
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 10 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1485053
This article is part of the Research Topic Therapeutic Mechanism of Osteosarcoma View all 10 articles
Objective: The objective of this study is to conduct a bibliometric analysis on examining the current condition, areas of interest, and rising trends of immunotherapy for osteosarcoma (ITFOS), as well as its importance in associated research domains.
Methods: An extensive collection of academic papers on the use of ITFOS was obtained from the Web of Science between January 1, 2000 and October 20, 2023. Then, using a variety of tools like HisCite, VOSviewer, CiteSpace, and the bibliometrix package, a bibliometric study was carried out. This study included the collection of information on country, institution, author, journal, and keywords.
Results: A comprehensive analysis was undertaken on a total of 616 publications obtained from 247 journals, encompassing the contributions of 3725 authors affiliated with 831 institutes spanning across 43 countries/regions. Notably, China exhibited the highest quantity of published 277 (44.99%) articles on ITFOS. The most productive institution was Zhejiang University, with 26 (4.22%) publications. The author with the highest publication output was Tsukahara, Tomohide from Japan with 15 (2.44%) publications. The article with the most citation was “DOI: 10.1200/JCO.2014.58.0225”. Frontiers in Immunology demonstrated the highest level of productivity, having published a total of 31 (5.03%) articles. The most frequently used were “osteosarcoma,” “immunotherapy,” and “cancer,”. Meanwhile, “sequencing”, “prognostic signature” and “immune microenvironment“ have been identified as the research frontiers for the forthcoming years.
Conclusion: This paper provides a thorough evaluation of current research trends and advancements in ITFOS. It includes relevant research findings and emphasizes collaborative efforts among authors, institutions, and countries.
Osteosarcoma is the most common primary malignant bone tumor characterized by highly aggressive and metastatic behavior, accounting for approximately 56% of primary malignant bone tumors (1). It predominantly affects children and adolescents with a median age of 16 years and occurs most frequently in the metaphysis of long bones, including the distal femur and proximal tibia (2). According to the 5th edition of the World Health Organization (WHO) classification of bone and soft tissue tumors, osteosarcoma is classified into several subtypes, including low-grade central osteosarcoma, conventional osteosarcoma, telangiectatic osteosarcoma, small cell osteosarcoma, parosteal osteosarcoma, periosteal osteosarcoma, high-grade surface osteosarcoma, and secondary osteosarcoma (3). Surgical intervention is an important treatment modality for osteosarcoma. With the introduction of neoadjuvant chemotherapy, the combination of chemotherapy and surgery has improved the 5-year survival rate to 70% for non-metastatic osteosarcoma patients. However, for advanced and recurrent osteosarcoma patients, despite the incorporation of various chemotherapy regimens, the treatment outcomes have remained poor based on decades of research, with a 5-year survival rate of only 20% (4, 5). Consequently, there is an urgent need to explore novel treatment approaches that can fundamentally improve the prognosis of osteosarcoma. In recent years, researchers have made significant progress in the study of tumor immune responses, leading to the development of targeted therapies focusing on T cells or their receptors in the tumor microenvironment. One such example is the use of immune checkpoint inhibitors, such as PD-1/PD-L1 monoclonal antibodies, which have shown promising therapeutic effects in various types of tumors, including melanoma, lung cancer, and breast cancer (6–9). These successes have reignited the enthusiasm for tumor immunotherapy research. In the case of osteosarcoma, researchers have increasingly conducted basic research and clinical trials exploring the potential of immunotherapy due to the challenges faced in achieving standardized treatment for osteosarcoma patients.
In the field of science, bibliometric analyses are frequently used to evaluate published research and predict future trends. The field of bibliometrics examines the relationships between scientific fields, countries, organizations, authors, and publications by using mathematical and statistical approaches (9, 10). In recent years, significant advancements have been made in the study of ITFOS, yet a bibliometric analysis of this research is lacking. This study aims to conduct a bibliometric analysis of ITFOS research. By utilizing knowledge maps, scientists can efficiently analyze large datasets and gain insights into the development and emerging trends in this field. This methodology enhances the ability to identify research hotspots and allows for a comprehensive examination of research patterns. Furthermore, the analysis may offer valuable insights for future research projects and decision-making processes.
Using the Web of Science Core Collection (WoSCC), a literature search was carried out at Dalian Municipal Central Hospital on October 20th, 2023. Use the following search parameters to find results: (((TS = (Osteosarcoma OR Osteosarcomas OR Osteosarcoma Tumor OR Osteosarcoma Tumors OR Tumor, Osteosarcoma OR Tumors, Osteosarcoma OR Sarcoma, Osteogenic OR Osteogenic Sarcomas OR Sarcomas, Osteogenic OR Osteogenic Sarcoma)) AND TS=(immunotherapy)) AND DT= (Article OR Review)) AND LA=(English). Articles that mentioned ITFOS or its synonyms in their title, abstract, or keywords were found as a result of the search query. Articles and reviews published between January 1, 2000, and October 20, 2023 were the only document kinds included in the search; publications earlier than January 1, 2000, case reports, meeting abstracts, editorial materials, and other document types were not included. Documents written in the English language were the only ones that met the inclusion criterion.
On October 20, 2023, a literature search query was performed in order to retrieve data from the WoSCC. The information retrieved covered a wide range of features of the literature, including authorship, title, source, sponsorship, citation count, accession number, abstract, address, document type, and cited references. To aid further analysis, the data was collected in both txt and BibTex formats. Web of Science was used to obtain the H-index of the top 10 authors with the most publication output. Furthermore, the 2022 impact factor and Journal Citation Report category quartile of the ten key journals relevant to ITFOS were obtained from Web of Science.
HisCite (version 12.03.17), VOSviewer (version 1.6.18), CiteSpace (version 6.1.R3), and the bibliometrix package (version 3.2.1; https://cran.r-project.org/web/packages/bibliometrix/) based on R language (version 4.1.2) were used to analyze the bibliometric data. HisCite was used to calculate the total number of publications and citations for producing countries, institutions, and authors. In addition, using HisCite, the top ten papers with the highest citation count in ITFOS were discovered. The yearly count of publications was calculated using HisCite and graphically represented in the R programming language using the ggplot2 package (version 3.3.6; https://github.com/tidyverse/ggplot2). VOSviewer was used to identify the top 10 keywords with the highest occurrence, bibliometric coupling within journals, and clustering of the top 54 keywords. CiteSpace was also used to create a dual-map overlay of the journals connected with ITFOS. CiteSpace was used to assess the level of collaborative centrality among countries/regions, institutions, and authors. Following that, trend topic detection within the bibliomatrix program was used as an alternate methodology. This program was also used to build visual representations of publication volume and collaborative relationship networks. When analyzing different subjects, it’s important to choose appropriate tools based on their specific characteristics. For example, in clustering diagrams for keywords, countries, and authorses,sti algorithmic influence is minimalcei focus on the compactness and aesthetics, making VOSviewer a suitable choice. In contrast, Citespace is better for citation bursts, co-citation networks, and timelines. The bibliometrix R package provides intuitive visualizations for trend topics, while the interactive tool biblioshiny has limited interactivity. However, bibliometrix also allows for custom visualizations, such as keyword time heatmaps. These tool differences necessitate careful selection based on research objectives. To mitigate discrepancies from using different tools, we analyze identified trends or clusters with multiple tools and compare the results. If consistent trends are found across tools for the same keyword or topic, we consider these findings robust. In our final reports, we prioritize keywords or topics identified as hotspots across various analyses to ensure the reliability and generalizability of the results.
A comprehensive search was conducted in the WoSCC database, resulting in the identification of 616 publications pertaining to ITFOS. The search period spanned from January 1, 2000, to October 20, 2023 (Figure 1).
Among these publications, 469 were categorized as original articles, while 147 were classified as review articles. Notably, the frequency of ITFOS-related publications exhibited an irregular pattern, albeit showing an overall upward trend in terms of total citations (Figure 2).
It is worth mentioning that the proportion of original articles consistently surpassed that of review articles on an annual basis. The cumulative collection of published articles has garnered a total of 14355 citations, resulting in an average of 23.31 citations per article, which holds significant scholarly value.
