- 1Respiratory Disease Center, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Integrative Traditional Chinese and Western Medicine, Graduate School, Beijing University of Chinese Medicine, Beijing, China
This case report highlights the immune-related adverse events (irAEs) that occurred during the treatment of esophageal cancer with Tislelizumab and discusses management strategies, indicating that photodynamic therapy (PDT) may be an optimal adjunctive treatment option. Following Tislelizumab therapy, the patient demonstrated significant tumor reduction; however, subsequent irAEs related to immunotherapy emerged, including eyelid muscle weakness and myocardial and skeletal muscle injury. Methylprednisolone successfully alleviated these symptoms, with early intervention being crucial for controlling irAEs. The patient then underwent PDT, which not only further helped manage irAEs but also inhibited tumor progression. This case underscores the specific adverse reactions, such as eyelid ptosis, skeletal muscle, and myocardial damage associated with Tislelizumab, and the importance of early corticosteroid intervention. It also emphasizes the significance of PDT as an adjunctive treatment for controlling tumors and alleviating immune-related adverse reactions.
Introduction
Immune checkpoint inhibitors have widespread clinical applications and have demonstrated durable antitumor responses across various cancer types (1). However, these agents can inadvertently activate non-tumor-specific T cells, leading to a range of irAEs affecting multiple organs. Commonly affected systems include the lungs, heart, skin, and gastrointestinal tract (2). Due to the non-specific nature of irAEs, diagnosis, and differentiation in clinical practice can be challenging.
