens may constitute another significant factor contributing to the diminished efficacy of EPIT in older children and adults.
This review offers several advantages. Firstly, it represents the initial meta-analysis that evaluates the effects and safety of EPIT for food allergy in children, providing robust evidence to support EPIT as an innovative allergen immunotherapy. Secondly, all included studies are RCTs, enhancing the credibility of our findings due to their high quality. Thirdly, we conducted subgroup analyses based on various study characteristics to investigate potential sources of significant heterogeneity observed in local adverse effects. There are several noteworthy constraints in our study. Firstly, the included studies had a limited number and sample size, potentially introducing random errors. Secondly, the EPIT patches utilized in this study were exclusively sourced from Viaskin (DBV Technologies), which also influenced the trial design, potentially limiting the generalizability of the findings to other EPIT products developed by different manufacturers. Additionally, there is a lack of studies investigating EPIT for food allergies beyond peanut and milk. Thirdly, due to variations in EPIT time and dosage across different studies, conducting subgroup analysis to determine the most suitable intervention time and dose posed challenges. Another limitation is that certain studies only encompassed a subset of young adults.
In conclusion, this systematic review and meta-analysis provide compelling evidence suggesting that EPIT may potentially induce desensitization of peanut allergy in children. The efficacy of EPIT for cow milk allergy remains uncertain, and current evidence does not support the utilization of EPIT treatment with milk patches in pediatric clinical practice; however, it is noteworthy that EPIT also exhibits mild to moderate side effects. Consequently, the safety profile of EPIT is considered favorable. Given the limited number of studies and variations in research methodologies, these findings should be cautiously interpreted. Further well-designed RCTs with larger sample sizes are warranted to investigate the benefits and adverse events associated with EPIT in pediatric populations as well as other allergic conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
BC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HG: Conceptualization, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. XL: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. ZZ: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. SW: Data curation, Investigation, Methodology, Software, Writing – review & editing. FT: Conceptualization, Formal analysis, Project administration, Software, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the National Science Foundation of China (Grant Number 82071353).
Acknowledgments
We thank the authors of the studies included in the meta-analysis and the participants in these studies for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1510653/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis of desensitization.
Supplementary Figure 2 | Sensitivity analysis of TRAEs.
Supplementary Figure 3 | Sensitivity analysis of LARs.
Supplementary Figure 4 | Sensitivity analysis of SARs.
Supplementary Figure 5 | Sensitivity analysis of SAEs.
References
1. Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. (2014) 69:992–1007. doi: 10.1111/all.12423
2. Santos AF, Riggioni C, Agache I, Akdis CA, Akdis M, Alvarez-Perea A, et al. EAACI guidelines on the diagnosis of IgE-mediated food allergy. Allergy. (2023) 78:3057–76. doi: 10.1111/all.15902
3. Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. (2011) 128:e9–17. doi: 10.1542/peds.2011-0204
4. Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food 2001-2006. J Allergy Clin Immunol. (2007) 119:1016–8. doi: 10.1016/j.jaci.2006.12.622
5. Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. (2001) 107:191–3. doi: 10.1067/mai.2001.112031
6. Baseggio Conrado A, Ierodiakonou D, Gowland MH, Boyle RJ, Turner PJ. Food anaphylaxis in the United Kingdom: analysis of national data 1998-2018. BMJ. (2021) 372:n251. doi: 10.1136/bmj.n251
7. Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. (2010) 125:1322–6. doi: 10.1016/j.jaci.2010.03.029
8. Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. (2008) 121:166–71. doi: 10.1016/j.jaci.2007.10.012
9. Fong AT, Katelaris CH, Wainstein B. Bullying and quality of life in children and adolescents with food allergy. J Paediatr Child Health. (2017) 53:630–5. doi: 10.1111/jpc.13570
10. Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. (2014) 134:1016–25 e43. doi: 10.1016/j.jaci.2014.05.013
