- 1State Key Laboratory of Biocontrol, Guangdong Provincial Key Laboratory for Aquatic Economic Animals and Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), School of Life Sciences, Sun Yat-sen University, Guangzhou, China
- 2Algae Health Science Co., Ltd., Kunming, China
- 3Laboratory of Aquatic Animal Nutrition and Feed, Fisheries College, Guangdong Ocean University, Zhanjiang, China
This study examined the effects of Haematococcus pluvialis on the growth performance, innate immunity, and gut microbiota of Litopenaeus vannamei under different water temperature conditions. Feeding regimens included a 20% fishmeal diet (control), a low-fish meal (LFM) diet with 10% fishmeal and an LFM diet supplemented with 0.03% H. pluvialis. These diets were administered to six groups of L. vannamei at normal (30°C) (NT) and low (20°C) (LT) temperatures (NT_C, NT_LFM, NT_LFM_HP, LT_C, LT_LFM, and LT_LFM_HP) over 8 weeks. The weight gain rate of L. vannamei in group NT_LFM_HP was significantly higher compared to group NT_LFM. Astaxanthin levels and body pigmentation intensity in L. vannamei were significantly increased in the NT_LFM_HP and LT_LFM_HP groups. Moreover, hepatopancreatic antioxidant capacities, such as superoxide dismutase (SOD) activity and total antioxidant capacity (T-AOC), were lower in normal-temperature groups compared to the low-temperature groups. Nevertheless, antioxidant capacity was significantly higher in both the NT_LFM_HP and LT_LFM_HP groups compared to the control group. Meanwhile, the expression levels of antioxidants were significantly higher at lower temperatures compared to higher temperatures, with the NT_LFM_HP and LT_LFM_HP groups exhibiting the highest expression levels. Additionally, the mRNA levels of genes associated with the Toll and IMD pathways indicated immunoregulatory effects in the organism. The expression levels of immune genes were significantly higher at lower temperatures, especially in the NT_LFM_HP and LT_LFM_HP groups compared to the control groups. Notably, significant differences in gut microbial composition were observed in the NT_LFM_HP group compared to other groups, with variations influenced by temperature and fishmeal content. Specifically, Vibrionaceae abundance was significantly lower in the LT_LFM_HP group compared to the control group. The results also revealed that the abundance of Actinomarinales was significantly higher in low-temperature groups, with the LT_LFM_HP group displaying the greatest increase. Overall, these findings suggest that L. vannamei may be susceptible to reduced fishmeal levels, potentially impacting growth and immune function. Furthermore, H. pluvialis supplementation may assist L. vannamei in acclimating to prolonged low-temperature conditions.
1 Introduction
As is well documented, Litopenaeus vannamei (L. vannamei) is an economically important aquaculture species worldwide. Fishmeal is considered the optimal source of protein in shrimp feed owing to its palatability, balanced nutritional composition, and ease of digestion and absorption. However, the price of fishmeal has increased in recent years. To cope with this change, it is essential to develop suitable protein sources to replace fishmeal. In low fishmeal diets, functional additives are introduced to ensure the growth performance and health of aquatic animals. Meanwhile, aquaculture has suffered from environmental destruction over the past decades (1). The extensive administration of antibiotics to enhance disease resistance in shrimp (2) resulted in numerous adverse effects (3). Consequently, the use of pure natural functional additives has become a trend. Shrimp body pigmentation influences consumer preference. However, shrimp are unable to synthesize astaxanthin, and earlier studies have reported that shrimp can use astaxanthin from their diet to optimize body pigmentation. Thus, natural sources of astaxanthin, such as Haematococcus pluviali (H. pluvialis), have emerged as prominent functional additives. Known to be the richest natural source of astaxanthin, H. pluvialis contains astaxanthin levels ranging from 1.0% to 7.0% (4). Therefore, the introduction of astaxanthin to feed can improve growth performance in L. vannamei (5), mitigate hepatopancreatic damage in white shrimp (6), improve immune levels in crayfish (7), and improve body color in Japanese shrimp (8).
The growth, health, and immunity of aquatic organisms are considerably impacted by water temperature. For instance, L. vannamei is a poikilothermic species whose growth, health, and immunity are regulated by fluctuations in environmental temperature (9). Indeed, temperature stress has been documented to significantly impact shrimp growth and immunity (10, 11), thereby promoting susceptibility to bacterial, fungal, viral, and parasitic infections and economic losses (12). The nutritional metabolism and immune function of shrimp are directly affected by the gastrointestinal tract, which plays a vital role in nutrient absorption and disease resistance. Throughout the growth of shrimp, the structure of the gut flora significantly changes (13, 14). According to earlier studies, alterations in temperature can affect the gut flora of shrimp, thereby influencing their growth and immune performance (15, 16).
To the best of our knowledge, no studies have investigated the effects of H. pluvialis on L. vannamei under different water temperatures. Given that other aquatic organisms are influenced by the multiple regulatory mechanisms of H. pluvialis, this study aimed to investigate the effects of H. pluvialis on the growth performance, antioxidant capacity, immune activity, and gut microbiology of L. vannamei at distinct water temperatures.
2 Materials and methods
2.1 Experimental diets
Prior to diet preparation, all raw materials were crushed using a grinder and subsequently sieved using a 60-mesh sieve. After sieving, the raw materials were weighed based on the diet formula outlined in Table 1. Briefly, the weighed raw materials were thoroughly mixed in a plastic zip-lock bag and then transferred to a commercial mixer for mixing and blending, during which fish oil, soya lecithin, and water were added and mixed. After mixing, the ingredients were extruded into long strips using a twin-screw extruder and subsequently transferred to a pelletizer to produce pellets for the trials. Next, diets were steam-conditioned at 60°C, then removed and air-dried until the moisture content of the diet was reduced to approximately 10%. Following packing into plastic ziplock bags, the diets were stored in a refrigerator at -20°C. Different feeding regimes, specifically the 20% fishmeal group, the 10% fishmeal group, and the 10% fishmeal + 0.03% H. pluvialis group, were administered to L. vannamei (NT_C, NT_LFM, NT_LFM_HP, LT_C, LT_LFM, and LT_LFM_HP) at 30°C and 20° water temperature environments.
2.2 Feeding experiments
In Lingshui Li Autonomous County, L. vannamei was used as the test animal for an 8-week culture experiment. Shrimp larvae were temporarily raised on commercial feed for 10 weeks before being used in the culture experiment. A total of 720 shrimp with an average weight of approximately 0.63 g were randomly selected from the temporarily reared shrimp and assigned to 24 cement tanks, each containing 30 shrimp. Sewage pipes were installed at the bottom of each cement tank for effluent discharge, and water pipes were installed in the cement tanks for seawater replenishment. The water was changed uniformly and regularly according to predetermined water quality indices, with 80% of the water replaced at each interval. All seawater used in the aquaculture tanks was sand-filtered, precipitated, sterilized, and filtered prior to use. The cement tanks were fitted with an air tube and air stone to maintain 24-hour continuous aeration.
Feeding rates were initially set at 5% of shrimp body weight and adjusted based on satiation by observing bait residues after feeding. All groups were fed three times daily, and dead shrimp, bait residues, feces, and other bottom waste were aspirated by suction, and dead shrimp were weighed and counted. During the 8-week aquaculture experiment, the temperatures of the water were maintained at 30°C for the normal-temperature groups and 20°C for the low-temperature groups. A chiller and a water recycling system were used to adjust water temperature, which was recorded every 4 hours. Throughout the experiment, natural light cycles were maintained.
2.3 Sampling
During the 8-week culture period, 5 g of feces from each cement tank were collected and stored in a refrigerator at -20°C for apparent digestibility analysis. All shrimp were fasted for 24 hours before sampling. Shrimp in each tank was counted and weighed. The body length, weight, and hepatopancreatic weight of five shrimp from each group were measured and recorded. A total of five shrimp were randomly selected from each group and stored at -20°C for whole shrimp crude nutrient analysis. Frozen hepatopancreas samples harvested from four shrimp were analyzed for enzyme activity and gene expression. Intestinal samples were collected from four shrimp and frozen for intestinal flora analysis. For H&E staining and sectioning, hepatopancreas samples collected from two shrimp were used. For comparison, five live shrimp from each group were boiled under identical conditions.
2.4 Determination of proximate composition
According to the guidelines of the Association of Official Analytical Chemists (AOAC), moisture, crude protein, and crude lipid content were determined from dried samples. After drying at 105°C, moisture content was determined, following which the samples were crushed. Next, a fully automated Dumas nitrogen tester (N Pro (DT Ar/He Basic), Gerhardt GMBH & CO.KG, Germany) was employed to determine crude protein content from 0.08 g of sample. The crude lipid content was determined using the Soxhlet extraction method on an automatic lipid analyzer (Soxtec System HT6, Tecator, Sweden) with light petroleum reflux extraction on approximately 0.5 g of the sample. Shrimp shell samples were freeze-dried and ground to extract astaxanthin and measure astaxanthin levels. Ionophore atomic emission spectrometry (ICP-AES) was utilized to quantify metal Y-ions in feces, and apparent digestibility was calculated. The methods for extracting astaxanthin in shrimp shells were performed as described in a previous study (50).
