
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 19 December 2024
Sec. Inflammation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1490653
This article is part of the Research Topic The Role of Inflammation and Immune Control in Digestive Disease and Therapeutic Approaches View all 21 articles
Background: Irritable bowel syndrome (IBS) is a common gastrointestinal disease. Recently, an increasing number of studies have shown that Toll-like receptor 4 (TLR4), widely distributed on the surface of a variety of epithelial cells (ECs) and immune sentinel cells in the gut, plays a vital role in developing IBS.
Objectives: We sought to synthesize the existing literature on TLR4 in IBS and inform further study.
Methods: We conducted a systematic search of the PubMed, Embase (Ovid), Scopus, Web of Science, MEDLINE, and Cochrane Library databases on June 8, 2024, and screened relevant literature. Critical information was extracted, including clinical significance, relevant molecular mechanisms, and therapeutic approaches targeting TLR4 and its pathways.
Results: Clinical data showed that aberrant TLR4 expression is associated with clinical manifestations such as pain and diarrhea in IBS. Aberrant expression of TLR4 is involved in pathological processes such as intestinal inflammation, barrier damage, visceral sensitization, and dysbiosis, which may be related to TLR4, NF-κB, pro-inflammatory effects, and CRF. Several studies have shown that many promising therapeutic options (i.e., acupuncture, herbs, probiotics, hormones, etc.) have been able to improve intestinal inflammation, visceral sensitization, intestinal barrier function, intestinal flora, defecation abnormalities, and depression by inhibiting TLR4 expression and related pathways.
Conclusion: TLR4 plays a crucial role in the development of IBS. Many promising therapeutic approaches alleviate IBS through TLR4 and its pathways. Strategies for targeting TLR4 in the future may provide new ideas for treating IBS.
Irritable bowel syndrome (IBS), a functional gastrointestinal disease, is characterized by recurring abdominal pain and alterations in stool frequency or shape (1). Using Bristol stool grade, IBS patients are classified based on their abnormal defecation patterns into diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), mixed IBS (IBS-M) and unclassified IBS (2). Globally, the incidence of IBS varies, approximately 10.1% (9.8%-10.5%) using the Rome III criteria and 3.8% (3.6%-4.0%) using the Rome IV criteria (3). Although IBS is not associated with increased mortality (4), it significantly impacts health-related quality of life, social functioning and psychosocial factors (5–8). Moreover, it imposes a substantial social and economic burden (1, 9, 10). Most costs incurred by IBS patients are due to productivity loss, and direct healthcare expenses are driven by IBS-related comorbidities (11). However, its underlying pathophysiological mechanisms are not fully understood, possibly involving factors like gut-brain axis dysfunction, stress, visceral hypersensitivity (VHS), altered gut motility, barrier function destruction, gut microbiome disorders, intestinal inflammation, immune activation, genetic factors, etc. (1). And as a consequence, no medical therapy is proven to alter the natural history of IBS, usually focus on alleviating symptoms. Therefore, new targets for IBS prevention and treatment have become important. In recent years, increasing numbers of studies have demonstrated that the Toll-like receptor (TLR) 4 plays a pivotal role in developing IBS (12, 13).
TLRs, members of the transmembrane pattern recognition receptor family, play an essential role in innate immune responses and bridge innate and acquired immunity. TLRs are involved in mucosal immune response, barrier function, cell adhesion, cell proliferation and migration, protection from pathogens, repair of epithelial cell injury, etc. (14). Thus, dysregulation of the TLRs signaling pathway contributes to the development and progression of various diseases such as autoimmune diseases, cancer, infections and chronic inflammation (15). Almost all TLRs are expressed in the small intestine and colon intestinal epithelial cells (ECs) (16).
TLR4 is one of the earliest transmembrane pattern recognition receptor family members to be studied. TLR4 is expressed in humans and mice’s colon and ileum crypts (17). TLR4 signaling is essential for maintaining intestinal homeostasis, and its hyperactivation is a crucial driver of many disease states affecting gastrointestinal function (18). Early life stress affects susceptibility to IBS by modulating TLR4 (19). One study found that TLR4 mRNA expression was associated with the intensity of abdominal pain in IBS-D patients (20). TLR4 can promote the up-regulation of pro-inflammatory cytokines by activating the transcription factors nuclear factor-kappa B (NF-κB) via the adaptor protein myeloid differentiation primary response gene 88 (MyD88), ultimately affecting the intestinal barrier, VSH (13, 21). In addition, increased intestinal permeability in IBS patients promotes the activation of TLR-dependent immune responses.
There are no relevant review articles on the role of TLR4 in the pathological mechanisms of IBS. We have collected the current essential studies on the expression of TLR4 in IBS, the mechanism of action, and the drugs targeting TLR4 for the treatment of IBS through a scoping review, which may be instructive for a complete understanding of the biological function of TLR4 in the development of IBS.
Six common life-science databases (PubMed, EMBASE, Scopus, Web of Science, MEDLINE, and Cochrane Library) were applied to identify those studies that met the review criteria. In the PubMed database, we searched the eligible studies by using the following keywords: (((((((Toll-Like Receptor 4 [MeSH Terms]) OR (TLR4 [Title/Abstract])) OR (TLR-4 [Title/Abstract])) OR (Toll Like Receptor 4 [Title/Abstract])) OR (Toll-4 Receptor [Title/Abstract])) OR (Toll 4 Receptor [Title/Abstract])) OR (TLR4 Receptor[Title/Abstract])) OR (Receptor, TLR4[Title/Abstract]) AND ((((((irritable bowel syndrome [MeSH Terms])) OR (Irritable Bowel Syndromes [Title/Abstract])) OR (Syndrome, Irritable Bowel [Title/Abstract])) OR (Syndromes, Irritable Bowel [Title/Abstract])) OR (Colon, Irritable [Title/Abstract])) OR (Irritable Colon [Title/Abstract]). Reference lists of relevant publications were searched to identify more relevant studies. There were no restrictions on the language and date of publication. The literature was last searched on 7 June 2023.
The study reports the expression of the TLR4 gene in irritable bowel syndrome compared to healthy controls, the pathogenesis involved in TLR4 in IBS, and therapeutic approaches targeting TLR4 for the treatment of IBS are included in the study. Study subjects included humans, rat rats, mouse mice, and cell cells. There are no language or publication status restrictions. Meetings and lack of full-text literature were excluded.
Study selection occurred in three stages: First, duplicate publications were immediately eliminated. Second, two researchers independently reviewed titles and abstracts of the literature, and literature that did not meet the inclusion or exclusion criteria was discarded. Articles whose abstracts needed to provide more information to determine whether they were excluded were included directly in the full-text review stage. Third, full-text reviews were conducted independently by two researchers. Disagreements throughout the process were resolved by discussion or input from a third reviewer if required.
Two researchers worked together independently to extract data from articles that fit the topic of this study and then exchanged them for validation. A third researcher resolved the differences. Variables included clinical sample size, diagnostic criteria, study object, disease modelling method, the status of TLR4, effect on IBS, associated genes or pathways, and the main findings of the independent study.
Six database searches identified 800 citations. After removing duplicates, 513 unique citations were screened for titles and abstracts. Of these citations, 110 met the criteria for full-text review. We excluded 70 studies because they did not have relevant data, could not be found in full text, contained insufficient information to assess the relationship between IBS and TLR4, or were conference literature. Finally, 40 studies were included. This whole process is outlined in Figure 1.
Based on the 40 included studies, we summarized the mechanisms and pathways of TLR4 involvement in IBS (Figure 2).
