- Department of Medical Microbiology, University of Gondar, Gondar, Ethiopia
Bacterial and viral infections cause a huge burden to healthcare settings worldwide, and mortality rates associated with infectious microorganisms have remained high in recent decades. Despite tremendous efforts and resources worldwide to explore diagnostic biomarkers, rapid and easily assayed indicators for the diagnosis of bacterial and viral infections remain a challenge. B7 homolog 3 (B7-H3), a member of the B7 family of immunoregulatory proteins, is overexpressed in patients with septicemia, meningitis, pneumonia, and hepatitis. Therefore, B7-H3 could be used as a potential clinical indicator and therapeutic target for bacterial and viral infections caused by H. pylori, S. pneumoniae, M. pneumoniae, hepatitis B virus (HBV), viral hemorrhagic septicemia virus (VHSV), respiratory syncytial virus (RSV), and human immunodeficiency virus (HIV). Moreover, the interplay between infectious microorganisms and B7-H3 and exploration of the functional roles of the B7-H3 molecule could aid in the development of novel strategies for disease diagnosis and immunotherapy.
Introduction
Bacterial and viral infections are common in children and adults. Fever is a common symptom of infectious diseases, suggesting systemic inflammation in response to bacterial or viral infections. The non-specific nature of signs and symptoms in febrile patients makes clinical differentiation of infections challenging, particularly in identifying severe diseases such as septicemia, meningitis, and pneumonia. For optimal treatment, early diagnostic biomarkers indicating bacterial or viral infections are required to reduce mortality from serious infections. Despite the difficulty in distinguishing between infection, inflammation, and autoimmunity, biomarkers in combination with the symptoms of the patient support physicians in considering proper diagnosis and treatment (1, 2). Although white blood cell counts (WBCs), C-reactive protein, and inflammatory cytokines are useful diagnostic indicators of infections, more rapid and easily assayed indicators can advance diagnosis (3).
B7 homolog 3 (B7-H3, CD276) is a member of the B7 family of immunoregulatory proteins, sharing 20–27% amino acid identity with other B7 family members (4, 5). It is a type I transmembrane protein that primarily functions as a negative immunoregulatory protein (6). B7-H3 is a novel protein structurally related to the B7 family of ligands, characterized by a single extracellular IgV- and IgC-like domain in the transmembrane region and a highly diverse cytoplasmic tail. The predominant form of human B7-H3, 4IgB7-H3, contains tandemly duplicated IgV-IgC domains due to exon duplication, which generates two isoforms: 2IgB7-H3 and 4IgB7-H3. Additionally, serine- and arginine-rich splicing factor 3 (SRSF3) plays a role in the splicing of B7-H3 by binding directly to exons 4 and/or 6 (7–10).
B7-H3 expression and its impact on tumor immune microenvironment
Several studies have shown that B7-H3 can be detected in immune cells, such as activated macrophages, dendritic cells, monocytes, myeloid-derived suppressor cells, activated T cells, and various types of cells in non-lymphoid tissues, including tumor cells, epithelial cells, fibroblast-like synoviocytes, osteoblasts, and human serum. B7-H3 is overexpressed in tumor tissues and shows limited expression in normal tissues (6, 11, 12). Confocal microscopy of fibroblast-like synoviocytes and T-cell co-cultures showed B7-H3 localization at the T-cell–fibroblast-like synoviocyte contact point (13). Most human intratumoral neutrophils express high levels of B7-H3, and locally enriched B7-H3+ neutrophils are positively correlated with increased granulocyte-macrophage colony-stimulating factor levels (14).
B7-H3 is an immunoregulatory ligand that affects immune responses through both immunological and non-immunological pathways (15) and exerts either inhibitory or stimulatory effects on immune cell activation (16). Activated CD4+ and CD8+ T cells express a putative receptor that recognizes B7-H3 molecules (17). The expression of B7-H3 favors an immunosuppressive microenvironment by promoting IL-10 and TGF-β1 (18). The VC and VCVC forms of human B7-H3 inhibit CD4+ T cell activation, proliferation, and cytokine production (19), as well as attenuate NK cell-mediated killing (20). B7-H3 expression inhibits the activation of CD4+ T cells, CD8+ T cells, γδT cells, CAR-T cells, Vδ2 T cells, Th17 cells, CD3+ T cells, macrophages, neutrophils, and dendritic cells, as well as the secretion of IFN-γ, IL-2, and perforin/granzyme B (21–24).
B7-H3 regulates the differentiation of tumor-associated macrophages, promotes the polarization of type 2 macrophages, and switches the M1 phenotype to the M2 phenotype (25). B7-H3 recruits macrophages into the tumor microenvironment (26) and contributes to CCL2–CCR2–M2 macrophage axis-mediated immunosuppression (27). Zhou et al. reported that the high expression of B7-H3 in human prostate cancer tissues is negatively correlated with CD8+ tumor-infiltrating lymphocytes (28). However, some studies have reported that human patients with high B7-H3 expression show increased numbers of immune cells, including CD8+ T cells, CD4+ T cells, natural killer cells, plasmacytoid dendritic cells, and increased interferon-γ production (4, 29, 30).
