- 1Department of Plastic and Aesthetic Surgery, Affiliated Hospital of Shaanxi University of Chinese Medicine, Shaanxi, China
- 2Department of Burns and Plastic Surgery, 969th Hospital of PLA Joint Logistic Support Force, Inner Mongolia, China
- 3Department of Proctology, Affiliated Hospital of Shaanxi University of Chinese Medicine, Shaanxi, China
- 4The First Clinical Medical College of Shaanxi University of Chinese Medicine, Shaanxi, China
Background: Immune checkpoint inhibitors (ICIs) are an emerging tumor treatment pathway after traditional surgery, chemoradiotherapy, and targeted therapy. They have proven to be effective in a variety of cancers, but may not respond to non-target populations. Inflammatory markers such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), derived neutrophil lymphocyte ratio (dNLR), and neutrophil count (ANC) have been shown to be strongly associated with tumor prognosis, but their prognostic significance remains controversial. We therefore performed a meta-analysis to explore the association between NLR, PLR, LMR, dNLR, ANC and prognostic and clinicopathological factors in melanoma patients treated with ICIs.
Methods: A comprehensive search was conducted in Pubmed, Embase, Web Of Science and Cochrane databases, and the last search time was July 2024. To estimate the prognostic value of NLR, PLR, LMR, dNLR, ANC for PFS and OS, hazard ratio (HR) and corresponding 95% confidence interval (CI) estimates were used.
Results: This meta-analysis ultimately included 22 cohort studies involving 3235 melanoma patients. Meta-analysis results showed that high levels of NLR in melanoma patients receiving ICIs were associated with poorer OS and PFS, Merging the HR respectively OS [HR = 2.21, 95% CI (1.62, 3.02), P < 0.001], PFS [HR = 1.80, 95% CI (1.40, 2.30), P < 0.001]; High levels of PLR were associated with poor OS and PFS, and the combined HR was OS[HR=2.15,95%CI(1.66,2.80),P < 0.001] and PFS[HR=1.67,95%CI(1.31,2.12),P < 0.001]. High levels of dNLR were associated with poor OS and PFS, with combined HR being OS[HR=2.34,95%CI(1.96,2.79),P < 0.001] and PFS[HR=2.05,95%CI(1.73,2.42),P < 0.001], respectively. High ANC was associated with poor OS and PFS, and combined HR was OS[HR=1.95,95%CI(1.16,3.27),P < 0.001] and PFS[HR=1.63,95%CI(1.04,2.54),P=0.032], respectively. Increased LMR was associated with prolonged OS and PFS, with combined HR being OS[HR=0.36, 95%CI(0.19,0.70),P < 0.001] and PFS[HR=0.56,95%CI(0.40,0.79),P=0.034], respectively.
Conclusion: In melanoma patients treated with ICIs, elevated levels of NLR, PLR, dNLR, and ANC were associated with poorer overall survival OS and PFS. Conversely, a high LMR correlated with improved OS and PFS. Subgroup analyses indicated that dNLR may be linked to a worse prognosis in melanoma patients. In summary, inflammatory markers such as NLR, PLR, LMR, dNLR, and ANC serve as effective biomarkers for the prognostic assessment of melanoma patients following ICI treatment. These markers provide valuable insights for treatment decision-making in the realm of melanoma immunotherapy, and we anticipate further high-quality prospective studies to validate our findings in the future.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, identifier CRD42024573406.
Introduction
Skin cancer is the most common type of cancer worldwide, with over 1.5 million new cases reported in 2020. Melanoma, which arises from the malignant transformation of melanocytes in the skin, mucous membranes, and other tissues, is the most prevalent form of skin cancer, accounting for approximately one-fifth of all skin cancer cases. In 2020, there were 325,000 new melanoma cases and 57,000 melanoma-related deaths globally. The incidence and mortality rates are higher in men than in women. By 2040, the number of new melanoma cases is projected to increase to 510,000, representing a 50% increase, while melanoma-related deaths are expected to rise to 96,000, indicating a 68% increase (1).Due to its high malignancy and tendency to metastasize through lymphatic and hematogenous routes, the overall efficacy of conventional surgical and radiotherapeutic interventions is limited. As a result, the mortality rate for patients with advanced melanoma exceeds 75%, and the 5-year survival rate is less than 15% (2, 3).Immunotherapy signifies a departure from traditional chemotherapy and targeted therapy by emphasizing the activation of the anti-tumor response of immune cells, rather than directly targeting and destroying cancer cells. Its primary objective is to bolster the immune system’s capacity to eradicate malignant cells. Immune checkpoint blockade (ICB) is an immunotherapeutic strategy that utilizes immune checkpoint inhibitors (ICIs) to prevent immune checkpoints, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed death 1 (PD-1), lymphocyte activation gene 3 (LAG-3), and T-cell immunoglobulin and ITIM domain (TIGIT), from binding to their respective ligands. This approach seeks to reinvigorate suppressed immune cells and enhance their capacity to eliminate tumors, thereby restoring their anti-tumor efficacy (4). In recent years, immune checkpoint inhibitors (ICIs) have demonstrated remarkable efficacy in tumor treatment by enhancing the signaling cascade of T-cell function, thereby promoting immune activation and causing damage to tumor tissues, and several studies have confirmed that ICIs have shown good anticancer activity in a variety of cancers such as melanoma (5), gastric cancer (6), non-small-cell lung cancer (7), renal cell carcinoma (8), esophageal cancer (9), and hepatocellular carcinoma (10). ICIs have demonstrated considerable promise in the treatment of malignant melanoma, significantly enhancing both overall survival (OS) and progression-free survival (PFS) among patients with advanced melanoma. A cohort study (11) involving 16,831 patients revealed that the overall survival of stage IV melanoma patients undergoing first-line ICI treatment was markedly improved. Specifically, the overall survival for patients treated with ICIs was 43.7 months, in contrast to 16.1 months for those receiving chemotherapy or targeted therapy, with a 2% year-on-year decline in mortality rates for melanoma patients between 2016 and 2020 (12). Currently, the U.S. FDA has approved three ICIs that target distinct molecules: PD-1, PD-L1, and CTLA-4. The approved drugs include Ipilimumab, Pembrolizumab, Nivolumab, Cemiplimab, Atezolizumab, and Durvalumab.
While immune checkpoint inhibitors, particularly PD-1 antibodies, have demonstrated promising anti-tumor effects in the clinical treatment of melanoma, some patients do not respond to this therapy. A study of a clinical trial of PD-1 antibodies in advanced solid tumors by Suzanne L Topalian (13) demonstrated that the cumulative response rate in patients with non-small-cell lung cancer was 18%, melanoma 28%, and renal cell carcinoma 27%. This indicates that a significant number of tumor patients do not benefit from immune checkpoint blockade therapy. In addition, ICIs, while activating T cells to attack the tumor, may also trigger irAEs, affecting patients’ quality of life and treatment compliance (14). Therefore, identifying biomarkers to predict the efficacy of immune checkpoint inhibitors (ICIs) and accurately screening patients who will benefit from them represents an urgent challenge in the field of immunotherapy. Current established immunotherapy biomarkers include PD-L1 expression, tumor mutation burden (TMB), and microsatellite instability (MSI). However, these biomarkers encounter several issues, including high testing costs, difficulties in sampling advanced patients, and the absence of uniform and clear cut-off values, which limit their clinical utility (15). Immunosuppression in the tumor microenvironment is a known factor that promotes tumor growth and cancer cell migration, induced by systemic and chronic inflammation, and mediated by several circulating cells (16),numerous studies have demonstrated that chronic inflammation, mediated by inflammatory cytokines, significantly influences tumor development. Tumorigenesis, progression, metastasis, and prognosis are closely linked to the body’s inflammatory state and immune function. Systemic inflammation facilitates tumor growth and metastasis through the production of pro-inflammatory molecules by innate immune cells, as well as the activation of oncogenic signaling pathways (17). A substantial body of research has established the predictive significance of inflammatory markers such as NLR (18), PLR (19), and LMR (20) in melanoma. A study conducted by Schneider et al. (21) demonstrated that an NLR of ≥4, a PLR of ≥145, and an LMR of <2 were significantly associated with a reduced incidence of overall survival (OS). These markers of preoperative peripheral inflammation function as indicators of poor prognosis in melanoma patients undergoing surgical intervention. Furthermore, a meta-analysis by Zhan et al. (22) corroborated these findings, indicating that elevated preoperative NLR is linked to poor prognosis in melanoma patients, suggesting that NLR may play a critical role in the prognostic assessment of this patient population. Several studies have demonstrated that peripheral blood markers, including NLR, dNLR, PLR, LMR, and ANC, can reflect systemic inflammation, and that they are noninvasive, economical, simple, inexpensive, and easily available, and have been used to reflect the immune and inflammatory status of patients with various malignant tumors (23–25), This is advantageous for clinical diagnosis and prognostic assessment of cancer. Currently, the role of inflammatory markers in predicting the survival response of melanoma to ICIs remains contentious, and there is a lack of systematic meta-analyses focusing on inflammatory markers such as NLR, dNLR, PLR, LMR, and ANC. Therefore, we conducted this meta-analysis to evaluate the prognostic significance of these inflammatory markers in melanoma patients undergoing ICI treatment. The objective is to enhance the ability of clinicians to accurately predict the response of melanoma patients to ICIs, thereby facilitating personalized treatment options and establishing a foundation for future research.
Materials and methods
The protocol has been registered in the International Prospective Register of Systematic Reviews data base (PROSPERO: CRD42024573406).
Literature search strategy
Two researchers (OY NAD LSF) independently conducted the search using Pubmed, Embase, Web Of Science, and Cochrane databases. Mesh words in PubMed are used to expand the search scope and include: Melanoma, Malignant Melanoma, Melanomas, Neutrophil-to-lymphocyte ratio, Platelet-lymphocyte ratio, “lymphocyte-monocyte ratio”, “Derived neutrophil to lymphocyte ratio”, “Absolute neutrophil count”, “Immune Check point Inhibitor”, “Immune Check point Blockers”, “PD-L1Inhibitors”, “Programmed Death-Ligand1Inhibitors”, “Ipilimumab”, “Pembrolizumab”, “Tremelimumab”, “Nivolumab”. There are no restrictions on language and type of research in the search strategy, and the last time to search is July 1, 2024. The two researchers screened the papers based on title, abstract and inclusion criteria. Two researchers respectively extracted and reviewed the basic information of relevant literature, research objectives, results and follow-up data. If there was any disagreement, third-party experts would evaluate it. The systematic review was conducted in accordance with the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA) guidelines.
Inclusion and exclusion criteria
The inclusion criteria are as follows:
(1) Clinically diagnosed melanoma patients who have received ICIs treatment;
(2) To report the effects of high and low expression of inflammatory markers NLR, PLR, LMR, dNLR, ANC on patient outcomes, using hazard ratio (HR) and 95% confidence interval (CI) studies;
(3)They were divided into exposed group (high expression of inflammatory markers) and non-exposed group (low expression of inflammatory markers) according to their exposure.