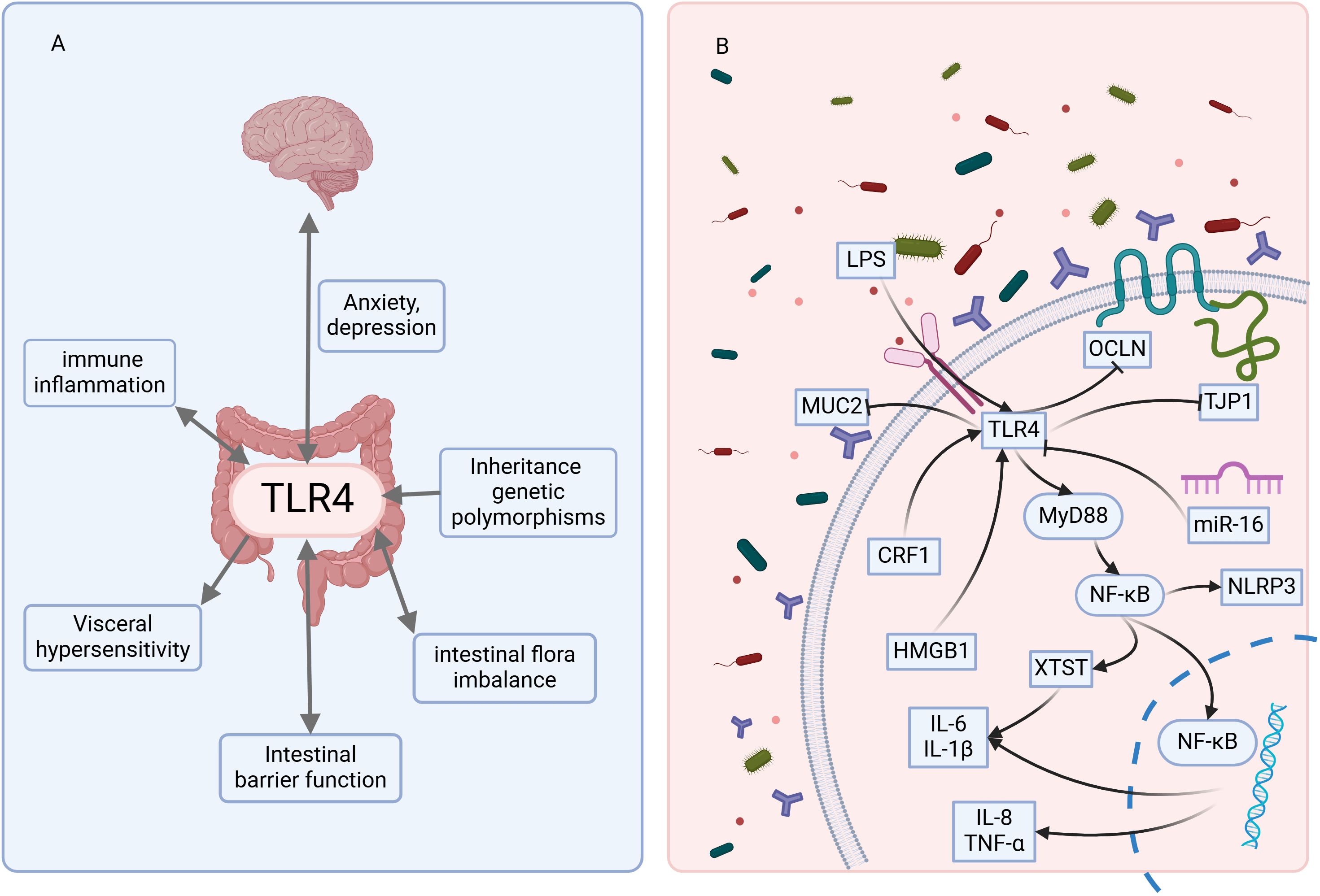
Figure 2. The mechanisms and pathways of TLR4 involvement in IBS. (A) indicates that TLR4 is involved in multiple pathogenic mechanisms of IBS. (B) indicates that TLR4 is involved in the molecular pathways involved in developing IBS. LPS, lipopolysaccharide; MUC2, Mucin 2; TJP1, Tight junction protein 1; OCLN, Occludin; HMGB1, High mobility group box 1; CRF1, Corticotropin-releasing factor receptor subtype 1. The figure was created using BioRender mapping software (https://BioRender.com).
A total of 13 clinical research articles describing the expression of TLR4 in patients with IBS were identified in the literature search; 11 (11/13, 84.62%) articles showed that the expression of TLR4 was up-regulated in IBS compared with health control (HC), and only 2 (2/13, 15.38%) of them suggested that no change in TLR4 was found in IBS (Table 1). In addition to changes in TLR4 expression, the expression of TLR2, TLR5, TLR9, and inflammatory factors (interleukin (IL)-1α, IL-1β, IL-6 and IL-8) were up-regulated and anti-inflammatory factor (IL-10) was down-regulated (Table 1).
Ten studies reported the molecular mechanisms underlying the role of TLR4 in IBS (Table 2). The development of IBS disease was found to involve the TLR4/MyD88/NF-κB signaling pathway primarily. Activation of TLR4 and its signaling pathway can contribute to IBS development by promoting inflammation and mediating visceral sensitization and stool abnormalities (Table 2). MicroRNA was involved in IBS; research indicated that miR-16 inhibited the TLR4/NF-κB/X-inactive specific transcript (XIST) axis to relieve IBS-D (13).
We searched 18 studies that provided the data of specific treatments-targeted TLR4 for IBS (Table 3). Treatments included herbal medicine, moxibustion, electroacupuncture (EA), probiotics and its postbiotic (PB) element, diet, hormones, etc. (Table 3). These treatments decreased inflammation, attenuated VHS and depressive-like behavior, and improved abnormal defecation and intestinal flora through TLR4/MyD88/NF-κB signaling pathway and TLR4/NF-κB/NLR family pyrin domain-containing protein 3 (NLRP3) pathway (Table 3).
By summarizing the literature on the progress of TLR4 in IBS disease research, it is clear that TLR4 plays a vital role in developing IBS. TLR4 is involved in the pathogenesis of IBS, including low-grade inflammation, increased visceral sensitivities, intestinal barrier damage, intestinal flora dysbiosis, defecation abnormalities, etc.
TLR4 expression was upregulated in IBS patients (20, 22–28, 30–32). However, the results of TLR4 expression in different subtypes were inconsistent. Belmonte et al. reported significant differences by subtype with a 2-fold increase in IBS-M, more significant than in IBS-D (22). While Shukla et al. found the most significant increase in IBS-D (30). Five IBS-D-only publications, including one PI-IBS D, showed upregulation of TLR4 (20, 24, 26, 27, 32). Only one article on IBS-D patients suggests no change in TLR4 (29).
In two clinical studies of IBS that showed no significant change in TLR4 (29, 33), the disease was diagnosed using the Rome III diagnostic criteria. One specimen was peripheral blood serum, and the other study took mucosal tissues from multiple sites in the gut. Overall, there were no significant differences from the other studies in terms of IBS diagnostic criteria, IBS subtypes, sample types, or anatomic locations. Analyze why the results of these two studies are inconsistent with those of other studies. Yoshimoto et al.’s study had the presence of taking anti-flatulent and antidepressant drugs, which may have influenced the results. Disease heterogeneity is a primary reason for inconsistent results, such as concomitant symptom involvement, with one study showing that TLR4 expression was higher in IBS patients with concomitant depression (31). In addition, the sample sizes in these pieces of literature are small, which can lead to excessive random errors in the results. Future large-scale studies are needed to investigate the expression pattern of TLR4 in IBS, whether there are differences in expression between subtypes and the effect of concomitant symptoms such as anxiety and depression on TLR4 expression in IBS. This will help us to understand the disease, the relationship between the different subtypes, and the management of the disease. Other factors that may have influenced the results were genetic, environmental, etc.
Low-grade mucosal inflammation and immune dysfunction are some of the main pathogenic mechanisms of IBS. Several clinical studies have found elevated levels of pro-inflammatory cytokines (such as IL-1α, IL-1β, IL-6, IL-8, IL-17, TNF-α, CXCL-11 and CXCR-3)and reduced levels of the anti-inflammatory cytokine IL-10 in patients with IBS (22, 24, 29–31, 59–62). There was a correlation between inflammatory factors and IBS symptoms and quality of life (60). Meanwhile, mRNA levels of TLR-4 in IBS patients were positively correlated with the inflammatory factor IL-6 (30).Activation of TLR4 induces the expression of IL-1, IL-6 and IL-8 (63). NF-κB, which TLR4 can activate, is a central mediator in the induction of pro-inflammatory genes and plays a role in both innate and adaptive immune cells (64). Three studies showed that inflammatory factor production may be caused by the TLR4/MyD88/NF-κB pathway in an animal model of IBS-D, which promotes the development of IBS-D (34–36). Inflammatory factor (IL-6, IL-1β) expression was found to be attenuated by inhibition of the TLR4/NF-κB/XIST pathway in LPS induced damage to human normal colonic ECs (13). Furthermore, Belmonte et al. found that the imbalance between elevated levels of TLR4 and the impaired expression of PPARγ, a potential inhibitor of colonic inflammation, suggests an altered response to luminal bacteria leading to colonic inflammation (22).
Then how does TLR4 specifically participate in IBS disease progression through immune cells? In the intestine, TLR4 is expressed on antigen-presenting cells (e.g., macrophages and dendritic cells) and lymphocytes (65). In normal physiology, immune cell expression of TLR4 is required for B cell recruitment, dendritic cell maturation, and triggering of T cell responses to invading pathogens (18). A Mendelian randomization study shows a significant genetic correlation between immune cell phenotype and IBS (66). In IBS, alterations in lymphocyte populations, including B and T lymphocyte counts and activation levels, are associated with increased colonic MCs in IBS patients (67). Colonic mast cells are more numerous in IBS, and their activation degranulation can modulate visceral sensitivity and epithelial barrier function by releasing neuroactive mediators (53, 55, 68, 69). In clinical trials, mast cell stabilizers or histamine 1 receptor antagonists improved IBS symptoms and quality of life (20, 70). In an experiment of IBS supernatant-induced degranulation of BMMCs (bone marrow-derived MCs (BMMCs)), it was found that TLR4 activation led to degranulation and histamine production and that a TLR4 inhibitor (TAK-242) attenuated degranulation of BMMCs (71). In animal experiments, the potential mechanism of visceral hypersensitivity has also been found to involve the expression of TLR4 in MCs of colonic tissues (58). It indicates that TLR4 may influence intestinal function and visceral sensitization responses by regulating mast cell degranulation. However, no evidence of TLR4 regulation of other immune cells in IBS was found. Most of the studies were unclear in their observation of cell specificity and failed to provide a distinction between epithelial cells and immune cells expressing TLR4, thus preventing in-depth analysis of the specific mechanism (18).
