- Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, New York, NY, United States
Neuroendocrine neoplasms of the thymus (tNENs), including typical carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma, and small cell carcinoma, are rare tumors with scarce clinical and pathological data available in the literature. They share many common features with neuroendocrine neoplasms in other organs, such as those in the lungs, while demonstrating some distinct clinical and pathological features. This review aims to give an updated overview of each category of tNENs, focusing primarily on the pathologic diagnosis and differential diagnosis of these tumors.
Introduction
Neuroendocrine neoplasms of the thymus (tNENs) consist of a spectrum of rare neuroendocrine epithelial tumors, accounting for less than 5% of total primary tumors in the thymus and mediastinum, and about 0.4-0.9% of neuroendocrine neoplasms of all organs (1–3). Due to the rarity of the tumors, clinical and pathological data are scarce.
The current World Health Organization (WHO) classification of tNENs follows its pulmonary counterpart and separates these tumors into typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC), and small cell carcinoma (SmCC) (Table 1). Recent whole-genome sequencing data on tNENs demonstrated three distinct molecular subgroups with low, intermediate, and high copy number instability (CNI) scores, respectively. The majority of TCs with excellent clinical prognosis fell into the CNI-low subgroup and all SCCs with worse prognosis fell into the CNI-high subgroup. The ACs and LCNECs were found variably located in all three subgroups, which corresponds to the variable morphologic features and clinical prognosis of these tumors. Based on these findings, a grading system to incorporate morphology with molecular characteristics has been proposed for patient stratification and prognostication (4).
The tNEN are morphologically indistinguishable from pulmonary neuroendocrine neoplasms (pNENs) in each category (5). The differentiation from pNENs relies almost entirely on clinical information and radiological studies. Currently available epidemiology data show that around 25% of carcinoids of both thymus and lung are associated with multiple endocrine neoplasia type 1 (MEN-1) (6), but with male predominance in the thymus and female predominance in the lung (7). AC is the most common NEN in the thymus, while TC and SmCC are the dominant types in the lung. Numerous studies have shown that cigarette smoking plays a critical role in the pathogenesis of SmCC and LCNEC in the lungs, while a close relationship between smoking and tNENs has not been demonstrated.
Typical carcinoid
TC is also known as low-grade neuroendocrine tumor (NET) or grade 1 NET. About 20% of tNENs are TCs. Half of the patients with TC present with chest pain, cough, dyspnea, and other respiratory symptoms. Around 30% of the patients demonstrate paraneoplastic manifestations such as Cushing syndrome due to adrenocorticotropic hormone (ACTH)-like hormone production (8) and hypercalcemia due to parathyroid hormone-related peptide (PTHrP) production (9). Hyperparathyroidism is commonly seen in patients with MEN-1 (10). Inappropriate production of antidiuretic hormone or atrial natriuretic peptide has also been described in patients with thymic TC (11). Carcinoid syndrome is rarely seen in association with thymic TC (2, 7).
Radiologically and macroscopically, thymic TC is difficult to distinguish from other thymic neoplasms such as thymoma. They are usually unencapsulated, with well-defined borders or with invasive growth patterns. The tumor size ranges from 2 cm to 20 cm. TC with paraneoplastic manifestations such as Cushing syndrome tend to be discovered early with smaller tumor sizes. The cut surface of TC is grey-white and firm, commonly with slight gritty consistency due to calcifications (1). The cut surface of the oncocytic variant is usually tan-brown (1, 7).
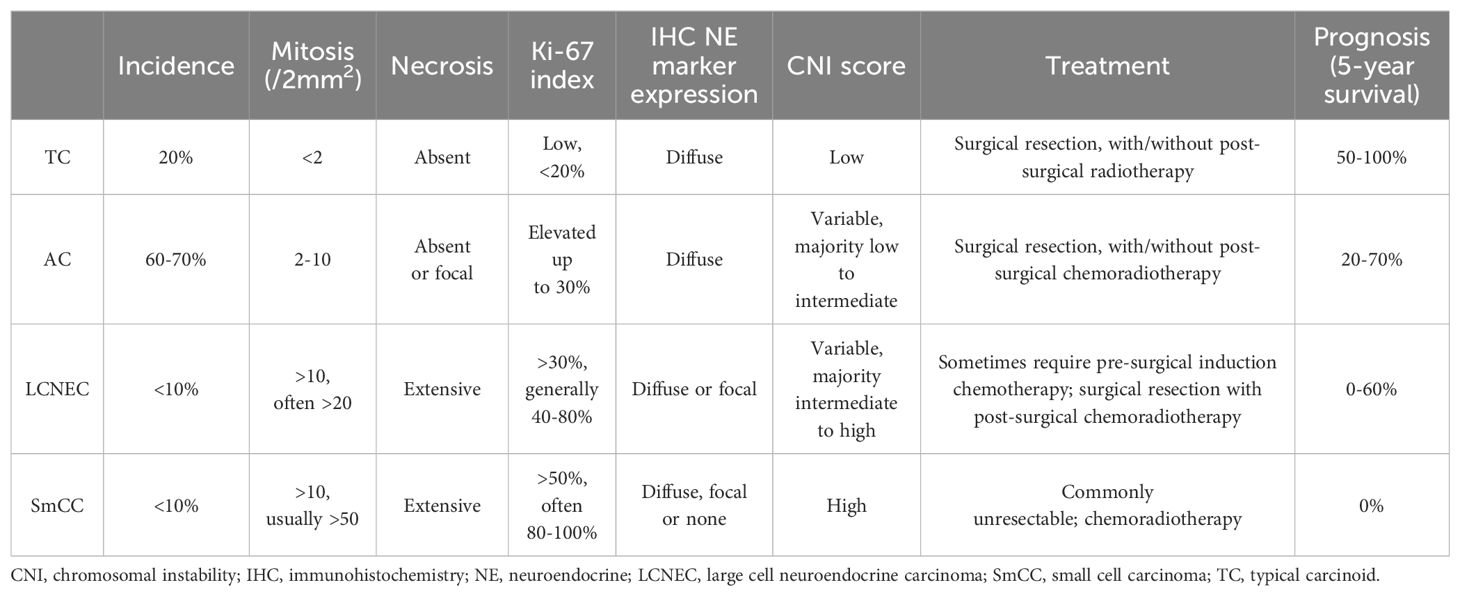
Microscopically, thymic TC consists of tumor cells with neuroendocrine cytologic features, including moderate to abundant pale eosinophilic cytoplasm and finely granular, salt-and-pepper nuclear chromatin. The tumor nuclei are round to oval with minimal pleomorphism. Mitotic activity is less than 2 mitoses/2 mm2, which corresponds to 10 high power fields (HPF) in most microscopes but varies depending upon the manufacturer and model of the microscope. No necrosis is present. Tumor cells are arranged in trabeculae, ribbons, cords, rosettes, glandular structures, or solid nests, with fine fibrous septa and rich, delicate vasculatures (Figures 1A-E). Rare variants of TC include spindle cell (12), oncocytic (1), mucinous (13), angiomatoid (14), and sarcomatous with myoid, chondroid, or osseous differentiation (15, 16). Lymphovascular invasion is common. Metastasis in regional lymph nodes is common (up to 50% of patients at presentation). Distant metastasis can also happen. Bones and lungs are the most common metastatic locations, followed by liver and other abdominal organs (1, 2).