Tislelizumab is a classic immune PD-1 checkpoint inhibitor that plays a significant role in the treatment of various cancers, including lung cancer, esophageal cancer, and hepatocellular carcinoma. For Tislelizumab, common immune-related adverse reactions include pneumonia, hypothyroidism, arthralgia, and vitiligo (3). Cardiac, ocular muscle neurological, and skeletal muscle toxicities are relatively rare, and there have been no reported cases of ocular muscle damage.
When severe adverse reactions occur with immune checkpoint inhibitors, the next steps in treatment are often controversial. Given the significant synergistic effects of PDT with anti-PD-1 agents, which can enhance the efficacy of PD-1 therapy and potentially inhibit the progression of adverse reactions, PDT emerges as a critical option for managing severe irAEs following immunotherapy.
We report a case of a 79-year-old female patient with advanced mid-lower esophageal cancer who experienced significant tumor shrinkage following treatment with Tislelizumab. However, after the first cycle of Tislelizumab, the patient developed pneumonia, and after the second cycle, she experienced multi-organ dysfunction, including ptosis, proximal muscle damage, and myocardial injury. Following discontinuation of the PD-1 inhibitor and administration of systemic corticosteroids, the patient’s symptoms improved. Subsequently, PDT was administered for esophageal cancer, leading to significant tumor control and complete resolution of adverse events.
Case description
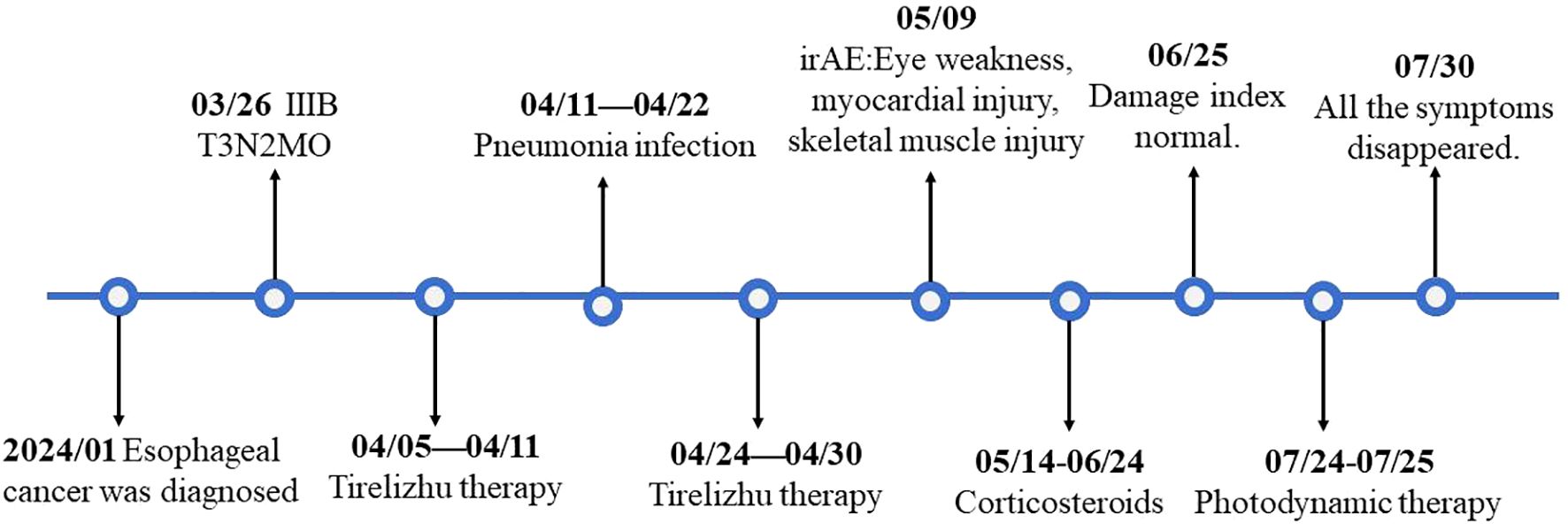
A 79-year-old Asian female patient with a history of over 30 years of hypertension, type 2 diabetes, and coronary heart disease presented to our department on March 26, 2024. The patient had been diagnosed with esophageal squamous cell carcinoma following an esophagogastroduodenoscopy and biopsy performed in January 2024 at another hospital. A