Increased intestinal permeability in patients with IBS ranges from 2% - 62% (72). The increased intestinal permeability in IBS is associated with abdominal pain and visceral sensitivity and exposes neurological and immune components to luminal microbes (73, 74). In IBS patients, TLR4 is strongly associated with barrier function-related genes, including protease-activated receptor 2, OCLN, and TJP1, suggesting potential functional relationships (75). Xi et al. found that overexpression of TLR4 led to down-regulation of TJP1 and OCLN, whereas inhibition of TLR4 expression led to up-regulation of TJP1 and OCLN in the IBS-D cell model (13). Singh P. et al. found that a high-FODMAP diet leads to colonic barrier loss and mast cell activation and that TLR4 receptors on MCs are critical for this high-FODMAP mediated loss of the colonic barrier (76). In vivo and in vitro experiments have revealed that activation of TLR4 increases intestinal permeability by down-regulating phosphorylated OCLN expression in the intestinal epithelial barrier, increasing myosin light chain kinase protein expression and kinase activity (77, 78). MUC2, a glycoprotein, forms the mucus layer of the intestinal barrier (79). In vitro experiment, silencing of TLR4 in the TLR4-expressing rat intestinal epithelioid cell line 6 induced MUC2 production, whereas overexpression of TLR4 in human Caco-2 cells, which generally do not express TLR4, resulted in the loss of their normal MUC2-producing phenotype (80). In the same in vivo experiments, increased permeability of the gut in villin-TLR4 mice (increased TLR4 signaling), about the significantly lower expression of epithelial cell-cell adhesion genes in colonic ECs, including junctional adhesion molecule A and cadherin-1, and a decreased depression of TJP1 although not significantly (81). In conclusion, aberrant expression of TLR4 is closely related to intestinal barrier function and is involved in barrier damage in IBS.
In a healthy state, the gut microbiota interacts closely with intestinal epithelial cells and the immune system to regulate inflammation and maintain the development of intestinal barrier and immune system (82, 83). Intestinal dysbiosis, an imbalance in the intestinal microbiota due to various internal and external factors, is an important causative factor in IBS (72, 84). Several Meta-analyses of altered intestinal flora in patients with IBS have shown the presence of intestinal dysbiosis in patients with IBS, mainly characterized by lower levels of lactobacilli and bifidobacteria compared to healthy controls (85, 86). Flora dysbiosis may cause loss of intestinal integrity and increased intestinal permeability, which can lead to penetration of the epithelial barrier by bacterial products and metabolites, thereby triggering an inflammatory response (87). Increased intestinal permeability may also increase bacterial dissemination (81). In addition, intestinal dysbiosis affects intestinal motility, increases VHS, and regulates the gut-brain axis (88, 89). TLRs recognize specific microbial components of commensal and pathogenic bacteria and play a role in immune tolerance to commensal bacteria and defense against pathogens (90). Altered microbiota profiles may affect TLR expression and immune activation in IBS (30). Guo et al. found that the diversity of intestinal mucosal colonizing flora and the two dominant bacterial genera (Mycobacterium avium and Clostridium spp.) were significantly reduced in IBS-D patients compared with that of healthy people, while the mucosal immune-related receptors TLR2 and TLR4 were significantly over-expressed, and there was a correlation between the reduction of these two genera and the high expression of TLR (26). Similarly, another study found that TLR4 expression in IBS-D was negatively associated with the microbial relative abundance of the Lactobacillus and Escherichia/Shigella genera, whereas it was positively associated with the relative abundance of the genera Megasphera and Sutterella and the class Betaproteobacteria (27). Both studies suggest a correlation between TLR4 and gut flora. Bacterial invasion can activate pro-inflammatory responses through TLR4-induced TIRAP/MyD88 and TRAM/TRIF signaling cascades (91). Thus, intestinal dysbiosis may induce immune disorders by activating the natural and acquired immune systems by activating intestinal mucosal TLR4 proteins, triggering inflammation and ultimately leading to IBS-D (26). Targeting TLR4 may benefit by restoring epithelial function and changing the microbiota (81). Future studies are needed to explore the mechanisms of gut flora dysbiosis and the role of the TLR4 pathway in IBS disease.
VHS, which refers to internal organs like the gastrointestinal tract exhibiting amplified perception of pain in response to stimuli, is a frequent complaint among individuals with IBS. The inflammatory consequences of TLR activation on glial cells (mainly microglia and astrocytes), sensory neurons, and other cell types affect injury perception processing and lead to pain (92). TLR4 expression in colonic tissues is associated with VHS reactions (58). Correlation analysis shows that TLR4 mRNA expression correlates with the intensity of abdominal pain in IBS-D patients (20). LPS Activation of TLR4 increases the production of pro-inflammatory cytokines, which activate visceral sensory neurons to induce visceral hypersensitivity (93). The results of eight studies have shown that VHS in IBS may be mediated through the TLR4, TLR4/NF-κB, TLR4/MyD88/NF-κB, TLR4/NF-κB/CBS, HMGB1/TLR4 pathways (12, 13, 35, 37–41). Genetically altered (i.e., TLR4 knockout and point-mutant) mice and rats with down-regulated TLR4 expression exhibit analgesia and low expression levels of cytokines, such as TNF-α and IL-1β (94). Tang et al. found that MS was associated with increased VHS, microglial TLR4, and inflammatory factors IL-1β and TNF-α expression in Tlr4 +/+ mice; however, MS did not alter VHS, IL-1β and TNF-α expression in Tlr4 -/- mice (12). Increased IL-1β and TNF-α proteins released by microglia via the TLR4/MyD88/NF-κB signaling pathway induced neonatal stress-induced VHS and pain (35). Administration of OT peripherally reduced VHS or visceral pain in human samples and animal models (39). OT pretreatment inhibited these increases as TLR4 signaling elicited a cellular response that released the downstream effectors MyD88, NF-κB, IL-1β, and TNF-α, thereby inducing VHS (39). TLR4 signaling and the pro-inflammatory cytokines TNF-α and IL-1β may be involved in neuroglial interactions in the pathogenesis of VHS reactions (41). TLR4 deficiency reduced visceral pain and prevented the development of chronic psychosocial stress-induced VHS. Administration of TLR4 antagonists, such as TAK-242 and CLI-095, counteracted chronic stress, neonatal colonic inflammation, and neonatal CRD-induced VHS (38, 40, 95). Furthermore, rats with a FODMAP diet resulted in impaired gut barrier function and increased sensitivity to colorectal distension, its LPS or fecal supernatants induced VHS, and this was blocked by small interfering RNA inhibition of TLR4 mRNA, suggesting that TLR4 activation by fecal LPS could mediate VHS (96). In conclusion, the generation of VHS is closely related to TLR4, NF-κB, and pro-inflammatory effects, which may be an essential way to improve abdominal pain in IBS patients.
Abnormal defecation is one of the main symptoms of IBS, including changes in fecal water content and bowel motility. TLRs, especially TLR2 and TLR4, significantly affect post-infection and lipopolysaccharide-mediated regulation of gastrointestinal motility (97). The muscle contractility induced by acetylcholine was significantly lower in TLR2 (-/-) and TLR4 (-/-) concerning WT mice (98). Gastrointestinal motility was significantly delayed in mice that do not express TLR4 or Myd88 compared to wild-type mice (99). These studies suggest that TLR4 is involved in intestinal motility. In patients with IBS, a positive correlation was found between mRNA levels of TLR-4 and weekly stool frequency in IBS patients (30). High expression of TLR4 in the IBS model results in shorter bowel intervals, higher fecal water content, and greater urgency (13, 27).
IBS affects psychosocial factors, including general and gut-related anxiety, depression, and somatization (8, 100). Some of these affections are bidirectional, and psychosocial factors can aggravate IBS symptoms and the disease progression, and vice versa (101, 102). Up to the minute, two Mendelian randomization studies revealed a bidirectional causal relationship between IBS and cerebral cortex structures, confirming the two-way communication along the brain-gut axis (103, 104). Moreover, there are longitudinal interactions from childhood into adulthood. A Swedish prospective longitudinal birth-cohort study found that health-related quality of life deterioration and psychological distress of adolescence are associated with new cases of adult IBS, and undergoing an abdominal pain–related adolescent gut-brain interaction disorder is associated with new-onset adult psychological distress (105). Growing evidence suggests that TLRs are associated with the pathophysiology of major depressive disorder, among which multiple linear regression analysis revealed that TLR4 was an independent risk factor relating to the severity of major depression (106, 107). TLRs (including TLR4) are upregulated in major depressive disorder patients, while antidepressant treatment downregulates TLRs expression, suggesting that TLRs are critical mediators for antidepressant therapy (108). Clinical studies have found that patients with IBS and depression have higher levels of TLR4 expression and IL-6, accompanied by a decrease in IL-10, which indicates that TLR participates in the inflammation reaction in IBS and depression (31). Erick J et al. found that chronic restraint stress induces anxiety-like behaviors, while blockade of the HMGB1/TLR4 pathway reverses chronic restraint stress-induced anxiety-like behaviors (37). Some drugs can treat depression through TLR4 and its signaling pathway. XYS can improve depressive-like behavior in rats by suppressing the activation of the TLR4/NLRP3 inflammasome signaling pathway (57). In the LPS-induced depression model, the use of raspberry ketone supplementation can alleviate depressive behavior through mitigated gut inflammation by inhibiting the TLR-4/NF-κB pathway (109).
