- 1College of Integrated Traditional Chinese and Western Medicine, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, China
- 2Department of Gynecology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 3State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, Jiangsu, China
- 4Center for New Drug Safety Evaluation and Research, China Pharmaceutical University, Nanjing, Jiangsu, China
Background: Immune checkpoint inhibitors (ICIs) combined with standard therapy (ST) have emerged as a novel treatment strategy for recurrent or advanced cervical cancer (r/a CC). However, the available data from phase 3 clinical trials have yielded mixed results. This study aims to evaluate the therapeutic efficacy and safety of adding ICIs to ST in the treatment of r/a CC.
Methods: Data from four phase 3 clinical trials (KEYNOTE-826, CALLA, BEATcc, and ENGOT-cx11/GOG-3047/KEYNOTE-A18), involving 2,857 patients, were analyzed. Meta-analyses were conducted to combine hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS), odds ratios (ORs) for the objective response rate (ORR), and relative risks (RRs) for adverse events (AEs).
Results: The addition of ICIs to ST significantly improved PFS (HR, 0.67; 95% CI, 0.60-0.75), OS (HR, 0.66; 95% CI, 0.58-0.75), and ORR (OR, 1.48; 95% CI, 1.13-1.94) compared to ST alone. However, there was a modest increase in grade 3-5 AEs (RR, 1.08; 95% CI, 1.03-1.13) with the combined therapy.
Conclusion: This meta-analysis indicates that the combination of ICIs with ST in the treatment of r/a CC not only demonstrates superior efficacy over ST alone but also maintains a comparable toxicity profile, offering strong evidence for an effective and relatively safe treatment approach for managing this disease.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024593895.
Introduction
Cervical cancer (CC) remains a significant global health issue, being the fourth most common cancer in women worldwide (1). For patients with recurrent or advanced cervical cancer (r/a CC), current standard therapies (ST), such as chemotherapy (CT), targeted therapy, and concurrent chemoradiotherapy (CCRT), offer limited benefits, underscoring the need for innovative treatments (2–4). Immunotherapy, especially immune checkpoint inhibitors (ICIs), has shown promise in various cancers and is increasingly being explored for CC treatment (5–8).
Recently, four phase 3 clinical trials have assessed the incorporation of ICIs into first-line ST for r/a CC. The KEYNOTE-826 trial evaluated the efficacy of pembrolizumab in combination with CT as a first-line treatment for r/a CC (9, 10). The results demonstrated a significant improvement in both progression-free survival (PFS) and overall survival (OS) compared to CT alone. This finding underscores the potential role of immunotherapy in the treatment of CC and supports its consideration in clinical practice. The CALLA trial investigated the addition of durvalumab to CCRT for the treatment of locally advanced cervical cancer (la CC) (11). Unfortunately, the study did not meet its primary endpoints of improving PFS or OS compared to CCRT alone. These results indicate that further research is necessary to determine the role of immunotherapy in the context of la CC treatment. The BEATcc trial assessed the efficacy of atezolizumab combined with platinum-based CT and bevacizumab as a first-line treatment for metastatic (stage IVB), persistent, or recurrent CC (12). The results showed a significant improvement in PFS and OS compared to CT and bevacizumab alone. This outcome highlights the potential of immunotherapy, in conjunction with traditional CT and targeted therapy, as a valuable treatment option for r/a CC, supporting its integration into clinical practice. The ENGOT-cx11/GOG-3047/KEYNOTE-A18 trial evaluated the therapeutic potential of combining pembrolizumab with CCRT as a first-line treatment for newly diagnosed, high-risk la CC (13, 14). The findings revealed a notable enhancement in both PFS and OS for patients receiving combination therapy compared to those treated with CCRT alone. These results reinforce the idea that immunotherapy plays a crucial role in the management of la CC and suggest its integration into standard clinical care. While KEYNOTE-826, BEATcc, and ENGOT-cx11/GOG-3047/KEYNOTE-A18 yielded positive results regarding their design, CALLA failed to meet its primary endpoint in the intention-to-treat (ITT) population. Additionally, the individual studies lacked sufficient power to analyze various clinically relevant subgroups.
Given the need in the treatment landscape of r/a CC, we conducted a meta-analysis of phase 3 clinical trials comparing the combination of ICIs with ST versus ST alone in patients with r/a CC. This study aims to provide insights into the clinical benefits and risks associated with the addition of ICIs to ST, empowering clinicians with robust data to inform their treatment decisions and patient management strategies.
Methods
Data sources
For this meta-analysis, data were sourced from four published phase 3 clinical trials: KEYNOTE-826, CALLA, BEATcc, and ENGOT-cx11/GOG-3047/KEYNOTE-A18. We extracted necessary data directly from the original publications of these trials and cross-referenced it with information available in clinical trial registries such as ClinicalTrials.gov to ensure consistency in trial design and reporting of outcomes. To guarantee the accuracy and completeness of the data, we also reviewed associated conference abstracts and supplementary materials. The data included primary and secondary endpoint results, along with key metrics for assessing treatment efficacy and safety.
Data extraction and assessment of risk of bias
Data pertinent to the study objectives were extracted by a primary investigator and subsequently verified for accuracy by an independent secondary reviewer. The information extracted included, where available, the title of the clinical trial, the date of publication, sample size, study design, therapeutic regimens for both the experimental and control groups, characteristics of the participants, hazard ratios (HRs) along with their corresponding 95% confidence intervals (CIs) for OS and PFS, odds ratios (ORs) with associated 95% CIs for objective response rate (ORR), and the incidence of any adverse events (AEs), as well as the number of patients experiencing grade 3-5 AEs. The assessment of risk of bias was conducted using the Cochrane Collaboration’s Risk of Bias assessment tool (15).
