- 1College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2College of Integrated Chinese and Western Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Experimental Teaching and Practical Training Center, Heilongjiang University of Chinese Medicine, Harbin, China
The cGAS-STING signaling pathway is a critical component of the innate immune response, playing a significant role in various diseases. As a central element of this pathway, STING responds to both endogenous and exogenous DNA stimuli, triggering the production of interferons and pro-inflammatory cytokines to enhance immune defenses against tumors and pathogens. However, dysregulated activation of the STING pathway is implicated in the pathogenesis of multiple diseases, including autoinflammation, viral infections, and cancer. Traditional Chinese Medicines (TCMs), which have a long history of use, have been associated with positive effects in disease prevention and treatment. TCM formulations (e.g., Lingguizhugan Decoction, Yi-Shen-Xie-Zhuo formula) and active compounds (e.g., Glabridin, Ginsenoside Rd) can modulate the cGAS-STING signaling pathway, thereby influencing the progression of inflammatory, infectious, or oncological diseases. This review explores the mechanisms by which TCMs interact with the cGAS-STING pathway to regulate immunity, focusing on their roles in infectious diseases, malignancies, and autoimmune disorders.
Highlights
● Traditional Chinese Medicine (TCM) has a rich history of preventing and treating many diseases. This review explores how TCM modulates the cGAS-STING signaling pathway and its therapeutic potential.
● To elucidate the intricate relationship between the STING pathway and different diseases, and to analyze TCM as a potential agonist or inhibitor of the STING pathway.
● By conducting an extensive literature review, we explore the key proteins within the cGAS-STING pathway and their significance as biomarkers in TCM-based immunomodulation and the treatment of various diseases.
1 Introduction
Immune system homeostasis is vital to overall health, as proper immune regulation ensures normal physiological functions, while dysregulation can lead to various diseases (1, 2). The innate immune system play a crucial role in recognizing pathogen-associated molecular patterns and danger-associated molecular patterns through pathogen recognition receptors. These receptors form as the first line of defense against bacterial and viral infections, as well as aseptic inflammatory, by triggering the production of pro-inflammatory and anti-viral cytokines (3).
The cGAS-STING signaling pathway, as an important element of innate immunity, has garnered significant attention in recent years for its role in maintaining immune system homeostasis (4). This pathway plays a crucial role in antitumor immunity, and inflammatory and infectious diseases, as it recognizes various sources of cytoplasmic DNA, including bacterial, viral, and mitochondrial DNA (5). Upon detection of cytoplasmic DNA, cGAS generates cyclic GMP-AMP (2′3′-cGAMP), which activates STING. This process leads to TANK-binding kinase 1 (TBK1) phosphorylation (pTBK1) and induces type I interferon (IFN-I) transcription (6–8). In antiviral infections, STING acts through an IFN-I-driven immune response (7, 9). Activation of cGAS-STING enhances the ability of immune cells to target antigens through multiple pathways, thereby defending against pathogen invasion (10, 11). However, structural or functional abnormalities in this pathway may also contribute to the development of autoimmune and inflammatory diseases, such as systemic lupus erythematosus (SLE) and non-alcoholic fatty liver disease (NAFLD). Although cGAS is able to sense double-stranded DNA (dsDNA), it is unable to distinguish between its own DNA and exogenous DNA (12). Prolonged stimulation of aberrant DNA can lead to the overactivation activation of the STING pathway, resulting in excessive synthesis and release of IFN-I and inflammatory cytokines, which drive the progression of inflammatory or autoimmune diseases (13). In addition, tumor-derived dsDNA activates the STING pathway in antigen-presenting cells, promoting interferon production. This process facilitates dendritic cell maturation, T cell recruitment, and enhances anti-tumor responses (14).
Traditional Chinese Medicine (TCM) has a long history, traditionally employed for the prevention and treatment of a wide spectrum of diseases. Studies have shown that TCM has a bidirectional regulatory effect in immunomodulation, both activating the immune system and suppressing excessive immune responses (15). For example, ginseng contains a variety of active ingredients (e.g., ginsenosides, ginseng polysaccharides) that have immunomodulatory effects. A randomized controlled trial showed that ginseng polysaccharide for 8-14 weeks enhanced the cytotoxic activity of NK cells and up-regulated serum TNF-α levels (16). TCM has been associated with potential benefits in adjunct cancer therapy, particularly in alleviating clinical symptoms, extending patient survival, and modulating immune functions (17–19). TCM principles identify ‘yang deficiency’ as the underlying cause of breast cancer. The classic anti-neoplastic formula, Yanghe Decoction (YHD), has been shown in contemporary research to decrease myeloid-derived suppressor cells (MDSCs) and suppress the tumor microenvironment’s iNOS and ARG-1 expression. At the same time, it enhances the immune response by increasing the number of natural killer T cells (NKTs) and CD4 T cells (20). Unlike chemotherapy, traditional Chinese medicine (TCM) not only directly targets tumor cells but also effectively boosts the immune response. For instance, the TCM formula Shugan Jianpi Decoction has been shown to suppress the proliferation of MDSCs and enhance the inflammatory regulatory functions of NKT cells (21). In addition, certain herbal components, such as astragaloside derived from Huangqi (Radix Astragali), can significantly promote IFN-γ secretion from T cells and enhance T cell immunoreactivity (22).
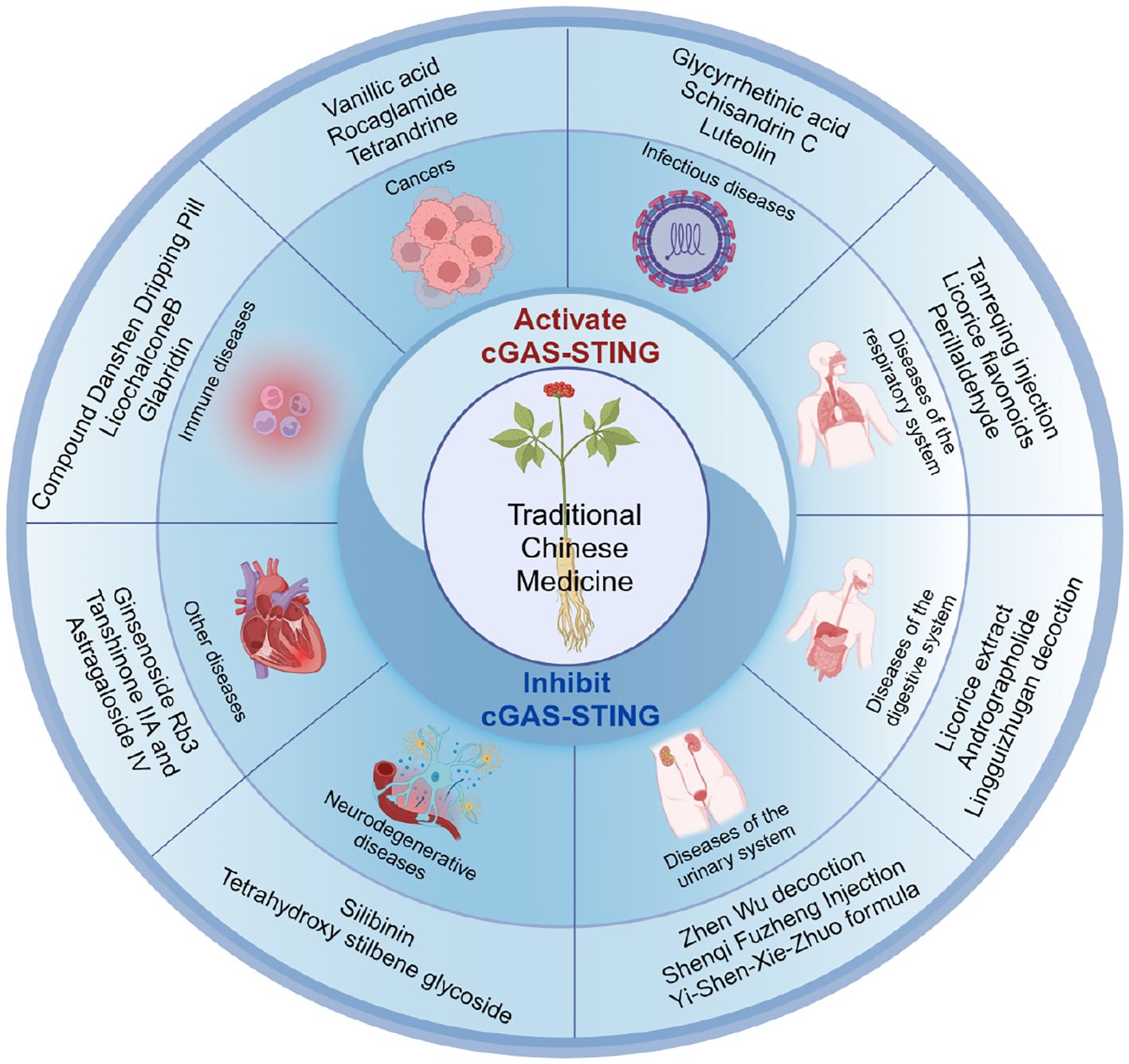
Increasing attention has been given to the immunomodulatory potential of TCM, particularly the ability of TCM active compounds or formulations to address diseases such as inflammation, infection, and cancer, by modulating the cGAS-STING signaling pathway (Figure 1). By regulating this pathway, Chinese medicines may enhance the body’s immune response to pathogens and tumors, as well as inhibit excessive immune responses. However, these effects are primarily observed in preclinical studies investigating underlying mechanisms of disease treatment. Further well-designed clinical trials are necessary to confirm these findings.
Figure 1. A VOSviewer analysis of key terms related to TCM and the cGAS-STING signaling pathway underscores their significance as prominent and actively explored research areas. Created with VOSviewer.
Despite the growing recognition of the cGAS-STING signaling pathway’s significance in immunomodulation, comprehensive reviews on how TCMs target this pathway remain scarce. This paper aims to address this gap by summarizing recent advancements in understanding the cGAS-STING pathway’s role in immune regulation and the potential mechanisms underlying herbal interventions. The findings are expected to serve as a valuable reference for future in-depth mechanistic research.
2 Structural characterization of STING and its application in clinical therapy
Innate immunity serves as the body’s first line of defense against invading pathogens (23). The stimulator of interferon genes (STING) is a critical protein that mediates various DNA receptors in this system. STING, also referred to as ERIS, MYPS, and MITA, is a conserved transmembrane protein encoded by the TMEM173 gene. Predominantly localized in the endoplasmic reticulum, it is also found on Golgi and mitochondrial membranes (24). STING consists of 379 amino acids and contains an N-terminal transmembrane region and a C-terminal cytoplasmic globular structural domain, which interacts with another STING molecule to form an intact dimer (25).
STING detects cytoplasmic dsDNA and serves as a direct sensor for endogenous cyclic dinucleotides (CDNs) (26). Activation of cGAS, a nucleotidyltransferase that senses cytoplasmic non-self DNA, catalyzes the production of 2′3′-cGAMP, a CDN composed of adenosine and guanosine (Figure 2) (27, 28). In addition to cGAMP, STING can be triggered by bacterial-derived CDNs, such as cyclic di-AMP and cyclic di-GMP (29). CDNs and 2′3′-cGAMP bind to STING in the endoplasmic reticulum, facilitating the dimerization and translocation of STING to the perinuclear region (29, 30). STING recruits TBK1 and IκB kinase (IKK) during translocation, which then relocates to the perinuclear region. These kinases phosphorylate interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), which activates the expression of IFN-I and pro-inflammatory cytokines (31–33). IFN-I enhances immune responses by promoting the activation and function of immune cells such as dendritic cells, T cells, and natural killer cells (34).