2.5 Determination of hepatopancreatic enzyme activities
After thawing hepatopancreatic tissues, samples were collected and added to PBS solution for grinding. The levels of hepatopancreatic enzyme activity indices were determined by centrifuging at 4000 rpm for 20 min at 4°C and collecting the supernatant. Superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and lipid oxidation (MDA) levels were measured using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6 Total RNA extraction and real-time quantitative PCR
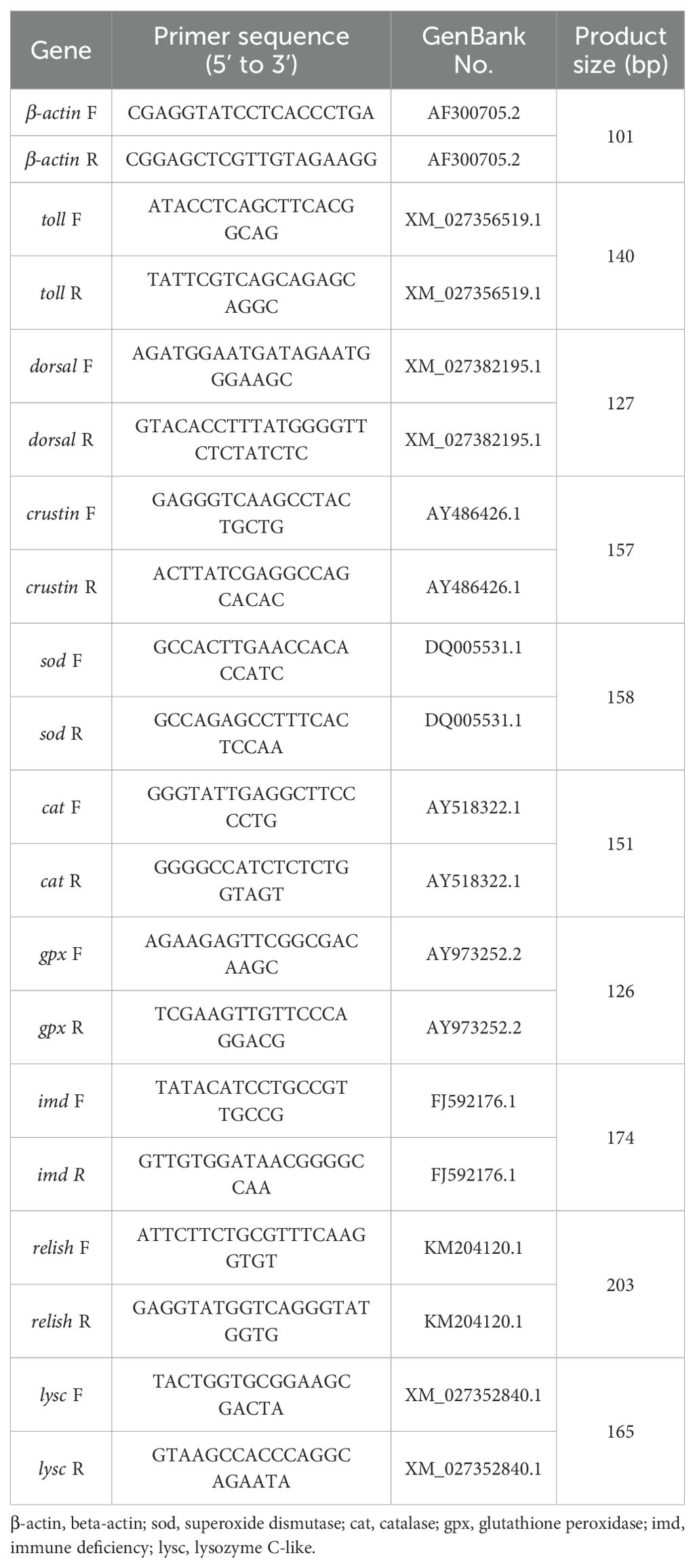
Real-time quantitative PCR (qRT-PCR) was performed for all gene expression assays in the present study. With the Evo MMLV Reverse transcription reagent kit (Accurate Biology, Hunan, China), total hepatopancreatic RNA was extracted using the TRIzol method, and cDNA was synthesized via reverse transcription. Moreover, qRT-PCR was performed on a Roche real-time quantitative PCR system (LightCycler 480 II, Roche Diagnostics, Basel, Switzerland). Conditions for the reaction were as follows: pre-denaturation at 95°C for 40 amplification cycles (denaturation at 95°C for 5 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s). A melting curve was plotted (95°C for 20 s, 60°C for 20 s, followed by continuous maintenance at 95°C), and the samples were cooled to 4°C. Shrimp primers used in this study are presented in Table 2. The β-actin gene served as an internal reference gene for gene expression analysis, and relative expression levels were calculated using the 2-ΔΔct method.
2.7 Morphological microscopy of hepatopancreatic tissues
Hepatopancreatic samples were initially fixed in a 4% paraformaldehyde solution for 24 hours and subsequently transferred to a 70% ethanol solution. Prior to sectioning, the samples were subjected to graded dehydration, paraffin embedding, and fixation, following which the sections were sliced into approximately 3.0 μm thick sections using a slicer. Afterward, the sections were subjected to hematoxylin and eosin staining and examined and imaged under a Nikon orthostatic microscope (Eclipse Ni-E, Nikon, Japan). Finally, the resulting images were analyzed utilizing NIS-Elements viewer software (National Institutes of Health, Bethesda, USA).
2.8 16S rRNA gene sequencing and microbiota analysis
The genomic DNA of intestinal microorganisms in the sample was extracted and analyzed for purity and concentration using 1% agarose gel electrophoresis. The purified genomic DNA was subsequently dispatched to Shanghai Majorbio Bio‐pharm Technology Co., Ltd (51) for further processing.
Paired-end (PE) reads generated from Illumina sequencing were initially aligned based on their overlapping regions. Subsequently, sequence quality was assessed and filtered, followed by sample differentiation for operational taxonomic unit (OTU) clustering and species taxonomy analysis to facilitate the calculation of various diversity indices. OTU-based diversity index analyses and sequencing depth detection were conducted, while taxonomic information enabled statistical analyses of community structure at different taxonomic levels. Data analyses were conducted utilizing the Meggie BioCloud platform (https://cloud.majorbio.com). Specifically, mothur software was employed to compute alpha diversity metrics such as Chao 1 and Shannon index, while the Wilcoxon rank sum test was utilized to assess variations in alpha diversity among groups (52). Intergroup differences in alpha diversity were evaluated using an algorithm based on the Bray-Curtis distance, coupled with PCoA (Principal Coordinate Analysis) analysis to examine the similarities in microbial community structures across samples. Additionally, the PERMANOVA non-parametric test was utilized in conjunction with LEfSe (Linear Discriminant Analysis Effect Size) analysis (LDA>2, P<0.05) to assess significant variations in microbial community structure among groups, identifying bacterial taxa with differing abundances from phylum to genus level (53).
2.9 Statistical analysis
Parameters were calculated using the following formulae: Initial body weight (IBW, g)=initial total wet weight/initial number of tails; Final body weight (FBW, g)=final total wet weight/final number of tails; Weight gain (WG, %)=100×(final body weight-initial body weight)/initial body weight; Specific growth rate (SGR, %/day)=100×(Ln final mean weight-Ln initial mean weight)/number of days; Food intake (FI, g/shrimp)=total food intake/total number of shrimp; Feed conversion ratio (FCR)=dry diet fed/wet weight gain; Conversion factor (CF, g/cm3)=100×wet weight/(body length)3; Hepatosomatic intake (HSI, %)=100×hepatopancreas weight/wet weight; Survival rate (SR, %)=100×number of terminal surviving tails/number of initial tails; Apparent Digestibility (AD, %)=100× (Y2O3 intake-Y2O3 output)/Y2O3 intake.
Experimental data were expressed as “mean ± standard error”. Two-way ANOVA with Bonferroni multiple comparisons were used to analyze data across treatment groups at the same temperature. Student’s t-test was conducted to analyze data in the same treatment groups at different temperatures. P < 0.05 was considered statistically significant.
3 Results
3.1 Growth performance, feed utilization, and morphometric parameters
Following the conclusion of the 8-week period, the growth performance, feed utilization, and morphometric parameters for the six groups of L. vannamei were individually recorded, as detailed in Table 3. The weight gain rate and specific growth rate of L. vannamei in the NT_C, NT_LFM, and NT_LFM_HP groups were significantly higher compared to the LT_C LT_LFM and LT_LFM_HP groups (P<0.05). Likewise, the weight gain rate of L. vannamei in group NT_LFM_HP was significantly higher compared to group NT_LFM (P<0.05). In contrast, the weight gain rate of L. vannamei in group NT_LFM_HP was comparable to that in group NT_C. Interestingly, no significant differences were noted in the survival rates of L. vannamei across treatment groups (P>0.05). Conversely, variations in feed efficiency and apparent digestibility were observed between the normal and low-temperature groups (P<0.05).
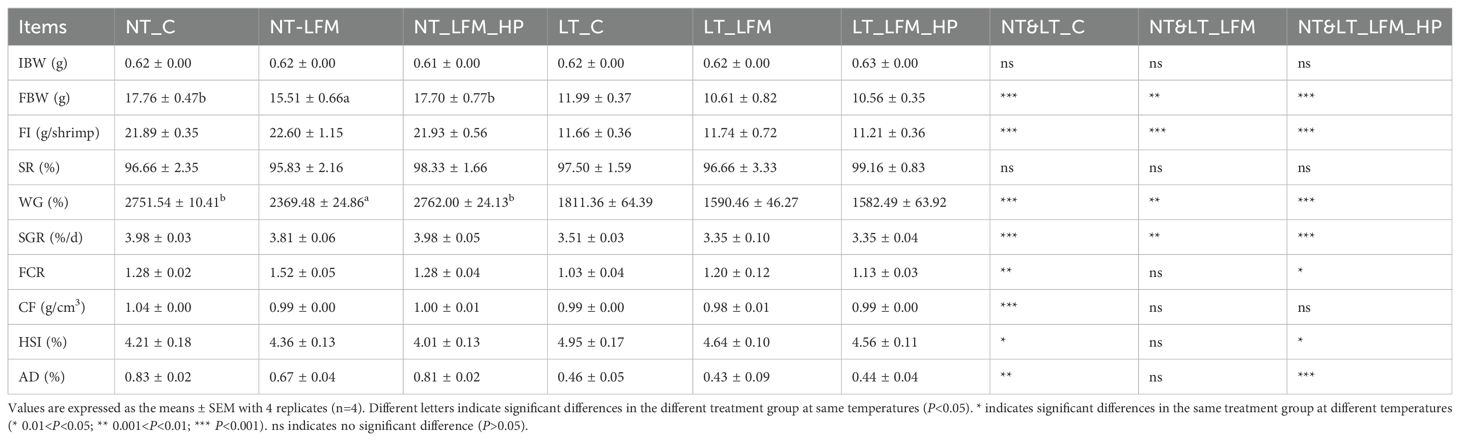
Table 3. Effect of the addition of H. pluvialis at varying water temperatures on growth performance and feed utilisation of L. vannamei.
3.2 Muscle proximate composition
The moisture, crude protein, and crude lipid content of L. vannamei muscle within each experimental group are outlined in Table 4. The findings revealed no significant differences in these parameters across the groups (P>0.05).
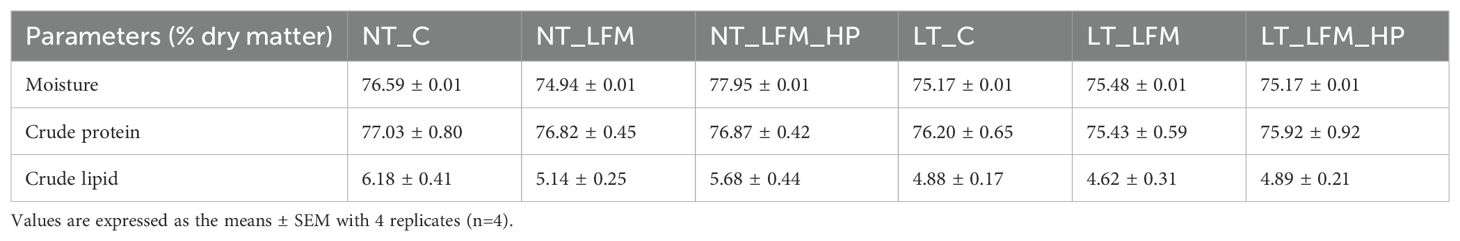
Table 4. Effect of the addition of H. pluvialis at varying water temperatures on the muscle composition of L. vannamei (% dry weigNT).