From January 1, 2000, to October 20, 2023, scholarly articles on ITFOS were disseminated across 43 countries/regions spanning six continents. Notable collaboration was observed among East Asia, North America, and Western Europe (Figure 3).
This study highlights the top 10 countries in terms of productivity in publishing articles on ITFOS. China emerged as the most prolific country, contributing 277 (44.99%) articles, followed by USA with 192 (31.17%) articles, Japan with 68 (11.04%) articles, Italy with 32 (5.19%) articles, and Germany with 25 (4.06%) articles. It is noteworthy that articles originating from the United States received the highest total number of citations, amounting to 6826, while articles from Germany had the highest average number of citations per article, with 57.16 (Table 1).
Through an extensive investigation, a total of 616 publications were identified, authored by 3725 individuals affiliated with 831 institutes across 43 countries/regions. Among the identified institutes, Zhejiang University in the China emerged as the most prolific, contributing 26 (4.22%) publications. This was followed by the Central South University, China with 22 (3.57%) publications, University of Texas MD Anderson Cancer Center, USA with 21 (3.41%) publications, Sapporo Medical University in Japan with 20 (3.25%) publications, and Shanghai Jiao Tong University, China with18 (2.92%) publications. Notably, among the top ten institutions, six were located in the China, three in USA, and one in Japan (Table 2).
The study also revealed four distinct clusters of institutional collaboration, with Zhejiang University, University of Texas MD Anderson Cancer Center, Nationwide Children’s Hospital and National Cancer Institute (NCI) exhibiting the highest level of collaboration (Figure 4A). Regarding authors, the most prolific author identified was Tsukahara, Tomohide, Japan with 15 (2.44%) publications, followed by Sato, Noriyuki, Japan with 13 (2.11%) publications, and Torigoe, Toshihiko, Japan, with 13 (2.11%) publications. Among the top ten most productive authors, four were affiliated with institutions in Japan, two with institutions in China, one with an institution in USA, one with an institution in Germany, and one with an institution in Australia. Notably, Gottschalk, Stephen from Germany had the highest H-index of 64 (Table 3).
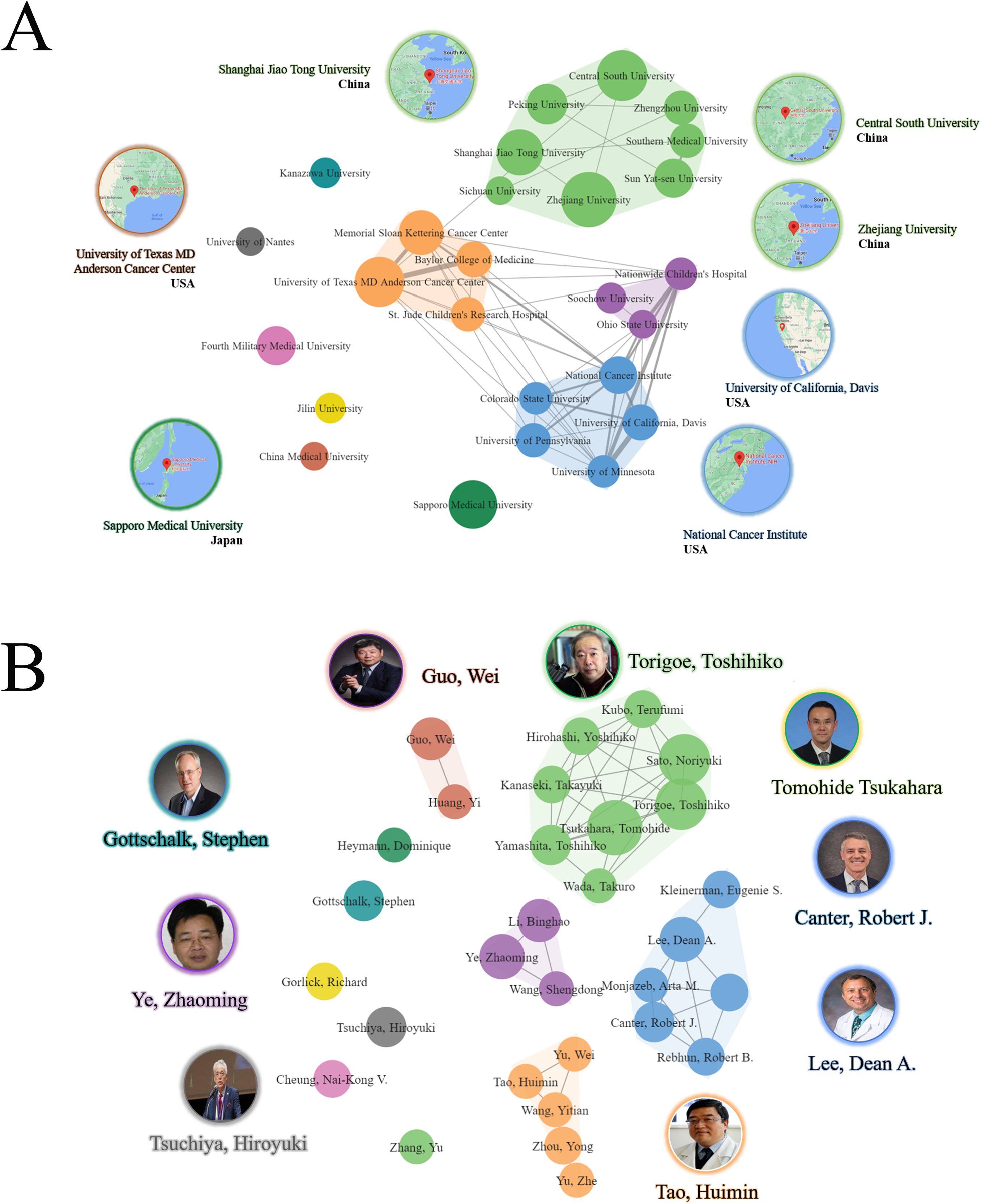
Figure 4. Collaborative clustering of institutions and authors. (A) Collaborative clustering of institutions. (B) Collaborative clustering of authors.
The authors demonstrated a notable level of cooperation, as evidenced by the presence of five clusters. Torigoe, Toshihiko, Tsukahara, Tomohide, Canter, Robert J., Tao Huimin, and Lee, Dean A. displayed a significant degree of collaborative centrality (Figure 4B).
A total of 43 journals have published research on ITFOS. Among these journals, Frontiers in Immunology demonstrated the highest productivity, publishing 31 (5.03%) articles related to ITFOS. This was followed by Frontiers in Oncology with 25 (4.07%) articles, Cancers with 20 (3.25%) articles, Journal for Immunotherapy of Cancer with 15 (2.44%) articles, and International Journal of Molecular Sciences with 11 (1.79%) articles. Notably, Clinical Cancer Research achieved the highest average citation rate, with an average of 74.40 citations per article (Table 4).
The dual-map overlay revealed a single citation pathway among the numerous inter-domain linkages between journals. Interestingly, publications in Molecular/Biology/Immunology were primarily referenced by publications in Molecular/Biology/Genetics (Figure 5A). Bradford’s Law, a bibliometric principle, describes the distribution of scientific literature in a specific field. It suggests that a few core information sources or journals contribute significantly to the published research in that field. In the context of ITFOS research, three clusters were identified, Frontiers in Immunology, Cancers, and Oncoimmunology emerged as the top three influential journals (Figure 5B).