subsequent computed tomography (CT) scan indicated no metastasis to other sites. Positron emission tomography-computed tomography (PET-CT) showed no metastatic disease(Figure 1A), and the surgical staging was determined to be stage IIIB (cT3N2M0). Upon evaluation, the patient exhibited significant dysphagia, dizziness, and fatigue, and had been bedridden for a long time, with a Karnofsky Performance Status (KPS) score of 40. Immunohistochemical analysis revealed CK5/6 and P63 positivity. Due to the absence of surgical indications and the patient’s inability to meet the baseline requirements for chemotherapy, she received monotherapy with the immune checkpoint inhibitor Tislelizumab (200 mg Q21, administered via intravenous infusion). The first cycle of treatment began on April 11, 2024, with injections administered every 21 days. After treatment, the patient developed a significant fever due to a pulmonary infection. The symptoms resolved following intravenous administration of cefoperazone-sulbactam and meropenem. Although the patient was eligible for adjuvant chemotherapy, it was not administered due to the patient’s low KPS score. On April 24, 2024, after the fever subsided, the patient received a second cycle of Tislelizumab (200 mg, intravenous infusion), during which no significant abnormalities were observed (Figure 2).
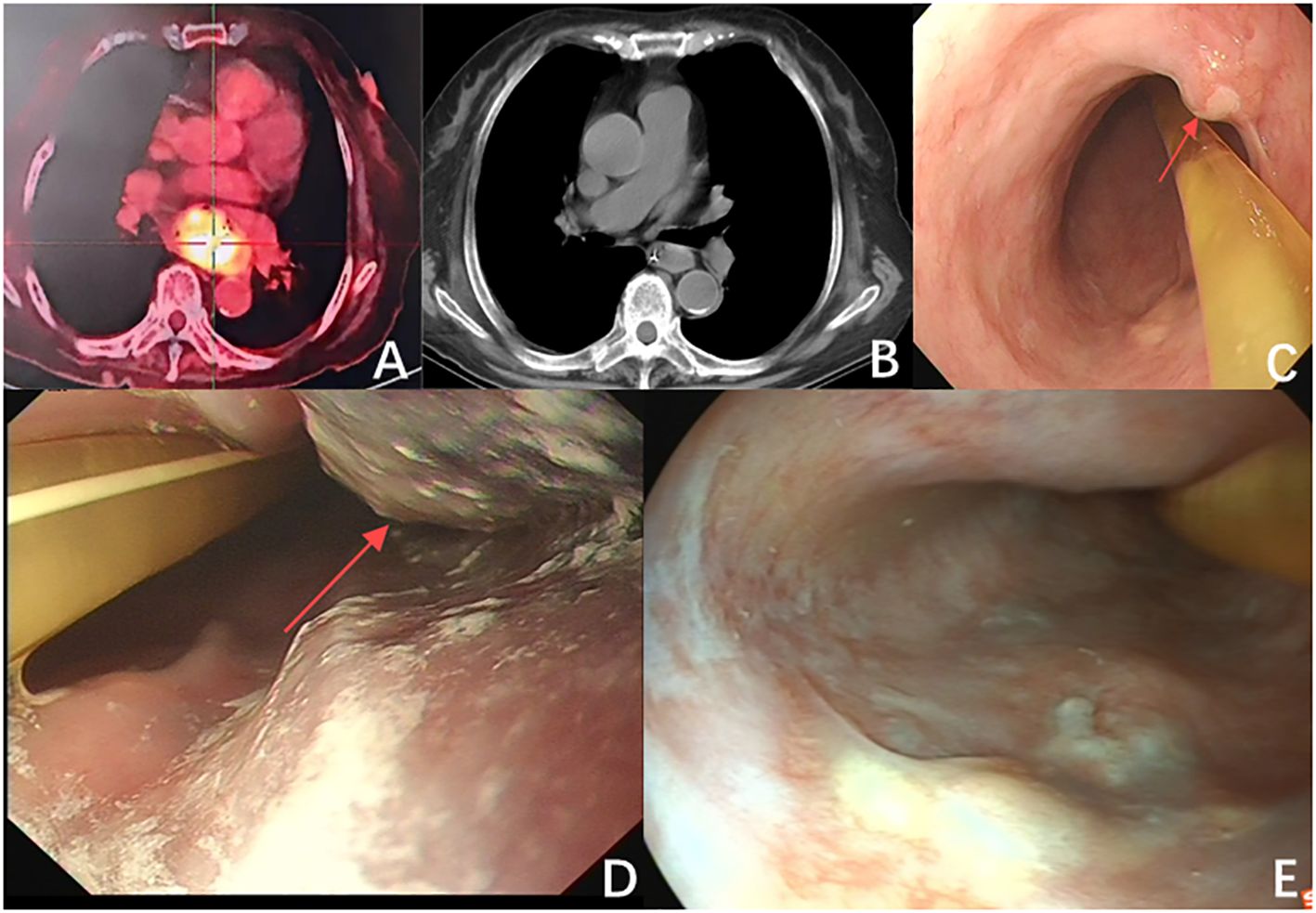
Figure 1. (A) The largest cross-section of the tumor before immunotherapy measures 5.5 cm × 3.5 cm (red arrow). (B) The tumor size is significantly reduced after immunotherapy (red arrow). (C) Endoscopic examination after immunotherapy showed a significant reduction in tumor size, with a diameter of approximately 1.2 cm (red arrow). (D) The tumor after the first photodynamic therapy (red arrow). (E) The follow-up two months post-treatment indicated that the patient’s tumor showed no significant progression, and there was notable scarring following photodynamic therapy.
