- Eye Center, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Demyelinating optic neuritis (DON) is a rare but sight-threatening ophthalmic condition which occasionally occurs after human papilloma virus (HPV) vaccination. We herein report a case of previously healthy 13-year-old girl who developed a bilateral refractory DON three days after the first dose of bivalent HPV vaccine. The patient experienced bilateral severe visual loss three days after HPV vaccination, and her vision was quickly deteriorated to no light perception one day after the onset of DON. Ophthalmic examination revealed sluggish pupillary light reflex and swollen optic disc in both eyes, and an emergent orbital MRI examination revealed bilateral hyperintensity and enlargement of the intraorbital optic nerve with contrast enhancement. Serological tests for aquaporin-4 IgG antibody, myelin oligodendrocyte glycoprotein IgG antibody, and other common autoantibodies were all negative. The patient showed poor response to 10 days of intravenous methylprednisolone pulse therapy (500 mg, 250 mg, and 125 mg twice per day for 4, 4, and 2 days, respectively). Hence, three-dosed subcutaneous satralizumab was used in the acute stage of DON as an adjunct therapy. Her vision gradually improved after satralizumab therapy, and increased to 20/20 and 20/32 in the right and left eye at the 3-month follow-up. To the best of our knowledge, this is the first case report of satralizumab therapy in the AQP-4 Ab and MOG-Ab dual seronegative isolated DON. Our study indicates that satralizumab may be a safe and efficient adjunct therapy which can be used in the acute stage of the refractory DON poorly responding to steroid pulse therapy.
Introduction
Human papilloma virus (HPV) vaccines including a bivalent vaccine (HPV2), a quadrivalent HPV (HPV4), and a nine-valent (HPV9) are worldwidely used in adolescent girls and adult women to prevent HPV infection and subsequent cervical cancer (1, 2). Although the safety of HPV vaccines has been proved by several large-scaled clinical studies, serious adverse events including central neural system (CNS) autoimmune inflammations after HPV vaccination are occasionally reported (1–5), which give rise to a public concern on the safety of HPV vaccines.
Optic neuritis (ON) is a rare but sight-threatening ophthalmic condition which occasionally occurs in the infectious, post-infection, or post-vaccination cases. It is reported that demyelinating optic neuritis (DON) is the most predominant clinical manifestation of CNS autoimmune inflammations in cases after vaccination. 1-5 A survey study based on the Regional Health Care Information Platform in Ningbo, China revealed an incidence of 1.8% (346/19328 cases) for DON in females receiving HPV4 or HPV2 vaccination (6). However, our literature research based on Pubmed database reveals that detailed clinical characteristics and natural course of DON after HPV vaccination are currently reported in only several cases (7–9).
Although most DON cases after vaccination show good response to steroid pulse therapy (7, 8, 10), there still exist a few cases who poorly respond to steroid pulse therapy and need other adjunct therapy such as plasma exchange to rescue their vision (9, 10). We herein report a case of a previously healthy girl who developed a bilateral refractory DON three days after the first dose of HPV2, satralizumab is used as an adjunct therapy in the acute stage of DON in this case due to her poor response to steroid pulse therapy.
Patient presentation
A 13-year-old previously healthy girl was taken to the local hospital by her patients due to severe visual loss in both eyes accompanied by ocular pain when moving her eyes for four days. Initial ophthalmic examination showed sluggish pupillary light reflex and swollen optic disc in both eyes, and an emergent orbital MRI examination revealed long-segment hyperintensity and enlargement of the intraorbital optic nerve with contrast enhancement in both eyes (Figures 1A–F), as well as an isolated finger-like slightly hyperintense lesion vertical to left lateral ventricle (Figure 1G), and several spotty hyperintense subcortical lesions in bilateral parietal lobes (Figure 1H). Her post medical record was unremarkable. However, the girl just received the first dose of HPV2 (Yuxi Zerun Biotechnology Company, China) three days before her visual loss. The girl was diagnosed as bilateral DON and received 500mg of methylprednisolone intravenous injection per day for 3 days. Unfortunately, her vision in both eyes continuously deteriorated to no light perception one day after the initial steroid therapy, and got no improvement after 3 days’ steroid pulse therapy.