(4) Chinese and English literature;
(5) Outcome measures were overall survival (OS) or progression-free survival (PFS);
(6) The included study design was randomized controlled trial, observational study, cross-sectional study, retrospective study or prospective study.
The exclusion criteria are as follows:
(1) The type of disease research or intervention is not consistent;
(2) No prognostic survival information was provided;
(3) Outcome indicators cannot be extracted;
(4) repeated publication or incomplete information;
(5) non-comparative studies, animal experiments, reviews, letters, guidelines, case reports, pathological mechanisms, conference abstracts, expert opinions, editorials, reviews;
(6) Documents in other languages.
Data extraction
Two researchers independently screened the literature according to the inclusion and exclusion criteria, information was independently extracted using a standardized data extraction form, cross-checked individually by both researchers, and disagreements were resolved through discussion. Studies were excluded if relevant data were not available. For each study, the following information was collected: (1) study characteristics: first author, country, year of publication, type of study, study duration, tumor clinical stage, type of survival analysis, immune checkpoint inhibitors used, and critical values; (2) patient baseline: number of patients, age, and gender; and (3) Research results: If the HR values of OS and PFS are described in this paper, they are extracted directly; if OS and PFS are described in Kaplan-Meier graphs, Engauge Digitizer is used for conversion extraction.
Literature quality assessment
The quality of the included cohort studies was independently assessed using the Newcastle-OttawaScale (NOS), which consists of three metrics: cohort selection, comparability, and outcome assessment. The modified NOS is a 9-point scale, with low-quality studies scoring 1-3, moderate-quality 4-6, and high-quality 7-9. Scoring was done independently by two investigators, and third-party experts were consulted to resolve any large differences between their scores or if this affected the study’s inclusion in the final analysis.
Statistical analysis
StataSE15.0 software was used for statistical analysis to calculate the combined HR and 95% confidence interval (95% CI), and P<0.05 showed a significant difference between the two groups. Heterogeneity was evaluated using I² values,I²≤30%,30%<I²<75% and ≥75% were considered to indicate low, medium and high heterogeneity, respectively.I²<50% was analyzed using a fixed-effects model, while I²≥50% was analyzed using a random-effects model. Sensitivity analysis was carried out on the results with large heterogeneity, one study in the merger was excluded one by one, the combined effect size and heterogeneity changes of the remaining literature were evaluated, and the source of heterogeneity was analyzed. Begg’s funnel plot and Egger’s test were used to evaluate whether there was publication bias, and no publication bias existed if P > 0.05.
Results
Literature search results
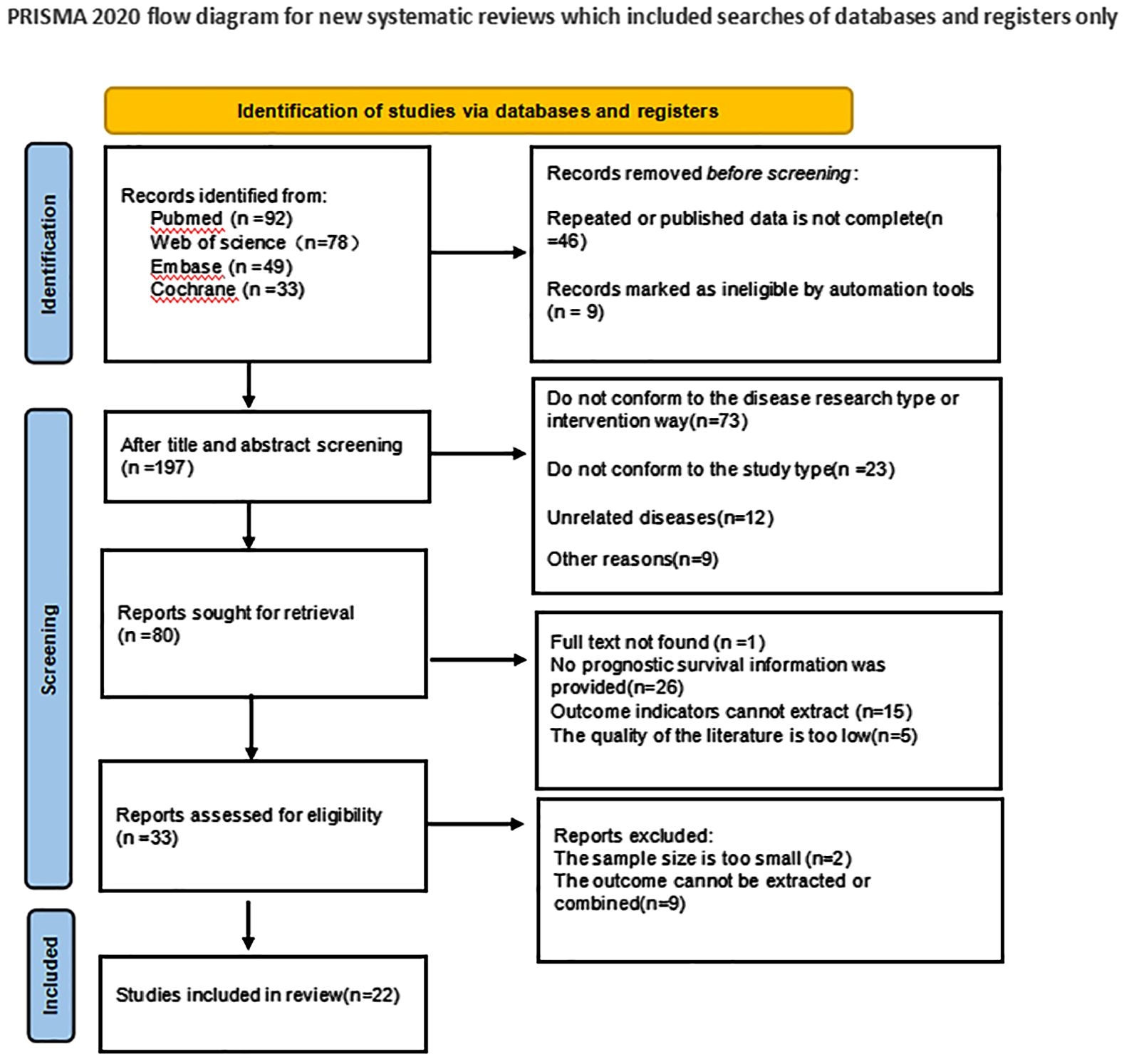
In the initial literature search, a total of 252 articles were searched. 46 duplicate studies were excluded; After reading the title and abstract of the article, 172 studies were excluded according to the exclusion criteria, and 80 studies were initially included. We then read the full text and excluded 58 studies that did not meet the inclusion criteria. Finally, 22 studies were included in the meta-analysis. The literature screening process and results are shown in Figure 1.
Basic characteristics of the included studies
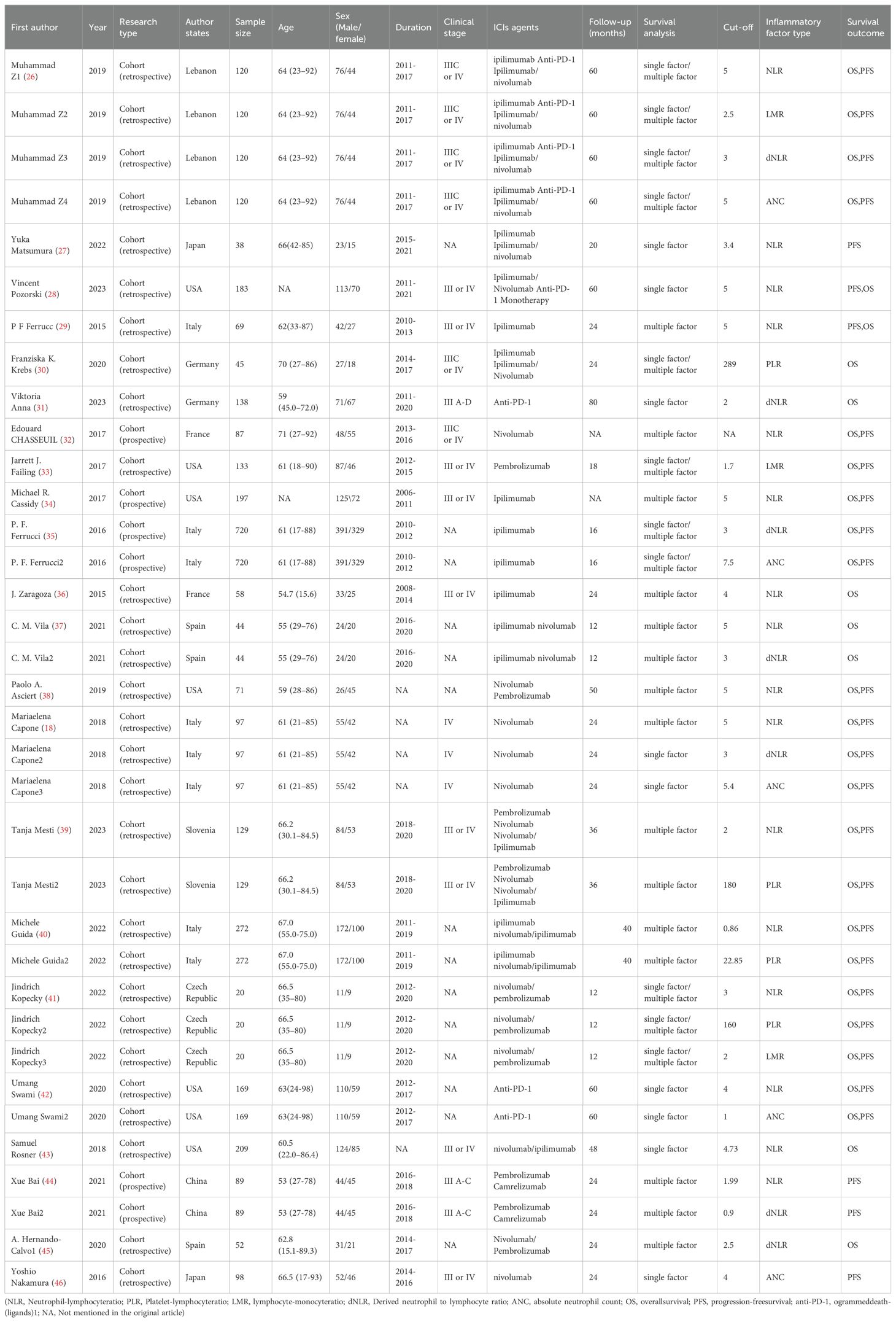
As shown in Table 1, the 22 studies included evaluated a total of 3235 patients with melanoma after ICIs treatment. All 22 studies were cohort studies, of which 18 were retrospective cohort studies and 4 were prospective cohort studies. Multiple inflammatory markers were studied in one study in the included literature, so we numbered different inflammatory markers in the same literature. Study characteristics, patient baseline, and study results of the included studies are shown in Table 1.