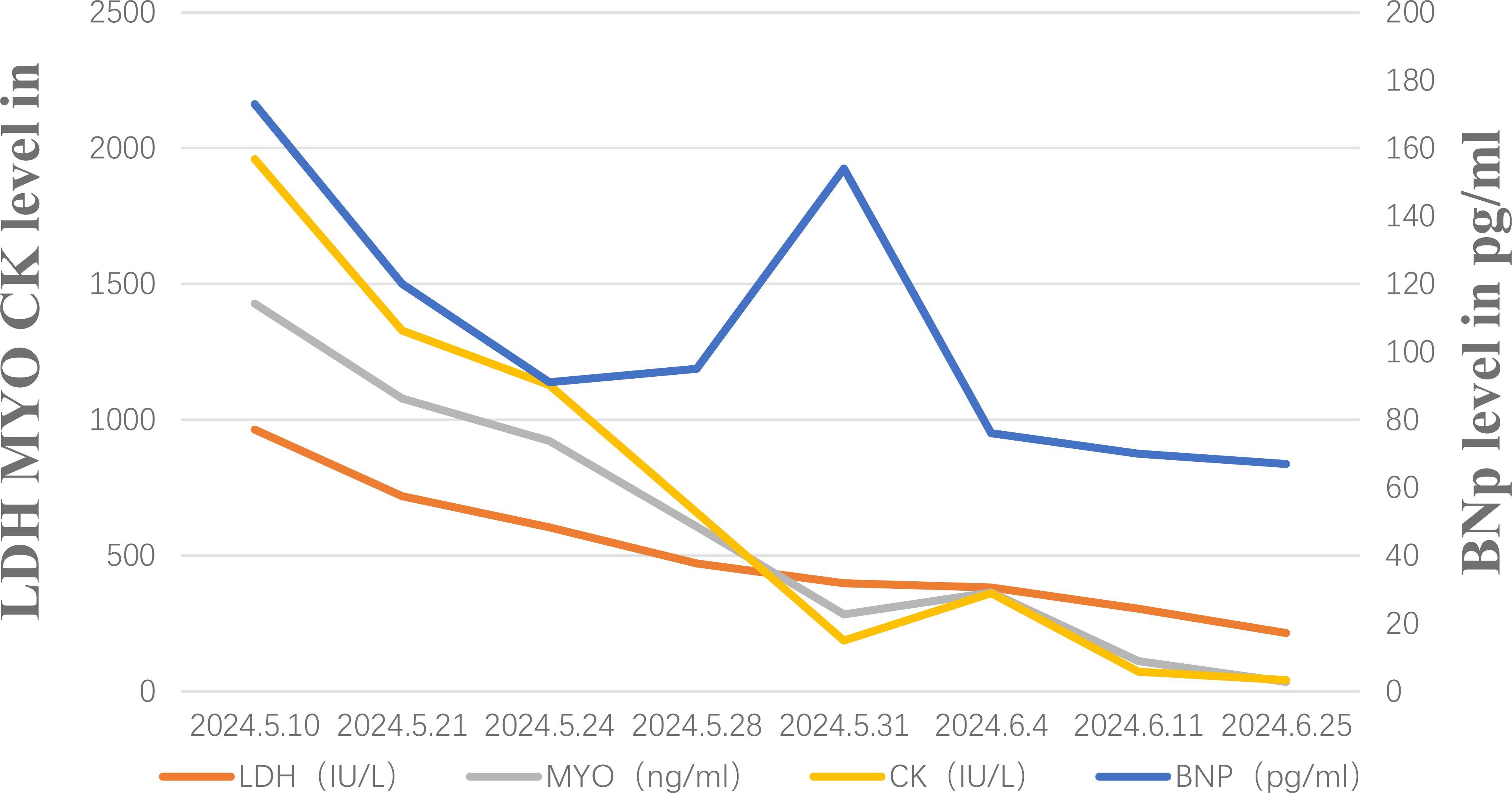
Unfortunately, after discharge, the patient experienced severe bilateral eyelid ptosis (Figure 3A), recurrent palpitations, and upper limb proximal muscle pain. On May 9, 2024, the patient was readmitted to the hospital. Subsequent examinations included an MRI of the brain, as well as assessments of the eyes and heart. The MRI showed no significant changes, the ophthalmological examination indicated no notable vision abnormalities, and the cardiac ultrasound did not reveal any significant worsening of the condition. Since ocular changes and cardiac damage are not common adverse reactions associated with PD-1 inhibitors, differentiation was particularly challenging (4). Further investigations included assessments of the endocrine, circulatory, respiratory systems, and muscle damage. Laboratory results showed elevated MYO: 1428 μg/L (normal range 10-70), BNP: 173 pg/ml (normal range 28.3–37.5), CK: 1127.6 U/L (normal range 40-200), LDH: 604.6 U/L (normal range 109-245), and positive Titin antibody. Electromyography indicated decreased compound muscle action potentials on both sides and peripheral nerve damage in both lower limbs. Given the patient’s severe metabolic disease, which is considered a high-risk factor for irAEs, and taking into account the patient’s medication and medical history, we are inclined to diagnose the irAE as grade 4.
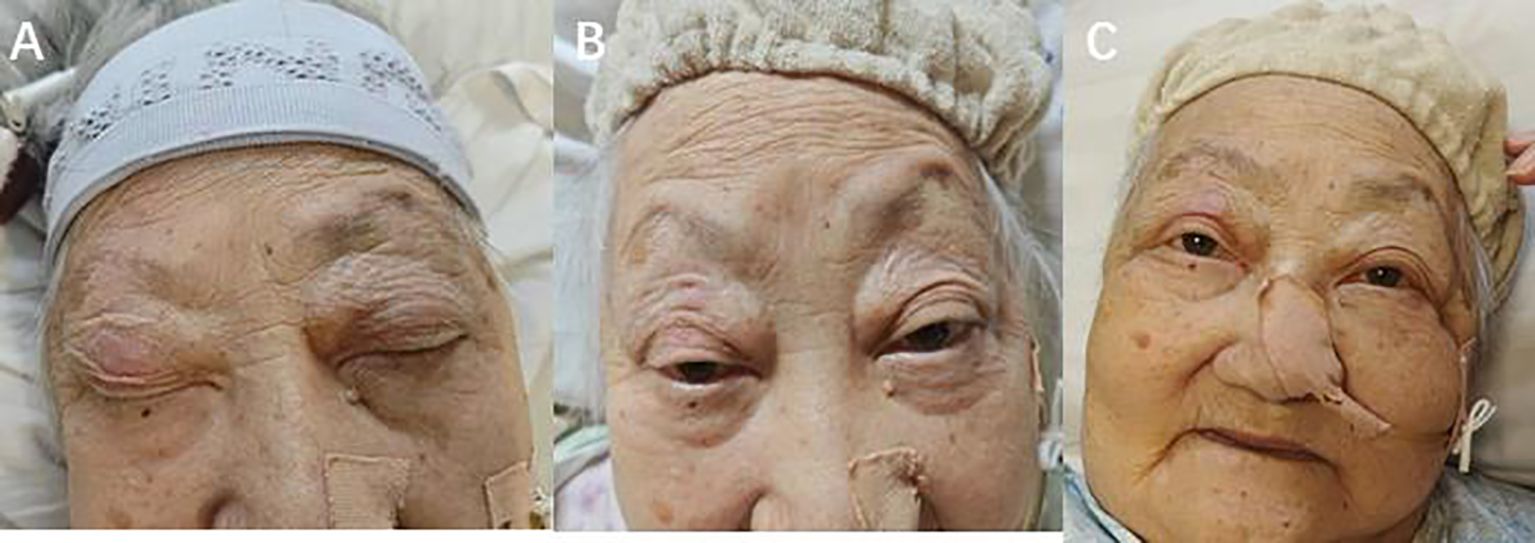
Figure 3. (A) The patient suffered from an immune adverse reaction and eyelid weakness. (B) The patient’s eyelids returned to normal after photodynamic therapy and corticosteroid therapy (C) 2 months after photodynamic therapy, the patient did not experience any adverse reactions.