Statistical analysis
For the efficacy analysis, HRs with 95% CIs for OS and PFS, as well as ORs with 95% CIs for ORR, were computed for each study to derive an overall estimate. In the context of safety analysis, relative risks (RRs) with 95% CIs for AEs were calculated for each study to obtain a comprehensive estimation. The I² statistic and the Cochrane Q test were utilized to evaluate between-study heterogeneity. An I² value exceeding 50% and a p-value below 0.1 from the Q test signified considerable heterogeneity, necessitating the use of a random-effects model. In contrast, a fixed-effect model was applied when these criteria were not satisfied. A funnel plot was created, and Egger’s test was conducted to evaluate publication bias. All statistical analyses were conducted using R (v4.2.2), with statistical significance set at a two-tailed p-value < 0.05.
Results
Characteristics of the four phase 3 trials
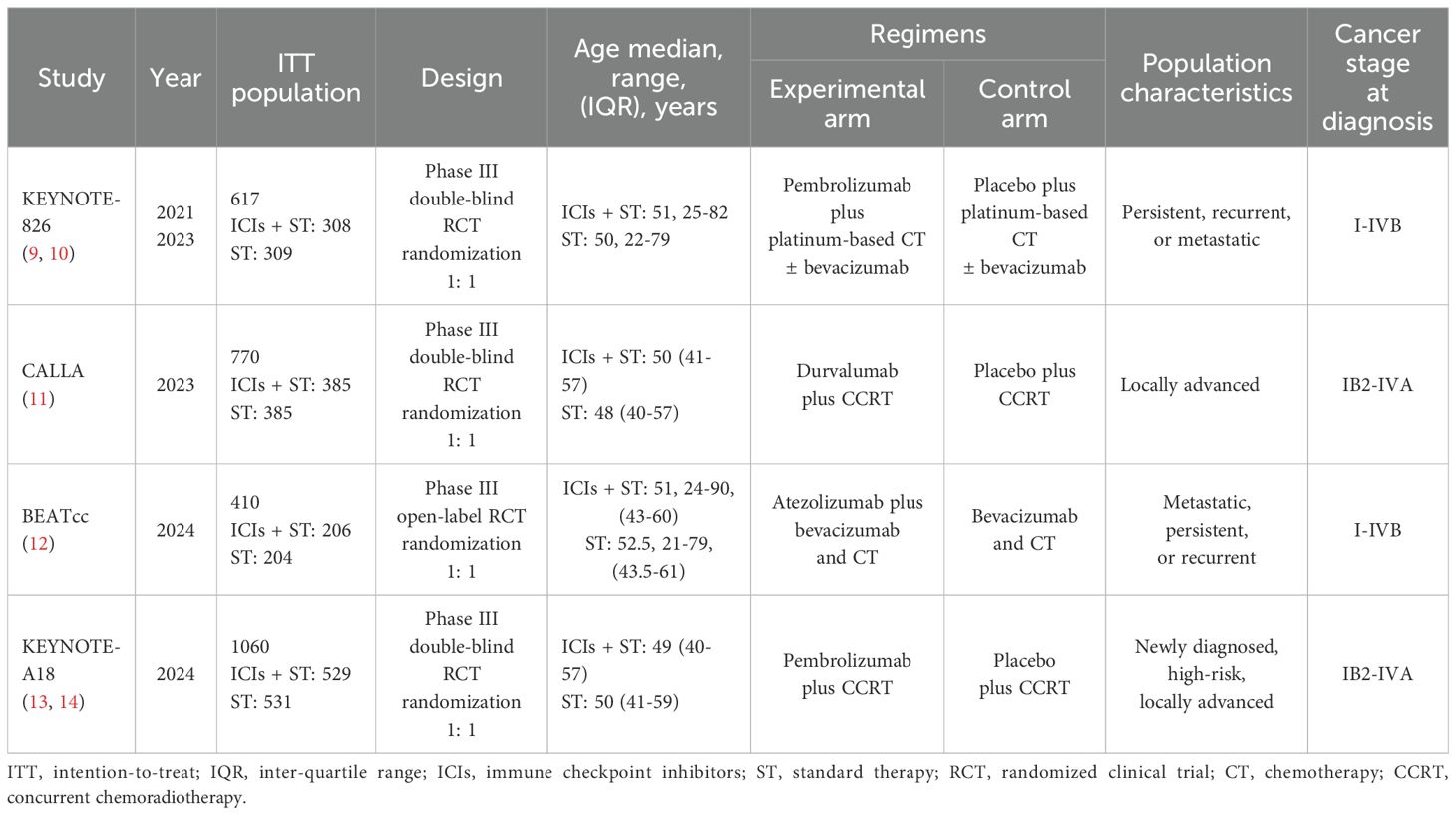
Among the four included studies, one employed an open-label design, while the remaining three utilized a double-blind design. A cumulative total of 2,857 patients diagnosed with r/a CC were analyzed. Of these, 1,428 patients were treated with ICIs in combination with ST, and 1,429 patients received ST alone. The experimental treatment regimens comprised pembrolizumab, durvalumab, and atezolizumab, each in conjunction with ST. The control arm regimens consisted of placebo plus ST, which included platinum-based CT ± bevacizumab, CCRT, and bevacizumab plus CT. The main characteristics of the four trials are summarized in Table 1.