3.3 Astaxanthin content
As displayed in Figure 1, significant differences were observed in coloration between live and cooked shrimp, with the NT_LFM_HP and LT_LFM_HP groups exhibiting darker pigmentation compared to the NT_LFM and LT_LFM groups. Furthermore, as depicted in Figure 2, the astaxanthin content in shrimp shells was highest in the NT_LFM_HP and LT_LFM_HP groups.
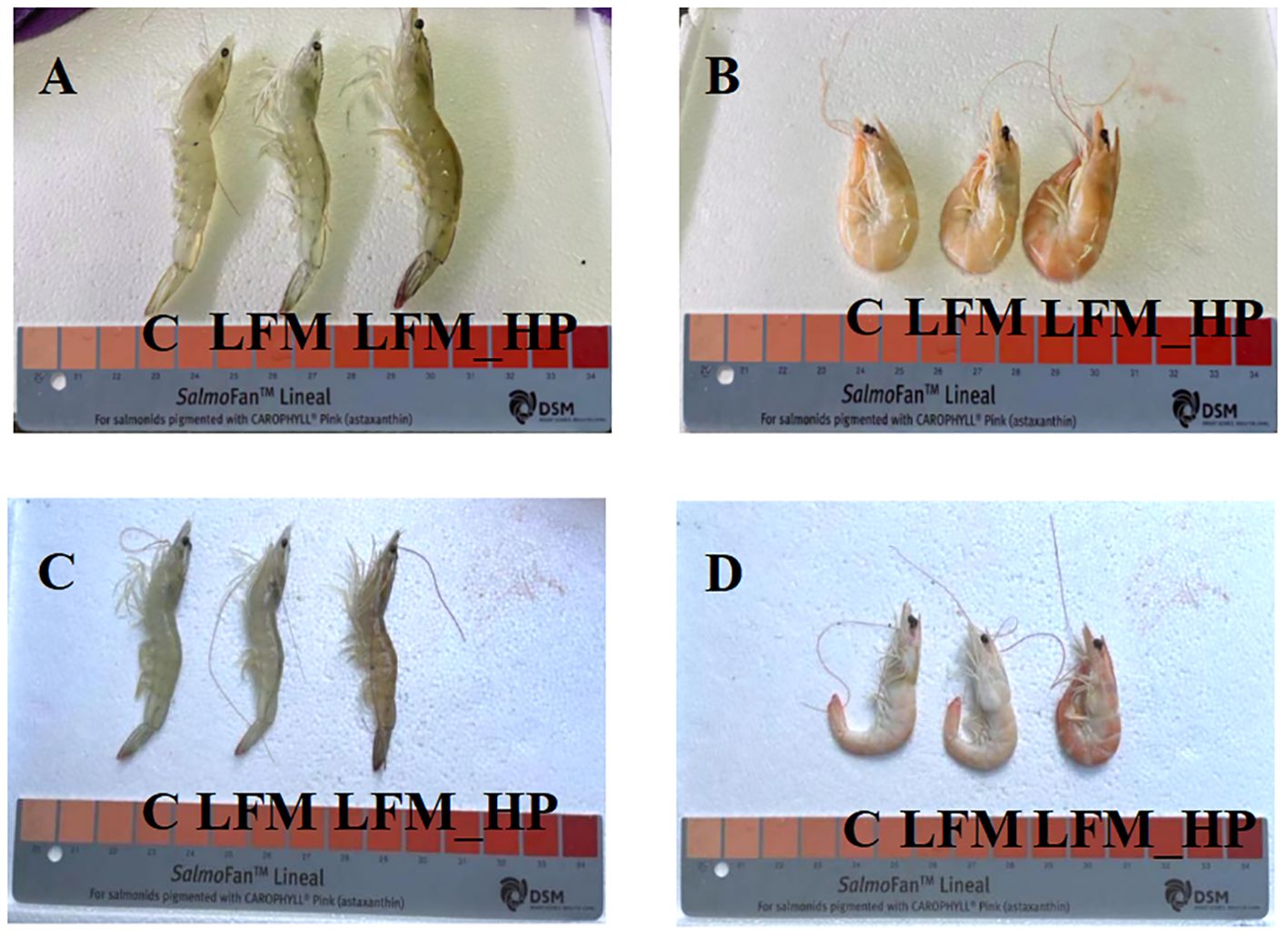
Figure 1. Effect of the addition of H. pluvialis at varying water temperatures on the body colour of L. vannamei. (A) Normal water temperature group of live shrimp; (B) Normal water temperature group after cooking shrimp; (C) Low water temperature group of live shrimp; (D) Low water temperature group after cooking shrimp.
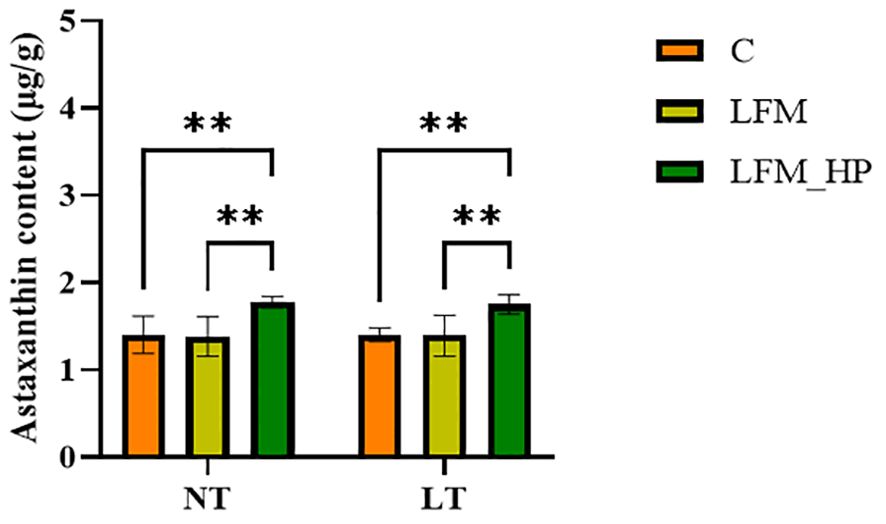
Figure 2. Effect of the addition of H. pluvialis at varying water temperatures on the astaxanthin content of shrimp shells of L. vannamei. Data represent the means ± SEM (n = 4). * indicates significant differences in the same treatment group at different temperatures (** 0.001<P<0.01).
3.4 Hepatopancreas morphology
Figure 3 illustrates the comparison of hepatopancreatic tissue sections across groups. The hepatic tubules within the hepatopancreas of groups NT_C, NT_LFM, NT_LFM_HP, LT_C, LT_LFM, and LT_LFM_HP displayed normal morphology, with normal B cells, R cells, and E cells and the absence of lesions.
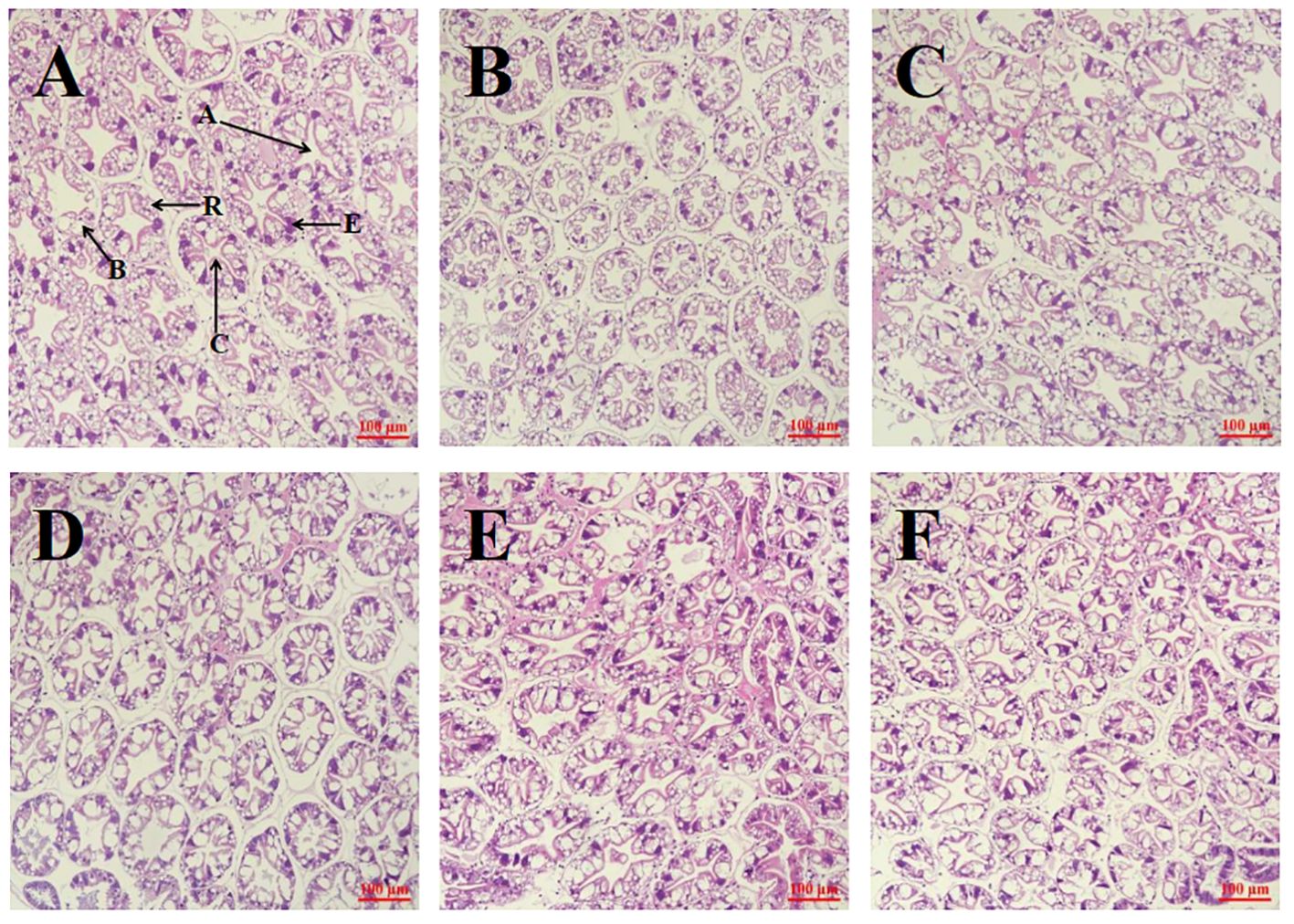
Figure 3. Effect of the addition of H. pluvialis at varying water temperatures on the hepatopancreas morphology of L. vannamei. Scale bar: 100 μm. Magnification: 20×. (A) NT_C group; (B) NT_LFM group; (C) NT_LFM_HP group; (D) LT_C group; (E) LT_LFM group; (F) LT_LFM_HP group. (A: stellate lumen, C: basement membrane, B: B cell, R: R cell, E: E cell).