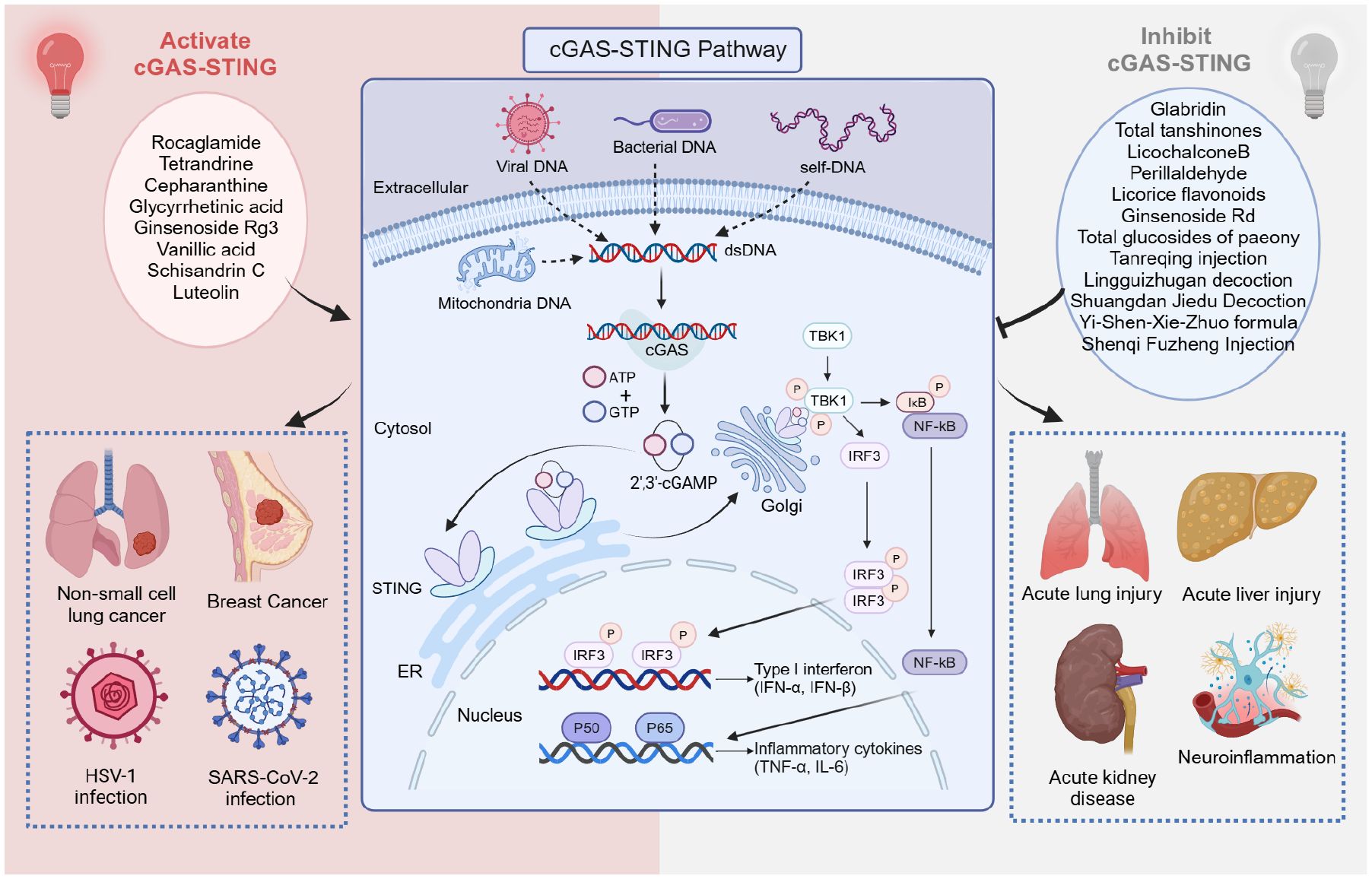
Figure 2. Mechanisms of cGAS-STING pathway activation, Chinese medicine inhibits or activates cGAS-STING signaling pathway to treat various diseases. Created with BioRender.com.
The cGAS-STING pathway is a conserved innate immune mechanism that responds to pathogenic infections, DNA damage, and aberrant cell activities like uncontrolled replication or senescence (35). In cancer therapy, activation of STING enhances tumor antigen presentation and promotes antitumor immunity, making it an attractive immunotherapeutic target (36). Studies have shown that cGAS-STING agonists not only induce tumor cell senescence but also boost adaptive anticancer immunity and combine efforts with immunotherapy (37, 38). Clinical trials have explored two main types of STING agonists: cyclic dinucleotides (CDNs, e.g., ADU-S100) and non-CDN (e.g., DMXAA) (39, 40). For instance, ADU-S100, the first CDN derivative in clinical trials, has demonstrated the ability to stimulate IFN-β production by human immune cells, showcasing its therapeutic potential (41). Additionally, cGAS activity is critical for the success of immune checkpoint blockade therapies, with STING agonists showing promise in enhancing vaccine efficacy for tumors resistant to PD-1 inhibitors (42).
In addition to cancer therapy, the cGAS/STING pathway plays a vital role in viral infections. Many DNA viruses, such as herpes simplex virus (HSV) and hepatitis B virus (HBV), are able to inhibit viral replication by initiating IFN-I production through activation of this pathway (43, 44). This suggests that STING agonists may have broad-spectrum antiviral potential (45). For example, DMXAA is a potent antiviral agent in mice; α-Mangostin, a flavonoid with antimicrobial properties, has been shown to possess antiviral properties and anti-DENV and HBV replicative activity in cellular experiments (46–48). However, numerous viruses such as HSV, Human CMV (HCMV), etc. have evolved mechanisms to circumvent this pathway (49–51). For instance, Epstein-Barr virus (EBV) suppresses localized innate immunity by targeting STING for degradation through the E3 ubiquitin ligase TRIM29 (52).
However, despite its protective roles, excessive activation of STING can lead to autoimmune conditions like Aicardi-Goutières syndrome (AGS) and SLE (53–55). In these diseases, both autologous DNA and mitochondrial DNA may be misrecognized by cGAS, leading to the activation of STING and an excessive IFN-I response (56). To address this, inhibitors targeting STING or cGAS have emerged as therapeutic candidates. Compounds like Acrinamin and Oxychloroquine show potential in blocking cGAS activation, offering new avenues for treating autoimmune disorders (57).
Although several STING agonists and inhibitors have been developed, they have had limited effect in clinical translation. TCM offers a valuable resource for the development of STING modulators, including inhibitors and activators. This underscores TCM’s potential to address various diseases through immunomodulatory mechanisms, providing a robust foundation for future research and drug development (Figure 2).
3 Role of Chinese medicines in immunomodulation
Chinese medicines play a vital bidirectional role in immunomodulation, both activating the immune system and suppressing excessive immune responses. This regulation is achieved by influencing various aspects such as immune cells, cytokines, and immune organs. Studies have shown that certain Chinese medicines can effectively regulate the production of immune cells and cytokines (58, 59). Certain herbal medicines boost innate immune system activity, while others act on cellular subpopulations of adaptive immunity (60).
Chinese medicines act by enhancing the function of various immune cells, including macrophages, dendritic cells, NK cells, T cells, and B cells. For example, Ganoderma lucidum polysaccharide (PS-G), the primary active compound in Ganoderma lucidum, has been shown to promote activation and maturation of dendritic cells derived from human monocytes (61). Herbal medicines also regulate T lymphocyte activity by stimulating their proliferation and differentiation, promoting cytotoxic T lymphocyte production, and modulating the TH1/TH2 balance as well as the function of T helper (TH) cell subsets (59). For example, polysaccharides from Cordyceps sinensis enhance the expression of transcription factors such as T-bet, GATA-3, and RoR-γt in TH cells, thereby increasing the number of TH1, TH2, and TH17 cells (62). B lymphocytes, the main cells of humoral immunity, depend on antigen stimulation to release antibodies (63). Research indicates that polysaccharides from Dendrobium huoshanense and Atractylodes macrocephala Koidz significantly increase B lymphocyte populations, thereby strengthening humoral immunity (64, 65).
In addition, herbal medicines can regulate the production of specific cytokines, including interferons (IFN-α, IFN-β, IFN-γ), tumor necrosis factor (TNF-α), and interleukins (e.g., IL-1, IL-2, IL-4), which are pivotal in immune and inflammatory processes. For example, polysaccharides from Atractylodes macrocephala and Astragalus membranaceus markedly upregulated IFN-γ expression in vitro experiments (66, 67).
The diversity of immunomodulatory components in TCM provides a wide range of therapeutic potential for clinical applications. These components are mainly divided into two categories: anti-inflammatory and immune-enhancing. The anti-inflammatory category includes phenolic acids (e.g., vanillic acid, salvianolic acid B), flavonoids (e.g., luteolin, glabridin), volatile oils (e.g., perillaldehyde, zingiber officinale), lignans (e.g., schisandrin C, asarinin), and alkaloids (e.g., rocaglamide, tetrandrine), while the immune-enhancing category mainly consists of polysaccharides (e.g., lycium barbarum polysaccharides, astragalus membranaceus polysaccharides) and glycosides (e.g., ginsenoside Rg3, ginsenoside Rd) (68–72). These components regulate the body’s immune response through different pathways, enabling TCM to demonstrate unique advantages in the treatment of immune-related diseases.
Additionally, TCM is closely related to the concept of “medicine and food,” i.e., certain species offer both nutritional benefits and therapeutic effects (73). With the growing emphasis on preventive care and holistic health in recent years, many TCM ingredients have been incorporated into daily diets as functional foods or dietary supplements and have become an important part of alternative therapies (74, 75).
4 The role of TCM in modulating the cGAS/STING pathway in clinical diseases
In recent years, activators and inhibitors of the cGAS-STING pathway have attracted widespread attention, but clinical translation still faces challenges. TCM, as a valuable cultural heritage of the Chinese nation, has shown promising potential in modulating immune-related diseases. Several active compounds have been found to effectively modulate the cGAS/STING signaling pathway and improve diseases. These include ginsenoside Rg3 and ginsenoside Rd, which are derived from Panax ginseng; glabridin and licochalcone B, obtained from Glycyrrhiza uralensis; perillaldehyde, isolated from Perilla frutescens; and schisandrin C, extracted from Schisandra chinensis (Figure 2). Natural products have been valued as indispensable resources for discovering novel therapeutic molecules and are instrumental in managing diseases (76–78). The mechanisms and clinical applications of TCM in modulating this pathway will be summarized below, categorized by different types of diseases.
4.1 Immune diseases
Normal activation of the cGAS-STING pathway can trigger immune responses and enhance the ability of immune cells to eliminate antigens and defend against pathogens. Nevertheless, excessive or abnormal activation of this pathway may trigger immune dysregulation, which in turn leads to the development of autoimmune diseases such as SLE and NAFLD. Research has demonstrated that TREX1 deficiency has a close association with various autoimmune diseases (e.g., AGS, SLE) and that in TREX1-deficient mouse models, deletion of cGAS or STING can ameliorate these disease phenotypes (79–81).
TCM has demonstrated promise in modulating the cGAS-STING pathway. For instance, total tanshinones, the main active ingredient of Salvia miltiorrhiza, can block STING-IRF3 binding, thereby suppressing aberrant pathway activation and alleviating autoimmune conditions associated with TREX1 deficiency TREX1 deficiency (82). Perillaldehyde (PAH), another TCM ingredient, is a natural monoterpenoid extracted from Perilla frutescens, has demonstrated the ability to inhibit STING pathway activation significantly (83). By targeting cGAS proteins, PAH reduces the interferon response, offering a potential therapeutic approach for cGAS-mediated autoimmune diseases (84).
Glabridin, an active ingredient in licorice, specifically inhibits the cGAS-STING pathway by decreasing the levels of IFN-I, IL-6, and TNF-α, thereby alleviating immune disorders triggered by TREX1 deficiency (85). In addition, Licochalcone B and Licochalcone D also showed significant anti-inflammatory effects by inhibiting STING downstream signaling and improved symptoms of inflammatory diseases, such as colitis, in experimental models (86, 87).
In addition, Compound Danshen Dropping Pills, widely utilized for managing cardiovascular conditions such as angina pectoris and acute myocardial infarction, have successfully completed Phase III clinical trials with the U.S. Food and Drug Administration (88–90). This TCM formulation has shown efficacy in reducing multi-organ inflammatory responses in TREX1-deficient mice by disrupting STING-TBK1 interactions and blocking cGAS-STING pathway activation, highlighting its therapeutic potential for inflammatory conditions, including obesity-induced insulin resistance (91).
4.2 Cancers
Tumorigenesis is a complex, multistep process, and conventional cancer research usually focuses on a single target (92). However, due to their diversity and complexity, the therapeutic effects are often limited. TCM has unique advantages in tumor therapy through holistic regulation and multi-target intervention (93, 94).
Ginsenoside Re, derived from ginseng, can regulate the host immune system and exert anticancer effects through multiple pathways (95). In non-small cell lung cancer (NSCLC), ginsenoside Re exerts antitumor effects by inhibiting the epithelial-mesenchymal transition (EMT) process. It does so through the inhibition of the AMPKα1/STING positive feedback loop and the reduction of M2-like macrophage formation (96). In addition, Rocaglamide (RocA), a compound extracted from Aglaia odorata, promotes the leakage of mitochondrial DNA (mtDNA) into the cytoplasm and activates the cGAS-STING pathway. This process increases tumor infiltration of NK cells and significantly enhances anti-tumor immunity in NSCLC (97).
Tetrandrine, derived from Stephania tetrandra S. Moore, is a bisbenzylisoquinoline alkaloid with the ability to inhibit tumor proliferation and angiogenesis (98). Tetrandrine activates the STING/TBK1/IRF3 pathway, promoting CCL5 and CXCL10 production. This enhances the infiltration of macrophages, dendritic cells, and CD8 T cells in the tumor microenvironment, significantly inhibiting the growth of NSCLC (99).
Vanillic acid is a phenolic compound present in TCMs such as Angelica sinensis and ginseng, with antioxidant and antimicrobial properties (100). It promotes macrophage polarization to the M1 type through activation of the STING pathway and enhances tumor cell apoptosis and anti-tumor immune response (101).
Breast cancer is a common tumor in women with high morbidity and mortality rates (102). Formononetin, an active ingredient in red clover and astragalus, inhibits the proliferation of BC cells by interfering with PD-L1 and inhibiting the activation of the STING-NF-κB pathway (103). Ginsenoside Rg3 inhibits tumor growth by inhibiting angiogenesis, inducing apoptosis, and other mechanisms. When combined with STING agonists, Rg3 can induce tumor-associated macrophages to polarize from M2 to M1 and improve the tumor microenvironment, effectively inhibiting the growth and invasion of triple-negative breast cancer (104).