The quality assessment of the included studies
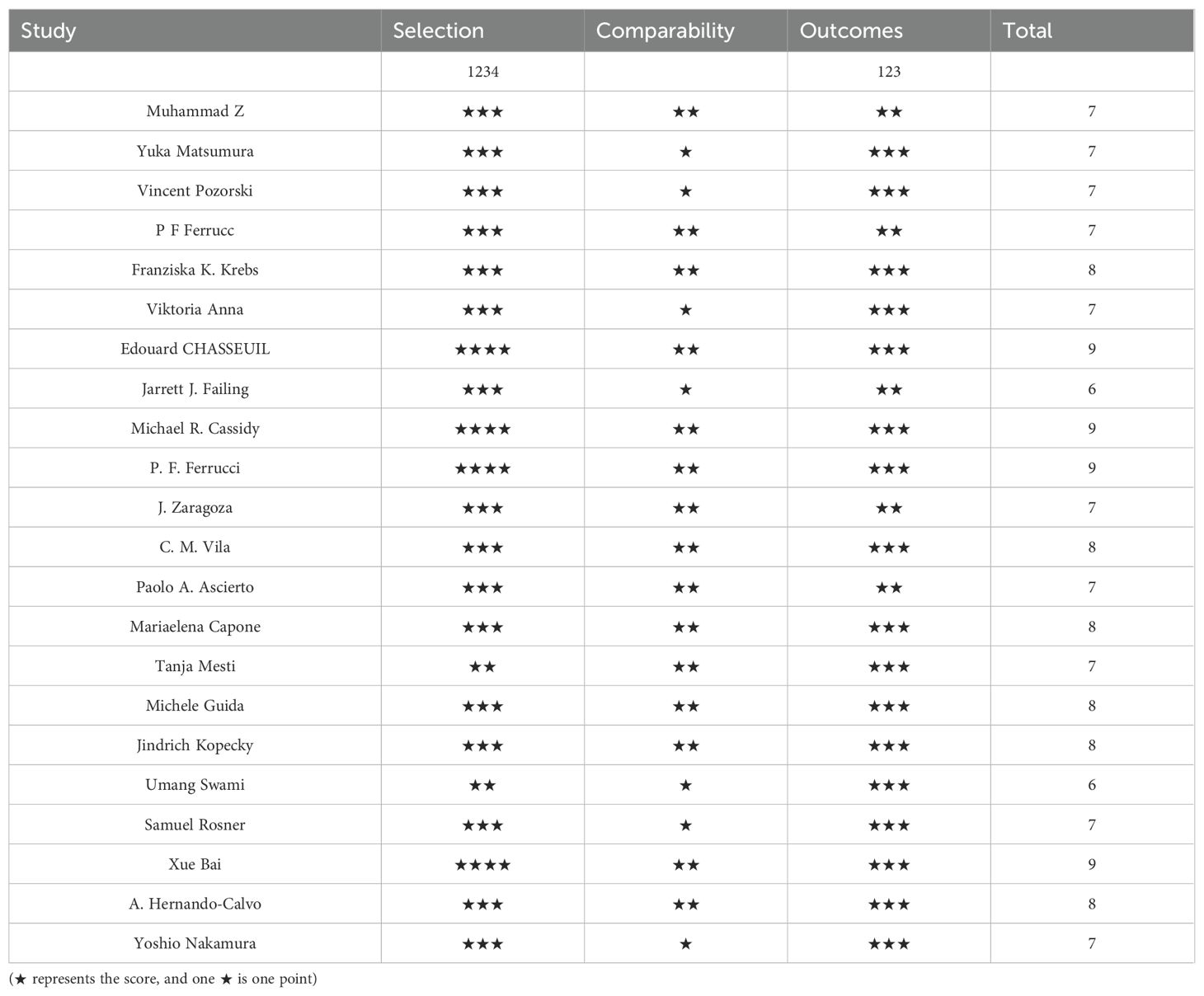
The quality of the included cohort studies was evaluated using the Newcastle-OttawaScale(NOS)for quality and the overall quality was rated as good, with the results shown in Table 2.
Meta-analysis results
Overall survival
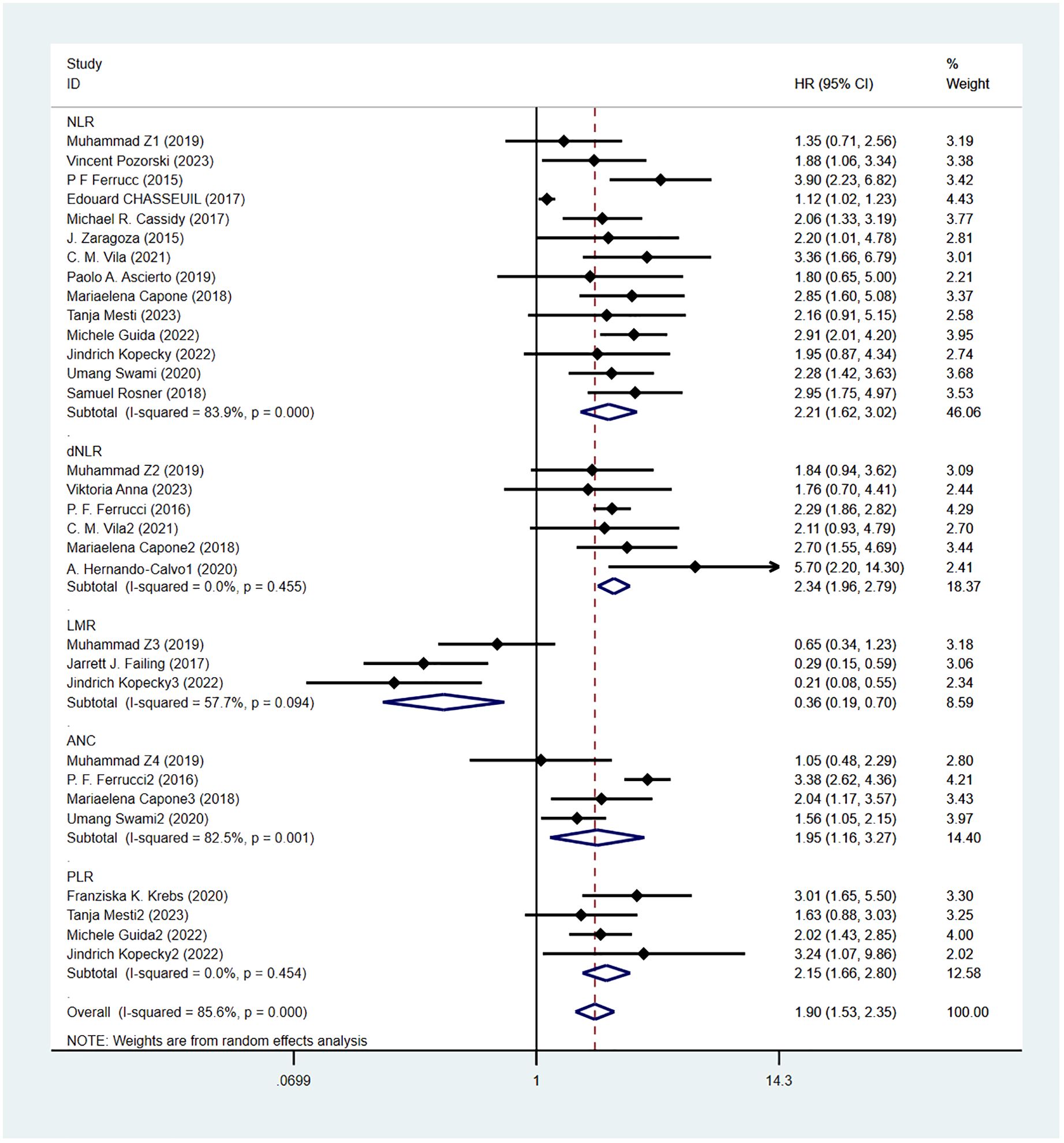
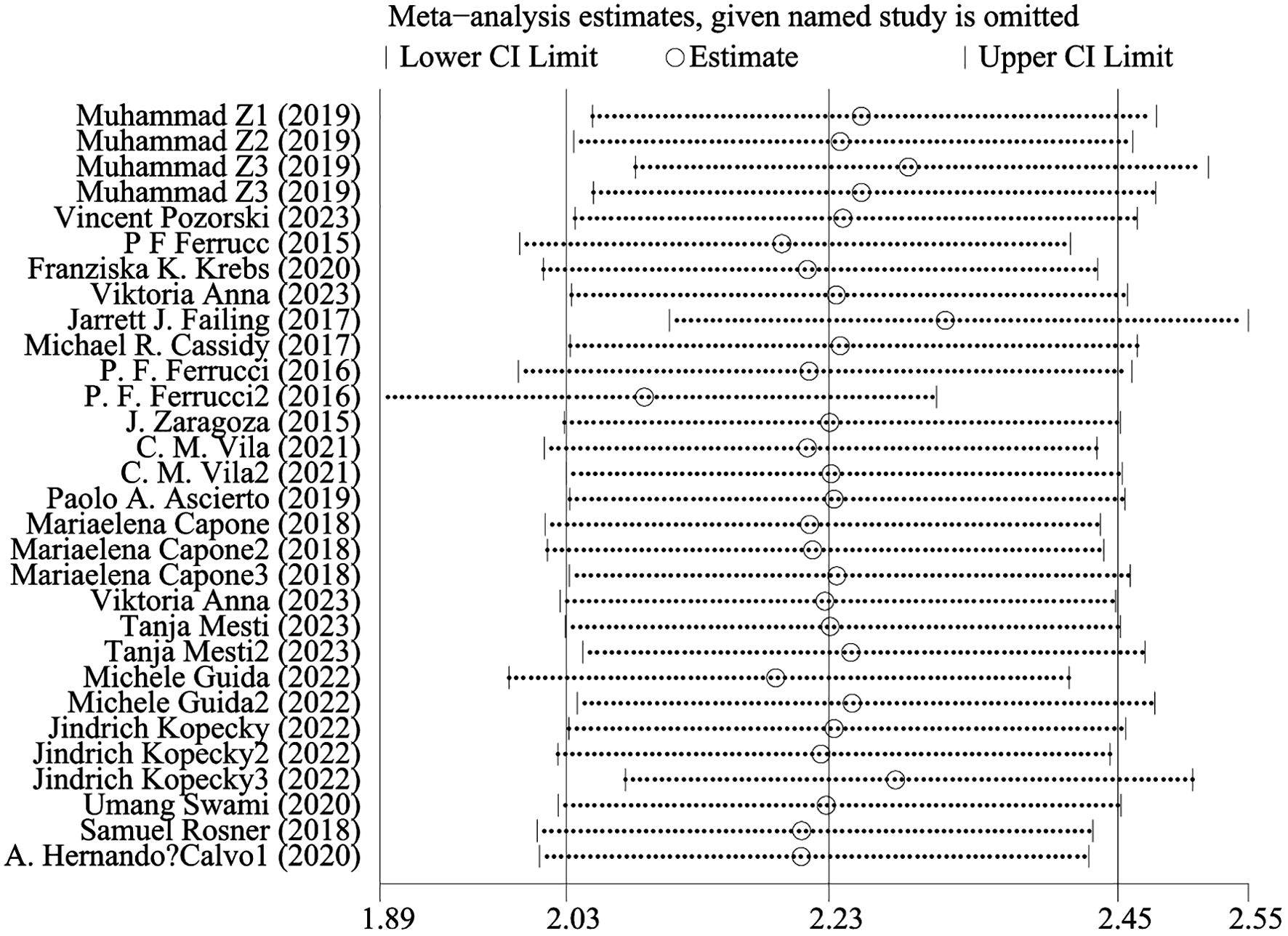
Overall survival was reported in 20 studies. Figure 2 shows the risk-ratio forest plots identified in 20 studies. A random-effects model was used for meta-analysis, taking into account the large heterogeneity between studies (P < 0.001,I²=89.2%). Analysis results showed that high levels of NLR, PLR, dNLR, ANC were associated with poor OS: [HR = 2.21, 95% CI (1.62, 3.02), P < 0.001], [HR = 2.15, 95% CI (1.66, 2.80), P < 0.001], [HR = 2.34, 95% CI (1.96, 2.79), P < 0.001], [HR = 1.95, 95% CI (1.16,3.27),P < 0.001]; In addition, high levels of LMR were associated with OS benefit [HR=0.36, 95%CI (0.19,0.70),P < 0.001]. Considering the existence of large heterogeneity, sensitivity analysis was carried out, and it was found that Umang Swami2 and Edouard CHASSEUIL were the sources of heterogeneity. After excluding the two studies, the heterogeneity of NLR group decreased from 84% to 0%, which may be due to differences in research types and survival analysis methods. The critical value of NLR is not mentioned in the article.
