- 1Cellular and Molecular Diagnostics (Molecular Biology Group), ICMR-National Institute of Cancer Prevention and Research, Noida, India
- 2Symbiosis School of Biological Sciences, Symbiosis International (Deemed University) (SIU), Pune, India
- 3Department of Zoology, Meerut College, C.C.S. University, Meerut, India
- 4Laboratory Oncology Unit, Dr. BRA-IRCH, All India Institute of Medical Sciences, New Delhi, India
- 5Depatment of Life Sciences, School of Basic Sciences and Research (SBSR), Sharda University, Greater Noida, India
- 6Division of Clinical Oncology, ICMR-National Institute of Cancer Prevention and Research, Noida, India
Background: Breast cancer has the highest mortality rate among all cancers affecting females worldwide. Several new effective therapeutic strategies are being developed to minimize the number of breast cancer-related deaths and improve the quality of life of breast cancer patients. However, resistance to conventional therapies in breast cancer patients remains a challenge which could be due to several reasons, including changes in the tumor microenvironment. Attention is being diverted towards minimizing the resistance, toxicity, and improving the affordability of therapeutics for better breast cancer management. This includes personalized medicine, target-specific drug delivery systems, combinational therapies and artificial intelligence based screening and disease prediction. Nowadays, researchers and clinicians are also exploring the use of combinatorial immunotherapies in breast cancer patients, which have shown encouraging results in terms of improved survival outcomes. This study attempts to analyze the role of combinational immunotherapies in breast cancer patients, and offer insights into their effectiveness in breast cancer management.
Methodology: We conducted a systematic review and meta-analysis for which we selected the randomized clinical trials (RCTs) focused on completed Phase I/II/III/IV clinical trials investigating combination immunotherapies for breast cancer. The analysis aimed to assess the efficacy of combination therapies in comparison to mono-therapies, focusing on overall survival (OS), and progression-free survival (PFS).
Results: We observed that, combination immunotherapies significantly (P<0.05) improved OS as compared to single-drug therapies in the Phase I with overall Risk ratio (RR) of 16.17 (CI 2.23,117.50), Phase II with an overall RR of 19.19 (CI 11.76,31.30) and for phase III overall RR 22.27 (CI 13.60,36.37). In the case of PFS, it was significant with RR: 12.35 (CI 2.14, 71.26) in Phase I RR 6.10 (CI 4.31, 8.64) in phase II, RR 8.95 (CI 6.09, 13.16) in phase III and RR 14.82 (CI 6.49, 33.82) in Phase IV of clinical trials.
Conclusion: The observed improvements in overall survival and progression-free survival suggest that combination immunotherapies could serve as a better approach to breast cancer management.
1 Introduction
As per Globocan 2022, among all cancers, breast cancer is one of the leading causes of death in females (1–3), due to various confounding factors, such as age, lifestyle, use of oral contraceptives, lack of physical activities, obesity, high Body Mass Index including epigenetic changes resulting into complexities, heterogenicity, and drug resistance have necessitated the use of a wide range of immunotherapeutic drugs, targeted radiation, and chemotherapies (4–7). The advent of the genomics era has significantly revolutionized the generation of cancer therapeutics. A better understanding of cancer genetics and epigenetics is crucial for the development of effective cancer prevention strategies, precision diagnostics, and therapeutic regimens (8). Targeted drug therapies, gene therapy, and cancer vaccines are available as part of cancer treatment. However, over the time, cancer cells develop resistance to these treatments or undergo genetic changes, making them less effective and increasing the risk of mortality. Finding new strategies to overcome these challenges is the need of the hour to improve cancer treatment outcomes (9, 10). To address these challenges, attempts are being made to develop new treatment approaches, such as precision medicine, personalized therapies, and combination therapy, to enhance treatment outcomes (11, 12).