4.3 Infectious diseases
The cGAS-STING pathway has played a crucial antiviral role during evolution, and its activation is closely linked to antiviral cellular responses (105). cGAMP synthesis is the critical first step in initiating cGAS-mediated antiviral effects. The downstream effects mainly include the synthesis of antiviral type I interferon and related genes (106, 107). TCM provides a rich source of natural compounds, and many herbs show antiviral, anti-inflammatory, and immunomodulatory effects, making them potential candidates for the development of antiviral drugs.
Schisandra chinensis (Turcz.) Baill., a long-established TCM, has been shown to modulate host immunity and exhibit anticancer, antiviral, and hepatoprotective effects (108, 109). Its active ingredient, Schisandrin C, was found to inhibit HBV replication by promoting the interaction between TBK1 and STING, enhancing the activation of the cGAS-STING pathway and promoting the expression of IFN-β and interferon-stimulated genes (110). Another active ingredient is luteolin, a natural flavonoid found in various plants (111). Research indicates that luteolin combats HSV-1 by activating the cGAS-STING pathway, thereby enhancing antiviral interferon production (112). Liuwei Wuling Tablet consists of various ingredients, including Schisandra chinensis and chasteberry, which have been shown to nourish the kidneys and liver while also exhibiting antiviral activity (113). The combination of Schisandrin C and Luteolin has been found to inhibit HBV replication and attenuate HBV infection by activating the cGAS-STING pathway (114).
Glycyrrhetinic acid (GA), a major constituent of licorice, exhibited anti-inflammatory, antioxidant, and antiviral effects during the COVID-19 pandemic (115, 116). GA was found to inhibit SARS-CoV-2 infection by activating cGAS-STING pathway (117). Cepharanthine (CEP) has demonstrated inhibitory effects against viruses such as HIV, SARS, and HSV-1 (118). CEP promotes cellular autophagy, thereby inhibiting HSV-1 infection (119). Euphorbia fischeriana Steud is a perennial herb whose root has traditionally been utilized in TCM to treat diseases such as cancer, edema, and ascites. Dpo, a compound isolated from the root of E. fischeriana, has been found to activate antiviral innate immune responses by targeting STING and utilizing the IRFs/ELF4 pathway (120). Similarly, Ginsenoside Rg3 has been shown to stimulate a type I interferon response via the cGAS-STING signaling axis. This response is supported by gut-derived short-chain fatty acids like acetate and propionate, offering protection against enteroviral infections (104).
In the context of sepsis—a severe systemic inflammatory condition triggered by bacterial or fungal infections and often leading to multiple organ dysfunction—Glycyrrhiza uralensis polysaccharides have demonstrated protective effects. These are achieved by disrupting the interactions between STING, TBK1, and IRF3, thereby reducing cGAS-STING pathway activation and mitigating sepsis-related damage (121).
4.4 Diseases of the respiratory system
Acute lung injury (ALI) is a serious lung disease recognized globally, manifesting as a persistent acute inflammatory response that is associated with high morbidity and mortality (122). Despite significant advances in therapy, treating ALI remains a major clinical challenge. The cGAS-STING pathway plays a vital role in the pathogenesis of ALI, affecting immune response, apoptosis, vascular permeability, and oxidative stress, which exacerbate inflammation and tissue damage (123). Various herbal medicines can improve ALI or pulmonary fibrosis by modulating this pathway (Table 1).
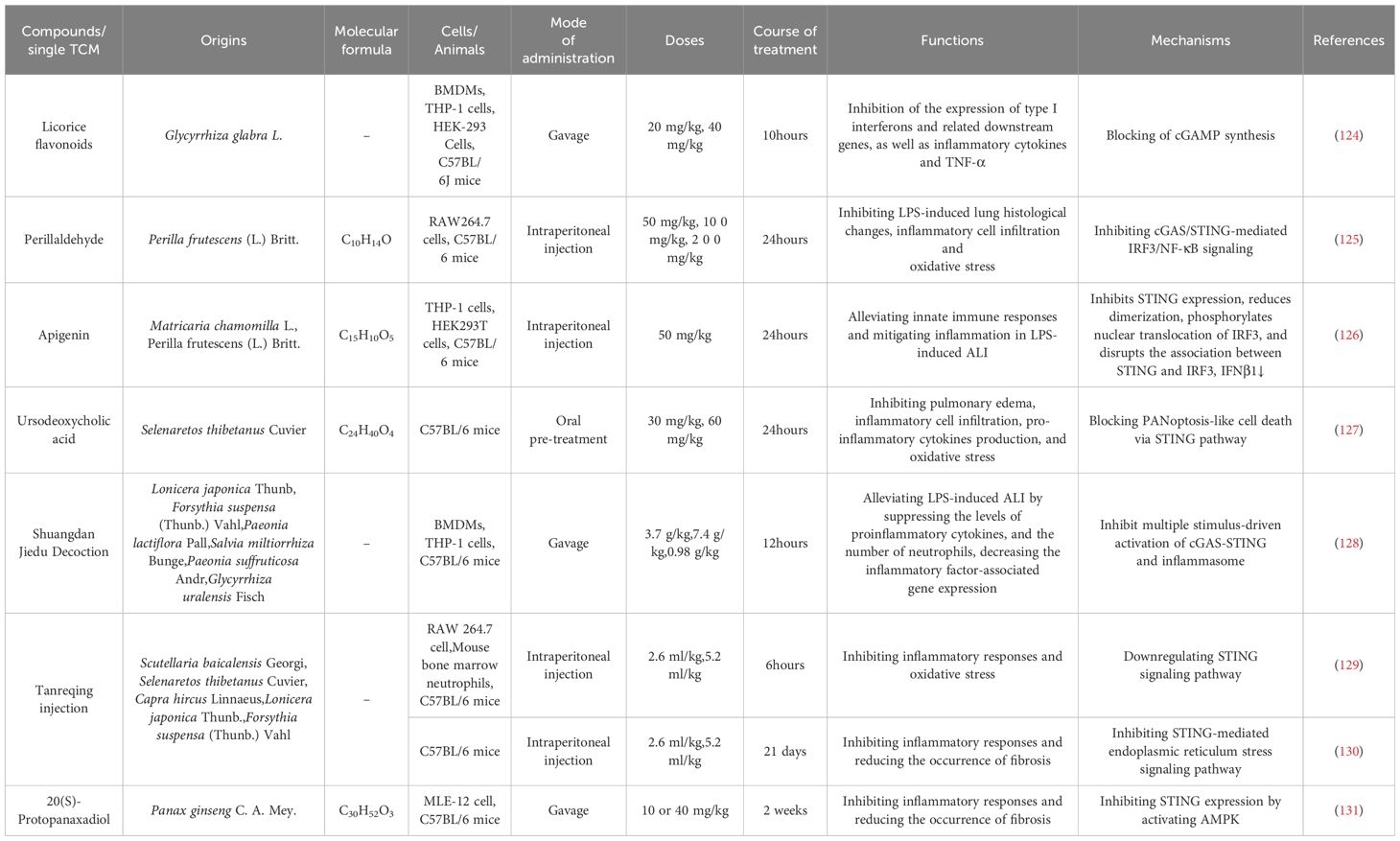
Table 1. Traditional Chinese medicine can treat or alleviate respiratory diseases by regulating the cGAS-STING signaling pathway.
For example, licorice flavonoids possess anti-inflammatory activity and inhibit cGAMP synthesis, thereby preventing overactivation of the cGAS-STING pathway and ameliorating lipopolysaccharide (LPS)-induced ALI (124). Perillaldehyde alleviated acute lung injury by inhibiting the cGAS-STING-mediated IRF3/NF-κB pathway (125). Additionally, apigenin and ursodeoxycholic acid (UDCA) have demonstrated efficacy in alleviating ALI by inhibiting STING-related signaling pathways. Apigenin attenuates the LPS-induced inflammatory response by inhibiting the STING/IRF3 pathway, whereas UDCA mitigates sepsis-induced lung injury by blocking cell death via the STING pathway (126, 127). Traditional Chinese medicine compound preparations, such as Shuangdan Jiedu Decoction and Tanreqing injection (TRQ), have also significantly ameliorated LPS-induced ALI and other respiratory-related diseases by regulating the STING pathway through multiple mechanisms (128, 129). TRQ is a proprietary Chinese medicine that is commonly used for lung diseases such as pneumonia and idiopathic pulmonary fibrosis (IPF) (132–134). Clinical evidence suggests that TRQ can alleviate the development of pulmonary fibrosis and improve lung function in patients (130). Recent studies have shown that 20(S)-Protopanaxadiol, isolated from ginseng, and TRQ can improve pulmonary fibrosis by modulating the cGAS-STING pathway (130, 131).
4.5 Diseases of the digestive system
Liver fibrosis is a chronic liver disease triggered by various factors, including excessive alcohol consumption, viral infections (HBV and HCV), and non-alcoholic steatohepatitis (NASH) (135–137). Recent studies have shown that the cGAS-STING pathway plays an important role in the pathological process of liver fibrosis, and various traditional Chinese medicines can exert anti-fibrotic effects by regulating this pathway.
Naringenin, an anti-inflammatory flavonoid extracted from citrus plants, has been shown to directly bind to cGAS (138). It reduces inflammatory factors secreted by hepatic stellate cells by inhibiting the cGAS-STING pathway, thereby alleviating liver fibrosis (139). Licorice extract improved hepatic inflammation and fibrosis in a mouse model of NASH, with its mechanism of action including inhibition of the cGAS-STING pathway (140). Oroxylin A, a baicalin derivative, activated the cGAS-STING pathway, promoted the secretion of cytokine IFN-β, induced hepatic stellate cell senescence, and acted as an antifibrotic agent (141).
Modulation of the cGAS-STING pathway by TCM can also alleviate acute liver injury. For example, total glucosides of paeon, on the other hand, reduced hepatic inflammation in an acute liver injury (ALI) model by inhibiting the STING-IRF3 interaction (142). Ginsenoside Rd protects mice from CCl4-induced ALI by inhibiting the cGAS-STING pathway and reducing iron death (143).
Lingguizhugan Decoction (LGZG) is a traditional Chinese herbal decoction that has been used for many years in the treatment of metabolic disorders and has been effective in alleviating obesity and dyslipidemia (144, 145). LGZG significantly reduced high-fat diet (HFD)-induced hepatic lipid deposition by inhibiting the STING-TBK1-NF-κB pathway in hepatic macrophages (146).
Additionally, drug-induced liver injury is a leading cause of acute liver injury and liver transplantation (147). Studies have shown that jujuboside B ameliorated acetaminophen-induced liver injury by upregulating Nrf2 protein expression and inhibiting the cGAS-STING pathway (148). Similarly, rhodopsin protected hepatocytes from APAP-induced toxicity by regulating Nrf2 and NLRP3 inflammatory vesicles, while inhibiting the cGAS-STING pathway (149).
Andrographolide, derived from Andrographis paniculata, has been shown to ameliorate chemotherapeutic drug-induced gastrointestinal mucosal inflammation by down-regulating the cGAS-STING pathway (150). Naringin can also attenuate intestinal ischemia-reperfusion injury by inhibiting the cGAS-STING pathway (151).
4.6 Diseases of the urinary system
Acute kidney injury (AKI) is a global health problem. Although cisplatin is an effective chemotherapeutic agent, its nephrotoxicity limits clinical use (152). Therefore, there is a need for nephroprotective drugs that are safe and do not compromise the antitumor effect. TCMs are widely used for preventing and treating renal diseases. From the Western medicine perspective, cisplatin triggers AKI primarily due to drug toxicity or edema, while from the TCM perspective, its pathogenesis involves spleen and kidney qi deficiency, damp-heat underflow, and blood stasis (153). Various traditional Chinese medicines and compound formulas can effectively alleviate cisplatin-induced AKI by regulating the cGAS/STING pathway.
Yi-Shen-Xie-Zhuo formula (YSXZF) is a Chinese herbal formula composed of four herbs: Astragali Radix (Huangqi), Alismatis Rhizoma (Zexie), Paeoniae Radix Rubra (Chishao), Sargassum (Haizao). Studies have shown that YSXZF can inhibit the cGAS/STING pathway, reduce the expression of inflammatory factors such as TNF-α, IL-3, and IL-1β, and decrease IRF1 activity, which in turn reduces the inflammatory response and prevents acute kidney injury (154). Shenqi Fuzheng Injection (SQFZ) consists of extracts from Codonopsis Radix and Astragali Radix, both of which possess anti-tumor and anti-inflammatory effects. It has been found that SQFZ can effectively inhibit the cGAS/STING pathway, attenuate cisplatin-induced nephrotoxicity, and improve the effectiveness of chemotherapeutic agents (155).