Progression-free survival
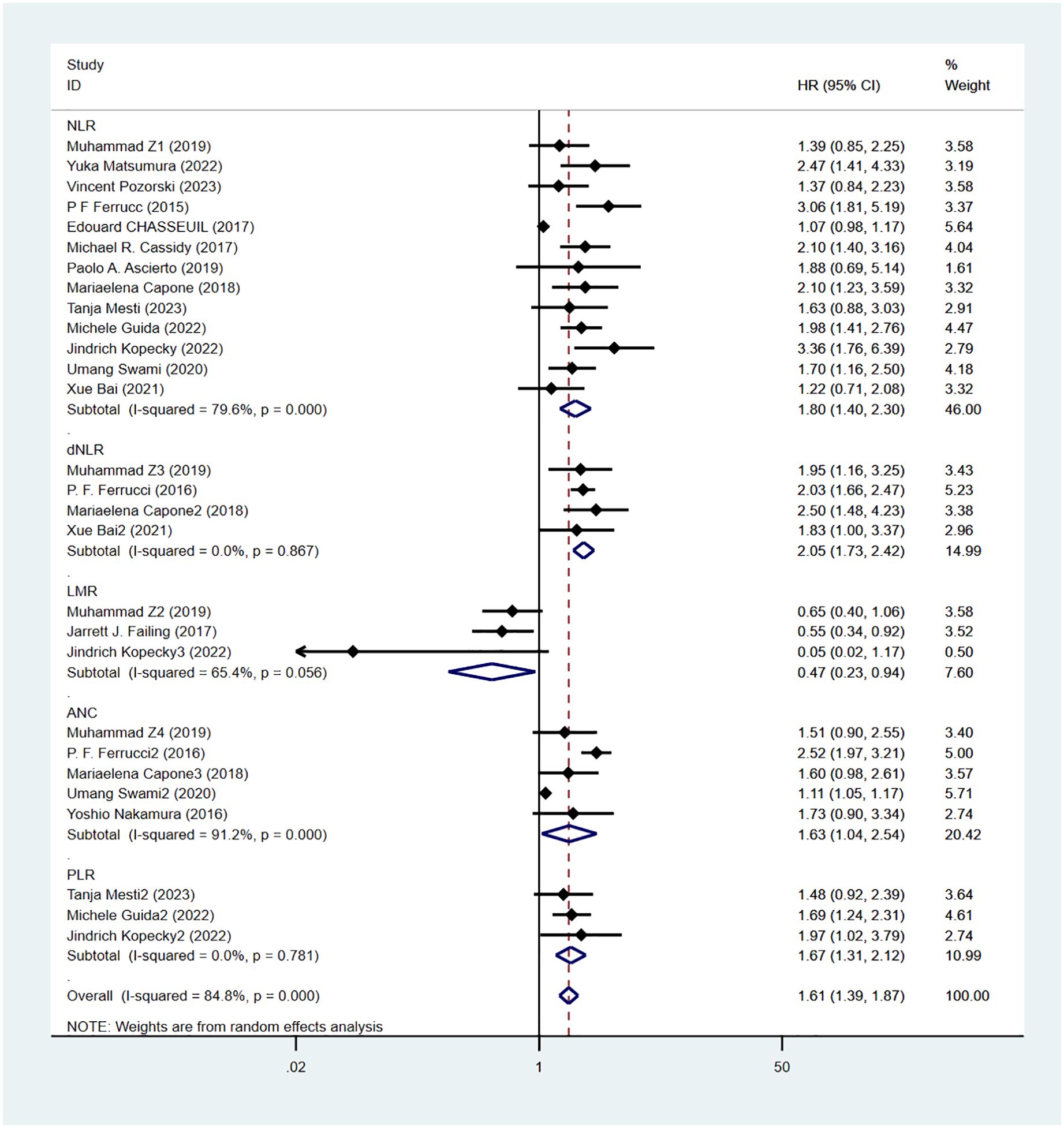
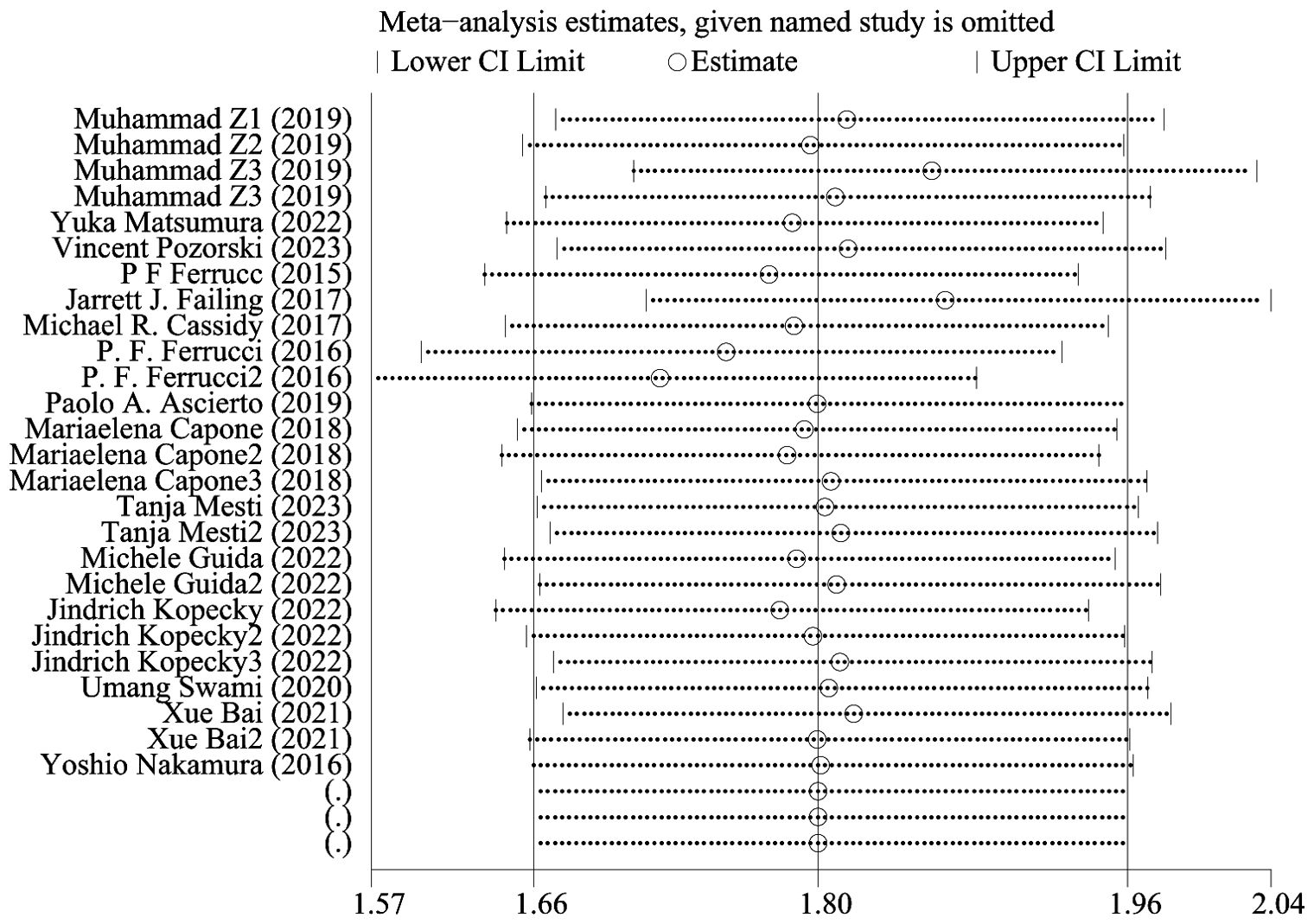
Progression-free survival was reported in 16 studies. Figure 3 shows the risk-ratio forest plots identified in 16 studies. Considering the large heterogeneity between studies (P < 0.01,I²=84.8%), a random effects model was used for meta-analysis. Analysis results showed that high levels of NLR, PLR, dNLR, ANC were all associated with poor PFS: [HR = 1.80, 95% CI (1.40, 2.30), P < 0.001], [HR = 1.67, 95% CI (1.31, 2.12), P < 0.001], [HR = 2.05, 95% CI (1.73, 2.42), P < 0.001], [HR = 1.63, 95% CI (1.04,2.54),P=0.032]; In addition, high levels of LMR were associated with PFS benefit [HR=0.56,95%CI(0.40,0.79),P=0.034]. Sensitivity analysis was also used to explore the sources of heterogeneity, and it was found that Umang Swami2 and Edouard CHASSEUIL were the sources of heterogeneity. After excluding the two studies, the heterogeneity of the NLR group decreased from 79.6% to 21%, and the reasons were analyzed as above.
Sensitive analysis
Figure 4 shows OS sensitivity analysis. After deleting Umang Swami2 and Edouard CHASSEUIL, the model is robust and reliable. Figure 5 shows the sensitivity analysis of PFS. The sensitivity is low, indicating that the model is robust and reliable.