11. Christine YYW, Nicki YHL, Patrick SCL, Ka Hou C. Immunotherapy of food allergy: a comprehensive review. J Clin Rev Allergy Immunol. (2017) 57:55–73. doi: 10.1007/s12016-017-8647-y
12. Muraro A, Tropeano A, Giovannini M. Allergen immunotherapy for food allergy: Evidence and outlook. Allergol Select. (2022) 6:285–92. doi: 10.5414/ALX02319E
13. Guillaume P, Guillaume L. Oral immunotherapy for food allergy: Translation from studies to clinical practice? J World Allergy Organ J. (2023) 16:100747. doi: 10.1016/j.waojou.2023.100747
14. Sakura S, Ken-Ichi N, Noriyuki Y, Motohiro E. Current perspective on allergen immunotherapy for food allergies. J Allergol Int. (2024) 73:501514. doi: 10.1016/j.alit.2024.08.002
15. Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. (1997) 99:744–51. doi: 10.1016/s0091-6749(97)80006-1
16. Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. (2019) 393:2222–32. doi: 10.1016/S0140-6736(19)30420-9
17. Akdis CA, Barlan IB, Bahceciler N, Akdis M. Immunological mechanisms of sublingual immunotherapy. Allergy. (2006) 61 Suppl:8111–4. doi: 10.1111/j.1398-9995.2006.01159.x
18. Emily CM, Robert AW. Sublingual (SLIT) versus oral immunotherapy (OIT) for food allergy. Curr Allergy Asthma Rep. (2014) 14:486. doi: 10.1007/s11882-014-0486-9
19. Edwin HK, Andrew B J, Corinne AK, Yamini VV, Lauren H, Ping Y, et al. Desensitization and remission after peanut sublingual immunotherapy in 1- to 4-year-old peanut-allergic children: A randomized, placebo-controlled trial. J Allergy Clin Immunol. 153:173–81. doi: 10.1016/j.jaci.2023.08.032
20. Corinne AK, Pamela AF-G, Ananth T, John TS, Robert GH, Stephen B, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. (2011) 129:448–55. doi: 10.1016/j.jaci.2011.10.023
21. Hervé P, Dioszeghy V, Matthews K, Bee K, Campbell D, Sampson H. Recent advances in epicutaneous immunotherapy and potential applications in food allergy. J Frontiers in allergy. (2023) 4:1290003. doi: 10.3389/falgy.2023.1290003
22. Pordel S, Rezaee M, Moghadam M, Sankian M. The hydrogel based allergen-coated gold nanoparticles for topical administration: A possible epicutaneous immunotherapy in pollen-sensitized mice? J Immunological investigations. (2024) 53:523–39. doi: 10.1080/08820139.2023.2298397
23. Oliver P, Jean B, Stephen RD, Jörg K-T, Mark L, Graham R, et al. One hundred and ten years of Allergen Immunotherapy: A journey from empiric observation to evidence. Allergy. (2021) 77:454–68. doi: 10.1111/all.15023
24. Gabriela S, Andreas UF, Thomas MK. Epicutaneous/transcutaneous allergen-specific immunotherapy: rationale and clinical trials. Curr Opin Allergy Clin Immunol. (2010) 10:582–6. doi: 10.1097/ACI.0b013e32833f1419
25. Lanser BJ, Leung DYM. The current state of epicutaneous immunotherapy for food allergy: a comprehensive review. Clin Rev Allergy Immunol. (2018) 55:153–61. doi: 10.1007/s12016-017-8650-3
26. Scheurer S, Toda M. Epicutaneous immunotherapy. Allergol Immunopathol (Madr). (2017) 45 Suppl:125–9. doi: 10.1016/j.aller.2017.09.007
27. Bird JA, Sanchez-Borges M, Ansotegui IJ, Ebisawa M, Ortega Martell JA. Skin as an immune organ and clinical applications of skin-based immunotherapy. World Allergy Organ J. (2018) 11:38. doi: 10.1186/s40413-018-0215-2
28. Xiong L, Lin J, Luo Y, Chen W, Dai J. The efficacy and safety of epicutaneous immunotherapy for allergic diseases: A systematic review and meta-analysis. Int Arch Allergy Immunol. (2020) 181:170–82. doi: 10.1159/000504366
29. Banatwala U, Nasir MM, Javed R, Ahmed A, Farhan SA, Ajam A. From skin to solution: exploring epicutaneous immunotherapy for peanut allergy-A systematic review and meta-analysis. Clin Rev Allergy Immunol. (2024) 66:125–37. doi: 10.1007/s12016-024-08990-8
30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
31. Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. RoB2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 28:366. doi: 10.1136/bmj.l4898
32. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
33. Dupont C, Kalach N, Soulaines P, Legoue-Morillon S, Piloquet H, Benhamou PH. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. (2010) 125:1165–7. doi: 10.1016/j.jaci.2010.02.029
34. Fleischer DM, Greenhawt M, Sussman G, Begin P, Nowak-Wegrzyn A, Petroni D, et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA. (2019) 321:946–55. doi: 10.1001/jama.2019.1113
35. Greenhawt M, Sindher SB, Wang J, O’Sullivan M, du Toit G, Kim EH, et al. Phase 3 trial of epicutaneous immunotherapy in toddlers with peanut allergy. N Engl J Med. (2023) 388:1755–66. doi: 10.1056/NEJMoa2212895
36. Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol. (2017) 139:1242–52 e9. doi: 10.1016/j.jaci.2016.08.017
37. Petroni D, Begin P, Bird JA, Brown-Whitehorn T, Chong HJ, Fleischer DM, et al. Varying doses of epicutaneous immunotherapy with viaskin milk vs placebo in children with cow’s milk allergy: A randomized clinical trial. JAMA Pediatr. (2024) 178:345–53. doi: 10.1001/jamapediatrics.2023.6630
38. Sampson HA, Shreffler WG, Yang WH, Sussman GL, Brown-Whitehorn TF, Nadeau KC, et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: A randomized clinical trial. JAMA. (2017) 318:1798–809. doi: 10.1001/jama.2017.16591
39. Spergel JM, Elci OU, Muir AB, Liacouras CA, Wilkins BJ, Burke D, et al. Efficacy of epicutaneous immunotherapy in children with milk-induced eosinophilic esophagitis. Clin Gastroenterol Hepatol. (2020) 18:328–36 e7. doi: 10.1016/j.cgh.2019.05.014
40. Alvarez-Florian L, Paula-Garcia W, Gutierrez-Brito G, Vaisberg V, Vakharia H, Moralez G, et al. The efficacy and safety of epicutaneous immunotherapy for milk allergy: A systematic review. Principles Pract Clin Res J. (2023) 9:12–6. doi: 10.21801/ppcrj.2023.91.3
41. Gernez Y, Nowak-Węgrzyn A. Immunotherapy for food allergy: are we there yet? J The Journal of Allergy. (2017) 5:250–72. doi: 10.1016/j.jaip.2016.12.004
42. Ling-Jen W, Shu-Chi M, Ming-I L, Tseng-Chen S, Bor-Luen C, Cheng-Hui L. Clinical manifestations of pediatric food allergy: a contemporary review. J The journal of allergy. (2021) 62:180–99. doi: 10.1007/s12016-021-08895-w
43. Vickery B, Vereda A, Casale T, Beyer K, du Toit G, Hourihane J, et al. AR101 oral immunotherapy for peanut allergy. J The New England Journal of Medicine. (2018) 379:1991–2001. doi: 10.1056/NEJMoa1812856
44. Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Benhamou P, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. (2011) 186:5629–37. doi: 10.4049/jimmunol.1003134
45. Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, Benhamou P, et al. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy. (2010) 40:659–67. doi: 10.1111/j.1365-2222.2009.03430.x
46. Li W, Zhang Z, Saxon A, Zhang K. Prevention of oral food allergy sensitization via skin application of food allergen in a mouse model. J Allergy. (2012) 67:622–9. doi: 10.1111/j.1398-9995.2012.02798.x
47. Kaori Zaiki F, Mariko Akita F, Kazuma M, Noriyasu O, Koichi N, Kazuhiko H, et al. Physiological skin characteristics of infants and children compared to those of women. Cureus. (2022) 13:e19904. doi: 10.7759/cureus.19904
48. Georgios NS, Pierre-Francois R, Elise B-A, Imane L, Thierry O. Skin maturation from birth to 10 years of age: Structure, function, composition and microbiome. Exp Dermatol. (2023) 32:1420–9. doi: 10.1111/exd.14843
49. Akutsu N, Ooguri M, Onodera T, Kobayashi Y, Katsuyama M, Kunizawa N, et al. Functional characteristics of the skin surface of children approaching puberty: age and seasonal influences. Acta Derm Venereol. (2009) 89:21–7. doi: 10.2340/00015555-0548
50. Jessica S, Scott S, Robert W. The natural history of food allergy. J Allergy Clin Immunol Pract. (2016) 4:196–203. doi: 10.1016/j.jaip.2015.11.024
51. Minji K, Ji Young L, Hea-Kyoung Y, Ho Jeong W, Kyunga K, Jihyun K, et al. The natural course of immediate-type cow’s milk and egg allergies in children. Int Arch Allergy Immunol. (2019) 181:103–10. doi: 10.1159/000503749
52. Maria S, Scott HS, Robert AW, Stacie MJ, Donald YML, Alice KH, et al. Early epitope-specific IgE antibodies are predictive of childhood peanut allergy. J Allergy Clin Immunol. (2020) 146:1080–8. doi: 10.1016/j.jaci.2020.08.005
53. Mayte S-F, Maria S, Henry TB, Rohit R, Robert G, George D, et al. Evolution of epitope-specific IgE and IgG(4) antibodies in children enrolled in the LEAP trial. J Allergy Clin Immunol. (2021) 148:835–42. doi: 10.1016/j.jaci.2021.01.030
Keywords: epicutaneous immunotherapy, food allergy, desensitization, adverse reaction, children
Citation: Chen B, Gao H, Li X, Zou Z, Wu S and Tang F (2024) Epithelial immunotherapy for food allergy in children: a systematic review and meta-analysis. Front. Immunol. 15:1510653. doi: 10.3389/fimmu.2024.1510653
Received: 13 October 2024; Accepted: 06 December 2024;
Published: 23 December 2024.
Edited by:
Danijela Apostolovic, Karolinska Institutet (KI), SwedenReviewed by:
Dominique M.A Bullens, KU Leuven, BelgiumIvana Prodic, Institute of Virology, Vaccines and Sera “Torlak”, Serbia
Copyright © 2024 Chen, Gao, Li, Zou, Wu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xihong Li, bGl4aWhvbmdoeGV5QDE2My5jb20=; Hu Gao, ZHJnYW9odUAxNjMuY29t
 Bin Chen
Bin Chen Hu Gao
Hu Gao Xihong Li
Xihong Li Zhuan Zou
Zhuan Zou Shanshan Wu1,2
Shanshan Wu1,2