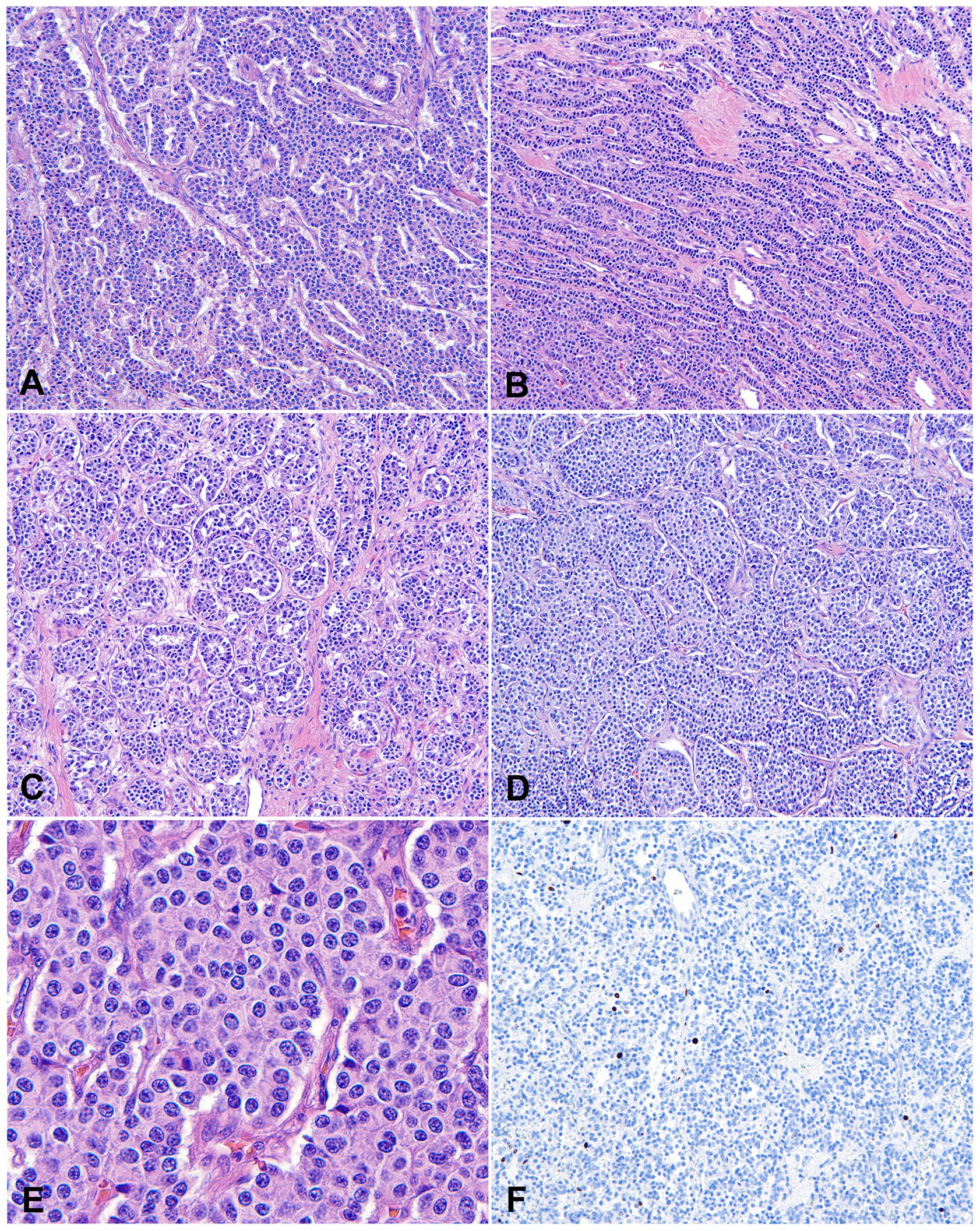
Figure 1. Typical carcinoid (TC). (A-D) Low to intermediate magnification photomicrographs of TCs show that tumor cells are arranged in trabeculae (A), cords and ribbons (B), glandular structures (C), or solid nests (D), with fine fibrous septa and rich, delicate vasculatures. Magnification (A-D): 40X. (E) High magnification view of TC comprising bland tumor cells with a moderate amount of pale eosinophilic cytoplasm, round to oval nuclei, and finely granular, salt-and-pepper chromatin. The cells are arranged in organoid solid nests separated with delicate vascular stroma. There is no mitosis or necrosis. Magnification (E): 400X. H&E stain (A-E). (F) The Ki-67 index is low (<2%).