Efficacy analysis
PFS in ITT population
In the absence of significant between-study heterogeneity (I² = 31%), a fixed-effect model was utilized to derive the pooled estimate of PFS. The combined analysis showed that adding ICIs to ST significantly improved PFS compared to ST alone (HR, 0.67; 95% CI, 0.60-0.75; Figure 1A).
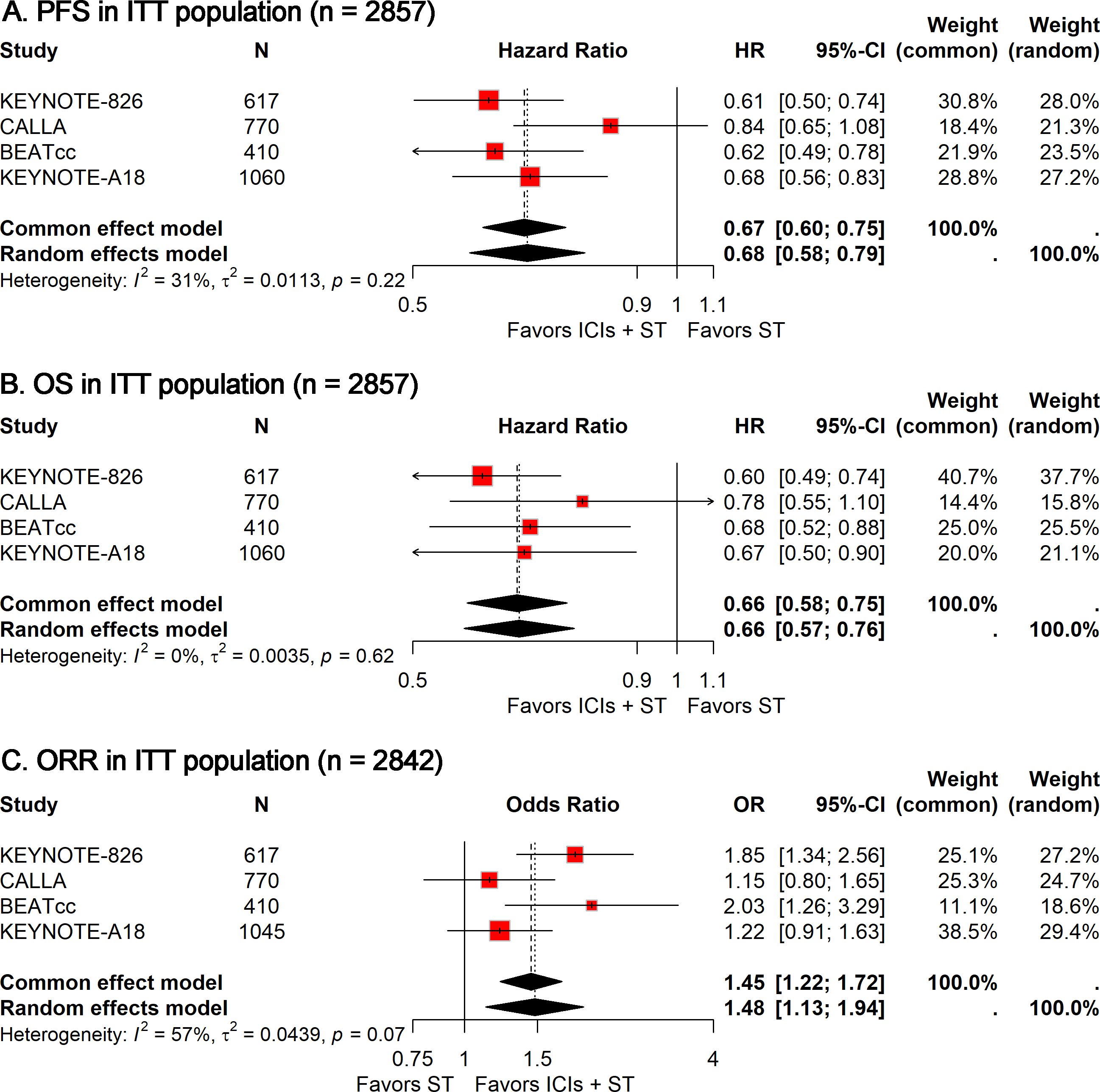
Figure 1. Forest plots comparing ICIs plus ST to ST alone in the ITT population for PFS (A), OS (B), and ORR (C). PFS, progression-free survival; OS, overall survival; ORR, objective response rate; ICIs, immune checkpoint inhibitors; ST, standard therapy; ITT, intention-to-treat.
OS in ITT population
Similarly, no heterogeneity (I² = 0) was observed across these studies. The meta-analysis suggested that combining ICIs with ST led to a significant extension of OS compared to ST alone (HR, 0.66; 95% CI, 0.58-0.75; Figure 1B).
ORR in ITT population
Given the significant heterogeneity observed among studies (I² = 57%), a random-effects model was used to compute the combined OR with 95% CI. The meta-analysis suggested that the addition of ICIs to ST significantly enhanced the ORR compared to ST alone (OR, 1.48; 95% CI, 1.13-1.94; Figure 1C).
Safety analysis
Grade 3-5 AEs
Among the 1,425 patients treated with ICIs plus ST, 1,029 (72.2%) experienced grade 3-5 AEs, compared to 949 out of 1,422 patients (66.7%) in the ST alone group. The meta-analysis showed that adding ICIs to ST was linked to a small rise in the risk of grade 3-5 AEs (RR, 1.08; 95% CI, 1.03-1.13; Figure 2).

