
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 22 November 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1504948
This article is part of the Research Topic Microbiota-Immune Interactions: A New Frontier in Cancer Treatment Optimization View all 5 articles
Hepatocellular carcinoma, a common malignancy of the digestive system, typically progresses through a sequence of hepatitis, liver fibrosis, cirrhosis and ultimately, tumor. The interaction between gut microbiota, the portal venous system and the biliary tract, referred to as the gut-liver axis, is crucial in understanding the mechanisms that contribute to the progression of hepatocellular carcinoma. Mechanisms implicated include gut dysbiosis, alterations in microbial metabolites and increased intestinal barrier permeability. Imbalances in gut microbiota, or dysbiosis, contributes to hepatocellular carcinoma by producing carcinogenic substances, disrupting the balance of the immune system, altering metabolic processes, and increasing intestinal barrier permeability. Concurrently, accumulating evidence suggests that gut microbiota has the ability to modulate antitumor immune responses and affect the efficacy of cancer immunotherapies. As a new and effective strategy, immunotherapy offers significant potential for managing advanced stages of hepatocellular carcinoma, with immune checkpoint inhibitors achieving significant advancements in improving patients’ survival. Probiotics play a vital role in promoting health and preventing diseases by modulating metabolic processes, inflammation and immune responses. Research indicates that they are instrumental in boosting antitumor immune responses through the modulation of gut microbiota. This review is to explore the relationship between gut microbiota and the emergence of hepatocellular carcinoma, assess the contributions of probiotics to immunotherapy and outline the latest research findings, providing a safer and more cost-effective potential strategy for the prevention and management of hepatocellular carcinoma.
Hepatocellular carcinoma (HCC), recognized as the leading form of liver cancer, is a prevalent malignancy of the digestive system and represents a significant global health challenge (1). HCC is among the top six cancers diagnosed around the world and stands as a major contributor to cancer mortality, underscoring the urgent need for improved preventive and therapeutic strategies (2). In recent years, immunotherapy for HCC has made significant strides, with an increasing number of immunotherapeutic agents emerging as viable first- and second-line treatment options. These therapies have demonstrated efficacy in controlling tumor progression and extending patient survival (3, 4).
Immune checkpoint inhibitors (ICIs) work by reversing tumor-induced immune suppression in HCC, thereby restoring and enhancing the T cells’ ability to target and eliminate cancer cells. Currently, the most widely used drugs in liver cancer immunotherapy are programmed cell death protein 1(PD-1) and programmed cell death-Ligand 1(PD-L1) inhibitors. They block the interaction between PD-1 and PD-L1, thereby reactivating tumor-specific T cells, enhancing their cytotoxic function, and promoting the destruction of tumor cells. Once the anti-tumor immune cycle is established, a sustained immune response can be achieved. Substantial evidence has demonstrated the association between gut microbiota and cancer (5). Gut microbiota is pivotal in regulating responses to cancer immunotherapy (6–11), while the microbial communities within the tumor microenvironment (TME) have been shown to enhance therapeutic efficacy (12).
Probiotics are preparations formulated using the principles of microecology, incorporating beneficial probiotics or growth-promoting substances that are harmless to the host, produced through specialized processes. These preparations can function as biochemical barriers, improving liver and intestinal function and enhancing immune responses. Probiotics have demonstrated wide-ranging clinical applications (Figure 1), such as treating recurrent Clostridium difficile infection (CDI) (13), alleviating functional constipation (14), managing non-alcoholic fatty liver disease (NAFLD) (15) and so on (16–19). Additionally, probiotics can be utilized to regulate blood pressure (20), modulate blood lipid levels (21), alleviate gastrointestinal symptoms in patients with Parkinson disease and indirectly impact motor symptoms, and improve Beck Depression Inventory (BDI) scores in individuals with major depressive disorder (22–24). Emerging evidence suggests that probiotics may enhance the efficacy of HCC immunotherapy through gut microbiota modulation, representing a promising novel therapeutic approach for HCC management.

Figure 1. Clinical application of probiotics Created in BioRender. c, c. (2024) https://BioRender.com/z26s686.
The gut-liver axis, a bidirectional communication system formed by gut microbiota, the portal venous system and the biliary tract, underlies the physiological interactions between gut microbiota and the liver (24). Originally, it described the immune response in cirrhotic patients to gut-derived microbes and food antigens in the bloodstream. Over the past decade, this concept has expanded to include a variety of diseases characterized by interactions between the gut, liver, and microbiota. Current discussions of the gut-liver axis often focus on the potential for gut-derived substances and microbiota-mediated signaling to influence liver pathology (25). Dysbiosis of gut microbiota can disrupt intestinal epithelial homeostasis and compromise the intestinal barrier, allowing bacterial components to enter the portal vein and systemic circulation. As a result, the liver is exposed to toxins, metabolites and by-products, which may trigger inflammation, cause hepatotoxicity, and directly contribute to liver carcinogenesis. Furthermore, gut microbiota is closely involved in the development of viral hepatitis and non-alcoholic steatohepatitis (NASH), both of which may lead to liver cirrhosis and significantly elevate the risk of HCC (26–28).
During the occurrence and development of HCC, different dietary structures, lifestyles and environmental factors can lead to changes in gut microbiota. Dietary cholesterol induced gut microbiota metabolites alteration including increased taurocholic acid and decreased 3-indolepropionic acid (29). A high-fiber diet can enrich beneficial microbiota and deplete potential detrimental ones in both humans and mice (30). Additionally, lifestyle changes also affect gut microbiota balance. Circadian rhythm disorders alter gut microbiota metabolic patterns, while increased stress responses elevate intestinal permeability (31). Furthermore, environmental factors such as air pollutants may interfere with the composition and function of gut microbiota through direct or indirect pathways, producing the carcinogenic metabolites (32). The following section will further elaborate on the mechanisms by which gut microbiota influences the initiation and development of HCC.
Gut microbiota plays a direct role in shaping the inflammatory microenvironment of the liver during HCC development and progression. By modifying the liver’s inflammatory status, gut microbiota can promote HCC progression. In Nlrp6-deficient mice, significant shifts in the microbiota were observed, characterized by a decrease in beneficial bacteria such as Akkermansia muciniphila and an increase in potentially harmful bacteria like Lactobacillus. These changes resulted in impaired intestinal barrier function, liver dysfunction and an elevated tumor burden. Harmful bacteria were found to facilitate HCC progression by activating neutrophil-mediated suppressive immune cells and impairing the function of CD8+ T cells, thereby disrupting the liver’s immune microenvironment (33, 34).