The immunohistochemical profile of thymic TC is similar to its pulmonary counterpart. All cases of TC are diffusely positive for the common neuroendocrine markers such as synaptophysin, chromogranin A and INSM1. Most (over 80%) are positive for cytokeratins such as AE1/AE3 and Cam 5.2 (1). The Ki-67 index is generally less than 20% (Figure 1F), although it is not one of the diagnostic criteria in the current WHO classification of tNENs. In small and crushed biopsy specimens, the Ki-67 staining can help rule out high-grade NENs such as SmCC, which show a high Ki-67 index of over 50%, often 80-100%. Molecular studies show low mutation burdens with a high prevalence of mutations in chromatin remodeling and histone modification-related genes and few chromosomal aberrances in TC (2).
The most encountered differential diagnosis of thymic TC is thymic/mediastinal involvement of pulmonary TC. As mentioned in the introduction, clinical information and radiology studies are usually sufficient to tell the primary location of the tumor. Histological features of thymic TC are identical to those of pulmonary TC. A minority (around 30%) of thymic TC show focal or diffuse reactivities to PAX-8, and they are almost always negative for TTF-1. Pulmonary TC is the opposite, with most (70 -90%) of them reactive to TTF-1 but usually nonreactive to PAX-8 (17, 18). Due to the variable expression levels of TTF-1 in pulmonary TC, a negative TTF-1 test alone cannot be used to favor a thymic origin. Other differential diagnoses can be encountered in some variants of thymic TC. For example, spindle cell TC can be mistaken for type A thymoma; mucinous variant needs to be differentiated from metastatic mucinous adenocarcinoma; sarcomatous variant can mimic true sarcomas such as synovial sarcoma. Most of these differentials can be solved using a brief panel of immunohistochemical stains, for example, pancytokeratin for type A thymoma, CK20 and CDX-2 for metastatic mucinous adenocarcinoma, and TLE-1 or CD99 and SS18-SSX for synovial sarcoma.
The primary treatment choice of thymic TC is surgical resection. Postsurgical radiotherapy has also been shown to improve survival. Chemotherapy alone or postsurgical chemotherapy has not demonstrated consistent benefits on survival rates. The 5-year survival rate of thymic TC ranges from 50-100%, with a median survival of 126 months (7, 19, 20). A recent retrospective analysis of 146 patients with thymic neuroendocrine neoplasms did not identify a significant difference in disease-free survival between thymic TC and thymic AC; however, there was a clear difference between the carcinoid categories and the neuroendocrine carcinomas (21).
Atypical carcinoid
AC, also known as intermediate-grade NETs or grade 2 NETs, is the most common type of tNENs. Around 40-50% of tNENs are AC. The clinical presentations, radiological manifestations, immunoprofile, and molecular alterations of AC are similar to those of TC (22). AC differs from TC only by microscopic features and clinical prognosis. AC is defined by increased mitotic counts (2-10 mitoses/2 mm2) and/or spot necrosis (Figures 2A, B). The Ki-67 index is elevated compared to TC, oftentimes up to 30%, although it is not useful to tell AC from TC AC is also characterized by a low mutational burden, but a slight overall increase from TC. Complete surgical resection or generous incisional/excisional biopsy specimen, extensive gross sampling, and careful microscopic examination are usually required to establish the diagnosis of AC. AC usually cannot be differentiated from TC on cytology specimens or small biopsies. In such instances, the diagnosis should be rendered as carcinoid tumor, with a note detailing the mitotic counts and presence or absence of necrosis in the limited samples, stating that the differential includes TC and AC, and recommending complete surgical resection for further evaluation.
