- 1School of Health and Rehabilitation Sciences, Division of Medical Laboratory Science, The Ohio State University, Columbus, OH, United States
- 2Gilead Sciences Inc., Foster City, CA, United States
- 3Case Western Reserve University, University Hospitals of Cleveland, Cleveland, OH, United States
Background: Heightened levels of inflammatory markers are linked to increased morbidity/mortality in people with HIV (PWH) and often remain elevated after virologic suppression by antiretroviral therapy (ART). As new combinations of ART become available, an evaluation of their effects on immune activation and inflammation is warranted. Additionally, it remains unknown whether transient increases in viral load (“blips”) during ART are associated with increases in inflammation.
Methods: We utilized cryopreserved samples from treatment-naïve PWH enrolled in two Phase 3 clinical trials investigating the efficacy and safety of bictegravir, emtricitabine and tenofovir alafenamide (B/F/TAF) or dolutegravir, abacavir, and lamivudine (DTG/ABC/3TC) or DTG + F/TAF over a 5-year window (GS-US-380-1489/1490). At week 144, participants were offered the option to switch to open label B/F/TAF for an additional 96 weeks. We measured levels of interleukin-6 (IL-6), C-reactive protein (hsCRP), D-dimer, soluble CD14 (sCD14), and tumor necrosis factor-α receptor 1 (TNFR1) from available baseline, week 24, 48, 144, and 240 samples (B/F/TAF, N=123; DTG/ABC/3TC, N=62; DTG+F/TAF, N=58). Additional samples from PWH who experienced a viral blip (n=44, defined as a single HIV-1 RNA >50c/mL) were also analyzed and paired with the most recent available suppressed sample before the blip. Longitudinal biomarker changes were assessed using a constrained mixed effects linear regression model adjusting for covariates.
Results: Baseline demographics and selected laboratory characteristics were similar across groups. Levels of D-dimer, sCD14, and TNFR1 decreased significantly from baseline in all treatment arms, with no significant differences between arms at any timepoint. Biomarker levels also remained stable following ART-switch at week 144. No significant changes in hsCRP or IL-6 were observed versus baseline in any arm at any timepoint. A significant association was observed between sCD14 and increasing viral load (p=0.022) in viral blips; D-dimer also increased with blips in the B/F/TAF arm.
Conclusions: Viral suppression was associated with reductions in most inflammatory markers in PWH, with no significant differences among the three ART regimens during the 144-week randomized period. These decreases were sustained after the open label switch to B/F/TAF. Viral blips were associated with increases in monocyte activation (sCD14). Further analysis is needed to confirm these findings and determine the potential impact on clinical outcomes.
Introduction
Treatment with combination antiretroviral therapy (ART) increases the expected lifespan of people with HIV (PWH), however, even when ART is successful at lowering HIV levels below the standard assay limit of detection, elevated levels of inflammation, immune activation, and hypercoagulation may persist (1–4). Chronic inflammation likely contributes to an increased risk of several comorbid conditions in PWH, including cardiovascular disease, insulin resistance and type II diabetes, osteoporosis, neurocognitive dysfunction, and frailty (2). Multiple mechanisms may contribute to inflammation, immune activation, and hypercoagulation in PWH, including low level viral replication, co-pathogens, microbial translocation, ART-related toxicities, oxidative stress, and altered lipid profiles (2). The Strategies for Management of Anti-Retroviral Therapy (SMART) trial explored the consequences of continuous viral suppression by ART versus limiting ART exposure in PWH. Viral suppression was beneficial within both treatment arms, but continuous inhibition had superior benefit over intermittent suppression for AIDS- and non-AIDS events and mortality (5, 6). Plasma levels of interleukin (IL)-6, high-sensitivity C-reactive protein (hsCRP), D-dimer, and soluble CD14 (sCD14) were independent predictors of morbidity and mortality in this study (5–7). Other inflammatory markers, including tumor necrosis factor receptors 1 and 2 (TNFR1 and TNFR2) (8–10) are also predictive of morbidity and mortality in PWH. Further, combinations of biomarkers (e.g. IL-6 and D-dimer and/or IL-6 and TNFR1) (11, 12) may have enhanced predictive value for adverse health outcomes compared to levels of individual proteins.
Long lived cells infected with HIV-1, including memory CD4+ T cells and macrophages, may contribute to persistent production of HIV-1 associated RNA molecules and proteins (13, 14). Activation of these latently infected cells may contribute to measurable levels of HIV-1 viremia, typically >50 copies/mL, often referred to as viral “blips.” These blips could result from an interruption in suppressive ART or through mechanisms that induce viral replication. The levels to which persistent HIV-1 replication products and/or virologic blips contribute to chronic inflammation in PWH have not been thoroughly investigated (13, 14).
As new ART drugs become available, assessment of the virologic, immunologic, and metabolic effects of these drugs in well-controlled clinical trials is needed. Improved ART combinations should rapidly and consistently decrease plasma HIV-1 levels, have minimal effects on metabolic profiles, and improve immune cell function while minimizing persistent immune activation and inflammation in PWH. Studies GS-US-380-1489,1490 evaluated the efficacy and safety of currently recommended INSTI plus two NRTI ART combinations bictegravir (B), emtricitabine (F), tenofovir alafenamide (TAF), compared to dolutegravir (DTG), F and TAF, or DTG, abacavir (ABC) and lamivudine (3TC) (15–18) in treatment naïve PWH. These studies demonstrated that B/F/TAF is a well-tolerated and effective ART regimen over a duration of 5 years, with rapid suppression of viral replication and minimal effects on lipid profiles, bone density, and kidney function (19). Here, using a subset of participants from these parent studies, we explored the longitudinal effects of these ART regimens on five soluble markers of inflammation and immune activation that have been previously linked to morbidity and mortality in PWH (4–8, 11).