Gut dysbiosis is a key characteristic across all stages of chronic liver disease, and compromised intestinal barrier integrity is directly linked to the initiation and progression of HCC. When the intestinal barrier is disrupted, harmful substances—including bacteria, toxins, microbial metabolites and microbe-associated molecular patterns (MAMPs)—can translocate across the mucosal barrier, further aggravating dysbiosis (35, 36). This imbalance not only generates carcinogenic substances but also fosters liver fibrosis, cirrhosis and HCC progression by dysregulating immune responses, altering microbial metabolic pathways and increasing intestinal permeability (37–40).
Additionally, gut microbiota-derived metabolites have an significant impact on the initiation and progression of HCC (35). For example, a reduction in butyrate-producing bacteria can weaken the intestinal mucosal barrier, facilitating the progression of HCC (41). Besides, one study shows that butyrate supplementation specifically induces hepatocellular carcinoma cell apoptosis through activation of calcium signaling pathway and reactive oxygen species (ROS) generation. Significant inhibition of HCC proliferation and metastasis can be achieved through either butyrate supplementation or the depletion of SCAD gene (ACADS), which encodes a key enzyme for butyrate metabolism (42). Gut microbiota can also transform primary bile acids into secondary bile acids. Through the enterohepatic circulation, they then recirculated back to the liver (43). Secondary bile acids are not only hepatotoxic but also induce DNA damage and possess carcinogeni-c potential (26). In mouse models of oncogene-induced HCC and human HCC samples, studies utilizing gene expression profiling, metabolomic analysis and immunohistochemistry have identified SIRT5 as an important metabolic regulator in HCC development. It has been observed that SIRT5 expression is decreased in human HCC samples, while bile acid levels are elevated and correlated with increased M2 macrophage polarization. Another study shows that in mice, the loss of SIRT5, in conjunction with oncogenic signaling, leads to enhanced bile acid production due to hyper-succinylation in hepatocytes. Elevated bile acids serve as signaling molecules that activate the nuclear receptor FXR, promoting M2 macrophage polarization (44),In the TME, M2 macrophages are typically associated with tumor growth, invasion and metastasis. Research has shown that M2 macrophages release exosomes containing miR-23a-3p. These exosomes are then transferred to HCC cells and human umbilical vein endothelial cells. After being absorbed, the exosomes trigger several changes in these cells. They induce epithelial-mesenchymal transition (EMT), promote the formation of new blood vessels, and increase blood vessel permeability. Through these mechanisms, the exosomes ultimately enhance HCC metastasis. Moreover, the immunosuppressive properties of M2 macrophages enable tumor cells to evade immune surveillance, facilitating immune escape and further accelerating HCC progression (45). In liver cancer animal models, gut microbiota and TLR4 activation have been found to accelerate HCC progression by promoting cell proliferation and inhibiting apoptosis (46).
In conclusion, gut microbiota influences the liver’s inflammatory microenvironment through various direct and indirect mechanisms. The compromise of the intestinal barrier worsens the translocation of bacterial toxins and components, resulting in dysbiosis while increased levels of microbiota-derived metabolites, such as secondary bile acids, contribute to the initiation and progression of HCC.
Gut microbiota regulates liver immune responses through metabolites, including MAMPs, short-chain fatty acids and bile acids, which in turn modulate antitumor immunity (47, 48). One study has shown that gut microbiota-mediated bile acid metabolism can increase the abundance of CXCR6+ NKT cells in the liver, exerting antitumor effects in HCC. CXCL16 produced by liver sinusoidal endothelial cells attracts CXCR6+ NKT cells to this specific area, enhancing the presence of NKT cells in the liver and inhibiting tumor proliferation. A positive correlation has been observed between primary bile acid levels and CXCL16 expression, while secondary bile acids are negatively correlated (49).
In recent years, immunotherapy for HCC has made remarkable progress, with ICIs emerging as a primary therapeutic approach for advanced HCC. ICIs act by targeting co-inhibitory molecules such as PD-1/PD-L1, thereby enhancing T cell-mediated immune responses and preventing tumor immune evasion. Gut microbiota and its metabolites are essential in modulating T cell functions, profoundly affecting the efficacy of immunotherapy (50, 51). Microbial metabolites not only regulate antitumor immune responses but also potentiate the effectiveness of ICIs by reshaping the TME and modulating the immunogenicity of tumor cells.
Research has shown that oral administration of Bifidobacterium can enhance the maturation of dendritic cells (DCs) and increase the activation and accumulation of CD8+ T cells within the TME, thereby restoring the anticancer effectiveness of PD-L1 inhibitors. A study involving 65 patients with unresectable HCC or advanced cholangiocarcinoma, who switched to PD-1 inhibitors following first-line chemotherapy (gemcitabine plus cisplatin), indicated that the composition of gut microbiota is significantly related to the clinical responses of immunotherapy. In the clinical benefit group (CBR), 74 bacterial taxa were significantly enriched, whereas 40 taxa were enriched in the non-clinical benefit group (NCB). Patients in the CBR group with elevated levels of Alistipes sp. Marseille-P5997 and Lachnospiraceae bacterium-GAM79 exhibited longer overall survival (OS) and progression-free survival (PFS). Conversely, higher abundances of Veillonellaceae in the NCB group were associated with shorter PFS and OS (52). This study highlights the close link between gut microbiota and clinical responses to PD-1 inhibitors, suggesting that certain bacterial taxa could be potential biomarkers for predicting immunotherapy outcomes and survival benefits.
Gut microbiota synthesizes and converts small-molecule metabolites, such as inosine, short-chain fatty acids (SCFAs) and tryptophan, which circulate systemically to regulate antitumor immune responses. Inosine enhances tumor cell immunogenicity, activates immune cells and serves as a carbon source for CD8+ T cells, while SCFAs help maintain intestinal barrier integrity, inhibit tumor cell proliferation and induce apoptosis. Research has demonstrated that supplementation with Lactobacillus significantly suppresses HCC development in mice, primarily through the secretion of valeric acid and other SCFAs, thereby exerting antitumor effects in the liver (53). Furthermore, gut microbiota metabolite butyrate enhances the efficacy of cancer treatments by regulating intracellular calcium homeostasis and promoting the generation of ROS (42). Fecal microbiota composition and bile acid levels have also been correlated with the clinical response to immunotherapy in patients with unresectable HCC (54). In a study of 52 patients with solid tumors, higher concentrations of acetic acid, butyric acid, propionic acid and valeric acid in fecal samples showed longer progression-free survival (PFS), suggesting that SCFA levels may serve as potential biomarkers for predicting the efficacy of PD-1 inhibitors (55).