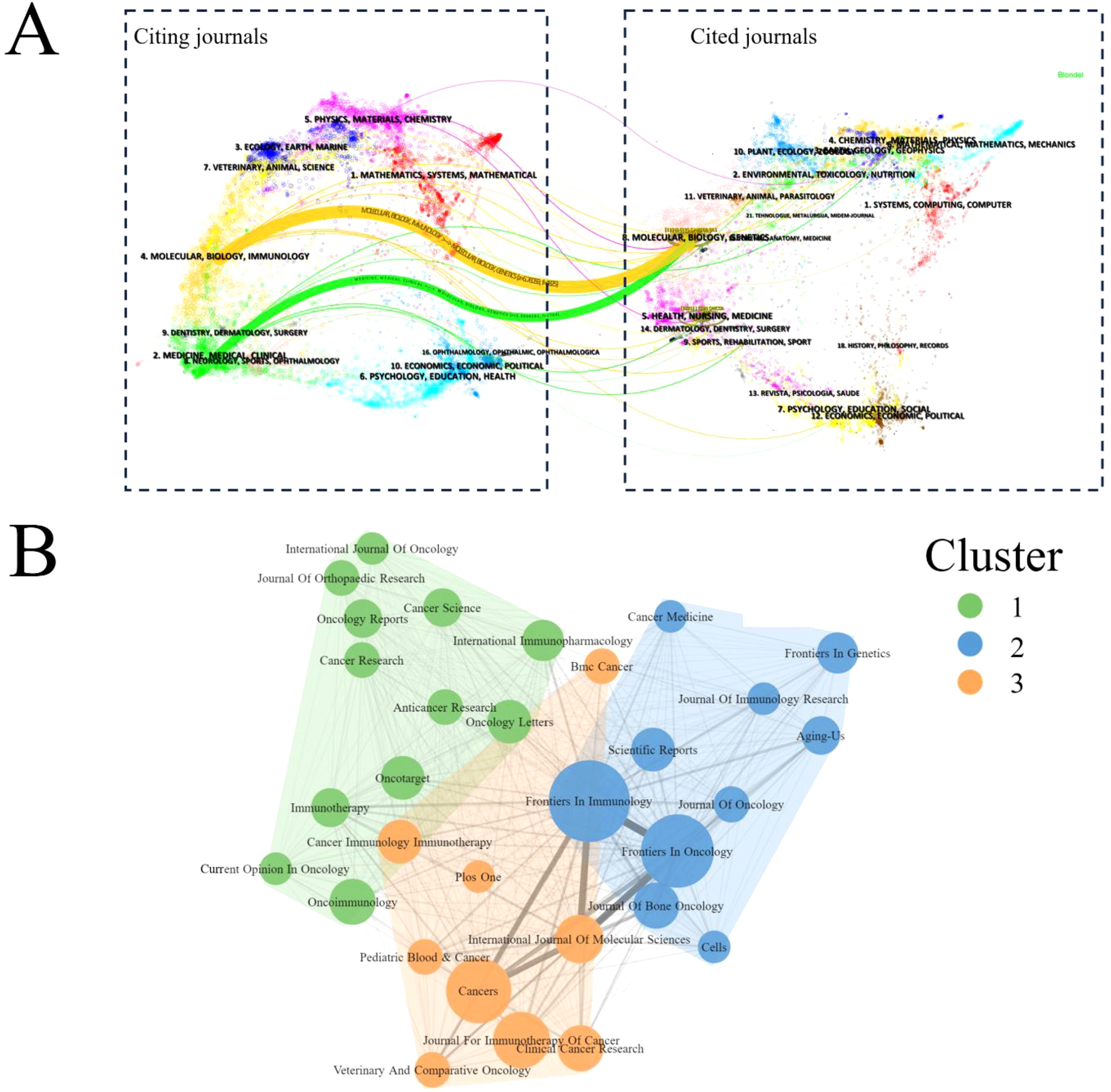
Figure 5. Analysis of journals. (A) The dual-map overlay of journals publishing studies on ITFOS. Citing journals are on the left, cited journals are on the right, and lines represent the citation relationship. (B) Bibliometric coupling within journals. Three clusters were identified based on journals that had published more than five articles.
Clusters located on the right-hand side, characterized by a higher incidence of red nodes, indicate a greater prevalence of recent references. The clusters labeled “#0” (TME) and “#2” (tumor-infiltrating lymphocytes) were found to be the most temporally proximate (Figure 6A). A list of the top 10 papers with the most citations can be found in (Table 5). The article with the most citation was “DOI: 10.1200/JCO.2014.58.0225”. Ahmed et al. conducted a study that evaluated the safety and efficacy of HER2-CAR T cells in cancer patients. They found that these cells can persist for 6 weeks without significant toxicities, paving the way for future studies that combine HER2-CAR T cells with other immunomodulatory approaches to enhance their expansion and persistence (11).
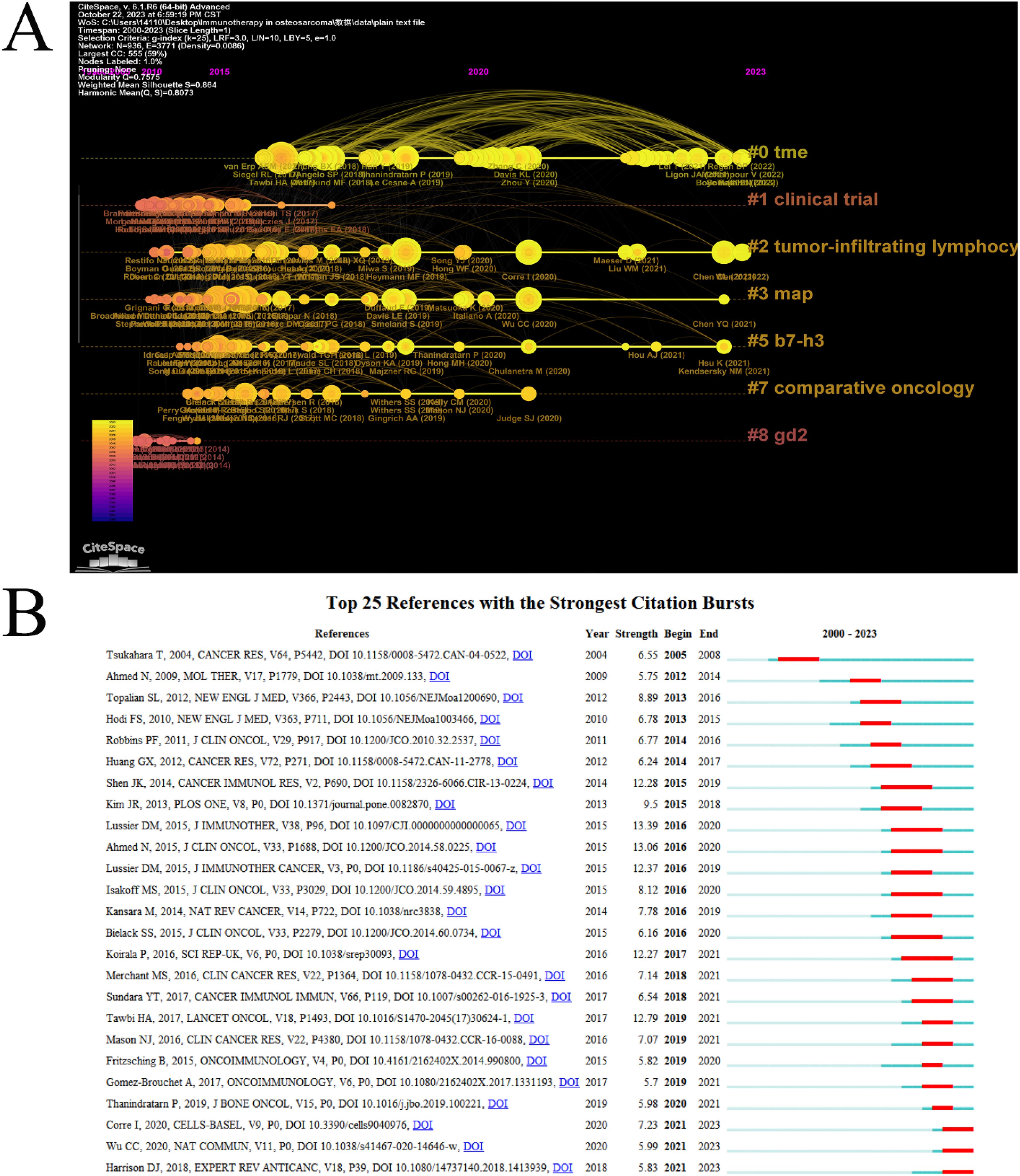
Figure 6. Analysis of citations and references. (A) Timeline of co-cited references related to ITFOS. (B) Reference burst detection of the top 25 references with the strongest emergent strength.
Moreover, reference burst detection was employed to identify research frontiers and emerging reference. The study examined the top 25 references with the most robust emergent properties. The reference “ doi: 10.3390/cells9040976” (21) “ doi: 10.1038/s41467-020-14646-w” (22) and “ doi: 10.1080/14737140.2018.1413939” (23) were identified as the most emergent reference in 2023 (Figure 6B).