The development and worsening of symptoms in irritable bowel syndrome is known to be closely related to stress, which induces visceral hypersensitivity and altered colonic motility and plays a vital role in the pathophysiology of disease development (110, 111). CRF, expressed in the brain and colon, is a significant mediator of the brain-gut axis stress response and mediates stress-induced enhancement of colonic motility and VHS, suggesting that CRF is a critical component of IBS (112). CRF receptor subtype 1 (CRF1), CRF2, and TLR4 were found to be upregulated in peripheral blood samples of IBS patients, especially in patients with concomitant depression (31). Persistent activation of the CRF1 system at central or peripheral sites may be one of the underlying causes of diarrhea and abdominal pain symptoms in IBS (112). Colonic TLR4 expression is downregulated in CRF-deficient mice and is more susceptible to colitis (113). One study found that CRF induces VHS and colonic hyperpermeability via TLR4 and cytokine systems, and these changes are dependent on CRF1 (114). They also found that LPS-induced VHS is mediated through CRF, TLR4, and pro-inflammatory factor pathways (115). Moreover, LPS increases colonic CRF expression at the gene and protein level (116) and activates peripheral CRF receptors (115). The CRF-TLR4-inflammatory cytokine system also affects the gut microbiota. Evidence suggests that activation of the CRF-TLR4-inflammatory cytokine system is followed by impairment of the intestinal barrier, which may alter the microbiota (93). In conclusion, CRF and TLR4-pro-inflammatory cytokine signaling generates a vicious cycle of mutual activation, leading to intestinal barrier damage and ecological dysregulation, affecting intestinal motility, and inducing a visceral hypersensitivity response that leads to IBS symptoms.
MiRNAs regulate the pathophysiological mechanisms of IBS, and searching for relevant miRNA biomarkers as diagnostic and therapeutic candidates for IBS is a hot topic (117, 118). Only one publication reported that miR-16 in IBS regulates defecation intervals, stool water content, and VHS by targeting the TLR4/NF-κB/XIST pathway (13). It has been reported that miR-16 targets and inhibits TLR4 in the LPS-induced inflammatory pathway, and this alteration can be reversed by the lncRNA SNHG16 (119). In addition, miR-16 can down-regulate the expression of NF-κB, NLRP3, and other inflammatory factors by targeting TLR4, thereby attenuating inflammation in the LPS-induced acute lung injury model (120). Given that most miRNAs have many-to-many relationships with target genes, more studies are needed to clarify the molecular mechanisms of miRNAs in IBS on the TLR4 pathway.
Genetics contributes to the development of IBS disease. As early as 2001, it was proposed that identical twins have a significantly higher concordance of IBS than dizygotic twins (121). SNPs are the most common type of sequence variation in genomes. It has been shown that TLR9 rs5743836 (A/g) gene polymorphism may be associated with IBS-D phenotype (122). However, this review did not retrieve complete text reports on TLR4 gene polymorphism in IBS. A study of SNPs in the TLR4 gene in inflammatory bowel disease shows that TLR4 D299G polymorphism is significantly associated with inflammatory bowel disease in North Indian populations and regulates the transcription of inflammatory cytokines during ulcerative colitis, leading to abnormal immune responses (123). The results suggest that SNPs in TLR4 are associated with immune inflammation, which is the primary pathogenesis of IBS. Its role in IBS needs to be clarified, and future studies can look for its role in IBS regarding TLR4 gene polymorphisms.
We included 18 studies in this review, which reported specific treatments for IBS by targeting TLR4. All these studies found that inhibition of TLR4 was one of the essential mechanisms underlying the improved IBS exerted by specific treatments. Multiple studies have found that chronic psychosocial stress or early-life stress impacts susceptibility to IBS by modulating TLR4, etc. (19, 37, 124, 125). KD might be beneficial in psychiatric disorders like stress, anxiety, depression, mood disorders, etc., given its ability to remodel the gut microbiota and antioxidant and anti-inflammatory effects, consequently impacting the brain-gut axis (126, 127). Chimienti et al. found that feeding animals with KD can reduce inflammation and oxidative stress, restore mitochondrial function and baseline autophagy, and thus reduce the harmful effects of stress in an animal model of IBS (43). In addition to diet therapy, EA and moxibustion therapy also have significant therapeutic effects on IBS (128, 129). These therapies may be practical to decrease patients’ pain, and fewer side effects will be sought.
Regarding the mechanism, the study showed that moxibustion improved diarrhea symptoms and VHS and alleviated inflammation of IBS-D through inhibited TLR4/MyD88/NF-κB signaling pathway (44). At the same time, EA-reduced visceral sensitivity of IBS may be involved in the suppression of TLR4 expression in the MCs of colonic tissues, which inhibits mast cell activation in colonic tissues and reduces the levels of inflammatory factors in serum that participate in the process of VHS (58). Multiple systematic reviews and meta-analyses have shown that herbal medicine effectively relieves IBS symptoms (130–132). TLR4 was the most critical type of TLRs regulated by phytochemicals (133). Herbal compound prescriptions, such as Wumei Pill, STW 5-II, QingHuaZhiXie Prescription, Sancao Lichang Decoction, CKF, Xiaoyaosan, improvement of inflammation, defecation abnormalities, VHS and depressive behavior in IBS, via suppressed TLR4, TLR4/MyD88/NF-κB or TLR4/NF-κB/NLRP3 signal pathway (45–47, 54, 55, 57). Flavonoids, mostly found as natural pigments in fruits, vegetables, and seeds of edible plants, have significant therapeutic activities, such as anti-inflammatory and antioxidant effects, and enhance intestinal barrier function (133–135). GSE, LE, and Apigenin, natural flavonoids, could diminish inflammation, maintain tight junction integrity, and improve visceral sensitization and colonic hypermobility with IBS by inhibiting TLR4 and TLR4/MyD88/NF-κB pathway (42, 51, 53). Other herbs that act on TLR4, such as SHE and BHS, may also improve IBS symptoms (48, 56). Increasingly, evidence has shown that gut microbiota dysbiosis plays a vital role in IBS pathogenesis (86, 136). Probiotic use may offer particular utility in managing IBS through its metabolic activity, immunomodulatory, and cross-feeding effects (137). LC-DG and its PB, Sb, and probiotic combination LT could decrease TLR4, attenuate inflammation, improve colonic hypermotility, and prevent epithelial barrier impairment of IBS (24, 50, 52). In addition to probiotics, MT is also closely linked to the gut microbiota, mitigated colonic microbiota dysbiosis, and intestinal inflammation in IBS animal models by inhibiting the activation of the TLR4/NF-κB pathway (49). Overall, the 18 studies suggest that multiple therapies targeting TLR4 can reduce IBS-related symptoms. TLR4 is viable as a potential therapeutic target.
TLR4 may play a vital role in the pathogenesis of IBS, and its abnormal activation may lead to dysregulation of the intestinal inflammatory response, which in turn affects the intestinal function, leading to hypersensitivity of the intestinal nervous system and aggravation of abdominal pain and discomfort. The structure of the intestinal flora of patients with BS may change, and the overgrowth of certain bacteria may lead to the activation of TLR4, which in turn may further affect the balance of the intestinal flora, forming a vicious circle and exacerbating the symptoms of IBS. Polymorphisms in the TLR4 gene may be associated with the risk of developing IBS. In addition, environmental factors such as diet and stress may also indirectly affect the development and symptoms of IBS by influencing the expression and function of TLR4. From the current evidence, changes in TLR4 and the development of IBS may be causative, and more clinical and basic studies are needed to elucidate the exact causal relationship between TLR4 and IBS.