3.5 Hepatopancreas biochemical parameters
3.5.1 Antioxidant enzyme activities
Figure 4 illustrates the impact of H. pluvialis supplementation on the hepatopancreatic antioxidant enzyme activities in L. vannamei shrimp under varying water temperatures. The findings demonstrated that, following exposure to elevated temperatures, total antioxidant capacity (T-AOC) was significantly higher in the NT_LFM_HP group compared to the NT_C and NT_LFM groups (P<0.05). Similarly, SOD activity was significantly higher in the NT_LFM_HP group compared to the NT_C and NT_LFM groups (P<0.05). After the inclusion of H. pluvialis, MDA levels were significantly lower in the NT_LFM_HP group compared to the NT_C and NT-LF groups (P<0.05). Following a decrease in temperature, SOD activity was significantly higher in the LT_C group compared to the LT_LFM_HP group (P<0.05). Besides, MDA levels were significantly lower in the LT_LFM_HP groups compared to the LT_C group (P<0.05).
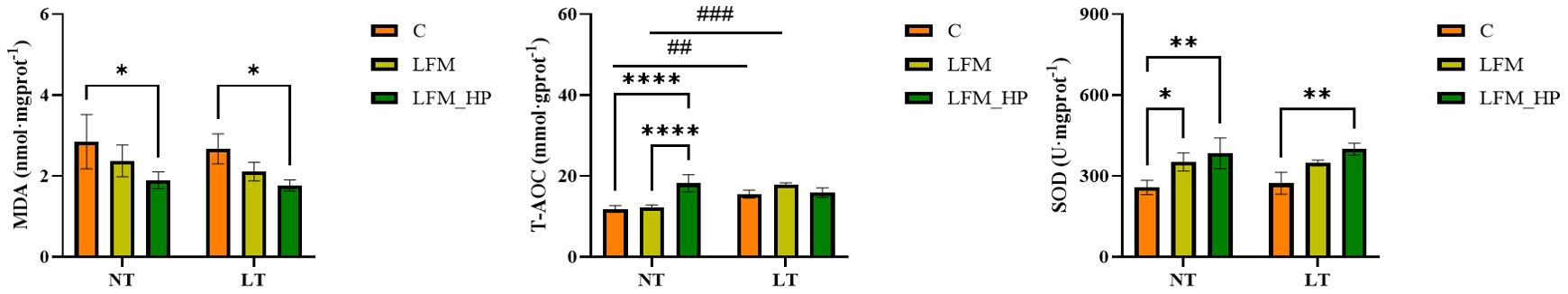
Figure 4. Effect of incorporating H. pluvialis at varying water temperatures on the hepatopancreatic antioxidant enzyme activities of L. vannamei. MDA, malondialdehyde; T-AOC, total antioxidant capacity; SOD, superoxide dismutase. Data represent the means ± SEM (n = 4).* indicates significant differences in the same treatment group at different temperatures (* 0.01<P<0.05; ** 0.001<P<0.01; **** P<0.0001). # indicates significant differences in the same treatment group at different temperatures (## 0.001<P<0.01; ### P<0.001).
3.5.2 Expression of antioxidant-related genes
The gene expression levels of antioxidant-related genes in the hepatopancreas of L. vannamei are depicted in Figure 5. Significantly lower expression levels of cat and sod were observed in the NT_LFM and LT_LFM groups compared to the NT_LFM_HP and LT_LFM_HP groups (P<0.05). Additionally, the relative expression of gpx was higher in the NT_LFM_HP and LT_LFM_HP groups compared to the NT_LFM and LT_LFM groups.
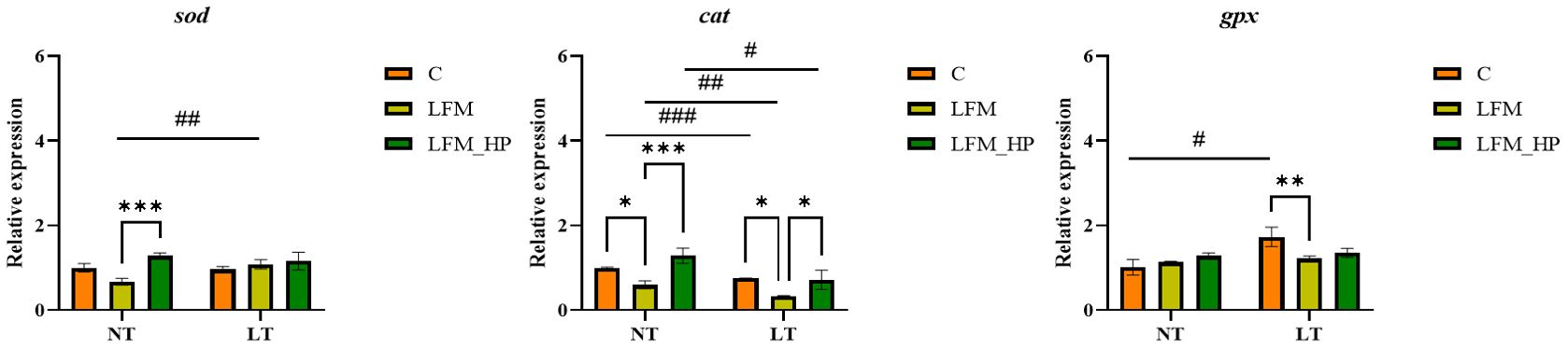
Figure 5. Effect of the addition of H. pluvialis at varying water temperatures on the expression of antioxidant genes in the hepatopancreas of L. vannamei. Data represent the means ± SEM (n = 4). * indicates significant differences in the same treatment group at different temperatures (* 0.01<P<0.05; ** 0.001<P<0.01; *** P<0.001). # indicates significant differences in the same treatment group at different temperatures (# 0.01<P<0.05; ## 0.001<P<0.01; ### P<0.001).
3.6 Immune response
The Toll and IMD signaling pathways were identified as pivotal elements of the innate immune response in shrimp, as evidenced by the expression of relevant genes depicted in Figures 6, 7.
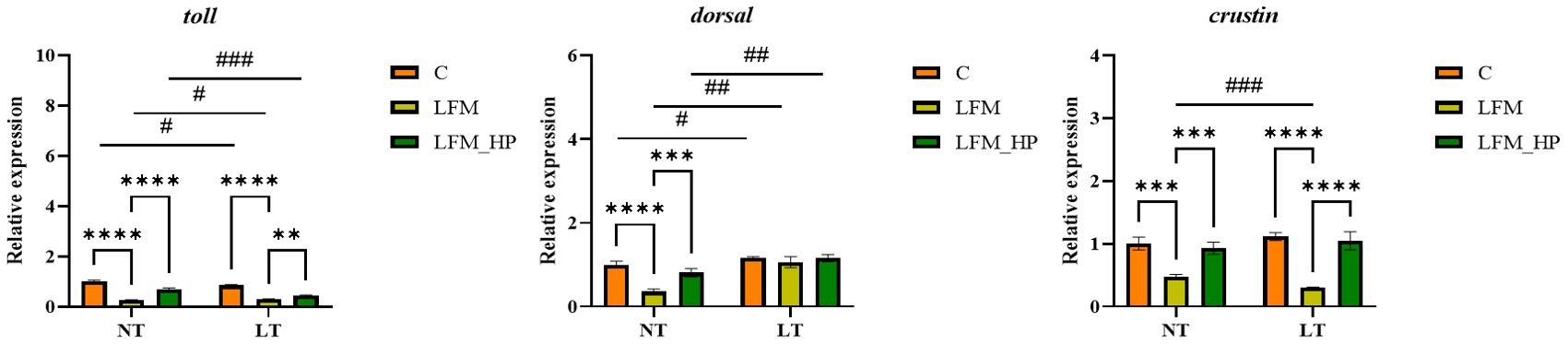
Figure 6. Effect of the addition of H. pluvialis at varying water temperatures on the expression of immune genes related to the Toll pathway of L. vannamei. Data represent the means ± SEM (n = 4). * indicates significant differences in the same treatment group at different temperatures (** 0.001<P<0.01; *** P<0.001; **** P<0.0001). # indicates significant differences in the same treatment group at different temperatures (# 0.01<P<0.05; ## 0.001<P<0.01; ### P<0.001).
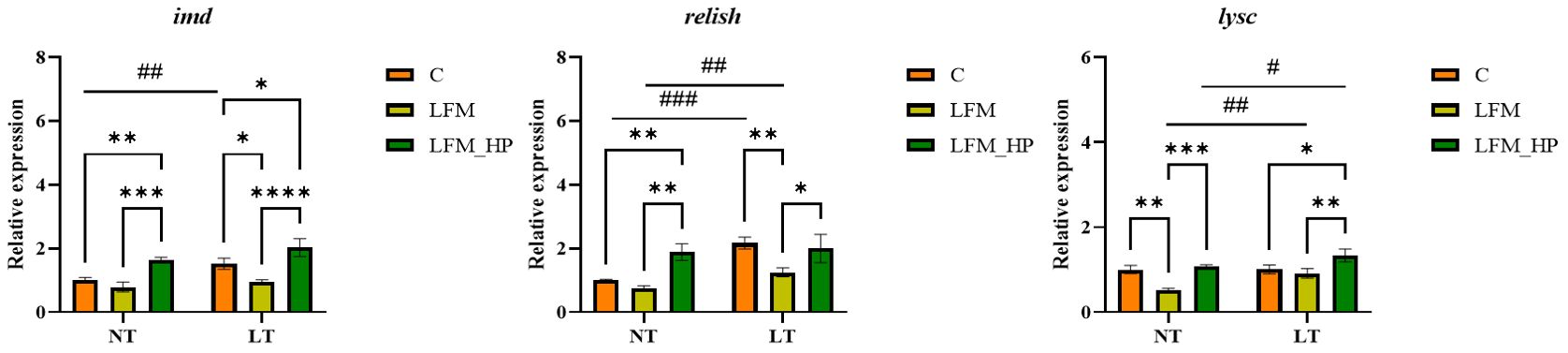
Figure 7. Effect of the addition of H. pluvialis at varying water temperatures on the expression of immune genes related to the IMD pathway of L. vannamei. Data represent the means ± SEM (n = 4). * indicates significant differences in the same treatment group at different temperatures (* 0.01<P<0.05; ** 0.001<P<0.01; *** P<0.001; **** P<0.0001). # indicates significant differences in the same treatment group at different temperatures (# 0.01<P<0.05; ## 0.001<P<0.01; ### P<0.001).
In the Toll pathway, the relative expression level of toll mRNA was significantly higher in the NT_C and LT_C groups compared to the NT_LFM, NT_LFM_HP, LT_LFM, and LT_LFM_HP groups (P<0.05). The highest relative expression of toll was observed in the NT_C group. At the same time, dorsal mRNA expression levels were significantly higher in the LT_LFM_HP group compared to the other groups (P<0.05), while crustin mRNA expression levels were highest in the LT_C group, and the differences between the groups under different temperature conditions were significant (P<0.05).