The role of B7-H3 in modulating immune responses during bacterial infections
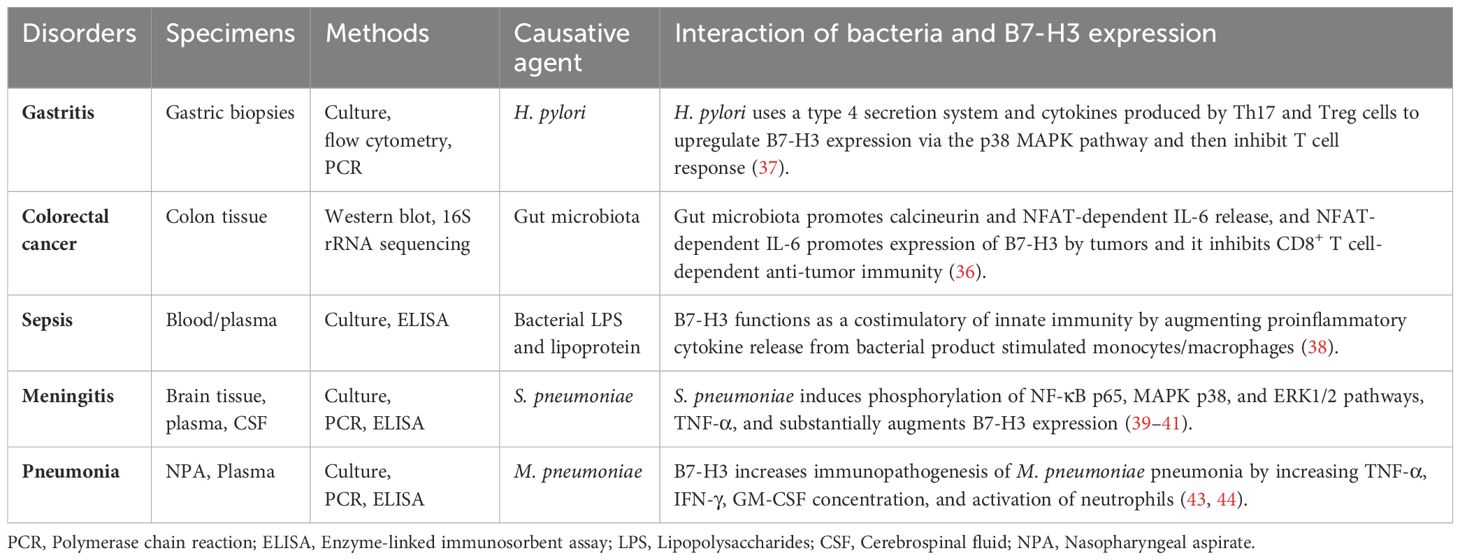
Several studies have shown that microorganisms have a profound impact on many aspects of cell function and are involved in many diseases (31–33). An infection happens when bacteria, viruses, or fungi invade the body, and damage the host. While the immune system works to eliminate these invaders, sometimes the pathogens overpower the body’s defenses, resulting in illness (34). The gut microbiota plays a crucial role in modulating the immune system. However, its dysbiosis induces chronic inflammation. For example, E. coli and B. fragilis are known to promote inflammation in the gut that leads to DNA damage and tumor formation (35). In mouse model, a microbiota-dependent pathway crosstalk between myeloid cells, T cells, and tumor cells that inhibits CD8+ T cell-dependent anti-tumor immunity through the co-inhibitory protein B7-H3 (34).
Bacterial sensing by myeloid cells promotes calcineurin- and NFAT-dependent IL-6 release. This IL-6, in turn, promotes the expression of co-inhibitory B7-H3 by tumors, which inhibits CD8+ T cell-dependent antitumor immunity, whereas B7-H3 blockade elicits protective T cell responses (36). Helicobacter pylori infection induces B7-H3 expression in human gastric epithelial cells through the type 4 secretion system components, CagA, and cell wall peptidoglycan fragments. These are recognized by the intracellular pattern recognition receptor NOD1, which activates the MAPKs and NF-κB pathways. During H. pylori infection, patients exhibit a mixed Th1/Th2 response, with increased circulating Treg and Th17 cells. Human biopsy samples from patients with gastritis and gastric tumors show increased B7-H3 expression and Th2 responses in H. pylori strains associated with gastritis (37).
B7-H3 functions as a costimulatory molecule in innate immunity by augmenting the release of proinflammatory cytokines from monocytes and macrophages stimulated by bacterial cell wall products, contributing to the development of sepsis. B7-H3 enhances human sepsis through bacterial lipopolysaccharide (LPS)- and lipoprotein-induced NF-κB activation and inflammatory responses. However, blocking B7-H3 in vivo attenuated LPS-induced proinflammatory cytokine release and reduced endotoxin shock-related lethality. Furthermore, human patients diagnosed with sepsis exhibit significantly higher levels of plasma soluble B7-H3 than healthy individuals. Stimulation of human monocytes with LPS and inflammatory cytokines leads to substantial release of soluble B7-H3 (38).
Circulating B7-H3 levels in cerebrospinal fluid (CSF) and plasma were higher in children with bacterial meningitis than in the control group. Additionally, circulating TNF-α levels in CSF and plasma were higher in the bacterial meningitis group than in the control group. On admission, circulating B7-H3 levels in the plasma and CSF of patients with bacterial meningitis were positively correlated with TNF-α, IFN-γ, and white blood cell counts, making them useful markers for distinguishing bacterial from aseptic meningitis and for evaluating the intensity of the infectious inflammatory process in the central nervous system (39).
The costimulatory protein B7-H3 contributes to the development and progression of pneumococcal meningitis by augmenting the innate immunity-associated inflammatory response in a TLR2-dependent manner. B7-H3 enhances the formation of the MyD88-IRAK immunocomplex in the brains of S. pneumoniae-infected mice and significantly augments S. pneumoniae-induced activation of TLR2 downstream of the NF-κB p65 and MAPK p38 pathways. This exacerbates mouse brain damage by intensifying inflammatory responses (40, 41).