We presented the fifteen most frequently appearing keywords in ITFOS research after consolidating synonymous terms, with “ osteosarcoma “ being the most commonly referenced (Table 6).
An analysis of keyword co-occurrence among the top 43 keywords revealed the presence of five distinct clusters. The cluster consisting of “osteosarcoma,” “immunotherapy,” “cancer,” “chemotherapy,” and “blockade” exhibited the highest frequency of occurrence (Figure 7A). Furthermore, an examination of the trend topics from 2000 to 2023 identified “sequencing”, “prognostic signature” and “immune microenvironment “ as the research frontiers for the upcoming years (Figure 7B).
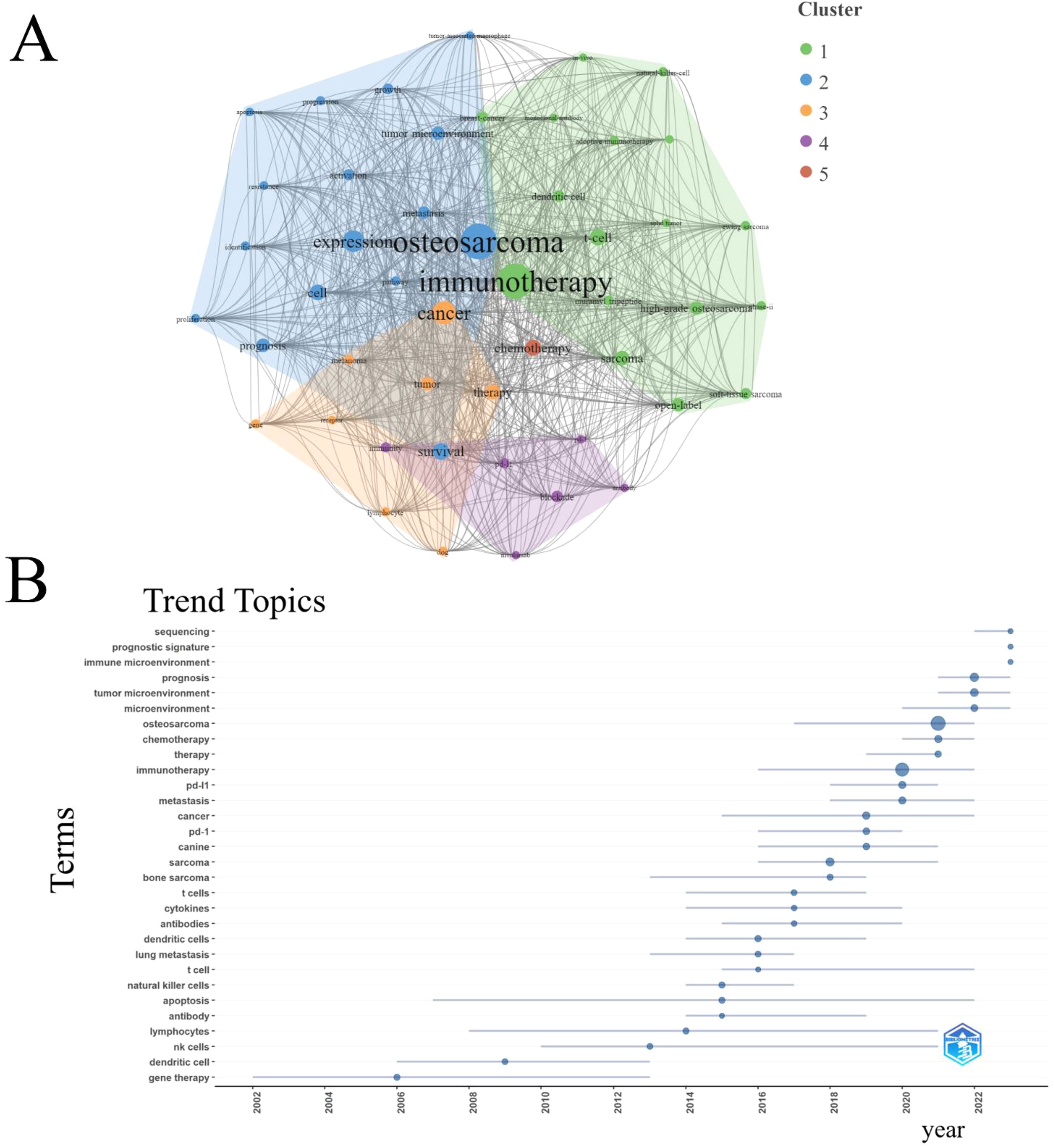
Figure 7. Analysis of keywords. (A) Clustering of the top 43 keywords with the highest number of occurrences. (B) Trend topics from 2000 to 2023.
As early as 1891, the potential of immunotherapy for the treatment of osteosarcoma was recognized (24). In recent years, new immunotherapy approaches have emerged rapidly, and the scope of treatment has been expanding, bringing a hopeful outlook for breakthroughs in osteosarcoma treatment, which has been stagnant for several decades (25, 26). Currently, a substantial body of preclinical experiments supports the significant potential of immunotherapy in the treatment of osteosarcoma. Furthermore, several clinical trials have been initiated, involving various aspects such as T cells and immune checkpoint inhibitors (27, 28). Therefore, we conducted a bibliometric analysis and found that China exhibited the highest quantity of published 277 (44.99%) articles on ITFOS. The most productive institution was Zhejiang University, with 26 (4.22%) publications. China and Zhejiang University lead in publication counts. Possible reasons include: Osteosarcoma is a highly heterogeneous malignant tumor with a complex pathogenesis involving multiple biological processes, prompting researchers to explore it in depth. The study of osteosarcoma involves multiple disciplines, including oncology, molecular biology, immunology, and pharmacology, which fosters interdisciplinary collaboration and innovation. Advances in technology, such as genomics, transcriptomics, and immunotherapy, provide new research tools and platforms that enhance the progress of osteosarcoma research. Furthermore, international collaboration is also significant, with many research institutions and teams engaging in joint efforts in the field, sharing resources and data to facilitate rapid scientific outcomes. Additionally, increased funding support from governments and academic institutions has led to growing attention on osteosarcoma research, enabling the implementation of related projects and boosting research output. Lastly, active academic exchanges through frequent conferences and forums offer researchers opportunities to share and showcase their findings, further promoting scientific development. The study also revealed four distinct clusters of institutional collaboration, with Zhejiang University, University of Texas MD Anderson Cancer Center, Nationwide Children’s Hospital and National Cancer Institute (NCI) exhibiting the highest level of collaboration. The benefits of closer collaboration between research institutions include: Resource sharing: Collaborative institutions can share equipment, data, and funding, which enhances research efficiency and reduces costs. Improved research quality: Through collaboration, research teams can gain broader perspectives and specialized skills, thereby increasing the rigor and credibility of their research. Accelerated translation of results: Close collaboration can shorten the time required to translate research findings into practical applications, facilitating rapid technology dissemination. Enhanced impact: Collaboration enables research institutions to better expand the influence of their findings and enhance their reputation within the academic community and society at large. The author with the highest publication output was Tsukahara, Tomohide from Japan with 15 (2.44%) publications. Frontiers in Immunology demonstrated the highest level of productivity, having published a total of 31 (5.03%) articles. The most frequently used keywords were “osteosarcoma,” “immunotherapy,” and “cancer,”. The trend in keyword frequency is identified by observing its growth or decline over time, thereby marking keywords with increasing frequency (emerging trends) or decreasing frequency (declining trends). “sequencing”, “prognostic signature” and “immune microenvironment” have been identified as the research frontiers for the forthcoming years. As predicted, research on “sequencing” (29, 30), “prognostic signature” and “immune microenvironment” (16, 31–36) in osteosarcoma has gained momentum, with the number of published studies increasing annually. These topics are expected to remain prominent in the near future. Zhong et al. developed a signature (ZNF583, CGNL1, CXCL13) to predict overall survival in osteosarcoma patients, focusing on the anoikis subcluster. This signature showed strong performance in external validation, with stratification revealing significant prognostic differences. It was identified as an independent prognostic factor (29). Wu et al. found that the TMEindex is a promising biomarker for predicting prognosis in osteosarcoma patients, assessing their response to immune checkpoint inhibitor (ICI) therapy, and distinguishing molecular and immune characteristics (34). The application of immunotherapy in osteosarcoma primarily involves two aspects: enhancing the patient’s own immune system response to the tumor and exogenously boosting the immune function of the patient.