The patient started intravenous infusions of 40 mg methylprednisolone sodium succinate for 12 days. After this treatment, the ability to open and close the left eyelid gradually improved, and muscle pain subsided. The regimen was then switched to oral methylprednisolone at a dose of 32 mg, with a taper of 4 mg per week. By June 25, most clinical indicators had returned to normal (Figure 4), although there was still some residual muscle weakness in the right eyelid. Following two cycles of Tislelizumab, the tumor showed significant shrinkage compared to initial measurements (Figure 1B). However, due to the patient’s intolerance to both chemotherapy and immunotherapy and considering her weakened immune status, localized PDT was selected as the next treatment option (5).
We performed a gastroscopy, which revealed a tumor approximately 1.2 cm in size (Figure 1C). After evaluation, we administered Hiporfin at a dose of 2 mg/kg. Forty-eight hours later, on July 24 and 25, photodynamic therapy (Shenzhen Laser Medical Technology, PDT630-A) was performed (Figure 1D). Using a 3 cm optical fiber, light irradiation was applied with an energy density of 100 W/cm², delivering 150 J of energy. The duration of irradiation was 900 seconds (630 J) and 1200 seconds (945 J), respectively. Five days later, the patient’s ptosis completely resolved (Figure 3B), and all adverse reactions disappeared. We conducted a two-month follow-up with the patient, during which no adverse reactions were observed. After this period, an endoscopic examination revealed no significant tumor progression, and the patient’s condition showed favorable recovery (Figures 1E, 3C).
Discussion
We describe the case of a patient with advanced esophageal cancer who developed irAEs after receiving two cycles of immune checkpoint inhibitor (ICI) monotherapy with Tislelizumab. While the tumor size was significantly reduced, the patient developed rare bilateral eyelid muscle weakness following the second cycle of ICI treatment, accompanied by cardiac and skeletal muscle injury.
The exact biological mechanisms behind the site and severity of irAE damage with immune checkpoint inhibitors (ICIs) are not fully understood, resulting in significant variability in irAE presentations.PD-1 inhibitors, commonly used among ICIs, tend to have adverse reactions primarily affecting cardiovascular and pulmonary functions, in contrast to CTLA-4 inhibitors, which mainly cause endocrine-related adverse effects (6). Previous literature has documented common irAEs associated with PD-1 inhibitors, such as pneumonia and arthralgia, which aligns with the findings of this study (2). Notably, the patient also experienced myocardial damage, skeletal muscle injury, and eyelid muscle weakness. The incidence of myocardial damage is reported to be <1% (7), and skeletal muscle injury is often associated with myocardial damage, with an incidence of <4% (8). There are few reports documenting adverse reactions such as corneal ulcers associated with PD-1 inhibitors (9), but there have been no reports to date regarding eyelid muscle weakness caused by Tislelizumab.
