- 1Department of Dermatology, Venereology and Allergology, University Hospital Würzburg, Würzburg, Germany
- 2Department of Dermatology, University Hospital, Goethe University Frankfurt, Frankfurt, Germany
Background: Merkel cell carcinoma (MCC) is a rare but highly aggressive cutaneous malignancy. Immune checkpoint inhibition (ICI) with PD-(L)1 blockade has significantly improved treatment outcomes in metastatic disease. In patients with primary resistance to PD-(L)1 inhibition, a high overall response rate (ORR) of 50% to later-line ipilimumab plus nivolumab (IPI/NIVO) has been demonstrated. However, clinical data on patients with progression after an initial response to IPI/NIVO are still lacking.
Methods: Clinical data of three metastatic MCC patients who were re-exposed to IPI/NIVO after progression were retrospectively evaluated.
Results: Two of the three patients showed primary resistance to avelumab with progressive disease, while one patient showed complete response (according to RECIST V.1.1). All three patients received combined ICI with IPI/NIVO as subsequent therapy, resulting in an ORR of ∼ 67%. However, all three patients progressed during follow-up and were re-exposed to IPI/NIVO. With a follow-up period ranging from 6.5 to 37.1 months, no PFS event has been detected. ORR for IPI/NIVO re-exposition was equal to that of initial IPI/NIVO treatment.
Conclusion: In this retrospective follow-up analysis, we observed a response rate of 67% and long-lasting responses after re-exposition to combined ICI in metastatic MCC patients with progression after initial response or disease control upon their first IPI/NIVO treatment. An important observation from this small analysis is that primary resistance to PD-L1 inhibition may result in a better response to IPI/NIVO.
Introduction
Merkel cell carcinoma (MCC) is a rare but aggressive non-melanoma skin cancer that primarily affects elderly patients (1). For unresectable or metastatic disease, immune checkpoint inhibition (ICI) with the programmed cell death protein 1 (PD-1) inhibitor pembrolizumab or the programmed cell death 1 ligand 1 (PD-L1) inhibitor avelumab have replaced chemotherapy as first-line systemic therapy (2–4). Despite high response rates ranging from 56% to 62%, a significant subset of patients exhibits either primary or acquired resistance to PD-(L)1 blockade (4). For patients with primary resistance to PD-L1 inhibition with avelumab, we recently reported a high overall response rate (ORR) of 50% to combined ICI with ipilimumab plus nivolumab (IPI/NIVO) as subsequent later-line therapy in a multicenter study of the prospective skin cancer registry ADOREG (5). However, median progression-free survival (PFS) was 5.1 months, with 1-year and 2-year PFS landmarks of 42.9% and 26.8%, respectively. These results reflect the urgent clinical need for further subsequent therapy options in case of disease progression or relapse under or after combined ICI with IPI/NIVO. In this retrospective analysis, we report three patients who relapsed or progressed after combined ICI and subsequently were re-exposed to IPI/NIVO.
Patients and methods
Clinical data of three patients with metastatic MCC who progressed during PD-L1 inhibition with avelumab and were later on treated with IPI/NIVO were retrospectively collected. Data were obtained from electronic medical records by chart review. Due to the retrospective nature of the study and the collection of anonymous patient data, informed consent was waived by the Ethics Committee of the University of Würzburg. Two patients had been reported previously and were included with additional follow-up (5, 6). Progression-free survival (PFS) was calculated from the first course of ICI until tumor assessment which showed progressive disease (PD) toward avelumab (PFS1) or the 1st course of IPI/NIVO treatment (PFS2). PFS and overall survival (OS) for IPI/NIVO re-exposition were calculated from the first course of the IPI/NIVO re-exposition to the last tumor assessment or the last consultation (PFS3 and OS).