Figure 2. Forest plot of grade 3-5 AEs comparing ICIs plus ST to ST alone in the ITT population. AEs, adverse events; ICIs, immune checkpoint inhibitors; ST, standard therapy; ITT, intention-to-treat.
Subgroup analysis
To achieve a more profound understanding of the efficacy of ICIs combined with ST in patients with r/a CC, we conducted several stratified analyses based on patient characteristics and treatment regimens.
In light of the observed heterogeneity within the r/a CC cohort and significant variations based on PD-L1 status, we performed a targeted subgroup analysis to ascertain whether PD-L1 status could serve as a biomarker for the efficacy of ICIs plus ST. The analyses revealed that the combination of ICIs with ST significantly improved PFS (HR, 0.68; 95% CI, 0.56-0.84; Figure 3A), OS (HR, 0.66; 95% CI, 0.57-0.77; Figure 3B), and ORR (OR, 1.73; 95% CI, 1.15-2.59; Figure 3C) in the PD-L1-positive population. Conversely, no significant statistical disparities were detected in the PD-L1-negative population (Figure 3). However, it is crucial to acknowledge the limited number of PD-L1-negative patients, which necessitates cautious interpretation of these data.
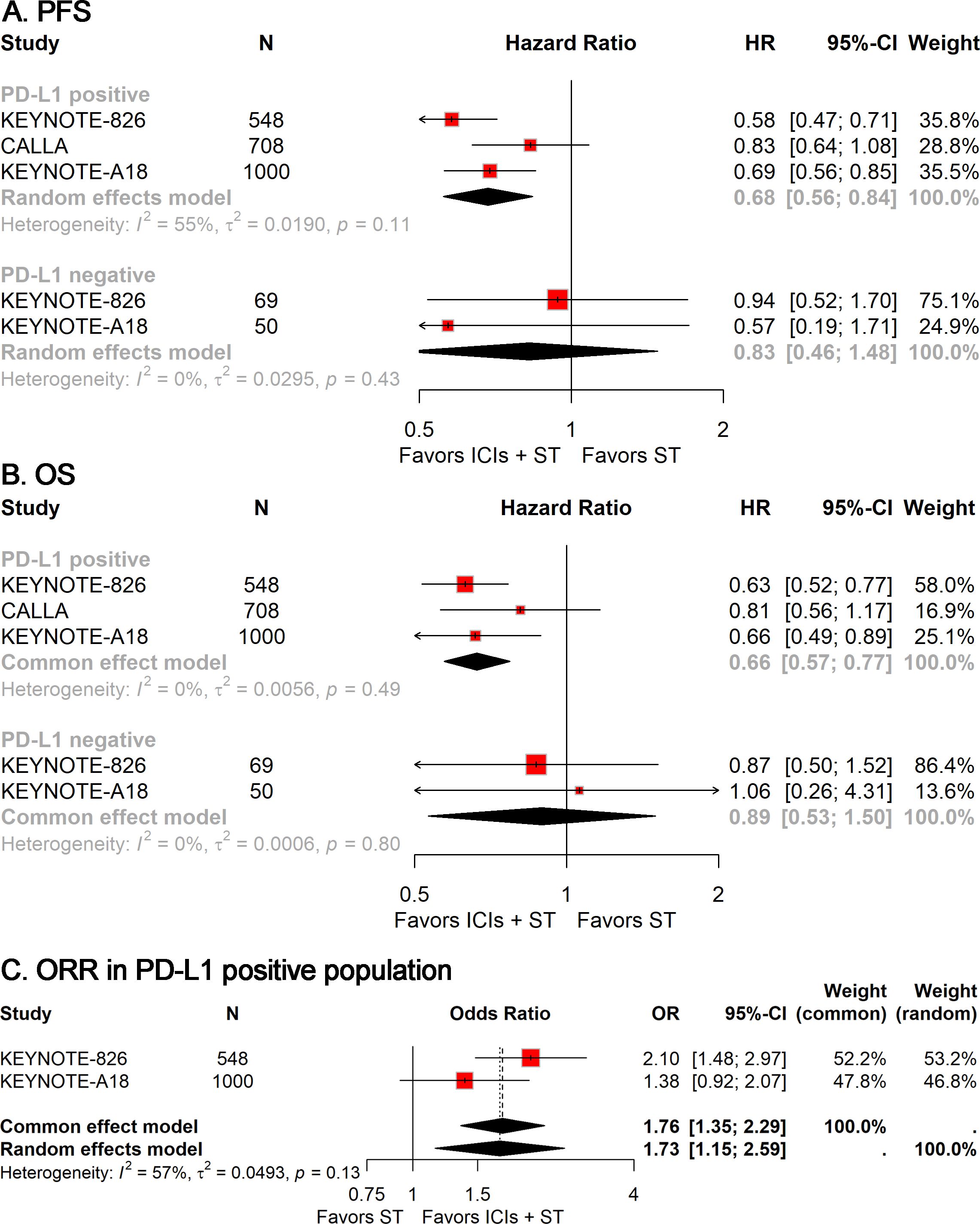
Figure 3. Forest plots of subgroup analysis stratified by PD-L1 status, showing results for PFS (A), OS (B), and ORR (C). PFS, progression-free survival; OS, overall survival; ORR, objective response rate.