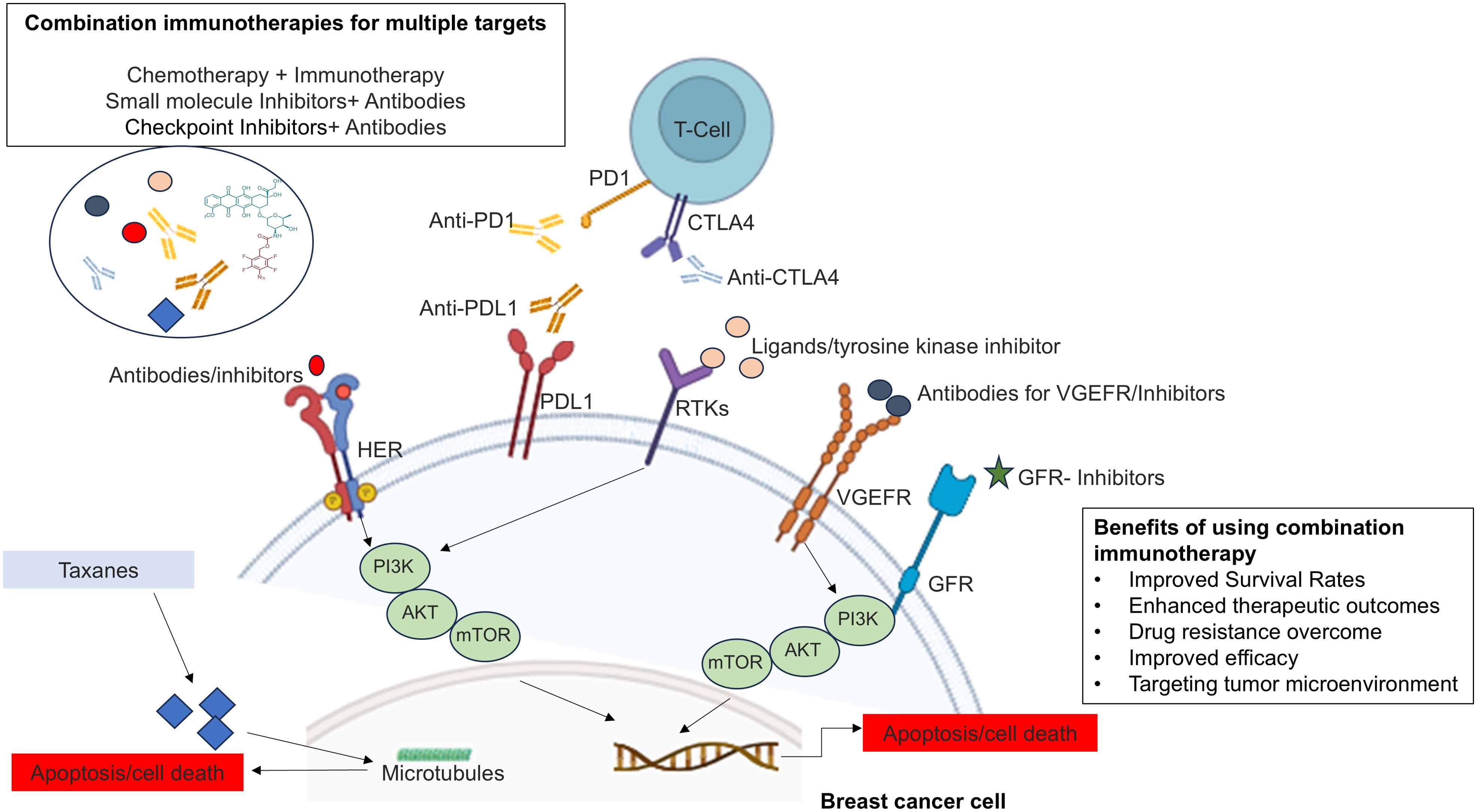
Conventional therapies for treating breast cancer patients exhibit varying response rates depending upon the stages and receptor profiles of breast cancer, as well as genetic changes in cancer cells (13, 14). These reasons highlight the complexity of cancer treatment outcomes and underscore the need for personalized and tailored approaches to improve the chances of successful responses in each patient (8). Ongoing research has led to innovative combination drug therapies, such as combination immunotherapy, where more than one molecule targets different immune response pathways or different pathways to improve the effectiveness of treatment, overcome drug resistance, and reduce the likelihood of relapse. The integration of innovative therapies with existing treatments offers a potential pathway to significantly improve survival rates and reduce the overall burden of breast cancer (15, 16). The results of combination therapies have the potential to improve treatment outcomes and offer a more comprehensive approach to manage complex diseases such as breast cancer (17–20), and may reduce the mortality rate of breast cancer (Figure 1).
Moreover, the breast tumor microenvironment (TME) in breast cancer is a critical determinant of tumor progression, metastasis, and therapy resistance. Its complex interplay of cellular and non-cellular components creates a supportive niche for tumor growth and poses significant challenges to effective treatment. Targeting the TME, in addition to the cancer cells themselves, represents a promising strategy for overcoming resistance and improving therapeutic outcomes in breast cancer (21). Literature also suggests that combination immunotherapy offers a multifaceted approach to overcome therapy resistance in the tumor microenvironment. By targeting various components of the TME—such as immune suppression, stromal interactions, hypoxia, and antigen presentation, combination therapies can enhance the effectiveness of immunotherapy and lead to more durable responses in breast cancer. This strategy not only improves the efficacy of treatment but also addresses the underlying mechanisms of resistance, potentially leading to better clinical outcomes (22).
The emergence of personalized medicine and combination therapies has become a pivotal strategy in modern cancer treatment. Personalized medicine tailors treatment to the individual characteristics of each patient, including genetic, biomarker, and phenotypic information, allowing for more precise and effective interventions. This approach is particularly important in breast cancer, where heterogeneity among patients requires targeted therapies that addresses specific tumor profiles. The integration of personalized medicine with combination therapies enhances treatment efficacy, reduces the likelihood of resistance, and improves patient outcomes by offering a more comprehensive and tailored approach to cancer management (23, 24).
Hence, to know the effectiveness and impact of combination immunotherapy, the current systematic review and meta-analysis was focused extensively on the completed clinical trials of phases I/II/III and IV in breast cancer, where immunotherapies are used in combination. The study revealed significant outcomes in terms of overall survival (OS), and progression-free survival (PFS) in combination immunotherapies. The results of this study hold the potential to improve cancer treatment and provide insights to develop new therapies, which can ultimately improve cancer patient outcomes, especially in breast cancer. This study may also open new avenues of research in combinational immunotherapies in breast cancer with different types of stages and receptor profiles, as well as other cancers that are hard to treat due to several genetic changes and drug resistance.