Results
Patient demographics
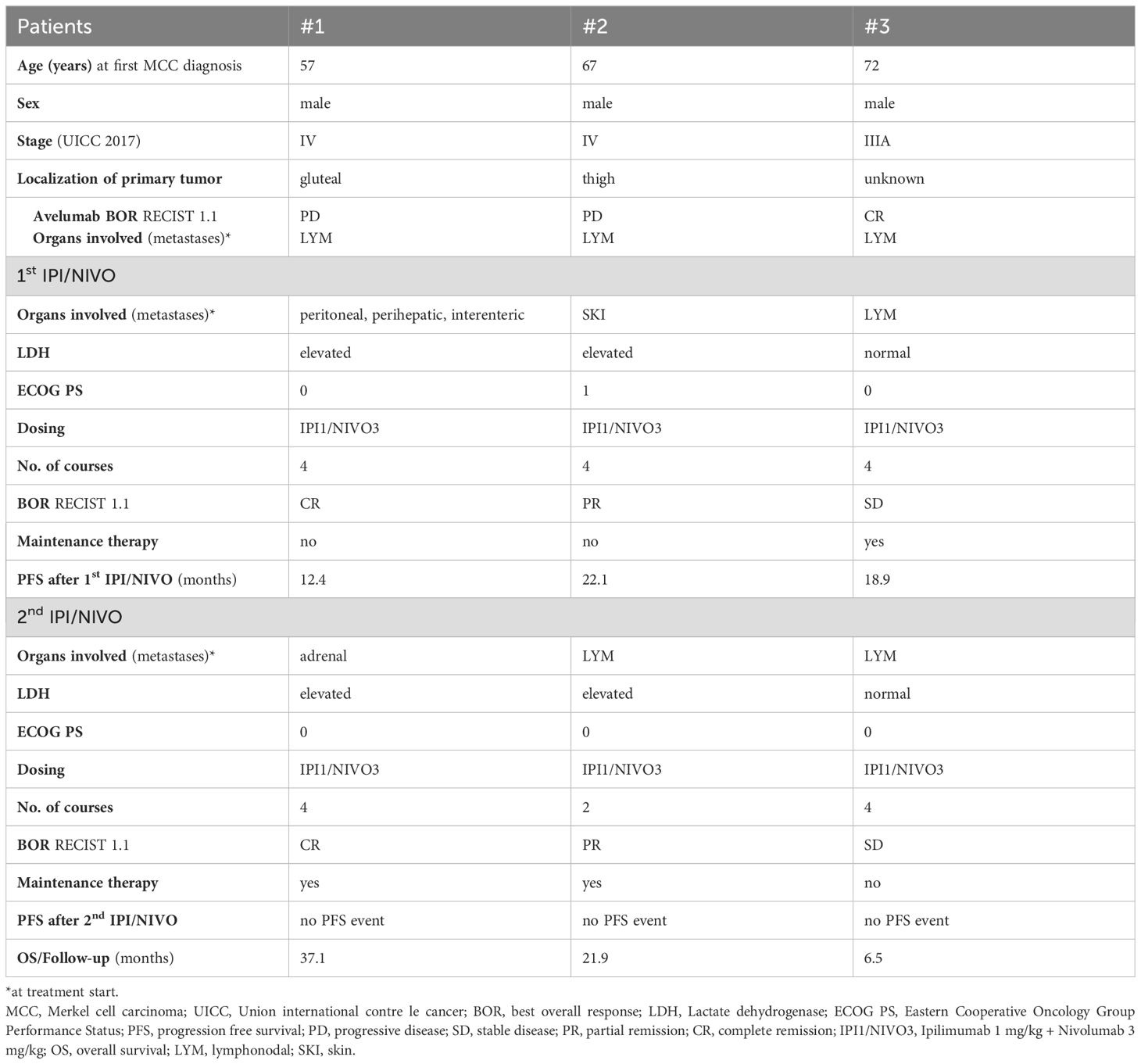
Three male patients with metastatic MCC, stage III-IV (UICC 2017), were included in our analysis. The age at first MCC diagnosis ranged from 57 to 72 years. None of the patients were immunosuppressed, either due to a pre-existing hemato-oncological disease or medication. Patient demographics and outcome are summarized in Table 1.