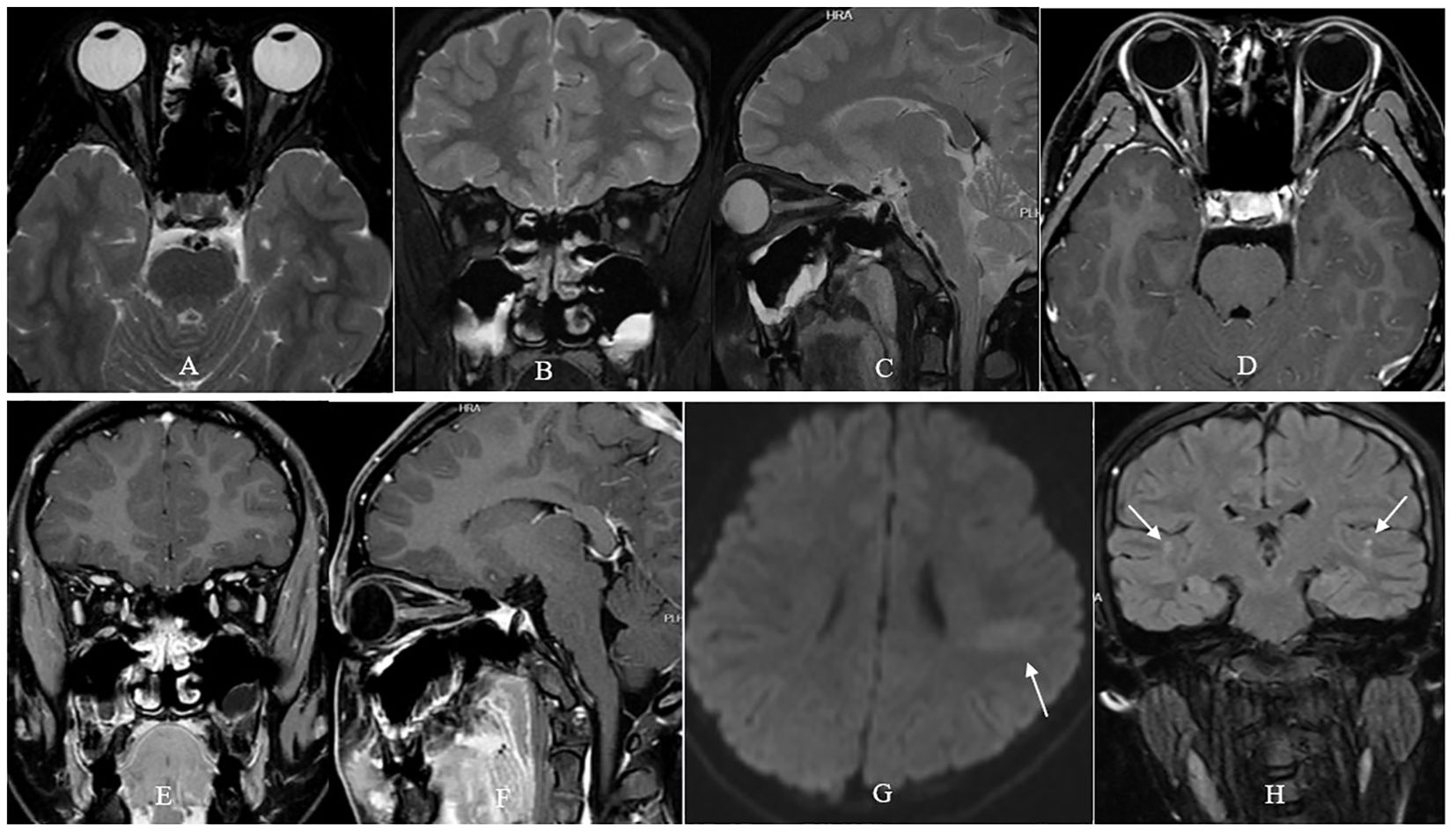
Figure 1. Orbital MRI examination of the patient. T2- fat suppression sequence showed long-segment hyperintensity and enlargement of the intraorbital optic nerve in both eyes (A–C). Contrast-enhanced T1- fat suppression sequence showed long-segment contrast-enhancement of the intraorbital optic nerve in both eyes (D–F). Fluid attenuated inversion recovery sequence showed an isolated finger-like slightly hyperintense lesion vertical to left lateral ventricle [arrow, (G)], and several spotty hyperintense lesions in bilateral parietal lobes [arrow, (H)].