2 Materials and methodology
2.1 Literature search strategy
A systematic review and meta-analysis study was performed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for ensuring transparency, rigor, and consistency (Figure 2) (25, 26). The literature search was done through the database “Clinicaltrials.gov.in” and PubMed as per the PRISMA guidelines. The keywords used to identify the completed studies on “Clinicaltrials.gov.in” and PubMed were “Combination therapy”, Combinational Immunotherapy” in “breast cancer”.
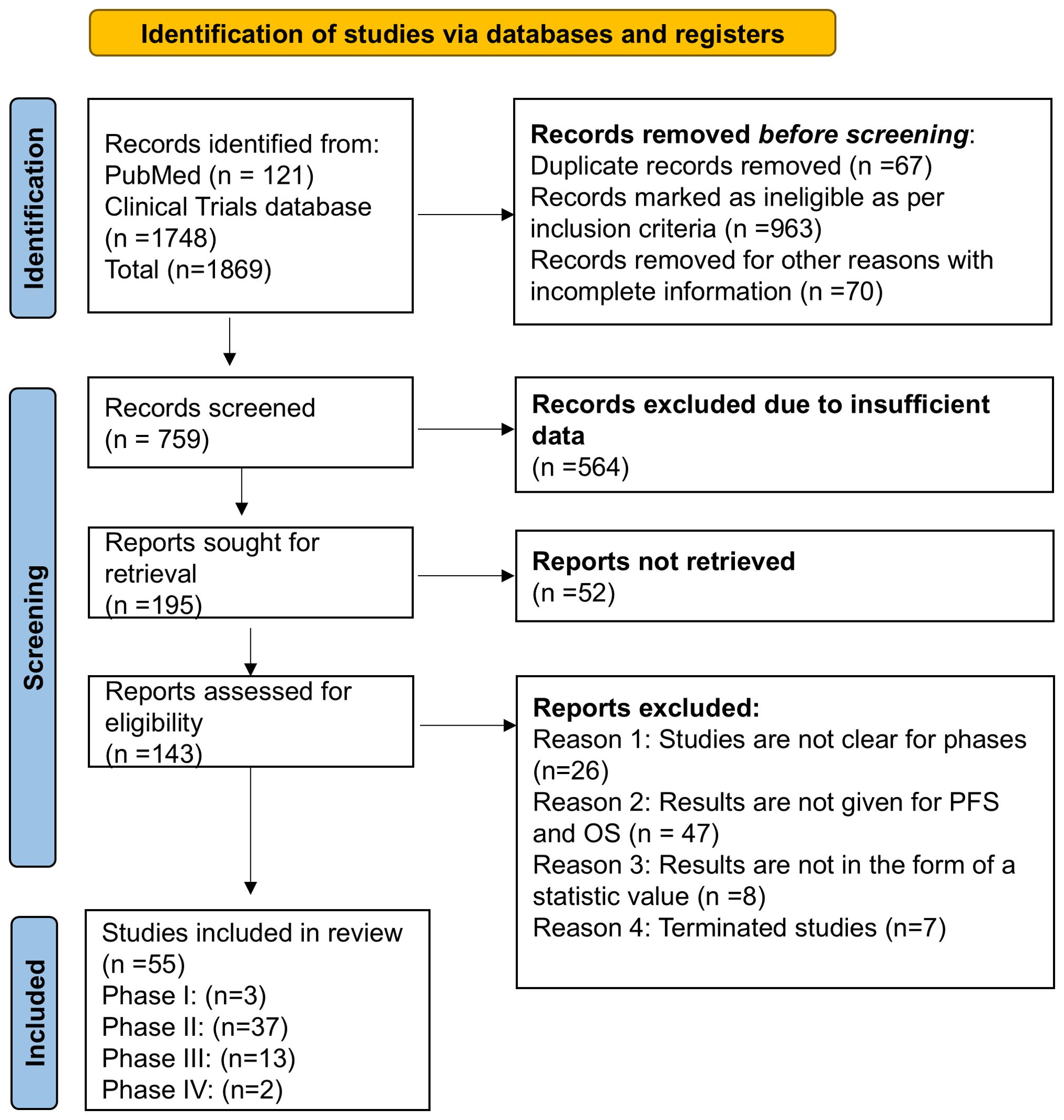
Figure 2. PRISMA flowchart for searching the clinical database and selection process for overall survival and progression-free survival in completed clinical trial phase I/II/III/IV in breast cancer.
The patients, intervention, comparison, outcome, and study design (PICOS) were followed to design the study.
a. Patients: The studies included known breast cancer patients (females only).
b. Interventions: Those studies were included that have an intervention with a drug combination with an immunotherapy drug.
c. Comparators: The included studies were focused on immunotherapy compared with combination therapy (chemotherapy/radiation/inhibitors/hormonal therapy/endocrine therapy/immunotherapy + immunotherapy).
d. Outcome Measures: Overall survival (OS) and progression-free survival (PFS).
e. Study design: Only randomized controlled trials (RCTs) were included.
2.2 Data retrieval
Screening of the studies was performed by the two authors (SS & JR) on the basis of inclusion and exclusion criteria, and their results were evaluated. A final decision was made and compared with the third author’s (VK) opinion. Only those studies that have statistical analysis for overall survival (OS) and progression-free survival (PFS) in patients treated with single immunotherapy versus a combination of immunotherapy with other molecules (two or more) were selected.