Pre-therapies
As first-line treatment for metastatic disease, all three patients received the PD-L1 inhibitor avelumab (10 mg per kilogram body weight, mg/kg). The number of courses ranged from two to 21. Two patients showed PD, while one patient showed a complete response (CR) according to RECIST V.1.1 in the first tumor assessment after therapy initiation. The patient who showed complete response to avelumab progressed after 13.4 months while still being on treatment. Treatment-related immune-related adverse events (irAE) of grade II and III (according to Common Toxicity Criteria of Adverse Events, CTCAE 4.03) were observed in only one of three patients. The patient developed pneumonitis grade II and hepatitis grade III after two courses of avelumab and was treated with methylprednisolone.
One patient underwent surgery and radiotherapy after having progressed under avelumab. Another patient received chemotherapy with carboplatin plus etoposide, but showed PD in the first tumor assessment (RECIST V.1.1.) after therapy initiation in between avelumab and IPI/NIVO treatment. The remaining patient received neither systemic therapy nor locoregional treatment in the interim.
All three patients received IPI/NIVO (flipped dosing IPI 1 mg/kg plus NIVO 3 mg/kg) as subsequent later-line therapy. Lactate dehydrogenase (LDH) was elevated in two of three patients. Eastern Cooperative Oncology Group Perfomance Status (ECOG PS) ranged from 0-1. All three patients received four courses of IPI/NIVO. Two out of three patients (1 CR; 1 partial response (PR)) responded to combined IPI/NIVO according to RECIST V.1.1 resulting in an ORR of ∼ 67%. The 3rd patient showed stable disease (SD) and, unlike the two responders, received maintenance therapy with nivolumab 480 mg q4w. No irAE were detected during the 1st IPI/NIVO treatment. PFS2 was 12.2, 22.1 and 18.9 months, respectively.
Of note, the patient who achieved CR after combined IPI/NIVO relapsed early and was initially treated with surgery (PFS 12.2 months).
Ipilimumab plus nivolumab re-exposition
After relapse or progression, all three patients were re-exposed to combined ICI with IPI/NIVO (flipped dosing IPI 1 mg/kg plus NIVO 3 mg/kg). LDH was elevated in two out of three patients and ECOG PS was 0. Two patients received four courses of IPI/NIVO, while one patient received only two courses due to immune-related hepatitis grade III and immune-related pneumonitis grade II. ORR was consistent to the 1st IPI/NIVO treatment with ∼ 67% (1 CR, 1 PR, 1 SD) according to RECIST V.1.1. The two responders received maintenance therapy with nivolumab. In the patient with SD, we opted against maintenance therapy and instead chose radiotherapy for his stable lymph node metastases.
Follow-up
After the IPI/NIVO re-exposition, no PFS event has been detected so far. Follow-up/OS was 37.1, 21.9 and 6.5 months, respectively (data cut-off 30.06.2024).