To improve the bioavailability of active ingredients in traditional Chinese medicine, recent studies have explored the use of nanotechnology. For example, baicalein (5,6,7-trihydroxyflavone, BA) possesses antioxidant and antitumor effects, but its poor water solubility and low bioavailability limit its clinical application. Self-assembly of silk fibroin peptide (SFP) into nanofibers encapsulating baicalein (SFP/BA NFs) enhances its in vivo efficacy, inhibits cisplatin-induced DNA damage and cGAS/STING pathway activation, and exerts a nephroprotective effect to prevent AKI (156).
Similarly, naringenin (NGN) has poor water solubility, limiting its application. To address this, researchers have developed NGN-loaded silk fibroin peptide nanofibers (SFP/NGN NFs). Cisplatin-induced mitochondrial damage leads to the release of mtDNA and activation of the cGAS-STING pathway, which in turn triggers the expression of inflammatory factors, such as IL-6 and TNF-α. SFP/NGN NFs effectively attenuated cisplatin-induced acute kidney injury by facilitating mitochondrial autophagy, decreasing the release of mtDNA and inhibiting the cGAS-STING pathway (157).
In addition, Zhen Wu decoction (a prescription composed of five herbs: Radix Aconiti lateralis Preparata, Poria, Radix Paoniae alba, ginger, and Rhizoma Atractylodis macrocephalae, which are decocted together for extraction) inhibited renal fibrosis by activating NRF2 and TFAM in renal tubules and promoting mitochondrial bioenergy production (158).
4.7 Neurodegenerative diseases
Neurodegenerative diseases are a group of chronic neurological disorders characterized by a progressive loss of neurons and an abnormal accumulation of specific proteins in the brain, accompanied by a decline in cognitive and motor function (159). This group includes Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) (160). Among these, Alzheimer’s disease (AD) is the most common neurodegenerative disorder worldwide, manifesting as severe cognitive decline (161).
As an anti-aging traditional Chinese medicine, Polygonum multiflorum has received widespread attention for its role in diseases such as AD, PD, and MS (162). Studies have shown that tetrahydroxy stilbene glucoside (TSG), the main active ingredient of Polygonum multiflorum, possesses significant anti-inflammatory, anti-aging, and memory-improving effects (163). TSG prevents neuroinflammation by modulating the cGAS-STING pathway, leading to significant improvement in cognitive decline in AD patients. In addition, TSG can reduce the formation of NLRP3 inflammatory vesicles by inhibiting the activation of the cGAS-STING pathway, thereby reducing the neuroinflammatory response and demonstrating its potential therapeutic value in Alzheimer’s disease (164).
Silibinin, an active ingredient extracted from the TCM silymarin, has attracted attention for its neuroprotective effects in AD models. Research has found that silibinin administration, downregulated the levels of IL-1β, TNF-α and IFN-β, as well as STING and IRF3, ameliorating depression/anxiety-like behaviors of Parkinson’s disease mouse model (165). While these findings suggest that silibinin may modulate the cGAS-STING pathway, it is important to note that the inhibition of pro-inflammatory cytokines such as IL-1β and TNF-α could also involve other signaling pathways, including NLRP3 inflammasome activation, NF-κB signaling, and the MAPK pathway (166, 167). And silibinin exerts significant neuroprotective effects by downregulating iron death injury and STING-mediated neuroinflammation, particularly in the STZ-induced sporadic AD model. This provides an important basis for silymarin as a potential drug for the treatment of AD (168). Given the multi-target nature of TCM, further studies are needed to clarify the mechanisms underlying silibinin’s effects on neuroinflammation and behavioral outcomes.
4.8 Other diseases
In addition to autoimmune diseases, tumors, and viral infections, a variety of Chinese herbal medicines can ameliorate other diseases by impacting the cGAS-STING pathway, potentially in conjunction with other molecular targets. For example, atherosclerosis is a chronic inflammatory disease of the arterial lining (169). Tetrandrine was found to inhibit the STING/TBK1/NF-κB pathway, reducing inflammation in macrophages attacked by oxidized low-density lipoprotein, and attenuating atherosclerosis in HFD-fed ApoE mice (170).
Myocardial ischemia-reperfusion injury (MIRI) is a major challenge in the treatment of acute myocardial infarction, primarily caused by oxidative stress and inflammatory responses induced by blood reperfusion (171, 172). Astragalus membranaceus (Fisch.) Bunge and Salvia miltiorrhiza Bunge are representative herbs used for replenishing Qi and activating blood circulation in traditional Chinese medicine, respectively. According to the compatibility theory of traditional Chinese medicine (173, 174), they are often used in combination (175). Astragaloside IV (As-IV) and Tanshinone IIA (Ta-IIA) are the primary active components of Astragalus membranaceus and Salvia miltiorrhiza, respectively. Research has indicated that the combined use of As-IV and Ta-IIA significantly reduces oxidative stress and apoptosis in cardiomyocytes by enhancing the inhibition of cGAS/STING signaling, thereby improving the therapeutic effect on MIRI (176).
In skin flap transplantation, ischemia/reperfusion (I/R) injury is the main cause of flap necrosis (177, 178). Ginsenoside Rb3, an active component of ginseng, has been shown to reduce leukocyte-endothelial cell adhesion and improve local microcirculation by inhibiting the phosphorylation of IRF3 in the STING pathway, effectively alleviating I/R injury in transregional flaps (179).
In addition, overactivation of the cGAS-STING pathway is closely related to cellular senescence. Liuwei Dihuang (LWDH), a classic Chinese herbal formula, shows potential for anti-endothelial cellular senescence. Studies have shown that LWDH reverses LPS-induced endothelial cell senescence by inhibiting the activation of the cGAS-STING pathway and blocking the interaction between JPX and STING. This provides a new approach for preventing and treating vascular endothelial cell aging (180).
In conclusion, TCM has demonstrated significant therapeutic potential in diseases such as atherosclerosis, myocardial ischemia-reperfusion injury, skin flap transplantation injury, and cellular senescence by modulating the cGAS/STING pathway. These studies provide a new scientific basis for the application of TCM in the treatment of modern diseases, as well as insights for the clinical development of more targeted TCM.
5 cGAS-STING pathway key proteins as biomarkers for TCM in immunomodulation and treatment of various diseases
Due to their multi-component and multi-target characteristics, traditional quality control methods have difficulty comprehensively assessing the safety and efficacy of Chinese medicines (181–183). To cope with these challenges, biomarkers have shown significant potential as tools for quality evaluation of TCM in recent years. Using technologies such as metabolomics, biomarkers can more comprehensively assess the systemic effects and compatibility of TCM. The components of schisandrol A, schisandrin A, gomisin N, and schisandrin B can be used as biomarkers for evaluating the quality standard of Schisandra chinensis (Turcz.) Baill (184). In addition, biomarkers can evaluate the clinical efficacy of TCM, such as NF2 and PPP1CA in CDDP, which are thought to be associated with its vasodilatory effects (185).
Biomarkers are equally important in disease treatment. In viral infections, IFN, a central factor in the antiviral response, has emerged as a potential therapeutic target for infections such as HCV and HBV (186). IL-6 plays an important role in the acute inflammatory response, and changes in its level correlate with the severity of infection. Especially in COVID-19, elevated IL-6 levels are closely associated with disease progression, suggesting its potential as a marker for monitoring treatment efficacy (187).
The cGAS-STING pathway is an important part of the innate immune system and has emerged as a potential therapeutic target for a variety of diseases in recent years (188–190). cGAS recognizes intracellular DNA and activates STING proteins, which in turn initiates downstream signaling pathways and induces antiviral and pro-inflammatory factors (191). cGAS-activated signaling molecules, such as TBK1 and IRF3, play a key role in immunoregulation (10). These proteins are not only key regulators of disease progression, but they may also be important biomarkers for evaluating therapeutic effects.
Traditional Chinese medicine plays an immunomodulatory role in the treatment of many diseases by modulating the cGAS-STING pathway. For example, total glucosides of paeony can alleviate liver inflammation caused by acute liver injury by inhibiting the STING-IRF3 interaction (142). Tetrahydroxy stilbene glucoside from Polygonum multiflorum was found to reduce neuroinflammation by inhibiting the cGAS-STING pathway, thereby improving cognitive function in patients with Alzheimer’s disease (164). In addition, ginsenoside Rb3 was effective in ameliorating ischemia-reperfusion injury in skin flap transplantation by inhibiting the STING-mediated inflammatory response (179). These studies demonstrated the modulatory effects of TCM on key proteins in the cGAS-STING pathway, suggesting that these proteins can be used as biomarkers of TCM therapy for assessing efficacy and potential for individualized treatment.
In summary, key proteins such as cGAS, STING, TBK1, and IRF3 play important roles in the occurrence and development of diseases. By regulating the expression and activity of these proteins, TCM can effectively regulate immune responses and treat a variety of diseases. Therefore, the key proteins in the cGAS-STING pathway can not only be used as targets for TCM to regulate immune and inflammatory responses, but they also have the potential to serve as biomarkers for clinical therapeutic effects. This provides a new direction for the future application of TCM in precision medicine.
6 Summary and prospect
TCM holds an important position in the field of medicine due to its unique bidirectional immunomodulatory ability, which can activate the immune system to enhance the body’s defense, while also moderately inhibiting excessive immune responses and reducing inflammation and autoimmune diseases. The cGAS-STING pathway, an important component of the innate immune system, plays a key role in defending against viral and bacterial infections, modulating cellular damage, inflammatory responses, autophagy, and tumor immunity (56, 192). Therefore, the cGAS-STING pathway has become a potential drug target for treating inflammatory diseases, tumors, and immune dysregulation. TCM has shown unique potential in modulating this pathway, providing new strategies for the treatment of a variety of diseases.
Currently, the development of activators and inhibitors of the cGAS-STING pathway is a research priority. Although STING agonists have shown promising results in preclinical antitumor studies, their clinical translation faces many challenges. For example, modified CDN compounds are rapidly degraded in vivo due to poor metabolic stability, which affects the durability of their efficacy (193). In addition, the low cellular uptake rate of CDN makes it difficult for the drug to efficiently enter target cells, which in turn limits its antitumor effects (194). While most studies rely on intra-tumor drug delivery, there is a lack of delivery technologies that can be applied on a large scale, further limiting the potential application of STING agonists in clinical therapy. In addition, the limited targeting of STING agonists may lead to off-target effects, triggering unnecessary immune activation and increasing autoimmune risks (195). Thus, improving the targeting and safety of STING agonists remains a critical issue for realizing their clinical applications. Regarding STING inhibitors, although compounds such as H-151, C-176, BB-Cl-amidine, and sulforaphane have been reported to inhibit the activation of the cGAS-STING pathway, their therapeutic potential remains limited (196–200). H-151, as the most promising STING inhibitor, inhibits palmitoylation by binding to the Cys91 site of the STING protein (196). However, studies on it are still at the animal experiment stage. Therefore, the development of clinically applicable STING inhibitors in inflammatory and autoimmune diseases remains an urgent topic.
Chinese medicines show remarkable potential in modulating the cGAS-STING pathway, especially in the treatment of inflammatory diseases. For example, compounds such as perilla aldehyde, ursodeoxycholic acid, total glucosides of paeony, and andrographolide affect the activity of this pathway through different mechanisms. Perillaldehyde has been shown to inhibit the innate immune response induced by cytosolic DNA by inhibiting cGAS activity and to attenuate the inflammatory response by reducing the release of inflammatory factors through inhibition of downstream signaling after STING activation. Ursodeoxycholic acid, on the other hand, inhibits the production of pro-inflammatory cytokines by blocking PANoptosis-like cell death through inhibition of the STING pathway. Various active components in licorice, such as glabridin, licorice flavonoids, and licorice chalcone B, can inhibit cGAS-STING-mediated inflammatory responses by modulating the cGAS-STING pathway, thereby exerting therapeutic effects on inflammatory diseases. TRIM29 has been reported to contribute to the pathogenesis of viral myocarditis by enhancing ROS-mediated oxidation of TBK1, thereby inhibiting its function (201). Both TRIM29 and TRIM18 play pivotal roles in the progression of various virus infections, including viral enteritis, viral myocarditis, and various organ inflammations (52, 202, 203). Studies suggest that TCM, with its rich repertoire of antiviral herbal compounds (such as quercetin and ginsenosides), may offer therapeutic potential in treating these infectious diseases. TCM may modulate immune responses by downregulating the expression of TRIM29 and TRIM18, thereby mitigating the inflammatory damage caused by these viral infections. These findings provide important clues for the development of novel herbal therapies based on the cGAS-STING pathway and open up new directions for immunomodulation in a variety of diseases.
Studying the targeting of the cGAS-STING pathway by TCM reflects the unique advantages of TCM in immunomodulation, providing both a scientific basis for modernizing traditional medicine and a new strategy for immunotherapy. However, while studies have demonstrated the potential of TCM in modulating the cGAS-STING pathway, more high-quality research is needed to validate these effects for true clinical applications. Meanwhile, an in-depth understanding of the mechanism of action of TCM can help promote the modernization of TCM and enhance its value for clinical application (204). In the future, with in-depth studies on the mechanisms of the cGAS-STING pathway, TCM may become an effective tool for modulating immune and inflammatory responses, bringing new hope for the treatment of a variety of diseases.