The girl was then referred to our Neuro-ophthalmology clinic for further consultation. At presentation in our hospital, ophthalmic examination revealed no light perception in both eyes, and clear cornea, quiet anterior chamber, dilated pupil of 8 mm and absent pupillary light reflex, clear lens and vitreous, and swollen optic disc in both eyes (Figures 2A, B). Visual field test showed diffuse depression in both eyes (Figures 2C, D). Spectral-domain optical coherence tomography (SD-OCT) revealed an average ganglion cell complex thickness of 99 μm in both eyes. Serological tests including antinuclear antibodies, antiphospholipid antibodies, antineutrophil cytoplasmic antibodies, and angiotensin converting enzyme were all negative. Commercial serological tests for aquaporin-4 IgG antibody (AQP-4 Ab) and myelin oligodendrocyte glycoprotein IgG antibody (MOG-Ab) by cell-based indirect dual-immunofluorescence assay were both negative. Microbiological screening tests for treponema pallidum, tubercle bacillus, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, cytomegalovirus, rubella virus, herpes viruses and toxoplasma were all negative.
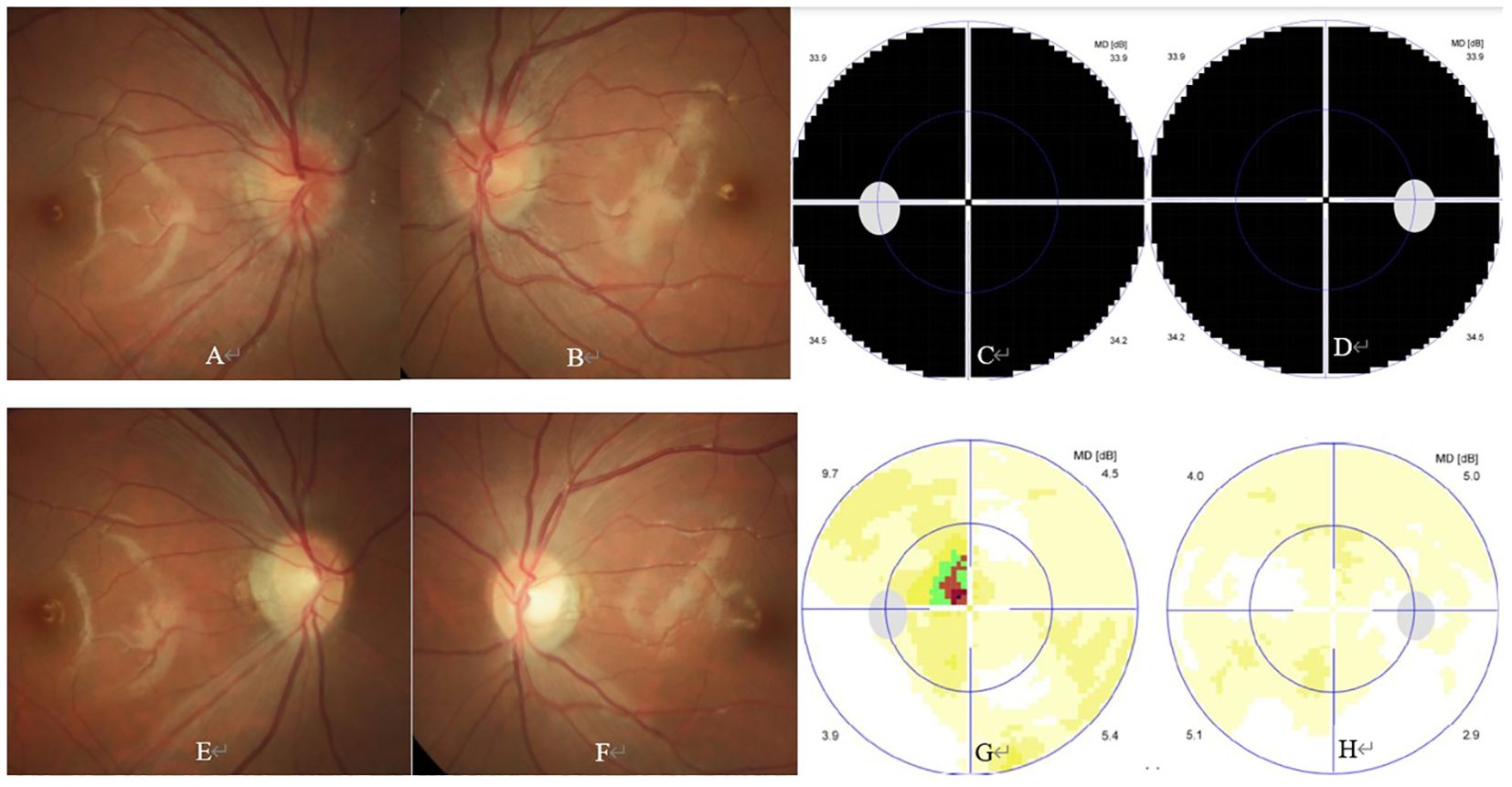
Figure 2. Ophthalmic and paraophthalmic examinations of the patient. At presentation, ophthalmic examination showed bilateral swollen optic disc (A, B). Visual field test showed diffuse depression in both eyes (C, D). At the 3-month follow-up, ophthalmic examination showed bilateral temporal optic disc pallor (E, F). Visual field test showed small central scotoma in the left eye (G), and normal result in the right eye (H).