Materials and methods
Study design and sample selection
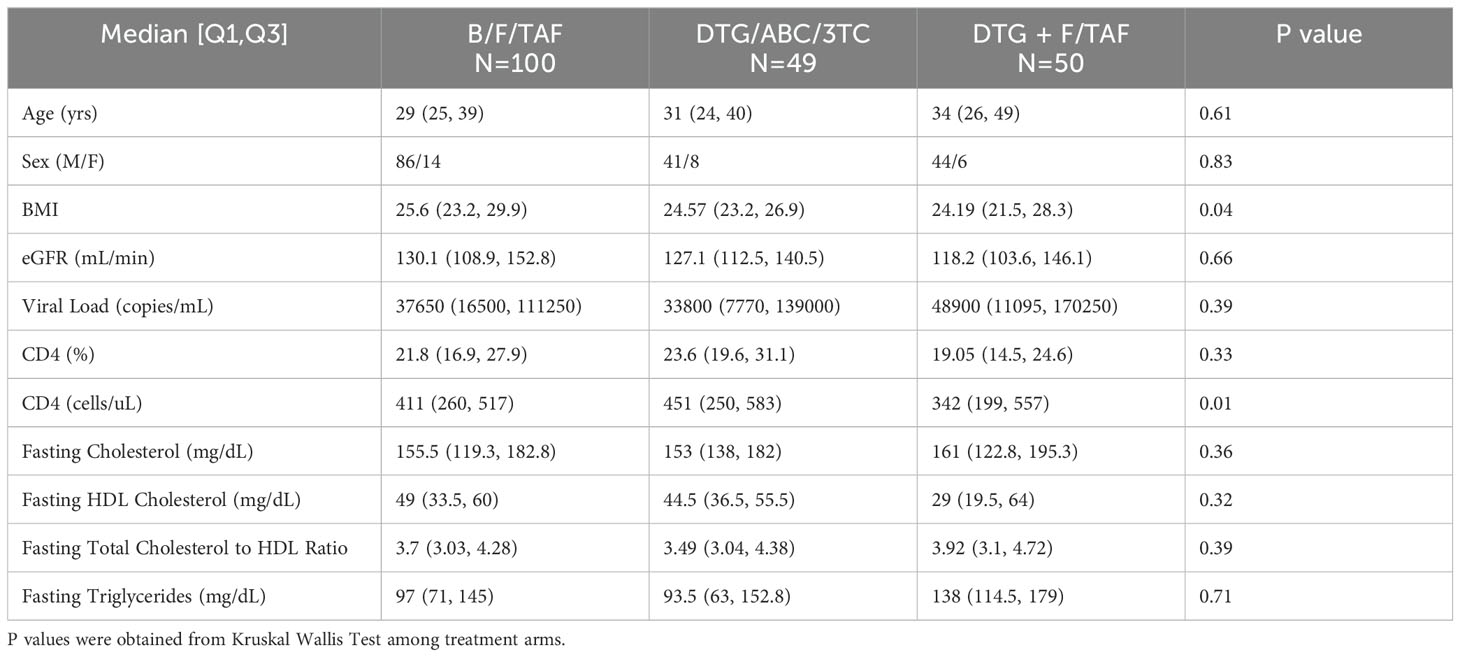
We utilized cryopreserved samples in a convenience sampling of US participants from the two Phase 3 clinical studies that enrolled treatment-naïve PWH and randomized them to receive either bictegravir, emtricitabine and tenofovir alafenamide (B/F/TAF) or dolutegravir, abacavir, and lamivudine (DTG/ABC/3TC) in Study 1489 or DTG + F/TAF in Study 1490 (15–18). All participants were switched to B/F/TAF at week 144 for a 96-week open label extension (OLE). Samples from baseline, week 24, 48, 144, and 240, were used from participants who had consistently suppressed viremia (HIV-1 RNA <50 copies/mL at week 24). Samples from participants with baseline viral loads (VL) of HIV-1 RNA >5log were over selected to assure significant decreases in markers of immune activation following ART initiation (N=50 in each arm). As well, all samples from eligible female participants were included for the analysis to be more representative of sex as well as to explore sex as a biological variable. Participants who failed to complete the trial to week 240 (Wk 96 OLE), had co-infection with hepatitis C virus (HCV), or who developed an underlying comorbid condition, such as COVID diagnosis, cancer diagnosis and statin initiation, that may complicate their inflammatory profiles were excluded. Finally, samples were selected to balance selected patient characteristics (age, sex, CD4+ T cell count at ART initiation), across treatment arms which may play a role in immune profiles.
Measurement of plasma biomarkers
Plasma samples were identified and shipped on dry ice for measurement of markers of immune activation and inflammation using enzyme-linked immunosorbent assays (ELISA) at Ohio State University (Columbus, Ohio, USA). Measured biomarkers included soluble CD14 (sCD14), high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), tumor necrosis factor receptor 1 (TNF-R1) (R&D Systems, Minneapolis, Minnesota, USA) and D-dimer (Diagnostica Stago, Parsippany, New Jersey, USA). To the extent possible, assays were performed by the same person each time, limiting operator variability and each assay plate was from the same manufacturer and lot. In brief, samples were diluted using the dilution factors that have been identified during our previous studies (20–24). Standard curves were run in duplicate per plate, and samples were re-run at a different dilution if values were out of range or if the duplicated sample values were not within manufacturer’s assay range. A threshold of CV<20% for repeats was applied to all technical duplicates. Each sample was thawed once and used on all plates run on that day.
Statistical analyses
Longitudinal biomarker changes were assessed from log-transformed values, using a constrained mixed-effects linear regression model (LMEM; R package “lme4”) with time as categorical values and adjusting for covariates (sex and baseline viral load). Associations between inflammation biomarkers and viral load were tested with a B-spline LMEM, with the R packages “lme4” and “spline”, adjusting for baseline viral load, treatment arm, and time. Baseline associations between CD4+ T cell count/CD4+ percentage and biomarker levels were performed using Spearman’s Correlation. Associations of longitudinal changes in CD4+ T cell count/CD4+ percentage and biomarker levels were tested with LEME using “lme4”, adjusting for changes of viral load from baseline, treatment arm, and time. Significance was determined by a false discover rate (FDR) < 0.05 unless otherwise stated. Nonparametric tests (Wilcoxon Signed Rank Test or Wilcoxon Rank Sum Test and Kruskal Wallis Test) were used to compare between groups where appropriate and as indicated. All analyses were performed in R (version 4.2.1).