2.2.1 Inclusion criteria
Studies were included to compare the results of “patients treated with one therapy versus a combination of immunotherapies with another molecule” of “randomized control clinical trials Phase I/II/III/IV” and “completed” in breast cancer.
Additionally, only those studies that had (a) statistical median values with 95% CI intervals results of OS and PFS and (b) studies that had a combination of immunotherapies or combination of any therapy with immunotherapy were included.
2.2.2 Exclusion criteria
Studies were excluded on the basis of pre-determined exclusion criteria listed below:
a. Any duplicate study.
b. Studies other than breast cancer.
c. Results posted only for single therapy in breast cancer.
d. Terminated clinical trials studies.
e. Studies that did not have statistical median values and 95% CI intervals.
f. Studies that did not have outcomes in the form of OS and PFS.
2.2.3 Quality assessment
Quality assessment of all the included studies has been done via CONSORT questionnaire for the randomized clinical trial. All included studies hold a quality score ranging from 22 to 25, which indicates that these were of high quality for the purpose of meta-analysis (27) (Supplementary Table 1).
We also assessed the risk of bias for randomized controlled trials (RCTs) using the Cochrane Collaboration’s Risk of Bias (RoB) tool in Review Manager software (version 5.3) (https://community.cochrane.org/help/tools-and-software/revman-5). The evaluation covered seven key domains: random sequence generation (to identify selection bias), allocation concealment (to detect selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases (such as funding sources). The results of this assessment are shown in Supplementary Figure 3.
2.3 Statistical analysis
The OS and PFS of patients treated with the combination of immunotherapies (with another molecule or multiple immunotherapy) versus single immunotherapy alone were investigated with the help of statistical median value with 95% confidence intervals. The statistical data of our outcome was observed and determined through overall RR and heterogeneity (I2 statistics) in the form of percentage value. All the statistical analysis has been carried out using RevMan 5.3 software in which p<0.05 was considered significant.
3 Results
3.1 Search criteria and study selection
The initial search focused on retrieving the studies from 2013 to 2024, where 1869 studies were identified, and on the basis of inclusion and exclusion criteria, 143 were found eligible studies. After screening and sorting of studies, 55 were selected for OS and PFS in breast cancer, where phase I-03 (OS-03 and PFS-03) phase II-34,(OS-34 and PFS found in only 28 studies) phase III-14, (OS-13 and PFS found in all 14 studies) and phase IV-02 (PFS-02 and OS was not found) studies were included in the current study (Figure 2 and Table 1). Additionally, studies were excluded if the results did not have OS, PFS, and 95% confidence intervals.
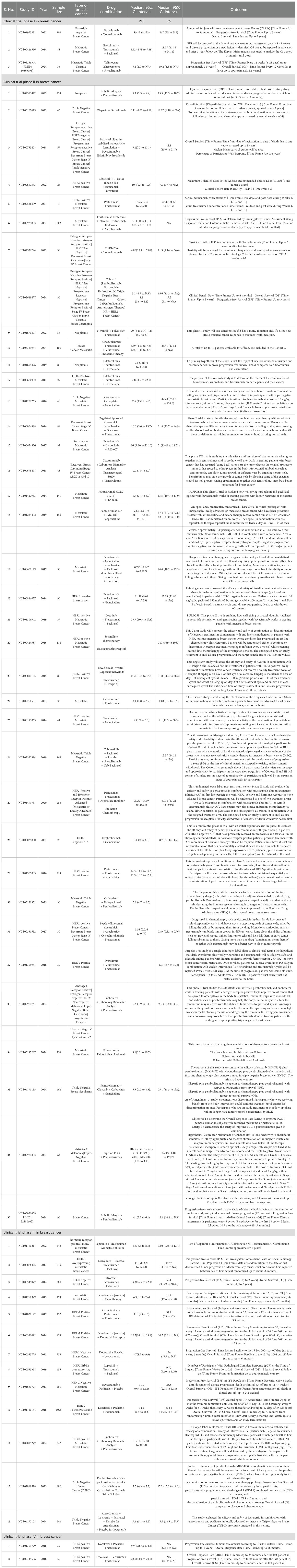
Table 1. Details of all breast cancer randomized clinical trials for combinational immunotherapies included for the analysis (Source: Clinicaltrials.gov.in and PubMed).
3.2 Analysis of breast cancer in different phase clinical trials
3.2.1 Overall survival
In the current meta-analysis, we have analyzed the overall survival (OS) in selected studies in phase I/II/III/IV RCTs where patients receiving a combination of immunotherapy or immunotherapy with other molecules exhibited a significant difference compared to those receiving one immunotherapy alone.
The meta-analysis revealed a high level of heterogeneity in overall survival with an overall Risk Ratio of 16.17 [(CI 2.23,117.50 (overall significance P< 0.0001)] for clinical trial phase 1, 19.19 [CI 11.76,31.30.00 (overall significance P<0.00001)] for phase II, and 22.27 [CI 13.64,36.37 (with overall significance P<0.00001)] for phase III with 95% CI interval (Figures 3A–C). For phase IV trials, OS data was not found in selected studies. Results of OS suggest that combination immunotherapy is highly significant in comparison to monotherapy or single immunotherapy in improving breast cancer management.