The diagnosis of bilateral DON was confirmed. The patient was then admitted into hospital, and intravenous injection of methylprednisolone 500 mg twice per day for 4 days, followed by 250 mg twice per day for 4 days, and then 125 mg twice per day for 2 days, was prescribed. Her visual acuity slowly improved from no light perception in both eyes to hand motion in the right eye and finger counting in the left eye respectively 3 days after admission, but kept stable in the following 7 days. Plasma exchange or immunoadsorption therapy was then highly recommended to rescue her vision, but was declined by the patient and her parents. Intravenous immunoglobulin injection was also refused by the patient and her parents when they were informed that immunoglobulin therapy might not help improving her visual function (11, 12).
After detailed communications with the patient and her parents on the severity of her ocular condition, and other alternative therapy especially the off-label use of monoclonal antibody therapy such as rituximab, ofatumumab, inebilizumab, and satralizumab, as well as their advantages and disadvantages, the patient and her patients finally chose satralizumab as an acute-staged adjunct therapy for this refractory DON. Ten days after her admission (2 weeks after the onset of DON), when the last dose of intravenous injection of methylprednisolone (125 mg) was completed, the girl received the first dose injection of satralizumab 120 mg subcutaneously, no side effects were reported after satralizumab injection. The girl was then discharged with a prescription of oral methylprednisolone 40 mg per day which was slowly tapering. The second and third dose of satralizumab was uneventfully injected 2 and 4 weeks after its initial injection, respectively.