The human immune system is highly complex, with various immune cells and factors working together to defend against external threats such as infections and tumors. Tumor cells can escape immune surveillance through mechanisms such as antigen concealment, downregulation of human leukocyte antigen (HLA) expression, release of inhibitory cytokines, recruitment of Treg cells, generation of bone marrow-derived suppressor cells, and promotion of tumor-associated M2 macrophages (37, 38). Therefore, enhancing the patient’s own immune function aims to eliminate tumor immune escape and reawaken the recognition of tumor cells by the body’s immune system, ultimately leading to tumor cell clearance.
Tumor vaccines aim to induce an anti-tumor immune response in the human body by exposing tumor antigens. Currently, the most mature technology for tumor vaccines is the cervical cancer vaccine. Cervical cancer is predominantly induced by human papillomavirus (HPV), so cervical cancer vaccines are divided into preventive vaccines (targeting HPV infection) and therapeutic vaccines (exposing tumor antigens) (39, 40). Dendritic cell (DC) vaccines, as the main type of tumor vaccine, have been applied in various types of tumor treatment to eliminate tumor cells in refractory tumors. DC vaccines are commonly used in osteosarcoma treatment (41, 42).
Recently, the role of innate immune cells in controlling tumor progression has been established. Innate immunity inhibits tumor progression by directly recognizing and killing tumor cells and triggering a strong adaptive immune response (43, 44). The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway has gained significant attention due to its ability to activate the production of type I interferon, thereby enhancing anti-tumor immune responses (45). The application of STING (DMXAA, CDN, MSA-2, etc.) agonists in the treatment of osteosarcoma may be an effective strategy (46).
The occurrence and development of tumors are closely related to changes in the surrounding tumor tissue environment, which occurs simultaneously. Tumor cells functionally shape the tumor microenvironment by secreting various cytokines, chemokines, and other factors. This microenvironment, in turn, influences the occurrence and development of tumors, known as the tumor microenvironment. The tumor microenvironment consists of various cells (such as macrophages, neutrophils, dendritic cells, bone marrow-derived suppressor cells, NK cells, T cells, B cells, tumor-associated fibroblasts) and abundant extracellular matrix (such as collagen, fibronectin, laminin, proteoglycans), as well as various cytokines (such as IL-1β, IL-6, IFN-γ, TGF-β) (47–49). Research on the tumor microenvironment of osteosarcoma has been ongoing, and in recent years, numerous studies have demonstrated that osteosarcoma-derived extracellular vesicle (EV) play a significant role in promoting widespread immune suppression. These EV can inhibit the activity of T cells and NK cells through various pathways, and can even induce T cell apoptosis. Furthermore, they enhance the activity of bone marrow-derived suppressor cells (MDSCs) to support immune evasion by osteosarcoma cells. These research findings highlight the importance of immune suppression mechanisms in the osteosarcoma microenvironment and provide new insights for the development of therapeutic strategies targeting this microenvironment (50).
In recent years, researchers have developed targeted antibodies against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its ligand PD-L1. These immune checkpoint inhibitors have shown promising therapeutic effects in malignant tumors such as melanoma, lung cancer (51–53). PD-1/PD-L1 as immune therapy targets have ushered in a new era of immunotherapy and accelerated research on immune treatment for osteosarcoma. Studies have shown that blocking the interaction between PD-1 and PD-L1 with antibodies significantly improves the responsiveness of osteosarcoma to cytotoxic T lymphocytes (CTLs), leading to reduced tumor burden and increased survival rates in mouse models of metastatic osteosarcoma (54). Koirala et al (15) reported a significant association between PD-L1 expression and the presence of T cells, dendritic cells, and natural killer cells in osteosarcoma. While all examined immune cell types were present in osteosarcoma samples, only infiltration of dendritic cells (28.3% vs. 83.9%, p = 0.001) and macrophages (45.5% vs. 84.4%, p = 0.031) was correlated with worse five-year event-free survival (EFS). Furthermore, PD-L1 expression was significantly associated with poorer five-year EFS (25.0% vs. 69.4%, p = 0.014). In addition, blockade of the PD-1/PD-L1 axis has also been shown to enhance the chemotherapeutic efficacy of cisplatin in osteosarcoma (55). However, there are differing opinions as well. Le et al. conducted a phase II clinical trial in 17 patients with advanced osteosarcoma and found a progression-free survival rate of only 13.3% at 6 months. They concluded that the efficacy of PD-1 inhibition in immunotherapy for osteosarcoma is limited (56). CTLA-4 is expressed on the surface of regulatory T cells (Tregs) and memory T cells. Overexpression of CTLA-4 can competitively inhibit the CD28 co-stimulatory signal required for optimal T cell activation, leading to a loss of anti-cancer activity. Additionally, binding of CTLA-4 to CD80/86 on dendritic cells can result in functional suppression of dendritic cells (57).Ipilimumab, a monoclonal antibody targeting CTLA-4 developed in 2011, was approved by the U.S. Food and Drug Administration (FDA) as the first-line immunotherapy for the treatment of melanoma (58). A phase I clinical trial demonstrated that 25% of osteosarcoma patients achieved disease stabilization following treatment with ipilimumab (59).
Enhancement of patient immune function through the infusion of immune cells, also known as adoptive cell-transfer therapy (ACT), is a primary approach for augmenting immune function (60). Tumor cells may downregulate the expression of their own HLA and tumor antigens, rendering them unrecognizable by T cells. Engineered T cells with high affinity for tumor-specific antigens, known as chimeric antigen receptor T cells (CAR-T cells) can recognize tumor cells independent of HLA presentation (61, 62). CAR-T cells have undergone extensive clinical trials for the treatment of hematological malignancies, achieving significant breakthroughs. In a clinical trial, CD22 CAR-T cells demonstrated an 80% response rate (24/30 patients) in the treatment of refractory or relapsed B-cell acute lymphoblastic leukemia, providing a valuable window of time for subsequent hematopoietic stem cell transplantation (63). Majzner et al (12) discovered that B7-H3 CAR T cells exhibited significant antitumor activity in vivo, leading to the regression of established solid tumors in xenograft models, including osteosarcoma, medulloblastoma, and Ewing sarcoma. Their findings revealed that the effectiveness of B7-H3 CAR T cells relied heavily on the high density of the target antigen on tumor tissues. Conversely, the activity of these CAR T cells was substantially reduced against target cells expressing low levels of the antigen. This observation suggests the potential for a therapeutic window, despite the low-level expression of B7-H3 in normal tissues. In osteosarcoma, primary bone tumors typically exhibit low mutation burden and are accompanied by rare naturally occurring anti-tumor T cells. Therefore, CAR-T cell therapy may be an effective strategy (64). Other adoptive cell transfer therapy approaches include CAR-NK cells and CAR-tumor infiltrating lymphocytes (TILs). Unlike T cells, NK cells are innate immune cells with cytotoxic and immunoregulatory functions (65, 66).