The course of treatment is shown in Figure 1 for all three patients (Figure 1).
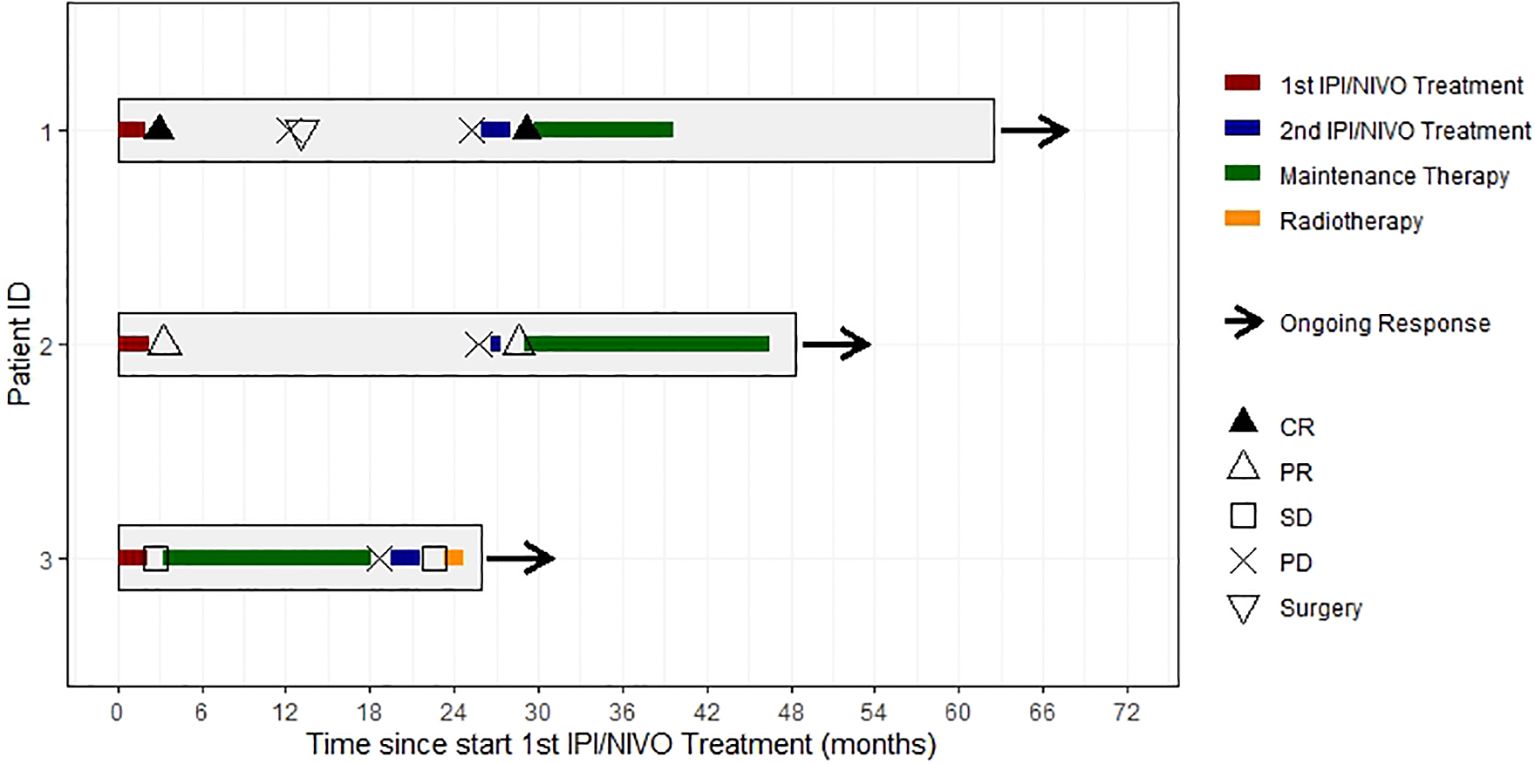
Figure 1. Swimmer’s plot showing individual courses of treatment, response and follow-up of all 3 patients. CR, complete response; PR, partial response; SD, stable disease. PD, progressive disease.
Discussion
In this single-center analysis of three metastatic MCC patients who were re-exposed to combined ICI with IPI/NIVO, we observed a renewed therapy response in two out of three patients. In this small cohort, the ORR to the 2nd IPI/NIVO treatment was equal to the 1st IPI/NIVO treatment. In Europe, PD-L1 inhibition with avelumab is the only approved systemic treatment for locally advanced or metastatic disease. Unfortunately, data on subsequent therapies in case of disease progression or relapse have this far been reported in rather heterogenous and small patient cohorts (7, 8). In a retrospective multicenter analysis of anti-PD-1 refractory melanoma patients, ORR to combined ICI with IPI/NIVO was only 21%, with a one-year overall survival rate of 55% (9). We recently reported a high ORR of 50% with durable responses in a multicenter analysis of 14 metastatic MCC patients with primary resistance to the PD-L1 inhibitor avelumab who were subsequently treated with combined ICI with IPI/NIVO. In this context, primary (not acquired) resistance to first-line PD-L1 (not PD-1) inhibition seems to be crucial for successful subsequent IPI/NIVO treatment since LoPiccolo et al. and Shalhout et al. reported little to no benefit to later-line combined immunotherapy with IPI/NIVO in rather heterogenous cohorts of PD-1/PD-L1 refractory MCC patients (7, 8). Although median OS was not reached, 63.3% of the patients remained alive at the 3-year landmark; median PFS was only 5.1 months, with a relapse in more than 50% of the treated patients at the 1-year landmark (5). Interestingly, 4/7 reported patients (2/4 with maintenance therapy) who primarily responded to IPI/NIVO showed PD or relapsed during follow-up. These data indicate an urgent clinical need not only for subsequent therapies after PD-L1/PD-1 failure but also in later line settings with relapse or PD under or after combined ICI with IPI/NIVO. In our current analysis, PFS after the 1st IPI/NIVO treatment ranged from 12.4 months to 22.1 months, while only one out of three patients received maintenance therapy with nivolumab. Based on the analysis of this small cohort and the literature, a re-challenge with IPI/NIVO appears particularly promising in patients with primary resistance to PD-L1 inhibition and primary deep PR or CR to IPI/NIVO. It remains uncertain if a maintenance therapy with nivolumab could have prevented relapse or disease progression in our two responders after the 1st IPI/NIVO treatment.