At the 2-week, 4-week, and 3-month follow-up, the general condition of the girl was unremarkable, and her visual acuity gradually improved to 20/400 and 20/400; 20/40 and 20/67, and 20/20 and 20/32 in the right and left eye, respectively. At the 3-month follow-up, ophthalmic examination revealed normal anterior segment and temporal optic disc pallor in both eyes (Figures 2E, F). Visual field test showed normal result and small central scotoma in the right and left eye, respectively (Figures 2G, H). SD-OCT revealed an average ganglion cell complex thickness of 65 μm in the right eye and 61 μm in the left eye, respectively. The patient is still under follow-up, but receiving neither steroid nor satralizumab therapy now.
Discussion
DON is a rare but sight-threatening ocular condition after vaccination. Many vaccines such as influenza, HPV, or severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) vaccine were reported to be related to DON occurrence (1–10). Although many clinical studies have reported that vaccination does not increase the risk of DON occurrence, the temporal relationship between vaccination and the onset of DON indicates that vaccine may be a trigger of an initial onset or a relapse of DON (1–6, 10).
DON can occur isolatedly or as an ocular manifestation of CNS autoimmune inflammations after vaccination. Chang et al. reported two episodes of DON which occurred 7 days and 3 days after the first and second HPV vaccination respectively in a 30-year-old woman. Serological tests after the second episode of DON revealed AQP-4 Ab positive in their case (7). DiMario et al. reported permanent bilateral visual loss and left hemiparesis which occurred 10 days after the second dose of HPV vaccination in a 16-year-old girl. Serological tests revealed AQP-4 and other autoantibodies all negative in above case (9).
As for our case, one ON attack and orbital MRI examination revealed bilateral optic nerve enlargement and enhancement, an isolated finger-like hyperintense lesion vertical to left lateral ventricle and several spotty hyperintense subcortical lesions which indicating multiple spacial attacks implied a diagnosis of multiple sclerosis (MS). However, according to 2017 McDonald criteria, there still need MRI findings indicating multiple temporal attacks or cerebrospinal fluid oligoclonal band positive test result, to make a diagnosis of definite MS. Unfortunately, this case lacked of MRI findings indicating multiple temporal attacks and refused lumbar puncture which meant that no cerebrospinal fluid test was performed. Hence, currently we still lack of enough evidence to make a diagnosis of definite MS-ON.
Moreover, the case in this study showed severe bilateral ON (no light perception in both eyes) and poor response to two rounds of steroid pulse therapy, which was not consistent to a typical MS-ON manifestation (typical MS-ON manifested as unilateral optic neuritis, mild visual loss and good response to steroid therapy), but to an AQP-4 Ab negative neuromyelitis optica spectrum disorder (NMOSD)-ON. Hence, long-term follow-up is needed to further evaluate it is an isolated DON, or MS-ON, or even an AQP-4 Ab negative NMOSD-ON.
Currently, the exact pathogenesis of post-vaccination DON is still not clear. Possible mechanisms include the immediate immune disturbance and subsequent inflammatory cytokines release after vaccination, as well as the production of autoimmune antibodies including AQP-4 Ab and MOG-Ab induced by viral or bacterial vaccines via molecular mimicking or random exposure of antigens during inflammation after vaccination (7–10, 13, 14). Previous investigations have revealed that HPV vaccination can induce AQP-4 Ab positive or AQP-4 Ab and MOG-Ab double negative DON (7–10). Considering that DON in this case occurred only 3 days after the first dose of HPV2 vaccination, it is reasonable to speculate that DON in this case was probably caused by an immediate immune disturbance and subsequent inflammatory cytokines release after HPV vaccination, since autoimmune antibodies such as AQP-4 Ab or MOG-Ab cannot be produced within 7 days after vaccination (13, 14). This hypothesis is supported by the negative serological test results for AQP-4 Ab, MOG-Ab, and other common autoantibodies in this study.