To examine the impact of clinical characteristic variations on the efficacy of ICIs plus ST in patients with r/a CC, we conducted multiple subgroup analyses based on patient attributes, including age, race, Eastern Cooperative Oncology Group (ECOG) Performance Status, and disease status. In the subgroup of patients under 65 years of age, the addition of ICIs to ST significantly improved PFS (HR, 0.68; 95% CI, 0.62-0.75; Figure 4A) and OS (HR, 0.62; 95% CI, 0.55-0.70; Figure 4B). In contrast, for patients aged 65 years and older, the addition of ICIs to ST significantly improved PFS (HR, 0.63; 95% CI, 0.46-0.87; Figure 4A), whereas no significant differences in OS were observed (Figure 4B). Subgroup analyses based on race and ECOG status indicated that the addition of ICIs to ST significantly improved both PFS and OS irrespective of patient race (White and others) (Figures 4C, D) and ECOG status (0 and 1) (Figures 4E, F). Among patients with metastatic disease, the addition of ICIs to ST did not significantly improve either PFS or OS (Figures 4G, H). However, in patients with non-metastatic disease, the addition of ICIs to ST significantly enhanced both PFS (HR, 0.59; 95% CI, 0.48-0.71; Figure 4G) and OS (HR, 0.58; 95% CI, 0.48-0.70; Figure 4H).
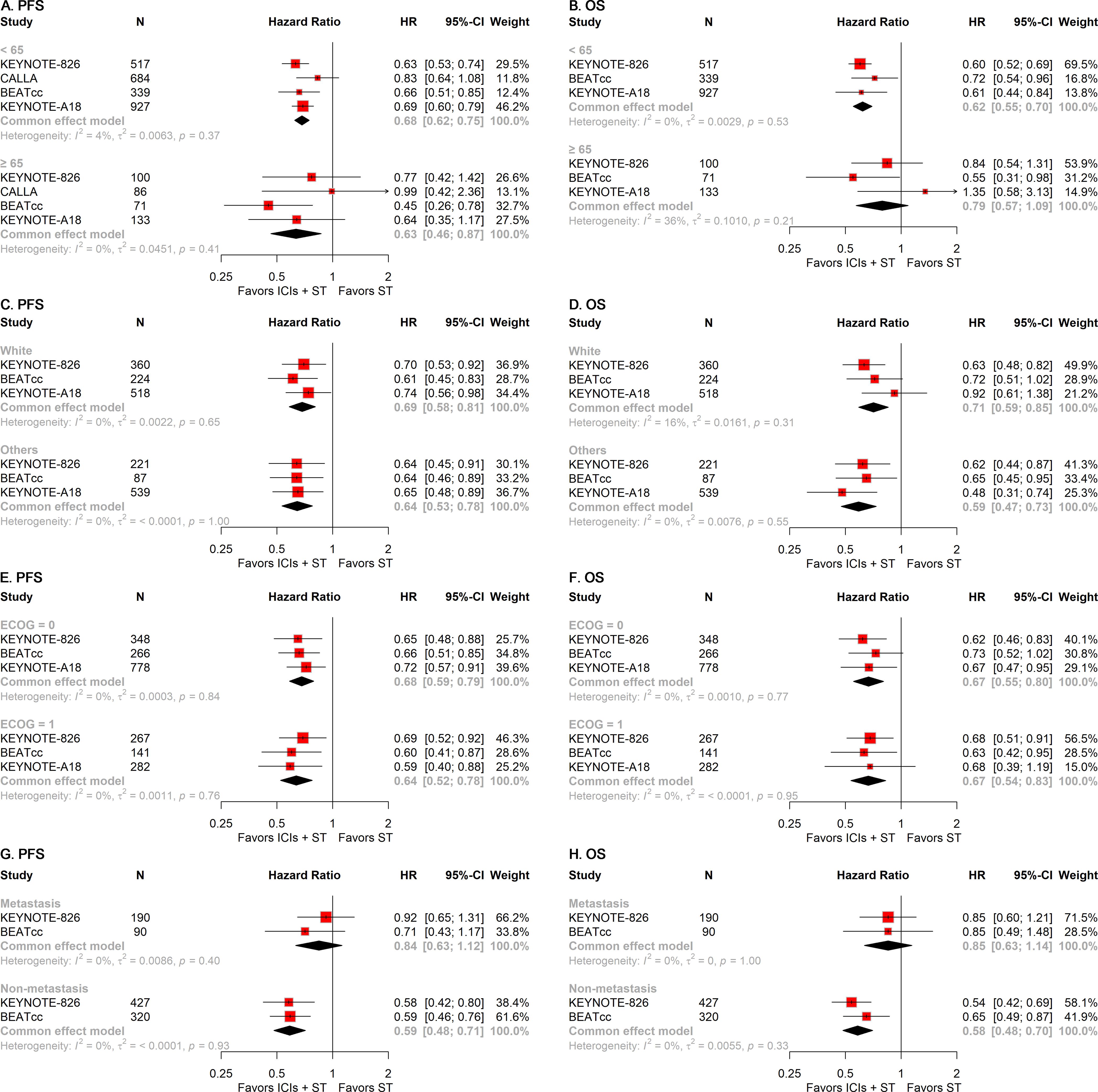
Figure 4. Forest plots of subgroup analysis stratified by clinical characteristics. Results for age on PFS (A) and OS (B). Results for race on PFS (C) and OS (D). Results for ECOG performance status on PFS (E) and OS (F). Results for disease status on PFS (G) and OS (H). PFS, progression-free survival; OS, overall survival; ECOG, Eastern Cooperative Oncology Group.