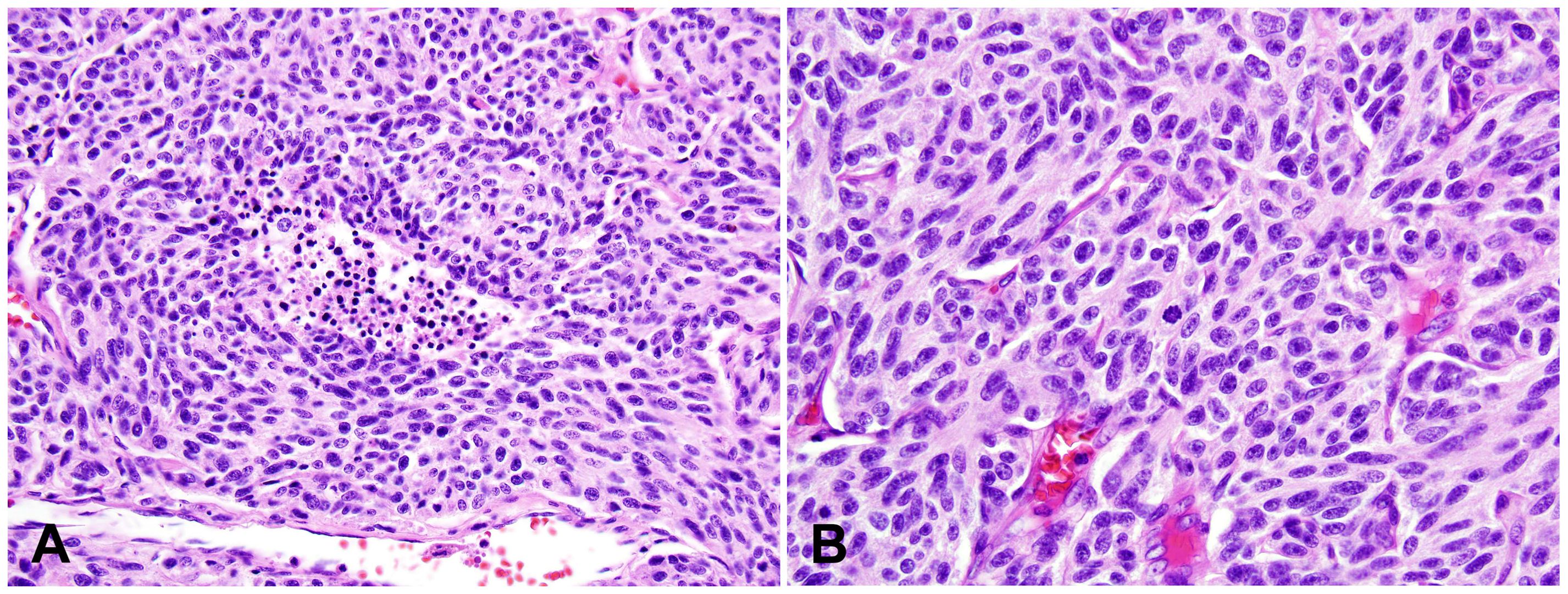
Figure 2. Atypical carcinoid (AC). (A) Intermediate magnification photomicrograph of AC with a small focus of comedo-type necrosis within the tumor nest. Magnification: 100X. (B) High magnification view of AC with a mitotic figure in the center of this photo. Tumor cells show neuroendocrine cytologic features, including a moderate amount of eosinophilic cytoplasm and finely granular nuclear chromatin. Magnification: 400X. H&E stain (A, B).
The treatment options of AC are similar to those of TC. The rates of lymph node and distant metastasis are higher than those of TC. The 5-year survival of thymic AC is variable, ranging from 20% to 87.5%, with a median survival of 59 months (1, 19, 20, 22–26).
Large cell neuroendocrine carcinoma
Thymic LCNEC is more common in males, with a median age of 57 years. Ture thymic LCNEC is rare. The indistinct diagnostic criteria may have attributed to the higher incidence rates in some reports (27–29). LCNEC is a high-grade tumor with neuroendocrine morphological features and reactivities to at least one of the neuroendocrine immunohistochemical markers (Figures 3A, B). The neuroendocrine architectures of LCNEC are often less prominent or organized compared to those of TC and AC. The reactivities to immunohistochemical markers need to be strong and diffuse in cases of LCNEC with less prominent neuroendocrine architectures, since weak and nonspecific staining of some neuroendocrine markers are not uncommon in other tumors such as adenocarcinoma and thymic carcinoma with neuroendocrine differentiation. The tumor cells show nonsmall cell cytological features, including moderate to abundant cytoplasm and pleomorphic nuclei with coarse chromatin and prominent nucleoli. High mitotic count (>10, often >20 mitoses/2 mm2), frequent apoptosis, and extensive necrosis are commonly seen. The Ki-67 index is high (>30%, generally 40-80%).
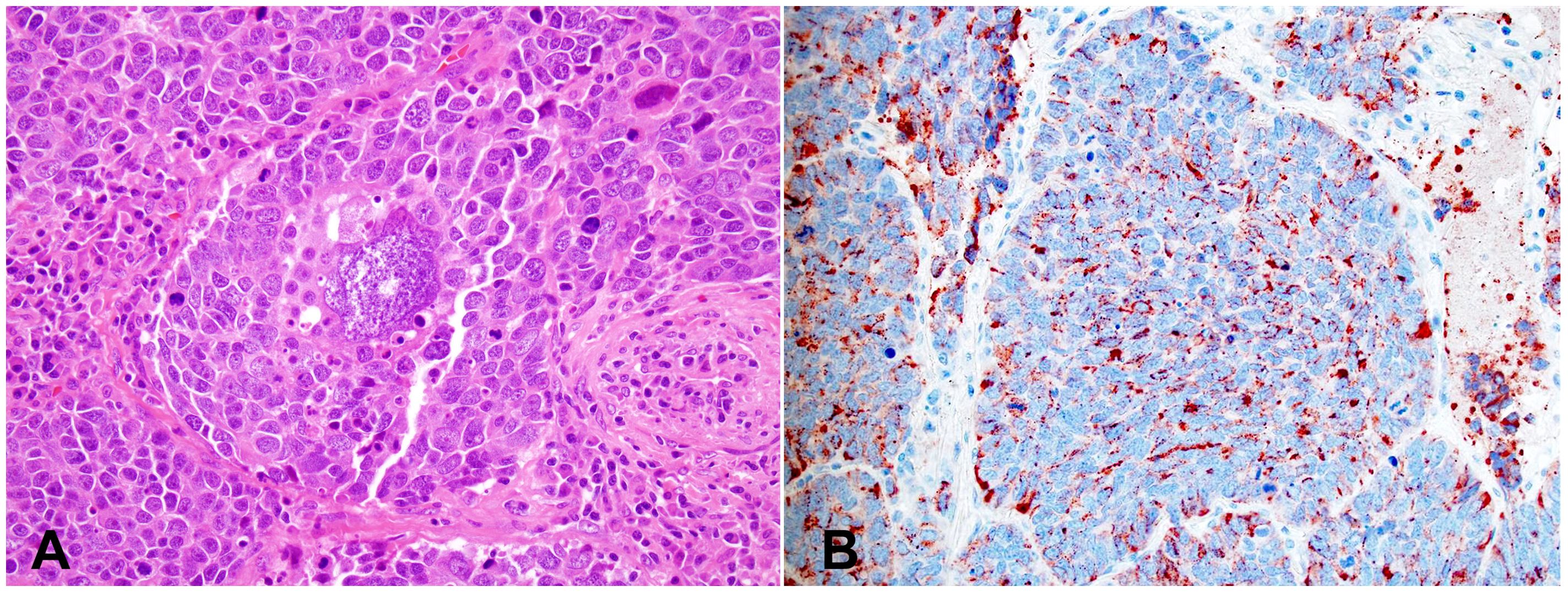
Figure 3. Large cell neuroendocrine carcinoma (LCNEC). (A) High magnification view of LCNEC comprising large pleomorphic tumor cells with a moderate amount of eosinophilic cytoplasm, coarse chromatin, and prominent nucleoli. Frequent mitoses and apoptotic bodies are seen. The tumor shows a nested growth pattern with focal peripheral palisading. H&E stain. (B) Immunohistochemical stain of Chromogranin A is diffusely positive in the tumor cells. Magnification (A, B): 400X. Photographs are courtesy of Jeffrey L. Myers, MD, University of Michigan, Ann Arbor, MI.