Results
Characteristics of study participants
We utilized cryopreserved samples from a sampling of treatment-naïve PWH enrolled in two Phase 3 clinical studies investigating the efficacy and safety of B/F/TAF, DTG/ABC/3TC and DTG+F/TAF over a 5-year window (GS-US-380-1489/1490, Figure 1). At wk 144, participants were offered a switch to open label B/F/TAF. In general, selected participant demographic and clinical characteristics were balanced across treatment arms (Table 1). Participants in the DTG+F/TAF arm were observed to have lower baseline CD4+ T cell counts compared to baseline CD4+ T cell counts in the other two treatment arms (p=0.01), however viral loads were generally balanced (p=0.34).
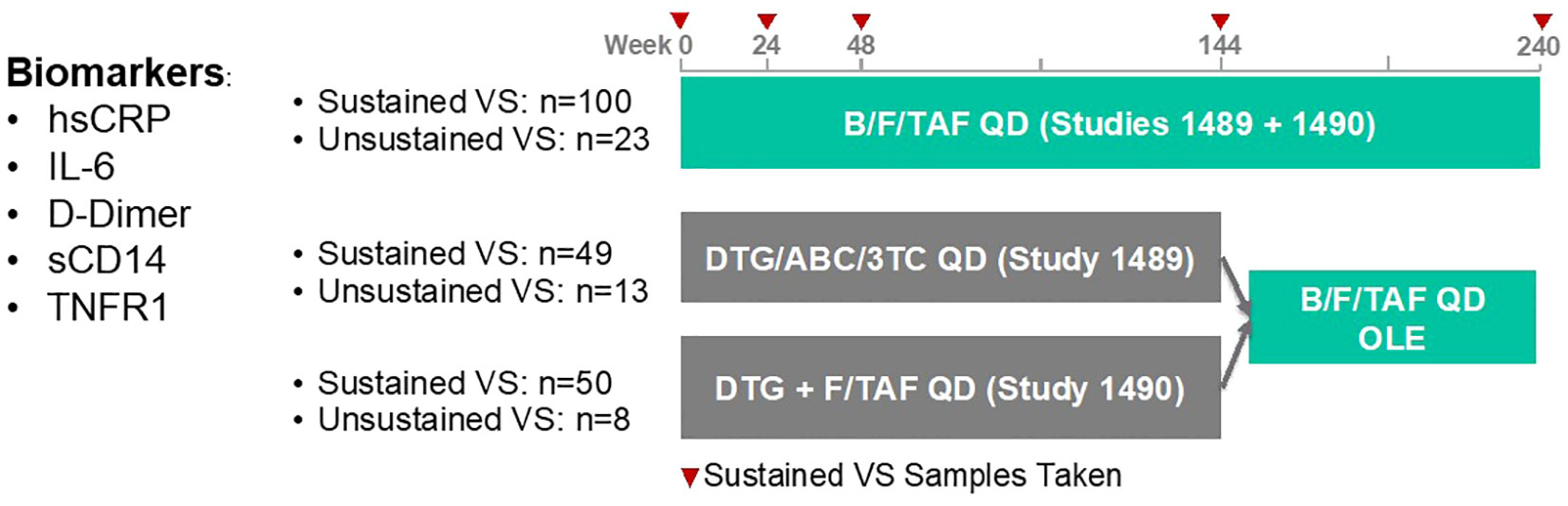
Figure 1. GS-US-380-1489/90 clinical study design and plan for retrospective biomarker analysis. We utilized cryopreserved samples from a sampling of treatment-naïve PWH in the United States who are enrolled in two Phase 3 clinical studies investigating the efficacy and safety of B/F/TAF, DTG/ABC/3TC and DTG+F/TAF over a 5-year window. Samples from sustained VS participants with baseline viral loads (VL) > 100,000 cp/mL were over selected. Participants with an underlying comorbid condition that complicated their inflammatory profiles were excluded.
Changes in inflammatory markers following ART initiation
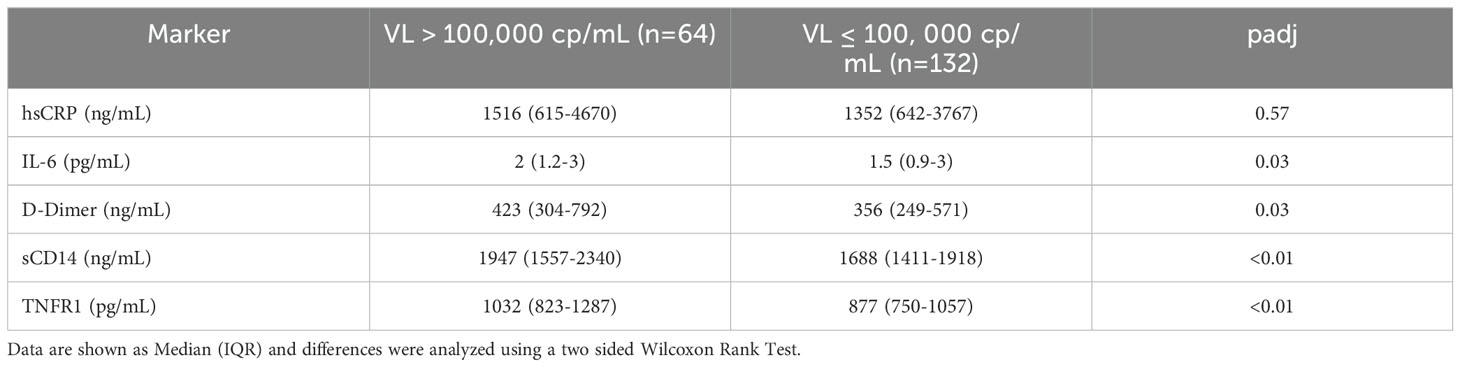
Serum levels of IL-6, hsCRP, D-dimer, sCD14, and TNFR1 were measured by ELISA in participants at baseline and selected time points. Levels of all biomarkers at baseline tended to be highest in the DTG+F/TAF group. Significant decreases (FDR<0.05) in levels of sCD14, D-dimer and TNFR1 from baseline were observed for each study arm and reductions were largely maintained throughout the randomized treatment timepoints as well as the open label extension (OLE, Figures 2A–C). IL-6 and hsCRP showed a tendency to decrease from baseline by week 48 across study arms (Figures 2D, E) but did not generally exhibit significant differences after multiple testing correction. No significant differences were observed in biomarker changes between treatment arms. As expected, most inflammatory markers were significantly higher (p<0.03 for all but hsCRP) at baseline in participants with high VLs (>100,000 copies/mL, N=64) compared to levels in participants who had lower VLs (<100,000 copies/mL, N=132) (Table 2). Nevertheless, changes in inflammatory markers were similar in participants with high and low VLs at baseline, with the magnitude of biomarker decreases largest in the high VL group (Supplementary Figure S1, S2).