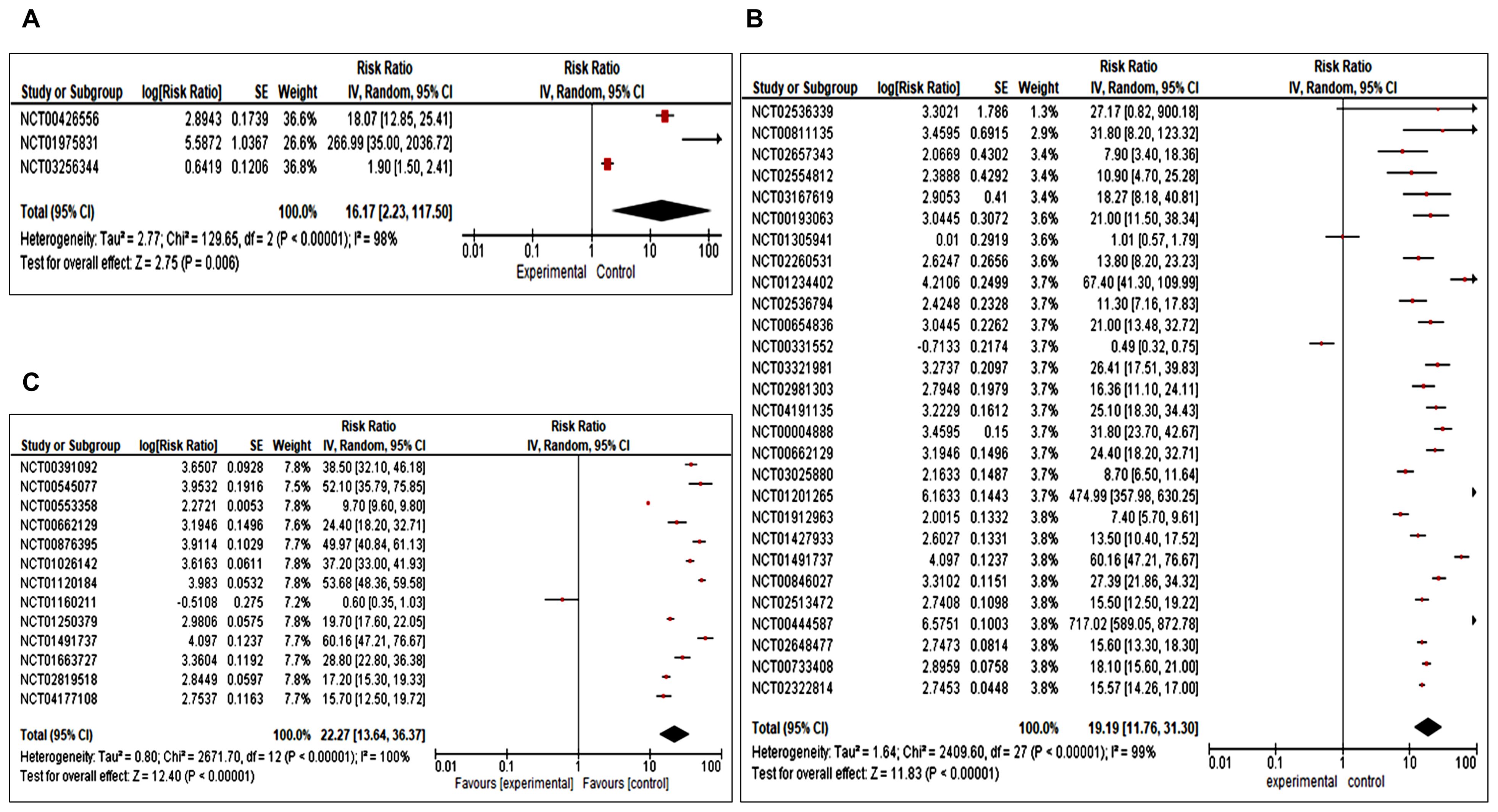
Figure 3. (A–C) Forest plot for a completed clinical trial comparing the effect of combination immunotherapies on overall survival (A) for phase I, (B) for phase II and (C) for phase III.
3.2.2 Progression-free survival
We also analyzed progression-free survival in all four phases I, II, III, and IV RCTs. We observed Risk Ratio of 12.35 [CI 2.14, 71.26 (overall significance P<0.0001) for phase I, 6.10 (CI 4.31, 8.64 (overall significance P<0.00001)] for phase II, 8.95 [CI 6.09, 13.16 (overall significance P<0.00001)] for phase III and 14.82 [CI 6.49, 33.82 (overall significance P<0.00001)] for phase IV (Figures 4A–D).