B7-H3 plays a role in S. pneumoniae infection-induced pneumococcal meningitis by amplifying the inflammatory response, worsening blood-brain barrier disruption, and aggravating the clinical disease status via a TLR2-dependent mechanism. B7-H3 augments proinflammatory cytokine and chemokine production, upregulates NF-κB, TLR2, p65, and MAPK p38 phosphorylation, and enhances the nuclear transactivation of NF-κB p65 at the TNF-α and IL-6 promoters in S. pneumoniae-stimulated microglial cells of the mice (42).
Soluble B7-H3 levels were significantly higher in human patients with M. pneumoniae pneumonia (MPP) compared to control subjects. Furthermore, soluble B7-H3 plays an important role in MPP by increasing TNF-α concentrations and neutrophil activation (43). Elevated levels of soluble B7-H3 were found in both mild and severe MPP in pediatric patients compared with control patients. Moreover, significantly higher levels of soluble B7-H3 were detected in patients with severe MPP compared with those with mild MPP. The receiver operating characteristic (ROC) curve showed that soluble B7-H3 had a severity prediction capacity for mild and severe MPP and was positively associated with IFN-γ and GM-CSF in patients with severe MPP. Additionally, elevated levels of soluble B7-H3 were found in acute-phase MPP patients compared with control subjects, while significantly lower levels of plasma soluble B7-H3 were observed in recovery-phase MPP patients compared with acute-phase patients (44).
Children with MPP and pleural effusion had higher levels of soluble B7-H3 and IL-36 than control subjects. The concentration of soluble B7-H3 in bronchoalveolar lavage fluid was strongly associated with IL-36 levels, the duration of fever, and length of hospital stay (45). Additionally, children with MPP had higher levels of soluble B7-H3 and IL-17 than controls, especially during the acute stage of MPP. Children with MPP and pleural effusion had higher levels of soluble B7-H3 than those without pleural effusion, and these levels were positively correlated with the number of fever days (46). During H. pylori, S. pneumoniae, and M. pneumoniae infections, elevated B7-H3 levels were detected in human biopsy, CSF, and serum samples. The interplay between bacteria and B7-H3 expression in different disorders has been documented in several studies (36–41, 43, 44) and the results are presented in Table 1.
B7-H3 as a modulator of immune responses in viral infections
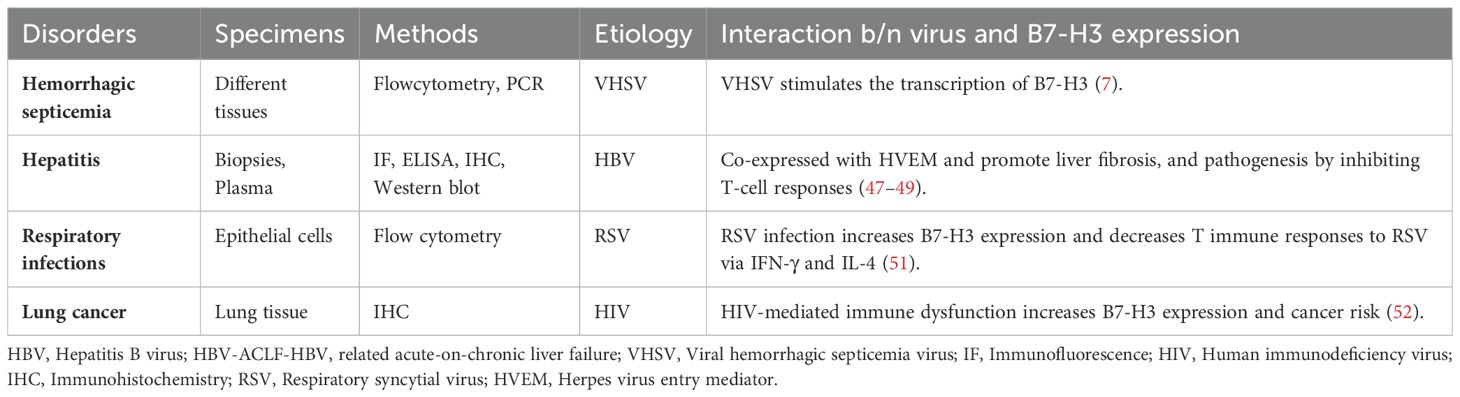
Costimulatory molecules are important regulators of the immune response and participate in the regulation of liver pathology during hepatitis B virus (HBV) infection. The costimulatory protein B7-H3 is upregulated after HBV infection and contributes to the progression and poor prognosis of HBV infection by triggering inhibitory signals in effector T cells. The membrane and soluble forms of B7-H3 are expressed on Treg cells and monocytes and are positively correlated with the frequency of Treg cells in patients with acute hepatitis B (AHB), chronic hepatitis B (CHB), and hepatocellular carcinoma (HCC) associated with HBV infection. Soluble B7-H3 levels are higher in the late tumor-node-metastasis (TNM) stages of HCC. Moreover, B7-H3 expression positively correlates with aspartate aminotransferase and alanine aminotransferase levels in chronic HBV infection. Immunohistochemistry tests show that higher membrane B7-H3 expression is associated with larger tumor size, later TNM stages, and worse prognosis in HBV-HCC (47).