Publication bias
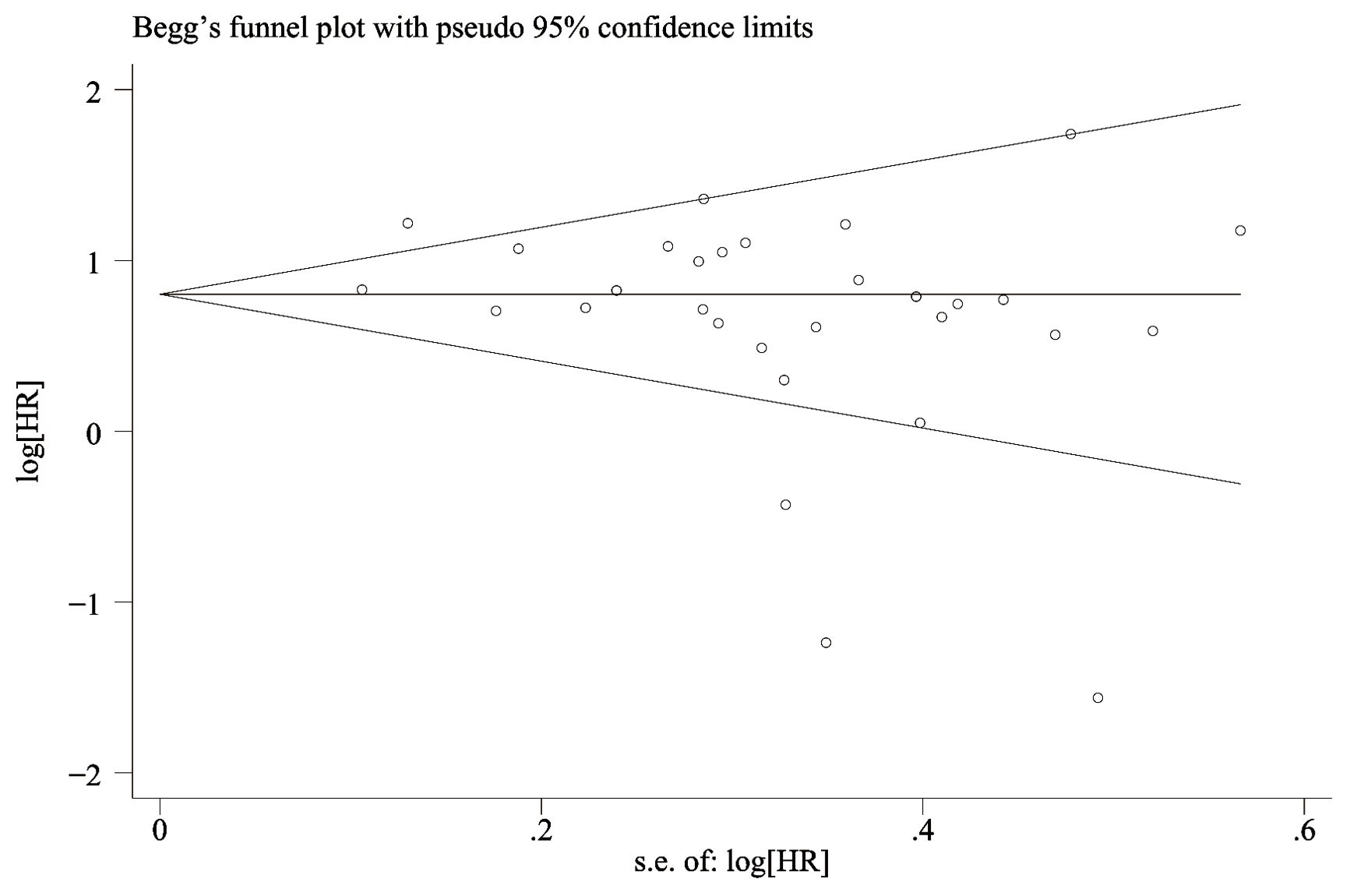
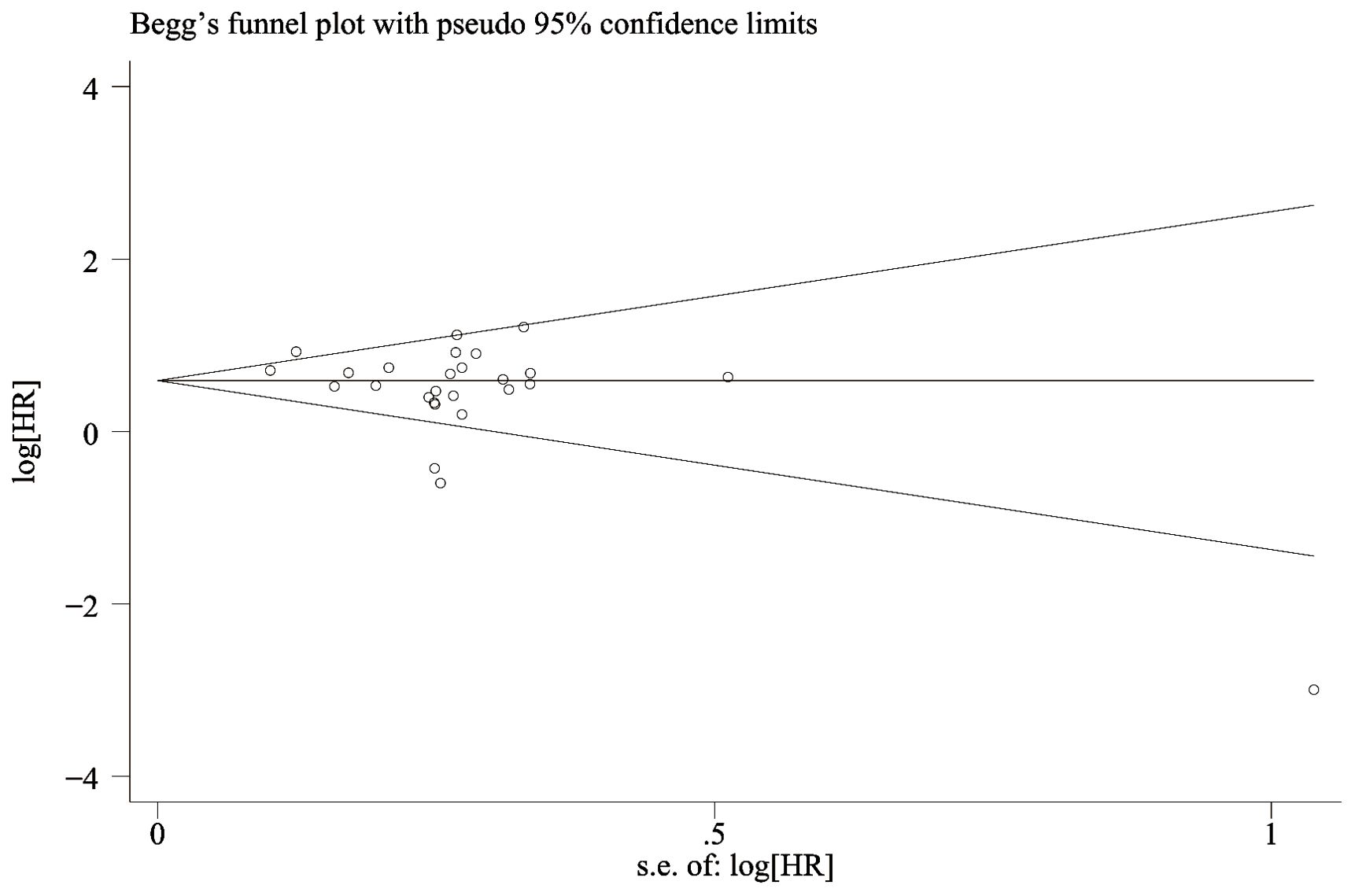
The funnel-plot of OS and PFS was evaluated for publication bias, and the results showed that the overall survival rate (Figure 6) Egger’s P=0.066 and Begg’s P=0.134, indicating no significant publication bias. There was no significant asymmetry in the shape of the funnel plot, and all studies were within 95%CI range. PFS (Figure 7) Egger’s P=0.062 and Begg’s P=0.724 indicate that there is no significant publication bias (P > 0.05). There was no significant asymmetry in the shape of the funnel plot, and all studies were within 95%CI range. ,
Subgroup analysis
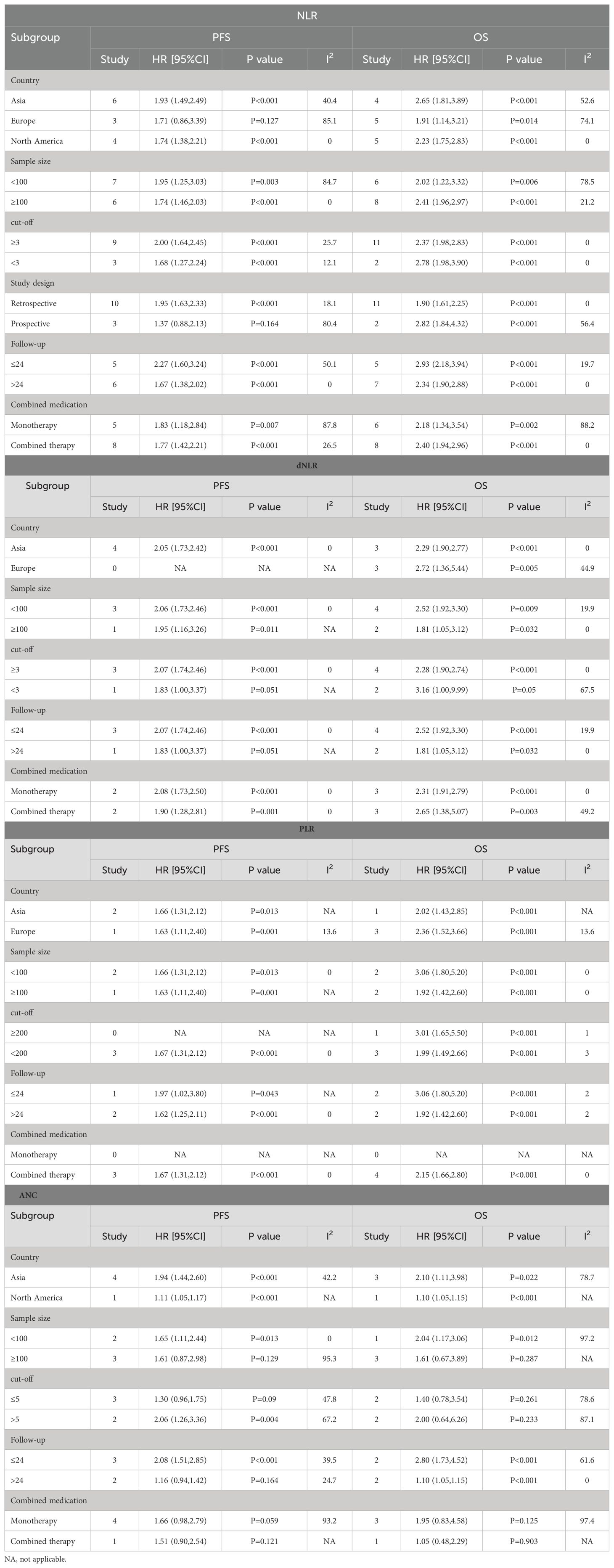
To determine the source of OS and PFS heterogeneity, we conducted subgroup analysis for NLR, dNLR, PLR, and ANC, respectively. Considering that the sample size of the LMR group was only 3 studies, no subgroup analysis was conducted for the LMR group. The results show that high NLR is an important prognostic factor affecting patients’ OS and PFS, and different regions and study types may be the source of heterogeneity, which is similar to the conclusion of our sensitivity analysis. High dNLR and PLR were important prognostic factors for OS and PFS, independent of country, sample size, cut-off value, study type, follow-up duration, and combination drugs. High ANC was also a prognostic factor for OS and PFS. Subgroup analysis showed that sample size, critical value and drug combination were the causes of high heterogeneity, and the results of subgroup analysis were shown in Table 3.