In summary, gut bacteria and their metabolites can affect the efficacy of immunotherapies such as ICIs, and some microbial communities may serve as predictive biomarkers for prognosis or treatment response in HCC patients (56). Additionally, gut microbiota may also influence the efficacy of other immunotherapies, such as adoptive cell transfer (ACT) therapy and cell-based therapies (57, 58).
Research has demonstrated that dysbiosis promotes cancer progression by altering TME. Probiotics, due to their ability to modulate gut microbiota and enhance immune responses, have emerged as a new strategy to improve the effectiveness of cancer immunotherapy. They effectively inhibiting tumor growth, including HCC (59). Compared with traditional HCC treatments, probiotics exhibit unique characteristics in regulating anti-tumor immunity (Table 1). Probiotics significantly impact the gut-liver axis by regulating gut microbial composition, enhancing intestinal barrier integrity, modulating immune reactions and influencing key metabolic pathways (60–62, 73).

Table 1. Comparison of Mechanisms and Effects on Gut Microbiota between Probiotics and Conventional Treatments for HCC.
Probiotics can reduce the risk of HCC through a variety of mechanisms (Figure 2). First, they regulate gut microbiota, preventing endotoxemia caused by dysbiosis and maintaining the integrity of the intestinal epithelial barrier, thus preventing the translocation of bacteria and MAMPs into the circulatory system. Additionally, probiotics produce helpful metabolites, which can alleviate oxidative stress in the liver of HCC patients by upregulating antioxidant enzyme expression (74, 75). Gut-derived lipopolysaccharide (LPS) can activate hepatic Kupffer cells, maintaining a pro-inflammatory and tumor-promoting environment via Toll-like receptor 4 (TLR4), which is often overexpressed in HCC tumor tissues. Probiotics protect the intestinal mucosa, reducing LPS translocation and thereby inhibiting HCC progression (76). A preclinical study demonstrated that fimbriae encoded by Bifidobacterium bifidum PRL2010 can induce the production of tumor necrosis factor-alpha (TNF-α). TNF-αplays an important part in both antitumor and anti-infective responses, it can promote immune cell communication without triggering inflammation (63). Prohep, a novel probiotic formulation, has been shown to significantly reduce tumor size and weight by approximately 40% in mouse models of HCC. In the TME, Th17 cells primarily secrete interleukin-17 (IL-17), a key factor in HCC progression and angiogenesis. Probiotics were found to significantly reduce the levels of IL-17 and Th17 cells, inhibiting tumor angiogenesis and resulting in tumor shrinkage. Immunohistochemistry revealed that the reduction in Th17 cells was primarily due to decreased migration from the gut and peripheral blood. This suggests that reducing Th17 cell recruitment and IL-17 secretion effectively impairs tumor angiogenesis and limits tumor growth. Probiotics also reshape gut microbiota. They promote the growth of beneficial bacteria such as Spirochaetes and Prevotella, which can produce anti-inflammatory metabolites, promote the differentiation of regulatory T cells (Treg/Tr1) and suppress Th17 polarization. In short, this research offers new insights into how probiotics modulate gut microbiota, regulate T cell differentiation, and influence pro-inflammatory factors in the TME, showing their preventive role in maintaining gut health (64). Additionally, a retrospective study of 1,267 chronic hepatitis B (HBC) patients found that patients who took ≥28 cumulative defined daily doses (cDDD) of probiotics had a lower risk of developing HCC compared to those who took <28 cDDD. The results suggested that probiotics might reduce the risk of HCC in CHB patients in a dose-dependent manner (77). Furthermore, another study has demonstrated that Bifidobacterium longum (BL) supplementation may improve postoperative outcomes in HCC patients through the enhancement of the tryptophan-5-hydroxytryptamine (5-HT) metabolic pathway, elevation of secondary bile acid levels and increase in SCFAs levels. These metabolic alterations were associated with improved liver function recovery and better clinical outcomes in HCC patients following surgery (78).
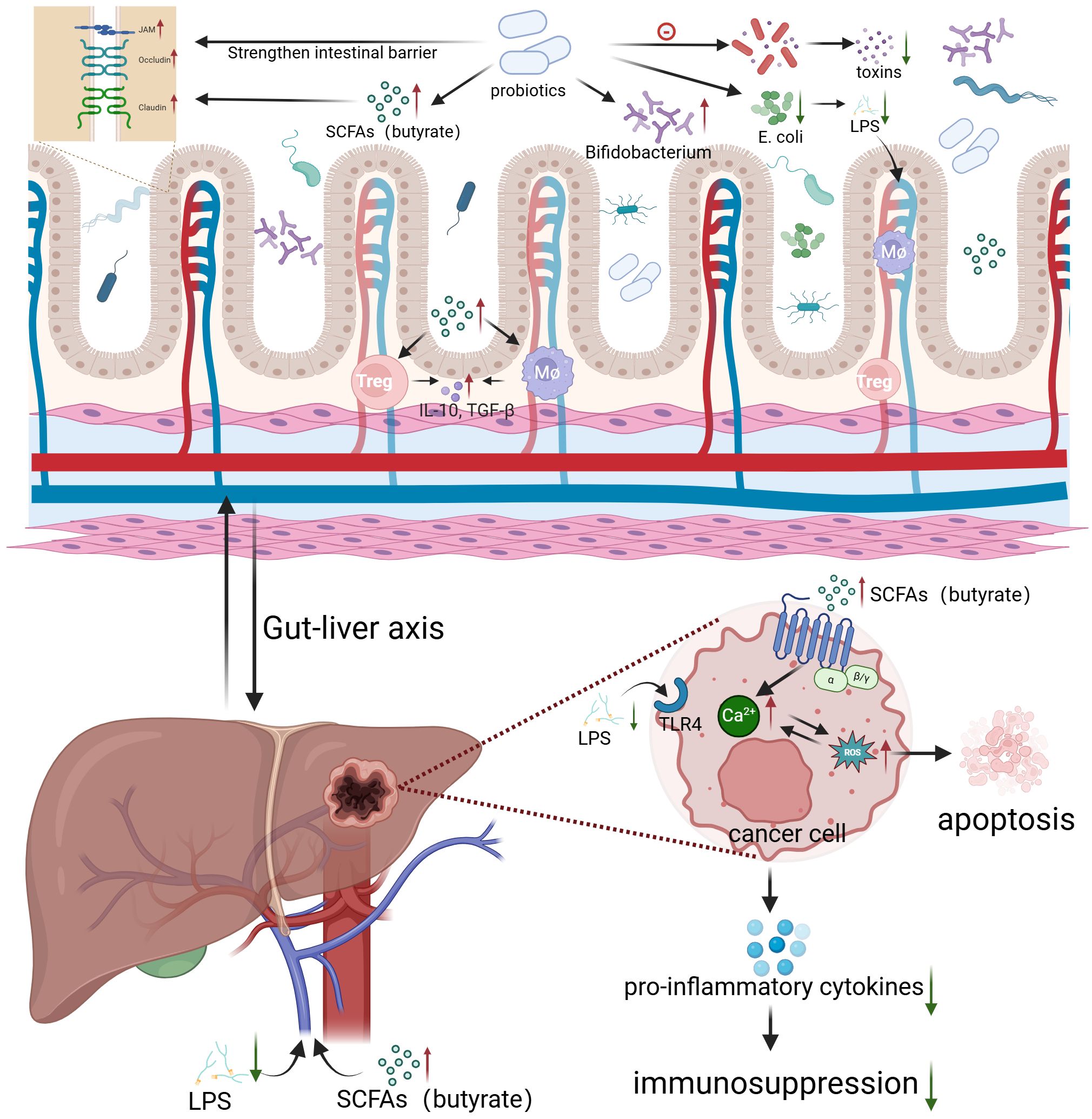
Figure 2. Probiotics regulate gut microbiota to inhibit HCC growth Created in BioRender. c, c. (2024) https://BioRender.com/f31p175.