TLR4 is significantly up-regulated in IBS, correlating with clinical manifestations, and is accompanied by up-regulation of pro-inflammatory factors and down-regulation of anti-inflammatory factors. Pathogenesis involved in IBS, such as intestinal barrier damage, intestinal dysbiosis, abnormal intestinal peristalsis, increased visceral sensitization, and anxiety behaviors, are related to the interactions among TLR4, the NF-κB pathway, the pro-inflammatory effects, and the CRFs. Various therapies such as herbs, acupuncture, probiotics, and their associated PB can treat IBS by targeting TLR4 and its pathway. In conclusion, TLR4 may be a promising target for treating IBS, and more clinical studies will be needed to evaluate therapeutic approaches targeting this pathway.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
XW: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. LW: Supervision, Writing – review & editing. ZW: Data curation, Formal analysis, Methodology, Writing – original draft. CW: Conceptualization, Supervision, Writing – review & editing, Writing – original draft, Investigation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Research and Development Project of Sichuan Provincial Science and Technology Department (No.2022YFS0026).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
IBS, Irritable bowel syndrome; IBS-D, Diarrhea-predominant IBS; IBS-C, Constipation-predominant IBS; IBS-M, Mixed IBS; VHS, Visceral hypersensitivity; TLR, Toll-like receptor; ECs, Epithelial cells; NF-κB, Nuclear factor-kappa B; MyD88, Myeloid differentiation primary response gene 88; HC, Health control; IL, Interleukin; XIST, X-inactive specific transcript; EA, Electroacupuncture; PB, Postbiotic; NLRP3, NLR family pyrin domain-containing protein 3; PI-IBS D, Post infectious IBS-D; CRD, Colorectal distension; CI, Colonic irritation; NMS, Neonatal maternal separation; WAS, Water avoidance stress; OT, Oxytocin; GSE, Grape seed extract; KD, Ketogenic Diet; LC-DG, Lactobacillus casei DG; SHE, Serpylli herba extract; MT, Melatonin; Sb, Saccharomyces boulardii; LE, Lens culinaris Medik extract; LT, Lactibiane Tolerance; MCs, Mast cells; CKF, Chang-Kang-Fang; BHS, Berberis heteropoda Schrenk roots; XYS, Xiaoyaosan; SNP, Single-nucleotide polymorphism.
1. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. (2020) 396:1675–88. doi: 10.1016/S0140-6736(20)31548-8
2. Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and rome IV. Gastroenterology. 150:1262–79. doi: 10.1053/j.gastro.2016.02.032
3. Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. (2021) 160:99–114.e3. doi: 10.1038/ajg.2010.40
4. Chang JY, Locke RGI, McNally MA, Halder SL, Schleck CD, Zinsmeister AR, et al. Impact of functional gastrointestinal disorders on survival in the community. Off J Am Coll Gastroenterol ACG. (2010) 105:822–32. doi: 10.1186/s12955-017-0611-2
5. Buono JL, Carson RT, Flores NM. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. (2017) 15:35.
6. Frändemark Å, Törnblom H, Jakobsson S, Simrén M. Work productivity and activity impairment in irritable bowel syndrome (IBS): A multifaceted problem. Off J Am Coll Gastroenterol ACG. (2018) 113:1540–9. doi: 10.1038/s41395-018-0262-x
7. Sugaya N. Work-related problems and the psychosocial characteristics of individuals with irritable bowel syndrome: an updated literature review. Biopsychosoc Med. (2024) 18:12. doi: 10.1186/s13030-024-00309-5
8. Patel P, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P, et al. Irritable bowel syndrome is significantly associated with somatisation in 840 patients, which may drive bloating. Aliment Pharmacol Ther. (2015) 41:449–58. doi: 10.1111/apt.2015.41.issue-5
9. Goodoory VC, Ng CE, Black CJ, Ford AC. Direct healthcare costs of Rome IV or Rome III-defined irritable bowel syndrome in the United Kingdom. Aliment Pharmacol Ther. (2022) 56:110–20. doi: 10.1111/apt.16939
10. Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. (2014) 40:1023–34. doi: 10.1111/apt.2014.40.issue-9
11. Bosman MHMA, Weerts ZZRM, Snijkers JTW, Vork L, Mujagic Z, Masclee AAM, et al. The socioeconomic impact of irritable bowel syndrome: an analysis of direct and indirect health care costs. Clin Gastroenterol Hepatol. (2023) 21:2660–9. doi: 10.1016/j.cgh.2023.01.017
12. Tang HL, Zhang G, Ji NN, Du L, Chen BB, Hua R, et al. Toll-like receptor 4 in paraventricular nucleus mediates visceral hypersensitivity induced by maternal separation. Front Pharmacol. (2017) 8:309/full. doi: 10.3389/fphar.2017.00309/full
13. Xi M, Zhao P, Li F, Bao H, Ding S, Ji L, et al. MicroRNA-16 inhibits the TLR4/NF-κB pathway and maintains tight junction integrity in irritable bowel syndrome with diarrhea. J Biol Chem. (2022) 298:102461. doi: 10.1016/j.jbc.2022.102461
14. Leulier F, Lemaitre B. Toll-like receptors — taking an evolutionary approach. Nat Rev Genet. (2008) 9:165–78. doi: 10.1038/nrg2303
15. Giambra V, Pagliari D, Rio P, Totti B, Di Nunzio C, Bosi A, et al. Gut microbiota, inflammatory bowel disease, and cancer: the role of guardians of innate immunity. Cells. (2023) 12:2654. doi: 10.3390/cells12222654
16. Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. (2010) 10:131–44. doi: 10.1038/nri2707
17. Bogunovic M, Davé SH, Tilstra JS, Chang DTW, Harpaz N, Xiong H, et al. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. (2007) 292:G1770–1783. doi: 10.1152/ajpgi.00249.2006
18. Bruning EE, Coller JK, Wardill HR, Bowen JM. Site-specific contribution of Toll-like receptor 4 to intestinal homeostasis and inflammatory disease. J Cell Physiol. (2021) 236:877–88. doi: 10.1002/jcp.v236.2
19. Zhou GQ, Huang MJ, Yu X, Zhang NN, Tao S, Zhang M. Early life adverse exposures in irritable bowel syndrome: new insights and opportunities. Front Pediatr. (2023) 11:1241801/full. doi: 10.3389/fped.2023.1241801/full
20. Lobo B, Ramos L, Martínez C, Guilarte M, González-Castro AM, Alonso-Cotoner C, et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study. United Eur Gastroenterol J. (2017) 5:887–97. doi: 10.1177/2050640617691690
21. Hyo-Jin K, Kim H, Jeong-Hyung L, Hwangbo C. Toll-like receptor 4 (TLR4): new insight immune and aging. Immun Ageing. (2023) 20:1–11. doi: 10.1186/s12979-023-00383-3
22. Belmonte L, Youmba SB, Bertiaux-Vandaële N, Antonietti M, Lecleire S, Zalar A, et al. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PloS One. (2012) 7:e42777. doi: 10.1371/journal.pone.0042777
23. Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EMM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Off J Am Coll Gastroenterol ACG. (2011) 106:329–36. doi: 10.1038/ajg.2010.438
24. Compare D, Rocco A, Coccoli P, Angrisani D, Sgamato C, Iovine B, et al. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. (2017) 17:53. doi: 10.1186/s12876-017-0605-x
25. Dlugosz A, Zakikhany K, Acevedo N, D’Amato M, Lindberg G. Increased expression of toll-like receptors 4, 5, and 9 in small bowel mucosa from patients with irritable bowel syndrome. BioMed Res Int. (2017) 2017:9624702. doi: 10.1155/2017/9624702
26. Guo W, Liu P, Dong L, Wang J. The correlation study between the changes of intestinal mucosa predominant bacteria and Toll-like receptor 2, Toll-like receptor 4 gene expressions in diarrhea-predominant irritable bowel syndrome pafients. Chin J Intern Med. (2016) 55:541–3. doi: 10.3760/cma.j.issn.0578-1426.2016.07.011
27. Jalanka J, Lam C, Bennett A, Hartikainen A, Crispie F, Finnegan LA, et al. Colonic Gene Expression and Fecal Microbiota in Diarrhea-predominant Irritable Bowel Syndrome: Increased Toll-like Receptor 4 but Minimal Inflammation and no Response to Mesalazine. J Neurogastroenterol Motil. (2021) 27:279–91. doi: 10.5056/jnm20205
28. Koçak E, Akbal E, Köklü S, Ergül B, Can M. The colonic tissue levels of TLR2, TLR4 and nitric oxide in patients with irritable bowel syndrome. Intern Med Tokyo Jpn. (2016) 55:1043–8. doi: 10.2169/internalmedicine.55.5716