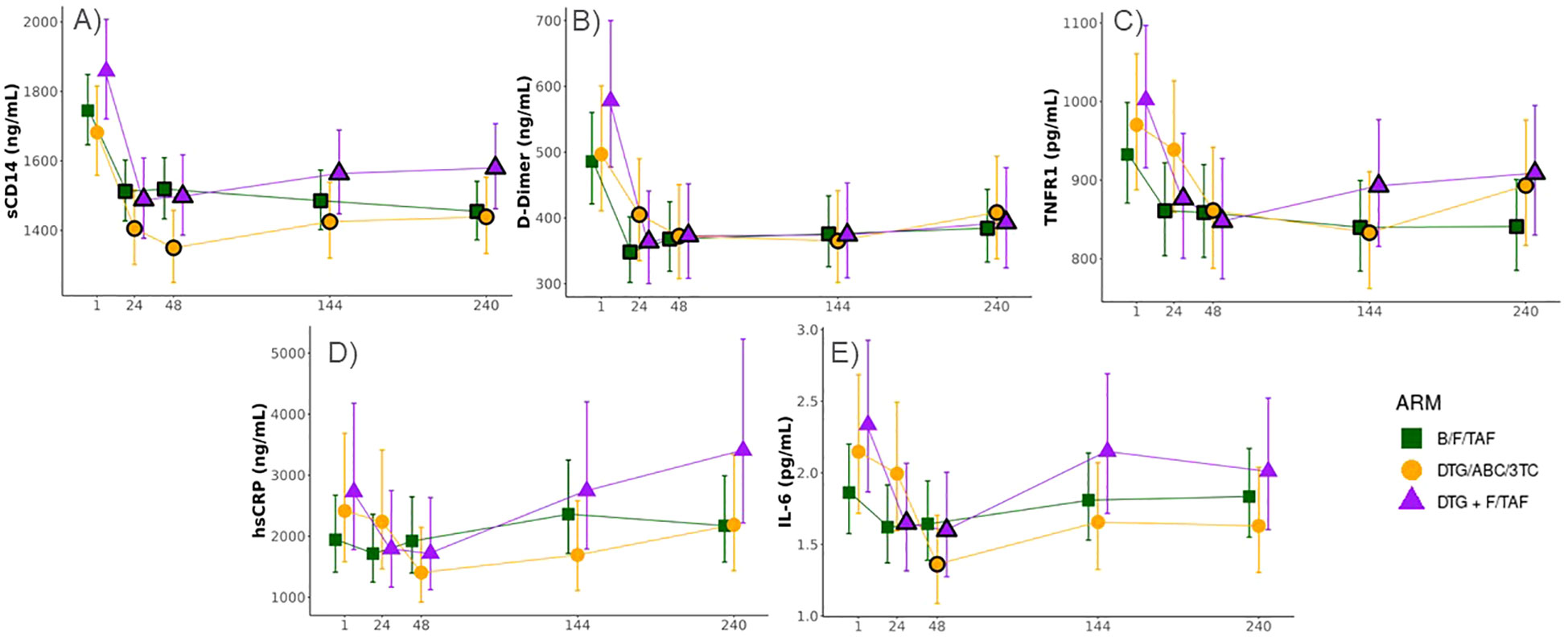
Figure 2. Significant decreases in biomarkers observed across all ART treatment arms in PWH. Longitudinal changes in serum biomarkers associated with inflammation (Least-square means ± 95%CI; statistical differences against baseline and between Week 144 and Week 240 were determined from log2 transformed data and a constrained mixed effects linear regression model adjusted for sex and baseline viral load). Levels of (A) sCD14, (B) D-Dimer, (C) TNFR1, (D) hsCRP, and (E) IL-6 were measured by enzyme linked immunosorbent assay (ELISA). Black borders on the symbols denote a significant difference from baseline at a false discovery rate (FDR) < 0.05.
We also sought to investigate relative longitudinal changes in these inflammatory biomarkers with changes in CD4+ T cell count and CD4+ percentage. Correlations were observed for all biomarkers and CD4+ T cell count and %CD4+ T-cells at baseline (Supplementary Table S1). Decreases of inflammatory biomarkers were inversely associated with increases in CD4+ T cell counts and %CD4+ T cells over time (Figures 3A, B, Supplementary Table S2A, B), independent of treatment arm and adjusted for changes in viral load. The strongest relationships were observed for IL-6 (p= 0.038 and 0.05, respectively), with a 1% decrease in IL-6 corresponding to a 68.03 cell increase in CD4+ T cells/µL (Supplementary Table S2A) and a 1.65% increase in %CD4 T-cells (Supplementary Table S2B). A similar pattern was observed between changes in CD4 T cell counts and hsCRP (p=0.04) and sCD14 (p<0.01) and changes in CD4% and D-Dimer (p=0.03), although the effect magnitudes on CD4 counts and CD4% were substantially smaller (Figure 3 and Supplementary Table S2A, B).