Under the current WHO classification, a subset of LCNEC is described as AC-like tumors with increased mitotic counts above the threshold of AC (between 11 to 20 mitoses/2mm2). These tumors show similar although slightly higher mutation burdens and chromosomal alterations compared to AC and TC. Still, these molecular profiles are significantly different from those of the high-grade NET, including LCNEC and SmCC (4, 30). This subset of tumors is predicted to behave less aggressively than the other LCNEC, resembling the concept of grade 3 NET of the pancreas (31–33). However, there are insufficient data to define this grade 3 NET category in the thymus or the lungs. Ongoing and future studies may supply evidence to separate this subset from LCNEC in the future. The current recommendation for these tumors is to add a comment to describe the AC-like morphological features and mitotic counts, and to suggest less aggressive managements than ordinary LCNEC.
The main differential diagnosis of LCNEC is thymic carcinoma with neuroendocrine differentiation. The latter does not demonstrate any neuroendocrine architecture. Immunohistochemistry can also be helpful in this differential. The thymic carcinomas with neuroendocrine differentiation usually only show focal and weak neuroendocrine immunoreactivities, and they are commonly positive for CD117, CD5, and p63. In contrast, neuroendocrine immunoreactivities in LCNEC are generally more diffuse compared to those in thymic carcinoma, although focal expression of NE markers is sufficient for diagnosing LCNEC when the NE architecture is as prominent. LCNEC can be focally positive for CD117 but generally negative for CD5 and p63 (1, 34, 35).
The prognosis of LCNEC is worse than that of TC and AC. The 5-year survival rate varies from 0% to 66% in the literature (4, 20, 35, 36).
Small cell carcinoma
SmCC is the uncommon but most aggressive form of tNENs, accounting for less than 10% of all tNENs (1, 20, 35, 37). Most patients with SmCC present with symptoms such as chest pain, weight loss, night sweating, and superior vena cava syndrome. Paraneoplastic manifestations such as Cushing syndrome and myasthenic syndrome are extremely rare (38, 39). Thymic SmCC is equally seen in males and females. The pathogenesis is unknown, and smoking has not been proved as a risk factor.
Macroscopically, SmCC is usually large in size (most >10 cm) with invasive growth. Tumor frequently invades into adjacent structures such as lung, pleura, pericardium, and large vessels. The microscopic features are like SmCC of all other organs. The size of the tumor cell is about three times the size of a resting lymphocyte. Tumor cells show a high nuclear-to-cytoplasmic ratio with nuclear molding and crushing artifacts (Figures 4A, B). The nuclei are oval with fine, granular chromatin and small, inconspicuous nucleoli. Mitotic counts are high (usually >50 mitoses/2mm2). Numerous apoptotic bodies and extensive necrosis are standard features of SmCC. Most cases of thymic SmCC stain positively for cytokeratins, typically in a peri-nuclear dot-like pattern (Figure 4C). Patchy reactivities to neuroendocrine markers such as synaptophysin and chromogranin A are common but are not required for the diagnosis. The Ki-67 index is high (commonly 80-100%, Figure 4D).