29. Linsalata M, Riezzo G, D’Attoma B, Clemente C, Orlando A, Russo F. Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: a case-control study. BMC Gastroenterol. (2018) 18:167. doi: 10.1186/s12876-018-0888-6
30. Shukla R, Ghoshal U, Ranjan P, Ghoshal UC. Expression of toll-like receptors, pro-, and anti-inflammatory cytokines in relation to gut microbiota in irritable bowel syndrome: the evidence for its micro-organic basis. J Neurogastroenterol Motil. (2018) 24:628–42. doi: 10.5056/jnm18130
31. Jizhong S, Qiaomin W, Chao W, Yanqing L. Corticotropin-releasing factor and toll-like receptor gene expression is associated with low-grade inflammation in irritable bowel syndrome patients with depression. Gastroenterol Res Pract. (2016) 2016:7394924. doi: 10.1155/2016/7394924
32. Wang XM, Liu YL. Signal transduction pathway of colonic Toll-like receptor 4 in patients with irritable bowel syndrome. Zhonghua Yi Xue Za Zhi. (2008) 88:3415–7. doi: 10.3321/j.issn:0376-2491.2008.48.008
33. Yoshimoto T, Oshima T, Huang X, Tomita T, Fukui H, Miwa H. Microinflammation in the intestinal mucosa and symptoms of irritable bowel syndrome. J Gastroenterol. (2022) 57:62–9. doi: 10.1007/s00535-021-01838-4
34. Bao LL, Cui LH. Exprossion of TLR4 and NF-κB in rats model induced with IBS-D and its mechanism. Med J Chin Peoples Lib Army. (2019) 44:648–51. doi: 10.11855/j.issn.0577-7402.2019.08.04
35. Chen ZY, Zhang XW, Yu L, Hua R, Zhao XP, Qin X, et al. Spinal toll-like receptor 4-mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. Eur J Pain. (2015) 19:176–86. doi: 10.1002/ejp.534
36. He X, Cui LH, Wang XH, Yan ZH, Li C, Gong SD, et al. Modulation of inflammation by toll-like receptor 4/nuclear factor-kappa B in diarrhea-predominant irritable bowel syndrome. Oncotarget. (2017) 8:113957–65. doi: 10.18632/oncotarget.23045
37. Rodriguez-Palma EJ, Velazquez-Lagunas I, Salinas-Abarca AB, Vidal-Cantu GC, Escoto-Rosales MJ, Castaneda-Corral G, et al. Spinal alarmin HMGB1 and the activation of TLR4 lead to chronic stress-induced nociceptive hypersensitivity in rodents. Eur J Pharmacol. (2023) 952:175804. doi: 10.1016/j.ejphar.2023.175804
38. Wu J, Li T, Mao G, Cha X, Fei S, Miao B. The involvement of Pellino-1 downregulation in the modulation of visceral hypersensitivity via the TLR4/NF-κB pathway in the rat fastigial nucleus. Neurosci Lett. (2022), 787:136815. doi: 10.1016/j.neulet.2022.136815
39. Xu S, Qin B, Shi A, Zhao J, Guo X, Dong L. Oxytocin inhibited stress induced visceral hypersensitivity, enteric glial cells activation, and release of proinflammatory cytokines in maternal separated rats. Eur J Pharmacol. (2018) 818:578–84. doi: 10.1016/j.ejphar.2017.11.018
40. Yuan B, Tang WH, Lu LJ, Zhou Y, Zhu HY, Zhou YL, et al. TLR4 upregulates CBS expression through NF-κB activation in a rat model of irritable bowel syndrome with chronic visceral hypersensitivity. World J Gastroenterol. (2015) 21:8615–28. doi: 10.3748/wjg.v21.i28.8615
41. Zhang G, Yu L, Chen ZY, Zhu JS, Hua R, Qin X, et al. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav Immun. (2016) 55:93–104. doi: 10.1016/j.bbi.2015.12.022
42. Arie H, Nozu T, Miyagishi S, Ida M, Izumo T, Shibata H. Grape seed extract eliminates visceral allodynia and colonic hyperpermeability induced by repeated water avoidance stress in rats. Nutrients. (2019) 11:2646. doi: 10.3390/nu11112646
43. Chimienti G, Orlando A, Lezza AMS, D’Attoma B, Notarnicola M, Gigante I, et al. The ketogenic diet reduces the harmful effects of stress on gut mitochondrial biogenesis in a rat model of irritable bowel syndrome. Int J Mol Sci. (2021) 22:3498. doi: 10.3390/ijms22073498
44. Chu HR, Wang Y, Tong L, Wu SB, Wu LB, Li N, et al. Effect of moxibustion on TLR4/MyD88/NF-κB signaling pathway in colon of diarrhea-predo-minant irritable bowel syndrome rats. Zhen Ci Yan Jiu Acupunct Res. (2020) 45:633–9. doi: 10.13702/j.1000-0607.190950
45. Ding X, Sun X, Wang Z, Zheng Q, Yu X, Hao W, et al. The effects of Wumei pill on TLRs/NF-kB signaling pathway in rats with diarrhea-predominant irritable bowel syndrome. Pak J Zool. (2019) 51:57–65. doi: 10.17582/journal.pjz/2019.51.1.57.65
46. Elbadawi M, Ammar RM, Rabini S, Klauck SM, Efferth T. Modulation of intestinal corticotropin-releasing hormone signaling by the herbal preparation STW 5-II: possible mechanisms for irritable bowel syndrome management. Pharmaceuticals. (2022) 15:1121. doi: 10.3390/ph15091121
47. Huang H, Zhao P, Xi M, Li F, Ji L. Mechanism of qingHuaZhiXie prescription regulating TLR4-IECs pathway in the intervention of diarrhea predominant irritable bowel syndrome. Evid Based Complement Alternat Med. (2021) 2021:5792130. doi: 10.1155/2021/5792130
48. Ruiz-Malagón AJ, Rodríguez-Sanchez MJ, Rodríguez-Sojo MJ, Vezza T, Pischel I, Algieri F, et al. Intestinal anti-inflammatory and visceral analgesic effects of a Serpylli herba extract in an experimental model of irritable bowel syndrome in rats. Front Pharmacol. (2022) 13:967644/full. doi: 10.3389/fphar.2022.967644/full
49. Lin R, Wang Z, Cao J, Gao T, Dong Y, Chen Y. Role of melatonin in murine “restraint stress”-induced dysfunction of colonic microbiota. J Microbiol. (2021) 59:500–12. doi: 10.1007/s12275-021-0305-7
50. Liu J, Ren H, Yuan F, Shao M, Luo H. The effects of Saccharomyces boulardii on rat colonic hypermotility induced by repeated water avoidance stress and the potential mechanism. PeerJ. (2022) 10:e14390. doi: 10.7717/peerj.14390
51. Maqoud F, Orlando A, Tricarico D, Antonacci M, Di Turi A, Giannelli G, et al. Anti-inflammatory effects of a novel acetonitrile–water extract of lens culinaris against LPS-induced damage in caco-2 cells. Int J Mol Sci. (2024) 25:3802. doi: 10.3390/ijms25073802
52. Nébot-Vivinus M, Harkat C. Multispecies probiotic protects gut barrier function in experimental models. World J Gastroenterol. (2014) 20:6832–43. doi: 10.3748/wjg.v20.i22.6832
53. Xia Y, Peng S, Lin M, Duan H, Yuan F, Shao M, et al. Apigenin attenuates visceral hypersensitivity in water avoidance stress rats by modulating the microbiota-gut-brain axis and inhibiting mast cell activation. BioMed Pharmacother. (2023) 167:115562. doi: 10.1016/j.biopha.2023.115562
54. Zhang P, Ma Y, Wang Z, Tang D. The effect and mechanism of sancao lichang decoction on diarrhea- predominant irritable bowel syndrome by regulating tlr4/myd88/nf-Kb pathway. doi: 10.2174/1386207326666230301104248
55. Zhang S, Tian D, Xia Z, Yang F, Chen Y, Yao Z, et al. Chang-Kang-Fang alleviates diarrhea predominant irritable bowel syndrome (IBS-D) through inhibiting TLR4/NF-κB/NLRP3 pathway. J Ethnopharmacol. (2024) 330:118236. doi: 10.1016/j.jep.2024.118236
56. Zhu HM, Li L, Li SY, Yan Q, Li F. Effect of water extract from Berberis heteropoda Schrenk roots on diarrhea-predominant irritable bowel syndrome by adjusting intestinal flora. J Ethnopharmacol. (2019) 237:182–91. doi: 10.1016/j.jep.2019.03.045
57. Zhu HZ, Liang YD, Hao WZ, Ma QY, Li XJ, Li YM, et al. Xiaoyaosan exerts therapeutic effects on the colon of chronic restraint stress model rats via the regulation of immunoinflammatory activation induced by the TLR4/NLRP3 inflammasome signaling pathway. Evid-Based Complement Altern Med ECAM. (2021) 2021:6673538. doi: 10.1155/2021/6673538
58. Yang J, Shang B, Shi H, Zhu S, Lu G, Dai F. The role of toll-like receptor 4 and mast cell in the ameliorating effect of electroacupuncture on visceral hypersensitivity in rats. Neurogastroenterol Motil. (2019) 31:e13583. doi: 10.1111/nmo.2019.31.issue-6
59. Seyedmirzaee S, Hayatbakhsh MM, Ahmadi B, Baniasadi N, Rafsanjani AMB, Nikpoor AR, et al. Serum immune biomarkers in irritable bowel syndrome. Clin Res Hepatol Gastroenterol. (2016) 40:631–7. doi: 10.1016/j.clinre.2015.12.013
60. Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. CYTOKINE. (2017) 93:34–43. doi: 10.1016/j.cyto.2017.05.005
61. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta analysis of IL-6 (-G174C) and circulating IL-6 levels. CYTOKINE. (2017) 99:132–8. doi: 10.1016/j.cyto.2017.08.017
62. Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, et al. Immune activation in patients with irritable bowel syndrome. GASTROENTEROLOGY. (2007) 132:913–20. doi: 10.1053/j.gastro.2007.01.046
63. Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. (1997) 388:394–7. doi: 10.1038/41131
64. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:1–9. doi: 10.1038/sigtrans.2017.23
65. Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol Baltim Md. (1950) 177:3273–82. doi: 10.4049/jimmunol.177.5.3273
66. Chai WH, Ma Y, Li JJ, Guo F, Wu YZ, Liu JW. Immune cell signatures and causal association with irritable bowel syndrome: A mendelian randomization study. World J Clin Cases. (2024) 12:3094–104. doi: 10.12998/wjcc.v12.i17.3094
67. Hasler WL, Grabauskas G, Singh P, Owyang C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol Motil. (2022) 34:e14339. doi: 10.1111/nmo.14339
68. Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. (2004) 126:693–702. doi: 10.1053/j.gastro.2003.11.055
69. Bashashati M, Moossavi S, Cremon C, Barbaro MR, Moraveji S, Talmon G, et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol Motil. (2018) 30:e13192. doi: 10.1111/nmo.2018.30.issue-1
70. Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. (2010) 59:1213–21. doi: 10.1136/gut.2010.213108
71. Shimbori C, De Palma G, Baerg L, Lu J, Verdu EF, Reed DE, et al. Gut bacteria interact directly with colonic mast cells in a humanized mouse model of IBS. Gut Microbes. (2022) 14:2105095. doi: 10.1080/19490976.2022.2105095
72. JohnBritto JS, Di Ciaula A, Noto A, Cassano V, Sciacqua A, Khalil M, et al. Gender-specific insights into the irritable bowel syndrome pathophysiology. Focus on gut dysbiosis and permeability. Eur J Intern Med. (2024) 125:10–8. doi: 10.1016/j.ejim.2024.03.011
73. King AJ, Chang L, Li Q, Liu L, Zhu Y, Pasricha PJ, et al. NHE3 inhibitor tenapanor maintains intestinal barrier function, decreases visceral hypersensitivity, and attenuates TRPV1 signaling in colonic sensory neurons. Am J Physiol-Gastrointest LIVER Physiol. (2024) 326:G543–54. doi: 10.1152/ajpgi.00233.2023
74. Long Y, Du L, Kim JJ, Chen B, Zhu Y, Zhang Y, et al. MLCK-mediated intestinal permeability promotes immune activation and visceral hypersensitivity in PI-IBS mice. Neurogastroenterol Motil. (2018) 30:e13348. doi: 10.1111/nmo.2018.30.issue-9
75. Polster A, Öhman L, Tap J, Derrien M, Le Nevé B, Sundin J, et al. A novel stepwise integrative analysis pipeline reveals distinct microbiota-host interactions and link to symptoms in irritable bowel syndrome. Sci Rep. (2021) 11:5521. doi: 10.1038/s41598-021-84686-9
76. Singh P, Grabauskas G, Zhou SY, Gao J, Zhang Y, Owyang C. High FODMAP diet causes barrier loss via lipopolysaccharide-mediated mast cell activation. JCI Insight. (2021) 6. doi: 10.1172/jci.insight.146529
77. Li X, Wang C, Nie J, Lv D, Wang T, Xu Y. Toll-like receptor 4 increases intestinal permeability through up-regulation of membrane PKC activity in alcoholic steatohepatitis. ALCOHOL. (2013) 47:459–65. doi: 10.1016/j.alcohol.2013.05.004
78. Nighot M, Al-Sadi R, Guo S, Rawat M, Nighot P, Watterson MD, et al. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am J Pathol. (2017) 187:2698–710. doi: 10.1016/j.ajpath.2017.08.005
79. Schoultz I, Keita AV. The intestinal barrier and current techniques for the assessment of gut permeability. CELLS. (2020) 9:1909. doi: 10.3390/cells9081909
80. Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, et al. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. (2012) 143:708–U234. doi: 10.1053/j.gastro.2012.05.053
81. Dheer R, Santaolalla R, Davies JM, Lang JK, Phillips MC, Pastorini C, et al. Intestinal epithelial toll-like receptor 4 signaling affects epithelial function and colonic microbiota and promotes a risk for transmissible colitis. Infect Immun. (2016) 84:798–810. doi: 10.1128/IAI.01374-15
82. Van de Wiele T, Van Praet JT, Marzorati M, Drennan MB, Elewaut D. How the microbiota shapes rheumatic diseases. Nat Rev Rheumatol. (2016) 12:398–411. doi: 10.1038/nrrheum.2016.85
83. Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. (2017) 5:e1373208. doi: 10.1080/21688370.2017.1373208
84. Shaikh SD, Sun N, Canakis A, Park WY, Weber HC. Irritable bowel syndrome and the gut microbiome: A comprehensive review. J Clin Med. (2023) 12:2558. doi: 10.3390/jcm12072558
85. Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig LIVER Dis. (2017) 49:331–7. doi: 10.1016/j.dld.2017.01.142
86. Wang L, Alammar N, Singh R, Nanavati J, Song Y, Chaudhary R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: A systematic review and meta-analysis of case-control studies. J Acad Nutr Diet. (2020) 120:565–86. doi: 10.1016/j.jand.2019.05.015
87. Linsalata M, Riezzo G, Clemente C, D’Attoma B, Russo F. Noninvasive biomarkers of gut barrier function in patients suffering from diarrhea predominant-IBS: an update. Dis Markers. (2020) 2020:2886268. doi: 10.1155/2020/2886268
88. Principi N, Cozzali R, Farinelli E, Brusaferro A, Esposito S. Gut dysbiosis and irritable bowel syndrome: The potential role of probiotics. J Infect. (2018) 76:111–20. doi: 10.1016/j.jinf.2017.12.013
89. Wollny T, Daniluk T, Piktel E, Wnorowska U, Bukłaha A, Głuszek K, et al. Targeting the gut microbiota to relieve the symptoms of irritable bowel syndrome. Pathogens. (2021) 10:1545. doi: 10.3390/pathogens10121545
90. de Kivit S, Tobin MC, Forsyth CB, Keshavarzian A, Landay AL. Regulation of intestinal immune responses through TLR activation: implications for pro- and prebiotics. Front Immunol. (2014) 5:60. doi: 10.3389/fimmu.2014.00060
91. Dmytriv TR, Storey KB, Lushchak VI. Intestinal barrier permeability: the influence of gut microbiota, nutrition, and exercise. Front Physiol. (2024) 15:1380713/full. doi: 10.3389/fphys.2024.1380713/full
92. Liu X, Yang W, Zhu C, Sun S, Wu S, Wang L, et al. Toll-like receptors and their role in neuropathic pain and migraine. Mol BRAIN. (2022) 15:73. doi: 10.1186/s13041-022-00960-5
93. Nozu T, Okumura T. Pathophysiological commonality between irritable bowel syndrome and metabolic syndrome: role of corticotropin-releasing factor-toll-like receptor 4-proinflammatory cytokine signaling. J Neurogastroenterol Motil. (2022) 28:173–84. doi: 10.5056/jnm21002
94. Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. (2005) 102:5856–61. doi: 10.1073/pnas.0501634102
95. Tramullas M, Finger BC, Moloney RD, Golubeva AV, Moloney G, Dinan TG, et al. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry. (2014) 76:340–8. doi: 10.1016/j.biopsych.2013.11.004
96. Zhou SY, Gillilland M, Wu X, Leelasinjaroen P, Zhang G, Zhou H, et al. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest. (2018) 128:267–80. doi: 10.1172/JCI92390
97. Nam Y, Min YS, Sohn UD. Recent advances in pharmacological research on the management of irritable bowel syndrome. Arch Pharm Res. (2018) 41:955–66. doi: 10.1007/s12272-018-1068-5
98. Layunta E, Forcén R, Grasa L. TLR2 and TLR4 modulate mouse ileal motility by the interaction with muscarinic and nicotinic receptors. Cells. (2022) 11:1791. doi: 10.3390/cells11111791
99. Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology. (2012) 143:1006. doi: 10.1053/j.gastro.2012.06.034
100. Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, clinical, and psychological characteristics of individuals with self-reported irritable bowel syndrome based on the rome IV vs rome III criteria. Clin Gastroenterol Hepatol. (2020) 18:392–398.e2. doi: 10.1016/j.cgh.2019.05.037
101. Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. GUT. (2012) 61:1284–90. doi: 10.1136/gutjnl-2011-300474
102. Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. (2016) 44:592–600. doi: 10.1111/apt.2016.44.issue-6
103. Xu Z, Ning F, Zhang X, Wang Q, Zhang Y, Guo Y, et al. Deciphering the brain-gut axis: elucidating the link between cerebral cortex structures and functional gastrointestinal disorders via integrated Mendelian randomization. Front Neurosci. (2024) 18:1398412/full. doi: 10.3389/fnins.2024.1398412/full