Author contributions
HZ: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft. HF: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft. YZ: Data curation, Investigation, Writing – original draft. NF: Data curation, Investigation, Writing – original draft. CZ: Software, Validation, Writing – original draft. YFL: Software, Validation, Writing – review & editing. YS: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. YPL: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China, grant numbers 82104568, 82074030, and 82374050.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. (2010) 140:805–20. doi: 10.1016/j.cell.2010.01.022
2. Ma H-D, Deng Y-R, Tian Z, Lian Z-X. Traditional Chinese medicine and immune regulation. Clin Rev Allergy Immunol. (2013) 44:229–41. doi: 10.1007/s12016-012-8332-0
3. Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: A cell biological perspective. Annu Rev Immunol. (2015) 33:257–90. doi: 10.1146/annurev-immunol-032414-112240
4. Kwon J, Bakhoum SF. The cytosolic DNA-sensing cgas-sting pathway in cancer. Cancer Discovery. (2020) 10:26–39. doi: 10.1158/2159-8290.CD-19-0761
5. Zhang X, Wu J, Liu Q, Li X, Li S, Chen J, et al. Mtdna-sting pathway promotes necroptosis-dependent enterocyte injury in intestinal ischemia reperfusion. Cell Death Dis. (2020) 11:1050. doi: 10.1038/s41419-020-03239-6
6. Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. Sting-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. (2014) 41:830–42. doi: 10.1016/j.immuni.2014.10.017
7. Ishikawa H, Barber GN. Sting is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. (2008) 455:674–8. doi: 10.1038/nature07317
8. Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. Sting is a direct innate immune sensor of cyclic Di-Gmp. Nature. (2011) 478:515–8. doi: 10.1038/nature10429
9. Guo F, Han Y, Zhao X, Wang J, Liu F, Xu C, et al. Sting agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob Agents Chemother. (2015) 59:1273–81. doi: 10.1128/AAC.04321-14
10. Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cgas-sting signalling. Nat Rev Mol Cell Biol. (2020) 21:501–21. doi: 10.1038/s41580-020-0244-x
11. Luo W, Zou X, Wang Y, Dong Z, Weng X, Pei Z, et al. Critical role of the cgas-sting pathway in doxorubicin-induced cardiotoxicity. Circ Res. (2023) 132:e223–e42. doi: 10.1161/CIRCRESAHA.122.321587
12. Gao D, Li T, Li X-D, Chen X, Li Q-Z, Wight-Carter M, et al. Activation of cyclic Gmp-Amp synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci. (2015) 112:E5699–E705. doi: 10.1073/pnas.1516465112
13. Chauvin SD, Stinson WA, Platt DJ, Poddar S, Miner JJ. Regulation of Cgas and sting signaling during inflammation and infection. J Biol Chem. (2023) 299:104866. doi: 10.1016/j.jbc.2023.104866
14. Liu Z, Wang D, Zhang J, Xiang P, Zeng Z, Xiong W, et al. Cgas-sting signaling in the tumor microenvironment. Cancer Lett. (2023) 577:216409. doi: 10.1016/j.canlet.2023.216409
15. Levander OA, Whanger PD. Deliberations and evaluations of the approaches, endpoints and paradigms for selenium and iodine dietary recommendations. J Nutr. (1996) 126:2427S–34S. doi: 10.1093/jn/126.suppl_9.2427S
16. Cho YJ, Son HJ, Kim KS. A 14-week randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy and safety of ginseng polysaccharide (Y-75). J Transl Med. (2014) 12:283. doi: 10.1186/s12967-014-0283-1
17. Guo Q, Li J, Lin H. Effect and molecular mechanisms of traditional Chinese medicine on regulating tumor immunosuppressive microenvironment. BioMed Res Int. (2015) 2015:261620. doi: 10.1155/2015/261620
18. Liu J, Wang S, Zhang Y, Fan HT, Lin HS. Traditional Chinese medicine and cancer: history, present situation, and development. Thorac Cancer. (2015) 6:561–9. doi: 10.1111/1759-7714.12270
19. Ye L, Jia Y, Ji KE, Sanders AJ, Xue K, Ji J, et al. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol Lett. (2015) 10:1240–50. doi: 10.3892/ol.2015.3459
20. Mao D, Feng L, Gong H. The antitumor and immunomodulatory effect of Yanghe decoction in breast cancer is related to the modulation of the Jak/Stat signaling pathway. Evid Based Complement Alternat Med. (2018) 2018:8460526. doi: 10.1155/2018/8460526
21. Taniguchi M, Seino K, Nakayama T. The Nkt cell system: bridging innate and acquired immunity. Nat Immunol. (2003) 4:1164–5. doi: 10.1038/ni1203-1164
22. Wan CP, Gao LX, Hou LF, Yang XQ, He PL, Yang YF, et al. Astragaloside Ii triggers T cell activation through regulation of Cd45 protein tyrosine phosphatase activity. Acta Pharmacol Sin. (2013) 34:522–30. doi: 10.1038/aps.2012.208
23. Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. (2011) 1:226–32. doi: 10.1016/j.coviro.2011.07.002
24. Pryde DC, Middya S, Banerjee M, Shrivastava R, Basu S, Ghosh R, et al. The discovery of potent small molecule activators of human sting. Eur J Med Chem. (2021) 209:112869. doi: 10.1016/j.ejmech.2020.112869
25. Shang G, Zhang C, Chen ZJ, Bai XC, Zhang X. Cryo-Em structures of sting reveal its mechanism of activation by cyclic Gmp-Amp. Nature. (2019) 567:389–93. doi: 10.1038/s41586-019-0998-5
26. Ding CY, Song ZL, Shen AC, Chen TT, Zhang A. Small molecules targeting the innate immune Cgas-Sting-Tbk1 signaling pathway. Acta Pharm Sin B. (2020) 10:2272–98. doi: 10.1016/j.apsb.2020.03.001
27. Sun LJ, Wu JX, Du FH, Chen X, Chen ZJJ. Cyclic Gmp-Amp synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. (2013) 339:786–91. doi: 10.1126/science.1232458
28. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, et al. Cgas produces a 2′-5′-linked cyclic dinucleotide second messenger that activates sting. Nature. (2013) 498:380–+. doi: 10.1038/nature12306
29. Marinho FV, Benmerzoug S, Oliveira SC, Ryffel B, Quesniaux VFJ. The emerging roles of sting in bacterial infections. Trends Microbiol. (2017) 25:906–18. doi: 10.1016/j.tim.2017.05.008
30. Dobbs N, Burnaevskiy N, Chen DD, Gonugunta VK, Alto NM, Yan N. Sting activation by translocation from the Er is associated with infection and autoinflammatory disease. Cell Host Microbe. (2015) 18:157–68. doi: 10.1016/j.chom.2015.07.001
31. Li AP, Yi M, Qin S, Song YP, Chu Q, Wu KM. Activating cgas-sting pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. (2019) 12:1–12. doi: 10.1186/s13045-019-0721-x
32. Corrales L, McWhirter SM, Dubensky TW, Gajewski TF. The host sting pathway at the interface of cancer and immunity. J Clin Invest. (2016) 126:2404–11. doi: 10.1172/Jci86892
33. Burdette DL, Vance RE. Sting and the innate immune response to nucleic acids in the cytosol. Nat Immunol. (2013) 14:19–26. doi: 10.1038/ni.2491
34. Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. (2013) 34:67–73. doi: 10.1016/j.it.2012.10.004
35. Decout A, Katz JD, Venkatraman S, Ablasser A. The Cgas–sting pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. (2021) 21:548–69. doi: 10.1038/s41577-021-00524-z
36. Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct activation of sting in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. (2015) 11:1018–30. doi: 10.1016/j.celrep.2015.04.031
37. Vasiyani H, Wadhwa B, Singh R. Regulation of cgas-sting signalling in cancer: approach for combination therapy. Biochim Biophys Acta (BBA) Reviews Cancer. (2023) 1878:188896. doi: 10.1016/j.bbcan.2023.188896
38. Pan X, Zhang W, Guo H, Wang L, Wu H, Ding L, et al. Strategies involving sting pathway activation for cancer immunotherapy: mechanism and agonists. Biochem Pharmacol. (2023) 213:115596. doi: 10.1016/j.bcp.2023.115596
39. Woon ST, Reddy CB, Drummond CJ, Schooltink MA, Baguley BC, Kieda C, et al. A comparison of the ability of Dmxaa and xanthenone analogues to activate Nf-Kappab in murine and human cell lines. Oncol Res. (2005) 15:351–64. doi: 10.3727/096504005776449743
40. Eckstein F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. (2014) 24:374–87. doi: 10.1089/nat.2014.0506
41. Meric-Bernstam F, Sweis RF, Hodi FS, Messersmith WA, Andtbacka RH, Ingham M, et al. Phase I dose-escalation trial of Miw815 (Adu-S100), an intratumoral sting agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. (2022) 28:677–88. doi: 10.1158/1078-0432.CCR-21-1963
42. Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, et al. Cgas is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci. (2017) 114:1637–42. doi: 10.1073/pnas.1621363114
43. Lucas-Hourani M, Dauzonne D, Jorda P, Cousin G, Lupan A, Helynck O, et al. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PloS Pathog. (2013) 9:e1003678. doi: 10.1371/journal.ppat.1003678
44. Shin HJ, Kim C, Cho S. Gemcitabine and nucleos(T)Ide synthesis inhibitors are broad-spectrum antiviral drugs that activate innate immunity. Viruses. (2018) 10(4):211. doi: 10.3390/v10040211
45. Paulis A, Tramontano E. Unlocking sting as a therapeutic antiviral strategy. Int J Mol Sci. (2023) 24:7448. doi: 10.3390/ijms24087448
46. Kim S, Li L, Maliga Z, Yin Q, Wu H, Mitchison TJ. Anticancer flavonoids are mouse-selective sting agonists. ACS Chem Biol. (2013) 8:1396–401. doi: 10.1021/cb400264n
47. Yongpitakwattana P, Morchang A, Panya A, Sawasdee N, Yenchitsomanus PT. Alpha-mangostin inhibits dengue virus production and pro-inflammatory cytokine/chemokine expression in dendritic cells. Arch Virol. (2021) 166:1623–32. doi: 10.1007/s00705-021-05017-x
48. Tarasuk M, Songprakhon P, Chieochansin T, Choomee K, Na-Bangchang K, Yenchitsomanus PT. Alpha-mangostin inhibits viral replication and suppresses nuclear factor kappa B (Nf-Kappab)-mediated inflammation in dengue virus infection. Sci Rep. (2022) 12:16088. doi: 10.1038/s41598-022-20284-7
49. Christensen MH, Jensen SB, Miettinen JJ, Luecke S, Prabakaran T, Reinert LS, et al. Hsv-1 icp27 targets the Tbk1-activated sting signalsome to inhibit virus-induced type I Ifn expression. EMBO J. (2016) 35:1385–99. doi: 10.15252/embj.201593458
50. Fu YZ, Su S, Gao YQ, Wang PP, Huang ZF, Hu MM, et al. Human cytomegalovirus tegument protein Ul82 inhibits sting-mediated signaling to evade antiviral immunity. Cell Host Microbe. (2017) 21:231–43. doi: 10.1016/j.chom.2017.01.001
51. Zhang K, Huang Q, Li X, Zhao Z, Hong C, Sun Z, et al. The cgas-sting pathway in viral infections: A promising link between inflammation, oxidative stress and autophagy. Front Immunol. (2024) 15:1352479. doi: 10.3389/fimmu.2024.1352479
52. Xing J, Zhang A, Zhang H, Wang J, Li XC, Zeng M-S, et al. Trim29 promotes DNA virus infections by inhibiting innate immune response. Nat Commun. (2017) 8:945. doi: 10.1038/s41467-017-00101-w
53. Kato Y, Park J, Takamatsu H, Konaka H, Aoki W, Aburaya S, et al. Apoptosis-derived membrane vesicles drive the cgas-sting pathway and enhance type I Ifn production in systemic lupus erythematosus. Ann Rheum Dis. (2018) 77:1507–15. doi: 10.1136/annrheumdis-2018-212988
54. Gray EE, Treuting PM, Woodward JJ, Stetson DB. Cutting edge: Cgas is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi-Goutières syndrome. J Immunol. (2015) 195:1939–43. doi: 10.4049/jimmunol.1500969
55. Ding C, Song Z, Shen A, Chen T, Zhang A. Small molecules targeting the innate immune Cgas-Sting-Tbk1 signaling pathway. Acta Pharm Sin B. (2020) 10:2272–98. doi: 10.1016/j.apsb.2020.03.001