To gain further insights into the treatment modalities, we performed stratified analyses according to the type of ICIs and ST employed in the treatment regimens. The addition of either anti-PD-1 or anti-PD-L1 to ST was associated with significant improvements in PFS (anti-PD-1: HR, 0.64; 95% CI, 0.55-0.75; anti-PD-L1: HR, 0.72; 95% CI, 0.54-0.96; Figure 5A) and OS (anti-PD-1: HR, 0.62; 95% CI, 0.53-0.74; anti-PD-L1: HR, 0.71; 95% CI, 0.58-0.88; Figure 5B). Similarly, the inclusion of ICIs in ST was linked to superior PFS (CT ± bevacizumab: HR, 0.61; 95% CI, 0.53-0.71; CCRT: HR, 0.74; 95% CI, 0.63-0.87; Figure 5C) and OS (CT ± bevacizumab: HR, 0.63; 95% CI, 0.54-0.74; CCRT: HR, 0.71; 95% CI, 0.57-0.89; Figure 5D), regardless of the specific ST regimen used.
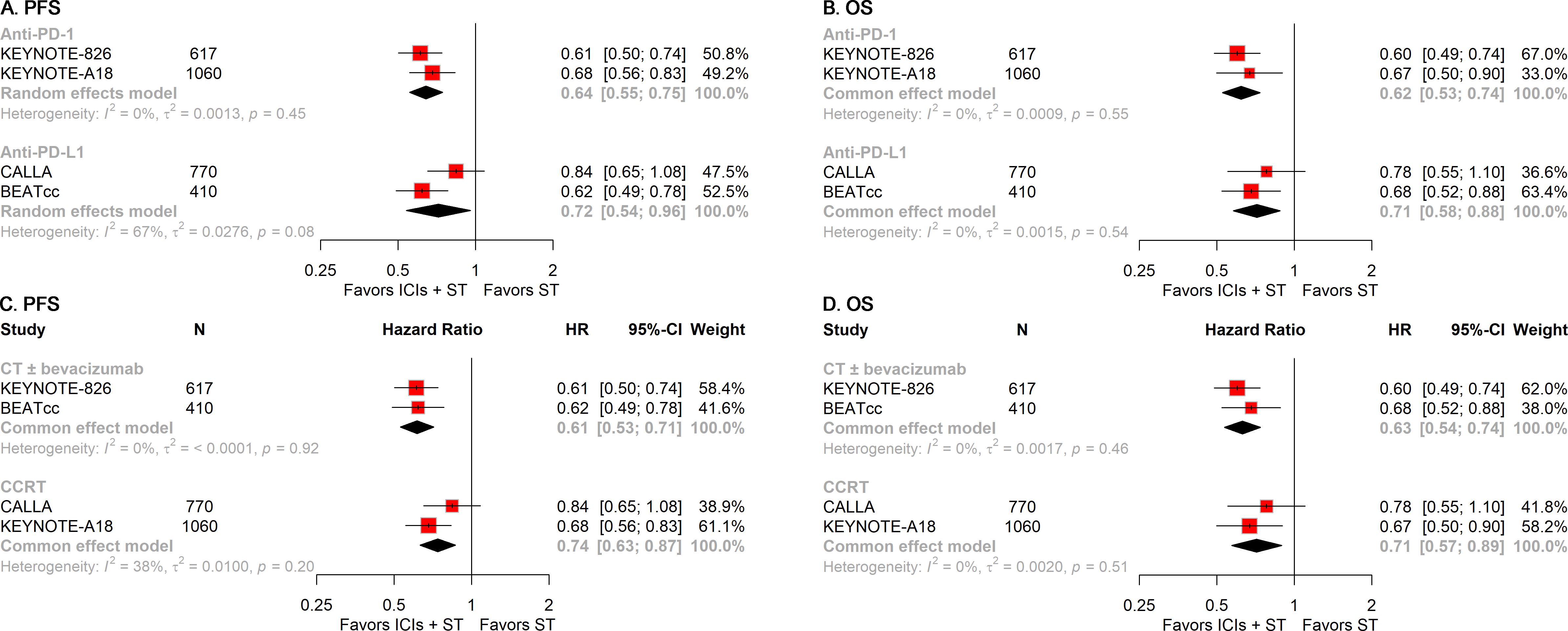
Figure 5. Forest plots of subgroup analysis stratified by treatment regimens. Results for the type of ICIs used on PFS (A) and OS (B). Results for the regimens of ST employed on PFS (C) and OS (D). PFS, progression-free survival; OS, overall survival; ICIs, immune checkpoint inhibitors; ST, standard therapy.
Risk of bias and sensitivity analysis
Among the four trials, three were conducted as double-blind trials, whereas one was conducted as an open-label trial. Consequently, the open-label trial was assessed as having a high risk of performance bias, an unclear risk of detection bias, and a low risk of selection, attrition, and reporting biases. The remaining studies were all evaluated as having a low risk of bias across all assessed criteria. The risk of bias assessment is graphically summarized in Supplementary Figure S1. The funnel plot, along with Egger’s test (P = 0.2984), did not indicate significant publication bias (Supplementary Figure S2). Sensitivity analysis for PFS, OS, and ORR confirmed the robustness of the pooled results (Supplementary Figure S3).