104. Li Z, Ma Q, Deng Y, Rolls ET, Shen C, Li Y, et al. Irritable bowel syndrome is associated with brain health by neuroimaging, behavioral, biochemical, and genetic analyses. Biol Psychiatry. (2024) 95:1122–32. doi: 10.1016/j.biopsych.2023.12.024
105. Sjölund J, Kull I, Bergström A, Ljótsson B, Törnblom H, Olén O, et al. Quality of life and bidirectional gut-brain interactions in irritable bowel syndrome from adolescence to adulthood. Clin Gastroenterol Hepatol. (2024) 22:858–866.e6. doi: 10.1016/j.cgh.2023.09.022
106. Wu MK, Huang TL, Huang KW, Huang YL, Hung YY. Association between toll-like receptor 4 expression and symptoms of major depressive disorder. Neuropsychiatr Dis Treat. (2015) 11:1853–7. doi: 10.2147/NDT.S88430
107. Hung YY, Kang HY, Huang KW, Huang TL. Association between toll-like receptors expression and major depressive disorder. Psychiatry Res. (2014) 220:283–6. doi: 10.1016/j.psychres.2014.07.074
108. Hung YY, Huang KW, Kang HY, Huang GYL, Huang TL. Antidepressants normalize elevated Toll-like receptor profile in major depressive disorder. Psychopharmacol (Berl). (2016) 233:1707–14. doi: 10.1007/s00213-015-4087-7
109. Liu Y, Dai C, Wang C, Wang J, Yan W, Luo M, et al. Raspberry ketone prevents LPS-induced depression-like behaviors in mice by inhibiting TLR-4/NF-κB signaling pathway via the gut-brain axis. Mol Nutr Food Res. (2024) 68. doi: 10.1002/mnfr.202400090
110. Schaper SJ, Stengel A. Emotional stress responsivity of patients with IBS-a systematic review. J Psychosom Res. (2022) 153:110694. doi: 10.1016/j.jpsychores.2021.110694
111. Fukudo S. Stress and visceral pain: Focusing on irritable bowel syndrome. PAIN. (2013) 154:S63–70. doi: 10.1016/j.pain.2013.09.008
112. Taché Y, Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil. (2015) 21:8–24. doi: 10.5056/jnm14162
113. Chaniotou Z, Giannogonas P, Theoharis S, Teli T, Gay J, Savidge T, et al. Corticotropin-releasing factor regulates TLR4 expression in the colon and protects mice from colitis. Gastroenterology. (2010) 139:2083–92. doi: 10.1053/j.gastro.2010.08.024
114. Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Altered colonic sensory and barrier functions by CRF: roles of TLR4 and IL-1. J Endocrinol. (2018) 239:241–52. doi: 10.1530/JOE-18-0441
115. Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Lipopolysaccharide induces visceral hypersensitivity: role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor in rats. J Gastroenterol. (2017) 52:72–80. doi: 10.1007/s00535-016-1208-y
116. Yuan PQ, Wu SV, Wang L, Tache Y. Corticotropin releasing factor in the rat colon: Expression, localization and upregulation by endotoxin. Peptides. (2010) 31:322–31. doi: 10.1016/j.peptides.2009.11.012
117. Chen H, Xu Z, Zhao H, Cao J, Wang R, He J, et al. Global research states and trends of micro RNA in irritable bowel syndrome: a bibliometric analysis. Clin Exp Med. (2024) 24:149. doi: 10.1007/s10238-024-01396-y
118. Bravo-Vázquez LA, Medina-Ríos I, Márquez-Gallardo LD, Reyes-Muñoz J, Serrano-Cano FI, Pathak S, et al. Functional implications and clinical potential of microRNAs in irritable bowel syndrome: A concise review. Dig Dis Sci. (2023) 68:38–53. doi: 10.1007/s10620-022-07516-6
119. Wang W, Lou C, Gao J, Zhang X, Du Y. LncRNA SNHG16 reverses the effects of miR-15a/16 on LPS-induced inflammatory pathway. BioMed Pharmacother. (2018) 106:1661–7. doi: 10.1016/j.biopha.2018.07.105
120. Yang Y, Yang F, Yu X, Wang B, Yang Y, Zhou X, et al. miR-16 inhibits NLRP3 inflammasome activation by directly targeting TLR4 in acute lung injury. BioMed Pharmacother. (2019) 112:108664. doi: 10.1016/j.biopha.2019.108664
121. Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: Heredity and social learning both contribute to etiology. Gastroenterology. (2001) 121:799–804. doi: 10.1053/gast.2001.27995
122. Camilleri M, Carlson P, McKinzie S, Zucchelli M, D’Amato M, Busciglio I, et al. Genetic susceptibility to inflammation and colonic transit in lower functional gastrointestinal disorders: preliminary analysis. Neurogastroenterol Motil. (2011) 23:935–E398. doi: 10.1111/j.1365-2982.2011.01749.x
123. Meena NK, Verma R, Verma N, Ahuja V, Paul J. TLR4 D299G polymorphism modulates cytokine expression in ulcerative colitis. J Clin Gastroenterol. (2013) 47:773. doi: 10.1097/MCG.0b013e31828a6e93
124. Tao E, Wu Y, Hu C, Zhu Z, Ye D, Long G, et al. Early life stress induces irritable bowel syndrome from childhood to adulthood in mice. Front Microbiol. (2023) 14:1255525. doi: 10.3389/fmicb.2023.1255525
125. Louwies T, Mohammadi E, Greenwood-Van Meerveld B. Epigenetic mechanisms underlying stress-induced visceral pain: Resilience versus vulnerability in a two-hit model of early life stress and chronic adult stress. Neurogastroenterol Motil. (2023) 35:e14558. doi: 10.1111/nmo.14558
126. Rawat K, Singh N, Kumari P, Saha L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev Neurosci. (2021) 32:143–57. doi: 10.1515/revneuro-2020-0078
127. Brietzke E, Mansur RB, Subramaniapillai M, Balanzá-Martínez V, Vinberg M, González-Pinto A, et al. Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci Biobehav Rev. (2018) 94:11–6. doi: 10.1016/j.neubiorev.2018.07.020
128. Dai YQ, Weng H, Wang Q, Guo XJ, Wu Q, Zhou L, et al. Moxibustion for diarrhea-predominant irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. (2022) 46:101532. doi: 10.1016/j.ctcp.2021.101532
129. Wu IXY, Wong CHL, Ho RST, Cheung WKW, Ford AC, Wu JCY, et al. Acupuncture and related therapies for treating irritable bowel syndrome: overview of systematic reviews and network meta-analysis. Ther Adv Gastroenterol. (2019) 12. doi: 10.1177/1756284818820438
130. Zheng H, Jin S, Shen YL, Peng WY, Ye K, Tang TC, et al. Chinese herbal medicine for irritable bowel syndrome: A meta-analysis and trial sequential analysis of randomized controlled trials. Front Pharmacol. (2021) 12:694741. doi: 10.3389/fphar.2021.694741
131. Wu YB, Dai YK, Zhang L, Pan HG, Chen WJ, Li RL, et al. Pharmacological treatments of Chinese herbal medicine for irritable bowel syndrome in adults: A network meta-analysis of randomized controlled trials. PloS One. (2021) 16:e0255665. doi: 10.1371/journal.pone.0255665
132. Hawrelak JA, Wohlmuth H, Ptatinson M, Myers SP, Goldenberg JZ, Harnett J, et al. Western herbal medicines in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Complement Ther Med. (2020) 48:102233. doi: 10.1016/j.ctim.2019.102233
133. Esmaealzadeh N, Ram M, Abdolghaffari A, Marques AM, Bahramsoltani R. Toll-like receptors in inflammatory bowel disease: A review of the role of phytochemicals. Phytomedicine. (2024) 123:155178. doi: 10.1016/j.phymed.2023.155178
134. Zulkefli N, Zahari CNMC, Sayuti NH, Kamarudin AA, Saad N, Hamezah HS, et al. Flavonoids as potential wound-healing molecules: emphasis on pathways perspective. Int J Mol Sci. (2023) 24:4607. doi: 10.3390/ijms24054607
135. Xiong HH, Lin SY, Chen LL, Ouyang KH, Wang WJ. The interaction between flavonoids and intestinal microbes: A review. FOODS. (2023) 12:320. doi: 10.3390/foods12020320
136. Pimentel M, Lembo A. Microbiome and its role in irritable bowel syndrome. Dig Dis Sci. (2020) 65:829–39. doi: 10.1007/s10620-020-06109-5
Keywords: irritable bowel syndrome, toll-like receptor 4, inflammation, visceral hypersensitivity, treatment
Citation: Wan X, Wang L, Wang Z and Wan C (2024) Toll-like receptor 4 plays a vital role in irritable bowel syndrome: a scoping review. Front. Immunol. 15:1490653. doi: 10.3389/fimmu.2024.1490653
Received: 03 September 2024; Accepted: 02 December 2024;
Published: 19 December 2024.
Edited by:
Shuai Wang, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Cong Zhang, The First People’s Hospital of Foshan, ChinaCopyright © 2024 Wan, Wang, Wang and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaomin Wan, d2NtMDIyMEAxMjYuY29t; Zhiling Wang, d2FuZ196aGlfbGluZ0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.