Despite the convincing efficacy of later-line IPI/NIVO in metastatic MCC patients who showed primary resistance to PD-L1 inhibition, the recently reported ORR of 100% to 1st line IPI/NIVO in patients with advanced MCC by Kim et al. substantiates considerations to prefer combined ICI with IPI/NIVO to PD-L1 respectively PD-1 monotherapy in the first-line setting (10). However, combined ICI with IPI/NIVO is associated with a high rate of severe irAE (11). In our analysis, IPI/NIVO was surprisingly well tolerated, with only one out of three patients experiencing irAEs of the grades II and III. Interestingly, this patient has tolerated the 1st IPI/NIVO treatment without any irAE and showed the same irAE during re-challenge with IPI/NIVO as during first-line PD-L1 inhibition with avelumab. Notably, all three patients received IPI 1 mg/kg plus NIVO 3 mg/kg (“flipped dose”, chosen according to the CheckMate-358 study, NCT02488759). Since the toxicity and rate of severe irAE of combined ICI seems to depend mainly on the dosing of IPI, using the “flipped dose” in this cohort might explain the low rate of severe irAE at least in part (12).
Our analysis has some limitations. The main limitation is the very small number of patients, as well as the retrospective data collection. Based on the data of three patients, a formal analysis of PFS and OS is not reasonable. Therefore, the course of treatment of our patients was alternatively presented as descriptive analysis (Figure 1).
In conclusion, this retrospective follow-up analysis of metastatic MCC patients who relapsed or progressed during or after later-line combined ICI with IPI/NIVO showed a renewed response, with durable responses to re-exposition with IPI/NIVO, primarily in former IPI/NIVO responders. An important observation from this small analysis is that primary resistance to PD-L1 inhibition with avelumab is likely to result in a better response to IPI/NIVO. To confirm this observation, a prospective randomized trial on the efficacy of IPI/NIVO, possibly also as 1st line therapy in this entity, is desirable.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the University of Würzburg. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the study.
Author contributions
VG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. PS: Formal analysis, Investigation, Resources, Validation, Visualization, Writing – review & editing. MG: Investigation, Resources, Validation, Writing – review & editing. AG: Investigation, Resources, Validation, Writing – review & editing. BS: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This publication was supported by the Open Access Publication Fund of the University of Würzburg. VG is supported by the BZKF Young Scientist Fellowship.
Acknowledgments
VG and PS are supported by TWINSIGHT, a Clinician Scientist Program at the Medical Faculty, University of Würzburg, which is funded by the Else Kröner-Fresenius Foundation.