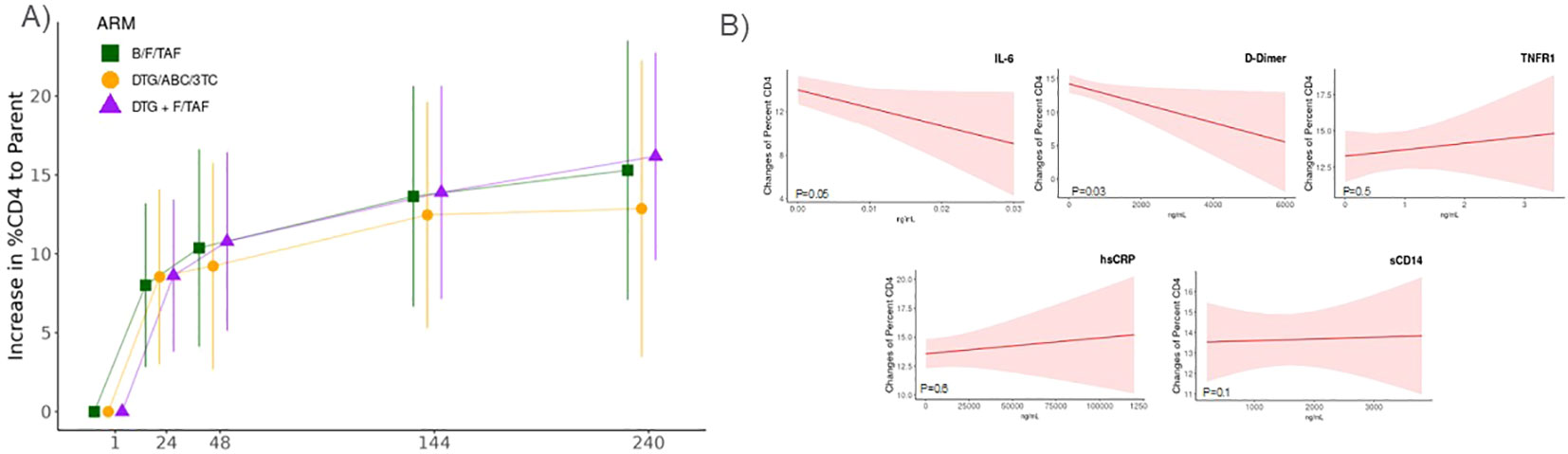
Figure 3. Changes in inflammatory markers were associated with increases in CD4+ T cell percentages. Association of changes in CD4+ T cell percentages and inflammatory biomarker levels derived from a linear mixed effect model adjusting for difference to baseline viral load, treatment arm, and time. (A) CD4+ percentage increases overtime in each treatment arm. (B) Changes in CD4+ T cell percentages (least-square means ± 95%CI) in relation to levels IL-6, D-Dimer, TNFR1, sCD14, or hsCRP that were measured by enzyme linked immunosorbent assay (ELISA).
Changes in inflammatory markers associated with viral blips
Next, we focused on a subgroup of participants from across all arms who experienced a period of intermittent viremia, or HIV-1 viral blips. A subset of samples from PWH who experienced a viral blip (n=44, defined as single 50 c/mL > HIV-1 RNA < 1000 c/mL) during treatment were analyzed and paired with the most recent suppressed sample before the blip. Associations between inflammation biomarkers and viral load were tested with a B-spline LMEM adjusting for baseline viral load, treatment arm, and time. A significant positive association (p=0.02) was observed between increasing viral load and sCD14 during blips (Figure 4A). Wilcoxon Signed Rank Test was then used to compare biomarker levels between paired control and blips samples. Levels of D-dimer were found to be significantly higher (p=0.04) in those with transient increases in viral load in the B/F/TAF arm (Figure 4B), but statistically significant differences were not detected in participants in the other arms.
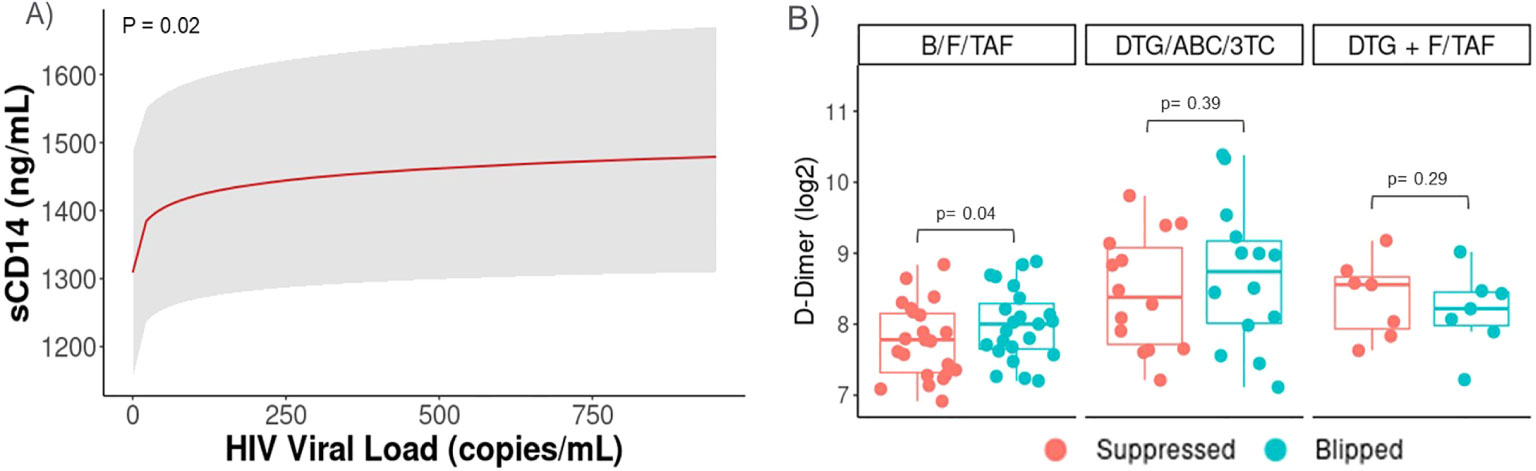
Figure 4. Transient viral elevations (Blips) were associated with increases in sCD14 and D-dimer levels. Data from PWH who experienced a viral blip (n=44, defined as single 50c/mL> HIV-1 RNA < 1000c/mL) during treatment were also analyzed and paired with the most recent suppressed sample before the blip. Participants with viral blips in each arm were included (B/F/TAF, n=23, DTG/ABC/3TC, n=13, and DTG+F/TAF, n=8). Associations between inflammation biomarkers and viral load were tested with a B-spline LMEM adjusting for baseline viral load, treatment arm, and time. Least-squares means and confidence intervals shown for (A) sCD14 and median (IQR) shown for (B) D-Dimer, with p-values tested by Wilcoxon Signed Rank Test.