Since the 1970s, although there has been some improvement in the overall treatment of osteosarcoma, the therapeutic options remain limited, particularly for recurrent and metastatic osteosarcoma. Osteosarcoma cannot be cured with a single treatment. Experts from the SARC028 clinical trial indicate that resistance to immunotherapy may stem from PTEN inactivation, resulting in hyperactivation of the PI3K-AKT pathway. This highlights the necessity of a combination treatment strategy (67). Surgical interventions and conventional chemotherapy often yield unsatisfactory results (68, 69). In recent years, the rapid advancement of immunotherapy techniques, methods, and new drugs has brought new opportunities for the stagnant field of osteosarcoma treatment (70, 71). However, these opportunities come with challenges. Many preclinical studies suggest that immunotherapy may benefit osteosarcoma patients, but clinical trials of single-agent therapies often yield disappointing results, hindering effective treatment. Several promising drugs have faced setbacks, primarily because trials included advanced cases of osteosarcoma that had relapsed or metastasized after conventional chemotherapy. In such cases, patients often have severely compromised immune systems, limiting immunotherapy’s effectiveness. Additionally, the immune microenvironment in tumor patients is complex and dynamic, with tumor cells employing various mechanisms to evade immune therapies, making single-agent approaches often ineffective (36, 72). Meazza et al. confirmed the significance of complete surgical remission and noted a promising (though improvable) survival rate in this patient cohort, highlighting a potential role for immunotherapy using IL-2 and LAK/NK cell activation (73). Boye et al. conducted a phase 2 study of pembrolizumab in advanced osteosarcoma, which was well-tolerated but showed no significant antitumor activity. Future trials should explore combination strategies with immunomodulatory agents in patients selected by molecular response profiles (74). Tang et al. conducted a study showing that amrelizumab combined with adriamycin, cisplatin, methotrexate, and ifosfamide in neoadjuvant treatment for resectable osteosarcoma was safe and tolerable. While this combination may not enhance tumor necrosis rate (TNR), the long-term survival benefits require further investigation (75). Wang et al. found that co-delivering a gel with the αPD-1 checkpoint inhibitor significantly enhances effectiveness in an orthotopic osteosarcoma model. Immunophenotyping data indicate a notable increase in T-cell infiltration and improved anti-tumor immunity at the whole-animal level (76). These findings highlight the necessity for combination therapies that integrate various immunotherapeutic approaches along with surgery, chemotherapy, targeted small molecules, and other novel treatments to create optimal treatment regimens. This strategy is crucial for achieving breakthroughs in the comprehensive treatment of osteosarcoma patients. Numerous clinical trials based on this approach have yielded promising results. Additionally, challenges such as personalized immunotherapy selection, immunotherapy resistance, and drug toxicity should also be addressed (Figure 8).
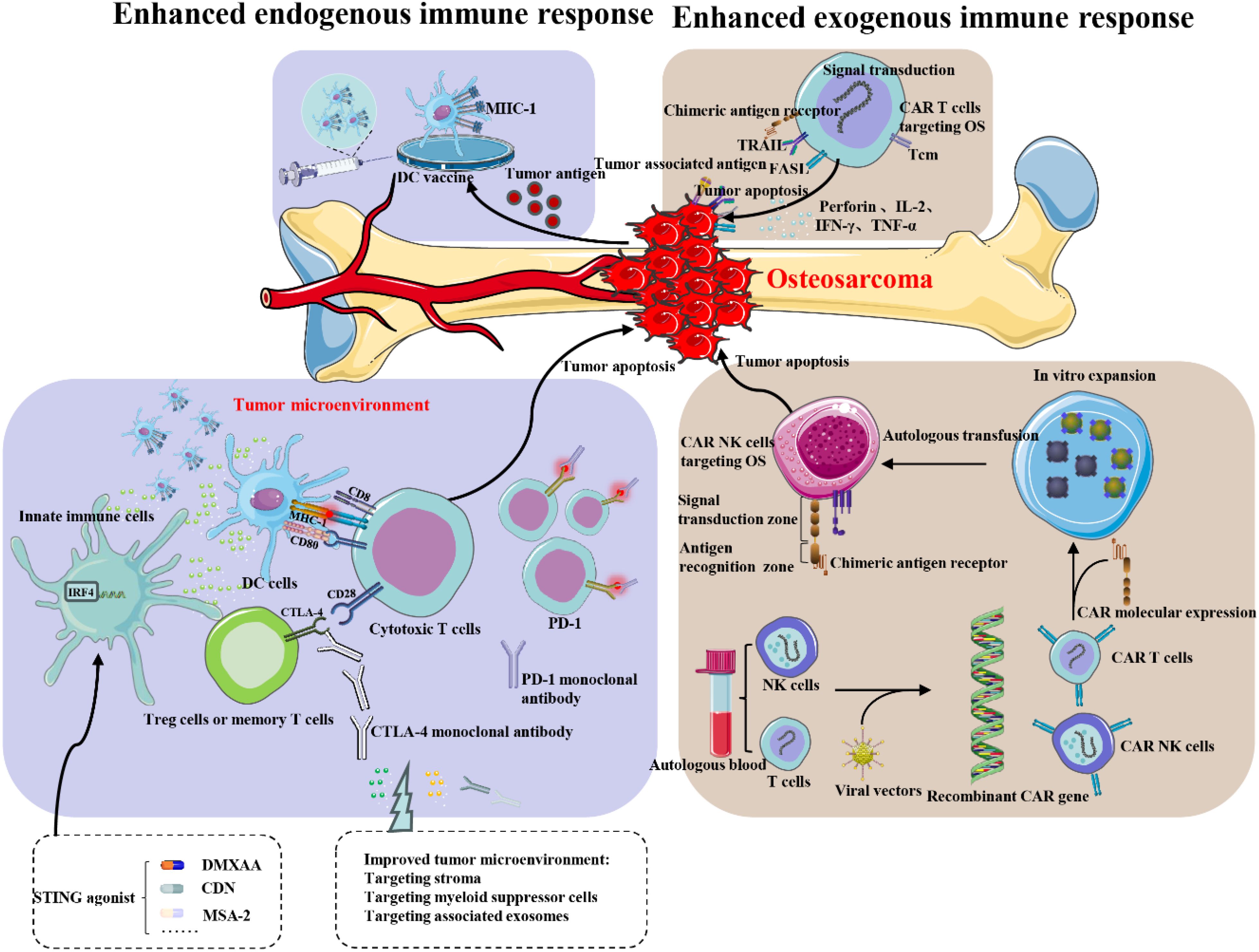
Figure 8. Targeted immunotherapy for osteosarcoma. The application of immunotherapy in osteosarcoma primarily involves two aspects: enhancing the patient’s own immune system response to the tumor and exogenously boosting the immune function of the patient. OS, osteosarcoma; MHC-I, major histocompatibility complex; DC cells, dendrite cells; Tcm, central memory T cell.
This study presents a comprehensive bibliometric review of the field of immunotherapy for osteosarcoma, encompassing an evaluation of its overall scope, advancements, significant contributions, and emerging trends. Researchers are advised to prioritize recent and highly cited references and topics of interest. However, it is important to acknowledge certain limitations in this bibliometric analysis. Our data source relies solely on Web of Science (WOS), primarily due to the compatibility of bibliometric tools and the feasibility of data processing. We previously considered merging data from multiple databases, such as Scopus, MEDLINE, and Cochrane, to enhance literature coverage. However, we encountered several significant challenges during implementation: differences in citation data formats, the complexity of data deduplication, insufficient tool compatibility, and high resource and time costs. Still, it is worth noting that our analysis was limited to articles exclusively sourced from the WoS Core Collection, potentially limiting the breadth of our findings. Furthermore, the exclusion of recently published articles may be attributed to a temporal delay. Lastly, despite the algorithm’s objective execution of the analysis, we observed an inherent subjective bias in the interpretation of the data.