In conclusion, probiotics can facilitate a more favorable TME through modulation of the gut microbiota. By enhancing anti-tumor immune responses, probiotics represent a promising therapeutic strategy for HCC immunotherapy.
Despite the remarkable efficacy of ICIs in HCC treatment, primary resistance to PD-1/PD-L1 blockade remains a significant challenge, with clinical benefit limited to only 15-20% of HCC patients (79–81). Recent studies have revealed that gut microbiota is closely associated with ICI efficacy, and its dysbiosis may be a key contributor to immunotherapy resistance (49, 60). At present, while clinical research on the use of probiotics in HCC immunotherapy remains relatively limited, modulating the gut microbiota through probiotics may represent a promising strategy to overcome ICI resistance and enhance immunotherapeutic outcomes.
Recent clinical studies have provided compelling evidence for the role of gut microbiota modulation in cancer immunotherapy. One phase I trial has shown that fecal microbiota transplantation(FMT)capsules derived from healthy donors, which represent another type of probiotics, are safe and can improve immune responses in melanoma patients who are refractory to PD-1 inhibitors. In responders, changes in gut microbiota composition following FMT were characterized by an enrichment of beneficial bacteria and a depletion of deleterious bacteria. This microbial modulation was associated with enhanced immune responses, including increased activated ICOS+CD8+ T cells and CD8+ MAIT cells, along with reduced immunosuppressive M-MDSCs. This novel therapeutic approach demonstrates safety and represents a promising treatment modality, though its clinical potential requires further evaluation in randomized clinical trials (65). Further evidence comes from a study analyzing clinical data from patients with non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC) and urothelial carcinoma. This study compared the outcomes of patients who received antibiotics before and after ICI treatment with those who did not. The results demonstrated that regulating gut microbiota, particularly through the supplementation of beneficial bacteria like Akkermansia muciniphila, significantly improves the anticancer efficacy of PD-1/PD-L1 inhibitors. This suggests a promising strategy for incorporating probiotics as an adjunctive treatment in clinical practice. During immunotherapy, careful management of antibiotic use is essential to avoid compromising the efficacy of ICIs, when antibiotics are necessary, probiotics may aid in restoring gut microbiota, thereby maintaining therapeutic efficacy (9).These findings offer valuable insights for HCC immunotherapy, indicating that proper management of antibiotic use and regulation of gut microbiota could further enhance treatment outcomes.
Clinical observations have indicated that patients undergoing immunotherapy showed improvements in symptoms following adjunctive probiotic treatment, with significant enhancements in functional and quality-of-life scores. Besides, although ICIs have greatly advanced cancer therapy, their associated side effects remain a challenge, and modulation of gut microbiota could potentially help mitigate these adverse effects (82). However, there is a need for more extensive and rigorously designed studies to confirm these findings (83). There has been substantial exploration of probiotics use to treat other tumor types, and large amounts of related clinical trials are currently underway, particularly in the context of combining neoadjuvant chemotherapy with immunotherapy (Table 2). The clinical application of specific bacterial strains to regulate the microbiota in cancer treatment warrants further extensive investigation. If the “optimal” microbiota composition can be identified, it may be possible to modulate the patient’s gut microecology using specialized probiotics, prebiotics, or selective antibiotics. Overall, modulating gut microbiota shows great potential to enhance the antitumor effects of immunotherapy while mitigating treatment-related side effects. Probiotics hold promise as a safe and promising adjunctive strategy in the treatment of HCC, though continued research is essential to fully understand and optimize their therapeutic application (58, 76, 84).