Discussion
Persistent elevation in levels of markers of immune activation and inflammation are associated with morbidity and mortality in PWH, including the increased risk of cardiometabolic disease that has been reported in this population (2, 25). There are likely multiple potential mediators of chronic immune activation in this population, including low level HIV-1 replication, co-infections, microbial translocation, and alterations in proinflammatory lipid profiles (2, 25). Many of these mediators can drive activation of innate (i.e., monocytes, macrophages, natural killer cells) and adaptive (i.e., T and B lymphocytes) immune cells, triggering intracellular signaling cascades (e.g., NFκB, the inflammasome) that modulate gene and protein expression and increase production of pro-inflammatory molecules. Several strategies to reduce chronic immune activation in PWH have been explored, including the optimization of ART regimens. Here, in a comparison of three different ART regimens, those containing DTG, TAF and/or BIC, we report that each of these regimens reduce levels of several inflammatory markers (sCD14, TNFR1, D-dimer) that have been associated with morbidity and mortality in PWH (5, 7, 8) and that reductions in these molecules persisted for over 5 years.
Newer ART drugs with improved profiles for viral suppression and reduced toxicity are being developed. Studies with these new agents have also focused on comparisons of the speed, magnitude, and durability of reductions in immune activation and inflammation between drugs and drug regimens (1). We have previously reported that changes in immune activation markers were not different in ART- naïve PWH initiating a regimen with either tenofovir disoproxil fumarate (TDF) or TAF (22). As newer reverse transcriptase inhibitors become available, similar studies should be performed.
Integrase inhibitors may decrease inflammation and immune activation more than other antiretroviral classes. Mechanisms for this difference could include findings that integrase inhibitors may be more lipid neutral (26) and concentrate at higher levels in enterocytes (27), resulting in improved gut barrier integrity and reduced translocation of microbial products from the gut lumen. Reducing microbial translocation and limiting adverse lipid effects may improve chronic inflammation in PWH. We have reported rapid declines in markers of immune activation (including IL-6, hsCRP, D-dimer, TNFR1, and sCD14) in ART naïve participants initiating a raltegravir (RAL) based regimen (20). In a study where ART- naïve participants were randomized to elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (EVG/c/TDF/FTC) or efavirenz/TDF/FTC (EFV/TDF/FTC) changes in inflammatory markers were compared in a subset of participants with suppressed viremia at week 48 (28, 29). Initiation of the integrase inhibitor-based regimen resulted in greater and more rapid declines in hsCRP, sCD14, and the vascular inflammation marker Lp-PLA2; the magnitude of changes in other markers (IL-6, TNFR1, and sCD163) was similar between arms. In ACTG 5260s, where ART-naïve participants were randomized to receive TDF/FTC plus open-label RAL, atazanavir/ritonavir (ATV/r), or darunavir/ritonavir (DRV/r) (26) the changes in inflammatory markers across groups were inconsistent; hsCRP decreased with ATV/r and RAL by 96 weeks; IL-6 decreased with RAL, but not with ATV/r and DRV/r; D-dimer decreased with ATV/r and DRV/r, but was unchanged with RAL; markers of T cell activation and sCD163, but not sCD14, decreased in all groups (30). In the SPIRAL study, where participants were switched from a boosted protease inhibitor (PI) to RAL or maintained their boosted PI regimen, switching to RAL resulted in greater decreases in multiple inflammatory markers, including IL-6, hsCRP, and D-dimer (31). Further studies are needed as innovative drugs emerge, including the relatively newer integrase inhibitors (e.g., DTG, BIC, cabotegravir), to determine if certain integrase inhibitors may be more effective at reducing immune activation than others. In this study, we did not find a significant difference between the longitudinal changes in inflammatory marker levels when comparing participants receiving DTG to those receiving BIC. We cannot discount the small sample size of our study and the potential small effects size of the treatment arm leading to low power. The current study was also not designed to test whether integrase-based regimens are more effective at reducing inflammation compared to other regimens, but as noted above, this has been and can continue to be explored in future studies.
While no significant changes in IL-6 levels from baseline were observed, a decrease in the levels of this pro-inflammatory cytokine may have important consequences for overall immune health. Here, we report a significant relationship among increasing CD4+ T cell counts and %CD4+ T cells and decreasing levels of IL-6 among participants. Pro-inflammatory cytokines, including IL-6 and IL-1β, can drive CD4+ T cell proliferation, dysfunction, and death (32, 33). In a recent study in suppressed PWH on ART, testing the blockade of the biological effects of IL-6 by tocilizumab, multiple markers of immune activation and CD4+ T cell cycling and PD-1 expression were decreased, reducing inflammation and potentially improving CD4+ T cell function (23). In our study, we also found a relationship between decreases in levels of TNFR1 and %CD4+ T cell increases, but the magnitude of this effect was much smaller than that of IL-6, highlighting the potential biological significance of decreasing IL-6 in PWH.
Persistent low-level HIV-1 viremia has been associated with chronic inflammation (13, 14) but the immunologic consequences of intermittent low-level viremia (i.e., virologic blips) are less clear. Here, we report a significant association between intermittent viremia and levels of sCD14 across participants in all arms and a relationship between blips and D-dimer levels in participants within the B/F/TAF arm. We have shown relationships among sCD14, monocyte expression of the procoagulant molecule tissue factor, and D-dimer levels in PWH with suppressed and unsuppressed viremia (21, 24). Further, HIV-1 could directly induce the expression of tissue factor on CD14DimCD16+ monocytes (24) providing a plausible mechanism whereby increased levels of HIV-1 or viral replication products could drive monocyte activation and coagulation. Intermittent viremia may be caused by a number of factors, including inconsistent ART use, activation of a latently infected CD4+ T cell resulting in clonal expansion, or exacerbation of low level HIV-1 replication in tissue sites that becomes detectable in the circulation (34). We cannot determine the exact causes of virologic blips among participants in this study, but future studies designed to explore the causes and immunologic consequences of virologic blips are appropriate.