Luan et al. found that abundant plasma-soluble B7-H3 positively correlated with liver fibrosis in children with chronic HBV infection (46). Soluble B7-H3 originates from the hepatocyte membrane and promotes hepatic inflammation and hepatitis progression. A functional study showed that immobilized B7-H3 fusion protein inhibits TCR-induced proliferation and IFN-γ secretion by T cells (48). Immunohistochemical analysis detected B7-H3 in all HBV-related acute and chronic liver failure (HBV-ACLF) human biopsy samples. B7-H3 is found on cell membranes and in the cytoplasm of HBV-ACLF samples, and its expression is predominantly observed in infiltrating inflammatory cells and damaged bile ducts (49). B7-H3 was co-expressed with the herpes virus entry mediator in human liver tissues, and with B and T lymphocyte attenuators and the herpes virus entry mediator (50).
Human respiratory tract epithelial cells express a wide range of B7 molecules, and B7-H3 is strongly expressed in unstimulated tracheal, bronchial, and alveolar epithelial cells. Respiratory syncytial virus (RSV) infection of tracheal, bronchial, and alveolar epithelial cells upregulates B7-H3 expression. The high expression of B7-H3 following RSV infection is regulated by IFN-γ and IL-4, which may be involved in decreasing T cell antiviral immune responses to RSV and RSV-associated wheezing. On RSV-infected alveolar epithelial cells, IFN-γ treatment decreases B7-H3, while IL-4 treatment increases B7-H3 expression (51).
Evidence shows that B7-H3 mRNA is broadly expressed in both lymphoid and non-lymphoid organs. Viral hemorrhagic septicemia virus (VHSV) induces the transcription of B7-H3 in the fish liver during the early hours, and it is expressed later in the fish head, kidney, spleen, intestine, and gill tissues. Flow cytometric analysis of leukocytes revealed that 85.1% of granulocytes and 3.1% of lymphocytes expressed B7-H3 molecules on their cell surfaces. The co-inhibitory molecule B7-H3 participates in regulating cell-mediated immune responses during VHSV infection (7).
Individuals with human immunodeficiency virus (HIV) infection have a higher incidence of various malignancies, and HIV-mediated immune dysfunction may lead to chronic immune activation, decreased tumor surveillance, and subsequently increased cancer risk. HIV-infected patients with lung cancer had significantly higher B7-H3 tumor expression levels than HIV-uninfected controls. B7-H3 expression was 92% in lung cancer samples from HIV-infected cases, compared to 69% in samples from HIV-uninfected cases (52). These studies suggest that B7-H3 may play a role in viral pathogenesis and could offer a promising approach for the diagnosis and treatment of viral infections. Previously, the interaction between different virus and B7-H3 protein has been reported in different diseases (7, 47–49, 51, 52) (Table 2).
Emerging receptors for B7-H3: current candidates and future research directions
The putative receptors for B7-H3 remain under investigation, but several candidates have emerged. The T-cell immunoreceptor with Ig and ITIM domains (TIGIT) has been suggested as an inhibitory receptor that could interact with B7-H3, contributing to immune evasion in tumors (53). The herpesvirus entry mediator (HVEM), another B7 family member, has also been proposed as a potential receptor, potentially influencing T-cell activation (54). Ongoing research is exploring whether B7-H3 might interact with other immune checkpoint receptors, such as PD-1 or CTLA-4 (55). Overall, while several receptor candidates exist, further research is needed to fully elucidate B7-H3’s potential receptors and their broader implications in immune modulation.
B7-H3 versus traditional markers: a new frontier in early disease diagnosis and treatment
B7-H3 has shown greater specificity to some bacterial and viral infections, particularly in patients with septicemia, meningitis, pneumonia, and hepatitis, thereby reducing the risk of false positives and negatives. Studies indicate that B7-H3 is significantly overexpressed in H. pylori, S. pneumoniae, M. pneumoniae, HBV, VHSV, RSV, and HIV compared to traditional markers, making it more reliable for early detection and disease progression monitoring (56). Unlike some traditional markers, B7-H3 also holds promise as a therapeutic target, opening possibilities for combined diagnostic and therapeutic strategies (57). However, many traditional markers, such as WBCs, C-reactive protein, and cytokines, have lower sensitivity in the early stages of disease, which may delay diagnosis and intervention. Moreover, traditional markers may not provide the same level of specificity in distinguishing between closely related conditions, leading to potential diagnostic ambiguities. While the implementation of B7-H3 in clinical settings is still being refined, early studies suggest that its integration into diagnostic workflows would not significantly increase complexity or cost, particularly with the development of standardized assays. B7-H3’s dual potential as both a diagnostic marker and a therapeutic target offers a long-term advantage (55), potentially streamlining patient care through personalized medicine approaches.
B7-H3’s role in differentiating diseases with overlapping symptoms
Febrile diseases often share common symptoms such as fever, fatigue, and malaise, which complicates early differentiation between infectious, autoimmune, or malignant conditions. B7-H3’s expression pattern offers a significant advantage in distinguishing between these conditions in the early stages, where other traditional markers may lack specificity. For example, B7-H3’s elevated expression in bacteria, and virus-infected patients allows for earlier detection when other markers remain inconclusive due to overlapping clinical presentations. We also highlight that B7-H3 can improve diagnostic accuracy during the initial symptomatic phase, facilitating prompt and targeted treatment (58, 59). This ability to provide early and reliable differentiation underlines B7-H3’s strength as a promising superior diagnostic tool.