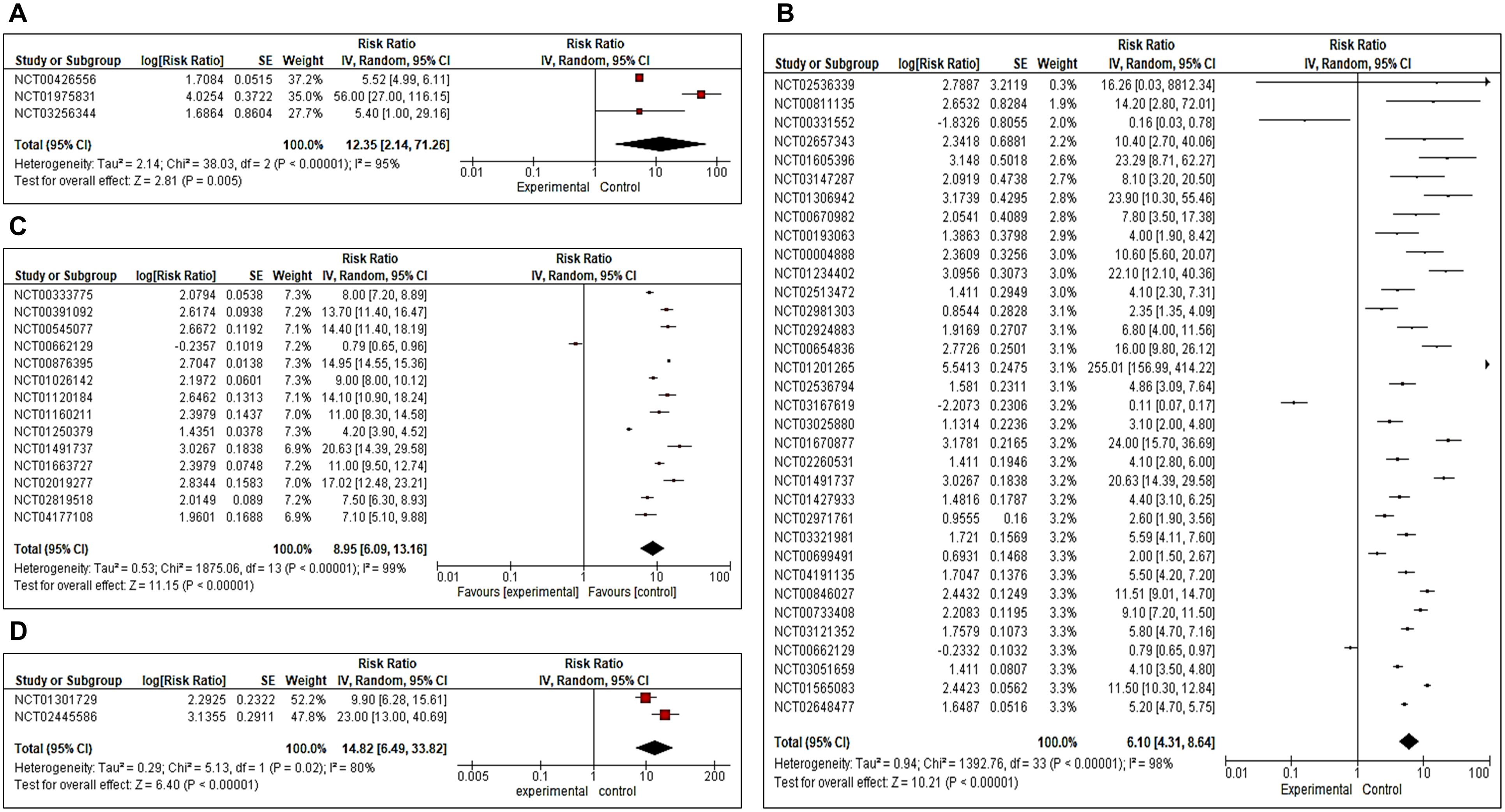
Figure 4. (A–D) Forest plot for a completed clinical trial comparing the effect of combination immunotherapies on progression-free survival (A) for phase II, (B) for phase II, (C) for phase III and (D) for Phase IV.
In addition, funnel plots of overall survival (Supplementary Figures 1A–C) and progression-free survival (Supplementary Figures 2A–D) were also analyzed to check the publication biases of the study. Apart from this, we have also analyzed the risk of bias through the Cochrane risk of Bias (RoB) tool in Review Manager software (version 5.3) and found a low risk of bias for eligible included studies (Supplementary Figure 3). Overall, the findings of the current study suggest that combination immunotherapies significantly enhance both overall survival and progression-free survival outcomes compared to single immunotherapy and better disease outcomes were observed.
4 Discussion
Combinatorial therapies have enabled healthcare professionals to address the limitations of traditional treatments by integrating multiple treatment modalities, such as chemotherapy, targeted therapies, immunotherapies, and radiation, in a coordinated manner for improved outcomes. Prior evidence has shown how hypo-fractioned radiotherapy was utilized in conjunction with immunotherapy to induce cancer cell death (28). Additionally, Bashraheel et al. found that combining targeted therapies like immune checkpoint inhibitors (ICIs), ligand-targeted therapeutics (LTT) or tumor-targeted superantigens (TTS) have more profound effects in treating cancer (8). Further, several other studies have also explored the effect of trastuzumab deruxtecan in solid tumors (29). Pegram et al. (1999) observed that combining trastuzumab with cisplatin led to significantly higher response rates compared to each agent when used individually. Similarly, another study explored the impact of the combination of everolimus and endocrine therapy among postmenopausal women grappling with endocrine-resistant HR+, HER2− breast cancer. This combination showed notable enhancements in progression-free survival (PFS) and objective response rates, in comparison to endocrine therapy alone (30). Moreover, meta-analysis studies have determined the efficacy of PD-1/PD-L1 inhibitors in clinical trials, highlighting their potential as effective immunotherapeutic agents across various cancer types, drug combinations, stages of treatment, and therapeutic schedules (31).