While this study provides insights into the relationships among changes in immune activation and CD4+ T cells during 5 years of ART treatment, and potential linkages between intermittent viremia and immune cell activation in PWH, there are limitations that should be considered. We did not find statistically significant changes in levels of IL-6 or hsCRP in study participants. This may be due, in part, because the baseline levels of these factors fell within the normal range (34, 36). Participants initiated ART with reasonably high levels of CD4+ T cell numbers, resulting in lower levels of IL-6 and hsCRP compared to other studies that show relationships between pre-ART levels of inflammation and morbidity/mortality (4, 5, 8). The relatively small sample size of participants who experienced a virologic blip and the indeterminant nature of the cause(s) of these blips should also be considered. Further, while all biomarkers measured in this study fell, to varying degrees, by week 48, many of the markers had slight upward trajectories at later timepoints. Week 240 for many participants fell during the onset of the COVID-19 pandemic and while we excluded for COVID diagnoses we cannot confirm whether or not all cases were captured, which could reasonably have influenced markers of immune activation and inflammation in this population. Even with these considerations, this work demonstrates the success of ART in blocking viral replication and improving immune profiles in PWH and suggests that viral blips may contribute to monocyte activation and coagulation in this population. Understanding the causes and immunologic consequences of persistent and intermittent HIV viremia should be explored further.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The Office of Responsible Research Practices at OSU determined this study was exempt as no direct participant interactions occurred or identifiable information was provided. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NF: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – original draft. SH: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. CC: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing. KA: Data curation, Investigation, Methodology, Writing – review & editing. MC: Data curation, Investigation, Methodology, Writing – review & editing. JL: Conceptualization, Project administration, Resources, Writing – review & editing. BN: Conceptualization, Project administration, Resources, Writing – review & editing. KW: Conceptualization, Project administration, Resources, Writing – review & editing. JW: Conceptualization, Project administration, Resources, Writing – review & editing. BD: Conceptualization, Data curation, Formal analysis, Resources, Writing – review & editing. GM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by a collaborative study between N.F. and Gilead. The commercial funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. Further support provided by the Clinical and Translational Science Collaborative of Northern Ohio which is funded by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health, UM1TR004528 (to GM).
Acknowledgments
The authors would like to thank Peisong Han and Zhiwei Zhang for their guidance on the statistical approaches.
Conflict of interest
Authors SH, CC, JL, BN, KW, JW, and BD were employed by the company Gilead Sciences Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1488799/full#supplementary-material
Supplementary Figure 1 | Levels of inflammatory markers overtime in participants with high baseline viral load. Longitudinal changes in serum biomarkers associated with inflammation (least-square means ± 95%CI; statistical differences against baseline and between Week 144 and Week 240 were determined from log2 transformed data and a constrained mixed effects linear regression model adjusted for baseline viral load and sex Levels of A) sCD14, B) D-Dimer, C) TNFR1, D) hsCRP, and E) IL-6 were measured by enzyme linked immunosorbent assay (ELISA). Black borders on the symbols denote a significant difference from baseline at a false discovery rate (FDR) < 0.05.
Supplementary Figure 2 | Levels of inflammatory markers overtime in participants with low baseline viral load. Longitudinal changes in serum biomarkers associated with inflammation (Least-square means ± 95%CI; statistical differences against baseline and between Week 144 and Week 240 were determined from log2 transformed data and a constrained mixed effects linear regression model adjusted for baseline viral load and sex). Levels of A) sCD14, B) D-Dimer, C) TNFR1, D) hsCRP, and E) IL-6 were measured by enzyme linked immunosorbent assay (ELISA). Black borders on the symbols denote a significant difference from baseline at a false discovery rate (FDR) < 0.05.
Supplementary Table 1 | Baseline biomarker levels are negatively associated with CD4% and CD4 Counts. Levels of plasma biomarkers were measured by enzyme linked immunosorbent assay (ELISA) and spearman correlations between baseline biomarkers and measures of CD4+ T cells are shown.
Supplementary Table 2 | Increases in IL-6 from Baseline is associated with significant reduction in the increase in A) CD4+ T cell counts and B) percent CD4+ T cells. There were no differences between treatment arms.