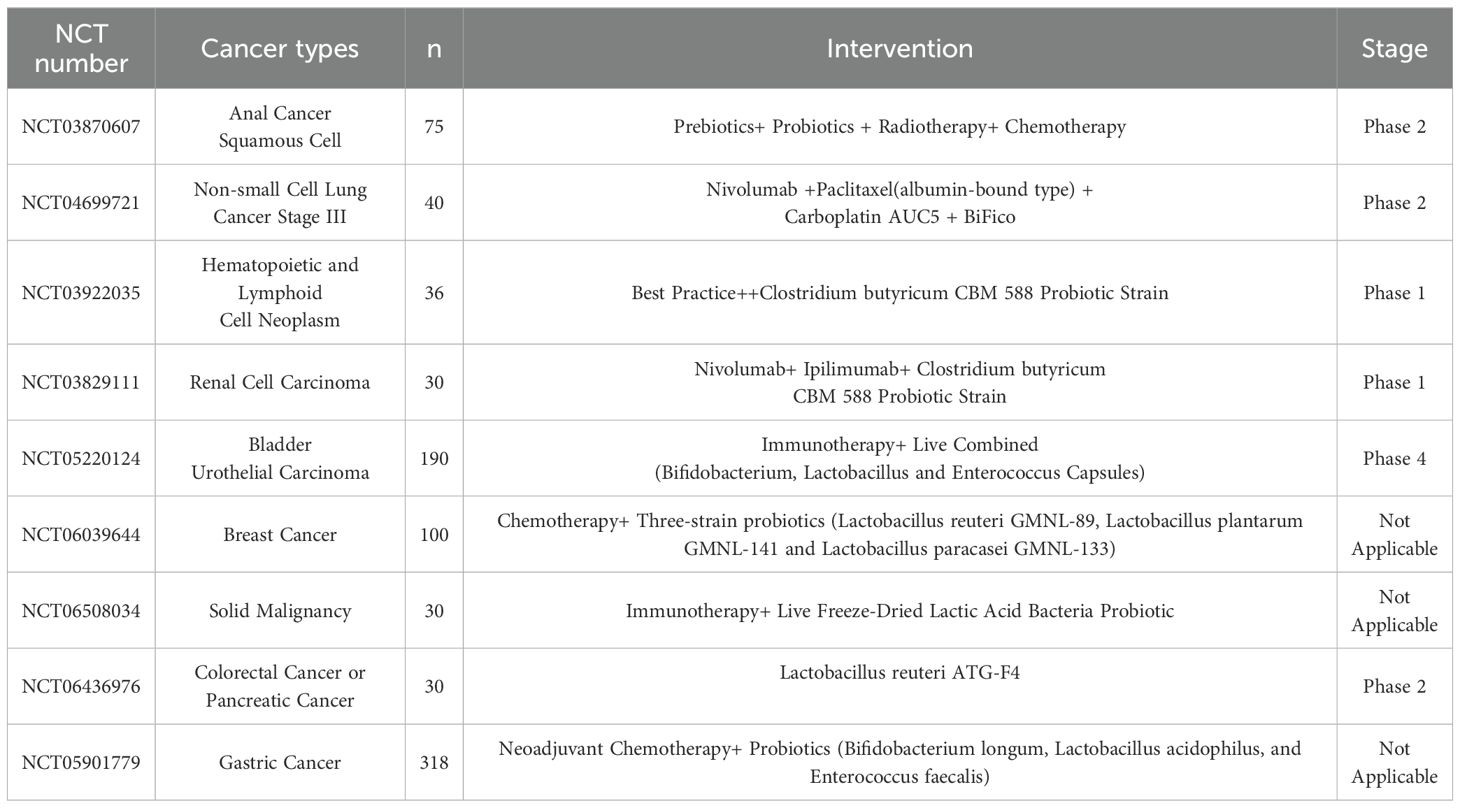
Table 2. Clinical Trials Investigating the Effects of Probiotics in Combination with Anticancer Drugs for Cancer Therapy www.clinicaltrials.gov.
In the TME of HCC, the tumor immune barrier (TIB) obstructs the infiltration of immune cells like T cells, thereby diminishing the efficacy of immunotherapy. This finding offers valuable insights for the use of probiotics in HCC immunotherapy. By modulating the gut-liver axis and the immune system, probiotics may disrupt the TIB structure, thereby enhancing the cytotoxic activity of immune cells against tumors. Moreover, probiotics may reduce SPP1 secretion by influencing macrophage polarization, potentially alleviating TIB-mediated resistance to immunotherapy. Combining probiotics with ICIs (such as PD-1 inhibitors) could create synergistic effects, improving HCC patient responses to immunotherapy. Through personalized analysis of a patient’s gut microbiota and tumor immune microenvironment, probiotics have the potential to emerge as a critical adjunctive approach to boost immunotherapy efficacy (85).
Research has demonstrated a positive correlation between gut microbiota diversity and the efficacy of ICIs. Therefore, modulating gut microbiota—particularly by increasing diversity through probiotics—may help establish a more favorable microenvironment for HCC immunotherapy. This strategy not only enhances patient responses to ICIs but may also mitigate treatment-related side effects by promoting immune system balance. The potential of combining probiotics with ICIs, especially in patients unresponsive to current therapies, merits further investigation (56).
Moreover, gut microbiota signatures (Gut OncoMicrobiome Signatures, GOMS) are crucial for predicting both the efficacy and resistance of ICIs. By precisely modulating gut microbiota, it may be possible to optimize treatment regimens, thereby improving survival rates and clinical outcomes for HCC patients. In the future, the development and application of probiotics tailored to the individual gut microbiota profiles of patients could drive the implementation of personalized treatments, further enhancing the efficacy of HCC immunotherapy (86).
In recent years, the role of gut microbiota in liver-related diseases has been extensively explored. Through the gut-liver axis, gut microbiota impacts various stages of HCC development, including microbial dysbiosis, changes in microbial metabolites and immunomodulatory functions. The widespread use of probiotics in both non-tumorous and tumorous diseases has been well-documented, especially in the treatment of disorders affecting the digestive, neuropsychiatric, and circulatory systems. Currently, numerous clinical trials are underway, recruiting patients to investigate how probiotics can modulate gut microbiota to enhance antitumor immunity and improve the safety and efficacy of cancer immunotherapy.
Despite promising preclinical results in enhancing cancer immunotherapy efficacy, the application of probiotics in immunocompromised HCC patients raises several safety concerns that warrant careful consideration (51). These patients are more susceptible to infections, necessitating rigorous safety screening and monitoring of probiotic strains to prevent harmful genetic transfer to other bacteria. Additionally, probiotics intervention may lead to dysbiosis, causing gastrointestinal symptoms such as abdominal pain and diarrhea (87). Moreover, when combined with ICIs in HCC treatment, probiotics may also cause overactive immune responses, increasing the risk of immune-related adverse events (irAEs) and autoimmune reactions. To mitigate these risks in HCC immunotherapy, systematic preventive measures should be implemented, including pre-treatment immune function assessment, development of individualized probiotic dosing regimens, regular monitoring of gut microbiota changes and establishment of comprehensive adverse events early warning mechanisms. Future research should focus on optimizing the clinical application of probiotics in HCC immunotherapy through identification of novel strains, determination of optimal dosing strategies, rigorous safety assessments and large-scale clinical trials to document therapeutic efficacy (88). Except for the safety concerns, the translation of microbiota-based therapies from animal models to clinical applications in HCC immunotherapy faces several significant challenges (89). First, there are marked differences between animals and humans in gut microbiota composition, immune system complexity and gut-liver axis interactions. These differences may significantly impact therapeutic efficacy (90). Second, multiple technical challenges need to be addressed. These include standardization of probiotic production, quality control, optimization of delivery methods and maintenance of viability during storage and transportation. Third, clinical implementation must consider individual variations among patients, which include gut microbiota composition, immune responses, concurrent medications and dietary habits. Current clinical research has several limitations: There is a lack of standardized methods for microbiota analysis, clinical endpoint indicators are not unified across studies and Long-term safety data remains insufficient. Moreover, the precise mechanisms underlying probiotic-mediated immunomodulation require more comprehensive investigation (60, 91).