When PD-1 inhibitors are used, the inhibition of the PD-1/PD-L1 pathway results in the suppression of regulatory T cells (Tregs) and an increase in cytokines (such as TNF, IFN-γ, IL-2, IL-17, CXCL10), which can significantly activate and proliferate T cells, leading to a risk of immune overactivation (2). Compared to other PD-1 inhibitors, Tislelizumab possesses multiple mutations in its Fc hinge region, preventing binding to FcγR and thereby inhibiting the phagocytic activation of macrophages, which reduces T cell clearance (10). This allows for maximal preservation of T cell immunity, but it also results in more severe irAEs. Due to the low tumor-selectivity of T cell immunity activated by PD-1 inhibitors, there is potential for cross-reactivity with normal tissues, resulting in the misidentification of normal tissues as antigens and subsequent immune attacks on them. Prior studies have identified a high frequency of T cell receptor sequences shared between skeletal muscle, myocardium, and tumor infiltrates, suggesting the presence of highly variable complementary determining region 3 (CDR3) sequences (11). Furthermore, the substantial release of cytokines contributes to widespread inflammatory responses, which may also be potential causes of irAEs. However, given the rarity of ocular adverse reactions and the lack of clarity in relevant research, it remains uncertain whether there is a shared receptor influence on ocular irAEs.
Given the unclear mechanisms behind multi-organ irAEs, and the absence of consensus guidelines for managing such cases despite established guidelines for single-organ irAEs (12), treatment largely relies on clinical experience. Early use of corticosteroids is crucial for achieving favorable outcomes in patients. Although immunoglobulin and plasma exchange are also recommended in the guidelines (13), the patients declined these treatments for economic reasons. After administering corticosteroids, their symptoms significantly improved, leading us to discontinue the use of other immunosuppressants, such as cyclophosphamide, for managing irAEs. However, due to the patient’s low KPS score and severe adverse reactions, recovery has been slow, presenting challenges for subsequent esophageal cancer treatment. After re-administration of ICIs, the estimated recurrence rate of adverse reactions is 28.8% (14), making further immune activation therapy deemed unsuitable for this patient. Considering the patient’s physical tolerance and personal wishes, paclitaxel and trastuzumab should only be administered after irAEs have completely resolved and the patient’s condition has improved, making local photodynamic therapy a more feasible option.
Photodynamic therapy may serve as an important adjunctive treatment following ICIs. First, the combination of PD-1 inhibitors and PDT can significantly enhance the immune response against tumors. Second, PDT may alleviate the side effects associated with immunosuppressants. Compared to other adjunctive therapies, photodynamic therapy is more suitable for the current patients. Existing clinical data indicate that photodynamic therapy can significantly inhibit tumor progression and is one of the important palliative treatments for advanced stages of the disease, often serving as adjuvant therapy following radiotherapy, chemotherapy, and immunotherapy (21–24). Recent foundational studies have demonstrated the efficacy of combining ICIs with PDT (18, 20, 25).PDT enhances local dendritic cell cross-presentation of tumor antigens and induces immunogenic cell death (ICD) in tumor cells, thereby mediating CD8+ T cell immune responses (15). This activation is typically suppressed by regulatory T cells (Tregs) (16). The use of ICIs inhibits Treg cells, further enhancing the immune response facilitated by PDT. On the other hand, various photosensitizers used in photodynamic therapy can increase tumor sensitivity to PD-L1 antibody checkpoint inhibition, thereby enhancing the immune response against the tumor (17, 18). The synergistic mechanisms of PDT and ICIs are related to the enrichment of immune cells and the formation of immune memory. Existing studies suggest that their combination may promote immune cell infiltration by altering the expression of ALAS2, ITGA10, and ADAM12 (19). This activation of tumor immune memory can lead to the destruction of tumor cells while simultaneously inhibiting distal tumor growth and preventing metastasis and recurrence (20).
Although PDT does not alter the cross-reactivity of normal tissues due to T cell low immune selectivity, it enhances T cell immune selectivity and directs T cells towards tumor sites, providing potential for controlling irAEs. By inducing immunogenic cell death (ICD) in tumor cells, PDT activates tumor-specific immunity and improves T cell selectivity (26), reducing attacks on normal cells. At the same time, PDT induces acute inflammatory responses, leading to the rapid release of pro-inflammatory mediators and chemokines from cancer cell membranes, damaged endothelial cells, and tumor stroma, including chemokines MIP-2 and KC, as well as pro-inflammatory mediators such as IL-1, IL-6, and TNF. These factors guide neutrophil recruitment to the tumor site and also direct T cells toward inflammatory and tumor regions (27, 28). Some patients may even exhibit a significant decrease in systemic T cell counts following recruitment, leading some studies to suggest that PDT can inhibit excessive systemic immune responses (29). Recent reports indicate that various carriers, including porphyrin-based metal-organic frameworks (pMOFs) and lipid-based (LP) micelle nanocarriers, can effectively reduce irAEs associated with ICIs and reverse resistance to immunotherapy (10, 30, 31). In summary, PDT holds promise as an important adjunctive approach in the treatment of tumors with ICIs.