Challenges and limitations of B7-H3 as a clinical diagnostic biomarker
B7-H3 has several limitations as a clinical diagnostic assay. Its broad expression across various diseases, including cancer, autoimmune disorders, and infections, reduces its specificity, making it difficult to determine if upregulation is caused by a particular pathology or a general immune response (60). Additionally, B7-H3 expression can vary significantly across different cancers and even within cancer subtypes, complicating its use as a reliable marker (61). Its overlap with other immune checkpoint molecules, such as PD-L1 and B7-H4 (62), further challenges the ability to distinguish its specific role. Moreover, the lack of standardized clinical assays and established cut-off values for B7-H3 detection limits its widespread clinical use. B7-H3 is also dynamically regulated by immune signals, which can lead to fluctuating expression levels during disease progression, increasing the risk of inconsistent diagnostic results. Furthermore, its dual role in both immune activation and suppression complicates interpretation, as it may signal different biological processes depending on the context. Finally, while B7-H3 shows promise as a therapeutic target, its predictive value for treatment response remains unclear, limiting its use in guiding therapy decisions. Therefore, B7-H3 would likely need to be combined with other biomarkers to improve diagnostic accuracy.
Current ongoing clinical trials targeting B7-H3 and their mechanisms
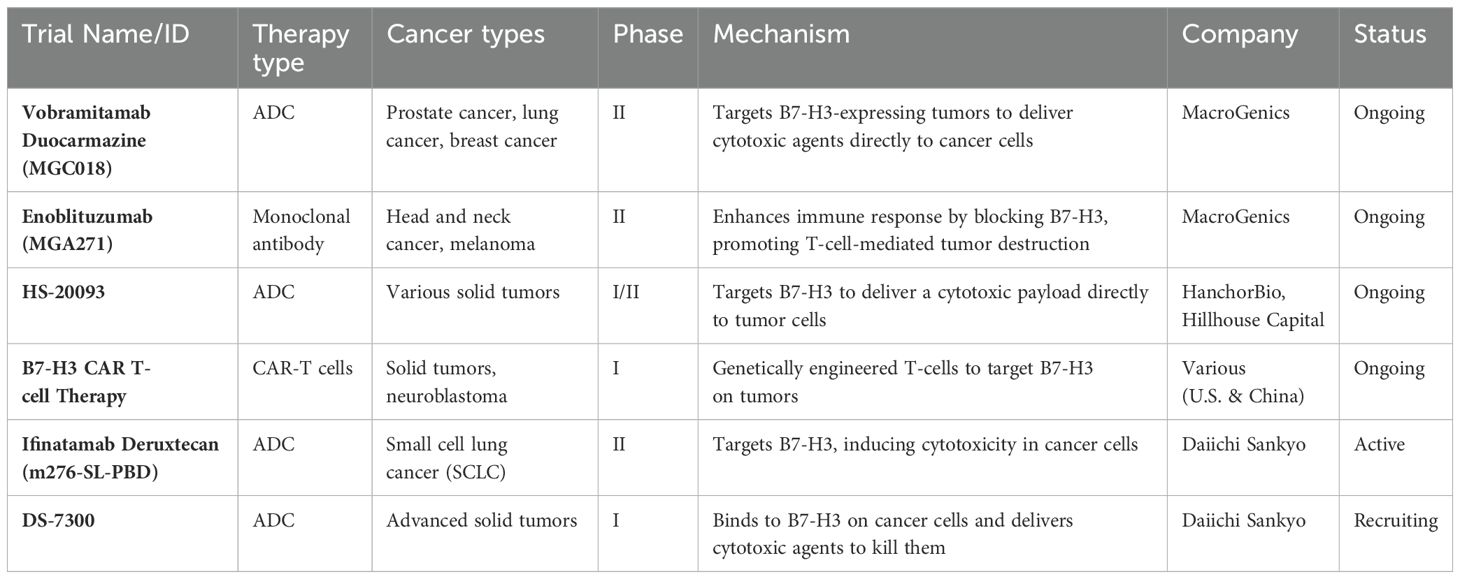
Although B7-H3 has not been extensively studied in bacterial and viral infections, its role in immune suppression may hold potential relevance for future infection-related research. By inhibiting immune checkpoints like B7-H3, it might be possible to enhance the body’s ability to fight not only tumors but also infections. However, at present, there are no ongoing trials directly targeting B7-H3 for bacterial or viral infections. Currently, clinical trials targeting B7-H3 primarily focus on cancer rather than on bacterial or viral infections (63). Several antibody-drug conjugates (ADCs) and chimeric antigen receptor (CAR)-T cell trials exploring therapies targeting B7-H3 for different cancer types are listed in Table 3.
Conclusion and future perspectives
Taken together, published evidence suggests that B7-H3 might contribute to the progression of bacterial and viral infections by triggering inhibitory signals in effector T cells and is associated with poor prognosis during these infections. Many patients with suspected febrile disease are present with similar or overlapping clinical symptoms, which makes early diagnosis difficult. A novel biomarker for infection in febrile patients is needed to help physicians make the correct diagnosis and initiate appropriate treatment to improve patient outcomes. Herein, we review the discovery of novel protein biomarkers to improve current diagnostics and accelerate early and personalized treatment decisions. Therefore, B7-H3 could be utilized as a potential diagnostic marker in addition to white blood cell counts, C-reactive protein, and inflammatory cytokines, and as a potential therapeutic target against bacterial and viral infections. In-depth studies should be conducted to explore the role of B7-H3 in these infections.