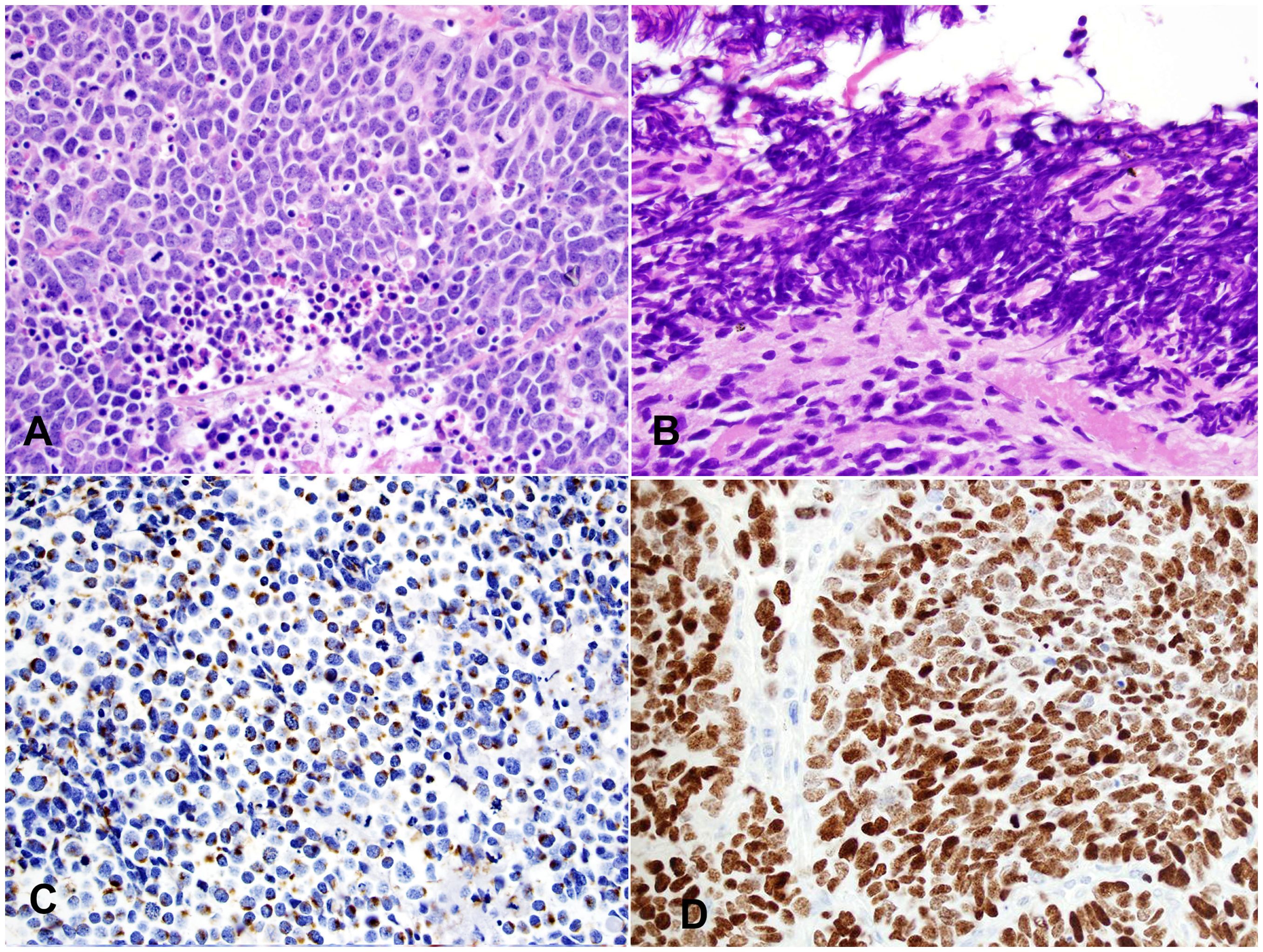
Figure 4. Small cell carcinoma (SmCC). (A) High magnification view of SmCC showing densely packed small to intermediate-sized tumor cells with scant cytoplasm, dense chromatin, and occasional inconspicuous nucleoli. Numerous mitoses and apoptotic bodies are seen. (B) One area of the tumor shows a prominent crushing artifact, a common feature of SmCC. H&E stain (A, B). (C) The tumor cells show characteristic cytoplasmic perinuclear dot-like staining for cytokeratin Cam 5.2. (D) The Ki-67 index is high (almost 100%). Magnification (A-D): 400X.
The main differential diagnosis of thymic SmCC is metastasis or direct invasion from pulmonary SmCC. Clinical information and radiographic findings are the keys to this differential. TTF-1 immunohistochemical stain is not helpful in this differential since thymic SmCC, just like SmCC from other extrapulmonary organs, can be positive for TTF-1 (40). Other differential diagnoses include mediastinal T lymphoblastic leukemia/lymphoma (TLL) and sarcomas such as Ewing’s sarcoma and rhabdomyosarcoma. Immunohistochemistry studies and fluorescence in situ hybridization (FISH) tests are helpful to exclude those differentials, e.g., CD45, TDT, and CD3 for TLL, CD99, TLE1, and EWSR1 translocation for Ewing’s sarcoma, and myogenin and myoD1 for rhabdomyosarcoma. SmCC invariably demonstrates high mutational burdens characterized by severe gene alterations such as biallelic inactivation of RB1 and TP53.
Most patients with thymic SmCC present with advanced clinical stages and are inoperable. The overall response to chemoradiotherapy is limited. The prognosis of thymic SmCC is poor. The reported 5-year survival rate is 0%, with a median survival of only 14 months (20, 35, 41–43).
Conclusion and future perspectives
All categories of tNENs have been described in other organs, although tNENs show some subtle distinctions such as the underlying risk factors (relationship to smoking) and relative frequency of each tumor type. Limited available data on tNENs have demonstrated similar clinical behavior to their respective counterparts, such as pulmonary and pancreatic NETs. It is reasonable to predict that future updates on the classification and clinical management of tNENs will follow the leads of NETs of other organs. However, many open questions on the biologic aspects of tNENs remain to be studied to better understand and treat these rare tumors.
Author contributions
PB: Data curation, Writing – original draft, Writing – review & editing. CZ: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol. (2000) 114:100–10. doi: 10.1309/3PDN-PMT5-EQTM-H0CD
2. Goto K, Kodama T, Matsuno Y, Yokose T, Asamura H, Kamiya N, et al. Clinicopathologic and DNA cytometric analysis of carcinoid tumors of the thymus. Mod Pathol. (2001) 14:985–94. doi: 10.1038/modpathol.3880423
3. Bohnenberger H, Dinter H, Konig A, Strobel P. Neuroendocrine tumors of the thymus and mediastinum. J Thorac Dis. (2017) 9:S1448–57. doi: 10.21037/jtd.2017.02.02
4. Dinter H, Bohnenberger H, Beck J, Bornemann-Kolatzki K, Schutz E, Kuffer S, et al. Molecular classification of neuroendocrine tumors of the thymus. J Thorac Oncol. (2019) 14:1472–83. doi: 10.1016/j.jtho.2019.04.015
5. Ströbel AMM P, Marom EM, Pelosi G. Thymic neuroendocrine neoplasms. In: WHO Classification of Tumours: Thoracic tumours, 5th ed. Lyon, France: International Agency for Research on Cancer (2021). Board WCoTE, ed.