This paper reports a case of adverse reactions caused by monotherapy with Tislelizumab. The patient received two cycles of Tislelizumab monotherapy. After the second round of ICI treatment, the patient developed rare bilateral eyelid muscle weakness, accompanied by signs of cardiac and skeletal muscle injury. This presents as a rare case of bilateral eyelid muscle weakness. Due to the complexity of malignant tumors, Tislelizumab-induced irAEs are difficult to identify in clinical practice. Early administration of corticosteroids has demonstrated good efficacy, potentially helping to prevent the rapid progression of multi-organ irAEs. Currently, treatment protocols for ICI adverse reactions remain controversial, particularly in conjunction with subsequent tumor therapies. Photodynamic therapy may represent a promising option, as it not only synergistically enhances the efficacy of immunosuppressants in significantly inhibiting tumors but also provides beneficial effects in alleviating ICI-induced adverse reactions. However, this study has a relatively short follow-up period and is limited by its single-case report nature. Future clinical data will be needed to further validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Dongzhimen Hospital of Beijing University of Chinese Medicine (Project No: 2024DZMEC-443-01). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LL: Investigation, Methodology, Writing – original draft. LB: Data curation, Writing – review & editing. NK: Data curation, Project administration, Writing – review & editing. YY: Data curation, Formal analysis, Writing – review & editing. HZ: Funding acquisition, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study did not receive any funding during the research process, but Leimai Company is willing to cover the Article Processing Fee. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Choi YY, Kim H, Shin S-J, Kim HY, Lee J, Yang H-K, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. (2019) 270:309–16. doi: 10.1097/SLA.0000000000002803
2. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
3. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
4. Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk factors and biomarkers for immune-related adverse events: A practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. (2022) 13:779691. doi: 10.3389/fimmu.2022.779691
5. Yano T, Wang KK. Photodynamic therapy for gastrointestinal cancer. Photochem Photobiol. (2020) 96:517–23. doi: 10.1111/php.13206
6. Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. (2007) 116:2062–71. doi: 10.1161/CIRCULATIONAHA.107.709360
7. Patel RP, Parikh R, Gunturu KS, Tariq RZ, Dani SS, Ganatra S, et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Oncol Rep. (2021) 23:79. doi: 10.1007/s11912-021-01070-6
8. Anquetil C, Salem J-E, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune checkpoint inhibitor-associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation. (2018) 138:743–5. doi: 10.1161/CIRCULATIONAHA.118.035898
9. Xiang X, Lin W, Guan X, Zhou B, Yuan Y, Silva D, et al. Corneal ulcer development due to sintilimab-anlotinib combination therapy-induced dry eye: a case report. Transl Cancer Res. (2024) 13:2571–9. doi: 10.21037/tcr-23-1952
10. Zhang L, Geng Z, Hao B, Geng Q. Tislelizumab: A modified anti-tumor programmed death receptor 1 antibody. Cancer Control. (2022) 29:10732748221111296. doi: 10.1177/10732748221111296
11. Cheng F, Loscalzo J. Autoimmune cardiotoxicity of cancer immunotherapy. Trends Immunol. (2017) 38:77–8. doi: 10.1016/j.it.2016.11.007
12. Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. (2017) 5:95. doi: 10.1186/s40425-017-0300-z
13. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9:e002435. doi: 10.1136/jitc-2021-002435
14. Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. (2020) 6:865–71. doi: 10.1001/jamaoncol.2020.0726
15. Canti G, Calastretti A, Bevilacqua A, Reddi E, Palumbo G, Nicolin A. Combination of photodynamic therapy + immunotherapy + chemotherapy in murine leukiemia. Neoplasma. (2010) 57:184–8. doi: 10.4149/neo_2010_02_184
16. Kleinovink JW, Fransen MF, Löwik CW, Ossendorp F. Photodynamic-immune checkpoint therapy eradicates local and distant tumors by CD8+ T cells. Cancer Immunol Res. (2017) 5:832–8. doi: 10.1158/2326-6066.CIR-17-0055
17. Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic therapy mediated by nontoxic core-shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc. (2016) 138:16686–95. doi: 10.1021/jacs.6b09538
18. Zhou Y, Zhang W, Wang B, Wang P, Li D, Cao T, et al. Mitochondria-targeted photodynamic therapy triggers GSDME-mediated pyroptosis and sensitizes anti-PD-1 therapy in colorectal cancer. J Immunother Cancer. (2024) 12:e008054. doi: 10.1136/jitc-2023-008054
19. Gong B, Wang L, Zhang H, Wang Q, Li W. Amplifying T cell-mediated antitumor immune responses in nonsmall cell lung cancer through photodynamic therapy and anti-PD1. Cell Biochem Funct. (2024) 42:e3925. doi: 10.1002/cbf.3925
20. Lou J, Aragaki M, Bernards N, Chee T, Gregor A, Hiraishi Y, et al. Repeated photodynamic therapy mediates the abscopal effect through multiple innate and adaptive immune responses with and without immune checkpoint therapy. Biomaterials. (2023) 292:121918. doi: 10.1016/j.biomaterials.2022.121918
21. Luketich JD, Christie NA, Buenaventura PO, Weigel TL, Keenan RJ, Nguyen NT. Endoscopic photodynamic therapy for obstructing esophageal cancer: 77 cases over a 2-year period. Surg Endosc. (2000) 14:653–7. doi: 10.1007/s004640000144
22. Wang X-Y, Maswikiti EP, Zhu J-Y, Ma Y-L, Zheng P, Yu Y, et al. Photodynamic therapy combined with immunotherapy for an advanced esophageal cancer with an obstruction post metal stent implantation: A case report and literature review. Photodiagnosis Photodyn Ther. (2022) 37:102671. doi: 10.1016/j.pdpdt.2021.102671
23. Rupinski M, Zagorowicz E, Regula J, Fijuth J, Kraszewska E, Polkowski M, et al. Randomized comparison of three palliative regimens including brachytherapy, photodynamic therapy, and APC in patients with Malignant dysphagia (CONSORT 1a) (Revised II). Am J Gastroenterol. (2011) 106:1612–20. doi: 10.1038/ajg.2011.178
24. Yano T, Muto M, Minashi K, Iwasaki J, Kojima T, Fuse N, et al. Photodynamic therapy as salvage treatment for local failure after chemoradiotherapy in patients with esophageal squamous cell carcinoma: a phase II study. Int J Cancer. (2012) 131:1228–34. doi: 10.1002/ijc.27320
25. Hao Y, Chung CK, Gu Z, Schomann T, Dong X, Veld RVHI‘t, et al. Combinatorial therapeutic approaches of photodynamic therapy and immune checkpoint blockade for colon cancer treatment. Mol BioMed. (2022) 3:26. doi: 10.1186/s43556-022-00086-z
26. Brackett CM, Gollnick SO. Photodynamic therapy enhancement of anti-tumor immunity. Photochem Photobiol Sci. (2011) 10:649–52. doi: 10.1039/c0pp00354a
27. New JY, Li B, Koh WP, Ng HK, Tan SY, Yap EH, et al. T cell infiltration and chemokine expression: relevance to the disease localization in murine graft-versus-host disease. Bone Marrow Transplant. (2002) 29:979–86. doi: 10.1038/sj.bmt.1703563
28. Jalili A, Makowski M, Switaj T, Nowis D, Wilczynski GM, Wilczek E, et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin Cancer Res. (2004) 10:4498–508. doi: 10.1158/1078-0432.CCR-04-0367
29. Jolles CJ, Ott MJ, Straight RC, Lynch DH. Systemic immunosuppression induced by peritoneal photodynamic therapy. Am J Obstet Gynecol. (1988) 158:1446–53. doi: 10.1016/0002-9378(88)90380-8
30. Chen Q, He Y, Wang Y, Li C, Zhang Y, Guo Q, et al. Penetrable nanoplatform for “Cold” Tumor immune microenvironment reeducation. Adv Sci (Weinh). (2020) 7:2000411. doi: 10.1002/advs.202000411
Keywords: esophageal cancer, Tislelizumab, immune-related adverse events, photodynamic therapy, PD-1 inhibitors
Citation: Li L, Bian L, Kou N, Yuan Y and Zou H (2024) Special immune-related adverse events and subsequent photodynamic therapy in tislelizumab treatment for esophageal cancer: a case report. Front. Immunol. 15:1497259. doi: 10.3389/fimmu.2024.1497259
Received: 16 September 2024; Accepted: 07 November 2024;
Published: 25 November 2024.
Edited by:
Mohd Wajid Ali Khan, University of Hail, Saudi ArabiaReviewed by:
Shahper Nazeer Khan, University of Manitoba, CanadaAzharuddin Sajid Syed Khaja, University of Hail, Saudi Arabia
Copyright © 2024 Li, Bian, Kou, Yuan and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heng Zou, em91aGFuZzc1QDEyNi5jb20=
 Longzhao Li
Longzhao Li Lingjie Bian
Lingjie Bian Na Kou1
Na Kou1