In the IMD pathway, the relative expression level of imd mRNA was significantly higher in the NT_LFM_HP and LT_LFM_HP groups compared to the NT_C, NT_LFM, LT_C, and LT_LFM groups (P<0.05). Additionally, the relative expression level of relish was higher in the LT_LFM_HP group compared to the NT_LFM_HP group. The mRNA expression levels of lysc in the LT_LFM_HP group were significantly higher compared to the other five groups (P<0.05), with the lowest expression observed in the NT_LFM group. Specifically, the relative expression of L. vannamei lysc mRNA in the LT_LFM_HP group was markedly higher compared to the remaining groups (P<0.05), with the lowest expression detected in the NT_LFM group.
3.7 Gut microbiota analysis
Sequences were grouped into operational taxonomic units (OTUs) at a 97% similarity threshold. The constructed Venn diagram delineated that the NT_C, NT_LFM, NT_LFM_HP, LT_C, LT_LFM, and LT_LFM_HP groups contained 8, 52, 81, 8, 5, and 10 unique Operational Taxonomic Units (OTUs), respectively. As shown in Figure 8, significant differences were noted in the microbial community structure due to differences in water temperature and the presence of H. pluvialis. Figure 9 illustrates the analysis of alpha diversity within the shrimp gut microbiome, as determined by sequencing results of the Ace, Chao1, Shannon, and Simpson diversity indices. The findings signaled that the coverage index for each treatment group exceeded 0.998. The beta diversity analysis visualized through PCoA plots (Figure 10) demonstrated the impact of varying water temperatures (normal and low) on variations in the intestinal microbiota of L. vannamei following exposure to H. pluvialis. As anticipated, the composition of the NT_LFM_HP group was significantly different compared to the other groups (P<0.05).
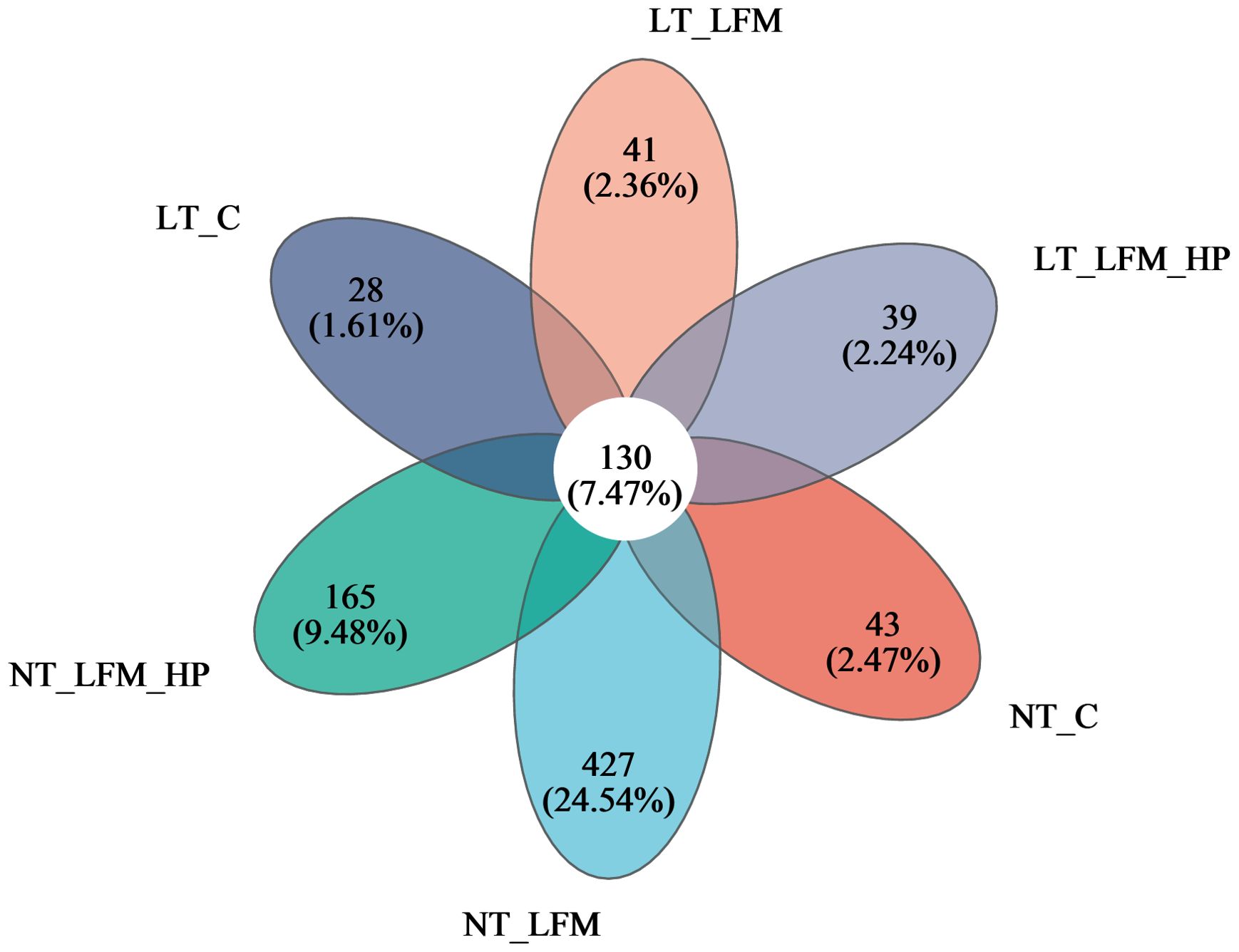
Figure 8. Venn diagram of OTUs Comparison of gut microbiota of the addition of H. pluvialis at varying water temperatures in L. vannamei.
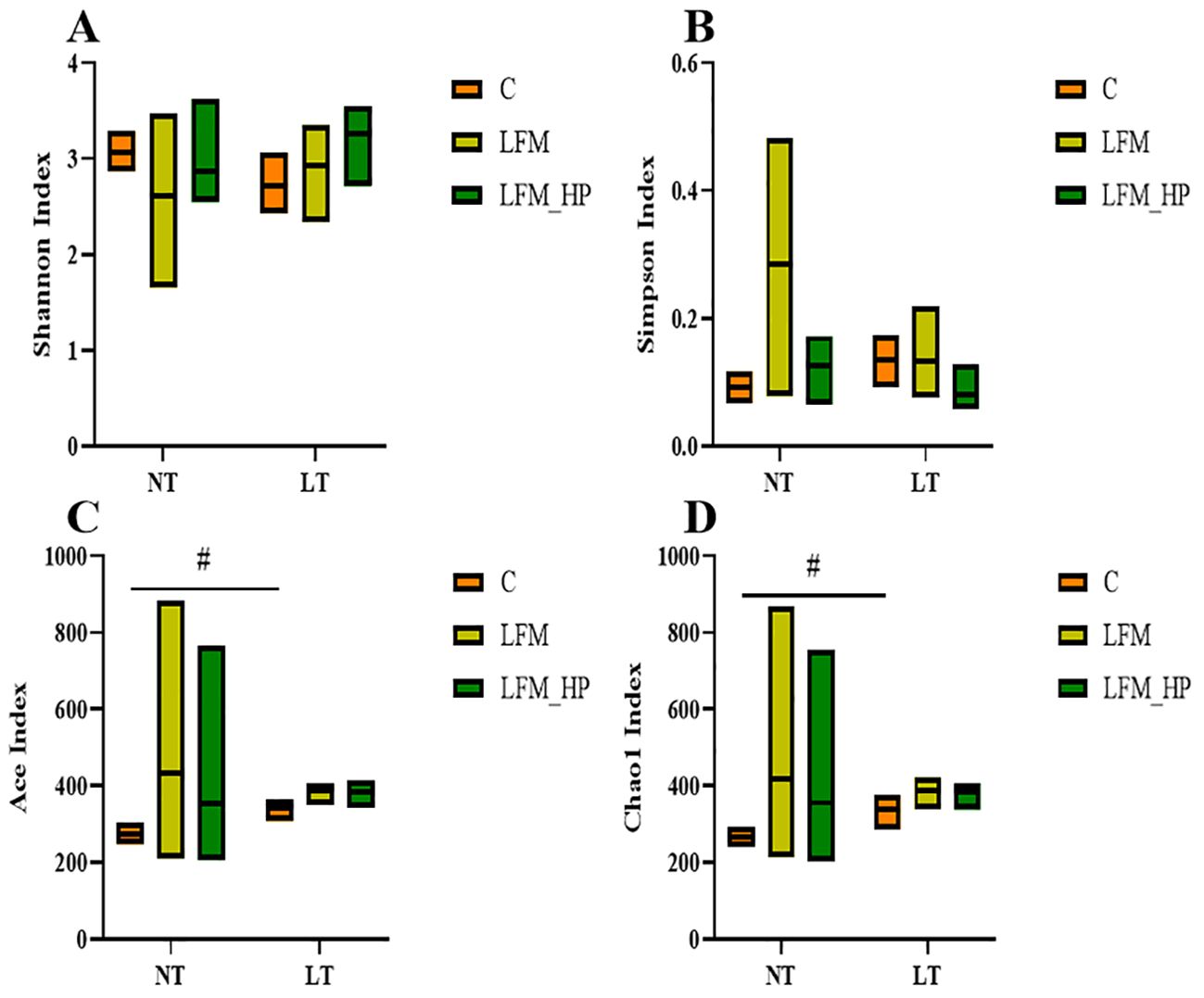
Figure 9. Alpha diversity index statistics of gut microbiota of the addition of (H) pluvialis at varying water temperatures in L. vannamei. Data represent the means ± SEM (n = 4). # indicates significant differences in the same treatment group at different temperatures (P<0.05). (A) Shannon index; (B) Simpson index; (C) Ace index; (D) Chao1 index.