In order to evaluate the impact of combination immunotherapy vs single therapy, we performed a meta-analysis of the interventional studies with statistical data on survival outcomes in completed phase I/II/III/IV clinical trials in breast cancer. We focused on clinical trials that reported statistical interpretation of the trial in terms of Risk Ratio with 95% confidence intervals (CI) and observed that combination immunotherapies offered better overall survival (OS), and progression-free survival (PFS) outcomes to single immunotherapy. The studies were observed to be significant, with high heterogeneity in breast cancer (p<0.005) for OS and PFS. The strength of this study lies in the fact that it included only the completed phase I/II/III/IV clinical trials, providing a comprehensive assessment of the efficacy and specificity of the combination immunotherapies in breast cancer. This meta-analysis has provided us with evidence-based analysis of how combination immunotherapies are effective in overcoming the different challenges faced in cancer treatment, especially in breast cancer.
4.1 Limitations
Despite having 55 eligible studies for data analysis, there were limited number of studies in phase I and IV clinical trial and insufficient data for overall survival in phase IV. Additionally, data on various other survival outcome measures, such as recursion-free survival (RFS), time-to-time progression (TTP), and disease-free survival (DFS) was lacking. Further, randomized controlled trials will be necessary to validate these outcomes.
5 Conclusion and future prospects
Overall, our meta-analysis indicates that combinational immunotherapies involving two or more drugs or combining drugs with immune checkpoint inhibitors significantly increase overall survival (OS) and progression-free survival (PFS) in breast cancer as compared to single (one) immunotherapy. Notably, these findings provide valuable insights into the efficacy of combination immunotherapies, which can guide clinicians in making evidence-based decisions for improved breast cancer management. The future combination immunotherapies hold great potential, with numerous opportunities to enhance treatment efficacy, overcome drug resistance, and improve the quality of life in breast cancer patients particularly in complex and resistant cancer cases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SS: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. VK: Data curation, Formal analysis, Writing – original draft. JR: Data curation, Formal analysis, Methodology, Writing – review & editing. NM: Supervision, Writing – review & editing. SK: Writing – review & editing. AK: Writing – review & editing. MA: Writing – review & editing. S: Writing – review & editing. PS: Writing – review & editing. EG: Writing – review & editing. PT: Writing – review & editing. SH: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge the support of the Indian Council of Medical Research (ICMR), New Delhi.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1469441/full#supplementary-material
References
1. Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, Boyer TG, et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr Rev. (2022) 43:678–719. doi: 10.1210/endrev/bnab039
2. Moo TA, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET Clin. (2018) 13:339–54. doi: 10.1016/j.cpet.2018.02.006
3. Nazir SU, Kumar R, Dil A, Rasool I, Bondhopadhyay B, Singh A, et al. Differential expression of ets-1 in breast cancer among North Indian population. J Cell Biochem. (2019) 120:14552–61. doi: 10.1002/jcb.28716
4. Bates SE. Epigenetic therapies for cancer. N Engl J Med. (2020) 383:650–63. doi: 10.1056/NEJMra1805035
5. Guedan S, Ruella M, June CH. Emerging cellular therapies for cancer. Annu Rev Immunol. (2019) 37:145–71. doi: 10.1146/annurev-immunol-042718-041407
6. Halder J, Pradhan D, Kar B, Ghosh G, Rath G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomedicine. (2022) 40:102494. doi: 10.1016/j.nano.2021.102494
7. Bondhopadhyay B, Sisodiya S, Chikara A, Khan A, Tanwar P, Afroze D, et al. Cancer immunotherapy: A promising dawn in cancer research. Am J Blood Res. (2020) 10:375–85.
8. Bashraheel SS, Domling A, Goda SK. Update on targeted cancer therapies, single or in combination, and their fine tuning for precision medicine. BioMed Pharmacother. (2020) 125:110009. doi: 10.1016/j.biopha.2020.110009
9. Igarashi Y, Sasada T. Cancer vaccines: toward the next breakthrough in cancer immunotherapy. J Immunol Res. (2020) 2020:5825401. doi: 10.1155/2020/5825401
10. Sisodiya S, Kasherwal V, Khan A, Roy B, Goel A, Kumar S, et al. Liquid biopsies: emerging role and clinical applications in solid tumours. Transl Oncol. (2023) 35:101716. doi: 10.1016/j.tranon.2023.101716
11. Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. (2015) 93:52–79. doi: 10.1016/j.ejpb.2015.03.018
12. Arabi F, Mansouri V, Ahmadbeigi N. Gene therapy clinical trials, where do we go? An overview. BioMed Pharmacother. (2022) 153:113324. doi: 10.1016/j.biopha.2022.113324