Meta regression
According to the results of the forest map, the NLR group was the main cause of heterogeneity. Therefore, we conducted a meta-regression analysis on the NLR group. Table 4 shows the results of univariate and multivariate meta-regression, and studies the factors affecting OS. The results of multivariate analysis suggested that study type may be the source of heterogeneity (P < 0.05), which is the same as the results of our sensitivity analysis and subgroup analysis.
Discussion
Melanoma is a malignant tumor that originates from primitive nerve cells and results from the over proliferation of abnormal melanocytes. While it primarily occurs in the skin, melanoma can also be found in other locations, including the eyes, ears, meninges, gastrointestinal tract, oral cavity, genitals, and the mucous membranes of the sinuses (47), In most populations, cutaneous melanomas represent over 90% of melanoma diagnoses. Malignant melanoma can develop from benign cutaneous melanomas and may be triggered by various factors, including ultraviolet radiation and genetic predisposition. Ultraviolet radiation, known to induce DNA mutations, is regarded as the primary environmental factor contributing to melanoma development (48). Its clinical characteristics are highly invasive, highly malignant, frequently recurring and easily metastasized, and its incidence is on the rise worldwide (49). Currently, the clinical diagnosis and treatment of melanoma are conducted by a multidisciplinary team (MDT) within the framework of integrative oncology. The primary treatment strategy involves extensive surgical resection, while immunotherapy, chemotherapy, and radiotherapy serve as the principal modalities for patients with deeply metastasized tumors or those whose cancer has spread to the lymph nodes (50). Due to melanoma’s high susceptibility to metastasis, most patients are diagnosed at intermediate to advanced stages, where surgical treatment becomes less effective and sensitivity to radiotherapy is low. This results in poor efficacy and the development of drug resistance. The objective remission rate (ORR) for first-line chemotherapy regimens based on the alkylating agents dacarbazine and temozolomide is less than 20%, with a 5-year overall survival (OS) rate of under 5%. Other chemotherapeutic agents have not demonstrated improved long-term survival, underscoring the urgent need for novel therapeutic approaches (51). The hallmarks of melanoma include mutations in genes associated with the mitogen-activated protein kinase (MAPK) pathway or the over-activation of proteins, which result in increased tumor cell proliferation and invasive capabilities, as well as immunosuppression within the tumor microenvironment (TME). The immune effects in the TME are primarily mediated by adaptive immune cells, which undergo a series of proliferation and differentiation processes in response to antigen stimulation. The effector T cells produced are capable of specifically binding to target cells within the organism, leading to their cleavage and subsequent death, thereby achieving the antitumor effect (52) (53).The latest ECSO guidelines recommend immune checkpoint blockade, either with anti-PD-1 alone or in combination with anti-CTLA-4, as a first-line treatment for patients with unresectable stage III and IV melanoma. Immune checkpoint inhibitors (ICIs) enhance the host’s immune response to tumors by inhibiting negative immunomodulatory molecules on T cells, thereby activating the immune system. This therapeutic approach has significantly improved clinical outcomes (54). Immunotherapy has significantly improved survival rates for melanoma patients, with a 5-year overall survival rate of up to 93.5%. Specifically, the overall survival rates are 73.9% for stage III and 35.1% for stage IV melanoma patients (55). The results of several Meta-analyses have also demonstrated better survival rates with ICIs in the treatment of progressive melanoma (56) (57). CTLA-4, an inhibitory receptor expressed by T cells, is a cellular antigen primarily derived from human cells. The T cell receptor recruits phosphatases that inhibit the activation of transcription factors and ubiquitin ligases associated with T cell activity, thereby attenuating signaling (58).Additionally, CTLA-4, another immune transmembrane receptor found on T lymphocytes, is upregulated during T cell activation and provides negative regulation of the immune system. It competes with CD28 for binding to B7 ligands. When CTLA-4 binds to B7 instead of CD28, it results in a loss of immune-reactive enzyme activity in T cells (59). CTLA-4 inhibitors target the binding of CTLA-4 to its ligands, CD80 and CD86, thereby blocking the interaction between CTLA-4 and these ligands. This blockade enhances antitumor T-cell activity. Approved drugs in this category include tremelimumab and ipilimumab (60). In the development of melanoma, tumor cells induce T-cell catabolism by binding to PD-1, a process that is activated through the phosphorylation of PD-1 and its ligand PD-L1 by the protein tyrosine kinase Lck. This interaction subsequently recruits the tyrosine phosphatase Shp2, which mediates the dephosphorylation of the T-cell receptor (TCR) and CD28, thereby inhibiting T-cell-associated signaling. Consequently, tumor cells proliferate by evading T-cell-mediated killing, contributing to the progression of melanoma (61) (62).PD-1/PD-L1 inhibitors bind to PD-1 or PD-L1, respectively, blocking the interaction between these two proteins. This action restores the recognition and cytotoxic effects of immune cells, thereby reducing the incidence of immune escape by tumor cells. Consequently, T cells are induced to exert their killing effects, leading to the elimination of tumor cells. Approved drugs in this category include nivolumab and pembrolizumab. In a study by Larkin J et al. (63) comparing the efficacy of nivolumab and dacarbazine in the treatment of unresectable stage III or IV melanoma, nivolumab treatment showed a significant clinical benefit, with a higher ORR and a higher median OS in the nivolumab group compared to the chemotherapy group (31.7% vs. 10.6%, respectively, and 16 months vs. 14 months).
Although immune checkpoint inhibitors, such as anti-CTLA-4 and PD-1, have demonstrated promising anti-tumor effects in the clinical treatment of melanoma, a subset of patients does not respond to these immunotherapeutic agents. Clinical trials indicate that the overall response rate to PD-1 antibody treatment for solid tumors is approximately 20%, while the response rate for combinations of multiple immune checkpoint inhibitors has not surpassed 50% (64, 65). It was found that tumor BRAF, NRAS, HRAS gene mutation status, Ki67, P16, PTEN protein expression levels, miRNA, lncRNA non-coding RNA mutation sites are potential prognostic predictors of malignant melanoma (66, 67), However, clinical testing for the aforementioned markers is expensive and controversial regarding their prognostic accuracy, rendering them unsuitable for routine screening of melanoma patients. Therefore, identifying cost-effective and easily accessible biomarkers is particularly important. Analyzing these biomarkers in combination can assist in identifying patient groups that will benefit most from immunotherapy and optimizing drug regimens. Inflammatory-related factors, such as cytokines, inflammatory cells, and chemokines, are present in the microenvironment of all early-stage tumors, and the persistent inflammatory response may significantly drive tumorigenesis (68). The overexpression of inflammatory mediators promotes angiogenesis, induces cellular mutations and DNA damage, triggers inflammatory cascades, and results in tissue atrophy. The inflammatory state of the body leads to an impaired immune response, which contributes, either directly or indirectly, to tumorigenesis, invasion, and metastasis (69, 70). Therefore, predictive biomarkers that reflect the inflammatory response, such as neutrophil, lymphocyte, and platelet counts, may be useful in clinical decision-making for managing melanoma patients. These markers are economically accessible and noninvasive. Although many studies have examined the prognostic significance of inflammatory markers in melanoma patients treated with ICIs, the results remain controversial. Therefore, we conducted this meta-analysis, which showed that levels of NLR, PLR, dNLR, and ANC were all independent predictors of OS and PFS in melanoma patients treated with ICIs: High level of NLR was associated with poor OS and PFS, and the combined HR was OS[HR=2.21,95%CI(1.62,3.02), P < 0.001] and PFS[HR=1.80,95%CI(1.40,2.30), P < 0.001], respectively. High levels of PLR were associated with poor OS and PFS, and the combined HR was OS[HR=2.15,95%CI(1.66,2.80), P < 0.001] and PFS[HR=1.67,95%CI(1.31,2.12), P < 0.001]. High levels of dNLR were associated with poor OS and PFS, with combined HR being OS[HR=2.34,95%CI(1.96,2.79), P < 0.001] and PFS[HR=2.05,95%CI(1.73,2.42), P < 0.001], respectively. High ANC was associated with poor OS and PFS, and combined HR was OS[HR=1.95,95%CI(1.16,3.27), P < 0.001] and PFS[HR=1.63,95%CI(1.04,2.54),P=0.032], respectively. Increased LMR was associated with prolonged OS and PFS, with combined HR being OS[HR=0.36, 95%CI(0.19,0.70), P < 0.001] and PFS[HR=0.56,95%CI(0.40,0.79), P=0.034], respectively. Meanwhile, the analysis showed that high dNLR resulted in worse OS and PFS. Subgroup analyses showed that region, threshold, and whether monotherapy or combination were factors contributing to differences and bias, and that differences in the thresholds of inflammatory markers may cause differences in the sensitivity of prognostic prediction. The results of a study by Ari VanderWalde et al. (71) validated our analysis: nabulizumab in combination with ibritumomab significantly improved progression-free survival compared with ibritumomab alone, with objective remission rates of 28% and 9%, respectively. The NLR serves a dual role in both the promotion and suppression of cancer within the tumor microenvironment. Neutrophils can be classified into two distinct phenotypes based on their function: high-density neutrophils (HDNs) and low-density neutrophils (LDNs) (72). The HDN phenotype enhances antitumor effects by directly targeting tumor cells and stimulating T-cell-mediated immunity. Conversely, the LDN phenotype suppresses antitumor T-cell responses through various mechanisms: it releases arginases that degrade antitumor factors, produces leukotrienes that promote metastasis-initiating cells, and induces angiogenesis via the release of vascular endothelial growth factor (VEGF). These actions undermine the immune system, allowing tumor cells to evade detection and thereby facilitating tumor progression and metastasis (73, 74). Tumorigenesis is characterized as a chronic inflammatory process. During the initial stages of inflammation, neutrophils predominantly display the HDN phenotype, whereas the LDN phenotype becomes more prevalent as inflammation subsides. As a result, an elevated ANC serves as a poor prognostic indicator. Lymphocytes, which are responsible for mediating both cellular and humoral immune responses, play a pivotal role in defending against pathogens, eliminating tumor cells, and regulating immune responses through the induction of apoptosis and the inhibition of tumor cell proliferation and migration (75).Elevated levels of the NLR and the dNLR indicate a poor prognosis. The PLR reflects the role of platelets, which are produced by megakaryocytes in the bone marrow. Platelets are among the first cells to aggregate at the site of injury and play a significant role in tumor metastasis as ‘first responders. Their hemostatic function is compromised and exploited by tumor cells within the tumor microenvironment. Platelets adhere to tumor cells to form aggregates, which protect these cells from high-flow shear stress and immune attacks, thereby promoting tumor progression, invasion, and metastasis (76, 77). An elevated PLR indicates a poorer prognosis. The LMR assesses the balance between lymphocytes and monocytes. Monocytes release various pro-inflammatory cytokines, including interleukins IL-1, IL-6, IL-10, and TNF-α, which are linked to reduced survival and a worse prognosis in patients with malignant tumors (78). An elevated PLR is associated with a poorer prognosis. The LMR evaluates the balance between lymphocytes and monocytes. Monocytes secrete a variety of pro-inflammatory cytokines, such as interleukins IL-1, IL-6, IL-10, and TNF-α, which have been correlated with reduced survival and a worse prognosis in patients with malignant tumors (79). Therefore, the higher LMR its level, suggests that the better the immune status of the body, the better the ability to monitor tumor cells, the better the prognosis. Increasing research evidence suggests that ICIs play a crucial role in the treatment of advanced melanoma, demonstrating irreplaceable functions in various contexts, whether as monotherapy or in combination therapy. The development of safe, effective, economical, and highly specific immunotherapy circulating biomarkers can not only dynamically monitor the therapeutic effects of ICIs in patients but also provide timely feedback on the efficacy of immune drugs. Furthermore, these biomarkers can flexibly predict clinical outcomes for patients undergoing immunotherapy and facilitate the early identification of benefit groups, thereby enhancing the clinical application value of ICIs.