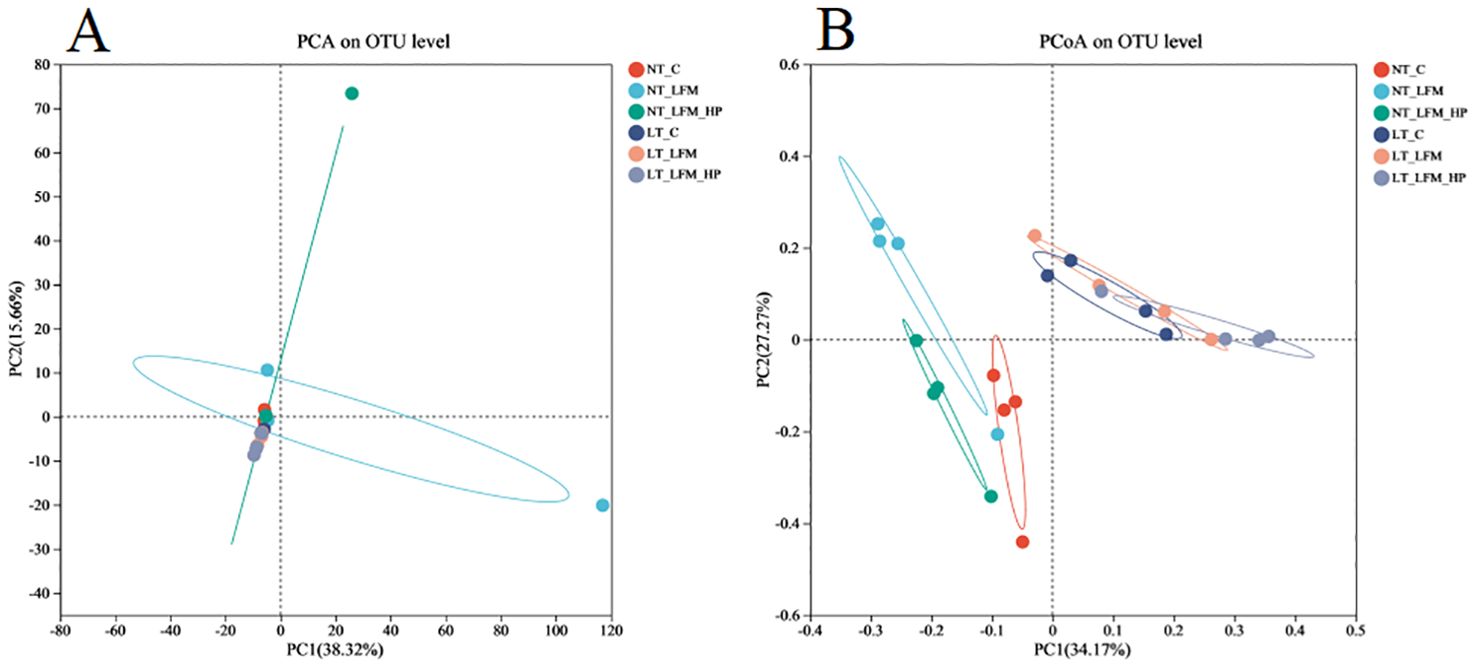
Figure 10. Beta diversity analysis of gut microbiota of the addition of H. pluvialis at varying water temperatures in L. vannamei. (A) PCA on OTU level; (B) PCoA on OTU level.
Figure 11 illustrates the distribution of gut bacteria across treatment groups, categorized at the phylum, class, family, and genus levels. Additionally, the relative abundance of species was depicted at each taxonomic level. Figure 11A displays the relative abundance of gut bacterial phyla, primarily composed of Bacteroidota, Proteobacteria, Actinobacteriota, and Verrucomicrobia. Figure 11B displays the relative abundance of gut bacterial classes in L. vannamei, with major bacteria identified as Bacteroidia, Alphaproteobacteria, Gammaproteobacteria, Acidimicrobiia, Verrucomicrobia, and Actinobacteria. Figure 11C displays the relative abundance of gut bacterial families, predominantly composed of Flavobacteraceae, Rhodobacteraceae, Vibrionaceae, and Actinomarinales. Figure 11D displays the relative abundance of gut bacterial genera, including Flavobacteriales, Rhodobacteraceae, Ruegeria, Spongiimonas, Vibrio, Actinomarinales, and Haloferula.
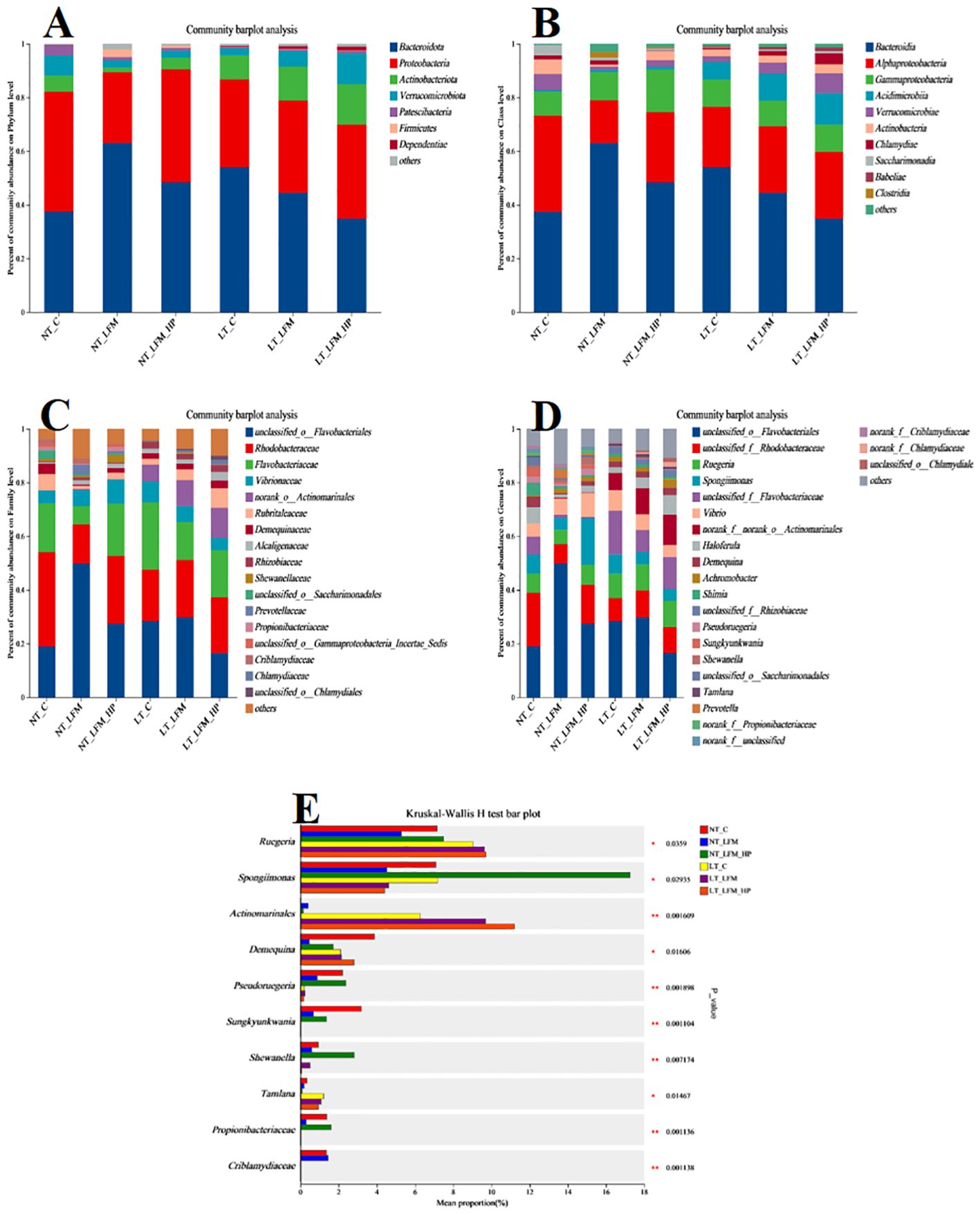
Figure 11. Relative abundance of bacterial community at (A) phylum, (B) class, (C) family, and (D) genus levels of gut microbiota of the addition of H. pluvialis at varying water temperatures in L. vannamei. (E) The significant difference among different treatments at the genus level.
4 Discussion
L. vannamei is considered a significant economically cultivated species (17). The growth, development, and metabolic performance of aquatic organisms are influenced by various environmental factors, with water temperature playing a critical role (18). Shrimp, as poikilothermic organisms, exhibit variations in body temperature in response to environmental conditions, which in turn impacts their metabolism and physiological regulatory mechanisms (19). Of note, nutrition, feeding, and feed utilization play crucial roles in commercial aquaculture due to the significant cost associated with feed (20). Consequently, microalgae have garnered considerable attention as a highly nutritious functional green additive in aquafeeds (21). Indeed, incorporating microalgae into aquafeeds has the potential to partially substitute fishmeal and enhance growth, performance, and immunity (22–25).
In the present study, the weight gain rate and specific growth rate of shrimp in the NT_C, NT_LFM, and NT_LFM_HP groups were significantly higher compared to the LT_C, LT_LFM, and LT_LFM_HP groups. Moreover, the weight gain rate of shrimp in the NT_LFM_HP group was higher than that of the NT_LFM group, with no significant difference observed between NT_LFM_HP and NT_C groups, suggesting that both temperature and H. pluvialis supplementation influenced shrimp feed intake and subsequently impacted their weight gain and specific growth rates, consistent with the findings of prior investigations indicating that L. vannamei exhibited enhanced growth rates within a specific temperature range (26). Noteworthily, no significant differences were noted in the survival rates of L. vannamei across treatment groups, whereas variations in feed conversion ratios were observed between the normal- and low-temperature treatment cohorts. In line with the findings of previous studies, at a temperature of 20°C, both the feeding and growth levels of L. vannamei were diminished (27). The inclusion of H. pluvialis did not yield statistically significant changes in the body composition and hepatopancreas of L. vannamei across the experimental groups. Additionally, the morphology of the hepatopancreas and hepatic ducts remained normal in all treatment groups, with B, R, and E cells displaying typical morphology and the absence of evident lesions. Overall, L. vannamei in the normal-temperature environment with H. pluvialis supplemtation exhibited favorable growth and physiological outcomes. Comparable findings have been documented in studies involving Pseudosciaena crocea (P. crocea) (28) and Trachinotus ovatus (T. ovatus) (29).
Body color is a significant factor in evaluating the quality of crustaceans. H. pluvialis, a microalgae known for its high astaxanthin content, has garnered interest in meeting the demand for natural pigments in aquaculture (30). Astaxanthin concentrations and body pigmentation intensity in L. vannamei were significantly higher in the NT_LFM_HP and LT_LFM_HP groups, consistent with similar observations in L. vannamei and Marsupenaeus japonicus (M. japonicus) (31, 32).