Although most post-vaccination DON shows good response to steroid pulse therapy, there exist a few case reports indicating that some AQP-4 Ab seropositive or AQP-4 Ab and MOG-Ab dual seronegative cases showed poor or no response to steroid pulse therapy, and needed other adjunct therapies such as plasma exchange or immunoadsorption to rescue their vision (7–10). However, if plasma exchange or immunoadsorption therapy is not available, for example, because of plasma shortage, unaffordable expenses, or allergic reaction, monoclonal antibody such as rituximab and satralizumab is an alternative for adjunct therapy for the refractory DON and NMOSD (15, 16).
Interleukin-6 (IL-6) is one of the most important pro-inflammatory cytokines, and can facilitate the production of the acute-phase inflammatory proteins, the maturation of B cells and production of antibodies, as well as the maturation of cytotoxic T cells. It is reported that IL-6 plays an important role in the pathogenesis of NMOSD, and several studies revealed that serum IL-6 level significantly increased in NMOSD cases during relapse, and that cerebrospinal fluid IL-6 level significantly increased in an acute disseminated encephalomyelitis case at its initial onset (17, 18). Satralizumab is a humanized monoclonal antibody specific for IL-6 receptor, and has been approved to significantly reduce the relapse rate in AQP4-Ab positive NMOSD cases in several clinical trials (15, 16, 19). However, the efficiency of satralizumab therapy in AQP4- Ab seronegative NMOSD cases is still under discussion. A phase 3 clinical trial revealed that satralizumab monotherapy could not decrease the relapse rate in AQP4- Ab seronegative NMOSD cases (19).
To the best of our knowledge, this is the first case report of satralizumab therapy for the AQP-4 Ab and MOG-Ab dual seronegative isolated DON. Findings in this case indicate that satralizumab may be a safe and efficient adjunct therapy which can be used in the acute stage of the refractory DON which shows poor or no response to steroid pulse therapy, even though the DON is AQP-4 Ab and MOG-Ab dual seronegative. However, a large-scaled randomized control study is needed to further evaluate the necessity and efficacy of satralizumab in acute-staged DON therapy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Second Affiliated Hospital of Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sridhar G, Tian F, Forshee R, Kulldorff M, Selvam N, Sutherland A, et al. Evaluation of optic neuritis following human papillomavirus vaccination. Hum Vaccin Immunother. (2017) 13:1705–13. doi: 10.1080/21645515.2017.1310788
2. Mouchet J, Salvo F, Raschi E, Poluzzi E, Antonazzo IC, De Ponti F, et al. Human papillomavirus vaccine and demyelinating diseases-A systematic review and meta-analysis. Pharmacol Res. (2018) 132:108–18. doi: 10.1016/j.phrs.2018.04.007
3. Liu EY, Smith LM, Ellis AK, Whitaker H, Law B, Kwong JC, et al. Quadrivalent human papillomavirus vaccination in girls and the risk of autoimmune disorders: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. (2018) 190:E648–55. doi: 10.1503/cmaj.170871
4. Grimaldi-Bensouda L, Papeix C, Hamon Y, Benichou J, Abenhaim L. Association between vaccination and the risk of central demyelination: results from a case-referent study. J Neurol. (2023) 270:4678–86. doi: 10.1007/s00415-023-11822-y
5. Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. (2014) 13:215–24. doi: 10.1016/j.autrev.2013.10.003
6. Liu Z, Zhang L, Yang Y, Meng R, Fang T, Dong Y, et al. Active surveillance of adverse events following human papillomavirus vaccination: feasibility pilot study based on the Regional Health Care Information Platform in the city of Ningbo, China. J Med Internet Res. (2020) 22:e17446. doi: 10.2196/17446