13. Rezayatmand H, Razmkhah M, Razeghian-Jahromi I. Drug resistance in cancer therapy: the pandora's box of cancer stem cells. Stem Cell Res Ther. (2022) 13:181. doi: 10.1186/s13287-022-02856-6
14. Karami Fath M, Azargoonjahromi A, Kiani A, Jalalifar F, Osati P, Akbari Oryani M, et al. The role of epigenetic modifications in drug resistance and treatment of breast cancer. Cell Mol Biol Lett. (2022) 27:52. doi: 10.1186/s11658-022-00344-6
15. Plana D, Palmer AC, Sorger PK. Independent drug action in combination therapy: implications for precision oncology. Cancer Discovery. (2022) 12:606–24. doi: 10.1158/2159-8290.CD-21-0212
16. Tsvetkova D, Ivanova S. Application of approved cisplatin derivatives in combination therapy against different cancer diseases. Molecules. (2022) 27(8):2466. doi: 10.3390/molecules27082466
17. Fulgenzi CAM, D'Alessio A, Airoldi C, Scotti L, Demirtas CO, Gennari A, et al. Comparative efficacy of novel combination strategies for unresectable hepatocellular carcinoma: A network metanalysis of phase iii trials. Eur J Cancer. (2022) 174:57–67. doi: 10.1016/j.ejca.2022.06.058
18. Latif F, Bint Abdul Jabbar H, Malik H, Sadaf H, Sarfraz A, Sarfraz Z, et al. Atezolizumab and pembrolizumab in triple-negative breast cancer: A meta-analysis. Expert Rev Anticancer Ther. (2022) 22:229–35. doi: 10.1080/14737140.2022.2023011
19. Rosen VM, Guerra I, McCormack M, Nogueira-Rodrigues A, Sasse A, Munk VC, et al. Systematic review and network meta-analysis of bevacizumab plus first-line topotecan-paclitaxel or cisplatin-paclitaxel versus non-bevacizumab-containing therapies in persistent, recurrent, or metastatic cervical cancer. Int J Gynecol Cancer. (2017) 27:1237–46. doi: 10.1097/IGC.0000000000001000
20. Mannucci E, Bonifazi A, Monami M. Comparison between different types of exercise training in patients with type 2 diabetes mellitus: A systematic review and network metanalysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2021) 31:1985–92. doi: 10.1016/j.numecd.2021.02.030
21. Akinsipe T, Mohamedelhassan R, Akinpelu A, Pondugula SR, Mistriotis P, Avila LA, et al. Cellular interactions in tumor microenvironment during breast cancer progression: new frontiers and implications for novel therapeutics. Front Immunol. (2024) 15:1302587. doi: 10.3389/fimmu.2024.1302587
22. Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. (2020) 30:507–19. doi: 10.1038/s41422-020-0337-2
23. Al Meslamani AZ. The future of precision medicine in oncology. Expert Rev Precis Med Drug Dev. (2023) 8:43–7. doi: 10.1080/23808993.2023.2292988
24. Subhan MA, Parveen F, Shah H, Yalamarty SSK, Ataide JA, Torchilin VP. Recent advances with precision medicine treatment for breast cancer including triple-negative sub-type. Cancers (Basel). (2023) 15(8):2204. doi: 10.3390/cancers15082204
25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
26. Janani M, Poorkhani A, Amiriani T, Donyadideh G, Ahmadi F, Jorjanisorkhankalateh Y, et al. Association of future cancer metastases with fibroblast activation protein-A: A systematic review and meta-analysis. Front Oncol. (2024) 14. doi: 10.3389/fonc.2024.1339050
27. Falci SG, Marques LS. Consort: when and how to use it. Dental Press J Orthod. (2015) 20:13–5. doi: 10.1590/2176-9451.20.3.013-015.ebo
28. Herrera FG, Irving M, Kandalaft LE, Coukos G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. (2019) 20:e417–e33. doi: 10.1016/S1470-2045(19)30401-2
29. Indini A, Rijavec E, Grossi F. Trastuzumab deruxtecan: changing the destiny of her2 expressing solid tumors. Int J Mol Sci. (2021) 22(9):4774. doi: 10.3390/ijms22094774
30. Brufsky AM. Managing postmenopausal women with hormone receptor-positive advanced breast cancer who progress on endocrine therapies with inhibitors of the pi3k pathway. Breast J. (2014) 20:347–57. doi: 10.1111/tbj.12278
Keywords: Combinational therapy, immunotherapy, breast cancer, systematic review, meta-analysis
Citation: Sisodiya S, Kasherwal V, Rani J, Mishra N, Kumar S, Khan A, Aftab M, Shagufta, Singh P, Gupta E, Tanwar P and Hussain S (2024) Impact of combinatorial immunotherapies in breast cancer: a systematic review and meta-analysis. Front. Immunol. 15:1469441. doi: 10.3389/fimmu.2024.1469441
Received: 23 July 2024; Accepted: 16 September 2024;
Published: 16 October 2024.
Edited by:
Mohd Wajid Ali Khan, University of Hail, Saudi ArabiaReviewed by:
Priyanka S. Rana, Case Western Reserve University, United StatesLuciana Rodrigues Carvalho Barros, University of São Paulo, Brazil
Copyright © 2024 Sisodiya, Kasherwal, Rani, Mishra, Kumar, Khan, Aftab, Shagufta, Singh, Gupta, Tanwar and Hussain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Showket Hussain, c2hvd2tldC5odXNzYWluQGdvdi5pbg==
 Sandeep Sisodiya
Sandeep Sisodiya Vishakha Kasherwal
Vishakha Kasherwal Jyoti Rani1,3
Jyoti Rani1,3 Neetu Mishra
Neetu Mishra Mehreen Aftab
Mehreen Aftab Pranay Tanwar
Pranay Tanwar Showket Hussain
Showket Hussain