In spite of these challenges, the close connection between gut microbiota and the liver presents significant opportunities for microbiome-based approaches in HCC diagnosis, treatment and prevention (92). Based on previous findings in melanoma research, oral administration of microbiome capsules, a novel probiotic derived from fecal bacteria of either healthy donors or HCC patients who responded well to ICIs therapy, may enhance immunotherapy efficacy in HCC patients with suboptimal immune responses through modulation of the gut microbiota. Additionally, probiotics show promise in alleviating adverse effects associated with antineoplastic drugs, as well as promote liver function recovery. Future research should comprehensively monitor factors that modulate gut microbiota, including diet, medication, lifestyles, environment, the host immune system and population-specific microbiome variations across different geographical regions. To effectively evaluate the efficacy and safety of probiotics in HCC immunotherapy, it is essential to well design clinical trials that incorporate knowledge from microbiology, immunology, metabolomics, and epidemiology. Several key research priorities should be addressed. First, establishing standardized systems for probiotic screening and evaluation in collaboration with regulatory authorities is crucial for clinical translation. Second, developing innovative delivery systems and formulations could significantly improve bacterial colonization efficiency and therapeutic outcomes. Third, precision medicine approaches should be developed to select optimal bacterial combinations based on individual microbiome profiles, incorporating patient stratification strategies and artificial intelligence-driven bioinformatics analysis. Fourth, the molecular mechanisms by which probiotics modulate the tumor immune microenvironment need to be elucidated through multi-omics integration approaches. Finally, large-scale, multicenter randomized controlled trials with standardized protocols are needed to provide robust evidence supporting the clinical application of probiotics in HCC immunotherapy. In conclusion, probiotics demonstrate broad application prospects in HCC immunotherapy, yet require systematic research efforts and standardized approaches to fully realize their therapeutic potential in clinical practice.
PC: Writing – original draft, Writing – review & editing. CY: Visualization, Writing – original draft, Writing – review & editing. KR: Writing – review & editing, Visualization. MX: Writing – review & editing. CP: Writing – review & editing. XY: Writing – review & editing. LL: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (Grant No. 2021YFA1301104 and Grant No. 2023YFC2506005).
We acknowledge BioRender.com for the creation of the figures used in this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the assistance of ChatGPT (GPT-4 model, OpenAI) in proofreading and enhancing the clarity of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. (2010) 7:448–58. doi: 10.1038/nrgastro.2010.100
2. Konyn P, Ahmed A, Kim D. The current trends in the health burden of primary liver cancer across the globe. Clin Mol Hepatol. (2023) 29:358–62. doi: 10.3350/cmh.2023.0092
3. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2021) 18:525–43. doi: 10.1038/s41575-021-00438-0
4. Qin S, Ren Z, Feng Y-H, Yau T, Wang B, Zhao H, et al. Atezolizumab plus Bevacizumab versus Sorafenib in the Chinese Subpopulation with Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer. (2021) 10:296–308. doi: 10.1159/000513486
5. Zitvogel L, Daillère R, Roberti MaríaP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. (2017) 15:465–78. doi: 10.1038/nrmicro.2017.44
6. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
7. Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Sci (New York NY). (2015) 350:1079–84. doi: 10.1126/science.aad1329
8. Ae F, La C JK, Tw F YX, Ep F, Ay K. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia (New York NY). (2017) 19:848–55. doi: 10.1016/j.neo.2017.08.004
9. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
10. Matson V, Jessica Fessler 1, Bao R, Chongsuwat T, Zha Y, Alegre M-L, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Sci (New York NY). (2018) 359:104–8. doi: 10.1126/science.aao3290
11. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Sci (New York NY). (2018) 359:97–103. doi: 10.1126/science.aan4236
12. Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Sci (New York NY). (2017) 357:1156–60. doi: 10.1126/science.aah5043
13. Louie T, Golan Y, Khanna S, Bobilev D, Erpelding N, Fratazzi C, et al. VE303, a defined bacterial consortium, for prevention of recurrent clostridioides difficile infection: A randomized clinical trial. JAMA. (2023) 329:1356–66. doi: 10.1001/jama.2023.4314
14. Lai H, Li Y, He Y, Chen F, Mi B, Li J, et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: a double-blinded randomized placebo trial. Gut Microbes. (2023) 15. doi: 10.1080/19490976.2023.2197837
15. Ma Y-Y, Li L, Yu C-H, Shen Z, Chen L-H, Li Y-M. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. (2013) 19:6911–8. doi: 10.3748/wjg.v19.i40.6911
16. Goodoory VC, Khasawneh M, Black CJ, Quigley EMM, Moayyedi P, Ford AC. Efficacy of probiotics in irritable bowel syndrome: systematic review and meta-analysis. Gastroenterology. (2023) 165:1206–18. doi: 10.1053/j.gastro.2023.07.018
17. Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. (2020) 11. doi: 10.1038/s41467-020-18414-8
18. Lukasik J, Dierikx T, Besseling-van der Vaart I, de Meij T, Szajewska H. Multispecies probiotic for the prevention of antibiotic-associated diarrhea in children: A randomized clinical trial. JAMA Pediatr. (2022) 176:860–6. doi: 10.1001/jamapediatrics.2022.1973
19. Depommier C, Everard A, Druart Céline, Plovier H, Hul MV, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. (2019) 25:1096–103. doi: 10.1038/s41591-019-0495-2
20. Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension (Dallas Tex : 1979). (2014) 64. doi: 10.1161/HYPERTENSIONAHA.114.03469
21. Wang S, Ren H, Zhong H, Zhao X, Li C, Ma J, et al. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: a double blinded placebo controlled randomized study. Gut Microbes. (2022) 14. doi: 10.1080/19490976.2021.2003176