This study also aimed to examine the antioxidant properties of H. pluvialis at varying water temperatures in L. vannamei. Carotenoids, non-enzymatic compounds within the antioxidant system, interact with reactive oxygen species (ROS) to mitigate oxidative damage in tissues (33). Superoxide dismutase (SOD) and catalase (CAT) are integral components of an organism’s antioxidant system. Collectively, they play essential roles in scavenging reactive oxygen species, alleviating oxidative stress, modulating the levels of reactive oxygen species, and preserving redox homeostasis (34, 35). In the current investigation, the incorporation of H. pluvialis in feeding groups (NT_LFM_HP, LT_LFM_HP) reduced MDA levels, indicative of diminished lipid peroxidation and heightened antioxidant potential, thereby enhancing the capacity to eliminate lipid hydroperoxides in shrimp. Additionally, T-AOC and SOD levels were increased, indicating that the inclusion of H. pluvialis may significantly enhance the ability of shrimp to counteract oxygen-free radicals and promote antioxidant defenses. A study investigating Penaeus monodon (P. monodon) concluded that the inclusion of astaxanthin could enhance antioxidant enzyme activity (36). Analysis of mRNA expression levels of antioxidant-related genes in L. vannamei revealed up-regulation of the cat, sod, and gpx genes in groups fed H. pluvialis (NT_LFM_HP, LT_LFM_HP), indicating that H. pluvialis supplementation may mitigate the decrease in antioxidant capacity caused by low fishmeal diets. Several studies have established that incorporating H. pluvialis into the diet could up-regulate the expression levels of antioxidant genes (5, 37, 38), leading to increased SOD enzyme activity in L. vannamei cultured at low temperatures compared to those raised at normal temperatures, indicating that prolonged exposure to low temperatures in aquaculture may mitigate oxidative stress and improve antioxidant capacity. The majority of recent studies explored changes in temperature in aquaculture experiments and temperature stress and did not focus on long-term low-temperature aquaculture. Therefore, we theorize that long-term low-temperature aquaculture may enhance the organism’s antioxidant capacity.
In the context of innate immunity in invertebrates, various signaling pathways are activated to regulate immunity through in vivo signaling. The Toll and IMD signaling pathways have been extensively investigated in relation to the development of the innate immune system in crustaceans (39). This study further investigated the mRNA levels of genes involved in the immune pathway, specifically focusing on the Toll pathway. The relative expression levels of the toll, dorsal, and crustin genes were significantly higher in the H. pluvialis supplementation group compared to the control group. In the IMD signaling pathway, the expression levels of imd, relish, and lysc were elevated in the H. pluvialis supplementation group. Similarly, in the IMD immune pathway, the relative mRNA expression levels of imd, relish, and lysc were up-regulated, indicating that exposure to H. pluvialis at varying temperatures may attenuate the impact of low fishmeal diets on shrimp immunocompetence. Additionally, in crayfish, the administration of astaxanthin modulated non-specific immunity levels following exogenous challenges (40). Studies have demonstrated that the inclusion of astaxanthin in the diet of M. japonicus boosted the immune response (41). Additionally, research indicated that H. pluvialis may contribute to the activation of the phenoloxidase cascade, eventually leading to enhanced immunity (42, 43). In this study, the impact of varying water temperatures on the immune system was examined. Our findings indicated that in the Toll signaling immune pathway, the normal water temperature group exhibited lower immune function compared to the low water temperature group. Additionally, in the IMD signaling immune pathway, the low water temperature group displayed significantly higher relative mRNA expression levels of the imd, relish, and lysc genes compared to the normal water temperature group. We postulate that shrimp cultured in a prolonged low-temperature environment may regulate their own immune system, thereby sustaining stable life and health. Moreover, we theorize that under extended low-temperature conditions, shrimp may autonomously modulate its immune response to maintain consistent vital functions.
The growth and health of organisms are influenced by gut microbial communities (44), while water temperature, a critical environmental factor in aquaculture, impacts the compositional structure and dynamic balance of these communities. This study investigated the gut microbiota of L. vannamei and identified significant differences between the NT_LFM_HP group and the remaining groups. The analysis of sample complexity revealed a significant difference in the number of operational taxonomic units (OTUs) between the NT_LFM, NT_LFM_HP, and other groups, suggesting that variations in temperature and the presence of H. pluvialis may impact the composition of gut microbiota, leading to distinct microbial communities. Beta diversity analysis unveiled that the gut sample points of the NT_LFM_HP group exhibited greater dissimilarity from the sample points of the other groups, with the microbial communities in the NT_LFM_HP group significantly differing from those in the other groups. This observation suggested that H. pluvialis influenced the relative abundance of dominant operational taxonomic units (OTUs). The relative abundance plot of the gut flora composition of L. vannamei indicated that Proteobacteria, Actinobacteria, and Bacteroidetes were the predominant gut microbial species, consistent with the findings of previous studies (45–47). The findings also indicated that reducing fishmeal content (NT_LFM, LT_LFM) resulted in a higher abundance of Flavobacteraceae. Importantly, H. pluvialis supplementation (NT_LFM_HP, LT_LFM_HP) promoted this increase, suggesting that the presence of H. pluvialis alleviated the detrimental impact of harmful microorganisms such as Flavobacterium. It is worthwhile emphasizing that the results uncovered significant differences in the abundance of Vibrionaceae and Actinomarinales between low- and normal-temperature environments, with Vibrionaceae being more abundant in normal-temperature environments and Actinomarinales being more abundant in low-temperature environments. This suggested that Actinomarinales may play a decisive role in enhancing shrimp immunity by resisting bacterial invasion and improving immune parameters (48, 49). Furthermore, the findings of this study suggested that exposure to low temperatures may decrease the abundance of detrimental intestinal flora, potentially enhancing shrimp resistance to pathogens through the modulation of intestinal flora composition. Furthermore, the research indicated that cultivating shrimp in low water temperatures could promote immune responses in non-specific immune assays, thereby supporting their overall health and survival.
5 Conclusion
The study incorporated varying water temperatures and fishmeal levels, including a group with 20% fishmeal and another with 10% fishmeal supplemented with H. pluvialis. The findings exposed that L. vannamei growth and immune response were negatively affected by reducing fishmeal levels, whereas H. pluvialis supplementation improved growth, antioxidant capacity, and immune function. Additionally, under long-term low-temperature conditions, L. vannamei demonstrated enhanced resistance to external pathogens through immune system modulation. Overall, H. pluvialis, as a natural additive, exerted positive effects on aquatic animal nutrition, including promoting growth, improving immunity, and improving biochemical indicators. Taken together, these results collectively highlighted the extensive application potential of H. pluvialis in the aquatic feed industry owing to its unique characteristics.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SL: Conceptualization, Data curation, Writing – original draft. MC: Data curation, Writing – review & editing. XC: Investigation, Writing – review & editing. YML: Investigation, Writing – review & editing. YFL: Investigation, Writing – review & editing. PZ: Investigation, Writing – review & editing. XH: Investigation, Writing – review & editing. BT: Supervision, Writing – review & editing. JN: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research received funding from multiple sources, including the National Key Research and Development Program of China (2023YFD2401705), Project of Algae health Science Co., Ltd. (HT99982023-0347), Project of Science and Technology of Guangxi Province (AA23062047), Project of National Natural Science Foundation of China (32172982), and Project of China Agriculture Research System of MOF and MARA 48 (CARS 48). The funders had no role in the study design, data collection and analysis, decision to publish, and preparation of the manuscript.
Conflict of interest
Authors YML, YFL, PNZ and XYH were employed by Algae Health Science Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang Y, Yu J, Su Y, Du Y, Liu Z. Long-term changes of water quality in aquaculture-dominated lakes as revealed by sediment geochemical records in Lake Taibai (Eastern China). Chemosphere. (2019) 235:297–307. doi: 10.1016/j.chemosphere.2019.06.179
2. Chen J, Sun R, Pan C, Sun Y, Mai B, Li QX. Antibiotics and food safety in aquaculture. J Agric Food Chem. (2020) 68:11908–19. doi: 10.1021/acs.jafc.0c03996
3. Santos L, Ramos F. Analytical strategies for the detection and quantification of antibiotic residues in aquaculture fishes: A review. Trends Food Sci Technol. (2016) 52:16–30. doi: 10.1016/j.tifs.2016.03.015
4. Bauer A, Minceva M. Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid–liquid chromatography. RSC Adv. (2019) 9:22779–89. doi: 10.1039/C9RA03263K
5. Liu XH, Wang BJ, Li YF, Wang L, Liu JG. Effects of dietary botanical and synthetic astaxanthin on E/Z and R/S isomer composition, growth performance, and antioxidant capacity of white shrimp, Litopenaeus vannamei, in the nursery phase [For this article an Erratum has been published. Invertebrate Survival J. (2018) 15:131–40. doi: 10.25431/1824-307X/isj.v15i1.131-140
6. Yu Y, Liu Y, Yin P, Zhou W, Tian L, Liu Y, et al. Astaxanthin attenuates fish oil-related hepatotoxicity and oxidative insult in juvenile pacific white shrimp (Litopenaeus vannamei). Mar Drugs. (2020) 18:218. doi: 10.3390/md18040218
7. Cheng Y, Wu S. Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkii. Aquaculture. (2019) 512:734341. doi: 10.1016/j.aquaculture.2019.734341
8. Angell A, de Nys R, Mangott A, Vucko MJ. The effects of concentration and supplementation time of natural and synthetic sources of astaxanthin on the colouration of the prawn Penaeus monodon. Algal Res. (2018) 35:577–85. doi: 10.1016/j.algal.2018.09.031