The field of immunotherapy for osteosarcoma has undergone significant evolution over time, revealing a notable trend. Notably, China emerged as the leading contributor. Among institutions, Zhejiang University exhibited the highest level of productivity. The author with the highest publication output was Tsukahara, Tomohide from Japan. The article with the most citation was “DOI: 10.1200/JCO.2014.58.0225”. Frontiers in Immunology emerged as the most productive journal. The most frequently occurring keywords in ITFOS research include “osteosarcoma,” “immunotherapy,” and “cancer.” Furthermore, “sequencing,” “prognostic signature,”. and “immune microenvironment” have been identified as emerging research frontiers for the future. To drive notable progress in the comprehensive treatment of osteosarcoma patients, the incorporation of multifaceted therapeutic approaches is imperative. This entails the integration of combination therapies that encompass multiple immunotherapeutic modalities, alongside the inclusion of surgical interventions, chemotherapy, targeted small molecule drugs, and novel therapeutic strategies. The strategic amalgamation of these diverse treatment modalities is poised to have a pivotal impact on advancing the field of osteosarcoma treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YH: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. RY: Investigation, Software, Supervision, Writing – original draft. SN: Methodology, Project administration, Supervision, Validation, Writing – original draft. ZS: Investigation, Resources, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
2. Cersosimo F, Lonardi S, Bernardini G, Telfer B, Mandelli GE, Santucci A, et al. Tumor-associated macrophages in osteosarcoma: from mechanisms to therapy. Int J Mol Sci. (2020) 21:5207. doi: 10.3390/ijms21155207
3. Choi JH, Ro JY. The 2020 WHO classification of tumors of bone: an updated review. Adv Anat Pathol. (2021) 28:119–38. doi: 10.1097/PAP.0000000000000293
4. Jiang ZY, Liu JB, Wang XF, Ma YS, Fu D. Current status and prospects of clinical treatment of osteosarcoma. Technol Cancer Res Treat. (2022) 21:15330338221124696. doi: 10.1177/15330338221124696
5. Meyers PA, Healey JH, Chou AJ, Wexler LH, Merola PR, Morris CD, et al. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. (2011) 117:1736–44. doi: 10.1002/cncr.v117.8
6. Patil NS, Nabet BY, Müller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. (2022) 40:289–300.e284. doi: 10.1136/jitc-2022-SITC2022.0435
7. Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. (2015) 37:764–82. doi: 10.1016/j.clinthera.2015.02.018
8. Zhang R, Yang Y, Dong W, Lin M, He J, Zhang X, et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc Natl Acad Sci U S A. (2022) 119:e2114851119. doi: 10.1073/pnas.2114851119
9. Liu B, He X, Wang Y, Huang JW, Zheng YB, Li Y, et al. Bibliometric analysis of γδ T cells as immune regulators in cancer prognosis. Front Immunol. (2022) 13:874640. doi: 10.3389/fimmu.2022.874640
10. Xu Y, Jiang Z, Kuang X, Chen X, Liu H. Research trends in immune checkpoint blockade for melanoma: visualization and bibliometric analysis. J Med Internet Res. (2022) 24:e32728. doi: 10.2196/32728
11. Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. (2015) 33:1688–96. doi: 10.1200/JCO.2014.58.0225
12. Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. (2019) 25:2560–74. doi: 10.1158/1078-0432.CCR-18-0432
13. Nakatsuka S, Oji Y, Horiuchi T, Kanda T, Kitagawa M, Takeuchi T, et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol. (2006) 19:804–14. doi: 10.1038/modpathol.3800588
14. Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr Opin Pediatr. (2016) 28:26–33. doi: 10.1097/MOP.0000000000000298
15. Koirala P, Roth ME, Gill J, Piperdi S, Chinai JM, Geller DS, et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep. (2016) 6:30093. doi: 10.1038/srep30093
16. Zhou Y, Yang D, Yang Q, Lv X, Huang W, Zhou Z, et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat Commun. (2020) 11:6322. doi: 10.1038/s41467-020-20059-6
17. Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. (2017) 13:357–68. doi: 10.2217/fon-2016-0261
18. Lu YC, Parker LL, Lu T, Zheng Z, Toomey MA, White DE, et al. Treatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol. (2017) 35:3322–9. doi: 10.1200/JCO.2017.74.5463
19. Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. (2021) 500:1–10. doi: 10.1016/j.canlet.2020.12.024
20. Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. (2020) 15:709–40. doi: 10.1016/j.jtho.2020.01.005
21. Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment: A complex but targetable ecosystem. Cells. (2020) 9:976. doi: 10.3390/cells9040976
22. Wu CC, Beird HC, Andrew Livingston J, Advani S, Mitra A, Cao S, et al. Immuno-genomic landscape of osteosarcoma. Nat Commun. (2020) 11:1008. doi: 10.1038/s41467-020-14646-w
23. Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. (2018) 18:39–50. doi: 10.1080/14737140.2018.1413939
24. Coley WB. II. Contribution to the knowledge of sarcoma. Ann Surg. (1891) 14:199–220. doi: 10.1097/00000658-189112000-00015
25. Sutherland TE, Dyer DP, Allen JE. The extracellular matrix and the immune system: A mutually dependent relationship. Science. (2023) 379:eabp8964. doi: 10.1126/science.abp8964
26. Panez-Toro I, Muñoz-García J, Vargas-Franco JW, Renodon-Cornière A, Heymann MF, Lézot F, et al. Advances in osteosarcoma. Curr Osteoporos Rep. (2023) 21:330–43. doi: 10.1007/s11914-023-00803-9
27. Park JA, Cheung NV. Promise and challenges of T cell immunotherapy for osteosarcoma. Int J Mol Sci. (2023) 24:12520. doi: 10.3390/ijms241512520
28. Li H, Sui X, Wang Z, Fu H, Wang Z, Yuan M, et al. A new antisarcoma strategy: multisubtype heat shock protein/peptide immunotherapy combined with PD-L1 immunological checkpoint inhibitors. Clin Transl Oncol. (2021) 23:1688–704. doi: 10.1007/s12094-021-02570-4
29. Zhong C, Yang D, Zhong L, Xie W, Sun G, Jin D, et al. Single-cell and bulk RNA sequencing reveals Anoikis related genes to guide prognosis and immunotherapy in osteosarcoma. Sci Rep. (2023) 13:20203. doi: 10.1038/s41598-023-47367-3
30. Huang Y, Cao D, Zhang M, Yang Y, Niu G, Tang L, et al. Exploring the impact of PDGFD in osteosarcoma metastasis through single-cell sequencing analysis. Cell Oncol (Dordr). (2024) 47:1715–33. doi: 10.1007/s13402-024-00949-3
31. Li Z, Xue Y, Huang X, Xiao G. Stratifying osteosarcoma patients using an epigenetic modification-related prognostic signature: implications for immunotherapy and chemotherapy selection. Transl Cancer Res. (2024) 13:3556–74. doi: 10.21037/tcr-23-2300
32. Chen W, Liao Y, Sun P, Tu J, Zou Y, Fang J, et al. Construction of an ER stress-related prognostic signature for predicting prognosis and screening the effective anti-tumor drug in osteosarcoma. J Transl Med. (2024) 22:66. doi: 10.1186/s12967-023-04794-0
33. Cillo AR, Mukherjee E, Bailey NG, Onkar S, Daley J, Salgado C, et al. Ewing sarcoma and osteosarcoma have distinct immune signatures and intercellular communication networks. Clin Cancer Res. (2022) 28:4968–82. doi: 10.1158/1078-0432.CCR-22-1471
34. Wu C, Gong S, Duan Y, Deng C, Kallendrusch S, Berninghausen L, et al. A tumor microenvironment-based prognostic index for osteosarcoma. J BioMed Sci. (2023) 30:23. doi: 10.1186/s12929-023-00917-3
35. Zhang Y, Gan W, Ru N, Xue Z, Chen W, Chen Z, et al. Comprehensive multi-omics analysis reveals m7G-related signature for evaluating prognosis and immunotherapy efficacy in osteosarcoma. J Bone Oncol. (2023) 40:100481. doi: 10.1016/j.jbo.2023.100481
36. Wu C, Tan J, Shen H, Deng C, Kleber C, Osterhoff G, et al. Exploring the relationship between metabolism and immune microenvironment in osteosarcoma based on metabolic pathways. J BioMed Sci. (2024) 31:4. doi: 10.1186/s12929-024-00999-7
37. Li W, Hao Y, Zhang X, Xu S, Pang D. Targeting RNA N(6)-methyladenosine modification: a precise weapon in overcoming tumor immune escape. Mol Cancer. (2022) 21:176. doi: 10.1186/s12943-022-01652-3
38. Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X, et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. (2023) 22:48. doi: 10.1186/s12943-023-01744-8
39. Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, et al. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. (2020) 471:88–102. doi: 10.1016/j.canlet.2019.11.039
40. Li M, Zhao C, Zhao Y, Li J, Wei L. Immunogenicity, efficacy, and safety of human papillomavirus vaccine: Data from China. Front Immunol. (2023) 14:1112750. doi: 10.3389/fimmu.2023.1112750
41. Dyson KA, Stover BD, Grippin A, Mendez-Gomez HR, Lagmay J, Mitchell DA, et al. Emerging trends in immunotherapy for pediatric sarcomas. J Hematol Oncol. (2019) 12:78. doi: 10.1186/s13045-019-0756-z
42. Sun C, Ma X, Zhou C, Zhang Z, Guo J. Irreversible electroporation combined with dendritic cell-based vaccines for the treatment of osteosarcoma. Anticancer Res. (2023) 43:3389–400. doi: 10.21873/anticanres.16514
43. Cui J, Chen Y, Wang HY, Wang RF. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother. (2014) 10:3270–85. doi: 10.4161/21645515.2014.979640
44. Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. (2015) 33:445–74. doi: 10.1146/annurev-immunol-032414-112043
45. Pu F, Chen F, Liu J, Zhang Z, Shao Z. Immune regulation of the cGAS-STING signaling pathway in the tumor microenvironment and its clinical application. Onco Targets Ther. (2021) 14:1501–16. doi: 10.2147/OTT.S298958
46. Withers SS, Moeller CE, Quick CN, Liu CC, Baham SM, Looper JS, et al. Effect of stimulator of interferon genes (STING) signaling on radiation-induced chemokine expression in human osteosarcoma cells. PLoS One. (2023) 18:e0284645. doi: 10.1371/journal.pone.0284645
47. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. (2021) 20:131. doi: 10.1186/s12943-021-01428-1
48. Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. (2020) 470:126–33. doi: 10.1016/j.canlet.2019.11.009
49. Kao KC, Vilbois S, Tsai CH, Ho PC. Metabolic communication in the tumour-immune microenvironment. Nat Cell Biol. (2022) 24:1574–83. doi: 10.1038/s41556-022-01002-x
50. Pu F, Chen F, Zhang Z, Liu J, Shao Z. Information transfer and biological significance of neoplastic exosomes in the tumor microenvironment of osteosarcoma. Onco Targets Ther. (2020) 13:8931–40. doi: 10.2147/OTT.S266835
51. Willsmore ZN, Coumbe BGT, Crescioli S, Reci S, Gupta A, Harris RJ, et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur J Immunol. (2021) 51:544–56. doi: 10.1002/eji.202048747
52. Reda M, Ngamcherdtrakul W, Nelson MA, Siriwon N, Wang R, Zaidan HY, et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat Commun. (2022) 13:4261. doi: 10.1038/s41467-022-31926-9
53. Campbell KM, Amouzgar M, Pfeiffer SM, Howes TR, Medina E, Travers M, et al. Prior anti-CTLA-4 therapy impacts molecular characteristics associated with anti-PD-1 response in advanced melanoma. Cancer Cell. (2023) 41:791–806.e794. doi: 10.1016/j.ccell.2023.03.010
54. Lussier DM, O’Neill L, Nieves LM, McAfee MS, Holechek SA, Collins AW, et al. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother. (2015) 38:96–106. doi: 10.1097/CJI.0000000000000065
55. Liu X, He S, Wu H, Xie H, Zhang T, Deng Z. Blocking the PD-1/PD-L1 axis enhanced cisplatin chemotherapy in osteosarcoma in vitro and in vivo. Environ Health Prev Med. (2019) 24:79. doi: 10.1186/s12199-019-0835-3
56. Le Cesne A, Marec-Berard P, Blay JY, Gaspar N, Bertucci F, Penel N, et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: results from the PEMBROSARC study. Eur J Cancer. (2019) 119:151–7. doi: 10.1016/j.ejca.2019.07.018
57. Kim GR, Choi JM. Current understanding of cytotoxic T lymphocyte antigen-4 (CTLA-4) signaling in T-cell biology and disease therapy. Mol Cells. (2022) 45:513–21. doi: 10.14348/molcells.2022.2056
58. Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. (2010) 11:155–64. doi: 10.1016/S1470-2045(09)70334-1
59. Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res. (2016) 22:1364–70. doi: 10.1158/1078-0432.CCR-15-0491
60. Inozume T. Adoptive cell transfer therapy for melanoma. Exp Dermatol. (2023) 32:250–5. doi: 10.1111/exd.14707
61. Pan K, Farrukh H, Chittepu V, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. (2022) 41:119. doi: 10.1186/s13046-022-02327-z
62. Chohan KL, Siegler EL, Kenderian SS. CAR-T cell therapy: the efficacy and toxicity balance. Curr Hematol Malig Rep. (2023) 18:9–18. doi: 10.1007/s11899-023-00687-7
63. Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, et al , et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. (2019) 33:2854–66. doi: 10.1038/s41375-019-0488-7
64. Zhang Q, Zhang Z, Liu G, Li D, Gu Z, Zhang L, et al. B7-H3 targeted CAR-T cells show highly efficient anti-tumor function against osteosarcoma both in vitro and in vivo. BMC Cancer. (2022) 22:1124. doi: 10.1186/s12885-022-10229-8
65. Quamine AE, Olsen MR, Cho MM, Capitini CM. Approaches to enhance natural killer cell-based immunotherapy for pediatric solid tumors. Cancers (Basel). (2021) 13:2796. doi: 10.3390/cancers13112796
66. Lu Y, Zhang J, Chen Y, Kang Y, Liao Z, He Y, et al. Novel immunotherapies for osteosarcoma. Front Oncol. (2022) 12:830546. doi: 10.3389/fonc.2022.830546
67. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. (2017) 18:1493–501. doi: 10.1016/S1470-2045(17)30624-1
68. Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. (2015) 33:2279–87. doi: 10.1200/JCO.2014.60.0734
69. Blay JY, Penel N, Toulmonde M, Valentine T, Chaigneauf L, Rios M, et al. Long term survival in adult osteosarcoma patients treated with a two-drug regimen: Final results of the OSAD93 phase II study of the FSG-GETO. Eur J Cancer. (2024) 208:114228. doi: 10.1016/j.ejca.2024.114228
70. Xie L, Liang X, Xu J, Liu K, Sun K, Li Y, et al. Exploratory study of an anti-PD-L1/TGF-β antibody, TQB2858, in patients with refractory or recurrent osteosarcoma and alveolar soft part sarcoma: a report from Chinese sarcoma study group (TQB2858-Ib-02). BMC Cancer. (2023) 23:868. doi: 10.1186/s12885-023-11390-4
71. Zhou Y, Li M, Zhang B, Yang C, Wang Y, Zheng S, et al. A pilot study of multi-antigen stimulated cell therapy-I plus camrelizumab and apatinib in patients with advanced bone and soft-tissue sarcomas. BMC Med. (2023) 21:470. doi: 10.1186/s12916-023-03132-x
72. Zhu T, Han J, Yang L, Cai Z, Sun W, Hua Y, et al. Immune microenvironment in osteosarcoma: components, therapeutic strategies and clinical applications. Front Immunol. (2022) 13:907550. doi: 10.3389/fimmu.2022.907550
73. Meazza C, Cefalo G, Massimino M, Daolio P, Pastorino U, Scanagatta P, et al. Primary metastatic osteosarcoma: results of a prospective study in children given chemotherapy and interleukin-2. Med Oncol. (2017) 34:191. doi: 10.1007/s12032-017-1052-9
74. Boye K, Longhi A, Guren T, Lorenz S, Næss S, Pierini M, et al. Pembrolizumab in advanced osteosarcoma: results of a single-arm, open-label, phase 2 trial. Cancer Immunol Immunother. (2021) 70:2617–24. doi: 10.1007/s00262-021-02876-w
75. Tang Q, Zhang X, Zhu X, Xu H, Song G, Lu J, et al. Camrelizumab in combination with doxorubicin, cisplatin, ifosfamide, and methotrexate in neoadjuvant treatment of resectable osteosarcoma: A prospective, single-arm, exploratory phase II trial. Cancer Med. (2024) 13:e70206. doi: 10.1002/cam4.v13.18
Keywords: osteosarcoma, immune function, immunotherapy, immune checkpoint inhibitor, targeted therapy
Citation: Hu Y, Yang R, Ni S and Song Z (2025) Bibliometric analysis of targeted immunotherapy for osteosarcoma-current knowledge, hotspots and future perspectives. Front. Immunol. 15:1485053. doi: 10.3389/fimmu.2024.1485053
Received: 23 August 2024; Accepted: 24 December 2024;
Published: 10 February 2025.
Edited by:
Tong-Chuan He, University of Chicago Medicine, United StatesReviewed by:
Saroj Kumari, Nation Institute of Immunology, IndiaCopyright © 2025 Hu, Yang, Ni and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxiang Hu, aG9vc2hhd256ZWVlQGRtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.