This Meta-analysis of ours has some significant strengths: (1) it is the first assessment of the prognostic predictive ability of peripheral blood inflammatory markers NLR, dNLR, PLR, LMR, and ANC in melanoma patients treated with ICIs, and the search of the literature is more comprehensive; and (2) the data were analyzed in subgroups meticulously, and the findings were fully discussed. However, it is undeniable that there are some shortcomings in our study:(1) there is some heterogeneity among studies, which may be caused by the fact that some studies did not specify the cutoff value;(2)Most of the included studies were retrospective cohort studies, which may introduce confounding bias. Furthermore, the levels of inflammatory markers can be influenced by other conditions, such as infections, and these confounding factors may impact the results. Therefore, we anticipate the emergence of more large-scale, well-designed prospective studies in the future to validate our findings. It is recommended that original studies employ more rigorous designs and methodologies to minimize bias and error. Additionally, researchers should be encouraged to publish their study data and methods for verification and reanalysis by others. Finally, establishing a uniform standard for cutoff values of inflammatory markers is essential to mitigate the risk of bias.
Conclusion
In melanoma patients receiving immune checkpoint inhibitors (ICIs), the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), derived neutrophil-to-lymphocyte ratio (dNLR), and absolute neutrophil count (ANC) may serve as effective biomarkers for prognostic prediction. These metrics offer valuable insights for therapeutic decision-making in the context of melanoma immunotherapy. However, further high-quality prospective studies are necessary to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author contributions
OY: Writing – original draft, Software, Methodology, Data curation. SFL: Writing – original draft, Data curation. QG: Writing – original draft, Data curation. YS: Writing – original draft, Methodology. JL: Writing – original draft, Methodology. WZ: Writing – original draft, Data curation. SL: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding by National Natural Science Foundation of China, Surface Project, 81971854, “Mechanisms by which BANCR regulates glucose metabolism and tumor stemness in melanoma by interfering Notch2/Nur77 expression through spongification of miR-145-5p as competitive endogenous RNA”, supported research.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. (2022) 158:495–503. doi: 10.1001/jamadermatol.2022.0160
2. Dzwierzynski WW. Melanoma risk factors and prevention. Clin Plast Surg. (2021) 48:543–50. doi: 10.1016/j.cps.2021.05.001
3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
4. Li G, Choi JE, Kryczek I, Sun Y, Liao P, Li S, et al. Intersection of immune and oncometabolic pathways drives cancer hyperprogression during immunotherapy. Cancer Cell. (2023) 41:304–322.e7. doi: 10.1016/j.ccell.2022.12.008
5. Knight A, Karapetyan L, Kirkwood JM. Immunotherapy in melanoma: recent advances and future directions. Cancers (Basel). (2023) 15:1106. doi: 10.3390/cancers15041106
6. Fei S, Lu Y, Chen J, Qi J, Wu W, Wang B, et al. Efficacy of PD-1 inhibitors in first-line treatment for advanced gastroesophageal junction and gastric cancer by subgroups: A systematic review and meta-analysis. Chemotherapy. (2023) 68:197–209. doi: 10.1159/000531457
7. Kagamu H. Immunotherapy for non-small cell lung cancer. Respir Investig. (2024) 62:307–12. doi: 10.1016/j.resinv.2024.01.011
8. Monteiro FSM, Soares A, Rizzo A, Santoni M, Mollica V, Grande E, et al. The role of immune checkpoint inhibitors (ICI) as adjuvant treatment in renal cell carcinoma (RCC): A systematic review and meta-analysis. Clin Genitourin Cancer. (2023) 21:324–33. doi: 10.1016/j.clgc.2023.01.005
9. Yap DWT, Leone AG, Wong NZH, Zhao JJ, Tey JCS, Sundar R, et al. Effectiveness of immune checkpoint inhibitors in patients with advanced esophageal squamous cell carcinoma: A meta-analysis including low PD-L1 subgroups. JAMA Oncol. (2023) 9:215–24. doi: 10.1001/jamaoncol.2022.5816
10. Lei Z, Ma W, Si A, Zhang Y, Yang F, Yu Q, et al. Effect of different PD-1 inhibitor combination therapies for unresectable intrahepatic cholangiocarcinoma. Aliment Pharmacol Ther. (2023) 58:611–22. doi: 10.1111/apt.17623
11. Lamba N, Ott PA, Iorgulescu JB. Use of first-line immune checkpoint inhibitors and association with overall survival among patients with metastatic melanoma in the anti–PD-1 era. JAMA Network Open. (2022) 5:e2225459–e2225459. doi: 10.1001/jamanetworkopen.2022.25459
12. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
13. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
14. Dimitriou F, Cheng PF, Saltari A, Schaper-Gerhardt K, Staeger R, Haunerdinger V, et al. A targetable type III immune response with increase of IL-17A expressing CD4 T cells is associated with immunotherapy-induced toxicity in melanoma. Nat Cancer. (2024) 5:1390–408. doi: 10.1038/s43018-024-00810-4
15. Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, Boland G, et al. NCCN guidelines® Insights: melanoma: cutaneous, version 2.2021. J Natl Compr Canc Netw. (2021) 19:364–76. doi: 10.6004/jnccn.2021.0018
16. Mallardo D, Fordellone M, White A, Ottaviano M, Sparano F, Bailey M, et al. CD39 and LDHA affects the prognostic role of NLR in metastatic melanoma patients treated with immunotherapy. [J] .J Transl Med. (2023) 21:610. doi: 10.1186/s12967-023-04419-6
17. Aguilar-Cazares D, Chavez-Dominguez R, Marroquin-Muciño M, Perez-Medina M, Benito-Lopez JJ, Camarena A, et al. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front Endocrinol (Lausanne). (2022) 13:929572. doi: 10.3389/fendo.2022.929572
18. Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. (2018) 6:74. doi: 10.1186/s40425-018-0383-1
19. Feng Z, Weihong G. Prognostic value of the platelet-to-lymphocyte ratio in patients with melanoma: A meta-analysis. Front Oncol. (2020) 10:1116. doi: 10.3389/fonc.2020.01116
20. Wang Y, Zhang H, Yang Y, Zhang T, Ma X. Prognostic value of peripheral inflammatory markers in preoperative mucosal melanoma: A multicenter retrospective study. Front Oncol. (2019) 9:995. doi: 10.3389/fonc.2019.00995
21. Schneider M, Schäfer N, Bode C, Borger V, Eichhorn L, Giordano FA, et al. Prognostic value of preoperative inflammatory markers in melanoma patients with brain metastases. J Clin Med. (2021) 10:634. doi: 10.3390/jcm10040634
22. Hui Z, Jian-Ying Ma, Qi-Chao J. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in melanoma patients: A meta-analysis. Clin Chim Acta. (2018) 484:136–40. doi: 10.1016/j.cca.2018.05.055
23. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. (2019) 8:886–94. doi: 10.21037/tlcr.2019.11.16
24. Karan C, Yaren A, Demirel BC, Dogan T, Ozdemir M, Demiray AG, et al. Pretreatment PLR is preferable to NLR and LMR as a predictor in locally advanced and metastatic bladder cancer. Cancer Diagn Progn. (2023) 3:706–15. doi: 10.21873/cdp.10275
25. Gawiński C, Mróz A, Roszkowska-Purska K, Sosnowska I, Derezińska-Wołek E, Michalski W, et al. A prospective study on the roles of the lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in patients with locally advanced rectal cancer. Biomedicines. (2023) 11:3048. doi: 10.3390/biomedicines11113048
26. Afzal MZ, Sarwar T, Shirai K. Prognostic significance of hematological indices in Malignant melanoma treated with immune checkpoint inhibitors. J Immunother. (2019) 42:251–64. doi: 10.1097/CJI.0000000000000272
27. Matsumura Y, Kawarada Y, Matsuo M, Yokota K, Mizoguchi H, Akiyama M, et al. Retrospective analysis of neutrophil-to-lymphocyte ratio in patients with melanoma who received ipilimumab monotherapy or ipilimumab in combination with nivolumab in Japan. Biol Pharm Bull. (2023) 46:427–31. doi: 10.1248/bpb.b22-00750
28. Pozorski V, Park Y, Mohamoud Y, Tesfamichael D, Emamekhoo H, Birbrair A, et al. Neutrophil-to-eosinophil ratio as a biomarker for clinical outcomes in advanced stage melanoma patients treated with anti-PD-1 therapy. Pigment Cell Melanoma Res. (2023) 36:501–11. doi: 10.1111/pcmr.13109
29. Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. (2015) 112:1904–10. doi: 10.1038/bjc.2015.180
30. Krebs FK, Trzeciak ER, Zimmer S, Özistanbullu D, Mitzel-Rink H, Meissner M, et al. Immune signature as predictive marker for response to checkpoint inhibitor immunotherapy and overall survival in melanoma. Cancer Med. (2021) 10:1562–75. doi: 10.1002/cam4.3710
31. Schildbach VAS, Horn S, Hidalgo-Gadea G, Johannis W, Mauch C, Franklin C. C-reactive protein and lymphocyte-to-monocyte ratio predict recurrence in stage III melanoma patients with microscopic sentinel lymph node metastasis. Cancers (Basel). (2023) 15:702. doi: 10.3390/cancers15030702
32. Chasseuil E, Saint-Jean M, Chasseuil H, Peuvrel L, Quéreux G, Nguyen JM, et al. Blood predictive biomarkers for nivolumab in advanced melanoma. Acta Derm Venereol. (2018) 98:406–10. doi: 10.2340/00015555-2872
33. Failing JJ, Yan Y, Porrata LF, Markovic SN. Lymphocyte-to-monocyte ratio is associated with survival in pembrolizumab-treated metastatic melanoma patients. Melanoma Res. (2017) 27:596–600. doi: 10.1097/CMR.0000000000000404