9. Wang Z, Qu Y, Zhuo X, Li J, Zou J, Fan L. Investigating the physiological responses of Pacific white shrimp Litopenaeus vannamei to acute cold-stress. PeerJ. (2019) 7:e7381. doi: 10.7717/peerj.7381
10. Tang Y, Tao P, Tan J, Mu H, Peng L, Yang D, et al. Identification of bacterial community composition in freshwater aquaculture system farming of Litopenaeus vannamei reveals distinct temperature-driven patterns. Int J Mol Sci. (2014) 15:13663–80. doi: 10.3390/ijms150813663
11. Topuz M, Kır M. Critical temperatures and aerobic metabolism in post-larvae of Pacific white shrimp Litopenaeus vannamei (Boone, 1931). (2023) 193:607–14. doi: 10.1007/s00360-023-01522-4
12. Millard RS, Ellis RP, Bateman KS, Bickley LK, Tyler CR, van Aerle R, et al. How do abiotic environmental conditions influence shrimp susceptibility to disease? A critical analysis focussed on White Spot Disease. J Invertebrate Pathol. (2021) 186:107369. doi: 10.1016/j.jip.2020.107369
13. Xie J-J, Liu Q, Liao S, Fang H-H, Yin P, Xie S-W, et al. Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. (2019) 90:456–65. doi: 10.1016/j.fsi.2019.04.301
14. Xiong J, Yu W, Dai W, Zhang J, Qiu Q, Ou C. Quantitative prediction of shrimp disease incidence via the profiles of gut eukaryotic microbiota. Appl Microbiol Biotechnol. (2018) 102:3315–26. doi: 10.1007/s00253-018-8874-z
15. Al-Masqari ZA, Guo H, Wang R, Yan H, Dong P, Wang G, et al. Effects of high temperature on water quality, growth performance, enzyme activity and the gut bacterial community of shrimp (Litopenaeus vannamei). Aquaculture Res. (2022) 53:3283–96. doi: 10.1111/are.15836
16. Liu J, Wang K, Wang Y, Chen W, Jin Z, Yao Z, et al. Strain-specific changes in the gut microbiota profiles of the white shrimp Litopenaeus vannamei in response to cold stress. Aquaculture. (2019) 503:357–66. doi: 10.1016/j.aquaculture.2019.01.026
17. Zhang C, Guo C-Y, Shu K-H, Xu S-L, Wang D-L. Comparative analysis of the growth performance, vitality, body chemical composition and economic efficiency of the main cultivated strains of Pacific white shrimp (Litopenaeus vannamei) in coastal areas of China. Aquaculture. (2024) 587:740856. doi: 10.1016/j.aquaculture.2024.740856
18. Lutterschmidt WI, Hutchison VH. The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can J Zoology. (1997) 75. doi: 10.1139/z97-782
19. Zhang L, Sha Z, Cheng J. Time-course and tissue-specific molecular responses to acute thermal stress in Japanese mantis shrimp oratosquilla oratoria. Int J Mol Sci. (2023) 24:11936. doi: 10.3390/ijms241511936
20. Hoseinifar SH, Dadar M, Ringø E. Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: The functional feed additives scenario. Aquaculture Res. (2017) 48:3987–4000. doi: 10.1111/are.13368
21. Roy SS, Pal R. Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. Proc Zool Soc. (2015) 68:1–8. doi: 10.1007/s12595-013-0089-9
22. Cerezuela R, Guardiola FA, Meseguer J, Esteban MÁ. Enrichment of gilthead seabream (Sparus aurata L.) diet with microalgae: effects on the immune system. Fish Physiol Biochem. (2012) 38:1729–39. doi: 10.1007/s10695-012-9670-9
23. Reyes-Becerril M, Angulo C, Estrada N, Murillo Y, Ascencio-Valle F. Dietary administration of microalgae alone or supplemented with Lactobacillus sakei affects immune response and intestinal morphology of Pacific red snapper (Lutjanus Peru). Fish Shellfish Immunol. (2014) 40:208–16. doi: 10.1016/j.fsi.2014.06.032
24. Reyes-Becerril M, Guardiola F, Rojas M, Ascencio-Valle F, Esteban MÁ. Dietary administration of microalgae Navicula sp. affects immune status and gene expression of gilthead seabream (Sparus aurata). Fish Shellfish Immunol. (2013) 35:883–9. doi: 10.1016/j.fsi.2013.06.026
25. Shah MR, Lutzu GA, Alam A, Sarker P, Kabir Chowdhury MA, Parsaeimehr A, et al. Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol. (2018) 30:197–213. doi: 10.1007/s10811-017-1234-z
26. Ponce-Palafox J, Martinez-Palacios CA, Ross LG. The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture. (1997) 157:107–15. doi: 10.1016/S0044-8486(97)00148-8
27. Bórquez-Lopez RA, Casillas-Hernandez R, Lopez-Elias JA, Barraza-Guardado RH, Martinez-Cordova LR. Improving feeding strategies for shrimp farming using fuzzy logic, based on water quality parameters. Aquacultural Eng. (2018) 81:38–45. doi: 10.1016/j.aquaeng.2018.01.002
28. Li M, Wu W, Zhou P, Xie F, Zhou Q, Mai K. Comparison effect of dietary astaxanthin and Haematococcus pluvialis on growth performance, antioxidant status and immune response of large yellow croaker Pseudosciaena crocea. Aquaculture. (2014) 434:227–32. doi: 10.1016/j.aquaculture.2014.08.022
29. Zhao W, Fang H-H, Liu Z-Z, Huang M-Q, Su M, Zhang C-W, et al. A newly isolated strain of Haematococcus pluvialis JNU35 improves the growth, antioxidation, immunity and liver function of golden pompano (Trachinotus ovatus). Aquaculture Nutr. (2021) 27:342–54. doi: 10.1111/anu.13188
30. Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. (2003) 21:210–6. doi: 10.1016/S0167-7799(03)00078-7
31. Chien Y-H, Shiau W-C. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate. J Exp Mar Biol Ecol. (2005) 318:201–11. doi: 10.1016/j.jembe.2004.12.016
32. Ju ZY, Deng D-F, Dominy WG, Forster IP. Pigmentation of Pacific White Shrimp, Litopenaeus vannamei, by Dietary Astaxanthin Extracted from Haematococcus pluvialis. J World Aquaculture Soc. (2011) 42:633–44. doi: 10.1111/j.1749-7345.2011.00511.x
33. He H, Huang N, Cao R, Meng L. Structures, Antioxidation Mechanism, and Antioxidation Test of the Common Natural Antioxidants in Plants (2015). Available online at: https://www.semanticscholar.org/paper/Structures%2C-Antioxidation-Mechanism%2C-and-Test-of-in-He-Huang/6513dfd3177302211dea2a83811791e71b5696ea (Accessed August 28, 2024).
34. Duan Y, Zhang Y, Dong H, Zhang J. Effect of desiccation on oxidative stress and antioxidant response of the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. (2016) 58:10–7. doi: 10.1016/j.fsi.2016.09.004
35. Long X, Wu X, Zhao L, Liu J, Cheng Y. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab. Eriocheir sinensis. Aquaculture. (2017) 473:545–53. doi: 10.1016/j.aquaculture.2017.03.010
36. Pan C-H, Chien Y-H, Hunter B. The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. J Exp Mar Biol Ecol. (2003) 297:107–18. doi: 10.1016/j.jembe.2003.07.002
37. Chuchird N, Rorkwiree P, Rairat T. Effect of dietary formic acid and astaxanthin on the survival and growth of Pacific white shrimp (Litopenaeus vannamei) and their resistance to Vibrio parahaemolyticus. SpringerPlus. (2015) 4:440. doi: 10.1186/s40064-015-1234-x
38. Wang H, Dai A, Liu F, Guan Y. Effects of dietary astaxanthin on the immune response, resistance to white spot syndrome virus and transcription of antioxidant enzyme genes in Pacific white shrimp Litopenaeus vannamei. Iranian J Fisheries Sci. (2015) 14:699–718. doi: 10.22092/ijfs.2018.114476
39. Li C, Wang S, He J. The two NF-κB pathways regulating bacterial and WSSV infection of shrimp. Front Immunol. (2019) 10:1785. doi: 10.3389/fimmu.2019.01785
40. Li F, Huang S, Lu X, Wang J, Lin M, An Y, et al. Effects of dietary supplementation with algal astaxanthin on growth, pigmentation, and antioxidant capacity of the blood parrot (Cichlasoma citrinellum × Cichlasoma synspilum). J Ocean Limnol. (2018) 36:1851–9. doi: 10.1007/s00343-019-7172-7
41. Wang W, Ishikawa M, Koshio S, Yokoyama S, Dawood MAO, Hossain Md S, et al. Interactive effects of dietary astaxanthin and cholesterol on the growth, pigmentation, fatty acid analysis, immune response and stress resistance of kuruma shrimp (Marsupenaeus japonicus). Aquaculture Nutr. (2019) 25:946–58. doi: 10.1111/anu.12913
42. Ngo HT, Nguyen TT, Nguyen QM, Tran AV, Do HT, Nguyen AH, et al. Screening of pigmented Bacillus aquimaris SH6 from the intestinal tracts of shrimp to develop a novel feed supplement for shrimp. J Appl Microbiol. (2016) 121:1357–72. doi: 10.1111/jam.13274
43. Yeh S-T, Lee C-S, Chen J-C. Administration of hot-water extract of brown seaweed Sargassum duplicatum via immersion and injection enhances the immune resistance of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. (2006) 20:332–45. doi: 10.1016/j.fsi.2005.05.008
44. Hou D, Huang Z, Zeng S, Liu J, Wei D, Deng X, et al. Intestinal bacterial signatures of white feces syndrome in shrimp. Appl Microbiol Biotechnol. (2018) 102:3701–9. doi: 10.1007/s00253-018-8855-2
45. Qiao F, Liu YK, Sun YH, Wang XD, Chen K, Li TY, et al. Influence of different dietary carbohydrate sources on the growth and intestinal microbiota of Litopenaeus vannamei at low salinity. . Aquaculture Nutr. (2017) 23:444–52. doi: 10.1111/anu.12412
46. Rungrassamee W, Klanchui A, Maibunkaew S, Chaiyapechara S, Jiravanichpaisal P, Karoonuthaisiri N. Characterization of intestinal bacteria in wild and domesticated adult black tiger shrimp (Penaeus monodon). PloS One. (2014) 9:e91853. doi: 10.1371/journal.pone.0091853
47. Suo Y, Li E, Li T, Jia Y, Qin JG, Gu Z, et al. Response of gut health and microbiota to sulfide exposure in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. (2017) 63:87–96. doi: 10.1016/j.fsi.2017.02.008
48. Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L. Probiotics in aquaculture: The need, principles and mechanisms of action and screening processes. Aquaculture. (2008) 274:1–14. doi: 10.1016/j.aquaculture.2007.11.019
49. Zhang M, Sun Y, Chen K, Yu N, Zhou Z, Chen L, et al. Characterization of the intestinal microbiota in Pacific white shrimp, Litopenaeus vannamei, fed diets with different lipid sources. Aquaculture. (2014) 434:449–55. doi: 10.1016/j.aquaculture.2014.09.008
50. McBeth JW. Carotenoids from nudibranchs. Comp Biochem Physiol Part B: Comp Biochem. (1972) 41:55–68. doi: 10.1016/0305-0491(72)90007-7
51. Xu K, Ren Y, Zhao S, Feng J, Wu Q, Gong X, et al. Oral d-ribose causes depressive-like behavior by altering glycerophospholipid metabolism via the gut-brain axis. Commun Biol. (2024) 7:1–14. doi: 10.1038/s42003-023-05759-1
52. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. (2009) 75:7537–41. doi: 10.1128/AEM.01541-09
Keywords: Litopenaeus vannamei, Haematococcus Pluvialis, temperature, growth performance, innate immunity
Citation: Lin S, Chen M, Chen X, Li Y, Liu Y, Zhang P, Hou X, Tan B and Niu J (2024) Supplemental effects of Haematococcus pluvialis in a low-fish meal diet for Litopenaeus vannamei at varying temperatures: growth performance, innate immunity and gut bacterial community. Front. Immunol. 15:1501753. doi: 10.3389/fimmu.2024.1501753
Received: 25 September 2024; Accepted: 25 November 2024;
Published: 10 December 2024.
Edited by:
Samad Rahimnejad, University of Murcia, SpainReviewed by:
Yun Wang, Chinese Academy of Fishery Sciences (CAFS), ChinaYong-Jun Chen, Southwest University, China
Copyright © 2024 Lin, Chen, Chen, Li, Liu, Zhang, Hou, Tan and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beiping Tan, YnB0YW5AMTI2LmNvbQ==; Jin Niu, bml1ajNAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Sihan Lin
Sihan Lin Mengdie Chen1†
Mengdie Chen1† Beiping Tan
Beiping Tan Jin Niu
Jin Niu