22. Patusco R, Ziegler J. Role of probiotics in managing gastrointestinal dysfunction in children with autism spectrum disorder: an update for practitioners. Adv Nutr (Bethesda Md). (2018) 9:637–50. doi: 10.1093/advances/nmy031
23. Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr (Edinburgh Scotland). (2019) 38:522–8. doi: 10.1016/j.clnu.2018.04.010
24. Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. (2016) 87:1274–80. doi: 10.1212/WNL.0000000000003127
25. Albillos Agustín, Gottardi Ade, Rescigno María. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
26. Pabst O, Hornef MW, Schaap FG, Cerovic V, Clavel T, Bruns T. Gut-liver axis: barriers and functional circuits. Nat Rev Gastroenterol Hepatol. (2023) 20. doi: 10.1038/s41575-023-00771-6
27. Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. (2013) 499:97–101. doi: 10.1038/nature12347
28. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. (2019) 25:377–88. doi: 10.1038/s41591-019-0377-7
29. Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. (2019) 11. doi: 10.15252/emmm.201809302
30. Zhang X, Coker OO, Chu ESh, Fu K, Lau HCH, Wang Y-X, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. (2021) 70. doi: 10.1136/gutjnl-2019-319664
31. Yang Qi, Liang Qi, Balakrishnan B, Belobrajdic DP, Feng Q-J, Zhang W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients. (2020) 12. doi: 10.3390/nu12020381
32. Bolshette N, Ibrahim H, Reinke H, Asher G. Circadian regulation of liver function: from molecular mechanisms to disease pathophysiology. Nat Rev Gastroenterol Hepatol. (2023) 20:695–707. doi: 10.1038/s41575-023-00792-1
33. Roje B, Zhang B, Mastrorilli E, Kovačić A, Sušak L, Ljubenkov I, et al. Gut microbiota carcinogen metabolism causes distal tissue tumours. Nature. (2024) 632:1137–44. doi: 10.1038/s41586-024-07754-w
34. Schneider KM, Mohs A, Gui W, Galvez EJC, Candels LS, Hoenicke L, et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun. (2022) 13. doi: 10.1038/s41467-022-31312-5
35. Ma J, Li J, Jin C, Yang J, Zheng C, Chen K, et al. Association of gut microbiome and primary liver cancer: A two-sample Mendelian randomization and case-control study. Liver Int. (2023) 43:221–33. doi: 10.1111/liv.15466
36. Schwabe RF, Greten TF. Gut microbiome in HCC – Mechanisms, diagnosis and therapy. J Hepatol. (2020) 72:230–8. doi: 10.1016/j.jhep.2019.08.016
37. Lu H, Ren Z, Li A, Zhang H, Jiang J, Xu S, et al. Deep sequencing reveals microbiota dysbiosis of tongue coat in patients with liver carcinoma. Sci Rep. (2016) 6. doi: 10.1038/srep33142
38. Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. (2018) 15:397–411. doi: 10.1038/s41575-018-0011-z
39. Lx Y, Rf S. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. (2017) 14:527–39. doi: 10.1038/nrgastro.2017.72
40. Zhou An, Tang Li, Zeng S, Lei Y, Yang S, Tang Bo. Gut microbiota: A new piece in understanding hepatocarcinogenesis. Cancer Lett. (2020) 474:15–22. doi: 10.1016/j.canlet.2020.01.002
41. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
42. Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. (2019) 68:1014–23. doi: 10.1136/gutjnl-2017-315084
43. Che Y, Chen G, Guo Q, Duan Y, Feng H, Xia Q. Gut microbial metabolite butyrate improves anticancer therapy by regulating intracellular calcium homeostasis. Hepatology. (2023) 78:88. doi: 10.1097/HEP.0000000000000047
44. Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. (2018) 15:111–28. doi: 10.1038/nrgastro.2017.119
45. Sun R, Zhang Z, Bao R, Guo X, Gu Y, Yang W, et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J Hepatol. (2022) 77:453–66. doi: 10.1016/j.jhep.2022.02.030
46. Lu Y, Han G, Zhang Y, Zhang L, Li Z, Wang Q, et al. M2 macrophage-secreted exosomes promote metastasis and increase vascular permeability in hepatocellular carcinoma. Cell communication signaling : CCS. (2023) 21. doi: 10.1186/s12964-022-00872-w
47. Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. (2015) 47:505–11. doi: 10.1038/ng.3252
48. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. (2016) 535:65–74. doi: 10.1038/nature18847
49. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. (2018) 68:280–95. doi: 10.1016/j.jhep.2017.11.014
50. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Sci (New York NY). (2018) 360. doi: 10.1126/science.aan5931
51. Zhao L-Y, Mei J-X, Yu G, Lei L, Zhang W-H, Liu K, et al. Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications. Signal transduction targeted Ther. (2023) 8. doi: 10.1038/s41392-023-01406-7
52. Xie J, Liu M, Deng X, Tang Y, Zheng S, Ou X, et al. Gut microbiota reshapes cancer immunotherapy efficacy: Mechanisms and therapeutic strategies. iMeta. (2024) 3:e156. doi: 10.1002/imt2.156
53. Mao J, Wang D, Long J, Yang X, Lin J, Song Y, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. (2021) 9:e003334. doi: 10.1136/jitc-2021-003334
54. Laua HC-H, Zhanga X, Jia F, Lina Y, Liangb W, Lia Q, et al. Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid. EBioMedicine. (2024) 100. doi: 10.1016/j.ebiom.2023.104952
55. Lee P-C, Wu C-J, Hung Y-W, Lee C-J, Chao Y, Hou M-C, et al. Association of gut microbiota and metabolites with tumor response to immune checkpoint inhibitors in patients with unresectable hepatocellular carcinoma. JCO. (2021) 39:e16165–5. doi: 10.1200/JCO.2021.39.15_suppl.e16165
56. Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA network Open. (2020) 3. doi: 10.1001/jamanetworkopen.2020.2895
57. Zhu C, Zhang C, Wang S, Xun Z, Zhang D, Lan Z, et al. Characterizations of multi-kingdom gut microbiota in immune checkpoint inhibitor-treated hepatocellular carcinoma. J immunother Cancer. (2024) 12. doi: 10.1136/jitc-2023-008686
58. Lu Y, Yuan X, Wang M, He Z, Li H, Wang Ji, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. (2022) 15. doi: 10.1186/s13045-022-01273-9
59. Zhu R, Lang T, Yan W, Zhu X, Huang X, Yin Q, et al. Gut microbiota: influence on carcinogenesis and modulation strategies by drug delivery systems to improve cancer therapy. Advanced Sci. (2021) 8:2003542. doi: 10.1002/advs.202003542
60. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. (2019) 25:716–29. doi: 10.1038/s41591-019-0439-x
61. Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J immunother Cancer. (2019) 7:193. doi: 10.1186/s40425-019-0650-9