6. Teh BT, McArdle J, Chan SP, Menon J, Hartley L, Pullan P, et al. Clinicopathologic studies of thymic carcinoids in multiple endocrine neoplasia type 1. Med (Baltimore). (1997) 76:21–9. doi: 10.1097/00005792-199701000-00002
7. Soga J, Yakuwa Y, Osaka M. Evaluation of 342 cases of mediastinal/thymic carcinoids collected from literature: a comparative study between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg. (1999) 5:285–92.
8. de Perrot M, Spiliopoulos A, Fischer S, Totsch M, Keshavjee S. Neuroendocrine carcinoma (carcinoid) of the thymus associated with Cushing's syndrome. Ann Thorac Surg. (2002) 73:675–81. doi: 10.1016/s0003-4975(01)02713-8
9. Yoshikawa T, Noguchi Y, Matsukawa H, Kondo J, Matsumoto A, Nakatani Y, et al. Thymus carcinoid producing parathyroid hormone (PTH)-related protein: report of a case. Surg Today. (1994) 24:544–7. doi: 10.1007/BF01884576
10. Teh BT, Zedenius J, Kytola S, Skogseid B, Trotter J, Choplin H, et al. Thymic carcinoids in multiple endocrine neoplasia type 1. Ann Surg. (1998) 228:99–105. doi: 10.1097/00000658-199807000-00015
11. Okada S, Ohshima K, Mori M. The Cushing syndrome induced by atrial natriuretic peptide-producing thymic carcinoid. Ann Intern Med. (1994) 121:75–6. doi: 10.7326/0003-4819-121-1-199407010-00025
12. Levine GD, Rosai J. A spindle cell varient of thymic carcinoid tumor. A clinical, histologic, and fine structural study with emphasis on its distinction from spindle cell thymoma. Arch Pathol Lab Med. (1976) 100:293–300.
13. Ohchi T, Tanaka H, Shibuya Y, Shibusa T, Inuzuka M, Satoh M, et al. Thymic carcinoid with mucinous stroma: a case report. Respir Med. (1998) 92:880–2. doi: 10.1016/s0954-6111(98)90394-8
14. Moran CA, Suster S. Angiomatoid neuroendocrine carcinoma of the thymus: report of a distinctive morphological variant of neuroendocrine tumor of the thymus resembling a vascular neoplasm. Hum Pathol. (1999) 30:635–9. doi: 10.1016/s0046-8177(99)90087-4
15. Kuo TT. Carcinoid tumor of the thymus with divergent sarcomatoid differentiation: report of a case with histogenetic consideration. Hum Pathol. (1994) 25:319–23. doi: 10.1016/0046-8177(94)90205-4
16. Paties C, Zangrandi A, Vassallo G, Rindi G, Solcia E. Multidirectional carcinoma of the thymus with neuroendocrine and sarcomatoid components and carcinoid syndrome. Pathol Res Pract. (1991) 187:170–7. doi: 10.1016/S0344-0338(11)80767-3
17. Weissferdt A, Tang X, Wistuba II, Moran CA. Comparative immunohistochemical analysis of pulmonary and thymic neuroendocrine carcinomas using PAX8 and TTF-1. Mod Pathol. (2013) 26:1554–60. doi: 10.1038/modpathol.2013.111
18. Oliveira AM, Tazelaar HD, Myers JL, Erickson LA, Lloyd RV. Thyroid transcription factor-1 distinguishes metastatic pulmonary from well-differentiated neuroendocrine tumors of other sites. Am J Surg Pathol. (2001) 25:815–9. doi: 10.1097/00000478-200106000-00015
19. Crona J, Bjorklund P, Welin S, Kozlovacki G, Oberg K, Granberg D. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. a study from a single tertiary referral centre. Lung Cancer. (2013) 79:289–93. doi: 10.1016/j.lungcan.2012.12.001
20. Strobel P, Zettl A, Shilo K, Chuang W, Nicholson AG, Matsuno Y, et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: a multi-institutional clinicopathologic study. Genes Chromosomes Cancer. (2014) 53:738–49. doi: 10.1002/gcc.22183
21. Fang W, Filosso PL, Roden AC, Gu Z, Liu Y, Agzarian J, et al. Clinicopathological features and current treatment outcomes of neuroendocrine thymic tumours. Eur J Cardiothorac Surg. (2021) 59:1004–13. doi: 10.1093/ejcts/ezaa453
22. Valli M, Fabris GA, Dewar A, Chikte S, Fisher C, Corrin B, et al. Atypical carcinoid tumour of the thymus: a study of eight cases. Histopathology. (1994) 24:371–5. doi: 10.1111/j.1365-2559.1994.tb00539.x
23. Filosso PL, Yao X, Ahmad U, Zhan Y, Huang J, Ruffini E, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg. (2015) 149:103–9.e2. doi: 10.1016/j.jtcvs.2014.08.061
24. Midorikawa K, Miyahara S, Nishino N, Ueda Y, Waseda R, Shiraishi T, et al. Analysis of 25 surgical cases of thymic neuroendocrine tumors and thymic carcinoma. J Cardiothorac Surg. (2024) 19:225. doi: 10.1186/s13019-024-02723-w
25. Chen Y, Zhang J, Zhou M, Guo C, Li S. Real-world clinicopathological features and outcome of thymic neuroendocrine tumors: a retrospective single-institution analysis. Orphanet J Rare Dis. (2022) 17:215. doi: 10.1186/s13023-022-02366-x