Author contributions
AT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I thank the University of Gondar for providing us with an opportunity to conduct this mini-review.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADCs, Antibody-drug conjugates; B7-H3, B7 homolog 3; CAR-T cells, Chimeric antigen receptor T cells; NK, Natural killer cell; LPS, lipopolysaccharide; CSF, cerebrospinal fluid; MPP, M. pneumoniae pneumonia; HBV, hepatitis B virus; AHB, acute hepatitis B; CHB, chronic hepatitis B; HCC, hepatocellular carcinoma; TNM, tumor-node-metastasis; HBV-ACLF, HBV-related acute and chronic liver failure; RSV, respiratory syncytial virus; VHSV, viral hemorrhagic septicemia virus; HIV, human immunodeficiency virus.
References
1. Hatherill M, Tibby Sm, Sykes K, Turner C, Murdoch IA. Diagnostic markers of infection: comparison of procalcitonin with C reactive protein and leucocyte count. Archives of Disease in Childhood. (1999) 81(5):417–21. doi: 10.1136/adc.81.5.417
2. Zandstra J, Jongerius I, Kuijpers TW. Future biomarkers for infection and inflammation in febrile children. Front Immunol. (2021) 12:631308. doi: 10.3389/fimmu.2021.631308
3. Yusa T, Tateda K, Ohara A, Miyazaki S. New possible biomarkers for diagnosis of infections and diagnostic distinction between bacterial and viral infections in children. J Infect Chemother. (2017) 23:96–100. doi: 10.1016/j.jiac.2016.11.002
4. Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3:A costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. (2001) 2:269–74. doi: 10.1038/85339
5. Flem-Karlsen K, Fodstad O, Tan M, Nunes-Xavier CE. B7-H3 in cancer - beyond immune regulation. Trends Cancer. (2018) 4:401–4. doi: 10.1016/j.trecan.2018.03.010
6. Oh Y, Park R, Kim SY, Park SH, Jo S, Kim TH, et al. B7-H3 regulates osteoclast differentiation via type I interferon-dependent IDO induction. Cell Death Dis. (2021) 12:971. doi: 10.1038/s41419-021-04275-6
7. Hwang JY, Jeong JM, Kwon MG, Seo JS, Hwang SD, Son MH, et al. Olive flounder CD276 (B7-H3) a coinhibitory molecule for T cells: Responses during viral hemorrhagic septicemia virus (VHSV) stimulation. Fish Shellfish Immunol. (2018) 73:228–33. doi: 10.1016/j.fsi.2017.12.021
8. Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, et al. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. (2003) 82:365–77. doi: 10.1016/S0888-7543(03)00126-5
9. Sun J, Fu F, Gu W, Yan R, Zhang G, Shen Z, et al. Origination of new immunological functions in the costimulatory molecule B7-H3: the role of exon duplication in evolution of the immune system. PloS One. (2011) 6:e24751. doi: 10.1371/journal.pone.0024751
10. Zhang C, Chen Y, Li F, Yang M, Meng F, Zhang Y, et al. B7-H3 is spliced by SRSF3 in colorectal cancer. Cancer Immunol Immunother. (2021) 70:311–21. doi: 10.1007/s00262-020-02683-9
11. Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. (2008) 123:538–46. doi: 10.1111/j.1365-2567.2007.02723.x
12. Zhou WT, Jin WL. B7-H3/CD276: an emerging cancer immunotherapy. Front Immunol. (2021) 12:701006. doi: 10.3389/fimmu.2021.701006
13. Tran CN, Thacker SG, Louie DM, Oliver J, White PT, Endres JL, et al. Interactions of T cells with fibroblast-like synoviocytes: role of the B7 family costimulatory ligand B7-H3. J Immunol. (2008) 180:2989–98. doi: 10.4049/jimmunol.180.5.2989
14. Li ZY, Wang JT, Chen G, Shan ZG, Wang TT, Shen Y, et al. Expression, regulation and clinical significance of B7-H3 on neutrophils in human gastric cancer. Clin Immunol. (2021) 227:108753. doi: 10.1016/j.clim.2021.108753
15. Zhang W, Zhang W, Gui L, Yan X, Zhou X, Ma Y, et al. Expression and prognosis of the B7 family in acute myeloid leukemia. Ann Transl Med. (2021) 9:1530. doi: 10.21037/atm-21-4255
16. Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. (2016) 22:3425–31. doi: 10.1158/1078-0432.CCR-15-2428
17. Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. (2002) 168:6294–7. doi: 10.4049/jimmunol.168.12.6294
18. Han S, Wang Y, Shi X, Zong L, Liu L, Zhang J, et al. Negative roles of B7-H3 and B7-H4 in the microenvironment of cervical cancer. Exp Cell Res. (2018) 371:222–30. doi: 10.1016/j.yexcr.2018.08.014
19. Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. (2013) 21:707–17. doi: 10.1016/j.str.2013.03.003
20. Lee CC, Ho KH, Huang TW, Shih CM, Hsu SY, Liu AJ, et al. A regulatory loop among CD276, miR-29c-3p, and Myc exists in cancer cells against natural killer cell cytotoxicity. Life Sci. (2021) 277:119438. doi: 10.1016/j.lfs.2021.119438
21. Xu J, Huang B, Xiong P, Feng W, Xu Y, Fang M, et al. Soluble mouse B7-H3 down-regulates dendritic cell stimulatory capacity to allogenic T cell proliferation and production of IL-2 and IFN-gamma Cell Mol Immunol. (2006) 3(3):235–40. doi: https://www.cmi.ustc.edu.cn/3/3/235.pdf
22. Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, et al. B7-H3 inhibits the IFN-gamma-dependent cytotoxicity of Vgamma9Vdelta2 T cells against colon cancer cells. Oncoimmunology. (2020) 9:1748991. doi: 10.1080/2162402X.2020.1748991
23. Si S, Wang L, Cao H, Xu Y, Zhan Q. Co-deficiency of B7-H3 and B7-H4 identifies high CD8 + T cell infiltration and better prognosis in pancreatic cancer. BMC Cancer. (2022) 22:211. doi: 10.1186/s12885-022-09294-w
24. Long C, Li G, Zhang C, Jiang T, Li Y, Duan X, et al. B7-H3 as a target for CAR-T cell therapy in skull base chordoma. Front Oncol. (2021) 11:659662. doi: 10.3389/fonc.2021.659662
25. Mao Y, Chen L, Wang F, Zhu D, Ge X, Hua D, et al. Cancer cell-expressed B7-H3 regulates the differentiation of tumor-associated macrophages in human colorectal carcinoma. Oncol Lett. (2017) 14:6177–83. doi: 10.3892/ol.2017.6935
26. Durlanik S, Fundel-Clemens K, Viollet C, Huber HJ, Lenter M, Kitt K, et al. CD276 is an important player in macrophage recruitment into the tumor and an upstream regulator for PAI-1. Sci Rep. (2021) 11:14849. doi: 10.1038/s41598-021-94360-9
27. Miyamoto T, Murakami R, Hamanishi J, Tanigaki K, Hosoe Y, Mise N, et al. B7-H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol Res. (2022) 10:56–69. doi: 10.1158/2326-6066.CIR-21-0407
28. Zhou Q, Li K, Lai Y, Yao K, Wang Q, Zhan X, et al. B7 score and T cell infiltration stratify immune status in prostate cancer. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002455
29. Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. (2004) 173:5445–50. doi: 10.4049/jimmunol.173.9.5445
30. Yim J, Koh J, Kim S, Song SG, Ahn HK, Kim YA, et al. Effects of B7-H3 expression on tumour-infiltrating immune cells and clinicopathological characteristics in non-small-cell lung cancer. Eur J Cancer. (2020) 133:74–85. doi: 10.1016/j.ejca.2020.03.033
31. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
32. Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. (2017) 20:145–55. doi: 10.1038/nn.4476
33. Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. (2017) 279:70–89. doi: 10.1111/imr.2017.279.issue-1
34. Hanlon G, Hodges NA. Essential microbiology for pharmacy and pharmaceutical science. John Wiley & Sons (2012).
35. Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. (2013) 13:800–12. doi: 10.1038/nrc3610
36. Peuker K, Strigli A, Tauriello DVF, Hendricks A, von Schonfels W, Burmeister G, et al. Microbiota-dependent activation of the myeloid calcineurin-NFAT pathway inhibits B7H3- and B7H4-dependent anti-tumor immunity in colorectal cancer. Immunity. (2022) 55:701–17 e7. doi: 10.1016/j.immuni.2022.03.008
37. Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Helicobacter pylori elicits B7H3 expression on gastric epithelial cells: Implications in local T cell regulation and subset development during infection. Clin Oncol Res. (2019) 2. doi: 10.31487/j.COR
38. Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, et al. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. (2010) 185:3677–84. doi: 10.4049/jimmunol.0904020
39. Chen X, Zhang G, Li Y, Feng X, Wan F, Zhang L, et al. Circulating B7-H3(CD276) elevations in cerebrospinal fluid and plasma of children with bacterial meningitis. J Mol Neurosci. (2009) 37:86–94. doi: 10.1007/s12031-008-9133-z
40. Chen X, Meng X, Foley NM, Shi X, Liu M, Chai Y, et al. Activation of the TLR2-mediated downstream signaling pathways NF-kappaB and MAPK is responsible for B7-H3-augmented inflammatory response during S. pneumoniae infection. J Neuroimmunol. (2017) 310:82–90. doi: 10.1016/j.jneuroim.2017.07.002
41. Chen X, Li Y, Blankson S, Liu M, Huang D, Redmond HP, et al. B7-H3 Augments Inflammatory Responses and Exacerbates Brain Damage via Amplifying NF-kappaB p65 and MAPK p38 Activation during Experimental Pneumococcal Meningitis. PloS One. (2017) 12(1):e0171146. doi: 10.1371/journal.pone.0171146
42. Chen X, Quinn EM, Ni H, Wang J, Blankson S, Redmond HP, et al. B7-H3 participates in the development of experimental pneumococcal meningitis by augmentation of the inflammatory response via a TLR2-dependent mechanism. J Immunol. (2012) 189:347–55. doi: 10.4049/jimmunol.1103715
43. Chen ZR, Zhang GB, Wang YQ, Yan YD, Zhou WF, Zhu CH, et al. Soluble B7-H3 elevations in hospitalized children with Mycoplasma pneumoniae pneumonia. Diagn Microbiol Infect Dis. (2013) 77:362–6. doi: 10.1016/j.diagmicrobio.2013.09.006
44. Xu Y, Yu L, Hao C, Wang Y, Zhu C, Ji W, et al. Plasma soluble B7-H3 levels for severity evaluation in pediatric patients with Mycoplasma pneumoniae pneumonia. Int Immunopharmacol. (2019) 73:163–71. doi: 10.1016/j.intimp.2019.05.014