Conflict of interest
VG has received honoraria from Bristol-Myers Squibb BMS and Pierre Fabre Pharmaceuticals and reports travel support from Novartis, Pierre Fabre Pharmaceuticals, BMS, Merck Sharp & Dohme MSD, Sanofi Genzyme and SUN Pharmaceuticals Industries, outside the submitted work. PS has received honoraria from BMS and reports travel support from Novartis, Pierre Fabre Pharmaceuticals, BMS, Sanofi-Aventis and SUN Pharmaceuticals Industries, outside the submitted work. MG received honoraria for invited talks and advisory boards from Almirall, Janssen, Lilly and Novartis and travel support from Janssen and UBC, outside the submitted work. AG served as consultant and/or has received honoraria or travel costs from Almirall, Amgen, BMS, Immunocore, MSD, Novartis, Pierre Fabre Pharmaceuticals, Pfizer, Roche and Sanofi Genzyme, outside the submitted work. BS has received honoraria from Pierre Fabre Pharmaceuticals, SUN Pharma, Sanofi Genzyme and reports travel support from Pierre Fabre Pharmaceuticals, outside the submitted work.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbe C, Veness M, et al. Merkel cell carcinoma. Nat Rev Dis Primers. (2017) 3:17077. doi: 10.1038/nrdp.2017.77
2. Nghiem PT, Bhatia S, Lipson EJ, KudChadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med. (2016) 374:2542–52. doi: 10.1056/NEJMoa1603702
3. Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, KudChadkar RR, Brohl AS, et al. Durable tumor regression and overall survival in patients with advanced merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. (2019) 37:693–702. doi: 10.1200/JCO.18.01896
4. D’Angelo SP, Russell J, Lebbé C, Chmielowski B, Gambichler T, Grob J-J, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol. (2018) 4:e180077–e180077. doi: 10.1001/jamaoncol.2018.0077
5. Glutsch V, Schummer P, Kneitz H, Gesierich A, Goebeler M, Klein D, et al. Ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma: a multicenter study of the prospective skin cancer registry ADOREG. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2022-005930
6. Glutsch V, Kneitz H, Gesierich A, Goebeler M, Haferkamp S, Becker JC, et al. Activity of ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma. Cancer Immunol Immunother. (2021) 70:2087–93. doi: 10.1007/s00262-020-02832-0
7. LoPiccolo J, Schollenberger MD, Dakhil S, Rosner S, Ali O, Sharfman WH, et al. Rescue therapy for patients with anti-PD-1-refractory Merkel cell carcinoma: a multicenter, retrospective case series. J Immunother Cancer. (2019) 7:170. doi: 10.1186/s40425-019-0661-6
8. Shalhout SZ, Emerick KS, Kaufman HL, Silk AW, Thakuria M, Miller DM. A retrospective study of ipilimumab plus nivolumab in anti-PD-L1/PD-1 refractory merkel cell carcinoma. J Immunother. (2022) 45:299–302. doi: 10.1097/CJI.0000000000000432
9. Zimmer L, Apuri S, Eroglu Z, KottsChade LA, Forschner A, Gutzmer R, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. (2017) 75:47–55. doi: 10.1016/j.ejca.2017.01.009
10. Kim S, Wuthrick E, Blakaj D, Eroglu Z, Verschraegen C, Thapa R, et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carcinoma: a randomised, open label, phase 2 trial. Lancet. (2022) 400:1008–19. doi: 10.1016/S0140-6736(22)01659-2
11. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. doi: 10.1056/NEJMoa1504030
12. Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. (2019) 20:948–60. doi: 10.1016/S1470-2045(19)30151-2
Keywords: Merkel cell carcinoma, immunotherapy, resistance, relapse, ipilimumab, nivolumab
Citation: Glutsch V, Schummer P, Goebeler M, Gesierich A and Schilling B (2024) Re-exposition to ipilimumab plus nivolumab in metastatic Merkel cell carcinoma. Front. Immunol. 15:1495004. doi: 10.3389/fimmu.2024.1495004
Received: 11 September 2024; Accepted: 19 November 2024;
Published: 05 December 2024.
Edited by:
Paul Nathan, Mount Vernon Cancer Centre, United KingdomReviewed by:
Selma Ugurel, Essen University Hospital, GermanyAnu Sharma, St. Jude Children’s Research Hospital, United States
Copyright © 2024 Glutsch, Schummer, Goebeler, Gesierich and Schilling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerie Glutsch, R2x1dHNjaF9WQHVrdy5kZQ==
 Valerie Glutsch
Valerie Glutsch Patrick Schummer
Patrick Schummer Matthias Goebeler
Matthias Goebeler Anja Gesierich
Anja Gesierich Bastian Schilling
Bastian Schilling