26. Huang C, Sun YG, Wu QJ, Ma C, Jiao P, Wang Y, et al. Surgical treatment of intermediate to high grade thymic neuroendocrine neoplasms: case series of five patients and literature review. Transl Cancer Res. (2022) 11:3535–47. doi: 10.21037/tcr-22-1150
27. Shoji T, Fushimi H, Takeda S, Tanio Y. Thymic large-cell neuroendocrine carcinoma: a disease neglected in the ESMO guideline? Ann Oncol. (2011) 22:2535. doi: 10.1093/annonc/mdr415
28. Ahn S, Lee JJ, Ha SY, Sung CO, Kim J, Han J. Clinicopathological analysis of 21 thymic neuroendocrine tumors. Korean J Pathol. (2012) 46:221–5. doi: 10.4132/KoreanJPathol.2012.46.3.221
29. Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg. (2010) 251:1117–21. doi: 10.1097/SLA.0b013e3181dd4ec4
30. Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res. (2016) 22:3618–29. doi: 10.1158/1078-0432.CCR-15-2946
31. Marchio C, Gatti G, Massa F, Bertero L, Filosso P, Pelosi G, et al. Distinctive pathological and clinical features of lung carcinoids with high proliferation index. Virchows Arch. (2017) 471:713–20. doi: 10.1007/s00428-017-2177-0
32. Quinn AM, Chaturvedi A, Nonaka D. High-grade neuroendocrine carcinoma of the lung with carcinoid morphology: A study of 12 cases. Am J Surg Pathol. (2017) 41:263–70. doi: 10.1097/PAS.0000000000000767
33. Kasajima A, Konukiewitz B, Oka N, Suzuki H, Sakurada A, Okada Y, et al. Clinicopathological profiling of lung carcinoids with a Ki67 index > 20. Neuroendocrinology. (2019) 108:109–20. doi: 10.1159/000495806
34. Chetty R, Batitang S, Govender D. Large cell neuroendocrine carcinoma of the thymus. Histopathology. (1997) 31:274–6. doi: 10.1046/j.1365-2559.1997.2380849.x
35. Tiffet O, Nicholson AG, Ladas G, Sheppard MN, Goldstraw P. A clinicopathologic study of 12 neuroendocrine tumors arising in the thymus. Chest. (2003) 124:141–6. doi: 10.1378/chest.124.1.141
36. Cardillo G, Rea F, Lucchi M, Paul MA, Margaritora S, Carleo F, et al. Primary neuroendocrine tumors of the thymus: a multicenter experience of 35 patients. Ann Thorac Surg. (2012) 94:241–5; discussion 245-6. doi: 10.1016/j.athoracsur.2012.03.062
37. Gal AA, Kornstein MJ, Cohen C, Duarte IG, Miller JI, Mansour KA. Neuroendocrine tumors of the thymus: a clinicopathological and prognostic study. Ann Thorac Surg. (2001) 72:1179–82. doi: 10.1016/s0003-4975(01)03032-6
38. Hashimoto S, Hayasaka K, Suzuki K, Endoh M, Yanagawa N, Shiono S. Thymic small cell carcinoma associated with lambert-eaton myasthenic syndrome. Ann Thorac Surg. (2020) 109:e347–8. doi: 10.1016/j.athoracsur.2019.08.080
39. Hekimgil M, Hamulu F, Cagirici U, Karabulut B, Ozgen AG, Soyda S, et al. Small cell neuroendocrine carcinoma of the thymus complicated by Cushing's syndrome. Report of a 58-year-old woman with a 3-year history of hypertension. Pathol Res Pract. (2001) 197:129–33. doi: 10.1078/0344-0338-00023
40. Bi Y, Deng Y, Li S, Zhou X, Chen Y, Ma D, et al. Immunophenotypic and prognostic analysis of PAX8 and TTF-1 expressions in neuroendocrine carcinomas of thymic origin: A comparative study with their pulmonary counterparts. J Surg Oncol. (2016) 114:697–702. doi: 10.1002/jso.24393
41. Moran CA, Suster S. Thymic neuroendocrine carcinomas with combined features ranging from well-differentiated (carcinoid) to small cell carcinoma. A clinicopathologic and immunohistochemical study of 11 cases. Am J Clin Pathol. (2000) 113:345–50. doi: 10.1309/Q01U-60BL-VEV4-TWR1
42. Kuo TT, Chang JP, Lin FJ, Wu WC, Chang CH. Thymic carcinomas: histopathological varieties and immunohistochemical study. Am J Surg Pathol. (1990) 14:24–34. doi: 10.1097/00000478-199001000-00003
Keywords: neuroendocrine neoplasm, thymus, carcinoid, large cell neuroendocrine carcinoma, small cell carcinoma
Citation: Barone PD and Zhang C (2024) Neuroendocrine neoplasms of the thymus. Front. Immunol. 15:1465775. doi: 10.3389/fimmu.2024.1465775
Received: 16 July 2024; Accepted: 14 August 2024;
Published: 29 August 2024.
Edited by:
Yong Fan, Allegheny Health Network, United StatesReviewed by:
Liang Lu, Dartmouth Hitchcock Medical Center, United StatesHaodong Xu, Wake Forest University School of Medicine, United States
Jian Jing, University of Colorado Anschutz Medical Campus, United States
Copyright © 2024 Barone and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Zhang, ZmpyOTAwN0BtZWQuY29ybmVsbC5lZHU=
 Paul D. Barone
Paul D. Barone Chen Zhang
Chen Zhang