45. Chen Z, Zhao X, Zhang X, Zhang G, Sun H, Jiang W, et al. Increased concentrations of soluble B7-H3 and interleukin 36 in bronchoalveolar lavage fluid of Children with Mycoplasma pneumoniae pneumonia. BMC Infect Dis. (2016) 16:212. doi: 10.1186/s12879-016-1555-6
46. Li QL, Wu YY, Sun HM, Gu WJ, Zhang XX, Wang MJ, et al. The role of miR-29c/B7-H3/Th17 axis in children with Mycoplasma pneumoniae pneumonia. Ital J Pediatr. (2019) 45:61. doi: 10.1186/s13052-019-0655-5
47. Gao F, Xu JC, Zhu L, Chen H, Zhu XY, You XR, et al. Clinical significance of B7-H3 expression during the progression of hepatitis B virus infection. Viral Immunol. (2018) 31:668–75. doi: 10.1089/vim.2018.0102
48. Luan Y, Ju J, Luo L, Zhang Z, Wang J, Zhu DM, et al. Potential role of soluble B7-H3 in liver immunopathogenesis during chronic HBV infection. J Viral Hepat. (2012) 19:23–31. doi: 10.1111/j.1365-2893.2010.01421.x
49. Guo G, Cao D, Xu H, Ruan Z, Fei L, Xie Z, et al. The characteristic expression of B7-H3 and B7-H4 in liver biopsies from patients with HBV-related acute-on-chronic liver failure. Pathol Int. (2012) 62:665–74. doi: 10.1111/j.1440-1827.2012.02856.x
50. Xu H, Cao D, Guo G, Ruan Z, Wu Y, Chen Y. The intrahepatic expression and distribution of BTLA and its ligand HVEM in patients with HBV-related acute-on-chronic liver failure. Diagnos Pathol. (2012) 7:1–9. doi: 10.1186/1746-1596-7-142
51. Stanciu LA, Bellettato Cm, Laza-Stanca V, Coyle Aj, Papi A, Johnston SL. Expression of programmed death-1 ligand (PD-L) 1, PD-L2, B7-H3, and inducible costimulator ligand on human respiratory tract epithelial cells and regulation by respiratory syncytial virus and type 1 and 2 cytokines.J Infect Dis. (2006) 193(3):404–12. doi: 10.1086/499275
52. Scilla KA, Zandberg DP, Bentzen SM, Mainor C, Heath J, Ioffe OB, et al. Case-control study of PD-1, PD-L1 and B7-H3 expression in lung cancer patients with and without human immunodeficiency virus (HIV) infection. Lung Cancer. (2018) 123:87–90. doi: 10.1016/j.lungcan.2018.06.028
53. Jin H-s, Ko M, Choi D-s, Kim JH, Lee D-h, Kang S-H, et al. CD226hiCD8+ T cells are a prerequisite for anti-TIGIT immunotherapy. Cancer Immunol Res. (2020) 8:912–25. doi: 10.1158/2326-6066.CIR-19-0877
54. Yasuma-Mitobe K, Matsuoka M. The roles of coinhibitory receptors in pathogenesis of human retroviral infections. Front Immunol. (2018) 9:2755. doi: 10.3389/fimmu.2018.02755
55. Getu AA, Tigabu A, Zhou M, Lu J, Fodstad Ø, Tan M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol Cancer. (2023) 22:43. doi: 10.1186/s12943-023-01751-9
56. Barichello T, Generoso JS, Singer M, Dal-Pizzol F. Biomarkers for sepsis: more than just fever and leukocytosis—a narrative review. Crit Care. (2022) 26:14. doi: 10.1186/s13054-021-03862-5
57. Koumprentziotis I-A, Theocharopoulos C, Foteinou D, Angeli E, Anastasopoulou A, Gogas H, et al. New emerging targets in cancer immunotherapy: the role of B7-H3. Vaccines. (2024) 12:54. doi: 10.3390/vaccines12010054
58. Song J. Biologics and biosimilars: clinical applications and biomarker testing. In: Biologics and Biosimilars. CRC Press (2022). p. 405–66. doi: 10.1201/9780429485626-25
59. Van den Bruel A, Thompson MJ, Haj-Hassan T, Stevens R, Moll H, Lakhanpaul M, et al. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. Bmj. (2011) 342. doi: 10.1136/bmj.d3082
60. Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer immunology Immunother. (2012) 61:1327–41. doi: 10.1007/s00262-012-1293-6
61. Kim NI, Park MH, Cho N, Lee JS. Comparison of the clinicopathologic features and T-cell infiltration of B7-H3 and B7-H4 expression in triple-negative breast cancer subtypes. Appl Immunohistochemistry Mol Morphology. (2022) 30:246–56. doi: 10.1097/PAI.0000000000001001
62. Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung Cancer (SCLC). J immunotherapy cancer. (2019) 7:1–9. doi: 10.1186/s40425-019-0540-1
Keywords: immunoregulatory protein, B7-H3, diagnosis, bacteria, virus, infection
Citation: Tigabu A (2024) Immunoregulatory protein B7-H3 upregulated in bacterial and viral infection and its diagnostic potential in clinical settings. Front. Immunol. 15:1472626. doi: 10.3389/fimmu.2024.1472626
Received: 29 July 2024; Accepted: 30 September 2024;
Published: 21 October 2024.
Edited by:
Daniel P. Potaczek, University of Marburg, GermanyReviewed by:
Xiaozhen Zhang, Zhejiang University, ChinaEszter Lázár-Molnár, The University of Utah, United States
Copyright © 2024 Tigabu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abiye Tigabu, YWJ0eTEyQGdtYWlsLmNvbQ==
 Abiye Tigabu
Abiye Tigabu