Discussion
Currently, the integration of ICIs into ST has emerged as a predominant area of research for patients diagnosed with r/a CC. However, published phase 3 trials have yielded conflicting results, leading to ongoing debate regarding the efficacy of ICIs combined with ST in treating r/a CC. The KEYNOTE-826 trial is a landmark study that evaluated pembrolizumab in conjunction with platinum-based CT and/or bevacizumab. The results demonstrated a significant improvement in both PFS and OS compared to CT alone. The CALLA trial explored the addition of durvalumab to CCRT; unfortunately, the study did not meet its primary endpoints compared to CCRT alone. The BEATcc trial investigated the incorporation of atezolizumab into a regimen of bevacizumab plus CT. The results showed a significant improvement in PFS and OS compared to CT and bevacizumab alone. In parallel, the ENGOT-cx11/GOG-3047/KEYNOTE-A18 trial assessed the efficacy of adding pembrolizumab to CCRT for r/a CC. The findings revealed a notable enhancement in both PFS and OS for patients receiving the combination therapy compared to those treated with CCRT alone. Therefore, we included these four phase 3 trials encompassing a total of 2,857 participants to evaluate the efficacy and safety of ICIs in combination with ST as a first-line treatment for patients with r/a CC. Our findings provide high-quality, evidence-based recommendations for the clinical management of patients with r/a CC and highlight crucial considerations for treating patients with different PD-L1 statuses.
While ICIs plus ST offer promising efficacy, it is important to acknowledge the increased toxicity associated with this treatment approach. Our meta-analysis revealed a slight increase in grade 3-5 AEs with ICIs plus ST compared to ST alone. These findings highlight the importance of carefully monitoring and managing AEs in patients undergoing ICIs plus ST. Clinicians should weigh the potential benefits of ICIs plus ST against the risk of increased toxicity when considering this treatment option for r/a CC patients.
To gain deeper insights into the efficacy of ICIs plus ST in the treatment of r/a CC, we conducted several specific subgroup analyses focusing on PD-L1 status, clinical characteristics, and treatment regimens. These subgroup analyses suggest that the combination of ICIs and ST holds particular therapeutic promise in the following patient populations: i) PD-L1-positive patients. The combination therapy of ICIs and ST demonstrated a significant improvement in patients with PD-L1-positive r/a CC, indicating that PD-L1 status is a critical biomarker for identifying patients who are likely to benefit the most from this treatment approach. ii) Patients aged less than 65 years. Our data revealed that the benefits of ICIs plus ST in terms of PFS and OS were more pronounced in patients under the age of 65. This suggests that younger patients may have a more favorable response to this combination therapy. iii) Non-metastatic patients. The combination of ICIs and ST appeared to be more effective in patients with non-metastatic disease compared to those with metastatic disease. This finding highlights the potential for ICIs to improve outcomes in patients with less advanced forms of the disease. These findings have significant therapeutic implications, as they can guide clinicians in personalizing treatment strategies for r/a CC patients. By focusing on these specific populations, clinicians can optimize the use of ICIs and ST, potentially leading to improved patient outcomes and minimizing the risk of unnecessary treatment-related AEs in those who may not benefit as much.
The potential of CCRT to enhance anticancer immune responses by promoting the release of cancer antigens is widely recognized (16). However, the optimal CCRT regimen to synergize with ICIs remains an open question, prompting ongoing research to identify treatment strategies that effectively mobilize and activate tumor-specific T cells while mitigating immune suppression. The results from the KEYNOTE-A18 study did not align with those observed in the CALLA study, highlighting the need for further investigation into the interplay between CCRT and ICIs in r/a CC. Three possibilities may explain the differences observed between CALLA and KEYNOTE-A18: i) Differences in drugs. Durvalumab is a PD-L1 inhibitor, while pembrolizumab is a PD-1 inhibitor. This raises the question of whether a PD-1 antibody targeting the T-cell surface has a more direct regulatory effect on the immune system compared to a PD-L1 antibody targeting the tumor cell surface, potentially leading to better treatment outcomes (17). ii) Patient population. The CALLA study enrolled a relatively high proportion of patients with early-stage disease (IB2-IIB). Patients with early-stage disease generally experience favorable outcomes with CCRT alone, which could narrow the survival gap between experimental and control groups, making it difficult to observe significant statistical differences in PFS between the two study arms (18). iii) Radiation dose regimen. The radiation dose regimen specified in the CALLA study might be more conservative compared to KEYNOTE-A18. Clinically, achieving a tumor-killing dose (radical dose) with radiotherapy is crucial for local control and patient prognosis (19). Unlike CT, immunotherapy may not achieve satisfactory tumor control as monotherapy (20). Therefore, adding immunotherapy to a regimen with an insufficient tumor-killing dose may not fully exploit the long-lasting benefits of immunotherapy. These considerations underscore the complexity of integrating CCRT with ICIs and highlight the necessity for further research to optimize treatment strategies for r/a CC.
There are some limitations in this meta-analysis. Firstly, a discrepancy exists in the techniques utilized to assess PD-L1 across the trials. Specifically, two trials employed the PD-L1 IHC 22C3 pharmDx assay, characterizing PD-L1 expression by a combined positive score (CPS) ≥ 1 (9, 10, 13). In contrast, the CALLA trial assessed PD-L1 expression according to the tumor area positivity (TAP) score using the VENTANA PD-L1 (SP263) assay, with TAP ≥ 1% serving as the criterion (11). This discrepancy in PD-L1 assessment poses a significant challenge in clinical studies exploring immunotherapy for r/a CC. To address this issue, there is a need for better harmonization of PD-L1 testing across clinical trials to ensure consistency and comparability of results. Secondly, the reporting of AEs was inconsistent across the included studies, and only those AEs reported in all trials were included in this meta-analysis. This limits our comprehensive understanding of potential AEs associated with the treatment regimens.