34. Cassidy MR, Wolchok RE, Zheng J, Panageas KS, Wolchok JD, Coit D, et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine. (2017) 18:56–61. doi: 10.1016/j.ebiom.2017.03.029
35. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. (2016) 27:732–8. doi: 10.1093/annonc/mdw016
36. Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. (2016) 174:146–51. doi: 10.1111/bjd.14155
37. Vila CM, Moreno FA, Estébanez MM, Ares GR, Villacampa G, Dashti P, et al. Exploratory analysis of clinical benefit of ipilimumab and nivolumab treatment in patients with metastatic melanoma from a single institution. Clin Transl Oncol. (2022) 24:319–30. doi: 10.1007/s12094-021-02692-9
38. Ascierto PA, Capone M, Grimaldi AM, Mallardo D, Simeone E, Madonna G, et al. Proteomic test for anti-PD-1 checkpoint blockade treatment of metastatic melanoma with and without BRAF mutations. J Immunother Cancer. (2019) 7:91. doi: 10.1186/s40425-019-0569-1
39. Mesti T, Grašič Kuhar C, Ocvirk J. Biomarkers for outcome in metastatic melanoma in first line treatment with immune checkpoint inhibitors. Biomedicines. (2023) 11:749. doi: 10.3390/biomedicines11030749
40. Guida M, Bartolomeo N, Quaresmini D, Quaglino P, Madonna G, Pigozzo J, et al. Basal and one-month differed neutrophil, lymphocyte and platelet values and their ratios strongly predict the efficacy of checkpoint inhibitors immunotherapy in patients with advanced BRAF wild-type melanoma. J Transl Med. (2022) 20:159. doi: 10.1186/s12967-022-03359-x
41. Kopecky J, Kubecek O, Priester P, Vosmikova H, Cermakova E, Kyllarova A. Prognostic value of blood cell count-derived ratios in BRAF-mutated metastatic melanoma. BioMed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2022) 166:393–404. doi: 10.5507/bp.2021.053
42. Swami U, Chennamadhavuni A, Borcherding N, Bossler AD, Mott SL, Garje R, et al. Multivariable analysis of 169 cases of advanced cutaneous melanoma to evaluate antibiotic exposure as predictor of survival to anti-PD-1 based immunotherapies. Antibiotics (Basel). (2020) 9:740. doi: 10.3390/antibiotics9110740
43. Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. (2018) 7:690–7. doi: 10.1002/cam4.1356
44. Bai X, Dai J, Li C, Cui C, Mao L, Wei X, et al. Risk models for advanced melanoma patients under anti-PD-1 monotherapy-ad hoc analyses of pooled data from two clinical trials. Front Oncol. (2021) 11:639085. doi: 10.3389/fonc.2021.639085
45. Hernando-Calvo A, García-Alvarez A, Villacampa G, Ortiz C, Bodet D, García-Patos V, et al. Dynamics of clinical biomarkers as predictors of immunotherapy benefit in metastatic melanoma patients. Clin Transl Oncol. (2021) 23:311–7. doi: 10.1007/s12094-020-02420-9
46. Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget. (2016) 7:77404–15. doi: 10.18632/oncotarget.12677
47. Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. (2021) 384:2229–40. doi: 10.1056/nejmra2034861
48. Long GV, Swetter SM, Menzies AM, Gershenwald JE, Scolyer RA. Cutaneous melanoma. Lancet. (2023) 402:485–502. doi: 10.1016/S0140-6736(23)00821-8
49. Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 world health organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med. (2020) 144:500–22. doi: 10.5858/arpa.2019-0561-RA
50. Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. (2019) 20:1366–79. doi: 10.1080/15384047.2019.1640032
51. Teimouri F, Nikfar S, Abdollahi M. Efficacy and side effects of dacarbazine in comparison with temozolomide in the treatment of Malignant melanoma: a meta-analysis consisting of 1314 patients. Melanoma Res. (2013) 23:381–9. doi: 10.1097/CMR.0b013e3283649a97
52. Lugović-Mihić L, Ćesić D, Vuković P, Novak Bilić G, Šitum M, Špoljar S. Melanoma development: current knowledge on melanoma pathogenesis. Acta Dermatovenerol Croat. (2019) 27:163–8.
53. Dong Y, Chen Z, Yang F, Wei J, Huang J, Long X. Prediction of immunotherapy responsiveness in melanoma through single-cell sequencing-based characterization of the tumor immune microenvironment. Transl Oncol. (2024) 43:101910. doi: 10.1016/j.tranon.2024.101910
54. Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U, ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2019) 30:1884–901. doi: 10.1093/annonc/mdz411
55. SEER Web Site. Melanoma of the skin–cancer stat facts[DB/OL] (2023). Available online at: https://seer.cancer.gov/statfacts/html/melan.html (Accessed March 16, 2023).
56. Mahdiabadi S, Momtazmanesh S, Karimi A, Rezaei N. Immune checkpoint inhibitors in advanced cutaneous melanoma: a systematic review and meta-analysis of efficacy and review of characteristics. Expert Rev Anticancer Ther. (2023) 23:1281–93. doi: 10.1080/14737140.2023.2278509
57. Li Y, Liang X, Li H, Chen X. Comparative efficacy and safety of immune checkpoint inhibitors for unresectable advanced melanoma: A systematic review and network meta-analysis. Int Immunopharmacol. (2023) 115:109657. doi: 10.1016/j.intimp.2022.109657
58. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
59. Kudo M. Combination cancer immunotherapy with molecular targeted agents/anti-CTLA-4 antibody for hepatocellular carcinoma. Liver Cancer. (2019) 8:1–11. doi: 10.1159/000496277
60. Fu J, Mao L, Jiao Y, Mei D, Chen Y. Elucidating CTLA-4's role in tumor immunity: a comprehensive overview of targeted antibody therapies and clinical developments. Mol Divers. (2024). doi: 10.1007/s11030-024-10917-6
61. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. (2014) 515:568–71. doi: 10.1038/nature13954
62. Xia L, Liu Y, Wang Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist. (2019) 24:S31–41. doi: 10.1634/theoncologist.2019-IO-S1-s05
63. Larkin J, Minor D, D'Angelo S, Neyns B, Smylie M, Miller WH Jr, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in checkMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol. (2018) 36:383–90. doi: 10.1200/JCO.2016.71.8023
64. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. (2018) 175:313–26. doi: 10.1016/j.cell.2018.09.035
65. Kim TK, Vandsemb EN, Herbst RS, Chen L. Adaptive immune resistance at the tumor site: mechanisms and therapeutic opportunities. Nat Rev Drug Discovery. (2022) 21:529–40. doi: 10.1038/s41573-022-00493-5
66. Moran B, Silva R, Perry AS, Gallagher WM. Epigenetics of Malignant melanoma. Semin Cancer Biol. (2018) 51:80–8. doi: 10.1016/j.semcancer.2017.10.006
67. Abbas O, Miller DD, Bhawan J. Cutaneous Malignant melanoma: update on diagnostic and prognostic biomarkers. Am J Dermatopathol. (2014) 36:363–79. doi: 10.1097/DAD.0b013e31828a2ec5
68. Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. (2017) 387:61–8. doi: 10.1016/j.canlet.2016.01.043
69. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. (2016) 27:732–8. doi: 10.1093/annonc/mdw016
70. Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, Machet L. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma [J. Br J Dermatol. (2016) 174:146–51. doi: 10.1111/bjd.14155
71. VanderWalde A, Bellasea SL, Kendra KL, Khushalani NI, Campbell KM, Scumpia PO, et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: a randomized phase 2 trial. Nat Med. (2023) 29:2278–85. doi: 10.1038/s41591-023-02498-y
72. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumor progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
73. Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. (2019) 40:228–42. doi: 10.1016/j.it.2019.01.006
74. Zhang H, Houghton AM. Good cops turn bad: The contribution of neutrophils to immune-checkpoint inhibitor treatment failures in cancer. Pharmacol Ther. (2021) 217:107662. doi: 10.1016/j.pharmthera.2020.107662
75. Życzkowski M, Kaletka Z, Rajwa P, Stelmach P, Bogacki R, Łach-Wojnarowicz O, et al. Mean platelet vol⁃ ume⁃to⁃lymphocyte ratio:a novel biomarker associated with overall survival in patients with nonmetastatic clear cell renal cell carcinoma treated with nephrectomy. Int Urol Nephrol. (2020) 52:885⁃891.
76. Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. (2017) 16:137. doi: 10.1186/s12943-017-0707-7
77. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus[J. Cancer Metastasis Rev. (2017) 36:249–62. doi: 10.1007/s10555-017-9673-1
78. Torisu-Itakura H, Lee JH, Huynh Y, Ye X, Essner R, Morton DL. Monocytederived IL-10 expression predicts prognosis of stage IV melanoma patients. J Immunotherapy. (2007) 30:831–8. doi: 10.1097/cji.0b013e318158795b
Keywords: melanoma, immune checkpoint inhibitors, inflammatory markers, survival, meta-analysis
Citation: Ou Y, Liang S, Gao Q, Shang Y, Liang J, Zhang W and Liu S (2024) Prognostic value of inflammatory markers NLR, PLR, LMR, dNLR, ANC in melanoma patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review. Front. Immunol. 15:1482746. doi: 10.3389/fimmu.2024.1482746
Received: 18 August 2024; Accepted: 30 September 2024;
Published: 18 October 2024.
Edited by:
Vijay Kumar, Morehouse School of Medicine, United StatesReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusXiao Long, Peking Union Medical College Hospital (CAMS), China
Copyright © 2024 Ou, Liang, Gao, Shang, Liang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sha Liu, MTMzNTkyOTg4ODRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yan Ou1†
Yan Ou1† Qiangqiang Gao
Qiangqiang Gao Sha Liu
Sha Liu