56. Wang MM, Zhao Y, Liu J, Fan RR, Tang YQ, Guo ZY, et al. The role of the cgas-sting signaling pathway in viral infections, inflammatory and autoimmune diseases. Acta Pharmacol Sin. (2024) 45:1997–2010. doi: 10.1038/s41401-023-01185-5
57. An J, Woodward JJ, Sasaki T, Minie M, Elkon KB. Cutting edge: antimalarial drugs inhibit Ifn-B Production through blockade of cyclic Gmp-Amp synthase-DNA interaction. J Immunol. (2015) 194:4089–93. doi: 10.4049/jimmunol.1402793
58. Huang CF, Lin SS, Liao PH, Young SC, Yang CC. The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol Immunol. (2008) 5:23–31. doi: 10.1038/cmi.2008.3
59. Jiang MH, Zhu LA, Jiang JG. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin Ther Tar. (2010) 14:1367–402. doi: 10.1517/14728222.2010.531010
60. Borchers AT, Sakai S, Henderson GL, Harkey MR, Keen CL, Stern JS, et al. Shosaiko-to and other Kampo (Japanese herbal) medicines: A review of their immunomodulatory activities. J Ethnopharmacol. (2000) 73:1–13. doi: 10.1016/s0378-8741(00)00334-2
61. Lin YL, Liang YC, Lee SS, Chiang BL. Polysaccharide purified from ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the Nf-Kappab and P38 mitogen-activated protein kinase pathways. J Leukoc Biol. (2005) 78:533–43. doi: 10.1189/jlb.0804481
62. Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, et al. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr Polymers. (2020) 235:115957. doi: 10.1016/j.carbpol.2020.115957
63. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. (2008) 112:1570–80. doi: 10.1182/blood-2008-02-078071
64. Xie S-Z, Liu B, Ye H-Y, Li Q-M, Pan L-H, Zha X-Q, et al. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr Polymers. (2019) 206:149–62. doi: 10.1016/j.carbpol.2018.11.002
65. Li W, Guo S, Xu D, Li B, Cao N, Tian Y, et al. Polysaccharide of Atractylodes macrocephala koidz (Pamk) relieves immunosuppression in cyclophosphamide-treated geese by maintaining a humoral and cellular immune balance. Molecules. (2018) 23(4):932. doi: 10.3390/molecules23040932
66. Xu W, Fang S, Cui X, Guan R, Wang Y, Shi F, et al. Signaling pathway underlying splenocytes activation by polysaccharides from Atractylodis macrocephalae koidz. Mol Immunol. (2019) 111:19–26. doi: 10.1016/j.molimm.2019.03.004
67. Hwang J, Zhang W, Dhananjay Y, An E-K, Kwak M, You S, et al. Astragalus membranaceus polysaccharides potentiate the growth-inhibitory activity of immune checkpoint inhibitors against pulmonary metastatic melanoma in mice. Int J Biol Macromol. (2021) 182:1292–300. doi: 10.1016/j.ijbiomac.2021.05.073
68. Zhong H, Han L, Lu RY, Wang Y. Antifungal and immunomodulatory ingredients from traditional Chinese medicine. Antibiotics-Basel. (2023) 12(1):48. doi: 10.3390/antibiotics12010048
69. Fu Y, Zhou X, Wang L, Fan W, Gao S, Zhang D, et al. Salvianolic acid B attenuates liver fibrosis by targeting Ecm1 and inhibiting hepatocyte ferroptosis. Redox Biol. (2024) 69:103029. doi: 10.1016/j.redox.2024.103029
70. Zhou H-l, Deng Y-m, Xie Q-m. The modulatory effects of the volatile oil of ginger on the cellular immune response in vitro and in vivo in mice. J Ethnopharmacol. (2006) 105:301–5. doi: 10.1016/j.jep.2005.10.022
71. Zeng Q, Zhou T-t, Huang W-j, Huang X-t, Huang L, Zhang X-h, et al. Asarinin attenuates bleomycin-induced pulmonary fibrosis by activating Pparγ. Sci Rep. (2023) 13:14706. doi: 10.1038/s41598-023-41933-5
72. Gao LL, Ma JM, Fan YN, Zhang YN, Ge R, Tao XJ, et al. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int J Biol Macromol. (2021) 183:1379–92. doi: 10.1016/j.ijbiomac.2021.05.066
73. Hou Y, Jiang JG. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. (2013) 4:1727–41. doi: 10.1039/c3fo60295h
74. Ma A, Zou F, Zhang R, Zhao X. The effects and underlying mechanisms of medicine and food homologous flowers on the prevention and treatment of related diseases. J Food Biochem. (2022) 46:e14430. doi: 10.1111/jfbc.14430
75. Song DX, Jiang JG. Hypolipidemic components from medicine food homology species used in China: pharmacological and health effects. Arch Med Res. (2017) 48:569–81. doi: 10.1016/j.arcmed.2018.01.004
76. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. (2020) 83:770–803. doi: 10.1021/acs.jnatprod.9b01285
77. Xu L, Zhang X, Yang W, Li H, Wang J, Wang L, et al. Advanced technology based on poly(Deep eutectic solvent) core–shell nanomaterials enriched with Fructus choerospondias phenols for efficient defense against Uvb-induced ferroptosis. Chem Eng J. (2024) 498:155224. doi: 10.1016/j.cej.2024.155224
78. Wang L, Li M, Wang Y, Xu L, He X, Li H, et al. Harnessing two-dimensional magnetic poly(Deep eutectic solvents) for matrix solid-phase dispersion extraction of polyphenols from roselle: promoting antiphotoaging strategy. Chem Eng J. (2024) 496:154019. doi: 10.1016/j.cej.2024.154019
79. Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. (2008) 134:587–98. doi: 10.1016/j.cell.2008.06.032
80. Grieves JL, Fye JM, Harvey S, Grayson JM, Hollis T, Perrino FW. Exonuclease Trex1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease. Proc Natl Acad Sci U.S.A. (2015) 112:5117–22. doi: 10.1073/pnas.1423804112
81. Wu J, Dobbs N, Yang K, Yan N. Interferon-independent activities of mammalian sting mediate antiviral response and tumor immune evasion. Immunity. (2020) 53:115–26 e5. doi: 10.1016/j.immuni.2020.06.009
82. Li C, Wen J, Zhan X, Shi W, Ye X, Yao Q, et al. Total tanshinones ameliorates cgas-sting-mediated inflammatory and autoimmune diseases by affecting sting-Irf3 binding. Chin Med. (2024) 19:107. doi: 10.1186/s13020-024-00980-4
83. Bumblauskiene L, Jakstas V, Janulis V, Mazdzieriene R, Ragazinskiene O. Preliminary analysis on essential oil composition of Perilla L. cultivated in Lithuania. Acta Pol Pharm. (2009) 66:409–13. Available at: https://hdl.handle.net/20.500.12512/85784
84. Chu L, Li CH, Li YX, Yu QY, Yu HS, Li CH, et al. Perillaldehyde inhibition of Cgas reduces Dsdna-induced interferon response. Front Immunol. (2021) 12:655637. doi: 10.3389/fimmu.2021.655637
85. Wen J, Mu W, Li H, Yan Y, Zhan X, Luo W, et al. Glabridin improves autoimmune disease in Trex1-deficient mice by reducing type I interferon production. Mol Med. (2023) 29:167. doi: 10.1186/s10020-023-00754-y
86. Luo W, Song Z, Xu G, Wang H, Mu W, Wen J, et al. Licochalconeb inhibits cgas-sting signaling pathway and prevents autoimmunity diseases. Int Immunopharmacol. (2024) 128:111550. doi: 10.1016/j.intimp.2024.111550
87. Zhang Y, Liu Y, Jiang B, Chen L, Hu J, Niu B, et al. Targeting sting oligomerization with licochalcone D ameliorates sting-driven inflammatory diseases. Sci China Life Sci. (2024), 1–4. doi: 10.1007/s11427-024-2703-6
88. Yang Y, Feng K, Yuan L, Liu Y, Zhang M, Guo K, et al. Compound Danshen dripping pill inhibits hypercholesterolemia/atherosclerosis-induced heart failure in Apoe and Ldlr dual deficient mice via multiple mechanisms. Acta Pharm Sin B. (2023) 13:1036–52. doi: 10.1016/j.apsb.2022.11.012
89. Lei W, Li X, Li L, Huang M, Cao Y, Sun X, et al. Compound Danshen dripping pill ameliorates post ischemic myocardial inflammation through synergistically regulating Mapk, Pi3k/Akt and Ppar signaling pathways. J Ethnopharmacol. (2021) 281:114438. doi: 10.1016/j.jep.2021.114438
90. Liao W, Ma X, Li J, Li X, Guo Z, Zhou S, et al. A review of the mechanism of action of dantonic® for the treatment of chronic stable angina. Biomed Pharmacother. (2019) 109:690–700. doi: 10.1016/j.biopha.2018.10.013
91. Shi W, Xu G, Gao Y, Yang H, Liu T, Zhao J, et al. Compound Danshen dripping pill effectively alleviates cgas-sting-triggered diseases by disrupting sting-Tbk1 interaction. Phytomedicine. (2024) 128:155404. doi: 10.1016/j.phymed.2024.155404
92. Wang X, Wang N, Cheung F, Lao L, Li C, Feng Y. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med. (2015) 13:142–64. doi: 10.1016/S2095-4964(15)60171-6
93. Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. (2014) 12:331–5. doi: 10.1016/S2095-4964(14)60038-8
94. Wang J, Luo ZJ, Lin LZ, Sui XB, Yu LL, Xu C, et al. Anoikis-associated lung cancer metastasis: mechanisms and therapies. Cancers. (2022) 14(19):4791. doi: 10.3390/cancers14194791
95. Gao XY, Liu GC, Zhang JX, Wang LH, Xu C, Yan ZA, et al. Pharmacological properties of ginsenoside re. Front Pharmacol. (2022) 13:754191. doi: 10.3389/fphar.2022.754191
96. Tang X, Zhu M, Zhu Z, Tang W, Zhang H, Chen Y, et al. Ginsenoside re inhibits non-small cell lung cancer progression by suppressing macrophage M2 polarization induced by Ampkalpha1/sting positive feedback loop. Phytother Res. (2024) 1–19. doi: 10.1002/ptr.8309
97. Yan X, Yao C, Fang C, Han M, Gong C, Hu D, et al. Rocaglamide promotes the infiltration and antitumor immunity of Nk cells by activating cgas-sting signaling in non-small cell lung cancer. Int J Biol Sci. (2022) 18:585–98. doi: 10.7150/ijbs.65019
98. Bhagya N, Chandrashekar KR. Autophagy and cancer: can tetrandrine be a potent anticancer drug in the near future? Biomed Pharmacother. (2022) 148:112727. doi: 10.1016/j.biopha.2022.112727
99. Tan Y, Zhu QC, Yang ML, Yang F, Zeng Q, Jiang ZB, et al. Tetrandrine activates sting/Tbk1/Irf3 pathway to potentiate anti-Pd-1 immunotherapy efficacy in non-small cell lung cancer. Pharmacol Res. (2024) 207:107314. doi: 10.1016/j.phrs.2024.107314
100. Punvittayagul C, Chariyakornkul A, Jarukamjorn K, Wongpoomchai R. Protective role of vanillic acid against diethylnitrosamine- and 1,2-dimethylhydrazine-induced hepatocarcinogenesis in rats. Molecules. (2021) 26:2718. doi: 10.3390/molecules26092718
101. Zhu M, Tang XY, Zhu ZR, Gong ZY, Tang WJ, Hu Y, et al. Sting activation in macrophages by vanillic acid exhibits antineoplastic potential. Biochem Pharmacol. (2023) 213:115618. doi: 10.1016/j.bcp.2023.115618
102. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
103. Liu H, Wang Z, Liu Z. Formononetin restrains tumorigenesis of breast tumor by restraining sting-Nf-Kappab and interfering with the activation of Pd-L1. Discovery Med. (2024) 36:613–20. doi: 10.24976/Discov.Med.202436182.58
104. Fu Q, Lu Z, Chang Y, Jin T, Zhang M. Ginseng extract (Ginsenoside Rg3) combined with sting agonist reverses Tam/M2 polarization to inhibit Tnbc evolution. Ind Crops Prod. (2024) 222:119589. doi: 10.1016/j.indcrop.2024.119589
105. Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. (2011) 472:481–5. doi: 10.1038/nature09907
106. Diner Elie J, Burdette Dara L, Wilson Stephen C, Monroe Kathryn M, Kellenberger Colleen A, Hyodo M, et al. The innate immune DNA sensor Cgas produces a noncanonical cyclic dinucleotide that activates human sting. Cell Rep. (2013) 3:1355–61. doi: 10.1016/j.celrep.2013.05.009
107. Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic Gmp-Amp is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. (2012) 339(6121):826–30. doi: 10.1126/science.1229963
108. Kopustinskiene DM, Bernatoniene J. Antioxidant effects of Schisandra chinensis fruits and their active constituents. Antioxidants-Basel. (2021) 10:620. doi: 10.3390/antiox10040620
109. Nowak A, Zakłos-Szyda M, Błasiak J, Nowak A, Zhang Z, Zhang B. Potential of Schisandra chinensis (Turcz.) baill. In human health and nutrition: A review of current knowledge and therapeutic perspectives. Nutrients. (2019) 11:333. doi: 10.3390/nu11020333