Conclusion
This meta-analysis is the inaugural study to elucidate that the integration of ICIs into ST represents a potent and relatively low-risk therapeutic strategy for individuals with r/a CC, offering robust support for the management of this malignancy. The synergistic effect of ICIs and ST is particularly pronounced within certain subsets of patients, including those with high PD-L1 expression, those younger than 65 years, and those with a non-metastatic disease state. The assessment of PD-L1 expression serves as a valuable biomarker in identifying patients likely to experience enhanced therapeutic gains from the combined regimen of ICIs and ST. The implications of these findings for clinical decision-making are significant, highlighting the need for further research to optimize the integration of ICIs with ST in the treatment of r/a CC. As such, these data have the potential to inform future clinical guideline development, particularly with regard to the incorporation of ICIs into standard ST protocols.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XZ: Writing – original draft, Methodology, Software, Visualization. JS: Methodology, Software, Visualization, Writing – original draft. MH: Data curation, Software, Writing – review & editing. RL: Funding acquisition, Project administration, Resources, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors extended their appreciation to the databases that provided the invaluable data resources for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to polish the Manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1507977/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Kitagawa R, Katsumata N, Shibata T, Kamura T, Kasamatsu T, Nakanishi T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol. (2015) 33:2129–35. doi: 10.1200/JCO.2014.58.4391
3. Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomized, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. (2017) 390:1654–63. doi: 10.1016/S0140-6736(17)31607-0
4. Mileshkin LR, Moore KN, Barnes EH, Gebski V, Narayan K, King MT, et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): an international, open-label, randomized, phase 3 trial. Lancet Oncol. (2023) 24:468–82. doi: 10.1016/S1470-2045(23)00147-X
5. Jiang Z, Ouyang Q, Sun T, Zhang Q, Teng Y, Cui J, et al. Toripalimab plus nab-paclitaxel in metastatic or recurrent triple-negative breast cancer: a randomized phase 3 trial. Nat Med. (2024) 30:249–56. doi: 10.1038/s41591-023-02677-x
6. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer. JAMA. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
7. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1853–65. doi: 10.1016/S0140-6736(23)00727-4
8. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. New Engl J Med. (2022) 386:449–62. doi: 10.1056/NEJMoa2111380
9. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. (2021) 385:1856–67. doi: 10.1056/NEJMoa2112435
10. Monk BJ, Colombo N, Tewari KS, Dubot C, Caceres MV, Hasegawa K, et al. First-line pembrolizumab + Chemotherapy versus placebo + Chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. J Clin Oncol. (2023) 41:5505–11. doi: 10.1200/JCO.23.00914
11. Monk BJ, Toita T, Wu X, Vázquez Limón JC, Tarnawski R, Mandai M, et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): a randomized, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1334–48. doi: 10.1016/S1470-2045(23)00479-5
12. Oaknin A, Gladieff L, Martínez-García J, Villacampa G, Takekuma M, De Giorgi U, et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomized, open-label, phase 3 trial. Lancet. (2024) 403:31–43. doi: 10.1016/S0140-6736(23)02405-4
13. Lorusso D, Xiang Y, Hasegawa K, Scambia G, Leiva M, Ramos-Elias P, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomized, double-blind, phase 3 clinical trial. Lancet. (2024) 403:1341–50. doi: 10.1016/S0140-6736(24)00317-9
14. Lorusso D, Xiang Y, Hasegawa K, Scambia G, Gálvez MHL, Elias PR, et alPembrolizumab plus chemoradiotherapy for high-risk locally advanced cervical cancer: Overall survival results from the randomized, double-blind, phase III ENGOT-cx11/GOG-3047/KEYNOTE-A18 study. Ann Oncol. (2024) 35:S544–95. doi: 10.1016/j.annonc.2024.08.771
15. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. (2011) 343:d5928–d. doi: 10.1136/bmj.d5928
16. Chen Daniel S, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
17. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer. JAMA Oncol. (2020) 6:375–84. doi: 10.1001/jamaoncol.2019.5367
18. Tan TH, Soon YY, Cheo T, Ho F, Wong LC, Tey J, et al. Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: A systematic review and meta-analysis. Radiotherapy Oncol. (2018) 129:10–7. doi: 10.1016/j.radonc.2018.02.027
19. Tanderup K, Fokdal LU, Sturdza A, Haie-Meder C, Mazeron R, van Limbergen E, et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiotherapy Oncol. (2016) 120:441–6. doi: 10.1016/j.radonc.2016.05.014
Keywords: immune checkpoint inhibitors, cervical cancer, efficacy, safety, meta-analysis
Citation: Zhang X, Shen J, Huang M and Li R (2024) Efficacy and safety of adding immune checkpoint inhibitors to first-line standard therapy for recurrent or advanced cervical cancer: a meta-analysis of phase 3 clinical trials. Front. Immunol. 15:1507977. doi: 10.3389/fimmu.2024.1507977
Received: 08 October 2024; Accepted: 20 November 2024;
Published: 06 December 2024.
Edited by:
Subhash Kumar Tripathi, Seattle Children’s Research Institute, United StatesReviewed by:
Sivasankaran M. Ponnan, Seattle Children’s Hospital, United StatesMansoor-Ali Vaali-Mohammed, King Saud University, Saudi Arabia
Copyright © 2024 Zhang, Shen, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongxia Li, bGlyb25neGlhMjAwMEAxNjMuY29t
†These authors have contributed equally to this work
 Xinmiao Zhang
Xinmiao Zhang Jinhai Shen
Jinhai Shen Mengfan Huang3,4
Mengfan Huang3,4 Rongxia Li
Rongxia Li