References
1. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. (2017) 14:93–100. doi: 10.1007/s11904-017-0356-x
2. Gabuzda D, Jamieson BD, Collman RG, Lederman MM, Burdo TH, Deeks SG, et al. Pathogenesis of aging and age-related comorbidities in people with HIV: highlights from the HIV ACTION workshop. Pathog Immun. (2020) 5:143–74. doi: 10.20411/pai.v5i1.365
3. Psomas C, Younas M, Reynes C, Cezar R, Portales P, Tuaillon E, et al. One of the immune activation profiles observed in HIV-1-infected adults with suppressed viremia is linked to metabolic syndrome: The ACTIVIH study. EBioMedicine. (2016) 8:265–76. doi: 10.1016/j.ebiom.2016.05.008
4. Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. (2014) 210:1248–59. doi: 10.1093/infdis/jiu254
5. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PloS Med. (2008) 5:e203. doi: 10.1371/journal.pmed.0050203
6. Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. (2008) 197:1133–44. doi: 10.1086/586713
7. Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. (2011) 203:780–90. doi: 10.1093/infdis/jiq118
8. Kalayjian RC, Machekano RN, Rizk N, Robbins GK, Gandhi RT, Rodriguez BA, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. (2010) 201:1796–805. doi: 10.1086/652750
9. Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. (2010) 33:2244–9. doi: 10.2337/dc10-0633
10. McComsey GA, Kitch D, Sax PE, Tierney C, Jahed NC, Melbourne K, et al. Associations of inflammatory markers with AIDS and non-AIDS clinical events after initiation of antiretroviral therapy: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Acquired Immune Deficiency Syndromes (1999). (2014) 65:167–74. doi: 10.1097/01.qai.0000437171.00504.41
11. Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PloS One. (2016) 11:e0155100. doi: 10.1371/journal.pone.0155100
12. Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci. (2015) 70:1542–7. doi: 10.1093/gerona/glv107
13. Kilroy JM, Leal AA, Henderson AJ. Chronic HIV transcription, translation, and persistent inflammation. Viruses. (2024) 16:751. doi: 10.3390/v16050751
14. Crespo-Bermejo C, de Arellano ER, Lara-Aguilar V, Valle-Millares D, Gomez-Lus ML, Madrid R, et al. Persistent low-Level viremia in persons living with HIV undertreatment: An unresolved status. Virulence. (2021) 12:2919–31. doi: 10.1080/21505594.2021.2004743
15. Orkin C, Antinori A, Rockstroh JK, Moreno-Guillen S, Martorell CT, Molina JM, et al. Switch to bictegravir/emtricitabine/tenofovir alafenamide from dolutegravir-based therapy. AIDS (London England). (2024) 38:983–91. doi: 10.1097/QAD.0000000000003865
16. Orkin C, DeJesus E, Sax PE, Arribas JR, Gupta SK, Martorell C, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV. (2020) 7:e389–400. doi: 10.1016/S2352-3018(20)30099-0
17. Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink HJ, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. (2017) 390:2073–82. doi: 10.1016/S0140-6736(17)32340-1
18. Stellbrink HJ, Arribas JR, Stephens JL, Albrecht H, Sax PE, Maggiolo F, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. (2019) 6:e364–e72. doi: 10.1016/S2352-3018(19)30080-3
19. Sax PE, Arribas JR, Orkin C, Lazzarin A, Pozniak A, DeJesus E, et al. Bictegravir/emtricitabine/tenofovir alafenamide as initial treatment for HIV-1: five-year follow-up from two randomized trials. EClinicalMedicine. (2023) 59:101991. doi: 10.1016/j.eclinm.2023.101991
20. Funderburg NT, Andrade A, Chan ES, Rosenkranz SL, Lu D, Clagett B, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naive patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PloS One. (2013) 8:e83514. doi: 10.1371/journal.pone.0083514
21. Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. (2010) 115:161–7. doi: 10.1182/blood-2009-03-210179
22. Funderburg NT, McComsey GA, Kulkarni M, Bannerman T, Mantini J, Thornton B, et al. Equivalent decline in inflammation markers with tenofovir disoproxil fumarate vs. Tenofovir alafenamide. EBioMedicine. (2016) 13:321–7. doi: 10.1016/j.ebiom.2016.10.009
23. Funderburg NT, Shive CL, Chen Z, Tatsuoka C, Bowman ER, Longenecker CT, et al. Interleukin 6 blockade with tocilizumab diminishes indices of inflammation that are linked to mortality in treated human immunodeficiency virus infection. Clin Infect Dis. (2023) 77:272–9. doi: 10.1093/cid/ciad199
24. Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. (2012) 120:4599–608. doi: 10.1182/blood-2012-05-433946
25. Dirajlal-Fargo S, Funderburg N. HIV and cardiovascular disease: the role of inflammation. Curr Opin HIV AIDS. (2022) 17:286–92. doi: 10.1097/COH.0000000000000755
26. Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med. (2014) 161:461–71. doi: 10.7326/M14-1084
27. Patterson KB, Prince HA, Stevens T, Shaheen NJ, Dellon ES, Madanick RD, et al. Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure. AIDS (London England). (2013) 27:1413–9. doi: 10.1097/QAD.0b013e32835f2b49
28. Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis. (2015) 212:345–54. doi: 10.1093/infdis/jiv004
29. Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. (2012) 379:2439–48. doi: 10.1016/S0140-6736(12)60917-9
30. Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, et al. Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis. (2015) 61:651–60. doi: 10.1093/cid/civ327
31. Martinez E, D’Albuquerque PM, Llibre JM, Gutierrez F, Podzamczer D, Antela A, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS (London England). (2012) 26:2315–26. doi: 10.1097/QAD.0b013e328359f29c
32. Shive CL, Clagett B, McCausland MR, Mudd JC, Funderburg NT, Freeman ML, et al. Inflammation perturbs the IL-7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquired Immune Deficiency Syndromes (1999). (2016) 71:483–92. doi: 10.1097/QAI.0000000000000913
33. Shive CL, Mudd JC, Funderburg NT, Sieg SF, Kyi B, Bazdar DA, et al. Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis. (2014) 210:619–29. doi: 10.1093/infdis/jiu125
34. Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS. (2016) 11:417–23. doi: 10.1097/COH.0000000000000287
35. Pagana KD, Pagana TJ, Pagana TN. Mosby’s diagnostic and laboratory test reference. Sixteenth Vol. xxiv. . St. Louis, Missouri: Elsevier (2023). p. 1031.
Keywords: HIV-1, antiretroviral therapy, inflammation, intermittent viremia, monocyte activation
Citation: Funderburg NT, Huang SSY, Cohen C, Ailstock K, Cummings M, Lee JC, Ng B, White K, Wallin JJ, Downie B and McComsey GA (2024) Changes to inflammatory markers during 5 years of viral suppression and during viral blips in people with HIV initiating different integrase inhibitor based regimens. Front. Immunol. 15:1488799. doi: 10.3389/fimmu.2024.1488799
Received: 30 August 2024; Accepted: 24 October 2024;
Published: 12 November 2024.
Edited by:
Mariza Gonçalves Morgado, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Christina K. Psomas, Hôpital Européen Marseille, FranceFrancesca Cossarini, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2024 Funderburg, Huang, Cohen, Ailstock, Cummings, Lee, Ng, White, Wallin, Downie and McComsey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas T. Funderburg, bmljaG9sYXMuZnVuZGVyYnVyZ0Bvc3VtYy5lZHU=
 Nicholas T. Funderburg
Nicholas T. Funderburg Susie S. Y. Huang
Susie S. Y. Huang Calvin Cohen
Calvin Cohen Kate Ailstock1
Kate Ailstock1 Kirsten White
Kirsten White Bryan Downie
Bryan Downie Grace A. McComsey
Grace A. McComsey