110. Zhao J, Xu G, Hou XR, Mu WQ, Yang HJ, Shi W, et al. Schisandrin C enhances cgas-sting pathway activation and inhibits Hbv replication. J Ethnopharmacol. (2023) 311:116427. doi: 10.1016/j.jep.2023.116427
111. Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. (2008) 8:634–46. doi: 10.2174/156800908786241050
112. Wang Y, Li F, Wang Z, Song X, Ren Z, Wang X, et al. Luteolin inhibits herpes simplex virus 1 infection by activating cyclic guanosine monophosphate-adenosine monophosphate synthase-mediated antiviral innate immunity. Phytomedicine. (2023) 120:155020. doi: 10.1016/j.phymed.2023.155020
113. Lei YC, Li W, Luo P. Liuweiwuling tablets attenuate acetaminophen-induced acute liver injury and promote liver regeneration in mice. World J Gastroenterol. (2015) 21:8089–95. doi: 10.3748/wjg.v21.i26.8089
114. Wu ZX, Zhao XM, Li RS, Wen XR, Xiu Y, Long MJ, et al. The combination of schisandrin C and luteolin synergistically attenuates hepatitis B virus infection via repressing Hbv replication and promoting cgas-sting pathway activation in macrophages. Chin Med Uk. (2024) 19:48. doi: 10.1186/s13020-024-00888-z
115. Yi Y, Li JH, Lai XY, Zhang M, Kuang Y, Bao YO, et al. Natural triterpenoids from licorice potently inhibit Sars-Cov-2 infection. J Adv Res. (2022) 36:201–10. doi: 10.1016/j.jare.2021.11.012
116. Zheng WJ, Huang XF, Lai YN, Liu XH, Jiang Y, Zhan SF. Glycyrrhizic acid for Covid-19: findings of targeting pivotal inflammatory pathways triggered by Sars-Cov-2. Front Pharmacol. (2021) 12:631206. doi: 10.3389/fphar.2021.631206
117. Qi H, Ma QH, Feng W, Chen SM, Wu CS, Wang YN, et al. Glycyrrhetinic acid blocks Sars-Cov-2 infection by activating the cgas-sting signalling pathway. Brit J Pharmacol. (2024) 181(20):3976–92. doi: 10.1111/bph.16473
118. Bailly C. Cepharanthine: an update of its mode of action, pharmacological properties and medical applications. Phytomedicine. (2019) 62:152956. doi: 10.1016/j.phymed.2019.152956
119. Liu Y, Tang Q, Rao ZL, Fang Y, Jiang XN, Liu WJ, et al. Inhibition of Herpes Simplex Virus 1 by Cepharanthine Via Promoting Cellular Autophagy through up-Regulation of Sting/Tbk1/P62 Pathway. Antivir Res. (2021) 193:105143. doi: 10.1016/j.antiviral.2021.105143
120. Chen JX, Du HQ, Cui S, Liu T, Yang G, Sun HP, et al. E. Fischeriana root compound Dpo activates antiviral innate immunity. Front Cell Infect Mi. (2017) 7:456. doi: 10.3389/fcimb.2017.00456
121. Hui S, Kan W, Qin S, He P, Zhao J, Li H, et al. Frontiers | Glycyrrhiza uralensis polysaccharides ameliorates cecal ligation and puncture-induced sepsis by inhibiting the cgas-sting signaling pathway. Front Pharmacol. (2024) 15. doi: 10.3389/fphar.2024.1374179
122. Song L, Li G, Guan W, Zeng Z, Ou Y, Zhao T, et al. Design, synthesis and anti-inflammatory activity study of lansiumamide analogues for treatment of acute lung injury. BioMed Pharmacother. (2023) 166:115412. doi: 10.1016/j.biopha.2023.115412
123. Comish PB, Liu MM, Huebinger R, Carlson D, Kang R, Tang D. The cgas-sting pathway connects mitochondrial damage to inflammation in burn-induced acute lung injury in rat. Burns. (2022) 48:168–75. doi: 10.1016/j.burns.2021.04.007
124. Wen J, Qin S, Li Y, Zhang P, Zhan X, Fang M, et al. Flavonoids derived from licorice suppress Lps-induced acute lung injury in mice by inhibiting the cgas-sting signaling pathway. Food Chem Toxicol. (2023) 175:113732. doi: 10.1016/j.fct.2023.113732
125. Wei JH, Liu ZJ, Sun HB, Xu L. Perillaldehyde ameliorates lipopolysaccharide-induced acute lung injury via suppressing the Cgas/sting signaling pathway. Int Immunopharmacol. (2024) 130:111641. doi: 10.1016/j.intimp.2024.111641
126. Zhou XW, Wang J, Tan WF. Apigenin suppresses innate immune responses and ameliorates lipopolysaccharide-induced inflammation via inhibition of sting/Irf3 pathway. Am J Chin Med. (2024) 52:471–92. doi: 10.1142/S0192415x24500204
127. He YQ, Deng JL, Zhou CC, Jiang SG, Zhang F, Tao X, et al. Ursodeoxycholic acid alleviates sepsis-induced lung injury by blocking panoptosis via sting pathway. Int Immunopharmacol. (2023) 125:111161. doi: 10.1016/j.intimp.2023.111161
128. Yao Q, Wen J, Chen S, Wang Y, Wen X, Wang X, et al. Shuangdan Jiedu decoction improved Lps-induced acute lung injury by regulating both cgas-sting pathway and inflammasome. J Ethnopharmacol. (2024) 336:118661. doi: 10.1016/j.jep.2024.118661
129. He Y-Q, Zhou C-C, Deng J-L, Wang L, Chen W-S. Tanreqing inhibits Lps-induced acute lung injury in vivo and in vitro through downregulating sting signaling pathway. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.746964
130. Deng J, He Y, Sun G, Yang H, Wang L, Tao X, et al. Tanreqing injection protects against bleomycin-induced pulmonary fibrosis via inhibiting sting-mediated endoplasmic reticulum stress signaling pathway. J Ethnopharmacol. (2023) 305:116071. doi: 10.1016/j.jep.2022.116071
131. Ren G, Lv W, Ding Y, Wang L, Cui Z, Li R, et al. Ginseng saponin metabolite 20(S)-protopanaxadiol relieves pulmonary fibrosis by multiple-targets signaling pathways. J Ginseng Res. (2023) 47(4):543–51. doi: 10.1016/j.jgr.2023.01.002
132. Chen X, Kang F, Lai J, Deng X, Guo X, Liu S. Comparative effectiveness of phlegm-heat clearing Chinese medicine injections for aecopd: A systematic review and network meta-analysis. J Ethnopharmacol. (2022)292:115043. doi: 10.1016/j.jep.2022.115043
133. Zhong Y, Mao B, Wang G, Fan T, Liu X, Diao X, et al. Tanreqing injection combined with conventional western medicine for acute exacerbations of chronic obstructive pulmonary disease: A systematic review. J Integr Complement Med. (2010) 16(12):1309–19. doi: 10.1089/acm.2009.0686
134. Tong Y, Wen J, Yang T, Li H, Wei S, Jing M, et al. Clinical efficacy and safety of tanreqing injection combined with antibiotics versus antibiotics alone in the treatment of pulmonary infection patients after chemotherapy with lung cancer: A systematic review and meta-analysis. Phytother Res. (2021) 35:122–37. doi: 10.1002/ptr.6790
135. Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. (2019) 65:37–55. doi: 10.1016/j.mam.2018.09.002
136. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. (2017) 12:153–86. doi: 10.1146/annurev-pathol-052016-100322
137. Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, et al. Pivotal role of Tnf-A in the development and progression of nonalcoholic fatty liver disease in a murine model. Horm Metab Res. (2018) 50:80–7. doi: 10.1055/s-0043-118666
138. Hernández-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol. (2018) 24:1679–707. doi: 10.3748/wjg.v24.i16.1679
139. Chen L, Xia S, Wang S, Zhou Y, Wang F, Li Z, et al. Naringenin is a potential immunomodulator for inhibiting liver fibrosis by inhibiting the cgas-sting pathway. J Clin Trans Hepatol. (2022) 11(1):26–37. doi: 10.14218/jcth.2022.00120
140. Luo W, Xu G, Song Z, Mu W, Wen J, Hui S, et al. Licorice extract inhibits the cgas-sting pathway and protects against non-alcoholic steatohepatitis. Front Pharmacol. (2023) 14. doi: 10.3389/fphar.2023.1160445
141. Sun Y, Weng J, Chen X, Ma S, Zhang Y, Zhang F, et al. Oroxylin a activates ferritinophagy to induce hepatic stellate cell senescence against hepatic fibrosis by regulating cgas-sting pathway. Biomed Pharmacother. (2023) 162:114653. doi: 10.1016/j.biopha.2023.114653
142. Xiu Y, Wang S, Zhang P, Li C, Wu Z, Wen J, et al. Total glucosides of paeony alleviates cgas-sting-mediated diseases by blocking the sting-Irf3 interaction. Chin J Natural Medicines. (2024) 22(5):402–15. doi: 10.1016/s1875-5364(24)60572-8
143. Li Y, Yu P, Fu W, Wang S, Zhao W, Ma Y, et al. Ginsenoside Rd inhibited ferroptosis to alleviate Ccl4-induced acute liver injury in mice via Cgas/sting pathway. Am J Chin Med. (2022) 51(01):91–105. doi: 10.1142/s0192415x23500064
144. Ding G, Yu G, Zhang J, Liang S, Liu L, Huang P, et al. The therapeutic effects of Ling Gui Zhu Gan Tang Mixture in 50 psychotic patients with obesity induced by the psychoactive drugs. J Tradit Chin Med. (2005) 25:25–8.
145. Dai L, Xu J, Liu B, Dang Y, Wang R, Zhuang L, et al. Lingguizhugan decoction, a Chinese herbal formula, improves insulin resistance in overweight/obese subjects with non-alcoholic fatty liver disease: A translational approach. Front Med. (2022) 16:745–59. doi: 10.1007/s11684-021-0880-3
146. Cao L, Xu E, Zheng RD, Zhangchen ZL, Zhong RL, Huang F, et al. Traditional Chinese medicine Lingguizhugan decoction ameliorate Hfd-induced hepatic-lipid deposition in mice by inhibiting sting-mediated inflammation in macrophages. Chin Med Uk. (2022) 17(7):1–16. doi: 10.1186/s13020-021-00559-3
147. Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. (2019) 5:58. doi: 10.1038/s41572-019-0105-0
148. Wang HF, Xu JS, Zong K, Liang ZW, Li RF, Xue JF, et al. Jujuboside B alleviates acetaminophen-induced hepatotoxicity in mice by regulating Nrf2-sting signaling pathway. Ecotoxicol Environ Saf. (2024) 269:115810. doi: 10.1016/j.ecoenv.2023.115810
149. Shen P, Han L, Chen G, Cheng Z, Liu Q. Emodin attenuates acetaminophen-induced hepatotoxicity via the cgas-sting pathway. Inflammation. (2021) 45:74–87. doi: 10.1007/s10753-021-01529-5
150. Wang Y, Wei B, Wang D, Wu J, Gao J, Zhong H, et al. DNA damage repair promotion in colonic epithelial cells by andrographolide downregulated Cgas-Sting pathway activation and contributed to the relief of Cpt-11-induced intestinal mucositis. Acta Pharm Sin B. (2021) 12(1):262–73. doi: 10.1016/j.apsb.2021.03.043
151. Gu L, Wang F, Wang Y, Sun D, Sun Y, Tian T, et al. Naringin Protects against Inflammation and Apoptosis Induced by Intestinal Ischemia–Reperfusion Injury through Deactivation of Cgas-Sting Signaling Pathway. Phytother Res. (2023) 37(8):3495–507. doi: 10.1002/ptr.7824
152. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. (2008) 73:994–1007. doi: 10.1038/sj.ki.5002786
153. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. (2010) 2(11):2490–518. doi: 10.3390/toxins2112490
154. Qi JY, Luo Q, Zhang QY, Wu MN, Zhang LL, Qin LS, et al. Yi-Shen-Xie-Zhuo formula alleviates cisplatin-induced Aki by regulating inflammation and apoptosis via the Cgas/sting pathway. J Ethnopharmacol. (2023) 309:116327. doi: 10.1016/j.jep.2023.116327
155. Ma Y, Bai B, Liu D, Shi R, Zhou Q. Shenqi fuzheng injection reduces cisplatin-induced kidney injury via Cgas/sting signaling pathway in breast cancer mice model. Breast Cancer: Targets Ther. (2024) 16:451–69. doi: 10.2147/bctt.s475860
156. Liu S, Gao X, Wang Y, Wang J, Qi X, Dong K, et al. Baicalein-loaded silk fibroin peptide nanofibers protect against cisplatin-induced acute kidney injury: fabrication, characterization and mechanism. Int J Pharm. (2022) 626:122161. doi: 10.1016/j.ijpharm.2022.122161
157. Liu S, Gao X, Yin Y, Wang J, Dong K, Shi D, et al. Silk fibroin peptide self-assembled nanofibers delivered naringenin to alleviate cisplatin-induced acute kidney injury by inhibiting Mtdna-cgas-sting pathway. Food Chem Toxicol. (2023) 177:113844. doi: 10.1016/j.fct.2023.113844
158. Zheng M, Hu Z, Wang Y, Wang C, Zhong C, Cui W, et al. Zhen Wu decoction represses renal fibrosis by invigorating tubular Nrf2 and Tfam to fuel mitochondrial bioenergetics. Phytomedicine. (2022) 108:154495. doi: 10.1016/j.phymed.2022.154495