62. Ossowski Iv, Pietilä TE, Rintahaka J, Nummenmaa E, Mäkinen V-M, Reunanen J, et al. Using recombinant Lactococci as an approach to dissect the immunomodulating capacity of surface piliation in probiotic Lactobacillus rhamnosus GG. PloS One. (2013) 8. doi: 10.1371/journal.pone.0064416
63. Turroni F, Serafini F, Foroni E, Duranti S, Motherway MO, Taverniti V, et al. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium-host interactions. Proc Natl Acad Sci United States America. (2013) 110:11151–6. doi: 10.1073/pnas.1303897110
64. Li J, Sung CYJ, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA. (2016) 113:E1306–15. doi: 10.1073/pnas.1518189113
65. Routy B, Lenehan JG, Jr WHM, Jamal R, Messaoudene M, Daisley BA, et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. (2023) 29:2121–32. doi: 10.1038/s41591-023-02453-x
66. Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med. (2018) 10:eaan3464. doi: 10.1126/scitranslmed.aan3464
67. She S, Shi J, Zhu J, Yang F, Yu J, Dai K. Impact of inflammation and the immune system on hepatocellular carcinoma recurrence after hepatectomy. Cancer Med. (2024) 13:e7018. doi: 10.1002/cam4.7018
68. Lu C, Gao Z, Wu Di, Zheng J, Hu C, Huang D, et al. Understanding the dynamics of TKI-induced changes in the tumor immune microenvironment for improved therapeutic effect. J immunother Cancer. (2024) 12. doi: 10.1136/jitc-2024-009165
69. Zhao Qi, Wu ZE, Li B, Li F. Recent advances in metabolism and toxicity of tyrosine kinase inhibitors. Pharmacol Ther. (2022) 237. doi: 10.1016/j.pharmthera.2022.108256
70. Sangro B, Chan SL, Meyer T, Reig María, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. (2020) 72:320–41. doi: 10.1016/j.jhep.2019.10.021
71. Tan J, Fan W, Liu T, Zhu B, Liu Y, Wang S, et al. TREM2+ macrophages suppress CD8+ T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma. J Hepatol. (2023) 79:126–40. doi: 10.1016/j.jhep.2023.02.032
72. Bian C, Wang Y, Yu A, Fu L, Zhang D, Zhu W, et al. Gut microbiota changes and biological mechanism in hepatocellular carcinoma after transarterial chemoembolization treatment. Front Oncol. (2022) 12:1002589. doi: 10.3389/fonc.2022.1002589
73. Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: A review of the literature. IJMS. (2019) 20:395. doi: 10.3390/ijms20020395
74. Thilakarathna W, Rupasinghe HPV, Ridgway N. Mechanisms by which probiotic bacteria attenuate the risk of hepatocellular carcinoma. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22052606
75. Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. Hepatobiliary Surg Nutr. (2018) 7:11–20. doi: 10.21037/hbsn.2017.12.07
76. Nicoletti A, Pompili M, Gasbarrini A, Ponziani FR. Going with the gut: probiotics as a novel therapy for hepatocellular carcinoma. Hepatobiliary Surg Nutr. (2019) 8:295–7. doi: 10.21037/hbsn.2019.01.16
77. Shi K, Zhang Q, Yi Z, Bi Y, Zeng X, Wang X. Association between probiotic therapy and the risk of hepatocellular carcinoma in patients with hepatitis B-related cirrhosis. Front Cell Infection Microbiol. (2023) 12:1104399. doi: 10.3389/fcimb.2022.1104399
78. Yu J, Zhu P, Shi L, Gao N, Li Y, Shu C, et al. Bifidobacterium longum promotes postoperative liver function recovery in patients with hepatocellular carcinoma. Cell Host Microbe. (2024) 32:131–144.e6. doi: 10.1016/j.chom.2023.11.011
79. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London England). (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
80. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. (2018) 19:940–52. doi: 10.1016/S1470-2045(18)30351-6
81. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
82. Marinelli L, Tenore GC, Novellino E. Probiotic species in the modulation of the anticancer immune response. Semin Cancer Biol. (2017) 46:182–90. doi: 10.1016/j.semcancer.2017.08.007
83. González Espinoza IR, Castro-Ponce A, Aparicio Tapia A, Juárez-Salazar G, Cortes Garcia CA, Aguilar C, et al. Effect of probiotics on quality of life for patients with cancer undergoing immunotherapy. JCO. (2024) 42:e24148–8. doi: 10.1200/JCO.2024.42.16_suppl.e24148
84. Temraz S, Nassar F, Kreidieh F, Mukherji D, Shamseddine A, Nasr R. Hepatocellular carcinoma immunotherapy and the potential influence of gut microbiome. IJMS. (2021) 22:7800. doi: 10.3390/ijms22157800
85. Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. (2023) 78:770–82. doi: 10.1016/j.jhep.2023.01.011
86. Thomas AM, Fidelle M, Routy B, Kroemer G, Wargo JA, Segata N, et al. Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy. Nat Rev Clin Oncol. (2023) 20:770–82. doi: 10.1038/s41571-023-00785-8
87. Dore MP, Bibbò S, Fresi G, Bassotti G, Pes GM. Side effects associated with probiotic use in adult patients with inflammatory bowel disease: A systematic review and meta-analysis of randomized controlled trials. Nutrients. (2019) 11. doi: 10.3390/nu11122913
88. Maftei N-M, Raileanu CR, Balta AA, Ambrose L, Boev M, Marin DB, et al. The potential impact of probiotics on human health: an update on their health-promoting properties. Microorganisms. (2024) 12:234. doi: 10.3390/microorganisms12020234
89. Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ. Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother. (2020) 129:110409. doi: 10.1016/j.biopha.2020.110409
90. Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Models Mech. (2015) 8:1–16. doi: 10.1242/dmm.017400
91. Araujo DV, Watson GA, Oliva M, Heirali A, Coburn B, Spreafico A, et al. Bugs as drugs: The role of microbiome in cancer focusing on immunotherapeutics. Cancer Treat Rev. (2021) 92:102125. doi: 10.1016/j.ctrv.2020.102125
Keywords: probiotics, gut microbiota, hepatocellular carcinoma, immunotherapy, therapeutic strategies
Citation: Chen P, Yang C, Ren K, Xu M, Pan C, Ye X and Li L (2024) Modulation of gut microbiota by probiotics to improve the efficacy of immunotherapy in hepatocellular carcinoma. Front. Immunol. 15:1504948. doi: 10.3389/fimmu.2024.1504948
Received: 01 October 2024; Accepted: 04 November 2024;
Published: 22 November 2024.
Edited by:
Lorenzo Mortara, University of Insubria, ItalyReviewed by:
Irfan Naseem Bandey, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2024 Chen, Yang, Ren, Xu, Pan, Ye and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanjuan Li, bGpsaUB6anUuZWR1LmNu; Xuewei Ye, MTEzMTkwMDdAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.