7. Chang H, Lee HL, Yeo M, Kim JS, Shin DI, Lee SS, et al. Recurrent optic neuritis and neuromyelitis optica-IgG following first and second human papillomavirus vaccinations. Clin Neurol Neurosurg. (2016) 144:126–8. doi: 10.1016/j.clineuro.2016.03.023
8. Michael NDB, Tuan Jaffar TN, Hussein A, Wan Hitam WH. Simultaneous bilateral optic neuritis following human papillomavirus vaccination in a young child. Cureus. (2018) 10:e3352. doi: 10.7759/cureus.3352
9. DiMario FJ Jr, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papilloma virus. J Child Neurol. (2010) 25:321–7. doi: 10.1177/0883073809349322
10. Anamnart C, Tisavipat N, Owattanapanich W, Apiwattanakul M, Savangned P, Prayoonwiwat N, et al. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: Case report and systematic review. Mult Scler Relat Disord. (2022) 58:103414. doi: 10.1016/j.msard.2021.103414
11. Du ZC, Lai CX, Qin YJ. Bilateral doughnut-shaped macular edema in a pregnant woman with neuromyelitis optica spectrum disorders. Am J Ophthalmol. (2024) 262:e1–2. doi: 10.1016/j.ajo.2024.03.013
12. Wu J, Zhang W, Liang S, Qin YL, Fan WQ. Pregnancy-associated neuromyelitis optical spectrum disorder combined with primary Sjögren’s syndrome: A critical illness case report. Beijing Da Xue Xue Bao Yi Xue Ban. (2023) 55:1118–24. doi: 10.19723/j.issn.1671-167X.2023.06.025
13. Sun CB, Liu Z. Incidence and clinical characteristics of covid-19 ophthalmopathy following the termination of Dynamic Zero COVID-19 Strategy in China. Neuro-Ophthalmology. (2024) 48(5):328-37. doi: 10.1080/01658107.2024.232511
14. Sun CB. Prevalence of serum MOG antibody and AQP4 antibody in optic neuritis after SARS-CoV-2 infection. Front Immunol. (2023) 14:129651. doi: 10.3389/fimmu.2023.129651
15. Li X, Wu W, Zeng Y, Wu W, Hou C, Zhu H, et al. Satralizumab as an adjunct treatment in refractory pediatric AQP4-antibody-positive neuromyelitis optica spectrum disorder: a case report. Front Immunol. (2023) 14:1257955. doi: 10.3389/fimmu.2023.1257955
16. Yoshida T, Watanabe O, Nomura M, Yoshimoto Y, Maki Y, Takashima H. Neuromyelitis optica spectrum disorder safely and successfully treated with satralizumab during pregnancy and breastfeeding: a case report. Front Neurol. (2023) 14:1322412. doi: 10.3389/fneur.2023.1322412
17. Haramati A, Rechtman A, Zveik O, Haham N, Brill L, Vaknin-Dembinsky A. IL-6 as a marker for NMOSD disease activity. J Neuroimmunol. (2022) 370:577925. doi: 10.1016/j.jneuroim.2022.577925
18. Virupakshaiah A, Moseley CE, Elicegui S, Gerwitz LM, Spencer CM, George E, et al. Life-threatening MOG antibody-associated hemorrhagic ADEM with elevated CSF IL-6. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200243. doi: 10.1212/NXI.0000000000200243
19. Traboulsee A, Greenberg BM, Bennett JL, Szczechowski L, Fox E, Shkrobot S, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. (2020) 19:402–12. doi: 10.1016/S1474-4422(20)30078-8
Keywords: human papilloma virus, bivalent HPV vaccine, optic neuritis, steroid pulse therapy, satralizumab
Citation: Sun C (2024) Case report: Satralizumab therapy for bilateral refractory optic neuritis following the first dose of bivalent human papilloma virus vaccine. Front. Immunol. 15:1499045. doi: 10.3389/fimmu.2024.1499045
Received: 20 September 2024; Accepted: 31 October 2024;
Published: 19 November 2024.
Edited by:
Honghao Wang, Guangzhou First People’s Hospital, ChinaReviewed by:
Indra Tri Mahayana, Gadjah Mada University, IndonesiaYouming Long, The Second Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2024 Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanbin Sun, c3Vuc2hpbmUyMDEyQHpqdS5lZHUuY24=
†ORCID: Chuanbin Sun, orcid.org/0000-0002-2767-6858
 Chuanbin Sun
Chuanbin Sun