159. Appel SH, Smith RG, Le WD. Immune-mediated cell death in neurodegenerative disease. Adv Neurol. (1996) 69:153–9.
160. Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. (2016) 12:719–32. doi: 10.1016/j.jalz.2016.02.010
161. Monteiro AR, Barbosa DJ, Remião F, Silva R. Alzheimer’s disease: insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem Pharmacol. (2023) 211:115522. doi: 10.1016/j.bcp.2023.115522
162. Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, et al. Traditional usages, botany, phytochemistry, pharmacology and toxicology of polygonum multiflorum thunb.: A review. J Ethnopharmacol. (2015) 159:158–83. doi: 10.1016/j.jep.2014.11.009
163. Gao D, Chen C, Huang R, Yang CC, Miao BB, Li L, et al. Tetrahydroxy stilbene glucoside ameliorates cognitive impairments and pathology in App/Ps1 transgenic mice. Curr Med Sci. (2021) 41:279–86. doi: 10.1007/s11596-021-2344-z
164. Gao D, Hao J-P, Li B-Y, Zheng C-C, Miao B-B, Zhang L, et al. Tetrahydroxy stilbene glycoside ameliorates neuroinflammation for Alzheimer’s disease via cgas-sting. Eur J Pharmacol. (2023) 953:175809. doi: 10.1016/j.ejphar.2023.175809
165. Liu X, Chen W, Wang C, Liu W, Hayashi T, Mizuno K, et al. Silibinin ameliorates depression/anxiety-like behaviors of Parkinson’s disease mouse model and is associated with attenuated sting-Irf3-Ifn-B Pathway activation and neuroinflammation. Physiol Behav. (2021) 241:113593. doi: 10.1016/j.physbeh.2021.113593
166. Tian L, Li W, Wang T. Therapeutic effects of silibinin on Lps-induced acute lung injury by inhibiting Nlrp3 and Nf-Kb signaling pathways. Microb Pathogen. (2017) 108:104–8. doi: 10.1016/j.micpath.2017.05.011
167. Chen J, Li D-L, Xie L-N, Ma Y-r, Wu P-P, Li C, et al. Synergistic anti-inflammatory effects of silibinin and thymol combination on Lps-induced Raw264.7 cells by inhibition of Nf-Kb and Mapk activation. Phytomedicine. (2020) 78:153309. doi: 10.1016/j.phymed.2020.153309
168. Liu P, Chen W, Kang Y, Wang C, Wang X, Liu W, et al. Silibinin ameliorates sting-mediated neuroinflammation via downregulation of ferroptotic damage in a sporadic Alzheimer’s disease model. Arch Biochem Biophys. (2023). doi: 10.1016/j.abb.2023.109691
169. Lan TH, Huang XQ, Tan HM. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. (2013) 22:401–7. doi: 10.1016/j.carpath.2013.01.003
170. Li W, Huang Z, Luo Y, Cui Y, Xu M, Luo W, et al. Tetrandrine alleviates atherosclerosis via inhibition of sting-Tbk1 pathway and inflammation in macrophages. Int Immunopharmacol. (2023) 119:110139. doi: 10.1016/j.intimp.2023.110139
171. Oseran AS, Yeh RW. Time to treatment in St-segment elevation myocardial infarction: identifying dangerous delays or diminishing returns? Jama. (2022) 328:2016–7. doi: 10.1001/jama.2022.19441
172. Chen J, Huang Q, Li J, Yao Y, Sun W, Zhang Z, et al. Panax ginseng against myocardial ischemia/reperfusion injury: A review of preclinical evidence and potential mechanisms. J Ethnopharmacol. (2023) 300:115715. doi: 10.1016/j.jep.2022.115715
173. Liu Y, Xue Q, Li A, Li K, Qin X. Mechanisms exploration of herbal pair of Huangqi-Danshen on cerebral ischemia based on metabonomics and network pharmacology. J Ethnopharmacol. (2020) 253:112688. doi: 10.1016/j.jep.2020.112688
174. Liu X, Lu J, Liu S, Huang D, Chen M, Xiong G, et al. Huangqi-danshen decoction alleviates diabetic nephropathy in Db/Db mice by inhibiting Pink1/Parkin-mediated mitophagy. Am J Transl Res. (2020) 12:989–98.
175. Rao S, Lin Y, Lin R, Liu J, Wang H, Hu W, et al. Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J Nanobiotechnol. (2022) 20:278. doi: 10.1186/s12951-022-01490-x
176. Zhai P, Chen Q, Wang X, Ouyang X, Yang M, Dong Y, et al. The combination of tanshinone Iia and astragaloside Iv attenuates myocardial ischemia–reperfusion injury by inhibiting the sting pathway. Chin Med Uk. (2024) 19:34. doi: 10.1186/s13020-024-00908-y
177. Mohan AT, Sur YJ, Zhu L, Morsy M, Wu PS, Moran SL, et al. The concepts of propeller, perforator, keystone, and other local flaps and their role in the evolution of reconstruction. Plast Reconstr Surg. (2016) 138:710e–29e. doi: 10.1097/prs.0000000000002610
178. Siemionow M, Arslan E. Ischemia/reperfusion injury: A review in relation to free tissue transfers. Microsurgery. (2004) 24:468–75. doi: 10.1002/micr.20060
179. Li YB, Liu HF, Zeng ZH, Lin H, Chen X, Yuan XL, et al. Ginsenoside Rb3 attenuates skin flap ischemia-reperfusion damage by inhibiting sting-Irf3 signaling. J Mol Histol. (2022) 53:763–72. doi: 10.1007/s10735-022-10081-x
180. Xu C, Cai T, Du X. Liuwei Dihuang prevents human umbilical vein endothelial cells senescence via the Jpx-Sting-Irf3 pathway. Combinatorial Chem High Throughput Screening. (2023). doi: 10.2174/1386207326666230901163717
181. Guo S, Wang J, Wang Y, Zhang Y, Bi K, Zhang Z, et al. Study on the multitarget synergistic effects of Kai-Xin-San against Alzheimer’s disease based on systems biology. Oxid Med Cell Longev. (2019) 2019:1707218. doi: 10.1155/2019/1707218
182. Pan JJ, He SY, Shao JY, Li N, Gong YQ, Gong XC. Critical pharmaceutical process identification considering chemical composition, biological activity, and batch-to-batch consistency: A case study of notoginseng total saponins. Chin Herb Med. (2020) 12:29–35. doi: 10.1016/j.chmed.2019.11.002
183. Wang D, Ding J, Feng X, Chai X, Yang J, Liu C, et al. Identification of Q-markers from Hedan tablet by employing “Spider-web” Mode and taking compounds’ Hepatotoxicity into account. Chin Herbal Medicines. (2022) 14(4):612–21. doi: 10.1016/j.chmed.2021.08.007
184. Zhang Y, Lv X, Liu R, Zhang M, Liu H, Gao H, et al. An integrated strategy for ascertaining quality marker of Schisandra chinensis (Turcz.) baill based on correlation analysis between depression-related monoaminergic metabolites and chemical components profiling. J Chromatogr A. (2019) 1598:122–31. doi: 10.1016/j.chroma.2019.03.056
185. Shen Q, Wu Y, Li Z, Wu X, Wang Y, Chen Y, et al. Quality assessment of traditional Chinese medicine using quality biomarkers: compound Danshen dripping pills as an example. Phytochem Anal. (2023) 34(5):580–93. doi: 10.1002/pca.3238
186. Koyama S, Ishii KJ, Coban C, Akira S. Innate immune response to viral infection. Cytokine. (2008) 43:336–41. doi: 10.1016/j.cyto.2008.07.009
187. Rong L, Perelson AS. Treatment of hepatitis C virus infection with interferon and small molecule direct antivirals: viral kinetics and modeling. Crit Rev Immunol. (2010) 30:131–48. doi: 10.1615/critrevimmunol.v30.i2.30
188. Liu Y, Pu F. Updated roles of cgas-sting signaling in autoimmune diseases. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1254915
189. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, et al. Cgas produces a 2’-5’-linked cyclic dinucleotide second messenger that activates sting. Nature. (2013) 498:380–4. doi: 10.1038/nature12306
190. Liu N, Pang X, Zhang H, Ji P. The cgas-sting pathway in bacterial infection and bacterial immunity. Front Immunol. (2022) 12:814709. doi: 10.3389/fimmu.2021.814709
191. Zhang X, Bai X-C, Chen ZJ. Structures and mechanisms in the cgas-sting innate immunity pathway. Immunity. (2020) 34(5):580–93. doi: 10.1016/j.immuni.2020.05.013
192. Fan N, Zhao F, Meng Y, Chen L, Miao L, Wang P, et al. Metal complex lipid-based nanoparticles deliver metabolism-regulating lomitapide to overcome Ctc immune evasion via activating sting pathway. Eur J Pharm Biopharm. (2024) 203:114467. doi: 10.1016/j.ejpb.2024.114467
193. Irvine DJ, Aung A, Silva M. Controlling timing and location in vaccines. Adv Drug Delivery Rev. (2020) 158:91–115. doi: 10.1016/j.addr.2020.06.019
194. Meric-Bernstam F, Sandhu SK, Hamid O, Spreafico A, Kasper S, Dummer R, et al. Phase Ib study of Miw815 (Adu-S100) in combination with spartalizumab (Pdr001) in patients (Pts) with advanced/metastatic solid tumors or lymphomas. J Clin Oncol. (2019) 37:2507. doi: 10.1200/JCO.2019.37.15_suppl.2507
195. Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide sting agonists to enhance cancer immunotherapy. Nat Nanotechnol. (2019) 14:269–78. doi: 10.1038/s41565-018-0342-5
196. Haag SM, Gulen MF, Reymond L, Gibelin A, Abrami L, Decout A, et al. Targeting sting with covalent small-molecule inhibitors. Nature. (2018) 559:269–73. doi: 10.1038/s41586-018-0287-8
197. Feng X, Ge J, Fu H, Miao L, Zhao F, Wang J, et al. Discovery of small molecule B-catenin suppressors that enhance immunotherapy. Bioorg Chem. (2023) 139:106754. doi: 10.1016/j.bioorg.2023.106754
198. Li S, Hong Z, Wang Z, Li F, Mei J, Huang L, et al. The cyclopeptide astin C specifically inhibits the innate immune Cdn sensor sting. Cell Rep. (2018) 25:3405–21 e7. doi: 10.1016/j.celrep.2018.11.097
199. Humphries F, Shmuel-Galia L, Jiang Z, Zhou JY, Barasa L, Mondal S, et al. Targeting sting oligomerization with small-molecule inhibitors. Proc Natl Acad Sci. (2023) 120:e2305420120. doi: 10.1073/pnas.2305420120
200. Wang M, Sooreshjani MA, Mikek C, Opoku-Temeng C, Sintim HO. Suramin potently inhibits Cgamp synthase, Cgas, in Thp1 cells to modulate Ifn-beta levels. Future Med Chem. (2018) 10:1301–17. doi: 10.4155/fmc-2017-0322
201. Wang J, Lu W, Zhang J, Du Y, Fang M, Zhang A, et al. Loss of Trim29 mitigates viral myocarditis by attenuating perk-driven Er stress response in male mice. Nat Commun. (2024) 15:3481. doi: 10.1038/s41467-024-44745-x
202. Wang J, Wang L, Lu W, Farhataziz N, Gonzalez A, Xing J, et al. Trim29 controls enteric Rna virus-induced intestinal inflammation by targeting Nlrp6 and Nlrp9b signaling pathways. Mucosal Immunol. (2024). doi: 10.1016/j.mucimm.2024.10.004
203. Fang M, Zhang A, Du Y, Lu W, Wang J, Minze LJ, et al. Trim18 is a critical regulator of viral myocarditis and organ inflammation. J Biomed Sci. (2022) 29:55. doi: 10.1186/s12929-022-00840-z
Keywords: cGAS-STING pathway, traditional Chinese medicine, immunity, disease, cancer, infection6 cGAS-STING pathway, infection
Citation: Zhi H, Fu H, Zhang Y, Fan N, Zhao C, Li Y, Sun Y and Li Y (2024) Progress of cGAS-STING signaling pathway-based modulation of immune response by traditional Chinese medicine in clinical diseases. Front. Immunol. 15:1510628. doi: 10.3389/fimmu.2024.1510628
Received: 13 October 2024; Accepted: 29 November 2024;
Published: 16 December 2024.
Edited by:
Thomas A. Kufer, University of Hohenheim, GermanyReviewed by:
Junji Xing, Houston Methodist Research Institute, United StatesRobert B. Levy, University of Miami, United States
Copyright © 2024 Zhi, Fu, Zhang, Fan, Zhao, Li, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujiao Sun, c3VueXVqaWFvQHRqdXRjbS5lZHUuY24=; Yingpeng Li, bGl5aW5ncGVuZ0B0anV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Hui Zhi1†
Hui Zhi1† Yingpeng Li
Yingpeng Li